Abstract
Myrtus, commonly called myrtle, is a genus of flowering plants in the Myrtaceae family. This study aimed to review myrtle’s pharmaceutical, food, and other uses. The pharmacological effects of myrtle for antioxidant, antibacterial, and anti-inflammatory activities, reduction of COVID-19 symptoms, anti-diabetic in the animal model, hepatoprotective in the rat model, antihypertensive, control of intestinal helminthiasis in mice model, inhibition of glucosyltransferase activity, protective effect on oxidative metabolism in the hypothyroidism model, and reducing the damage caused by skin burns are reviewed. In addition, the food uses of this plant such as improving the oxidative and microbial stability of products containing salmon, antimicrobial activity in meat and dairy products, flavoring in sea salt, microbial improvement of fresh fruits during post-harvest storage, animal nutrition, and bio-oil production are summarized.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41110-023-00194-y.
Keywords: Myrtle; Extract, Biological activity, Pharmaceutical activity, Medicinal food activity
Introduction
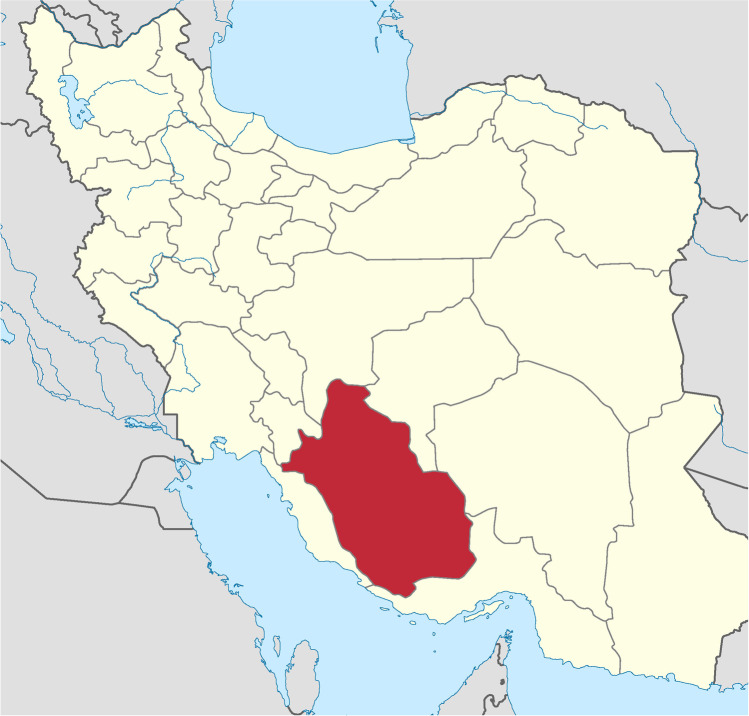
Myrtaceae family contains 100 genera and 3000 species. Myrtus communis L. (Myrtaceae) is one of the popular medicinal plants that have been used in the phytomedicine system since ancient Greece. Myrtus communis L. (myrtle) genus is an evergreen and fragrant perennial shrub that belongs to the Myrtaceae family [1–5]. Myrtle (Myrtus communis L.) is a wild shrub native in the Mediterranean and the Middle East, especially in Iran, southern Europe, northern Africa, western Asia, and the Indian subcontinent. It is also a symbol of love and immortality in the Mediterranean region [2, 5–8]. Myrtle is a native plant in Rostam region, Fars Province, in Iran (Fig. 1).
Fig. 1.
Map of Rostam region, province of Fars, Iran
Myrtle has evergreen leaves 2–5 cm long and becomes fragrant after crushing, and these leaves are highly bitter due to astringency [9]. The flowers of the myrtle plant are stellar, white or pink, and very fragrant. The round blueberry fruit of this plant contains several seeds [10] (Fig. 2). Myrtle fruit has a unique taste and could be in two different colors, black or white [3, 11]. The dark blue fruit of this plant mainly contains polyphenolic compounds and shows high antioxidant activity. The main constituents of myrtle white fruit contain unsaturated fatty acids such as myrtenyl acetate, linoleic acid, and oleic acid.
Fig. 2.

Photos of myrtle (Myrtus communis L.): a tree, b flower, c and d leaves, and e black fruits from Rostam region, province of Fars, Iran
Myrtle has been widely used in the pharmaceutical and food industries due to its high content of bioactive ingredients. A variety of medicinal uses are reported for the leaves and fruits of the myrtle plant. These include treatment of wounds, gastrointestinal tract, urinary tract disorders, diarrhea, dysentery, pulmonary disorders, rheumatism, deep sinuses, dyspepsia, anxiety, insomnia, diabetes, hypertension, and menstrual problems. This plant also has astringent, anti-inflammatory, anti-hemorrhoid, anti-skin disease, anti-pain, anti-infertility, anti-viral, antibacterial, antifungal, antioxidant, and neuroprotective properties [1, 3, 4, 12, 13]. According to literature, the crushed leaves of this plant are added to butter or oil ointment. Then, they have been employed in dermatosis treatment and hair and body care. The decoction of myrtle leaves was mixed with goat’s milk and warmed on charcoal. It was employed for liver diseases by the Algerian nomads of the Tassili region. Myrtle leaves are used as a common beverage, such as green tea. Its fresh or dried berries were used to treat mouth canker sores [12]. Previous studies investigated morphological and chemical characterization of two wild Tunisian myrtle (Myrtus communis L.) populations. Myrtle leaves have more antioxidant activity than other parts of the plant due to their having more phenolic compounds [14]. Also, it was found that the phenolic fraction of the myrtle leaf and the fruit was rich in tannins, while the stem was rich in flavonoids [9]
Star myrtle, a new species of Myrcia (Myrtaceae) from Espirito Santo in Brazil, was identified during classification studies [15]. Lemon myrtle (Backhousia citriodora) and anise myrtle (Syzygium anisatum) belong to the family Myrtaceae. They are native Australian plants. The leaves of this plant are collected to use in folk medicine, phytotherapy, and as a lemon flavoring agent in cuisines. Also, for the production of herbal tea with lemon flavor, the aromatic essential oil of lemon myrtle has been used for flavoring food and personal care products [16–19].
The annual market value of this farm is estimated at between 7 and 23 million Australian dollars [16]. According to a study carried out in Turkey on the physicochemical properties of myrtle berries, the fruit has 21.11 kcal calories per gram. It contains 4.17% protein, 17.41% fiber, 2 .37% fat, 8.64% sugar, 76.11% tannin, and 0.01% essential oil [3]. Production of liqueur from Myrtus communis berries, called Mirto, resulted in domesticated cultivars, expanding myrtle berry production [20]. The production of myrtle fruits is also traditionally used in the confectionery and beverage industries. Ripe fruit juices of this plant are the main element of the famous Italian soft drink “Chinotto” [16]. In Sardinia (Italy), where the plant’s liqueur is an important consumer product, an alcoholic beverage is produced by macerating white or red berries of myrtle in a mixed water-ethanol solvent. The organoleptic and biological properties of the beverage were suggested due to the high concentration of oenothein B in liqueur [3, 5, 13, 20]. The fatty acid composition for myrtle (Myrtus communis L.) bark samples was reported to be 13.5% oleic acid, 15.8% palmitic acid, and 61.1% linoleic acid. In contrast, the seed samples contained 9.79% oleic acid, 10.38% palmitic acid, and 75.5% linoleic acid [21]. Some biological activities of Myrtus communis L. berries seeds on gastrointestinal tract diseases such as ethnobotanical and phytochemical have been reviewed [22]. The influence of different drying methods on antioxidant properties of extractable phenolic compounds from lemon myrtle dried leaves has been reported [19]. Aqueous extracts of Myrtus communis L. populations of the southwest Zagros region of Iran were also evaluated. Although they have antibacterial properties against Escherichia coli, Bacillus subtilis, and Pseudomonas aeruginosa, no antifungal activity against Aspergillus oryzae was observed [23].
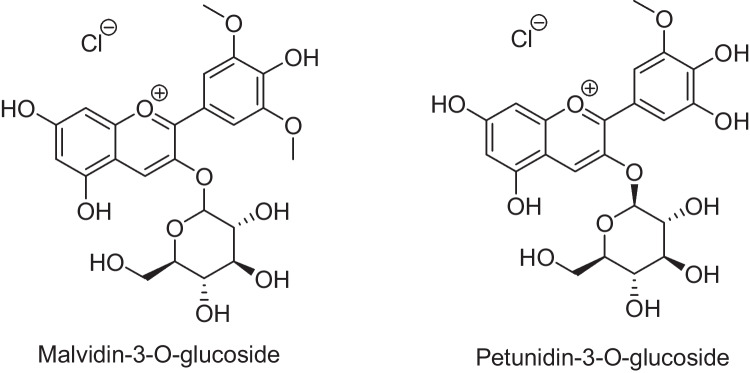
The anthocyanin profile in Myrtus communis berries from the Salento area was studied by high-performance liquid chromatography/electrospray ionization quadrupole time-of-flight mass spectrometry (LC/ESI-Q-TOF/MS) [24]. The study showed delphinidin 3-O-glucoside, petunidin 3-O-glucoside, and malvidin 3-O-glucoside with 31.5%, 25.8%, and 24.3%, respectively, as major anthocyanins of total pigments. The minor anthocyanins included cyanidin 3-O-glucoside (6.3%), delphinidin-pentose (4%), delphinidin-pentose (3.8%), peonidin 3-O-glucoside (2%), petunidin-pentose (1.6%), and petunidin-pentose (0.7%). Therefore, Myrtus communis berries of Salento are a significant precursor of natural antioxidants, which can be very useful in new biotechnological applications in the food and pharmaceutical industry. The physicochemical properties and antioxidant activity of commercial white myrtle berry liqueurs were also reported [25]. Asik and coworkers evaluated by optimizing the drying conditions of the spray (inlet air temperature: 120–180 °C) and the combination of wall materials (maltodextrin, gum arabic) to obtain myrtle berry extract powder with higher biological activities [26]. Saifullah et al. demonstrated microwave-assisted extraction as an effective extraction technique to extract gallic acid and hesperetin from the leaf of lemon myrtle (Backhousia citriodora) [27]. However, intraspecific diversity and environmental conditions can significantly affect the quantitative and qualitative levels of the main constituents of essential oils [5]. Also, much research has been carried out to preserve the natural genetic diversity of myrtle and design for efficient and logical exploitation of myrtle biomass to prevent further reduction of species’ genetic diversity [2, 27]. A comparative study was performed on preparing and evaluating the encapsulated extract’s physical and chemical properties in nanovesicles, including nanoliposomes and nanoniosomes, without cholesterol and toxic organic solvents [14]. According to the literature survey, some parts of the plant are common in the food industry for flavoring meat and preparing sauces. In rural areas, foods are flavored with myrtle smoke. Its leaves are also widely employed in the cosmetic and perfume industries [3]. In 2014, the pharmacological effects of Myrtus communis L. were summarized in a review [28]. The current mini-review target is to give an overview on the new developments and utilizations of myrtle extracts and other active ingredients of plant in biological, pharmaceutical, food nutrient, and clinical studies.
Phytochemical studies
The myrtle plant (M. communis L.) contains fiber, sugar, antioxidants, and many biologically active compounds. The commonly known volatile phytochemical compounds of myrtle (M. communis L.) are terpenoids, flavonoids, phenolics, tannins, and fatty acids (Tables 1 and 2). The chemical structure of some components of M. communis L. is shown in Figs. 3 and 4. The essential oil of M. communis, which was extracted from the leaves, branches, fruits, and flowers through steam distillation, is characterized by a yellow or greenish-yellow color with a refreshing smell.
Table 1.
The major phytoconstituents content of M. communis L. essential oils in different plant parts
| Chemical class | Major essential oil compound | Content (%) | M. communis L. parts | Reference(s) |
|---|---|---|---|---|
| Terpenes | α-Pinene | 10–60 |
Leaf Stem Flower |
[29–31] |
| Limonene | 9–17.1 |
Leaf Stem Flower |
[29, 30] | |
| Myrcene | 0.6–2.09 | Flower | [30] | |
| p-Cymene | 2.5–3.5 | Stem | [30] | |
| α-Caryophyllene | 0.2–0.3 | Stem | [30] | |
| Germacrene D | 2.5 | Stem | [30] | |
| Terpenoids | 1,8-Cineole | 12–34 |
Leaf Steam Flower |
[4, 29–31] |
| Linalool | 2.01–7.0 |
Stem Flower |
[29, 30] | |
| Myrtenol | 0.1–0.6 | Stem | [30]. | |
| 4.01 | Flower | [29] | ||
| Nerol | 0.15 | Stem | [30] | |
| Geraniol | 1.5–2 | Flower | [29, 30] | |
| Caryophyllene oxide | 1.5 | Stem | [30] | |
| Spathulenol | 0.6 | Flower | [29, 30] | |
| Phenylpropanoids | Methyleugenol | 4–4.5 | Flower | [30] |
Table 2.
The major phytoconstituents content of M. communis L. extract from different plant parts
| Chemical class | Content from different parts (%) | Chemical subclasses | Major compound(s) | References |
|---|---|---|---|---|
| Phenolic acids |
Leaf (12–15) Stem (38–40) Flower (38–40) Berries (8–10) |
- |
Gallic acid Caffeic acid Syringic acid Vanillic acid Ferulic acid |
[30] |
| Tannins |
Leaf (79–82) Stem (trace) Flower (60) Berries (53–56) |
Hydrolysable tannins | Gallotannins | [30] |
| Proanthocyanidins |
Malvidin-3-O-arabinoside Petunidin-3-O-arabinoside Delphinidin-3-O-arabinoside Peonidin-3-O-glucoside Cyanidin-3-O-glucoside Malvidin-3-O-glucoside Petunidin-3-O-glucoside |
[30] | ||
| Flavonoids |
Leaf (8–10) Stem (61–63) Flower (trace) Berries (35–39) |
Flavanols | Catechin | [30] |
| Flavonols |
Quercetin-3-d-rahmnoside Quercetin-3-d-galactoside Quercetin Myricetin-3-O-ramnoside Myricetin-3-O-galactoside Myricetin |
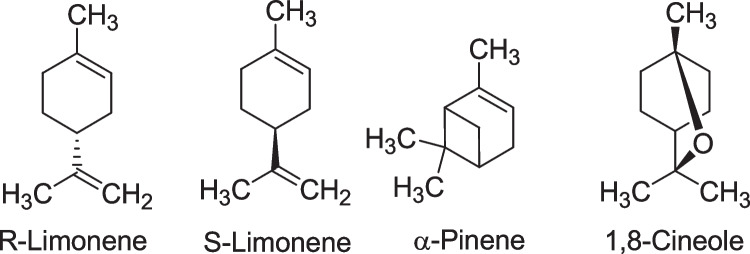
Fig. 3.
Chemical structure of most common monoterpenes and sesquiterpenoids in myrtle-derived essential oils
Fig. 4.
Chemical structure of most common anthocyanins present in myrtle berry
Myrtus species are very rich in essential oil, phenolic acids, flavonoid, tannin, anthocyanin pigments, and fatty acids. Myrtle (M. communis L.) leaves contained small amounts of phenolic acids (caffeic, ellagic, and gallic acids) and a flavonoid, i.e., quercetin 3-O-galactoside and quercetin 3-O-rhamnoside). The flavonoids such as galloyl derivatives of catechin and myricetin derivatives were reported in large quantities. The hydrolyzable tannins, polyphenolic compounds, and myricetin glycosides were isolated from the leaves. In addition, the major terpenoids and their derivatives such as α-pinene, α-terpineol, linalool, 1,8-cineole, geranyl butyrate, geraniol, caryophyllene oxide, and neryl acetate from the oil sample of myrtle leaves were demonstrated.
α-Pinene, 1,8-cineole, limonene, and linalool were reported as the major compounds in the essential oil of myrtle leaves from Iran. The herb leaves is rich in flavonoids such as quercetin, catchin, and myricetin derivatives. It was found that the flowers of this medicinal plant in Morocco contained a 1.75% essential oil, which included the components of α-pinene (48.54%), 1,2-cineol (14.75%), myrtinal (5.01%), myrtinol (4.01%), myrtinal acetate (3.45%), myrcene (2.09%), linalool (2.01%), and geraniol (1.67%) [32]. Oligomeric compounds such as myrtucommulone A, myrtucommulone B, semi-myrtocommulone, and nonprenylated acylphloroglucinols have been reported in myrtle leaves [29]. Chemical composition of two myrtle oil samples taken from different regions was analyzed by gas chromatography-flame ionization detector (GC-FID), gas chromatography-mass spectrometry (GC-MS), and nuclear magnetic resonance (NMR) spectroscopy. The monoterpene derivatives were found as the main compounds: α-pinene (50.8% and 33.6%), 1,8-cineole (21.9% and 13.3%), linalool (2.7% and 14.8%), and linalyl acetate (0.5% and 9.5%) [29].
Myrtle fruit (berries) contains tannins, anthocyanins, fats, and organic acids, and their amounts depend on the solvent extraction and the fruit’s ripening time. Also, the amount of these compounds differs depending on the plant parts. Generally, the most common compounds in myrtle leaves, stem, and flowers are α-pinene (10-60%) and 1,8-cineole (12-34%) [30].
The chemical composition of berry extract and myrtle leaf has been studied. In both species of M. nivellei and M. communis, the main constituents were gallic acid derivatives, flavonol derivatives, and hydroxybenzoic acids. In the extract of M. nivellei, some compounds such as 2-hydroxy-1,8-cineole-β-D-glucopyranoside, 2-hydroxy-1,8-cineole, 2-O-α-L-arabinofuranosyl (1-6)-β-D-glucopyranoside, rugosin A, and rugosin B have been reported, which have not been mentioned in M. communis. The flavonoids and volatile compounds of essential oils of myrtle leaves and berries, including terpenoids, especially α-pinene, 1,8-cineole, geranyl acetate, and linalool, can influence their biological properties [31].
Phenolic compounds, flavonoids, and anthocyanins are the main phytochemicals in berries. The seeds contain 12–15% fatty oil (fixed oil), including oleic, linoleic, myristic, palmitic, linolenic, and lauric acid glycerides. Based on the studies conducted on the analysis of the fatty acids of myrtle fruits, fourteen fatty acids are detected, and oleic acid is the predominant fatty acid (67.07%), followed by palmitic acid (10.24%), and stearic acid (8.19%). The yield and quality of the oil depend on the production area, harvesting season, and the length of distillation. Moreover, the yield of essential oil (w/w) from various parts of the plant is different. Yields of water-distilled oils for the plant are leaf, 0.4–0.5%; flowers, 0.4%; unripe fruits, 0.5%; and ripe fruits, 0.02%. Terpenoid compounds, such as myrtenyl acetate, 1,8-cineole, limonene, and linalool were observed in the oil of leaves (0.19–0.37%), fruits (0.03–0.13%), and flowers (0.21–0.26%) in different proportions. Also, the essential components were 1,8-cineole, α-pinene, methyl eugenol, terpineol, trans-carveol, cis-carveol, geraniol, methyl geranate, α-terpinyl acetate, neryl acetate, beta-caryophyllene, myrcene, sabinene, myrcene, p-cymene, c-terpinene, linalyl acetate, car-3-ene, phellandrene, methyl eugenol, methyl butyrate, methyl benzoate, benzyl alcohol, isobutyl butyrate, myrtenyl acetate, limonene, α-terpineol, linalool, eucalyptol, p-cymol, beta-pinene, geranyl, camphene, butyl butyrate, and myrtenol. Citric acid, malic acid, resin, tannin, fixed oil, sugar, flavonoids, anthocyanin arabinosides, anthocyanin glucosides, kaempferol, quercetin, myricetin 3-o-glucoside, myristin 3 rutinoside, esculin, scopoletin, caffeic acid, myricetin 3-o-rhamnoside or myricitrin, esculetin-6-oglucoside or esculin, hesperetin 7-o-rhamnoglucoside or hesperidin, and hesperetin-2-o-methylcalcone-4-o-rhamnoglucoside were detected in berries. The leaves contain tannins, flavonoids, coumarins, myrtucommulone A and B, semimyrtucomulone, galloyl glucosides, ellagitannins, galloylquinic acids, caffeic, gallic, and ellagic acids. Tannins, alkaloids, glycosides, reducing sugars, fixed oil, gallic acids, phenolic acids, quercetin, and patuletin were also reported in the myrtle root extraction [33].
In a study, the hydroethanolic extract of dry myrtle leaves was performed in the range of 50 to 200 °C, and the GC-MS analysis showed the main compounds as follows: 1,2,3-Benzenetriol (40.62%), a polyphenol; 3,5,7-Trihydroxy-2-isopropyl-chromen-4-one (28.37%), a flavonol; and 5-Hydroxymethylfurfural (14.29%), a furan [34].
Mohamed et al. investigated the chemical composition of myrtle leave essential oil in the Eastern province of Saudi Arabia by GC analysis. Essential oil contained 48 components; however, 86.4% peak area included 28 identified and quantified components. Major components were identified as monoterpene hydrocarbons (77.9%), with 1,8-cineol (25.9%), α-pinene (14.2%), linalool (4.6%), and its oxide (3.2%), verbenol (4.6%), and carveol (5.3%). Although their topically formulated essential oil exhibited anti-inflammatory activity regarding the reduction of Prostaglandin E2 concentration in inflamed tissue, the anti-inflammatory efficiency was less than a positive control by ibuprofen. Myrtle essential oil showed a notable inhibitory effect on T47D cell lines’ proliferation. Their results demonstrated that myrtle essential oil could be a promising agent in complementary therapy [35].
Biological and pharmaceutical activities of myrtle
The bioactive compounds of Myrtus communis L. leaves and berries extracted by ultrasound-assisted extraction were identified by Pereira et al. [36]. The major components in the leaves were in glycoside forms, including myricetin-galactoside-gallate, quercetin-galactoside-gallate, and myricetin. The ingredients in the berries were oenothein B, galloyl-HHDP-glucose, digalloyl HHDP-glucose, quinic acid 3,5-di-O-gallate, cyanidin-3-O-glucoside, peonidin-3-O-monoglucoside, and myricetin galactoside-gallate. Then, the antioxidant efficiency of extracts was investigated. The values of antioxidants capacity recorded by the oxygen radical antioxidant capacity (ORAC) and Trolox equivalent antioxidant capacity (TEAC) methods were in good agreement with the polyphenol content. In addition, it was indicated that myrtle’s high antioxidant capacity was attributed to the flavonols and flavanol compounds and not to the anthocyanins.
The antimicrobial activity of leaf extracts of Myrtus communis against pathogenic bacterial strains (Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi, and Staphylococcus aureus) and fungi strains (Fusarium oxysporum and Aspergillus niger) was reported [37]. The results showed that bacterial infections caused by Escherichia coli and Staphylococcus aureus strains could be treated by the hexane and methanol extract of Myrtus communis, and chloroform extract could be employed to treat the fungal disease caused by Fusarium oxysporum.
Benchikh and coworkers investigate the antioxidant activity of the methanol extract and its fractions. The highest total phenolic, tannins, and flavonoids could be obtained by methanol, water, and ethyl acetate extraction, respectively [38]. The most increased inhibiting activity for the oxidation of β-carotene/linoleic acid was induced by chloroform (93.95%) and ethyl acetate extracts (90.29%), respectively. The ethyl acetate extract exhibited an excellent antioxidant activity using 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging (IC50 = 0.004 mg/mL). In contrast, the aqueous extract showed high activity in the hydroxyl radical scavenging test (IC50 = 0.08 mg/mL) and reducing power (EC50 = 0.03 mg/mL).
Nirmal et al. studied the antibacterial activity of lemon myrtle and anise myrtle essential oil in water nanoemulsion against two gram-positive (Staphylococcus aureus, Listeria monocytogenes) and two gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa) [18]. Although anise myrtle essential oil and its nanoemulsion did not show any inhibitory activity against the bacteria mentioned above, lemon myrtle essential oil and its nanoemulsion displayed inhibitory activity against Staphylococcus aureus, Listeria monocytogenes, and Escherichia coli.
The antibacterial activity of myrtle extract was studied in vitro [39]. In addition, the phytoniosome of myrtle extract was assessed against Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Micrococcus luteus, and Bacillus subtilis. The results demonstrated that all the strains were more sensitive to niosome containing myrtle extract than free myrtle extract.
Beikzadeh and coworkers reported the cellulose acetate electrospun nanofibers encapsulating lemon myrtle essential oil as a robust and sustainable antimicrobial system [40]. The antibacterial efficiency of lemon myrtle essential oil-loaded cellulose acetate electrospun nanofibers was determined by minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) against Escherichia coli and Staphylococcus aureus, which showed 100% efficiency even at 2 wt. % concentration of lemon myrtle essential oil. In addition, antibacterial activity was observed even after 2 months of storage.
The antibacterial effect of aqueous and methanol extract of Myrtus communis was investigated against Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia [41]. Both Myrtus communis extracts showed antibacterial activity against all bacteria mentioned above. Furthermore, it was indicated that aqueous extract was more appropriate than alcoholic extract regarding time and cost-efficiency.
Gao et al. investigated the particle size and grinding effect on physicochemical and functional properties of downy rose-myrtle powders. The total polyphenols and anthocyanins were determined [42]. Then, the antiradical activity was evaluated by a 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assay after the intestinal phase of digestion. The results showed that the antiradical activity of the crude powder samples was further increased, while those of the superfine powder remained unchanged. A good correlation was also detected between the antioxidant activity of the aqueous and methanol downy rose-myrtle extracts and the total polyphenolic content (r = 0.9316, P < 0.01) or the total anthocyanin content (r = 0.8956, P < 0.01).
A myrtle case study was carried out by electron paramagnetic resonance (EPR) spin trapping under forced aging conditions on the oxidative stability of plant hydroalcoholic extracts [43]. This study confirmed the hypothesis that ellagic acid is the main compound responsible for the oxidative stability of myrtle hydroalcoholic extracts. Furthermore, in addition to the expected antioxidant behavior, this extract showed a pro-oxidant effect that helped to create radical species.
Cheikh et al. evaluated the antioxidant activity of alginate nanocomposite biofilms containing sepiolite modified with polyphenols from myrtle berries extract [44]. The results showed that incorporating different concentrations from myrtle berries extract improved the antioxidant powers by the ABTS method compared to that of the Trolox standard for the films compared with control film Alg/Sep.
The antibacterial activity of Myrtus communis ethanolic leaf extract was evaluated by minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and inhibition-zone size against gram-positive bacteria [45]. Myrtle ethanolic extract showed a significant inhibitory effect against gram-positive and acid-fast bacteria, while it showed no effect on the growth of gram-negative bacteria.
The anti-inflammatory of lemon myrtle extract was examined using the lipopolysaccharide (LPS)-induced RAW model system and anti-oxidative activities of alcoholic lemon myrtle extract by Shim et al. [46]. The results revealed that both lemon myrtle leaf and volatile stem oils showed high antioxidant by DPPH assay. In addition, the ABTS radical scavenging activity was comparatively higher than the DPPH radical scavenging activity. The use of lemon myrtle extract as a potential therapeutic agent was demonstrated with the potent anti-inflammatory effects that can be used to treat inflammatory bowel disease.
Anti-inflammatory and cytotoxic activities of essential oil from myrtle leaves were evaluated by Mohamed and his group [35]. It was found that anti-inflammatory and cytotoxic activities, assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) on HCT and T47D cell lines, could be used as a protective agent in complementary therapy.
Recently, the acceleration of the healing process of patients clinically suspected of COVID-19 pneumonia and the decreasing hospital admission and other related complications using M. communis was investigated [47]. It was reported that consumption of M. communis as a potent anti-viral agent in the first days reduced the signs and symptoms of the disease of COVID-19 and respiratory distress and improved health.
Tas et al. studied the hypoglycemic, hypolipidemic, and oxidative stress inhibitory efficiency of Myrtus communis L. fruits hydroalcoholic extract in vivo in normoglycemic and streptozotocin-induced diabetic rats [48]. It was found that insulin secretion was increased in the diabetic rats, whereas their blood glucose and lipid levels decreased.
The anti-diabetic activity type I and II of hydroalcoholic extract of Myrtus communis (myrtle) fruits were also reported by Talebianpoor and coworkers [49]. Streptozotocin-induced and dexamethasone-induced diabetic rats showed that the extract reduced the serum levels of glucose, triglyceride, urine volume, urine protein, and malondialdehyde at the end of the 45 days.
Very recently, Khodaie et al. evaluated the effect of Myrtus communis aqueous extract-containing gel on wound healing in streptozotocin-induced diabetic rats [50]. These results indicate that the gel containing myrtle aqueous extract has wound healing potential in diabetic conditions. Therefore, it was suggested to use a topical gel containing aqueous extract 6% for treating and healing diabetic wounds.
The chemical composition and hepatoprotective effect of essential oil from Myrtus communis L. flowers against CCl4-induced acute hepatotoxicity in rats [51]. The study suggested that M. communis contains promising substances to counteract the CCl4 intoxication, which may be efficient in preventing hepatotoxicity complications.
The effect of Myrtus communis extract on serum cytokines in angiotensin-dependent hypertensive rats was evaluated by Cevikelli-Yakut et al. [52]. Further research is needed regarding the protective agents of Myrtus communis extract against tissue injury caused by inflammatory cytokines generated in hypertension. Lemon myrtle (Backhousia citriodora) extract displayed the highest GTF-inhibitory activity and inhibited S. mutans biofilm, but myrtle extracts did not inhibit cell growth. They also investigated the effect of the leaves, flowers, and roots of (Myrtus communis L.) essential oil on oxidative metabolism in an HT model induced by propylthiouracil (PTU) in rats. The results showed that M. communis L. oil plays a protective and supportive role in HT.
The effect of standardized extract of Myrtus communis L. (myrtle) against bleomycin-induced pulmonary fibrosis was also investigated by Fekri and coworkers [53]. The inflammation and fibrosis were improved in the myrtle regime group in the early phase, especially fibrosis, based on the biochemical and histopathological results.
The myrtle efficiency for vaginal suppositories in patients with cervicovaginal human papillomavirus (HPV) infection was investigated [54]. The results showed that the myrtle vaginal suppository was effective in HPV clearance compared with placebo.
Ozcan et al. studied the oral or topical myrtle (Myrtus communis L.) protective role against burn-induced damage [55]. Their findings showed that topical and oral utilization of myrtle significantly reduced burn-induced damage in the skin.
The Myrtus communis L. solution effect versus ketoconazole shampoo in dandruff treatment was demonstrated [56]. The 90 individuals were randomized into two equal groups, whereas the treatment group received Myrtus communis L. solution and a placebo shampoo. In contrast, the control group received ketoconazole shampoo and a placebo solution for 1 month. Both groups showed remarkable improvements (P<0.001). Moreover, no significant differences were displayed between the groups regarding efficacy, satisfaction rate, and side effects (P>0.05 for each outcome).
A comparative study was performed to evaluate the effect of Myrtus communis herbal and anti-hemorrhoid ointments on hemorrhoid symptoms, quality of life (primary outcomes), treatment satisfaction, and side effects (secondary outcomes) [57]. The treatment and control groups received the Myrtus communis herbal and the anti-hemorrhoid ointment twice a day with an interval of 12 ± 2 h, by a drug applicator through the rectum for 4 weeks. The results showed that the severity of all hemorrhoid symptoms decreased in both two groups, and no statistically significant difference was between the two groups (P>0.05).
The influence of fatty acid composition and the protective mechanism of aqueous extract of Myrtle berries seeds against an alcohol-induced peptic ulcer in rats was studied [58]. The results demonstrated that protective effects of myrtle berries seeds (MBSs) aqueous extract against alcohol-induced gastric and duodenal ulceration were mainly due to antioxidant properties of polyunsaturated fatty acids (PUFAs) and high levels of phenolic compounds. One year later, he and coworkers showed that the erythrocytes osmotic stability disturbance, hematological, and biochemical toxicity due to chronic alcohol consumption could be abolished by aqueous extract of myrtle berries seeds (MBSs) [22]. Very recently, Jabri et al. investigated the role of myrtle berry seeds on helminthiasis treatment regarding its anti-inflammatory and nematicidal properties and the scavenging activity for reactive oxygen species [59]. It was found that the total mean adult worms were drastically decreased by administration of myrtle (Myrtus communis L.) berry seeds aqueous extract (100 mg/kg, b.w., p.o.) compared with the infected and non-treated group. Moreover, the plant extract efficiency was comparable to Albendazole, a standard drug. Their results approve the potential of Myrtus communis berry seeds aqueous extract as an excellent natural aqueous extract for intestinal helminthiasis control.
The healing effect of three plant species (Myrtus Communis L., Camellia sinensis L., Zataria multiflora Boiss.) on intraoral ulcers in rats was investigated [60]. It was found that Myrtus communis L. has the highest healing effects on oral wound compared with other plant species.
The protective activity of Myrtus communis in the scopolamine-induced Alzheimer's model through cholinergic receptors was evaluated [61]. The results exhibited that the Myrtus communis administration remarkably improved the scopolamine-induced reduction of spatial memory and increased brain-derived neurotrophic factor (BDNF), M1, and acetylcholine (Ach) receptor expression levels in the different brain regions. Also, Myrtus communis regulation was demonstrated on cholinergic function, neurotransmitters, and galectin (GAL) in inflammations.
Khosropour et al. investigated the anti-inflammatory activity of Myrtus communis hydroalcoholic extract and its essential oil on acetic acid-induced colitis in rats [62]. This study showed the healing activity of Myrtus communis on colitis, probably due to its anti-inflammatory and antioxidant activities. Myrtus communis beneficial effect was proximate to Prednisolone and Mesalazine. Myrtus communis extract and essential oil were effective at lower doses; however, a dose-response relationship requires further basic tests and clinical trials to introducing Myrtus communis extract and essential oil to the pharmaceutical society.
The effect of the extracts from myrtle liqueur processing waste, rich in tannins and flavonoids, on modulating stem cell pluripotency in vitro under oxidative stressing conditions by hydrogen peroxide treatment was reported [63]. Cells were treated with extracts for 12–24 and 48 h and then senescence-induced. Finally, the reactive oxygen species (ROS) production was recorded. The expression of inflammatory cytokines, sirtuin-dependent epigenetic changes, and the stem cell pluripotency modifications were evaluated by the real-time polymerase chain reaction (PCR). The results prove that industrial myrtle waste obtained from its berries could protect cells from oxidative stress damage due to its antioxidant and antisenescence activity. It can be employed in the formulation of food or pharmaceutical fields with antioxidant, anti-aging, and anti-inflammatory properties.
Nourzadeh and coworkers compared antimicrobial activity of chlorhexidine, sodium hypochlorite, and extracts obtained from Eucalyptus galbie and Myrtus communis L. by methanol against Enterococcus faecalis (E. faecalis) [64]. Both extracts showed good efficiency for destroying E. faecalis; however, their antibacterial activity was lower than sodium hypochlorite. Thus, these natural extracts seem to be promising agents for endodontics treatment.
Recently, Gorjian et al. conducted a comparative study on antioxidant and antimicrobial properties of different formulation of the encapsulated myrtle extract of nanoliposome and nanoniosome [14]. As-obtained nanovesicles were temperature and pH-sensitive. Thermal phase transition of encapsulated myrtle improved, and myrtle extract release rate could be controlled. The myrtle extract-encapsulated nanoliposomes showed higher release rates and more robust antioxidant activity compared with those nanoniosomes. Also, the nanoliposome formulations showed the lowest MIC and the highest zone inhibition against Staphylococcus aureus, Escherichia coli, and Salmonella enteritidis compared to those of nanoniosome formulations.
Streptococcus mutans in human dental plaques can cause dental caries, and glucosyltransferases (GTFs) are involved in the formation of dental plaque. Therefore, the dental caries can be minimized by control of their activity. It was indicated that the highest GTF-inhibitory activity was observed in the presence of lemon myrtle (Backhousia citriodora) extract with an IC50 = 0.14 mg/mL [65]. It was found that the cell growth was not prevented by the extracts.
Medicinal food activities of myrtle
The effect of extra virgin olive oil (EVOO) enriched by myrtle phenolic extracts was studied as an antioxidant on iron-mediated lipid peroxidation under simulated intestinal conditions (pH 7.4) [66]. Their results showed that Myrtus communis phenolic compounds could interact with EVOO to inhibit phospholipid peroxidation. Thus, it was indicated that this system can be a potential functional food.
In the same year, the effects of modified atmosphere in cold storage on the liqueur quality of myrtle fruit (Myrtus communis L.) was studied [67]. It was indicated that the storage of myrtle fruit at oxygen concentration between 60 and 80% at 2 °C for 3 weeks had a good effect on the quality and increased the total phenolic and anthocyanin concentration.
One year later, Fadil et al. studied the combined treatment of Thymus vulgaris L., Rosmarinus officinalis L., and Myrtus communis L. essential oils against Salmonella typhimurium for optimization antibacterial activity via a mixture design methodology [68]. Formulations containing 55% essential oil of T. vulgaris L. and 45% of M. communis L. were considered the optimized formulations to increase the susceptibility of Salmonella typhimurium.
In addition, the prevention effect of flavored sea salts with Mediterranean fruits and herbs on cholesterol and phospholipid membrane oxidation and generation of cell-free radicals was investigated [69]. Their results showed that the salts flavored with myrtle, rosemary, and a mixture of herbs/plants preserved liposomes from Cu2+-induced oxidation.
The antibacterial efficiency of the optimal formula of clove, cinnamon, lavender, and myrtle essential oils against Escherichia coli was studied [70]. The combination validation results exhibited efficiency, restricting the E. coli viability to 1 ×106 CFU·mL−1. In addition, the as-obtained formula was also considered to protect milk (and dairy products) from E. coli contamination in the dairy industry.
Tibaoui et al. investigated the fatty acid profile, physicochemical properties, and oxidative stability of ewe’s sausage affected by distilled myrtle (Myrtus communis) leaves’ intake [71]. This study exhibited that the distilled myrtle did not significantly affect lamb sausage’s physicochemical and sensory properties, while it increased oxidative lipid stability.
The reduced respiration and transpiration of the fresh loquat (Eriobotrya japonica L.) fruits during storage through exposing them to the volatile essential oils of myrtle (Myrtus communis L.) leaves was reported [72]. One control and four different treatments were conducted, including (a) water vapor (2 min), (b) myrtle leaves (3% w/w), (c) myrtle leaf vapor (2 min), and (d) myrtle leaf vapor (10 min). The best results for reducing of weight loss, decay incidence, and browning index were obtained through exposure to myrtle leaves (3 wt. %) and myrtle leaf vapor (2 min).
The anti-proliferative and anti-quorum sensing potential of Myrtus communis L. essential oil was evaluated for the improved microbial stability of salmon-based products [73]. Their results demonstrated the role of myrtle essential oil as a new generation antimicrobial in the food industry.
A study was conducted in vitro and in vivo to control the post-harvest diseases caused by Penicillium spp. on mandarin fruit during storage with myrtle leaf phenolic extracts [74]. Their finding displayed that myrtle extracts could strongly inhibit the conidial germination of the pathogens. In addition, it was suggested that myrtle extracts could act as a possible natural alternative to synthetic fungicides.
Toxicity studies
It was mentioned that Myrtucommulone, a unique nonprenylated acylphloroglucinol in the leaves of M. communis., causes cell death of different cancer cell lines (EC50 = 3–8 μmol) with apoptotic features such as activation of caspase-3, 8, and 9, cleavage of poly (ADP-ribose) polymerase (PARP), and release of nucleosome to cytosol and DNA fragmentation [75]. It was less toxic to human blood mononuclear cells or immediate skin fibroblasts (EC50 cell death = 20–50 μmol). M. communis up to 30 μmol barely induced PARP, caspase-3, 8, and 9 processing in human PBMC. Myrtucommulone apoptosis is by the intrinsic rather than extrinsic death pathway. Therefore, M. communis caused loss of mitochondrial membrane potential in MM6 cells and release of cytochrome c from mitochondria. Interestingly, caspase-9-deficient Jurkat cells were resistant to myrtucommulone-induced cell death, and no processing of PARP or caspase-8 was evident. Finally, myrtucommulone induced apoptosis in cancer cell lines, with marginal cytotoxicity for non-transformed cells, through the cytochrome c/Apaf-1/mitochondrial caspase-9 pathway [75].
The toxicity study of Australian essential oil of Backhousia citriodora (lemon myrtle), in vitro based on The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS), was performed. Cytotoxicity testing in laboratory conditions showed that lemon myrtle oil and citral both had highly toxic effects on human cell lines: HepG2 (a cell line derived from hepatocarcinoma); F1-73 (a fibroblast cell line derived from normal skin); and primary cell cultures of human skin fibroblasts. Cytotoxicity IC50 values (50% inhibitory concentration) were also reported to range from 0.008–0.014% (w/v) at 4 h to 0.003–0.012% (w/v) at 24 h of exposure. In this study, the no observed adverse effect level (NOAEL) for lemon myrtle oil was determined to be 0.5 mg/L per 24-h exposure, and the RfD (reference dose) was 0.01 mg/L. A product containing 1% lemon myrtle oil has low toxicity and could potentially be used in the formulation of topical antimicrobial products [75].
In a study, the effect of different concentrations of myrtle extract (ME) and niosomal myrtle extract (nME) (0.1–7 μg/mL) for 24 h on the viability of 3T3 cells (these cells were planted in 96-well plates with a density of 5000 cells per well) was investigated for 4 h in a 37 °C incubator by the MTT assay, a colorimetric method to determine cell viability in cytotoxicity and proliferation studies. The results showed that the F5 formula of nME with a concentration of 7 (μg/mL) exhibited less toxicity on cells, which could be due to the encapsulation of ME. It was mentioned by other researchers that the empty niosome composed of nonionic surfactants and Chol had not any toxic effect on treated HeLa and A549 cell lines at concentrations of 0.2–10 mM [39].
In another research, cytotoxic activity was determined using the MTT method on HCT and T47D cell lines. Myrtle oil showed a dose-dependent inhibitory effect on the proliferation of both cell lines. However, its effect on T47D cells was more obvious, so that cell viability was 15% in T47D and 43% in HCT cells after 24 h of treatment with 800 μg/ml of oil. These findings suggest that myrtle oil exerts anti-inflammatory and cytotoxic effects, which can be used as a protective agent in complementary therapy [35].
Conservation strategy studies
Estimation of the conservation value of myrtle was investigated by modeling some long-term consequences of CO2 fertilization for global forests and forest industries in a case study in the Dooreh forest area of Lorestan Province, Iran. Increases in CO2 concentration (mol L−1) have implications for climate change because they directly affect global temperature levels. In this context, forests are a potential source of CO2 emissions, especially due to deforestation. In this study, forecast of forest area and forest reserves and production, consumption, prices, and trade of various products from fuel wood to paper and cardboard were obtained with the global forest products model (GFPM) for each scenario, with and without CO2 fertilization from 2011 to 2065. The results showed that CO2 fertilization increased wood storage and led to a decrease in the price of wood, which in turn caused a decrease in the average price of final products and higher global consumption. However, production and added value in industries decreased in some regions due to relative competitive advantages and different regional effects of CO2 fertilization. Overall, the main effect of CO2 fertilization was to increase the level of global forest reserves in 2065 by 9–10% for the A2 and B2 scenarios and 20% for the A1B scenario. Also, the general results showed that the increase in forest reserves caused by fertilization was partially offset by the stimulation of wood supply, which led to a decrease in the price of wood and an increase in harvesting [76].
A study of integrated site conservation strategies for endangered New Zealand Myrtaceae species was conducted. The current threat of myrtle rust (Austropuccinia psidii) to a number of endemic, socially, and economically important New Zealand Myrtaceae species requires that site conservation be used to supplement in situ populations. In this study, seed banking options were determined by evaluating seed drought tolerance, cultivation in laboratory conditions, freezing of pollen, and freezing of zygotic embryos of resistant Syzygium maire. Therefore, a desiccation experiment was conducted on six species of Myrtaceae: Lophomyrtus bullata, L. obcordata, Metrosideros diffusa, M. umbellata, M. bartlettii, and Syzygium maire. The results showed that the seeds and embryos of S. maire confirmed the extreme sensitivity to drying, while the seeds of other species were drought tolerant. Zygotic embryos of S. maire were successfully frozen using the encapsulation-dehydration method, and pollen freezing was successful for M. excelsa following drying to about 5% moisture. Also, the efficiency of manual pollination in the production of live seeds was tested, and tissue culture protocols were successfully developed for selected Myrtaceae. In addition, photoautotrophic micropropagation techniques were developed for L. scoparium in this study. Therefore, the importance of comprehensive conservation strategies to ensure future access to unique New Zealand Myrtaceae germplasm as a key component in the long-term management response to the threat posed by A. psidii was highlighted [27].
Azizi and coworkers evaluated the drought resistance mechanism and the drought mitigation potential of common myrtle essential oil on growth, seedling survival, physiology, and biochemical traits under soil water deficit by single and dual inoculations of Arbuscular mycorrhizal fungi (AMF) and plant growth-promoting rhizobacteria (PGPR) boosts [77]. It was indicated that the dual inoculations significantly increase myrtle drought resistance by increasing water and nutrients and stimulating antioxidant defenses. Furthermore, it was shown that dual inoculations were the most effective and low-cost method for optimizing myrtle cultivation and restoration programs.
Agrimonti et al. investigates the biological conservation strategies in the two regions of Sardinia and Calabria of Italy, a molecular genetic approach to myrtle (Myrtus communis L.). It is significant that reductions in the size and number of wild populations are often associated with the loss of genetic diversity and reproductive potential. Therefore, in this study, fluorescent amplified fragment length polymorphisms (fAFLPs) were used to assess the genetic diversity within and among natural populations of myrtle from Sardinia and Calabria to gain new understanding into their adaptation and survival potential. The results showed that a significant amount of variation (31.15%) was attributed to the different between the population groups of Sardinia and Calabria, which indicates the genotypic differentiation between the populations of these two regions. The Analysis of MOlecular VAriance (AMOVA) analysis showed that the genetic diversity within populations (51.86%) was higher than among populations (16.99%), as previously reported for outcrossing species. Also, intra-population genetic diversity assessed by unbiased expected heterozygosity (HE) estimates ranged from 0.0595 to 0.2595. These values was related to the population increase (r = 0.918; P < 0.01) with two reproductive parameters: seed germination (r = 0.793; P < 0.01) and the number of seeds in the fruit (r = 0.631; P < 0.05). Therefore, a medium gene flow among Sardinian and Calabrian myrtle populations (1.2719 and 1.0478, respectively) counteracts the low level of genetic diversity observed in some populations and prevents their differentiation and isolation [78].
In a study, Gossia gonoclada (angle-stemmed myrtle) was listed as threatened under the Environment and Biodiversity Conservation Act 1999 (Cwlth) (EPBC Act) in force from 16 July 2000. Under state and territory legislation, these species can be listed as threatened. Small population and limited distribution are the main factors that make this species eligible for the endangered list. Among the key threats affecting angular-stemmed myrtle are the spread of myrtle rust, excessive land use activities, and the spread of weeds. One of Logan City Council’s conservation actions is to protect the angle-stemmed myrtle, including the protection of all Gossia gonoclada trees and their habitat through vegetation management. Among them, tree planting is considered part of vegetation projects [79].
Other activities of myrtle
The effects of dietary myrtle (Myrtus communis) on skin mucus immune parameters and mRNA level of growth, antioxidant, and immune-related genes in zebrafish (Danio rerio) were evaluated by Safari and coworkers [80]. Their study showed that dietary administration of myrtle improved mucosal immune parameters and modified mRNA levels of selected genes.
The effect of whole exhausted Myrtus communis L. berries, a by-product of commercial liquor production, was studied on milk production traits and blood metabolites supplied to dairy ewes [81]. No effects were observed in composition, milk yield, and coagulation properties; however, milk urea decreased with increasing the exhausted myrtle berries in the diet. These results proved a positive effect of exhausting myrtle berries on dietary nitrogen use. Furthermore, although the blood urea and protein count in the blood decreased with the inclusion of exhausted myrtle berries in the diet, no influence was displayed on the liver, kidney, and hematologic parameters. Furthermore, exhausted myrtle berries could be used in the diet of dairy ewes without adverse effects on milk production and health level.
Taee and coworkers investigated the effect of dietary myrtle (Myrtus communis L.) on bactericidal activity and non-specific immune parameters of the rainbow trout (Oncorhynchus mykiss) fingerling skin mucus [8]. The results showed that the highest skin mucus soluble protein level and alkaline phosphate activity were observed in fish groups fed with 1 and 1.5% Myrtle (P < 0.05). On the contrary, the treatment and control groups showed no notable difference in skin mucus lysozyme activity (P > 0.05). Furthermore, the antibacterial activity against Escherichia coli, Staphylococcus aureus, and Salmonella enterica failed in treatments and control groups. However, the antimicrobial activity against Aeromonas hydrophila and Yersinia ruckeri was demonstrated in 1 and 1.5% myrtle treatment groups. Therefore, they indicated that dietary myrtle plays a beneficial role on the fingerling rainbow trout mucosal immune parameters.
The responses of muscle satellite cells to lemon myrtle supplementation combined with electrical stimulation in disuse-induced skeletal muscle atrophy were evaluated by Takuwa and coworkers [82]. The results showed that the combination of myrtle supplementation and electrical stimulation suppressed the reduction of fiber cross-section compared to each factor alone. Furthermore, the number of myonuclei increased along with the activation of muscle satellite cells.
The effect of the dietary supplementation of exhausted myrtle berries (EMB), containing polyphenols, was evaluated on the metabolism and microbial population in the ruminal fluid of Sarda dairy ewes [83]. The supplementation of EMB displayed no effect on rumen pH (P < 0.05); however, total volatile fatty acids (VFA) reduced (P < 0.05). The results demonstrate that EMB fed to sheep affected the rumen microbiota structure and reduced the abundance of the Butyrivibrio group and the rumen ammonia accumulation. Furthermore, the dietary nitrogen balance was improved by using EMB as a feed additive in dairy sheep.
Lemon myrtle waste was proved as a valuable feedstock for pyrolysis with high volatile chemicals and low ash content [84]. The maximum pyrolytic degradation was determined at 355 °C by thermogravimetric analysis/derivative thermogravimetric (TGA/DTA). Gas chromatography-mass spectrometry (GC-MS) analysis showed that the bio-oil contained large amounts of acetic acid, phenol, 3-methyl-1,2-cyclopentanedione, 1,2-benzenediol, guaiacol, 2-furanmethanol, and methyl dodecanoate. A drop in the % peak areas for organic acids and ketones, along with an increase in the % peak area for guaiacols and anhydrosugars, was observed in higher pyrolysis temperatures.
Buffa et al. investigated the effect of diets containing different dried by-products from grape marc (GM), tomato pomate (TP), and exhausted myrtle berries (EMBs) on milk and blood plasma antioxidant capacity of dairy ewes [85]. Sarda ewes were fed by four diets, including (a) control (no by-product), 100 g of GM per day, 100 g of TP per day, and 75 g of EMBs per day. In addition, the milk fatty acid profile was investigated by gas chromatography. The results showed that all by-products did not modify the nutritional indexes of milk fat. Instead, they increased the milk antioxidant capacity; however, further studies are required to determine the optimal inclusion level of TP and EMB in sheep diet.
The corrosion inhibitor activity of essential oils of myrtle (MEO) and rosemary (REO) were demonstrated as eco-friendly cathodic inhibitors in 3 wt. % sodium chloride solution [86]. Open circuit potential (OCP), potentiodynamic polarization (PDP), and electrochemical impedance spectroscopy (EIS) evaluated the copper corrosion behavior. The results demonstrated that both EOs increased cathodic inhibitor capacity, and REO was superior to MEO. Furthermore, it was indicated that the adsorption of EOs on the cupper surface is mainly physical and followed by a Langmuir isotherm.
The effects of myrtle (Myrtus communis L.) essential oils as dietary antioxidant supplementation were investigated on carcass and meat quality of goat meat [87]. A MEO dietary supplement to goat diets could improve goat carcass traits and meat quality characteristics without negative effects on its fatty acid profile.
The antibacterial effect of rose myrtle (Rhodomyrtus tomentosa) seed extract was investigated in vitro against three acute hepatopancreatic necrosis disease (AHPND) bacterial strains [88]. In addition, its efficiency against AHPND in shrimp was demonstrated in vivo. The results indicated that R. tomentosa extract could be an alternative route to minimize AHPND in shrimp with reduced drug/chemical residues in shrimp products.
Atik et al. separated the essential oil from the leaves, flowers, and roots of Myrtus communis L. [89]. Then, they investigated the effect of the as-obtained essential oil on oxidative metabolism in a hypothyroidism (HT) model induced by propylthiouracil (PTU) in rats. Their result showed an increase in antioxidant activity among groups supplemented with myrtle essential oil.
Outlook future
The antioxidant, antimicrobial, anti-diabetic, antihypertensive, and anti-cancer properties of different parts of the myrtle plant have been confirmed in this review. Therefore, the myrtle plant has promising properties and potential applications in the food and pharmaceutical industries. It can be a versatile plant for industrials such as soft drinks, dairy products, and meat. Most research has been conducted on Myrtus communis L.; however, only a few studies reported on lemon myrtle (Backhousia citriodora) and anise myrtle (Syzygium anisatum). Therefore, more studies and research should be performed to find more efficient and low-cost extraction methods at low temperatures and determine the extract composition and characterization by appropriate spectroscopic techniques. In general, the literature survey exhibits that myrtle can be used as a valued precursor in the biological and pharmaceutical fields of food chemistry. Therefore, a step can be taken in the food industry by preparing medicinal diet-based plant foods using the myrtle plant and myrtle waste.
Supplementary Information
(DOCX 36 kb)
Abbreviations
- AHPND
Acute hepatopancreatic necrosis disease
- A. actinomycetemcomitans
Actinobacillus actinomycetemcomitans
- AAPH
2,2′-Azobis(2-methylpropionamidine)dihydrochloride
- ABTS
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- Ach
Acetylcholine
- ALP
Alkaline phosphatase
- AMOVA
Analysis of MOlecular VAriance
- ARE
Arylesterase
- B. subtilis
Bacillus subtilis
- BDNF
Brain-derived neurotrophic factor
- b.o
Body weight
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- E. aerogenes
Enterococcus aerogenes
- E. coli
Escherichia coli
- E. faecalis
Enterococcus faecalis
- EIS
Electrochemical impedance spectroscopy
- EMBs
Exhausted myrtle berries
- EOs
Essential oils
- EPR
Electron paramagnetic resonance
- EVOO
Extra virgin olive oil
- fAFLPs
Fluorescent amplified fragment length polymorphisms
- GAL
Galectin
- GC-MS
Gas chromatography-mass spectrometry
- GFPM
The global forest products model
- GM
Grape marc
- GTFs
Glucosyltransferases
- HPPD
2,2-Diphenyl-1-picryl-hydrazyl-hydrate
- HT
Hypothyroidism
- K. pneumoniae
Klebsiella pneumoniae
- LC/ESI-Q-TOF/MS
High-performance liquid chromatography/electrospray ionization quadrupole time-of-flight mass spectrometry
- LPS
Lipopolysaccharide
- M. luteus
Micrococcus luteus
- M. smegmatis
Mycobacterium smegmatis
- MBE
Myrtle berries extract
- MBSs
Myrtle berries seeds
- MBC
Minimum bactericidal concentration
- MC
Myrtus communis L.
- MIC
Minimum inhibitory concentration
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
- NO
Nitric oxide
- NOAEL
No observed adverse effect level
- OCP
Open circuit potential
- ORAC
Oxygen radical antioxidant capacity
- P. aeruginosa
Pseudomonas aeruginosa
- P. gingivalis
Porphyromonas gingivalis
- P. intermedia
Prevotella intermedia
- PARP
Poly (ADP-ribose) polymerase
- PCR
Polymerase chain reaction
- PDP
Potentiodynamic polarization
- p.o or PO
By mouth
- PON
Paraoxonase
- PSA
Plasma scavenging activity
- PUFAs
Polyunsaturated fatty acids
- PTU
Propylthiouracil
- RfD
Reference dose
- ROS
Reactive oxygen species
- S. aureus
Staphylococcus aureus
- S. epidermidis
Staphylococcus epidermidis
- S. marcescens
Serratia marcescens
- S. mutans
Streptococcus mutans
- S. typhi
Salmonella typhi
- SASA
Superoxide anion (O2•−) scavenging activity
- TBARS
Thiobarbituric acid reactive substance
- TGA/DTA
Thermogravimetric analysis/derivative thermogravimetric
- TP
Tomato pomate
- TEAC
Trolox equivalent antioxidant capacity
- VFA
Volatile fatty acids
Author contribution
Hayedeh Gorjian: investigation, data collection, writing-original draft. Nader Ghaffari Khaligh: conceptualization, resources, visualization, validation, writing-review and editing.
Funding
This reasearch was funded by the Universiti Malaya International Collaboration Grant (ST018-2022), Malaysia.
Data Availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Competing interest
The authors declare no competing interests.
References
- 1.Ghafouri F, Rahimmalek M. Genetic structure and variation in different Iranian myrtle (Myrtus communis L.) populations based on morphological, phytochemical and molecular markers. Ind Crop Prod. 2018;123:489–499. doi: 10.1016/j.indcrop.2018.06.086. [DOI] [Google Scholar]
- 2.Mele C, Corona L, Melito S, Raggi L, Mulas M. The genetic diversity of selections and wild populations of myrtle revealed by molecular geographic contexts. Ind Crop Prod. 2019;132:168–176. doi: 10.1016/j.indcrop.2019.02.018. [DOI] [Google Scholar]
- 3.Siracusa L, Napoli E, Tuttolomondo T, Licata M, La Bella S, Gennaro MC, Ruberto G. A two-year bio-agronomic and chemotaxonomic evaluation of wild Sicilian myrtle (Myrtus communis L.) berries and leaves. Chem Biodivers. 2019;16(3):e1800575. doi: 10.1002/cbdv.201800575. [DOI] [PubMed] [Google Scholar]
- 4.Sisay M, Gashaw T. Ethnobotanical, ethnopharmacological, and phytochemical studies of Myrtus communis Linn: a popular herb in Unani system of medicine. J Evid Based Complement Altern Med. 2017;22(4):1035–1043. doi: 10.1177/2156587217718958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Usai M, Marchetti M, Culeddu N, Mulas M. Chemotaxonomic evaluation by volatolomics analysis of fifty-two genotypes of Myrtus communis L. Plants. 2020;9(10):1288. doi: 10.3390/plants9101288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dejam M, Farahmand Y. Essential oil content and composition of myrtle (Myrtus communis L.) leaves from South of Iran. J Essent Oil-Bear Plants. 2017;20(3):869–872. doi: 10.1080/0972060X.2014.981599. [DOI] [Google Scholar]
- 7.Shahbazian D, Karami A, Eshghi S, Maggi F. Variation in the essential oil yields and compositions of Myrtle (Myrtus communis L.) populations collected from natural habitats of Southern Iran. J Essent Oil Res. 2018;30(5):369–378. doi: 10.1080/10412905.2018.1486745. [DOI] [Google Scholar]
- 8.Taee HM, Hajimoradloo A, Hoseinifar SH, Ahmadvand H (2017) Dietary myrtle (Myrtus communis L.) improved non-specific immune parameters and bactericidal activity of skin mucus in rainbow trout (Oncorhynchus mykiss) fingerlings. Fish Shellfish Immunol. 64:320–4. 10.1016/j.fsi.2017.03.034 [DOI] [PubMed]
- 9.Aidi Wannes W, Saidani Tounsi M, Marzouk B (2019) Morphological and chemical characterization of two wild Tunisian myrtle (Myrtus communis L.) populations. Trends Phytochem Res 3(4):231–42
- 10.Rashed KN, Phytochemical Z. and bioactivities of Myrtus communis L.: a review. Plant Sci 2021;4(2):133–6. 10.32439/ps.v4i2.133-136
- 11.Giampieri F, Cianciosi D, Forbes-Hernández TY. Myrtle (Myrtus communis L.) berries, seeds, leaves, and essential oils: new undiscovered sources of natural compounds with promising health benefits. Food Front. 2020;1(3):276–295. doi: 10.1002/fft2.37. [DOI] [Google Scholar]
- 12.Hennia A, Nemmiche S, Guerreiro A, Faleiro ML, Antunes MD, Aazza S, Miguel MG. Antioxidant and antiproliferative activities of Myrtus communis l. essential oils from different Algerian regions. J Essent Oil-Bear Plants. 2019;22(6):1488–1499. doi: 10.1080/0972060X.2019.1687335. [DOI] [Google Scholar]
- 13.Medda S, Fadda A, Dessena L, Mulas M. Quantification of total phenols, tannins, anthocyanins content in Myrtus communis L. and antioxidant activity evaluation in function of plant development stages and altitude of origin site. Agronomy. 2021;11(6):1059. doi: 10.3390/agronomy11061059. [DOI] [Google Scholar]
- 14.Gorjian H, Amiri ZR, Milani JM, Khaligh NG. Preparation and characterization of the encapsulated myrtle extract nanoliposome and nanoniosome without using cholesterol and toxic organic solvents: a comparative study. Food Chem. 2021;342:128342. doi: 10.1016/j.foodchem.2020.128342. [DOI] [PubMed] [Google Scholar]
- 15.Gaem PH, Scaravelli FS, Valdemarin KS, Lucas E, Mazine FF. Star myrtle, a new Myrcia (Myrtacae) from Espírito Santo, Brazil. Brittonia. 2021;73:1–7. doi: 10.1007/s12228-021-09667-8. [DOI] [Google Scholar]
- 16.Flamini G, Pistelli L, Ascrizzi R, Pistelli L, Zinnai A. The influence of ripeness stage and growth area on myrtle-leaved orange (chinotto) peel essential oil composition. Biochem Syst Ecol. 2020;91:104071. doi: 10.1016/j.bse.2020.104071. [DOI] [Google Scholar]
- 17.Heim RHJ, Wright IJ, Allen AP, Geedicke I, Oldeland J. Developing a spectral disease index for myrtle rust (Austropuccinia psidii) Plant Pathol. 2019;68(4):738–745. doi: 10.1111/ppa.12996. [DOI] [Google Scholar]
- 18.Nirmal NP, Mereddy R, Li L, Sultanbawa Y. Formulation, characterisation and antibacterial activity of lemon myrtle and anise myrtle essential oil in water nanoemulsion. Food Chem. 2018;254:1–7. doi: 10.1016/j.foodchem.2018.01.173. [DOI] [PubMed] [Google Scholar]
- 19.Saifullah M, McCullum R, McCluskey A, Vuong Q. Effects of different drying methods on extractable phenolic compounds and antioxidant properties from lemon myrtle dried leaves. Heliyon. 2019;5(12):e03044. doi: 10.1016/j.heliyon.2019.e03044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franco AM, Tocci N, Guella G, Dell’Agli M, Sangiovanni E, Perenzoni D, Manca G. Myrtle Seeds (Myrtus communis L.) as a rich source of the bioactive ellagitannins oenothein B and eugeniflorin D2. ACS Omega. 2019;4(14):15966–15974. doi: 10.1021/acsomega.9b02010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akyuz M, GuzelL A, Elmastas M. Fatty acid composition and antioxidant capacity of Myrtus (Myrtus communis L.). Malays. Appl Biol J. 2019;48(5):101–112. [Google Scholar]
- 22.Jabri MA, Marzouki L, Sebai H. Ethnobotanical, phytochemical and therapeutic effects of Myrtus communis L. berries seeds on gastrointestinal tract diseases: a review. Arch Physiol Biochem. 2018;124(5):390–396. doi: 10.1080/13813455.2017.1423504. [DOI] [PubMed] [Google Scholar]
- 23.Salimi Beni A, Kocheki Shahmokhtar M, Masoumias A, Khajehsharifi H. Phytochemical and biological studies of some myrtus (Myrtus communis L.) populations of south west region of Zagros (Iran) Nat Prod Chem Res. 2017;5(290):2329–6836. doi: 10.4172/2329-6836.1000290. [DOI] [Google Scholar]
- 24.Scorrano S, Lazzoi MR, Mergola L, Di Bello MP, Del Sole R, Vasapollo G. Anthocyanins profile by Q-TOF LC/MS in Myrtus communis berries from Salento area. Food Anal Methods. 2017;10(7):2404–2411. doi: 10.1007/s12161-017-0813-6. [DOI] [Google Scholar]
- 25.Serreli G, Jerković I, Gil KA, Marijanović Z, Pacini V, Tuberoso CIG. Phenolic compounds, volatiles and antioxidant capacity of white myrtle berry liqueurs. Plant Foods Hum Nutr. 2017;72(2):205–210. doi: 10.1007/s11130-017-0611-8. [DOI] [PubMed] [Google Scholar]
- 26.Asik S, Atbakan Kalkan T, Topuz A. Optimization of spray drying condition and wall material composition for myrtle extract powder using response surface methodology. Drying Technol. 2021;39(12):1–14. doi: 10.1080/07373937.2021.1914077. [DOI] [Google Scholar]
- 27.Nadarajan J, van der Walt K, Lehnebach CA, Saeiahagh H, Pathirana R. Integrated ex situ conservation strategies for endangered New Zealand Myrtaceae species. NZ J Bot. 2021;59(1):72–89. doi: 10.1080/0028825X.2020.1754245. [DOI] [Google Scholar]
- 28.Alipour G, Dashti S, Hosseinzadeh H. Review of pharmacological effects of Myrtus communis L. and its active constituents. Phytother Res. 2014;28(8):1125–1136. doi: 10.1002/ptr.5122. [DOI] [PubMed] [Google Scholar]
- 29.Asgarpanah J, Ariamanesh A (2015) Phytochemistry and pharmacological properties of Myrtus communis L. Indian J Trad Knowl 1(1).82-87. http://nopr.niscpr.res.in/bitstream/123456789/32031/1/IJTK%201(1)%2082-87.pdf
- 30.Aleksic V, Knezevic P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol Res. 2014;169(4):240–254. doi: 10.1016/j.micres.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Miraj S, Kiani S (2016) A review study of therapeutic effects of Myrtus communis. Pharm Lett 8(9):281–5. http://scholarsresearchlibrary.com/archive.html. Accessed 15 Feb 2023
- 32.Derwich E, Benziane Z, Chabir R, Taouil R. Characterisation of volatiles and evaluation of antioxidant activity of the flowers essential oils of Myrtus communis L from Morocco. Int J Curr Pharm Res. 2011;3(3):17–23. [Google Scholar]
- 33.Sumbul S, Ahmad MA, Asif M, Akhtar M (2011) Myrtus communis Linn.-a review. Indian J Tradit Knowl. 2(4):395–402. https://www.researchgate.net/publication/287539126_Myrtus_communis_Linn_-_A_review. Accessed 15 Feb 2023
- 34.Gorjian H, Amiri ZR, Milani JM, Khaligh NG. Influence of nanovesicle type, nanoliposome and nanoniosome, on antioxidant and antimicrobial activities of encapsulated myrtle extract: a comparative study. Food Bioproc Tech. 2022;15:144–164. doi: 10.1007/s11947-021-02747-3. [DOI] [Google Scholar]
- 35.Mohamed ME, Mohafez OM, Khalil HE, Alhaider IA. Essential oil from Myrtle leaves growing in the eastern part of Saudi Arabia: components, anti-inflammatory and cytotoxic activities. J Essent Oil-Bear Plants. 2019;22(4):877–892. doi: 10.1080/0972060X.2019.1645046. [DOI] [Google Scholar]
- 36.Pereira P, Cebola MJ, Oliveira MC, Gil MGB. Antioxidant capacity and identification of bioactive compounds of Myrtus communis L. extract obtained by ultrasound-assisted extraction. J Food Sci Technol. 2017;54:4362–4369. doi: 10.1007/s13197-017-2907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Besufekad SY, Mekdes M, Abebech M, Delesa D, Tekalign D, Demitu K, Birtukan B (2017) The antimicrobial activity of leaf extracts of Myrtus communis. J Microb Biochem Tech 9(6):290–2. https://www.walshmedicalmedia.com/open-access/the-antimicrobial-activity-of-leaf-extracts-of-myrtus-communis-16928.html. Accessed 15 Feb 2023
- 38.Benchikh F, Amira S, Benabdallah H. The evaluation of antioxidant capacity of different fractions of Myrtus communis L. leaves. Annu Res Rev Biol. 2018;22:1–14. doi: 10.9734/ARRB/2018/39217. [DOI] [Google Scholar]
- 39.Raeiszadeh M, Pardakhty A, Sharififar F, Mehrabani M. Phytoniosome: a novel drug delivery for myrtle extract. Iran J Pharm Res. 2018;17(3):804–817. [PMC free article] [PubMed] [Google Scholar]
- 40.Beikzadeh S, Akbarinejad A, Swift S, Perera J, Kilmartin PA, Travas-Sejdic J. Cellulose acetate electrospun nanofibers encapsulating Lemon Myrtle essential oil as active agent with potent and sustainable antimicrobial activity. React Funct Polym. 2020;157:104769. doi: 10.1016/j.reactfunctpolym.2020.104769. [DOI] [Google Scholar]
- 41.Rahimvand L, Niakan M, Naderi NJ. The antibacterial effect of aquatic and methanolic extract of Myrtus communis on Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Prevotella intermedia. Iran J Microbiol. 2018;10(4):254–257. [PMC free article] [PubMed] [Google Scholar]
- 42.Gao X, Zhu D, Liu Y, Zha L, Chen D, Guo H. Physicochemical properties and anthocyanin bioaccessibility of downy rose-myrtle powder prepared by superfine grinding. Int J Food Prop. 2019;22(1):2022–2032. doi: 10.1080/10942912.2019.1702999. [DOI] [Google Scholar]
- 43.Sanna D, Mulas M, Molinu MG, Fadda A. Oxidative stability of plant hydroalcoholic extracts assessed by EPR spin trapping under forced ageing conditions: a myrtle case study. Food Chem. 2019;271:753–761. doi: 10.1016/j.foodchem.2018.07.156. [DOI] [PubMed] [Google Scholar]
- 44.Cheikh D, Martín-Sampedro R, Majdoub H, Darder M. Alginate bionanocomposite films containing sepiolite modified with polyphenols from myrtle berries extract. Int J Biol Macromol. 2020;165:2079–2088. doi: 10.1016/j.ijbiomac.2020.10.052. [DOI] [PubMed] [Google Scholar]
- 45.Mir MA, Bashir N, Alfaify A, Oteef MD. GC-MS analysis of Myrtus communis extract and its antibacterial activity against Gram-positive bacteria. BMC Complement Med Ther. 2020;20(1):86. doi: 10.1186/s12906-020-2863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shim SY, Kim JH, Kho KH, Lee M. Anti-inflammatory and anti-oxidative activities of lemon myrtle (Backhousia citriodora) leaf extract. Toxicol Rep. 2020;7:277–281. doi: 10.1016/j.toxrep.2020.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azimi M, Hasheminasab FS. Evaluating the E cacy and safety of the myrtle (Myrtus Communis) in treatment and prognosis of patients suspected to novel coronavirus (COVID-19) pneumonia: Study protocol for a randomized controlled trial. Trial. 2019;21:978. doi: 10.5455/medscience.2019.08.9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tas S, Tas B, Bassalat N, Jaradat N (2018) In vivo, hypoglycemic, hypolipidemic and oxidative stress inhibitory activities of Myrtus communis L. fruits hydroalcoholic extract in normoglycemic and streptozotocin-induced diabetic rats. Biomed Res 29(13):2727–34. https://www.alliedacademies.org/abstract/invivo-hypoglycemic-hypolipidemic-and-oxidative-stress-inhibitory-activities-of-myrtus-communis-l-fruits-hydroalcoholic-extract-in-10561.html. Accessed 15 Feb 2023
- 49.Talebianpoor MS, Talebianpoor MS, Mansourian M, Vafaiee Nejad T. Antidiabetic activity of hydroalcoholic extract of Myrtus communis (Myrtle) fruits in streptozotocin induced and dexamethasone induced diabetic rats. Pharm Res. 2019;11(2):115–120. doi: 10.4103/pr.pr_160_18. [DOI] [Google Scholar]
- 50.Khodaie SA, Emadi F, Naseri M, Kamalinejad M, Riahi SM, Alijaniha F, Roghani M. The effect of Myrtus communis aqueous extract-containing gel on wound healing in streptozotocin-induced diabetic rats. Curr Drug Discov Technol. 2021;18(4):542–547. doi: 10.2174/1570163817666200712163956. [DOI] [PubMed] [Google Scholar]
- 51.Hsouna AB, Dhibi S, Dhifi W, Mnif W, Hfaiedh N. Chemical composition and hepatoprotective effect of essential oil from Myrtus communis L. flowers against CCl4-induced acute hepatotoxicity in rats. RSC Adv. 2019;9(7):3777–3787. doi: 10.1039/C8RA08204A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cevikelli-Yakut ZA, Ertas B, Sen A, Sener G. Effect of Myrtus communis extract on serum cytokines in angiotensin dependent hypertensive rats. Medicine. 2020;9(2):404–407. doi: 10.5455/medscience.2019.08.9204. [DOI] [Google Scholar]
- 53.Fekri MS, Mandegary A, Sharififar F, Poursalehi HR, Nematollahi MH, Izadi A, Samareh Fekri M. Protective effect of standardized extract of Myrtus communis L. (myrtle) on experimentally bleomycin-induced pulmonary fibrosis: biochemical and histopathological study. Drug Chem Toxicol. 2018;41(4):408–414. doi: 10.1080/01480545.2018.1459670. [DOI] [PubMed] [Google Scholar]
- 54.Nikakhtar Z, Hasanzadeh M, Hamedi SS, Najafi MN, Tavassoli AP, Feyzabadi Z, Saki A. The efficacy of vaginal suppository based on myrtle in patients with cervicovaginal human papillomavirus infection: a randomized, double-blind, placebo trial. Phytother Res. 2018;32(10):2002–2008. doi: 10.1002/ptr.6131. [DOI] [PubMed] [Google Scholar]
- 55.Ozcan O, Ipekci H, Alev B, Ustundag UV, Ak E, Sen A, Akbay TT. Protective effect of Myrtle (Myrtus communis) on burn induced skin injury. Burns. 2019;45(8):1856–1863. doi: 10.1016/j.burns.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 56.Chaijan MR, Handjani F, Zarshenas M, Rahimabadi MS, Tavakkoli A. The Myrtus communis L. solution versus ketoconazole shampoo intreatment of dandruff: a double blinded randomized clinical trial. J Pak Med Assoc. 2018;68:715–720. [PubMed] [Google Scholar]
- 57.Malekuti J, Mirghafourvand M, Samadi K, Abbasalizadeh F, Khodaei L. Comparison of the effect of Myrtus communis herbal and anti-hemorrhoid ointments on the hemorrhoid symptoms and quality of life in postpartum women with grade I and II internal hemorrhoid: a triple-blinded randomized controlled clinical trial. J Complement Integr Med. 2019;16(4):20180147. doi: 10.1515/jcim-2018-0147. [DOI] [PubMed] [Google Scholar]
- 58.Jabri MA, Rtibi K, Tounsi H, Hosni K, Marzouki L, Sakly M, Sebai H. Fatty acid composition and mechanisms of the protective effects of myrtle berry seed aqueous extract in alcohol-induced peptic ulcer in rat. Can J Physiol Pharmacol. 2017;95(5):510–521. doi: 10.1139/cjpp-2016-0094. [DOI] [PubMed] [Google Scholar]
- 59.Jabri MA, Hajaji S, Rtibi K, Sebai H. Role of anti-inflammatory, reactive oxygen species scavenging activity and nematicidal properties of Myrtle berry seeds on Helminthiasis treatment. J Med Food. 2021;24(4):377–384. doi: 10.1089/jmf.2020.0089. [DOI] [PubMed] [Google Scholar]
- 60.Hashemipour MA, Lotfi S, Torabi M, Sharifi F, Ansari M, Ghassemi A, Sheikhshoaie S (2017) Evaluation of the effects of three plant species (Myrtus Communis L., Camellia Sinensis L., Zataria Multiflora Boiss.) on the healing process of intraoral ulcers in rats. J Dent 18(2):127–35. https://pubmed.ncbi.nlm.nih.gov/28620637/. Accessed 17 Feb 2023 [PMC free article] [PubMed]
- 61.Aykac A, Ozbeyli D, Uncu M, Ertaş B, Kılınc O, Şen A, Orun O, Sener G. Evaluation of the protective effect of Myrtus communis in scopolamine-induced Alzheimer model through cholinergic receptors. Gene. 2019;689:194–201. doi: 10.1016/j.gene.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 62.Khosropour P, Sajjadi SE, Talebi A, Minaiyan M. Antiinflammatory effect of Myrtus communis hydroalcoholic extract and essential oil on acetic acid–induced colitis in rats. J Rep Pharm Sci. 2019;8(2):204–210. doi: 10.4103/jrptps.JRPTPS_8_19. [DOI] [Google Scholar]
- 63.Cruciani S, Santaniello S, Fadda A, Sale L, Sarais G, Sanna D, Maioli M. Extracts from myrtle liqueur processing waste modulate stem cells pluripotency under stressing conditions. Biomed Res Int. 2019;2019. 10.1155/2019/5641034. [DOI] [PMC free article] [PubMed]
- 64.Nourzadeh M, Amini A, Fakoor F, Raoof M, Sharififar F. Comparative antimicrobial efficacy of Eucalyptus galbie and Myrtus communis L. extracts, chlorhexidine and sodium hypochlorite against Enterococcus faecalis. Iran Endod J. 2017;12(2):205–210. doi: 10.22037/iej.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yabuta Y, Mukoyama H, Kaneda Y, Kimura N, Bito T, Ichiyanagi T, Watanabe F. A lemon myrtle extract inhibits glucosyltransferases activity of Streptococcus mutans. Biosci Biotechnol Biochem. 2018;82(9):1584–1590. doi: 10.1080/09168451.2018.1478714. [DOI] [PubMed] [Google Scholar]
- 66.Dairi S, Carbonneau MA, Galeano-Diaz T, Remini H, Dahmoune F, Aoun O, Madani K. Antioxidant effects of extra virgin olive oil enriched by myrtle phenolic extracts on iron-mediated lipid peroxidation under intestinal conditions model. Food Chem. 2017;237:297–304. doi: 10.1016/j.foodchem.2017.04.106. [DOI] [PubMed] [Google Scholar]
- 67.Fadda A, Sarais G, Lai C, Sale L, Mulas M. Control of postharvest diseases caused by Penicillium spp. with myrtle leaf phenolic extracts: in vitro and in vivo study on mandarin fruit during storage. J Sci Food Agric. 2021;101(10):4229–4240. doi: 10.1002/jsfa.11062. [DOI] [PubMed] [Google Scholar]
- 68.Fadil M, Fikri-Benbrahim K, Rachiq S, Ihssane B, Lebrazi S, Chraibi M, Farah A. Combined treatment of Thymus vulgaris L., Rosmarinus officinalis L. and Myrtus communis L. essential oils against Salmonella typhimurium: optimization of antibacterial activity by mixture design methodology. Eur J Pharm Biopharm. 2018;126:211–220. doi: 10.1016/j.ejpb.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 69.Rosa A, Putzu D, Atzeri A, Cesare Marincola F, Sarais G. Sea salts flavored with Mediterranean herbs and fruits prevent cholesterol and phospholipid membrane oxidation and cell free radical generation. Eur J Lipid Sci Technol. 2018;120(4):1700323. doi: 10.1002/ejlt.201700323. [DOI] [Google Scholar]
- 70.Falleh H, Ben Jemaa M, Djebali K, Abid S, Saada M, Ksouri R. Application of the mixture design for optimum antimicrobial activity: combined treatment of Syzygium aromaticum, Cinnamomum zeylanicum, Myrtus communis, and Lavandula stoechas essential oils against Escherichia coli. J Food Process Preserv. 2019;43(12):e14257. doi: 10.1111/jfpp.14257. [DOI] [Google Scholar]
- 71.Tibaoui S, Essid I, Smeti S, Bertolin JR, Joy M, Atti N. Fatty acid profile, physiochemical properties and oxidative stability of ewe’s sausage as affected by distillated myrtle (Myrtus communis) leaves’ intake. Int J Food Sci Technol. 2020;55(3):1151–1161. doi: 10.1111/ijfs.14549. [DOI] [Google Scholar]
- 72.Bahadırlı NP, Kahramanoğlu İ, Wan C. Exposure to volatile essential oils of myrtle (Myrtus communis L.) leaves for improving the postharvest storability of fresh loquat fruits. J Food Qual. 2020;2020. 10.1155/2020/8857669.
- 73.Myszka K, Sobieszczańska N, Olejnik A, Majcher M, Szwengiel A, Wolko Ł, Juzwa W. Studies on the anti-proliferative and anti-quorum sensing potentials of Myrtus communis L. essential oil for the improved microbial stability of salmon-based products. LWT. 2020;127:109380. doi: 10.1016/j.lwt.2020.109380. [DOI] [Google Scholar]
- 74.Fadda A, Palma A, D'Aquino S, Mulas M. Effects of myrtle (Myrtus communis L.) fruit cold storage under modified atmosphere on liqueur quality. J Food Process Preserv. 2017;41(1):e12776. doi: 10.1111/jfpp.12776. [DOI] [Google Scholar]
- 75.Hayes AJ, Markovic B. Toxicity of Australian essential oil Backhousia citriodora (Lemon myrtle). Part 1. Antimicrobial activity and in vitro cytotoxicity. Food Chem Toxicol. 2002;40(4):535–543. doi: 10.1016/S0278-6915(01)00103-X. [DOI] [PubMed] [Google Scholar]
- 76.Buongiorno J. Modeling some long-term implications of CO2 fertilization for global forests and forest industries. Forest Ecosys. 2015;2(1):1–13. doi: 10.1186/s40663-015-0054-3. [DOI] [Google Scholar]
- 77.Azizi S, Kouchaksaraei MT, Hadian J, Abad ARFN, Sanavi SAMM, Ammer C, Bader MKF. Dual inoculations of arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria boost drought resistance and essential oil yield of common myrtle. Forest Ecol Manag. 2021;497:119478. doi: 10.1016/j.foreco.2021.119478. [DOI] [Google Scholar]
- 78.Agrimonti C, Bianchi R, Bianchi A, Ballero M, Poli F, Marmiroli N. Understanding biological conservation strategies: a molecular-genetic approach to the case of myrtle (Myrtus communis L.) in two Italian regions: Sardinia and Calabria. Conserv Gen. 2007;8(2):385–396. doi: 10.1007/s10592-006-9177-y. [DOI] [Google Scholar]
- 79.Mawson PR, Majer JD. The Western Australian threatened species scientific committee: lessons from invertebrates. 1999. [Google Scholar]
- 80.Safari R, Hoseinifar SH, Van Doan H, Dadar M. The effects of dietary Myrtle (Myrtus communis) on skin mucus immune parameters and mRNA levels of growth, antioxidant and immune related genes in zebrafish (Danio rerio) Fish Shellfish Immunol. 2017;66:264–269. doi: 10.1016/j.fsi.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 81.Nudda A, Correddu F, Atzori AS, Marzano A, Battacone G, Nicolussi P, Pulina G. Whole exhausted berries of Myrtus communis L. supplied to dairy ewes: effects on milk production traits and blood metabolites. Small Rumin Res. 2017;155:33–38. doi: 10.1016/j.smallrumres.2017.08.020. [DOI] [Google Scholar]
- 82.Takuwa M, Matsumoto T, Hirabayashi T, Ikeji T, Ono K, Honda S, Fujino H. Responses of muscle satellite cells to Lemon Myrtle S. supplementation combined with electrical stimulation in disuse-induced skeletal muscle atrophy. FASEB J. 2018;32(S1):lb374. doi: 10.1096/fasebj.2018.32.1_supplement.lb483. [DOI] [Google Scholar]
- 83.Correddu F, Fancello F, Chessa L, Atzori AS, Pulina G, Nudda A. Effects of supplementation with exhausted myrtle berries on rumen function of dairy sheep. Small Rumin Res. 2019;170:51–61. doi: 10.1016/j.smallrumres.2018.11.003. [DOI] [Google Scholar]
- 84.Bakar MSA, Ahmed A, Jeffery DM, Hidayat S, Sukri RS, Mahlia TMI, Park YK. Pyrolysis of solid waste residues from Lemon Myrtle essential oils extraction for bio-oil production. Bioresour Technol. 2020;318:123913. doi: 10.1016/j.biortech.2020.123913. [DOI] [PubMed] [Google Scholar]
- 85.Buffa G, Tsiplakou E, Mitsiopoulou C, Pulina G, Nudda A. Supplementation of by-products from grape, tomato and myrtle affects antioxidant status of dairy ewes and milk fatty acid profile. J Anim Physiol Anim Nutr. 2020;104(2):493–506. doi: 10.1111/jpn.13315. [DOI] [PubMed] [Google Scholar]
- 86.Dhouibi I, Masmoudi F, Bouaziz M, Masmoudi M. A study of the anti-corrosive effects of essential oils of rosemary and myrtle for copper corrosion in chloride media. Arab J Chem. 2021;14(2):102961. doi: 10.1016/j.arabjc.2020.102961. [DOI] [Google Scholar]
- 87.Smeti S, Tibaoui S, Bertolín JR, Yagoubi Y, Mekki I, Joy M, Atti N. Effects of myrtle (Myrtus communis L.) essential oils as dietary antioxidant supplementation on carcass and meat quality of goat meat. J Anim Physiol Anim Nutr. 2021;105(3):452–461. doi: 10.1111/jpn.13483. [DOI] [PubMed] [Google Scholar]
- 88.Dang LT, Nguyen HT, Hoang HH, Lai HN, Nguyen HT. Efficacy of rose myrtle Rhodomyrtus tomentosa seed extract against acute hepatopancreatic necrosis disease in Pacific white leg shrimp Penaeus vannamei. J Aquat Anim Health. 2019;31(4):311–319. doi: 10.1002/aah.10080. [DOI] [PubMed] [Google Scholar]
- 89.Atik H, Bülbül T, Özdemir V, Avci G, Bülbül A. Effect of myrtle (Myrtus communis L.) essential oil on oxidant–antioxidant balance in rats with propylthiouracil-induced hypothyroidism. J Food Biochem. 2020;44(12):e13498. doi: 10.1111/jfbc.13498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 36 kb)
Data Availability Statement
Not applicable.