Summary
Background
Clinical guidelines advise osimertinib as preferred first line treatment for advanced epidermal growth factor receptor (EGFR) mutated non-small cell lung cancer (NSCLC) with deletions in exon 19 (del19) or exon 21 L858R mutation. However, for first-line osimertinib the real world overall survival (OS) in mutation subgroups remains unknown. Therefore, the aim of this study was to evaluate the real-world OS of those patients treated with different generations of EGFR-tyrosine kinase inhibitors (TKI), and to identify predictors of survival.
Methods
Using real-world data from the Netherlands Cancer Registry (NCR) we assessed patients diagnosed with stage IV NSCLC with del19 or L858R mutation between January 1, 2015, and December 31, 2020, primarily treated with then regularly available TKIs (including osimertinib).
Findings
Between January 1, 2015, and December 31, 2020, 57,592 patients were included in the NCR. Within this cohort we identified 1109 patients, 654 (59%) with del19 and 455 (41%) with L858R mutations, respectively; 230 (21%) patients were diagnosed with baseline brain metastases (BM). Patients were treated with gefitinib (19%, 213/1109), erlotinib (42%, 470/1109), afatinib (15%, 161/1109) or osimertinib (24%, 265/1109). Median OS was superior for del19 versus L858R (28.4 months (95% CI 25.6–30.6) versus 17.7 months (95% CI 16.1–19.5), p < 0.001. In multivariable analysis, no difference in survival was observed between various TKIs in both groups. Only in the subgroup of patients with del19 and baseline BM, a benefit was observed for treatment with osimertinib.
Interpretation
In this nationwide real-world cohort, survival of Dutch patients with advanced NSCLC and an EGFR del19 mutation was superior versus those harboring an L858R mutation. Osimertinib performed only better as first-line treatment in patients with del19 and BM.
Funding
None.
Keywords: Non-small cell lung cancer, Epidermal growth factor receptor, Tyrosine kinase inhibitor
Research in context.
Evidence before this study
We searched PubMed for studies published up to August 2022, using the terms “osimertinib AND observational AND overall survival”, restricted to English articles, resulting in 37 articles. These studies mainly focused on second line treatment, real world series without first line osimertinib, sequencing of EGFR TKI, and reported only progression free survival of first line treatment with osimertinib, due to limited follow up with recent adaption to first line osimertinib for EGFR mutated NSCLC. For first-line osimertinib the real-world overall survival (OS) in mutation subgroups remains unknown.
Added value of this study
This is one of the largest cohorts from Western Europe reporting real-world treatment effects of EGFR TKI on OS, including first line osimertinib, stratifying between patients with del19 or L858R mutation.
Implications of all the available evidence
Based on real-world evidence from our large, nationwide cohort, we showed that individual patient characteristics may be of influence on treatment choice, as we did not observe differences in OS between first-, second-, en third-generation EGFR TKI.
Introduction
In patients with advanced non-small cell lung cancer (NSCLC), the presence of a common epidermal growth factor receptor (EGFR) mutation is prognostically favorable as these patients have a superior overall survival (OS) compared with patients with wild type EGFR status.1 Treatment with first- and second-generation tyrosine kinase inhibitors (TKIs) showed superior results regarding progression free survival (PFS) compared to chemotherapy in both clinical trials as well as real world data, wheras OS was similar, probably due to cross-over in later treatment lines.2,3 Third-generation TKI have been compared to first- and second-generation TKIs mainly in clinical trials.4, 5, 6, 7
The most frequent detected mutations in EGFR are deletions in exon 19 (del19) and a single point mutation in exon 21 (L858R), together they account for 90% of EGFR mutations. It has been demonstrated that these common mutations are associated with better outcomes than uncommon EGFR mutations.8,9
Randomized trials comparing first- and second-generation EGFR TKI with chemotherapy as first-line therapy concluded that treatment with TKI significantly prolonged OS, in which the benefit was more pronounced for patients with del19 mutations compared to those with L858R mutations.10,11 This difference in OS between the two groups could mean either a different prognosis due to the structure of the mutation itself leading to a more aggressive biological behavior or be reflective of differences in efficacy of the used TKI for each mutation type.12 Resistance to targeted therapy due to genetic alterations, cell lineage plasticity, and the tumor microenvironment is different between del19 and L858R mutations.13 When the first- and second-generation TKI gefitinib and afatinib were compared directly in the LUX-lung 7 trial, OS did not differ between patients with del19 or L858R mutations, suggesting that both generations of TKI perform equally in patients within these common mutations.14 The third-generation EGFR TKI osimertinib was compared directly to the first-generation TKI erlotinib or gefitinib in the FLAURA study. In a post hoc analysis, a superior OS in patients with del19 treated with osimertinib was found, with a hazard rate (HR) of 0.68 (95% confidence interval (CI) 0.51–0.90), compared to a HR of 1.00 (95% CI 0.71–1.40) in patients with L858R mutation.15
The incidence of brain metastases (BM) is higher in patients with EGFR mutations compared to wild-type EGFR.16 Whereas the penetration of earlier generation TKI through the blood brain barrier is limited, the biological availability of osimertinib in the brain is better, showing a prolonged time to cerebral progression in patients with BM at start of treatment, although the efficacy on OS in the FLAURA trial was equal between patients with or baseline without BM (screening was not mandated).15,17, 18, 19
Whereas RCTs are the gold standard for evidence-based medicine, they are often not representative for clinical practice due to selection and exclusion criteria.20 On the other hand, real-world studies are prone to bias but may provide information about patient groups that are underrepresented in RCTs (poor performance status, elderly, BM) and can have sufficient sample size to allow subgroup analyses.21
At the end of 2019, as the third-generation EGFR TKI osimertinib was found superior compared to earlier generation TKI's in clinical trials, osimertinib was implemented as first line treatment in the Netherlands.4 Till date, there is limited real-world evidence that supports the benefit of osimertinib on OS found in clinical trials. Therefore, the aim of this study was to evaluate whether the introduction of osimertinib improved the OS of Dutch patients with advanced NSCLC harboring an EGFR del19 or L858R mutation compared to treatment with earlier-generation EGFR TKIs and whether the survival benefit was influenced by mutation type and the presence of baseline BM.
Methods
All patients diagnosed with any type of cancer are registered in the Netherlands Cancer Registry (NCR). A standardized real-world dataset is collected from patient records consisting of basic patient and disease characteristics, including histology, TNM stage, World Health Organization (WHO) performance score (PS), site(s) of metastasis, and type of first-line treatment. Information on OS is obtained by annual linkage with the population registry. Data on treatment response, progression of disease, coexisting mutations such as TP53 and STK11, second-line treatment, and cause of death are not available. Mutation analyses were predominantly performed with Next Generation Sequencing (83%).22
From the NCR, we assessed patients diagnosed with stage IV NSCLC with del19 or L858R mutation between January 1, 2015, and December 31, 2020, primarily treated with then regularly available TKIs (including osimertinib). The primary endpoint of this study was OS, calculated from the day of starting TKI, with follow up until February 1, 2022.
Considering its observational nature, this study did not require approval from an accredited medical ethics committee (MEC) or the Central Committee on Research involving Human Subjects (CCMO). However, the study has been reviewed and approved by the Privacy Review Board of the NCR (application number K21.320).
Statistical analyses were performed using StataSE 17. Patient characteristics were summarized using descriptive statistics and variation in the proportion of EGFR subtypes was assessed with chi-square tests. Survival was estimated by the Kaplan–Meier method and variation between subgroups was assessed with log-rank tests. The prognostic contribution of type of mutation and type of TKI was assessed by multivariable Cox regression and represented by HR and 95% confidence intervals (95% CI). The proportional hazard assumption was tested using log–log plots and independent prognostic factors have been determined using the backwards selection method. The final model included age, WHO PS, and number of organs with metastases as significant covariates. A p-value of <5% was considered as significant. A subgroup analysis was performed for patients with known baseline BM.
Results
The Netherlands has a population of about 17 million inhabitants, mainly white and including approximately 6% inhabitants from Asian descent.23 In the period 2015–2020, a total of 57,592 patients were diagnosed with NSCLC and registered in the NCR. We assessed 1109 patients with a median age of 68 years (interquartile range (IQR) 60–75) including 68% women. Baseline brain imaging (comprising MRI in 86%) was performed in 400 (36%) patients, diagnosing 230 (58%) patients with BM. Median time from diagnosis to start of TKI was 23 days. Before start of TKI, local treatment of these metastasis was performed in 74 of 230 (32%) patients, 66 with radiotherapy (Stereotactic Body Radio Therapy (SBRT) or Whole Brain Radio Therapy (WBRT)), 4 with surgery and 4 with combined modality. The use of upfront local treatment for BM decreased sharply after 2018, from 44% to 19%. In patients with BM treated with osimertinib, local treatment of BM was performed in 17% of cases compared with 39% in patients treated with other TKIs. The use of osimertinib increased with time from 3% in 2018 to 18% in 2019 and 94% of all prescribed first line EGFR TKI in 2020.
With respect to type of mutation, 654 (59%) patients had an EGFR del19 and 455 (41%) L858R mutation (Table 1). Patient characteristics were similar between the two mutations except for WHO PS 2 or higher, which was more frequent in patients with del19 (15.0% versus 8.1%).
Table 1.
Patient characteristics (n total = 1109).
| Del19 N |
% | L858R N |
% | p-value | |
|---|---|---|---|---|---|
| Age | |||||
| 18–59 | 165 | 25.2 | 96 | 21.1 | 0.07 |
| 60–69 | 209 | 32.0 | 140 | 30.8 | |
| 70–79 | 212 | 32.4 | 150 | 33.0 | |
| 80+ | 68 | 10.4 | 69 | 15.2 | |
| Gender | |||||
| Men | 218 | 33.3 | 137 | 30.1 | 0.26 |
| Women | 436 | 66.7 | 318 | 69.9 | |
| Histology | |||||
| Adenocarcinoma | 616 | 94.2 | 419 | 92.1 | 0.17 |
| Large NOS | 38 | 5.8 | 36 | 7.9 | |
| TKI | |||||
| Gefitinib | 115 | 17.6 | 98 | 21.5 | 0.10 |
| Erlotinib | 269 | 41.1 | 201 | 44.2 | |
| Afatinib | 103 | 15.8 | 58 | 12.8 | |
| Osimertinib | 167 | 25.5 | 98 | 21.5 | |
| WHO PS | |||||
| 0 | 241 | 36.9 | 165 | 36.3 | 0.003 |
| 1 | 177 | 27.1 | 152 | 33.4 | |
| 2+ | 98 | 15.0 | 37 | 8.1 | |
| Unknown | 138 | 21.1 | 101 | 22.2 | |
| TNM M | |||||
| 1A | 179 | 27.4 | 110 | 24.2 | 0.23 |
| 1B/C | 475 | 72.6 | 345 | 75.8 | |
| Period | |||||
| 2015–2017 | 266 | 42.2 | 202 | 44.4 | 0.22 |
| 2018–2020 | 388 | 57.8 | 253 | 55.6 | |
| Brain metastases | |||||
| Yes | 137 | 21.0 | 93 | 20.4 | 0.84 |
| No | 517 | 79.1 | 362 | 79.6 | |
| Number of metastatic organs | |||||
| 1 | 285 | 43.6 | 215 | 47.3 | 0.39 |
| 2 | 207 | 31.7 | 128 | 28.1 | |
| 3+ | 162 | 24.8 | 112 | 24.6 | |
| PD-L1 | |||||
| 0 | 195 | 29.8 | 127 | 27.9 | 0.79 |
| 1–49 | 110 | 16.8 | 72 | 16.4 | |
| 50+ | 72 | 11.0 | 56 | 11.5 | |
| Unknown | 277 | 42.4 | 200 | 44.0 |
TKI: tyrosine kinase inhibitor, WHO PS: World Health Organization performance score.
At the time of data cutoff, 70% of patients had deceased (41% in the osimertinib subgroup versus 79% in the other TKI group). Median follow-up was 28 months for the whole series and 17 months for patients treated with osimertinib.
The median OS was 22.8 months (95% CI 21.1–24.8) while three- and five-year survival rates were 31% (95% CI 28–34) and 12% (95% CI 10–15), respectively. Survival decreased with increasing age and poorer PS (Table 2). Survival was better for patients with stage M1A and those with less than 3 organs affected by metastases compared to more advanced disease. Survival of patients with del19 was significantly superior than for L858R, median OS 28.4 (95% CI 25.6–30.6) versus 17.7 months (95% CI 16.1–19.5), p < 0.001 (Fig. 1). The presence of baseline BM had no significant impact on survival, with a three-year survival rates of 27% versus 32%, p = 0.20. In patients with BM, local treatment did not influence survival, with three-year survival rates of 28% versus 26% (p = 0.70), respectively.
Table 2.
Overall survival (median and 3-year) by subgroups.
| n | Median (months) | 3-year (%) | p-value | |
|---|---|---|---|---|
| Age | ||||
| 18–59 | 261 | 25.9 | 35 | <0.001 |
| 60–69 | 349 | 24.7 | 33 | |
| 70–79 | 362 | 21.8 | 31 | |
| 80+ | 137 | 16.9 | 18 | |
| Gender | ||||
| Men | 355 | 20.6 | 27 | 0.15 |
| Women | 754 | 24.4 | 33 | |
| Histology | ||||
| Adeno | 1035 | 23.2 | 31 | 0.24 |
| Large NOS | 74 | 19.2 | 26 | |
| TKI | ||||
| Gefitinib | 213 | 19.7 | 26 | 0.29 |
| Erlotinib | 470 | 23.2 | 32 | |
| Afatinib | 161 | 23.3 | 34 | |
| Osimertinib | 265 | 22.8 | – | |
| WHO PS | ||||
| 0 | 406 | 26.8 | 34 | <0.001 |
| 1 | 329 | 23.2 | 32 | |
| 2+ | 135 | 13.1 | 16 | |
| Unknown | 239 | 21.2 | 32 | |
| TNM M | ||||
| 1A | 289 | 28.5 | 41 | <0.001 |
| 1B/C | 820 | 21.2 | 27 | |
| Period | ||||
| 2015–2017 | 468 | 21.0 | 29 | 0.10 |
| 2018–2020 | 641 | 24.1 | 32 | |
| Brain metastases | ||||
| Yes | 230 | 21.0 | 27 | 0.20 |
| No | 879 | 23.3 | 32 | |
| Number of metastatic organs | ||||
| 1 | 500 | 26.4 | 37 | <0.001 |
| 2 | 335 | 24.0 | 31 | |
| 3+ | 274 | 17.7 | 18 | |
| Mutation | ||||
| Del19 | 654 | 28.4 | 37 | <0.001 |
| L858R | 455 | 17.7 | 22 | |
| PD-L1 | ||||
| 0 | 322 | 27.6 | 37 | 0.07 |
| 1–49 | 182 | 21.8 | 25 | |
| 50+ | 128 | 20.8 | 32 | |
| Unknown | 477 | 21.2 | 29 |
TKI: tyrosine kinase inhibitor, WHO PS: World Health Organization performance score.
Fig. 1.
Overall survival by type of mutation in patients with stage IV EGFR mutated NSCLC, treated with first line TKI.
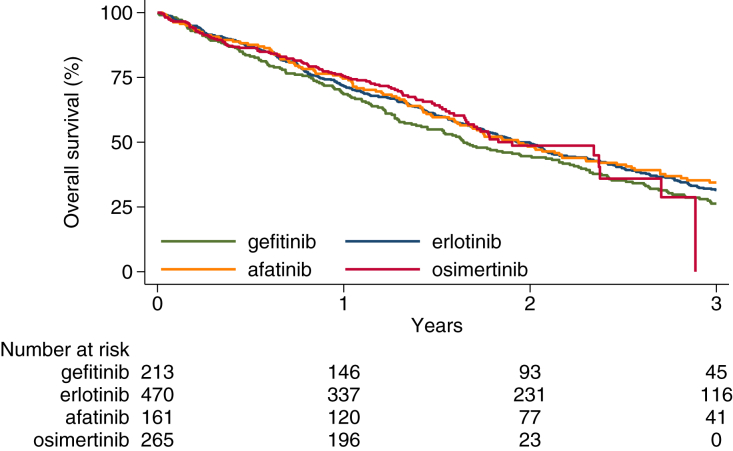
Univariable analysis did not show a significant difference in OS between the various TKIs (Table 2 and Fig. 2). Age, WHO PS and number of organs affected by metastases were identified as independent prognostic factors in multivariable analysis. When controlling for these factors in multivariable analysis, again no difference in survival was observed between the individual TKIs (Table 3). In the subgroup of patients with BM and del19, a benefit was observed for treatment with osimertinib while survival was significantly worse for gefitinib in patients with BM and L858R. Subgroup analysis in patients with BM treated with osimertinib confirmed a significant difference in survival between del19 and L858R (Fig. 3).
Fig. 2.
Overall survival by type of TKI in patients with stage IV EGFR mutated NSCLC treated with first line TKI.
Table 3.
Prognostic impact of type of TKI, stratified by type of EGFR mutation, controlling for age, WHO performance score and number of metastatic organs involved.
| TKI | Del19 |
L858R |
||||
|---|---|---|---|---|---|---|
| n | HR | 95% CI | n | HR | 95% CI | |
| Overall | ||||||
| Erlotinib | 115 | 1 | – | 98 | 1 | – |
| Gefitinib | 269 | 1.11 | 0.86–1.42 | 201 | 1.12 | 0.86–1.45 |
| Afatinib | 103 | 1.00 | 0.76–1.32 | 58 | 1.20 | 0.87–1.66 |
| Osimertinib | 167 | 0.82 | 0.60–1.12 | 98 | 1.02 | 0.73–1.41 |
| Brain metastases | ||||||
| Erlotinib | 60 | 1 | – | 36 | 1 | – |
| Gefitinib | 17 | 0.96 | 0.49–1.87 | 18 | 1.99∗ | 1.05–3.76 |
| Afatinib | 13 | 0.87 | 0.41–1.85 | 13 | 1.71 | 0.83–3.52 |
| Osimertinib | 50 | 0.54∗ | 0.30–0.99 | 26 | 1.60 | 0.80–3.20 |
TKI: tyrosine kinase inhibitor; HR: hazard ratio; CI: confidence interval; ∗: p-value < 0.05.
Fig. 3.
Overall survival by type of mutation in patients with stage IV EGFR mutated NSCLC and brain metastases, who received first-line treatment with osimertinib.
Discussion
In this Dutch nationwide real-world cohort, we confirmed the previously described superior OS for patients with an EGFR del19 versus an L858R mutation.11 OS benefit for patients treated with the third-generation TKI osimertinib as first line therapy was not different compared with first- and second-generation TKI. However, subgroup analysis revealed a benefit of osimertinib in patients with del19 and baseline BM, suggesting that the efficacy of the various TKIs may vary depending on tumor characteristics.
Similar findings were observed in the FLAURA trial, in which first-line osimertinib was compared with the first-generation TKIs gefitinib and erlotinib. Overall a major PFS benefit (HR 0.46 (95% CI 0.37–0.57)) was found, but with a borderline significant OS benefit (HR 0.80 (95% CI 0.64–1.00)). Subgroup analysis suggested that this OS benefit was restricted to del19 patients (HR 0.68 (95% CI 0.51–0.90)), no OS benefit was observed among L858R patients (HR 1.00 (95% CI 0.71–1.40)).15 Furthermore, in the FLAURA trial only an OS benefit was found for non-Asian patients (HR 0.54 (95% CI 0.38–0.77). In contrast to our results, no advantage with osimertinib was found for patients with baseline BM (HR 0.83 (95% CI 0.53–1.30)). Pursuing this approach, we also stratified the OS analyses by type of mutation but did not find significant variation between the individual TKIs in our mainly white population. However, despite including more than 1000 patients, the confidence intervals are relatively wide as osimertinib was only introduced in recent years and follow-up for patients treated with osimertinib was relatively short. Second-line treatment with osimertinib after first- or second-generation TKI was available during the study period for patients with T790M resistance and may have diluted the comparison. A concurrent study reported that 42% of Dutch patients with EGFR mutated NSCLC were diagnosed with resistance mutations upon progression, and that 75% of patients receiving second-line therapy were treated with a TKI.22 However, other, smaller real-world studies evaluating the introduction of first line osimertinib also failed to find an OS benefit and this may reflect the poor results of second-line treatment after progression on osimertinib.24,25 Regrettably, information on date and type of progression and type of second-line treatment was not available within the Netherlands Cancer Registry.
Subgroup analysis of patients with BM suggests that OS does vary between TKIs, depending on the type of EGFR mutation. Gefitinib and erlotinib are almost identical in chemical structure, but some substituents are different, and this may have consequences in selective binding. The chemical structure of afatinib and osimertinib differs significantly from first-generation agents.26 Also, the pharmacokinetics of the agents are different, with respect to the area under the drug plasma concentration–time curve and maximum drug concentration at steady state, and the influence of smoking status, drug interactions and negative influence of acid-suppressant therapies. Of the agents reported here, osimertinib is least affected by these factors.27
Whereas gefitinib and erlotinib bind competitively and reversibly to the ATP-binding site of the EGFR tyrosine kinase domain, afatinib and osimertinib form irreversible covalent bonds to the ATP-binding site, hereby irreversibly blocking activation. Osimertinib is also capable of targeting the T790M mutation and its metabolites penetrate the blood brain barrier better than previous generation TKIs.27 These factors suggest that osimertinib has superior pharmacokinetic properties compared to gefitinib, erlotinib and afatinib, especially for patients with BM. However, in our study this was only confirmed for patients with a del19 mutation, but the relatively small number of patients limit clinical application of these findings.
Several studies suggest that BM of patients with del19 are biologically different from those in L858R patients. Takano et al. reported that BM in patients with L858R are spread differently within the brain, as they are located more often closer to the brain surface and located in the caudate, cerebellum, and temporal lobe compared to del19.28 Sekine et al. found that patients with del19 were more prone to miliary BM compared to L858R patients.29 Moreover, CNS progression appears to be earlier in L858R patients than del19 patients.30
Over the years, cranial radiotherapy is increasingly deferred when there is an option to treat patients with TKI that may cross the blood brain barrier. However, for patients with L858R mutation and BM, a retrospective Chinese study analyzing 61 patients showed that median OS was significantly better (29.2 versus 18.8 months) if osimertinib was combined with cranial radiotherapy.31 This can also be suggested from our data as, although the number of patients was low, 40% of patients with L858R and BM died within 4 months, the minority of these patients received local treatment for their BM before start of systemic treatment. It is not clear whether these patients died from neurological progression (i.e. no CNS response), or that there was a high need to immediately starting TKI to control extracranial disease, but without success. RCTs evaluating osimertinib versus osimertinib plus stereotactic radiotherapy are currently under investigation.32,33 Another treatment option for these patients might be dose escalation to 160 mg daily. This strategy was analyzed in the phase I BLOOM trial as upfront treatment (n = 41) and in a recent retrospective multi-center study after progressive disease on regular dose of 80 mg (n = 105). Only minor toxicity was observed with 160 mg, but efficacy with escalating the dose from 80 to 160 mg was limited, and prospective trials evaluating a dose escalation strategy are absent.34,35
Real-world studies about the treatment and survival of EGFR mutated NSCLC have their pros and cons.20 Data were derived from a national registry and included octogenarians (12%) and patients with performance status 2 or higher (12%). BM were diagnosed in 21% of patients and the real prevalence may even be higher as only 36% of patients received brain imaging before treatment start. To our knowledge, this is one of the largest cohort studies from Western Europe reporting real-world treatment effects of first-, second- and third-generation TKI, stratifying between patients with del19 or L858R mutation. However, due to its retrospective design, we did not have information about method of testing, clinical information on therapy response (e.g., response rate or PFS), treatment duration, toxicity, resistance mechanisms, subsequent treatments and data on quality of life, thus lacking the details of data from RCT's. However, we provided results that are more generalizable to the average patient in Europe. Also, multiplicity should be taken in account for our subgroup analysis. For a proper interpretation of OS findings, the availability and use of second-line treatment should have been incorporated. As a second-line treatment, osimertinib was formally approved for patients with EGFR T790M mutation in 2017 but it may have been available earlier in clinical trials or early access programs. As a first-line treatment, osimertinib was made available through an expanded access program as of 2019 and got reimbursed in the Netherlands in 2020.
As we did not observe a clear survival benefit for patients treated with first line osimertinib, this study challenges the current guideline with respect to appropriate sequencing of TKI and other treatment options. Of note, as the median follow-up of osimertinib treated patients is still limited in this study, it might be possible that there are more long-term survivors on osimertinib compared to previous generation TKI. Approximately half of the patients treated with first- or second-generation TKI can be treated with osimertinib upon progression due to an acquired T790M mutation. Notably, there appears to be no difference in the occurrence of the T790M co-mutation between del19 and L858R patients.36 For these patients, the quality of life may be preserved for a longer period, as those treated with upfront osimertinib mainly rely on cytotoxic chemotherapy upon progression, with decreased treatment time on TKI.37 When analyzing the toxicity profiles of osimertinib and erlotinib/gefitinib in the FLAURA trial, patients treated with osimertinib experienced less grade ≥3 adverse events than those treated with first-generation TKI, however, these rates become equal with expanded follow up.4,15 In a network meta-analysis performed by Holleman et al., gefitinib, erlotinib, and osimertinib were associated with fewer toxicities compared to afatinib, whereas the increased toxicity of afatinib was also shown in the large retrospective study by Pluzanski et al.38,39 However, data on any grade toxicity and quality of life and more specific BM related quality of life over multiple treatment lines is lacking.
Combination therapy with other anti-cancer drugs is currently investigated. Preclinical data showed that L858R is correlated with a higher expression of vascular endothelial growth factor (VEGF) compared to del19.40 And whereas patients with del19 profit more from TKI monotherapy compared to those with an L858R mutation, the addition of the VEGF inhibitor ramucirumab to erlotinib treatment in the RELAY trial seems to be more effective in L858R in terms of PFS, equalizing the difference with del19 patients. Although the OS data from that trial are still immature, a hypothesis-generating trend to a better OS was observed in L858R patients treated with erlotinib plus ramucirumab.41,42 This was also observed in the ARTEMIS-CTONG1591 trial comparing erlotinib plus bevacizumab with monotherapy erlotinib. In addition, in this trial a potential benefit was found for the patients with baseline BM, as the effect of the combination therapy is approaching statistical significancy albeit with immature OS data (HR 0.62 (95% CI 0.38–1.01)).43 However, for these combination regimens, a comparison with osimertinib is not available in first line and in second line no superiority for osimertinib plus bevacizumab versus monotherapy osimertinib was shown.44 Also, the effect might be affected by for example baseline TP53 co-mutation or smoking status.42,45 Another study showed that addition of chemotherapy to gefitinib enhanced OS in patients with del19, whereas this was not significant for L858R, at cost of a higher rate of grade ≥3 treatment-related adverse events.46 The results of the addition of immune checkpoint inhibition to TKI treatment are disappointing.47 Currently, platinum and pemetrexed added to concurrent osimertinib is being evaluated in the first-line setting in the FLAURA2 (ClinicalTrials.gov Identifier: NCT04035486) and TAKUMI (UMIN000024438) trials.48 Next, although a phase 2 trial recently failed to show benefit of addition of bevacizumab to osimertinib, it is still under investigation (ClinicalTrials.gov Identifier: NCT04181060).49,50 These trials may lead to new treatment options and sequences, but due to the heterogeneity of the patient population and evolving options for biomolecular testing, optimal treatment for specific subgroups cannot readily be evaluated.
Conclusion
Dutch patients with stage IV EGFR mutated NSCLC harboring a del19 mutation have superior OS compared to patients with a L858R mutation. A survival benefit of the introduction of first line treatment with osimertinib was observed for a subgroup of patients with del19 and BM as compared to other TKI, but not for other subgroups. This finding needs to be substantiated in larger real-world populations.
Contributors
Rolof Gijtenbeek: Formal analysis, Investigation, Writing – original draft, Visualization, Project Administration.
Ronald Damhuis: Conceptualization, Methodology, Data Curation, Formal analysis, Writing – review & editing.
Anthonie van der Wekken: Supervision, Formal analysis, Writing – review & editing.
Lizza Hendriks: Formal analysis, Writing – review & editing.
Harry Groen: Conceptualization, Methodology, Supervision, Formal analysis, Writing – review & editing.
Wouter van Geffen: Supervision, Investigation, Formal analysis, Writing – review & editing.
Data sharing statement
After publication of this study, deidentified data can be made available upon request, after approval of the NCR.
Declaration of interests
RG: none.
RD: none.
AW: has received grants or contracts from AstraZeneca, Pfizer, Boehringer Ingelheim, Takeda and Roche, outside the submitted work; consulting fees from AstraZeneca, Novartis, Boehringer Ingelheim, Roche, Janssen, Pfizer, Lilly, Takeda and Merck, outside the submitted work; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Pfizer, outside the submitted work; and support for attending meetings and/or travel received from Lilly, outside the submitted work; and has a leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid, for CPCT, all payments were made to the institution, outside the submitted work.
LH: has received grants or contracts from Roche Genentech, AstraZeneca, Boehringer Ingelheim, Takeda, and Beigene (under negotiation) (all paid to the institution) outside the submitted work, speakers fee from MSD and Lilly (paid to the institution), fees for educational webinars from Benecke, Medtalks, VJOncology (self) and high5oncology (paid to the institution), outside the submitted work. Support for attending meetings was received from Roche Genentech, and advisory boards from BMS, Eli Lilly, Roche Genentech, Pfizer, Takeda, MSD, Merck, Novartis, Boehringer Ingelheim, Amgen, Janssen (except Roche, all paid to the institution), outside the submitted work. Mentorship program funded by AstraZenica and interview sessions funded by Roche Genentech, Bayer, Lilly (all paid to the institution), outside the submitted work. Local PI of clinical trials: AstraZeneca, Novartis, BMS, MSD, Merck, GSK, Takeda, Blueprint Medicines, Roche Genentech, Janssen Pharmaceuticals, Mirati, Abbvie, Gilead.
HG: has received grants or contracts from Roche and Boehringer Ingelheim (paid to the institution), outside the submitted work; and consulting fees from Lilly, Novartis, Roche/Genentech, and AstraZeneca (except for Lilly, paid to the institution), outside the submitted work.
WG: has unpaid roles for ERS and NVALT, outside the submitted work; and there have been trials run by his department funded by Roche and Amgen.
Acknowledgements
Funding: none.
References
- 1.Kuijpers C.C.H.J., Hendriks L.E.L., Derks J.L., et al. Association of molecular status and metastatic organs at diagnosis in patients with stage IV non-squamous non-small cell lung cancer. Lung Cancer. 2018;121:76–81. doi: 10.1016/j.lungcan.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Kuan F.C., Kuo L.T., Chen M.C., et al. Overall survival benefits of first-line EGFR tyrosine kinase inhibitors in EGFR-mutated non-small-cell lung cancers: a systematic review and meta-analysis. Br J Cancer. 2015;113:1519–1528. doi: 10.1038/bjc.2015.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y., Appius A., Pattipaka T., Feyereislova A., Cassidy A., Ganti A.K. Real-world management of patients with epidermal growth factor receptor (EGFR) mutation-positive non–small-cell lung cancer in the USA. PLoS One. 2019;14:e0209709. doi: 10.1371/journal.pone.0209709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soria J.-C., Ohe Y., Vansteenkiste J., et al. Osimertinib in untreated EGFR -mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 5.Lu S., Dong X., Jian H., et al. AENEAS: a randomized phase III trial of aumolertinib versus gefitinib as first-line therapy for locally advanced or metastatic non-small-cell lung cancer with EGFR exon 19 deletion or L858R mutations. J Clin Oncol. 2022;40:3162. doi: 10.1200/JCO.21.02641. JCO2102641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Y., Chen G., Wang X., et al. Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study. Lancet Respir Med. 2022;10:1019. doi: 10.1016/s2213-2600(22)00168-0. [DOI] [PubMed] [Google Scholar]
- 7.Kelly R.J., Shepherd F.A., Krivoshik A., Jie F., Horn L. A phase III, randomized, open-label study of ASP8273 versus erlotinib or gefitinib in patients with advanced stage IIIB/IV non-small-cell lung cancer. Ann Oncol. 2019;30:1127. doi: 10.1093/annonc/mdz128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leduc C., Merlio J.P., Besse B., et al. Clinical and molecular characteristics of non-small-cell lung cancer (NSCLC) harboring EGFR mutation: results of the nationwide French Cooperative Thoracic Intergroup (IFCT) program. Ann Oncol. 2017;28:2715–2724. doi: 10.1093/annonc/mdx404. [DOI] [PubMed] [Google Scholar]
- 9.Graham R.P., Treece A.L., Lindeman N.I., et al. Worldwide frequency of commonly detected EGFR mutations. Arch Pathol Lab Med. 2018;142:163–167. doi: 10.5858/arpa.2016-0579-CP. [DOI] [PubMed] [Google Scholar]
- 10.Yang J.C.H., Wu Y.L., Schuler M., et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–151. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 11.Sheng M., Wang F., Zhao Y., et al. Comparison of clinical outcomes of patients with non-small-cell lung cancer harbouring epidermal growth factor receptor exon 19 or exon 21 mutations after tyrosine kinase inhibitors treatment: a meta-analysis. Eur J Clin Pharmacol. 2016;72:1–11. doi: 10.1007/s00228-015-1966-0. [DOI] [PubMed] [Google Scholar]
- 12.Shigematsu H., Gazdar A.F. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer. 2006;118:257–262. doi: 10.1002/ijc.21496. [DOI] [PubMed] [Google Scholar]
- 13.Chua K.P., Teng Y.H.F., Tan A.C., et al. Integrative profiling of T790M-negative EGFR-mutated NSCLC reveals pervasive lineage transition and therapeutic opportunities. Clin Cancer Res. 2021;27:5939–5950. doi: 10.1158/1078-0432.CCR-20-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paz-Ares L., Tan E.-H., O'Byrne K., et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol. 2017;28:270–277. doi: 10.1093/annonc/mdw611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramalingam S.S., Vansteenkiste J., Planchard D., et al. Overall survival with osimertinib in untreated, EGFR -mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 16.Li L., Luo S., Lin H., et al. Correlation between EGFR mutation status and the incidence of brain metastases in patients with non-small cell lung cancer. J Thorac Dis. 2017;9:2510. doi: 10.21037/jtd.2017.07.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reungwetwattana T., Nakagawa K., Cho B.C., et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018;36:3290–3297. doi: 10.1200/JCO.2018.78.3118. [DOI] [PubMed] [Google Scholar]
- 18.Varrone A., Varnäs K., Jucaite A., et al. A PET study in healthy subjects of brain exposure of 11 C-labelled osimertinib - a drug intended for treatment of brain metastases in non-small cell lung cancer. J Cereb Blood Flow Metab. 2020;40:799–807. doi: 10.1177/0271678X19843776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colclough N., Chen K., Johnstrom P., et al. Preclinical comparison of the blood-brain barrier permeability of osimertinib with other EGFR TKIs. Clin Cancer Res. 2021;27:189–201. doi: 10.1158/1078-0432.CCR-19-1871. [DOI] [PubMed] [Google Scholar]
- 20.Nazha B., Yang J.C.H., Owonikoko T.K. Benefits and limitations of real-world evidence: lessons from EGFR mutation-positive non-small-cell lung cancer. Future Oncol. 2021;17:965–977. doi: 10.2217/fon-2020-0951. [DOI] [PubMed] [Google Scholar]
- 21.Gijtenbeek R.G.P., Damhuis R.A.M., Groen H.J.M., van der Wekken A.J., van Geffen W.H. Nationwide real-world cohort study of first-line tyrosine kinase inhibitor treatment in epidermal growth factor receptor-mutated non–small-cell lung cancer. Clin Lung Cancer. 2020;21:E647–E653. doi: 10.1016/j.cllc.2020.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Steeghs E.M.P., Groen H.J.M., Schuuring E., et al. Mutation-tailored treatment selection in non-small cell lung cancer patients in daily clinical practice. Lung Cancer. 2022;167:87–97. doi: 10.1016/j.lungcan.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Statistics Netherlands StatLine. 2016. https://opendata.cbs.nl/statline/#/CBS/nl/dataset/37296ned/table?ts=1560279178833
- 24.Bazhenova L., Minchom A., Viteri S., et al. Comparative clinical outcomes for patients with advanced NSCLC harboring EGFR exon 20 insertion mutations and common EGFR mutations. Lung Cancer. 2021;162:154–161. doi: 10.1016/j.lungcan.2021.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Lee C.S., Ahmed I., Miao E., et al. A real world analysis of first line treatment of advanced EGFR mutated non-small cell lung cancer: a multi-center, retrospective study. J Oncol Pharm Pract. 2021 doi: 10.1177/10781552211020798. [DOI] [PubMed] [Google Scholar]
- 26.Todsaporn D., Mahalapbutr P., Poo-arporn R.P., Choowongkomon K., Rungrotmongkol T. Structural dynamics and kinase inhibitory activity of three generations of tyrosine kinase inhibitors against wild-type, L858R/T790M, and L858R/T790M/C797S forms of EGFR. Comput Biol Med. 2022;147 doi: 10.1016/j.compbiomed.2022.105787. [DOI] [PubMed] [Google Scholar]
- 27.Solassol I., Pinguet F., Quantin X. FDA- and EMA-approved tyrosine kinase inhibitors in advanced EGFR-mutated non-small cell lung cancer: safety, tolerability, plasma concentration monitoring, and management. Biomolecules. 2019;9 doi: 10.3390/biom9110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takano K., Kinoshita M., Takagaki M., et al. Different spatial distributions of brain metastases from lung cancer by histological subtype and mutation status of epidermal growth factor receptor. Neuro Oncol. 2016;18:716–724. doi: 10.1093/neuonc/nov266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sekine A., Kato T., Hagiwara E., et al. Metastatic brain tumors from non-small cell lung cancer with EGFR mutations: distinguishing influence of exon 19 deletion on radiographic features. Lung Cancer. 2012;77:64–69. doi: 10.1016/j.lungcan.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Heon S., Yeap B.Y., Britt G.J., et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010;16:5873–5882. doi: 10.1158/1078-0432.CCR-10-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhai X., Li W., Li J., et al. Therapeutic effect of osimertinib plus cranial radiotherapy compared to osimertinib alone in NSCLC patients with EGFR-activating mutations and brain metastases: a retrospective study. Radiat Oncol. 2021;16:233. doi: 10.1186/s13014-021-01955-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. NCT04908956. Osimertinib and locally ablative radiotherapy in patients with synchronous oligo-metastatic EGFR mutant NSCLC (STEREO) 2021. https://clinicaltrials.gov/ct2/show/NCT04908956
- 33. NCT03769103. Study of osimertinib + SRS vs osimertinib alone for brain metastases in EGFR positive patients with NSCLC. 2018. https://clinicaltrials.gov/ct2/show/NCT03769103
- 34.Yang J.C.H., Kim S.-W., Kim D.-W., et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: the BLOOM study. J Clin Oncol. 2019;38:538–547. doi: 10.1200/JCO.19.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piper-Vallillo A.J., Rotow J.K., Aredo J.v., et al. High-dose osimertinib for CNS progression in EGFR+ NSCLC: a multi-institutional experience. JTO Clin Res Rep. 2022;3 doi: 10.1016/j.jtocrr.2022.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park S., Lee S.Y., Kim D., et al. Comparison of epidermal growth factor receptor tyrosine kinase inhibitors for patients with lung adenocarcinoma harboring different epidermal growth factor receptor mutation types. BMC Cancer. 2021;21:52. doi: 10.1186/s12885-020-07765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khoon Lee C., Novello S., Rydén A., Mann H., Mok T. Patient-reported symptoms and impact of treatment with osimertinib versus chemotherapy in advanced non-small-cell lung cancer: the AURA3 trial. J Clin Oncol. 2018;36:1853–1860. doi: 10.1200/JCO.2017.77.2293. [DOI] [PubMed] [Google Scholar]
- 38.Holleman M.S., van Tinteren H., Groen H.J.M., Al M.J., Uyl-de Groot C.A. First-line tyrosine kinase inhibitors in EGFR mutation-positive non-small-cell lung cancer: a network meta-analysis. Onco Targets Ther. 2019;12:1413–1421. doi: 10.2147/OTT.S189438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pluzanski A., Krzakowski M., Kowalski D., Dziadziuszko R. Real-world clinical outcomes of first-generation and second-generation epidermal growth factor receptor tyrosine kinase inhibitors in a large cohort of European non-small-cell lung cancer patients. ESMO Open. 2020;5:1–8. doi: 10.1136/esmoopen-2020-001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan X.H., Yang J., Wang X.Y., Zhang X.L., Qin T.T., Li K. Association between EGFR/KRAS mutation and expression of VEGFA, VEGFR and VEGFR2 in lung adenocarcinoma. Oncol Lett. 2018;16:2105–2112. doi: 10.3892/ol.2018.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landre T., des Guetz G., Chouahnia K., Duchemann B., Assié J.B., Chouaid C. First-line angiogenesis inhibitor plus erlotinib versus erlotinib alone for advanced non-small-cell lung cancer harboring an EGFR mutation. J Cancer Res Clin Oncol. 2020;146:3333–3339. doi: 10.1007/s00432-020-03311-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakagawa K., Nadal E., Garon E.B., et al. RELAY Subgroup analyses by EGFR Ex19del and Ex21L858R mutations for Ramucirumab plus erlotinib in metastatic non-small cell lung cancer. Clin Cancer Res. 2021;27:5258. doi: 10.1158/1078-0432.CCR-21-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Q., Xu C.R., Cheng Y., et al. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): a multicenter phase 3 study. Cancer Cell. 2021;39:1279–1291.e3. doi: 10.1016/j.ccell.2021.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Soo R.A., Han J.Y., Dafni U., et al. A randomised phase II study of osimertinib and bevacizumab versus osimertinib alone as second-line targeted treatment in advanced NSCLC with confirmed EGFR and acquired T790M mutations: the European Thoracic Oncology Platform (ETOP 10-16) BOOSTER trial. Ann Oncol. 2022;33:181–192. doi: 10.1016/j.annonc.2021.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Dafni U., Soo R.A., Peters S., et al. Impact of smoking status on the relative efficacy of the EGFR TKI/angiogenesis inhibitor combination therapy in advanced NSCLC—a systematic review and meta-analysis. ESMO Open. 2022;7:100507. doi: 10.1016/j.esmoop.2022.100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hosomi Y., Morita S., Sugawara S., et al. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol. 2020;38:115–123. doi: 10.1200/JCO.19.01488. [DOI] [PubMed] [Google Scholar]
- 47.Creelan B.C., Yeh T.C., Kim S.W., et al. A phase 1 study of gefitinib combined with durvalumab in EGFR TKI-naive patients with EGFR mutation-positive locally advanced/metastatic non-small-cell lung cancer. Br J Cancer. 2021;124:383–390. doi: 10.1038/s41416-020-01099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Planchard D., Feng P.-H., Karaseva N., et al. Osimertinib plus platinum–pemetrexed in newly diagnosed epidermal growth factor receptor mutation-positive advanced/metastatic non-small-cell lung cancer: safety run-in results from the FLAURA2 study. ESMO Open. 2021;6 doi: 10.1016/j.esmoop.2021.100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.NCT04181060. Osimertinib with or without bevacizumab as initial treatment for patients with EGFR-mutant lung cancer - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04181060; 2020
- 50.Kenmotsu H., Wakuda K., Mori K., et al. Randomized phase 2 study of osimertinib plus bevacizumab versus osimertinib for untreated patients with nonsquamous NSCLC harboring EGFR mutations: WJOG9717L study. J Thorac Oncol. 2022;17:1098–1108. doi: 10.1016/j.jtho.2022.05.006. [DOI] [PubMed] [Google Scholar]