Abstract
Cancer cells accumulate genetic mutations in coding proteins that may be presented by HLA as neoantigenic peptides (peptide HLA, pHLA). T cells scan for neoantigenic pHLA by the T-cell receptor (TCR):CD3 complex. This complex has the dual function of binding pHLA, by the TCR, and triggering T-cell activation by CD3. Checkpoint therapy activates exhausted T cells to kill cancer cells and generally work best against tumors with high neoantigen burden and in patients with neoantigenic-reactive T cells. TCR T-cell engagers (TCE) are a novel class of immunotherapy that bypasses these two requirements by redirecting polyclonal T cells, regardless of their native specificity, to kill a cancer cell independent of neoantigen burden. This is accomplished through deconstructing the membrane-bound TCR:CD3 complex into a soluble bispecific protein comprised of a targeting domain (TCR) and activating domain (usually anti-CD3 single-chain variable fragment). The pool of targets for TCR TCE is larger than for antibody therapeutics and includes >90% of human intra- or extracellular proteins. Most tumor-associated antigens for solid tumors are intracellular and accessible only by a TCR therapeutic. Tebentafusp, a TCR TCE directed to a peptide derived from the gp100 melanoma protein presented by HLA*A02:01, demonstrated a survival benefit in metastatic uveal melanoma (mUM). This survival benefit highlights the promise of TCR TCEs because mUM is a solid tumor with a very low neoantigen burden and has poor response to checkpoints and chemotherapy. Other TCR TCE programs are now in clinical studies for a broader range of tumors.
Introduction
Checkpoint therapies activate tumor-specific T cells to kill immunogenic tumors. However, many patients with immunogenic tumors do not respond to checkpoints and low neoantigen burden tumors are generally insensitive (1). A promising way to address this unmet need would be to redirect any T-cell, regardless of specificity, to kill tumors independent of mutation burden.
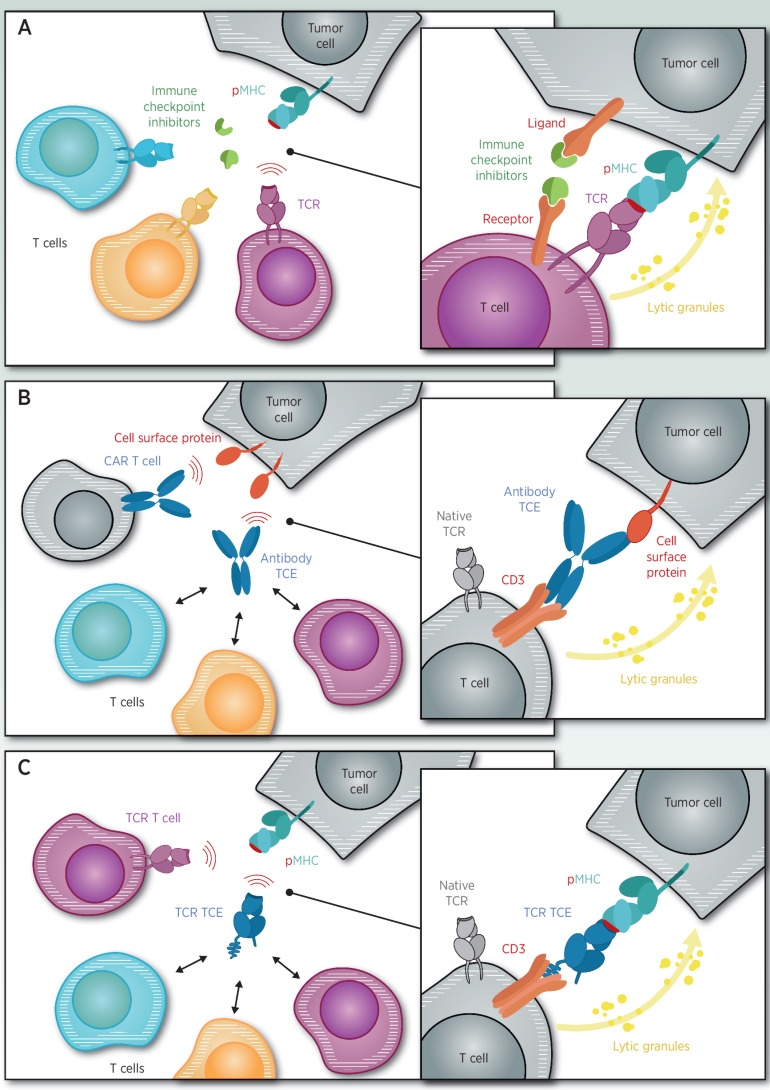
Redirecting T cells is achieved in vivo through T-cell engagers (TCE) or ex vivo by genetically manipulating T cells, for example, adoptive T-cell therapy (Fig. 1). TCEs are bispecific soluble proteins comprised of a targeting domain, either T-cell receptor (TCR) or antibody, fused to a modular effector domain that can be tuned to activate (usually via CD3 activation) or inhibit the immune system (2). Adoptive T-cell therapy entails genetic insertion of a chimeric antibody receptor or TCR specific for a tumor antigen into T cells (3). The engineered T cells are then infused with supportive cytokines into a patient after lymphoablative chemotherapy.
Figure 1.
A, Immune checkpoints block inhibitory receptors on tumors or T cells, resulting in activation of tumor-specific T cells. B, Antibody-based TCE or antibody-modified adoptive T-cell (CAR-T) target cell surface proteins on cancer cells. C, T-cell receptor–based TCE or TCR-modified adoptive T cells (TCR-T) target peptide-HLA on cancer cells.
As a pharmacologic class, TCEs have several attractive features compared with adoptive T-cell therapy. TCEs do not require chemotherapy or supportive cytokines and may be combined with any standard of care or used in the adjuvant setting. TCEs are “off-the-shelf” molecules with simpler manufacturing enabling repeat dosing. As soluble proteins, TCEs have more predictable and tunable serum kinetics that is reflected in a more predictable adverse event profile. Finally, the TCE mechanism favors polyclonal T cells activation and recruitment (4) that could trigger a secondary natural response, including antigen spreading (5) or even cytokine-mediated cell killing (6) of adjacent tumor cells that do not express the target of the TCE.
There are currently two marketed TCEs: Blinatumomab (CD19xCD3) and tebentafusp (gp100xCD3). Blinatumomab targets B-cell leukemia via an antibody fragment that binds the B-cell lineage antigen CD19 (7). Tebentafusp targets melanoma via a soluble TCR (sTCR) that recognizes an HLA*A02:01 presented 9-amino acid peptide, derived from processing of the intracellular melanocyte lineage protein gp100 (8)
TCR TCEs Enable Targeting of Solid and Hematologic Tumors
mAbs have proven successful to treat numerous cancers. However, antibodies only bind to extracellular accessible proteins that comprise <10% of the proteome (9). Because the majority of cancer cell surface proteins are also on normal cells, antibody-based therapy may induce on-target, off-tumor toxicity. Antibody TCE requires a high number of 103–104 targets/cell, further restricting the target pool (10–12). Antibody TCEs have had success in hematologic tumors by targeting high-abundance cell surface lineage antigens; on-target killing of antigen-positive healthy cells is managed by supportive care. However, the major burden of cancer lies in solid tumors where there are fewer cell surface proteins not expressed on vital tissue.
T cells recognize infected or cancer cells through a transmembrane TCR that recognizes HLA presentation of 8–15 amino acid peptides. TCR therapeutics provide access to >90% of the proteome, including intracellular proteins, and have sensitivity to as few as 10 peptide-HLA (pHLA) per cell (13, 14). Therefore, a TCR TCE would be of particular interest for solid tumors because most of the specific proteins are intracellular and not accessible by antibodies. Recently, tebentafusp validated TCR TCEs by demonstrating an overall survival (OS) benefit in a randomized phase 3 trial in metastatic uveal melanoma (mUM), a tumor that has a low tumor mutation burden and is metastatic to the liver, an organ that generally responds poorly to checkpoint blockade-based immunotherapy (15, 16).
Target Selection Begins with the Peptide HLA
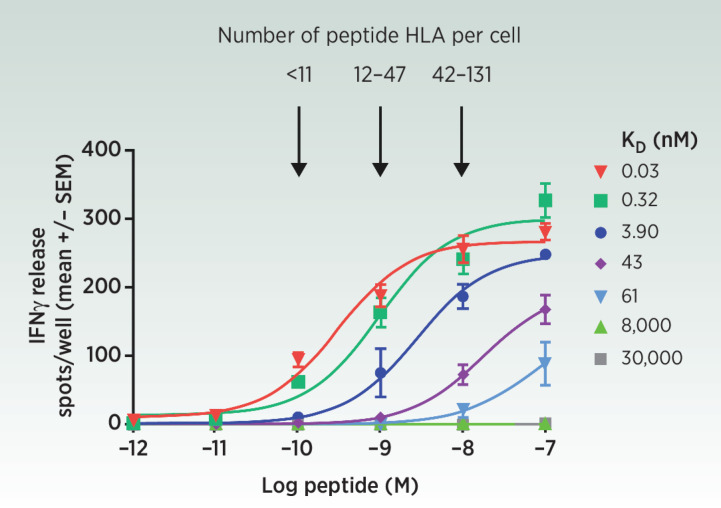
The first major challenge is to identify a peptide HLA (pHLA) presented on a diseased cell relative to normal tissue. We have found that peptides predicted by commonly available algorithms to be processed into pHLA are not always detected by mass spectrometry (MS). Until these algorithms improve, validation by MS is the gold standard (17). Quantification of pHLA is also important because TCR TCE activity increases as a function of pHLA levels (Fig. 2).
Figure 2.
ImmTAC redirect and activate polyclonal T cells against as few as 11 peptide-HLA per cell (56) as measured by IFNγ release, a marker of T-cell activation. This sensitivity is achieved only if the TCR end of the ImmTAC is engineered from its natural micromolar affinity to picomolar strength.
The two most advanced protein targets for TCE are lineage antigens and cancer–testes antigen class, CTA Table 1. Although gp100 is restricted to melanomas, CTAs are expressed more broadly in a variety of solid and hematologic tumors (18). In general, CTA expression is limited to testes and ovary germline cells with minimal to no expression in other vital tissues.
Table 1.
Clinical stage T-cell receptor (Target x CD3) T-cell engagers.
| Class of target | Name | Target | Design | Status | NCT or EudraCT | Sponsor |
|---|---|---|---|---|---|---|
| Lineage antigens | Tebentafusp (IMC-gp100) | gp100 | Full-length alpha and beta chains Stcr | OS benefit in metastatic uveal melanoma (8). Approved for metastatic and unresectable uveal melanoma | NCT03070392 | Immunocore |
| RG6007 | WT1 | TCR mimetic antibody | Phase 1 (multiple tumors) | NCT04580121 | Roche | |
| IMC-C103C | MAGE-A4 | Full-length alpha and beta chains sTCR | Clinical activity in ovarian and HNSCC cancer. Phase 1 (multiple tumors; ref. 55) | NCT03973333 | Immunocore and Genentech | |
| IMC-F106C | PRAME | Full-length alpha and beta chains sTCR | Phase 1 (multiple tumors) | NCT04262466 | Immunocore | |
| Cancer–testes antigens | RG8129 | MAGE-A4 | TCR mimetic antibody | Phase 1 (multiple tumors) | NCT01694472 | Roche |
| IMA401 | MAGE-A4 | Single chain of alpha and beta variable chain sTCR | Phase 1 (multiple tumors) | NCT05359445 | Immatics and Bristol-Myers Squibb | |
| IMC-NYESO | NYESO | Full-length alpha and beta chains sTCR | Discontinued | NCT03515551 | Immunocore and GSK | |
| Viral antigens | IMV | HBV | Full-length alpha and beta chains sTCR | Phase 1 (chronic HBV) | 2019–004212–64 | Immunocore |
| Differentially expressed | ABBV-184 | Survivin | Single chain of alpha and beta variable chain sTCR | Discontinued | NCT04272203 | AbbVie |
Differentially expressed proteins offer a large pool of targets. Redirecting T cells to target such antigens would need to account for on-target, off-tumor toxicity from normal tissue expression. Because of exquisite sensitivity to even low antigen levels, TCR TCE should be calibrated in their potency to reduce the risk to antigen-positive normal cells.
Cancers with a viral etiology are attractive for TCR TCEs because the targets should be highly specific. An HBVxCD3 TCR TCE is currently in phase 1 for chronic HBV but could be studied in HBV-positive hepatocellular carcinoma (19). TCR-engineered T cells targeting HPV have shown activity providing rationale for an HPV TCR TCE (20).
Oncogenes are highly specific for the tumor and drive tumor pathogenesis. Preclinical reports confirm the technical feasibility of TCR bispecific targeting of KRAS and p53 oncogenes (21, 22, 23). However, neoantigens tend to be present at very low pHLA density (1–2 pHLA per cell for p53) and 99% are private for each patient (24). Neoantigen oncogene driver peptides are generally restricted to less prevalent HLA subtypes.
TCR TCEs are restricted by HLA. Some non-classical HLA such as HLA-E and MR1 may be amenable for universal TCRs. However, these non-classical HLA are expressed in normal cells, and in the absence of antigen specificity may introduce the risk of off-tumor toxicity (25). Identifying tumor-specific antigens presented by these HLAs is a challenge. For MR1 and CD1 that present metabolites and lipids, respectively, being able to screen for patients whose tumors harbor the small-molecule antigens will also be a challenge as they will not be amenable to standard IHC.
Deconstructing the Natural TCR into an sTCR Bispecific
Antibodies are ideal substrates for engineered medicines. Following antigen challenge, B cells undergo post-thymic maturation in vivo and secrete high affinity and specificity, soluble antibodies. In contrast, TCRs are a more challenging substrate for soluble therapeutics for several reasons (16, 18).
First, the natural TCR is tethered to the membrane. An sTCR requires correctly pairing two polypetides, alpha and beta, in the correct conformation. The most advanced, and only clinically validated to date, is the immune mobilizing monoclonal TCRs against cancer (ImmTAC) platform that uses an internally hidden disulfide bond to link the full polypeptides in an optimized formation (26). An alternative approach just entering Phase 1 uses an sTCR comprised of only the variable domains of the alpha and beta polypeptide (27)
Second, the properties that enable a natural TCR to have very high sensitivity for low pHLA are not consistent with ideal characteristics of an sTCR. For example, the natural TCR has very low affinity (micromolar) for pHLA and rapid binding kinetics that allows serial triggering of clustered TCRs (avidity). However, a wild-type, very low affinity sTCR TCE with rapid release from a pHLA would translate into low activity (ref. 13; Fig. 2). Therefore, the native TCR must be engineered into a very high-affinity sTCR.
Third, T cells do not undergo in vivo post thymic maturation. The only options for generating very high, sub-nanomolar affinity and specific sTCRs are ex vivo phage, yeast, and mammalian display libraries technology (25, 28). Although each approach has advantages, only phage display engineers a million-fold affinity improvement to generate low picomolar affinity TCRs (29). For ImmTAC molecules, which use the phage display system, low picomolar affinity TCRs are required to redirect T cells to kill target cells with very low pHLA levels of 10/cell (13).
Fourth, engineering high-affinity TCRs without introducing crossreactivity is a challenge due to inherent binding promiscuity of natural TCRs. Thymic selection prevents circulation of T cells that react to “healthy” proteins and even the T cells that emerge may bind to 106 different pHLA (30). Engineering TCRs ex vivo outside thymic selection introduces the risk of crossreactivity to different pHLA on vital tissue. Two TCR-Ts against MAGE-A3 resulted in severe and fatal cardiac or neurologic toxicity due to crossreactivity with cardiac titin or an EPS8L2 in neuronal cells (31–33). Phage display and structure-guided mutagenesis can increase both the affinity and specificity of an sTCR by several orders of magnitude (34, 35).
In vitro, and in the absence of target, ImmTAC molecules do not trigger cytokine release at therapeutic concentrations due to high specificity for the target pHLA and a bispecific format with picomolar affinity TCR compared with nanomolar affinity anti-CD3 scFv. This 1,000-fold differential may represent an optimal ratio that increases the safety window by favoring binding to tumor pHLA rather than spontaneous T-cell activation in the absence of target pHLA, which may also minimize T-cell activation to off-target pHLA.
Finally, non-human primate toxicology studies are not relevant because these TCRs are human HLA restricted and peptidome processing differs between species. Therefore, we have developed a paradigm to de-risk a TCR TCE in vitro through ruling out allogenicity (ability to react to non-targeted HLA subtypes), identifying cross-reactive peptides (alanine and X-scanning), testing reactivity to normal donor and iPSC-derived tissue-specific cells in vitro and for spontaneous cytokine release from peripheral blood (36).
Mechanism of Action
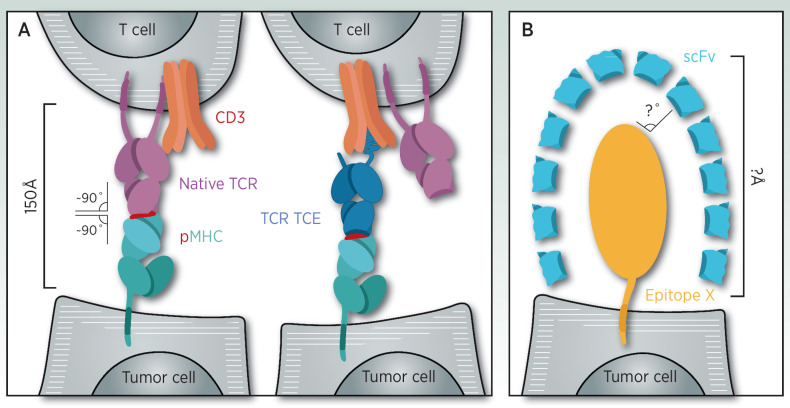
TCRs bind to pHLA in a canonical fashion through a perpendicular 150 Å bridge (Fig. 3). This bridge is the contact around which other receptors on the T-cell surface organize to strengthen the synapse (37–40). Through this geometric construct, a T-cell can kill a cell presenting few pHLA.
Figure 3.
Orchestration of the T-cell synapse centers around the native TCR that binds to the target cell pHLA in a canonical fashion. TCR TCEs are designed to recreate this optimized synapse, including binding conformation, geometry, and distance (A). Antibody TCE binds surface proteins and may not recreate the canonical T-cell synapse (B).
TCR TCEs have sensitivity to 2–10 pHLA targets/cell, perhaps reflecting their ability to bind to pHLA in a canonical fashion that mimics that natural TCR and recreates the geometry of a natural T-cell synapse (41). In contrast, antibody bispecifics and CAR-T bring the T-cell to non-HLA surface proteins outside of the pHLA synapse, which may not have the optimized size, distance or geometric angle and may not support optimal co-factor binding. This may explain in part why antibody-based therapeutics requires 103–104 targets/cell for effective targeting (11, 12).
Tebentafusp is highly specific for the gp100 peptide presented by HLA-A*02:01 and redirects donor, polyclonal T cells with picomolar potency to kill gp100+ melanoma cell lines in vitro (42). The specificity of tebentafusp is highlighted by the low reactivity to melanoma cell lines that do not express gp100. Activated T cells release polycytokines, including IL2, TNFα, and IFNγ (4, 13). IFNs may upregulate HLA potentially increasing activity in a feed forward loop (43). CD8 T cells are redirected to a greater degree than CD4 T cells, perhaps reflecting their natural preference for Class 1 HLA (4).
Co-incubation with T regulatory cells does not block T-cell redirection by ImmTAC molecules (4). Although macrophages can blunt the reaction, exogenous IL-2 can overcome this inhibition (44). Tebentafusp redirects T cells to kill both PD-L1–negative and –positive tumor cell lines, although this is blunted if the T cells are PD-1 positive. The latter can be reversed with the addition of an anti-PD1 checkpoint (45). Therefore, TCR-based TCEs could be synergistic with anti-PD(L)1 checkpoints in some tumors.
TCE may trigger an immune cascade that results in killing of adjacent tumor cells that do not express the target antigen. In vitro, tebentafusp induced cell killing that led to cross-presentation of tumor antigen that could stimulate epitope spreading (5). In addition, T cells activated by TCE may release cytokines that kill adjacent, antigen negative tumor cells (6).
Clinical Validation with Tebentafusp in mUM
Tebetanfusp was tested in metastastic cutaneous and mUM, both of which overexpress gp100. Within a day of receiving tebentafusp, patients have an increase in plasma cytokines and chemokines, including IL6, IFNγ, and IL2 (46, 47). T cells transiently decrease in peripheral circulation presumably due to redistribution following the cytokine and chemokines. By day 16, the majority of evaluable patients have an increase in T-cell infiltration and IFN signature gene expression in tumors (43, 47). This marked T-cell infiltration is remarkable because mUM is an immune cold tumor, with low tumor mutation burden, and is highly metastatic to the liver, which is an immunosuppressive organ. In contrast, checkpoint inhibitors are known to be less effective in tumors with this phenotype.
The plasma half-life of tebentafusp is only 8 hours (8). However, the pharmacodynamic effect on T cells and cytokines remains evident for several days. The median duration of response to tebentafusp is almost 10 months (48), underscoring the disconnect between plasma PK and pharmacodynamics. Tebentafusp, which has a long residence time to pHLA in vitro, may bind to the tumor cell for longer than is detected in the peripheral blood. In addition, the impact of antigen spreading in creating a wider and deeper immune response may also contribute to clinical benefit.
The majority of patients treated with tebentafusp experienced treatment-related adverse events (AE) of rash and cytokine release syndrome (CRS). These generally occur within a day or two of the first several doses and resolve quickly following pre-specified management algorithms. These AEs, which decrease in severity and frequency with repeated dosing, are consistent with the mechanism of action.
Rash likely results from tebentafusp-redirecting T cells to cutaneous melanocytes whereas CRS follows from T-cell activation (49). In vitro, the degree of T-cell redirection against healthy donor melanocytes differs based on individual donor T cells and melanocytes, suggesting that immune fitness and differential pHLA density may be important. Early rash on tebentafusp appears to be a marker of baseline performance or immune status rather than a predictive marker of OS benefit (8, 50). The degree of plasma cytokine induction or clinical CRS does not appear to correlate with OS benefit (51).
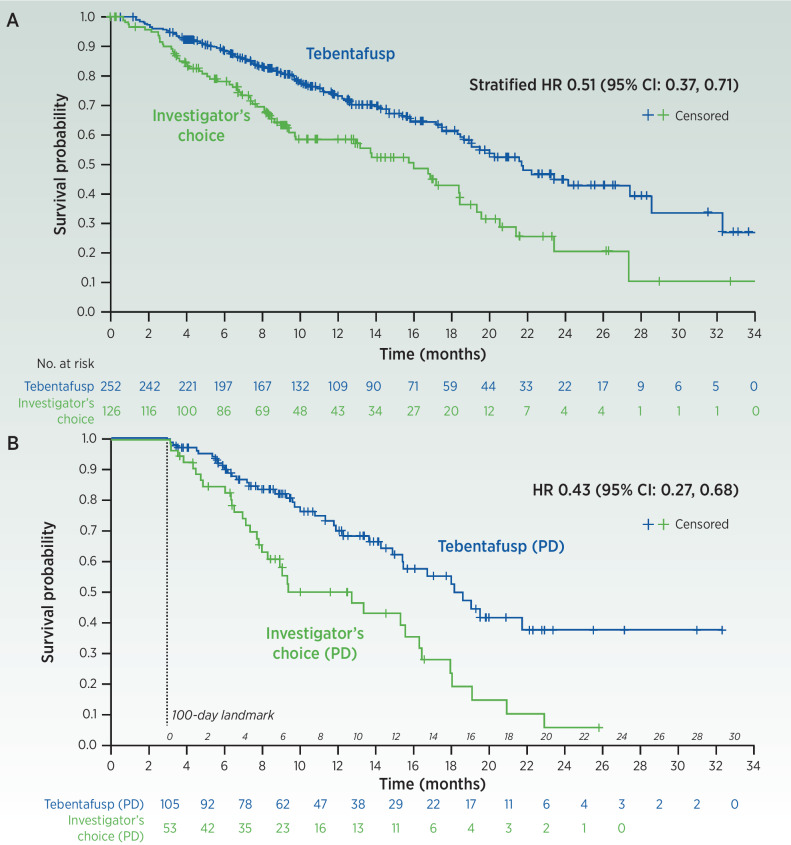
In a phase 3 study of previously untreated mUM, patients were randomized to tebentafusp versus investigator's choice (IC) of either pembrolizumab (82% patients), ipilimumab or chemotherapy. Tebentafusp resulted in a highly statistically and clinically meaningful OS benefit, HR, 0.51 (Fig. 4A; ref. 8). The Kaplan–Meier OS curves separated early, suggesting rapidity of treatment effect, and remained separated suggesting durability. This landmark phase 3 survival marks several firsts, including the first TCR therapy, the first TCE in a solid tumor (in this case often a cold solid tumor) and the first in mUM.
Figure 4.
Overall survival (OS) in the intention-to-treat population (A). OS in landmark analysis of patients who had best response of progressive disease and were alive at day 100 (B). (From New England Journal of Medicine, Nathan et al. (8), Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma, 385, 1196. Copyright 2021 Massachusetts Medical Society. Reprinted/adapted with permission from Massachusetts Medical Society.)
Progression-free survival (PFS) was statistically significant, HR, 0.73, although the degree of benefit was not as strong as OS. This is in contrast with cutaneous melanoma checkpoint trials where PFS is usually stronger than OS. The objective response rate (ORR) per RECIST radiographic criteria was 9% for tebentafusp versus 5% for the IC arm. Together, the modest ORR and PFS benefit indicate radiographic assessment of response underestimated a much larger OS benefit. This disconnect was consistent with anecdotal reports from early trials where some patients with radiographic progressive disease (PD) had long OS.
In a phase 2 mUM trial, one third of patients with OS longer than 1 year surprisingly had a best radiographic response of PD (52). This observation raised the question of whether an increase in the radiographic tumor size whereas on tebentafusp was due to necrosis and inflammation rather than tumor growth, that is, pseudoprogression. Of the 60 patients with best response of radiographic PD, 25 (42%) had OS greater than 1 year, suggesting a significant proportion of presumed pseudoprogression.
The contribution of the marked pseudoprogression on tebentafusp was confirmed in a post hoc analysis of the Phase 3 trial limited to patients with a best radiographic response of PD. In this subset, tebentafusp was associated with a very strong OS benefit, HR, 0.43 (Fig. 4B). Just over half of tebentafusp-treated patients with a best response of PD received tebentafusp beyond initial progression.
Once patients discontinued therapy, they often received subsequent therapy that was mostly checkpoint inhibitors. In an OS analysis starting from subsequent therapy, patients who had prior tebentafusp appear to have better OS compared with prior IC (53). These data raise two non-exclusive hypotheses. Tebentafusp recruits polyclonal T cells and a rich cytokine milieu in the presence of some tumor killing may foster antigen spreading that is enhanced by subsequent checkpoints. Alternatively, the initial progression event resulting in tebentafusp discontinuation may have been pseudoprogression and the patient may have carried over benefit.
A majority of mUM tumor cells harbor one of several mutations, primarily GNAQ/11. In the phase 2 study, baseline mutation ctDNA levels were highly correlated with tumor burden (51). About 70% of evaluable patients had any decrease in ctDNA by week 9 and 14% had complete clearance. The degree of ctDNA reduction was highly correlated with the degree of OS benefit. Approximately 1/3 of patients with a best response of PD had at least a 0.5 log reduction in ctDNA at time of initial progression, supporting the hypothesis of pseudoprogression in a significant number of these patients.
In a retrospective analysis of gp100 protein IHC expression from the phase 3 study, the OS from tebentafusp was independent of whether patients had high or low total gp100 protein (54). One potential explanation is that tebentafusp, with in vitro sensitivity to very low pHLA/cell, may outstrip the sensitivity of IHC, requiring >103 molecules per cell. Alternatively, even a small percentage of gp100+ cells may be sufficient for tebentafusp to trigger an initial immune response that could result in IFN-induced upregulation (43), cytokine-mediated tumor stasis or killing (6), or epitope spreading (5). Finally, even a low percentage of positive tumor cells may be sufficient for tebentafusp to slow tumor growth kinetics.
Conclusion
Tebentafusp demonstrated that targeting a single pHLA with a TCR TCE is sufficient to prolong OS in mUM, a checkpoint insensitive and very low tumor mutational burden tumor. This OS benefit was observed in the face of high rate of pseudoprogression, modest radiographic RECIST response rate, short tebentafusp plasma half-life of 8 hours, and heterogenous gp100 tumor expression by IHC.
Seventy percent of evaluable tebentafusp-treated patients had ctDNA reduction, suggesting that a much larger percentage of patients may have benefit than indicated by radiographic RECIST measurements. Whether this is specific for gp100 or more broadly applicable remains open. By recruiting polyclonal T cells, TCR TCE may engender a secondary immune response, including non-contact dependent cancer cell killing (6) or antigen spreading that may lead to bystander cell killing.
Sensitivity to TCR TCEs is likely multifactorial integrating host immunity and tumor variables, including pHLA density. Resistance mechanisms are likely similar to those for checkpoints, including loss of proteosomal processing or peptide presentation. These may be mitigated by antigen spreading and the recruitment of innate immunity.
The next step for TCR TCE, including expanding beyond gp100, moving earlier in the disease setting, and beyond HLA-A*02 restriction. IMC-C103C (MAGEA4xCD3) showed early signs of durable partial responses in ovarian and head and neck cancer, demonstrating activity beyond gp100 and melanoma (55). TCR TCEs are an intriguing platform for earlier-stage disease and as adjuvant therapy. This platform can be combined with standards of care; combination with checkpoints, IL2 and chemotherapy should be studied. Finally, expanding to other HLA genotypes or being able to develop non-HLA restricted TCRs is of high interest.
Acknowledgments
The authors would like to acknowledge and thank the following for critical review: Milos Aleksic, Mohammed Dar, Jane Harper, Stephen Harper, Bahija Jallal, Peter Kirk, Nathaniel Liddy, Michelle McCully, Carole Perot, Koustubh Ranade, Ian Robertson, and Sarah Stanhope.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authors' Disclosures
D.M. Berman reports non-financial support from Immunocore during the conduct of the study as well as personal fees from Immunocore outside the submitted work. J.I. Bell reports personal fees from Immunocore during the conduct of the study as well as personal fees from Oxford University, Oxford Sciences Enterprise, and Janssen Immunology Advisory Board outside the submitted work.
References
- 1. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. New Engl J Medicine 2017;377:2500–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goebeler M-E, Bargou RC. T-cell–engaging therapies—BiTEs and beyond. Nat Rev Clin Oncol 2020;17:418–34. [DOI] [PubMed] [Google Scholar]
- 3. Morotti M, Albukhari A, Alsaadi A, Artibani M, Brenton JD, Curbishley SM, et al. Promises and challenges of adoptive T-cell therapies for solid tumours. Brit J Cancer 2021;124:1759–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boudousquie C, Bossi G, Hurst JM, Rygiel KA, Jakobsen BK, Hassan NJ. Polyfunctional response by ImmTAC (IMCgp100) redirected CD8 +and CD4 +T cells. Immunology 2017;152:425–38. [Google Scholar]
- 5. Bossi G, Buisson S, Oates J, Jakobsen BK, Hassan NJ. ImmTAC-redirected tumour cell killing induces and potentiates antigen cross-presentation by dendritic cells. Cancer Immunol Immunother 2014;63:437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kamakura D, Asano R, Kawai H, Yasunaga M. Mechanism of action of a T-cell–dependent bispecific antibody as a breakthrough immunotherapy against refractory colorectal cancer with an oncogenic mutation. Cancer Immunol Immunother 2021;70:177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. New Engl J Medicine 2014;371:1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nathan P, Hassel JC, Rutkowski P, Baurain J-F, Butler MO, Schlaak M, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. New Engl J Med 2021;385:1196–206. [DOI] [PubMed] [Google Scholar]
- 9. Bausch-Fluck D, Hofmann A, Bock T, Frei AP, Cerciello F, Jacobs A, et al. A mass spectrometric-derived cell surface protein Atlas. PLoS ONE 2015;10:e0121314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ellerman D. Bispecific T-cell engagers: towards understanding variables influencing the in vitro potency and tumor selectivity and their modulation to enhance their efficacy and safety. Methods 2019;154:102–17. [DOI] [PubMed] [Google Scholar]
- 11. Bluemel C, Hausmann S, Fluhr P, Sriskandarajah M, Stallcup WB, Baeuerle PA, et al. Epitope distance to the target cell membrane and antigen size determine the potency of T cell-mediated lysis by BiTE antibodies specific for a large melanoma surface antigen. Cancer Immunol Immunother 2010;59:1197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stone JD, Aggen DH, Schietinger A, Schreiber H, Kranz DM. A sensitivity scale for targeting T cells with chimeric antigen receptors (CARs) and bispecific T-cell Engagers (BiTEs). Oncoimmunology 2014;1:863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liddy N, Bossi G, Adams KJ, Lissina A, Mahon TM, Hassan NJ, et al. Monoclonal TCR-redirected tumor cell killing. Nat Med 2012;18:980–7. [DOI] [PubMed] [Google Scholar]
- 14. Lowe KL, Cole D, Kenefeck R, OKelly I, Lepore M, Jakobsen BK. Novel TCR-based biologics: mobilising T cells to warm ‘cold’ tumours. Cancer Treat Rev 2019;77:35–43. [DOI] [PubMed] [Google Scholar]
- 15. Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T-cell elimination. Nat Med 2021;27:152–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Osorio JC, Arbour KC, Le DT, Durham JN, Plodkowski AJ, Halpenny DF, et al. Lesion-level response dynamics to programmed cell death protein (PD-1) blockade. J Clin Oncol 2019;37:3546–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stopfer LE, Gajadhar AS, Patel B, Gallien S, Frederick DT, Boland GM, et al. Absolute quantification of tumor antigens using embedded MHC-I isotopologue calibrants. Proc National Acad Sci 2021;118:e2111173118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simpson AJG, Caballero OL, Jungbluth A, Chen Y-T, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 2005;5:615–25. [DOI] [PubMed] [Google Scholar]
- 19. Fergusson JR, Wallace Z, Connolly MM, Woon AP, Suckling RJ, Hine DW, et al. Immune-mobilizing monoclonal T-cell receptors mediate specific and rapid elimination of hepatitis B–infected cells. Hepatology 2020;72:1528–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nagarsheth NB, Norberg SM, Sinkoe AL, Adhikary S, Meyer TJ, Lack JB, et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat Med 2021;27:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andrew W, Andrew P, Vijaykumar K, Annabelle H, Jaafar H, Sylvie M, et al. 882 Selective affinity-enhanced T-cell receptor bispecific targeting of KRAS G12D neoantigen driven cancers. J Immunother Cancer 2021;9:A924. [Google Scholar]
- 22. Douglass J, Hsiue EH-C, Mog BJ, Hwang MS, DiNapoli SR, Pearlman AH, et al. Bispecific antibodies targeting mutant RAS neoantigens. Sci Immunol 2021;6:eabd5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hsiue EH-C, Wright KM, Douglass J, Hwang MS, Mog BJ, Pearlman AH, et al. Targeting a neoantigen derived from a common TP53 mutation. Science 2021;371:eabc8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parkhurst MR, Robbins PF, Tran E, Prickett TD, Gartner JJ, Jia L, et al. Unique neoantigens arise from somatic mutations in patients with gastrointestinal cancers. Cancer Discov 2019;9:1022–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robinson RA, McMurran C, McCully ML, Cole DK. Engineering soluble T-cell receptors for therapy. Febs J 2021;288:6159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boulter JM, Glick M, Todorov PT, Baston E, Sami M, Rizkallah P, et al. Stable, soluble T-cell receptor molecules for crystallization and therapeutics. Protein Eng Des Sel. 2003;16:707–11. [DOI] [PubMed] [Google Scholar]
- 27. Bunk S, Hofmann M, Unverdorben F, Hutt M, Pszolla G, Schwöbel F, et al. Effective targeting of PRAME-positive tumors with bispecific T-cell–engaging receptor (TCER®) molecules. Blood 2019;134:3368–. [Google Scholar]
- 28. Richman SA, Kranz DM. Display, engineering, and applications of antigen-specific T-cell receptors. Biomol Eng 2007;24:361–73. [DOI] [PubMed] [Google Scholar]
- 29. Li Y, Moysey R, Molloy PE, Vuidepot A-L, Mahon T, Baston E, et al. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol 2005;23:349–54. [DOI] [PubMed] [Google Scholar]
- 30. Wooldridge L, Ekeruche-Makinde J, van den Berg HA, Skowera A, Miles JJ, Tan MP, et al. A single autoimmune T-cell receptor recognizes more than a million different peptides. J Biological Chem 2012;287:1168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, et al. Identification of a Titin-Derived HLA-A1–presented peptide as a cross-reactive target for engineered MAGE A3–directed T cells. Sci Transl Med 2013;5:197ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martin AD, Wang X, Sandberg ML, Negri KR, Wu ML, Warshaviak DT, et al. Re-examination of MAGE-A3 as a T-cell therapeutic target. J Immunother 2021;44:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 2013;36:133–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holland CJ, Crean RM, Pentier JM, de Wet B, Lloyd A, Srikannathasan V, et al. Specificity of bispecific T-cell receptors and antibodies targeting peptide-HLA. J Clin Invest 2020;130:2673–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hellman LM, Foley KC, Singh NK, Alonso JA, Riley TP, Devlin JR, et al. Improving T-cell receptor on-target specificity via structure-guided design. Mol Ther 2019;27:300–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harper J, Adams KJ, Bossi G, Wright DE, Stacey AR, Bedke N, et al. An approved in vitro approach to preclinical safety and efficacy evaluation of engineered T-cell receptor anti-CD3 bispecific (ImmTAC) molecules. PLoS ONE 2018;13:e0205491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T-cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol 2003;4:749–55. [DOI] [PubMed] [Google Scholar]
- 38. Chen L, Flies DB. Molecular mechanisms of T-cell co-stimulation and co-inhibition. Nat Rev Immunol 2013;13:227–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dickopf S, Buldun C, Vasic V, Georges G, Hage C, Mayer K, et al. Prodrug-Activating Chain Exchange (PACE) converts targeted prodrug derivatives to functional bi- or multispecific antibodies. Biol Chem 2022;403:495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Laugel B, van den Berg HA, Gostick E, Cole DK, Wooldridge L, Boulter J, et al. Different T-cell receptor affinity thresholds and CD8 coreceptor dependence govern cytotoxic T lymphocyte activation and tetramer binding properties. J Biol Chem 2007;282:23799–810. [DOI] [PubMed] [Google Scholar]
- 41. Dickopf S, Georges GJ, Brinkmann U. Format and geometries matter: structure-based design defines the functionality of bispecific antibodies. Comput Struct Biotechnology J 2020;18:1221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Orloff M, Sacco JJ, Nathan P, Holland C, Cohen C, Harper J, et al. Abstract 3295: vitiligo and other clinical melanocyte-related adverse events following tebentafusp (IMCgp100) exposure in patients with uveal melanoma. Cancer Res 2020;80;3295. [Google Scholar]
- 43. Butler MO, Stanhope S, Naidoo R, Leach E, Kaur S, Collins L, et al. Abstract 517: tebentafusp induces transient systemic inflammation and modifies the micro-environment to sensitize uveal melanoma tumors to cytotoxic CD8 cells. Cancer Res 2021;81:517.33479028 [Google Scholar]
- 44. Khanolkar R, Naidoo R, Güç E, Leach E, Stanhope S, Gascoyne D, et al. 571 IL2 combination with ImmTAC overcomes CD163 +TAM-like M2 macrophage inhibition of ImmTAC-mediated T-cell killing of tumor cells. J Immunother Cancer 2021;9:A600. [Google Scholar]
- 45. Petrovic K, Depoil D, Gascoyne DM, Page K, Curnock A, Benlahrech A. 1016P ImmTAC redirect exhausted tumor-infiltrating T cells: an effect enhanced by pembrolizumab against PD-L1 +tumors. Ann Oncol 2021;32:S856. [Google Scholar]
- 46. Middleton MR, Steven NM, Evans TJ, Infante JR, Sznol M, Mulatero C, et al. Safety, pharmacokinetics and efficacy of IMCgp100, a first-in-class soluble TCR-antiCD3 bispecific T-cell redirector with solid tumour activity: results from the FIH study in melanoma. J Clin Oncol 2016;34:3016. [Google Scholar]
- 47. Carvajal RD, Nathan P, Sacco JJ, Orloff M, Hernandez-Aya LF, Yang J, et al. Phase I study of safety, tolerability, and efficacy of tebentafusp using a step-up dosing regimen and expansion in patients with metastatic uveal melanoma. J Clin Oncol 2022;40:1939–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sacco JJ, Carvajal R, Butler MO, Shoushtari AN, Hassel JC, Ikeguchi A, et al. 64MO A phase (ph) II, multi-center study of the safety and efficacy of tebentafusp (tebe) (IMCgp100) in patients (pts) with metastatic uveal melanoma (mUM). Ann Oncol 2020;31:S1442–3. [Google Scholar]
- 49. Stäger R, Stanhope S, Greenshields-Watson A, Collins L, Ramelyte E, Kolm I, et al. 1772P demonstration of T-cell redirection and immune activation in skin rash following tebentafusp treatment. Ann Oncol 2021;32:S1215. [Google Scholar]
- 50. Hassel JC, Rutkowski P, Baurain J-F, Butler MO, Schlaak M, Sullivan R, et al. Co-primary endpoint of overall survival for tebentafusp (tebe)-induced rash in a phase 3 randomized trial comparing tebe versus investigator's choice (IC) in first-line metastatic uveal melanoma. J Clin Oncol 2021;39:9527. [Google Scholar]
- 51. Salama AKS, Cheshuk V, Siveke J, Berrocal A, Abdullah SE, Lockwood S, et al. 1014P Characterization of cytokine release syndrome (CRS) following treatment with tebentafusp in previously untreated patients with metastatic uveal melanoma. Ann Oncol 2021;32:S855. [Google Scholar]
- 52. Butler MO, Sato T, Carvajal RD, Sacco JJ, Abdullah SE, Holland C, et al. Abstract CT038: kinetics of radiographic response for tebentafusp (tebe) in previously treated metastatic uveal melanoma (mUM) patients (pts) achieving prolonged survival. Cancer Res 2021;81:CT038. [Google Scholar]
- 53. Orloff M, Carvajal RD, Shoushtari AN, Sacco JJ, Schlaak M, Watkins C, et al. Overall survival in patients who received checkpoint inhibitors after completing tebentafusp in a phase 3 randomized trial of first-line metastatic uveal melanoma. J Clin Oncol 2021;39:9526. [Google Scholar]
- 54. Leach E, Stanhope S, Naidoo R, Abdullah S, Collins L, Ranade K. 868 Overall survival on tebentafusp in metastatic uveal melanoma (mUM) across the range of tumor gp100 expression levels. J Immunother Cancer 2021;9:A909. [Google Scholar]
- 55. Davar D, Sweis RF, Blumenschein G, Gutierrez R, Melero I, Chen HA, et al. 91P Phase I dose escalation of IMC-C103C, a CD3×MAGE-A4 T-cell receptor (TCR) bispecific protein. Ann Oncol 2021;32:S1411–3. [Google Scholar]
- 56. Bossi G, Gerry AB, Paston SJ, Sutton DH, Hassan NJ, Jakobsen BK. Examining the presentation of tumor-associated antigens on peptide-pulsed T2 cells. Oncoimmunology 2013;2:e26840. [DOI] [PMC free article] [PubMed] [Google Scholar]