Abstract
Purpose:
A novel single-dose regimen of 300 mg tremelimumab in combination with durvalumab [Single Tremelimumab Regular Interval Durvalumab (STRIDE)] has demonstrated a favorable benefit-risk profile in the phase I/II Study 22 (NCT02519348) and phase III HIMALAYA study (NCT03298451). This study evaluated the pharmacokinetics, exposure–response, and exposure–pharmacodynamics relationships of tremelimumab in patients with unresectable hepatocellular carcinoma (uHCC).
Patients and Methods:
A previous tremelimumab population pharmacokinetic model was validated using data from parts 2 and 3 of Study 22. Exposure–response analyses explored relationships of tremelimumab exposure with efficacy and safety. Pharmacokinetics and pharmacodynamics relationships were evaluated using linear and nonlinear regression models.
Results:
The observed pharmacokinetics of tremelimumab in uHCC were consistent with predictions; no significant covariates were identified. Tremelimumab exposure was not significantly associated with adverse events, objective response rate, or progression-free survival. Overall survival (OS) was longer for patients with tremelimumab exposure, minimum serum drug concentration (Cmin1) ≥ median versus Cmin1 < median (18.99 months vs. 10.97 months), but this exposure-survival analysis might be confounded with baseline characteristics of albumin level and neutrophil to lymphocyte ratio, which had a significant impact on OS (P = 0.0004 and 0.0001, respectively). The predicted Cmin1 of tremelimumab in STRIDE regimen (12.9 μg/mL) was greater than the estimated concentration of tremelimumab eliciting half-maximal increases (EC50 = 5.24 μg/mL) in CD8+Ki67+ T-cell counts.
Conclusions:
Our findings support novel insights into tremelimumab pharmacokinetics and exposure–response relationships in HCC and support the clinical utility of the STRIDE regimen in patients with uHCC.
Translational Relevance.
A novel single-dose regimen of 300 mg tremelimumab in combination with durvalumab (STRIDE) demonstrated a favorable benefit-risk profile in patients with unresectable hepatocellular carcinoma (uHCC) in the phase I/II Study 22, which was confirmed by the results of the phase III HIMALAYA study of uHCC. This analysis of Study 22 is the first to evaluate the pharmacokinetics, exposure–response, and exposure–pharmacodynamics relationships of tremelimumab in patients with uHCC. Tremelimumab exposure was not significantly associated with adverse events, objective response rate, or progression-free survival. The predicted minimum serum tremelimumab concentration in STRIDE was greater than the estimated concentration of tremelimumab, eliciting half-maximal increases in CD8+Ki67+ T cells, which supports the hypothesis that the STRIDE regimen induces a substantial and relevant immune response in patients with uHCC. These data present key insights into tremelimumab pharmacokinetics and exposure–response relationships in patients with uHCC and support the clinical utility of the STRIDE regimen.
Introduction
Liver cancer is the sixth most common cancer and third leading cause of cancer-related death worldwide (1, 2). Hepatocellular carcinoma (HCC) is the most common type of liver cancer. In the United States, 45% of patients with HCC present with cancer that has spread to regional lymph nodes, or with cancer that has metastasized (3, 4). Treatment options for unresectable HCC (uHCC) include embolization for intermediate stage disease, and systemic therapy for advanced disease, including the tyrosine kinase inhibitors, sorafenib and lenvatinib (5, 6). The 5-year survival rates in 2012 to 2018 for treatment of uHCC were low at 12.8% for cancer that had spread to regional lymph nodes and 3.1% for cancer that had metastasized (4). However, immunotherapies have been approved as a treatment option for uHCC (6), giving hope for improved 5-year survival rates.
Upregulation of immune checkpoint molecules is known to contribute to the immunosuppressive environment of HCC and enable escape from the antitumor immune response. Immune checkpoint inhibitors (ICI), including cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed cell death-1 (PD-1), and programmed cell death ligand-1 (PD-L1), have therefore been a key area of investigation for novel treatment strategies in HCC (7). Pembrolizumab, an anti-PD-1 antibody, was granted accelerated approval by the US FDA in 2018 for the treatment of patients with HCC who have been previously treated with sorafenib based on the results of the phase II KEYNOTE-224 study (8–10). Similarly, nivolumab, another PD-1 inhibitor, in combination with ipilimumab, a CTLA-4 inhibitor, was also granted accelerated approval by the FDA in 2020 for patients with uHCC who have previously been treated with sorafenib (9, 11, 12). In 2020, the combination of atezolizumab, an anti-PD-L1 antibody, and bevacizumab, a VEGF inhibitor, was approved by the FDA for the treatment of patients with unresectable or metastatic HCC who have not received prior systemic therapy (9, 13).
Tremelimumab, an anti-CTLA-4 antibody, and durvalumab, an anti-PD-L1 antibody, are novel immunotherapies under investigation for the treatment of HCC and have shown efficacy and tolerable safety profiles as monotherapies in HCC (14, 15). As CTLA-4 and PD-L1 function independently of each other to promote immunosuppression in HCC (7), there is strong rationale to combine these therapies to produce improved antitumor efficacy. This combination strategy is under investigation in multiple tumor types, including non–small cell lung cancer (NSCLC; refs. 16–18), and bladder cancer (19, 20). Despite their promising efficacy in multiple tumor types, ICIs are frequently associated with immune-mediated adverse events which appear to be more common with anti-CTLA-4 therapy than with anti-PD-1/PD-L1 therapy (21). In addition, combination strategies are associated with greater toxicity, compared with single-agent ICIs (21). Establishing combination dosing regimens that aim to limit toxicity while delivering antitumor efficacy greater than the efficacy of monotherapy is supported by an understanding of the pharmacokinetics (PK), pharmacodynamics (PD), and exposure–response relationships of these agents.
Study 22 is a multipart phase I/II study of tremelimumab and durvalumab as monotherapy or in combination in immunotherapy-naïve patients with uHCC who had progressed on, were intolerant to, or refused, sorafenib (NCT02519348; ref. 22). Part 1 of the study involved a safety run-in and efficacy gating of tremelimumab 75 mg plus durvalumab 1,500 mg once every 4 weeks for a total of 4 doses, followed by durvalumab monotherapy every 4 weeks (T75+D). In part 2 of the study, patients were randomized to receive either durvalumab 1,500 mg every 4 weeks (durvalumab monotherapy); tremelimumab 750 mg every 4 weeks for a total of 7 doses then once every 12 weeks (tremelimumab monotherapy); the T75+D regimen; or a safety run-in of a novel combination regimen: a single dose of tremelimumab 300 mg with durvalumab 1,500 mg, followed by durvalumab 1,500 mg every 4 weeks [Single Tremelimumab Regular Interval Durvalumab (STRIDE) or T300+D]. In part 3 of the study, patients were randomized to receive either durvalumab monotherapy, tremelimumab monotherapy, the T75+D regimen, or the STRIDE combination regimen. Results from parts 2 and 3 of the study showed that the STRIDE regimen had the highest objective response rate (ORR; 24.0%) compared with durvalumab (10.6%) or tremelimumab (7.2%) monotherapies, or T75+D (9.5%). STRIDE also demonstrated the longest median overall survival (OS; 18.7 months) compared with durvalumab (13.6 months) or tremelimumab (15.1 months) monotherapies, or T75+D (11.3 months; ref. 22). The frequency of Grade ≥ 3 treatment-related adverse events (TRAE) was highest with tremelimumab monotherapy (43.5%) and second highest with STRIDE (37.8%). Treatment-related deaths occurred in 3 patients who received durvalumab monotherapy, 1 patient who received T75+D, and 1 patient who received STRIDE (22). These results suggest that the novel STRIDE regimen provides the most favorable benefit-risk profile compared with either monotherapy or T75+D. On the basis of the findings of Study 22, the STRIDE regimen was further evaluated in the phase III HIMALAYA study (NCT03298451) in patients with uHCC who had not been previously treated with systemic therapy (23). The recently reported results of the HIMALAYA study demonstrated that the STRIDE regimen significantly improved OS versus sorafenib [HR, 0.78; 96% confidence interval (CI), 0.65–0.92; P = 0.0035], and durvalumab monotherapy was noninferior to sorafenib for OS (HR, 0.86; 96% CI, 0.73–1.03; ref. 23).
The objective of the analysis reported here was to evaluate the PK of tremelimumab and the relationships between tremelimumab PK exposure and safety, efficacy, and PD in patients treated with tremelimumab monotherapy, the T75+D regimen, or the STRIDE regimen in parts 2 and 3 of Study 22.
Patients and Methods
Patient population and study design
Patients included in these analyses were immune checkpoint therapy-naïve patients aged ≥18 years (≥20 years in Japan) with histologically confirmed uHCC who had progressed on, were intolerant to, or refused, sorafenib (22). In Study 22, patients were randomized in part 2a to receive durvalumab monotherapy, tremelimumab monotherapy, or the T75+D regimen; were allocated in part 2b to receive the STRIDE regimen; or were randomized in part 3 across the four treatment arms. Enrollment to the T75+D arm was closed following a protocol amendment (22). Data obtained from all tremelimumab-containing arms of Study 22 were used to assess the PK of tremelimumab and analyze tremelimumab exposure–response relationships, including efficacy, safety, and PD.
Detailed study design and patient eligibility criteria for Study 22 were published previously (22). The primary objective of Study 22 was to assess safety, the secondary objective was to assess efficacy, and PK/PD evaluations were exploratory objectives. Study 22 was conducted in accordance with the Declaration of Helsinki and was consistent with International Conference on Harmonisation and Good Clinical Practice guidelines, and applicable regulatory requirements. Written informed consent from participants was obtained before performing any protocol-related procedures.
PK assessments
Measurement of tremelimumab concentration in serum samples was performed using validated immunoassays. For PK analysis, blood samples were obtained from patients’ pre-dose and 10 minutes after end of infusion (EOI) at week 1 day 1 and week 13 day 1 (±3 days), pre-dose at week 5 day 1 (± 3 days), and week 25 day 1 (±3 days) of the study treatment period. Blood samples collected at the week 25 visit served as the 90-day post-last dose tremelimumab sample for all subjects assigned to the T75+D regimen who completed the full four-cycle course of tremelimumab. Blood samples were obtained from patients receiving the STRIDE regimen for tremelimumab PK at pre-dose and 10 minutes after EOI at week 1 day 1, anytime at week 5 day 1, and anytime at week 13 day 1, which also served as the 90-day post-last dose tremelimumab sample. In addition, blood samples were also obtained from Japanese patients receiving the STRIDE regimen in part 2b for tremelimumab PK at pre-dose and 10 minutes after EOI at week 2 day 1 (±1 day), week 3 day 1, and week 4 day 1 (±1 day).
The data from patients receiving tremelimumab as monotherapy or in combination with durvalumab in Study 22 were used to validate a previous tremelimumab population PK model based on multiple tumor types (24). A post hoc analysis was performed to assess the impact of existing covariates in the model, as well as additional covariates of interest, on individual PK parameters to evaluate fixed-dose regimens for tremelimumab, either as monotherapy or in combination with durvalumab. Predicted tremelimumab concentrations for validation subjects were obtained by fixing the parameters in the structural and variance model to the parameter estimates in the final model using post hoc Bayesian forecasting with NONMEM 7 (version 7.4.3; ICON, Ellicott City, Maryland, USA), with the run set at MAXEVEL = 0. Tremelimumab serum concentration–time profiles were simulated using the Bayesian post hoc individual PK parameters following first tremelimumab dose. The model-predicted Cmin1 and steady-state AUCss, Cmax,ss, and Cmin,ss were computed for each patient in Study 22. Cmin1 is the minimum tremelimumab concentration at day 28. AUCss is the AUC versus time curve from day 84 to day 112, calculated using the linear up/log down variant of the trapezoidal rule. Cmax,ss is the maximum tremelimumab concentration from day 84 to 112 and Cmin,ss is the minimum tremelimumab concentration at day 112. Because the STRIDE regimen includes only a single dose of tremelimumab, AUCss, Cmax,ss, and Cmin,ss steady-state parameters for tremelimumab could not be computed for the STRIDE regimen thus only the model-predicted Cmin1 was computed for the STRIDE regimen.
Exposure–response assessments
Safety endpoints used in the exposure–response analysis were Grade 3/4 TRAEs, Grade 3/4 drug-related AEs of special interest (AESI), and AEs leading to treatment discontinuation. AESIs include, but are not limited to, events with a potential inflammatory or immune-mediated mechanism and which may require more frequent monitoring and/or interventions such as steroids, immunosuppressants, and/or hormone replacement therapy.
Efficacy endpoints used in the exposure–response analysis were OS, progression-free survival (PFS), and ORR. Tremelimumab serum concentration–time profiles for each patient in Study 22 were simulated using the Bayesian post hoc individual PK parameters following actual tremelimumab dose. The model-predicted Cmin1 (as described above) were computed and explored with the corresponding efficacy or safety data for exposure–response analysis.
The exposure–response relationships for the time-to-event variables of OS and PFS were explored by Kaplan–Meier (KM) estimates and were analyzed by Cox proportional hazard models if an exposure–response trend was observed:
where hi(t) is the hazard function for subject i at time t, h0(t) is the baseline hazard function, θX is the coefficient for a binary covariate Χi, θϕ is the coefficient for a continuous covariate ϕi, centered at the median covariate value ϕmed, and θPK is the coefficient for the exposure value PKi. Continuous covariates and exposure metrics could be used as their original values or transformed values (e.g., log-transformed) if deemed appropriate depending on their distribution.
The confounding effects of baseline characteristics, including Eastern Cooperative Oncology Group performance status, baseline lactate dehydrogenase, baseline tumor size per investigator (sum of the longest diameters of target lesions, measured with CT or MRI), baseline alpha-fetoprotein, liver disease type (hepatitis B virus, hepatitis C virus, uninfected, or unknown), baseline serum albumin level, baseline neutrophil-to-lymphocyte ratio (NLR), combination therapy, and treatment arm, on exposure-response relationships were further investigated, if necessary, via covariate testing in the Cox proportional hazard model (25). Cox proportional hazard modeling is an effective approach to assess confounding factors in exposure–response analyses (26), and has been used in previous studies of the antibody–drug conjugate, trastuzumab emtansine to identify baseline factors impacting apparent exposure–OS relationships (27). Covariates were selected using a forward addition and backward elimination method based on the significance level of P < 0.05.
The exposure–response relationships for ORR and safety endpoints were evaluated as binary outcomes (yes/no), where objective responders are those patients whose best overall response was either confirmed complete response or confirmed partial response, otherwise, patients are classified as non-objective responders. Boxplots of exposures stratified by response outcomes were generated. The probability of events was calculated and plotted across exposure quantiles in tremelimumab-treated patients. If an apparent relationship was evident based on the exploratory analysis, the binary outcome would be further analyzed with linear logistic regression models:
where logit is the logit transform and Pri is the probability of response for patient i. θX is the coefficient for a binary covariate Χi, θϕ is the coefficient for a continuous covariate ϕi, centered at the median covariate value ϕmed, and θPK is the coefficient for the exposure value PKi.
PD assessments
The data from patients receiving tremelimumab as monotherapy or in combination with durvalumab in Study 22 was used to explore PD relationships with durvalumab monotherapy as a control. Flow cytometry-based immunophenotyping assays were used to quantify circulating lymphocyte subsets as reported in Kelley and colleagues (22). PD analyses focused on CD8+Ki67+ T cells as (i) this population is known to be a pharmacodynamic and/or a predictive biomarker in patients treated with PD-(L)1 inhibitors and anti-CTLA-4 therapies alone or in combination (28–30) and (ii) while multiple populations were statistically associated with radiographic response (complete response or partial response) in Study 22, all were populations in which CD8+Ki67+ T cells are highly prevalent (Supplementary Table S1). PD was assessed as change from baseline (%CFB) on day 15 of proliferating CD8+Ki67+ T cells, and PD relationships were explored through linear and nonlinear regression models.
Data availability
Data from the Study 22 clinical trial are available at: https://www.clinicaltrials.gov/ct2/show/results/NCT02519348.
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Anonymized datasets may be available on request. Data for studies directly listed on Vivli can be requested through Vivli at https://search.vivli.org/; data for studies not listed on Vivli may be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. The request will undergo an internal review process, and if approved, data will be prepared and shared with specified accessors named on the request form for 12 months via Vivli Secure Research Environment.
Results
Patient characteristics
Of the 228 patients who were enrolled in Study 22 and received tremelimumab, either as monotherapy or in combination with durvalumab, 220 were included in the PK analysis (66/69 in the tremelimumab monotherapy arm, 80/84 in the T75+D arm, and 74/75 in the STRIDE arm), 216 were included in the exposure–response analysis (65/69 in the tremelimumab monotherapy arm, 79/84 in the T75+D arm, and 72/75 in the STRIDE arm), and 165 were included in the PD analysis (23/69 in the tremelimumab monotherapy arm, 51/104 in the durvalumab monotherapy arm, 31/84 in the T75+D arm, and 60/75 in the STRIDE arm; Supplementary Fig. S1). Baseline characteristics for all patients enrolled in parts 2 and 3 of Study 22 have been previously reported by Kelley and colleagues (22) and were generally consistent across the three tremelimumab-containing treatment arms. In the exposure–response analysis population, median baseline tumor size (range) was 67 (13–270) mm, 63 (11–324) mm, and 75 (12–288) mm in the tremelimumab monotherapy arm, T75+D arm, and the STRIDE arm, respectively. Median baseline NLR (range) was 3.2 (0.6–13.2), 3.0 (1.1–12.7), and 3.5 (1.2–253.3), in the tremelimumab monotherapy arm (n = 53/65), T75+D arm (n = 65/79), and the STRIDE arm (n = 52/72), respectively. Median baseline albumin level (g/L; range) was 38 (28–48), 37 (27–50), and 37 (26–48) in the tremelimumab monotherapy arm (n = 65/65), T75+D arm (n = 79/79), and the STRIDE arm (n = 72/72), respectively.
Tremelimumab PK
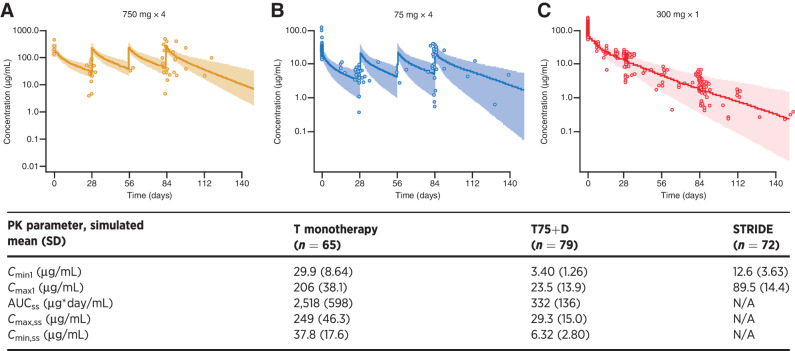
Observed versus predicted tremelimumab exposure is shown in Fig. 1. The observed tremelimumab PK data in patients with uHCC were generally consistent with predictions from a historical population PK model based on other solid tumor types (24). A post hoc covariate analysis indicated that the existing covariate–parameter relationships in the model, including body weight on clearance and central volume, baseline albumin on clearance, and combination therapy on time-varying clearance, described well the Study 22 data. For the tremelimumab exposure metric Cmin1, the difference in geometric mean of the model-predicted value from the population geometric mean estimate at the top 10th percentile and the bottom 10th percentile of the covariate distribution, or across covariate categories, was up to 28.9%, 25.9%, and 3.44% relative change in exposure compared with the reference value for body weight, baseline albumin level, and combination therapy, respectively (Supplementary Fig. S2). Additional covariates evaluated in the post hoc covariate analysis, including race, region of enrollment, country of enrollment, renal function, and antidrug antibody (ADA) to tremelimumab status, did not show a statistically significant impact on the PK of tremelimumab. Moreover, ADA status did not have a significant impact on clearance (P = 0.148, ANOVA).
Figure 1.
Observed versus predicted tremelimumab exposure of (A) tremelimumab 750 mg at steady state after 4 doses, (B) tremelimumab 75 mg at steady state after four doses, and (C) tremelimumab 300 mg after first dose (PK analysis population). N = 216. Symbols are the observed tremelimumab concentrations. Line and shaded area are the model-predicted median and 95% CI. AUCss, area under the concentration vs. time curve from day 84 to day 112 following actual tremelimumab dose in Study 22 and calculated using the linear up/log down variant of the trapezoidal rule; CI, confidence interval; Cmax1, maximum tremelimumab concentration after first dose; Cmax,ss, maximum tremelimumab concentration from day 84 to 112; Cmin1, minimum tremelimumab concentration at day 28; Cmin,ss, minimum tremelimumab concentration at day 112; N, number of patients included in the analysis; N/A, not applicable; STRIDE (single dose of tremelimumab 300 mg with durvalumab 1,500 mg, followed by durvalumab 1,500 mg once every 4 weeks); T, tremelimumab monotherapy (tremelimumab 750 mg every 4 weeks for a total of 7 doses then once every 12 weeks); T75+D, tremelimumab 75 mg plus durvalumab 1,500 mg once every 4 weeks for a total of 4 doses, followed by durvalumab monotherapy once every 4 weeks.
Tremelimumab exposure–response relationships
For the exposure-safety analysis that included 216 patients in Study 22, the exposure ranges appeared to be similar in patients who experienced AEs relative to those who did not. The probability of AE plots and logistic regression models showed no evident exposure–response relationships between tremelimumab Cmin1 and the probability of Grade 3/4 TRAEs (P = 0.797), or AEs leading to treatment discontinuation (P = 0.885; Fig. 2A and C). The probability of Grade 3/4 AESIs trends to be significant with higher tremelimumab exposure in the pooled tremelimumab cohort, as measured by Cmin1 (P = 0.037; Fig. 2B).
Figure 2.
Tremelimumab exposure-safety analysis for Cmin1 and (A) Grade 3/4 treatment-related AEs, (B) Grade 3/4 treatment-related AESI, and (C) AEs leading to treatment discontinuation (exposure–response analysis population); N = 216. Open blue circles are the observed events. Filled black circles are the observed probability of events and the error bars are the standard errors (calculated as sqrt [P × (1 − P)/n], where P is probability of response and n is the number of patients in each quantile bin) for quantiles [at 100 × (1/4)th percentiles, vertical dotted lines] of exposures (plotted at the median value within each quantile). The red solid line is the model-predicted probability, and the shaded area is the 95% prediction interval. AE, adverse event; AESI, adverse event of special interest; Cmin1, minimum tremelimumab concentration at day 28; N, number of patients included in the analysis.
In the exposure–ORR analysis, the median values of Cmin1 were similar between responders and nonresponders based on 208 patients in Study 22. The probability of response plots for ORR indicated that none of the tremelimumab exposure metrics had a statistically significant exposure–ORR relationship. As shown in Fig. 3, the probability of ORR peaks in the intermediate quantile of the Cmin1 tremelimumab exposure metric. In addition, the proportion of patients achieving complete response or partial response was greatest in the STRIDE arm [26.5% (yes/no: 18/50)] compared with the tremelimumab monotherapy arm [7.94% (yes/no: 5/58)] or the T75+D arm [10.4% (yes/no: 8/69)].
Figure 3.
Tremelimumab exposure-ORR analysis at Cmin1 (exposure–response analysis population); N = 208. Open blue circles reflect the observed events. Filled black circles are the observed probability of ORR and the error bars are the standard errors (calculated as sqrt [P*(1 − P)/n], where P is probability of response and n is the number of patients in each quantile bin) for quantiles [at 100 × (1/5)th percentiles, vertical dotted lines] of exposures (plotted at the median value within each quantile). The red lines are smooth curves (loess) to show the relationship between two variables. Cmin1, minimum tremelimumab concentration at day 28; N, number of patients included in the analysis. ORR, objective response rate.
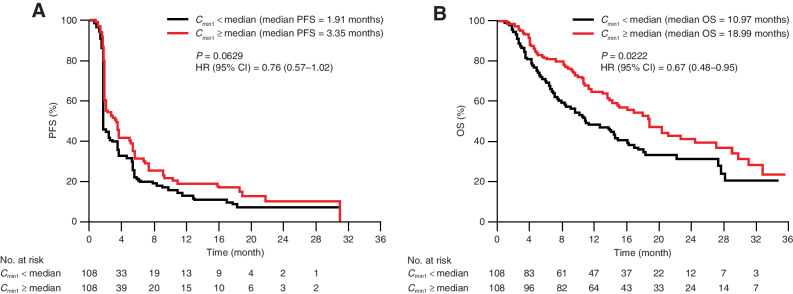
Median PFS (95% CI) was numerically longer with tremelimumab exposure where Cmin1 ≥ median compared with Cmin1 < median [3.35 (95% CI, 2.04–5.29) vs. 1.91 (95% CI, 1.81–3.48) months] in the KM plot, suggesting there is no clear association between tremelimumab exposure and PFS (Fig. 4A). In addition, the Cox proportional hazard model showed no statistically significant relationship between PFS and any of the tremelimumab exposure metrics.
Figure 4.
Tremelimumab exposure-efficacy analysis stratified by Cmin1 for (A) PFS and (B) OS (exposure-response analysis population); N = 216. Median Cmin1 was 12 μg/mL. CI, confidence interval; Cmin1, minimum tremelimumab concentration at day 28; HR, hazard ratio; N, number of patients included in the analysis; OS, overall survival; PFS, progression-free survival.
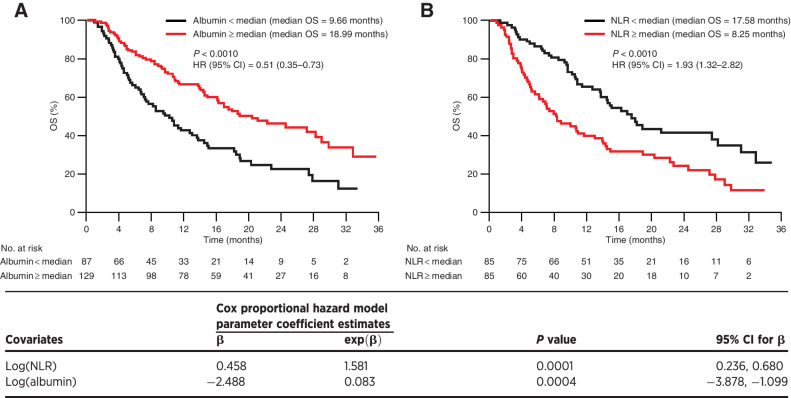
Median OS (95% CI) was longer for patients with tremelimumab exposure Cmin1 ≥ median compared with Cmin1 <median [18.99 (95% CI, 14.88–29.14) vs. 10.97 (95% CI, 8.38–16.33) months; Fig. 4B]. As tremelimumab exposure appeared to impact OS of patients with uHCC, baseline characteristics were assessed for their impact on OS to determine if the increase in median OS was directly attributed to tremelimumab exposure or other confounding factors. The Cox proportional hazard model analysis showed that baseline serum albumin level and NLR had a significant impact on OS in tremelimumab-treated patients in Study 22. Median OS was significantly longer in patients with higher baseline albumin (≥ 37.0 g/L, cut-off) compared with lower baseline albumin [<37.0 g/L; 18.99 (95% CI, 16.16–29.14) vs. 9.66 (95% CI, 7.13–13.77) months; P = 0.0004]. Median OS was significantly longer in patients with a lower baseline NLR (<3.22, cut-off) compared with a higher baseline NLR [≥3.22; 17.58 (95% CI, 13.77–31.18) vs. 8.25 (95% CI, 6.14–14.00) months; P = 0.0001; Fig. 5]. The Cox proportional hazard model analysis showed that after accounting for confounding baseline characteristics of baseline albumin level, and NLR, tremelimumab Cmin1 was not a significant factor for OS hazard. Therefore, the observed trend of longer median OS with higher tremelimumab exposure might be confounded with baseline characteristics of higher albumin level and a lower NLR. However, higher median NLRs at baseline were observed for both the T monotherapy and STRIDE arms compared with the T75+D arm. Within treatment arm comparisons adjusting for these baseline variables could not be further explored due to small sample size.
Figure 5.
OS stratified by baseline covariates (A) albumin and (B) NLR (exposure–response analysis population). Median baseline albumin level = 37.0 g/L, N = 216. Median baseline NLR = 3.22, N = 170. Low NLR and low albumin are the reference in the Cox proportional hazard models. CI, confidence interval; HR, hazard ratio; N, number of patients included in the analysis; NLR, neutrophil to lymphocyte ratio; OS, overall survival.
Tremelimumab PD
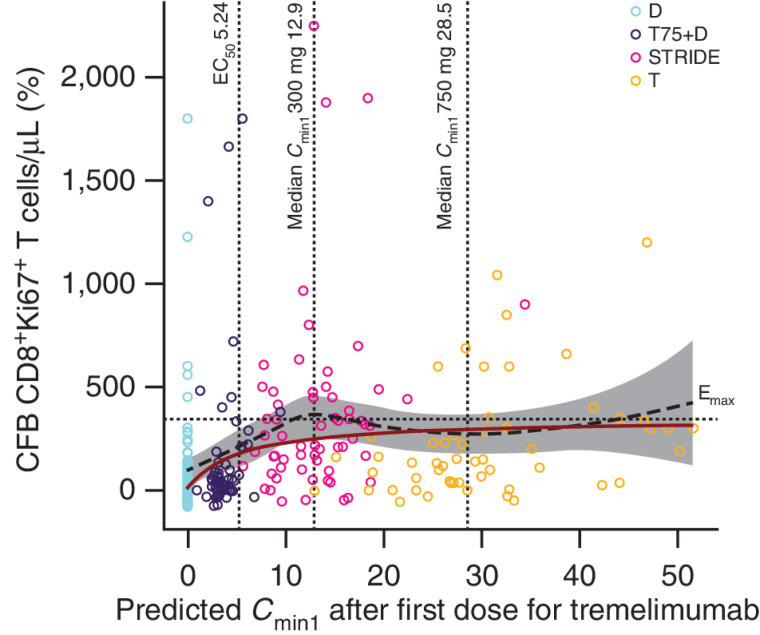
A saturable PD relationship was observed between tremelimumab exposure and %CFB in CD8+Ki67+ T-cell count (Fig. 6). Analysis of %CFB in CD8+Ki67+ T-cell count on day 15 suggested an Emax model best described the relationship with tremelimumab exposure (Cmin1), where durvalumab monotherapy was used as a control. The Emax model predicted a 250%CFB in CD8+Ki67+ T-cell count for STRIDE and 295%CFB in CD8+Ki67+ T-cell count for tremelimumab 750 mg monotherapy at median Cmin1 and median CD8+Ki67+ T-cell count. In the PD model, the EC50 of tremelimumab was 5.24 μg/mL (95% CI, 1.37–20.1) and the Emax was 341% (95% CI, 208%–474%). The predicted Cmin1 of tremelimumab in the T75+D arm (75 mg, multiple doses) was below the estimated EC50, whereas the predicted median Cmin1 in the STRIDE (300 mg, single dose) and tremelimumab monotherapy (750 mg, multiple doses) arms were much higher than the EC50, at 12.9 and 28.5 μg/mL, respectively (Fig. 6). In addition, across all four treatment arms, the maximum effect of tremelimumab, described by the %CFB in CD8+Ki67+ T cells, was greater in patients with lower baseline CD8+Ki67+ T-cell counts and lower in patients with higher baseline CD8+Ki67+ T-cell counts (Supplementary Fig. S3).
Figure 6.
CFB on day 15 of proliferating CD8+Ki67+ T cells versus predicted tremelimumab exposure (PD analysis population); N = 165. The black dashed line and gray-shaded area represent a loess smooth curve and 95% CI for the observed data. The dark red line is the model-predicted response (%CFB) at median CD8+Ki67+ T-cell count. The horizontal dotted line indicates the estimated Emax. The vertical lines indicate the predicted EC50 and the median Cmin1 after first dose (day 28) for 300 and 750 mg tremelimumab. T-cell response to durvalumab is included in the graph as a baseline measurement. Durvalumab monotherapy was included in this analysis as a control. CFB, change from baseline; Cmin1, minimum tremelimumab concentration at day 28; D, durvalumab monotherapy (durvalumab 1,500 mg once every 4 weeks); Emax, maximal effect of tremelimumab on CD8+Ki67+ T-cell counts; EC50, concentration of tremelimumab eliciting half-maximal increases in CD8+Ki67+ T-cell counts; N, number of patients included in the analysis; PD, pharmacodynamics; STRIDE, Single Tremelimumab Regular Interval Durvalumab (single dose of tremelimumab 300 mg with durvalumab 1,500 mg, followed by durvalumab 1,500 mg once every 4 weeks); T, tremelimumab monotherapy (tremelimumab 750 mg once every 4 weeks for a total of seven doses then once every 12 weeks); T75+D, tremelimumab 75 mg plus durvalumab 1,500 mg once every 4 weeks for a total of four doses, followed by durvalumab monotherapy once every 4 weeks; T300+D.
Discussion
This is the first study to demonstrate the PK of tremelimumab in a cohort of patients with uHCC treated with tremelimumab monotherapy or in combination with durvalumab. In this study, observed tremelimumab PK concentrations in tremelimumab-treated patients were consistent with previously predicted profiles, and no additional statistically significant PK parameter–covariate relationships were identified. These results are consistent with a previous analysis of tremelimumab PK in patients with mesothelioma, in which no covariates were found to be clinically relevant in terms of effect on tremelimumab exposure (31).
Tremelimumab has demonstrated a tolerable safety profile across different tumor types, when administered alone or in combination with durvalumab (28, 32). In Study 22, the three dosing regimens of tremelimumab: tremelimumab monotherapy (750 mg), T75+D (75 mg), and STRIDE (300 mg), all demonstrated manageable toxicity in patients with uHCC, with a low rate of TRAEs leading to discontinuation (22). Although a higher frequency of TRAEs, serious TRAEs, and TRAEs requiring steroids was observed for tremelimumab-containing arms compared with the durvalumab monotherapy arm (22), exposure-safety analyses showed that there was no evident clinically relevant exposure–response relationship between tremelimumab and safety outcomes based on the selected safety endpoints from Study 22. To our knowledge, tremelimumab exposure-safety relationships have not been previously reported for any tumor type. The lack of significant exposure–safety relationships is generally consistent with the current understanding in the field of exposure–safety relationships for ICIs, primarily supported by data on PD-1 and PD-L1 inhibitors (33–37).
Exposure-efficacy analyses indicated no significant association between tremelimumab exposure and ORR or PFS. Results of this study suggested that a higher tremelimumab exposure may be associated with longer OS compared with lower tremelimumab exposure. However, when incorporating the impact of covariates, tremelimumab exposure was no longer a significant factor for OS. Various factors, including baseline patient characteristics and treatment response, have been observed to confound exposure–response relationships of several ICIs, including atezolizumab, nivolumab, pembrolizumab, and tremelimumab (38, 39). Exposure–survival response relationship may also be confounded by the use of subsequent therapies; however, this was not assessed in the current analysis. Analysis of data from a phase III study in which patients with advanced melanoma were treated with 15 mg/kg tremelimumab every 90 days demonstrated an association between slower tremelimumab clearance and longer OS (24). However, this finding was possibly confounded by the impact of covariates, similar to the results of the current study. A recent analysis suggested that higher tremelimumab exposure may be associated with longer median OS in patients with advanced mesothelioma (31). However, potential confounders of the relationship were assessed by evaluating patient characteristics in the exposure quartiles and it was determined that the observed exposure–OS relationship was likely attributed to unbalanced prognostic factors in the patient population (31). Evaluation of the impact of prognostic factors on apparent exposure–response relationships is critical to avoid incorrect dosage of mAb for the treatment of cancer (26, 38).
The present study identified a significant association between higher baseline albumin levels and longer OS, which impacted the apparent relationship between tremelimumab exposure and OS. ICIs have been found to show a reverse causal relationship between PK exposure and clearance, a covariate for efficacy, that is, patients that achieve favorable efficacy outcomes show reduced drug clearance during the course of treatment (26, 38, 39). Lower albumin levels have previously been associated with higher clearance of ICIs, including ipilimumab (40), durvalumab (41), and pembrolizumab (42), and therefore higher albumin may be important to tremelimumab exposure parameters in the present study. In addition, low serum albumin levels correlate with increased parameters of HCC aggressiveness, such as larger tumor diameters, increased tumor multifocality, and greater prevalence of portal vein thrombosis (43). Therefore, abnormally low levels of albumin, a reflection of liver decompensation and dysfunction (43), are a potential indicator of poor prognosis in patients with uHCC, irrespective of tremelimumab exposure. Systemic inflammation has been found to be a prognostic factor in people with HCC (44, 45). Low baseline NLR (a measure of systemic inflammation) was also significantly associated with longer OS in this study, which is consistent with previous reports that low baseline NLR confers better prognosis compared with high baseline NLR in patients with uHCC (46, 47). Low baseline NLR was defined in this study as lower than the median of 3.22, which is consistent with a previous report that an NLR of approximately 3 to 4.5 is an appropriate cut-off for predicting survival outcomes for patients with uHCC (46). Although albumin levels and NLR were shown to have a significant impact on OS in the current analysis, the highest median NLR was observed in the STRIDE arm, which suggests other factors could have influenced these findings, and further exploration may be warranted. Therefore, albumin levels or NLR alone might not represent a clinically relevant indicator of response in this patient population. However, given the association of albumin levels with response in the current analysis, it would be interesting to investigate ALBI grade, a strong and accurate prognostic indicator for HCC (48, 49), as a clinical indicator of response to tremelimumab treatment.
Although, data from Study 22 suggest that there is a potential for higher concentrations of tremelimumab to drive a more durable response, it is not feasible to adequately perform this analysis from the Study 22 dataset due to the limited patient numbers per dose group and potential cofounding factors.
Although tremelimumab exposure levels were observed to be minimally associated with efficacy, there were observations of a tremelimumab dose response in that the OS of the STRIDE arm was elevated above that observed in the T75+D arm and the durvalumab monotherapy arm. Thus, PK alone did not adequately describe the relationship between tremelimumab and efficacy. It has been postulated that the inherent variability in activities of immune-modulating therapies in heterogenous patient populations makes exposure metrics minimally informative (50). Instead, PD read-outs may be more adequate to assess exposure–response relationships. In addition, PD read-outs can confirm mechanistic hypotheses of immune-modulating therapies and identify potential predictive biomarkers of response.
Pharmacodynamic analysis may suggest a biological rationale for the apparent dose-dependent response of tremelimumab. Tremelimumab dose-dependent elevations in CD8+Ki67+ T cells were observed in patients with NSCLC, highlighting the importance of this cell population as a pharmacodynamic biomarker of tremelimumab (28). This study demonstrated a saturable relationship between tremelimumab exposure and expansion of CD8+Ki67+ T cells in patients with uHCC, in which the median Cmin1 of STRIDE was found to be higher than that of T75+D, and well above the EC50 estimate for tremelimumab. Higher tremelimumab exposure levels resulted in no greater CD8+Ki67+ T-cell expansion levels and no increased survival benefit. In addition, the maximum effect of tremelimumab was inversely associated with baseline CD8+Ki67+ T-cell counts of patients, suggesting that the magnitude of tremelimumab-induced increase is likely more important than an absolute count value. Thus, these findings expand on previous analyses and may indicate that a critical threshold of CD8+ T-cell expansion is required for positive clinical response in uHCC. In addition, for uHCC, increased tremelimumab dose may elicit more AEs and treatment disruptions (22). Taken together, these findings support the hypothesis that the STRIDE regimen induces a substantial and relevant immune response in patients with uHCC that likely contributes to the improved patient outcomes observed with this regimen without the safety issues typically observed with higher or more frequent doses of tremelimumab (22, 23).
Although there is a minimal pharmacokinetic tremelimumab exposure–response relationship, pharmacodynamic analysis of tremelimumab with an Emax model shows a correlation with response. On the basis of these findings, we hypothesize that for ICI therapies, such as tremelimumab plus durvalumab, studying the PK of these treatments may not be sufficient on its own, and it may be critical to assess the PK/PD relationship of these treatments in early phase clinical development.
Overall, the exposure-response and PK/PD analysis in Study 22 provide insights on the mode of action of tremelimumab and supports the favorable benefit-risk profile of the STRIDE regimen, as confirmed in the phase III HIMALAYA study (23). Additional data from the phase III HIMALAYA study will provide further insights regarding the exposure–response relationships. In Study 22, the STRIDE regimen resulted in a more favorable benefit-risk ratio compared with T75+D and tremelimumab monotherapy, in terms of efficacy and safety (22), and data from the phase III HIMALAYA study support that the novel STRIDE regimen offers a well-tolerated and effective treatment option for patients with uHCC (23).
Supplementary Material
Supplementary Figure S1. Flow diagram of patients included in the PK analysis, exposure-response analysis, and PD analysis datasets
Supplementary Figure S2. The effect of covariates, body weight, baseline albumin level, and combination therapy, on tremelimumab exposure metric Cmin1 (PK analysis population)
Supplementary Figure S3. CFB of proliferating CD8+Ki67+ T cells vs predicted tremelimumab exposure (PD analysis population) in patients with lower and higher baseline CD8+Ki67+ T-cell counts
Supplementary Data for publication
Acknowledgments
The authors would like to thank the patients who participated in Study 22, their families, and the investigators and study site personnel. Medical writing support, under the direction of the authors, was provided by Claire Tinderholm, PhD, CMC Connect, a division of IPG Health Medical Communications, funded by AstraZeneca, in accordance with Good Publication Practice (GPP 2022) guidelines. This study was funded by AstraZeneca.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
X. Song reports other support from CMC Connect during the conduct of the study; other support from AstraZeneca outside the submitted work; and also reports employment with AstraZeneca and reports ownership of AstraZeneca stock. R.K. Kelley reports grants from AstraZeneca during the conduct of the study; grants from Agios, Bayer, BMS, Eli Lilly, Exelixis, EMD Serono, Ipsen, Merck, Genentech/Roche, Relay Therapeutics, Surface Oncology, and Taiho outside the submitted work. A.A. Khan reports employment with AstraZeneca and ownership of AstraZeneca stock. N. Standifer reports personal fees from AstraZeneca outside the submitted work. D. Zhou reports other support from AstraZeneca during the conduct of the study; other support from AstraZeneca outside the submitted work. K. Lim reports other support from AstraZeneca during the conduct of the study; other support from AstraZeneca outside the submitted work. R. Krishna reports employment with Certara USA, Inc. and was a paid consultant for work described in the manuscript. L. Liu reports personal fees from AstraZeneca during the conduct of the study. K. Wang reports consulting fees from AstraZeneca. P. McCoon reports other support from AstraZeneca outside the submitted work. A. Negro reports a patent for tremelimumab pending. P. He reports other support from AstraZeneca during the conduct of the study. M. Gibbs reports other support from AstraZeneca during the conduct of the study; other support from AstraZeneca outside the submitted work. J.F. Kurland reports personal fees from AstraZeneca during the conduct of the study; personal fees from AstraZeneca outside the submitted work; also has a patent for tremelimumab—dosing strategies pending. G.K. Abou-Alfa reports grants from Arcus, Astra Zeneca, BioNtech, BMS, Celgene, Flatiron, Genentech/Roche, Genoscience, Incyte, Polaris, Puma, QED, Silenseed, and Yiviv and personal fees from Adicet, Alnylam, Astra Zeneca, Autem, Beigene, Berry Genomics, Boehringer Ingelheim, Celgene, Cend, CytomX, Eisai, Eli Lilly, Exelixis, Flatiron, Genentech/Roche, Genoscience, Helio, Helsinn, Incyte, Ipsen, Merck, Nerviano, Newbridge, Novartis, QED, Redhill, Rafael, Servier, Silenseed, Sobi, Vector, and Yiviva during the conduct of the study; also has a patent for PCT/US2014/031545 filed on March 24, 2014, and priority application Serial No.: 61/804,907; Filed: March 25, 2013 issued.
Authors' Contributions
X. Song: Conceptualization, formal analysis, writing–original draft, writing–review and editing. R.K. Kelley: Conceptualization, formal analysis, supervision, writing–original draft, writing–review and editing. A.A. Khan: Conceptualization, formal analysis, writing–original draft, writing–review and editing. N. Standifer: Conceptualization, data curation, formal analysis, writing–original draft, writing–review and editing. D. Zhou: Formal analysis, writing–original draft, writing–review and editing. K. Lim: Formal analysis, writing–original draft, writing–review and editing. R. Krishna: Formal analysis, writing–original draft, writing–review and editing. L. Liu: Formal analysis, writing–original draft, writing–review and editing. K. Wang: Formal analysis, writing–original draft, writing–review and editing. P. McCoon: Formal analysis, writing–original draft, writing–review and editing. A. Negro: Conceptualization, formal analysis, writing–original draft, writing–review and editing. P. He: Conceptualization, formal analysis, writing–original draft, writing–review and editing. M. Gibbs: Formal analysis, writing–original draft, writing–review and editing. J.F. Kurland: Conceptualization, formal analysis, writing–original draft, writing–review and editing. G.K. Abou-Alfa: Conceptualization, formal analysis, supervision, writing–original draft, writing–review and editing.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 2. Global Cancer Observatory. GLOBOCAN liver factsheet. 2020. Available from:https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf. Accessed February 15, 2022. [Google Scholar]
- 3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 4. National Institutes of Health. Cancer stat facts: liver and intrahepatic bile duct cancer. 2021. Available from: https://seer.cancer.gov/statfacts/html/livibd.html. Accessed July 6, 2021.
- 5. European Association for the Study of Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 6. Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2021;19:541–65. [DOI] [PubMed] [Google Scholar]
- 7. Fu Y, Liu S, Zeng S, Shen H. From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J Exp Clin Cancer Res 2019;38:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940–52. [DOI] [PubMed] [Google Scholar]
- 9. Luo X-Y, Wu K-M, He X-X. Advances in drug development for hepatocellular carcinoma: clinical trials and potential therapeutic targets. J Exp Clin Cancer Res 2021;40:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. US Food and Drug Administration. Keytruda (pembrolizumab) prescribing information. 2021. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125514s066lbl.pdf. Accessed May 17, 2021.
- 11. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol 2020;6:e204564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. US Food and Drug Administration. Opdivo (nivolumab) prescribing information. 2022.
- 13. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894–905. [DOI] [PubMed] [Google Scholar]
- 14. Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013;59:81–8. [DOI] [PubMed] [Google Scholar]
- 15. Wainberg ZA, Segal NH, Jaeger D, Lee K-H, Marshall J, Antonia SJ, et al. Safety and clinical activity of durvalumab monotherapy in patients with hepatocellular carcinoma (HCC). J Clin Oncol 2017;35(suppl 15):Abs 4071. [Google Scholar]
- 16. Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn M-J, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol 2020;6:661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson M, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K, et al. PL02.01 Durvalumab ± tremelimumab + chemotherapy as first-line treatment for mNSCLC: results from the phase 3 POSEIDON study. J Thorac Oncol 2021;16:S844. [Google Scholar]
- 18. Senan S, Okamoto I, Lee G-wChen Y, Niho S, Mak G, et al. Design and rationale for a phase III, randomized, placebo-controlled trial of durvalumab with or without tremelimumab after concurrent chemoradiotherapy for patients with limited-stage small-cell lung cancer: the ADRIATIC study. Clin Lung Cancer 2020;21:e84–e8. [DOI] [PubMed] [Google Scholar]
- 19. Powles T, Drakaki A, Teoh JY-C, Grande E, Fontes-Sousa M, Porta C, et al. A phase 3, randomized, open-label, multicenter, global study of the efficacy and safety of durvalumab (D) + tremelimumab (T) + enfortumab vedotin (EV) or D + EV for neoadjuvant treatment in cisplatin-ineligible muscle-invasive bladder cancer (MIBC) (VOLGA). J Clin Oncol 2022;40(6_suppl):TPS579. [Google Scholar]
- 20. Galsky MD, Necchi A, Sridhar SS, Ogawa O, Angra N, Hois S, et al. A phase III, randomized, open-label, multicenter, global study of first-line durvalumab plus standard of care (SoC) chemotherapy and durvalumab plus tremelimumab, and SoC chemotherapy versus SoC chemotherapy alone in unresectable locally advanced or metastatic urothelial cancer (NILE). J Clin Oncol 2021;39(6_suppl):TPS504. [Google Scholar]
- 21. Davies M, Duffield EA. Safety of checkpoint inhibitors for cancer treatment: strategies for patient monitoring and management of immune-mediated adverse events. Immunotargets Ther 2017;6:51–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J Clin Oncol 2021;39:2991–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid 2022;1:EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 24. Wang E, Kang D, Bae K-S, Marshall MA, Pavlov D, Parivar K. Population pharmacokinetic and pharmacodynamic analysis of tremelimumab in patients with metastatic melanoma. J Clin Pharmacol 2014;54:1108–16. [DOI] [PubMed] [Google Scholar]
- 25. Cox DR. Regression models and life-tables. J R Stat Soc Series B Methodol 1972;34:187–202. [Google Scholar]
- 26. Kawakatsu S, Bruno R, Kågedal M, Li C, Girish S, Joshi A, et al. Confounding factors in exposure-response analyses and mitigation strategies for monoclonal antibodies in oncology. Br J Clin Pharmacol 2021;87:2493–501. [DOI] [PubMed] [Google Scholar]
- 27. Li C, Wang B, Chen S-C, Wada R, Lu D, Wang X, et al. Exposure-response analyses of trastuzumab emtansine in patients with HER2-positive advanced breast cancer previously treated with trastuzumab and a taxane. Cancer Chemother Pharmacol 2017;80:1079–90. [DOI] [PubMed] [Google Scholar]
- 28. Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 2016;17:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1–targeted therapy in lung cancer patients. Proc Natl Acad Sci 2017;114:4993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mankor JM, Disselhorst MJ, Poncin M, Baas P, Aerts JGJV, Vroman H. Efficacy of nivolumab and ipilimumab in patients with malignant pleural mesothelioma is related to a subtype of effector memory cytotoxic T cells: Translational evidence from two clinical trials. eBioMedicine 2020;62:103040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baverel P, Roskos L, Tatipalli M, Lee N, Stockman P, Taboada M, et al. Exposure-response analysis of overall survival for tremelimumab in unresectable malignant mesothelioma: the confounding effect of disease status. Clin Transl Sci 2019;12:450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kelly RJ, Lee J, Bang YJ, Almhanna K, Blum-Murphy M, Catenacci DVT, et al. Safety and efficacy of durvalumab and tremelimumab alone or in combination in patients with advanced gastric and gastroesophageal junction adenocarcinoma. Clin Cancer Res 2020;26:846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stroh M, Winter H, Marchand M, Claret L, Eppler S, Ruppel J, et al. Clinical pharmacokinetics and pharmacodynamics of atezolizumab in metastatic urothelial carcinoma. Clin Pharmacol Ther 2017;102:305–12. [DOI] [PubMed] [Google Scholar]
- 34. US Food and Drug Administration. Clinical pharmacology and biopharmaceutics review(s): durvalumab. 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761069Orig1s000ClinPharmR.pdf. Accessed May 17, 2021.
- 35. Wang X, Feng Y, Bajaj G, Gupta M, Agrawal S, Yang A, et al. Quantitative characterization of the exposure-response relationship for cancer immunotherapy: a case study of nivolumab in patients with advanced melanoma. CPT Pharmacometrics Syst Pharmacol 2017;6:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chatterjee M, Turner DC, Felip E, Lena H, Cappuzzo F, Horn L, et al. Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol 2016;27:1291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Centanni M, Moes DJA, Trocóniz IF, Ciccolini J, van Hasselt J. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin pharmacokinet 2019;58:835–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dai HI, Vugmeyster Y, Mangal N. Characterizing exposure-response relationship for therapeutic monoclonal antibodies in immuno-oncology and beyond: challenges, perspectives, and prospects. Clin Pharmacol Ther 2020;108:1156–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu C, Yu J, Li H, Liu J, Xu Y, Song P, et al. Association of time-varying clearance of nivolumab with disease dynamics and its implications on exposure response analysis. Clin Pharmacol Ther 2017;101:657–66. [DOI] [PubMed] [Google Scholar]
- 40. Sanghavi K, Zhang J, Zhao X, Feng Y, Statkevich P, Sheng J, et al. Population pharmacokinetics of ipilimumab in combination with nivolumab in patients with advanced solid tumors. CPT Pharmacometrics Syst Pharmacol 2020;9:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baverel PG, Dubois VFS, Jin CY, Zheng Y, Song X, Jin X, et al. Population pharmacokinetics of durvalumab in cancer patients and association with longitudinal biomarkers of disease status. Clin Pharmacol Ther 2018;103:631–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li H, Sun Y, Yu J, Liu C, Liu J, Wang Y. Semimechanistically based modeling of pembrolizumab time-varying clearance using 4 longitudinal covariates in patients with non-small cell lung cancer. J Pharm Sci 2019;108:692–700. [DOI] [PubMed] [Google Scholar]
- 43. Carr BI, Guerra V. Serum albumin levels in relation to tumor parameters in hepatocellular carcinoma patients. Int J Biol Markers 2017;32:e391–e6. [DOI] [PubMed] [Google Scholar]
- 44. Pinato DJ, Stebbing J, Ishizuka M, Khan SA, Wasan HS, North BV, et al. A novel and validated prognostic index in hepatocellular carcinoma: The inflammation based index (IBI). J Hepatol 2012;57:1013–20. [DOI] [PubMed] [Google Scholar]
- 45. Muhammed A, Fulgenzi CAM, Dharmapuri S, Pinter M, Balcar L, Scheiner B, et al. The systemic inflammatory response identifies patients with adverse clinical outcome from immunotherapy in hepatocellular carcinoma. Cancers 2021;14:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tada T, Kumada T, Hiraoka A, Michitaka K, Atsukawa M, Hirooka M, et al. Neutrophil-to-lymphocyte ratio is associated with survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Liver Int 2020;40:968–76. [DOI] [PubMed] [Google Scholar]
- 47. Bruix J, Cheng A-L, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol 2017;67:999–1008. [DOI] [PubMed] [Google Scholar]
- 48. Pinato DJ, Kaneko T, Saeed A, Pressiani T, Kaseb A, Wang Y, et al. Immunotherapy in hepatocellular cancer patients with mild to severe liver dysfunction: adjunctive role of the ALBI Grade. Cancers 2020;12:1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pinato DJ, Sharma R, Allara E, Yen C, Arizumi T, Kubota K, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol 2017;66:338–46. [DOI] [PubMed] [Google Scholar]
- 50. Vicini P, Standifer N, Hickling TP. Recruiting the immune system against dease: lessons for clinical and systems pharmacology. CPT Pharmacometrics Syst Pharmacol 2019;8:436–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Flow diagram of patients included in the PK analysis, exposure-response analysis, and PD analysis datasets
Supplementary Figure S2. The effect of covariates, body weight, baseline albumin level, and combination therapy, on tremelimumab exposure metric Cmin1 (PK analysis population)
Supplementary Figure S3. CFB of proliferating CD8+Ki67+ T cells vs predicted tremelimumab exposure (PD analysis population) in patients with lower and higher baseline CD8+Ki67+ T-cell counts
Supplementary Data for publication
Data Availability Statement
Data from the Study 22 clinical trial are available at: https://www.clinicaltrials.gov/ct2/show/results/NCT02519348.
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Anonymized datasets may be available on request. Data for studies directly listed on Vivli can be requested through Vivli at https://search.vivli.org/; data for studies not listed on Vivli may be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. The request will undergo an internal review process, and if approved, data will be prepared and shared with specified accessors named on the request form for 12 months via Vivli Secure Research Environment.



![Figure 2. Tremelimumab exposure-safety analysis for Cmin1 and (A) Grade 3/4 treatment-related AEs, (B) Grade 3/4 treatment-related AESI, and (C) AEs leading to treatment discontinuation (exposure–response analysis population); N = 216. Open blue circles are the observed events. Filled black circles are the observed probability of events and the error bars are the standard errors (calculated as sqrt [P × (1 − P)/n], where P is probability of response and n is the number of patients in each quantile bin) for quantiles [at 100 × (1/4)th percentiles, vertical dotted lines] of exposures (plotted at the median value within each quantile). The red solid line is the model-predicted probability, and the shaded area is the 95% prediction interval. Cmin1, minimum tremelimumab concentration at day 28; N, number of patients included in the analysis.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c24e/9932581/89535a5bee9d/754fig2.jpg)
![Figure 3. Tremelimumab exposure-ORR analysis at Cmin1 (exposure–response analysis population); N = 208. Open blue circles reflect the observed events. Filled black circles are the observed probability of ORR and the error bars are the standard errors (calculated as sqrt [P*(1 − P)/n], where P is probability of response and n is the number of patients in each quantile bin) for quantiles [at 100 × (1/5)th percentiles, vertical dotted lines] of exposures (plotted at the median value within each quantile). The red lines are smooth curves (loess) to show the relationship between two variables. Cmin1, minimum tremelimumab concentration at day 28; N, number of patients included in the analysis.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c24e/9932581/a9173d1209e6/754fig3.jpg)