Abstract
Introduction
When lenvatinib is administered to people with hepatocellular carcinoma (HCC), tumor blood flow is reduced due to the inhibition of the vascular endothelial growth factor receptor (VEGFR) and fibroblast growth factor receptor (FGFR). Few studies have examined the decrease in tumor blood flow with respect to changes in tumor blood vessels (TBVs) in clinical practice. We investigated the mechanism of tumor blood flow control by investigating changes in the diameter of relatively large TBVs in large-sized lesions with high blood flow.
Methods
From January 2011 to October 2021, patients receiving lenvatinib for unresectable intrahepatic HCC at Toranomon Hospital, Tokyo, Japan, were considered for inclusion. We investigated the TBV diameter in the arterial phase of dynamic computed tomography before treatment and its change over time (2–12 weeks after lenvatinib initiation). The relationship between changes in TBV diameter and prognosis was also examined.
Results
Of 114 patients treated with lenvatinib for HCC, 26 patients who had intrahepatic lesions with a tumor diameter of 30 mm or more enrolled in the study. The median tumor and TBV diameters before treatment were 58 mm and 2.55 mm, respectively. Twenty-five patients (96%) had a shrinkage in TBV diameter 2–12 weeks after lenvatinib administration. The maximum TBV diameter shrinkage of 20% or more was observed in 19 patients (73%), and progression-free survival was prolonged in these patients compared to the group with less than 20% TBV diameter shrinkage (p = 0.039).
Discussion/Conclusion
Due to the antiangiogenic effect of lenvatinib, a shrinkage in the TBV diameter of HCC was observed. The shrinkage of TBV may be regarded as a process of normalization of TBVs. The shrinkage of TBVs in imaging analysis may be associated with improved prognosis; however, additional studies are still required.
Keywords: Hepatocellular carcinoma, Lenvatinib, Tumor blood vessels, Normalization of tumor blood vessels
Introduction
Hepatocellular carcinoma (HCC) is the most common type of liver cancer and the third leading cause of cancer [1]. Lenvatinib, a multi-tyrosine kinase inhibitor that targets vascular endothelial growth factor receptors (VEGFR) 1–3, fibroblast growth factor receptors (FGFR) 1–3, rearranged during transfection (RET), mast/stem cell growth factor receptor kit (SCFR), and platelet-derived growth factor receptor (PDGFR) beta [2, 3], is available for the treatment of unresectable HCC. In vivo studies have shown that lenvatinib strongly inhibits VEGFR and FGFR, resulting in a marked normalization of tumor blood vessels (TBVs) [4].
Tumor cells require oxygen and nutrients provided by blood flow to survive and grow. For efficient transport of oxygen and nutrients, cells must be within the oxygen diffusion limit of 100 μm from nearby blood vessels. In addition to metabolic stress such as hypoxia, angiogenesis in tumors is promoted through inflammation, mechanical stress, and gene mutation. Angiogenesis is governed by a balance between pro-angiogenic and antiangiogenic factors. Among the angiogenic molecules, vascular endothelial growth factor (VEGF) promotes the survival and proliferation of vascular endothelial cells, increases the display of adhesion molecules on these cells, and increases vascular permeability [5]. Therefore, new blood vessels need to be mobilized by angiogenesis for a tumor to grow to a certain size [6].
TBVs are structurally and functionally abnormal. VEGF is overexpressed in tumors, causing an active state of angiogenesis. Cancer treatments aimed at inhibiting VEGF have been developed through in vivo research. However, it is difficult to evaluate the effect on the human intratumoral environment in vivo over time. We speculated that the angiogenesis-inhibiting effect of lenvatinib could be evaluated by focusing on the TBV diameter, if the HCC with high blood flow was large enough to evaluate TBVs.
Few studies have investigated the decrease in tumor blood flow with respect to changes in TBVs in clinical practice. By capturing changes in HCC TBV diameter, we evaluated how the intratumoral environment affects the course of treatment. We investigated whether the angiogenesis-inhibiting effect of lenvatinib is clinically effective by measuring changes in the diameter of TBVs that are large enough to be evaluated by dynamic computed tomography (dynamic CT).
Materials and Methods
Study Population and Inclusion Criteria
From January 2011 to October 2021, 114 patients receiving systemic anticancer treatment with lenvatinib for unresectable HCC at the Department of Hepatology, Toranomon Hospital, Tokyo, Japan, were considered for inclusion. The following inclusion criteria were used: (1) triple-phase dynamic CT was performed within 1 month prior to initiation of lenvatinib, (2) the tumor in the arterial phase on dynamic CT had a tumor diameter of at least 30 mm, (3) the TBVs inside the tumor were visible and measurable before and after treatment, (4) triple-phase dynamic CT was performed to evaluate the treatment response 2–12 weeks after the initiation of lenvatinib, (5) Child-Pugh class A liver function at the time of lenvatinib initiation, (6) Barcelona Clinic Liver Cancer (BCLC) stage A to C tumor(s), (7) unresectable HCC with the patient not wanting to undergo local ablation or chemoembolization therapy for various reasons (i.e., tumor size, number and location, extrahepatic metastasis, transarterial chemoembolization [TACE] refractoriness, and other complications), (8) no previous treatment with lenvatinib, (9) a treatment interval of ≥28 days since any previous therapy with a tyrosine kinase inhibitor (TKI; sorafenib or regorafenib), and (10) an observation period of ≥4 weeks. In total, 26 patients met the 10 inclusion criteria.
All procedures in the study were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and complied with the 1975 Declaration of Helsinki. The study was approved by the Institutional Review Board of Toranomon Hospital, Tokyo, Japan (protocol number; 1438-H/B).
HCC Diagnosis
The diagnosis of HCC was based predominantly on the imaging findings using dynamic CT. All patients underwent unenhanced and four-phase dynamic CT using a 64-row multidetector CT scanner (Aquilion 64; Canon Medical Systems, Tochigi, Japan) or an 80-row multidetector CT scanner (Aquilion ONE; Canon Medical Systems) according to a protocol reported elsewhere [7]. A liver nodule was diagnosed as HCC when a dynamic study showed hyperattenuation in the arterial phase and washout in the portal or delayed phase.
Imaging Findings for HCC and Definitions of TBVs
The tubular structure inside the tumor that was visible and measurable in the arterial phase of dynamic CT with slice thicknesses of 5 mm before treatment was defined as the TBV, and the maximum diameter of the TBV was defined as the TBV diameter in the tumor. When multiple TBV diameters were confirmed in multiple tumors, the largest TBV diameter was defined as the patient's TBV diameter. It was necessary that the TBV could be measured after treatment in the same slice in the arterial phase of dynamic CT with slice thicknesses of 5 mm. The shrinkage rate of the TBV diameter was calculated by comparing the TBV diameter over time with the pretreatment TBV diameter. The maximum value of the TBV shrinkage rate was set as the maximum TBV diameter shrinkage rate observed 2–12 weeks after lenvatinib administration. In the cases where the changes in TBVs were followed, the blood vessel diameter of the right hepatic artery was measured at the same time to evaluate normal vascular structure changes.
The TBVs were assessed independently by two expert hepatologists (Y. Kawamura and N. Muraishi) who were blinded to the clinical data. Discrepancies between any two of these examiners were resolved by a consensus review that included input from an additional reviewer (K. Ikeda). The TBV diameter of each tumor was measured at a workstation (SYNAPS; Fuji Film Medical, Tokyo, Japan).
Lenvatinib Treatment and Assessment of Adverse Events
Lenvatinib (Lenvima®; Eisai, Tokyo, Japan) was administered orally to the majority of patients at either 8 mg/day for patients <60 kg or 12 mg/day for patients ≥60 kg. Treatment was discontinued when any unacceptable or serious adverse event (AE) or significant clinical progression of the tumor was observed. According to the guidelines for the administration of lenvatinib, the dose should be reduced or the treatment interrupted when a patient develops grade ≥3 severe AEs or any unacceptable grade 2 drug-related AE. AEs were assessed using the National Cancer Institute's Common Terminology Criteria for Adverse Events version 4.0 [8]. In accordance with the manufacturer's guidelines, if a drug-related AE occurred, dose reduction or temporary interruption was maintained until the symptom resolved to grade 1 or 2.
Evaluation of Treatment Response
Treatment response was evaluated in accordance with the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [9]. We assessed the best tumor response over 2–12 weeks. In this study, mRECIST was predominantly used as a progressive disease criterion to indicate progression-free survival (PFS), and PFS using mRECIST was adopted to investigate the relationships between changes in tumor vessel diameter and prognosis.
Tumor assessments were generally performed every 4–8 weeks using dynamic CT. The treatment response was assessed independently by an expert hepatologist (Y. Kawamura) and an expert hepatobiliary surgeon (J. Shindoh) who were blinded to the clinical data. Discrepancies between these two examiners were resolved by a consensus review including an additional reviewer (K. Ikeda).
Definition of TACE Failure/Refractoriness
TACE failure was defined as an insufficient response after at least two consecutive TACE procedures, based on the response evaluation CT or magnetic resonance imaging performed after 1–3 months, even after the chemotherapeutic agent was changed and/or the feeding artery was reanalyzed. In addition, the identification of a greater number of lesions in the liver than that recorded at the previous TACE procedure (other than the nodule being treated) was included in the classification of TACE failure/refractoriness [10].
Assessment of Hepatic Functional Reserve
The Child-Pugh classification [11] and ALBI grade [12] were used to assess the hepatic functional reserve. The modified albumin-bilirubin grade was based on the ALBI score, calculated from the serum albumin and total bilirubin concentrations using the following formula: [ALBI score = (log10 bilirubin [µmol/L] × 0.66) + (albumin [g/L] × −0.085)], and defined by the following cutoff values: ≤ −2.60 = grade 1; −2.60 to −2.27 = grade 2a; −2.27 to −1.39 = grade 2b; and > −1.39 = grade 3 [13].
Follow-Up Protocol
Physicians examined the patients every 1–2 weeks after the initiation of lenvatinib, and biochemical laboratory and urine tests were also performed. After the initiation of lenvatinib, the patients underwent dynamic CT to evaluate their early treatment response during the 2–12-week period. Dynamic CT or magnetic resonance imaging was performed every 1–3 months after the first evaluation of the best treatment response.
Statistical Analyses
Statistical analyses were performed using the IBM SPSS software program (version 27.0; SPSS Inc., IL, USA). Differences in background features between each parameter were analyzed by Mann-Whitney U test and Friedman test. The PFS and overall survival (OS) after the introduction of lenvatinib were estimated by the Kaplan-Meier method, with the values compared using the log-rank test. p values <0.05 were considered to indicate statistical significance.
Results
Study Population Overview
Table 1 summarizes the clinical profile and laboratory data of the 26 HCC patients with follow-up evaluation of the TBV. The male:female ratio was 2.71:1. Hepatitis C virus antibody was detected in 38.5% of patients. Overall, 24 patients (92%) received an initial dose of lenvatinib according to body weight, while 2 patients (8%) received a reduced starting dose. In addition, 2 patients (8%) received an elevated starting dose of lenvatinib according to their body weight, as they were enrolled in a Global Phase II study with fixed dosing (12 mg). Regarding the liver function, 14 (54%) patients had a Child-Pugh score of 5, and 3 patients (12%) had a modified albumin-bilirubin grade of 1. Based on the pretreatment image analysis, the median tumor diameter was 58.0 mm, with 17 of 26 patients (65%) presenting with Barcelona Clinic Liver Cancer stage C disease. Thirteen patients (50%) had macrovascular invasion and 6 (23%) had extrahepatic metastasis. In addition, 14 patients (54%) had TACE failure/refractoriness. The median levels of alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP) were 123.4 μg/L and 787.0 AU/L, respectively. The median tumor diameter was 58 mm, and the median TBV diameter before treatment was 2.55 mm.
Table 1.
Clinical profiles and laboratory data of patients with follow-up evaluation of the TBVs
| Patient characteristics and laboratory dataa,b | ||
| Patients, n | 26 | |
| Sex (male:female) | 19:7 | |
| Age, years | 73 (48–85) | |
| Body weight (<60 kg:≥60 kg) | 13:13 | |
| HCV:HBV:NonBNonC | 10:2:14 | |
| Platelet count, ×104/mL | 17.6 (7.9–37.1) | |
| Albumin, g/dL | 3.7 (3.0–4.5) | |
| Total bilirubin, mg/dL | 0.7 (0.3–2.9) | |
| Prothrombin activity, % | 83.1 (44.9–124.8) | |
| AST, IU/L | 40 (16–351) | |
| AFP, µg/L | 123.4 (1.7–55,372.0) | |
| DCP, AU/L | 787.0 (14.0–96,035.0) | |
| Child-Pugh score 5:6 | 14:12 | |
| mALBI score (1:2a:2b:3) | 3:15:8:0 | |
| Initial dose of lenvatinib, 4 mg:8 mg:12 mg | 1:11:14 | |
| Reduced starting dose of lenvatinib | 2 | |
| History of TKI treatment, n (%) | 3 (12) | |
|
| ||
| Tumor characteristics | ||
| Largest tumor diameter, mm | 58 (30–127) | |
| TBV diameter before lenvatinib treatment, mm | 2.55 (1.67–4.83) | |
| Macrovascular invasion, n (%) | 13 (50) | |
| Extrahepatic metastasis, n (%) | 6 (23) | |
| BCLC stage B:C | 9:17 | |
| TACE failure/refractoriness, n (%) | 14 (54) | |
AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; AST, aspartate aminotransferase; DCP, des-γ-carboxy prothrombin; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IU, international units; mALBI, modified albumin-bilirubin; NonB, NonC, neither HBV nor HCV infection present; TACE, transarterial chemoembolization; TBV, tumor blood vessel.
Data expressed as median (range).
The median duration of lenvatinib administration was 3.7 months, and the median observation period was 11.5 months. The median 8-week relative dose intensity (RDI), defined as the actual dose divided by the standard dose (8 mg/day or 12 mg/day depending on body weight), was 75.9%.
Shrinkage Rate of TBV Diameter after the Initiation of Lenvatinib
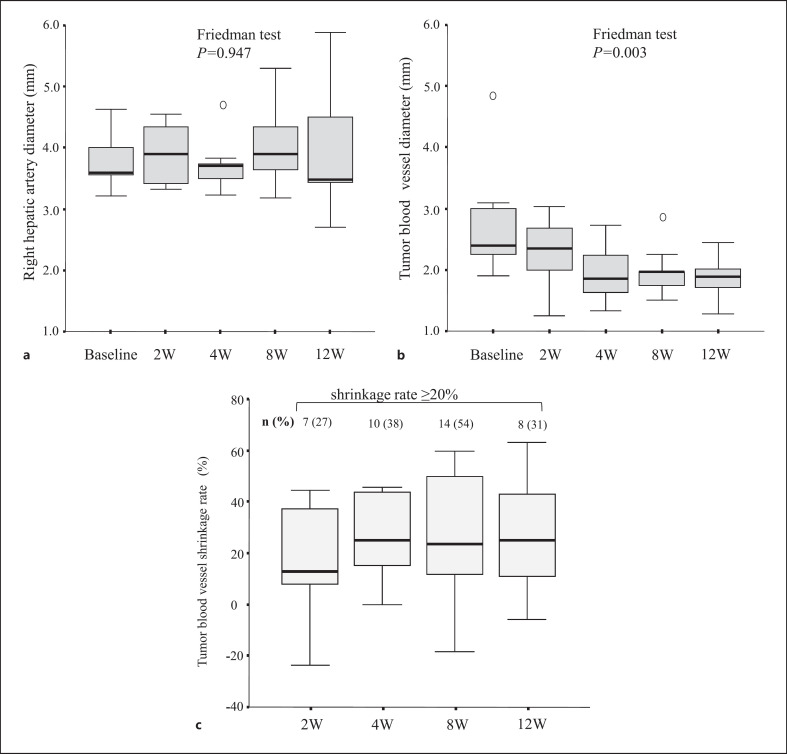
We compared the TBV diameter of the target tumors after the initiation of lenvatinib. Figure 1a and b show the changes in right hepatic artery diameter and TBV diameter before and after treatment. The diameter of the right hepatic artery as a normal vascular structure did not change significantly (p = 0.947), but the diameter of the tumor vessel decreased significantly (p = 0.003).
Fig. 1.
Box plots of the changes in blood vessel diameter before and after lenvatinib treatment. a The changes in the right hepatic artery diameter. b The decrease in the TBV diameter. c The shrinkage rate of TBV diameter over time. The number and percentage of participants with a shrinkage rate of 20% or greater at each time point are shown at the top of the plot.
Figure 1c shows the shrinkage rate of TBV diameter over time. The median maximum shrinkage rate of TBV diameter was 24.7%. The maximum shrinkage rate of TBV diameter was high during weeks 4–8. The maximum shrinkage rate of TBV diameter of 20% or more was observed in 19 patients (73%). Table 2 summarizes the clinical profiles and laboratory data for cases divided into groups based on the maximum shrinkage rate of TBV diameter. There were no differences in patient or tumor backgrounds between cases with a maximum shrinkage rate of more than 20% and those with a maximum shrinkage rate of less than 20%.
Table 2.
Clinical profile and laboratory data for cases divided into groups based on the maximum shrinkage rate of the tumor blood vessel (TBV) diameter
| Tumor characteristicsa, b | Maximum shrinkage rate of TBV diameter ≥20% (n = 19) | Maximum shrinkage rate of TBV diameter <20% (n = 7) | p value |
|---|---|---|---|
| Largest tumor diameter, mm | 53.8 (30–127) | 62.5 (37.6–90) | 0.563 |
| TBV diameter before lenvatinib treatment, mm | 2.95 (1.85–4.83) | 2.23 (1.67–2.55) | 0.775 |
| Macrovascular invasion, n (%) | 9 (47) | 4 (57) | 0.500 |
| Extrahepatic metastasis, n (%) | 5 (26) | 1 (14) | 1.000 |
| BCLC stage B:C | 6:13 | 3:4 | 0.661 |
| TACE failure/refractoriness, n (%) | 10 (53) | 4 (57) | 1.000 |
BCLC, Barcelona Clinic Liver Cancer; TACE, transarterial chemoembolization; TBV, tumor blood vessel. Data expressed as median (range).
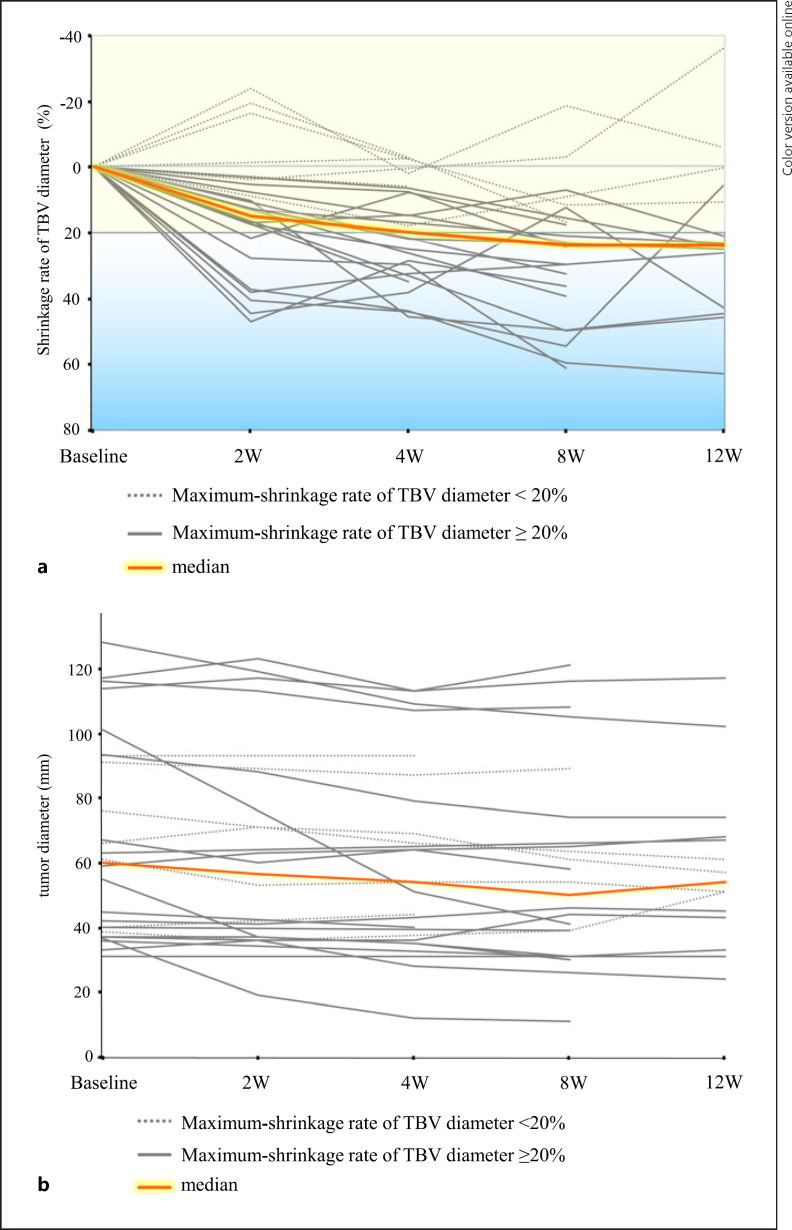
Figure 2a shows the time course of the shrinkage rate of TBV diameter. Twenty-five patients (96%) had a reduction in TBV diameter 2–12 weeks after lenvatinib administration. Figure 2b shows changes in tumor size over time. Tumor diameter did not change appreciably during the 12 weeks after lenvatinib administration compared with the changes in TBV diameter.
Fig. 2.
a Time course of the TBV diameter shrinkage rate after lenvatinib administration. Dashed gray lines indicate cases in which the maximum shrinkage rate of the TBV diameter was less than 20%. Solid gray lines indicate cases in which the maximum shrinkage rate of the TBV diameter was greater than or equal to 20%. b Changes in tumor diameter up to 12 weeks after lenvatinib administration. Dashed gray lines indicate cases in which the maximum shrinkage rate of the TBV diameter was less than 20%. Solid gray lines indicate cases in which the maximum shrinkage rate of the TBV diameter was greater than or equal to 20%.
Relationship between Maximum Shrinkage Rate of TBV Diameter after Lenvatinib Treatment and Patient Prognosis
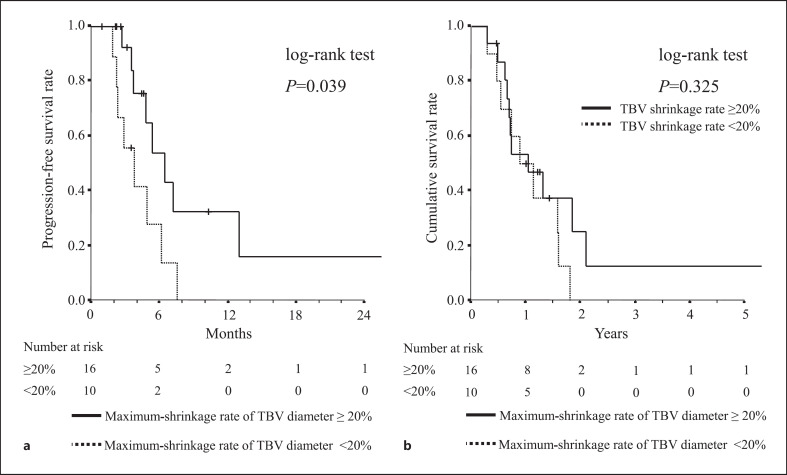
Figure 3 shows patient survival stratified according to the maximum shrinkage rate of TBV diameter after the initiation of lenvatinib. Survival is plotted with respect to the maximum shrinkage rate of TBV diameter after the initiation of lenvatinib treatment. The median PFS in the group with a maximum shrinkage rate of TBV diameter of 20% or more was 6.2 months but only 2.9 months in the group with a maximum shrinkage rate of less than 20% (Fig. 3a), showing a significant difference between the two groups (p = 0.009). There was no significant difference between the two groups in cumulative OS (p = 0.439) (Fig. 3b).
Fig. 3.
Survival among patients stratified by the maximum shrinkage rate of the TBV diameter after lenvatinib initiation. The solid black line indicates the ≥20% maximum shrinkage rate group and the dashed line indicates the <20% maximum shrinkage rate group. a Progression-free survival (PFS) in both groups. b Cumulative overall survival (OS) in both groups.
Relationship between Maximum Shrinkage Rate of TBV Diameter and 8-Week RDI
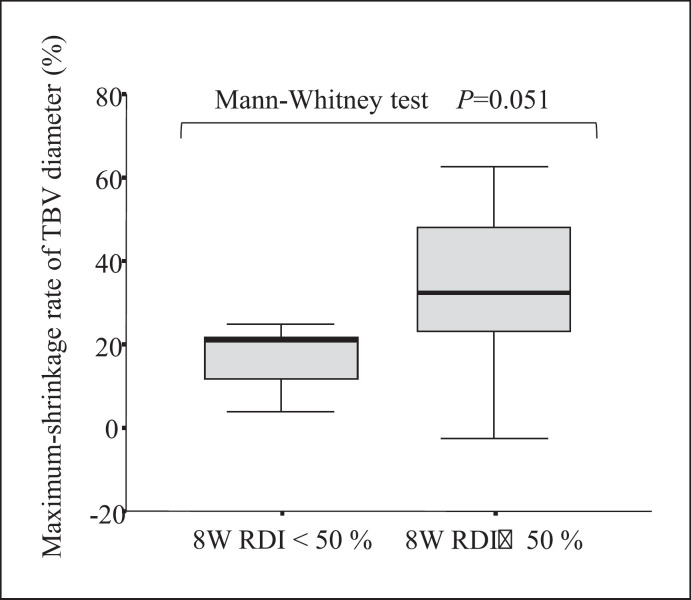
The relationship between the 8-week RDI and the maximum shrinkage rate of TBV diameter was evaluated because the 2- or 4-week RDI remained high. Figure 4 shows that the group with the 8-week RDI of 50% or more had a higher maximum shrinkage of TBV diameter compared to the group with the 8-week RDI of less than 50% (p = 0.051).
Fig. 4.
Box plot showing the distribution of the maximum shrinkage rate of TBVs with the 50% RDI at 8 weeks as the boundary.
Discussion/Conclusion
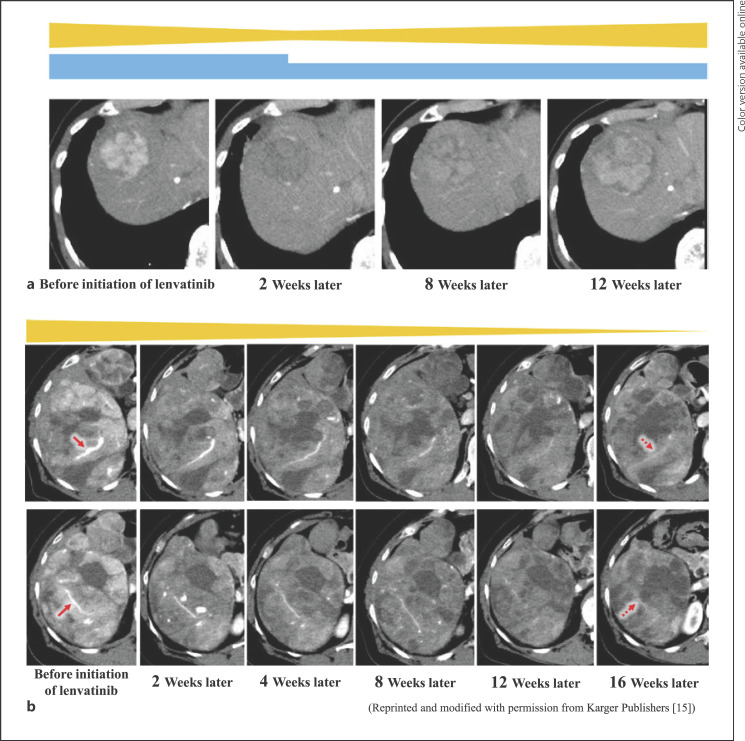
There appear to be two clinical types of changes in TBVs after lenvatinib treatment. It was expected that there would be a functional change in which blood flow returned rapidly to the TBVs when a dose reduction or withdrawal of lenvatinib was performed and a structural change in which the blood flow remained low even after a dose reduction or withdrawal of lenvatinib. Case images were presented for the temporal changes in tumor blood flow in each pattern in which TBVs undergo functional and structural changes (Fig. 5a, b). Figure 5a shows the case of a 77-year-old woman with a nonviral liver background. In her case, the functional change of the TBV caused the resumption of blood flow by dose reduction after the intratumoral blood flow decreased with lenvatinib therapy. The initial dose of lenvatinib was 8 mg. There was a decrease in tumor blood flow 2 weeks later, but 8 weeks later, due to renal dysfunction, the dose reduction of lenvatinib was 4 mg 2 days on/1 day off, resulting in resumption of tumor blood flow. Figure 5b shows the case of a 65-year-old woman with a nonviral liver background. In her case, the structural change of the TBV for bulky HCC caused a remarkable shrinkage during lenvatinib treatment [14]. The macroscopic TBV diameter shrank from 8 to 12 weeks.
Fig. 5.
Dynamic CT arterial phase images of the functional and structural changes of the TBV caused by the resumption of blood flow after dose reduction during lenvatinib treatment. a Case of a 77-year-old woman with HCC. The initial dose of lenvatinib was 8 mg. The blue bar indicates the dose of lenvatinib, and the yellow bar indicates the image-predicted tumor blood flow. Functional changes in the TBV caused the resumption of blood flow by dose reduction during lenvatinib treatment. Dynamic CT arterial phase images. b Case of a 65-year-old woman with HCC. The macroscopic TBV diameter shrank from 8 to 12 weeks. The yellow bar indicates the image-predicted tumor blood flow. Structural changes in the TBV for bulky HCC caused a marked shrinkage during lenvatinib treatment. Dynamic CT arterial phase images. Reprinted and modified with permission from Karger Publishers [14].
In a recent in vivo study, there were interesting results that captured changes caused by lenvatinib in the intratumoral vascular structure using CT scans and immune tissue staining. Une et al. reported a decrease in tumor vessel density 2 weeks after administering lenvatinib to mice. In addition, they evaluated blood vessels in the tumor by double immunostaining with anti-cluster of differentiation 31 (CD31) and anti-α-smooth muscle actin (α-SMA) on day 4 of lenvatinib administration. By observing the ratio of pericyte coverage in TBVs in mice, changes in tumor vascular structure were observed microscopically beginning only 4 days after administering lenvatinib. CT scans showed no significant decrease in the volume density of TBVs at this point. In addition, the authors reported a significant decrease in the volume density of TBVs on day 14 of lenvatinib administration [4].
Therefore, we propose that the shrinkage of the TBV diameter that we confirmed in patients over a short period of time may be a process of normalizing the abnormally dilated TBVs. We speculate that the macroscopic change in TBV diameter resulting from the progress of normalization of TBVs may be applicable to the pattern of structural changes in the clinical TBVs. On the other hand, microscopic changes in tumor vascular structure may correspond to functional changes in the clinical pattern of tumor vasculature. The normalization of TBVs that occurs from an early stage is considered to be a unique feature of lenvatinib treatment. In addition, since the diameter of the right hepatic artery, as a normal vascular structure, did not change significantly, we conclude that lenvatinib did not affect the existing vascular structure and caused a vasodilatory effect only in the sense of the normalization of TBVs.
Another interesting result is that PFS improved in cases where the TBV diameter decreased significantly. There is a possibility that the prognosis improved due to complicated reasons including the anti-epidermal growth factor receptor (EGFR) action of lenvatinib. Nevertheless, some researchers have suggested that the normalization of TBVs promoted by lenvatinib may also contribute to improving the short-term prognosis as a manifestation of antitumor effects. However, it was also found that RDI was involved in this process. The RDI should be considered as a therapeutic prognostic factor for lenvatinib [15]. In this study, the initial RDI was kept high in many cases, but the maximum shrinkage rate of the TBV diameter was significantly lower in the cases where the 8-week RDI was less than 50%. In cases where the dose of lenvatinib was reduced, it is possible that the VEGFR inhibitory effect was not sufficient to cause the normalization of TBVs. Although not fully shown, the RDI may affect the prognosis because it is involved in the inhibition of angiogenesis and the normalization of TBVs. By contrast, no significant relationship was found between the shrinkage of TBV diameter and the effect of improving OS. We speculated that these results may be related to the effects of subsequent treatment.
Several limitations associated with the present study warrant mention. First, it was a retrospective, single-center, cohort study that evaluated a small number of patients. Second, the follow-up period of the trial was shorter than that of the global Phase III REFLECT trial [16] (median follow-up period of 11.5 months in our study vs. 27.7 months in REFLECT). It was therefore not possible to perform a high-quality prognostic analysis. Third, the diagnosis of HCC was based essentially on the image analysis findings. In addition, evaluation of VEGF expression in plasma and VEGFR expression in tumors was not performed. A large-scale study is required for the detailed evaluation of changes in TBV diameter by lenvatinib. It may also be necessary to evaluate and confirm changes in the intratumoral environment pathologically or biochemically. Fourth, it is also necessary to consider the limitations of CT measurements. Since it is difficult to determine tumor necrosis by CT, the exclusion criteria in this study excluded highly effective cases of lenvatinib treatment in which the TBVs disappeared extensively with tumor necrosis, beyond the normalization of the TBVs [5]. In addition, very small TBVs may be overestimated by CT with blurred margins. Obviously, small TBVs that could not be evaluated as blood vessels by CT were not included in the study. In another study using cone-beam CT, factors that could make feeder measurement of HCC difficult were blood vessel diameters of less than 1 mm and tumor diameters of less than 15 mm [17]. In our study, blood vessels smaller than 1 mm were not tracked, and TBVs that were judged to be difficult to follow were excluded so that the results would not be distorted. Therefore, multicenter studies should be conducted to address the bias and unreliability that can occur when measuring TBVs. In conclusion, due to the antiangiogenic effect of lenvatinib, a reduction in the tumor vessel diameter of people being treated with lenvatinib for HCC was observed. The TBV shrinkage may occur in the process of normalization of TBVs.
Statement of Ethics
This retrospective, noninterventional study was approved by the Institutional Review Board, Toranomon Hospital (protocol number; 1438-H/B). The study was performed in accordance with the Declaration of Helsinki. Because the data were anonymized and the opt-out option was disclosed on our institution's homepage (https://toranomon.kkr.or.jp/crc/files/uploads/2020/06/rinken_1438HB-3.pdf), the requirement for additional informed consent to participate in this study was deemed unnecessary according to the Japanese national regulations “Ethical Guidelines for Medical and Health Research Involving Human Subjects” (https://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdf).
Conflict of Interest Statement
Yusuke Kawamura, MD, PhD, and Junichi Shindoh, MD, PhD, report honoraria from Eisai Co., Ltd., and Chugai Pharmaceutical Co., Ltd. Hiromitsu Kumada, MD, PhD, reports honoraria from Eisai Co., Ltd. The other authors declare no conflicts of interest.
Funding Sources
This work was supported, in part, by grants from the Ministry of Health, Labor and Welfare in Japan, the Japan Agency for Medical Research and Development, and the Okinaka Memorial Institute for Medical Research.
Author Contributions
Nozomu Muraishi, MD, and Yusuke Kawamura, MD, PhD: study concept and design, acquisition of data, statistical analysis, and drafting of manuscript. Norio Akuta, MD, PhD; Junichi Shindoh, MD, PhD; Masaru Matsumura, MD, PhD; Satoshi Okubo, MD, PhD; Shunichiro Fujiyama, MD; Tetsuya Hosaka, MD; Satoshi Saitoh, MD; Hitomi Sezaki, MD; Fumitaka Suzuki, MD, PhD; Yoshiyuki Suzuki, MD, PhD; Yasuji Arase, MD, PhD; Masaji Hashimoto, MD, PhD; and Hiromitsu Kumada, MD, PhD: acquisition of data. Kenji Ikeda, MD, PhD: acquisition of data, statistical analysis, and study supervision. Ichiro Yasuda, MD, PhD: study supervision.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Funding Statement
This work was supported, in part, by grants from the Ministry of Health, Labor and Welfare in Japan, the Japan Agency for Medical Research and Development, and the Okinaka Memorial Institute for Medical Research.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015 Mar 1;136((5)):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Okamoto K, Kodama K, Takase K, Sugi NH, Yamamoto Y, Iwata M, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013 Oct 28;340((1)):97–103. doi: 10.1016/j.canlet.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and child-pugh A liver function a proof-of-concept study. Cancers. 2019 Jul 31;11((8)):1084. doi: 10.3390/cancers11081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Une N, Takano-Kasuya M, Kitamura N, Ohta M, Inose T, Kato C, et al. The anti-angiogenic agent lenvatinib induces tumor vessel normalization and enhances radiosensitivity in hepatocellular tumors. Med Oncol. 2021;38((6)):60. doi: 10.1007/s12032-021-01503-z. [DOI] [PubMed] [Google Scholar]
- 5.Jain RK. Normalization of tumor vasculature an emerging concept in antiangiogenic therapy. Science. 2005 Jan 7;307((5706)):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407((6801)):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 7.Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Kasuya K, Sano T, et al. Pretreatment heterogeneous enhancement pattern of hepatocellular carcinoma may be a useful new predictor of early response to lenvatinib and overall prognosis. Liver Cancer. 2020;9((3)):275–292. doi: 10.1159/000505190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Cancer Institute Division of Cancer Treatment and Diagnosis. Cancer therapy evaluation program. Adverse events/CTCAE [accessed 2018 Dec 23]. Available from https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40.
- 9.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010 Feb;30((1)):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 10.Kudo M, Matsui O, Izumi N, Kadoya M, Okusaka T, Miyayama S, et al. Transarterial chemoembolization failure/refractoriness JSH-LCSGJ criteria 2014 update. Oncology. 2014;87((Suppl 1)):22–31. doi: 10.1159/000368142. [DOI] [PubMed] [Google Scholar]
- 11.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60((8)):646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 12.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015 Feb 20;33((6)):550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiraoka A, Michitaka K, Kumada T, Izumi N, Kadoya M, Kokudo N, et al. Validation and potential of albumin-bilirubin grade and prognostication in a nationwide survey of 46681 hepatocellular carcinoma patients in Japan the need for a more detailed evaluation of hepatic function. Liver Cancer. 2017 Nov;6((4)):325–336. doi: 10.1159/000479984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Okubo S, Tominaga L, et al. Lenvatinib-transarterial chemoembolization sequential therapy as an effective treatment at progression during lenvatinib therapy for advanced hepatocellular carcinoma. Liver Cancer. 2020 Dec;9((6)):756–770. doi: 10.1159/000510299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirino S, Tsuchiya K, Kurosaki M, Kaneko S, Inada K, Yamashita K, et al. Relative dose intensity over the first four weeks of lenvatinib therapy is a factor of favorable response and overall survival in patients with unresectable hepatocellular carcinoma. PLoS One. 2020;15((4)):e0231828. doi: 10.1371/journal.pone.0231828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudo M, Finn RS, Qin S, Han K-H, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma a randomised phase 3 non-inferiority trial. Lancet. 2018;391((10126)):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 17.Iwazawa J, Ohue S, Hashimoto N, Muramoto O, Mitani T. Clinical utility and limitations of tumor-feeder detection software for liver cancer embolization. Eur J Radiol. 2013 Oct;82((10)):1665–1671. doi: 10.1016/j.ejrad.2013.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.