Abstract
Amazonian Camu camu fruit (Myrciaria dubia (Kunth) McVaugh) has been called a “superfruit” due to its high levels of bioactive and antioxidant compounds such as polyphenols, carotenoids, and vitamin C. The biofunctional properties of camu camu fruit (including pulp, peel, and seeds) have been well established through several in vitro and in vivo studies. Several reports confirmed the nutritious and biofunctional value of camu camu extracts or its food-derived products, exhibiting antioxidant, antihyperglycemic, antihypertensive, and antiobesity activity, contributing to quality life improvement. Other studies showed antimicrobial, anti-inflammatory, antiproliferative, antihepatotoxic, antihemolytic, antimutagenic, and cell rejuvenation bioactivities. This Review summarizes the bioactive profile of camu camu fruit through the understanding of some physiological modulation processes and its contribution to the Amazon bioeconomy under the development of biofunctional food ingredients exhibiting health benefits.
Introduction
Camu camu (Myrciaria dubia (Kunth) McVaugh) is a Myrtaceae fruit commonly found in the Amazon region from Brazil, Peru, Colombia, and Venezuela.1 Its round berries are about 2.5 cm in diameter, containing one to four seeds. When immature, M. dubia fruit epicarp is green; then, during the ripening process, the color changes to red-purple because of the presence of anthocyanins and other phenolic compounds.2 Camu camu is considered a complex matrix of fatty acids, some amino acids, volatile compounds, minerals, and vitamins such as vitamin C.3,4 Bioactive molecules such as anthocyanins (cyanidin-3-O-glucoside and delphinidin-3-O-glucoside), flavonols (myricetin, quercetin), ellagitannins (ellagic acid and derivatives), proanthocyanidins, and carotenoids (lutein, β-carotene, violaxanthin, and luteoxanthin) are also part of camu camu chemical profile.5,6
The most common noncommunicable diseases, such as cardiovascular disease, obesity, and diabetes mellitus type 2, are usually treated with traditional medication. However, it is known that consuming fruits and vegetables, rich in vitamin C, carotenoids, and/or polyphenols, decrease the risk of those diseases because they act as natural antioxidants. An example is the edible fruits belonging to the Myrtaceae family, which has attracted attention in the past few years.7 Because of that, the chemical profile of bioactive compounds contained in some tropical fruits, such as camu camu (M. dubia), has led to the research of natural sources to treat and prevent some metabolic diseases in replacement of drug-based treatment.8 In this way, it has been verified that M. dubia has high health-beneficial properties that potentially can prevent some noncommunicable chronic diseases, such as type 2 diabetes, metabolic syndrome, obesity, and hypertension,9,10 and conditions related to oxidative stress such as inflammations, mutations, and chromosome damage, among others.8,9,11
The total weight of camu camu comprises 60% pulp and 40% subproducts such as seeds and peel.2 Even its seeds represent 20% of the total weight.11 Whole parts of M. dubia fruits have shown significant bioactive compounds that contribute to the functional characterization of camu camu.5 Thus, the evaluation of the chemical profile of the whole or any part of the M. dubia fruit promotes the preserving capacity, food stability, and nutritional value itself, allowing the development of sustainable processes and products with high added value, making the commercial cultivation of camu camu a feasible dietary, functional, and economical alternative.2,12
In the past decade, the food industry and its final customers have been more interested in developing, producing, and consuming food products from exotics and native origins that have beneficial health claims. This quality standard requires a proper assessment of fruits and biochemical compounds to determine functional biological properties. This Review aims to provide updated information about the biofunctional properties reported in the peel, seeds, and pulp of camu camu fruit that allow its uses as a food product focused on its antioxidant capacity and antihyperglycemic, antihypertensive, and antiobesity activities.
Methodology
The literature in this Review included original English-language research papers published between January 2010 and November 2022 in peer-reviewed journals and reviewed using electronic databases, including SciFinder, Science Direct, Google Scholar, and Wiley, as well as the official Web sites of health organizations to report current figures on the suffering of diseases. The search results were obtained using keywords such as “Myrciaria dubia”, “camu camu health”, “bioactives in camu camu”, or “camu camu food”, yielding a total of 70 publications. This Review includes two main sections highlighting the chemical composition of the fruit, especially bioactive compounds, and its biofunctional properties with health benefits. Similarly, for the first time, biofunctionalities associated with the prevention of metabolic syndrome diseases are discussed among the pulp, peel, and seed of the fruit. Other health-linked and toxic bioactivities are summarized in this Review. Moreover, this Review emphasizes the nutritional and biofunctional value of M. dubia according to its location, maturity stages, part of the fruit, and food-derived products. Since the interest in the health-promoting properties of camu camu has grown in recent years, this Review is focused on both in vitro and in vivo publications.
Main Groups of Biofunctional Compounds of Camu Camu
Phenolic compounds, vitamin C, and carotenoids have been reported as the major bioactive compounds in M. dubia pulp, peel, and seeds. Previous studies reported that external factors such as origin, climatic conditions, maturity stage, harvest time, soil irrigation, soil type, and direct exposure to light and temperature influenced the total composition of bioactive compounds in camu camu.13−15 It is known that maturity is intrinsically related to significant quantities of bioactive compounds in camu camu; consequently, more biofunctional activities are expected.7 Furthermore, the steady decrease of total phenolic compounds, carotenoids, and ascorbic acid contents is due to the catabolic reactions during the fruit ripening.6 For that reason, assessing camu camu from different harvest regions is essential for knowing an accurate chemical composition. Subsequently, drying techniques have been applied to fresh camu camu allowing longer fruit shelf life and providing adequate bioactive compound stability.5,14 For instance, dehydration techniques such as freeze-drying and spray-drying have been employed to generate new food-healthy alternatives based on camu camu biofunctional properties,2 some of them addressed during this Review.
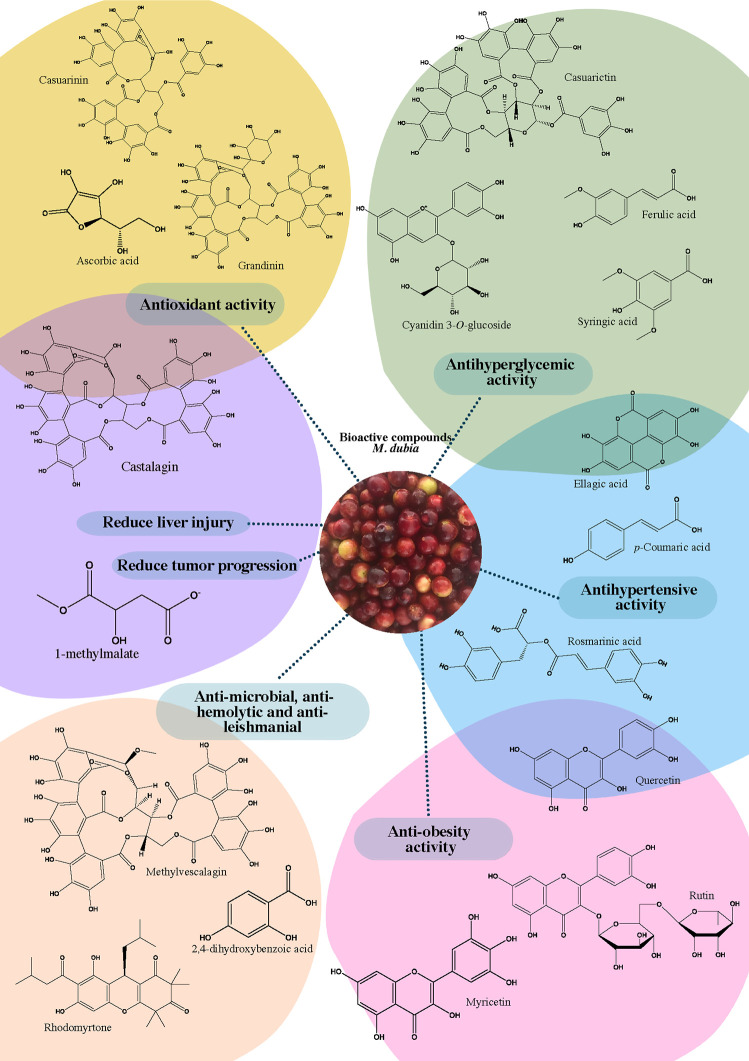
On the other hand, the extractions of bioactive compounds in M. dubia have usually been performed with an organic solvent. The compounds could be analyzed by titrimetry, spectrophotometric methods (such as Folin-Ciocalteu), liquid chromatography–mass spectrometry (LC-MS), and finally, statistical analysis that allows one to identify and quantify those responsible for biofunctional activities.16−18 Each step involves advantages and disadvantages. For example, during extraction, the solvent system, extraction technique, and physicochemical properties of the sample directly affect the stability and preservation of the bioactive compounds.5 Analytical methods such as titrimetric techniques allow one to determine the vitamin C content, and spectrophotometric methods allow one to quantify the phenolic and carotenoid content. Both techniques are fast, noncomplex, and economical, but vitamin C is easily oxidized, which induces measurement errors, and spectrophotometric techniques do not allow the identification of bioactive compounds.16,18 Although liquid chromatography presents more accuracy, selectivity, and sensitivity, it is still a higher operational cost technique and is limited in sample throughput.18 In that sense, adequate performance during the analysis allows a better biochemical understanding of the fruit. A more detailed description of the most outstanding bioactive compounds in camu camu is presented in Figure 1.
Figure 1.
Promising bioactive compounds related with camu camu (Myrciaria dubia) fruit biofunctionalities.
Carotenoids
The total carotenoid content in camu camu is shown in Table 1. The content of carotenoids has been reported in camu camu pulp as 619.98 ± 68.01 mg β-carotene/100 g8 and in peel-seeds as 14.34 ± 0.72 mg equivalent β-carotene/100 g.19 The carotenoid profile was characterized by containing 16 different compounds, such as all-trans-lutein (601.9 ± 92.5 μg/100 g) as the major carotenoid, followed by β-carotene (142.3 ± 23.7 μg/100 g) and violaxanthin (115.6 ± 62.4 μg/100 g).20 Data showed that carotenoids concentration in camu camu tended to decrease with maturity in the pulp and the peel. Carotenoids concentration was lower in camu camu pulp than in peel and decreased during the ripening process from ca. 6.00 to 1.00 mg/100 g in the peel6 and from ca. 300 to 133 μg/100 g in the pulp.21 The same studies showed that, although no yellow color was visually detected in the camu camu peel, carotenoids were present in the fruit.6 It is known that carotenoids are potential health-promoting compounds in preventing and treating some diseases, such as cardiovascular diseases (CVDs) and cancer.15 Further studies in carotenoid bioavailability are expected to define its influence on camu camu health-promoting properties.
Table 1. Bioactive Compounds in Camu Camu (Myrciaria dubia) Fruita.
| fruit part | total carotenoids | vitamin C | total polyphenols | total ellagic acid | total anthocyanins | reference |
|---|---|---|---|---|---|---|
| Pulp | ||||||
| Spray-dried pulp | n.r. | 3.51 ± 0.97 g AA/100 g | 48.54 ± 0.28 mg/100 g | 5.60 ± 0.11 mg/100 g | 19.63 ± 0.60 mg C3G/100 g | Fracassetti et al., 20135 |
| Frozen pulp | n.r. | 1889 ± 68 mg/100 g | 24,900 ± 140 mg CAE/100 g | 737 ± 18 mg/100 g | n.d. | de Souza Schmidt Gonçalves et al., 201437 |
| Fresh pulp | n.r. | 150.3 ± 0.2 AAE mg/g | 81.6 ± 6.5 GAE mg/g | n.r. | n.r. | Fujita et al., 20159 |
| Fresh pulp | 0.10 mg/100 g | 4752.23 mg AA/100 g | 12798.80 mg GAE/100 g | n.r. | 170.00 mg/100 g | Neves et al., 20156 |
| Freeze-dried pulp | n.r. | 123.0 ± 0.1 AAE mg/g | 67.1 ± 6.4 GAE mg/g | n.r. | n.r. | Fujita et al., 20159 |
| Lyophilized and reconstituted | 2919 ± 55 mg/100 g | n.r. | 141.2 ± 3.9 mg/g | n.r. | 13.6 ± 1.3 μg/g | Grigio et al., 201730 |
| Lyophilized | n.r. | n.r. | 4.32 ± 0.03 mg/g | n.r. | 1.0 ± 0.001 mg C3G/g | Conceição et al., 20202 |
| Ripe fresh pulp | 133 μg/100 g | 6.289 mg/100 g | 7.916 g CAE/100 g | n.r. | 2513.28 μg/100 g | Grigio et al., 202121 |
| Peel | ||||||
| Fresh peel | 0.60 mg/100 g | 5178.49 mg AA/100 g | 14854.00 mg GAE/100 g | n.r. | 170.00 mg/100 g | Neves et al., 20156 |
| Lyophilized and reconstituted | 3068 ± 135 mg/100 g | n.r. | 98.1 ± 0.6 mg/g | n.r. | 107.2 ± 2.6 μg/g | Grigio et al., 201730 |
| Ripe genotype-44 | 105.88 ± 0.25 mg/100 g | 24.02 ± 0.18 g/100 g | 881.46 ± 88.68 mg GAE/100 g | n.r. | 165.91 ± 0.39 mg/100 g | Souza et al., 201823 |
| Ripe peel | 619.98 ± 68.01 mg β-carotene/100 g | 1109.62 ± 56.5 mg/100 g | 13348.97 ± 99.94 mg GAE/100 g | 472.80 ± 30.96 mg/100 g | 223.14 ± 6.89 mg C3G/100 g | Azevedo et al., 20198 |
| Seeds | ||||||
| Lyophilized and reconstituted | 551 ± 31 mg/100 g | n.r. | n.d. | n.r. | n.d. | Grigio et al., 201730 |
| Dried seeds | n.r. | n.r. | 385.63 ± 12.73 mg/100 g | 4.31 ± 0.02 mg/100 g | 4.03 ± 0.02 mg/100 g | Carmo et al., 201911 |
| Aqueous extract | n.r. | n.r. | 2502 ± 46 mg GAE/100 g | 16.12 ± 0.04 mg/100 g | n.d | Fidelis et al., 202013 |
| Ethanolic extract | n.r. | n.r. | 1353 ± 19 mg GAE/100 g | 16.12 ± 0.36 mg/100 g | 3.51 ± 0.00 mg C3G/100 g | Fidelis et al., 202013 |
| Pulp + Peel | ||||||
| Lyophilized and reconstituted | 4426 ± 90 mg/100 g | n.r. | 130.6 ± 2.9 mg/g | n.r. | 50.7 ± 1.5 μg/g | Grigio et al., 201730 |
| Aqueous extract | 197.22 ± 49.5 mg/100 g | 1100.54 ± 18.7 mg/100 g | 14 749.93 ± 5.51 mg GAE/100 g | 425.44 ± 23.47 mg/100 g | 209 ± 5.02 mg C3G/100 g | Azevedo et al., 20198 |
| Peel + Seeds | ||||||
| Dried | n.r. | 9.04 ± 0.95 g AA/100 g | 672.49 ± 0.55 mg/100 g | 76.49 ± 0.49 mg/100 g | n.d. | Fracassetti et al., 20135 |
| Fresh | 5694.6 ± 2.9 μg/100 g | 458.2 ± 9.2 mg AA/g | 3738.0 ± 20.8 mg GAE/100 g | n.r. | 4.1 ± 0.3 mg eq. C3G/g | de Azevedo et al., 201432 |
| Freeze-dried | 1467.9 ± 1.6 μg/100 g | 11.8 ± 0.3 mg AA/g | 2160.2 ± 54.4 mg GAE/100 g | n.r. | 1.9 ± 0.1 mg eq. C3G/g | de Azevedo et al., 201432 |
| Fresh | 14.34 ± 0.72 mg equivalent β-carotene/100 g | n.r. | 10011.06 ± 585.26 mg GAE/100 g | n.r. | 21.10 ± 0.94 mg/100 g | da Costa Araújo et al., 202219 |
n.d. = not detected. n.r. = not reported. AA = Ascorbic Acid. AAE = Ascorbic Acid Equivalent. GAE = Gallic Acid Equivalent. EAC = Ellagic Acid Conjugate. CAE = Chlorogenic Acid Equivalent. C3G = Cyanidin-3-glucoside.
Vitamin C
It is a water-soluble nutrient that performs essential biological activities such as collagen production, wound healing, iron absorption, reduced susceptibility to infection, and prevention of mucosal bleeding.17 Likewise, vitamin C bioactivity is determined by the action of both ascorbic acid (AA) and its oxidation product, dehydroascorbic acid (DHAA).16 Compared to other Amazonian fruits, such as açaí (Euterpe oleracea) (84.0 ± 10 mg AA/100 g), acerola (Malpighia emarginata) (1357 ± 9.5 mg AA/100 g), bacuri (Platonia insigni) (2.4 ± 0.3 mg AA/100 g), murta (Ugni molinae) (181.0 ± 1.8 mg AA/100 g), or jaboticaba (Myrciaria jaboticaba) (238 ± 2.2 mg AA/100 g),22 camu camu is one the main natural sources of vitamin C (1882 ± 43.2 mg/100 g). Some values of AA content in camu camu pulp, peel, and seeds are reported in Table 1. No AA content has been reported in camu camu seeds, and some studies reported more AA content in the camu camu peel than pulp.5
As vitamin C content depends on the maturation stage of camu camu, several studies have reported the variation of vitamin C content during the ripening process.6,21 In fact, an increment of ascorbic acid was reported during camu camu maturation from 2004.66 to 2605.76 mg/100 g fruit6 and from semimature (5799 mg/100 g pulp) to mature stages (6289 mg/100 g pulp).21 Moreover, the analysis of the vitamin C content in unripe camu camu pitted fruit and unripe skin displayed significant differences with the ripe stage (p ≤ 0.05).23 Other studies evaluated vitamin C content according to genotypes in Brazil. Therefore, genotypes 17, 38, and 44 of green (90% to 100% green), semiripe (10% to 80% red), and ripe (above 80% red) stages in camu camu peel were assessed. Genotype 44 had the highest ascorbic acid content in the ripe stage; its value is shown in Table 1. Semiripe (17.06 g/100 g) and ripe stages (24.02 g/100 g) were also measured. On the other hand, genotype 17 had the highest ascorbic acid content at the green stage (17.79 g/100 g) compared to the different genotypes at the same maturity stage.23
Other reports have shown the influence of extraction methods and drying techniques on the vitamin C content in camu camu. First, five acid solvents were used to determine vitamin C (AA + DHAA) content in camu camu pulp and peel. Acid extraction carried by 0.05 M sulfuric acid proved to be the solvent with the highest extraction efficiency of vitamin C. In the same study, among five different origin camu camu samples (Iguape, Sete Barras, Peru, Embrapa, and Brazil-commercial), Peru camu camu pulp (38.37 ± 2.13 g/100 g DW) and Embrapa camu camu peel (13.56 ± 0.42 g/100 g DW) presented the highest AA content. Likewise, Embrapa camu camu peel (2.16 ± 0.10 g/100 g DW) presented the highest DHAA content, but no content was determined in any camu camu pulp.16 Second, thermosonication was used to extract vitamin C from camu camu nectars (17 g of camu camu pulp, 17 g of sugar, and 66 g of mineral water). The research showed that extraction conditions of 40 °C/30 min presented the highest AA content (835.78 ± 1.8 mg/100 g) with no significant differences with the control (no-thermosonication) (p ≤ 0.05). Nonetheless, as temperature and time increased, the AA content decreased because of its thermal sensitivity, and therefore, AA can be easily degraded.24
The influence of drying conditions on the AA content was assessed using lyophilization and spray-drying techniques. Microencapsulates from camu camu pulp (Manaus and Sao Paulo) were obtained at three different inlet temperatures (120, 150, and 180 °C) with three proportions (6%, 12%, and 18%) of carrier agents (maltodextrin (MD) and Arabic gum (AG)) by using spray-drying. Likewise, camu camu pulp was freeze-dried. The highest AA values were 62.0 ± 0.7 mg AAE/g DW (6% MD/180 °C), 137.5 ± 2.7 mg AAE/g DW (6% MD/120 °C), and 248.2 ± 1.2 mg AAE/g DW (freeze-dried), concluding that, as the drying temperature and carrier agent (%) increased, the AA content decreased.25 On the other hand, camu camu microencapulates were also produced using maltodextrin (MD), inulin (IN), and oligo-fructose (OL) as carrier agents at 20% and a drying temperature of 150 °C, obtaining AA content of 1124.17 ± 2.55, 935.13 ± 2.59, and 872.84 ± 6.25 mg/mL, for the three carrier agents, respectively. The authors concluded that MD presented greater encapsulating capacity as AA content exhibited fewer losses during drying.14 The high vitamin C content in camu camu makes relevant the development of new technologies that help to preserve health-promoting substances during food processing.
Phenolic Compounds
They are secondary metabolites responsible for the oxidative performance, acidity, taste, fragrance, and color of plants.26 These compounds are involved in the regulation against abiotic stresses and pathogens and are responsible for different health-promoting properties.1 Polyphenols from several plants have acted in the human body as modulators in analgesic, anticancer, antihypertensive, and antidiabetic processes.27 Similarly, anthocyanins, a polyphenol-type metabolite, are involved in reducing the risk of coronary heart disease and diabetes and have been demonstrated to possess antioxidant and anticancer activities acting in obesity control.27 The total phenolic content in camu camu pulp, seeds, and peel has been estimated in several studies, as shown in Table 1. Rufino et al.22 reported the highest total phenolic content value of camu camu fresh pulp (1176 ± 14.8 mg GAE/100 g) in comparison to açai (454 ± 44.6 mg GAE/100 g), acerola (1063 ± 53.1 mg GAE/100 g), and jaboticaba (440 ± 9.9 mg GAE/100 g). Moreover, cyanidin-3-glucoside (C3G) has been reported as the main anthocyanin in camu camu pulp and peel2,5 with a content of 182.56 ± 1.76 mg/100 mL.14
Fracassetti et al.5 have reported flavonols (3.05 ± 0.07 mg/100 g) in camu camu dried pulp, such as myricetin 3-O-hexoside, quercetin 3-O-hexoside, and myricetin; ellagitannins (16.10 ± 0.33 mg/100 g) such as vescalagin and castalagin; ellagic acids derivatives (9.75 ± 0.10 mg/100 g) such as ellagic acid (5.60 ± 0.11 mg/100 g) as the main phenolic compounds. Physical factors can affect the content of polyphenols in camu camu according to maturity stages; therefore, the phenolic compound content increases as the ripening process advances. An increment in the anthocyanin content from 7.37 to 170.00 mg/100 g has been reported in the camu camu pulp during fruit development; also, an increase of flavonol content from 38.96 to 60.75 mg quercetin/100 g and a higher concentration of phenolic compounds (12798.80 mg GAE/100g) in comparison to other maturity stages have been reported.6 The geographical region is another factor that affects the phenolic compound content. Cunha-Santos et al.28 studied the phenolic content of camu camu from the different areas of Brazil, finding maximum values of catechin (606.81 ± 37.68 mg/100 g DW), rutin (116.29 ± 16.43 mg/100 g DW), epicatechin (55.51 ± 7.82 mg/100 g DW), luteolin (29.83 ± 0.07 mg/100 g DW), quercetin (10.48 ± 0.91 mg/100 g DW), and p-coumaric acid (32.69 ± 0.91 mg/100 g DW) in those from Belem, concluding that variation of phenolic content is directly linked with the harvest soil conditions. Some processing factors, such as the thermosonication of camu camu nectars, revealed that total anthocyanin content (C3G) decreased from 4.67 ± 0.2 to 3.92 ± 0.3 mg/100 g, presenting the highest degradation at 85 °C for the 60 s, possibly due to the thermal instability of anthocyanins. In contrast, the total flavonoid and total phenolic content of camu camu nectars increased during thermosonication from 9.75 ± 0.41 to 14.21 ± 0.5 mg/100 g (at 40 °C/2 min) and from 829 ± 12 to 906 ± 1 mg GAE/L (85 °C/60 s), respectively.24
It is worth mentioning that camu camu byproducts such as seeds and peel contain promising bioactive compounds that suggest a potential as health-promoting raw materials.13,19,29,30 Proanthocyanins were only found in camu camu seeds (35.77 mg/100 g);5 this kind of compound has shown a protective role against cancers in humans.31 Likewise, the content of polyphenols and anthocyanins was higher in the peel than in the pulp fruit.6,8 In addition, total phenolic content was higher in seeds than in the pulp from Tarapoto, Peru, with values of 3320.79 ± 229.06 and 2475.81 ± 454.86 mg/100 g DW, respectively.28 Genotype studies indicated that the highest values of phenolic compounds and anthocyanins in the peel of ripe camu camu were found in genotypes 17 and 44 from Embrapa’s active germplasm bank (Brazil).23 Former studies also reported the content of syringic acid (7.0 ± 0.4 mg/100 g) and the flavonoids quercetin (0.9 ± 0.1 mg/100 g), myricetin (2.1 ± 0.5 mg/100 g), and catechin (12.4 ± 1.0 mg/100 g) in camu camu peel and seeds.32 Another report established the total phenolic content from camu camu seeds using ethanol, propanone, and water as extracting solvents. Indeed, the solvent employed influenced the content of polyphenols resulting in values of 40.00 ± 0.15 mg/100 g (aqueous extract), 44.92 ± 0.74 mg/100 g (ethanolic extract), and 24.68 ± 0.11 mg/100 g (propanone extract). The authors reported a phenolic profile of 16 compounds such as phenolic acids like gallic, caffeic, ferulic, and rosmarinic acid and flavonoids such as quercetin, catechin, and malvidin-3,5-diglucoside.13 These findings contradict previous results, where no anthocyanins, phenolic compounds, and proanthocyanins such as procyanidin A2 were determined in camu camu seeds.5,13,30 Phenolic compounds such as trans-resveratrol, methylvescalagin, and 2,4-dihydroxybenzoic acid exhibited a statistical correlation with in vitro antihemolytic activity, and their presence in camu camu seeds suggests a potential use for this byproduct.33 Other compounds reported in camu camu peels and seeds are acylphloroglucinols, such as rhodomyrtone, myrciarone A, myrciarone B, and isomyrtucommulone B, which were isolated with n-hexane and purified by counter-current partition with 90% acetonitrile and exhibited antimicrobial activity against Gram-positive bacteria.34
The strong acidity and bitterness of camu camu fruit is a disadvantage for its fresh consumption; however, different processed products such as beverages, juices, purees, dairies, and jams have been developed as alternatives to increasing M. dubia hedonic acceptation and therefore its nutritional and healthy value.2,12,35 For example, camu camu cookies were produced using byproducts (seeds, peel, and residual pulp) as ingredients in a proportion of 5–20% and drying at 50, 60, and 70 °C to enhance the total phenolic content. Identification of polyphenols showed the presence of 11 flavonoids in camu camu cookies as follows: the flavonols, such as myricetin 3-O-glucoside and myricetin-pentoside; flavones, such as luteolin 8-C-glucoside; anthocyanins, such as cyanidin 3-O-glucoside and cyanidin malonyl-diglucoside. Moreover, the content of total phenolic compounds increased with drying temperature from 23.9 ± 0.7 mg GAE/g (50 °C) to 47.9 ± 1.1 mg GAE/g (70 °C), which could be attributable to the heterogeneous composition of the byproducts or the influence of external fruit factors.36 The highest content of phenolic compounds in microencapsulated camu camu showed values of 43.8 ± 5.7 mg GAE/g DW (6% MD/150 °C) and 57.9 ± 2.9 mg GAE/g DW (6% AG/120 °C); in contrast, fresh camu camu displayed higher values of 100.0 ± 7.2 mg GAE/g, and lyophilized camu camu presented values of 97.1 ± 6.9 mg GAE/g DW. According to the authors, the increment of temperature and carrier agent (%) reduced the content of phenolic compounds from 56% to 75% (MD) and from 38% to 65% (AG).25 Other camu camu microencapsulates showed higher retention of anthocyanins using maltodextrin (MD) as the carrier agent from the fresh weight of 7.2–9.0 g anthocyanins/kg.14 In this regard, phenolic compounds are the secondary metabolites with more impact on the health-promoting properties of camu camu fruit.
Biofunctional Properties of Camu Camu with Health Benefits
Some biofunctional properties related to human health-promoting have been attributed to this Amazon fruit because of the high content of bioactive compounds. In general, these properties are linked with the prevention and treatment of cardiovascular and metabolic diseases that currently present a significant affectation on the daily activities of the human being.1,2,9,10,27,35,37 In the following section, some of them are summarized.
Antioxidant Activity
Three different single electron transfer methods have mainly been used to evaluate the antioxidant activity of camu camu: DPPH, ABTS, and FRAP, as observed in Table 2. These results showed higher antioxidant activity values compared to other fruits, such as maqui (Aristotelia chilensis) (6.76 ± 0.25 mmol Trolox/100 g), papaya (Carica papaya) (4.41 ± 0.28 mmol Trolox/100 g), and noni (Morinda citrifolia) (3.71 ± 0.17 mmol Trolox/100 g) for the DPPH method,38 as well as açaí (Euterpe oleraceae) (64.5 ± 19.2 μmol Trolox/g), acerola (Malpighia emarginata) (953 ± 34.1 μmol Trolox/g), jaboticaba (Eugenia jaboticaba) (317 ± 2.7 μmol Trolox/g), and Jambolão (Syzygium cumini) (125 ± 10.8 μmol Trolox/g) for the ABTS method.22 Data has shown that the antioxidant activity of camu camu tends to increase with maturity, presenting the highest values at the mature and semimature stages.6,21 Generally, this activity has been attributed to the vitamin C and phenolic compounds of camu camu, as they can scavenge free radicals and reduce transition metals.9,10,12,36In vivo studies showed that consuming frozen pulp extracts of camu camu significantly increased plasma antioxidant activity in Type 1 diabetic rats.37 Recent in vivo reports indicated a synergistic effect between bioactive compounds in camu camu (phenolic compounds, carotenoids, and vitamin C), generating a scavenging action against reactive oxygen species (ROS), for future antigenotoxic and antimutagenic effects as it was evidenced in rats.9
Table 2. Antioxidant Activity Reported for Camu Camu (Myrciaria dubia) Fruit Partsa.
| scavenging
capacity |
||||
|---|---|---|---|---|
| fruit description | DPPH | FRAP | ABTS | ref |
| Pulp | ||||
| Fresh mature | 5159.50 μmol Trolox eq./100 g | n.r. | n.r. | Neves et al., 20156 |
| Fresh | 1.32 ± 0.09 mmol Trolox/g | 1.23 ± 0.03 mmol Trolox/g | n.r. | Fujita et al., 20159 |
| Freeze-dried | 1.28 ± 0.09 mmol Trolox/g | 1.16 ± 0.11 mmol Trolox/g | n.r. | Fujita et al., 20159 |
| Lyophilized and reconstituted | 8.46 ± 0 mg/g | 11.7 ± 0.3 mg/g | n.r. | Grigio et al., 201730 |
| Nectar | 1360 ± 6 μmol Trolox | n.r. | n.r. | do Amaral Souza et al., 201924 |
| Ripe fresh pulp | 1.93 μmol TEAC/g | 2.167 μmol ferrous sulfate/g | n.r. | Grigio et al., 202121 |
| Peel | ||||
| Fresh mature | 5848.90 μmol Trolox eq./100 g | n.r. | n.r. | Neves et al., 20156 |
| Lyophilized and reconstituted | 8.53 ± 0 mg/g | 11.7 ± 0 mg/g | n.r. | Grigio et al., 201730 |
| Ripe skin | 1328.50 ± 14.40 μmol Trolox equivalents/g | n.r. | 1370.64 ± 30.36 μmol Trolox equivalents/g | Azevedo et al., 20198 |
| Seeds | ||||
| Acetone extract | 8.75 ± 0.05 μmol equivalent of Trolox/g of casuarinin | n.r. | 7.47 ± 0.02 μmol equivalent of Trolox/g of casuarinin | Kaneshima et al., 201641 |
| Lyophilized and reconstituted | 8.4 ± 0.2 mg/g | 2.45 ± 0 mg/g | n.r. | Grigio et al., 201730 |
| 50:50% aqueous:ethanolic extract | n.r. | 8076 ± 511 mg AAE/100 g | 4340 ± 117 mg AAE/100 g | Carmo et al., 201911 |
| Aqueous extract | 1963 ± 11 mg AAE/100 g | n.r. | n.r. | Fidelis et al., 202013 |
| Ethanolic extract | 1190 ± 84 mg AAE/100 g | n.r. | n.r. | Fidelis et al., 202013 |
| Propanone | 1336 ± 50 mg AAE/100 g | n.r. | n.r. | Fidelis et al., 202013 |
| Pulp + Peel | ||||
| Fresh | 167 ± 11 μmol TE/g | n.r. | n.r. | Chirinos et al., 201039 |
| Lyophilized and reconstitute | 11.7 ± 0.2 mg/g | 8.53 ± 0 mg/g | n.r. | Grigio et al., 201730 |
| Ripe pitted fruit | 1520.45 ± 112.79 μmol Trolox equivalents/g | n.r. | 1418.25 ± 17.65 μmol Trolox equivalents/g | Azevedo et al., 20198 |
| Peel + Seeds | ||||
| Fresh | 166.6 ± 1.1 μmol TE/g | n.r. | n.r. | de Azevedo et al., 201432 |
| Freeze-dried | 32.4 ± 1.6 μmol TE/g | n.r. | n.r. | de Azevedo et al., 201432 |
| Byproducts in cookies dried at 50 °C | 5.3 ± 0.2 mg/mL (EC50) | 8.8 ± 0.6 μmol TE/g | n.r. | das Chagas et al., 202136 |
n.d. = not detected. n.r. = not reported. TE = Trolox Equivalent. TEAC = Trolox Equivalent Antioxidant Capacity. AAE = Ascorbic Acid Equivalent.
According to former statistical analysis, the antioxidant activity of camu camu fruit from Peru, under the DPPH assay, exhibited the highest correlation with phenolic compound content (r2 = 0.931) compared to the ascorbic acid (r2 = 0.190).39 Other studies presented a high correlation with phenolic compounds (r2 = 0.991) and ascorbic acid (r2 = 0.979).6 Moreover, during the ripening, the content of flavan-3-ols and ellagic acid derivatives increased, possibly influencing the antioxidant capacity of camu camu. The authors concluded that phenolic compounds with hydroxyl groups in their chemical structure could donate electrons and hydrogen atoms to free radicals.39 In camu camu nectars, thermosonication decreases the antioxidant activity under the DPPH assay due to the thermal treatment, and during the ultrasonic processing, water solvolysis is produced, causing degradation of vitamin C and polyphenols as well as production of hydroxyl radicals, inducing the bioactive compound oxidation.24 Camu camu pulp was applied in Teff starch films. A concentration of 2.0% of camu camu extract in the final film showed radical scavenging activities against DPPH (100.00 ± 0.00%) and ABTS (89.19 ± 0.42%).40 This means that a possible synergistic effect can result from camu camu and other food matrices, thus enhancing its antioxidant activity, therefore increasing the number of applications in agreement with previous reports.39
Additionally, camu camu pulp microencapsulates exhibited a higher DPPH scavenging capacity of 0.29 ± 0.01 mmol Trolox/g (6% MD, 120 °C) and 0.77 ± 0.03 mmol Trolox/g (6% AG, 120 °C), in comparison to the FRAC scavenging capacity of 0.78 ± 0.02 mmol Trolox/g (6% MD, 120 °C) and 0.49 ± 0.03 mmol Trolox/g (6% AG, 120 °C), agreeing with higher polyphenol content. However, due to the thermal treatment during the drying process, some phenolic compounds suffered degradation, resulting in DPPH/FRAP scavenging capacity losses ranging from 42% to 73% compared to fresh pulp.25 To avoid this effect, the antioxidant activity of camu camu pulp microencapsulates was assessed in a soy milk beverage. After 72 h of fermentation, the antioxidant activity increased by around 90%, related to DPPH inhibition.35 In another study, microencapsulates of camu camu obtained with maltodextrin (MD) as carrier agent showed the highest value of DPPH antioxidant activity (92.72%), followed by oligo-fructose (85.32%) and inulin (82.43%) samples. Although there is the possibility of degradation of ascorbic acid or phenolics compounds during drying, the microencapsulation process can help to preserve and retain the main bioactive compounds of this fruit.14
The DPPH scavenging activity of camu camu byproducts (peel and seeds) was also evaluated. Results indicated a significant correlation between the antioxidant capacity of the camu camu samples with the bioactive compound content such as ascorbic acid (r2 = 0.999), total phenolic compounds (r2 = 0.955), anthocyanins (r2 = 0.905), carotenoids (r2 = 0.994), and proanthocyanins (r2 = 0.786).38 Likewise, camu camu peel and seeds exhibited higher DPPH scavenger activity (1036.4 ± 211.2 μmol Trolox/100 g) and FRAP (752.3 ± 41.0 μmol Trolox/100 g) values than camu camu pulp, 510.5 ± 156.1 μmol Trolox/100 g and 167.5 ± 54.4 μmol Trolox/100 g, respectively.5 These results could be linked to the amount of bioactive compounds in each sample.
In camu camu seeds, the mechanism of scavenging activity against DPPH radical was also assessed, exhibiting a higher value for the water extract (2838 mg AAE/100 g) compared to ethanol and propanone extracts. Moreover, a positive correlation between scavenging activity and bioactive compounds was established for total phenolic content (r = 0.8992), total flavonoids content (r = 0.9156), and condensed tannins content (r = 0.8975) with high significance (p ≤ 0.05).13 The antioxidant activities of aqueous, ethanolic, and their mixed extracts (25:75%, 50:50%, and 75:25%) of camu camu seeds were also evaluated, finding the highest DDPH and FRAP values with 50:50% aqueous:ethanolic extract as shown in Table 2. As mentioned before, the antioxidant activity of camu camu is intrinsically linked with the bioactive compound amount. Thus, the authors established that methylvescalagin is responsible for that bioactivity because it presented a significant positive correlation with DPPH (r = 0.9504) and FRAP (r = 0.9730) values in the camu camu seed extracts (p ≤ 0.05).33 Additionally, six tannins (casuarinin, castalagin, grandinin, methylvescalagin, stachyurin, and vescalagin) isolated from camu camu seeds and peel acetone extracts exhibited a high antioxidant capacity (Table 2). Even after the camu camu extracts were incorporated in yogurt, the antioxidant capacity showed high values, so the dairy matrix can potentialize the camu camu antioxidant activity.2,41 Furthermore, C-glycosidic ellagitannins from camu camu seeds such as grandinin, vescalagin, castalagin, methylvescalagin, stachyurin, and casuarinin were also isolated and purified, and its DPPH and ABTS antioxidant activities were measured, indicating that C-glycosidic ellagitannins exhibited stronger antioxidant activities than gallic acid and ascorbic acid.41
Antioxidant activity was also measured by ORAC (oxygen radical absorbance capacity) methodology, obtaining values of 337.1 ± 77.9 μmol Trolox/100 g for camu camu powered pulp.5 Likewise, ORAC activity increased with camu camu maturity stages, obtaining maximum values of 5036.5 μmol Trolox eq./100g for camu camu pulp and 5810.0 μmol Trolox eq./100g for camu camu peel, mainly attributed to ascorbic acid (r2 = 0.967) and total phenolic compounds (r2 = 0.983).6 In camu camu seeds, the ORAC value was 28.838 ± 1620 mg CE/100 g ((+)-catequin equivalents), enough to inhibit the rat’s brain lipid peroxidation at 10–65%.13 Finally, in the cookie application with 10% of the camu camu extract, the maximum ORAC activity reported was 25.38 ± 1.21 μmol TE/g; nonetheless, the authors reported that, although there was a drying process and heterogeneity of the samples, ORAC activity remained in the food product due to the presence of bioactive compounds.14 Therefore, vitamin C and phenolic compounds, such as ellagitannins and flavonoids, are responsible for the high antioxidant activity values in camu camu fruit.
Antihyperglycemic Activity
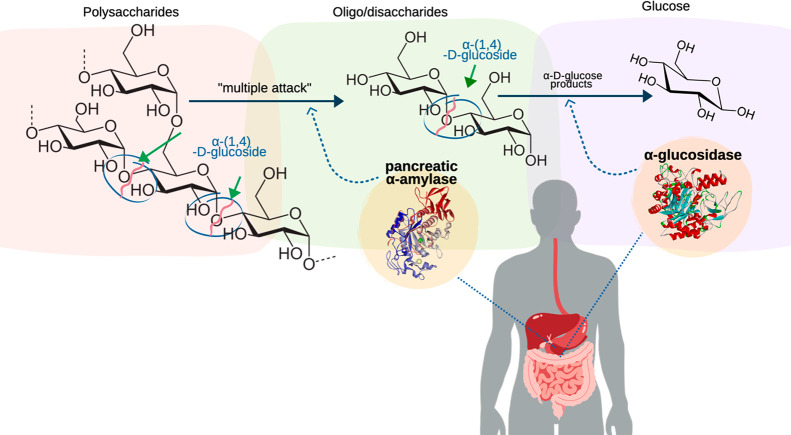
Hyperglycemia is caused by high levels of postprandial glucose in the blood. Generally, this disease is a consequence of the common, well-known metabolic syndrome diseases such as diabetes.41 According to the International Diabetes Federation, by 2045, the population with diabetes cases is estimated to reach over 700 million,42 being the seventh leading cause of death. The type 2 diabetes epidemic is a result of genetic and nongenetic factors. It can be linked to unhealthy sedentary lifestyles and excess nutrition from diets covered by refined carbohydrates and fats that cause metabolic breakdown through chronic oxygen malfunction and concurrently induce metabolic syndrome.32,43 Dietary carbohydrates usually contain polysaccharides whose chemical structure is characterized by chains of α-(1,4) bonds and amylopectin bonds, as well as branched glucans with α-(1,4) and α-(1,6) bonds. For absorption purposes in the human body, carbohydrates are hydrolyzed during food gastrointestinal digestion, beginning with catalysis by salivary enzymes and, subsequently, hydrolysis by intestinal enzymes. Pancreatic α-amylase (produced by the pancreas) acts on carbohydrates at the beginning of the small intestine, hydrolyzing them at α-(1,4) and α-(1, 6) bonds, producing oligosaccharides and disaccharides, as reaction products such as maltose, maltotriose, and dextrins.44 Then, the α-glucosidase (present in the small intestine) acts on the α-amylase hydrolysis products that cannot be absorbed, breaking them down into monosaccharides such as glucose, fructose, and galactose.45,46 Consequently, the inhibition against these enzymes helps to reduce postprandial blood glucose levels.32,43 The hydrolyzation catabolized by the intestinal enzymes is shown in Figure 2.
Figure 2.
Hydrolyzation steps of polysaccharides and oligosaccharides by the effect of pancreatic α-amylase and α-glucosidase enzymes.
Some medical drugs, such as acarbose, inhibit the action of these associated glucose metabolism enzymes and, in consequence, are currently used to diagnose patients with hyperglycemia and diabetes Type 1.43,45In vivo antidiabetic activity was proved using diabetic balb/c mice at doses of 100, 500, and 1000 mg/kg of lyophilized camu camu pulp diluted in distilled water (5:50 w/v). The results allowed one to conclude that all doses reduced the levels of plasmatic glucose; therefore, camu camu pulp exhibited an antidiabetic capacity.47 Likewise, 40 dyslipidemic rats consumed three doses of camu camu juices (0.4, 4.0, and 10 mL/kg) for 14 days. The final results showed a decrease in plasmatic lipoproteins, triacylglycerol, and total cholesterol, proving the potential hypolipidemic effect of camu camu juice.48 Some phenolic compounds such as soluble tannins and flavan-3-ols have been reported to be α-amylase inhibitors, and anthocyanins and flavonols are reported as α-glucosidase inhibitors.45 Subsequently, compounds such as (−)-3-O-galloylepicatechin, (−)-3-O-galloylcatechin, proanthocyanidins [(−)-epigallocatechin, (−)-epigallocatechin-3-O-gallate, (−)-epicatechin, (+)-catechin, (−)-epicatechin-3-O-gallate], naringenin, kaempferol, luteolin glycoside, and apigenin have shown α-amylase inhibition activity, while ellagic acid, caffeic acid, ferulic acid, (+)-catechin/(−)-epicatechin, pelargonidin-3-rutinoside, and cyanidin-diglucoside have shown α-glucosidase inhibition capacity in different plant materials.49
The high concentration of phenolic compounds in camu camu has shown antihyperglycemic activity that counteracts the second effects of glucose in the blood.9 The inhibition values of pancreatic α-amylase have been reported in other fruits from the Myrtaceae family, such as cambuci (Campomanesia phaea (O. Berg.)) (IC50 = 1.24 ± 0.02 μg CE/mL), jaboticaba (Eugenia jaboticaba) (IC50 = 7.28 ± 0.02 μg CE/mL),50 cagaita (Eugenia dysenterica DC) (IC50 = 3.8 mg of sample DW/mL), and araçá (Psidium guineensis Sw) (IC50 = 5.9 mg of sample DW/mL).51 Also, inhibition values of α-glucosidase have been reported for cambuci (Campomanesia phaea (O. Berg.)) (IC50 = 2.0 ± 0.3 μg CE/mL), cagaita (IC50 = 1.4 ± 0.2 μg CE/mL),50 araçá (Psidium cattleianum Sabine) (IC50 = 25.4 ± 0.7 μg/mL), pitanga (Eugenia uniflora L.) (IC50 = 66.3 ± 2.4 μg/mL),52 and jaboticaba (Eugenia jaboticaba) (IC50 = 14.30 ± 4.2 μg GAE/mL reaction).53
In freeze-dried camu camu pulp, the in vitro inhibitory activity for the α-amylase enzyme was reported as IC50 = 359 ± 105 μg/mL for the Amazonian sample and IC50 = 299 ± 152 μg/mL of reaction for the Sao Paulo sample, and α-glucosidase inhibition values were reported as IC50 = 5.57 ± 1.05 μg/mL for the Amazonian sample and IC50 = 2.98 ± 1.12 μg/mL of reaction for the Sao Paulo sample.9 Likewise, a high correlation between casuarictin (r = 0.969), ellagic acid (r = 0.965), and syringic acid (r = 0.818) with the α-amylase inhibitory activity values of the Amazonian sample was found. Freeze-dried samples displayed lower α-amylase inhibition values than acarbose (3.05 ± 0.25 μg/mL), but samples from Sao Paulo showed higher inhibitory activity than the Amazonian fruit. Certainly, the reported values can be the result of the drying conditions, such as temperature, that affect the stability of phenolic compounds.9 In contrast, both samples showed higher α-glucosidase inhibitory activity than acarbose (152 ± 47 μg/mL of reaction). On the other hand, dried camu camu seed extract exhibited antihyperglycemic activity, inhibiting α-amylase from 85% to 95% and α-glucosidase from 82% to 99%. A high statistical correlation was found with phenolic compounds of camu camu seeds, such as ferulic acid (r = 0.988), p-coumaric acid (r = 0.935), and ellagic acid (r = 0.934). As a modulator of enzymatic activity, the camu camu seeds extract was suggested to be incorporated into different food matrices to manage the early stages of type 2 diabetes.13
In clarified Brazilian camu camu fruit juice, a polyphenol extraction using solid phase extraction (SPE) with polyamide and C18 cartridges was done to measure the in vitro inhibitory activities against pancreatic α-amylase (IC50= 4.65 ± 0.05 and 0.56 ± 0.0 μg CE/mL) and α-glucosidase (IC50= 6.7 ± 0.0 and 2.7 ± 0.0 μg CE/mL), respectively. In the same study, the effect of camu camu juice on postprandial glycemia was also assessed. After consuming a 1-week carbohydrate meal, 23 healthy subjects consumed 50 g of white bread and 300 mL of camu camu juice, getting lower serum glucose levels than water as the control. These results prove that camu camu and its derived products exhibited an antihyperglycemic activity at in vitro and in vivo levels as well as the management of hyperglycemia complications.50 Moreover, microencapsulated camu camu exhibited more inhibitory capacity than acarbose against α-glucosidase with the highest values of IC50 = 5.13 ± 3.39 μg/mL for the Amazonian sample (6% MD/150 °C) and IC50 = 2.98 ± 1.12 μg/mL for the Sao Paulo sample (6% MD, 120 °C).9 As was expected, freeze-dried samples showed better inhibitory values than spray-dried samples. Actually, no camu camu sample showed more inhibition capacity than acarbose against α-amylase. The same authors evaluated the addition of camu camu powders (freeze-dried and spray-dried; 0.5 to 1% camu camu powder during 0, 24, 48, and 72 h) to a soymilk fermented beverage to assess the effect on the antihyperglycemic activity by the action of lactic acid bacteria.30 They found that freeze-dried samples showed higher enzyme inhibition activity than spray-dried samples and exhibited the most elevated α-amylase enzyme inhibitory after 24 and 48 h of fermentation and α-glucosidase inhibitory activity after 48 and 72 h (around 90%). In conclusion, higher concentrations of camu camu powders in soymilk significantly enhanced α-amylase and α-glucosidase inhibitory activities
These results agree with previous studies,32 suggesting that camu camu fresh and dried extract could be considered a natural antidiabetic source due to values of the inhibitory activities. This study assessed the antienzymatic activity of hot-dried (50 and 80 °C) and freeze-dried camu camu residue. Due to the drying conditions, the lyophilized extract showed a high percentage of inhibition of both enzymes, α-amylase (about 60%) and α-glucosidase (about 99%). However, there was no clear correlation between the inhibition values and the total phenolic content in the samples. Therefore, more research is necessary to establish the responsible compounds of α-amylase and α-glucosidase inhibitory activities.
Antihypertensive Activity
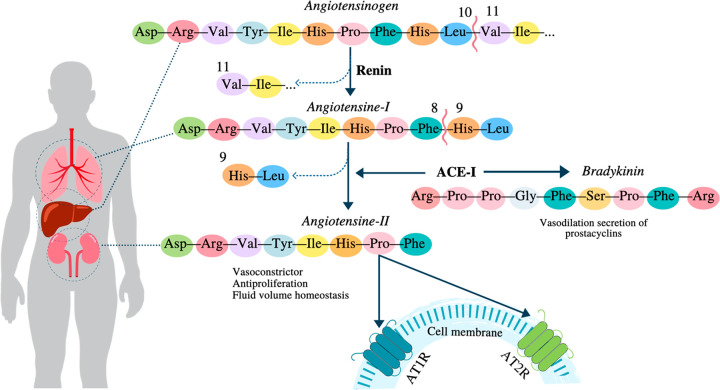
Hypertension is one of the significant risk factors for cardiovascular diseases (CVDs), causing side effects such as kidney damage and heart attack. This disease, suffered by 1 billion people worldwide, is defined as a blood pressure increase in the body.54 Additionally, it was one of the most critical comorbidities during the COVID-19 pandemic. When the kidney detects low blood pressure levels, it secretes renin (protease), responsible for converting angiotensinogen (hormone) into angiotensin-I, a decapeptide. Angiotensinogen is produced by the liver but is released into the bloodstream to carry out its metabolic action. In the lungs, angiotensin-I is converted into angiotensin-II (an octapeptide) by the activity of the enzyme ACE-I (angiotensin-converting enzyme-I), which is released by the kidneys. Angiotensin-II binds to two different types of receptors on cells, known as angiotensin receptors, AT1R and AT2R, and depending on the cell it interacts with (kidney cells or arteries), increases blood pressure by causing a vasoconstrictor effect, sympathetic activity, secretion of aldosterone, and reabsorption of sodium. In addition, ACE-I contributes to increased blood pressure by inactivating bradykinin, a potent antihypertensive peptide.55 This metabolic pathway is shown in Figure 3.
Figure 3.
Mechanism of angiotensin-converting enzyme (ACE-I) inhibitors.
Among the most common medicines for treating hypertension are captopril, lisinopril, or enalapril, which regulate high blood pressure by inhibiting ACE-I. It is reported that some phenolic compounds and flavonoids found in plants have a direct ACE-I inhibitory activity, reducing the risk of developing hypertension and normalizing blood pressure,53,55 for instance, tannic acid, benzoic acid, p-hydroxybenzoic acid, protocatechuic acid, gallic acid, vanillic acid, syringic acid, catechol, and pyrogallol, among others.54 Despite the phenolic compound profile in camu camu, few antihypertensive studies have been developed. Furthermore, no in vitro ACE-I inhibitory activity was found for camu camu pulp and its products from Sao Paulo and the Brazilian Amazon region.9 Although quercetin, ellagic acid, and chlorogenic acid were detected in camu camu, no ACE-I inhibitory or antihypertensive-related activity was reported. Nevertheless, in vivo data showed that dried camu camu pericarp extracts, administered to 20 participants using gelatin capsules, decreased the blood pressure levels and influenced the arterial diameter.56
Combining dried camu camu pulp powders in fermented soymilk samples displayed high ACE-I inhibitory activity (around 90%), where isoflavones and peptides of soymilk substrate possibly contributed to an increment of the fruit bioactivity.34 Likewise, aqueous, ethanolic, and propanone extracts from dried seeds of camu camu exhibited ACE-I inhibition between 28% and 40%. The potential of camu camu seed coat extracts in inhibiting ACE-I was also assessed, finding a positive correlation between some phenolic compounds and ACE-I inhibition capacity, as follows: quercetin (r = 0.852), p-coumaric acid (r = 0.935), rosmarinic acid (r = 0.881), rutin (r = 0.841), ellagic acid (r = 0.934), and caffeic acid (r = 0.925), compounds that were reported as responsible for the ACE-I inhibition. The high correlation value of ellagic acid and quercetin contradicts previously reported results.9 Therefore, further analysis with more concentrated extracts is suggested to characterize the responsible compounds of camu camu antihypertensive activity.
Antiobesity Activity
Obesity is one of the principal causes of metabolic syndrome and complications of chronic inflammatory etiology. Especially, abdominal obesity is a disease related to complex metabolic disorders such as insulin resistance, high blood pressure, dysglycemia, visceral adiposity, and dyslipidemia, which are directly interconnected with the increased risk of cardiovascular diseases.10 The prevalence of obesity and overweight is extremely high worldwide, and worrisome predictions indicate that more than 1 billion people will be obese by 2030.57 One way of treatment is adopting a healthy and balanced diet supplying the daily energy intake and making the individual adept at regular physical activities. The use of camu camu is increasingly imminent in preventing and treating this disease. Many antiobesity studies have been done in vivo with mice or rats during preclinical models, leading to a precise stage of further research in the biofunctional properties of camu camu.58,37
The effect of continuous exercise with or without the ingestion of Brazilian camu camu pulp was studied in a diet-induced obesity rat model.58,59 They used as variables the postprandial levels of cholesterol (HDL and LDL), triglycerides, glucose, body weight, feed intake, and visceral and epididymal fats of rats, concluding that there is a relation between the camu camu intake with physical exercise to reduce the body weight and the values of the visceral and epididymal fat deposits in the feces, heart, and liver, and inflammatory proteins in rats. In addition, the authors obtained adequate results of glucose, triglyceride, and HDL levels, while the exercise group lowered cholesterol and LDL levels, finding this experiment as promising.58
Other authors found a relationship between gut microbiota and consumption of camu camu in the treatment of obesity and associated immunometabolic disorders in high fat or high sucrose diet fed mice. Relevant results indicated that the crude extract of camu camu prevented weight gain, reduced fat accumulation, and blunted metabolic inflammation and endotoxemia. Treated mice improved glucose tolerance and insulin sensitivity and were also fully protected against hepatic steatosis. So, the authors concluded that camu camu prevents visceral and liver fat deposition through brown adipose tissue activation and increased energy expenditure, a mechanism dependent on the gut microbiota and linked to significant changes in the membrane bile acid pool size and composition.57 Another in vivo study with obese rats showed that camu camu juice derived from its pulp could reduce glucose, cholesterol, triglycerides, and insulin in the plasma of the rats having an intake of 25 mL of pulp juice daily for 12 weeks.37 The ingestion of camu camu juice decreased the inflammatory and oxidative markers in smoker individuals and the fasting glucose, total cholesterol, and LDL cholesterol levels in healthy individuals. Quercetin, quercetin glycosides, myricetin, rutin, ellagic acid, ellagitannins, and proanthocyanidins have been considered responsible compounds of antiobesity capacity found in camu camu juices. Some shown effects have been a reduction of body weight gain, dyslipidemia, hepatic steatosis, fecal cholesterol excretion, adiposity, fasting hyperglycemia, hyperinsulinemia, oxidative stress, and saturated lipids.1,37
Recent studies showed that the highest dose of the camu camu extract with 20% ascorbic acid (200 mg/kg/day CCE) significantly reduced body weight gain (p < 0.05) of diet-induced obese mice (six-hour-fasted) by oral administration after 5 weeks of treatment. Also, lower subcutaneous adipose tissue, insulin resistance index, triglycerides, and cholesterol content were evidenced. The lower CCE dose (62.5 mg/kg/day) only reduced the non-HDL cholesterol. Nonetheless, according to previous data, both doses of CCE exhibited a modulation of the gut microbiota, decreasing hyperglycemia and improving glucose tolerance.10 Finally, the impact of camu camu consumption in 58 obese participants was assessed against the metabolic syndrome indicators. Participants (20–59 years old) were divided into equal groups: control (placebo) and experimental (lyophilized camu camu with 442 mg of vitamin C). At the end of the experimentation, a decrease in blood pressure, triglycerides, abdominal circumference, and increment of HDL values were obtained in the experimental group compared to the control group. The authors mentioned that the practice of physical activity also contributed to the participants weight loss, concluding that healthy daily habits must accompany a bioactive compound-rich diet. In this regard, M. dubia and its bioactive compounds demonstrated an antiobesity capacity, reducing cholesterol, triglycerides, and weight levels in in vivo analysis.60
Other Health-Linked and Toxicity Bioactivities
After providing a report and analysis of the biofunctional properties of camu camu, particular focus has been put on the use of Myrciaria dubia fruit for the prevention or treatment of diseases related to metabolic syndromes such as type 2 diabetes and cardiovascular diseases. During the final part of this Review, some other biological and pharmacological activities were found. Antiallergenic, neuroprotective, antiproliferative, antihemolytic, and anti-inflammatory activities attributed to camu camu fruit have been reported and summarized in Table 3 as part of the main findings in M. dubia pulp, peel, and seeds.
Table 3. Health-Linked and Toxicity in Vivo and in Vitro Bioactivities of Camu Camu Fruit.
| bioactivity | fruit description | summary | ref |
|---|---|---|---|
| Antiallergic effect | Dried camu camu fruit | The 70% ethanol camu camu extract with 1% formic acid at 50 μg/mL displayed a reduction of histamine release and mast cell degranulation by A23187-induced allergies in RBL-2H3 cells. The effect was achieved through the modulation of H1 and H4 histamine receptors leading to a decrease in histidine decarboxylase (HDC) expression and therefore a diminishing in histamine release. | Do et al., 202261 |
| In vitro | |||
| Antitumor Activity | Fruit powder | Variation in the microbial composition of mice (n = 10/group) demonstrated antitumor activity and a stronger anti-PD-1 response by the oral supplementation of camu camu (polyphenol-rich). Likewise, showing immuno-therapeutic responses (promoting the growth of Ruminococcaceae and Alistipes) improved the CD8+/FOXP3+CD4+ ratio within the tumor microenvironment. Castalagin, an ellagitannin found in camu camu, was identified as the compound responsible for those effects. | Messaoudene et al., 202262 |
| In vivo/In vitro | In a preliminary human trial, two HIV patients with undetectable viral load receiving antiretroviral therapy were supplied with 1 g of camu camu capsules for 4 days. At the end of the trial, using fecal metagenomic analysis, a promotion in the growth of the immunostimulatory gut bacteria R. bromii was observed. | ||
| Oxidative stress and anti-inflammatory effect in skin diseases | Dried camu camu fruit | The 70% ethanol plus 1% of formic acid extract obtained from dried camu camu fruit at 10 μg/mL showed a protective effect against oxidative stress and inflammation in a high glucose-induced human keratinocytes model of skin damage. The anti-inflammatory mechanism proposed was a regulation in the production of cytokines and chemokines through different signaling pathways (NF-κB/AP-1, MAPK, and NFAT). In addition, the camu camu extract, at the same concentration, promoted the activation of the transcription factor Nrf2, which led to an increased level of the antioxidant enzyme, NAD(P)H:quinone oxidoreductase 1 (NQO1). | Do et al., 202163 |
| In vitro | |||
| Analgesic and Antiedematogenic | Pulp and peel | Juices at 10%, 25%, and 50% with distilled water presented no changes in the spontaneous locomotion of adult male mice (n = 128) during 12-h dark/12-h light cycles using juice at 50%. An oral antinociceptive effect was evidenced by formalin, hot plate, and complete Freund’s adjuvant tests. Camu camu juice exhibited antiedematogenic activity in paw edema. | da Silva et al., 202164 |
| In vivo | |||
| Antiproliferative and hepatotoxic | Peel, pulp, and seed ethanolic extracts | Four human tumor cell lines were assessed. Only camu camu peel showed inhibitory effects against cells, related to the content of apigenin and myricetin derivatives. Extracts showed no toxicity against the liver primary culture PLP2 (400 μg/mL), confirming that camu camu endorses their safe use in food systems. | Conceição et al., 20202 |
| In vitro | |||
| Antimalarial and antischistosomicidal effects | Seeds | Five extracts of camu camu seeds elaborated with different proportions of water and ethanol (100% water, 100% ethyl alcohol, 50% water + 50% ethyl alcohol, 25% water + 75% ethyl alcohol, and 75% water + 25% ethyl alcohol) were evaluated against Plasmodium falciparum, Schistosoma mansoni, and Leishmania amazonensis. The 50% water + 50% ethyl alcohol, 75% water + 25% ethyl alcohol, and 100% water extracts showed the highest growth inhibitory activity against malaria and schistosomiasis parasites. No hemolytic and antileishmanial activity was observed. The phenolic compounds, especially methylvescalagin and 2,4-dihydroxybenzoic acid, were associated with the activities presented by the extracts. | do Carmo et al., 202033 |
| In vitro | |||
| Antiproliferative, antihemolytic, anti-inflammatory | Seed extracts | Two tumor cell lines and intracellular reactive oxygen species (ROS) were assessed through antiproliferative effects. Seed extract showed antiproliferative activity at higher doses. The antihemolytic activity was assessed in isotonic and hypotonic conditions in relation to human erythrocytes, being dose-dependent at higher doses of seed extract. Finally, seed extract was toxic to cells at concentrations of 100, 300, and 1000 μg/mL, showing an anti-inflammatory activity and reduced the release of TNF-α cytokine in LPS-stimulated macrophages. | Fidelis et al., 202013 |
| In vitro | |||
| Nephroprotective effect | Alcoholic extract | The nephroprotective action of the alcoholic extract of camu camu was evaluated at three different doses (800, 1000, and 1200 mg/kg) in a gentamicin-induced nephrotoxicity rat model. The camu camu alcoholic extract, supplied by 8 days, showed low detrimental effects in the histopathological analysis. Moreover, a reduction in creatinine levels and gain in kidney weight was observed in the camu camu group in comparison with the gentamicin treated group. | Becerra et al., 201965 |
| In vivo | |||
| Antigenotoxic and antimutagenic | Juices from whole fruit (Prepared at 25% and 50%, and + 5 g/kg Ethanol) | Swiss male mice (n = 56), divided into 7 groups were employed by a 28-day treatment. Distilled water was used as a negative control. In liver and kidney tissues, ethanol samples were genotoxic until day 28, being antigenotoxic. In bone marrow, no cytotoxic effect was detected. Samples with the addition of ethanol showed antimutagenic activity, and data is associated with the content of phenolic compounds | da Silva et al., 201966 |
| In vivo | |||
| Immunological response | Fruit powder | A 5-week model with Nile tilapia was developed with oral camu camu administration (0, 50, 100, 250, and 500 mg/kg) to assess immune parameters, showing a significant increase in white blood cells counts in blood and exudate, burst respiratory activity, lysozyme activity, serum bactericidal activity, direct agglutination, and melanomacrophage center count (p < 0.05). No histopathological lesions were observed in intestine, kidney, spleen, and gills. | Yunis-Aguinaga et al., 201667 |
| In vivo | |||
| Cellular rejuvenation | Freeze-dried pulp and spray drying products | A 10-day model was developed with planarian animals to evaluate for cellular protection and rejuvenation. Compared to the control, camu camu extracts showed rapid regeneration and regrowth. Only freeze-dried camu camu showed superior regrowth in the tail of the animals. Arabic gum and phenolic compounds in the extract may stimulate regeneration. | Fujita et al., 20159 |
| In vivo | |||
| Suppression of d-galactosamine (GalN)-induced liver injury | Lyophilized fruit juice concentrate | The lyophilized camu camu fruit (powder) added to the diet (10%) (7 days) displayed an hepatoprotective effect in rats, suppressing the liver injury induced by the action of d-galactosamine. The active principle was isolated and identified as 1-methylmalate. This compound, at 250, 500, and 1000 mg/kg of body weight, suppressed the increased levels of bilirubin and the activity of ALT, AST, and LDH enzymes induced by GalN. | Akachi et al., 201068 |
| In vivo | |||
| Antimicrobial activity | Pulp, seeds, skin, leaves, bark, and skin + seed combined | Antimicrobial activity of camu camu extracts obtained from different organs has been established. Reports of growth inhibition against Gram positive (L. monocytogenes, S. aureus), Gram negative (E. coli, S. typhimurium, S. enteritidis, P. aeruginosa), yeasts (Candida albicans and Saccharomyces cerevisiae), and pathogenic protozoa (Leishmania spp. and Plasmodium spp.) are described. Some phenolic compounds (i.e., acylphloroglucinols, flavonoids, ellagitannins, C6C1 phenolic acids, and chalcones, among others) present in camu camu have shown antimicrobial activity, but the mechanism of action is waiting to be elucidated. | Barrios Renteria et al., 202269 and references therein |
| In vitro | |||
| Peels, pulp, seeds, and leaves | Eleven reports of antimicrobial activity against oral pathogens have been published. The extracts obtained from different camu camu organs have been evaluated by different methodologies. Activity has been mainly observed against Gram positive oral cavity flora (S. aureus, S. mutans, and S. sanguinis) and one yeast (C. albicans). | Pardo-Aldave et al., 201970 and references therein |
Conclusions and Outlook
As part of the Myrtaceae family, camu camu (Myciaria dubia) fruit is a promising starting material with multiple health benefits due to its high content of different bioactive compounds. This Review summarizes the publications related to assessing the role of biocomponents from camu camu (seeds, peel, and pulp) as health-promoting food ingredients, concluding that M. dubia is the second natural source of vitamin C in nature, and its variety of polyphenols could be associated with the prevention of metabolic syndrome diseases. However, new molecules of biological interest related to these effects still need to be elucidated, avoiding oxidation and degradation processes during its extraction and future food applications. Some molecules have been identified as responsible for certain activities, for instance, 1-methylmalate associated with suppression of d-galactosamine (GalN)-induced liver injury and Castalagin, with a reduction in tumor progression. Likely, more bioactive compounds are present in this fruit; therefore, additional in vitro and in vivo studies are needed to determine the relevant bioactive compounds, to identify novel targets, or to decipher its physiological mechanisms in the human body, which allows one to expand the number of applications of this promising fruit. To our knowledge, clinical trials with camu camu are being developed to prevent hypertension and diabetes, so the expected results would provide an understanding of the camu camu beneficial human health effects. Despite M. dubia antioxidant activity and its positive effects reducing the risk of hyperglycemia, hypertension, and obesity, it is worth mentioning that healthy lifestyles and a nutrient-based diet are also necessary complements for the prevention and treatment of noncommunicable diseases.
Formerly, indigenous populations consumed camu camu for health purposes to prevent the flu.38 In the last years, the bioeconomy of the Amazon region has been a national concern among the seven countries that make up the Amazon biome. For that reason, international cooperation programs incorporate some of the most prominent species, such as camu camu fruit. The aim is to support the employment of local farmers and economic income through sustainable production, working with indigenous communities, combating illegal mining and the climate crisis, and reducing the carbon footprint, leading to a scientific study and application in the food industry. Likewise, this application could be considered a healthy alternative, being incorporated as a natural ingredient and functional additive with high added value for nutraceutical purposes. Additionally, camu camu holds numerous possibilities of technological use as spray-dried microencapsulates, allowing the development of functional foods into diverse food matrices (beverages, candies, and dairy and bakery products, among others) for commercial use. In conclusion, the physicochemical, bioactive, and technological studies of M. dubia acknowledge the immersion of this fruit in new research approaches in consideration for its future applications in the food and pharmacological industry.
Acknowledgments
J.M.G.-C. thanks Ministerio de Ciencia, Tecnología e Innovación of Colombia (Minciencias) for grant Programa de Becas de Excelencia Doctoral del Bicentenario–Corte-I.
This work was supported by grants from the Fondo Nacional de Financiamiento para la Ciencia, la Tecnología y la Innovación, Francisco José de Caldas (contract No. 0459–2013), and Red Nacional para la Bioprospección de Frutas Tropicales-RIFRUTBIO.
The authors declare no competing financial interest.
References
- Donado-Pestana C. M; Moura M. H. C.; de Araujo R. L.; de Lima Santiago G.; de Moraes Barros H. R.; Genovese M. I. Polyphenols from Brazilian native Myrtaceae fruits and their potential health benefits against obesity and its associated complications. Curr. Opin. Food. Sci. 2018, 19, 42–49. 10.1016/j.cofs.2018.01.001. [DOI] [Google Scholar]
- Conceição N.; Albuquerque B. R.; Pereira C.; Corrêa R. C. G.; Lopes C. B.; Calhelha R. C.; Alves M. J.; Barros L.; Ferreira I. C. F. R. By-products of camu-camu [Myrciaria dubia (Kunth) McVaugh] as promising sources of bioactive high added-value food ingredients: Functionalization of yogurts. Molecules. 2020, 25, 70. 10.3390/molecules25010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campelo P. H.; Alves Filho E. G.; Silva L. M.A.; de Brito E. S.; Rodrigues S.; Fernandes F. A.N. Modulation of aroma and flavor using dielectric barrier discharge plasma technology in a juice rich in terpenes and sesquiterpenes. LWT - Food. Sci. Technol. 2020, 130, 109644. 10.1016/j.lwt.2020.109644. [DOI] [Google Scholar]
- Akter M. S.; Oh S.; Eun J.-B.; Ahmed M. Nutritional compositions and health promoting phytochemicals of camu-camu (Myrciaria dubia) fruit: A review. Food. Res. Int. 2011, 44, 1728–1732. 10.1016/j.foodres.2011.03.045. [DOI] [Google Scholar]
- Fracassetti D.; Costa C.; Moulay L.; Tomás-Barberán F. A. Ellagic acid derivatives, ellagitannins, proanthocyanidins and other phenolics, vitamin C and antioxidant capacity of two powder products from camu-camu fruit (Myrciaria dubia). Food. Chem. 2013, 139, 578–588. 10.1016/j.foodchem.2013.01.121. [DOI] [PubMed] [Google Scholar]
- Neves L. C.; Silva V. X. d.; Pontis J. A.; Flach A.; Roberto S. R. Bioactive compounds and antioxidant activity in pre-harvest camu-camu [Myrciaria dubia (H.B.K.) Mc Vaugh] fruits. Sci. Hortic. 2015, 186, 223–229. 10.1016/j.scienta.2015.02.031. [DOI] [Google Scholar]
- de Araujo F. F.; Neri-Numa I. A.; de Paulo Farias D.; da Cunha G. R. M. C.; Pastore G. M. Wild Brazilian species of Eugenia genera (Myrtaceae) as an innovation hotspot for food and pharmacological purposes. Food. Res. Int. 2019, 121, 57–72. 10.1016/j.foodres.2019.03.018. [DOI] [PubMed] [Google Scholar]
- Azevedo L.; de Araujo Ribeiro P. F.; de Carvalho Oliveira J. A.; Correia M. G.; Ramos F. M.; de Oliveira E. B.; Barros F.; Stringheta P. C. Camu-camu (Myrciaria dubia) from commercial cultivation has higher levels of bioactive compounds than native cultivation (Amazon Forest) and presents antimutagenic effects in vivo. J. Sci. Food Agric. 2019, 99, 624–631. 10.1002/jsfa.9224. [DOI] [PubMed] [Google Scholar]
- Fujita A.; Sarkar D.; Wu S.; Kennelly E.; Shetty K.; Genovese M. I. Evaluation of phenolic-linked bioactives of camu-camu (Myrciaria dubia McVaugh) for antihyperglycemia, antihypertension, antimicrobial properties and cellular rejuvenation. Food Res. Int. 2015, 77, 194–203. 10.1016/j.foodres.2015.07.009. [DOI] [Google Scholar]
- Abot A.; Brochot A.; Pomié N.; Wemelle E.; Druart C.; Régnier M.; Delzenne N. M.; de Vos W. M.; Knauf C.; Cani P. D. Camu-camu reduces obesity and improves diabetic profiles of obese and diabetic mice: A dose-ranging study. Metabolites 2022, 12, 301. 10.3390/metabo12040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo M. A. V. D.; Fidelis M.; Pressete C. G.; Marques M. J.; Castro-Gamero A. M.; Myoda T.; Granato D.; Azevedo L. Hydroalcoholic Myrciaria dubia (camu-camu) seed extracts prevent chromosome damage and act as antioxidant and cytotoxic agents. Food Res. Int. 2019, 125, 108551. 10.1016/j.foodres.2019.108551. [DOI] [PubMed] [Google Scholar]
- Santos I. L.; Miranda L. C. F.; da Cruz Rodrigues A. M.; da Silva L. H. M.; Amante E. R. Camu-camu [Myrciaria dubia (HBK) McVaugh]: A review of properties and proposals of products for integral valorization of raw material. Food Chem. 2022, 372, 131290. 10.1016/j.foodchem.2021.131290. [DOI] [PubMed] [Google Scholar]
- Fidelis M.; Araújo Vieira do Carmo M.; da Cruz T. M.; Azevedo L.; Myoda T.; Miranda Furtado M.; Boscacci Marques M.; Sant’Ana A. S.; Genovese M. I.; Young Oh W.; Wen M.; Shahidi F.; Zhangh L.; Franchin M.; de Alencar S. M.; Rosalen P. L.; Granato D. Camu-camu seed (Myrciaria dubia) – From side stream to an antioxidant, antihyperglycemic, antiproliferative, antimicrobial, antihemolytic, anti-inflammatory, and antihypertensive ingredient. Food Chem. 2020, 310, 125909. 10.1016/j.foodchem.2019.125909. [DOI] [PubMed] [Google Scholar]
- de Abreu Figueiredo J.; Andrade Teixeira M.; Henrique Campelo P.; Teixeira Lago A. M.; Pereira de Souza T.; Yoshida M. I.; Rodrigues de Oliveira C.; Pereira A. P. A.; Pastore G. M.; Sanches E. A.; Alvarenga Botrel D.; Vilela Borges S. Encapsulation of camu-camu extracts using prebiotic biopolymers: controlled release of bioactive compounds and effect on their physicochemical and thermal properties. Food Res. Int. 2020, 137, 109563. 10.1016/j.foodres.2020.109563. [DOI] [PubMed] [Google Scholar]
- Meléndez-Martínez A. J.; Mandić A. I.; Bantis F.; Böhm V.; Borge G. I. A.; Brnčić M.; Bysted A.; Cano M. P.; Dias M. G.; Elgersma A.; Fikselová M.; García-Alonso J.; Giuffrida D.; Gonçalves V. S. S.; Hornero-Méndez D.; Kljak K.; Lavelli V.; Manganaris G. A.; Mapelli-Brahm P.; Marounek M.; Olmedilla-Alonso B.; Periago-Castón M. J.; Pintea A.; Sheehan J. J.; Tumbas Šaponjac V.; Valšíková-Frey M.; Meulebroek L. V.; O’Brien N. A comprehensive review on carotenoids in foods and feeds: status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. 10.1080/10408398.2020.1867959. [DOI] [PubMed] [Google Scholar]
- Cunha-Santos E. C. M.; Viganó J.; Neves D. A.; Martínez J.; Godoy H. T. Vitamin C in camu-camu [Myrciaria dubia (H.B.K.) McVaugh]: evaluation of extraction and analytical methods. Food Res. Int. 2019, 115, 160–166. 10.1016/j.foodres.2018.08.031. [DOI] [PubMed] [Google Scholar]
- Doseděl M.; Jirkovský E.; Macáková K.; Krčmová L. K.; Javorská L.; Pourová J.; Mercolini L.; Remião F.; Nováková L.; Mladěnka P. Vitamin C—Sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients. 2021, 13, 615. 10.3390/nu13020615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Fernández O.; Domínguez R.; Pateiro M.; Munekata P. E. S.; Rocchetti G.; Lorenzo J. M. Determination of polyphenols using liquid chromatography–tandem mass spectrometry technique (LC–MS/MS): A Review. Antioxidants. 2020, 9, 479. 10.3390/antiox9060479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa Araújo P. A.; dos Santos Garcia V. A.; Osiro D.; de Souza França D.; Vanin F. M.; de Carvalho R. A. Active compounds from the industrial residue of dry camu-camu. Food Sci. Technol. 2022, 42, e0532 10.1590/fst.05321. [DOI] [Google Scholar]
- Zanatta C. F.; Mercadante A. Z. Carotenoid composition from the Brazilian tropical fruit camu-camu (Myrciaria dubia). Food Chem. 2007, 101, 1526–1532. 10.1016/j.foodchem.2006.04.004. [DOI] [Google Scholar]
- Grigio M. L.; de Moura E. A.; Alves Chagas E.; Berlingieri Durigan M. F.; Cardoso Chagas P.; Ferreira de Carvalho G.; Zanchetta J. J. Bioactive compounds in and antioxidant activity of camu- camu fruits harvested at different maturation stages during postharvest storage. Acta Sci. Agron. 2021, 43, e50997. 10.4025/actasciagron.v43i1.50997. [DOI] [Google Scholar]
- Rufino M. S. M.; Alves R. E.; de Brito E. S.; Pérez-Jiménez J.; Saura-Calixto F.; Mancini-Filho J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. 10.1016/j.foodchem.2010.01.037. [DOI] [Google Scholar]
- Souza A.; Oliveira T.; Mattietto R.; Nascimento W.; Lopes A. Bioactive compounds in the peel of camu-camu genotypes from Embrapa’s active germplasm bank. Food Sci. Technol, Campinas. 2018, 38, 67–71. 10.1590/1678-457x.33716. [DOI] [Google Scholar]
- do Amaral Souza F. d. C.; Gomes Sanders Moura L.; de Oliveira Bezerra K.; Paiva Lopes Aguiar J.; Moreira Mar J.; Sanches E. A.; Faccini dos Santos F.; Bakry A. M.; Nicolau Paulino B.; Campelo P. H. Thermosonication applied on camu–camu nectars processing: Effect on bioactive compounds and quality parameters. Food and Biopro Proc. 2019, 116, 212–218. 10.1016/j.fbp.2019.06.003. [DOI] [Google Scholar]
- Fujita A.; Souza V. B.; Daza L. D.; Fávaro-Trindade C. S.; Granato D.; Genovese M. I. Effects of spray-drying parameters on in vitro functional properties of camu-camu (Myrciaria dubia Mc. Vaugh): A typical Amazonian fruit. J. Food Sci. 2017, 82, 1083–1091. 10.1111/1750-3841.13668. [DOI] [PubMed] [Google Scholar]
- Erukainure O. L.; Sanni O.; Islam S.. Clerodendrum volubile: phenolics and applications to health. In Polyphenols: Mechanisms of Action in Human Health and Disease, 2nd ed.; Academic Press, Elsevier Inc., 2018; pp 53–68. [Google Scholar]
- Gomes J. V. P.; Rigolon T. C. B.; Souza M. S. d. S.; Alvarez-Leite J. I.; Lucia C. M. D.; Martino H. S. D.; Rosa C. d. O. B. The antiobesity effects of anthocyanins on mitochondrial biogenesis, inflammation and oxidative stress: A systematic review. Nutrition. 2019, 66, 192–202. 10.1016/j.nut.2019.05.005. [DOI] [PubMed] [Google Scholar]
- Cunha-Santos E. C. E.; Rodrigues-Silva C.; Ferreira Ferreira da Silveira T.; Teixeira Godoy H. Optimization of phenolic compounds extraction of different parts of camu-camu fruit from different geographic regions. Plant Foods Hum. Nutr. 2022, 77, 340–344. 10.1007/s11130-022-00985-0. [DOI] [PubMed] [Google Scholar]
- Lima S. R.; Azevedo de Carvalho A. P.; Conte-Junior C. A. Health from Brazilian Amazon food wastes: Bioactive compounds, antioxidants, antimicrobials, and potentials against cancer and oral diseases. Crit. Rev. Food. Sci. Nutr. 2022, 25, 1–23. 10.1080/10408398.2022.2101983. [DOI] [PubMed] [Google Scholar]
- Grigio M. L.; Chagas E. A.; Rathinasabapathi B.; Cardoso Chagas P.; Vieria da Silva A. R.; Moreira Sobral S. T.; Rodrigues de Oliveira R. Qualitative evaluation and biocompounds present in different parts of camu-camu (Myrciaria dubia) fruit. Afr. J. Food Sci. 2017, 11, 124–129. 10.5897/AJFS2016.1574. [DOI] [Google Scholar]
- Lee Y. Cancer chemopreventive potential of procyanidin. Toxicol Res. 2017, 33, 273–282. 10.5487/TR.2017.33.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Azevedo J. C. S.; Fujita A.; de Oliveira E. L.; Genovese M. I.; Correia R. T. P. Dried camu-camu (Myrciaria dubia H.B.K. McVaugh) industrial residue: A bioactive-rich Amazonian powder with functional attributes. Food Res. Int. 2014, 62, 934–940. 10.1016/j.foodres.2014.05.018. [DOI] [Google Scholar]
- do Carmo M. A. V.; Fidelis M.; Sanchez C. A.; Castro A. P.; Camps I.; Colombo F. A.; Marques M. J.; Myoda T.; Granato D.; Azevedo L. Camu-camu (Myrciaria dubia) seeds as a novel source of bioactive compounds with promising antimalarial and antischistosomicidal properties. Food Res. Int. 2020, 136, 109334. 10.1016/j.foodres.2020.109334. [DOI] [PubMed] [Google Scholar]
- Kaneshima T.; Myoda T.; Toeda K.; Fujimori T.; Nishizawa M. Antimicrobial constituents of peel and seeds of camu-camu (Myrciaria dubia). Biosci. Biotechnol. Biochem. 2017, 81 (8), 1461–1465. 10.1080/09168451.2017.1320517. [DOI] [PubMed] [Google Scholar]
- Fujita A.; Sarkar D.; Genovese M. I.; Shetty K. Improving anti-hyperglycemic and anti-hypertensive properties of camu-camu (Myrciaria dubia Mc. Vaugh) using lactic acid bacterial fermentation. Process Biochem 2017, 59, 133–140. 10.1016/j.procbio.2017.05.017. [DOI] [Google Scholar]
- das Chagas E. G. L.; Vanin F. M.; dos Santos Garcia V. A.; Yoshida C. M. P.; de Carvalho R. A. Enrichment of antioxidants compounds in cookies produced with camu-camu (Myrciaria dubia) coproducts powders. LWT – Food Sci. Technol. 2021, 137, 110472. 10.1016/j.lwt.2020.110472. [DOI] [Google Scholar]
- de Souza Schmidt Gonçalves A. E.; Lellis-Santos C.; Curi R.; Lajolo F. M.; Genovese M. I. Frozen pulp extracts of camu-camu (Myrciaria dubia McVaugh) attenuate the hyperlipidemia and lipid peroxidation of Type 1 diabetic rats. Food Res. Int. 2014, 64, 1–8. 10.1016/j.foodres.2014.05.074. [DOI] [PubMed] [Google Scholar]
- Gironés-Vilaplana A.; Baenas N.; Villaño D.; Speisky H.; García-Viguera C.; Moreno D. A. Evaluation of Latin-American fruits rich in phytochemicals with biological effects. J. Funct. Foods. 2014, 7, 599–608. 10.1016/j.jff.2013.12.025. [DOI] [Google Scholar]
- Chirinos R.; Galarza J.; Betalleluz-Pallardel I.; Pedreschi R.; Campos D. Antioxidant compounds and antioxidant capacity of Peruvian camu camu (Myrciaria dubia (H.B.K.) McVaugh) fruit at different maturity stages. Food Chem. 2010, 120, 1019–1024. 10.1016/j.foodchem.2009.11.041. [DOI] [Google Scholar]
- Ju A.; Song K. B. Development of teff starch films containing camu-camu (Myrciaria dubia Mc. Vaugh) extract as an antioxidant packaging material. Ind. Crops Prod. 2019, 141, 111737. 10.1016/j.indcrop.2019.111737. [DOI] [Google Scholar]
- Kaneshima T.; Myoda T.; Nakata M.; Fujimori T.; Toeda K.; Nishizawa M. Antioxidant activity of C-glycosidic ellagitannins from the seeds and peel of camu-camu (Myrciaria dubia). LWT – Food Sci. Technol. 2016, 69, 76–81. 10.1016/j.lwt.2016.01.024. [DOI] [Google Scholar]
- International Diabetes Federation . About Diabetes: Diabetes Facts & Figures; 2021; https://idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html (accessed 2022–11–07).
- Gothai S.; Ganesan P.; Park S.-Y.; Fakurazi S.; Choi D.-K.; Arulselvan P. Natural phyto-bioactive compounds for the treatment of type 2 diabetes: inflammation as a target. Nutrients. 2016, 8, 461. 10.3390/nu8080461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood N. A review of α-amylase inhibitors on weight loss and glycemic control in pathological states such as obesity and diabetes. Comp. Clin Pathol. 2016, 25, 1253–1264. 10.1007/s00580-014-1967-x. [DOI] [Google Scholar]
- Lankatillake C.; Luo S.; Flavel M.; Lenon G. B.; Gull H.; Huyng T.; Dias D. A. Screening natural product extracts for potential enzyme inhibitors: protocols, and the standardization of the usage of blanks in α-amylase, α-glucosidase, and lipase assays. Plant Methods. 2021, 17, 3. 10.1186/s13007-020-00702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti S. K.; Krishnan S.; Kumar A.; Kumar A. Antidiabetic phytoconstituents and their mode of action on metabolic pathways. Ther. Adv. Endocrinol. Metab. 2018, 9, 81–100. 10.1177/2042018818755019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña Hidalgo M.; Espinoza Campos F. O.; Ramirez M. D.; Villacrés-Vallejo J.; Vásquez Torres D. Efecto tóxico y antidiabético de tres plantas amazónicas en ratones balb/c inducidas con estreptozotocina. Ciencia Amazónica 2022, 9, 21–32. 10.22386/ca.v9i2.338. [DOI] [Google Scholar]
- Schwertz M. C.; Maia J. R. d. P.; de Sousa R. F. S.; Aguiar J. P. L.; Yuyama L. K. O.; Lima E. S. Hypolipidemic effect of camu-camu juice in rats. Rev. Nutr. Campinas 2012, 25, 35–44. 10.1590/S1415-52732012000100004. [DOI] [Google Scholar]
- Asgar A. Anti-diabetic potential of phenolic compounds: A review. Int. J. Food Prop. 2013, 16, 91–103. 10.1080/10942912.2011.595864. [DOI] [Google Scholar]
- Balisteiro D. M.; de Araujo R. L.; Giacaglia L. R.; Genovese M. I. Effect of clarified Brazilian native fruit juices on postprandial glycemia in healthy subjects. Food Res. Int. 2017, 100, 196–203. 10.1016/j.foodres.2017.08.044. [DOI] [PubMed] [Google Scholar]
- De Souza Schmidt Gonçalves A. E.; Lajolo F. M.; Genovese M. I. Chemical composition and antioxidant/antidiabetic potential of Brazilian native fruits and commercial frozen pulps. J. Agric. Food Chem. 2010, 58, 4666–4674. 10.1021/jf903875u. [DOI] [PubMed] [Google Scholar]
- Vinholes J.; Lemos G.; Barbieri R. L.; Franzon R. C.; Vizzotto M. In vitro assessment of the antihyperglycemic and antioxidant properties of araçá, butiá and pitanga. Food Biosci. 2017, 19, 92–100. 10.1016/j.fbio.2017.06.005. [DOI] [Google Scholar]
- Moura M. H. C.; Cunha M. G.; Alezandro M. R.; Genovese M. I. Phenolic-rich jaboticaba (Plinia jaboticaba (Vell.) Berg) extracts prevent high-fat-sucrose diet-induced obesity in C57BL/6 mice. Food Res. Int. 2018, 107, 48–60. 10.1016/j.foodres.2018.01.071. [DOI] [PubMed] [Google Scholar]
- Al Shukor N.; Van Camp J.; Gonzales G. B.; Staljanssens D.; Struijs K.; Zotti M. J.; Raes K.; Smagghe G. Angiotensin-converting enzyme inhibitory effects by plant phenolic compounds: a study of structure activity relationships. J. Agric. Food Chem. 2013, 61, 11832–11839. 10.1021/jf404641v. [DOI] [PubMed] [Google Scholar]
- Felkle D.; Jarczynski M.; Kaleta K.; Zięba K.; Nazimek K. The immunomodulatory effects of antihypertensive therapy: A review. Biomed. Pharmacother. 2022, 153, 113287. 10.1016/j.biopha.2022.113287. [DOI] [PubMed] [Google Scholar]
- Miyashita T.; Koizumi R.; Myoda T.; Sagane Y.; Niwa K.; Watanabe T.; Minami K. Data on a single oral dose of camu-camu (Myrciaria dubia) pericarp extract on flow-mediated vasodilation and blood pressure in young adult humans. Data Brief. 2018, 16, 993–999. 10.1016/j.dib.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anhê F. F.; Nachbar R. T.; Varin T. V.; Trottier J.; Dudonné S.; Le Barz M.; Perrine F.; Genevière P.; Barbier O.; Desjardins Y.; Roy D.; Marette A. Treatment with camu-camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut. 2019, 68, 453–464. 10.1136/gutjnl-2017-315565. [DOI] [PubMed] [Google Scholar]
- Vilaça do Nascimento O.; de Araújo Boleti A. P.; Schwertz M.; Lima E. S. Dietary supplementation with camu-camu and continuous exercises in the treatment of obesity. Rev. Nutr. 2018, 31, 25–33. 10.1590/1678-98652018000100003. [DOI] [Google Scholar]
- Nascimento O. V.; Boleti A. P. A; Yuyama L. K. O.; Lima E. S. Effects of diet supplementation with Camu-camu (Myrciaria dubia HBK McVaugh) fruit in a rat model of diet-induced obesity. An. Acad. Bras. Cienc. 2013, 85, 355–363. 10.1590/S0001-37652013005000001. [DOI] [PubMed] [Google Scholar]
- Salomão-Oliveira A.; de Souza Costa S.; Albuquerque Marinho H. Metabolic syndrome: Intake of camu-camu (Myrciaria dubia (Kunth) McVaugh). Int. J. Food Sci. Nutr. 2018, 3, 5–13. [Google Scholar]
- Do N. Q.; Zheng S.; Oh S.; Nguyen Q. T. N.; Fang M.; Kim M.; Choi J.; Kim M.-J.; Jeong J.; Yi T.-H. Anti-allergic effects of Myrciaria dubia (camu-camu) fruit extract by inhibiting histamine H1 and H4 receptors and histidine decarboxylase in RBL-2H3 cells. Antioxidants 2022, 11, 104. 10.3390/antiox11010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudene M.; Pidgeon R.; Richard C.; Ponce M.; Diop K.; Benlaifaoui M.; Nolin-Lapalme A.; Cauchois F.; Malo J.; Belkaid W.; Isnard S.; Fradet Y.; Dridi L.; Velin D.; Oster P.; Raoult D.; Ghiringhelli F.; Boidot R.; Chevrier S.; Kysela D. T.; Brun Y. V.; Falcone E. L.; Pilon G.; Oñate F. P.; Gitton-Quent O.; Le Chatelier E.; Durand S.; Kroemer G.; Elkrief A.; Marette A.; Castagner B.; Routy B. A natural polyphenol exerts antitumor activity and circumvents anti-PD-1 resistance through effects on the gut microbiota. Cancer Discovery 2022, 12, 1070–1087. 10.1158/2159-8290.CD-21-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do N. Q.; Zheng S.; Park B.; Nguyen Q. T. N.; Choi B.-R.; Fang M.; Kim M.; Jeong J.; Choi J.; Yang S.-J.; Yi T.-H. Camu-camu fruit extract inhibits oxidative stress and inflammatory responses by regulating NFAT and Nrf2 signaling pathways in high glucose-induced human keratinocytes. Molecules. 2021, 26, 3174. 10.3390/molecules26113174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva F. C.; Hübner de Souza A.; Kempfer Bassoli B.; Alves Prates G.; Daudt C.; de Oliveira Meneguetti D. U.; Corrêa A. P.; Berbigier de Oliveira I.; de Vargas Schons S.; Pessano Fialho M. F.; Silva Correa D.; Nascimento Picada J.; de Barros Falcão Ferraz A. Myrciaria dubia juice (camu-camu) exhibits analgesic and antiedematogenic activities in mice. J. Med. Food 2021, 24, 626–634. 10.1089/jmf.2020.0094. [DOI] [PubMed] [Google Scholar]
- Becerra B. K.; García D. J.; Becerra B. M.; Ruiz R. E.; Chávez R. L. Nephroprotective effect of camu camu (Myrciaria dubia) in a model of nephrotoxicity induced by gentamicin in rats. Rev. Chil Nutr. 2019, 46, 303–307. [Google Scholar]
- da Silva F. C.; Nascimento Picada J.; Faria Romão N.; de Oliveira Solla Sobral F.; Lemos D.; de Vargas Schons S.; de Mello T. L.; Melo Silva W.; da Silva Oliveira R.; Perboni Lucas C.; Pereira P.; Clasen Chaves V.; Reginatto F. H.; de Barros Falcão Ferraz A. Antigenotoxic and antimutagenic effects of Myrciaria dubia juice in mice submitted to ethanol 28- day treatment. J. Toxicol. Environ. Health Part A 2019, 82, 956–968. 10.1080/15287394.2019.1671279. [DOI] [PubMed] [Google Scholar]
- Yunis-Aguinaga J.; Fernandes D. C.; Eto S. F.; Claudiano G. S.; Marcusso P. F.; Marinho-Neto F. A.; Fernandes J. B. K.; de Moraes F. R.; de Moraes J. R. E. Dietary camu camu, Myrciaria dubia, enhances immunological response in Nile tilapia. Fish Shellfish Immunol. 2016, 58, 284–291. 10.1016/j.fsi.2016.08.030. [DOI] [PubMed] [Google Scholar]
- Akachi T.; Shiina Y.; Kawaguchi T.; Kawagishi H.; Morita T.; Sugiyama K. 1-Methylmalate from camu-camu (Myrciaria dubia) suppressed D-galactosamine-induced liver injury in rats. Biosci. Biotechnol. Biochem. 2010, 74, 573–578. 10.1271/bbb.90775. [DOI] [PubMed] [Google Scholar]
- Barrios Renteria J. C.; Mauricio-Sandoval E. A.; Espinoza L. A.; Cornelio-Santiago H. P.; Moreno-Quispe L. A.; Vega Portalatino E. J. Antimicrobial potential of camu camu (Myrciaria dubia) against bacteria, yeasts, and parasitic protozoa: a review. Rev. Fac. Nac. Agron. Medellín 2022, 75, 9989–9998. 10.15446/rfnam.v75n2.98010. [DOI] [Google Scholar]
- Pardo-Aldave K.; Pareja-Vásquez M.; Guillen A.; Ureta-Tapia J. M. Antimicrobial activity in vitro of camu-camu (Myrciaria dubia) against oral microorganisms: a systematic review. Rev. Peru Med. Exp. Salud Publica 2019, 36, 573–582. 10.17843/rpmesp.2019.364.4270. [DOI] [PubMed] [Google Scholar]