Abstract
Lactobacillus reuteri (L. reuteri), a type of Lactobacillus spp., is a gut symbiont that can colonize many mammals. Since it was first isolated in 1962, a multitude of research has been conducted to investigate its function and unique role in different diseases as an essential probiotic. Among these, the basic functions, beneficial effects, and underlying mechanisms of L. reuteri have been noticed and understood profoundly in intestinal diseases. The origins of L. reuteri strains are diverse, with humans, rats, and piglets being the most common. With numerous L. reuteri strains playing significant roles in different intestinal diseases, DSM 17938 is the most widely used in humans, especially in children. The mechanisms by which L. reuteri improves intestinal disorders include protecting the gut barrier, suppressing inflammation and the immune response, regulating the gut microbiota and its metabolism, and inhibiting oxidative stress. While a growing body of studies focused on L. reuteri, there are still many unknowns concerning its curative effects, clinical safety, and precise mechanisms. In this review, we initially interpreted the basic functions of L. reuteri and its related metabolites. Then, we comprehensively summarized its functions in different intestinal diseases, including inflammatory bowel disease, colorectal cancer, infection-associated bowel diseases, and pediatric intestinal disorders. We also highlighted some important molecules in relation to the underlying mechanisms. In conclusion, L. reuteri has the potential to exert a beneficial impact on intestinal diseases, which should be further explored to obtain better clinical application and therapeutic effects.
Keywords: Lactobacillus reuteri, intestinal diseases, gut microbiota, inflammatory bowel disease, colorectal cancer
Introduction
According to the World Health Organization, probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” (Mu et al., 2018). These probiotics, that have already been proven to exert a beneficial impact, include Saccharomyces boulardii, Lactobacillus spp., Bifidobacterium spp., Propionibacterium spp., Streptococcus spp., Bacillus spp., Enterococcus spp., and some specific strains of Escherichia colietc, in which Lactobacillus spp. is the most widely used in human nutrition (Kechagia et al., 2013; Markowiak and Slizewska, 2017).
Recently, numerous bodies of research demonstrated that probiotics are beneficial for various diseases, such as intestinal disorders, respiratory tract infections, vaginal diseases, and so on (Markowiak and Slizewska, 2017; Nami et al., 2018). With the development of the food and drug industries and the innovation of technology, an increasing number of emerging probiotic strains were developed and applied to different fields, including natural food preservatives, nutraceuticals, and so on (Nami et al., 2015, 2018). Some novel technologies can also enhance probiotic products' quality and sensory characteristics, which can contribute to the extensive application of probiotics (Kiani et al., 2021a,b).
Safety is also an essential issue during the process of investigating probiotics. An ocean of evidence indicated that the use of probiotics can cause some risks regarding systemic infections, deleterious metabolic activities, excessive immune stimulation in susceptible individuals, gastrointestinal side effects, and so on (Doron and Snydman, 2015). Some probiotic microorganisms can even transfer resistance genes to protect against antibiotics, which may be responsible for the development of the antibiotic resistance crisis (Daniali et al., 2020). Taken together, the application of probiotics is a double-edged sword. Before probiotics are used, we still need to carry out enough clinical trials and animal experiments to assess their benefits and harms.
Lactobacillus spp., which can be found in various food products, is one of the most widely used probiotics, and includes Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Lactobacillus casei, and Lactobacillus reuteri (L. reuteri) (Giraffa et al., 2010). Lactobacillus reuteri is a gut symbiont mainly colonized in the intestines of humans, rodents, pigs, and chickens (Oh et al., 2010; Walter et al., 2011; Rattanaprasert et al., 2019). Since first isolated in 1962, there have been a great number of studies conducted on L. reuteri to explore its functions and concrete mechanisms in different diseases, which cover gastrointestinal diseases, hypercholesterolemia, skin infection, allergic asthma, periodontitis, hand, foot, and mouth disease (HFMD), and so on (Prince et al., 2012; Ang et al., 2016; Giudice et al., 2016; Mu et al., 2018; Tachi et al., 2018; Theodoro et al., 2019; Wang et al., 2022). Among these, there is an increasingly prevalent trend that the investigations of L. reuteri in terms of intestinal diseases are becoming far and away the best area, which mainly concentrates on inflammatory bowel disease (IBD), colorectal cancer (CRC), children's functional bowel diseases, and so on.
Intestinal diseases, especially IBD, have become an increasing burden on the global healthcare system and society. The prevalence of IBD is expected to increase to 790 per 100,000 in 2025 (Morales et al., 2017). Of note, there will be over 1.5 million cases of IBD in China by 2025 (Kaplan, 2015). The increased use of biological therapies and the aging population will bring new challenges and complexities to public healthcare and society's economy (Kaplan, 2015). Moreover, IBD is one of the leading causes of CRC, which is the third most common cancer globally (Weitz et al., 2005; Keller et al., 2019). Although the incidence of CRC has decreased overall, it has been estimated that the incidence rates for colon and rectal cancers may increase by 90.0 and 124.2%, respectively, for patients between the ages of 20 and 34 by 2030 (Bailey et al., 2015). This phenomenon may be attributed to the fact that the pediatric overall prevalence of IBD has increased sharply (Ye et al., 2020). IBD, including ulcerative colitis (UC), and Crohn's disease (CD), is a type of chronic relapsing-remitting disease characterized by intestinal inflammation (Guo X. et al., 2021). Conventional treatment approaches include mesalazine, glucocorticoids, immunosuppressors, and so on. However, considering multiple investigations on gut microbiota and IBD (David et al., 2014; Jost et al., 2014; Thaiss et al., 2014; Ananthakrishnan, 2015), probiotics are expected to become an effective treatment for this disease. Notably, there is an abundance of studies on the fact that L. reuteri exhibits the following beneficial capacities: anti-inflammation, immune regulation, gut micro-ecology balance, gut barrier protection, metabolic control, and so on (Mu et al., 2018).
In this study, we comprehensively reviewed the literature concerning L. reuteri and its metabolites in the pathogenesis of several common intestinal diseases, which are illustrated in a separate section for a clearer understanding. Some basic introductions and future perspectives are also discussed in this review (Tables 1–3).
Table 1.
Therapeutic efficacy and potential mechanisms of Lactobacillus reuteri strains in various intestinal diseases in cell models.
| Lactobacillus reuteri strain | References | Experimental model/participant | Disease | Effect/outcome | Mechanism of action |
|---|---|---|---|---|---|
| I5007 | Marcinkiewicz et al. (2007) | LPS-induced human colon cell line HT-29 cells | IBD | Reduced pro-inflammatory cytokines levels | Inhibition of NF-κB pathway |
| NK33 | Mackos et al. (2016) | LPS-induced BV2 and SH-SY5Y cells | Anxiety/ depression and colitis | Inhibited IL-6 expression; increased LPS-suppressed CREB phosphorylation as well as BDNF expression | Inhibition of NF-κB pathway |
| MG5346 | Fong et al. (2020) | Human colorectal carcinoma RKO cells | CRC | Induced cell apoptosis | Activation of Caspase-9-Dependent Apoptosis pathway |
| LFCA-encoding L. reuteri CO21 (LR-LFCA) | Gao et al. (2017) | LPS-induced IPEC-J2 cells | Infectious bowel disease | Reduced oxidative stress and inflammatory factors | Activation of the NRF2/HO-1 pathway; inhibition of the NF-κB pathway |
| L26 Biocenol (CCM 8616) | Zhang et al. (2018) | ETEC-induced IPEC-1 cells | Infectious bowel disease | Attenuated overexpression of the gene and suppressed inflammatory responses | The underlying mechanisms remain unclear |
| ATCC PTA 6475 | Bell et al. (2022) | ETEC-induced IPEC-J2 cells | Infectious bowel disease | Protected the mucosal barrier and reduced inflammatory factors | The underlying mechanisms remain unclear |
| DSM 17938 | Bell et al. (2022) | ETEC-induced IPEC-J2 cells | Infectious bowel disease | Protected the mucosal barrier and reduced inflammatory factors | The underlying mechanisms remain unclear |
| 1563F | Bell et al. (2022) | ETEC-induced IPEC-J2 cells | Infectious bowel disease | Protected the mucosal barrier and reduced inflammatory factors | The underlying mechanisms remain unclear |
| LR1 | Martín-Cabrejas et al. (2017) | ETEC-induced IPEC-1 cells | Infectious bowel disease | Decreased the adhesion and invasion of the coliform in IPEC-1 cells; increased transcript abundance and protein contents of TJ proteins ZO-1 and occludin and enhanced epithelial barrier | Activation of MLCK pathway |
| ATCC PTA 6475 | Watschinger and Moschen (2022) | EPEC-induced human colon carcinoma HT-29 (ATCC HTB-38) and LS174T cells (ATCC CL-188) | Infectious bowel disease | Adherence of L. reuteri to HT-29 cells was strain-specific; inhibited EPEC binding to HT-29 but not LS174T cells; decreased EPEC adherence to small intestinal biopsy epithelium | The underlying mechanisms remain unclear |
| ATCC 53608 | Watschinger and Moschen (2022) | EPEC-induced human colon carcinoma HT-29 (ATCC HTB-38) and LS174T cells (ATCC CL-188) | Infectious bowel disease | Adherence of L. reuteri to HT-29 cells was strain-specific; inhibited EPEC binding to HT-29 but not LS174T cells; decreased EPEC adherence to small intestinal biopsy epithelium | The underlying mechanisms remain unclear |
| ATCC 55730 | Karimi et al. (2018) | Peritoneal macrophages | Infectious bowel disease | Activated macrophages and enhanced the ability of macrophages to phagocytose and to kill intracellular Salmonella Typhimurium; increased the secretion of NO in macrophages and enhanced the anti-inflammatory effect | The underlying mechanisms remain unclear |
IBD, inflammatory bowel disease; NF-κB, nuclearfactor-κB; LPS, lipopolysaccharide; BDNF, brain-derived neurotrophic factor; CREB, cAMP-response element binding protein; NRF2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase 1; TJ, tight junction; MLCK, myosin light-chain kinase; NO, nitric oxide; IPEC-1, Intestinal porcine epithelial cell; IPEC-J2, porcine jejunal epithelial cell; TJ, tight junction; ZO-1, zonula occluden-1; MLCK, myosin light-chain kinase.
Table 3.
Therapeutic efficacy and potential mechanisms of Lactobacillus reuteri strains in various intestinal diseases in humans.
| Lactobacillus reuteri strain | References | Experimental model/participant | Disease | Effect/outcome | Mechanism of action |
|---|---|---|---|---|---|
| RC-14 | Jang et al. (2018a) | Human | IBD | The proportion of CD4+ CD25 high T cells increased, but TNF-α+/IL-12+ monocytes and myeloid DC decreased | The underlying mechanisms remain unclear |
| ATCC 55730 | Jang H. M. et al. (2019) | Children(years range 6–18) | IBD | Improved mucosal inflammation and changed mucosal expression levels of some cytokines(IL-10 significantly increased whereas IL-1β, TNFα, and IL-8 significantly decreased) | The underlying mechanisms remain unclear |
| DSM 17938 | Gancarčíková et al. (2019) | Infants younger than 60 days | Colic | Decrease in daily crying time in infants with colic | Activation of FoxP3 |
| SGL01 | Hojsak (2019) | Infants aged 3–16 weeks | Colic | Number and duration of crying episodes decreased significantly | The underlying mechanisms remain unclear |
| FloraActive™ 12246 | Pärtty et al. (2018) | Infants aged 4–12 weeks | Colic | Decreased cry and fuss time | The underlying mechanisms remain unclear |
| ATCC 55730 | Roos et al. (2013) | Infants | Colic | Decreased crying time | The underlying mechanisms remain unclear |
| LR92 DSM 26866 | Skórka et al. (2017) | Pregnant women | Colic | Prevented the occurrence and reduce the severity of infantile colic | The underlying mechanisms remain unclear |
| DSM 17938 | Gerasimov et al. (2018) | Children aged 6 months−6 years | CFC | Exhibited significant improvement in defecation frequency | The underlying mechanisms remain unclear |
| DSM 17938 | Pourmirzaiee et al. (2020) | Children with a mean age 9.1 ± 3.8 years | FAP | Reduced the frequency and intensity of abdominal pain episodes | The underlying mechanisms remain unclear |
IBD, inflammatory bowel disease; DCs, dendritic cells; TNF-α, tumor necrosis factor-α; FoxP3, forkhead box P3; IL, interleukin; CFC, chronic functional constipation; FAP, functional abdominal pain.
Table 2.
Therapeutic efficacy and potential mechanisms of Lactobacillus reuteri strains in various intestinal diseases in animal models.
| Lactobacillus reuteri strain | References | Experimental model/participant | Disease | Effect/outcome | Mechanism of action |
|---|---|---|---|---|---|
| R28 | Xu et al. (2021) | PEG + DSS-induced C57BL/6 mice | IBD | Reduced diarrhea; reduced pro-inflammatory cytokines; enhanced the intestinal barrier | The underlying mechanisms remain unclear |
| ATCC PTA 4659 | Guarner et al. (2002) | DSS-induced C57BL/6J mice | IBD | Reduced pro-inflammatory cytokines; prevented the CD11b+Ly6G+ neutrophil recruitment; reduced the CD11b+CD11c+ DCs; Foxp3+CD4+ t cells decreased in MLNs | Upregulated HSPs family |
| 5454 | Shin and Kim (2018) | TNBS-induced C57BL/6J and BALB/c ByJ mice | IBD | Improved colitis; induced tolerogenic DCs and triggered IL-22 secretion | Induction of Tregs |
| ATTC PTA 6475 | Guo F. et al. (2021) | TNBS-induced BALB/c mice | IBD | Suppressed inflammation; promoted DCs maturation, stimulated IL-10 production | The underlying mechanisms remain unclear |
| I5007 | Marcinkiewicz et al. (2007) | DSS-induced C57BL/6 mice | IBD | Reduced weight loss, colon length shortening, and histopathological damage; restored the mucus layer; reduced pro-inflammatory cytokines levels; altered colonic microbiota and metabolic structural and functional composition | Inhibition of NF-κB pathway; stimulated the expression of MUC2, increased the number of goblet cells; the enrichment of KEGG pathways, such as ABC transporters and carbohydrate metabolism-related pathways |
| ATCC PTA 6475 | Liu et al. (2022) | DSS-induced C57BL/6 mice | IBD | Reduced weight loss; ameliorated the immunopathology and inflammatory status | Reduction of ILC3s |
| BR11 | Wang G. et al. (2020) | DSS-induced Sprague–Dawley rats | IBD | Improvement in crypt area; | The underlying mechanisms remain unclear |
| 23272 | Khalil et al. (2016) | Citrobacter rodentium-induced C57BL/6 mice and CCL2−/− mice | IBD | Reduced the stressor effects and histopathological damage | Down-regulation of the chemokine CCL2 |
| Clade II strain 6475 | Weger and Sandi (2018) | TNBS-induced BALB/c mice | IBD | Diminished weight loss, colonic injury, serum amyloid A (SAA) protein concentrations, and reduced uptake of [18F]FDG | Activation of hdc/l-histidine/histamine/H2R pathway |
| F-9-35 | Jang et al. (2018b) | DSS-induced ICR mice | IBD | Had less inflammatory Phenotype; reduced myeloperoxidase activity, and lower expression of proinflammatory genes (TNF-α, COX-2 and IL-6); alleviation of microbiota dysregulation | The underlying mechanisms remain unclear |
| NK33 | Mackos et al. (2016) | Immobilization stress (IS)-induced C57BL/6 mice | Anxiety/ depression and colitis | Alleviated the occurrence and development of anxiety/depression and colitis; suppressed infiltration of Iba1+ and LPS+/CD11b+ cells (activated microglia) into the hippocampus, and corticosterone, IL-6, and LPS levels in the blood | Inhibition of NF-κB pathway; increase of BDNF expression and CREB phosphorylation |
| ATCC PTA 6475 | He et al. (2022) | AOM-induced BALB/c mice, hdc−/− BALB/c mice | CRC | Reduced the number and size of colon tumors | Activation of hdc/histamine/H2R pathway |
| MG5346 | Fong et al. (2020) | Human colorectal cancer xenografts in BALB/c nude mice | CRC | Inhibited tumor growth | Activation of Caspase-9-Dependent Apoptosis pathway |
| LFCA-encoding L. reuteri CO21 (LR-LFCA) | Gao et al. (2017) | ETEC-induced piglets | Infectious bowel disease | Attenuated the weight loss and diarrhea incidence; improved the intestinal morphology, intestinal epithelial barrier and increased the expression of intestinal tight junction proteins; improved the gut microbiota; modulated gut immune responses; reduced oxidative stress and inflammatory factors | Activation of the NRF2/HO-1 pathway; inhibition of the NF-κB pathway |
| HCM2 | Asare et al. (2020) | ETEC-induced ICR mice | Infectious bowel disease | Inhibited the growth of ETEC and its ability to adhere to intestinal epithelial cells; preserved intestinal morphology; stabilized the gut microbiota | The underlying mechanisms remain unclear |
| TMW1.656 | Zhang et al. (2020) | Weanling piglets | Infectious bowel disease | Reduced the copy numbers of genes for E. coli and the heat-stable enterotoxin in feces, reduced the level of colonization of weaning piglets with ETEC | The underlying mechanisms remain unclear |
| LTH5794 | Zhang et al. (2020) | Weanling piglets | Infectious bowel disease | Reduced the copy numbers of genes for E. coli and the heat-stable enterotoxin in feces; reduced the level of colonization of weaning piglets with ETEC | The underlying mechanisms remain unclear |
| Lb11 | Xie et al. (2021) | Eggs and chickens | Infectious bowel disease | Inhibited the growth of Salmonella enteritidis | Reduction of the AcrAB-TolC efflux pump genes, outer membrane protein genes and antibiotic resistance genes |
| KUB-AC5 | Tkáčiková et al. (2020) | Salmonella enteritidis S003-induced broiler chickens (Ross 308) | Infectious bowel disease | Maintained the stabilization of gut microbiome; enhanced Lactobacillaceae levels in both the ileum and caecum and suppressed Enterobacteriaceae levels | The underlying mechanisms remain unclear |
| ATCC 55730 | Karimi et al. (2018) | Salmonella Typhimurium-induced C57BL/6 mice | Infectious bowel disease | Reduced weight loss; prolonged the survival of mice; inhibited the dissemination of S. typhimurium from the abdominal cavity to the spleen and liver | The underlying mechanisms remain unclear |
| CCM 8617 | Yi et al. (2018a) | Salmonella Typhimurium CCM 7205NAL-induced BALB/c mice | Infectious bowel disease | Reduced the growth of Salm. Typhimurium; alleviated the negative impact of Salm. Typhimurium; the liver showed marked reduction of overall inflammation, hepatocyte necrosis and size of typhoid nodules | The underlying mechanisms remain unclear |
| SLZX19-12 | Yang et al. (2015) | Salmonella Typhimurium SL1344-induced C57BL/6J mice | Infectious bowel disease | Lower loads of Salmonella in visceral organs, less colonic inflammation, and higher barrier integrity; more stable microbiota structure of the colon, in which the abundance of Alloprevotella was greatly enhanced | The underlying mechanisms remain unclear |
| ATCC 23272 | Walsham et al. (2016) | HRV-induced gnotobiotic pigs | Infectious bowel disease | Enhanced Th1 and Th2 cytokine responses to HRV infection; regulated TGF-β production to maintain immune homeostasis | The underlying mechanisms remain unclear |
| L26 BiocenolTM | Eaton et al. (2011) | PCV2-induced germ-free Balb/c mice | Infectious bowel disease | Enhanced the gut immune response and decreased the amount of PCV2 in feces and in the ileum | Up-regulated the gene expression of chemokines, IFN-γ, IgA and PIgR and increased the proportion of natural killer cells and the CD19+ lymphocytes in the MLN. |
| DSM 17938 | Kubota et al. (2020) | Newborn Sprague-Dawley rat pups | NEC | Increased the percentage of Foxp3+ T cells in the ileum while decreasing the percentage of cells in the MLN; Enhanced anti-inflammatory effect and regulated immune response | Activation of FoxP3+ Tregs |
IBD, inflammatory bowel disease; HSPs, heat shock proteins; PEG, polyethylene glycol; DSS, dextran sodium sulfate; DCs, dendritic cells; MLNs, mesenteric lymph nodes; FoxP3, forkhead box P3; TNBS, 2,4,6-trinitrobenzenesulfonic acid; Tregs, regulatory T cells; IL, interleukin; NF-κB, nuclearfactor-κB; MUC2, mucin 2; KEGG, kyoto encyclopedia of genes and genome; ILC3s, group 3 innate lymphoid cells; SAA, serum amyloid A; H2R, type 2 histamine receptor; TNF-α, tumor necrosis factor-α; HDC, histidine decarboxylase; AOM, azoxymethane; COX-2, cyclooxygenase-2; IS, immobilization stress; ETEC, enterotoxigenic Escherichia coli; EPEC, enteropathogenic Escherichia coli; CREB, cAMP-response element binding protein; LFCA, lactoferricin-lactoferrampin; IFN-γ, interferon-γ; IgA, immunoglobulin A; PIgR, polymeric Ig receptor; NEC, necrotizing enterocolitis; PCV2, porcine circovirus type 2; HRV, human rotavirus; TH1, helper T cell 1; TH2, helper T cell 2; TGF-β, transforming growth factor-β.
The basic function of L. reuteri and its metabolites
According to incomplete statistics, L. reuteri consists of dozens of strains that originated from different samples. In addition, each stain and its unique metabolite may play a distinct role in various intestinal diseases. It is well documented that L. reuteri plays multifaceted roles in regulating immune responses, modulating gut microbiota, boosting beneficial metabolites, protecting against oxidative stress, maintaining intestinal barrier (IEB) function and intestinal morphology, and so on (Yi et al., 2018b; Liu et al., 2019; Garg et al., 2020; Singh et al., 2021). In this study, we will list some representative strains to introduce concrete pathogenesis.
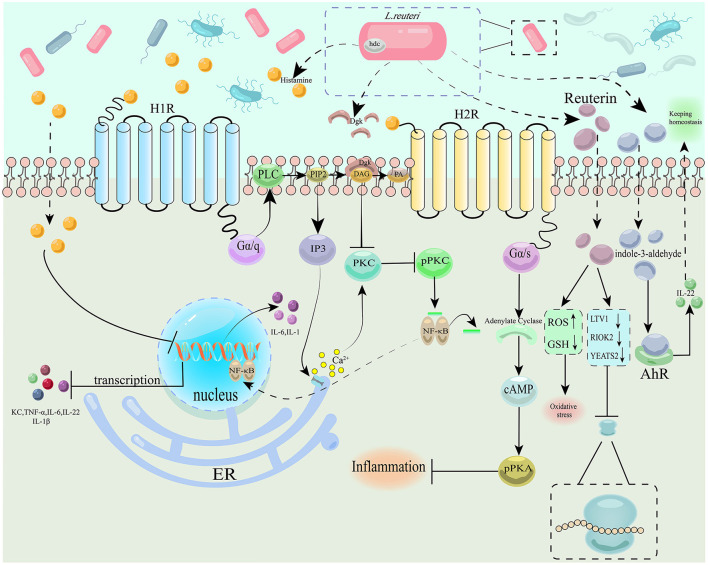
It is well established that histamine is beneficial to the intestine, whose synthesis and secretion require l-histidine decarboxylase and a l-histidine/histamine exchanger (Hemarajata et al., 2013; Spinler et al., 2014). The current study identified chloride channel (ClC)-family proton/chloride antiporters as a modulator in the process of histamine production (Hall et al., 2019). Lactobacillus reuteri 6475 is special among gut microbes due to it containing a complete chromosomal histidine decarboxylase (hdc) gene cluster (genes hdcA, hdcB, and hdcP) and thus having the genetic capacity to convert histidine to histamine (Spinler et al., 2014). Lactobacillus reuteri-derived histamine can suppress gut inflammation by activating type 2 histamine receptors (H2R) and restricting pro-inflammatory H1R (Schreiber et al., 2009; Preidis et al., 2012). One of the mechanisms can be attributed to the function of a soluble bacterial enzyme named diacylglycerol kinase (Dgk), secreted by the L. reuteri strain, which can diminish Protein Kinase C (PKC) phosphorylation and suppress the H1R signaling pathway in the intestinal epithelium (Ganesh et al., 2018). Lactobacillus reuteri 6475 was also related to folate metabolism, which was mediated by dihydrofolate synthase/folylpolyglutamate synthase type 2 (folC2). Notably, the folC2 mutant can yield diminished hdc gene cluster expression and thus reduced histamine production, hinting at a link between folate and histadine/histamine metabolism (Thomas et al., 2016). Tryptophan (Trp) metabolism is also essential for gut immune homeostasis. The Trp metabolites from L. reuteri are known as aryl hydrocarbon receptor (AhR) ligand—indole-3-aldehydes, which can contribute to activating the AhR-IL-22 axis and maintaining intestinal homeostasis (Zelante et al., 2013). Another experiment also disclosed that indole-activated AhR could reprogram intraepithelial CD4+ T cells into immunoregulatory T cells to perform regulatory functions (Cervantes-Barragan et al., 2017). Özçam et al. (2019) creatively identified a novel pathway by which L. reuteri activates AhR, which is independent of Trp metabolism, known as polyketide synthase (PKS) gene clusters in L. reuteri 2010 and R2lc.
As is well-known, the adhesive ability is significant for bacterial function in the intestines of the host. In vitro experiments showed that L. reuteri has the potential to enhance adhesion in HT-29 cells (Dudík et al., 2020). Given this, Gao et al. (2016) assessed the modulatory effects of two strains of L. reuteri—ZJ617 and ZJ615, with different adhesive abilities in in vivo experiments. Finally, the authors indicated that both of them can exert anti-inflammatory and anti-oxidative effects, plus metabolism regulation, including glucose and its derivatives, galactose, amino acid metabolism, biosynthesis of antibiotics, and mineral absorption (Gao et al., 2016). Furthermore, the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein of L. reuteri ZJ617 has been proven to work as an essential adhesion component in binding to the intestinal epithelial cells (Yang et al., 2020). In a harsh context, L. reuteri SH23 still retained its adhesive ability with the help of the Mub protein (Xu et al., 2021).
Maintaining the functions of the intestinal epithelium is a key point in protecting against bowel diseases. It was demonstrated that L. reuteri D8 has the ability to restore the epithelial damage caused by TNF by activating the Wnt/β-catenin pathway, thus stimulating the proliferation of the intestine, increasing the number of Paneth cells and increasing the expression of antimicrobial peptides (Wu et al., 2020). Lactobacillus reuteri 22 also was capable of promoting intestinal stem cell differentiation into goblet cells with increased mucin 2 (Muc-2) expression to ensure the functionality of the intestinal mucosal barrier (Xie et al., 2019). microRNAs (miRNAs) functioned as an agent during the anti-inflammatory course of L. reuteri I5007. It was able to maintain intestinal epithelial function by changing the miRNA expression of piglets, especially the PI3K-Akt and MAPK pathways, modulated by different signaling pathways (Wang Q. et al., 2020).
Association between L. reuteri and inflammatory bowel disease
With the acceleration of industrialization and changes in diet, IBD has become an emerging global disease, the incidence of which has risen considerably over the past several decades, both in the Western world and in developing countries (Kaplan, 2015). It is common knowledge that the gut microbiota's roles in the development and course of IBD are significant and enlightening. Given this, probiotics have become a hot topic both in the fundamental research and clinical practice of IBD (Martyniak et al., 2021). Research on L. reuteri is thus increasingly important.
Research on L. reuteri in fundamental fields of IBD
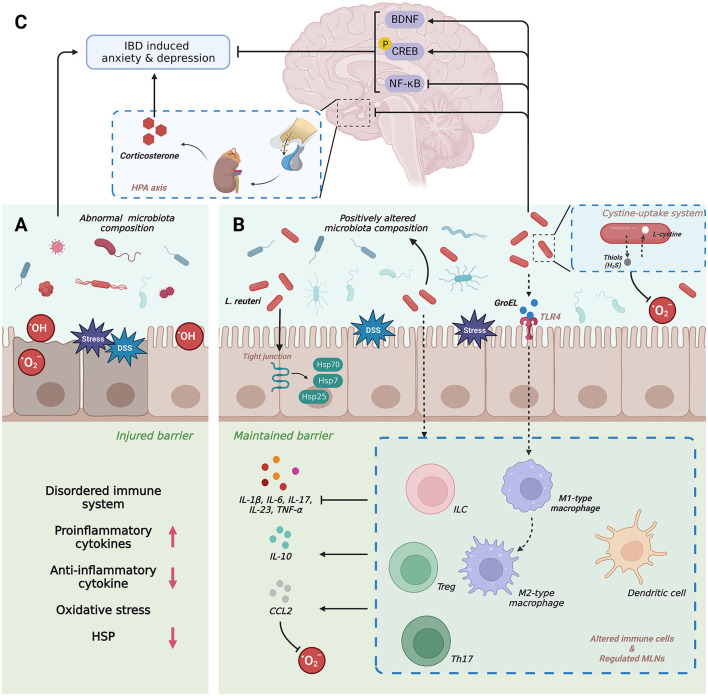
Fundamental study of the relationship between IBD and L. reuteri can be traced back to 2002. In that study, researchers initially found that a colonized L. reuteri strain can prevent the development of colitis in genetically susceptible mice (Guarner et al., 2002). One common feature of IBD is the disruption of the intestinal barrier and, subsequently, an uncontrollable inflammatory signal cascade (Shin and Kim, 2018; Guo F. et al., 2021). Consequently, a collection of studies focused on these two aspects. With the help of the 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (cFDA-SE) labeling technique, Wang et al. (2021) found that endogenous L. reuteri R28, isolated from mouse feces, can, significantly ameliorate diarrhea caused by polyethylene glycol (PEG) through its superior colonization in the intestinal environment, regulate the expression of pro-inflammatory factors in mice with colitis induced by PEG + dextran sulfate sodium (DSS), and enhance the intestinal barrier. Marcinkiewicz et al. (2007) used a chronic active colitis animal model to explore the function of Lactobacillus strains. Among these, L. reuteri was also found to perform anti-inflammatory activities. Macrophages play a key role in the establishment of chronic intestinal inflammation observed in IBD (Heinsbroek and Gordon, 2009). It is well-documented that macrophages have two phenotypes: pro-inflammatory M1-like and anti-inflammatory M2-like. Evidence suggests that GroEL purified from L. reuteri can promote macrophage switching to M2-like polarization from the M1-like phenotype to present its anti-inflammatory properties through the Toll-like receptor (TLR) 4 and the non-canonical pathway (Dias et al., 2021). The previous study by Liu et al. (2021) demonstrated that L. reuteri can strengthen the intestinal barrier by regulating the expression level of tight junction (TJ) protein and thus protect against colitis in mice. Further, the authors found that L. reuteri ATCC PTA 4659 plays an essential role in the anti-inflammatory effect and the related immune reactions by reducing the number of CD11b+CD11c+ dendritic cells (DCs) and regulating the function of mesenteric lymph nodes (MLNs) (Liu et al., 2022). Similarly, L. reuteri 5454 and ATTC PTA 6475 have anti-inflammatory and anti-infectious capacities in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced acute colitis in mice by promoting DC maturation, stimulating IL-10 production, and inducing the differentiation of Treg cells and Th17 cells (Hrdý et al., 2020; Engevik et al., 2021).
In addition to having anti-inflammatory effects, L. reuteri can also modulate gut microbiota and metabolic disorders in colitis in mice. Wang G. et al. (2020) first isolated L. reuteri I5007 from healthy weanling piglets and subsequently examined the effects of L. reuteri I5007 in suppressing colonic inflammation, improving colonic microbiota composition, and regulating the metabolic pathways through in vivo and in vitro models (Hou et al., 2014). In in vivo experiments, pretreatment with L. reuteri I5007 for 1 week can effectively decelerate DSS-induced weight loss, minimize the reduction in colon length, restore the function of goblet cells, and reduce the production of cytokines. Moreover, it was also beneficial to microbiota and metabolite composition. In vitro, the pretreatment of this strain was able to reduce the expression level of IL-1β and TNF-α in HT-29 cells challenged with lipopolysaccharides (LPS) for 4 h. In addition, the authors also revealed that L. reuteri treatment improves the DSS-disrupted gut microbial ecology, especially in the colon.
Inflammatory bowel disease is known to be a chronic inflammatory-immune disease, stimulating the exploration of immune-related mechanisms regulated by probiotics, in which L. reuteri can play an important role. It is currently widely known that immune checkpoint blockade (ICB) immunotherapy has become a promising cancer treatment (Postow et al., 2015; Khalil et al., 2016). However, it can also have some serious side effects, of which ICB-associated colitis is one of the most common complications (Michot et al., 2016). In the animal experiment of Wang et al. (2019) the authors finally concluded that direct administration of L. reuteri ATCC PTA 6475 can facilitate the immunopathology of ICB colitis and, inspiringly, does not exert an impact on the antitumor immunity of ICB by means of significantly decreasing the numbers of mucosal group 3 innate lymphoid cells (ILC3s) and the expression of IL17 and IL23. Nevertheless, the authors did not elaborate on the further mechanism for how L. reuteri lowered the numbers of ILC3s.
Some studies suggested that probiotic therapy can attenuate oxidative stress in rats, which is one of the main factors aggravating intestinal injury in IBD (Damiani et al., 2007; Sengül, 2011). In the experiment of Haydn et al., the authors concluded that neither wild-type BR11 nor a CyuC-deficient strain of L. reuteri could prevent the development of experimental colitis in rats. Hence, the authors stated that L. reuteri BR11 has the ability to reduce the severity of experimental IBD, owing to its unique antioxidant properties and cysteine/cystine-transport system (Turner et al., 1999; Atkins et al., 2012). However, in this study, researchers only explored the relationship between the cystine-uptake system and L. reuteri BR11. The further mechanism still requires investigation. Lactobacillus reuteri 23272 can also attenuate the effects of stressor exposure on pathogen-induced colitis by downregulating the chemokine CCL2, which was proven to be indispensable in Citrobacter rodentium-induced colitis (Mackos et al., 2016).
Anxiety disorder is a common disorder and can progress to depression, which has become a global disease (Baxter et al., 2014; Bandelow and Michaelis, 2015; Weger and Sandi, 2018). A systematic review concluded that patients with IBD have an approximately 20% prevalence rate of anxiety and a 15% prevalence rate of depression (Neuendorf et al., 2016). An ocean of research demonstrated that probiotics have the ability to reduce depression, accompanied by a series of mechanisms, in which L. reuteri was found to exert an anti-depressive impact on mice (Davis et al., 2016; Jang et al., 2018a,b). Based on the above, Jang H. M. et al. (2019) investigated the preventive and curative effects of L. reuteri NK33, isolated from healthy human feces, on immobilization stress (IS)-induced anxiety/depression and colitis in mice. The findings can be divided into two major parts: (1) L. reuteri NK33 exhibited an anti-inflammatory effect by inhibiting the expression of IL-6 and the activation NF-κB pathways in LPS-treated BV-2 cells. Further, L. reuteri NK33 could improve intestinal inflammation by restricting the expression of pro-inflammatory cytokines and the infiltration of inflammatory cells and enhancing the abundance of gut microbiota, such as Bacteroidetes, Firmicutes, and Actinobacteria in mice. (2) The treatment with L. reuteri NK33 can activate microglial cell infiltration into the hippocampus and induce hippocampal brain-derived neurotrophic factor (BDNF) expression and cAMP-response element binding protein (CREB) phosphorylation in IS-exposed mice as well as LPS-stimulated SH-SY5Y cells by suppressing the activation of the NF-κB pathway and HPA axis, thus suggesting that NK33 alleviated the suppression of NF-κB-mediated BDNF expression in the hippocampus, with the regulation of LPS infiltration into the brain, leading to the attenuation of anxiety and depression. At the same time, this research also uncovered the synergy between L. reuteri NK33 and Bifidobacterium adolescentis NK98.
Some vital molecules also play indispensable roles in treating IBD using L. reuteri, which deserve expounding. Heat shock proteins (HSPs), a type of highly conserved molecular chaperone, work as gatekeepers for intracellular proteins to maintain cell homeostasis (Liu et al., 2014a; Gupta et al., 2017). HSPs, activated by TJ protein, are vital in protecting the gut epithelium against oxidative stress and inflammation, regulating the immune response, and modulating bacterial functions (Liu et al., 2014a, 2022). The research of Liu H-Y. et al. also found that pretreatment with L. reuteri ATCC PTA 4659 can enhance the expression of two inducible HSPs, i.e., HSP7 and HSP25, in the distal colon of mice, at mRNA and/or protein levels, by increasing the mean fluorescence intensity (MFI) of HSP70 and HSP25 in both surface mucosa and the crypt as well as expanding their distribution when compared with the control animals. Furthermore, in the colon, the crypt HSP25 expression was negatively correlated with the bacterial load and the Ki67+ cell number (Liu et al., 2022).
Nevertheless, this study only revealed the expression of HSPs after treatment with L. reuteri without elucidating concrete mechanisms. Histamine, the vital molecular in histidine metabolism, played an essential role in the TNBS-induced mouse colitis model. Gao et al. (2017) demonstrated that hdc+ L. reuteri clade II strain 6475, isolated from breast milk, attenuates colonic inflammation through the activation of the histidine decarboxylase (hdc) gene and the histamine H2 receptor (H2R) and supplementation of dietary L-histidine (Gao et al., 2015). Regarding this topic, Hemarajata et al. (2013) reported that the L. reuteri-specific immunoregulatory (rsiR) gene, which originated from gene expression profiles of L. reuteri ATCC PTA 6475, is essential for TNF suppression and hdc gene expression. Further, the TNBS mouse model lacking the rsiR gene fails to exhibit anti-inflammatory effects (Hemarajata et al., 2013). MiR-142a-3p, a type of microRNAs (miRNAs), was found to alleviate colitis by promoting the growth of L. reuteri and its metabolite, further affecting the expression of inflammatory genes in intestinal epithelial cells (He et al., 2022) (Figure 1).
Figure 1.
(A–C) Schematic diagram depicting the pathogenic role and therapeutic potential of Lactobacillus reuteri in IBD. IBD, inflammatory bowel disease; BDNF, brain-derived neurotrophic factor; CREB, cAMP-response element binding protein; DSS, dextran sodium sulphate; HSPs, heat shock proteins; NF-κB, nuclearfactor-κB; IL, interlukin; TNF-α, tumor necrosis factor-κ; CCL, C-C motif chemokine 2; Th, T helper cell; TLR, Toll-like receptors; ILC, innate lymphoid cells; Treg, regulatory T cell; MLN, mesenteric lymph node.
The clinical applications of L. reuteri in IBD
Diet therapy has always been a focus in the treatment of IBD. In the study of Kim et al. (2020) the conclusion indicated that mango intake significantly reduced biomarkers of inflammation and modulated the intestinal microbiota, which significantly increased the abundance of L. reuteri (Kim et al., 2020). The experiment by Sun et al. (2018) also supported this idea. The authors found that the space flight–induced mutant L. reuteri F-9-35 has excellent potential for the prevention of UC as a dietary supplement compared with that of the wild type and milk alone (Sun et al., 2018). With the maturity of the probiotics industry, probiotics have also been added to the daily diet. For instance, one prospective study used L. reuteri RC-14–supplemented yogurt as atherapy and finally confirmed its anti-inflammatory effect on patients with IBD (Lorea Baroja et al., 2007). As for children with active distal UC, a randomized clinical trial showed the effectiveness of L. reuteri ATCC 55730 in improving mucosal inflammation and reducing the expression level of some iconic cytokines (Oliva et al., 2012).
Nevertheless, the number of clinical research studies on L. reuteri in IBD is less than that of fundamental studies. We attribute this phenomenon to the speculation that L. reuteri is composed of multiple stains. Each stain may have different functions that must be fully investigated in fundamental experiments.
Colorectal cancer
Colorectal cancer (CRC) ranks as the third most common type of cancer and the fourth leading cause of cancer-related deaths globally (Weitz et al., 2005). It is well-known that several risk factors have been associated with the occurrence and development of CRC, such as inflammatory bowel disease (IBD), age, and genetic, and environmental factors (Keller et al., 2019; Thanikachalam and Khan, 2019). Probiotic therapy has become a hotspot treatment for CRC (Ambalam et al., 2016; Fong et al., 2020). Researchers demonstrated that L. reuteri ATCC PTA 6475 had the ability to reduce the number and size of colon tumors, with the mechanism that administration of hdc+ L. reuteri led to hdc gene expression and histamine production in the gut to suppress chronic intestinal inflammation and colorectal tumorigenesis (Gao et al., 2017). In addition, the interaction between Sirt3 and L. reuteri was proven to be crucial in gut tumorigenesis (Zhang et al., 2018). The research conducted by Bell et al. (2022) acquired some convincing results. In vitro, the authors found fecal metabolites from wild-type mice and normal humans. Both can inhibit the proliferation of CRC cell lines but not repress the noncancerous cell line NCM460. Metabolomics finally identified reuterin as the most inhibitory compound. According to a previous study, we learned that reuterin was an antimicrobial produced by L. reuteri—an intermediate in glycerol metabolism to 1,3-Propanediol (Martín-Cabrejas et al., 2017; Asare et al., 2020; Zhang et al., 2020). In addition, reuterin at a dose of 25 μM could inhibit the growth of CRC cell lines (HCT116, SW480, RKO, and DLD1), but a higher concentration of reuterin (100 μM) had no effect on normal colon epithelial cells. In vivo, the authors concluded that L. reuteri was reduced in tumors compared with normal tissues.
Moreover, the authors confirmed this result by using public datasets and patient tissue samples. Based on metabolomics and gene-enriched analysis, it was found that L. reuteri growth was suppressed by the homocysteine degradative metabolites hydrogen sulfide and cystathionine. Lactobacillus reuteri growth could not be altered by supplementing with the antioxidant glutathione ethyl ester, which indicated that the oxidative stress pathway was not specific to L. reuteri. In a bid to explore the concrete mechanism, the authors treated an intestinal cell line with 100 μM reuterin for 24 h. With the help of metabolomics, transcriptomics, and proteomics, the authors observed upregulation of the nuclear factor erythroid 2–related factor 2 (NRF2), which played an essential role in the oxidative stress response. The most enriched pathway focuses on glutathione and glutamate metabolism. Quantification of oxidized L-glutathione confirmed the role reuterin played in oxidative stress. With the subsequent observation that acetylcysteine (NAC) inhibited the induction of NRF2-dependent oxidative stress genes, it was strongly confirmed that reuterin directly controls the redox balance of a cell in a glutathione (GSH)-dependent manner. Creatively, researchers found that sodium sulfide protected cells against reuterin-induced growth inhibition, along with the result that reuterin selectively bound to cysteine residues in numerous biological replicates, indicating the significantly different cysteine proteomics profile of reuterin. The NAD pathway was also involved in the oxidative stress process. Using RNA-sequencing analysis, the puromycin incorporation assay (SUnSET), and a cell inhibition experiment, the authors finally identified the inhibiting ribosomal assembly as an essential cytotoxic pathway of reuterin, in which YEATS2 target genes were found to be downregulated after treatment with reuterin.
Taken together, reuterin was capable of repressing colorectal cancer growth in vivo. This excellent study opened new avenues for researchers. Christina Watschinger and Alexander R. Moschen expressed their distinct opinions on this comprehensive research (Watschinger and Moschen, 2022). The authors further desired to determine how reuterin's selectivity is mediated and the mechanism by which reuterin accumulates in tumor cells outside the gut. All of these outstanding findings can contribute to enhancing the transformation from basic research to clinical application. Along with the experiments on living bacteria and their secrets, Kim et al. (2022) creatively reported the joint function of heat-killed L. reuteri MG5346 and L. casei MG4584 in human CRC. The authors ultimately demonstrated that both of these strains could play an antitumor role through the caspase-9-dependent apoptosis pathway (Figure 2).
Figure 2.
Schematic diagram depicting the pathogenic role and therapeutic potential of Lactobacillus reuteri in CRC. H1R, type 1 histamine receptor; H2R, type 2 histamine receptor; hdc, histidine decarboxylase; PLC, phospholipase C; PIP2, phosphatidylinositol 2; Dgk, diacylglycerol kinase; DAG, diacylglycerol; PA, phosphatidic acid; IP3, inositol triphosphate; PKC, protein kinase C; pPKC, phosphorylated protein kinase C; ER, endoplasmic reticulum; AhR, aryl hydrocarbon receptor; PKA, protein kinase A; pPKA, phosphorylated protein kinase A; KC, keratinocyte chemoattractant; ROS, reactive oxygen species; GSH, glutathione; LTV1, LTV1 ribosome biogenesis factor; RIOK2, right open reading frame kinase 2; YEATS2, YEATS domain-containing protein 2.
Infection-associated intestinal diseases
Enterotoxigenic Escherichia coli (ETEC) is a leading cause of infectious diarrhea in humans and animals. In the study of Xie et al., the authors creatively developed a new type of L. reuteri—a bovine lactoferricin-lactoferrampin (LFCA)-encoding L. reuteri CO21 (LR-LFCA) and finally demonstrated that LR-LFCA can function in the following three aspects in a newborn ETEC-infected piglet intestine model: (1) it could enhance gut immune responses by improving intestinal barrier function and gut microbiota composition, (2) it was able to protect the gut from oxidative stress by activating the NRF2/HO-1 pathway, and (3) it had the ability to inhibit the NF-κB pathway to perform its anti-inflammatory effect (Xie et al., 2021). Further, Tkáčiková et al. (2020) found that the pretreatment of L. reuteri L26 Biocenol (CCM 8616)-derived bacterial exopolysaccharides (EPSs) can attenuate the overexpression of the genes induced by ETEC infection to suppress inflammatory responses. Human-derived L. reuteri strains (ATCC PTA 6475, DSM 17938, and 1563F), a rat strain (R2LC), and piglet-derived L. reuteri (HCM2 and LR1) were also able to reduce the detrimental effect of ETEC (Karimi et al., 2018; Wang et al., 2018; Yi et al., 2018a). Reuteran and Levan, two metabolites produced by L. reuteri TMW1.656 and L. reuteri LTH5794, respectively, can also reduce the colonization of weanling piglets by ETEC (Yang et al., 2015). As for enteropathogenic E. coli (EPEC), there was evidence suggesting that L. reuteri ATCC PTA 6475 and ATCC 53608 significantly inhibited EPEC by targeting either the epithelium or the mucus layer, depending on the strain's specialty (Walsham et al., 2016). Notably, L. reuteri ATCC PTA 6475 was also effective in suppressing enterohemorrhagic E. coli (EHEC) (Eaton et al., 2011). With respect to Salmonella infections, it was demonstrated that L. reuteri Lb11, isolated from the chicken intestinal tract, can effectively prevent the formation of an efflux pump, inhibiting the production of multidrug-resistant Salmonella enteritidis in eggs (Hai and Huang, 2021). Lactobacillus reuteri KUB-AC5 can also protect against Salmonella infection in chickens (Nakphaichit et al., 2019). Jiang P. et al. (2019) revealed that L. reuteri ATCC 55730 can prevent mice from acquiring Salmonella Typhimurium by activating macrophages to produce nitric oxide. A combination of a phage cocktail and L. reuteri was able to ameliorate mouse colitis caused by S. Typhimurium by improving the intestinal barrier and colonic pathological damage, in which the metabolites of L. reuteri-acetate and reuterin played important roles (Eaton et al., 2011). Remarkably, glycerol supplementation had the ability to enhance L. reuteri ATCC PTA 6475 's protective effect against S. Typhimurium colonization (De Weirdt et al., 2012). Lactobacillus reuteri CCM 8617 and SLZX19-12 were also shown to exert vital impacts on S. Typhimurium (Gancarčíková et al., 2019; Wu et al., 2022). In regard to virus infection, some investigations have already indicated that L. reuteri ATCC 23272 functions as a significant modulator in gnotobiotic pigs infected with human rotavirus, and L. reuteri L26 Biocenol™ plays an essential role in protecting against porcine circovirus type 2 (Azevedo et al., 2012; Karaffová et al., 2017).
Pediatric intestinal diseases
Functional intestinal disorders
At present, the majority of the research for L. reuteri on functional intestinal disorders focuses on clinical research conducted on children and infants. Infantile colic, functional constipation (FC), functional abdominal pain (FAP), and irritable bowel syndrome (IBS) are the most common functional gastrointestinal disorders in children (Hojsak, 2019). In these fields, probiotics have proven to be promising therapeutic options (Pärtty et al., 2018). In 2013, a randomized DBPC trial showed no difference in microbiota between colicky infants with or without treatment using L. reuteri DSM 17938 (Roos et al., 2013). A systematic review also summarized the effects of L. reuteri ATCC 55730 and L. reuteri DSM 17938, concluding that none affected infantile colic relief (Skórka et al., 2017). However, several studies indicated that treatment with L. reuteri DSM 17938 can relieve infantile colic (Savino et al., 2018a,b, 2019; Turco et al., 2021). A combination containing heat-killed L. reuteri SGL01 and Bifidobacterium brevis SGB01 had better curative effects in infantile colic than ordinary dietary supplements (Vandenplas et al., 2017). Lactobacillus reuteri (FloraActive™) 12246, L. reuteri (American Type Culture Collection Strain 55730), and LR92 DSM 26866 all came into play in infantile colic (Savino et al., 2007; Gerasimov et al., 2018; Pourmirzaiee et al., 2020). Over 90% of child-associated constipation can be classified as FC, and some meaningful studies have been conducted in this area (Tambucci et al., 2018). Some reviews summarized the effectiveness of L. reuteri DSM 17938 in infants and children, with the conclusion that it is not recommended to use L. reuteri DSM 17938 routinely in the management of infants with constipation (Urbańska and Szajewska, 2014; Wegh et al., 2018). Similarly, some randomized controlled trials also found that L. reuteri DSM 17938 is not beneficial for the treatment of FC in children (Jadrešin et al., 2018; Wegner et al., 2018). Conversely, Kubota et al. (2020) noted a remarkable improvement in the defecation frequency with L. reuteri DSM 17938 and Magnesium Oxide in FC. Current research on FAP in children focuses on L. reuteri DSM 17938. Authors have found that L. reuteri DSM 17938 effectively alleviates pain and restores normal activities in children with FAP (Weizman et al., 2016; Maragkoudaki et al., 2017; Jadrešin et al., 2020; Trivić et al., 2021). With respect to IBS, the corresponding studies were marginal, and their findings all indicated that L. reuteri DSM 17938 is unable to improve the symptoms of IBS (Niv et al., 2005; Jadrešin et al., 2020). Another functional disorder in children, diarrhea, can also be relieved by L. reuteri DSM 17938 (Gutierrez-Castrellon et al., 2014). From the above interpretations, we can see that L. reuteri does not = have effective therapeutic results. Our point of view can be divided into two aspects: (1) this can be attributed to the limitation of sample size because nearly all studies' sample size was < 100 cases and (2) current trials are mostly centered on L. reuteri DSM 17938 and perhaps other strains would have some effects we still do not know. Thus, more trials need to be carried out in these areas.
Necrotizing enterocolitis
Necrotizing enterocolitis (NEC) is a common intestinal disease that occurs in premature infants and is the leading cause of short bowel syndrome in neonates (Neu and Walker, 2011). It is well documented that L. reuteri has become an effective treatment for this disease. Lactobacillus reuteri DSM 17938 can improve survival and reduce the incidence and severity of NEC by modulating the immune response and the induction and migration of Foxp3+ regulatory T cells (Tregs) (Liu et al., 2013). Further, this research team found that the anti-inflammatory effect of L. reuteri DSM 17938 on NEC relied on differential modulation of effector memory T cells and Foxp3+Tregs (Liu et al., 2014b). In 2018, the authors also discovered that TLR2 could play a part in alleviating NEC by means of activating DC (Hoang et al., 2018). Based on previous research results, the authors conducted their experiment on newborn mice by feeding experimental animals L. reuteri DSM 17938, concluding that oral administration of this probiotic can increase levels of tryptophan metabolites and purine nucleoside adenosine and can be beneficial to general health (Liu et al., 2019). Probiotic persistence is a major topic in probiotic therapy. Given this, Olson et al. (2018) have fully used biofilm's function to enhance the persistence of L. reuteri in the protection against NEC. Similarly, Al-Hadidi et al. (2021) also developed a new formulation of enterally delivered probiotics to improve probiotic survival through biofilm formation. Shelby et al. (2022) showed that, compared with the planktonic state of L. reuteri, its biofilm state significantly decreased the incidence of NEC through antibacterial and anti-inflammatory effects.
Conclusions and future perspectives
A growing number of studies showed that intestinal diseases can cause mounting healthcare bills and economic burdens. It is well-documented that gut microbiota plays an increasingly essential role in the treatment and prognosis of gut diseases. Lactobacillus reuteri is a common and well-studied microbe. Extensive investigations have been conducted in this area. However, we still have numerous unanswered questions. As a gut symbiont, L. reuteri can be colonized in the intestine of humans, rodents, pigs, and chickens and can perform multiple actions, including regulating immune responses, modulating gut microbiota, boosting beneficial metabolites, protecting against oxidative stress, maintaining intestinal barrier (IEB) function and intestinal morphology, and so on (Yi et al., 2018b; Liu et al., 2019; Garg et al., 2020; Singh et al., 2021). In this review, we first elucidated the basic function of L. reuteri and its related metabolites. Next, we systematically interpreted its function in different intestinal diseases, such as inflammatory bowel disease, colorectal cancer, infection-associated bowel disease, and pediatric intestinal disorders. We also emphasized some vital molecules in association with the underlying mechanisms. Cumulatively, L. reuteri is potentially beneficial to intestinal diseases, which should be further investigated in a bid to obtain better clinical application and therapeutic effects.
Although an increasing number of research studies on L. reuteri are well-studied by current researchers, there are still some key issues that are in doubt. First, there is a substantial gap between basic research and clinical applications based on the present literature data, probably owing to the unspecific mechanisms and doubtful safety of this microbe. Safety is an important issue for the wide application of probiotics. Conducting standardized safety assessments and finding effective methods to control the side effects of probiotics may be the future research direction. In addition, the paradoxical results of clinical research also restrict the development of L. reuteri, which may be attributed to the fact that L. reuteri has many distinct strains, and each strain may have its own unique function, for better or worse.
We need to fully evaluate the clinical effect of each strain and the mechanism underlying it. These issues may be addressed with the improvement of industrialized probiotics and experimental techniques. At last, the development of multiple omics analyses, especially metabonomics, allows us to investigate the functions of L. reuteri's metabolites, which may help us thoroughly investigate this field. Based on this, researchers can develop metabolites-targeted probiotic products, contributing to the refinement management of the probiotic industry.
Author contributions
ZY, JC, and YL: writing—original draft and visualization. QM, HL, QY, WS, and XR: conceptualization. XC: conceptualization and writing—review, editing, and supervision. All authors contributed to the article and approved the submitted version.
Funding Statement
This study was supported by the National Key R&D Program of China (No. 2019YFB1311505) and the Science and Technology Program of Tianjin (No. 21JCQNJC00990).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Al-Hadidi A., Navarro J., Goodman S. D., Bailey M. T., Besner G. E. (2021). Lactobacillus reuteri in its biofilm state improves protection from experimental necrotizing enterocolitis. Nutrients 13, 918. 10.3390/nu13030918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambalam P., Raman M., Purama R. K., Doble M. (2016). Probiotics, prebiotics and colorectal cancer prevention. Best Pract. Res. Clin. Gastroenterol. 30, 119–131. 10.1016/j.bpg.2016.02.009 [DOI] [PubMed] [Google Scholar]
- Ananthakrishnan A. N. (2015). Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 12, 205–217. 10.1038/nrgastro.2015.34 [DOI] [PubMed] [Google Scholar]
- Ang L. Y., Too H. K., Tan E. L., Chow T. K., Shek L. P., Tham E. H., et al. (2016). Antiviral activity of Lactobacillus reuteri protectis against coxsackievirus A and enterovirus 71 infection in human skeletal muscle and colon cell lines. Virol. J. 13, 111. 10.1186/s12985-016-0567-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asare P. T., Zurfluh K., Greppi A., Lynch D., Schwab C., Stephan R., et al. (2020). Reuterin demonstrates potent antimicrobial activity against a broad panel of human and poultry meat Campylobacter spp. isolates. Microorganisms 8, 78. 10.3390/microorganisms8010078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins H. L., Geier M. S., Prisciandaro L. D., Pattanaik A. K., Forder R. E. A., Turner M. S., et al. (2012). Effects of a Lactobacillus reuteri BR11 mutant deficient in the cystine-transport system in a rat model of inflammatory bowel disease. Dig. Dis. Sci. 57, 713–719. 10.1007/s10620-011-1943-0 [DOI] [PubMed] [Google Scholar]
- Azevedo M. S. P., Zhang W., Wen K., Gonzalez A. M., Saif L. J., Yousef A. E., et al. (2012). Lactobacillus acidophilus and Lactobacillus reuteri modulate cytokine responses in gnotobiotic pigs infected with human rotavirus. Benef. Microbes. 3, 33–42. 10.3920/BM2011.0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. E., Hu C.-Y., You Y. N., Bednarski B. K., Rodriguez-Bigas M. A., Skibber J. M., et al. (2015). Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 150, 17–22. 10.1001/jamasurg.2014.1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B., Michaelis S. (2015). Epidemiology of anxiety disorders in the 21st century. Dialogues Clin. Neurosci. 17, 327–335. 10.31887/DCNS.2015.17.3/bbandelow [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter A. J., Scott K. M., Ferrari A. J., Norman R. E., Vos T., Whiteford H. A., et al. (2014). Challenging the myth of an “epidemic” of common mental disorders: trends in the global prevalence of anxiety and depression between 1990 and 2010. Depress. Anxiety 31, 506–516. 10.1002/da.22230 [DOI] [PubMed] [Google Scholar]
- Bell H. N., Rebernick R. J., Goyert J., Singhal R., Kuljanin M., Kerk S. A., et al. (2022). Reuterin in the healthy gut microbiome suppresses colorectal cancer growth through altering redox balance. Cancer Cell 40, 185–200.e6. 10.1016/j.ccell.2021.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Barragan L., Chai J. N., Tianero M. D., Di Luccia B., Ahern P. P., Merriman J., et al. (2017). Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8αα(+) T cells. Science 357, 806–810. 10.1126/science.aah5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani C. R., Benetton C. A. F., Stoffel C., Bardini K. C., Cardoso V. H., Di Giunta G., et al. (2007). Oxidative stress and metabolism in animal model of colitis induced by dextran sulfate sodium. J. Gastroenterol. Hepatol. 22, 1846–1851. 10.1111/j.1440-1746.2007.04890.x [DOI] [PubMed] [Google Scholar]
- Daniali M., Nikfar S., Abdollahi M. (2020). Antibiotic resistance propagation through probiotics. Expert Opin. Drug Metab. Toxicol. 16, 1207–1215. 10.1080/17425255.2020.1825682 [DOI] [PubMed] [Google Scholar]
- David L. A., Maurice C. F., Carmody R. N., Gootenberg D. B., Button J. E., Wolfe B. E., et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. J., Doerr H. M., Grzelak A. K., Busi S. B., Jasarevic E., Ericsson A. C., et al. (2016). Lactobacillus plantarum attenuates anxiety-related behavior and protects against stress-induced dysbiosis in adult zebrafish. Sci. Rep. 6, 33726. 10.1038/srep33726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weirdt R., Crabbé A., Roos S., Vollenweider S., Lacroix C., van Pijkeren J. P., et al. (2012). Glycerol supplementation enhances L. reuteri's protective effect against S. Typhimurium colonization in a 3-D model of colonic epithelium. PLoS ONE 7, e37116. 10.1371/journal.pone.0037116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias A. M. M., Douhard R., Hermetet F., Regimbeau M., Lopez T. E., Gonzalez D., et al. (2021). Lactobacillus stress protein GroEL prevents colonic inflammation. J. Gastroenterol. 56, 442–455. 10.1007/s00535-021-01774-3 [DOI] [PubMed] [Google Scholar]
- Doron S., Snydman D. R. (2015). Risk and safety of probiotics. Clin. Infect. Dis. 60(Suppl 2), S129–S134. 10.1093/cid/civ085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudík B., Kinová Sepová H., Bilka F., Pašková L., Bilková A. (2020). Mucin pre-cultivated Lactobacillus reuteri E shows enhanced adhesion and increases mucin expression in HT-29 cells. Antonie Van Leeuwenhoek 113, 1191–1200. 10.1007/s10482-020-01426-1 [DOI] [PubMed] [Google Scholar]
- Eaton K. A., Honkala A., Auchtung T. A., Britton R. A. (2011). Probiotic Lactobacillus reuteri ameliorates disease due to enterohemorrhagic Escherichia coli in germfree mice. Infect. Immun. 79, 185–191. 10.1128/IAI.00880-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engevik M. A., Ruan W., Esparza M., Fultz R., Shi Z., Engevik K. A., et al. (2021). Immunomodulation of dendritic cells by Lactobacillus reuteri surface components and metabolites. Physiol. Rep. 9, e14719. 10.14814/phy2.14719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong W., Li Q., Yu J. (2020). Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene 39, 4925–4943. 10.1038/s41388-020-1341-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancarčíková S., Nemcová R., Popper M., Hrčková G., Sciranková L., Madar M., et al. (2019). The influence of feed-supplementation with probiotic strain Lactobacillus reuteri CCM 8617 and alginite on intestinal microenvironment of SPF mice infected with Salmonella Typhimurium CCM 7205. Probiotics Antimicrob. Proteins 11, 493–508. 10.1007/s12602-018-9413-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh B. P., Hall A., Ayyaswamy S., Nelson J. W., Fultz R., Major A., et al. (2018). Diacylglycerol kinase synthesized by commensal Lactobacillus reuteri diminishes protein kinase C phosphorylation and histamine-mediated signaling in the mammalian intestinal epithelium. Mucosal Immunol. 11, 380–393. 10.1038/mi.2017.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C., Ganesh B. P., Shi Z., Shah R. R., Fultz R., Major A., et al. (2017). Gut microbe-mediated suppression of inflammation-associated colon carcinogenesis by luminal histamine production. Am. J. Pathol. 187, 2323–2336. 10.1016/j.ajpath.2017.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C., Major A., Rendon D., Lugo M., Jackson V., Shi Z., et al. (2015). Histamine H2 receptor-mediated suppression of intestinal inflammation by probiotic Lactobacillus reuteri. MBio 6, e01358-15. 10.1128/mBio.01358-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K., Liu L., Dou X., Wang C., Liu J., Zhang W., et al. (2016). Doses Lactobacillus reuteri depend on adhesive ability to modulate the intestinal immune response and metabolism in mice challenged with lipopolysaccharide. Sci. Rep. 6, 28332. 10.1038/srep28332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S., Singh T. P., Malik R. K. (2020). In vivo implications of potential probiotic Lactobacillus reuteri LR6 on the gut and immunological parameters as an adjuvant against protein energy malnutrition. Probiotics Antimicrob. Proteins. 12, 517–534. 10.1007/s12602-019-09563-4 [DOI] [PubMed] [Google Scholar]
- Gerasimov S., Gantzel J., Dementieva N., Schevchenko O., Tsitsura O., Guta N., et al. (2018). Role of Lactobacillus rhamnosus (FloraActive™) 19070-2 and Lactobacillus reuteri (FloraActive™) 12246 in infant colic: a randomized dietary study. Nutrients 10, 1975. 10.3390/nu10121975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraffa G., Chanishvili N., Widyastuti Y. (2010). Importance of lactobacilli in food and feed biotechnology. Res. Microbiol. 161, 480–487. 10.1016/j.resmic.2010.03.001 [DOI] [PubMed] [Google Scholar]
- Giudice M. M. D., Maiello N., Allegorico A., Iavarazzo L., Capasso M., Capristo C., et al. (2016). Lactobacillus reuteri DSM 17938 plus vitamin D as ancillary treatment in allergic children with asthma. Ann. Allergy Asthma Immunol. 117, 710–712. 10.1016/j.anai.2016.09.004 [DOI] [PubMed] [Google Scholar]
- Guarner F., Casellas F., Borruel N., Antolín M., Videla S., Vilaseca J., et al. (2002). Role of microecology in chronic inflammatory bowel diseases. Eur. J. Clin. Nutr. 56, S34–S38. 10.1038/sj.ejcn.1601662 [DOI] [PubMed] [Google Scholar]
- Guo F., Cai D., Li Y., Gu H., Qu H., Zong Q., et al. (2021). How early-life gut microbiota alteration sets trajectories for health and inflammatory bowel disease. Front Nutr. 8, 690073. 10.3389/fnut.2021.690073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Huang C., Xu J., Xu H., Liu L., Zhao H., et al. (2021). Gut microbiota is a potential biomarker in inflammatory bowel disease. Front Nutr. 8, 818902. 10.3389/fnut.2021.818902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Chauhan N. R., Chowdhury D., Singh A., Meena R. C., Chakrabarti A., et al. (2017). Heat stress modulated gastrointestinal barrier dysfunction: role of tight junctions and heat shock proteins. Scand. J. Gastroenterol. 52, 1315–1319. 10.1080/00365521.2017.1377285 [DOI] [PubMed] [Google Scholar]
- Gutierrez-Castrellon P., Lopez-Velazquez G., Diaz-Garcia L., Jimenez-Gutierrez C., Mancilla-Ramirez J., Estevez-Jimenez J., et al. (2014). Diarrhea in preschool children and Lactobacillus reuteri: a randomized controlled trial. Pediatrics 133, e904–e949. 10.1542/peds.2013-0652 [DOI] [PubMed] [Google Scholar]
- Hai D., Huang X. (2021). Protective effect of Lactobacillus reuteri Lb11 from chicken intestinal tract against Salmonella enteritidis SE05 in vitro. Antonie Van Leeuwenhoek 114, 1745–1757. 10.1007/s10482-021-01625-4 [DOI] [PubMed] [Google Scholar]
- Hall A. E., Engevik M. A., Oezguen N., Haag A., Versalovic J. (2019). ClC transporter activity modulates histidine catabolism in Lactobacillus reuteri by altering intracellular pH and membrane potential. Microb. Cell Fact. 18, 212. 10.1186/s12934-019-1264-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Zhou X., Liu Y., Zhou L., Li F. (2022). Fecal miR-142a-3p from dextran sulfate sodium-challenge recovered mice prevents colitis by promoting the growth of Lactobacillus reuteri. Mol. Ther. 30, 388–399. 10.1016/j.ymthe.2021.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsbroek S. E., Gordon S. (2009). The role of macrophages in inflammatory bowel diseases. Expert Rev. Mol. Med. 11, e14. 10.1017/S1462399409001069 [DOI] [PubMed] [Google Scholar]
- Hemarajata P., Gao C., Pflughoeft K. J., Thomas C. M., Saulnier D. M., Spinler J. K., et al. (2013). Lactobacillus reuteri-specific immunoregulatory gene rsiR modulates histamine production and immunomodulation by Lactobacillus reuteri. J. Bacteriol. 195, 5567–5576. 10.1128/JB.00261-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T. K., He B., Wang T., Tran D. Q., Rhoads J. M., Liu Y., et al. (2018). Protective effect of Lactobacillus reuteri DSM 17938 against experimental necrotizing enterocolitis is mediated by Toll-like receptor 2. Am. J. Physiol. Gastrointest. Liver Physiol. 315, G231–G240. 10.1152/ajpgi.00084.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojsak I. (2019). Probiotics in functional gastrointestinal disorders. Adv. Exp. Med. Biol. 1125, 121–137. 10.1007/5584_2018_321 [DOI] [PubMed] [Google Scholar]
- Hou C., Wang Q., Zeng X., Yang F., Zhang J., Liu H., et al. (2014). Complete genome sequence of Lactobacillus reuteri I5007, a probiotic strain isolated from healthy piglet. J. Biotechnol. 179, 63–64. 10.1016/j.jbiotec.2014.03.019 [DOI] [PubMed] [Google Scholar]
- Hrdý J., Alard J., Couturier-Maillard A., Boulard O., Boutillier D., Delacre M., et al. (2020). Lactobacillus reuteri 5454 and Bifidobacterium animalis ssp. lactis 5764 improve colitis while differentially impacting dendritic cells maturation and antimicrobial responses. Sci. Rep. 10, 5345. 10.1038/s41598-020-62161-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadrešin O., Sila S., Trivić I., Mišak Z., Hojsak I., Kolaček S., et al. (2018). Lack of benefit of Lactobacillus reuteri DSM 17938 as an addition to the treatment of functional constipation. J. Pediatr. Gastroenterol. Nutr. 67, 763–766. 10.1097/MPG.0000000000002134 [DOI] [PubMed] [Google Scholar]
- Jadrešin O., Sila S., Trivić I., Mišak Z., Kolaček S., Hojsak I., et al. (2020). Lactobacillus reuteri DSM 17938 is effective in the treatment of functional abdominal pain in children: results of the double-blind randomized study. Clin. Nutr. 39, 3645–3651. 10.1016/j.clnu.2020.04.019 [DOI] [PubMed] [Google Scholar]
- Jang H. M., Jang S. E., Han M. J., Kim D. H. (2018a). Anxiolytic-like effect of Bifidobacterium adolescentis IM38 in mice with or without immobilisation stress. Benef. Microbes 9, 123–132. 10.3920/BM2016.0226 [DOI] [PubMed] [Google Scholar]
- Jang H. M., Lee H. J., Jang S. E., Han M. J., Kim D. H. (2018b). Evidence for interplay among antibacterial-induced gut microbiota disturbance, neuro-inflammation, and anxiety in mice. Mucosal Immunol. 11, 1386–1397. 10.1038/s41385-018-0042-3 [DOI] [PubMed] [Google Scholar]
- Jang H. M., Lee K. E., Kim D. H. (2019). The preventive and curative effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on immobilization stress-induced anxiety/depression and colitis in mice. Nutrients 11, 819. 10.3390/nu11040819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P., Yang W., Jin Y., Huang H., Shi C., Jiang Y., et al. (2019). Lactobacillus reuteri protects mice against Salmonella Typhimurium challenge by activating macrophages to produce nitric oxide. Microb. Pathog. 137, 103754. 10.1016/j.micpath.2019.103754 [DOI] [PubMed] [Google Scholar]
- Jost T., Lacroix C., Braegger C. P., Rochat F., Chassard C. (2014). Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environ. Microbiol. 16, 2891–2904. 10.1111/1462-2920.12238 [DOI] [PubMed] [Google Scholar]
- Kaplan G. G. (2015). The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 12, 720–727. 10.1038/nrgastro.2015.150 [DOI] [PubMed] [Google Scholar]
- Karaffová V., Csank T., Mudronová D., Király J., Revajová V., Gancarčíková S., et al. (2017). Influence of Lactobacillus reuteri L26 Biocenol™ on immune response against porcine circovirus type 2 infection in germ-free mice. Benef. Microbes 8, 367–378. 10.3920/BM2016.0114 [DOI] [PubMed] [Google Scholar]
- Karimi S., Jonsson H., Lundh T., Roos S. (2018). Lactobacillus reuteri strains protect epithelial barrier integrity of IPEC-J2 monolayers from the detrimental effect of enterotoxigenic Escherichia coli. Physiol. Rep. 6, e13514. 10.14814/phy2.13514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kechagia M., Basoulis D., Konstantopoulou S., Dimitriadi D., Gyftopoulou K., Skarmoutsou N., et al. (2013). Health benefits of probiotics: a review. ISRN Nutr. 2013, 481651. 10.5402/2013/481651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller D. S., Windsor A., Cohen R., Chand M. (2019). Colorectal cancer in inflammatory bowel disease: review of the evidence. Tech. Coloproctol. 23, 3–13. 10.1007/s10151-019-1926-2 [DOI] [PubMed] [Google Scholar]
- Khalil D. N., Smith E. L., Brentjens R. J., Wolchok J. D. (2016). The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 13, 273–290. 10.1038/nrclinonc.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani A., Nami Y., Hedayati S., Elieh Ali Komi D., Goudarzi F., Haghshenas B., et al. (2021b). Application of tarkhineh fermented product to produce potato chips with strong probiotic properties, high shelf-life, and desirable sensory characteristics. Front. Microbiol. 12, 657579. 10.3389/fmicb.2021.657579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani A., Nami Y., Hedayati S., Jaymand M., Samadian H., Haghshenas B., et al. (2021a). Tarkhineh as a new microencapsulation matrix improves the quality and sensory characteristics of probiotic Lactococcus lactis KUMS-T18 enriched potato chips. Sci. Rep. 11, 12599. 10.1038/s41598-021-92095-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Venancio V. P., Fang C., Dupont A. W., Talcott S. T., Mertens-Talcott S. U., et al. (2020). Mango (Mangifera indica L.) polyphenols reduce IL-8, GRO, and GM-SCF plasma levels and increase Lactobacillus species in a pilot study in patients with inflammatory bowel disease. Nutr. Res. 75, 85–94. 10.1016/j.nutres.2020.01.002 [DOI] [PubMed] [Google Scholar]
- Kim S. J., Kang C. H., Kim G. H., Cho H. (2022). Anti-tumor effects of heat-killed L. reuteri MG5346 and L. casei MG4584 against human colorectal carcinoma through Caspase-9-dependent apoptosis in xenograft model. Microorganisms 10, 533. 10.3390/microorganisms10030533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota M., Ito K., Tomimoto K., Kanazaki M., Tsukiyama K., Kubota A., et al. (2020). Lactobacillus reuteri DSM 17938 and magnesium oxide in children with functional chronic constipation: a double-blind and randomized clinical trial. Nutrients 12, 225. 10.3390/nu12010225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Dicksved J., Lundh T., Lindberg J. E. (2014a). Heat shock proteins: intestinal gatekeepers that are influenced by dietary components and the gut microbiota. Pathogens 3, 187–210. 10.3390/pathogens3010187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.-Y., Gu F., Zhu C., Yuan L., Zhu C., Zhu M., et al. (2022). Epithelial heat shock proteins mediate the protective effects of limosi Lactobacillus reuteri in dextran sulfate sodium-induced colitis. Front. Immunol. 13, 865982. 10.3389/fimmu.2022.865982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. Y., Giraud A., Seignez C., Ahl D., Guo F., Sedin F., et al. (2021). Distinct B cell subsets in Peyer's patches convey probiotic effects by limosi Lactobacillus reuteri. Microbiome 9, 198. 10.1186/s40168-021-01128-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Fatheree N. Y., Dingle B. M., Tran D. Q., Rhoads J. M. (2013). Lactobacillus reuteri DSM 17938 changes the frequency of Foxp3+ regulatory T cells in the intestine and mesenteric lymph node in experimental necrotizing enterocolitis. PLoS ONE 8, e56547. 10.1371/journal.pone.0056547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Tian X., He B., Hoang T. K., Taylor C. M., Blanchard E., et al. (2019). Lactobacillus reuteri DSM 17938 feeding of healthy newborn mice regulates immune responses while modulating gut microbiota and boosting beneficial metabolites. Am. J. Physiol. Gastrointest. Liver Physiol. 317, G824–G838. 10.1152/ajpgi.00107.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Tran D. Q., Fatheree N. Y., Marc Rhoads J. (2014b). Lactobacillus reuteri DSM 17938 differentially modulates effector memory T cells and Foxp3+ regulatory T cells in a mouse model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G177–G186. 10.1152/ajpgi.00038.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorea Baroja M., Kirjavainen P. V., Hekmat S., Reid G. (2007). Anti-inflammatory effects of probiotic yogurt in inflammatory bowel disease patients. Clin. Exp. Immunol. 149, 470–479. 10.1111/j.1365-2249.2007.03434.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackos A. R., Galley J. D., Eubank T. D., Easterling R. S., Parry N. M., Fox J. G., et al. (2016). Social stress-enhanced severity of Citrobacter rodentium-induced colitis is CCL2-dependent and attenuated by probiotic Lactobacillus reuteri. Mucosal Immunol. 9, 515–526. 10.1038/mi.2015.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragkoudaki M., Chouliaras G., Orel R., Horvath A., Szajewska H., Papadopoulou A., et al. (2017). Lactobacillus reuteri DSM 17938 and a placebo both significantly reduced symptoms in children with functional abdominal pain. Acta Paediatr. 106, 1857–1862. 10.1111/apa.13992 [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz J., Ciszek M., Bobek M., Strus M., Heczko P. B., Kurnyta M., et al. (2007). Differential inflammatory mediator response in vitro from murine macrophages to lactobacilli and pathogenic intestinal bacteria. Int. J. Exp. Pathol. 88, 155–164. 10.1111/j.1365-2613.2007.00530.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowiak P., Slizewska K. (2017). Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9, 21. 10.3390/nu9091021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Cabrejas I., Langa S., Gaya P., Rodríguez E., Landete J. M., Medina M., et al. (2017). Optimization of reuterin production in cheese by Lactobacillus reuteri. J. Food Sci. Technol. 54, 1346–1349. 10.1007/s13197-017-2563-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyniak A., Medyńska-Przeczek A., Wedrychowicz A., Skoczeń S., Tomasik P. J. (2021). Prebiotics, probiotics, synbiotics, paraprobiotics and postbiotic compounds in IBD. Biomolecules 11, 1903. 10.3390/biom11121903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michot J. M., Bigenwald C., Champiat S., Collins M., Carbonnel F., Postel-Vinay S., et al. (2016). Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur. J. Cancer 54, 139–148. 10.1016/j.ejca.2015.11.016 [DOI] [PubMed] [Google Scholar]
- Morales P. E., Bucarey J. L., Espinosa A. (2017). Muscle lipid metabolism: role of lipid droplets and perilipins. J. Diabetes Res. 2017, 1789395. 10.1155/2017/1789395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Q., Tavella V. J., Luo X. M. (2018). Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 9, 757. 10.3389/fmicb.2018.00757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakphaichit M., Sobanbua S., Siemuang S., Vongsangnak W., Nakayama J., Nitisinprasert S., et al. (2019). Protective effect of Lactobacillus reuteri KUB-AC5 against Salmonella enteritidis challenge in chickens. Benef. Microbes 10, 43–54. 10.3920/BM2018.0034 [DOI] [PubMed] [Google Scholar]
- Nami Y., Haghshenas B., Haghshenas M., Yari Khosroushahi A. (2015). Antimicrobial activity and the presence of virulence factors and bacteriocin structural genes in Enterococcus faecium CM33 isolated from ewe colostrum. Front. Microbiol. 6, 782. 10.3389/fmicb.2015.00782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nami Y., Haghshenas B., Yari Khosroushahi A. (2018). Molecular identification and probiotic potential characterization of lactic acid bacteria isolated from human vaginal microbiota. Adv Pharm Bull. 8, 683–695. 10.15171/apb.2018.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu J., Walker W. A. (2011). Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264. 10.1056/NEJMra1005408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuendorf R., Harding A., Stello N., Hanes D., Wahbeh H. (2016). Depression and anxiety in patients with inflammatory bowel disease: a systematic review. J. Psychosom. Res. 87, 70–80. 10.1016/j.jpsychores.2016.06.001 [DOI] [PubMed] [Google Scholar]
- Niv E., Naftali T., Hallak R., Vaisman N. (2005). The efficacy of Lactobacillus reuteri ATCC 55730 in the treatment of patients with irritable bowel syndrome–a double blind, placebo-controlled, randomized study. Clin. Nutr. 24, 925–931. 10.1016/j.clnu.2005.06.001 [DOI] [PubMed] [Google Scholar]
- Oh P. L., Benson A. K., Peterson D. A., Patil P. B., Moriyama E. N., Roos S., et al. (2010). Diversification of the gut symbiont Lactobacillus reuteri as a result of host-driven evolution. ISME J. 4, 377–387. 10.1038/ismej.2009.123 [DOI] [PubMed] [Google Scholar]
- Oliva S., Di Nardo G., Ferrari F., Mallardo S., Rossi P., Patrizi G., et al. (2012). Randomised clinical trial: the effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment. Pharmacol. Ther. 35, 327–334. 10.1111/j.1365-2036.2011.04939.x [DOI] [PubMed] [Google Scholar]
- Olson J. K., Navarro J. B., Allen J. M., McCulloh C. J., Mashburn-Warren L., Wang Y., et al. (2018). An enhanced Lactobacillus reuteri biofilm formulation that increases protection against experimental necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 315, G408–G419. 10.1152/ajpgi.00078.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özçam M., Tocmo R., Oh J.-H., Afrazi A., Mezrich J. D., Roos S., et al. (2019). Gut symbionts Lactobacillus reuteri R2lc and 2010 encode a polyketide synthase cluster that activates the mammalian aryl hydrocarbon receptor. Appl. Environ. Microbiol. 85, e01661-18. 10.1128/AEM.01661-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pärtty A., Rautava S., Kalliomäki M. (2018). Probiotics on pediatric functional gastrointestinal disorders. Nutrients 10, 1836. 10.3390/nu10121836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow M. A., Chesney J., Pavlick A. C., Robert C., Grossmann K., McDermott D., et al. (2015). Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372, 2006–2017. 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourmirzaiee M. A., Famouri F., Moazeni W., Hassanzadeh A., Hajihashemi M. (2020). The efficacy of the prenatal administration of Lactobacillus reuteri LR92 DSM 26866 on the prevention of infantile colic: a randomized control trial. Eur. J. Pediatr. 179, 1619–1626. 10.1007/s00431-020-03641-4 [DOI] [PubMed] [Google Scholar]
- Preidis G. A., Saulnier D. M., Blutt S. E., Mistretta T.-A., Riehle K. P., Major A. M., et al. (2012). Host response to probiotics determined by nutritional status of rotavirus-infected neonatal mice. J. Pediatr. Gastroenterol. Nutr. 55, 299–307. 10.1097/MPG.0b013e31824d2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince T., McBain A. J., O'Neill C. A. (2012). Lactobacillus reuteri protects epidermal keratinocytes from Staphylococcus aureus-induced cell death by competitive exclusion. Appl. Environ. Microbiol. 78, 5119–5126. 10.1128/AEM.00595-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattanaprasert M., van Pijkeren J.-P., Ramer-Tait A. E., Quintero M., Kok C. R., Walter J., et al. (2019). Genes involved in galactooligosaccharide metabolism in Lactobacillus reuteri and their ecological role in the gastrointestinal tract. Appl. Environ. Microbiol. 85, e01788-19. 10.1128/AEM.01788-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos S., Dicksved J., Tarasco V., Locatelli E., Ricceri F., Grandin U., et al. (2013). 454 pyrosequencing analysis on faecal samples from a randomized DBPC trial of colicky infants treated with Lactobacillus reuteri DSM 17938. PLoS ONE. 8, e56710. 10.1371/journal.pone.0056710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino F., Galliano I., Garro M., Savino A., Daprà V., Montanari P., et al. (2018a). Regulatory T cells and Toll-like receptor 2 and 4 mRNA expression in infants with colic treated with Lactobacillus reuteri DSM17938. Benef. Microbes 9, 917–925. 10.3920/BM2017.0194 [DOI] [PubMed] [Google Scholar]
- Savino F., Galliano I., Savino A., Daprà V., Montanari P., Calvi C., et al. (2019). Lactobacillus reuteri DSM 17938 probiotics may increase CC-chemokine receptor 7 expression in infants treated with for colic. Front Pediatr. 7, 292. 10.3389/fped.2019.00292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino F., Garro M., Montanari P., Galliano I., Bergallo M. (2018b). Crying time and RORγ/FOXP3 expression in Lactobacillus reuteri DSM17938-treated infants with colic: a randomized trial. J Pediatr. 192, 171–177.e1. 10.1016/j.jpeds.2017.08.062 [DOI] [PubMed] [Google Scholar]
- Savino F., Pelle E., Palumeri E., Oggero R., Miniero R. (2007). Lactobacillus reuteri (American Type Culture Collection Strain 55730) simethicone in the treatment of infantile colic: a prospective randomized study. Pediatrics 119, e124–e130. 10.1542/peds.2006-1222 [DOI] [PubMed] [Google Scholar]
- Schreiber O., Petersson J., Phillipson M., Perry M., Roos S., Holm L., et al. (2009). Lactobacillus reuteri prevents colitis by reducing P-selectin-associated leukocyte- and platelet-endothelial cell interactions. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G534–G542. 10.1152/ajpgi.90470.2008 [DOI] [PubMed] [Google Scholar]
- Sengül N. (2011). Işik S, Aslim B, Uçar G, Demirbag AE. The effect of exopolysaccharide-producing probiotic strains on gut oxidative damage in experimental colitis. Dig. Dis. Sci. 56, 707–714. 10.1007/s10620-010-1362-7 [DOI] [PubMed] [Google Scholar]
- Shelby R. D., Mar P., Janzow G. E., Mashburn-Warren L., Tengberg N., Navarro J. B., et al. (2022). Antibacterial and anti-inflammatory effects of Lactobacillus reuteri in its biofilm state contribute to its beneficial effects in a rat model of experimental necrotizing enterocolitis. J. Pediatr. Surg. 57, 1382–1390. 10.1016/j.jpedsurg.2021.09.001 [DOI] [PubMed] [Google Scholar]
- Shin W., Kim H. J. (2018). Intestinal barrier dysfunction orchestrates the onset of inflammatory host-microbiome cross-talk in a human gut inflammation-on-a-chip. Proc. Natl. Acad. Sci. USA. 115, E10539–E10547. 10.1073/pnas.1810819115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T. P., Tehri N., Kaur G., Malik R. K. (2021). Cell surface and extracellular proteins of potentially probiotic Lactobacillus reuteri as an effective mediator to regulate intestinal epithelial barrier function. Arch. Microbiol. 203, 3219–3228. 10.1007/s00203-021-02318-2 [DOI] [PubMed] [Google Scholar]
- Skórka A., Pieścik-Lech M., Kołodziej M., Szajewska H. (2017). To add or not to add probiotics to infant formulae? An updated systematic review. Benef. Microbes 8, 717–725. 10.3920/BM2016.0233 [DOI] [PubMed] [Google Scholar]
- Spinler J. K., Sontakke A., Hollister E. B., Venable S. F., Oh P. L., Balderas M. A., et al. (2014). From prediction to function using evolutionary genomics: human-specific ecotypes of Lactobacillus reuteri have diverse probiotic functions. Genome Biol. Evol. 6, 1772–1789. 10.1093/gbe/evu137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M.-C., Zhang F.-C., Yin X., Cheng B.-J., Zhao C.-H., Wang Y.-L., et al. (2018). Lactobacillus reuteri F-9-35 Prevents DSS-induced colitis by inhibiting proinflammatory gene expression and restoring the gut microbiota in mice. J. Food Sci. 83, 2645–2652. 10.1111/1750-3841.14326 [DOI] [PubMed] [Google Scholar]
- Tachi Y., Kozuka A., Hirai T., Ishizu Y., Honda T., Kuzuya T., et al. (2018). Impact of myosteatosis on skeletal muscle volume loss in patients with chronic liver disease. J Gastroenterol Hepatol. 10.1111/jgh.14133 [DOI] [PubMed] [Google Scholar]
- Tambucci R., Quitadamo P., Thapar N., Zenzeri L., Caldaro T., Staiano A., et al. (2018). Diagnostic tests in pediatric constipation. J. Pediatr. Gastroenterol. Nutr. 66, e89–e98. 10.1097/MPG.0000000000001874 [DOI] [PubMed] [Google Scholar]
- Thaiss C. A., Zeevi D., Levy M., Zilberman-Schapira G., Suez J., Tengeler A. C., et al. (2014). Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159, 514–529. 10.1016/j.cell.2014.09.048 [DOI] [PubMed] [Google Scholar]
- Thanikachalam K., Khan G. (2019). Colorectal cancer and nutrition. Nutrients 11, 164. 10.3390/nu11010164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoro L. H., Cláudio M. M., Nuernberg M. A. A., Miessi D. M. J., Batista J. A., Duque C., et al. (2019). Effects of Lactobacillus reuteri as an adjunct to the treatment of periodontitis in smokers: randomised clinical trial. Benef. Microbes 10, 375–384. 10.3920/BM2018.0150 [DOI] [PubMed] [Google Scholar]
- Thomas C. M., Saulnier D. M., Spinler J. K., Hemarajata P., Gao C., Jones S. E., et al. (2016). FolC2-mediated folate metabolism contributes to suppression of inflammation by probiotic Lactobacillus reuteri. Microbiol. Open 5, 802–818. 10.1002/mbo3.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkáčiková L., Mochnáčová E., Tyagi P., Kiššová Z., Bhide M. (2020). Comprehensive mapping of the cell response to E. coli infection in porcine intestinal epithelial cells pretreated with exopolysaccharide derived from Lactobacillus reuteri. Vet. Res. 51, 49. 10.1186/s13567-020-00773-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivić I., Niseteo T., Jadrešin O., Hojsak I. (2021). Use of probiotics in the treatment of functional abdominal pain in children-systematic review and meta-analysis. Eur. J. Pediatr. 180, 339–351. 10.1007/s00431-020-03809-y [DOI] [PubMed] [Google Scholar]
- Turco R., Russo M., Bruzzese D., Staiano A. (2021). Efficacy of a partially hydrolysed formula, with reduced lactose content and with Lactobacillus reuteri DSM 17938 in infant colic: a double blind, randomised clinical trial. Clin. Nutr. 40, 412–419. 10.1016/j.clnu.2020.05.048 [DOI] [PubMed] [Google Scholar]
- Turner M. S., Woodberry T., Hafner L. M., Giffard P. M. (1999). The bspA locus of Lactobacillus fermentum BR11 encodes an L-cystine uptake system. J. Bacteriol. 181, 2192–2198. 10.1128/JB.181.7.2192-2198.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbańska M., Szajewska H. (2014). The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur. J. Pediatr. 173, 1327–1337. 10.1007/s00431-014-2328-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenplas Y., Bacarea A., Marusteri M., Bacarea V., Constantin M., Manolache M., et al. (2017). Efficacy and safety of APT198K for the treatment of infantile colic: a pilot study. J. Comp. Eff. Res. 6, 137–144. 10.2217/cer-2016-0059 [DOI] [PubMed] [Google Scholar]
- Walsham A. D. S., MacKenzie D. A., Cook V., Wemyss-Holden S., Hews C. L., Juge N., et al. (2016). Lactobacillus reuteri inhibition of enteropathogenic Escherichia coli adherence to human intestinal epithelium. Front. Microbiol. 7, 244. 10.3389/fmicb.2016.00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J., Britton R. A., Roos S. (2011). Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc. Natl. Acad. Sci. USA. 108(Suppl 1), 4645–4652. 10.1073/pnas.1000099107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Chen Y., Fei S., Xie C., Xia Y., Colonisation A. i. L., et al. (2021). with endogenous Lactobacillus reuteri R28 and exogenous Lactobacillus plantarum AR17-1 and the effects on intestinal inflammation in mice. Food Funct. 12, 2481–2488. 10.1039/D0FO02624G [DOI] [PubMed] [Google Scholar]
- Wang G., Huang S., Cai S., Yu H., Wang Y., Zeng X., et al. (2020). Lactobacillus reuteri ameliorates intestinal inflammation and modulates gut microbiota and metabolic disorders in dextran sulfate sodium-induced colitis in mice. Nutrients 12, 2298. 10.3390/nu12082298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., He Y., Li X., Zhang T., Liang M., Wang G., et al. (2022). Lactobacillus reuteri CCFM8631 alleviates hypercholesterolaemia caused by the paigen atherogenic diet by regulating the gut microbiota. Nutrients 14, 1272. 10.3390/nu14061272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Sun Q., Wang J., Qiu X., Qi R., Huang J., et al. (2020). Identification of differentially expressed miRNAs after Lactobacillus reuteri treatment in the ileum mucosa of piglets. Genes Genomics 42, 1327–1338. 10.1007/s13258-020-00998-6 [DOI] [PubMed] [Google Scholar]
- Wang T., Teng K., Liu G., Liu Y., Zhang J., Zhang X., et al. (2018). Lactobacillus reuteri HCM2 protects mice against enterotoxigenic Escherichia coli through modulation of gut microbiota. Sci. Rep. 8, 17485. 10.1038/s41598-018-35702-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Zheng N., Luo Q., Jiang L., He B., Yuan X., et al. (2019). Probiotics Lactobacillus reuteri abrogates immune checkpoint blockade-associated colitis by inhibiting group 3 innate lymphoid cells. Front. Immunol. 10, 1235. 10.3389/fimmu.2019.01235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watschinger C., Moschen A. R. (2022). Lactobacillus reuteri-an old acquaintance takes on a new task in colorectal tumor surveillance. Cancer Cell 40, 125–127. 10.1016/j.ccell.2022.01.014 [DOI] [PubMed] [Google Scholar]
- Weger M., Sandi C. (2018). High anxiety trait: a vulnerable phenotype for stress-induced depression. Neurosci. Biobehav. Rev. 87, 27–37. 10.1016/j.neubiorev.2018.01.012 [DOI] [PubMed] [Google Scholar]
- Wegh C., Benninga M. A., Tabbers M. M. (2018). Effectiveness of probiotics in children with functional abdominal pain disorders and functional constipation: a systematic review. J. Clin. Gastroenterol. 52(Suppl 1), S10–S26. 10.1097/MCG.0000000000001054 [DOI] [PubMed] [Google Scholar]
- Wegner A., Banaszkiewicz A., Kierkus J., Landowski P., Korlatowicz-Bilar A., Wiecek S., et al. (2018). The effectiveness of Lactobacillus reuteri DSM 17938 as an adjunct to macrogol in the treatment of functional constipation in children. A randomized, double-blind, placebo-controlled, multicentre trial. Clin. Res. Hepatol. Gastroenterol. 42, 494–500. 10.1016/j.clinre.2018.03.008 [DOI] [PubMed] [Google Scholar]
- Weitz J., Koch M., Debus J., Höhler T., Galle P. R., Büchler M. W., et al. (2005). Colorectal cancer. Lancet 365, 153–165. 10.1016/S0140-6736(05)17706-X [DOI] [PubMed] [Google Scholar]
- Weizman Z., Abu-Abed J., Binsztok M. (2016). Lactobacillus reuteri DSM 17938 for the management of functional abdominal pain in childhood: a randomized, double-blind, placebo-controlled trial. J Pediatr. 174, 160–164.e1. 10.1016/j.jpeds.2016.04.003 [DOI] [PubMed] [Google Scholar]
- Wu H., Xie S., Miao J., Li Y., Wang Z., Wang M., et al. (2020). Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes 11, 997–1014. 10.1080/19490976.2020.1734423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Lin Z., Wang X., Zhao Y., Zhao J., Liu H., et al. (2022). Limosi Lactobacillus reuteri SLZX19-12 protects the colon from infection by enhancing stability of the gut microbiota and barrier integrity and reducing inflammation. Microbiol. Spectr. 10, e0212421. 10.1128/spectrum.02124-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S., Zhao S., Jiang L., Lu L., Yang Q., Yu Q., et al. (2019). Lactobacillus reuteri stimulates intestinal epithelial proliferation and induces differentiation into goblet cells in young chickens. J. Agric. Food Chem. 67, 13758–13766. 10.1021/acs.jafc.9b06256 [DOI] [PubMed] [Google Scholar]
- Xie W., Song L., Wang X., Xu Y., Liu Z., Zhao D., et al. (2021). A bovine lactoferricin-lactoferrampin-encoding Lactobacillus reuteri CO21 regulates the intestinal mucosal immunity and enhances the protection of piglets against enterotoxigenic Escherichia coli K88 challenge. Gut Microbes 13, 1956281. 10.1080/19490976.2021.1956281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Wu L., Pan D., Zeng X., Cai Z., Guo Y., et al. (2021). Adhesion characteristics and dual transcriptomic and proteomic analysis of Lactobacillus reuteri SH23 upon gastrointestinal fluid stress. J. Proteome Res. 20, 2447–2457. 10.1021/acs.jproteome.0c00933 [DOI] [PubMed] [Google Scholar]
- Yang J., Wang C., Liu L., Zhang M. (2020). Lactobacillus reuteri KT260178 supplementation reduced morbidity of piglets through its targeted colonization, improvement of cecal microbiota profile, and immune functions. Probiotics Antimicrob. Proteins 12, 194–203. 10.1007/s12602-019-9514-3 [DOI] [PubMed] [Google Scholar]
- Yang Y., Galle S., Le M. H., Zijlstra R. T., Gänzle M. G. (2015). Feed fermentation with reuteran- and levan-producing Lactobacillus reuteri reduces colonization of weanling pigs by enterotoxigenic Escherichia coli. Appl. Environ. Microbiol. 81, 5743–5752. 10.1128/AEM.01525-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Manne S., Treem W. R., Bennett D. (2020). Prevalence of inflammatory bowel disease in pediatric and adult populations: recent estimates from large national databases in the United States, 2007-2016. Inflamm. Bowel Dis. 26, 619–625. 10.1093/ibd/izz182 [DOI] [PubMed] [Google Scholar]
- Yi H., Wang L., Xiong Y., Wang Z., Qiu Y., Wen X., et al. (2018a). Lactobacillus reuteri LR1 improved expression of genes of tight junction proteins via the MLCK pathway in IPEC-1 cells during infection with enterotoxigenic Escherichia coli K88. Mediators Inflamm. 2018, 6434910. 10.1155/2018/6434910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H., Wang L., Xiong Y., Wen X., Wang Z., Yang X., et al. (2018b). Effects of Lactobacillus reuteri LR1 on the growth performance, intestinal morphology, and intestinal barrier function in weaned pigs. J. Anim. Sci. 96, 2342–2351. 10.1093/jas/sky129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T., Iannitti R. G., Cunha C., De Luca A., Giovannini G., Pieraccini G., et al. (2013). Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385. 10.1016/j.immuni.2013.08.003 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang X.-L., Zhou M., Kang C., Lang H.-D., Chen M.-T., et al. (2018). Crosstalk between gut microbiota and Sirtuin-3 in colonic inflammation and tumorigenesis. Exp. Mol. Med. 50, 1–11. 10.1038/s12276-017-0002-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Wang K., Oh J. H., Zhang S., van Pijkeren J. P., Cheng C. C., et al. (2020). A phylogenetic view on the role of glycerol for growth enhancement and reuterin formation in limosi Lactobacillus reuteri. Front. Microbiol. 11, 601422. 10.3389/fmicb.2020.601422 [DOI] [PMC free article] [PubMed] [Google Scholar]