Abstract
Objectives
To assess the effects of occupational inhalable exposures on rheumatoid arthritis (RA) development and their interactions with smoking and RA-risk genes, stratifying by presence of anticitrullinated protein antibodies (ACPA).
Methods
Data came from the Swedish Epidemiological Investigation of RA, consisting of 4033 incident RA cases and 6485 matched controls. Occupational histories were retrieved, combining with a Swedish national job-exposure matrix, to estimate exposure to 32 inhalable agents. Genetic data were used to define Genetic Risk Score (GRS) or carrying any copy of human leucocyte antigen class II shared epitope (HLA-SE) alleles. Associations were identified with unconditional logistical regression models. Attributable proportion due to interaction was estimated to evaluate presence of interaction.
Results
Exposure to any occupational inhalable agents was associated with increased risk for ACPA-positive RA (OR 1.25, 95% CI 1.12 to 1.38). The risk increased as number of exposed agents increased (Ptrend<0.001) or duration of exposure elongated (Ptrend<0.001). When jointly considering exposure to any occupational inhalable agents, smoking and high GRS, a markedly elevated risk for ACPA-positive RA was observed among the triple-exposed group compared with those not exposed to any (OR 18.22, 95% CI 11.77 to 28.19). Significant interactions were found between occupational inhalable agents and smoking/genetic factors (high GRS or HLA-SE) in ACPA-positive RA.
Conclusions
Occupational inhalable agents could act as important environmental triggers in RA development and interact with smoking and RA-risk genes leading to excessive risk for ACPA-positive RA. Future studies are warranted to assess preventive strategies aimed at reducing occupational hazards and smoking, especially among those who are genetically vulnerable.
Keywords: Arthritis, Rheumatoid; Anti-Citrullinated Protein Antibodies; Smoking; Epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Cigarette smoking has been shown to increase the risk of developing rheumatoid arthritis (RA), but little is known about the effects of occupational inhalable agents on RA.
WHAT THIS STUDY ADDS
Our results suggest that exposure to occupational inhalable agents increases the risk of developing RA and interacts with smoking and RA-risk genes leading to an excessive risk for anticitrullinated protein antibodies-positive RA.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our study emphasises the importance of occupational respiratory protections, particularly for individuals who are genetically predisposed to RA.
Background
Rheumatoid arthritis (RA) is a chronic autoimmune joint disorder characterised by painful and disabling polyarthritis, affecting commonly 0.3%–1.0% of the global population.1 2 External exposures such as smoking have been recognised as important environmental risk factors for RA, while human leucocyte antigen class II shared epitope (HLA-SE) alleles constitute the major genetic risk factors.3–6 A strikingly 21-fold increased risk of developing anticitrullinated protein/peptide antibodies positive (ACPA-positive) RA has been reported for smokers carrying two copies of HLA-SE alleles, leading to the formulation of an aetiological hypothesis where autoimmunity to citrullinated autoantigens occurs after activation of HLA-SE restricted immunity to autoantigens generated in lungs by smoking.7–9 Notably, the observations forming the basis of this hypothesis are made only for the ACPA-positive subtype.7 10 11
Over recent years additional environmental inhalable exposures have been linked to risk for RA, including silica dust, asbestos and textile dust, whereas studies on effects of air pollution have yielded variable results.12 13 However, there is still a lack of knowledge of the impact on risk for RA from many different environmental exposures affecting airways that occur in occupational situations worldwide. Furthermore, the studies on inhalant–RA associations that exist have rarely considered personal smoking habits or genetic backgrounds in the same context and only in few cases subdivided RA in serologically defined subsets.
From this background and provided that occupational environmental airway exposures constitute potentially modifiable causes of RA, we set out to investigate the impact of such exposures on risk for the two major subtypes of RA, taking also smoking and genetic constitution into account. For this purpose, we used data from the large case–control study, Epidemiological Investigation of RA (EIRA), which enabled us to investigate the associations between multiple occupational inhalable exposures and risk of RA, as well as their interactions with smoking and genetic variants.
Methods
Study base
The EIRA is a Swedish population-based case–control study that comprises participants over the age of 18 in southern/central regions of Sweden. Cases were newly onset patients with RA diagnosed by a rheumatologist, based on the American College of Rheumatology (ACR) 1987 criteria or the more recently introduced ACR/the Grading of Recommendations Assessment, Development and Evaluation 2010 criteria. Controls were randomly selected from the nationwide population register shortly after case identification and matched on age, sex and residential area. The year of first symptom onset was registered for cases and taken as the index year for matched controls. Information regarding demographics, work history and lifestyles was collected by self-administrated questionnaires, and blood samples were collected for anticitrulline antibody test and genotyping.
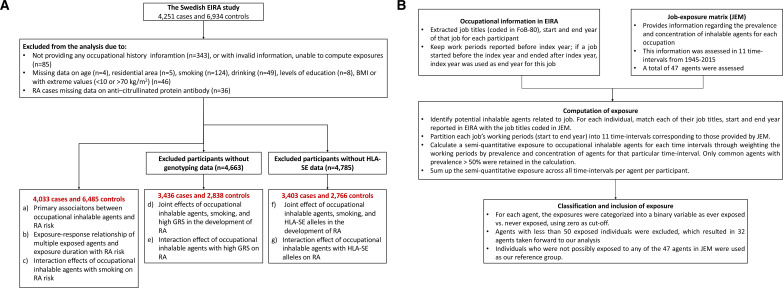
During the period of 1996–2017, 4251 cases and 6934 controls participated in EIRA. After excluding participants who missed information on occupational history or important covariates, 4033 cases and 6485 controls were available for questionnaire data; approximately 3400 cases and 2800 controls had concomitant genetic data. Exclusion criteria are shown in figure 1A.
Figure 1.
Flow chart of data handling in the current study. (A) Exclusion criteria of study participants. (B) Definition of exposure to occupational inhalable agents using the Swedish national job-exposure matrix (JEM). BMI, body mass index; GRS, Genetic Risk Score.
Patient and public involvement
No patients or members of the public were directly involved in the design or conduct of this study.
Exposure
The participants were asked to provide information on job titles, start year and end year for up to 14 working periods. To determine exposures to inhalable agents across different occupations and working periods, we applied a job-exposure matrix (JEM) developed for working conditions in Sweden, which contained assessment of prevalence and concentration of 47 inhalable agents.14 Details regarding the quantification of exposures are shown in figure 1B. We classified the exposures into binary variables as ever exposed versus never exposed (to a particular agent) with zero as cut-off. The participants who were possibly exposed to any of the 47 inhalable agents were classified as exposed to any agents. To ensure statistical power, we only retained agents with >50 exposed individuals in our agent-specific analysis, resulting in 32 agents. Our reference group consisted of individuals who were not exposed to any agents (0/47 agents in the JEM).
Covariates
Genetic risk score and HLA-SE alleles
We included participants of European ancestry for genetic analysis. To get an appropriate genetic metrics for a European population and avoid overlapping, we retrieved the genetic summary statistics for the European-ancestral subpopulations from the hitherto largest RA genome-wide association study (GWAS).15 We meta-analysed summary statistics of participating studies after excluding EIRA, which resulted in 13 264 RA cases and 42 879 controls. We then computed the Genetic Risk Score (GRS) for EIRA participants using LDpred2 software with weights from this RA meta-GWAS.16 Participants were classified into carrying high versus low genetic burden based on the median values of GRS among controls.
In addition to GRS, we complementarily incorporated and investigated the primary RA risk genes summarised as presence of HLA-SE alleles,17 18 with participants classified as carriers or non-carriers based on the presence of any copy of HLA-SE alleles.
Other covariates
Participants reported never smoked were classified as non-smokers, while those reported as current smokers, ex-smokers or non-regular smokers were classified as ever smokers. Alcohol consumption was defined as non-drinkers and ever drinkers. Body mass index (BMI) (kg/m2) was categorised into <20, 20–25 and >25. Levels of education were classified into primary education, secondary education and with a university degree. Residential areas were categorised into 16 counties. Age in years was included as a continuous variable. Sex was binary as male and female.
Statistical analysis
We first compared the basic characteristics of each of the two RA subtypes (ACPA-positive and ACPA-negative) with the controls, using t-tests for continuous variables and χ2 tests for categorical variables. We then estimated the association of exposure to each inhalable agent with the risk of developing overall RA as well as with ACPA-based subtypes through unconditional logistic regressions with adjustment for matching factors.
Occupational hazardous agents often coexist. To account for potential correlations among inhalable agents, we calculated Pearson’s correlation coefficients pair-wising all 32 agents and ‘clumped’ these agents with a significant P-threshold of 1.0×10−4 (0.05/496 pairs) and a correlation coefficient threshold of 0.4 (moderate correlation), through which a total of 16 independent collections of inhalable agents (therefore 16 index agents) were identified. These 16 index agents were used as main exposures in our subsequent analyses.
To understand the accumulated effect of inhalable agents, we classified participants into five groups based on their total numbers of exposures (exposed to 1, 2, 3, 4 or ≥5 of the 16 independent index agents) or quantiles of exposure duration (0–3.3, 3.3–8.0, 8.0–1.5, 1.5–24.0, 24.0–51.0 years of exposure to any agents). We evaluated an exposure–response relationship comparing each of the five exposed groups with the reference group (individuals not exposed to any agents).
To investigate the joint effect of inhalable agents, smoking and genetic predisposition (high GRS or carrying HLA-SE alleles), we categorised participants into seven groups based on their exposure status to any of the three factors (only exposed to one factor, only exposed to two factors or triple-exposed). We performed analysis comparing each of the seven exposed groups with the reference group (individuals not exposed to any occupational inhalable agents, non-smoker and with low GRS or non-HLA-SE-carriers). To explore the gene–environment (G×E) or E×E interaction effect among inhalable agents, smoking and genetic predisposition (high GRS or carrying HLA-SE alleles), we estimated the additive interaction defined as departure from the additivity of effects.19
All analyses were conducted using unconditional regression models, with age, sex, residential area, BMI, smoking, drinking and levels of education commonly included as covariates. All analyses were performed in RA overall as well as stratified into ACPA-based subtypes. For analysis involving genetic data, principal components 1-10 were additionally included to control for population stratification. To account for multiple testing, statistical significance was set at a stringent P-threshold of 1.6×10−3 (0.05/32), while suggestive significance was set at 1.6×10−3<P<0.05. More details for our methods were described in online supplemental methods.
ard-2022-223134supp001.pdf (1MB, pdf)
Results
Basic characteristics of RA cases and controls are shown in table 1. Compared with controls, both ACPA-positive and ACPA-negative cases were more likely to smoke, drink less, be overweight and be without a university degree. Compared with ACPA-negative cases, ACPA-positive cases were slightly younger, more likely to be women and smokers, had high GRS and more HLA-SE carriers. In terms of occupational inhalable agents, 73% of ACPA-positive cases and 72% of ACPA-negative cases were ever exposed, significantly higher than controls (67%).
Table 1.
Basic characteristics of the study participants
| Characteristics | Overall RA cases | Controls | P for ACPA-positive cases versus controls | P for ACPA-negative cases versus controls | P for ACPA-positive versus ACPA-negative cases | |
| ACPA-positive cases | ACPA-negative cases | |||||
| Total N | 2642 | 1391 | 6485 | |||
| Age, mean (SD) | 52.93 (12.44) | 55.18 (13.07) | 53.83 (12.82) | 0.002 | <0.001 | <0.001 |
| Women, N (%) | 1908 (72) | 946 (68) | 4609 (71) | 0.28 | 0.025 | 0.0058 |
| Education, N (%) | ||||||
| Primary education | 317 (12) | 190 (14) | 537 (8) | <0.001 | <0.001 | 0.18 |
| Secondary education | 1678 (64) | 846 (61) | 3760 (58) | |||
| University degree | 647 (24) | 355 (26) | 2188 (34) | |||
| Smoking status, N (%) | ||||||
| Non-smoker | 791 (30) | 539 (39) | 2951 (46) | <0.001 | <0.001 | <0.001 |
| Ever smoker | 1851 (70) | 852 (61) | 3534 (54) | |||
| Alcohol drinking, N (%) | ||||||
| Non-drinker | 254 (10) | 123 (9) | 400 6) | <0.001 | <0.001 | 0.46 |
| Ever drinker | 2388 (90) | 1268 (91) | 6085 (94) | |||
| BMI, N (%) | ||||||
| <20 kg/m2 | 188 (7) | 85 (6) | 378 (6) | 0.042 | <0.001 | 0.010 |
| 20–25 kg/m2 | 1195 (45) | 574 (41) | 3047 (47) | |||
| >25 kg/m2 | 1259 (48) | 732 (53) | 3060 (47) | |||
| Participants with genetic data, N (%) | 2271 (86) | 1165 (84) | 2838 (44) | <0.001 | <0.001 | 0.068 |
| High genetic predisposition to RA | 1949 (86) | 670 (58) | 1416 (50) | <0.001 | <0.001 | <0.001 |
| Participants with HLA-DRB1 genotypes available, N (%) | 2232 (84) | 1171 (84) | 2766 (43) | <0.001 | <0.001 | 0.84 |
| With any copy of HLA-SE allele, N (%) | 1886 (84) | 638 (54) | 1451 (52) | <0.001 | 0.26 | <0.001 |
| Ever exposed to any occupational inhalable agents, N (%) | 1928 (73) | 1007 (72) | 4371 (67) | <0.001 | <0.001 | 0.72 |
| Ever exposed to any occupational inhalable agent among women, N (%) | 1307 (69) | 656 (69) | 3003 (65) | 0.010 | 0.015 | 0.68 |
| Ever exposed to any occupational inhalable agent among men, N (%) | 621 (85) | 351 (79) | 1368 (73) | <0.001 | 0.012 | 0.015 |
ACPA, anticitrullinated protein antibodies; BMI, body mass index; RA, rheumatoid arthritis.
Primary associations of exposure to occupational inhalable agents and RA risk
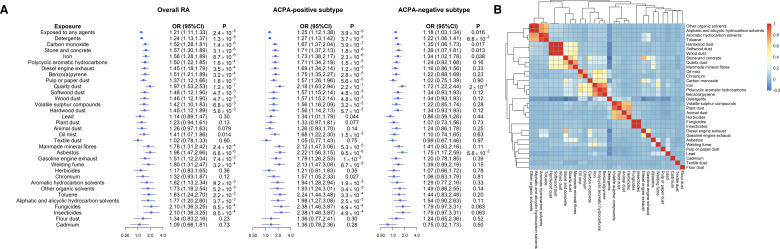
As shown in figure 2A and online supplemental table 1, exposure to any occupational inhalable agents was associated with a significantly increased risk of RA overall (OR 1.21, 95% CI 1.11 to 1.33, p=2.4×10−5). The association remained consistent in ACPA-positive subtype (OR 1.25, 95% CI 1.12 to 1.38, p=3.9×10−5), while attenuated in ACPA-negative subtype (OR 1.18, 95% CI 1.03 to 1.34, p=0.016). When stratified by sex, exposure to any occupational inhalable agents was associated with a higher risk of developing overall (OR 1.40, 95% CI 1.15 to 1.70, p=7.4×10−4) and ACPA-positive RA (OR 1.66, 95% CI 1.30 to 2.11, p=4.2×10−5) in men than in women (overall RA: OR 1.13, 95% CI 1.02 to 1.25, p=0.022; ACPA-positive RA: OR 1.13, 95% CI 1.00 to 1.27, p=0.05; Psex-difference<0.05) (online supplemental table 2).
Figure 2.
Primary associations between occupational inhalable agents and RA risk. (A) Associations between occupational inhalable agents and risk for RA. Results are shown for overall RA, as well as ACPA-positive and ACPA-negative subtypes. Estimates were adjusted for age, sex, residential area, smoking, alcohol drinking, education and body mass index. (B) Pairwise correlation of 32 occupational inhalable agents. ACPA, anticitrullinated protein antibodies; RA, rheumatoid arthritis.
When looking into each of the 32 inhalable agents (figure 2A and online supplemental table 1), we observed distinct association patterns in RA subtypes. The point estimates for all 32 agents in ACPA-positive RA were greater than the corresponding estimates in ACPA-negative RA. Specifically, 17 out of 32 agents were strongly associated with an increased risk of ACPA-positive RA (p<1.6×10−3, ORs raging from 1.25 to 2.38); meanwhile, none of the agents withstood Bonferroni correction (p<1.6×10−3) for ACPA-negative RA—the strongest association for ACPA-negative RA (in terms of significance) were found for quartz dust (p=2.0×10−3), followed by asbestos (p=5.8×10−3) and detergents (p=6.8×10−3).
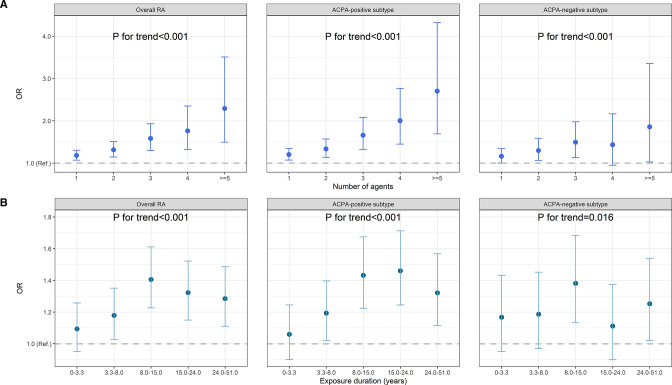
Multiple occupational hazards are likely coexist in the same work environment, reflected by our clustering plot (figure 2B). After clumping, 16 collections of agents that were mutually independent to each other were identified (online supplemental figure 1). The risk of developing RA increased as the numbers of exposed agents (out of the 16) increased (Ptrend<0.001 for overall RA and both subtypes) (figure 3A and online supplemental table 3) or as duration of exposure (to any agents) elongated (Ptrend<0.001 for overall and ACPA-positive RA) (figure 3B and online supplemental table 4).
Figure 3.
Exposure–response relationship between occupational inhalable agents and RA. (A) Participants were classified into exposed to 1, 2, 3, 4 or ≥5 agents out of the 16 independent agent collections and compared with non-exposed group (not exposed to any of the 47 agents). (B) Participants were classified into five subsets with exposure durations of 0–3.3, 3.3–8.0, 8.0–1.5, 1.5–24.0, 24.0–51.0 years (to any agents) and compared with non-exposed group (not exposed to any of the 47 agents). Results are shown for overall RA, as well as ACPA-positive and ACPA-negative subtypes. Estimates were adjusted for age, sex, residential area, smoking, alcohol drinking, education and body mass index. ACPA, anticitrullinated protein antibodies; RA, rheumatoid arthritis.
Joint effects of occupational inhalable agents, smoking and genetic predisposition on RA risk
When simultaneously analysing inhalable agents (exposed to any agent), smoking and high GRS, participants who were triple-exposed had a higher risk of developing RA overall compared with those who were not exposed to any of the three factors (OR 5.50, 95% CI 4.23 to 7.14, p=1.8×10−37). For ACPA-positive subtype, the estimated OR was 18.22 (95% CI 11.77 to 28.19, p=8.2×10−39), notably higher than an OR of 1.69 (95% CI 1.25 to 2.29, p=6.1×10−4) observed in ACPA-negative subtype (table 2).
Table 2.
The combined effects of occupational inhalable agents (exposed to any agents), smoking and high GRS with risk for RA
| Exposed to any agents | Smoking | High GRS | Overall RA | ACPA-positive subtype | ACPA-negative subtype | ||||||
| N (case/control) | OR (95% CI) | P value | N (case/control) | OR (95% CI) | P value | N (case/control) | OR (95% CI) | P value | |||
| – | – | – | 101/232 | 1.00 ref | 25/232 | 1.00 ref | 76/232 | 1.00 ref | |||
| + | – | – | 176/413 | 0.98 (0.73 to 1.33) | 0.91 | 61/413 | 1.46 (0.88 to 2.41) | 0.14 | 115/413 | 0.84 (0.60 to 1.18) | 0.31 |
| – | + | – | 139/228 | 1.46 (1.06 to 2.02) | 0.022 | 60/228 | 2.63 (1.57 to 4.40) | 2.4×10–4 | 79/228 | 1.07 (0.74 to 1.55) | 0.71 |
| – | – | + | 263/182 | 3.23 (2.37 to 4.40) | 8.8×10–14 | 193/182 | 9.92 (6.20 to 15.87) | 1.2×10–21 | 70/182 | 1.16 (0.79 to 1.71) | 0.45 |
| + | + | – | 401/549 | 1.70 (1.29 to 2.24) | 1.6×10–4 | 176/549 | 3.24 (2.05 to 5.11) | 4.9×10–7 | 225/549 | 1.23 (0.90 to 1.67) | 0.20 |
| – | + | + | 435/236 | 4.41 (3.30 to 5.89) | 1.0×10–23 | 338/236 | 14.80 (9.38 to 23.35) | 5.6×10–31 | 97/236 | 1.30 (0.91 to 1.86) | 0.16 |
| + | – | + | 554/413 | 3,00 (2.28 to 3.94) | 3.7×10–15 | 376/413 | 8.48 (5.43 to 13.24) | 5.2×10–21 | 178/413 | 1.26 (0.92 to 1.74) | 0.15 |
| + | + | + | 1367/585 | 5.50 (4.23 to 7.14) | 1.8×10–37 | 1042/585 | 18.22 (11.77 to 28.19) | 8.2×10–39 | 325/585 | 1.69 (1.25 to 2.29) | 6.1×10-4 |
Results are shown for overall RA, as well as ACPA-positive and ACPA-negative subtypes. Estimates were adjusted for age, sex, residential area, alcohol drinking, levels of education, body mass index and principal components 1–10.
ACPA, anticitrullinated protein antibodies; GRS, Genetic Risk Score; RA, rheumatoid arthritis.
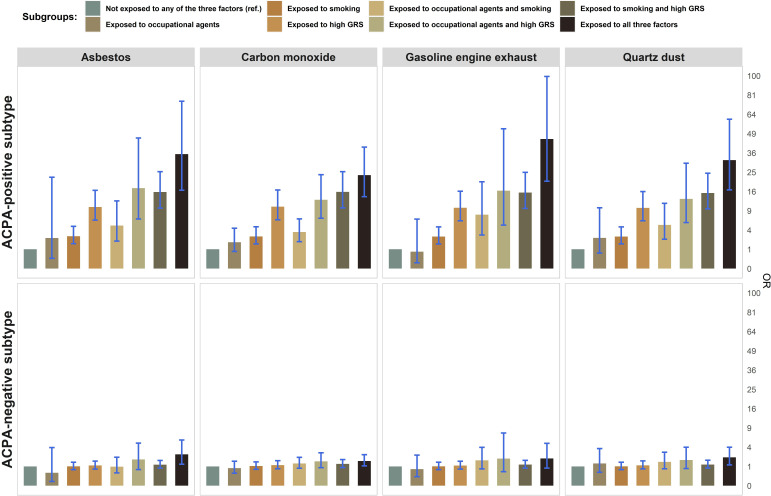
More importantly, across the 16 specific collections of agents, a large risk of developing RA in the triple-exposed group (number of individuals in this group >30 for robustness) was observed for ACPA-positive subtype with ORs ranging from 18.0 to 45.1, while the estimates for ACPA-negative subtype were weaker with ORs ranging from 0.85 to 2.64 (see results for four common collections in figure 4, for all 32 agents in online supplemental table 4). When replacing GRS with HLA-SE, a similar pattern was observed—a strikingly increased risk in the triple-exposed group was found exclusively in ACPA-positive subtype, with ORs ranging from 8.6 to 25.9 (online supplemental table 6).
Figure 4.
The combined effects of four common inhalable agents, smoking and high genetic risk score with risk for RA. Results are shown for ACPA-positive and ACPA-negative subtypes. Estimates were adjusted for age, sex, residential area, body mass index, levels of education, alcohol drinking and principal components 1–10. ACPA, anticitrullinated protein antibodies; RA, rheumatoid arthritis.
G×E and E×E interaction effects
Regarding G×E interaction for the 16 independent collections of inhalable agents, we observed an additive interaction between high GRS and gasoline engine exhaust (attributable proportion (AP) 0.52, 95% CI 0.23 to 0.80, p=4.1×10−4), asbestos (AP 0.44, 95% CI 0.13 to 0.74, p=0.0055), carbon monoxide (AP 0.23, 95% CI 0.01 to 0.45, p=0.042), fungicide (AP 0.56, 95% CI 0.19 to 0.93, p=0.0031) and quartz dust (AP 0.36, 95% CI 0.08 to 0.63, p=0.01), all restricted to ACPA-positive RA (online supplemental table 7). When replacing GRS with HLA-SE alleles, a similar synergistic effect remained for gasoline engine exhaust (AP 0.48, 95% CI 0.09 to 0.60, p=0.003), asbestos (AP 0.41, 95% CI 0.08 to 0.75, p=0.016), carbon monoxide (AP 0.29, 95% CI 0.07 to 0.51, p=0.01) and quartz dust (AP 0.42, 95% CI 0.15 to 0.68, p=0.0024), all restricted to ACPA-positive RA (online supplemental table 8). Despite the comparable APs (interaction effects) observed for GRS and HLA-SE alleles, participants with a high GRS generally had a larger point estimate for the risk of developing RA than those who carried HLA-SE alleles under exposure to asbestos (OR for high GRS vs HLA-SE: 14.20 vs 9.97), carbon monoxide (9.84 vs 7.04), gasoline engine exhaust (17.06 vs 10.79) and quartz dust (12.42 vs 9.48). On the contrary, no apparent G×E interaction was found for ACPA-negative RA (online supplemental tables 7–8).
Regarding agent–smoking (E×E) interaction, significant interactions between exposure to any agent and smoking were observed in RA overall (AP 0.19, 95% CI 0.08 to 0.31, p=0.0011) (online supplemental table 9). Interesting findings included a strong synergistic effect of smoking with the collection containing carbon monoxide (overall RA: AP 0.30, 95% CI 0.14 to 0.46, p=3.5×10−4; ACPA-positive subtype: AP 0.30, 95% CI 0.12 to 0.47, p=9.8×10−4) and detergents (overall RA: AP 0.26, 95% CI 0.14 to 0.37, p=1.4×10−5; ACPA-positive subtype: AP 0.25, 95% CI 0.12 to 0.37, p=1.3×10−4). Nevertheless, no apparent E×E interaction was found for ACPA-negative subtype (online supplemental table 9).
Discussion
Our study supports a general link between occupational inhalable agents and risk of RA, with clear restriction towards ACPA-positive rather than ACPA-negative RA and with higher ORs for risk in men than in women. We also observed an exposure–response relationship, in which the risk of ACPA-positive RA increased either with an increased duration or with an increased number of exposed agents. When taking smoking and genes into account, an 18-fold higher risk of developing ACPA-positive RA was observed in the triple-exposed group (any agent, smoking and high GRS) compared with the non-exposed reference group. Furthermore, a positive G×E interaction between inhalable agents and genetic predisposition was observed in our study, additionally supports a trigger role for the environmental exposures.
Inhalable exposures have long been proposed as important risk factors for RA, particularly for seropositive subtype.3 8 20 However, so far relatively few studies have investigated the inhalant–RA relationship, most of which only covered a limited number of exposures, rarely controlled for important confounders and usually lacked statistical power to stratify cases by seropositivity.12 Briefly, studies have reported silica, asbestos, textile dust, organic solvents, oil mist and pesticides to significantly affect RA risk.21–27 Our results, which comprehensively interrogated 32 agents summarising information from 10 518 individuals largely extend previous observations by providing novel dimensions. We successfully validated the hazardous role of quartz dust (silica), asbestos, toluene (organic solvents), oil mist and pesticides in RA, while additionally identified several common inhalable agents not investigated before in relation to RA, including detergents, carbon monoxide, pulp or paper dust, gasoline engine exhaust and welding fume. However, the effect of textile dust, reported as a risk factor in a Malaysian case–control study involving 910 female RA case and 910 age, sex matched controls,24 failed to be replicated in our Swedish population. Indeed, most individuals assessed as exposed to textile dust in our data worked as painting teachers, tailors and packers, in which both intensity and characteristics of textile exposure might differ from Malaysian textile workers.
Noteworthily, we observed a sex difference shown as exposure to occupational inhalable agents affect male patients with RA more severely than females. Indeed, men and women presented different exposure patterns.28 According to our data, men had an average longer duration of occupational exposure (11.6 vs 7.1 years, p<0.001) and were exposed to more agents considered hazardous. The top five exposures in men were detergents (percentage exposed: 33%), carbon monoxide (31%), stone and concrete (26%), iron (22%) and polycyclic aromatic hydrocarbons (17%), while the top exposures in women were detergents (51%), pulp or paper dust (5%) and carbon monoxide (4%). This underlying relationship was further confirmed in our exposure–response analysis showing that the risk of developing RA increased either with elongated exposure duration or with increased number of exposed agents. Taken together, our results corroborated with each other, emphasising occupational inhalable exposures as an important risk factor for RA development and reflecting a general effect of inhalants on RA pathogenesis as well as highlighting lung as an important site for triggering RA. Indeed, respiratory disorders, both acute and chronic, have been recognised as risk factors for RA, which might partially affect the link between occupational inhalable exposures and RA.29–32
Our study also reveals distinct inhalant–RA association patterns for ACPA-based subtypes, which extends previous epidemiological findings and further emphasise the effect of inhalable exposures possibly restricted in ACPA-positive RA.7 21 26 ACPA was found in the sputum of individuals who were seronegative but considered to be at risk for RA due to family history. In these individuals the ratio of autoantibody to total Ig was thus higher in sputum than in serum.33 Also in early ACPA-positive RA, levels of ACPA were higher in bronchoalveolar fluids than in serum.34 These evidence indicated a local production of RA-related autoantibodies, such as ACPA, in lungs and provided a possible explanation for the specific linkage of provocation by inhalable exposures to ACPA-positive RA. Typically, ACPA-positive RA has a worse prognosis with higher rates of erosive damage and is usually linked with more genetic and environmental risk factors as compared with ACPA-negative RA, which is believed to constitute a heterogeneous group of RA with so far unknown aetiologies.10 11 35 36
Calculation of additive interaction has been described as the most appropriate approach to identify ‘sufficient cause interactions’ and to inform on disease mechanism.37 38 We observed a significant interaction between exposure to asbestos, carbon monoxide, gasoline engine exhaust, and quartz dust and genetic predisposition for the risk of ACPA-positive RA. Notably though, high GRS combined with these agents contributed to a higher point-estimated risk of developing RA in the double-exposed subgroups than if combined with HLA-SE alleles. A possible explanation might be that the GRS which was constructed based on genome-wide genetic markers reflecting a larger part of RA heritability than HLA-SE and thereby has a better predictive ability. The higher-risk figures and interaction results for GRS as compared with HLA-SE may also indicate that GRS interacts with inhalable agents via molecular pathways not fully captured by investigations restricted to HLA-SE alleles.
Noteworthily, detergents (OR 1.27, 95% CI 1.13 to 1.42) and pulp or paper dust (OR 1.57, 95% CI 1.26 to 1.96), despite their strong primary associations with ACPA-positive RA, yielded APs close to 0 for the interaction with either high GRS (detergents: AP 0.00, 95% CI −0.17 to 0.17; pulp or paper dust: AP 0.02, 95% CI −0.33 to 0.36) or HLA-SE alleles (detergents: AP 0.07, 95% CI −0.10 to 0.25; pulp or paper dust: AP 0.06, 95% CI −0.3 to 0.42). These findings of different influences of gene–environment interactions between different inhaled agents imply that distinct pathogenetic pathways may be active after exposure to different inhaled occupational agents and that some of these pathways are different from those previously proposed for smoking.34 39
Despite the substantial advantages of our current study including its large sample size, multiple exposures, being able to account for personal smoking and genetic background as well as adjust for important confounders, we must acknowledge several limitations. First, information on occupation was retrospectively collected, which might introduce recall bias. We, however, expect such bias to be modest given our population-based design, recruitment of incident cases and the fact that occupational careers are important life events that are less likely to be neglected. Second, although JEM is a validated tool widely used to estimate job-exposure status in large-scale studies, it might lead to nondifferential misclassification, that is, participants who ever worked in any occupation assessed with a high probability of exposure (>50% in this study, not necessarily 100%) would be defined as exposed. However, such non-differential misclassification is expected to underestimate the associations meaning the true associations are even stronger. Third, because certain kinds of inhalable agents often coexist, it is difficult to identify the independent relationship for one of these agents with RA risk due to limited size of participants who were only exposed to one agent in this study. Finally, the sample size of ACPA-negative cases were relatively small, and further studies with larger samples are warranted to re-examine the potential associations between occupational inhalable agents and ACPA-negative RA.
To conclude, our study shows that inhalable, mainly occupational exposures act as important environmental risk factors in RA development, especially in ACPA-positive RA. The markedly increased risk for RA after exposure of smoking and occupational inhalable agents observed among individuals carrying genetic variants common in Swedish as well as in most Caucasian populations strongly suggests the implementation of broad preventive strategies such as quit smoking and mitigation of occupational hazards. Notably, it is likely that similar effects on major histocompatibility complex (MHC)–environmental interactions seen here for a Caucasian population may be present also in other, for example, Asian populations as MHC-class II smoking interactions and high risk for ACPA-positive RA has been described also for Asia.40 41 Emphasis on the assessment and implementation of preventive strategies is thus warranted in industries worldwide, something that may in the future also involve awareness of genetic vulnerability, for example, through family history or testing for genetic variants predisposing for RA. Overall, our data provide a novel and quite dramatic emphasis on the role of occupational exposures in the aetiology of seropositive RA, calling for extended measures to reduce these exposures as part of international collaborative efforts to reduce morbidities due to working life.
Footnotes
Handling editor: Josef S Smolen
LK, LA and XJ contributed equally.
Contributors: All authors have made substantial contributions to the conception and design of this study, analysis, interpretation of the results and drafting and revising the manuscript. XJ is the guarantor of the study.
Funding: The study was supported by the fundings from the Swedish Research Foundation for Health, Working Life and Welfare, the Swedish Research Council, the AFA foundation, Region Stockholm, King Gustaf V’s 80-year foundation, the Swedish Rheumatic Foundation. We would like to thank the participants in this study as well as the clinicians and nurses in the EIRA study group.
Competing interests: None declared.
Patient and public involvement: No patients or members of the public were directly involved in the design or conduct of this study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. All data and codes are available on request to the corresponding authors (xia.jiang@ki.se).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by ethical approval for EIRA granted by the Karolinska Institutet Ethics Committee, the Regional Stockholm Ethics Committee, and the Central Swedish Ethics Authority (Etikprövningnämnden). Participants gave informed consent to participate in the study before taking part.
References
- 1. Catrina AI, Svensson CI, Malmström V, et al. Mechanisms leading from systemic autoimmunity to joint-specific disease in rheumatoid arthritis. Nat Rev Rheumatol 2017;13:79–86. 10.1038/nrrheum.2016.200 [DOI] [PubMed] [Google Scholar]
- 2. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205–19. 10.1056/NEJMra1004965 [DOI] [PubMed] [Google Scholar]
- 3. Klareskog L, Padyukov L, Lorentzen J, et al. Mechanisms of disease: genetic susceptibility and environmental triggers in the development of rheumatoid arthritis. Nat Clin Pract Rheumatol 2006;2:425–33. 10.1038/ncprheum0249 [DOI] [PubMed] [Google Scholar]
- 4. Karlson EW, Chang S-C, Cui J, et al. Gene-environment interaction between HLA-DRB1 shared epitope and heavy cigarette smoking in predicting incident rheumatoid arthritis. Ann Rheum Dis 2010;69:54–60. 10.1136/ard.2008.102962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van der Helm-van Mil AHM, Verpoort KN, le Cessie S, et al. The HLA-DRB1 shared epitope alleles differ in the interaction with smoking and predisposition to antibodies to cyclic citrullinated peptide. Arthritis Rheum 2007;56:425–32. 10.1002/art.22373 [DOI] [PubMed] [Google Scholar]
- 6. Kim K, Jiang X, Cui J, et al. Interactions between amino acid-defined major histocompatibility complex class II variants and smoking in seropositive rheumatoid arthritis. Arthritis Rheumatol 2015;67:2611–23. 10.1002/art.39228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum 2006;54:38–46. 10.1002/art.21575 [DOI] [PubMed] [Google Scholar]
- 8. Holers VM, Demoruelle MK, Kuhn KA, et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat Rev Rheumatol 2018;14:542–57. 10.1038/s41584-018-0070-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McDermott GC, Doyle TJ, Sparks JA. Interstitial lung disease throughout the rheumatoid arthritis disease course. Curr Opin Rheumatol 2021;33:284–91. 10.1097/BOR.0000000000000787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verstappen M, van Steenbergen HW, de Jong PHP, et al. Unraveling heterogeneity within ACPA-negative rheumatoid arthritis: the subgroup of patients with a strong clinical and serological response to initiation of DMARD treatment favor disease resolution. Arthritis Res Ther 2022;24:4. 10.1186/s13075-021-02671-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han B, Diogo D, Eyre S, et al. Fine mapping seronegative and seropositive rheumatoid arthritis to shared and distinct HLA alleles by adjusting for the effects of heterogeneity. Am J Hum Genet 2014;94:522–32. 10.1016/j.ajhg.2014.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prisco LC, Martin LW, Sparks JA. Inhalants other than personal cigarette smoking and risk for developing rheumatoid arthritis. Curr Opin Rheumatol 2020;32:279–88. 10.1097/BOR.0000000000000705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adami G, Pontalti M, Cattani G, et al. Association between long-term exposure to air pollution and immune-mediated diseases: a population-based cohort study. RMD Open 2022;8:e002055. 10.1136/rmdopen-2021-002055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wiebert P, Lönn M, Fremling K, et al. Occupational exposure to particles and incidence of acute myocardial infarction and other ischaemic heart disease. Occup Environ Med 2012;69:651–7. 10.1136/oemed-2011-100285 [DOI] [PubMed] [Google Scholar]
- 15. Okada Y, Wu D, Trynka G, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 2014;506:376–81. 10.1038/nature12873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Privé F, Arbel J, Vilhjálmsson BJ. LDpred2: better, faster, stronger. Bioinformatics 2020;36:5424–31. 10.1093/bioinformatics/btaa1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raychaudhuri S, Sandor C, Stahl EA, et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat Genet 2012;44:291–6. 10.1038/ng.1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum 1987;30:1205–13. 10.1002/art.1780301102 [DOI] [PubMed] [Google Scholar]
- 19. Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol 1980;112:467–70. 10.1093/oxfordjournals.aje.a113015 [DOI] [PubMed] [Google Scholar]
- 20. Demoruelle MK, Deane KD, Holers VM. When and where does inflammation begin in rheumatoid arthritis? Curr Opin Rheumatol 2014;26:64–71. 10.1097/BOR.0000000000000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stolt P, Yahya A, Bengtsson C, et al. Silica exposure among male current smokers is associated with a high risk of developing ACPA-positive rheumatoid arthritis. Ann Rheum Dis 2010;69:1072–6. 10.1136/ard.2009.114694 [DOI] [PubMed] [Google Scholar]
- 22. Ilar A, Klareskog L, Saevarsdottir S, et al. Occupational exposure to asbestos and silica and risk of developing rheumatoid arthritis: findings from a Swedish population-based case-control study. RMD Open 2019;5:e000978. 10.1136/rmdopen-2019-000978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olsson AR, Skogh T, Axelson O, et al. Occupations and exposures in the work environment as determinants for rheumatoid arthritis. Occup Environ Med 2004;61:233–8. 10.1136/oem.2003.007971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Too CL, Muhamad NA, Ilar A, et al. Occupational exposure to textile dust increases the risk of rheumatoid arthritis: results from a Malaysian population-based case-control study. Ann Rheum Dis 2016;75:997–1002. 10.1136/annrheumdis-2015-208278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lundberg I, Alfredsson L, Plato N, et al. Occupation, occupational exposure to chemicals and rheumatological disease. A register based cohort study. Scand J Rheumatol 1994;23:305–10. 10.3109/03009749409099278 [DOI] [PubMed] [Google Scholar]
- 26. Sverdrup B, Källberg H, Bengtsson C, et al. Association between occupational exposure to mineral oil and rheumatoid arthritis: results from the Swedish EIRA case-control study. Arthritis Res Ther 2005;7:R1296–303. 10.1186/ar1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parks CG, Hoppin JA, De Roos AJ, et al. Rheumatoid arthritis in agricultural health study spouses: associations with pesticides and other farm exposures. Environ Health Perspect 2016;124:1728–34. 10.1289/EHP129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Biswas A, Harbin S, Irvin E, et al. Sex and gender differences in occupational hazard exposures: a scoping review of the recent literature. Curr Envir Health Rpt 2021;8:267–80. 10.1007/s40572-021-00330-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cullinan P, Muñoz X, Suojalehto H, et al. Occupational lung diseases: from old and novel exposures to effective preventive strategies. Lancet Respir Med 2017;5:445–55. 10.1016/S2213-2600(16)30424-6 [DOI] [PubMed] [Google Scholar]
- 30. Friedlander HM, Ford JA, Zaccardelli A, et al. Obstructive lung diseases and risk of rheumatoid arthritis. Expert Rev Clin Immunol 2020;16:37–50. 10.1080/1744666X.2019.1698293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kronzer VL, Westerlind H, Alfredsson L, et al. Respiratory diseases as risk factors for seropositive and seronegative rheumatoid arthritis and in relation to smoking. Arthritis Rheumatol 2021;73:61–8. 10.1002/art.41491 [DOI] [PubMed] [Google Scholar]
- 32. Ford JA, Liu X, Chu SH, et al. Asthma, chronic obstructive pulmonary disease, and subsequent risk for incident rheumatoid arthritis among women: a prospective cohort study. Arthritis Rheumatol 2020;72:704–13. 10.1002/art.41194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Willis VC, Demoruelle MK, Derber LA, et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum 2013;65:2545–54. 10.1002/art.38066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reynisdottir G, Karimi R, Joshua V, et al. Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheumatol 2014;66:31–9. 10.1002/art.38201 [DOI] [PubMed] [Google Scholar]
- 35. Willemze A, Trouw LA, Toes REM, et al. The influence of AcpA status and characteristics on the course of RA. Nat Rev Rheumatol 2012;8:144–52. 10.1038/nrrheum.2011.204 [DOI] [PubMed] [Google Scholar]
- 36. McHugh J. ACPA-negative RA divided into clinical subsets. Nat Rev Rheumatol 2019;15:384. 10.1038/s41584-019-0245-3 [DOI] [PubMed] [Google Scholar]
- 37. Andersson T, Alfredsson L, Källberg H, et al. Calculating measures of biological interaction. Eur J Epidemiol 2005;20:575–9. 10.1007/s10654-005-7835-x [DOI] [PubMed] [Google Scholar]
- 38. VanderWeele TJ. Sufficient cause interactions and statistical interactions. Epidemiology 2009;20:6–13. 10.1097/EDE.0b013e31818f69e7 [DOI] [PubMed] [Google Scholar]
- 39. Catrina AI, Ytterberg AJ, Reynisdottir G, et al. Lungs, joints and immunity against citrullinated proteins in rheumatoid arthritis. Nat Rev Rheumatol 2014;10:645–53. 10.1038/nrrheum.2014.115 [DOI] [PubMed] [Google Scholar]
- 40. Too CL, Yahya A, Murad S, et al. Smoking interacts with HLA-DRB1 shared epitope in the development of anti-citrullinated protein antibody-positive rheumatoid arthritis: results from the Malaysian epidemiological investigation of rheumatoid arthritis (MyEIRA). Arthritis Res Ther 2012;14:R89. 10.1186/ar3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Terao C, Ohmura K, Ikari K, et al. Effects of smoking and shared epitope on the production of anti-citrullinated peptide antibody in a Japanese adult population. Arthritis Care Res 2014;66:1818–27. 10.1002/acr.22385 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ard-2022-223134supp001.pdf (1MB, pdf)
Data Availability Statement
Data are available on reasonable request. All data and codes are available on request to the corresponding authors (xia.jiang@ki.se).