Abstract
Objective
Status epilepticus (SE) is a common neurological emergency that is defined as a prolonged seizure or a series of seizures which often leads to irreversible damage. Levetiracetam (LEV) and valproate (VPA) are second-line anti-seizure drugs that are frequently used in patients with established SE (ESE). This meta-analysis compared the efficacy and safety of LEV and VPA for the treatment of ESE.
Method
MEDLINE, EMBASE, Central Register of Controlled Trials (CENTRAL), and clinicaltrials.gov were searched by two authors, which identified six randomized controlled trials (RCTs) that compared LEV and VPA for ESE.
Results
The six RCTs included 1213 patients (LEV group, n = 593; VPA group, n = 620). Integrated patient data information display LEV was not superior to VPA in terms of clinical seizure termination (63.55% vs. 64.08%, respectively; relative risk [RR] = 1.03, 95% confidence interval [CI] = 0.94–1.11, p = 0.55), with no significant differences between LEV and VPA in terms of good functional outcome at discharge (Glasgow Outcome Scale [GOS] = 4 or 5), intensive care unit (ICU) admission, adverse events, and mortality. There was no statistically significant difference between the two drugs in different age groups. Previous multicenter studies have demonstrated that VPA was slightly more effective than LEV, whereas single-center studies showed the opposite results. In addition, LEV and VPA had similar rates of clinical seizure termination, ICU admission, and adverse events between the age subgroups (ages <18 and >18 years).
Conclusions
Levetiracetam (LEV) was not superior to valproate (VPA) in terms of efficacy or safety outcomes. In addition, children (<18 years) and adults (>18 years) might have similar responses to LEV and VPA. Additional RCTs are required to verify our results.
Keywords: Levetiracetam, Valproate, Established status epilepticus, Meta-analysis
Highlights
-
•
Levetiracetam (LEV) was not superior to valproate (VPA) in terms of efficacy outcomes.
-
•
There were no significant differences in the safety outcomes between LEV and VPA.
-
•
The efficacy of VPA was better in children than in adults, but the safety profile was opposite.
-
•
There is no difference in efficacy and safety between children and adults with LEV.
1. Introduction
Status epilepticus (SE) is one of the most severe manifestations of epilepsy and is associated with significant morbidity and mortality (annual prevalence: 17–23/100,000; mortality: 24%–26% in adults, 3%–6% in children, and 10% in all patients) [[1], [2], [3], [4]]. SE that continues after first-line treatment with benzodiazepines (BZDs) is called established SE (ESE; benzodiazepine-refractory SE). ESE is treated with second-line anti-seizure drugs, such as levetiracetam (LEV), valproate (VPA), phenytoin/fosphenytoin, and phenobarbital. If second-line drugs fail to achieve seizure control, seizures are termed refractory SE (RSE) [5]. RSE is associated with a mortality rate of 15%–30% [1,6] and nearly all of the survivors experience relapses [[7], [8], [9]]. If SE lasts for one day or longer after general anesthesia, the condition is termed super-refractory SE (SRSE) [10], which has a mortality rate of approximately 36%–40% [1,11,12].
BZDs are positive modulators of chloride (Cl−) channel and permeate γ-aminobutyric acid (GABA) receptors for the treatment of SE. However, about a third of patients do not respond to BZDs [13,14]. As a result, one-third of epileptic patients develop ESE, and for such patients, aggressive treatment is required to prevent progression to RSE or SRSE. Thus, the treatment of ESE is essential for a good prognosis of epileptic patients.
The failure of BZD treatment for SE prompted the development of second-line anti-seizure drugs. Nevertheless, there is no explicit evidence for selecting any single second-line anti-seizure drug. The selection of second-line anti-seizure drug depends on the personal choice of the treating doctor. At present, the commonly used second-line drugs for ESE are LEV, VPA, phenytoin, and fosphenytoin.
The mechanism of action of LEV, a pyrrolidone derivative and piracetam analog, in the treatment of ESE is not clear [5]. LEV acts on neurotransmitter release, synaptic vesicle protein 2 (SV2), and Ca2+ signaling, which might contribute to the treatment of SE [15]. VPA, a broad-spectrum anticonvulsant agent, has extremely complex pharmacological mechanisms, including various actions on GABA, N-methyl-d-aspartic acid (NMDA) receptor antagonism, and histone deacetylase inhibition [16].
The main purpose of this meta-analysis was to compare the efficacy and safety of LEV and VPA. Because longer duration of SE is associated with worse recovery and higher mortality [17], clinical seizure termination was used to evaluate the efficacy of drugs in the treatment of ESE. In addition, the Glasgow Outcome Scale (GOS) is usually recommended to assess the functional ability of ESE patient, with a GOS score of 4 or 5 representing good functional status in work and daily life. If a single second-line anti-seizure drug fails to control SE, patients are generally admitted to the intensive care unit (ICU) for further treatment. In addition, early termination of convulsive SE results in reduced risk of ICU admission [14,18]. Hence, ICU admission can be regarded as another marker of anti-seizure drug efficacy. Finally, we selected good functional ability at discharge (GOS = 4 or 5), ICU admission, adverse event rate, and mortality as the safety outcomes.
2. Method
2.1. Study protocol
We drafted a research protocol based on the Cochrane Collaboration format before initiating the project [19].
2.2. Eligibility criteria
We set the inclusion criteria as follows: (a) study type: RCT; (b) language restriction: available only in English; (c) participants: patients of all ages who have been diagnosed with ESE; (d) intervention: oral LEV therapy alone or oral VPA therapy alone; (e) outcomes: efficacy outcomes included clinical seizure termination and good functional ability at discharge (GOS = 4 or 5). Safety outcomes included good functional ability at discharge (GOS = 1–3), ICU admission, adverse events, and mortality. The included RCTs were requested to supply all the efficacy outcomes and at least one safety outcomes. Inclusion, exclusion criteria and study design, all efficacy and safety outcomes are reported in the online supplementary material (Table S1).
2.3. Information sources and search strategy
Two authors (SXW and XW) independently searched four databases (MEDLINE, EMBASE, Central Register of Controlled Trials [CENTRAL], and clinicaltrials.gov) for relevant meta-analysis, reviews, clinical trials, and case reports. The following search strategy was employed: (“levetiracetam” OR “valproate”) AND “established status epilepticus” in title, abstract or keywords. Additionally, the reference lists of RCTs, relevant systematic reviews and meta-analyses were also screened independently and manually to ensure a more comprehensive search.
2.4. Study selection and data collection
According to the eligibility criteria mentioned above, two reviewers (SXW and XW) independently reviewed all titles, abstracts, and full-text articles searched from the four databases and the reference lists of RCTs and relevant systematic reviews or meta-analyses. We excluded the duplicates and research articles for which the full text was not available. Discrepancies between the two authors were resolved by discussion or, if necessary, by a third author (XT) who did not participate in the data collection. After selection and evaluation, a total of 6 RCT articles were selected and included in the final research summary. Finally, the selected literature data, including countries, centers, publications, treatment group, patient gender composition, age range, and doses (mg/kg), are presented in Table 1.
Table 1.
Characteristics and interventions of the included studies.
| Study | Country | Centers (no.) | Publication | Treatment group (no. of participants) | Age range | Male (%) |
Mean age ±SD (year) |
Dosage (mg/kg) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LEV | VPA | LEV | VPA | LEV | VPA | ||||||
| Marson et al., 2021 | UK | 69 | The lancet | LEV (260) vs. VPA (260) | 5-95 y | 65 | 64 | 16.9 ± 11.8 | 17.1 ± 12.9 | 40 | 25 |
| Vignsesh et al., 2020 | India | 1 | India Pediatrics | LEV (32) vs. VPA (35) | 3 mo to 12 y | 56.2 | 60 | 4.83 ± 4.17 | 4.92 ± 3.67 | 20 | 20 |
| Kapur et al., 2019 (ESETT trial) | USA | 57 | New England Journal of Medicine | LEV (145) vs. VPA (121) | >2 y | 53.1 | 53.7 | 33.3 ± 26.0 | 32.2 ± 25.4 | 60 | 40 |
| Nene et al., 2019 | India | 1 | Seizure: European Journal of Epilepsy | LEV (58) vs. VPA (60) | >60 y | 58.6 | 65 | 68.5 ± 8.0 | 66.6 ± 6.7 | 20–25 | 20–25 |
| Misra et al., 2016 | India | 1 | International Journal of Neuroscience | LEV (38) vs. VPA (65) | >1 y | 55.3 | 67.7 | 39.2 ± 21.2 | 34.1 ± 21.3 | 20 | 30 |
| Mundlamuri et al., 2015 | India | 1 | Epilepsy Research | LEV (50) vs. VPA (50) | >15 y | 64 | 56 | 34.78 ± 13.64 | 33.12 ± 11.99 | 25 | 30 |
LEV: levetiracetam; VPA: valproate; ESETT: Established Status Epilepticus Treatment Trial.
2.5. Risk of bias
Review Manager software (version 5.3) was used to assess the risk of bias for individual studies. The biases included selection, performance, detection, attrition, reporting, and other potential biases. We applied the unified standard of the Cochrane Collaboration to assess the risk of bias of RCTs.
2.6. Summary measures and synthesis of results
We used Review Manager software (version 5.3) to evaluate the data from six RCTs. We categorized statistical heterogeneity (I2) as low (<30%), moderate (30%–50%), and substantial (>50%). For this paper, the heterogeneity is generally less than 50%, thus we analyzed the dichotomous outcomes using a fixed effects model and presented them as risk ratios (relative risk [RR]; 95% confidence interval [CI]). Due to the differences in prevalence of BZD-refractory SE among different LEV doses, age, and number of study centers, we performed subgroup analyses to compare the efficacy and safety of LEV and VPA among different age groups (<18 and >18 years) and LEV doses (LEV >30 and <30 mg/kg). Sensitivity analysis was performed to assess the stability of the consolidated results. P < 0.05 was deemed to be significant. All tests were two-tailed.
3. Results
3.1. Study identification and selection
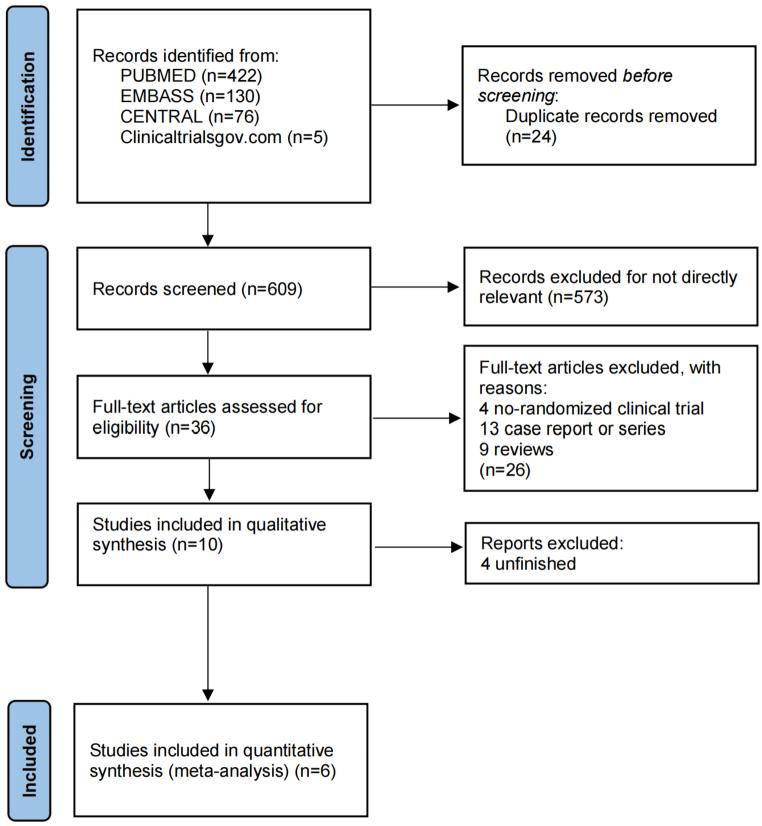
We identified a total of 633 records from MEDLINE, EMBASE, CENTRAL, and clinicaltrialsgov.com. We did not find any duplicates in the 609 records. After excluding documents that were not related to the study topic, 36 records were left. After excluding 1 non-randomized clinical trial, 7 case reports or series, 6 reviews, and 4 unfinished studies, six RCTs remained (Fig. 1). The six RCTs [[20], [21], [22], [23], [24], [25]] included 1213 patients (LEV group, n = 593; VPA group, n = 620) who were entered into the qualitative synthesis. Disagreement was determined by the discussion or by a third researcher who was not involved in the literature selection. The main characteristics of the five included studies are summarized in Table 1.
Fig. 1.
Study search, selection, and inclusion process.
3.2. Efficacy outcomes
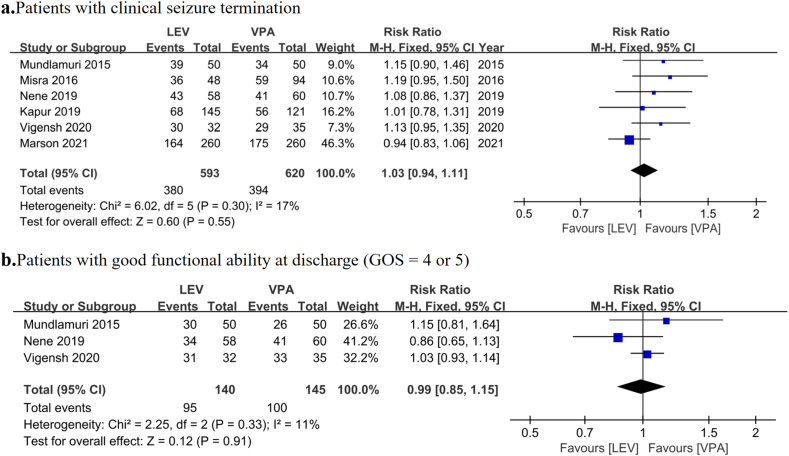
In terms of efficacy, two factors, including clinical seizure termination and good functional outcome at discharge (GOS = 4 or 5), were used to assess the treatment effectiveness. No significant differences were noted in clinical seizure termination (64.08% vs. 63.55% for LEV and VPA, respectively; RR = 1.03, 95% CI = 0.94–1.11, p = 0.55; Fig. 2a) and good functional ability at discharge (GOS = 4 or 5) (67.86% vs. 68.97% for LEV and VPA, respectively; RR = 0.99, 95% CI = 0.85–1.15, p = 0.91; Fig. 2b) between the LEV and VPA groups. However, the heterogeneity in clinical seizure termination was 17%. Sensitivity and subgroup analyses were performed to explore the source of statistical heterogeneity. The sensitivity analysis showed that the consolidated results were stable. The results of subgroup analysis are presented later in the manuscript.
Fig. 2.
Pooled relative risk of the efficacy outcomes. Diamond indicates the estimated relative risk (95% confidence interval) for all patients. a. Clinical seizure termination. b. Good functional ability at discharge (GOS = 4 or 5).
3.3. Safety outcomes
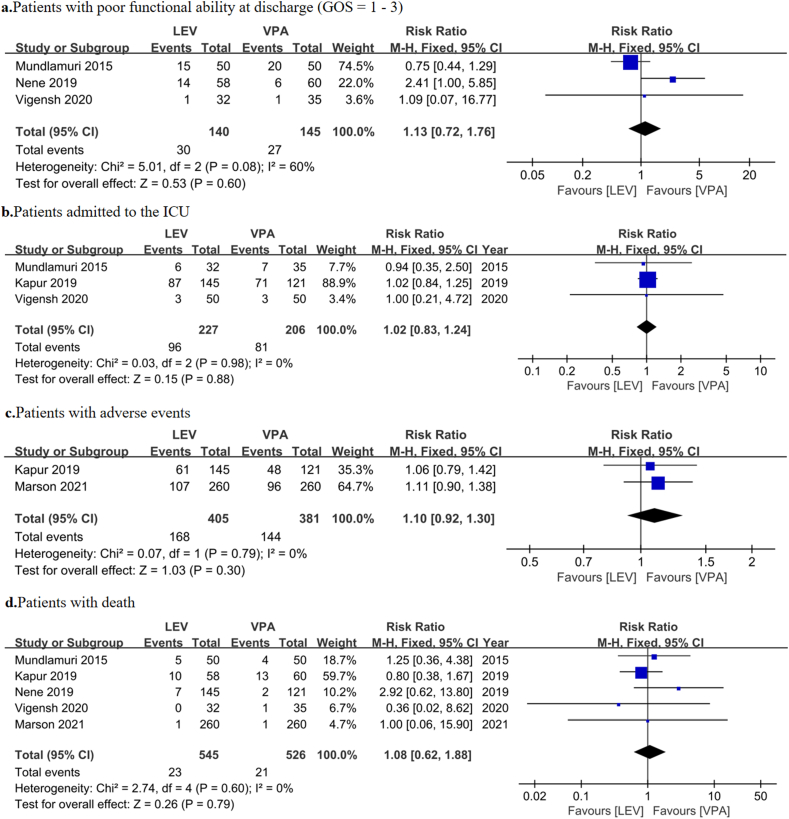
We evaluated the effectiveness of treatment based on the good functional ability at discharge (GOS = 1–3), ICU admission, adverse events, and mortality. There were no significant differences in good functional ability at discharge (GOS = 1–3) (21.43% vs. 18.62% for LEV and VPA, respectively; RR = 1.13, 95% CI = 0.72–1.76, p = 0.60; Fig. 3a), ICU admission (42.29% vs. 39.32% for LEV and VPA, respectively; RR = 1.02, 95% CI = 0.83–1.24, p = 0.88; Fig. 3b), adverse events (41.48% vs. 37.80% for LEV and VPA, respectively; RR = 1.10, 95% CI = 0.92–1.30, p = 0.30; Fig. 3c), and mortality (4.22% vs. 3.99% for LEV and VPA, respectively; RR = 1.08, 95% CI = 0.62–1.88, p = 0.79; Fig. 3d) between LEV and VPA groups.
Fig. 3.
Pooled relative risk of safety outcomes. Diamond indicates the estimated relative risk (95% confidence interval) for all patients.
3.4. Subgroup analysis
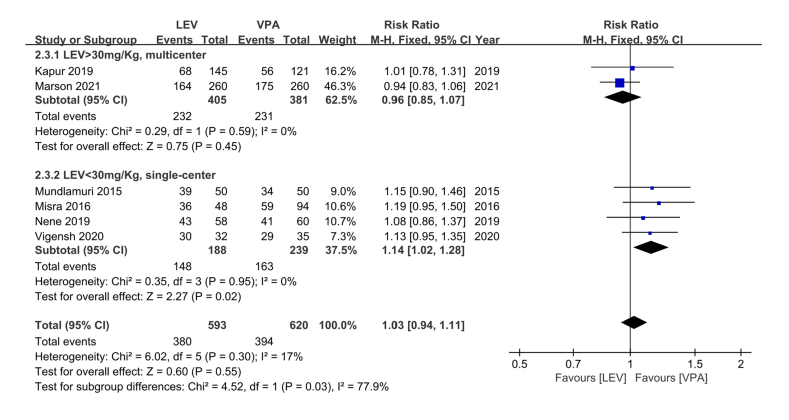
Subgroup analysis was performed to assess the efficacy and safety of LEV and VPA at different LEV doses (LEV >30 and <30 mg/kg) and age groups (children: <18 years, adults: >18 years). We used a dose cutoff of 30 mg/kg for LEV to divide the patients into two groups; these groups were used to compare single- and multi-center studies, as well as studies conducted in developing and developed countries. In the age groups, due to the absence of data regarding good functional ability at discharge (GOS = 4 or 5) and mortality in some included RCTs, the three factors (including clinical seizure termination, ICU admission, and adverse events) were used to evaluate the efficacy and safety of LEV and VPA. In addition, data from [25] included mainly juveniles so we categorized the data into the juvenile group. LEV demonstrated similar effectiveness in children and adults; however, compared with adults, VPA showed higher effectiveness in children, these differences were not statistically significant (children: 63.13% vs. 65.93% for LEV and VPA, respectively; RR = 0.97, 95% CI = 0.87–1.08, p = 0.55; adults: 63.14% vs. 58.17% for LEV and VPA, respectively; RR = 1.17, 95% CI 0.97–1.42, p = 0.13; Table 2). In the subgroup analysis of LEV doses and single- and multi-center studies, VPA was found to be superior to LEV in multicenter studies and at LEV dose >30 mg/kg. LEV was superior to VPA in single-center studies and LEV dose <30 mg/kg, however, the difference was not statistically significant (LEV >30 mg/kg: 57.28% vs. 60.63% for LEV and VPA, respectively; RR = 0.96, 95% CI 0.85–1.07, p = 0.45; LEV <30 mg/kg: 78.72% vs. 68.20% for LEV and VPA, respectively; RR = 1.14, 95% CI = 1.02–1.28, p = 0.02; Fig. 4). There was no difference in the incidence of adverse events between LEV and VPA in children; however, in adults, LEV had a higher incidence of adverse events compared with VPA, this difference was statistically not significant (children: 33.60% vs. 32.05% for LEV and VPA, respectively; RR = 1.05, 95% CI = 0.86–1.28, p = 0.64; adults: 24.26% vs. 15.87% for LEV and VPA, respectively; RR = 1.37, 95% CI = 0.97–1.95, p = 0.08; Table 2). The incidence of ICU admission was similar between different age groups (children: 50.43% vs. 48.08% for LEV and VPA, respectively; RR = 0.99, 95% CI = 0.78–1.27, p = 0.95; adults: 40% vs. 35.71% for LEV and VPA, respectively; RR = 1.06, 95% CI = 0.81–1.39, p = 0.66; Table 2). In addition, we found that the heterogeneity in clinical seizure termination in the subgroups was acceptable (children: I2 = 40%, adults: I2 = 16%; Table 2) Detailed results are shown in Figs. S1–S3.
Table 2.
Age subgroup analysis of LEV and VPA on efficacy and safety (including admission to ICU and adverse events) outcomes.
| Children (<18 years) |
Adults (>18 years) |
|||
|---|---|---|---|---|
| MD (95% CI)/RR [95% CI] | p value | MD (95% CI)/RR [95% CI] | p value | |
| Efficacy outcomes | ||||
| Clinical seizure termination | 0.97 [0.87, 1.08] | 0.55 | 1.11 [0.97, 1.11] | 0.13 |
| Safety outcomes | ||||
| Admission to ICU | 0.99 [0.78, 1.27] | 0.95 | 1.06 [0.81, 1.23] | 0.66 |
| Adverse events | 1.05 [0.86, 1.28] | 0.64 | 1.37 [0.97, 1.95] | 0.08 |
MD: mean difference; RR: relative risk; CI: confidence interval.
Fig. 4.
Subgroup analysis of levetiracetam (LEV) dose (similar to the comparison of single- and multi-center studies, as well as studies conducted in countries with different levels of economic development). Diamond indicates the estimated relative risk (95% confidence interval) for all patients.
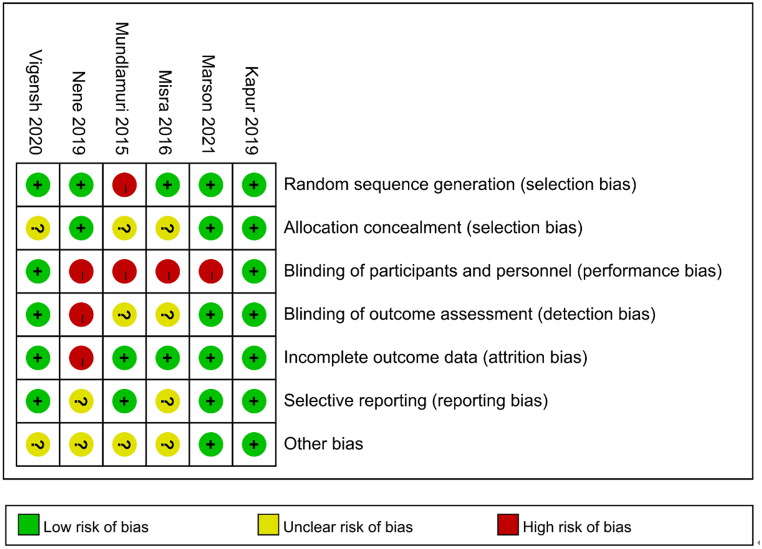
3.5. Risk of bias
The details of bias for the six RCTs are presented in Fig. 5. For random sequence generation, [24] demonstrated high risk of bias. For allocation concealment, there was unclear risk of bias in three RCTs [20, 23, 24]. For blinding of participants and personnel, high risk of bias existed in four RCTs [[22], [23], [24], [25]]. For blinding of outcome assessment, the risk of bias was high in [22] and unclear in [23, 24]. For incomplete outcome data, [20] had high risk of bias. For selective reporting, the risk of bias was unclear in two RCTs [22, 23]. In addition, there was unclear risk of bias in four RCTs [20, 23, 24] for other bias.
Fig. 5.
Risk of bias: A summary table for each risk of bias item for each study.
4. Discussion
By summarizing the data from six RCTs, we analyzed the effectiveness and safety of two drugs. We divided the data into juvenile and adult groups for subgroup analysis of effectiveness, incidence of adverse events, and incidence of ICU admission according to age. The LEV dose was used to categorize the data; this grouping overlapped with single- and multi-center comparisons, as well as regional economic development. According to the results of this meta-analysis, we found that there was no difference in the efficacy of the two drugs, the results of the meta-analysis showed no difference in efficacy between the LEV and VPA, which was inconsistent with previous studies that LEV was superior to VPA. However, in the subgroup analysis, we made some valuable discoveries, even though they were not statistically significant, such as: LEV was almost equally effective for ESE in the age groups; however, VPA was more effective in treating ESE in children than in adults (children: 65.93%, adults: 58.17%). Meanwhile, during the treatment of ESE, the incidence of adverse events was significantly lower in adults than in children, LEV had a slightly higher incidence of adverse events than VPA, but these results were not statistically significant (LEV, children: 33.59%, adults: 24.25%; VPA, children: 32.05%, adults: 15.87%). Safety analysis of the two drugs showed no significant differences between them.
When the duration of SE exceeds 30 min, BZD receptors may decrease gradually and the therapeutic effects of BZDs may be unsatisfactory [26]. However, it is often difficult for patients with SE to receive appropriate treatment within 30 min, which prompted the development of second-line drugs. LEV, a pyrrolidone derivative and piracetam analog [16], has effects on GABA turnover in the striatum and causes a reduction in the amino acid taurine (a low affinity agonist for GABA receptors) [27]. In addition, LEV can bind to SV2 receptors to affect synaptic release [[28], [29], [30]]. The effects of LEV on inhibiting Ca2+ channels may also play an important role in SE [31]. However, so far, the exact pharmacological mechanisms of LEV for ESE are not clear. Meanwhile, LEV was shown to have a rapid antiepileptic activity in animal experiments [32]. VPA, a unique antiepileptic drug, is effective against several seizure types and epileptic syndromes in children and adults [33]. VPA can improve the transmission capacity of GABA in specific brain regions by enhancing GABA synthesis and release [34]. In addition, the excitatory molecules, such as β-hydroxybutyric acid, and activation of NMDA glutamate receptors can be reduced by VPA to reduce the excitation of nerves [35]. In addition, voltage-gated ionic channels, including sodium, potassium, and calcium channels, can be blocked by VPA to prevent high-frequency neuronal firing [36]. Through a combination of the aforementioned mechanisms, VPA can control ESE effectively. In summary, LEV and VPA have significant therapeutic effects on ESE, and their effects and side effects have been confirmed after a long period of clinical use, which is why we selected it for this meta-analysis.
This meta-analysis was conducted to compare the efficacy and safety between LEV and VPA groups for ESE. The analysis of effectiveness of the two drugs revealed a heterogeneity of only 17%, but with a non-significant p value (p = 0.55). In the subgroup analysis for LEV dose, we found that the economically developed regions conducted multi-center studies with a dose of more than 30 mg, and showed that VPA was superior to LEV. The studies conducted in economically underdeveloped areas were single-center studies with LEV doses of less than 30 mg, and the results showed that LEV was more effective than VPA. This may be due to the fact that the current RCTs did not include a uniform duration of treatment. In the latest RCT, 520 patients were followed for a long duration, and it was found that VPA was significantly superior to LEV in the short term (1 year), but the efficacy of VPA and LEV gradually reached similar levels after 2–3 years of treatment. In the previous five RCTS, the duration of course of the two drugs was not mentioned, which may be because they were used for long term; therefore, there was no significant difference in efficacy. Finally, we divided the patients into two subgroups according to the mean age, and identified acceptable heterogeneity in clinical seizure termination in subgroup analysis (children: 40%; adults: 16%). Therefore, the conclusion that there are no significant differences in clinical seizure termination was credible. No distinct differences were observed between the groups in terms of adverse events and mortality. Additional RCTs are required on the efficacy, dose, and duration of LEV and VPA to enrich the data and obtain clinically useful results.
SE in children is usually associated with fever, drug ingestions, electrolyte imbalances, inborn errors of metabolism, low anticonvulsant levels, central nervous system infections, bacteremia, and various neuroimaging abnormalities (arteriovenous malformations, cortical malformations, stroke/hemorrhage, trauma, tumors, and hydrocephalus) [37]. The risk factors of SE in adults mainly include encephalitis, massive stroke, and large brain tumors [17]. In addition, the age distribution of SE follows a U-shaped curve. Children and older adults (aged ≥60 years) demonstrated the highest incidence of SE [38,39]. The severity of SE differs between children and adults [38], so we conducted subgroup analyses to assess the efficacy and safety of LEV and VPA in different age subgroups (children: <18 years, adults: >18 years). We identified slight differences in clinical seizure termination and adverse events among the subgroups, with VPA being more effective in treating ESE in children than in adults. In addition, the incidence and probability of adverse events of VPA were lower in adults than in children. Meanwhile, our finding showed that VPA was superior to LEV in children. However, the result was not definitive because of several known safety concerns of VPA (eg: cognitive issues in children born to mothers taking VPA, hepatotoxicity when given to children aging 0–2 years). Although the different dosages of LEV and VPA may lead to heterogeneity within the studies, the heterogeneity in the present meta-analysis was acceptable. However, additional clinical trials are needed to confirm the conclusions of this meta-analysis. To sum up, there is no significant difference between LEV and VPA in the treatment of ESE, unless economic considerations are taken into account, such as: LEV is more expensive than VPA in terms of the cost.
This is the first meta-analysis to compare the two drugs (i.e., VPA and LEV) based on RCTs only. Most previous meta-analyses and systematic reviews of second-line drugs in ESE included both RCTs and retrospective studies or analyzed the combined use of drugs. However, the present meta-analysis included only RCTs, which is the best strategy to compare the risk factors between two groups. In addition, some previous meta-analyses and systematic reviews focused on not only LEV and VPA, but also other antiepileptic drugs. There might be significant heterogeneity and risk of bias in the outcomes of these studies [[40], [41], [42]]. Meanwhile, some studies did not analyze the efficacy and safety of antiepileptic drugs among different age subgroups [[43], [44], [45]]. The present meta-analysis compares and analyzes the two drugs from different perspectives, with convincing results. In addition, ganaxolone, brivaracetam, and perampanel are also common second-line drugs for treatments of ESE [[46], [47], [48]]. The number of clinical trials of these drugs is not enough to form a valuable comparison. We hope that more RCTS of relevant aspects can be published to help us further explore more suitable drugs for ESE.
There were several limitations to this meta-analysis, which cannot be ignored. First, only six RCTs [[20], [21], [22], [23], [24], [25]] were included. This meta-analysis was performed on the basis of limited data, more RCTs are required to verify our results. Second, we could not analyze the time from SE onset to treatment, which may affect the disease prognosis. Third, because of the prolonged treatment cycle, patient loss may occur during treatment, which may introduce bias in the data. Finally, the doses of drugs used in different studies was not standardized and VPA had several known safety concerns. The aforementioned factors may introduce bias in the study and make the results less significant.
5. Conclusion
Overall, our present findings demonstrated that LEV was not superior to VPA for patients with established status epilepticus in terms of efficacy and safety. This conclusion differs from the previous view that LEV is superior to VPA in terms of cognition. In addition, children (<18 years) and adults (>18 years) might have similar responses to LEV and VPA. Additional RCTs are required to verify our results.
Declarations
Code availability
Not applicable.
Ethics approval
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Prof. Zhong Wang was supported by National Natural Science Foundation of China [81971171], Science and Technology Program of Suzhou [SLT201906], Suzhou Health Talents Training Project [GSWS2019002].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e13380.
Contributor Information
Xiaoou Sun, Email: sunxo76@163.com.
Zhong Wang, Email: wangzhong761@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Strzelczyk A., Ansorge S., Hapfelmeier J., Bonthapally V., Erder M.H., Rosenow F. Costs, length of stay, and mortality of super-refractory status epilepticus: a population-based study from Germany. Epilepsia. 2017;58(9):1533–1541. doi: 10.1111/epi.13837. [DOI] [PubMed] [Google Scholar]
- 2.Raspall-Chaure M., Chin R.F.M., Neville B.G., Bedford H., Scott R.C. The epidemiology of convulsive status epilepticus in children: a critical review. Epilepsia. 2007;48(9):1652–1663. doi: 10.1111/j.1528-1167.2007.01175.x. [DOI] [PubMed] [Google Scholar]
- 3.Logroscino G., Hesdorffer D.C., Cascino G., Hauser W.A., Coeytaux A., Galobardes B., Morabia A., Jallon P. Mortality after a first episode of status epilepticus in the United States and Europe. Epilepsia. 2005;46(Suppl 11):46–48. doi: 10.1111/j.1528-1167.2005.00409.x. [DOI] [PubMed] [Google Scholar]
- 4.Jallon P. Mortality in patients with epilepsy. Curr. Opin. Neurol. 2004;17(2):141–146. doi: 10.1097/00019052-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Falco-Walter J.J., Bleck T. Treatment of established status epilepticus. J. Clin. Med. 2016;5(5) doi: 10.3390/jcm5050049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brophy G.M., Bell R., Claassen J., Alldredge B., Bleck T.P., Glauser T., Laroche S.M., Riviello J.J., Jr., Shutter L., Sperling M.R., et al. Guidelines for the evaluation and management of status epilepticus. Neurocritical Care. 2012;17(1):3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 7.Hunter G., Young G.B. Status epilepticus: a review, with emphasis on refractory cases. Can. J. Neurol. Sci. 2012;39(2):157–169. doi: 10.1017/s0317167100013160. [DOI] [PubMed] [Google Scholar]
- 8.Mayer S.A., Claassen J., Lokin J., Mendelsohn F., Dennis L.J., Fitzsimmons B.F. Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch. Neurol. 2002;59(2):205–210. doi: 10.1001/archneur.59.2.205. [DOI] [PubMed] [Google Scholar]
- 9.Claassen J., Hirsch L.J., Emerson R.G., Bates J.E., Thompson T.B., Mayer S.A. Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus. Neurology. 2001;57(6):1036–1042. doi: 10.1212/wnl.57.6.1036. [DOI] [PubMed] [Google Scholar]
- 10.Shorvon S. Super-refractory status epilepticus: an approach to therapy in this difficult clinical situation. Epilepsia. 2011;52(Suppl 8):53–56. doi: 10.1111/j.1528-1167.2011.03238.x. [DOI] [PubMed] [Google Scholar]
- 11.Kalita J., Misra U.K., Singh V.K., Dubey D. Predictors and outcome of status epilepticus in cerebral venous thrombosis. J. Neurol. 2019;266(2):417–425. doi: 10.1007/s00415-018-9145-8. [DOI] [PubMed] [Google Scholar]
- 12.Novy J., Logroscino G., Rossetti A.O. Refractory status epilepticus: a prospective observational study. Epilepsia. 2010;51(2):251–256. doi: 10.1111/j.1528-1167.2009.02323.x. [DOI] [PubMed] [Google Scholar]
- 13.Chamberlain J.M., Okada P., Holsti M., Mahajan P., Brown K.M., Vance C., Gonzalez V., Lichenstein R., Stanley R., Brousseau D.C., et al. Lorazepam vs diazepam for pediatric status epilepticus: a randomized clinical trial. JAMA. 2014;311(16):1652–1660. doi: 10.1001/jama.2014.2625. [DOI] [PubMed] [Google Scholar]
- 14.Silbergleit R., Durkalski V., Lowenstein D., Conwit R., Pancioli A., Palesch Y., Barsan W., Investigators N. Intramuscular versus intravenous therapy for prehospital status epilepticus. N. Engl. J. Med. 2012;366(7):591–600. doi: 10.1056/NEJMoa1107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deshpande L.S., Delorenzo R.J. Mechanisms of levetiracetam in the control of status epilepticus and epilepsy. Front. Neurol. 2014;5:11. doi: 10.3389/fneur.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wasim M., Husain A.M. Nonconvulsive seizure control in the intensive care unit. Curr. Treat. Options Neurol. 2015;17(3):340. doi: 10.1007/s11940-015-0340-y. [DOI] [PubMed] [Google Scholar]
- 17.Abend N.S., Bearden D., Helbig I., McGuire J., Narula S., Panzer J.A., Topjian A., Dlugos D.J. Status epilepticus and refractory status epilepticus management. Semin. Pediatr. Neurol. 2014;21(4):263–274. doi: 10.1016/j.spen.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alldredge B.K., Gelb A.M., Isaacs S.M., Corry M.D., Allen F., Ulrich S., Gottwald M.D., O'Neil N., Neuhaus J.M., Segal M.R., et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N. Engl. J. Med. 2001;345(9):631–637. doi: 10.1056/NEJMoa002141. [DOI] [PubMed] [Google Scholar]
- 19.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vignesh V., Rameshkumar R., Mahadevan S. Comparison of phenytoin, valproate and levetiracetam in pediatric convulsive status epilepticus: a randomized double-blind controlled clinical trial. Indian Pediatr. 2020;57(3):222–227. [PubMed] [Google Scholar]
- 21.Kapur J., Elm J., Chamberlain J.M., Barsan W., Cloyd J., Lowenstein D., Shinnar S., Conwit R., Meinzer C., Cock H., et al. Randomized trial of three anticonvulsant medications for status epilepticus. N. Engl. J. Med. 2019;381(22):2103–2113. doi: 10.1056/NEJMoa1905795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nene D., Mundlamuri R.C., Satishchandra P., Prathyusha P.V., Nagappa M., Bindu P.S., Raghavendra K., Saini J., Bharath R.D., Thennarasu K., et al. Comparing the efficacy of sodium valproate and levetiracetam following initial lorazepam in elderly patients with generalized convulsive status epilepticus (GCSE): a prospective randomized controlled pilot study. Seizure. 2019;65:111–117. doi: 10.1016/j.seizure.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Misra U.K., Kalita J. A comparison of four antiepileptic drugs in status epilepticus: experience from India. Int. J. Neurosci. 2016;126(11):1013–1019. doi: 10.3109/00207454.2015.1095743. [DOI] [PubMed] [Google Scholar]
- 24.Mundlamuri R.C., Sinha S., Subbakrishna D.K., Prathyusha P.V., Nagappa M., Bindu P.S., Taly A.B., Umamaheswara Rao G.S., Satishchandra P. Management of generalised convulsive status epilepticus (SE): a prospective randomised controlled study of combined treatment with intravenous lorazepam with either phenytoin, sodium valproate or levetiracetam--Pilot study. Epilepsy Res. 2015;114:52–58. doi: 10.1016/j.eplepsyres.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Marson A., Burnside G., Appleton R., Smith D., Leach J.P., Sills G., Tudur-Smith C., Plumpton C., Hughes D.A., Williamson P., et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalised and unclassifiable epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet. 2021;397(10282):1375–1386. doi: 10.1016/S0140-6736(21)00246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J.W., Wasterlain C.G. Status epilepticus: pathophysiology and management in adults. Lancet Neurol. 2006;5(3):246–256. doi: 10.1016/S1474-4422(06)70374-X. [DOI] [PubMed] [Google Scholar]
- 27.Tong X., Patsalos P.N. A microdialysis study of the novel antiepileptic drug levetiracetam: extracellular pharmacokinetics and effect on taurine in rat brain. Br. J. Pharmacol. 2001;133(6):867–874. doi: 10.1038/sj.bjp.0704141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wasterlain C.G., Chen J.W. Mechanistic and pharmacologic aspects of status epilepticus and its treatment with new antiepileptic drugs. Epilepsia. 2008;49(Suppl 9):63–73. doi: 10.1111/j.1528-1167.2008.01928.x. [DOI] [PubMed] [Google Scholar]
- 29.Gillard M., Fuks B., Michel P., Vertongen P., Massingham R., Chatelain P. Binding characteristics of [3H]ucb 30889 to levetiracetam binding sites in rat brain. Eur. J. Pharmacol. 2003;478(1):1–9. doi: 10.1016/j.ejphar.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 30.Crowder K.M., Gunther J.M., Jones T.A., Hale B.D., Zhang H.Z., Peterson M.R., Scheller R.H., Chavkin C., Bajjalieh S.M. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A) Proc. Natl. Acad. Sci. U. S. A. 1999;96(26):15268–15273. doi: 10.1073/pnas.96.26.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukyanetz E.A., Shkryl V.M., Kostyuk P.G. Selective blockade of N-type calcium channels by levetiracetam. Epilepsia. 2002;43(1):9–18. doi: 10.1046/j.1528-1157.2002.24501.x. [DOI] [PubMed] [Google Scholar]
- 32.Mazarati A.M., Baldwin R., Klitgaard H., Matagne A., Wasterlain C.G. Anticonvulsant effects of levetiracetam and levetiracetam-diazepam combinations in experimental status epilepticus. Epilepsy Res. 2004;58(2–3):167–174. doi: 10.1016/j.eplepsyres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Romoli M., Mazzocchetti P., D'Alonzo R., Siliquini S., Rinaldi V.E., Verrotti A., Calabresi P., Costa C. Valproic acid and epilepsy: from molecular mechanisms to clinical evidences. Curr. Neuropharmacol. 2019;17(10):926–946. doi: 10.2174/1570159X17666181227165722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertram E. The relevance of kindling for human epilepsy. Epilepsia. 2007;48(Suppl 2):65–74. doi: 10.1111/j.1528-1167.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- 35.Beydoun A., Sackellares J.C., Shu V. Safety and efficacy of divalproex sodium monotherapy in partial epilepsy: a double-blind, concentration-response design clinical trial. Depakote Monotherapy for Partial Seizures Study Group. Neurology. 1997;48(1):182–188. doi: 10.1212/wnl.48.1.182. [DOI] [PubMed] [Google Scholar]
- 36.Biggs C.S., Pearce B.R., Fowler L.J., Whitton P.S. Regional effects of sodium valproate on extracellular concentrations of 5-hydroxytryptamine, dopamine, and their metabolites in the rat brain: an in vivo microdialysis study. J. Neurochem. 1992;59(5):1702–1708. doi: 10.1111/j.1471-4159.1992.tb11001.x. [DOI] [PubMed] [Google Scholar]
- 37.Riviello J.J., Jr., Ashwal S., Hirtz D., Glauser T., Ballaban-Gil K., Kelley K., Morton L.D., Phillips S., Sloan E., Shinnar S., et al. Practice parameter: diagnostic assessment of the child with status epilepticus (an evidence-based review): report of the quality standards subcommittee of the American academy of neurology and the practice committee of the child neurology society. Neurology. 2006;67(9):1542–1550. doi: 10.1212/01.wnl.0000243197.05519.3d. [DOI] [PubMed] [Google Scholar]
- 38.Leppik I.E. Status epilepticus in the elderly. Epilepsia. 2018;59(Suppl 2):140–143. doi: 10.1111/epi.14497. [DOI] [PubMed] [Google Scholar]
- 39.Leppik I.E., Boucher R., Wilder B.J., Murthy V.S., Rask C.A., Watridge C., Graves N.M., Rangel R.J., Turlapaty P. Phenytoin prodrug: preclinical and clinical studies. Epilepsia. 1989;30(Suppl 2):S22–S26. doi: 10.1111/j.1528-1157.1989.tb05821.x. [DOI] [PubMed] [Google Scholar]
- 40.Brigo F., Del Giovane C., Nardone R., Trinka E., Lattanzi S. Intravenous antiepileptic drugs in adults with benzodiazepine-resistant convulsive status epilepticus: a systematic review and network meta-analysis. Epilepsy Behav. 2019;101(Pt B) doi: 10.1016/j.yebeh.2019.106466. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez Fernandez I., Gainza-Lein M., Lamb N., Loddenkemper T. Meta-analysis and cost-effectiveness of second-line antiepileptic drugs for status epilepticus. Neurology. 2019;92(20):e2339–e2348. doi: 10.1212/WNL.0000000000007503. [DOI] [PubMed] [Google Scholar]
- 42.Brigo F., Bragazzi N., Nardone R., Trinka E. Direct and indirect comparison meta-analysis of levetiracetam versus phenytoin or valproate for convulsive status epilepticus. Epilepsy Behav. 2016;64(Pt A):110–115. doi: 10.1016/j.yebeh.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 43.Singh A., Stredny C.M., Loddenkemper T. Pharmacotherapy for pediatric convulsive status epilepticus. CNS Drugs. 2020;34(1):47–63. doi: 10.1007/s40263-019-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosati A., Ilvento L., Lucenteforte E., Pugi A., Crescioli G., McGreevy K.S., Virgili G., Mugelli A., De Masi S., Guerrini R. Comparative efficacy of antiepileptic drugs in children and adolescents: a network meta-analysis. Epilepsia. 2018;59(2):297–314. doi: 10.1111/epi.13981. [DOI] [PubMed] [Google Scholar]
- 45.Trinka E., Hofler J., Leitinger M., Rohracher A., Kalss G., Brigo F. Pharmacologic treatment of status epilepticus. Expet Opin. Pharmacother. 2016;17(4):513–534. doi: 10.1517/14656566.2016.1127354. [DOI] [PubMed] [Google Scholar]
- 46.Brigo F., Lattanzi S., Nardone R., Trinka E. Intravenous brivaracetam in the treatment of status epilepticus: a systematic review. CNS Drugs. 2019;33(8):771–781. doi: 10.1007/s40263-019-00652-0. [DOI] [PubMed] [Google Scholar]
- 47.Brigo F., Lattanzi S., Rohracher A., Russo E., Meletti S., Grillo E., Trinka E. Perampanel in the treatment of status epilepticus: a systematic review of the literature. Epilepsy Behav. 2018;86:179–186. doi: 10.1016/j.yebeh.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Lattanzi S., Riva A., Striano P. Ganaxolone treatment for epilepsy patients: from pharmacology to place in therapy. Expert Rev. Neurother. 2021;21(11):1317–1332. doi: 10.1080/14737175.2021.1904895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.