Abstract
Cutaneous squamous cell carcinoma (cSCC) and its premalignant precursor, actinic keratosis (AK), present a global health burden that is continuously increasing despite extensive efforts to promote sun safety. Chronic UV exposure is a recognized risk factor for the development of AK and cSCC. However, increasing evidence suggests that AK and cSCC is also associated with skin microbiome dysbiosis and, in particular, an overabundance of the bacterium Staphylococcus aureus (S. aureus). Studies have shown that S. aureus-derived toxins can contribute to DNA damage and lead to chronic upregulation of proinflammatory cytokines that may affect carcinogenesis. Eradication of S. aureus from AK lesions and restoration of a healthy microbiome may therefore represent a therapeutic opportunity to alter disease progression. Whilst antibiotics can reduce the S. aureus load, antibiotic resistant S. aureus pose an increasing global public health threat. The use of specific topically delivered probiotics has been used experimentally in other skin conditions to restore eubiosis, and could therefore also present a non-invasive treatment approach to decrease S. aureus colonization and restore a healthy skin microbiome on AK lesions. This article reviews mechanisms by which S. aureus may contribute to cutaneous carcinogenesis, and discusses hypotheses and theories that explore the therapeutic potential of specific bacterial species which compete with S. aureus in an attempt to restore microbial eubiosis in skin.
Keywords: Staphylococcus aureus, Cutaneous squamous cell carcinoma, Actinic keratosis, Staphylococcus epidermidis (S. epidermidis), Corynebacterium striatum (C. striatum), Cutibacterium acnes (C. acnes), UV damage and repair
Introduction
Cutaneous squamous cell carcinoma is an invasive keratinocyte cancer arising from the basal layer of the epidermis. A longitudinal analysis of the global burden of cSCC from 1990-2017 revealed a 310% increase in disease incidence, ranking cSCC as the sixth most frequently diagnosed neoplasm worldwide (1). cSCC are thought to result from the malignant transformation of actinic keratosis (AK). AK are characterized as scaly, rough skin lesions, typically less than 1cm in diameter (2). The presence of AK is indicative of cumulative UV-A/B exposure and a lack of regularly implemented sun protection (3, 4). Although the risk of malignant transformation of AK to cSCC is typically 0.0075%/lesion/year, 60% of cSCCs develop from AK lesions (4). AKs that have undergone treatment have a recurrence rate of 20-30% per lesion after 12 months (2, 5). Aside from originating from AKs, an alternative pathway has also been reported where cSCCs arise de novo from photo-damaged skin and are not associated with AK (5). A key determinant of progression of AKs or photo-damaged skin to cSCC is immune system competency. Immune suppressed patients, particularly organ transplant recipients, are 200 times more likely to develop cSCC when compared to an immunocompetent age-matched population (6).
cSCC is a multifactorial disease, with the best recognized risk factor being UV exposure, and is endemic amongst Caucasians in tropical and subtropical areas of the globe. UV exposure, particularly UV-B, is associated with epidermal erythema (sunburn), gene mutation, immunosuppression and cancer. The shorter wavelength and higher energy of UV-B enhances damage to DNA and is absorbed in the superficial layers of the skin, mainly the epidermis (7).
Whilst UV exposure contributes to the development of AK and progression of AK to cSCC, it is not currently possible to predict which AK lesions will progress to malignancy. However, 196 genes were found to be differentially expressed between AK and cSCC (8). These genes impact on the mitogen active protein kinase pathways, with overexpression of oncogenes MET, JUN, and PAK2 in cSCC compared to AK, and are associated with loss of differentiation, and gain of malignant properties associated with extracellular matrix remodeling and cell migration (8, 9).
The central dogma of cancer pathogenesis is that all cancers arise as a result of mutated somatic DNA (10). When compared with “normal” skin, skin with solar damage (including AK), skin with selective loss of pigmentation, and aged “normal” skin, each have a substantially higher rate of somatic mutations. These mutations are particularly observed in TP53, CDKN2A, KNSTRN, and NOTCH1-3 (11–13). NOTCH signaling, for example, is tumor suppressive for squamous cell carcinomas, and loss of this signaling can create a carcinogenic environment that promotes tumor growth (14). When examining the NOTCH genes in particular, Matrincorena et al. found that NOTCH1 was the most frequently mutated gene in sun exposed skin, with NOTCH 2 and NOTCH3 also harboring a significant excess of protein altering mutations (11). Martincorena et al. also identified that normal skin has a high frequency of driver mutations where 20% of cells appearing otherwise normal carried NOTCH mutations, thus indicating that there are other factors that trigger cells to become malignant.
In immune competent patients, the skin has a remarkable ability to combat malignant growth by eliminating abnormal tissue structures (15). Brown et al. demonstrated that mutations in the ß-catenin pathway and HrasG12V in hair follicle stem cells of mouse skin in vivo resulted in hair shaft-like outgrowths which extended out through the epidermis and ectopically into the dermis (15). These are known to promote tumorigenic tissue growth in the skin due to an impact on the WNT pathway. However, most of these outgrowths fully regressed within 4 weeks. The study demonstrated that envelopment of the outgrowth by normal cells consistently preceded the expulsion of mutant cells from the tissue, and blocking proliferation of non-mutated cells reduced lesion regression to 2%, compared to the 68% regression in controls. Comparable concepts also applied to mutated stem cells that build the upper epithelium, where large benign tumors regressed over time, and normal tissue architecture and function was re-established. Furthermore, hair follicle ablation was also corrected and restored. It thus appears that the skin is able to correct structural aberrancies caused by gene mutations or physical damage. As the skin has the capacity to eliminate genetically abnormal keratinocytes, there may be other factors besides genetic damage that can promote transformation of AK to cSCC.
Evidence of the microbiome as a potential driver of skin cancer progression
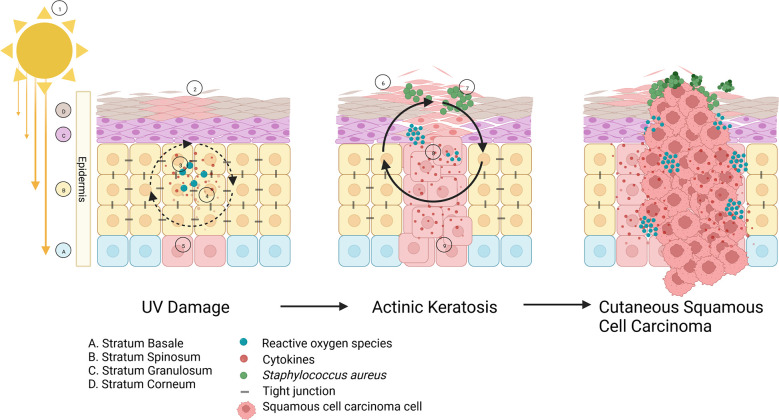
A possible determinant in the progression of AK to cSCC could be changes in the community of microorganisms (bacteria, viruses, and fungi) that reside on the epidermis and in the dermal layers. The skin microbiome assists in maintaining normal skin function by protecting against invading pathogens, interacting with the skin immune system, and processing the breakdown of dead skin cells and other skin products (16, 17). The microbiome of non-lesional photodamaged skin is substantially different from that of AK lesions (18, 19). Sun damage changes the skin in a variety of ways ( Figure 1 ). Chronic sun exposure causes inflammation, due to UV-A/B radiation penetrating the skin. This inflammation causes epidermal barrier dysfunction and trans-epidermal water loss, creating the red, dry, and scaly appearance associated with AKs (20). Patients with AK lesions often present with Staphylococcus as the dominant taxon on the skin, and a high portion of this taxon are S. aureus. S. aureus secrete toxins on the skin which can cause human keratinocytes to overexpress inflammatory factors that may promote skin carcinogenesis (21–24). While no causative relationship between S. aureus colonization and AK to cSCC progression has been established to date, this hypothesis presents an interesting interdisciplinary avenue to explore in the fields of oncology and microbiology. Other skin diseases such as atopic dermatitis are also associated with microbial dysbiosis, as the skin’s normal colonizers do not thrive in the altered environment, and pathogens or opportunistic pathogens colonize the affected area. Wood et al. noted that Malassezia and Cutibacterium (previously known as Propionibacterium) are associated with non-lesional photo-damaged skin, whereas S. aureus is associated with AK and cSCC lesions (19). A number of studies have suggested a link between the skin microbiota and cSCC progression, whereby S. aureus has been found in high relative abundance on AK lesions, but even more so on cSCCs (18, 19, 21–25). The presence of pathogenic bacteria on the skin can cause an inflammatory response, and inflammation is widely recognized as a factor in tumorigenesis, discussed in more detail below (26).
Figure 1.
The role of bacteria in AK to cSCC progression: ① Sun overexposure causes UV- A/B radiation to penetrate the epidermis, resulting in ② inflammatory cytokines accumulating in the area and causing erythema. ③ Reactive oxygen species (ROS) are produced and accrue, leading to oxidative stress. ④ The combination of these factors cause ⑤ DNA damage to keratinocytes and ⑥ skin barrier dysfunction including transepidermal water loss and increased pH. As more UV damage to the keratinocytes occurs, the skin barrier function deteriorates and hyperkeratosis can take place to compensate, forming an actinic keratosis (AK) lesion. Meanwhile, a change in skin physiology results in the healthy skin microbiome becoming dysbiotic, and instead enables pathogenic species such as ⑦ Staphylococcus aureus to proliferate and release toxins which can induce DNA damage and more ⑧ inflammation and ROS. ⑨ Squamous cell carcinomas begin developing in the basal layer of the epidermis, and penetrate through the stratum corneum creating a cSCC, that can spread into the basal epithelium.
Relationships between bacterially induced inflammation and tumorigenesis
Skin inflammation can be driven by a variety of factors that ultimately cause leukocytes, phagocytic cells and cytokines to accumulate in skin. Inflammation can induce DNA damage in proliferating cells as it generates reactive oxygen and nitrogen species (ROS and NOS) (27). Typically, in cSCC, UV-A/B exposure acts as an inflammatory stimulus, which causes ROS and NOS to react and form a mutagenic agent, peroxynitrite (27). ROS can cause significant damage to cellular components, including proteins, lipids, and DNA, and lead to apoptotic or necrotic cell death if not neutralized by an antioxidant (28). UV radiation is a potent inducer of ROS, which further accelerates intrinsic and photo-aging of the skin, therefore playing a key role in skin cancer pathogenesis (28). Chronic inflammation has been shown to induce a variety of cancers, including colorectal cancer from inflammatory bowel disease, oesophageal cancer from chronic acid reflux, and bladder cancer from chronic cystitis (29).
Krueger and colleagues investigated the relationship between the secretome of S. aureus strains isolated from AK and cSCC lesions, and the induction of pro-inflammatory cytokines produced by keratinocytes (22). Proinflammatory cytokines such as IL-6, IL-8, and TNF-α have previously been shown to promote tumor initiation and progression, and expression of these cytokines is associated with a poor clinical prognosis (30, 31). IL-6 in particular is a key factor for mediating tumor progression. Lederle and colleagues found that IL-6 activates STAT3 and directly stimulates proliferation of benign non-invasive HaCaT-ras A-5 cells in vitro (30). IL-6 also leads to overexpression of the collagenase MMP1, thereby enabling migration and invasion. IL-6 further induces a reciprocal cytokine network in tumor tissues, including IL-8, GM-CSF, and VEGF, which supports angiogenesis and leads to the recruitment of immunoregulatory cells that enable tumor progression (30). Krueger and colleagues demonstrated that the cytokines IL-6, IL-8, and TNF-α were overexpressed in HaCaT cells and primary human keratinocytes exposed to filter-sterilized culture supernatants from S. aureus, which was consistent with their findings in AK and cSCC biopsies (22). However, clinical isolates of S. aureus do not produce a homogenous secretome, and each strain induced a different level of inflammation in keratinocytes. Some S. aureus secretomes that were injected into murine skin produced a high level of IL-6 expression intradermally, and resulted in a significant increase in immune cell recruitment, including neutrophils, monocytes, and macrophages, when compared to mice injected with a low-level IL-6 inducing secretome (22). A study conducted by Nakagawa and colleagues investigated the role of the S. aureus produced toxin phenol-soluble modulin (PSM)α on inflammation of keratinocytes. They found that this toxin induced the release of various proinflammatory cytokines, including IL-1α and IL-36, and was further associated with the induction of chronically circulating IL-17 (32). IL-17 is a key player in the elimination and protection of cells against bacterial and fungal infection, and its dysregulation is known to be associated with early and late stages of skin cancer. IL-17 signaling also plays a role in wound healing through its association with epidermal growth factor receptor, fibroblast growth factor receptor, and NOTCH1, resulting in keratinocyte proliferation and repair (33, 34). Dysregulation can lead to chronic inflammation and tumorigenesis. These studies demonstrate that S. aureus induced inflammation has the potential to contribute to the development of AK lesions and their progression to cSCC.
Current treatment options for AK and cSCC
Gutzmer and colleagues conducted a comprehensive review on the current treatment options for AK and cSCC, characterizing the AK treatments as ‘lesion-directed’ or ‘field-directed’, and the cSCC treatments as ‘locoregional’ or ‘systemic’ (35). Lesion- directed AK treatments include cryosurgery, ablative and non-ablative laser techniques, and operative techniques (35). While these treatments are effective, some patients cannot undergo surgery due to poor general health, and some surgery causes loss of function, such as reduced eyelid movement, or cosmetic disfigurement to the affected area (36). Patients undergoing these procedures commonly experience pain, bleeding, scarring, erythema, hypopigmentation, and erosions.
The field-directed treatments include topical ointments such as 5-fluorouracil, imiquimod, ingenol mebutate gel, photodynamic therapy, fractional laser resurfacing, and alpha lipoic acid cream (35). The objective of field directed therapies is two-fold. It aims firstly to reduce the number of AKs and to prevent their recurrence. Secondly, it aims to reduce the onset of cSCCs in the future. Among all the immune- modulating and anti-mitotic topical creams only topical fluorouracil has a demonstrated capacity to reduce the risk of cSCC development for 12 months after a 2-4 week course (37). All topical therapies are associated with adverse skin reactions such as erythema, lesion formation, ulceration, and itching (38).
Hence, there is a clinical need for less invasive treatment options for AK that are designed to prevent or reduce the likelihood of progression to cSCC. With increasing recognition of the overall dominant role of the microbiome in health and disease, and a demonstrated microbial dysbiosis on AK and cSCC, it is reasonable to hypothesize that restoration of microbial eubiosis on AK may lead to clinical benefits. While this is an emerging research field, we will here review the current evidence mostly collected from other skin diseases that manipulation of the skin microbiome represents a potential non-invasive method to prevent AK colonization with proinflammatory bacteria that may promote progression of AK to cSCCs.
Antibiotic therapy
The use of topical antibiotics for skin infections is not a new concept, and common antibiotics such as mupirocin are regularly used for skin infections caused by staphylococci. Skin diseases associated with S. aureus colonization including atopic dermatitis (AD) often use this treatment method as colonization with this species results in disease exacerbation and skin barrier dysfunction (39–41). Antibiotics have been shown to decrease the severity of AD, however, there are increasing reports of antimicrobial resistance in S. aureus strains isolated from patients with AD (42–44). In particular, methicillin resistant S. aureus (MRSA) is becoming increasingly prevalent on children and adults with AD, and skin colonized with MRSA on AD affected sites is associated with a significant decrease in microbial diversity compared to skin colonized with methicillin sensitive S. aureus (MSSA) (45). Resistance of S. aureus to different antimicrobial agents is typically regional, as different countries have different antibiotic preferences to treat infection (46). Abdulgader and colleagues investigated antibiotic resistance in 212 S. aureus isolates obtained from hospital patients over a 6-year period in Cape Town South Africa, and found that 12% were mupirocin resistant, and 44% were MRSA (47). MRSA is a global concern due to the morbidity and mortality rates compared to MSSA, and the increasing prevalence of other antibiotic resistant S. aureus should prompt clinicians and researchers to promote and practice good antimicrobial stewardship. Another disadvantage of skin antibiotic treatment is that antibiotics will disturb the entire skin microbiome, while some bacteria are known to play beneficial roles in skin homeostasis. To our knowledge, there are no published studies on the use of antibiotic therapy as part of the management of AK and cSCC. It is likely that such treatments would inhibit S. aureus colonization but may also lead to antibiotic resistance and loss of a healthy skin microbiome that may be able to competitively exclude S. aureus.
Bleach baths
The use of sodium hypochlorite (bleach baths) has been a common practice in dermatology to treat AD (48). Similar to AK and cSCC, patients with AD experience an increase in S. aureus colonization on the skin associated with increased disease severity and disease exacerbations, and with reduction of commensals such as Streptococcus, Cutibacterium, and Corynebacterium during flare ups (49). Bleach typically exhibits an effect through non-specific antimicrobial action and is also able to reduce inflammation through inhibition of MAPK and NF-kB signaling (50). The reported effectiveness of bleach baths has been variable, particularly with respect to the reduction of S. aureus, likely reflecting variability in the skin microbiome (50–52). Kong et al. found that intermittent bleach treatment significantly reduced S. aureus allowing expansion of other bacterial populations and thereby increasing the overall diversity of the skin microbiome (52). Huang et al. found that bleach baths in combination with intranasal mupirocin decreased the S. aureus load, and was associated with an improved clinical condition of infection-prone AD patients, as assessed by eczema area and severity index scores (44). However, they also noted that bleach baths did not eradicate S. aureus. By contrast, no statistical difference was found between the use of 0.01% bleach baths and water baths with respect to barrier dysfunction, irritation, erythema, transepidermal water loss and pH in both healthy and AD patients (48). Sawada et al. found that bleach baths were only bactericidal at concentrations >0.03%, however this concentration is cytotoxic to human cells and is not clinically recommended (26). Thus, the use of bleach baths at the recommended concentration is safe but clinical outcomes and particularly the effect on S. aureus are uncertain.
Phage therapy
Phage therapy is a century old method used for the treatment of bacterial infection as an alternative to antibiotics (53). Phages are non-living biological entities that are classed as viruses that inject their genetic material into bacterial cells with high host specificity. This can result in the hijacking of the bacterial replication apparatus to produce phage progeny, ultimately resulting in the destruction of the host cell (53). With the rise of antimicrobial resistance, there has been a renewed interest in phage therapy. Phage treatments in experimental mouse infections have demonstrated efficacy and viability for several Gram-negative bacterial infections, including Acinetobacter baumanii, Pseudomonas aeruginosa, and Vibrio vulnificus (54, 55). A study by Capparelli et al. found that the administration of S. aureus-targeting phage to mice presented with a lethal dose of S. aureus had a 97% rescue rate, and the pathogen was completely eradicated after 4 days of treatment (56). Recently, Shimamori and colleagues demonstrated that a phage isolated from a sewage treatment plant (SaGU1) selectively targeted S. aureus and not S. epidermidis isolated from the skin of AD patients (57), which is important as the latter is usually considered part of a healthy skin microbiome (see below). However, the authors found that S. aureus developed resistance to SaGU1 between 14-24 hours post-inoculation and increasing the concentration of SaGU1 did not decrease the rate of resistance. Interestingly, when combining phage treatment with the secretome of S. epidermidis, the S. aureus did not regrow, indicating the effectiveness of S. epidermidis in combination with phage technology in controlling S. aureus colonization (57). However, phage therapy also has several disadvantages, including phages translocating into the blood through the intestinal epithelium when administered orally, which could negatively impact clinical outcome (58). It has also been suggested that phage therapy can induce intestinal barrier dysfunction known as ‘leaky gut syndrome’, which can have serious implications for disorders such as Crohn’s disease, inflammatory bowel disease, and type 1 diabetes (53, 59). This suggests that phage therapy is a potentially viable treatment for S. aureus eradication on AK when used in combination with probiotic strains with the additional benefit of not contributing to antimicrobial resistance. An unmet challenge is the provision of standard defined phage preparations, particularly if these are to be used as a regulated therapy.
Topical probiotics as a potential non-invasive treatment for skin diseases
Alteration of the AK and cSCC microbiome via the application of topical probiotics has not previously been attempted. However, there are a number of studies relating to topical probiotics for skin diseases such as AD, acne and eczema that are also characterized by Staphylococcus colonization (60). Benefits to local application of probiotics on the skin include competition with harmful skin microbiota, secretion of metabolites, reduction of skin pH, and formation of a barrier or a biofilm to protect the skin from foreign invaders (60). A study conducted by Khmaladze and colleagues applied a topical live probiotic ointment containing Lactobacillus reuteri to ex vivo skin models and examined the effect of L. reuteri on inflammation caused by UV-B radiation ( Table 1 ) (61). The authors found that the probiotic application reduced inflammation through reduction of proinflammatory IL-6 and IL-8, whilst also displaying antimicrobial activity against S. aureus, among other pathogenic strains. However, Lactobacillus strains are sensitive and susceptible to environmental perturbations, particularly heat, and therefore would not have sustainable shelf life. A study conducted by Nakatsuji et al. investigated the variability in coagulase-negative Staphylococcus (CoNS) showing antimicrobial activity against S. aureus in AD patients and healthy patients, and found that AD patients rarely possess CoNS with antimicrobial activity (62). The authors identified that two CoNS strains, S. epidermidis and S. hominis, produced strong and selective antimicrobial peptides against S. aureus ( Table 1 ). The authors isolated three S. hominis and two S. epidermidis strains from two of five AD subjects, which were formulated into a cream that was applied to AD patients. S. aureus abundance on the skin was measured before application, and 24 hours post-application. S. aureus was significantly decreased compared to control cream and untreated patients, demonstrating the potential of using topical probiotics to decrease S. aureus colonization on the skin. This research indicates that topical probiotics could be advantageous for modulating the AK microbiome, where a particular strain may competitively exclude a harmful pathogen, whilst allowing beneficial and commensal bacteria to recolonize the area.
Table 1.
Advantages and disadvantages of bacterial species for use as topical probiotics.
| Advantages | Disadvantages | |
|---|---|---|
| Staphylococci-specific antibiotics | Elimination of S. aureus on the skin | - Can procure antimicrobial resistance - Allows recolonization of other potential pathogens - Eliminates other Staphylococcal species |
| Lactobacillus reuteri | - Decreases inflammation (IL-6 and IL-8) - Antimicrobial activity against S. aureus |
- Low shelf life and specific storage conditions - Heat sensitive |
| Staphylococcus hominis | - Produces antimicrobial peptides that selectively kill S. aureus | - Potential pathogen - Can cause body odor |
| Staphylococcus epidermidis | - Common skin colonizer - Generation of 6-HAP - Generation of Esp that inhibits S. aureus biofilm formation |
- Opportunistic pathogen in immunocompromised patients - 6-HAP generation is a rare gene not commonly isolated from S. epidermidis |
| Corynebacterium striatum | - Decreases agr quorum sensing system - Decreases hemolytic activity - Decreases epithelial cell adhesion |
- Does not eliminate S. aureus |
| Cutibacterium acnes | - Generation of RoxP resulting in protection of skin against UV-induced free radicals - Common skin colonizer |
- Overabundance of C. acnes on the face and back can cause acne vulgaris |
Staphylococcus epidermidis
S. epidermidis is one of the most common human skin colonizers, and is typically a commensal species in the healthy population that has important functionality for maintaining the skin barrier and integrity (63) ( Table 1 ). Nakatsuji et al. found that certain strains of S. epidermidis produce a molecule known as 6-HAP, which in vitro can selectively inhibit proliferation of tumor lines (64). Additionally, it was found that mice colonized with 6-HAP producing strains had reduced incidence of UV-induced tumors compared to non-6-HAP producing strains. Another potentially important mechanism by which S. epidermidis may be able to prevent or reduce S. aureus colonization is via the protein factor serine protease Esp (65–67). S. epidermidis strains containing Esp have been shown to degrade 75 S. aureus proteins, 11 of which are needed for biofilm formation and colonization (68). This reduces the viability and pathogenic capability of S. aureus, whilst leaving the human host unharmed due to the commensal nature of S. epidermidis. However, S. epidermidis can be an opportunistic pathogen and cause nosocomial infections from medical devices, particularly in immunocompromised patients (69, 70). Some strains of S. epidermidis also possess antimicrobial resistance genes. Therefore it is imperative to evaluate potential strains of S. epidermidis to ensure transfer of these resistance genes does not occur (70). Thus, further experimental investigations on utilizing S. epidermidis as a topical probiotic for the treatment of AK and cSCC lesions are warranted.
Corynebacterium striatum
Corynebacterium striatum is another commensal bacterium residing on the human skin and on mucosal membranes ( Table 1 ). A study published by Ramsey et al. investigated the relationship between Corynebacterium striatum and S. aureus, and found that C. striatum modulated the behavior of S. aureus to exhibit commensal rather than pathogenic behavior (71). The analysis revealed that the transcriptome of S. aureus changed dramatically, including decreased expression of the accessory gene regulator (agr) quorum sensing system that controls a plethora of virulence factors (71, 72). S. aureus also displayed decreased hemolysin activity indicating a reduction in virulence factors, and increased epithelial cell adhesion indicating a commensal state. This could lead to potential treatment options for AKs and cSCCs due to the pathogenic nature of S. aureus that has been implicated with the disease. Modulation of the microbial community by means of bacterial behavioral changes rather than bacterial eradication could lead to better patient outcomes cosmetically and functionally due to a sustained healthy skin microbiome physiology.
Cutibacterium acnes
A commensal species, Cutibacterium acnes, is a regular skin colonizer that has demonstrated both beneficial effects and opportunistic pathogenic effects on human skin (73) ( Table 1 ). It encompasses 90% of the skin microbiome in predominantly oily areas such as the face and back (74). C. acnes has a variety of roles in maintenance of skin homeostasis, including the degradation of long chain fatty acids in sebum to short-chain fatty acids such as propionic acid, which acts as a natural antimicrobial agent on the skin as well as modulating skin pH (74). In terms of the pathogenic effects, C. acnes has demonstrated increased abundance on swabs of acne vulgaris and is thought to play a pro-inflammatory role as it is able to form biofilms and change the composition of sebum (74, 75). Whilst not the focus of this review, acne vulgaris is the most frequent diagnosis of skin disease in patients aged 5–44 years, and is an uncommon diagnosis in elderly populations due to the change in the nature of the skin (76). As AK and cSCC are most commonly diagnosed in the elderly population, it is unlikely that a C. acnes-based topical probiotic would result in acne vulgaris, however this would need to be investigated further. However, when investigating AD, a lack of C. acnes, and its inverse relationship with S. aureus, has been identified as a potential contributor to AD pathogenicity, as it has been suggested that patients with AD are deficient in the antimicrobial peptides produced by skin cells (62, 77). C. acnes demonstrates a high level of antioxidant activity due to the production of the Radical oxygenase of Propionibacterium acnes (RoxP), which appears to be a common property of this species (78). Of particular interest is the relationship between RoxP, UV radiation and cSCC, as RoxP has been shown to protect skin cells against UV radiation by preventing free radical generation (73, 79). A study conducted by Andersson and colleagues studied protein abundance on AKs and found that the concentration of RoxP and C. acnes was significantly lower on AK compared to healthy skin (73). The use of bacteria that produce a potent antioxidant would be highly beneficial in AK and early cSCC treatment as it could provide ongoing protection against UV-A/B radiation, as well as neutralizing free radicals and thereby decreasing the overall cellular damage.
Conclusion and future directions
There is mounting evidence that the progression of UV damaged skin to AK and cSCC is multifactorial. Well recognized factors include UV-A/B radiation and mutational burden associated with age, immune competency, and environmental factors. The role of pathogenic bacteria, specifically S. aureus, and microbial dysbiosis in this progression is under active investigation. Although the relationship between S. aureus and common skin conditions have been reported in the literature, the effect of S. aureus on AK progression to cSCC are not yet fully established. However, recent studies have demonstrated that S. aureus-derived toxins can induce tumor-promoting cytokines and reactive oxygen species.
While future studies are needed to determine the magnitude of contribution of microbial dysbiosis to AK and cSCC development, the ability to change the microbial profile by means of restoring a healthy skin microbiome and eliminating pathogenic bacteria, specifically S. aureus, has the potential to change the way that premalignancies are treated, and could also lead to new treatment methods for cSCC. While there will be challenges in preparing standardized bacterial products to use as therapy in research studies, the hypothesis that a healthy immune-supportive microbial community stabilized on AKs and cSCCs may result in improved patient outcomes and lower the rate of disease progression is warranted.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
JIB wrote the first draft of the manuscript. JIB, PH, IF and JC conceptualized the review. JIB, PH, IF, JC and KK revised and edited the manuscript.
Acknowledgments
Thank you to Dr Bernhard Paetzold for providing detailed information about Cutibacterium acnes and its secretome.
Funding Statement
We thank the funders for their ongoing support in our research. JC is supported by the Garnett Passe Rodney Williams Memorial Foundation and the Zelman Cowen Academic Initiatives. IHF holds grants from the National Health Medical Research Council (NHMRC) Investigator Fellowship and the Merchant Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Urban K, Mehrmal S, Uppal P, Giesey RL, Delost GR. The global burden of skin cancer: A longitudinal analysis from the global burden of disease study 1990-2017. JAAD Int (2021) 2:98–108. doi: 10.1016/j.jdin.2020.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Werner RN, Sammain A, Erdmann R, Hartmann V, Stockfleth E, Nast A. The natural history of actinic keratosis: A systematic review. Br J Dermatol (2013) 169(3):502–18. doi: 10.1111/bjd.12420 [DOI] [PubMed] [Google Scholar]
- 3. Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol (2004) 195(3):298–308. doi: 10.1016/j.taap.2003.08.019 [DOI] [PubMed] [Google Scholar]
- 4. Perera E, McGuigan S, Sinclair R. Cost for the treatment of actinic keratosis on the rise in Australia. F1000Res (2014) 3:184. doi: 10.12688/f1000research.4671.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berman B, Bienstock L, Kuritzky L, Mayeaux EJ, Jr., Tyring SK. Actinic keratoses: Sequelae and treatments. recommendations from a consensus panel. J Fam Pract (2006) 55(5):suppl 1–8. [PubMed] [Google Scholar]
- 6. Harwood CA, Toland AE, Proby CM, Euvrard S, Hofbauer GFL, Tommasino M, et al. The pathogenesis of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol (2017) 177(5):1217–24. doi: 10.1111/bjd.15956 [DOI] [PubMed] [Google Scholar]
- 7. Riffat F, Palme C, Veness M. Non-melanoma skin cancer of the head and neck preface. Non-Melanoma Skin Cancer Head Neck (2015), Xi–i. doi: 10.1007/978-81-322-2497-6 [DOI] [Google Scholar]
- 8. Lambert SR, Mladkova N, Gulati A, Hamoudi R, Purdie K, Cerio R, et al. Key differences identified between actinic keratosis and cutaneous squamous cell carcinoma by transcriptome profiling. Br J Cancer (2014) 110(2):520–9. doi: 10.1038/bjc.2013.760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Haren R, Feldman D, Sinha AA. Systematic comparison of nonmelanoma skin cancer microarray datasets reveals lack of consensus genes. Br J Dermatol (2009) 161(6):1278–87. doi: 10.1111/j.1365-2133.2009.09338.x [DOI] [PubMed] [Google Scholar]
- 10. Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature (2013) 499(7457):214–8. doi: 10.1038/nature12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martincorena I, Roshan A, Gerstung M, Ellis P, Van Loo P, McLaren S, et al. Tumor evolution. high burden and pervasive positive selection of somatic mutations in normal human skin. Science (2015) 348(6237):880–6. doi: 10.1126/science.aaa6806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aster JC, Pear WS, Blacklow SC. The varied roles of notch in cancer. Annu Rev Pathol (2017) 12:245–75. doi: 10.1146/annurev-pathol-052016-100127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmitz L, Novak B, Hoeh AK, Gambichler T, von Dobbeler C, Szeimies RM, et al. Somatic mutations in the kinetochore gene KNSTRN are associated with basal proliferating actinic keratosis and cutaneous squamous cell carcinoma. J Der Deutschen Dermatologischen Gesellschaft (2018) 16:25–6.doi: 10.1111/jdv.15615 [DOI] [PubMed] [Google Scholar]
- 14. Nowell CS, Radtke F. Notch as a tumour suppressor. Nat Rev Cancer (2017) 17(3):145–59. doi: 10.1038/nrc.2016.145 [DOI] [PubMed] [Google Scholar]
- 15. Brown S, Pineda CM, Xin TC, Boucher J, Suozzi KC, Park S, et al. Correction of aberrant growth preserves tissue homeostasis. Nature (2017) 548(7667):334–+. doi: 10.1038/nature23304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol (2018) 16(3):143–55. doi: 10.1038/nrmicro.2017.157 [DOI] [PubMed] [Google Scholar]
- 17. Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science (2014) 346(6212):954–9. doi: 10.1126/science.1260144 [DOI] [PubMed] [Google Scholar]
- 18. Kullander J, Forslund O, Dillner J. Staphylococcus aureus and squamous cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev (2009) 18(2):472–8. doi: 10.1158/1055-9965.Epi-08-0905 [DOI] [PubMed] [Google Scholar]
- 19. Wood DLA, Lachner N, Tan JM, Tang S, Angel N, Laino A, et al. A natural history of actinic keratosis and cutaneous squamous cell carcinoma microbiomes. Mbio (2018) 9(5):e01432–18 doi: 10.1128/mBio.01432-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Santiago JL, Muñoz-Rodriguez JR, Cruz-Morcillo MA, Villar-Rodriguez C, Gonzalez-Lopez L, Aguado C, et al. Characterization of permeability barrier dysfunction in a murine model of cutaneous field cancerization following chronic UV-b irradiation: Implications for the pathogenesis of skin cancer. Cancers (Basel) (2021) 13(16). doi: 10.3390/cancers13163935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krueger A, Mohamed A, Kolka CM, Stoll T, Zaugg J, Linedale R, et al. Skin cancer-associated s. aureus strains can induce DNA damage in human keratinocytes by downregulating DNA repair and promoting oxidative stress. Cancers (2022) 14(9). doi: 10.3390/cancers14092143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krueger A, Zaugg J, Chisholm S, Linedale R, Lachner N, Teoh SM, et al. Secreted toxins from staphylococcus aureus strains isolated from keratinocyte skin cancers mediate pro-tumorigenic inflammatory responses in the skin. Front Microbiol (2022) 12:789042. doi: 10.3389/fmicb.2021.789042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krueger A, Zaugg J, Lachner N, Bialasiewicz S, Lin LL, Gabizon S, et al. Changes in the skin microbiome associated with squamous cell carcinoma in transplant recipients. ISME Commun (2022) 2(1):13. doi: 10.1038/s43705-022-00095-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Madhusudhan N, Pausan MR, Halwachs B, Durdević M, Windisch M, Kehrmann J, et al. Molecular profiling of keratinocyte skin tumors links staphylococcus aureus overabundance and increased human β-Defensin-2 expression to growth promotion of squamous cell carcinoma. Cancers (Basel) (2020) 12(3). doi: 10.3390/cancers12030541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Voigt AY, Emiola A, Johnson JS, Fleming ES, Nguyen H, Zhou W, et al. Skin microbiome variation with cancer progression in human cutaneous squamous cell carcinoma. J Invest Dermatol (2022) 142(10):2773–2782.e2716. doi: 10.1016/j.jid.2022.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sawada Y, Tong Y, Barangi M, Hata T, Williams MR, Nakatsuji T, et al. Dilute bleach baths used for treatment of atopic dermatitis are not antimicrobial in vitro. J Allergy Clin Immunol (2019) 143(5):1946–8. doi: 10.1016/j.jaci.2019.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coussens LM, Werb Z. Inflammation and cancer. Nature (2002) 420(6917):860–7. doi: 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Narendhirakannan RT, Hannah MA. Oxidative stress and skin cancer: An overview. Indian J Clin Biochem (2013) 28(2):110–5. doi: 10.1007/s12291-012-0278-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weinberg RA. The biology of cancer (2 ed.). New York: W W Norton & Company; (2013). [Google Scholar]
- 30. Lederle W, Depner S, Schnur S, Obermueller E, Catone N, Just A, et al. IL-6 promotes malignant growth of skin SCCs by regulating a network of autocrine and paracrine cytokines. Int J Cancer (2011) 128(12):2803–14. doi: 10.1002/ijc.25621 [DOI] [PubMed] [Google Scholar]
- 31. Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest (2007) 117(5):1175–83. doi: 10.1172/jci31537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nakagawa S, Matsumoto M, Katayama Y, Oguma R, Wakabayashi S, Nygaard T, et al. Staphylococcus aureus virulent PSMα peptides induce keratinocyte alarmin release to orchestrate IL-17-Dependent skin inflammation. Cell Host Microbe (2017) 22(5):667–677.e665. doi: 10.1016/j.chom.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brevi A, Cogrossi LL, Grazia G, Masciovecchio D, Impellizzieri D, Lacanfora L, et al. Much more than IL-17A: Cytokines of the IL-17 family between microbiota and cancer [Mini review]. Front Immunol (2020) 11:565470. doi: 10.3389/fimmu.2020.565470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity (2019) 50(4):892–906. doi: 10.1016/j.immuni.2019.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gutzmer R, Wiegand S, Kölbl O, Wermker K, Heppt M, Berking C. Actinic keratosis and cutaneous squamous cell carcinoma. Dtsch Arztebl Int (2019) 116(37):616–26. doi: 10.3238/arztebl.2019.0616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bhatta AK, Wang PR, Keyal U, Zhao ZJ, Jie J, Zhu LD, et al. Therapeutic effect of imiquimod enhanced ALA-PDT on cutaneous squamous cell carcinoma. Photodiagnosis Photodyn Ther (2018) 23:273–80. doi: 10.1016/j.pdpdt.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 37. Weinstock MA, Thwin SS, Siegel JA, Marcolivio K, Means AD, Leader NF, et al. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: A randomized clinical trial. JAMA Dermatol (2018) 154(2):167–74. doi: 10.1001/jamadermatol.2017.3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stockfleth E, Begeault N, Delarue A. Intensity of local skin reactions during 5-fluorouracil treatment related to the number of actinic keratosis lesions: A Post hoc, exploratory analysis. Dermatol Ther (2022) 12(2):467–79. doi: 10.1007/s13555-021-00668-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leung DYM. Can antibiotics be harmful in atopic dermatitis? Br J Dermatol (2018) 179(4):807–8. doi: 10.1111/bjd.17023 [DOI] [PubMed] [Google Scholar]
- 40. Rajkumar J, Jetter N, Samuels K, Schwartz A. Combined antibiotic, steroid, and moisturizer for atopic dermatitis: A two-year case series of patient-reported outcomes. Pediatr Dermatol (2021) 38(3):623–8. doi: 10.1111/pde.14458 [DOI] [PubMed] [Google Scholar]
- 41. Tay ASL, Li CH, Nandi T, Chng KR, Andiappan AK, Mettu VS, et al. Atopic dermatitis microbiomes stratify into ecologic dermotypes enabling microbial virulence and disease severity. J Allergy Clin Immunol (2021) 147(4):1329–40. doi: 10.1016/j.jaci.2020.09.031 [DOI] [PubMed] [Google Scholar]
- 42. Bath-Hextall F, Bimie A, Ravenscroft J, William H. Interventions to reduce Staphylococcus aureus in the management of atopic eczema: An updated Cochrane review. Br J Dermatol (2010) 163:12–26. [DOI] [PubMed] [Google Scholar]
- 43. Fortina AB, Neri L. Antibiotic therapy in the management of atopic dermatitis. Giornale Italiano Di Dermatol E Venereol (2015) 150(3):321–5. [PubMed] [Google Scholar]
- 44. Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics (2009) 123(5):e808–814. doi: 10.1542/peds.2008-2217 [DOI] [PubMed] [Google Scholar]
- 45. Shi C, Xiao Y, Zhang Q. fficacy and safety of cefazolin versus antistaphylococcal penicillins for the treatment of methicillin-susceptible Staphylococcus aureus bacteremia: A systematic review and meta-analysis. BMC Infect Dis (2018) 15:508. doi: 10.1186/s12879-018-3418-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dadashi M, Hajikhani B, Darban-Sarokhalil D, van Belkum A, Goudarzi M. Mupirocin resistance in staphylococcus aureus: A systematic review and meta-analysis. J Glob Antimicrob Resist (2020) 20:238–47. doi: 10.1016/j.jgar.2019.07.032 [DOI] [PubMed] [Google Scholar]
- 47. Abdulgader SM, Lentswe T, Whitelaw A, Newton-Foot M. The prevalence and molecular mechanisms of mupirocin resistance in staphylococcus aureus isolates from a hospital in cape town, south Africa. Antimicrobial Resist Infect Control (2020) 9(1). doi: 10.1186/s13756-020-00707-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shi VY, Foolad N, Ornelas JN, Hassoun LA, Monico G, Takeda N, et al. Comparing the effect of bleach and water baths on skin barrier function in atopic dermatitis: A split-body randomized controlled trial. Br J Dermatol (2016) 175(1):212–4. doi: 10.1111/bjd.14483 [DOI] [PubMed] [Google Scholar]
- 49. Wong SM, Ng TG, Baba R. Efficacy and safety of sodium hypochlorite (bleach) baths in patients with moderate to severe atopic dermatitis in Malaysia. J Dermatol (2013) 40(11):874–80. doi: 10.1111/1346-8138.12265 [DOI] [PubMed] [Google Scholar]
- 50. Maarouf M, Shi VY. Bleach for atopic dermatitis. Dermatitis (2018) 29(3):120–6. doi: 10.1097/der.0000000000000358 [DOI] [PubMed] [Google Scholar]
- 51. Knowlden SA, Perez-Nazario N, Yoshida T, Wang D, De Benedetto A, Gill A, et al. Bleach baths repair skin barrier function without modifying Th2 biomarkers or skin dysbiosis in atopic dermatitis patients. J Invest Dermatol (2016) 136(8):B6–6. doi: 10.1016/j.jid.2016.05.031 [DOI] [Google Scholar]
- 52. Kong HDH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res (2012) 22(5):850–9. doi: 10.1101/gr.131029.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lin DM, Koskella B, Lin HC. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J Gastrointest Pharmacol Ther (2017) 8(3):162–73. doi: 10.4292/wjgpt.v8.i3.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Soothill JS. Treatment of experimental infections of mice with bacteriophages. J Med Microbiol (1992) 37(4):258–61. doi: 10.1099/00222615-37-4-258 [DOI] [PubMed] [Google Scholar]
- 55. Cerveny KE, DePaola A, Duckworth DH, Gulig PA. Phage therapy of local and systemic disease caused by vibrio vulnificus in iron-dextran-treated mice. Infect Immun (2002) 70(11):6251–62. doi: 10.1128/IAI.70.11.6251-6262.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Capparelli R, Parlato M, Borriello G, Salvatore P, Iannelli D. Experimental phage therapy against staphylococcus aureus in mice. Antimicrob Agents Chemother (2007) 51(8):2765–73. doi: 10.1128/AAC.01513-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shimamori Y, Mitsunaka S, Yamashita H, Suzuki T, Kitao T, Kubori T, et al. Staphylococcal phage in combination with staphylococcus epidermidis as a potential treatment for staphylococcus aureus-associated atopic dermatitis and suppressor of phage-resistant mutants. Viruses-Basel (2021) 13(1). doi: 10.3390/v13010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gorski A, Wazna E, Dabrowska BW, Dabrowska K, Switala-Jelen K, Miedzybrodzki R. Bacteriophage translocation. FEMS Immunol Med Microbiol (2006) 46(3):313–9. doi: 10.1111/j.1574-695X.2006.00044.x [DOI] [PubMed] [Google Scholar]
- 59. Tetz G, Tetz V. Bacteriophage infections of microbiota can lead to leaky gut in an experimental rodent model. Gut Pathog (2016) 8. doi: 10.1186/s13099-016-0109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Roudsari MR, Karimi R, Sohrabvandi S, Mortazavian AM. Health effects of probiotics on the skin. Crit Rev Food Sci Nutr (2015) 55(9):1219–40. doi: 10.1080/10408398.2012.680078 [DOI] [PubMed] [Google Scholar]
- 61. Khmaladze I, Butler E, Fabre S, Gillbro JM. Lactobacillus reuteri DSM 17938-a comparative study on the effect of probiotics and lysates on human skin. Exp Dermatol (2019) 28(7):822–8. doi: 10.1111/exd.13950 [DOI] [PubMed] [Google Scholar]
- 62. Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, et al. Antimicrobials from human skin commensal bacteria protect against staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med (2017) 9(378). doi: 10.1126/scitranslmed.aah4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brown MM, Horswill AR. Staphylococcus epidermidis-skin friend or foe? PloS Pathog (2020) 16(11). doi: 10.1371/journal.ppat.1009026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nakatsuji T, Chen TH, Butcher AM, Trzoss LL, Nam SJ, Shirakawa KT, et al. A commensal strain of staphylococcus epidermidis protects against skin neoplasia. Sci Adv (2018) 4(2):eaao4502. doi: 10.1126/sciadv.aao4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dubin G, Chmiel D, Mak P, Rakwalska M, Rzychon M, Dubin A. Molecular cloning and biochemical characterisation of proteases from staphylococcus epidermidis . Biol Chem (2001) 382(11):1575–82. doi: 10.1515/BC.2001.192 [DOI] [PubMed] [Google Scholar]
- 66. Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, et al. Staphylococcus epidermidis esp inhibits staphylococcus aureus biofilm formation and nasal colonization. Nature (2010) 465(7296):346–9. doi: 10.1038/nature09074 [DOI] [PubMed] [Google Scholar]
- 67. Moon JL, Banbula A, Oleksy A, Mayo JA, Travis J. Isolation and characterization of a highly specific serine endopeptidase from an oral strain of staphylococcus epidermidis . Biol Chem (2001) 382(7):1095–9. doi: 10.1515/Bc.2001.138 [DOI] [PubMed] [Google Scholar]
- 68. Sugimoto S, Iwamoto T, Takada K, Okuda K, Tajima A, Iwase T, et al. Staphylococcus epidermidis esp degrades specific proteins associated with staphylococcus aureus biofilm formation and host-pathogen interaction. J Bacteriol (2013) 195(8):1645–55. doi: 10.1128/Jb.01672-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rendboe AK, Johannesen TB, Ingham AC, Månsson E, Iversen S, Baig S, et al. The epidome - a species-specific approach to assess the population structure and heterogeneity of staphylococcus epidermidis colonization and infection. BMC Microbiol (2020) 20(1):362. doi: 10.1186/s12866-020-02041-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhou W, Spoto M, Hardy R, Guan C, Fleming E, Larson PJ, et al. Host-specific evolutionary and transmission dynamics shape the functional diversification of staphylococcus epidermidis in human skin. Cell (2020) 180(3):454–470.e418. doi: 10.1016/j.cell.2020.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ramsey MM, Freire MO, Gabrilska RA, Rumbaugh KP, Lemon KP. Staphylococcus aureus shifts toward commensalism in response to corynebacterium species. Front Microbiol (2016) 7:1230. doi: 10.3389/fmicb.2016.01230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Paulander W, Varming AN, Bojer MS, Friberg C, Baek K, Ingmer H. The agr quorum sensing system in staphylococcus aureus cells mediates death of sub-population. BMC Res Notes (2018) 11(1):503. doi: 10.1186/s13104-018-3600-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Andersson T, Bergdahl GE, Saleh K, Magnusdottir H, Stodkilde K, Andersen CBF, et al. Common skin bacteria protect their host from oxidative stress through secreted antioxidant RoxP. Sci Rep (2019) 9. doi: 10.1038/s41598-019-40471-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gueniche A, Perin O, Bouslimani A, Landemaine L, Misra N, Cupferman S, et al. Advances in microbiome-derived solutions and methodologies are founding a new era in skin health and care. Pathogens (2022) 11(2). doi: 10.3390/pathogens11020121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Agak GW, Qin M, Nobe J, Kim MH, Krutzik SR, Tristan GR, et al. Propionibacterium acnes induces an IL-17 response in acne vulgaris that is regulated by vitamin a and vitamin d. J Invest Dermatol (2014) 134(2):366–73. doi: 10.1038/jid.2013.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tuchayi SM, Makrantonaki E, Ganceviciene R, Dessinioti C, Feldman SR, Zouboulis CC. Acne vulgaris. Nat Rev Dis Primers (2015) 1(1):15029. doi: 10.1038/nrdp.2015.29 [DOI] [PubMed] [Google Scholar]
- 77. Rozas M, Hart de Ruijter A, Fabrega MJ, Zorgani A, Guell M, Paetzold B, et al. From dysbiosis to healthy skin: Major contributions of cutibacterium acnes to skin homeostasis. Microorganisms (2021) 9(3). doi: 10.3390/microorganisms9030628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Holland C, Mak TN, Zimny-Arndt U, Schmid M, Meyer TF, Jungblut PR, et al. Proteomic identification of secreted proteins of propionibacterium acnes. BMC Microbiol (2010) 10:230. doi: 10.1186/1471-2180-10-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mayslich C, Grange PA, Dupin N. Cutibacterium acnes as an opportunistic pathogen: An update of its virulence-associated factors. Microorganisms (2021) 9(2). doi: 10.3390/microorganisms9020303 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.