Abstract
Objectives
To quantify global, regional and country-specific estimates of epidemiology of systemic lupus erythematosus (SLE).
Methods
Four databases were systematically searched, and a Bayesian hierarchical linear mixed model was constructed to estimate the global, regional, and country-specific incidence and prevalence of SLE.
Results
112 studies met the inclusion criteria. The global SLE incidence and newly diagnosed population were estimated to be 5.14 (1.4 to 15.13) per 100 000 person-years and 0.40 million people annually, respectively. In women, the values were 8.82 (2.4 to 25.99) per 100 000 person-years and 0.34 million people annually, while in men, the estimates were 1.53 (0.41 to 4.46) per 100 000 person-years and 0.06 million people annually, respectively. Poland, the USA and Barbados had the highest estimates of SLE incidence. Regarding prevalence, the global SLE prevalence and affected population were estimated to be 43.7 (15.87 to 108.92) per 100 000 persons and 3.41 million people, respectively. In women, the values were 78.73 (28.61 to 196.33) per 100 000 persons and 3.04 million people, while in men the estimates were 9.26 (3.36 to 22.97) per 100 000 persons and 0.36 million people, respectively. The United Arab Emirates, Barbados and Brazil had the highest SLE prevalence. In addition to regional and sex differences, age and prevalence estimation method (period or point prevalence) differences could also lead to variations in epidemiological SLE findings.
Conclusions
Epidemiological data on SLE are lacking for 79.8% of countries worldwide. The epidemiology of SLE varies substantially between different sex and age groups and is distributed unequally among geographical regions; specifically, SLE occurs more frequently in high-income countries.
Keywords: Systemic Lupus Erythematosus, Epidemiology, Autoimmune Diseases
WHAT IS ALREADY KNOWN ON THIS TOPIC
The epidemiology estimates of systemic lupus erythematosus (SLE) vary substantially worldwide and are influenced by geographical regions and factors like the age and gender of population.
None of the previous studies systematically reviewed and provided quantitative global, regional, and country-specific estimates of SLE incidence and prevalence.
WHAT THIS STUDY ADDS
This is the first study using Bayesian hierarchical linear mixed model to estimate global, regional, and country-specific SLE incidence, prevalence, and population even in regions without SLE epidemiological data.
We provided the potential SLE epidemiological data in each country considering the influence of gender, age, diagnostic method and prevalence measure differences.
The incidence and prevalence of SLE vary substantially with sex as well as age and happen more frequently in high-income countries.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our epidemiology estimation can help advance SLE-related research and assess SLE natural history over time.
Our study can be used as a reference for future SLE epidemiology research especially for countries that lack SLE epidemiology study so far.
Introduction
Systemic lupus erythematosus (SLE), characterised by the presence of autoantibodies, is a multifactorial autoimmune disease with diverse clinical manifestations and can involve one or more organs.1 The substantial morbidity, chronic disease course and over-reliance on corticosteroid therapy all contribute to long-term organ damage, even leading to life-threatening systemic organ damage.2 3 However, the complexity of the clinical manifestations and challenge brought by diagnosis make SLE epidemiological studies difficult to conduct. Although anti-dsDNA and anti-Sm antibodies are highly specific for SLE, and the 2019 European Alliance of Associations for Rheumatology/American College of Rheumatology (EULAR/ACR) classification criteria for SLE include positive antinuclear antibody as an entry criterion,4 the risk of false positives resulting in diagnosis of SLE still relies on classification criteria instead of a singular diagnostic test. Currently, the epidemiological estimates of SLE vary substantially worldwide due to the variability in study design, environmental exposures, location and characteristics of the surveyed population, including race, sex and age.5 6 Moreover, the trends of SLE incidence and prevalence are not unified across studies,7–11 and there is a paucity of SLE epidemiological studies in developing countries.5 6 The Global Burden of Disease group is the only source of the global-based epidemiological data of SLE; however, the disease is included in the category of ‘other musculoskeletal disorders’, and the detailed information of SLE epidemiological data worldwide or in individual countries is still not available.12 A comprehensive understanding of the epidemiology of SLE is urgently needed to help us gain more insights towards understanding the disease and better manage healthcare resources.
In this systematic review and meta-analysis, we included studies published in the past 30 years on SLE epidemiology to establish a Bayesian hierarchical linear mixed model, which would enable us to estimate incidence and prevalence data for each individual country globally with or without SLE epidemiological data. The study also generated global, regional and country-specific estimates of the annual population with newly diagnosed SLE and the overall population with SLE. In all, by providing comprehensive, concise, objective, less biased and standardised estimates of SLE epidemiological data, we hope to increase the accessibility of SLE epidemiological data for researchers, especially those in regions that lack related studies. More importantly, we hope that the model could help manage the care for patients with SLE, reduce the life-long health impact and improve the survival and quality of life for these patients.
Methods
Search strategy and review guidelines
A total of four electronic databases (Medline, EMBASE, Web of Science and China National Knowledge Infrastructure) were systematically searched using the main search terms “systemic lupus erythematosus”, “incidence” and “prevalence” from 1 January 1992 to 7 May 2022. The references of all included studies and review articles were screened to identify any additional eligible studies. Detailed information extracted from each included trial is listed in online supplemental appendix 1. The search strategy, study selection and screening and data extraction methods are provided in online supplemental file 1. This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.
ard-2022-223035supp001.pdf (3.3MB, pdf)
Data analysis
The internal validity of trials was assessed using the appraisal tool for cross-sectional studies.13 For eligible duplicate studies, the studies with the most complete data on the variable of interest or the most robust data in terms of the methods used were included.
A Bayesian hierarchical linear mixed model, which is the gold-standard model for sparse and heterogeneous data, was applied to estimate the global, regional, and country-specific incidence and prevalence of SLE.14–17 Referring to the study by Parisi et al,15 the hierarchical model had four levels (global, super-regions, regions and countries); countries were mapped according to the Global Burden of Disease geographical and income classifications and were nested in 21 regions. These 21 regions were then nested in 7 super-regions, and the 7 super-regions composed the global regions (online supplemental table 9). In addition, the model also had four fixed covariates: age strata (children (aged <18 years), adults (aged ≥18 years) and the overall population (different age strata combined)), the type of diagnostic method (physician, dermatologist or self-reported diagnosis), sex (male, female, unclaimed) and the type of prevalence measure (point, period or lifetime prevalence).
Geographical clustering in the model was used to inform and generate estimates for countries with missing information. Although no regional-level or super-regional-level studies were available, we were able to use the model to get the regional and super-region estimates from country-level studies. For countries without available data, we estimated the prevalence/incidence of those countries according to the evidence from higher levels in the model. That is, when no country-level data were available, the regional estimate was used as country estimate, and under circumstance when both regional data and country-level data were deficient, the super-region estimate was regarded as corresponding country-level and regional-level estimates. A detailed report of information on countries with observed or missing data is listed in online supplemental tables 11-28.
ard-2022-223035supp002.pdf (1.2MB, pdf)
The Bayesian inference statistical model was fitted with 4 chains and 4000 iterations. We used the Hamiltonian Markov chain Monte Carlo method to sample from the posterior distribution over the parameters. To avoid divergent transitions and transitions that exceeded the maximum tree depth after warm-up, we set the target acceptance probability and maximum tree depth as 0.995 and 20, respectively. Based on the posterior distribution of the fitted model, we estimated the incidence and prevalence for different age strata (adults/children), different diagnosis methods, each country, each region and each super-region with 95% CIs. We also considered different types of prevalence in the prevalence model. All incidence data were normalised to 100 000 person-years, and all prevalence data were normalised to 100 000 persons. To obtain the number of people affected by SLE by country, we built binomial models for each country-specific incidence and prevalence estimate. The parameters of these binomial models were sampled from the posterior distribution of the fitted Bayesian model. The size of its population from different groups was based on the United Nations population structure for 2019.18 Since studies often hold different population structures from the real country, we weighted the prevalence, incidence and number of people affected according to the real population structure in the region. We evaluated the measures relative to the model by effective sample size and assessed the trace plots (online supplemental figure 2).
Subgroup analysis of classification criteria and diagnostic method was conducted using the same Bayesian hierarchical linear mixed model to further demonstrate the epidemiological estimation distortion brought by different classification criteria and self-report diagnosis. Detailed method and results were listed in online supplemental appendix 1.
The accuracy of the Bayesian model was evaluated through comparing model data with data from countries that have conducted multiple epidemiological studies and through comparing model predicted number with the actual number of patients with SLE. Detailed method and results were listed in online supplemental appendix 1.
All analyses were conducted by using R software (V.4.0.5).
Patient and public involvement
Adult patients were asked whether they had SLE during outpatient and epidemiological surveys conducted by our dermatologists. According to their answers and some literature, sex and age were included in the model as covariates. We introduced the research to some of the patients with SLE, and they asked questions about the study design and findings.
Results
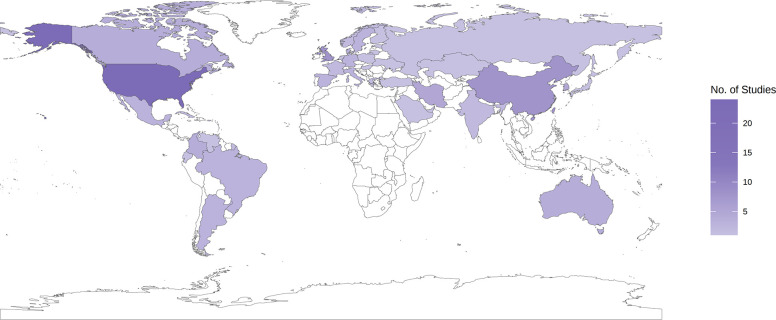
We identified 112 studies that reported the incidence or prevalence of SLE in the general population from 21 regions worldwide (table 1, online supplemental figure 1). Most of the studies (73; 65.2%) and the largest number of nationally representative studies were conducted in high-income countries. Fifty-three studies reported incidence, 66 studies reported prevalence, and 46 studies reported both prevalence and incidence (online supplemental table 6 and 7). A total of 79.5% of the included studies were judged as having a low-medium risk of bias (online supplemental table 8). Thirty-nine out of 193 (20.21%) countries in the world were covered (figure 1).
Table 1.
Distribution of studies on SLE epidemiology (n=113) in 21 regions
| Region | No of studies* |
| Europe, western | 32 |
| North America, high income | 28 |
| Asia, east | 8 |
| Latin America, central | 8 |
| North Africa and the Middle East | 8 |
| Asia Pacific, high income | 6 |
| Australasia | 4 |
| Caribbean | 4 |
| Europe, eastern | 3 |
| Latin America, southern | 3 |
| Latin America, tropical | 3 |
| Asia, south | 2 |
| Europe, central | 2 |
| Asia, central | 1 |
| Latin America, Andean | 1 |
SLE, systemic lupus erythematosus.
Figure 1.
Distribution of number of studies included in statistical analysis by country. Countries with no observed data are white.
Online supplemental tables 11-28 show summaries of our estimates of SLE incidence and prevalence. All incident SLE was diagnosed by a physician or dermatologist, and we estimated and reported the incidence of SLE in different age groups (adult/all) and sexes (online supplemental table 11-16). Regarding prevalence, only three lifetime prevalence data points were self-reported, and other prevalent SLE was diagnosed by a physician or dermatologist. The 1-year period prevalence was the most reported type of data and was chosen for our prevalence estimation. The detailed 1-year period prevalence and point prevalence of SLE in different age groups (adult/all) and sexes were reported in online supplemental tables 17-28.
Incidence of SLE
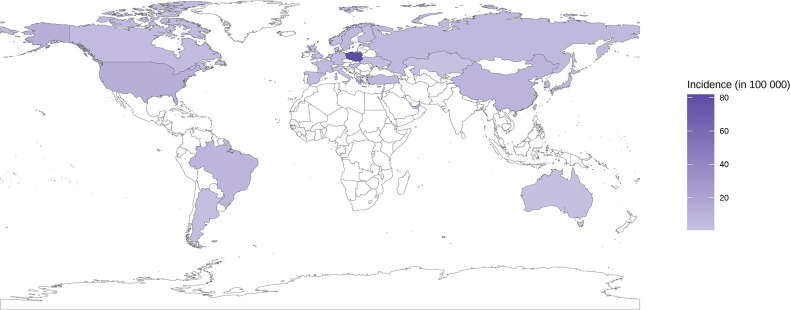
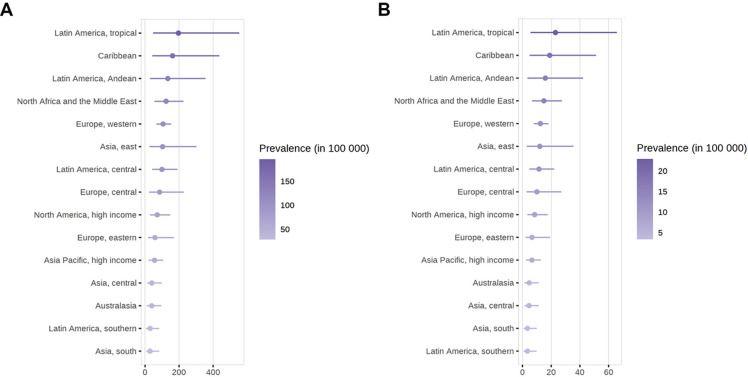
For the overall population, the global SLE incidence and newly diagnosed population were estimated to be 5.14 (1.4 to 15.13) per 100 000 person-years and 0.40 million annually, respectively. At the regional level, the incidence of SLE in the general population varied from 1.18 (0.16 to 3.68) per 100 000 person-years in central Asia to 13.74 (3.2 to 31.82) per 100 000 person-years in central Europe (figure 2). The incidence of SLE differed greatly by country. Regarding the general population, the top four countries with the highest estimates of SLE incidence were Poland (81.84, 80.33 to 83.51 per 100 000 person-years), the USA (12.13, 11.94 to 12.35 per 100 000 person-years), Barbados (10.37, 2.01 to 36.46 per 100 000 person-years) and China (8.57, 8.37 to 8.77 per 100 000 person-years). In contrast, Kazakhstan was the country with the lowest incidence worldwide (0.57, 0.17 to 1.24 per 100 000 person-years) (online supplemental table 11).
Figure 2.
Incidence of systemic lupus erythematosus for overall population by country.
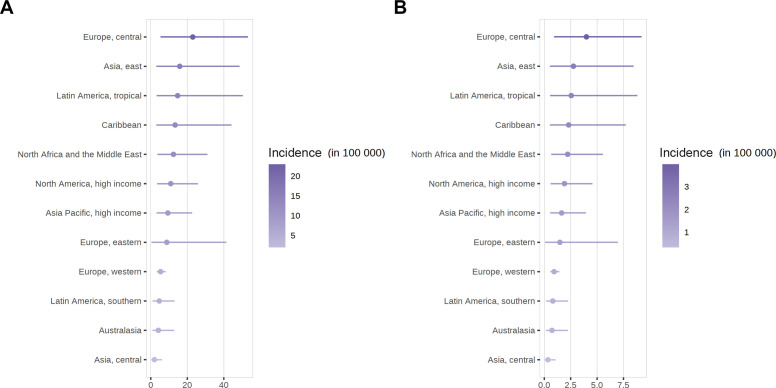
Women were more likely to have SLE than men. The global SLE incidence and newly diagnosed population in women were estimated to be 8.82 (2.4 to 25.99) per 100 000 person-years and 0.34 million annually, respectively. At the regional level, the incidence of SLE in women varied from 2.00 (0.27 to 6.22) per 100 000 person-years in central Asia to 22.99 (5.35 to 53.28) per 100 000 person-years in central Europe (figure 3). The countries with the highest female SLE incidence were Poland (136.48, 133.89 to 139.31 per 100 000 person-years), the USA (20.51, 20.17 to 20.87 per 100 000 person-years), the United Arab Emirates (18.72, 9.25 to 31.19 per 100 000 person-years) and Barbados (17.3, 3.35 to 60.74 per 100 000 person-years) (figure 3 and online supplemental table 14). The largest female populations with newly diagnosed SLE were reported in China (106 610, 104 214 to 109 161 annually), India (58 465, 15 909 to 172 281 annually), the USA (34 284, 33 609 to 34 958 annually) and Poland (26 624, 26 040 to 27 256 annually).
Figure 3.
(A) Crude incidence of SLE in female according to world regions. (B) Crude incidence of SLE in male according to world regions. SLE, systemic lupus erythematosus.
In men, the global SLE incidence and newly diagnosed population were estimated to be 1.53 (0.41 to 4.46) per 100 000 person-years and 0.06 million annually, respectively. The incidence of SLE in men varied from 0.34 (0.05 to 1.07) per 100 000 person-years in central Asia to 3.99 (0.93 to 9.22) per 100 000 person-years in central Europe (figure 3). The countries with the highest male SLE incidence were Poland (23.72, 23.2 to 24.33 per 100 000 person-years), the United Arab Emirates (3.96, 1.96 to 6.58 per 100 000 person-years), the USA (3.58, 3.51 to 3.66 per 100 000 person-years) and Barbados (2.99, 0.58 to 10.56 per 100 000 person-years) (figure 3 and online supplemental table 13). The largest male populations with newly diagnosed SLE were reported in China (19 458, 18 876 to 20 058 annually), India (10 971, 2940 to 31 982 annually), the USA (5864, 5671 to 6066 annually) and Poland (4352, 4187 to 4524 annually).
In addition to regional differences, age strata could also show variation. Based on our estimate, the SLE incidence in adults was approximately 1.42-fold higher than that in the total population. The global SLE incidence in adults was 7.31 (1.98 to 21.54) per 100 000 person-years, corresponding to approximately 0.38 million newly diagnosed cases in adults worldwide annually. In women, the SLE incidence in adults was approximately 1.4-fold higher than that in the total female population and reached 12.38 (3.36 to 36.55) per 100 000 person-years, indicating that approximately 0.68 million adult women worldwide were newly diagnosed with SLE annually. Moreover, the incidence of SLE in adult men was approximately 1.44-fold higher than that in the total male population and reached 2.2 (0.59 to 6.41) per 100 000 person-years; approximately 0.06 million adult men worldwide were newly diagnosed annually.
Prevalence of SLE
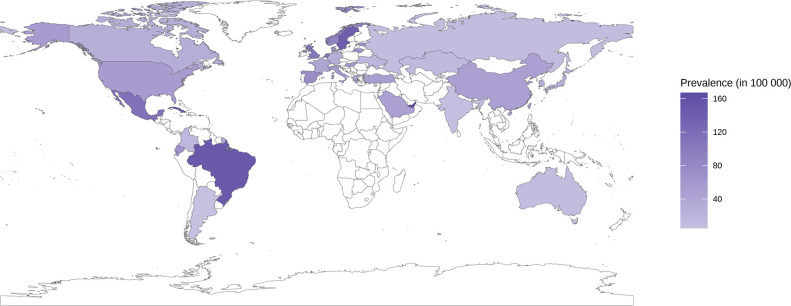
For the overall population, the global SLE prevalence and affected population were estimated to be 43.7 (15.87 to 108.92) per 100 000 persons and 3.41 million people, respectively. At the regional level, the prevalence of SLE in the general population varied from 15.9 (3.29 to 45.85) per 100 000 persons in southern Asia to 110.85 (26.74 to 314.1) per 100 000 persons in tropical Latin America (figure 4). The prevalence of SLE differed greatly by country. For the general population, the top four countries with the highest estimates of SLE prevalence were the United Arab Emirates (166.92, 139.01 to 198.54 per 100 000 persons), Barbados (163.31, 35.41 to 391.57 per 100 000 persons), Cuba (149.9, 26.05 to 424.12 per 100 000 persons) and Brazil (147.37, 38.19 to 351.48 per 100 000 persons). In contrast, Argentina was the country with the lowest prevalence worldwide (5.05, 4.22 to 6.06 per 100 000 persons) (online supplemental table 17).
Figure 4.
One-year period (physician or dermatologist diagnosed) prevalence of systemic lupus erythematosus for overall population by country.
Women were more likely to have SLE than men. The global SLE prevalence and affected population in women were estimated to be 78.73 (28.61 to 196.33) per 100 000 persons and 3.04 million people, respectively. At the regional level, the prevalence of SLE in women varied from 29.21 (6.04 to 84.23) per 100 000 persons in southern Latin America to 195.94 (47.25 to 554.5) per 100 000 persons in tropical Latin America (figure 5). The countries with the highest female SLE prevalence were the United Arab Emirates (410.3, 341.95 to 487.56 per 100 000 persons), Barbados (285.17, 61.9 to 683.25 per 100 000 persons), Cuba (266.62, 46.34 to 754.49 per 100 000 persons) and Brazil (251.86, 245.05 to 258.67 per 100 000 persons) (figure 5 and online supplemental table 21). The largest female population with SLE was reported in China (622 526, 616 988 to 628 178), Brazil (281 763, 66 454 to 670 851), the USA (159 914, 157 567 to 162 205) and Indonesia (131 214, 31 086 to 427 730).
Figure 5.
(A) Crude 1-year period (physician or dermatologist diagnosed) prevalence of SLE in female according to world regions. (B) Crude 1-year period (physician or dermatologist diagnosed) prevalence of SLE in male according to world regions. SLE, systemic lupus erythematosus.
For men, the global SLE prevalence and affected population were estimated to be 9.26 (3.36 to 22.97) per 100 000 persons and 0.36 million people, respectively. The prevalence of SLE in men varied from 3.44 (0.62 to 9.73) per 100 000 persons in southern Latin America to 22.96 (5.55 to 65.79) per 100 000 persons in tropical Latin America (figure 5). The countries with the highest male SLE prevalence were the United Arab Emirates (58.2, 48.54– to 69.28 per 100 000 persons), Barbados (33.4, 7.18 to 80.61 per 100 000 persons), Cuba (31.55, 5.45 to 89.33 per 100 000 persons) and Brazil (30.55, 7.23 to 73.12 per 100 000 persons) (figure 5 and online supplemental table 19). The largest male populations with SLE were reported in China (76 677, 75 631 to 77 816), Brazil (31 909, 7527 to 76 769), the USA (18 455, 18 057 to 18 839) and Indonesia (15 617, 3718 to 50 680).
In addition to regional differences, age and the method used to estimate prevalence (period or point prevalence) could also lead to variations. Based on our estimate, the SLE prevalence in adults was approximately 1.4-fold higher than that in the total population. The global SLE prevalence in adults was 61.08 (22.18 to 151.87) per 100 000 persons, corresponding to approximately 3.17 million adults worldwide. For women, the SLE prevalence in adult women was approximately 1.38-fold higher than that in the total female population and reached 108.75 (39.5 to 270.58) per 100 000 persons, which meant that approximately 2.84 million adult women worldwide were affected. Moreover, the prevalence of SLE in adult men was approximately 1.41-fold higher than that in the total male population and reached 13.04 (4.73 to 32.29) per 100 000 persons, indicating that approximately 0.34 million adult men worldwide were affected.
Discussion
Our systematic review is the first study to use a gold-standard model for sparse and heterogeneous data to estimate the global incidence and prevalence of SLE;14 15 our results complemented missing epidemiological data from most countries in the world. Importantly, the model estimated the potential SLE population in each country considering the influence of sex, age, diagnostic method and prevalence measurement method. The majority of studies included in the review were conducted in western Europe, high-income countries (North America), eastern Asia, central Latin America, North Africa and the Middle East. Few studies have been conducted in central Asia and Andean Latin America. The paucity of available data and the substantial variation in the prevalence estimates in several regions made a large-scale epidemiological SLE study necessary. The age and sex of the surveyed population as well as geographical location all influenced the incidence and prevalence of SLE. Specifically, adults, women and people living in countries/regions with high-income levels were more likely to suffer from SLE than children, men and people in lower-income communities.
In terms of the study design, the cases in most studies were diagnosed by physicians or dermatologists. However, 58.90% of the incidence studies and 58.67% of the prevalence studies did not report diagnostic criteria. For the remaining reported studies, the ACR classification criteria were the dominant diagnostic criteria. Given that the commonly used classification criteria for SLE, such as the ACR 1997,19 Systemic Lupus International Collaborating Clinics (SLICC) 201220 and EULAR/ACR 2019,4 were different and that the SLICC criteria might be more inclusive of subjects with SLE for clinical studies, potential bias could be introduced by differences in diagnostic criteria.21–23 Using the same model, we found the global SLE incidence and newly diagnosed population according to the ACR classification criteria were estimated to be 4.78 (1.46 to 12.11) per 100 000 person-years and 0.37 million people annually, which were slightly less than the estimations with no classification criteria limitations (5.14 (1.4 to 15.13) per 100 000 person-years and 0.40 million people annually) (online supplemental figure 5). The global SLE prevalence and affected population according to the ACR classification criteria were estimated to be 50.80 (17.64 to 117.05) per 100 000 person-years and 3.96 million people, which were slightly higher than the estimations with no classification criteria limitation (43.7 (15.87 to 108.92) per 100 000 person-years and 3.41 million people) (online supplemental figure 5). Even though the ACR classification criteria did not significantly alter the estimated SLE global incidence and prevalence but was limited by the small number of SLICC and EULAR/ACR defined studies, the impact of these two classification criteria was uncovered. Future SLE epidemiological studies should report the SLE diagnostic classification used to help further data analysis. The majority of the patients with SLE included in our study were diagnosed by physicians and dermatologists; only three studies that reported the lifetime prevalence used self-reported diagnosis, which was based on a physician’s former diagnosis rather than the patient’s self-judgement. The model estimated the global SLE prevalence and affected population excluding self-reported diagnoses, as 44.12 (14.75 to 99.91) per 100 000 person-years and 3.44 million people, respectively, which were slightly higher than the estimations including all diagnostic methods (43.7 (15.87 to 108.92) per 100 000 person-years and 3.41 million people) (online supplemental figure 6). Overall, in our study, differences in the diagnostic methods did not introduce much bias.
Further age-stratified analyses were impeded due to the limited number of studies and inconsistent age stratification among studies.24–26 Similarly, other interesting factors that may affect SLE epidemiology, such as variability in healthcare systems, public health and access to experts, were difficult to measure because almost all epidemiological studies do not provide related information. Since the affected population was estimated based on the prevalence and incidence, when only a few studies were available, population estimates might be subject to extreme values and need to be interpreted with caution. Moreover, countries were grouped and classified according to the Global Burden of Disease classification, which was mainly based on geography and income level. This categorisation might influence the income and population-related patterns emphasised in the study.14 15
Several studies reviewed the current global prevalence and incidence of SLE, but none of them showed detailed information in certain regions or countries using statistical models. Overall, the global incidence of SLE reported ranged from 1.5 to 11 per 100 000 person-years, and the global prevalence ranged from 13 to 7713.5 per 100 000 individuals.6 Similar to previous studies, our analysis suggested that women were more likely to be affected by SLE than men in all international regions, and a higher incidence and prevalence of SLE were reported in countries/regions with a higher income level.5 6 These income and population patterns might be attributed to better healthcare systems, easier access to experts, more comprehensive insurance records and higher levels of public awareness. However, since detailed ethnic data were not provided in the included studies, we could not exclude related factors such as income level, geographical location and climate to clarify the effect of ethnicity on the epidemiology of SLE. Similarly, we could not identify the association between the prevalence and latitude, humidity or temperature.27–30
We systematically reviewed and analysed all available SLE epidemiology studies published in the last 30 years to establish a statistical disease model for SLE. By providing estimations of global, regional, and country-specific epidemiology rates and affected populations, our results and interpretation provided more insights into the SLE disease paradigm and lay a foundation for population-based studies in the developing world, ultimately contributing to a reduction in the disease burden.
Footnotes
Handling editor: Josef S Smolen
Contributors: QL and JT conceived of the study. JT and QL developed the protocol. JT and DZ did the literature search. JT and DZ appraised study quality, and extracted and analyzed the data. DZ did the computational analysis and coding. JT, DZ, XY and QL interpreted the data. JT and DZ wrote the first draft of the article. XY and YH revised the first draft of the article. QL reviewed and critically evaluated the draft paper. QL is responsible for the overall content as the guarantor.
Funding: We acknowledge the CAMS Innovation Fund for Medical Sciences (No. 2021-I2M-1-059), non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (No. 2021-RC320-001, No. 2020-RC320-003), National Natural Science Foundation of China (No. 81830097) and the Special Program of National Natural Science Foundation of China (No. 32141004).
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study. Not applicable.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Not applicable.
References
- 1. Kiriakidou M, Ching CL. Systemic lupus erythematosus. Ann Intern Med 2020;172:ITC81–96. 10.7326/AITC202006020 [DOI] [PubMed] [Google Scholar]
- 2. Yen EY, Singh RR. Brief report: Lupus-An unrecognized leading cause of death in young females: a population-based study using nationwide death certificates, 2000-2015. Arthritis Rheumatol 2018;70:1251–5. 10.1002/art.40512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Durcan L, O'Dwyer T, Petri M. Management strategies and future directions for systemic lupus erythematosus in adults. Lancet 2019;393:2332–43. 10.1016/S0140-6736(19)30237-5 [DOI] [PubMed] [Google Scholar]
- 4. Aringer M, Costenbader K, Daikh D. 2019 European League against Rheumatism/American College of rheumatology classification criteria for systemic lupus erythematosus. Arthritis & Rheumatology 20192019;71:1400–12. 10.1002/art.40930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol 2016;12:605–20. 10.1038/nrrheum.2016.137 [DOI] [PubMed] [Google Scholar]
- 6. Barber MRW, Drenkard C, Falasinnu T, et al. Global epidemiology of systemic lupus erythematosus. Nat Rev Rheumatol 2021;17:515–32. 10.1038/s41584-021-00668-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bae EH, Lim SY, Han K-D, et al. Trend of prevalence and incidence of systemic lupus erythematosus in South Korea, 2005 to 2015: a nationwide population-based study. Korean J Intern Med 2020;35:652–61. 10.3904/kjim.2018.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leong P-Y, Huang J-Y, Chiou J-Y, et al. The prevalence and incidence of systemic lupus erythematosus in Taiwan: a nationwide population-based study. Sci Rep 2021;11:5631. 10.1038/s41598-021-84957-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tan Y, Yu F, Long J, et al. Frequency of systemic lupus erythematosus was decreasing among hospitalized patients from 2013 to 2017 in a national database in China. Front Med 2021;8:648727. 10.3389/fmed.2021.648727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwarting A, Friedel H, Garal-Pantaler E, et al. The burden of systemic lupus erythematosus in Germany: incidence, prevalence, and healthcare resource utilization. Rheumatol Ther 2021;8:375–93. 10.1007/s40744-021-00277-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alamanos Y, Voulgari PV, Siozos C, et al. Epidemiology of systemic lupus erythematosus in northwest Greece 1982-2001. J Rheumatol 2003;30:731–5. [PubMed] [Google Scholar]
- 12. Vos T, Lim SS, Abbafati C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. The Lancet 2020;396:1204–22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Downes MJ, Brennan ML, Williams HC, et al. Development of a critical appraisal tool to assess the quality of cross-sectional studies (axis). BMJ Open 2016;6:e011458. 10.1136/bmjopen-2016-011458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mariel MF, Christopher JP, Goodarz D. Bayesian estimation of population-level trends in measures of health status. Statistical Science 2014;29:18–25. [Google Scholar]
- 15. ed). Parisi R, Iskandar IYK, Kontopantelis E. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study.. In: BMJ (Clinical research. 93, 2020: m1590. 10.1136/bmj.m1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joseph L, Gyorkos TW, Coupal L. Bayesian estimation of disease prevalence and the parameters of diagnostic tests in the absence of a gold standard. Am J Epidemiol 1995;141:263–72. 10.1093/oxfordjournals.aje.a117428 [DOI] [PubMed] [Google Scholar]
- 17. Flor M, Weiß M, Selhorst T, et al. Comparison of Bayesian and frequentist methods for prevalence estimation under misclassification. BMC Public Health 2020;20:1135. 10.1186/s12889-020-09177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. United Nations, Department of Economic and Social Affairs, Population Division . World population prospects 2019: volume II: demographic profiles, 2019. [Google Scholar]
- 19. Hochberg MC. Updating the American College of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 20. Black SM, Walocko F, Li X, et al. Development of systemic lupus in patients with cutaneous lupus using the 2012 systemic lupus international collaborating clinics (SLICC) classification criteria for systemic lupus erythematosus. J Am Acad Dermatol 2021;85:200–2. 10.1016/j.jaad.2020.12.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aberle T, Bourn RL, Chen H, et al. Use of SLICC criteria in a large, diverse lupus registry enables SLE classification of a subset of ACR-designated subjects with incomplete lupus. Lupus Sci Med 2017;4:e000176. 10.1136/lupus-2016-000176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Inês L, Silva C, Galindo M, et al. Classification of systemic lupus erythematosus: systemic lupus international collaborating clinics versus American College of rheumatology criteria. A comparative study of 2,055 patients from a real-life, International systemic lupus erythematosus cohort. Arthritis Care Res 2015;67:1180–5. 10.1002/acr.22539 [DOI] [PubMed] [Google Scholar]
- 23. Low ESH, Krishnaswamy G, Thumboo J. Comparing the 1997 update of the 1982 American College of rheumatology (ACR-97) and the 2012 systemic lupus international collaborating clinics (SLICC-12) criteria for systemic lupus erythematosus (SLE) classification: which enables earlier classification of SLE in an urban Asian population? Lupus 2019;28:11–18. 10.1177/0961203318811599 [DOI] [PubMed] [Google Scholar]
- 24. Brinks R, Hoyer A, Weber S, et al. Age-Specific and sex-specific incidence of systemic lupus erythematosus: an estimate from cross-sectional claims data of 2.3 million people in the German statutory health insurance 2002. Lupus Sci Med 2016;3:e000181. 10.1136/lupus-2016-000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Somers EC, Marder W, Cagnoli P, et al. Population-Based incidence and prevalence of systemic lupus erythematosus: the Michigan lupus epidemiology and surveillance program. Arthritis Rheumatol 2014;66:369–78. 10.1002/art.38238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rees F, Doherty M, Grainge M, et al. The incidence and prevalence of systemic lupus erythematosus in the UK, 1999-2012. Ann Rheum Dis 2016;75:136–41. 10.1136/annrheumdis-2014-206334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng Y, Li M, Zhao J, et al. Chinese SLE Treatment and Research Group (CSTAR) registry:VIII. Influence of socioeconomic and geographical variables on disease phenotype and activity in Chinese patients with SLE. Int J Rheum Dis 2018;21:716–24. 10.1111/1756-185X.13057 [DOI] [PubMed] [Google Scholar]
- 28. Hasan T, Pertovaara M, Yli-Kerttula U, et al. Seasonal variation of disease activity of systemic lupus erythematosus in Finland: a 1 year follow up study. Ann Rheum Dis 2004;63:1498–500. 10.1136/ard.2003.012740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Szeto C-C, Mok H-Y, Chow K-M, et al. Climatic influence on the prevalence of noncutaneous disease flare in systemic lupus erythematosus in Hong Kong. J Rheumatol 2008;35:1031–7. [PubMed] [Google Scholar]
- 30. Hua-Li Z, Shi-Chao X, De-Shen T, et al. Seasonal distribution of active systemic lupus erythematosus and its correlation with Meteorological factors. Clinics 2011;66:1009–13. 10.1590/s1807-59322011000600015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ard-2022-223035supp001.pdf (3.3MB, pdf)
ard-2022-223035supp002.pdf (1.2MB, pdf)
Data Availability Statement
Data sharing not applicable as no datasets generated and/or analysed for this study. Not applicable.