This cohort study evaluates the long-term outcomes after rituximab treatment for systemic sclerosis and identifies potential response markers.
Key Points
Question
What is the long-term outcome of rituximab treatment for systemic sclerosis, and what are the potential markers of response?
Findings
In this cohort study of 29 patients, rituximab was associated with significantly improved skin sclerosis and lung function at a median follow-up of 96 weeks. Decrease in serum IgA and IgM levels was associated with greater improvement in skin sclerosis and lung function, respectively.
Meaning
The findings of this study suggest that rituximab treatment may provide long-term benefit for patients with systemic sclerosis, and serum immunoglobulins should be explored as potential response markers.
Abstract
Importance
Rituximab is emerging as a promising therapeutic option for systemic sclerosis (SSc), but its long-term outcomes and response markers are unknown.
Objective
To evaluate the long-term outcomes after rituximab treatment for SSc and identify potential response markers.
Design, Setting, and Participants
In this single-center cohort study, patients with SSc who continued to receive rituximab after the DESIRES trial were analyzed with a median follow-up of 96 weeks. Among the 43 patients who completed the DESIRES trial, 31 continued to receive rituximab, of which 29 with complete data were included in this study.
Exposures
Rituximab treatment.
Main Outcomes and Measures
A post hoc analysis of the clinical and laboratory data.
Results
In 29 patients with SSc (27 female [93%]; median [IQR] age, 48 [35-45] years), significant improvement in modified Rodnan skin score (MRSS) and percentage of predicted forced vital capacity (FVC%) were observed after 1 (median [IQR] change in MRSS, −7 [−8.5 to −4]; P < .001) and 3 (median [IQR] change in FVC% predicted, 1.85 [0.13-5.68]; P < .001) courses of rituximab, respectively, both of which were sustained during follow-up. High responders (MRSS improvement of ≥9; n = 16) experienced a greater decrease in serum levels of IgG (median [IQR] change in IgG, −125 [−207 to −83] vs 7 [−120 to 43]; P = .008) and IgA (median [IQR] change in IgA, −45 [−96 to −32] vs −11 [−20 to 3]; P < .001) compared with low responders (MRSS improvement of ≤8; n = 13). In particular, decrease in serum IgA levels significantly correlated with the improvement in MRSS (r = 0.64; P < .001). At the last follow-up, low IgM, low IgA, and low IgG was observed in 7, 1, and 1 patient, respectively, of which low IgM was associated with greater improvement in FVC% predicted (median [IQR] change in FVC% predicted, 7.2 [3.8-8.9] vs 3.6 [1.4-6.2]; P = .003).
Conclusions and Relevance
In this cohort study, rituximab treatment was associated with significantly improved skin and lung fibrosis in SSc in a long-term follow-up. Decrease in serum immunoglobulins was associated with greater clinical response.
Introduction
Systemic sclerosis (SSc) is a progressive fibrotic disorder affecting the skin and internal organs with unknown etiology.1 It has the worst prognosis among connective tissue diseases, with a 10-year mortality rate approximating 30%.2 Despite the high disease burden, there were few effective treatment options for SSc until recently.
Although the pathogenesis of SSc is unclear, growing evidence suggests that B cells play an important role in SSc by producing autoantibodies, secreting distinctive cytokines, and activating other immune cells.3,4,5 Consistent with this, B-cell depletion therapy with rituximab is emerging as a promising treatment for SSc.6,7,8 Rituximab is a chimeric monoclonal antibody that efficiently depletes circulating B cells by targeting the B-cell specific antigen CD20. In addition, data from other autoimmune diseases show varying degrees of decrease in serum immunoglobulin levels following rituximab treatment, which might be associated with clinical response.9,10,11
We previously conducted the double-blind, investigator-initiated, randomized, placebo-controlled DESIRES trial to assess the efficacy of rituximab in SSc.12,13 In this 48-week trial, rituximab was associated with the improvement of skin sclerosis. However, the long-term outcomes of rituximab in SSc is largely unknown. In addition, little information is available on response markers to evaluate and predict the outcomes of rituximab in SSc.
Here, we report a long-term follow-up of 29 patients with SSc who continued to receive rituximab after completion of the DESIRES trial. We also investigate potential markers of response by using clinical and laboratory data during the follow-up.
Methods
Study Design
The study design for the DESIRES trial has been previously reported (NCT04274257).12,13 Briefly, the DESIRES trial consisted of 2 phases: a randomized, double-blind, placebo-controlled period of 24 weeks and a subsequent open-label extension period of 24 weeks. In the double-blind phase, 56 patients were randomized to receive either intravenous rituximab (375 mg/m2) or matching placebo once per week for 4 weeks. Of 49 patients who completed the double-blind phase, 46 entered the open-label extension phase, where all patients received rituximab (375 mg/m2 once per week for 4 weeks). Among the 43 patients who completed the DESIRES trial, 31 continued to receive rituximab (375 mg/m2 once per week for 4 weeks every 6 months), of which 29 had complete data available and were therefore included in this study (Figure 1A). This study was approved by the ethics committee of the University of Tokyo Graduate School of Medicine and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Figure 1. Flow Diagram for the Study and Depletion of Circulating B Cells by Rituximab in Systemic Sclerosis (SSc).
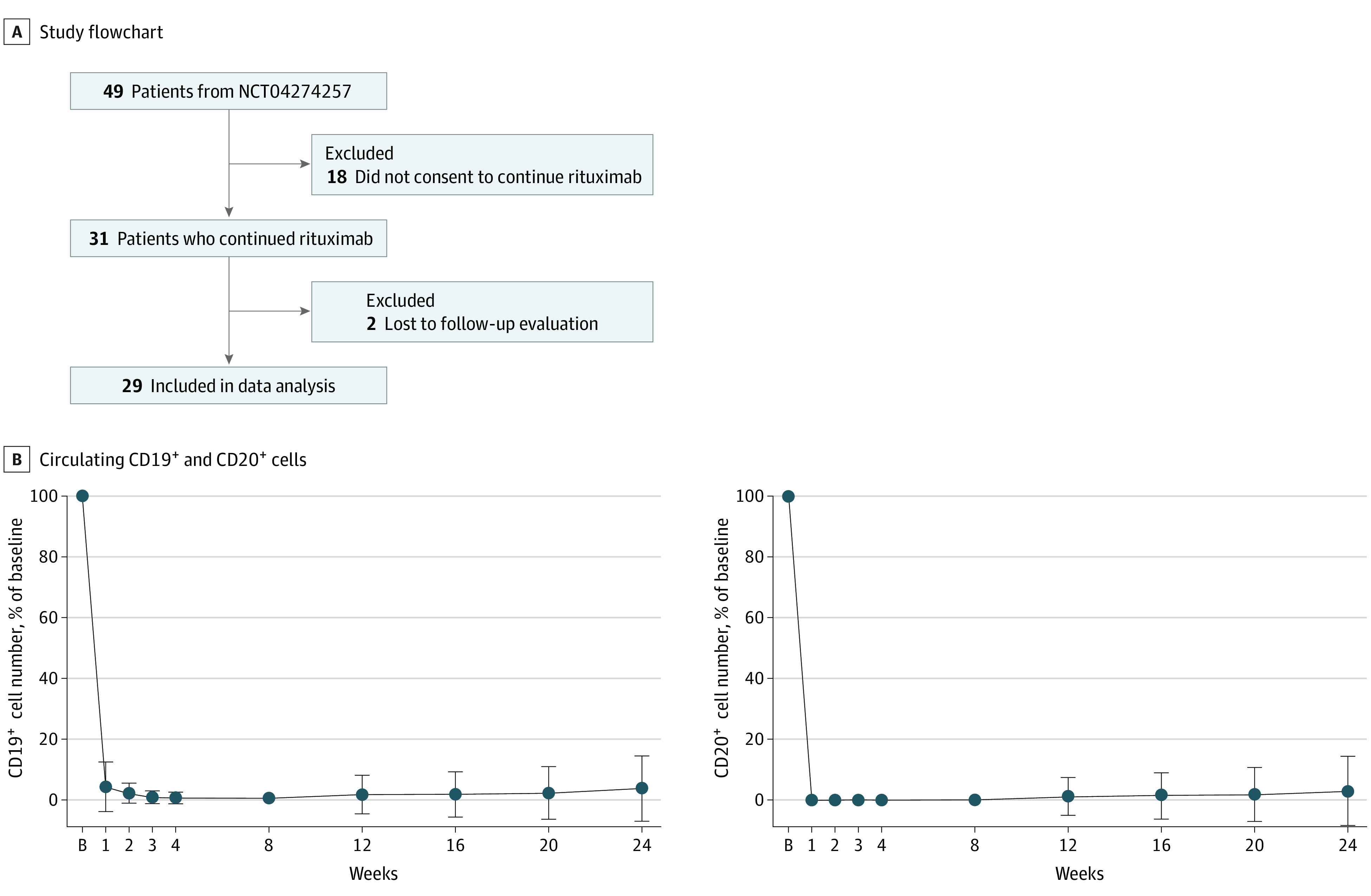
A, A total of 29 patients with SSc who completed the DESIRES trial and continued to receive rituximab with complete data were included in this study. B, Percentages of circulating CD19+ and CD20+ cell number of baseline (B) following the first course of rituximab. Data are presented as mean (SEM). Error bars represent SEM.
Patients
The criteria for the DESIRES trial have been described previously.12,13 Briefly, the inclusion criteria were (1) age of 20 to 79 years; (2) fulfillment of the 2013 American College of Rheumatology and European League Against Rheumatism classification criteria for SSc14; (3) modified Rodnan skin score (MRSS) of 10 or greater15; (4) not received corticosteroids equivalent to more than 10 mg/d of prednisolone within 2 weeks before the start of study treatment; and (5) not received imatinib, immunosuppressants, antifibrotic agents, high-dose intravenous immunoglobulin, or other investigational products within 4 weeks before the start of study treatment. Major exclusion criteria included (1) poor respiratory reserve (vital capacity <60%); (2) pulmonary hypertension or other serious disease complications associated with SSc; and (3) cyclophosphamide use within the past 2 years.
Assessments
Clinical and laboratory assessments were performed at baseline and at routine intervals until 24 weeks after the first infusion of each rituximab course. Skin sclerosis was assessed by MRSS. Lung function was evaluated by pulmonary function tests. Serum levels of Krebs von den Lungen-6 (KL-6; normal range: 0-500 U/mL) and surfactant protein-D (SP-D; normal range: 0-110 ng/mL), which are glycoproteins mainly produced by type II pneumocytes, were measured as established markers of interstitial lung disease (ILD) in patients with SSc.16,17 Laboratory examinations included white blood cell count, lymphocyte count, the number of CD19+ and CD20+ cells, and serum levels of IgG (normal range: 700-1600 mg/dL), IgM (normal range: 40-250 mg/dL), and IgA (normal range: 70-400 mg/dL). Patients with MRSS improvement of 9 or higher and 8 or lower were classified as high responders and low responders, respectively, based on the previous study on minimal important difference for MRSS.18 This classification resulted in 16 high responders and 13 low responders, enabling subsequent statistical analyses.
Statistical Analysis
Fisher exact test was performed to compare categorical variables. Mann-Whitney U test or Wilcoxon signed-rank test as appropriate was performed to compare continuous variables. Spearman correlation test was used for correlation analysis. P values of <.05 were considered statistically significant. GraphPad Prism, version 7.03 (GraphPad Software) was used for statistical analysis.
Results
Baseline Characteristics
Clinical characteristics of the study participants are summarized in the Table. The median (IQR) age was 48 (35-54) years with female predominance (27 of 29; 93%). Twenty-five patients (86%) had diffuse cutaneous form,19 and 24 (83%) had pulmonary fibrosis on high-resolution computed tomography.20 The patients received a median (IQR) of 4 (4-5) courses of rituximab and were followed up for a median (IQR) of 96 (96-120) weeks.
Table. Characteristics of the Study Cohort.
| Characteristic | Median (IQR) | P values | ||
|---|---|---|---|---|
| Total (n = 29) | LR (n = 13) | HR (n = 16) | ||
| Age, y | 48 (35-54) | 50 (35-54.5) | 46.5 (35.5-53.5) | .58 |
| Sex, No. (%) | ||||
| Women | 27 (93) | 13 (100) | 14 (88) | .49 |
| Men | 2 (7) | 0 | 2 (12) | |
| Disease duration, mo | 65 (30-135.5) | 77 (23-174.5) | 51.5 (38.5-122.3) | .44 |
| Disease type, No. (%) | ||||
| dcSSc | 25 (86) | 10 (77) | 15 (94) | .30 |
| lcSSc | 4 (14) | 3 (23) | 1 (6) | |
| No. of rituximab courses | 4.0 (4.0-5.0) | 4.0 (3.0-5.0) | 5.0 (4.0-5.8) | .04 |
| Follow-up period, wk | 96 (96-120) | 96 (72-120) | 120 (96-140) | .04 |
| Clinical features | ||||
| MRSS | 14 (10.5-16) | 11 (10-13.5) | 15.5 (14-18.8) | <.001 |
| MRSS improvement rate, % | 70 (50-77.5) | 55.6 (26.9-68.3) | 74.2 (67.9-84.7) | .01 |
| Pitting scars/digital ulcers, No. (%) | 25 (86) | 11 (85) | 14 (88) | >.99 |
| Raynaud phenomenon, No. (%) | 28 (97) | 13 (100) | 15 (94) | >.99 |
| Nail fold bleeding, No. (%) | 17 (59) | 6 (46) | 11 (69) | .27 |
| Telangiectasia, No. (%) | 9 (31) | 4 (31) | 5 (31) | >.99 |
| Calcinosis, No. (%) | 1 (3) | 1 (4) | 0 | >.99 |
| Lungs | ||||
| Pulmonary fibrosis, No. (%) | 24 (83) | 10 (77) | 14 (88) | .63 |
| FVC predicted, % | 80.1 (73.5-96.3) | 80.1 (64.5-97.7) | 79.6 (69.6-95.0) | .81 |
| Dlco, % | 79.7 (69.7-97.3) | 79.7 (69.3-96.4) | 81.2 (70.2-98.1) | .79 |
| Laboratory findingsa | ||||
| KL-6, U/mL | 410 (216-615) | 421 (172-615) | 378 (251-645) | >.99 |
| SP-D, ng/mL | 154 (79-194) | 156 (91-193) | 127 (69-225) | .62 |
| White blood cell count, /μL | 6200 (4600-7400) | 6300 (4500-6900) | 6150 (4725-8700) | .80 |
| Lymphocyte count, /μL | 1200 (900-1750) | 1400 (1100-1850) | 1000 (725-1650) | .15 |
| CD19+ cell number, /μL | 179 (118-278) | 213 (100-367) | 165 (121-234) | .50 |
| CD20+ cell number, /μL | 181 (111-258) | 215 (96-360) | 161 (114-226) | .48 |
| IgG, mg/dL | 1277 (1150-1510) | 1327 (1173-1510) | 1237 (1126-1604) | .63 |
| IgM, mg/dL | 119 (85-138) | 125 (91-157) | 106 (83-133) | .37 |
| IgA, mg/dL | 223 (181-283) | 207 (137-328) | 225 (187-277) | .45 |
| Antitopoisomerase I antibody, No. (%) | 18 (62) | 8 (62) | 10 (63) | >.99 |
| Anticentromere antibody, No. (%) | 4 (14) | 2 (15) | 2 (13) | >.99 |
| Anti-RNA polymerase III antibody, No. (%) | 1 (3) | 1 (8) | 0 | .45 |
| Concurrent medication | ||||
| Oral prednisolone, No. (%) | 15 (52) | 5 (38) | 10 (63) | .67 |
| Dose, mg/d | 4 (0-7.3) | 0 (0-5.5) | 4.5 (0-8.6) | .24 |
Abbreviations: dcSSc, diffuse cutaneous systemic sclerosis; Dlco, diffusing capacity of the lung for carbon monoxide; FVC, forced vital capacity; HR, high responders; KL-6, Krebs von den Lungen-6; lcSSc, limited cutaneous systemic sclerosis; LR, low responders; MRSS, modified Rodnan skin score; SP-D, surfactant protein-D.
SI conversion factors: To convert IgA, IgG, and IgM to g/L, multiply by 0.01; /μL to ×109/L, multiply by 0.001.
Of 6 patients who were negative for antitopoisomerase I antibody, anticentromere antibody, and anti-RNA polymerase III antibody, 2 patients (HR and LR) were positive for anti-U1-RNP antibody, 1 patient (HR) was positive for anti-U3-RNP antibody, and 1 patient (HR) was positive for anti-SS-A antibody. The remaining 2 patients (HR and LR) were negative for autoantibodies.
Degree of B-Cell Depletion
First, we measured the number of circulating CD19+ and CD20+ cells at baseline and at 1, 2, 3, 4, 8, 12, 16, 20, and 24 weeks after the first infusion of rituximab (Figure 1B). Consistent with previous reports,12,13 CD19+ cells were markedly depleted a week after the first infusion of rituximab (median [IQR] residual ratio, 4.3% [0.6%-4.1%]), followed by a further decrease until 8-week follow-up (median [IQR] residual ratio, 0% [0%-0.6%]) and a subtle increase thereafter (median [IQR] residual ratio, 0.7% [0%-1.3%] at 24-week follow-up). Of note, CD20+ cells were depleted more rapidly, deeply, and persistently than CD19+ cells, with median (IQR) residual ratio of 0% [0%-0%], 0% [0%-0%], and 0% [0%-0.3%] at 1-, 8-, and 24-week follow-up, respectively.
Outcomes of Rituximab Treatment for Skin and Lung Fibrosis
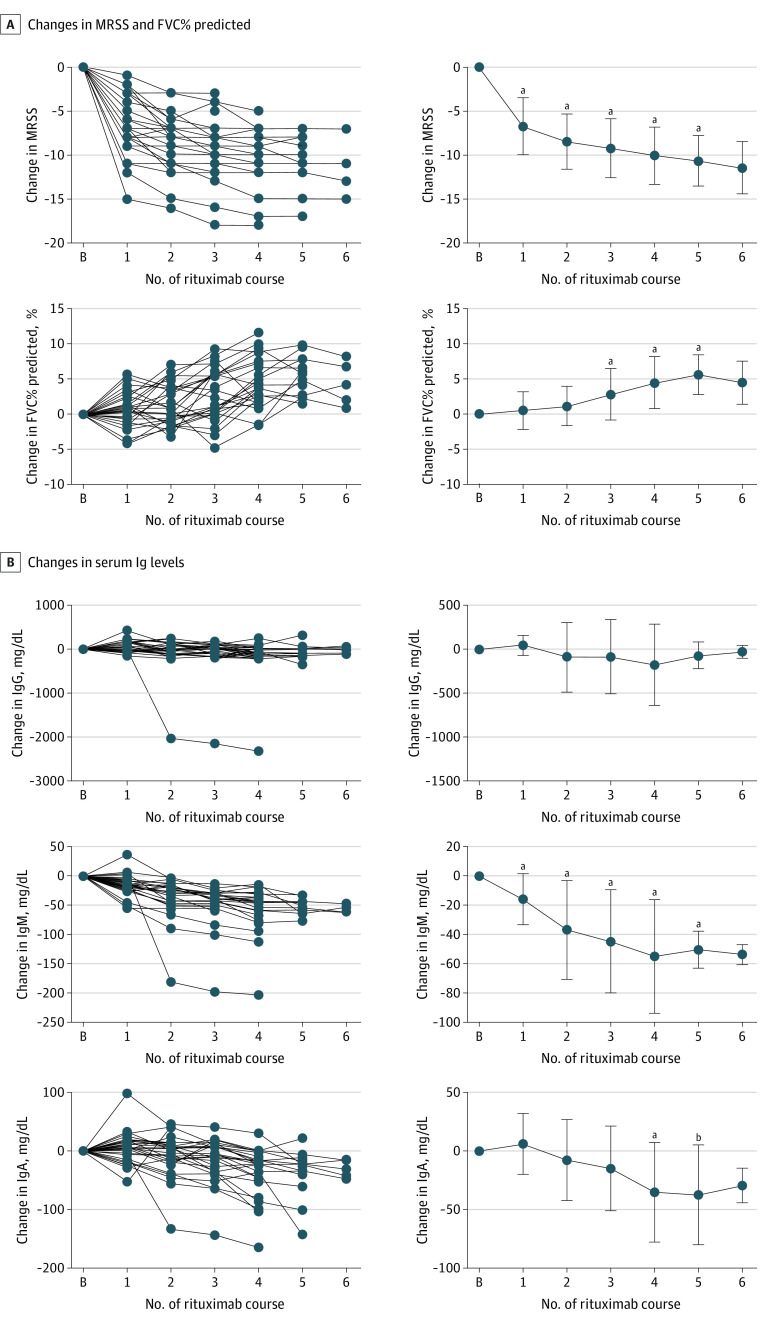
Next, we analyzed the long-term outcomes of rituximab treatment for skin sclerosis and ILD in SSc (Figure 2A; eFigure 1A in Supplement 1). We observed that MRSS significantly decreased after the first course of rituximab (median [IQR] change in MRSS, −7 [−8.5 to −4]; P < .001) and continued to decrease gradually during follow-up. Similarly, there was a gradual improvement in FVC% predicted, which reached statistical significance after 3 courses of rituximab (median [IQR] change in FVC% predicted, 1.85 [0.13-5.68]; P < .001). Given the close association between FVC% predicted and prognosis of SSc-associated ILD,20,21,22 this result suggests that rituximab provides long-term benefit for patients with SSc-associated ILD. Serum KL-6 levels decreased after repeated courses of rituximab (median [IQR] change in KL-6, −53 [−146 to 7]; P = .01 after 4 courses of rituximab, median [IQR] change in KL-6, −155 [−206 to 0]; P = .04 after 5 courses of rituximab), which was strongly influenced by 1 outlier with a marked decrease in KL-6 (eFigure 2A in Supplement 1). There were no significant changes in percentage of predicted diffusing capacity of the lung for carbon monoxide (%Dlco) and serum SP-D levels.
Figure 2. Outcomes of Rituximab Treatment for Skin Sclerosis and Interstitial Lung Disease in Systemic Sclerosis.
A, Longitudinal changes in MRSS and FVC% predicted from baseline during follow-up. B, Longitudinal changes in serum IgG, IgM, and IgA levels from baseline during follow-up. N = 29, 29, 29, 28, 25, 13, and 5 at baseline and 1st, 2nd, 3rd, 4th, 5th, and 6th course of rituximab, respectively. Data are presented for each individual (left) or as mean (SD) (right). Error bars represent SD. B indicates baseline; FVC, forced vital capacity; MRSS, modified Rodnan skin score.
aP < .001.
bP = .007.
Serum Immunoglobulin Levels During Follow-up
To investigate the association of repeated rituximab therapy with immunoglobulin levels, we evaluated serum levels of IgG, IgM, and IgA (Figure 2B), along with white blood cell count and lymphocyte count (eFigure 1B in Supplement 1). Serum IgM levels significantly decreased after the first course of rituximab (median [IQR] change in IgM, −23 [−49 to −5.5]; P < .001) and continued to decrease during follow-up. A similar result was obtained after excluding 1 outlier with a marked decrease in IgM (eFigure 2B in Supplement 1). There was also a gradual decrease in serum IgA levels, which reached a statistical significance after 4 courses of rituximab (median [IQR] change in IgA, −8 [−36 to 8.8]; P < .001). Serum IgG levels remained almost unchanged throughout the follow-up. No significant changes were observed in white blood cell count and lymphocyte count.
Association Between Clinical Response and Serum Immunoglobulin Levels
Given the heterogeneous outcomes of rituximab among patients with SSc (Figure 2A; eFigure 1A in Supplement 1), we next sought to characterize those with better response to rituximab (Table). Patients with MRSS improvement of 9 or greater and 8 or less during follow-up were classified as high responders (n = 16) and low responders (n = 13), respectively. High responders received more courses of rituximab than low responders (median [IQR], 5 [4-5.8] vs 4 [3-5]; P = .04). In addition, baseline MRSS was significantly higher in high responders than in low responders (median [IQR], 15.5 [14-18.8] vs 11 [10-13.5]; P < .001). The MRSS improvement rate relative to the baseline was also significantly higher in high responders compared with low responders (median [IQR], 74.2% [67.9%-84.7%] vs 55.6% [26.9%-68.3%]; P = .01). Other baseline characteristics were similar between the 2 groups.
Next, changes in various parameters were compared between the 2 groups during follow-up (Figure 3A; eFigure 3 in Supplement 1). High responders showed a significantly greater decrease in serum IgG and IgA levels compared with low responders (median [IQR] change in IgG, −125 [−207 to −83] vs 7 [−120 to 43]; P = .008; median [IQR] change in IgA, −45 [−96 to −32] vs −11 [−20 to 3]; P < .001). Subsequently, we analyzed the data based on another definition that high responders are those with MRSS improvement of more than 5 and 25% or greater (n = 25) and low responders are those with MRSS improvement of 5 or less or less than 25% (n = 4).23 Similar results were obtained regarding the baseline characteristics (eTable 1 in Supplement 1). However, there were no statistically significant differences in changes in parameters other than MRSS between the 2 groups, which might be attributed to low statistical power due to the small number in the low-responder group based on this definition (eFigure 4 in Supplement 1).
Figure 3. Serum Immunoglobulin Levels and Clinical Response to Rituximab in Systemic Sclerosis.
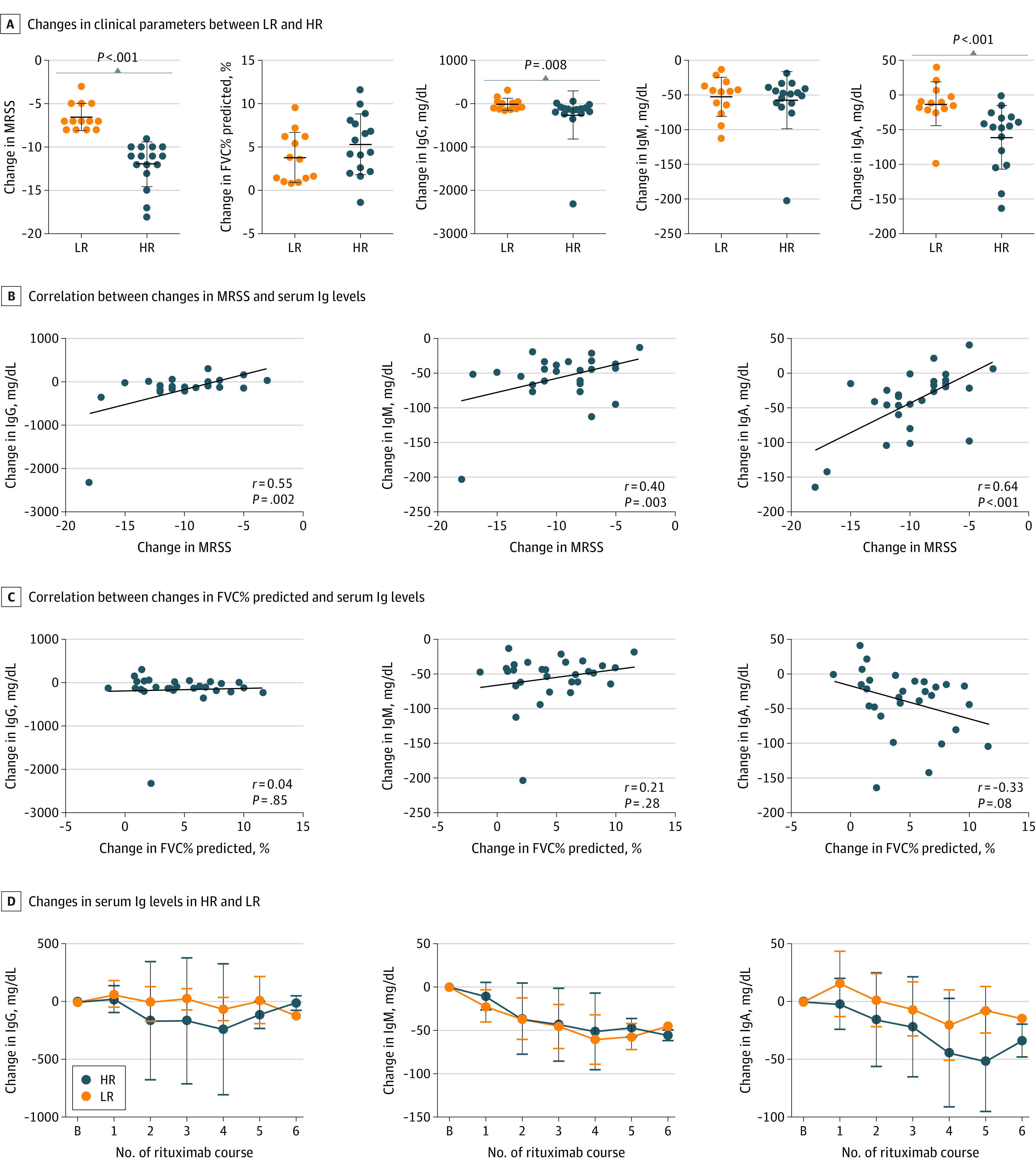
A, Changes in clinical parameters were compared between HR and LR during follow-up. P < .001 (MRSS); P = .11 (FVC% predicted); P = .008 (IgG); P = .77 (IgM); P < .001 (IgA). Error bars indicate SD, and longer horizontal lines in the middle of the bars indicate mean values. B and C, Correlation between changes in MRSS (B) or FVC% predicted (C) and serum immunoglobulin levels. The solid lines show the regression lines. D, Longitudinal changes in serum immunoglobulin levels in HR and LR. N = 29. Data are presented as mean (SD). Error bars represent SD. B indicates baseline; FVC, forced vital capacity; HR, high responders; LR, low responders; MRSS, modified Rodnan skin score.
There was a significant correlation between MRSS improvement and the decrease in serum IgA levels (r = 0.64, P < .001; Figure 3B). The MRSS improvement correlated with the decrease in serum IgG and IgM levels (IgG, r = 0.55, P = .002; IgM, r = 0.40, P = .003), but the result of IgM was heavily influenced by 1 outlier with a marked decrease in IgM (eFigure 5 in Supplement 1). There was a modest correlation between the improvement in FVC% predicted and the decrease in serum IgA levels (r = −0.33, P = .08; Figure 3C). Collectively, the decrease in serum IgA levels was associated with the outcome after rituximab treatment, especially for skin sclerosis.
We also observed the different dynamics of serum IgA levels between high and low responders throughout the follow-up period: high responders showed an early and significant decrease in serum IgA levels, whereas in low responders, serum IgA levels tended to increase after the first course of rituximab and were largely sustained during follow-up (Figure 3D).
Because patients in the cohort had relatively mild ILD, we analyzed the data of patients with FVC% predicted less than 80% at baseline (n = 14) and obtained similar results to the analysis of all patients (eFigure 6 in Supplement 1). As the sample size for the fifth and sixth course of rituximab was small, we also analyzed the results during the first 4 courses of rituximab and obtained similar results to those throughout all courses of rituximab (eTable 2, eFigure 7 in Supplement 1).
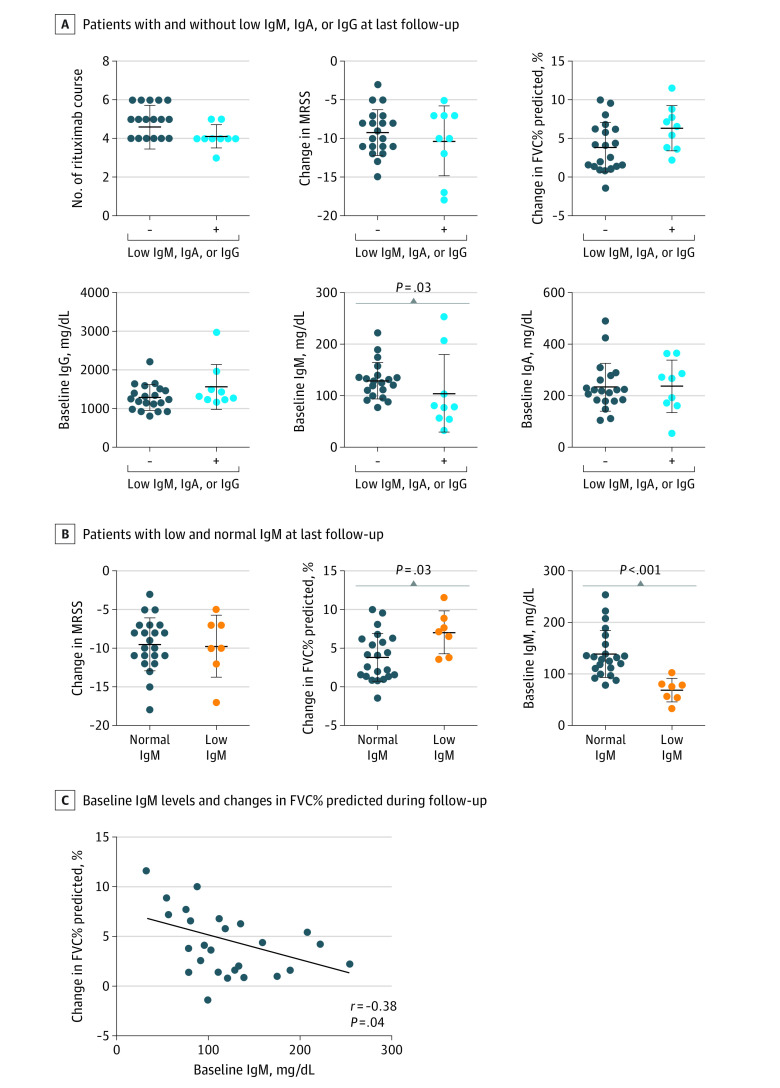
Low Serum IgM, IgA, and IgG Levels at the Last Follow-up
Finally, we evaluated serum immunoglobulin levels at the last follow-up and their association with clinical improvement. Seven patients (24%) had isolated low IgM, 1 (3%) had low IgA, and 1 (3%) had low IgG. No significant difference was observed in the number of rituximab courses between patients with and without low IgM, IgA, or IgG. Of note, patients with low IgM, IgA, or IgG tended to show greater improvement in FVC% predicted compared with those without (median [IQR] change in FVC% predicted, 6.6 [3.7-8.3] vs 3.4 [1.4-6.3]; P = .06), whereas MRSS improvement was similar between the 2 groups (median [IQR] change in MRSS, −10 [−14.5 to −7] vs −9.5 [−11 to −7.3]; P = .90; Figure 4A). In addition, serum IgM levels at baseline were significantly lower in patients with low IgM, IgA, or IgG at the last follow-up (median [IQR] baseline IgM, 79 [56-156] vs 127 [103-138]; P = .03; Figure 4A). Other laboratory data at baseline were similar between the 2 groups (eFigure 8A in Supplement 1).
Figure 4. Low Serum Immunoglobulin Levels Following Rituximab Therapy in Systemic Sclerosis.
A, The number of rituximab courses, changes in MRSS and FVC% predicted, and baseline immunoglobulin levels were compared between patients with and without low IgM, IgA, or IgG at last follow-up. B, The changes in MRSS and FVC% predicted and baseline IgM levels were compared between patients with low and normal IgM at last follow-up. C, Correlation between baseline IgM levels and changes in FVC% predicted during follow-up. The solid line shows the regression line. N = 29. Data are presented as mean (SD). Error bars indicate SD, and longer horizontal lines in the middle of the bars indicate mean values. FVC indicates forced vital capacity; MRSS, modified Rodnan skin score.
As the majority of patients with low IgM, IgA, or IgG had low IgM, we also compared the clinical response to rituximab between patients with and without low IgM at the last follow-up (Figure 4B; eFigure 8B in Supplement 1). Patients with low IgM showed greater improvement in FVC% predicted than those without (median [IQR] change in FVC% predicted, 7.2 [3.8-8.9] vs 3.6 [1.4-6.2]; P = .03). At baseline, serum IgM levels were significantly lower in patients with low IgM at the last follow-up (median [IQR] baseline IgM, 76 [55-81] vs 131 [108-163]; P < .001; Figure 4B). Furthermore, serum IgM levels at baseline were negatively correlated with the improvement in FVC% predicted at the last follow-up (r = −0.38, P = .04; Figure 4C). In addition, we analyzed the data of patients with FVC% predicted less than 80% at baseline and observed similar results to the analysis of all patients (eFigure 9 in Supplement 1). Similar results were also obtained in the analysis of the first 4 courses of rituximab (eFigure 10 in Supplement 1). Overall, these results indicate that low serum IgM levels at baseline were associated with greater improvement of ILD.
Safety
Adverse events occurred in 27 patients (93%) during the follow-up, of which upper respiratory tract infection was the most common. The only serious adverse event was the development of cholangitis, which was considered to be unrelated to the study medication. There were no adverse events that led to death. Further details are provided in eTable 3 in Supplement 1.
Discussion
This study examined the long-term outcomes of rituximab treatment for SSc with detailed clinical and laboratory data (Figure 1, Table). The results presented here demonstrate the improvement of MRSS and FVC% predicted following rituximab therapy, which was more pronounced after repeated courses (Figure 2). The longitudinal data also show that serum IgA and IgM levels gradually decreased, and that the decrease in serum IgA levels significantly correlated with MRSS improvement (Figure 3). At the last follow-up, more than 30% of patients developed low IgM, IgA, or IgG, of which low IgM was the most common and associated with greater improvement in FVC% predicted (Figure 4).
Both CD19+ and CD20+ cells were markedly depleted a week after the first infusion of rituximab, with the latter depleted more profoundly (Figure 1B). Considering that CD19+CD20− cells represent plasmablasts and plasma cells in peripheral blood,24 these subsets of B cells may explain the different dynamics of CD19+ and CD20+ cells.
In the DESIRES randomized clinical trial, we have shown the outcome of rituximab for skin sclerosis in SSc as a primary end point, which led to its approval for the treatment of SSc in Japan in 2021.12,13 The current work notably extends the findings of the DESIRES trial by demonstrating the overall linear improvement in MRSS during a median follow-up of 96 weeks (Figure 2). Our data also showed the improvement in FVC% predicted after repeated courses of rituximab, consistent with previous studies.6,7,8,25 Although improvement in FVC% predicted is small with fluctuation in each patient, this result suggests the disease-modifying properties of rituximab, considering that pulmonary function gradually deteriorates in the natural course of ILD in SSc.26
Although rituximab specifically targets circulating CD20+ B cells, previous studies have reported a variable degree of decrease in serum immunoglobulin levels in patients with other autoimmune diseases after receiving rituximab.9,10,11,27 This decrease is attributed to impaired formation of immunoglobulin-producing plasmablasts and plasma cells and/or susceptibility of their precursor B cells to rituximab. In this study, serum IgM levels started to decrease after the first course of rituximab, which was followed by a moderate decrease in serum IgA levels, while serum IgG levels were largely maintained (Figure 3). Since IgM is mainly produced by short-lived plasma cells,24 the early decrease in serum IgM levels suggests that the formation of short-lived plasma cells is impaired by rapid depletion of their precursor cells, such as naive and preswitched memory B cells. In line with this, unchanged serum IgG levels over time may be attributed to the stable storage of long-lived plasma cells in bone marrow that are unaffected by rituximab.24 In addition, recent studies show that serum IgA is mostly monomeric and produced by long-lived plasma cells in inflammatory sites as well as in bone marrow.28,29,30,31 Therefore, the decrease in serum IgA levels might reflect the reduced niche of IgA-producing long-lived plasma cells in inflammatory microenvironment after repeated courses of rituximab.
Limitations
There are several limitations to this study. First, the number of patients is limited. Second, this study involved a retrospective analysis of patients who chose to continue rituximab after the DESIRES trial, which may lead to selection bias and some discrepancies between the results of this study and those of the preceding trial. For instance, serum IgM levels significantly decreased after the first course of rituximab in this study (Figure 2B), whereas there were no significant differences in serum IgM levels between rituximab and placebo groups in the DESIRES trial.12,13 As patients with greater clinical improvement were more likely to choose to continue rituximab, this discrepancy may further support the association between decreased serum IgM levels and better response to rituximab. Another limitation is the lack of data on functional variables such as overall health scores, which would serve as a more comprehensive measure of treatment response.32 Moreover, comparing clinical and laboratory parameters between patients in the present cohort and those who did not continue rituximab after the DESIRES trial would also be helpful to better understand its long-term outcomes.
Conclusions
In this cohort study, rituximab was associated with significantly improved MRSS and FVC% predicted in patients with SSc at a median follow-up of 96 weeks, supporting its role as a disease-modifying therapy for SSc. This study also suggests that serum immunoglobulins may be a promising tool to evaluate and predict the outcomes of rituximab in SSc.
eFigure 1. Effect of rituximab on physiological and laboratory data in SSc.
eFigure 2. Longitudinal changes in serum KL-6 and IgM levels after excluding the outliers.
eFigure 3. Changes in clinical parameters and response to rituximab in SSc.
eFigure 4. Changes in clinical parameters in patients with MRSS improvement of > 5 and ≥ 25% and those with MRSS improvement of ≤ 5 or < 25%.
eFigure 5. Correlation between changes in clinical parameters and serum IgM levels after excluding the outlier.
eFigure 6. Changes in FVC% predicted and their correlation with serum immunoglobulin levels in patients with FVC% predicted < 80% at baseline.
eFigure 7. Serum immunoglobulin levels and clinical response during the first 4 courses of rituximab.
eFigure 8. Baseline laboratory data and low serum immunoglobulin levels following rituximab therapy in SSc.
eFigure 9. Correlation baseline IgM and ΔFVC% predicted in patients with FVC% predicted < 80% at baseline.
eFigure 10. Low IgM, IgA, or IgG after the first 4 courses of rituximab.
eTable 1. Baseline characteristics of the patients with MRSS improvement of > 5 and ≥ 25% and those with MRSS improvement of ≤ 5 or < 25%.
eTable 2. Baseline characteristics of high responders and low responders after 4 courses of rituximab.
eTable 3. Adverse events.
Data Sharing Statement
References
- 1.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360(19):1989-2003. doi: 10.1056/NEJMra0806188 [DOI] [PubMed] [Google Scholar]
- 2.Rubio-Rivas M, Royo C, Simeón CP, Corbella X, Fonollosa V. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44(2):208-219. doi: 10.1016/j.semarthrit.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 3.Hasegawa M, Hamaguchi Y, Yanaba K, et al. B-lymphocyte depletion reduces skin fibrosis and autoimmunity in the tight-skin mouse model for systemic sclerosis. Am J Pathol. 2006;169(3):954-966. doi: 10.2353/ajpath.2006.060205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Numajiri H, Kuzumi A, Fukasawa T, et al. B cell depletion inhibits fibrosis via suppression of profibrotic macrophage differentiation in a mouse model of systemic sclerosis. Arthritis Rheumatol. 2021;73(11):2086-2095. doi: 10.1002/art.41798 [DOI] [PubMed] [Google Scholar]
- 5.Fukasawa T, Yoshizaki A, Ebata S, et al. Single-cell-level protein analysis revealing the roles of autoantigen-reactive B lymphocytes in autoimmune disease and the murine model. Elife. 2021;10:e67209. doi: 10.7554/eLife.67209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daoussis D, Melissaropoulos K, Sakellaropoulos G, et al. A multicenter, open-label, comparative study of B-cell depletion therapy with rituximab for systemic sclerosis-associated interstitial lung disease. Semin Arthritis Rheum. 2017;46(5):625-631. doi: 10.1016/j.semarthrit.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 7.Sircar G, Goswami RP, Sircar D, Ghosh A, Ghosh P. Intravenous cyclophosphamide vs rituximab for the treatment of early diffuse scleroderma lung disease: open label, randomized, controlled trial. Rheumatology (Oxford). 2018;57(12):2106-2113. doi: 10.1093/rheumatology/key213 [DOI] [PubMed] [Google Scholar]
- 8.Ebata S, Yoshizaki A, Fukasawa T, et al. Rituximab therapy is more effective than cyclophosphamide therapy for Japanese patients with anti-topoisomerase I-positive systemic sclerosis-associated interstitial lung disease. J Dermatol. 2019;46(11):1006-1013. doi: 10.1111/1346-8138.15079 [DOI] [PubMed] [Google Scholar]
- 9.de la Torre I, Leandro MJ, Edwards JC, Cambridge G. Baseline serum immunoglobulin levels in patients with rheumatoid arthritis: relationships with clinical parameters and with B-cell dynamics following rituximab. Clin Exp Rheumatol. 2012;30(4):554-560. [PubMed] [Google Scholar]
- 10.Evangelatos G, Fragoulis GE, Klavdianou K, Moschopoulou M, Vassilopoulos D, Iliopoulos A. Hypogammaglobulinemia after rituximab for rheumatoid arthritis is not rare and is related with good response: 13 years real-life experience. Rheumatology (Oxford). 2021;60(5):2375-2382. doi: 10.1093/rheumatology/keaa617 [DOI] [PubMed] [Google Scholar]
- 11.Reddy V, Martinez L, Isenberg DA, Leandro MJ, Cambridge G. Pragmatic treatment of patients with systemic lupus erythematosus with rituximab: long-term effects on serum immunoglobulins. Arthritis Care Res (Hoboken). 2017;69(6):857-866. doi: 10.1002/acr.22993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebata S, Yoshizaki A, Oba K, et al. Safety and efficacy of rituximab in systemic sclerosis (DESIRES): a double-blind, randomised, placebo-controlled trial. Lancet Rheumatol. 2021;3(7):E489-E497. doi: 10.1016/S2665-9913(21)00107-7 [DOI] [PubMed] [Google Scholar]
- 13.Ebata S, Yoshizaki A, Oba K, et al. Safety and efficacy of rituximab in systemic sclerosis (DESIRES): open-label extension of a double-blind, investigator-initiated, randomised, placebo-controlled trial. Lancet Rheumatol. 2022;4(8):E546-E555. doi: 10.1016/S2665-9913(22)00131-X [DOI] [PubMed] [Google Scholar]
- 14.van den Hoogen F, Khanna D, Fransen J, et al. 2013 Classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2013;72(11):1747-1755. doi: 10.1136/annrheumdis-2013-204424 [DOI] [PubMed] [Google Scholar]
- 15.Khanna D, Furst DE, Clements PJ, et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J Scleroderma Relat Disord. 2017;2(1):11-18. doi: 10.5301/jsrd.5000231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato S, Nagaoka T, Hasegawa M, Nishijima C, Takehara K. Elevated serum KL-6 levels in patients with systemic sclerosis: association with the severity of pulmonary fibrosis. Dermatology. 2000;200(3):196-201. doi: 10.1159/000018382 [DOI] [PubMed] [Google Scholar]
- 17.Asano Y, Ihn H, Yamane K, et al. Clinical significance of surfactant protein D as a serum marker for evaluating pulmonary fibrosis in patients with systemic sclerosis. Arthritis Rheum. 2001;44(6):1363-1369. doi: [DOI] [PubMed] [Google Scholar]
- 18.Khanna D, Furst DE, Hays RD, et al. Minimally important difference in diffuse systemic sclerosis: results from the D-penicillamine study. Ann Rheum Dis. 2006;65(10):1325-1329. doi: 10.1136/ard.2005.050187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15(2):202-205. [PubMed] [Google Scholar]
- 20.Goh NS, Desai SR, Veeraraghavan S, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. 2008;177(11):1248-1254. doi: 10.1164/rccm.200706-877OC [DOI] [PubMed] [Google Scholar]
- 21.Moore OA, Proudman SM, Goh N, et al. Quantifying change in pulmonary function as a prognostic marker in systemic sclerosis-related interstitial lung disease. Clin Exp Rheumatol. 2015;33(4)(suppl 91):S111-S116. [PubMed] [Google Scholar]
- 22.Goh NS, Hoyles RK, Denton CP, et al. Short-term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol. 2017;69(8):1670-1678. doi: 10.1002/art.40130 [DOI] [PubMed] [Google Scholar]
- 23.Dobrota R, Maurer B, Graf N, et al. ; EUSTAR coauthors . Prediction of improvement in skin fibrosis in diffuse cutaneous systemic sclerosis: a EUSTAR analysis. Ann Rheum Dis. 2016;75(10):1743-1748. doi: 10.1136/annrheumdis-2015-208024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez-Andres M, Paiva B, Nieto WG, et al. ; Primary Health Care Group of Salamanca for the Study of MBL . Human peripheral blood B-cell compartments: a crossroad in B-cell traffic. Cytometry B Clin Cytom. 2010;78(suppl 1):S47-S60. doi: 10.1002/cyto.b.20547 [DOI] [PubMed] [Google Scholar]
- 25.Goswami RP, Ray A, Chatterjee M, Mukherjee A, Sircar G, Ghosh P. Rituximab in the treatment of systemic sclerosis-related interstitial lung disease: a systematic review and meta-analysis. Rheumatology (Oxford). 2021;60(2):557-567. doi: 10.1093/rheumatology/keaa550 [DOI] [PubMed] [Google Scholar]
- 26.Steen VD, Conte C, Owens GR, Medsger TA Jr. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum. 1994;37(9):1283-1289. doi: 10.1002/art.1780370903 [DOI] [PubMed] [Google Scholar]
- 27.Venhoff N, Effelsberg NM, Salzer U, et al. Impact of rituximab on immunoglobulin concentrations and B cell numbers after cyclophosphamide treatment in patients with ANCA-associated vasculitides. PLoS One. 2012;7(5):e37626. doi: 10.1371/journal.pone.0037626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gommerman JL, Rojas OL, Fritz JH. Re-thinking the functions of IgA(+) plasma cells. Gut Microbes. 2014;5(5):652-662. doi: 10.4161/19490976.2014.969977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keppler SJ, Goess MC, Heinze JM. The wanderings of gut-derived IgA plasma cells: impact on systemic immune responses. Front Immunol. 2021;12:670290. doi: 10.3389/fimmu.2021.670290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilmore JR, Gaudette BT, Gómez Atria D, et al. IgA plasma cells are long-lived residents of gut and bone marrow that express isotype- and tissue-specific gene expression patterns. Front Immunol. 2021;12:791095. doi: 10.3389/fimmu.2021.791095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isho B, Florescu A, Wang AA, Gommerman JL. Fantastic IgA plasma cells and where to find them. Immunol Rev. 2021;303(1):119-137. doi: 10.1111/imr.12980 [DOI] [PubMed] [Google Scholar]
- 32.Volkmann ER, Tashkin DP, Li N, Furst DE, Clements PJ, Elashoff RM. Development of a composite outcome measure for systemic sclerosis related interstitial lung disease. Rheumatology (Sunnyvale). 2015;5(2):154. doi: 10.4172/2161-1149.1000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Effect of rituximab on physiological and laboratory data in SSc.
eFigure 2. Longitudinal changes in serum KL-6 and IgM levels after excluding the outliers.
eFigure 3. Changes in clinical parameters and response to rituximab in SSc.
eFigure 4. Changes in clinical parameters in patients with MRSS improvement of > 5 and ≥ 25% and those with MRSS improvement of ≤ 5 or < 25%.
eFigure 5. Correlation between changes in clinical parameters and serum IgM levels after excluding the outlier.
eFigure 6. Changes in FVC% predicted and their correlation with serum immunoglobulin levels in patients with FVC% predicted < 80% at baseline.
eFigure 7. Serum immunoglobulin levels and clinical response during the first 4 courses of rituximab.
eFigure 8. Baseline laboratory data and low serum immunoglobulin levels following rituximab therapy in SSc.
eFigure 9. Correlation baseline IgM and ΔFVC% predicted in patients with FVC% predicted < 80% at baseline.
eFigure 10. Low IgM, IgA, or IgG after the first 4 courses of rituximab.
eTable 1. Baseline characteristics of the patients with MRSS improvement of > 5 and ≥ 25% and those with MRSS improvement of ≤ 5 or < 25%.
eTable 2. Baseline characteristics of high responders and low responders after 4 courses of rituximab.
eTable 3. Adverse events.
Data Sharing Statement