TO THE EDITOR:
End-stage hemophilic arthropathy usually warrants total joint arthroplasty, which is considered to be a major hemostatic challenge.1 Although gene transfer with adeno-associated virus (AAV) vector carrying a cassette coding for hyperactive mutant factor IX Padua (Arg338Leu) has shown repeated success in several clinical trials of gene therapy for patients with hemophilia B,2–4 the in vivo hemostatic effect of factor IX Padua has not been confirmed in the context of major hemostatic challenges.5 Here, we report on a patient with hemophilia B who received AAV-mediated factor IX–Padua gene transfer (BBM-H901) and subsequently underwent unilateral total knee arthroplasty without exogenous factor IX infusion during the perioperative period.
A 26-year-old man with severe hemophilia B received factor IX–Padua gene therapy with no vector-related adverse events during a 52-week follow-up interval.3 The transgene-derived factor IX:C level ranged from 51.4 to 85.0 IU per deciliter, measured by the one-stage method with the Dade Actin FSL activated partial-thromboplastin time (APTT) reagent on a Sysmex CS-5100 system (Siemens) (Fig. 1A). Although no bleeding events developed and there was no need for factor IX concentrate infusion after gene therapy, the hemophilic arthropathy worsened, especially in the right knee. The score on the Hemophilia Early Arthropathy Detection with Ultrasound in China scale (range, 0 to 78, with higher scores indicating worse joint health) increased from 35 to 43 at the 1-year visit (Table S1 in the Supplementary Appendix, available with full text of this letter at NEJM.org). Imaging of the right knee is shown in Figures S1 and S2.
Figure 1. Patient with Hemophilia B Who Underwent Total Knee Arthroplasty after Gene Therapy.
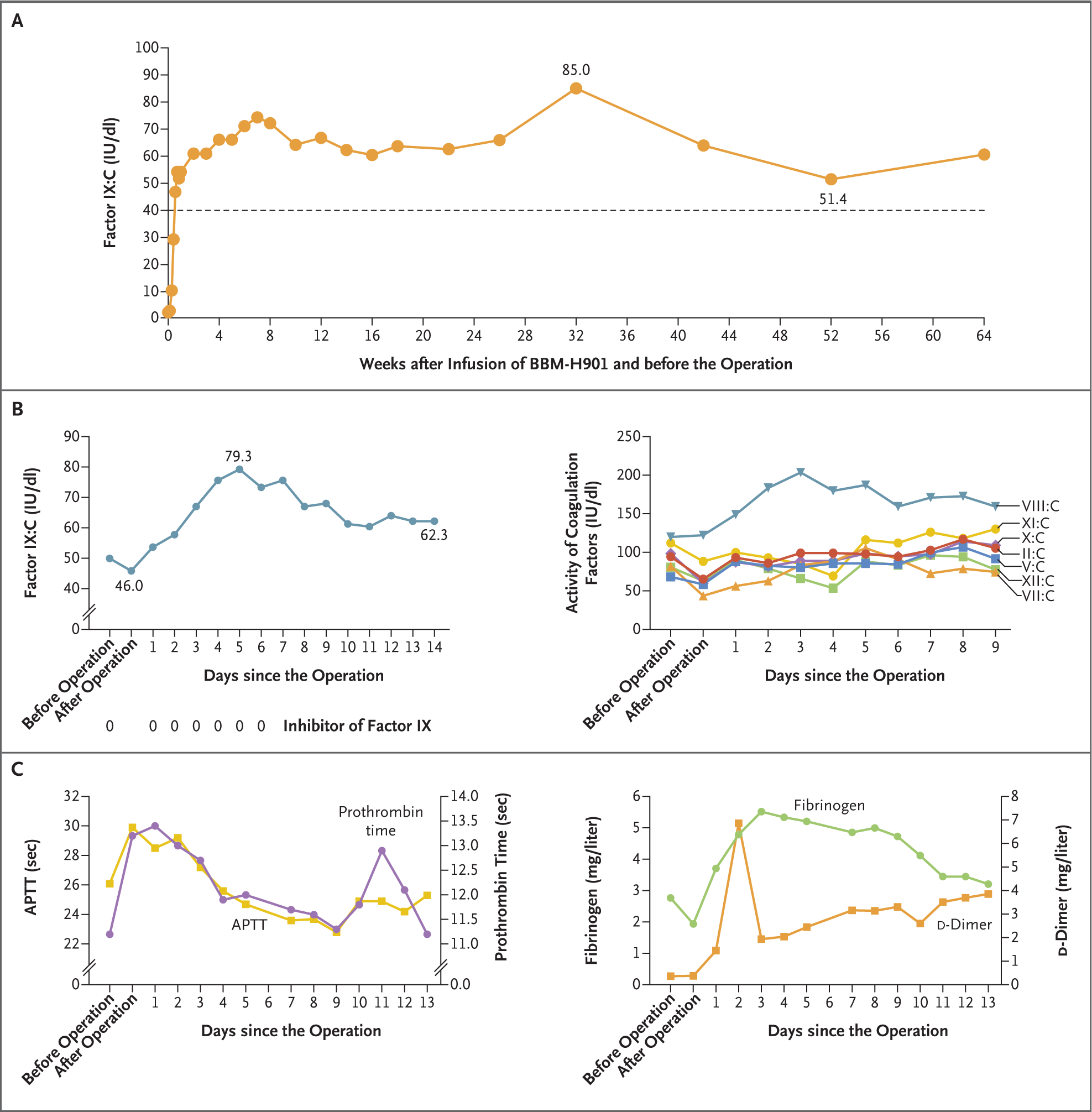
Panel A shows the factor IX:C level after the infusion of BBM-H901, a factor IX–Padua gene product, but before the operation. The activity level of the dotted line is 40 IU per deciliter. The left graph in Panel B shows the factor IX:C level measured by the one-stage method with the Dade Actin FSL activated partial-thromboplastin time (APTT) reagent and the detection of factor IX inhibitors during the perioperative period, and the right graph in Panel B shows the activity of other coagulation factors (factors II, V, VII, VIII, X, XI, and XII). The left graph in Panel C shows the changes in prothrombin time and APTT after the operation, and the right graph in Panel C shows the changes in fibrinogen and d-dimer levels.
The patient underwent a right total knee arthroplasty successfully on day 479 after vector infusion without factor IX concentrate infusion. Hemostasis was normal; intraoperative blood loss was estimated at 150 ml, similar to that in the general population undergoing the procedure. The surgical field showed no excess bleeding without a tourniquet. Postoperative radiographs are shown in Figure S4. Only physical thromboprophylaxis was used, and the patient underwent normal rehabilitation without the need for any factor IX concentrate infusion. The surgical wound healed well, and no infection occurred (Figs. S5 and S6).
The factor IX:C level was 50.1 IU per deciliter on the morning of the operation and decreased to 46.0 IU per deciliter after the operation on the same day. The excellent hemostatic effect suggests that endogenous factor IX–Padua activity correlates with in vivo hemostatic function. One day after the procedure, the factor IX:C level rose to 53.8 IU per deciliter. Changes in the factor IX:C level in the patient showed a strong sustained secretion capacity of transduced hepatocytes. An upward trend in the factor IX:C level was observed from day 1 to day 5 after the operation, and the peak factor IX:C level was 79.3 IU per deciliter. Then the factor IX:C level decreased gradually to a stable level of approximately 60 IU per deciliter (Fig. 1B). The factor IX:C level measured with another APTT reagent (Dade Actin) was a mean (±SD) of 1.58±0.13 times the value with the Dade Actin FSL reagent. Factor IX:C levels measured with the two reagents and thromboelastography results are shown in Table S2. After the operation, the other coagulation factors had an initial slight decrease in activity and then returned to baseline levels or increased (Fig. 1B). The same trend occurred in prothrombin time or APTT (Fig. 1C). Elevated fibrinogen and d-dimer levels were regarded by the investigators as part of the normal postoperative course (Fig. 1C). No factor IX inhibitor was detected by means of the Bethesda assay.
Overall, this case shows the in vivo hemostatic effect of factor IX Padua after gene therapy in a patient undergoing a procedure that is considered to be a major hemostatic challenge, and the effect correlates with the factor IX:C level determined with the one-stage method. Our case also supports that 50 IU per deciliter of factor IX:C, the lower limit of factor IX:C usually recommended for major surgery, is adequate to achieve good hemostasis during a major procedure. However, this is only a single case, and further study is needed to confirm the in vivo hemostatic effect of factor IX Padua and its correlation with factor IX:C levels.
Supplementary Material
Acknowledgments
Supported in part by grants from the National Key Research and Development Program of China (2019YFA0110802), the CAMS Innovation Fund for Medical Sciences (2021-I2M-003, 2021-I2M-1-073, and 2022-I2M-2-001), the Non-profit Central Research Institute Fund of the Chinese Academy of Medical Sciences (2020-PT310-011), and the Bayer Hemophilia Award Program (2021).
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Feng Xue, State Key Laboratory of Experimental Hematology, Tianjin, China
PanJing Wang, Peking Union Medical College, Beijing, China
Zhen Yuan, Shandong Provincial Qianfoshan Hospital, Jinan, China
Chao Shi, Shandong Provincial Qianfoshan Hospital, Jinan, China
Yunhai Fang, Shandong Blood Center, Jinan, China
Wei Liu, Blood Diseases Hospital, Tianjin, China
Yuhua Wang, Blood Diseases Hospital, Tianjin, China
Xiao Xiao, Belief BioMed, Shanghai, China
Renchi Yang, Blood Diseases Hospital, Tianjin, China
Lindsey A. George, University of Pennsylvania School of Medicine, Philadelphia, PA
Lei Zhang, State Key Laboratory of Experimental Hematology, Tianjin, China
References
- 1.Sackstein P, Cooper P, Kessler C. The role of total ankle replacement in patients with haemophilia and end-stage ankle arthropathy: a review. Haemophilia 2021;27:184–91. [DOI] [PubMed] [Google Scholar]
- 2.George LA, Sullivan SK, Giermasz A, et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N Engl J Med 2017;377:2215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xue F, Li H, Wu X, et al. Safety and activity of an engineered, liver-tropic adeno-associated virus vector expressing a hyperactive Padua factor IX administered with prophylactic glucocorticoids in patients with haemophilia B: a single-centre, single-arm, phase 1, pilot trial. Lancet Haematol 2022;9(7):e504–e513. [DOI] [PubMed] [Google Scholar]
- 4.Chowdary P, Shapiro S, Makris M, et al. Phase 1–2 trial of AAVS3 gene therapy in patients with hemophilia B. N Engl J Med 2022;387:237–47. [DOI] [PubMed] [Google Scholar]
- 5.George LA, Sullivan SK, High KA, Murphy JE, Smith L, Rupon J. Surgical experience with fidanacogene elaparvovec. Res Pract Thromb Haemost 2020;4:Suppl 1. abstract (https://abstracts.isth.org/abstract/surgical-experience-with-fidanacogene-elaparvovec/). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


