Abstract
Background:
Decolonization is considered a valuable means to limit Staphylococcus aureus infection rates. However, previous topical strategies targeting the nose or skin had limited success, while more comprehensive, oral antibiotic-based decolonization is ill-advised due to eradication of the microbiota and development of antibiotic resistance. Based on our recent findings that in mice probiotic Bacillus subtilis strongly diminished S. aureus at the main intestinal colonization site via specific bacterial interaction, we here tested a probiotic approach to control S. aureus colonization in humans.
Methods:
We performed a single-center, randomized, double-blind, placebo-controlled trial (Thai Clinical Trials Registry number, TCTR20210128003) in S. aureus-colonized individuals from the community in the Songkhla region of Thailand. Participants were allocated to groups by computer randomization and research coordinators were blinded as for group allocation. Participants received 250 mg of probiotic B. subtilis MB40 or placebo once daily for 30 days and S. aureus colonization was determined after the last dose was received.
Results:
The trial was performed between January 29 and June 30, 2021, with enrollment from January 29 to April 6, 2021. 84 participants with intestinal and 50 with nasal colonization were split in treatment and placebo groups of n=42 and n=25 each, respectively. Oral probiotic B. subtilis resulted in significant reduction of S. aureus in the stool (96.8%, P<0·0001) and nose (65.4%, P=0·0002). There were no differences in adverse effects or significant microbiome changes between the groups.
Interpretation:
B. subtilis probiotic eliminated more than 95% of the total S. aureus colonizing the human body without altering the microbiota.
Introduction
Staphylococcus aureus is a human pathogen that can cause a multitude of serious and often fatal infections. Treatment is complicated by widespread antibiotic resistance, such as in methicillin-resistant S. aureus (MRSA)1. In the U.S., S. aureus kills more people than any other antibiotic-resistant pathogen, with an annual death toll of 20,000 due to blood infections alone2. About one fourth to one third of the population are generally reported to be permanent asymptomatic carriers of S. aureus3,4. Because S. aureus infections usually originate from asymptomatic colonization5,6, decolonization has frequently been suggested to limit S. aureus infection rates7-9. S. aureus decolonization strategies have generally used antibiotics, which is inherently problematic due to the dangers associated with the destruction of the natural microbiota and the spread of antimicrobial resistance10,11. Most strategies have targeted the nares7,9, which are traditionally considered the most important S. aureus colonization site3, and some also included skin decolonization with antiseptics7. However, it is increasingly recognized that S. aureus also colonizes the intestine4, and there are several reports demonstrating that similar to nasal carriage, intestinal carriage is a source for infection6,12,13. Interestingly, one study showed that intestinal but not nasal carriage is associated with skin and soft tissue infections in children14. Notably, re-inoculation from intestinal carriage may explain previously reported rapid recolonization and limited clinical success of S. aureus decolonization attempts solely directed at the nose or skin4. Rarely, oral antibiotics have been given to achieve comprehensive systemic decolonization including that of the intestine, but given what we know now about the role of the natural intestinal microbiome in preventing overgrowth of pathogens, this is hardly considered an appropriate strategy and not recommended by the IDSA15.
Probiotics are live microorganisms which when administered in adequate amounts confer a health benefit on the host16. In contrast to antibiotics, in most instances they do not have deleterious influences on the microbiota and do not lead to resistance17. Several strains of Bacillus subtilis are classified as probiotics that are commercially available in mono-species form or as a component of mixed probiotic formulae. B. subtilis probiotic is taken as spores that germinate in the gut18, which compared to other probiotic microorganisms has the advantage of strongly increased survival during stomach passage. We previously showed that most strains of Bacillus spp. that we studied, including most strains of B. subtilis, secrete molecules that specifically inhibit S. aureus quorum-sensing, a mechanism we demonstrated is essential for S. aureus intestinal colonization19, and orally administered B. subtilis strongly diminished S. aureus intestinal colonization in mice19.
Prompted by our mechanistic findings, we here analyzed whether a regimen of B. subtilis (strain MB40) can decrease S. aureus colonization in humans and thereby overcome the problems related to topical decolonization efforts and the use of antibiotics. Our study presents a strategy for S. aureus colonization that is safe, without harm to the existing microbiota, and efficacious as in contrast to previous topical strategies it eradicates most of the S. aureus population colonizing humans. Furthermore, our data call for a categorical rethinking of S. aureus colonization dynamics and decolonization strategies.
Methods
Study design
We conducted a single-center, randomized, double-blind, placebo-controlled trial in the Songkhla region of Thailand between January 29, 2021 and June 30, 2021 (first and last enrollment, January 29 and April 6, 2021, respectively) at Prince of Songkla University, Hat Yai, Songkhla, Thailand, to assess the efficacy of B. subtilis (strain MB40) to reduce intestinal and nasal colonization in healthy individuals colonized with S. aureus. Ethics approval was obtained by the Human Research Ethic Committee (HREC), Faculty of Medicine, Prince of Songkla University (reference no. Zhs5-qWmp-qUCq-1xnX).
Participants
Eligible participants were at least 18 years of age without history of intestinal disease, antibiotic treatment or hospitalization within the last 3 months (90 days). Participants were excluded if they were currently pregnant, breast-feeding, had diarrhea, or were taking probiotic. Written informed consent was obtained from all participants. No effort was made to balance the groups on the basis of age, race or ethnic group, or sex.
611 participants were first screened for S. aureus and Bacillus spp. intestinal and nasal colonization. Nasal swabs and fecal samples were collected twice from every individual in a four-week interval and screened for S. aureus and Bacillus spp. by plating on mannitol salt agar, which is selective for staphylococci and bacilli and on which S. aureus and Bacillus spp. can easily be differentiated from each other and other microorganisms by their morphology. To that end, dilutions of nasal swabs (from both nares) or of 1 g fecal matter suspended in 1 ml in PBS were plated and grown overnight at 37 °C on mannitol salt agar (MSA) plates and entire plates of countable dilutions were counted. Representative colonies were confirmed for species identity using MALDI-TOF mass spectrometry and 16S rRNA sequencing. Participants were considered permanently colonized at a specific site (intestinal, nasal) by either S. aureus or Bacillus spp. if two positive samples (with at least one species-confirmed colony) were obtained at both time points. Six participants that showed only transient colonization by either S. aureus or Bacillus spp. were excluded from the study. Five were lost to follow-up. Presence of Bacillus or S. aureus at either colonization site was consistent at both tested time points in all remaining 600 individuals and colonization rates were highly correlated quantitatively between the two time points for both organisms (Supplementary Figure 1). Notably, S. aureus colonization of either intestine or nares occurred only in individuals that were not colonized with Bacillus (Supplementary Figure 1). These results showing a strong exclusion effect of Bacillus spp. On S. aureus confirmed those obtained in our previous more limited study19.
Eligible participants permanently colonized by either S. aureus or Bacillus spp. were also interviewed by a research assistant using a structured questionnaire that included the collection of demographic and socioeconomic data.
Randomization and masking
Among 115 individuals with S. aureus colonization, 84 had intestinal colonization, and 50 had nasal colonization, with 19 participants having both intestinal and nasal colonization. Following equal (1:1 ratio) randomization, 55 subjects (n = 30 colonized only in the intestine, n = 13 only in the nose, and n = 12 in both locations) were assigned to the treatment group, and 50 (n=35, n=18, and n=7, respectively) subjects were assigned to the control group (Figure 1A). The randomization code was computer-generated using Microsoft Excel, and randomization was performed in blocks of four.
Figure 1. Enrollment and randomization.
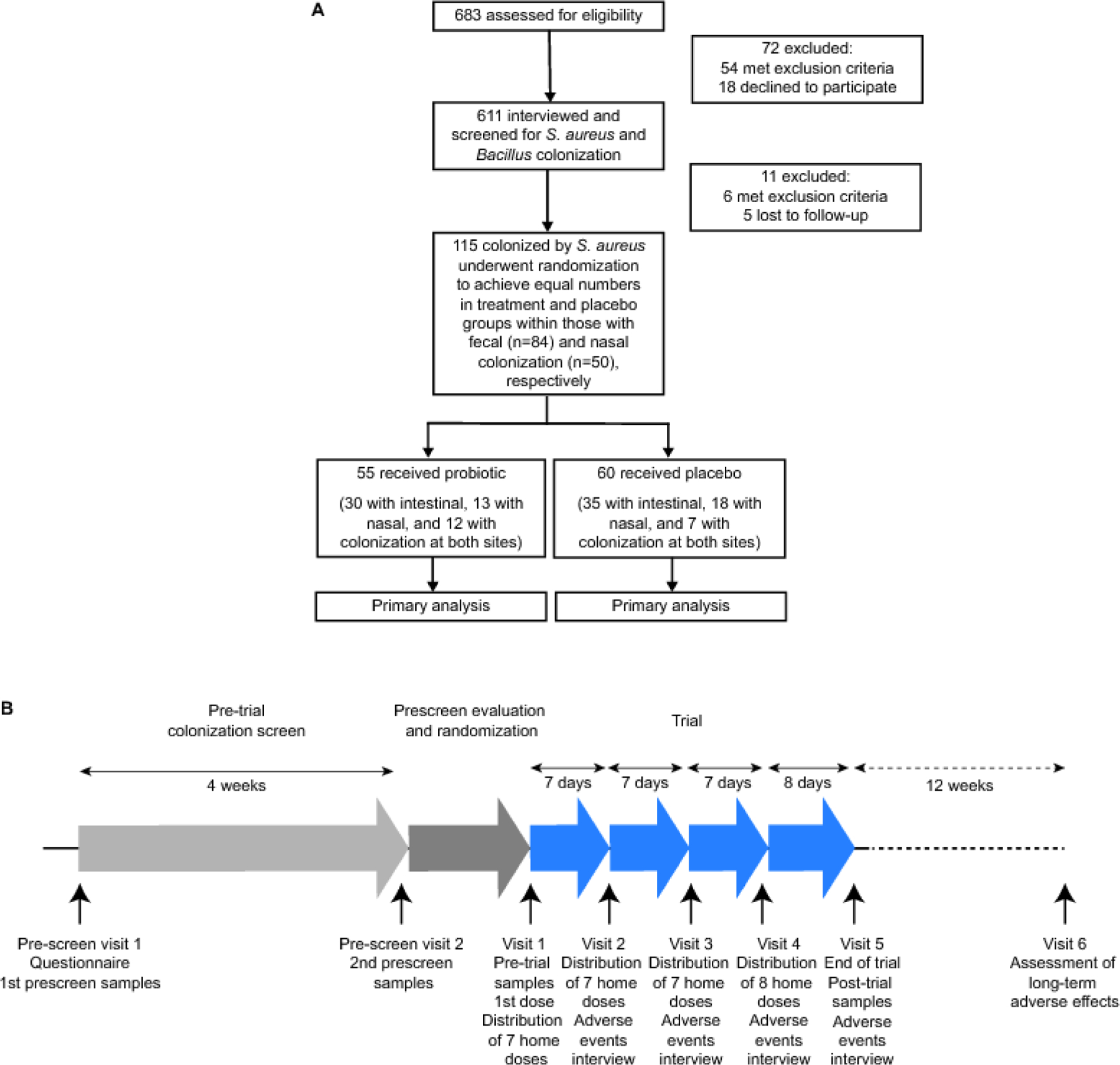
A, Enrollment and randomization flowchart. B, Timeline of pre-trial and trial procedures.
Participants received probiotic or placebo, which were indistinguishable in appearance and texture, in sealed, non-transparent medical zip bags. The bags were coded by numbers by a research assistant and handed to participants by another research assistant who did not have information on group allocation or contents of the bags. The research assistants who generated the sequence, enrolled participants, and assigned them to the trial did not have any involvement in trial analyses. The nurses in the research clinic assessing adverse effects and the individuals analyzing the data were blinded as for group allocation.
Procedures
Trial participants received a capsule (MySkinRecipes, GMP and GHP certified) that had been filled with 250 mg of spores of a B. subtilis strain previously tested for safety and general probiotic effects on gastrointestinal health in humans (OPTI-BIOME® B. subtilis strain MB40, BIO-CAT Microbials, Shakopee, Minnesota, USA)20, corresponding to a dose of 10 × 109 colony-forming units (CFU)/day once daily for 30 days, or 250 mg of maltodextrin (Chemipan Corp., Bangkok, Thailand) filled in the same type of capsules as a placebo. The OPTI-BIOME® probiotic formula (with strain B. subtilis MB40) was selected among several B. subtilis strains frequently used in commercially available B. subtilis probiotic or potentially probiotic formulae (Natto, R0179) based on its considerably higher production of fengycins, which are the active molecules in B. subtilis that inhibit the S. aureus quorum-sensing system we had found to be essential for S. aureus intestinal colonization19 (Supplementary Figure 2). The microbiological purity of the OPTI-BIOME® formula was confirmed directly before the start of the intervention. To that end, absence of contaminating microorganisms was ascertained by bacterial contamination screening (Enterobacteriaceae, Pseudomonas aeruginosa, Acinetobacter baumannii, Enterococcus spp., S. aureus). Briefly, 1 g of each probiotic formula was suspended in 1 ml PBS and diluted. For Enterobacteriaceae, P. aeruginosa, and A. baumannii, the suspension was cultured on MacConkey agar and incubated at 37 °C for 18 h. For Enterococcus spp., the suspension was cultured on bile esculin agar both with and without vancomycin (6 mg/l) and incubated at 37 °C for 24 h. For S. aureus, the suspension was cultured on MSA and incubated at 37 °C for 24 h.
After assignment to a trial group, participants received the first dose of B. subtilis or placebo orally at a research clinic. Participants were given doses for seven days to take at home and returned to the research clinic weekly to receive further daily doses. The last time they received 8 doses for a total of 30 treatment days. After completing the 30-day treatment, nasal swab and fecal samples were analyzed for S. aureus and Bacillus the next day. See Figure 1B for a timeline of pre-trial and trial procedures.
For microbiome analysis, genomic DNA from each fecal sample was extracted using a QIAamp DNA stool Minikit (Qiagen, Germantown, Maryland, USA) according to the manufacturer’s instructions. DNA was quantified using a Nanodrop spectrophotometer and paired-end sequencing of the 16S rRNA V3-V4 region was performed by PSOMAGEN (Rockville, Maryland, USA) using an Illumina MiSeq system. All obtained paired-end sequences were identified and quantified for the abundance of Operational Taxonomic Units (OTUs) using Quantitative Insights Into Microbial Ecology (QIIME 1.9.1). This study used the Nephele (release 1.6) platform. The sequences were assigned to OTUs with QIIME’s uclust-based open-reference OTU picking protocol and the Greengenes 13_8 reference sequence set at 99% similarity.
Outcomes
The primary outcome determined was colonization by S. aureus (continuous, mean decrease in CFU count) in the intestine (by fecal counts) and nares (by nasal swabs) after intervention (30-day regime of B. subtilis probiotic). Secondary outcomes determined were intestinal and nasal colonization by B. subtilis after intervention. Furthermore, participants underwent intestinal microbiome analysis.
Safety, adverse effects
Participants were requested to visit at the research clinic weekly for four weeks after receiving B. subtilis probiotic or placebo and report any adverse events. Participants were also requested to visit at the research clinic again at week 12 after completing the study and report any long-term adverse events.
Statistical analysis
The power analysis was based on a previous study that had shown that the mean density of S. aureus in fecal human samples was 5·1 ± 1·5 log10 CFU/g 21. We estimated that probiotic B. subtilis would reduce the number of S. aureus by 25% (to 3.82 ± 1·5 log10 CFU/g). The power calculation was performed using a program available online at https://clincalc.com/stats/samplesize.aspx by a continuous endpoint method (mean CFU) and two independent samples at 0·01 and 0·1 of the probability of a type-I and type-II error, respectively. Based on the power calculation, the required sample size was 41 per group. All enrolled participants were included in primary and safety analyses.
To estimate how many individuals had to be screened for S. aureus colonization in the pre-trial selection, we assumed a colonization rate in the Thai rural community where our study was performed of ~ 12·5% - 13% based on our previous study in the same community19. At a confidence level of 95% with an incidence rate of 12·75% and an allowable error of 2·5%, the required sample size for our initial screen was 684 participants.
Prism 8 for Mac OS was used for statistical analyses. The primary outcome (efficacy of decolonization) was analyzed by two-tailed Wilcoxon matched-pairs signed-rank tests within treatment and placebo groups, and by two-tailed unpaired Mann-Whitney tests comparing pre- and post-treatment differences in CFU between treatment and placebo groups. These non-parametric tests were used because groups did not show normal distribution by Anderson-Darling, D’Agostino & Pearson, Shapiro-Wilk, and Kolmogorov-Smirnov tests. Statistical analysis comparing adverse effects between treatment and placebo groups was by Fisher’s exact test. Further statistical analyses for secondary outcomes are indicated in the figure legends. All error bars show the standard deviation (SD) of the mean for non-logarithmic and the standard deviation of the geometric mean for logarithmic scales.
This trial was registered by the Thai Clinical Trials Registry, number TCTR20210128003.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
To assess the efficacy of oral B. subtilis in reducing S. aureus colonization, we conducted a single-center, randomized, double-blind, placebo-controlled trial between January 29, 2021 and June 30, 2021 (first and last enrollment, January 29 and April 6, 2021, respectively) in the Songkhla region of Thailand in healthy individuals that according to an initial screen (with 683 participants) were permanently colonized with S. aureus. Among 115 individuals with S. aureus colonization, 84 had intestinal colonization, and 50 had nasal colonization, with 19 participants having both intestinal and nasal colonization. Following equal (1:1 ratio) randomization, 55 subjects (n = 30 colonized only in the intestine, n = 13 only in the nose, and n = 12 in both locations) were assigned to the treatment group, and 50 (n=35, n=18, and n=7, respectively) subjects were assigned to the control group (Figure 1). Baseline characteristics of the participants of the trial are shown in Table 1. All participants were assessed for the primary outcome.
Table 1.
Participant baseline data
| Intestinal colonization | Nasal colonization | |||||
|---|---|---|---|---|---|---|
| Total (n=84) |
Probiotic (n=42) |
Placebo (n=42) |
Total (n=50) |
Probiotic (n=25) |
Placebo (n=25) |
|
| Male | 39 | 18 (46·15%) | 21 (53·85%) | 23 | 10 (43·48%) | 13 (56·52%) |
| Female | 45 | 24 (53·33%) | 21 (46·67%) | 27 | 15 (55·56%) | 12 (44·44%) |
| Average age | 36·20 ± 12·95 | 36·19 ± 14·03 | 36·21 ± 11·94 | 34·2 ± 11·07 | 34·32 ± 12·31 | 34·08 ± 9·94 |
| Occupation | ||||||
| General employee | 13 | 7 (53·85%) | 6 (46·15%) | 20 | 7 (35·00%) | 13 (65·00%) |
| Farmer | 17 | 5 (29·41%) | 12 (70·59%) | 2 | 1 (50·00%) | 1 (50·00%) |
| Federal employee | 5 | 1 (20·00%) | 4 (80·00%) | 2 | 2 (100·00%) | 0 (0·00%) |
| Grocer | 3 | 0 (0·00%) | 3 (100·00%) | 1 | 1 (100·00%) | 0 (0·00%) |
| Healthcare worker | 5 | 2 (40·00%) | 3 (60·00%) | 1 | 0 (0·00%) | 1 (100·00%) |
| Student | 27 | 18 (66·67%) | 9 (33·33%) | 14 | 8 (57·14%) | 6 (42·86%) |
| Unemployed | 9 | 6 (66·67%) | 3 (33·33%) | 7 | 5 (71·43%) | 2 (28·57%) |
| Veterinarian | 3 | 2 (66·67%) | 1 (33·33%) | 2 | 1 (50·00%) | 1 (50·00%) |
| Business owner | 2 | 1 (50·00%) | 1 (50·00%) | 1 | 0 (0·00%) | 1 (100·00%) |
| Smoking | ||||||
| ≥3 times/week | 39 | 25 (64·10%) | 14 (35·90%) | 15 | 9 (60·00%) | 6 (40·00%) |
| 1–2 times/week | 0 | 0 (0·00%) | 0 (0·00%) | 0 | 0 (0·00%) | 0 (0·00%) |
| Never | 45 | 17 (37·78%) | 28 (62·22%) | 35 | 16 (45·71%) | 19 (54·29%) |
| Alcohol consumption | ||||||
| ≥3 times/week | 3 | 2 (66·67%) | 1 (33·33%) | 3 | 2 (66·67%) | 1 (33·33%) |
| 1–2 times/week | 34 | 16 (47·06%) | 18 (52·94%) | 14 | 9 (64·29%) | 5 (35·71%) |
| Never | 47 | 24 (51·06%) | 23 (48·94%) | 33 | 14 (42·42%) | 19 (57·58%) |
| Underlying condition | ||||||
| Allergic rhinitis | 2 | 2 (100·00%) | 0 (0·00%) | 18 | 9 (50·00%) | 9 (50·00%) |
| Asthma | 3 | 1 (33·33%) | 2 (66·67%) | 4 | 2 (50·00%) | 2 (50·00%) |
| Diabetes | 2 | 2 (100·00%) | 0 (0·00%) | 0 | 0 (0·00%) | 0 (0·00%) |
| Hypertension | 1 | 0 (0·00%) | 1 (100·00%) | 1 | 1 (100·00%) | 0 (0·00%) |
Oral administration of probiotic B. subtilis resulted in significant reduction of S. aureus in the stool (96.8%; P<0·0001) and the nose (65.4%; P=0·0002), while there were no significant differences in the placebo groups (Table 2). Direct comparison of decolonization efficacies in the treatment versus placebo groups by analyzing reduction of colonization yielded highly significant differences (stool, P<0·0001; nose, P=0·0002) (Table 2, Figure 2A). We also detected significant reduction of nasal and stool CFU when separately analyzing individuals only colonized in the noses (P=0·0035) or intestines (P=0·0007), respectively (Table 2). In the analysis of individuals with both nasal and intestinal colonization, differences in the nose were significant (P=0·021) while for stool values they failed to reach significance (P=0·083) (Table 2). Of note, the latter analyzed groups, particularly the group with colonization at both sites, were small as not subject to previous power analysis like for the primary outcome, At the end of the intervention period, individuals in the treatment group all had Bacillus in their feces at ~ 103 to 105 CFU g−1 with a geometric mean of ~ 104 (9541 and a geometric SD factor of 4·033) (Supplementary Figure 3). (Note no trial participants had pre-trial colonization with Bacillus spp.). No Bacillus was found in the placebo group and representative Bacillus colonies obtained from the treatment group were confirmed by MALDI-TOF mass spectrometry to be B. subtilis, substantiating that they originated from the ingested probiotic. Bacillus spp. were never found in the noses of participants.
Table 2.
Trial results
| Stool CFU pre1 [95% CI] | Stool CFU post2 [95% CI] | % reduction (P value pre vs. post3) | P value OPTI-BIOME® vs. placebo4 | Nose CFU pre [95% CI] | Nose CFU post [95% CI] | % reduction (P value pre vs. post3) | P value OPTI-BIOME® vs. placebo4 | |
|---|---|---|---|---|---|---|---|---|
| All participants OPTI-BIOME ® | 20213 [12026, 28401] | 646 [261, 1031] | 96·8 (<0·0001) | 0·0001 | 1576 [1130, 2022] | 564 [265, 863] | 65·4 (<0·0001) | 0·0002 |
| All participants placebo | 15350 [9729, 20971] | 12532 [7547, 17571] | 19·4 (0·12) | 1306 [911, 1702] | 1116 [756, 1476] | 14·6 (0·084) | ||
| Participants only colonized in the intestine, OPTI-BIOME® | 18027 [8757, 27297] | 659 [150, 1168] | 96·3 (<0·0001) | 0·0007 | ND | ND | ND | ND |
| Participants only colonized in the intestine, placebo | 14581 [8673, 20490] | 12499 [6952, 18046] | 14·3 (0·33) | ND | ND | ND | ||
| Participants only colonized in the nose, OPTI-BIOME® | ND | ND | ND | ND | 1508 [1059, 1956] | 400 [176, 624] | 73·5 (0·0005) | 0·0035 |
| Participants only colonized in the nose, placebo | ND | ND | ND | 1288 [898, 1677] | 1048 [673, 1423] | 18·6 (0·13) | ||
| Participants colonized in nose and intestine, OPTI-BIOME® | 25680 [6591, 44769 | 612 [48, 1175] | 97·6 (0·0005) | 0·083 | 1650 [767, 2533] | 742 [131, 1353] | 55·0 (0·0005) | 0·021 |
| Participants colonized in nose and intestine, placebo | 19194 [−2017, 40406] | 12697 [−2537, 27931 | 34·0 (0·16) | 1354 [71, 2638] | 1291 [207, 2376] | 4·7 (0·61) |
pre, before intervention
post, after intervention
Wilcoxon matched-pairs signed-rank test
Mann-Whitney test of CFU differences in individuals at a given site
Figure 2. Trial results with colonization levels by individual and study interpretation.
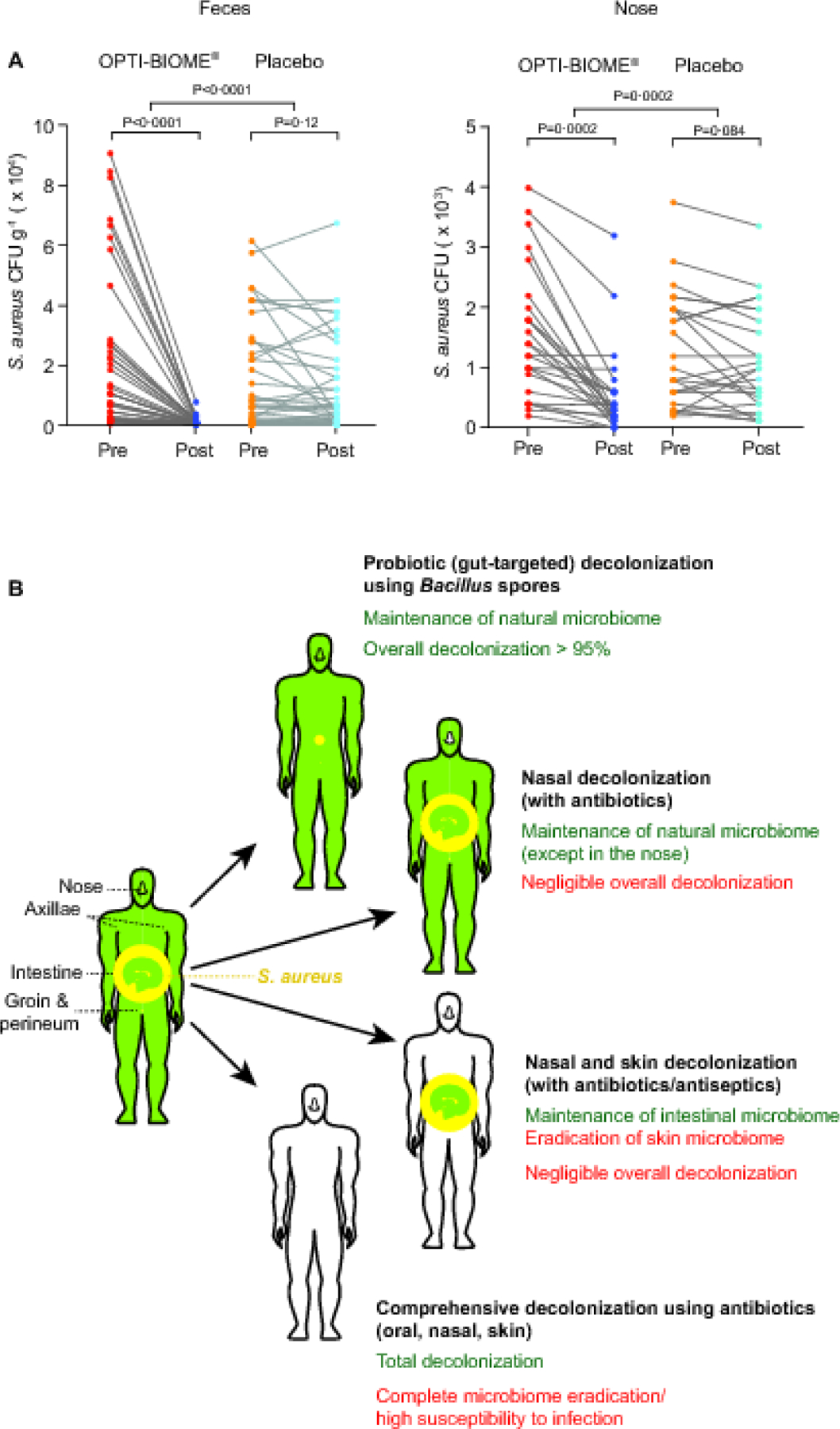
A, Pairwise comparison of pre- and post-intervention colonization levels for specific individuals and statistical analysis of the effect difference between treatment and placebo by comparing differences between pre-and post-levels by Mann-Whitney tests. Statistical analysis of difference between pre- and post-intervention data within a group is by Wilcoxon matched-pairs signed-rank test. All error bars show the mean ± SD. B, S. aureus distribution in the human body and comparison of decolonization strategies. The average abundance of S. aureus in S. aureus-colonized individuals in the intestine, nares, and predominant further skin colonization sites (axillae and groin/perineum) was estimated based on data obtained in this and previous studies. Decolonization efficacies were estimated based on data obtained in this study for probiotic-based decolonization and assuming 100% decolonization for antibiotic/antiseptic-based topical or antibiotic-based comprehensive/systemic (oral application combined with nasal and skin decolonization) methods at the targeted sites. The impact of probiotic treatment on S. aureus in the groin, perineum, and axillae was estimated at a relative decrease as measured for the nares. Yellow, S. aureus. Circle areas represent abundance. For extra-intestinal sites, note comparatively low S. aureus colonization as expressed by small yellow circles. Green, intact microbiome.
To confirm the absence of deleterious effects on the intestinal microbiome as well as the specificity of the S. aureus exclusion mechanism (as opposed to a general effect on the intestinal microbiome), we determined the composition of the intestinal microbiome in all participants that received B. subtilis probiotic before and after treatment. Common analyses for α- and β-diversity showed absence of significant microbiome alterations. Furthermore, relative abundances of the major phylae inhabiting the gut, which often show shifts under different diets or drug treatments, were not significantly changed. Moreover, we detected no changes in the most abundant OTUs on the genus level (Supplementary Figure 4) and only very few changes in any of the detected OTUs (Supplementary Figure 5). These results showing absence of significant overall changes in the intestinal microbiome caused by treatment with B. subtilis probiotic are in good accordance with and as expected by the specificity of the quorum-quenching effect of Bacillus fengycins on S. aureus as a comparatively negligible component of the intestinal microbiome regarding absolute quantity.
B. subtilis, including strain MB40, is being used as a probiotic with demonstrated benefits for gastro-intestinal health and shown in human studies to be safe20,22. Accordingly, in our study no severe adverse effects (severe watery diarrhea, severe vomiting, dermatitis, or eye irritation) were reported. Moderate adverse effects were rarely reported and were not significantly more frequent than in the placebo group (Table 3).
Table 3.
Adverse events
| Adverse events | Probiotic (n=55) | Placebo (n=60) | P value1 |
|---|---|---|---|
| Fever | 0 | 0 | 1·00 |
| Infection | 0 | 0 | 1·00 |
| Nausea/vomiting | 4 (7·3%) | 4 (6·7%) | 1·00 |
| Constipation | 3 (5·5%) | 2 (3·3%) | 0·67 |
| Headache | 0 | 0 | 1·00 |
| Muscle pain/cramp/spasm | 0 | 0 | 1·00 |
| Upset stomach/heartburn | 3 (5·5%) | 2 (3·3%) | 0·67 |
| Gas/bloating | 0 | 2 (3·3%) | 0·50 |
| Unusual stool (loose/discolored/more frequent) | 3 (5·5%) | 2 (3·3%) | 0·67 |
| Bad taste | 4 (7·3%) | 1 (1·7%) | 0·19 |
Two-tailed Fisher’s exact test
Discussion
In this study, we performed a randomized trial to analyze the value of a B. subtilis probiotic for S. aureus decolonization. Our decolonization strategy differed from previous approaches in two categorical features: First, we used a probiotic, which is generally considered safe and in contrast to previous strategies with antibiotics and antiseptics does not harm the existing microbiota. Furthermore, the specific decolonization agent that we used was selected based on our previous mechanistic results to virtually only interfere with staphylococcal colonization19, further minimizing effects on other members of the microbiota.
Second, our strategy was to target intestinal S. aureus colonization to eradicate a maximal number of the total colonizing S. aureus population in humans and to base our analysis of efficacy on quantitative rather than qualitative data. This contrasts previous decolonization strategies, which generally used topical antibiotic treatment of the nares and occasionally the skin and measured efficacy by analyzing how many participants showed S. aureus eradication over a certain detection threshold at those sites, notably often neglecting analysis of the feces7. Our study met the primary outcome of reducing S. aureus colonization in the intestine (P<0·0001 versus placebo) as well as the nares (P=0·0002 versus placebo). Colonization densities in the intestine were reduced by probiotic treatment by an average factor of ~ 31. As expected, reduction of colonization in the nares, as sites distal to the targeted intervention site, was much lower (factor ~ 3). This was of minor relevance for our goal to reduce overall S. aureus colonization of the human body (Figure 2B), but of major importance to our understanding of S. aureus colonization dynamics as discussed further below.
There were no severe adverse effects, and no other adverse effects were reported at rates significantly higher than in the placebo group. Furthermore, there were no significant effects on the overall composition of the intestinal microbiome. These results show safety and efficacy of the B. subtilis probiotic in reducing S. aureus human colonization, offering a previously unavailable method to eradicate the main, intestinal reservoir of S. aureus without the considerable dangers of pathogen overgrowth that are associated with systemic oral antibiotic treatment. Based on our data and those from previous studies on S. aureus CFU densities23-27, we estimate that the decolonization strategy we propose leads to at least ~ 95% decolonization, which contrasts previous strategies aimed at the nose and the skin that even with 100% eradication at those sites can only affect a small portion of the total S. aureus in the human body (Figure 2B). Furthermore, we here confirmed our previous findings19 showing complete correlation of Bacillus colonization with absence of S. aureus colonization in a human population as determined by analysis of fecal CFU. This suggests that prolonged intake of B. subtilis may have an even more pronounced effect than that observed in our trial, which was limited regarding the time of intervention and only used once-daily dosing. Bacillus is a transient colonizer, and it is thus not expected that the effect on S. aureus colonization persists long after cessation of oral administration. However, this probiotic strategy allows for long-term application due to the absence of harmful side effects, which contrasts antibiotic decolonization procedures that are similarly short termed in effect but hardly amenable to extended use for the abovementioned reasons. Finally, it is important to stress that based on the underlying mechanism that we established in mice19, similar efficacy can only be expected from B. subtilis strains that produce fengycins. According to our in vitro results, this feature is absent from several frequently used commercially available B. subtilis probiotic formulae, which contrasts the more widespread production of fengycins we previously detected in human isolates of that species19.
Our study also has important implications for our understanding of the relative importance of S. aureus colonization sites and the dynamics of S. aureus colonization. The average number of CFU we detected in only 1 g of feces of S. aureus-colonized individuals was ~ 1 log higher than that in a total nasal swab (Supplemental Figure 1), indicating that total S. aureus numbers in the gut greatly exceed those in the nose (by ~ 3 orders of magnitude given the average weight of human feces of ~ 100 g). While we are not aware of a previous study that measured S. aureus CFUs in the nares and feces in the same cohort of individuals, our numbers are in general accordance with previously obtained data on S. aureus CFU density in the nares and feces23-27 and emphasize the overwhelming importance of the intestinal colonization site for overall S. aureus colonization of the human body in quantitative terms. Furthermore, as mentioned above, we did not expect a pronounced impact of the gut-targeted decolonization on nasal colonization, but the significant reduction of S. aureus nasal CFU that we observed suggests a dominating role of the intestinal site for S. aureus colonization. In contrast, we are not aware of any study that reported reduction of intestinal CFU upon exclusively nose/skin-targeted decolonization, a scenario that also appears unlikely given the much greater abundance of S. aureus in the gut. These findings are of particular value, as studies analyzing the dynamic interdependence of different sites of S. aureus colonization are hardly possible in animals due to the limited extent and duration especially of experimental S. aureus nasal colonization28,29 and because the animals eat their feces. They indicate that intestinal S. aureus forms a reservoir for nasal S. aureus that may originate from repeated anal-to-nasal re-introduction. The higher over-time consistency we observed for intestinal versus nasal colonization (correlation coefficient of r=0.894 versus r=0.697; Supplementary Figure 1) is in further agreement with this idea. In that context it is noteworthy that we also detected significant reduction of nasal colonization in individuals in which we detected no previous S. aureus intestinal colonization as assessed by fecal CFU counting. However, in contrast to the direct analysis of nasal colonization by nasal swabs, that of intestinal colonization in the feces is only indirect. While fecal analysis is believed to give an overall adequate assessment of individual or post-intervention differences of intestinal colonization, underlying intestinal S. aureus may in some cases remain undetected.
Our study has limitations. First, among the non-intestinal S. aureus colonization sites we only analyzed the nose. We did so due to the traditional focus of S. aureus colonization studies on the nose and the comparatively lower colonization of other non-intestinal body sites24,27. Given that the S. aureus strain composition of those sites is similar30, indicating dynamic interdependence, it is likely that the relationship between intestinal colonization and that of those sites follows dynamics similar to those we have demonstrated for the nose.
Second, we performed our trial in a rural Thai population, because we wanted to confirm our previous more limited study on Bacillus spp./S aureus exclusion in the feces. We believe it is fair to assume a similar trial outcome in S. aureus carriers from a different geographic area, because the quorum-quenching effect of Bacillus on S. aureus is not strain-specific and we previously established considerable heterogeneity of the S. aureus strains colonizing Thai rural populations19. Third, the intervention groups had somewhat higher average baseline fecal and nasal CFU than the placebo groups. However, the differences were not significant (P=0.48, feces; P=0.40, nose, Mann-Whitney tests) and unlikely to have had more than a minor impact on the outcome.
In conclusion, our findings suggest that B. subtilis probiotic may be used to reduce S. aureus/MRSA colonization prevalence and thus may have clinical potential to lower infection rates for example in individuals with history of recurring S. aureus infections or in long-term care facilities, such as nursing homes, with notoriously increased S. aureus colonization and infection risks. While no known S. aureus decolonization procedure can achieve long-term protection from recolonization, the probiotic strategy – in contrast to any antibiotic-based strategy – offers the possibility for daily and long-term application as it does not harm the microbiota or triggers development of antibiotic resistance. Furthermore, our data provide support for the notion of a dominating role of the intestinal site for S. aureus colonization, suggesting that S. aureus decolonization efforts should generally focus on intestinal rather than, or at least in addition to, nasal colonization.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed from Jan 1, 2020 to Jan 1, 2022 for research studies published in any language with a primary goal of (1) decolonizing the human body from Staphylococcus aureus or (2) assessing S. aureus infection rates after decolonization but which included data on S. aureus decolonization efficiency. We used the search terms “decolonization/decolonisation” AND “Staphylococcus aureus”, and only included clinical trials in humans. Abstracts were screened by PP and MO for inclusion. We found 8 studies in which topical antibiotics or antiseptics were used fitting in category (1) and 4 in category (2). Most studies used 5-day protocols with mupirocin to decolonize the nares, sometimes combined with chlorhexidine or bleach skin washes. Three of those studies examined investigatory substances to decolonize the nares. We found no trials assessing decolonization of other sites in the analyzed time span.
Added value of this study
In this study, we show that orally administered probiotic Bacillus subtilis strongly diminishes S. aureus colonization of the human intestine without a significant effect on the microbiome, and even affects S. aureus numbers in the nose as a colonization site distal to the site of intervention. While previously employed topical approaches only affect a minor portion of the total S. aureus colonizing humans, this method achieves what was previously impossible, a reduction of a large portion (> 95%) of the total number of S. aureus colonizing humans without adverse side effects.
Implications of all the available evidence
The generally healthy probiotic method of decolonization that we propose could be of great value in settings with frequent S. aureus infections, such as nursing homes, long-term care hospitals, or surgical wards. Furthermore, our findings that indicate a pivotal role of the intestinal S. aureus colonization site call for a categorical rethinking of S. aureus colonization dynamics and the setup of S. aureus decolonization strategies.
Acknowledgements
This study was supported by The National Research Council of Thailand (grant number 256109A1710018, to S.K.) and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), U.S. National Institutes of Health (NIH) (project number ZIA AI000904 to M.O.). The authors wish to thank Dr. Lori E. Dodd, Biostatistics Research Branch, NIAID, and Dr. Huang Lin, Biostatistics and Bioinformatics Branch, National Institute of Child Health and Human Development (NICHD) for critically reading the manuscript and advice on statistics.
Funding:
National Research Council of Thailand, U.S. National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
We declare no competing interests except for funding as listed below. The B. subtilis probiotic formula (OPTI-BIOME®) was purchased from BIO-CAT Microbials. The suppliers did not have any influence on study design or interpretation.
Data sharing
The study protocol and de-identified participant data (questionnaires and summaries) are available from Michael Otto (motto@niaid.nih.gov) or Sunisa Khongthong (sunisa.p@RMUTSV.ac.th), respectively, upon request within one year after publication. Microbiome sequencing data were deposited at https://www.ncbi.nlm.nih.gov/bioproject/ under BioProject number PRJNA886398.
References
- 1.Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest 2003; 111(9): 1265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kourtis AP, Hatfield K, Baggs J, et al. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections - United States. MMWR Morb Mortal Wkly Rep 2019; 68(9): 214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 2005; 5(12): 751–62. [DOI] [PubMed] [Google Scholar]
- 4.Acton DS, Plat-Sinnige MJ, van Wamel W, de Groot N, van Belkum A. Intestinal carriage of Staphylococcus aureus: how does its frequency compare with that of nasal carriage and what is its clinical impact? Eur J Clin Microbiol Infect Dis 2009; 28(2): 115–27. [DOI] [PubMed] [Google Scholar]
- 5.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med 2001; 344(1): 11–6. [DOI] [PubMed] [Google Scholar]
- 6.Squier C, Rihs JD, Risa KJ, et al. Staphylococcus aureus rectal carriage and its association with infections in patients in a surgical intensive care unit and a liver transplant unit. Infect Control Hosp Epidemiol 2002; 23(9): 495–501. [DOI] [PubMed] [Google Scholar]
- 7.Sharara SL, Maragakis LL, Cosgrove SE. Decolonization of Staphylococcus aureus. Infect Dis Clin North Am 2021; 35(1): 107–33. [DOI] [PubMed] [Google Scholar]
- 8.Otto M Staphylococci in the human microbiome: the role of host and interbacterial interactions. Curr Opin Microbiol 2020; 53: 71–7. [DOI] [PubMed] [Google Scholar]
- 9.Simor AE, Daneman N. Staphylococcus aureus decolonization as a prevention strategy. Infect Dis Clin North Am 2009; 23(1): 133–51. [DOI] [PubMed] [Google Scholar]
- 10.Madden GR, Sifri CD. Antimicrobial Resistance to Agents Used for Staphylococcus aureus Decolonization: Is There a Reason for Concern? Curr Infect Dis Rep 2018; 20(8): 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz DJ, Langdon AE, Dantas G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med 2020; 12(1): 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhalla A, Aron DC, Donskey CJ. Staphylococcus aureus intestinal colonization is associated with increased frequency of S. aureus on skin of hospitalized patients. BMC Infect Dis 2007; 7: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srinivasan A, Seifried SE, Zhu L, et al. Increasing prevalence of nasal and rectal colonization with methicillin-resistant Staphylococcus aureus in children with cancer. Pediatr Blood Cancer 2010; 55(7): 1317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faden H, Lesse AJ, Trask J, et al. Importance of colonization site in the current epidemic of staphylococcal skin abscesses. Pediatrics 2010; 125(3): e618–24. [DOI] [PubMed] [Google Scholar]
- 15.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3): e18–55. [DOI] [PubMed] [Google Scholar]
- 16.Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014; 11(8): 506–14. [DOI] [PubMed] [Google Scholar]
- 17.Ouwehand AC, Forssten S, Hibberd AA, Lyra A, Stahl B. Probiotic approach to prevent antibiotic resistance. Ann Med 2016; 48(4): 246–55. [DOI] [PubMed] [Google Scholar]
- 18.Casula G, Cutting SM. Bacillus probiotics: spore germination in the gastrointestinal tract. Appl Environ Microbiol 2002; 68(5): 2344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piewngam P, Zheng Y, Nguyen TH, et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature 2018; 562(7728): 532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spears JL, Kramer R, Nikiforov AI, Rihner MO, Lambert EA. Safety Assessment of Bacillus subtilis MB40 for Use in Foods and Dietary Supplements. Nutrients 2021; 13(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray AJ, Pultz NJ, Bhalla A, Aron DC, Donskey CJ. Coexistence of vancomycin-resistant enterococci and Staphylococcus aureus in the intestinal tracts of hospitalized patients. Clin Infect Dis 2003; 37(7): 875–81. [DOI] [PubMed] [Google Scholar]
- 22.Cutting SM. Bacillus probiotics. Food Microbiol 2011; 28(2): 214–20. [DOI] [PubMed] [Google Scholar]
- 23.Nouwen JL, Ott A, Kluytmans-Vandenbergh MF, et al. Predicting the Staphylococcus aureus nasal carrier state: derivation and validation of a “culture rule”. Clin Infect Dis 2004; 39(6): 806–11. [DOI] [PubMed] [Google Scholar]
- 24.Solberg CO. A study of carriers of Staphylococcus aureus with special regard to quantitative bacterial estimations. Acta Med Scand Suppl 1965; 436: 1–96. [PubMed] [Google Scholar]
- 25.Lindberg E, Adlerberth I, Hesselmar B, et al. High rate of transfer of Staphylococcus aureus from parental skin to infant gut flora. J Clin Microbiol 2004; 42(2): 530–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindberg E, Adlerberth I, Matricardi P, et al. Effect of lifestyle factors on Staphylococcus aureus gut colonization in Swedish and Italian infants. Clin Microbiol Infect 2011; 17(8): 1209–15. [DOI] [PubMed] [Google Scholar]
- 27.Mermel LA, Cartony JM, Covington P, Maxey G, Morse D. Methicillin-resistant Staphylococcus aureus colonization at different body sites: a prospective, quantitative analysis. J Clin Microbiol 2011; 49(3): 1119–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiser KB, Cantey-Kiser JM, Lee JC. Development and characterization of a Staphylococcus aureus nasal colonization model in mice. Infect Immun 1999; 67(10): 5001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kokai-Kun JF. The Cotton Rat as a Model for Staphylococcus aureus nasal colonization in humans: cotton rat S. aureus nasal colonization model. Methods Mol Biol 2008; 431: 241–54. [DOI] [PubMed] [Google Scholar]
- 30.Albrecht VS, Limbago BM, Moran GJ, et al. Staphylococcus aureus Colonization and Strain Type at Various Body Sites among Patients with a Closed Abscess and Uninfected Controls at U.S. Emergency Departments. J Clin Microbiol 2015; 53(11): 3478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


