Abstract
Purpose:
Neoadjuvant anti–PD-1 therapy has shown promise for resectable non–small cell lung cancer (NSCLC). We reported the first phase I/II trial of neoadjuvant nivolumab in resectable NSCLC, finding it to be safe and feasible with encouraging major pathological responses (MPR). We now present 5-year clinical outcomes from this trial, representing to our knowledge, the longest follow-up data for neoadjuvant anti–PD-1 in any cancer type.
Patients and Methods:
Two doses of nivolumab (3 mg/kg) were administered for 4 weeks before surgery to 21 patients with Stage I–IIIA NSCLC. 5-year recurrence-free survival (RFS), overall survival (OS), and associations with MPR and PD-L1, were evaluated.
Results:
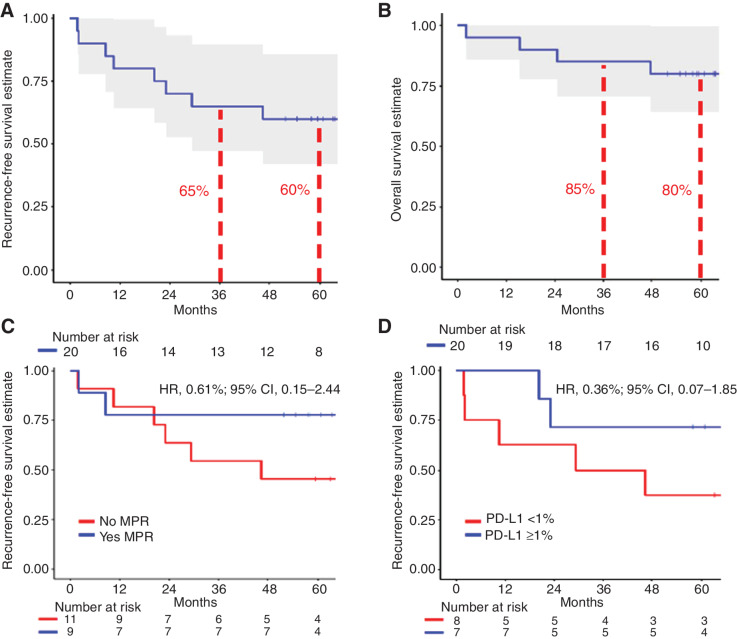
With a median follow-up of 63 months, 5-year RFS and OS rates were 60% and 80%, respectively. The presence of MPR and pre-treatment tumor PD-L1 positivity (TPS ≥1%) each trended toward favorable RFS; HR, 0.61 [95% confidence interval (CI), 0.15–2.44] and HR, 0.36 (95% CI, 0.07–1.85), respectively. At 5-year follow-up, 8 of 9 (89%) patients with MPR were alive and disease-free. There were no cancer-related deaths among patients with MPR. In contrast, 6/11 patients without MPR experienced tumor relapse, and 3 died.
Conclusions:
Five-year clinical outcomes for neoadjuvant nivolumab in resectable NSCLC compare favorably with historical outcomes. MPR and PD-L1 positivity trended toward improved RFS, though definitive conclusions are limited by cohort size.
Translational Relevance.
Neoadjuvant immune checkpoint blockade is increasingly being incorporated into the perioperative treatment setting for solid tumor malignancies, including resectable non–small cell lung cancer (NSCLC). However, long-term outcomes, specifically survival, after treatment with these agents are still maturing. In this report, we present the five-year clinical outcomes after neoadjuvant nivolumab for resectable NSCLC, representing, to our knowledge, the longest follow-up data available after neoadjuvant anti–PD-1 therapy in any cancer type. In addition to the durable clinical benefit of neoadjuvant nivolumab highlighted in this report, the examination of key subgroups and biomarkers may help clinicians and investigators as they look to navigate this rapidly evolving treatment landscape. Several questions still remain on how to further optimize perioperative outcomes for patients with resectable NSCLC, making long-term follow-up data, as reported here, particularly valuable.
Introduction
Lung cancer is the leading cause of cancer-related death worldwide (1). For patients with resectable disease, 5-year survival rates can range from 68%, for stage IB, to 36% for IIIA disease (2). Despite advances in treatments for advanced non–small cell lung cancer (NSCLC), breakthroughs have only recently emerged for early-stage disease (3–5). Results and overall survival (OS) from studies evaluating immune checkpoint blockade (ICB) in the peri-operative setting are still maturing.
Our group published the first clinical trial of neoadjuvant anti-programmed-cell death protein-1 (PD-1) therapy in any cancer type, finding it to be safe and feasible (6), with informative immunologic correlative follow-up data (7). In the current report, we present final clinical results from this study, representing, to our knowledge, the longest follow-up data for neoadjuvant anti–PD-1 therapy. In addition to long-term clinical outcomes, we present data on key subgroups, providing insight to researchers and clinicians navigating this rapidly evolving treatment setting.
Patients and Methods
Patient selection and study design
This open-label single-arm phase Ib/II study (NCT02259621) was conducted at Johns Hopkins University and Memorial Sloan-Kettering Cancer Center. Patients, ages ≥18 years, with resectable stage I (>4 cm)–IIIA NSCLC, were eligible. Staging was per American Joint Committee on Cancer 7th edition (8). Additional eligibility criteria have been reported (6).
Enrolled patients received two pre-operative doses of intravenous nivolumab (3 mg/kg of body weight) every 2 weeks. Surgical resection was planned approximately 4 weeks after the first dose.
This clinical trial and research study were conducted in accordance with the U.S. Common rule. Informed written consent was obtained from each subject or each subject’s guardian prior to clinical trial enrollment. All human investigations were performed after approval by an institutional review board in accordance with an assurance filed with and approved by the U.S. Department of Health and Human Services.
Study endpoints and biomarkers
Primary endpoints of this study were safety and feasibility both having been previously reported (6). This analysis is a description of five-year outcomes for all patents who successfully underwent definitive resection of their disease after neoadjuvant nivolumab. Key exploratory endpoints included pathological markers of response and assessments of recurrence-free survival (RFS) and OS, measured from date of surgery. Pathological response was measured as the percentage of residual viable tumor (%RVT) identified on routine hematoxylin and eosin staining (9). Pathological complete response (pCR) and major pathological response (MPR) were defined as 0% and ≤10% residual viable tumor, respectively. IHC was performed for pre-treatment tumor PD-L1 evaluation. PD-L1 staining was performed on formalin-fixed, paraffin-embedded (FFPE) tissue sections using the Dako PD-L1 IHC 28–8 pharmDx assay. Samples were considered to be PD-L1+ if ≥1% of tumor cells showed membranous PD-L1 expression.
For the 11 patients with tumor samples available for sequencing analysis, correlations between mean tumor mutational burden (TMB) and RFS/OS were assessed. Whole-exome sequencing was performed on pre-treatment tumor and matched normal samples. FFPE tumor samples underwent pathological review for confirmation of diagnosis and tumor purity assessment. Qiagen DNA FFPE and Qiagen DNA blood mini kit was used to extract DNA from patients’ tumors and matched peripheral blood, respectively. VariantDx software was used to identify somatic mutations in matched tumor and normal samples.
Statistical analysis
OS, RFS, and median follow-up are reported using the Kaplan–Meier and reverse Kaplan–Meier methods, respectively. Comparisons were made using the Cox proportional hazards regression model. Proportions are reported with exact 95% binomial confidence intervals (95% CI). Binomial probabilities are compared with Fisher's exact tests and reported with exact binomial 95% CIs. All P values reported are two-sided. Statistical analyses were performed using R version 4.1.0.
Data availability
The data generated in this study are available upon request from the corresponding author.
Results
Pathological, clinical, and safety outcomes
Twenty-one eligible patients were enrolled and planned for resection after receiving neoadjuvant nivolumab (Table 1), with one patient subsequently deemed inoperable. For the 20 patients who underwent definitive resection, data are reported here. With a median follow-up of 63 months, 5-year RFS and OS rates were 60% and 80%, respectively (Fig. 1A and B). The one patient deemed inoperable did not undergo resection due to primary progression of their disease and subsequently passed away from their cancer within 9 months of study enrollment.
Table 1.
Demographic data for all enrolled patients (n = 21) at baseline and based on pathological response (n = 20).
| Characteristic | All Patients (N = 21) | Patients with MPR (N = 9) | Patients without MPR (N = 11) |
|---|---|---|---|
| Median age at enrollment, years (range) | 67 (55–84) | 66 (57–79) | 67 (55–84) |
| Sex, number (%) | |||
| Female | 11 (52) | 6 (67) | 4 (36) |
| Male | 10 (48) | 3 (33) | 7 (64) |
| Histology, number (%) | |||
| Adenocarcinoma | 13 (62) | 6 (67) | 6 (55) |
| Squamous cell carcinoma | 6 (29) | 2 (22) | 4 (36) |
| Othera | 2 (10) | 1 (11) | 1 (9) |
| Clinical stage at diagnosisb, number (%) | |||
| I | 4 (19) | 2 (22) | 2 (18) |
| II | 10 (48) | 5 (56) | 5 (45) |
| IIIA | 7 (33) | 2 (22) | 4 (36) |
| Smoking status, number (%) | |||
| Never | 3 (14) | 1 (11) | 2 (18) |
| Former or Current | 18 (86) | 8 (89) | 9 (82) |
| PD-L1 statusc (%) | |||
| ≥1% | 7 (33) | 3 (33) | 4 (36) |
| <1% | 8 (38) | 2 (22) | 6 (55) |
| N/A | 6 (29) | 4 (44) | 1 (9) |
| Adjuvant Treatment (%) | |||
| Yes | 6 (29) | 2 (22) | 4 (36) |
| No | 15 (71) | 7 (78) | 7 (64) |
Note: One patient was deemed unresectable at time of surgery, without evaluable pathological response.
Abbreviations: MPR, Major pathological response; %, Percentage; PD-L1, Programmed death-ligand 1; N/A, Not available.
aOther histologic diagnoses included pleomorphic and adenosquamous carcinomas.
bClinical staging was per American Joint Committee on Cancer Tumor Node Metastases 7th edition.
cOn the basis of pre-treatment tumor PD-L1 expression (TPS < 1% vs. ≥1%). There were 5 patients where pre-treatment PD-L1 assessment was not available (N/A).
Figure 1.
A–D, Kaplan–Meier curves depicting the recurrence-free survival (A) and overall survival (B) for patients who underwent definitive resection after receiving neoadjuvant nivolumab. C, Depicts the recurrence-free survival for patients with or without major pathological response after neoadjuvant nivolumab. D, Shows the recurrence-free survival stratified by pre-treatment tumor PD-L1 expression. The dashed lines represent the 95% confidence intervals for each KM-curve. Abbreviations: Major pathological response, MPR; Programmed death-ligand 1, PD-L1; Hazard ratio, HR; Confidence Interval, CI.
As previously reported (6), rates of MPR and pCR were 45% and 10%, respectively. At 5-year follow-up, 8/9 (89%) patients with an MPR were alive and cancer-free. There was one death at 2 months in a patient with MPR without cancer recurrence, secondary to a traumatic head injury.
Six patients received standard-of-care adjuvant therapy (Table 1), as allowed per study protocol. Adjuvant therapy consisted of three to four cycles of cisplatin-based chemotherapy. Neither post-operative radiotherapy or targeted therapy was used as part of adjuvant treatment for this cohort.
Neoadjuvant nivolumab was associated with few side effects and did not lead to surgical delays, as had previously been reported (6). One late-onset grade 3 dermatologic immune-related adverse event (irAE) occurred 16 months after the patient's last dose of nivolumab. This event consisted of dermatitis herpetiformis with accompanying alopecia universalis, and was successfully managed with immunosuppression. No other late-onset irAEs have occurred.
Clinicopathologic subgroup analysis
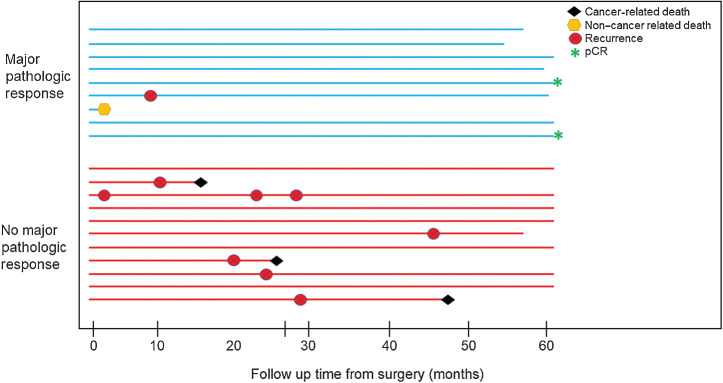
At the time of analysis, the hazard ratio (HR) for presence of MPR was in the direction of improved RFS (HR, 0.61; 95% CI, 0.15–2.44; Fig. 1C). Pre-treatment tumor PD-L1 positivity (TPS ≥1%) trended toward improved RFS (HR, 0.36; 95% CI, 0.07–1.85; Fig. 1D). Figure 2 illustrates recurrence and survival events between patients with or without MPR. The majority of recurrences, 6/7 (86%), occurred in patients without an MPR after neoadjuvant therapy. Among 6 patients with recurrence and available pre-treatment tumor PD-L1 assessment, 4 had PD-L1–negative disease (Supplementary Fig. S1). For the two patients with pCR, both remain alive and disease-free at five-year follow-up.
Figure 2.
Swimmer plot summarizing follow-up and recurrence patterns for patients with or without a major pathological response after neoadjuvant nivolumab. Follow-up is limited to 60 months. Abbreviation: Pathological complete response, pCR.
Patients with stage I/II disease had numerically favorable RFS compared with those with stage IIIA disease (HR, 0.42; 95% CI, 0.11–1.62; Supplementary Fig. S2A). To evaluate associations between degree of pathologic response and long-term clinical outcomes, we used an alternative cutoff value of 50% RVT, also referred to as partial pathologic response (10, 11), which showed a favorable association with RFS (HR, 0.36; 95% CI, 0.09–1.51; Supplementary Fig. S2B). Assessed as a continuous variable, increasing %RVT trended toward increasing risk for recurrence (HR, 2.9; 95% CI, 0.51–16.57).
Examining mean TMB, when assessed as a continuous variable, was not associated with improved RFS or OS (HR, 1.0; 95% CI, 0.99–1.01; HR, 1.01; 95% CI, 1.00–1.02, respectively).
Patterns of recurrence
In total, there were seven tumor recurrences within the observation period (Table 2). Four recurrence events (57%) occurred greater than 1 year after surgery. Intrathoracic recurrences occurred in 3/7 (43%) patients. Among these seven patients, four remain alive after treatment for NSCLC recurrence, among whom three received successful definitive local treatment for metachronous oligometastatic disease.
Table 2.
Summary of clinicopathologic features of 7 NSCLC recurrences among 20 patients treated with neoadjuvant nivolumab and surgical resection.
| AJCC stage | Histology | PD-L1 (%)a | Notable Mutations | %RVT | Adjuvant chemotherapy (Y/N) | RFS duration (mo) | Intrathoracic vs. Distant Recurrence (IT/D) | Additional Treatment | Alive (Y/N) |
|---|---|---|---|---|---|---|---|---|---|
| IA | Adenocarcinoma | 0 | — | 100 | N | 46.5 | IT | SBRT | Y |
| IIA | Adenocarcinoma | 0 | Kras G12c, STK11 | 75 | Y | 1.8 | D | Resection + SRS | Y |
| IIB | Adenocarcinoma | 0 | F11R-NRG1 fusion | 100 | N | 29.3 | IT | Carboplatin-pemetrexed | N |
| IIIA | Adenocarcinoma | N/A | — | 5 | N | 8.5 | IT | ChemoRT | Y |
| IIIA | Adenocarcinoma | 60 | Ros1 | 95 | N | 23.1 | D | Crizotinib/lorlatinib | Y |
| IIIA | Squamous | 0 | TP53 | 80 | N | 10.4 | D | Palliative XRT | N |
| IIIA | Squamous | 25 | — | 30 | N | 20.3 | D | Unknown | N |
Abbreviations: AJCC, American Joint Committee on Cancer; D, Distant; L, Local; N, No; PD-L1, Programmed death-ligand 1; %RVT, Percentage of residual viable tumor; RFS, Recurrence-free survival; Squamous, Squamous cell carcinoma; SBRT, Stereotactic Body Radiotherapy; SRS, Stereotactic radiosurgery; XRT, Radiotherapy; Y, Yes.
aThe percentage of tumor cells expressing cell surface programmed death-ligand 1 (PD-L1) from pre-treatment biopsy specimen.
Discussion
Safety, feasibility, and promising clinical data from neoadjuvant PD-1 pathway blockade have prompted a proliferation of treatment strategies incorporating ICB for resectable NSCLC. In 2022, the FDA-approved neoadjuvant nivolumab plus platinum-doublet chemotherapy for stage I–IIIA NSCLC, based on results from the CheckMate-816 phase III clinical trial (5). Additional studies are testing novel immunotherapeutic combinations (12). Given the paucity of long-term data, reports such as ours, detailing 5-year clinical outcomes from the earliest reported study of neoadjuvant nivolumab, provide key insights for patients and providers.
Neoadjuvant nivolumab led to favorable clinical outcomes, with a 5-year OS rate of 80%. With the caveat of small patient numbers, this highlights potential durable benefits of ICB, even when administered briefly before surgery. Additional long-term follow-up reports, studying neoadjuvant single-agent ICB, have shown similar favorable clinical outcomes, with three-year survival rates ranging from 80% to 88.5% (13, 14). Among patients who experienced disease recurrence, several were able to receive definitive local therapy leading to extended survival.
There was a notable difference in recurrence rate based on MPR (Supplementary Fig. S1), which corresponded with a trend in improved RFS (Fig. 1C). Of patients with MPR, there was one recurrence, compared with six recurrences among patients without MPR (Fig. 2). In addition, the two patients with a pCR remain alive and recurrence-free at 5-year follow-up. Indeed, differences in RFS based on MPR status, may in part be driven by the two patients with pCR who are included under this category. Available data from CheckMate-816 suggest that both pathological response thresholds may carry prognostic value (15), albeit questions still remain as to how this may translate into clinical decision making.
As an exploratory analysis, we evaluated an alternative pathological response threshold of 50% RVT that, in addition to MPR, showed a favorable association with RFS (Supplementary Fig. S2B), suggesting that cutoff points for pathological response beyond pCR and MPR may be worth further investigation (15). In planning future trials, prognostic markers, including pathological response or ctDNA clearance, will be critical in tailoring peri-operative management (16). Post-treatment ctDNA clearance has shown early promise as a biomarker of event-free survival (EFS) after neoadjuvant chemoimmunotherapy (17); however, conclusive interpretation may be limited by proportion of patients with available samples. How pre-surgical ctDNA clearance compares as a biomarker with post-surgical ctDNA negativity, also referred to minimal residual disease, remains an active question (18). In addition, identifying optimal treatment strategies for patients with suboptimal pathological response—whether it be adjuvant chemotherapy or extended ICB—remains an outstanding question.
In our trial, patients with PD-L1–positive tumors trended toward improved RFS (Fig. 1D). This is in contrast with the initial report (6), which noted a limited correlation between pathological response and pre-treatment PD-L1 status. This may be in part due to small cohort size, with pre-treatment PD-L1 assessment available for only 15 of the 20 patients. A larger prospective trial, evaluating neoadjuvant atezolizumab, did indicate a correlation between baseline PD-L1 level and MPR, particularly for patients with TPS ≥50% (14). Subsequent studies, incorporating neoadjuvant dual-ICB and chemoimmunotherapy have shown improvement in pathological response based on pre-treatment PD-L1 positivity (5, 19, 20). Specifically, results from CheckMate-816 (5), showed a significant association between PD-L1 positivity and both pathological response and EFS benefit from chemoimmunotherapy.
Although the original report of this cohort noted TMB to be predictive of pathological response to neoadjuvant nivolumab, this biomarker was not associated with improved RFS or OS. This finding is limited by availability of sequencing data, which was available for only 11 patients in our cohort. This is in contrast with a recent study evaluating neoadjuvant sintilimab (13), which noted improved disease-free survival for patients with “high” TMB. Indeed, there has been conflicting data regarding the role of TMB as a biomarker in predicting response to ICB in the metastatic setting (21–23). These conflicting findings may be in part due to variability among blood versus tissue-based assessments, sequencing platforms and established cutoff values (24). Further evaluation of TMB, in both the neoadjuvant and metastatic setting, is needed to assess its role as a predictive biomarker, either alone or integrated with additional histologic or immunologic markers (25).
As noted in our results, patients with stage I/II disease had favorable recurrence outcomes compared with those with IIIA disease after neoadjuvant ICB (Supplementary Fig. S2A). However, this may just represent the superior outcomes of a lower-risk group of patients and should be interpreted cautiously. In comparison, patients with IIIA disease treated on CheckMate-816, exhibited improved EFS with chemoimmunotherapy compared with chemotherapy alone (5). Although chemotherapy plus nivolumab will increasingly be used for resectable NSCLC, there may still be a future role for ICB-only strategies, perhaps in PD-L1 high tumors or lower-risk (I/II) disease. Identifying patients who may derive equivalent benefit from ICB alone versus chemoimmunotherapy, by incorporating predictive biomarkers, is an important goal in early- and late-stage cancers.
This study has limitations that affect our ability to draw definitive conclusions. The small cohort size makes it difficult to assign significance between histopathologic subgroups and long-term clinical outcomes. Analysis from ongoing, larger-scale trials will be needed to definitively answer the pertinent questions raised in this report.
In conclusion, neoadjuvant nivolumab monotherapy in NSCLC led to favorable long-term clinical outcomes, with a low rate of toxicity. Clinicians should be confident in using ICB in the pre-operative setting. Further long-term data evaluating the role of neoadjuvant single- and dual-agent ICB and neoadjuvant chemoimmunotherapy are awaited.
Supplementary Material
Recurrence per PDL1 and MPR
KM curvces for recurrence based on stage and RVT
Acknowledgments
This trial received grant support from a Stand Up To Cancer—Cancer Research Institute Cancer Immunology Translational Cancer Research grant, BMS, International Immuno-Oncology Network, LUNGevity Foundation, IASLC, Prevent Cancer Foundation, Lung Cancer Foundation of America, MacMillan Foundation, ECOG-ACRIN, NIH (CA121113, CA006973, CA180950, and R01 CA142779), Johns Hopkins University Cancer Center, Memorial Sloan Kettering Cancer center, among others. Stand Up To Cancer is a division of the Entertainment Industry Foundation. The indicated Stand Up To Cancer grant is administered by the American Association for Cancer Research.
This article is featured in Highlights of This Issue, p. 689
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
J.E. Reuss reports other support from Genentech/Roche, Sanofi/Genzyme, Personalis, and Guardant, grants from Genentech/Roche and Verastem, and personal fees from Astrazeneca outside the submitted work. J. Taube reports grants and other support from Bristol Myers Squibb and other support from Merck and Astra Zeneca during the conduct of the study; grants and other support from Akoya Biosciences and other support from Lunaphore outside the submitted work; and a patent for machine learning for irPRC pending. V. Anagnostou reports grants from Astra Zeneca, Personal Genome Diagnostics, Delfi Diagnostics, and Bristol–Myers Squibb during the conduct of the study; as well as reports a patent for 63/276,525 issued, 17/598,690 issued, 17/047,006 issued, 17/779,936 issued, 16/312,152 issued, and 16/341,862 issued. K.N. Smith reports a patent for MANAFEST licensed and with royalties paid from ManaT Bio, Inc.; and has received research support from BMS, AstraZeneca, and Enara Bio, and honoraria/travel support from Adaptive Biotechnologies. P.B. Illei reports grants from Bristol Myers Squibb during the conduct of the study; and personal fees from Bristol Myers Squibb, AstraZeneca, Janssen, Roche, Genentech, Sanofi, and Eli Lilly outside the submitted work. S.R. Broderick reports other support from Bristol Myers Squibb during the conduct of the study; other support from AstraZeneca outside the submitted work. D.R. Jones reports other support from AstraZeneca and Merck outside the submitted work. S.L. Topalian reports other support from Bristol Myers Squibb during the conduct of the study. S.L. Topalian also reports other support from Amgen, AstraZeneca, Compugen, DNAtrix, Dracen, Enara Bio, Immunomic Therapeutics, Janssen Pharmaceuticals, ManaT Bio, Inc., RAPT Therapeutics, Regeneron, Shattuck Labs, Tempest Therapeutics, Tizona LLC, Tieza, TRex Bio, and WindMIL; grants and other support from Bristol Myers Squibb; personal fees and other support from Dragonfly Therapeutics and Five Prime Therapeutics; and personal fees from Immunocore and Path AI outside the submitted work, and reports a patent for Bristol–Myers Squibb with royalties paid, a patent for Immunomic Therapeutics with royalties paid, and a patent for WindMIL with royalties paid. D.M. Pardoll reports grants from Bristol–Myers–Squibb during the conduct of the study; as well as reports a patent for Lag 3 issued, licensed, and with royalties paid from Bristol–Myers–Squibb. J.R. Brahmer reports grants and personal fees from Bristol Myers Squibb during the conduct of the study. J.R. Brahmer also reports personal fees from Merck, Genentech Roche, and Regeneron, grants and personal fees from AstraZeneca, and other support from RAPT therapeutics outside the submitted work; and J.R. Brahmer reports advisory board member relationships with Janssen and Sanofi, DSMB member relationships with Janssen, Sanofi, and GlaxoSmith Kline, and clinical trial funding from RAPT Therapeutics and Spectrum. J.E. Chaft reports grants from NCI P30 CA008748 during the conduct of the study; personal fees and other support from AZ, BMS, Genentech, Merck, and Novartis, and personal fees from Janssen, Regeneron/Sanofi, Guardant Health, Arcus biosciences, and Flame bioscience outside the submitted work. P.M. Forde reports grants from BMS, LUNGevity Foundation, Stand Up to Cancer Research Institute, and JHU SKCCC Cancer Center Support grant during the conduct of the study; as well as grants and personal fees from AstraZeneca, BMS, and Novartis, and personal fees from Genentech, Amgen, Daiichi, F Star, G1, Janssen, Iteos, Merck, Sanofi, and Surface outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
S. Rosner: Conceptualization, data curation, formal analysis, investigation, visualization, methodology, writing–original draft, project administration, writing–review and editing. J.E. Reuss: Conceptualization, data curation, formal analysis, supervision, funding acquisition, investigation, methodology, writing–original draft. M. Zahurak: Software, formal analysis, validation, visualization, methodology, writing–original draft, writing–review and editing. J. Zhang: Software, formal analysis. Z. Zeng: Software, formal analysis. J. Taube: Resources, software, supervision, funding acquisition, investigation, methodology. V. Anagnostou: Conceptualization, resources, software, formal analysis, supervision, funding acquisition, validation, methodology. K.N. Smith: Conceptualization, resources, software, formal analysis, supervision, funding acquisition, validation, methodology. J. Riemer: Investigation, project administration. P.B. Illei: Supervision, investigation. S.R. Broderick: Conceptualization, investigation. D.R. Jones: Conceptualization, investigation. S.L. Topalian: Conceptualization, supervision, writing–original draft. D.M. Pardoll: Conceptualization, resources, formal analysis, supervision, funding acquisition, methodology. J.R. Brahmer: Conceptualization, supervision, investigation. J.E. Chaft: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, investigation, writing–original draft, project administration. P.M. Forde: Conceptualization, resources, data curation, supervision, funding acquisition, investigation, methodology, writing–original draft, project administration, writing–review and editing.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39–51. [DOI] [PubMed] [Google Scholar]
- 3. Wu Y-L, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected EGFR-mutated non–small cell lung cancer. N Engl J Med 2020;383:1711–23. [DOI] [PubMed] [Google Scholar]
- 4. Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non–small cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet North Am Ed 2021;398:1344–57. [DOI] [PubMed] [Google Scholar]
- 5. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 2022;386:1973–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018;378:1976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reuss JE, Smith KN, Anagnostou V, Zhang J, Zahurak M, Caushi J, et al. Neoadjuvant nivolumab in resectable non–small cell lung cancer: extended follow-up and molecular markers of response. J Clin Oncol 2019;37:8524. [Google Scholar]
- 8. Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A, et al. AJCC Cancer Staging Handbook. 7 ed. Springer; 2010. p. 718. [Google Scholar]
- 9. Cottrell TR, Thompson ED, Forde PM, Stein JE, Duffield AS, Anagnostou V, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non–small cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol 2018;29:1853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tetzlaff MT, Messina JL, Stein JE, Xu X, Amaria RN, Blank CU, et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann Oncol 2018;29:1861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Menzies AM, Amaria RN, Rozeman EA, Huang AC, Tetzlaff MT, van de Wiel BA, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the international neoadjuvant melanoma consortium (INMC). Nat Med 2021;27:301–9. [DOI] [PubMed] [Google Scholar]
- 12. Campelo RG, Forde P, Weder W, Spicer J, He P, Hamid O, et al. P2.04–28 NeoCOAST: neoadjuvant durvalumab alone or with novel agents for resectable, early-stage (I–IIIA) non–small cell lung cancer. J Thorac Oncol 2019;14:S719. [Google Scholar]
- 13. Zhang F, Guo W, Zhou B, Wang S, Li N, Qiu B, et al. Three-year follow-up of neoadjuvant programmed cell death protein-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol 2022;17:909–20. [DOI] [PubMed] [Google Scholar]
- 14. Chaft JE, Oezkan F, Kris MG, Bunn PA, Wistuba II, Kwiatkowski DJ, et al. Neoadjuvant atezolizumab for resectable non–small cell lung cancer: an open-label, single-arm phase II trial. Nat Med 2022;28:2155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Provencio-Pulla M, Spicer J, Taube JM, Martin C, Spigel DR, Wang C, et al. Neoadjuvant nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) versus chemo for resectable (IB–IIIA) non–small cell lung cancer (NSCLC): association of pathological regression with event-free survival(EFS) in CheckMate 816. J Clin Oncol 2022;40:LBA8511. [Google Scholar]
- 16. Spigel DR, Peters S, Ahn M-J, Tsuboi M, Chaft J, Harpole D, et al. 93TiP MERMAID-2: phase III study of durvalumab in patients with resected, stage II–III NSCLC who become MRD+ after curative-intent therapy. J Thorac Oncol 2021;16:S745–6. [Google Scholar]
- 17. Provencio M, Serna-Blasco R, Nadal E, Insa A, García-Campelo MR, Casal Rubio JN, et al. Overall survival and biomarker analysis of neoadjuvant nivolumab plus chemotherapy in operable stage IIIA non–small cell lung cancer (NADIM phase II trial). J Clin Oncol 2022;40:2924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pellini B, Chaudhuri AA. Circulating tumor DNA minimal residual disease detection of non–small cell lung cancer treated with curative intent. J Clin Oncol 2022;40:567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reuss JE, Anagnostou V, Cottrell TR, Smith KN, Verde F, Zahurak M, et al. Neoadjuvant nivolumab plus ipilimumab in resectable non–small cell lung cancer. J Immunother Cancer 2020;8:e001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cascone T, William WN, Weissferdt A, Leung CH, Lin HY, Pataer A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non–small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 2021;27:504–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garassino M, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, Speranza G, et al. OA04.06 Evaluation of TMB in KEYNOTE-189: pembrolizumab plus chemotherapy vs. placebo plus chemotherapy for nonsquamous NSCLC. J Thorac Oncol 2019;14:S216–7. [Google Scholar]
- 22. Peters S, Dziadziuszko R, Morabito A, Felip E, Gadgeel SM, Cheema P, et al. Atezolizumab versus chemotherapy in advanced or metastatic NSCLC with high blood-based tumor mutational burden: primary analysis of BFAST cohort C randomized phase 3 trial. Nat Med. 2022;28:1831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Castro G, Rizvi NA, Schmid P, Syrigos K, Martin C, Yamamoto N, et al. NEPTUNE: phase III study of first-line durvalumab plus tremelimumab in patients with metastatic NSCLC. J Thorac Oncol 2022;18:106–19. [DOI] [PubMed] [Google Scholar]
- 24. Ricciuti B, Wang X, Alessi JV, Rizvi H, Mahadevan NR, Li YY, et al. Association of high tumor mutation burden in non–small cell lung cancers with increased immune infiltration and improved clinical outcomes of PD-L1 blockade across PD-L1 expression levels. JAMA Oncol 2022;8:1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mino-Kenudson M, Schalper K, Cooper W, Dacic S, Hirsch FR, Jain D, et al. Predictive biomarkers for immunotherapy in lung cancer: perspective from the International Association for the study of lung cancer pathology committee. J Thorac Oncol 2022;17:1335–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Recurrence per PDL1 and MPR
KM curvces for recurrence based on stage and RVT
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.