Abstract
Purpose of review:
There has been an increased prevalence of allergy. Due to this relatively rapid rise, changes in environmental exposures are likely the main contributor. In this review we highlight literature from the last 3 years pertaining to the role of air pollution, greenness, and the rural/farm lifestyle and their association with the development of allergic sensitization, atopic dermatitis, food allergy, and allergic rhinitis in infancy and childhood. Because asthma has a more complex pathophysiology, it was excluded from this review.
Recent findings:
Recent studies support a role for air pollution, greenness, and rural/farming lifestyle influencing atopic outcomes that continues to be defined. While many studies have examined singular environmental exposures, the interconnectedness of these exposures and others points to a need for future work to consider an individual’s whole exposure.
Summary:
Environmental exposures’ influence on atopic disease development remains an ongoing and important area of study.
Keywords: air pollution, greenness, rural/farm, atopic dermatitis, food allergy, allergic rhinitis
Introduction
Atopic dermatitis (AD), food allergy (FA), allergic rhinitis (AR), and asthma encompass allergic manifestations that have seen a rise in prevalence in many industrialized regions. AD, FA, and AR manifest in early infancy and childhood and have been reported to affect up to 20%, 10%, and 8% of children respectively(1–5). AD is a chronic relapsing-remitting inflammatory skin disease; FA is an abnormal immune response to food antigens following ingestion. AR is a seasonal or year-round allergic inflammatory response, mainly localized in the nasal mucosa, in response to inhaled allergens, including mold, dust mites, and pollen. There is a natural progression of these allergic diseases, referred to as the atopic march. Starting with AD, the atopic march progresses to FA shortly after, and later to AR and asthma in childhood(6). Notably, studies have demonstrated associations between the diagnosis of one allergic manifestation and the risk of subsequent diagnosis of another. For example, studies have shown an increased risk of FA in infants/children that have AD(7, 8).
From birth cohort studies, it has been shown that children with FA(9) or AD(10) are at an increased risk to develop AR and asthma. Findings from multiple studies suggest epicutaneous sensitization may also lead to the development of FA(11–13). Notably, in a randomized controlled trial of early oral egg introduction to combat egg allergy in moderate to severe eczema infants, ~30% of infants at 4 months were already sensitized to egg before known ingestion(14). Additionally, another study reported that increased environmental peanut exposure (EPE) measured in home dust was a risk factor for peanut sensitization and allergy among a cohort of children less than 15 months(15). Furthermore, the authors found a significant interaction between EPE and peanut sensitization in infants with a history of AD, which was further augmented in infants with severe AD(15). Thus, future treatments targeting the epithelial dysfunction that occurs in AD may be beneficial in preventing the development of FA(16). Currently there are no cures for AD, FA, or AR, and primary prevention strategies have been largely unsuccessful. For AD, disease management has included the use of emollients, moisturizers, and corticosteroids to improve and maintain skin barrier integrity and limit inflammation(17, 18), while for FA, food avoidance is the most effective method to evade an allergic response. There has also been active research on the use of allergen immunotherapy as a potential treatment for FA(19). Treatment for AR can include allergen avoidance or medications like antihistamines as well as immunotherapy. Despite these best efforts at disease management, the diagnosis of AD, FA, and AR can negatively impact the quality of life of allergic individuals and their families.
As is the case for many other diseases, the interplay of genetics and environment in the development of allergy continues to be debated. Familial aggregation(20–23) and twin studies(24–27) have demonstrated a genetic component to atopic disease development. Additionally, candidate gene association and genome-wide association studies have revealed several genes commonly altered in allergy, some of which are shared among allergic manifestations including FLG, IL4, SPINK5, HLA, STAT6(28–32). It is clear there is a role for genetics, but the relatively rapid rise in prevalence of allergy suggests recent changes in environmental exposures may be the main contributor. Globally, individuals have seen a dramatic change in their environment and lifestyle due to the rise of urbanization and industrialization(33). Various environmental factors have been implicated in allergy development; these factors may be collectively referred to as the exposome(34, 35). In this review, we will highlight human findings from the last 3 years on the roles of air pollution, greenness, and the farming/rural lifestyle on allergic sensitization, AD, FA, and AR in infants and children. For this review, asthma outcomes will not be reported.
Pollution
Global industrialization and urbanization have dramatically increased levels of air pollution, prompting concern for a myriad of potential health effects. Infants and children may be at increased risk of pollution’s harmful effects for several reasons. First, they are more likely to breathe through their mouths than adults, thus drawing more particulate matter into their lungs(36). Additionally, children have faster respiratory rates than adults, as well as more permeable respiratory tracts(37), and they generally tend to spend more time being physically active, which increases air intake, as well as more time outside(38). Together, these factors increase children’s contact to airborne pollutants and thus the potential for these pollutants to contact the skin and respiratory tract. Various components make up air pollution including particulate matter (PM), nitrogen oxide compounds (NOx), volatile organic compounds (VOCs), ozone (O3), environmental tobacco smoke (ETS), and traffic-related air pollution (TRAP). Particulate matter is generated by many sources, from vehicle emissions to industrial processes(39). These particles are categorized based on their diameter(40); two such categories are PM2.5 and PM10. Of the two, only PM2.5 has the capacity to penetrate skin due to its smaller size(39, 40), which has implications for atopic disease. VOCs are unstable carbon compounds, which arise from common household materials(40); examples include benzene and toluene. VOCs are generated by consumer and natural sources alike, with sources including cleaning supplies and plants(40). ETS comes from cigarettes and has long been associated with implications for health(41). Finally, TRAP encompasses all vehicle emissions, including PM, VOCs, NOx, SO2, CO, and O3(40). Recent literature has largely focused on the allergic impact of PM, but there have been some findings regarding other pollutants. A summary of the most recent findings can be found in Table 1.
Table 1.
Air pollution exposure and atopic outcomes (only papers from 2019 until present)
| Exposure | Author, Year | Country | Atopic outcome evaluated | Findings |
|---|---|---|---|---|
| Air pollution | Noh, 201961 | S. Korea | AD | Children with AD have variable responses to increased air pollution; overall, PM10, NO2, SO2, and CO correlated with increased AD symptoms. |
| Gallant, 202036 | Canada | Sensitization | Children who were exposed to ETS at 2 years of age were significantly more likely to have a positive skin prick test to any of 14 common allergens at 2 years old. | |
| Granum, 202056 | UK, France, Spain, Greece, Lithuania, Norway (multicenter) | AR, itchy rash, eczema, FA | Increased PMabs exposure correlated with decreased risk of AR. Early childhood exposure to pollutants did not correlate with AR risk. The authors did not find correlations between prenatal exposures and the allergic outcomes of AD or FA, nor did they find correlations between childhood exposures with any allergic outcome studied. | |
| Kuiper, 202064 | Norway, Sweden | AR | Paternal exposure to O3 (medium level) correlated with increased risk of offspring AR. Maternal exposure to PM10 (high level) also correlated with offspring’s increased risk of AR. | |
| Min, 202059 | S. Korea | AR, AD | Both NO2 and PM10 were associated with AD risk and symptoms. PM2.5 tended to correlate with AD symptoms and diagnosis as well, but this finding was not statistically significant. | |
| To, 202057 | Canada | AR, AD | The combined exposure of O3 and NO2 at birth correlated with risk for AD in a dose-dependent manner. In contrast, early PM2.5 exposure did not correlate with risk for allergic outcomes. | |
| Wang, 202065 | China | AR | Increased levels of pollutants (PM2.5, PM10, SO2, NO2, and CO) were associated with significantly more outpatient AR visits that same day; all associations were stronger for patients under the age of 15. | |
| Guo, 202167 | China | AR, AD | Increased levels of NO2, PM2.5, and PM10 during the second trimester of pregnancy increased a child’s risk of developing allergic disease. | |
| Hao, 202166 | China | AR | TRAP exposure in preschool children correlated with AR prevalence; more specifically, increases in PM10 and NO2 exposure increased one’s risk of developing AR. | |
| Melén, 202153 | Sweden, Germany, The Netherlands | Sensitization | There was not a consistent association of air pollution with sensitization to any common allergen up to age 16. Allergen-specific analyses suggested an increase in NO2 correlated with increased risks of birch sensitization. Additionally, PM2.5 exposure at birth may relate to sensitization to the grass allergen Phl p 1 and the cat allergen Fel d 1. | |
| Wang, 202137 | Taiwan | AD | Increased exposure to THCs, NMHCs, and CH4 (hydrocarbon air pollutants) correlated with higher rates of developing AD in childhood. | |
| Yao, 202143 | Taiwan | AD | Prenatal PM2.5 exposure from week 7–17 of gestation correlated with increased risk of developing AD in childhood, particularly during the 12th week. | |
| Hu, 202263 | China | AD | NO2 levels correlated with more outpatient visits for AD. | |
| Liu, 202268 | China | AR | Exposure to outdoor air pollution, in the year prior to conception, throughout pregnancy, and within the past 12 months of survey administration, correlated with aggravated AR symptoms in the fall. Specifically, symptoms were associated with pre-conception and prenatal SO2 and NO2 exposure, and recent PM10 and NO2 exposure. | |
| Park, 202239 | S. Korea | AD | Increased PM2.5, PM10, SO2, and CO all correlate with increased monthly AD visits; this was not seen for O3 nor NO2. | |
| Wu, 202269 | China | AR | Exposure to pollutants (PM2.5, PM10, NO2, SO2) were not correlated with increased risk of outpatient visit for AR in children under the age of 18. | |
| Ye, 202262 | China | AD | Increased levels of air pollutants (SO2, NO2, but not O3) were associated with increased AD outpatients. Patients less than 7 years old were most sensitive to air pollutants (including PM10, SO2, and NO2). Factors such as season or other pollutants were seen to significantly heighten the harmful effects of SO2 and NO2. |
AD, atopic dermatitis; AR, allergic rhinitis; FA, food allergy
It has been suggested that air pollutants can contribute to atopic disease through a variety of mechanisms, ranging from skin barrier dysfunction, oxidative stress induction, epigenetic modifications and even influence on immune cell responses(34, 37, 40, 42–44). For example, PM2.5 can directly and indirectly generate reactive oxygen species (ROS), which can ultimately trigger oxidative stress(45). It is hypothesized that the resulting damage from the oxidative stress may compromise skin barrier function(45). Another study found that when human nasal epithelial cells were exposed to PM2.5, decreased epithelial barrier integrity was observed(46), perhaps contributing to increased susceptibility to aeroallergens and inflammation. As for pollution’s potential effects on immune response, some studies have reported prenatal exposure to pollutants (specifically ETS and VOCs) may be associated with postnatal Th2 immune bias(37).
There is mixed data as to whether airborne pollutants contribute to allergic sensitization, as shown in Table 1(42). An older study previously found PM2.5 concentration and absorbance in outdoor air positively correlated with sensitization to outdoor inhalants (timothy grass, rye, birch, mugwort) at 6 years old. (47).Though the authors of this report did not examine the potential impact of PM10, it will still be important to elicit any difference between PM2.5 and PM10 in correlating with allergic sensitization. Meanwhile, it has been reported that tobacco smoke can negatively affect allergic sensitization outcomes. Gallant and Ellis observed ETS exposure at 2 years of age correlated with significantly higher odds of a positive skin prick test also at 2 years for any of 14 common allergens(36). This finding is consistent with previous literature suggesting early life and even prenatal exposure to secondhand smoke may increase a child’s risk for allergic disease(48–50). Similarly, a previous study with children from the Netherlands’ Prevention and Incidence Asthma and Mite Allergy (PIAMA) birth cohort found long-term exposure to TRAP was associated with food allergen sensitization at 4 years(51). However, two meta-analyses did not find a correlation between air pollution and sensitization to any common allergen in children up to 10 and 16 years old (52, 53). Yet, when further analysis was conducted on specific components affecting sensitization by 16 years, PM2.5 was positively associated with sensitization against grass antigen Phl p 1 and cat antigen Fel d1(53). From the studies highlighted here, it seems associations between sensitization and air pollution may be pollutant-specific.
There is limited research exploring the possible connection between air pollution and FA. A meta-analysis focused on ETS found that passive smoking, but not maternal smoking, correlated with an increased risk of FA in children and adolescents, and this increased risk became significant when the authors limited their analysis to five cohort studies(54). It is possible duration and timing of exposure to ETS factor into allergic outcomes. In this case, the cohort studies analyzed were longitudinal, following infants and children from anywhere from one to seven years. However, there are no recent reports specifically examining association between pollution and FA.
In contrast to FA, there has been plentiful research examining the potential connection between air pollutants, especially PM and AD. Several recent studies have supported the role of PM2.5 in contributing to risk of AD as well as exacerbating existing cases of AD(39, 55). Notably, Kim et al. investigated the impact of indoor PM2.5, which tends to be less studied than its outdoor equivalent. They reported a positive correlation between concentration of indoor PM2.5 and symptoms of AD in children younger than 18; this correlation was observed during the winter and spring (when indoor PM2.5 levels were higher), as well as when indoor temperatures were less than 25.5 degrees Celsius(55). As mentioned earlier, it is possible these effects could be explained in part by PM2.5’s ability to penetrate the epidermal barrier, as another study did not conclude an association between PM10 and risk of AD at 6 to 11 years old(56). However, To et al. did not see a correlation between early childhood exposure to PM2.5 and odds of AD(57). In terms of prenatal exposure, Yao et al. found the most sensitive time for prenatal PM exposure (as it relates to development of AD) is before the epidermal barrier has formed, prior to 20 weeks of gestation(43). Despite these inconsistencies, a recent meta-analysis by Wang et al. found an overall significant correlation between both long- and short-term exposure to PM2.5 and AD(58).
While PM has largely been the focus of recent research on pollution and AD, some studies have reported on the effects of other pollutants. Hydrocarbon compounds, VOCs, NO2, and ETS have generally been found to contribute to the risk of AD(37, 40, 50, 59), but there have been inconsistent findings regarding SO2, O3 and CO(39, 55, 57, 59, 60). In their study of early childhood exposure to pollutants and risk of AD, To et al. found an association between risk of AD and early-life O3 and NO2 exposure(57). Other recent research has focused more on the contribution of pollution to existing cases of AD, measuring changes in symptoms and visits to clinicians. A 2019 study from Noh et al. compared pollutant levels and their relationship to AD symptoms, reporting that increases in NO2, SO2, and CO levels each correlated with worsened symptoms among children under six, while increases in O3 did not(61). Another report, published by Ye et al. in 2022, found that daily increases in SO2 and NO2, but not O3, contributed to the number of outpatient visits for AD among children under 8 years old(62). A multicenter study published a 2022 report again supporting the correlation between NO2 levels and outpatient visits for childhood AD; notably, this report observed a correlation both in cold and warm months, considering seasonality as an additional variable(63). Together, these mixed findings underscore the need for more research on SO2, O3, and CO. Overall, further studies are needed to consider how the different pollutants may be functioning individually or in synergy. Also, there are important caveats to consider when defining each pollutant. For example, VOCs arise both from natural and synthetic sources, a difference which may impact whether VOCs yield protection against or exacerbate risk in the case for AD(40). Since the term VOC encompasses a variety of carbon-based compounds, perhaps specific properties of each compound differentially affect one’s risk of AD.
Finally, there have been several reports on possible connections between periods of exposure to air pollutants (pre-conception, prenatal, and in early life) and childhood AR outcomes (development and symptoms). In a multicenter study, Granum et al. studied prenatal and early childhood exposure (from birth until 6–11 years) to airborne pollutants, including NO2, PM10, PM2.5, and PMabs (soot particles)(56). Of the prenatal exposures, only PMabs was reported to have an association with risk of AR among 6-to-11-year-old children; interestingly, increased PMabs exposure correlated with decreased risk of AR. Early childhood exposure to pollutants did not correlate with AR risk(56). Kuiper et al. examined pre-conceptional exposures to air pollution, reporting that paternal childhood exposure to O3 and maternal childhood exposure to PM10 correlated with increased offspring risk of AR(64). Wang et al. reported a stronger correlation between ambient levels of PM2.5 and PM10, SO2, NO2, O3, and CO, and the number of outpatient AR visits for children up to 15 years old. All pollutants studied were found to correlate with increased risk of AR(65). In 2021, Hao et al. in a case-control study found that early exposure to TRAP (including PM10, NO2, O3, CO, and SO2) correlated with prevalence of AR in early childhood(66). Specifically, exposure to PM10 and NO2 heightened one’s chances of developing AR in males or in those with family stress(66). That same year, Guo et al. reported that the second trimester is a sensitive window during which exposure to NO2, PM10, and PM2.5 significantly increase a child’s odds of developing allergic disease, including AR(67). Most recently, Liu et al. reported among 3-to-6-year-old children that pre-conceptional and prenatal as well as current exposure to outdoor air pollutants significantly correlated with fall symptoms of AR(68). Wu et al. did not find an association between ambient air pollutants (PM2.5, PM10, NO2, and SO2) and increased risk of AR-related outpatient visits for children (69). Overall, these studies point towards a correlation between air pollution and childhood AR, but due to the variety of exposure periods and outcomes measured, more research is needed to fully elicit a connection.
Greenness
Greenness can be defined as the amount of flora in each region. This flora has numerous benefits for health, both physical and mental, via means such as promoting physical activity and reducing stress(70). The normalized difference vegetation index (NDVI) is a commonly used proxy for greenness, which uses visible and near-infrared light reflection (obtained from satellite imagery) to calculate the density of vegetation(71). It is important to acknowledge that NDVI fails to capture which species of flora are present(72, 73), limiting the ability to evaluate the potential impact of biodiversity on allergic outcomes. Vegetation varies across geography, and certain species may have greater effects than others. Biodiversity is important in the discussion of atopic disease; the biodiversity hypothesis proposes that diverse vegetative and microbial exposures benefit the gut microbiome and immune system development, and thus may contribute to protection against allergic disease(74, 75). There are still other limitations of NDVI. This assessment does not collect behavioral information(42), such as amount of time spent outside. Thus, NDVI should be used in combination with lifestyle questionnaires and/or other measures of outdoor exposure. Additionally, vegetation density may fluctuate seasonally, so researchers must take caution to consider seasonal values when applicable, rather than annual averages. An alternative, more recent proxy for greenness is Light Detection and Ranging (LiDAR) imagery(76, 77). Much like NDVI, LiDAR imagery analyzes extent of greenness using aerial images, but its laser-based method provides higher-resolution imagery and differentiates between types of land cover, from categories such as trees, grass, and bodies of water(72, 78).
There have been several proposed mechanisms for the role of greenness in atopic disease development. One is the type of greenness present; grass coverage and tree canopy coverage may impact the distribution and spread of pollen differently, affecting amounts of aeroallergen exposure(72). Notably, plants also naturally generate VOCs, which can be a component of air pollution(40, 79). Yet greenery may also be protective against the negative effects of air pollutants, via absorption(79), so it is difficult to conclude whether greenness ultimately exacerbates or mitigates the harms of air pollution and may vary by geographic location. Other explanations attempt to elicit a connection between greenness and the microbiome, drawing upon the biodiversity hypothesis(75). For example, a diverse array of plants and trees may expose individuals to an equally diverse array of microorganisms and aeroallergens that results in protection against sensitization and allergy(74).
Recent studies have reached differing conclusions as to whether greenness is protective factor or a risk factor for allergic disease, most recent findings are highlighted in Table 2. To fully understand where the discrepancies lie, it is important to break down findings by type of greenery (namely, grass versus trees) and by allergic outcome (pollen versus other types of sensitization). Additionally, as mentioned earlier, conflicting results may be due in part to methodological and analytical inconsistencies. Previous reviews have discussed the body of mixed evidence as to whether greenness is associated with allergic sensitization. In their 2018 systematic review, Lambert et al. (80) could not conclude whether an association exists. Since then, Markevych et al. used birth home addresses to find that living near more allergenic trees correlated with increased risk of aeroallergen sensitization (measured at 2, 6, 10, and 15 years), though their results for food sensitization specifically were inconsistent(81). Considering that grass and other greenery produce pollen, this might explain why higher amounts of greenery have sometimes been related to a higher risk of sensitization. Yet Gernes et al. found differing roles of grass and trees – proximity to grass, but not trees, was associated with an increased risk of sensitization at age 7(72). Additionally, indirect pathways of sensitization should not be ruled out; for instance, greenness may be associated with allergic sensitization by means of allergen cross-reactivity. For example, peanut allergen Ara h 5 has been found to share structural similarities to birch pollen allergen Bet v 2(82). Thus, exposure to pollen allergens may prompt an IgE-mediated response to similarly-structured food allergens(82).
Table 2.
Greenness exposure and atopic outcomes (only papers from 2019 until present)
| Exposure | Author, Year | Country | Atopic outcome evaluated | Findings |
|---|---|---|---|---|
| Greenness | Gernes, 201972 | U.S. | Sensitization, AR | Members of a high-risk cohort were at increased risk of sensitization to outdoor allergens when they lived near grassy areas. Grass vs. tree coverage impacted risk of allergic outcomes differently. A 10% increase in grass coverage correlated with increased risk of sensitization to grass pollen; this was not observed for increased tree coverage. When comparing measures of greenery (such as UGS or NDVI) it may be important to detect grass vs. tree. |
| Li, 201971 | China | AR, AD | No seasonal NDVI-based measure showed a statistically significant correlation with either AR or AD. The authors’ results suggested increased residential distance might decrease one’s risk of allergic symptoms, though data was not collected on participants’ park usage. | |
| Kuiper, 202064 | Norway, Sweden | AR | There were few associations between exposure to greenness and offspring AR. Parental exposure to high NDVI appeared to increase offspring’s risk of AR. | |
| Markevych, 202081 | Germany | AR, sensitization | Growing up in a tree-dense area (specifically allergenic trees) correlated with one’s risk of developing AR and aeroallergen sensitization; results were unclear for one’s risk of food sensitization. | |
| Parmes, 202079 | Italy, France, Solvenia, Poland | AR, AD | Increased greenspace coverage was significantly associated with increased odds of AR but not AD. Living near coniferous forests may increase one’s risk of AR. Their results showed a preliminary trend (not significant) that agricultural spaces may be protective factors for childhood allergic and respiratory disease. | |
| Dzhambov, 202185 | Austria, Italy | AR, AD | Lower residential naturalness and higher residential greyness correlated with more AR symptoms. | |
| Peters, 202274 | Australia | FA | There was an association between increased NDVI and risk of allergy to peanut and egg in children, but not between increased NDVI and risk of sesame allergy. The effect of increased NDVI was magnified by high pollution, but not by lower levels of pollution. |
AD, atopic dermatitis; AR, allergic rhinitis; FA, food allergy
Overall, there is limited research looking at greenness and FA(42). The Australian HealthNuts study was the first to explore whether residential greenness (as measured by NDVI) might be implicated in one’s risk of challenge-confirmed FA(74). The study found an association between increased NDVI and risk of allergy to peanut and egg in children(74). The researchers did not observe the same trend with sesame allergy, but their results may have been affected by the low rate of sesame allergy in their cohort(74). Additionally, the authors noted the effect of increased NDVI was augmented in areas of high pollution, but not in areas with lower levels of pollution(74); the interplay between greenness and air pollution makes it difficult to study either in isolation. The HealthNuts study is the only investigation of a connection between greenness and FA. It will be important to examine the effect of greenness on other food allergies, such as to cow’s milk, as well as the use of LiDAR in studies.
Recent reports have produced conflicting conclusions about greenness and risk of AD compared to previous literature. Previously, a study from 2015 found a short-term forest exposure correlated with decreased scoring AD (SCORAD) indices as well as decreased levels of Macrophage-Derived Chemokine (MDC)/CCL2 among 7-to-12-year-old urban children with AD(83). Additionally, a 2018 study of mothers and infants found that more greenness near the families’ residences helped mitigate the risk of prenatal pollution exposure on AD risk in infancy(84). In contrast, another study in 2019 by Li et al. found no correlation between either greenness (measured by NDVI) or distance to a park with AD in adolescents, most between the ages of 12 and 15(71). Research by Parmes et al. supported this finding(79). Further research is needed.
Regarding association between greenness and AR, there have been mixed findings. A 2019 study looked at AR outcomes by 7 years of age, including sensitivity to both outdoor and indoor allergens associated with AR(72). This study found no association between greenness and AR; however, their inclusion of indoor allergens may have influenced this result(72). In the aforementioned 2019 report from Li et al., the authors also looked at greenness (NDVI) and distance from the nearest park as they related to AR(71). While seasonal NDVI was not found to be associated with AR, distance from park inversely correlated with AR symptoms, though the authors did not collect data on participants’ park usage(71). A 2020 study by Kuiper et al. looked at maternal and paternal childhood exposure to greenness and found limited evidence for an association with offspring AR(64). Another 2020 study measured greenness via land cover data rather than NDVI and found that a 10% increase in greenspace correlated with increased odds of AR(79). Dzhambov et al. found in 2021 that less greenness (measured by NDVI and distance to nature, a new measure) correlated with aggravated AR symptoms in school children aged 8–12(85). Markevych et al. sought to define trees by allergenicity, reporting that children who grew up near trees, especially allergenic ones, were more likely to later develop AR(81). Overall, these findings contribute mixed conclusions to the discussion of greenness and AR.
Overall, the lack of a standardized, comprehensive measurement for greenness presents a challenge to researchers. Such methodological and analytical variation may partly explain inconsistencies in results among different studies. Future research should aim to optimize and standardize measures for greenness so that future studies may be compared more directly.
Farming/rural lifestyle
While the growing prevalence of AD, FA, and AR continues to be a concern, several studies have suggested that not all individuals may be at risk. Initial studies from Europe(86–88) and America(89, 90) have demonstrated an association of rural/farm living and protection against sensitization and allergy. The most recent work is highlighted in Table 3. Recently, several reports have looked at the rural versus urban environment impact on sensitization in South Africa. Two 2019 studies reported that urban infants from the South African Food Allergy (SAFFA) cohort were found to have an increased prevalence of food and aeroallergen sensitization compared to those in rural South Africa(91, 92). A later study examined which factors may contribute to a decreased prevalence of sensitization in rural children(93), finding that contact with farm animals was associated with decreased rates of food and aeroallergen sensitization(93). The Copenhagen Prospective Study on Asthma in Childhood (COPSAC) cohort from Denmark found that infants who spent their first year of life in an urban environment had an increased prevalence of aeroallergen sensitization at 6 years(94). However, food sensitization prevalence was not different between rural and urban children(94). Our pilot studies(95, 96) and follow-up studies compared atopic outcomes in infants from the traditional farming Old Order Mennonite (OOM) community of Western New York and infants, at high-risk for allergic disease, not living on farms. We observed an increased proportion of sensitization in non-farm infants to egg white compared to OOM infants at 12 months(97).
Table 3.
Rural/farm exposure and atopic outcomes (only papers from 2019 until present)
| Exposure | Author, Year | Country | Atopic outcome evaluated | Findings |
|---|---|---|---|---|
| Rural/farm | Botha, 201991 | S. Africa | Sensitization, FA | Food sensitization and FA is lower in the rural S. African children (1–3yr), raw egg white was the most common allergen urban infants were reactive to. |
| Botha, 201992 | S. Africa | Sensitization, FA, AD, AR | Increased prevalence of food and aeroallergen sensitization, AD, and AR in urban children (1–3yrs). | |
| Levin, 202093 | S. Africa | Sensitization, FA, AR, AD | Rural children with farm animal contact, lower prevalence of food and aeroallergen sensitization and FA compared with those without contact. Rural mother’s exposure to farm animals also associated with lower food sensitization and FA in children. Within the urban cohort, consumption of fermented milk associated with lower rates of AR and AD. | |
| Phillips, 202096 | U.S. | FA | Decreased prevalence of allergy against several foods including shellfish, tree nut, and egg in Old Order Mennonite (OOM) children (1–17yrs) compared to NHANES. | |
| Tong, 2020104 | China | AR | Living in towns or metropolis before 2 years old are risk factors for AR in 6–12yr children in the city of Wuhan. | |
| Steiman, 2020103 | U.S. | AD | Decreased prevalence of AD among farm children at 2yrs. Creation of farm exposure profiles based on diversity of farm animal contact of child and mother; found inverse relationship between exposure and AD in children. | |
| Lehtimaki, 202194 | Denmark | Eczema, sensitization, AR, FA | Infants who spent their 1st year of life in an urban environment had a higher prevalence of aeroallergen sensitization at age 6. Urbanized gut bacteria profile at 1 wk was associated with eczema, while the profile at 1 yr was associated with “any sensitization”. AR was no different after adjustments for lifestyle features and there were no differences in FA. | |
| Strieker, 2022106 | Germany | AR | At baseline (6–10yrs) there is a reduced prevalence of AR in children living on a farm. No additional protection is gained by continuing to live on farm after childhood, highlighting the importance of early farm exposure. | |
| Jarvinen, 202297 | U.S. | Sensitization, FA, AD | At 12 months, OOM infants were more sensitized to egg white compared to non-farm infants. In addition, there was an increased prevalence of AD and FA in non-farm infants. | |
| Tong, 2022105 | China | AR | Among 5 cities of the Hubei Province, living in a town or urban area before 2 years old is a risk factor for allergic rhinitis in 6–12yr children. Wuhan, the capital city was found to be a risk factor as well. |
AD, atopic dermatitis; AR, allergic rhinitis; FA, food allergy
Only recently have studies started to examine the relationship between rural/farm lifestyle and FA. In the SAFFA cohort, FA prevalence was increased in urban children(91, 92). Again, child contact with farm animals was associated with decreased rates of FA(93). Notably, maternal exposure to farm animals was also found to be protective against FA, suggestive of a prenatal farm effect that has been previously reported(93, 98, 99). Through surveys, we showed that individuals from the OOM community had a lower self-reported allergy to several foods, including shellfish, peanut, wheat, and fish, compared to non-farm individuals(96). More recently, we found a lower prevalence of FA by 12 months in OOM compared to non-farming infants; the majority of the non-farm infants with FA were allergic to egg(97).
While there are examples demonstrating the protection of the rural/farm lifestyle against AD(87, 88, 100), there are several observations where it appears this environment did not confer protection(86, 101, 102). Inconsistencies in this relationship can be due to variability in the type of rural or farm environment, types of farm animals, as well as whether studies examined the role of the maternal prenatal effect. Nonetheless, recently, in the SAFFA cohort, urban infants were found to have an increased prevalence of AD(92). Within the urban arm of the cohort, consumption of fermented milk appeared to protect against the development of AD(93). In the COPSAC cohort, the authors reported a trend towards increased eczema in urban infants by age 6, but this association was not significant after adjusting for lifestyle features (socioeconomics, pet ownership, older siblings, etc.)(94). Since the initial study of Amish children(89), there has been limited evaluation of the rural/farm environment and AD outcomes in America. Recently, authors found a reduced incidence of AD through the first 2 years of life in farm infants in the Wisconsin Infant Cohort Study (WISC) cohort(103). In this study, authors created patterns of farm exposure based on the diversity of farm animal contact, and they found that the more diverse the animal contact pre and post birth, the less likely infants were to develop AD(103). Similarly, in our own infant cohort, there was a lower prevalence of AD by 12 months in the OOM compared to non-farm counterparts(97).
In the SAFFA cohort, an increased prevalence of AR was also identified in the urban cohort(92). Similar to AD, consumption of fermented milk protected against AR among the urban children (92). In the COPSAC the authors initially found an increased prevalence of AR among urban infants; however, after adjusting for lifestyle features as mentioned previously there was no difference(94). Two recent studies examined AR prevalence and risk factors among 6-to-12-year-old children in China(104, 105) and demonstrated that living in a town/metropolis before the age of 2 was found to be a risk factor for AR(104, 105). Authors from the German GABRIEL study also reported a relationship between farm living and less symptoms of AR among 6-to-11-year-old children(106). Moreover, as this cohort aged into early adulthood (20–25 years), both farm and non-farm adults’ AR prevalence doubled. Yet, the prevalence remained higher among the non-farm adults(106). Notably, the authors found similar odds of AR between those who remained on the farm from childhood to adulthood and those who moved away after childhood, suggesting that the potential window of protection of the farm lifestyle is mainly in childhood(106).
The protective nature of the farm lifestyle continues to be explored. Practices such as the consumption of raw unpasteurized milk and as mentioned previously, contact with farm animals and their stables, have been reported as protective(86, 88, 107). These practices, among others, may result in more diverse and/or enriched microbial exposures, which have gradually been diminished in more westernized regions(108–110). For example, several studies have examined how the microbial composition in house dust relates to sensitization and allergy. In one study using samples from the SAFFA cohort, the authors found no difference in home dust fungal β-glucan among children with different environments (rural/urban) and with or without AD(111). However, they did find that house dust of children with AD from both rural and urban environments had lower levels of endotoxin; suggesting endotoxin exposure may play a role in protection against AD(111), which has already been suggested by earlier studies on asthma(90). Notably, house dust samples from this cohort were further examined by 16S sequencing(112). Compared to controls, house dust of children with AD had a decreased relative abundance of several taxa including Clostridiales, Ruminococcaceae, and Bacteroidales(112). Moreover, within the rural arm of this cohort, AD and control children’s house dust microbiomes clustered separately. The authors found a decreased abundance of these aforementioned taxa in house dust of rural children with AD, while there were no differences in the dust microbiomes between AD and control children from the urban environment(112).
There have been reports of altered gut microbiome in AD, FA, and AR(113–115), but the contribution of the rural/farm lifestyle is limited. Limited data suggest that farm lifestyle was associated with estimated gut microbiome age at 12 months that was protective against asthma in school-aged(116). We recently found that the gut microbiome in OOM infants at 2 months differed from that of non-farm infants(117). OOM infants were enriched with Bifidobacterium longum ssp. infantis (B. infantis)(117), which the gradual loss of this bacteria parallels the rise of allergic atopic disease in many industrialized regions(118, 119). It is thought that Bifidobacteria can induce various immunoregulatory effects protecting against sensitization and allergy(120). A 2021 report from the COPSAC cohort found differences between the gut microbiomes of rural and urban infants(94). Notably, the urbanized gut microbiome profile at 1 week was found to associate with eczema and sensitization at 1 year(94). Together, these microbial exposures from the dust and gut reflective of the biodiversity hypothesis(75) and could influence downstream immune responses during allergen encounters. Following up on human studies, Schuijs et al. found that mice treated farm dust extract were protected against allergy mediated by lung epithelial induction of NF-κB negative regulator A20(121). Thus, components in farm dust have the capacity to modulate immune signaling to blunt aberrant responses. Differential innate immune signaling(90) and expression of TLR receptors(98) have been described in farm children. Increased frequency and function regulatory T cells which can be utilized to regulate immune responses have been reported in farm-exposed infants/children as well(122, 123). Additionally, studies from the Swedish FARMFLORA birth cohort have suggested that farm exposure induces enhanced B cell maturation(124, 125). Overall, rural/farm exposure can induce a differential innate and adaptive immune responses leading to protection against sensitization and allergy development(108).
Following up on associations in human cohorts, animal studies have suggested that bioactive components (cytokines, microbes, proteins, etc.) found in raw cow’s milk induced anti-allergic responses otherwise diminished by pasteurization(126–128). One pilot study examined how children with cow’s milk allergy react to consumption of raw cow milk(129). In a small oral provocation pilot of 11 children, subjects were able to tolerate, on average, 50mL of raw milk compared to 8.6mL of pasteurized shop milk(129). Further studies are needed to examine the impact of raw cow’s milk on human cells and its capacity to induce anti-allergic responses.
Conclusion
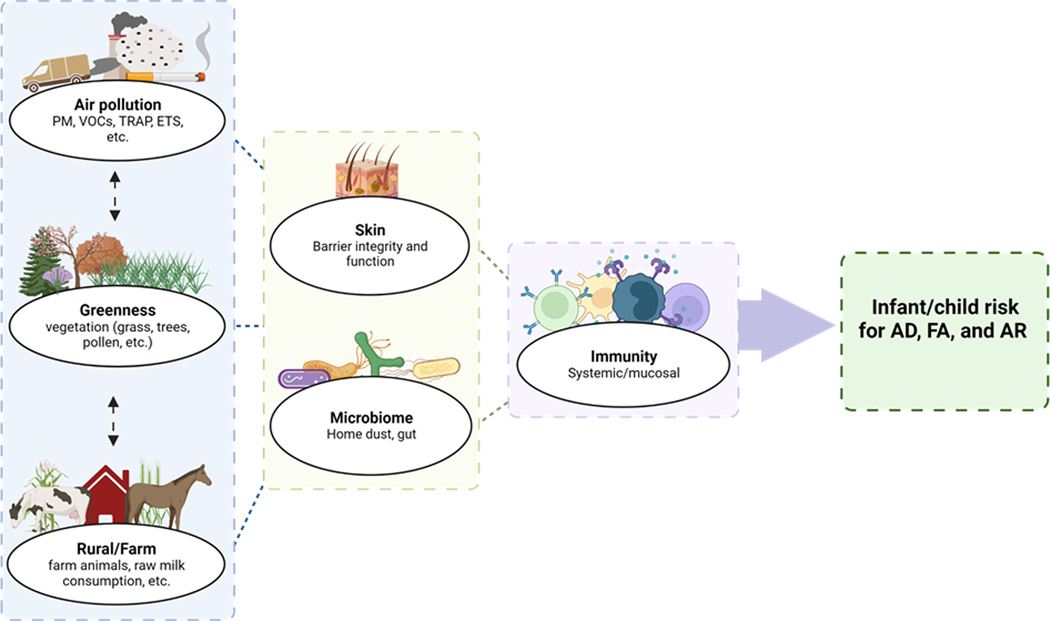
The growing prevalence of AD, FA, and AR continues to impact allergic individuals and their families. While there is a role for genetics, environmental exposures are strong determinants of the recent rise. Specific factors such as air pollution, greenness, and rural/farm lifestyle appear to have a role in sensitization, FA, AD, and AR, which continues to be defined (Figure 1). Most studies have examined the impact of singular environmental exposures. Importantly, as in the case of air pollution and greenness, those exposures can be further subdivided. While this research has provided useful information and has supported some associations, it is more likely that these environmental exposures and others work in conjunction. Recently, researchers have argued for the consideration of the exposome, an individual’s comprehensive set of environmental exposures since conception, including but not limited to external allergens and pollutants, host microbiota, and broader social, psychological, and economic factors(130, 131). The exposome approach would better account for the relationship between related variables; for example, the combined presence of grass, wind, and the spread of pollen may together contribute to allergic outcomes. Or a relationship may be observed between exposures; perhaps greenness mitigates air pollution. Alternatively, the rural/farm environment would likely contain a differential profile of air pollutants and greenness that may need to be accounted for. Furthermore, factors such as socioeconomic status may affect one’s environmental exposures. Financial resources may afford families the ability to live in less polluted, greener areas. In the case of the HealthNuts study, adjusting for socioeconomic status (SES) revealed that infants residing in high SES areas had lower risk for peanut allergy compared to their low SES counterparts(74). Considering the association between SES and diet, it is particularly important to control for SES when studying food sensitization and food allergy. Logistically, a systematic approach to environmental exposures may be difficult to analyze, but more attempts to capture comprehensive information are needed.
Figure 1. Environmental exposures’ impact on infant/child development of allergy.
The association between the risk for development of allergy and the select environmental exposures of air pollution, greenness, and rural/farm lifestyle continues to be explored. These exposures likely mediate their effect through interactions with the skin and microbiome and converge at the immune system. Figure created with BioRender.com
Conflicting findings can be explained by potential variations in how exposures are defined as well as limitations of currently available tools for exposure measurements - for example, NDVI and LiDAR(71, 76). Also, several of these environmental exposures may have a maternal prenatal effect that can influence infant/child allergic outcomes(36, 93) which needs to be considered. Gestation and early infancy are key periods of physiological development; it will be important to continue to elicit differences in effect between prenatal and postnatal environmental exposures. Identifying sensitive exposure windows even before birth could ultimately aid in establishing mechanistic explanations and perhaps even future interventions.
Footnotes
Conflict of Interest
Courtney M. Jackson reports grants from NIH, outside the submitted work;
Alexandra N. Kaplan no conflict of interest.
Kirsi M. Jarvinen reports grants from NIH, grants from Janssen R&D, grants from Bill and Melinda Gates Foundation, personal fees from DBV, personal fees from Janssen R&D, grants from Aimmune, personal fees from Up-To-Date, personal fees from Jovie, outside the submitted work; .
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
REFERENCES
Some recent publications are noted as (·) for important or (··) for very important.
- 1.Nutten S Atopic dermatitis: global epidemiology and risk factors. Annals of nutrition & metabolism. 2015;66 Suppl 1:8–16. [DOI] [PubMed] [Google Scholar]
- 2.Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet (London, England). 2006;368(9537):733–43. [DOI] [PubMed] [Google Scholar]
- 3.Loh W, Tang MLK. The Epidemiology of Food Allergy in the Global Context. International journal of environmental research and public health. 2018;15(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. The Journal of allergy and clinical immunology. 2011;127(3):668–76.e1-2. [DOI] [PubMed] [Google Scholar]
- 5.Aït-Khaled N, Pearce N, Anderson HR, Ellwood P, Montefort S, Shah J. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Allergy. 2009;64(1):123–48. [DOI] [PubMed] [Google Scholar]
- 6.Hill DA, Spergel JM. The atopic march: Critical evidence and clinical relevance. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2018;120(2):131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papapostolou N, Xepapadaki P, Gregoriou S, Makris M. Atopic Dermatitis and Food Allergy: A Complex Interplay What We Know and What We Would Like to Learn. Journal of clinical medicine. 2022;11(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eigenmann PA, Calza AM. Diagnosis of IgE-mediated food allergy among Swiss children with atopic dermatitis. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2000;11(2):95–100. [DOI] [PubMed] [Google Scholar]
- 9.Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC pediatrics. 2016;16:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustafsson D, Sjöberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitis--a prospective follow-up to 7 years of age. Allergy. 2000;55(3):240–5. [DOI] [PubMed] [Google Scholar]
- 11.Brough HA, Nadeau KC, Sindher SB, Alkotob SS, Chan S, Bahnson HT, et al. Epicutaneous sensitization in the development of food allergy: What is the evidence and how can this be prevented? Allergy. 2020;75(9):2185–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin PE, Eckert JK, Koplin JJ, Lowe AJ, Gurrin LC, Dharmage SC, et al. Which infants with eczema are at risk of food allergy? Results from a population-based cohort. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2015;45(1):255–64. [DOI] [PubMed] [Google Scholar]
- 13.Gray CL, Levin ME, du Toit G. Egg sensitization, allergy and component patterns in African children with atopic dermatitis. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2016;27(7):709–15. [DOI] [PubMed] [Google Scholar]
- 14.Palmer DJ, Metcalfe J, Makrides M, Gold MS, Quinn P, West CE, et al. Early regular egg exposure in infants with eczema: A randomized controlled trial. The Journal of allergy and clinical immunology. 2013;132(2):387–92.e1. [DOI] [PubMed] [Google Scholar]
- 15.Brough HA, Liu AH, Sicherer S, Makinson K, Douiri A, Brown SJ, et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. The Journal of allergy and clinical immunology. 2015;135(1):164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sweeney A, Sampath V, Nadeau KC. Early intervention of atopic dermatitis as a preventive strategy for progression of food allergy. Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology. 2021;17(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kader HA, Azeem M, Jwayed SA, Al-Shehhi A, Tabassum A, Ayoub MA, et al. Current Insights into Immunology and Novel Therapeutics of Atopic Dermatitis. Cells. 2021;10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hon KLE, Chan VPY, Leung AKC. Experimental Drugs with the Potential to Treat Atopic Eczema. Journal of experimental pharmacology. 2021;13:487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dantzer JA, Wood RA. Next-Generation Approaches for the Treatment of Food Allergy. Current allergy and asthma reports. 2019;19(1):5. [DOI] [PubMed] [Google Scholar]
- 20.Apfelbacher CJ, Diepgen TL, Schmitt J. Determinants of eczema: population-based cross-sectional study in Germany. Allergy. 2011;66(2):206–13. [DOI] [PubMed] [Google Scholar]
- 21.Wadonda-Kabondo N, Sterne JA, Golding J, Kennedy CT, Archer CB, Dunnill MG. Association of parental eczema, hayfever, and asthma with atopic dermatitis in infancy: birth cohort study. Archives of disease in childhood. 2004;89(10):917–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai HJ, Kumar R, Pongracic J, Liu X, Story R, Yu Y, et al. Familial aggregation of food allergy and sensitization to food allergens: a family-based study. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2009;39(1):101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatzler L, Panetta V, Illi S, Hofmaier S, Rohrbach A, Hakimeh D, et al. Parental hay fever reinforces IgE to pollen as pre-clinical biomarker of hay fever in childhood. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2014;25(4):366–73. [DOI] [PubMed] [Google Scholar]
- 24.Schultz Larsen F Atopic dermatitis: a genetic-epidemiologic study in a population-based twin sample. Journal of the American Academy of Dermatology. 1993;28(5 Pt 1):719–23. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Zhang S, Tsai HJ, Hong X, Wang B, Fang Y, et al. Genetic and environmental contributions to allergen sensitization in a Chinese twin study. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2009;39(7):991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sicherer SH, Furlong TJ, Maes HH, Desnick RJ, Sampson HA, Gelb BD. Genetics of peanut allergy: a twin study. The Journal of allergy and clinical immunology. 2000;106(1 Pt 1):53–6. [DOI] [PubMed] [Google Scholar]
- 27.van Beijsterveldt CE, Boomsma DI. Genetics of parentally reported asthma, eczema and rhinitis in 5-yr-old twins. The European respiratory journal. 2007;29(3):516–21. [DOI] [PubMed] [Google Scholar]
- 28.Kanchan K, Clay S, Irizar H, Bunyavanich S, Mathias RA. Current insights into the genetics of food allergy. The Journal of allergy and clinical immunology. 2021;147(1):15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson E, Mersha TB. Genetics of Food Allergy. Immunology and allergy clinics of North America. 2021;41(2):301–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Shobaili HA, Ahmed AA, Alnomair N, Alobead ZA, Rasheed Z. Molecular Genetic of Atopic dermatitis: An Update. International journal of health sciences. 2016;10(1):96–120. [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta J, Johansson E, Bernstein JA, Chakraborty R, Khurana Hershey GK, Rothenberg ME, et al. Resolving the etiology of atopic disorders by using genetic analysis of racial ancestry. The Journal of allergy and clinical immunology. 2016;138(3):676–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi BY, Han M, Kwak JW, Kim TH. Genetics and Epigenetics in Allergic Rhinitis. Genes. 2021;12(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore M, Gould P, Keary BS. Global urbanization and impact on health. International journal of hygiene and environmental health. 2003;206(4–5):269–78. [DOI] [PubMed] [Google Scholar]
- 34.Alkotob SS, Cannedy C, Harter K, Movassagh H, Paudel B, Prunicki M, et al. Advances and novel developments in environmental influences on the development of atopic diseases. Allergy. 2020;75(12):3077–86. [DOI] [PubMed] [Google Scholar]
- 35.Cecchi L, D’Amato G, Annesi-Maesano I. External exposome and allergic respiratory and skin diseases. The Journal of allergy and clinical immunology. 2018;141(3):846–57. [DOI] [PubMed] [Google Scholar]
- 36.Gallant MJ, Ellis AK. Prenatal and early-life exposure to indoor air-polluting factors and allergic sensitization at 2 years of age. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2020;124(3):283–7. [DOI] [PubMed] [Google Scholar]
- 37.Wang C, Wei CC, Wan L, Lin CL, Tsai JD. Association of exposure to hydrocarbon air pollution with the incidence of atopic dermatitis in children. Italian journal of pediatrics. 2021;47(1):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salvi S Health effects of ambient air pollution in children. Paediatric respiratory reviews. 2007;8(4):275–80. [DOI] [PubMed] [Google Scholar]
- 39.Park TH, Park S, Cho MK, Kim S. Associations of particulate matter with atopic dermatitis and chronic inflammatory skin diseases in South Korea. Clinical and experimental dermatology. 2022;47(2):325–34. [DOI] [PubMed] [Google Scholar]
- 40.Hendricks AJ, Eichenfield LF, Shi VY. The impact of airborne pollution on atopic dermatitis: a literature review. The British journal of dermatology. 2020;183(1):16–23. [DOI] [PubMed] [Google Scholar]
- 41.Rushton L Health Impact of Environmental Tobacco Smoke in the Horne. Reviews on environmental health. 2021;19(3–4):291–310. [DOI] [PubMed] [Google Scholar]
- 42.Peters RL, Mavoa S, Koplin JJ. An Overview of Environmental Risk Factors for Food Allergy. International journal of environmental research and public health. 2022;19(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.··. Yao TC, Huang HY, Pan WC, Wu CY, Tsai SY, Hung CY, et al. Association of prenatal exposure to fine particulate matter pollution with childhood eczema. Allergy. 2021;76(7):2241–5. COMMENT: Identified a sensitive prenatal window for exposure to PM and found a lower threshold for the negative effects of PM2.5.
- 44.Sbihi H, Boutin RC, Cutler C, Suen M, Finlay BB, Turvey SE. Thinking bigger: How early-life environmental exposures shape the gut microbiome and influence the development of asthma and allergic disease. Allergy. 2019;74(11):2103–15. [DOI] [PubMed] [Google Scholar]
- 45.Dijkhoff IM, Drasler B, Karakocak BB, Petri-Fink A, Valacchi G, Eeman M, et al. Impact of airborne particulate matter on skin: a systematic review from epidemiology to in vitro studies. Particle and fibre toxicology. 2020;17(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xian M, Ma S, Wang K, Lou H, Wang Y, Zhang L, et al. Particulate Matter 2.5 Causes Deficiency in Barrier Integrity in Human Nasal Epithelial Cells. Allergy, asthma & immunology research. 2020;12(1):56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Krämer U, et al. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. American journal of respiratory and critical care medicine. 2008;177(12):1331–7. [DOI] [PubMed] [Google Scholar]
- 48.Kantor R, Kim A, Thyssen JP, Silverberg JI. Association of atopic dermatitis with smoking: A systematic review and meta-analysis. Journal of the American Academy of Dermatology. 2016;75(6):1119–25.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thacher JD, Gruzieva O, Pershagen G, Neuman Å, Wickman M, Kull I, et al. Pre- and postnatal exposure to parental smoking and allergic disease through adolescence. Pediatrics. 2014;134(3):428–34. [DOI] [PubMed] [Google Scholar]
- 50.Thacher JD, Gruzieva O, Pershagen G, Neuman Å, van Hage M, Wickman M, et al. Parental smoking and development of allergic sensitization from birth to adolescence. Allergy. 2016;71(2):239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brauer M, Hoek G, Smit HA, de Jongste JC, Gerritsen J, Postma DS, et al. Air pollution and development of asthma, allergy and infections in a birth cohort. The European respiratory journal. 2007;29(5):879–88. [DOI] [PubMed] [Google Scholar]
- 52.Gruzieva O, Gehring U, Aalberse R, Agius R, Beelen R, Behrendt H, et al. Meta-analysis of air pollution exposure association with allergic sensitization in European birth cohorts. The Journal of allergy and clinical immunology. 2014;133(3):767–76.e7. [DOI] [PubMed] [Google Scholar]
- 53.Melén E, Standl M, Gehring U, Altug H, Antó JM, Berdel D, et al. Air pollution and IgE sensitization in 4 European birth cohorts-the MeDALL project. The Journal of allergy and clinical immunology. 2021;147(2):713–22. [DOI] [PubMed] [Google Scholar]
- 54.Saulyte J, Regueira C, Montes-Martínez A, Khudyakov P, Takkouche B. Active or passive exposure to tobacco smoking and allergic rhinitis, allergic dermatitis, and food allergy in adults and children: a systematic review and meta-analysis. PLoS medicine. 2014;11(3):e1001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.··. Kim YM, Kim J, Ha SC, Ahn K. Effects of Exposure to Indoor Fine Particulate Matter on Atopic Dermatitis in Children. International journal of environmental research and public health. 2021;18(21). COMMENT: Examined the effect of indoor concentration of PM, which is not as commonly studied as outdoor PM.
- 56.Granum B, Oftedal B, Agier L, Siroux V, Bird P, Casas M, et al. Multiple environmental exposures in early-life and allergy-related outcomes in childhood. Environment international. 2020;144:106038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.To T, Zhu J, Stieb D, Gray N, Fong I, Pinault L, et al. Early life exposure to air pollution and incidence of childhood asthma, allergic rhinitis and eczema. The European respiratory journal. 2020;55(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H, Li XB, Chu XJ, Cao NW, Wu H, Huang RG, et al. Ambient air pollutants increase the risk of immunoglobulin E-mediated allergic diseases: a systematic review and meta-analysis. Environmental science and pollution research international. 2022:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Min KD, Yi SJ, Kim HC, Leem JH, Kwon HJ, Hong S, et al. Association between exposure to traffic-related air pollution and pediatric allergic diseases based on modeled air pollution concentrations and traffic measures in Seoul, Korea: a comparative analysis. Environmental health : a global access science source. 2020;19(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. The Journal of investigative dermatology. 2013;133(7):1752–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noh SR, Kim JS, Kim EH, Jeon BH, Kim JH, Kim YM, et al. Spectrum of susceptibility to air quality and weather in individual children with atopic dermatitis. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2019;30(2):179–87. [DOI] [PubMed] [Google Scholar]
- 62.Ye C, Gu H, Li M, Chen R, Xiao X, Zou Y. Air Pollution and Weather Conditions Are Associated with Daily Outpatient Visits of Atopic Dermatitis in Shanghai, China. Dermatology (Basel, Switzerland). 2022:1–11. [DOI] [PubMed] [Google Scholar]
- 63.Hu Y, Jiang F, Tan J, Liu S, Li S, Wu M, et al. Environmental Exposure and Childhood Atopic Dermatitis in Shanghai: A Season-Stratified Time-Series Analysis. Dermatology (Basel, Switzerland). 2022;238(1):101–8. [DOI] [PubMed] [Google Scholar]
- 64.Kuiper IN, Markevych I, Accordini S, Bertelsen RJ, Bråbäck L, Christensen JH, et al. Associations of Preconception Exposure to Air Pollution and Greenness with Offspring Asthma and Hay Fever. International journal of environmental research and public health. 2020;17(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J, Lu M, An Z, Jiang J, Li J, Wang Y, et al. Associations between air pollution and outpatient visits for allergic rhinitis in Xinxiang, China. Environmental science and pollution research international. 2020;27(19):23565–74. [DOI] [PubMed] [Google Scholar]
- 66.Hao S, Yuan F, Pang P, Yang B, Jiang X, Yan A. Early childhood traffic-related air pollution and risk of allergic rhinitis at 2–4 years of age modification by family stress and male gender: a case-control study in Shenyang, China. Environmental health and preventive medicine. 2021;26(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo M, Wei L, Yan H, Duan Z, Niu Z, Xiao C. Exposure to ambient air pollution during trimesters of pregnancy and childhood allergic diseases in Wuhan, China. International journal of environmental health research. 2021:1–11. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y, Lu C, Li Y, Norbäck D, Deng Q. Outdoor Air Pollution and Indoor Window Condensation Associated with Childhood Symptoms of Allergic Rhinitis to Pollen. International journal of environmental research and public health. 2022;19(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu R, Guo Q, Fan J, Guo C, Wang G, Wu W, et al. Association between air pollution and outpatient visits for allergic rhinitis: Effect modification by ambient temperature and relative humidity. The Science of the total environment. 2022;821:152960. [DOI] [PubMed] [Google Scholar]
- 70.Hartig T, Mitchell R, de Vries S, Frumkin H. Nature and health. Annual review of public health. 2014;35:207–28. [DOI] [PubMed] [Google Scholar]
- 71.Li L, Hart JE, Coull BA, Cao SJ, Spengler JD, Adamkiewicz G. Effect of Residential Greenness and Nearby Parks on Respiratory and Allergic Diseases among Middle School Adolescents in a Chinese City. International journal of environmental research and public health. 2019;16(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gernes R, Brokamp C, Rice GE, Wright JM, Kondo MC, Michael YL, et al. Using high-resolution residential greenspace measures in an urban environment to assess risks of allergy outcomes in children. The Science of the total environment. 2019;668:760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lambert KA, Katelaris C, Burton P, Cowie C, Lodge C, Garden FL, et al. Tree pollen exposure is associated with reduced lung function in children. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2020;50(10):1176–83. [DOI] [PubMed] [Google Scholar]
- 74.··. Peters RL, Sutherland D, Dharmage SC, Lowe AJ, Perrett KP, Tang MLK, et al. The association between environmental greenness and the risk of food allergy: A population-based study in Melbourne, Australia. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2022;33(2):e13749. COMMENT: First study to look at the association between greenness and FA.
- 75.Haahtela T A biodiversity hypothesis. Allergy. 2019;74(8):1445–56. [DOI] [PubMed] [Google Scholar]
- 76.Caynes RJC, Mitchell MGE, Wu DS, Johansen K, Rhodes JR. Using high-resolution LiDAR data to quantify the three-dimensional structure of vegetation in urban green space. Urban Ecosystems. 2016;19(4):1749–65. [Google Scholar]
- 77.Song J-h, Han S-h, Yu K, Kim Y-i. Assessing the Possibility of Land-cover Classification Using Lidar Intensity Data. International Archives of Photogrammetry, Remote Sensing and Spatial Information Sciences. 2012;34. [Google Scholar]
- 78.Kwak DA, Lee WK, Kafatos M, Son Y, Cho HK, Lee SH. Estimation of effective plant area index for South Korean forests using LiDAR system. Science China Life sciences. 2010;53(7):898–908. [DOI] [PubMed] [Google Scholar]
- 79.Parmes E, Pesce G, Sabel CE, Baldacci S, Bono R, Brescianini S, et al. Influence of residential land cover on childhood allergic and respiratory symptoms and diseases: Evidence from 9 European cohorts. Environmental research. 2020;183:108953. [DOI] [PubMed] [Google Scholar]
- 80.Lambert KA, Bowatte G, Tham R, Lodge CJ, Prendergast LA, Heinrich J, et al. Greenspace and Atopic Sensitization in Children and Adolescents-A Systematic Review. International journal of environmental research and public health. 2018;15(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Markevych I, Ludwig R, Baumbach C, Standl M, Heinrich J, Herberth G, et al. Residing near allergenic trees can increase risk of allergies later in life: LISA Leipzig study. Environmental research. 2020;191:110132. [DOI] [PubMed] [Google Scholar]
- 82.Bublin M, Breiteneder H. Cross-reactivity of peanut allergens. Current allergy and asthma reports. 2014;14(4):426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seo SC, Park SJ, Park CW, Yoon WS, Choung JT, Yoo Y. Clinical and immunological effects of a forest trip in children with asthma and atopic dermatitis. Iranian journal of allergy, asthma, and immunology. 2015;14(1):28–36. [PubMed] [Google Scholar]
- 84.Lee JY, Lamichhane DK, Lee M, Ye S, Kwon JH, Park MS, et al. Preventive Effect of Residential Green Space on Infantile Atopic Dermatitis Associated with Prenatal Air Pollution Exposure. International journal of environmental research and public health. 2018;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dzhambov AM, Lercher P, Rüdisser J, Browning M, Markevych I. Allergic symptoms in association with naturalness, greenness, and greyness: A cross-sectional study in schoolchildren in the Alps. Environmental research. 2021;198:110456. [DOI] [PubMed] [Google Scholar]
- 86.Riedler J, Braun-Fahrländer C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet (London, England). 2001;358(9288):1129–33. [DOI] [PubMed] [Google Scholar]
- 87.Alfvén T, Braun-Fahrländer C, Brunekreef B, von Mutius E, Riedler J, Scheynius A, et al. Allergic diseases and atopic sensitization in children related to farming and anthroposophic lifestyle--the PARSIFAL study. Allergy. 2006;61(4):414–21. [DOI] [PubMed] [Google Scholar]
- 88.Illi S, Depner M, Genuneit J, Horak E, Loss G, Strunz-Lehner C, et al. Protection from childhood asthma and allergy in Alpine farm environments-the GABRIEL Advanced Studies. The Journal of allergy and clinical immunology. 2012;129(6):1470–7.e6. [DOI] [PubMed] [Google Scholar]
- 89.Holbreich M, Genuneit J, Weber J, Braun-Fahrländer C, Waser M, von Mutius E. Amish children living in northern Indiana have a very low prevalence of allergic sensitization. The Journal of allergy and clinical immunology. 2012;129(6):1671–3. [DOI] [PubMed] [Google Scholar]
- 90.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. The New England journal of medicine. 2016;375(5):411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Botha M, Basera W, Facey-Thomas HE, Gaunt B, Gray CL, Ramjith J, et al. Rural and urban food allergy prevalence from the South African Food Allergy (SAFFA) study. The Journal of allergy and clinical immunology. 2019;143(2):662–8.e2. [DOI] [PubMed] [Google Scholar]
- 92.Botha M, Basera W, Facey-Thomas HE, Gaunt B, Genuneit J, Gray CL, et al. Nutrition and allergic diseases in urban and rural communities from the South African Food Allergy cohort. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2019;30(5):511–21. [DOI] [PubMed] [Google Scholar]
- 93.Levin ME, Botha M, Basera W, Facey-Thomas HE, Gaunt B, Gray CL, et al. Environmental factors associated with allergy in urban and rural children from the South African Food Allergy (SAFFA) cohort. The Journal of allergy and clinical immunology. 2020;145(1):415–26. [DOI] [PubMed] [Google Scholar]
- 94.··. Lehtimäki J, Thorsen J, Rasmussen MA, Hjelmsø M, Shah S, Mortensen MS, et al. Urbanized microbiota in infants, immune constitution, and later risk of atopic diseases. The Journal of allergy and clinical immunology. 2021;148(1):234–43. COMMENT: A study of early life rural exposure on infant microbiome and childhood atopic outcomes.
- 95.Martina C, Looney RJ, Marcus C, Allen M, Stahlhut R. Prevalence of allergic disease in Old Order Mennonites in New York. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2016;117(5):562–3.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Phillips JT, Stahlhut RW, Looney RJ, Järvinen KM. Food allergy, breastfeeding, and introduction of complementary foods in the New York Old Order Mennonite Community. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2020;124(3):292–4.e2. [DOI] [PubMed] [Google Scholar]
- 97.Järvinen KM, Davis EC, Bevec E, Jackson CM, Pizzarello C, Catlin E, et al. Biomarkers of Development of Immunity and Allergic Diseases in Farming and Non-farming Lifestyle Infants: Design, Methods and 1 Year Outcomes in the “Zooming in to Old Order Mennonites” Birth Cohort Study. Frontiers in Pediatrics. 2022;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ege MJ, Bieli C, Frei R, van Strien RT, Riedler J, Ublagger E, et al. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. The Journal of allergy and clinical immunology. 2006;117(4):817–23. [DOI] [PubMed] [Google Scholar]
- 99.Pfefferle PI, Büchele G, Blümer N, Roponen M, Ege MJ, Krauss-Etschmann S, et al. Cord blood cytokines are modulated by maternal farming activities and consumption of farm dairy products during pregnancy: the PASTURE Study. The Journal of allergy and clinical immunology. 2010;125(1):108–15.e1-3. [DOI] [PubMed] [Google Scholar]
- 100.Douwes J, Cheng S, Travier N, Cohet C, Niesink A, McKenzie J, et al. Farm exposure in utero may protect against asthma, hay fever and eczema. The European respiratory journal. 2008;32(3):603–11. [DOI] [PubMed] [Google Scholar]
- 101.Horak E, Morass B, Ulmer H, Genuneit J, Braun-Fahrländer C, von Mutius E. Prevalence of wheezing and atopic diseases in Austrian schoolchildren in conjunction with urban, rural or farm residence. Wiener klinische Wochenschrift. 2014;126(17–18):532–6. [DOI] [PubMed] [Google Scholar]
- 102.Braun-Fahrländer C, Gassner M, Grize L, Neu U, Sennhauser FH, Varonier HS, et al. Prevalence of hay fever and allergic sensitization in farmer’s children and their peers living in the same rural community. SCARPOL team. Swiss Study on Childhood Allergy and Respiratory Symptoms with Respect to Air Pollution. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 1999;29(1):28–34. [DOI] [PubMed] [Google Scholar]
- 103.··. Steiman CA, Evans MD, Lee KE, Lasarev MR, Gangnon, Olson BF, et al. Patterns of farm exposure are associated with reduced incidence of atopic dermatitis in early life. The Journal of allergy and clinical immunology. 2020;146(6):1379–86.e6. COMMENT: First study demostrating farm -life protection against AD in America.
- 104.Tong H, Gao L, Deng Y, Kong Y, Xiang R, Tan L, et al. Prevalence of Allergic Rhinitis and Associated Risk Factors in 6 to 12 Years Schoolchildren From Wuhan in Central China: A Cross-sectional Study. American journal of rhinology & allergy. 2020;34(5):632–41. [DOI] [PubMed] [Google Scholar]
- 105.Tong X, Tong H, Gao L, Deng Y, Xiang R, Cen R, et al. A Multicenter Study of Prevalence and Risk Factors for Allergic Rhinitis in Primary School Children in 5 Cities of Hubei Province, China. International archives of allergy and immunology. 2022;183(1):34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.·. Strieker S, Weinmann T, Gerlich J, von Mutius E, Nowak D, Radon K, et al. Farm living and allergic rhinitis from childhood to young adulthood - prospective results of the GABRIEL study. The Journal of allergy and clinical immunology. 2022. COMMENT: Demostrated a role for farm exposure in childhood is protective against childhood and young adult AR.
- 107.Loss G, Apprich S, Waser M, Kneifel W, Genuneit J, Büchele G, et al. The protective effect of farm milk consumption on childhood asthma and atopy: the GABRIELA study. The Journal of allergy and clinical immunology. 2011;128(4):766–73.e4. [DOI] [PubMed] [Google Scholar]
- 108.von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nature reviews Immunology. 2010;10(12):861–8. [DOI] [PubMed] [Google Scholar]
- 109.Broussard JL, Devkota S. The changing microbial landscape of Western society: Diet, dwellings and discordance. Molecular metabolism. 2016;5(9):737–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrländer C, et al. Exposure to environmental microorganisms and childhood asthma. The New England journal of medicine. 2011;364(8):701–9. [DOI] [PubMed] [Google Scholar]
- 111.Trikamjee T, Basera W, Botha M, Facey-Thomas HE, Gaunt B, Genuneit J, et al. Associations between Environmental dust composition and Atopic Dermatitis in urban and rural settings. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2021;32(5):1013–21. [DOI] [PubMed] [Google Scholar]
- 112.Mahdavinia M, Greenfield LR, Moore D, Botha M, Engen P, Gray C, et al. House dust microbiota and atopic dermatitis; effect of urbanization. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2021;32(5):1006–12. [DOI] [PubMed] [Google Scholar]
- 113.Chen CC, Chen KJ, Kong MS, Chang HJ, Huang JL. Alterations in the gut microbiotas of children with food sensitization in early life. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2016;27(3):254–62. [DOI] [PubMed] [Google Scholar]
- 114.Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007;56(5):661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bisgaard H, Li N, Bonnelykke K, Chawes BL, Skov T, Paludan-Müller G, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. The Journal of allergy and clinical immunology. 2011;128(3):646–52.e1-5. [DOI] [PubMed] [Google Scholar]
- 116.Depner M, Taft DH, Kirjavainen PV, Kalanetra KM, Karvonen AM, Peschel S, et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nature medicine. 2020;26(11):1766–75. [DOI] [PubMed] [Google Scholar]
- 117.Seppo AE, Bu K, Jumabaeva M, Thakar J, Choudhury RA, Yonemitsu C, et al. Infant gut microbiome is enriched with Bifidobacterium longum ssp. infantis in Old Order Mennonites with traditional farming lifestyle. Allergy. 2021;76(11):3489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Henrick BM, Hutton AA, Palumbo MC, Casaburi G, Mitchell RD, Underwood MA, et al. Elevated Fecal pH Indicates a Profound Change in the Breastfed Infant Gut Microbiome Due to Reduction of Bifidobacterium over the Past Century. mSphere. 2018;3(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nature medicine. 2016;22(10):1187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Henrick BM, Rodriguez L, Lakshmikanth T, Pou C, Henckel E, Arzoomand A, et al. Bifidobacteria-mediated immune system imprinting early in life. Cell. 2021;184(15):3884–98.e11. [DOI] [PubMed] [Google Scholar]
- 121.Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science (New York, NY). 2015;349(6252):1106–10. [DOI] [PubMed] [Google Scholar]
- 122.Lluis A, Depner M, Gaugler B, Saas P, Casaca VI, Raedler D, et al. Increased regulatory T-cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. The Journal of allergy and clinical immunology. 2014;133(2):551–9. [DOI] [PubMed] [Google Scholar]
- 123.Schaub B, Liu J, Höppler S, Schleich I, Huehn J, Olek S, et al. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. The Journal of allergy and clinical immunology. 2009;123(4):774–82.e5. [DOI] [PubMed] [Google Scholar]
- 124.Lundell AC, Hesselmar B, Nordström I, Adlerberth I, Wold AE, Rudin A. Higher B-cell activating factor levels at birth are positively associated with maternal dairy farm exposure and negatively related to allergy development. The Journal of allergy and clinical immunology. 2015;136(4):1074–82.e3. [DOI] [PubMed] [Google Scholar]
- 125.Strömbeck A, Nordström I, Andersson K, Andersson H, Johansen S, Maglio C, et al. Allergic disease in 8-year-old children is preceded by delayed B cell maturation. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2017;47(7):918–28. [DOI] [PubMed] [Google Scholar]
- 126.Abbring S, Hols G, Garssen J, van Esch B. Raw cow’s milk consumption and allergic diseases - The potential role of bioactive whey proteins. European journal of pharmacology. 2019;843:55–65. [DOI] [PubMed] [Google Scholar]
- 127.Abbring S, Ryan JT, Diks MAP, Hols G, Garssen J, van Esch B. Suppression of Food Allergic Symptoms by Raw Cow’s Milk in Mice is Retained after Skimming but Abolished after Heating the Milk-A Promising Contribution of Alkaline Phosphatase. Nutrients. 2019;11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Abbring S, Blokhuis BRJ, Miltenburg JL, Olmedo K, Garssen J, Redegeld FA, et al. Direct Inhibition of the Allergic Effector Response by Raw Cow’s Milk-An Extensive In Vitro Assessment. Cells. 2020;9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abbring S, Kusche D, Roos TC, Diks MAP, Hols G, Garssen J, et al. Milk processing increases the allergenicity of cow’s milk-Preclinical evidence supported by a human proof-of-concept provocation pilot. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2019;49(7):1013–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wild CP. The exposome: from concept to utility. International journal of epidemiology. 2012;41(1):24–32. [DOI] [PubMed] [Google Scholar]
- 131.Moran TP. The External Exposome and Food Allergy. Current allergy and asthma reports. 2020;20(8):37. [DOI] [PMC free article] [PubMed] [Google Scholar]