Abstract
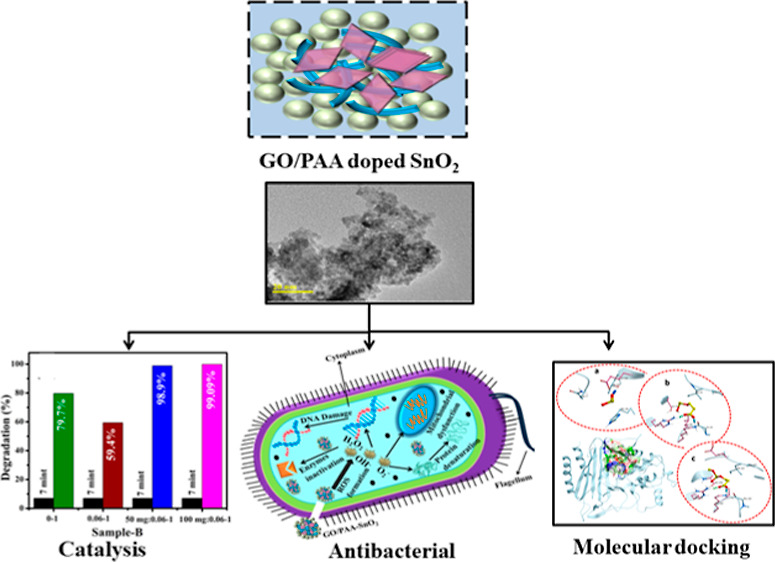
The present work demonstrates the systematic incorporation of different concentrations of graphene oxide (GO) into a fixed amount of polyacrylic acid (PAA)-doped SnO2 quantum dots (QDs) through a co-precipitation approach. The research aimed to evaluate the catalytic and antibacterial actions of GO/PAA-SnO2 QDs. Moreover, optical properties, surface morphologies, crystal structures, elemental compositions, and d-spacings of prepared QDs were examined. X-ray diffraction patterns revealed the tetragonal configuration of SnO2, and the crystallinity of QDs was suppressed upon dopants verified by the SAED patterns. Electronic spectra identified the blue shift by incorporating GO and PAA led to a reduction in band gap energy. Fourier transform infrared spectra showed the existence of rotational and vibrational modes associated with the functional groups during the synthesis process. A drastic increase in the catalytic efficacy of QDs was observed in the neutral medium by including dopants, indicating that GO/PAA-SnO2 is a promising catalyst. GO/PAA-SnO2 showed strong bactericidal efficacy against Escherichia coli (E. coli) at higher GO concentrations. Molecular docking studies predicted the given nanocomposites, i.e., SnO2, PAA-SnO2, and GO/PAA-SnO2, as potential inhibitors of beta-lactamaseE. coli and DNA gyraseE. coli.
1. Introduction
Urbanization and fast industrialization are responsible for the discharge of many toxic insoluble organic and inorganic impurities (heavy metals and dyes etc.), destroying aquatic life, animals, and plants.1,2 According to the World Health Organization (WHO), ∼2.3 million people die annually from polluted water that causes diseases like typhoid, diarrhea, cholera, hepatitis, and cancer.3,4 Per annum, 1/10th million different dyes are employed, 10–15% of which are discharged directly and indirectly into the environment, causing havoc to life.4,5 Fabric and paper industries apply different dyes; most of the dyes are aromatic compounds,6 and two major cationic dyes are methylene blue (MB)7 and rhodamine blue (RhB).8 Herein, MB is adopted and explored because of its toxicity, extensive usage, and high commitment to biotic degradation.9
To remove impurities from water, different methods like ozonation,10 membrane filtration,11 adsorption,12 catalytic reduction, and so forth13,14 have been used. But catalytic reduction has a great impact showing good catalytic activity, affordability, and high thermal stability.15−17 Nanomaterials possess fast adsorption, catalytic activity, and solubility attributed to the large surface area and small size. Different nanomaterials have been used to remove organic pollutants, inorganic anions, and various types of bacteria from water.18 Using water-containing bacteria causes diseases, such as eye infection, respiratory tract irritation, skin irritation, and mastitis.19 Mastitis has a significant economic impact on dairy products. Different factors, including host, environment, seasons, and specific agent parasites, such as Staphylococcus Aureus (S. aureus) and Escherichia Coli (E. coli) are responsible for it.20,21
Metal–organic frameworks (MOFs) and covalent organic frameworks have been considered as two types of organic materials, in the last few decades, which can be easily made and designed due to their properties.22 In recent years, researchers have found great interest in semiconductor nanomaterials because of their remarkable physical and chemical properties, high surface area, and less toxicity. MOFs or porous coordination polymers are crystalline porous materials composed of inorganic metal nodes linked by organic linkers via coordination bonds.23,24 Different types of semiconductor nanomaterials, such as Li2O3, SnO2, ZnO, MnO4, CdS, and so forth, are applicable; SnO2 has attracted great attention for purifying drain water. SnO2 is an n-type semiconductor with a wide band gap (Eg = 3.6 eV)25 and has remarkable properties, such as electrical conductivity rechargeability, high transmittance in the ultraviolet (UV)–visible (vis) region, low toxicity, and negligible biological effects. Various methods for degrading dyes have been adopted for synthesizing metal oxides, including hydrothermal, co-precipitation, sol–gel, and so forth.26 From these methods, the co-precipitation technique is used as it is eco-friendly, inexpensive, energy-efficient, and easy to use with a short preparation time.27
However, SnO2 has the limitation of a high recombination rate. To overcome this problem, different polymers like polyvinyl alcohol (PVA), polyvinylpyrrolidone (PVP), and polyacrylic acid (PAA) are used, which have great influence as dopants because of their low-cost and easy processing.28 Among these, PAA was used as it has the potential to remove dyes and metal ions from polluted water and overcome antibacterial activities due to the carboxylic group (COOH).29
Carbon-based materials like amorphous carbon, graphitized carbon, nanotubes, and graphene are extensively used as matrix materials with the advantages of flexibility, chemical stability, and structural heterogeneity.30 Graphene oxide (GO) is a 2D aromatic compound with a hexagonal lattice of sp2 carbon atoms with a long-range π-conjugation bond and a monolayer structure with oxygen functional groups, bearing on the basal planes and edges of GO sheets. GO is introduced to increase the concentration of charge carriers in SnO2 by increasing conductivity31 and a larger surface area. This work synthesized pristine SnO2 and doped it with different concentrations of GO and a fixed amount of PAA via co-precipitation methodology. The catalytic efficiency of the prepared samples was examined to remove the synthetic methylene blue (MB) dye, and bactericidal activity was investigated against E. coli by the agar well diffusion method. Molecular docking predictions were performed against enzymes selected from the cell wall and nucleic acid synthesis pathways, i.e., beta-lactamaseE. coli and DNA gyraseE. coli, respectively.
2. Experimental Work
2.1. Chemicals
Tin chloride dihydrate (SnCl2·2H2O, 99%), NaOH (99%), poly[acrylic acid (C3H4O2)]n, graphite powder (99.5%), sodium nitrate (NaNO3), potassium permanganate (KMnO4, 99.5%), hydrogen peroxide (H2O2), and 2, 2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) were purchased from Sigma-Aldrich (Germany). Sulfuric acid (H2SO4) and hydrochloric acid (HCl) were procured from Analar.
2.2. Synthesis of Graphene Oxide
For the synthesis of GO, a modified Hummer’s route was adopted.32 First, 2 g of graphite powder and 1 g of NaNO3 were dissolved in 46 mL of concentrated H2SO4 in an ice bath and stirred for 10 min. KMnO4 was introduced at >5 °C, turning the solution color from purple to yellow at magnetic stirring on an ice bath for 24 h. Further, 12 mL H2O2 was incorporated, and the solution was centrifuged at 7000 rpm for 7 min and washed with HCl and deionized (DI) water to obtain a residue. The accumulation was heated for 12 h to receive a powder that was converted into a fine powder by grinding, as shown in Figure 1a.
Figure 1.
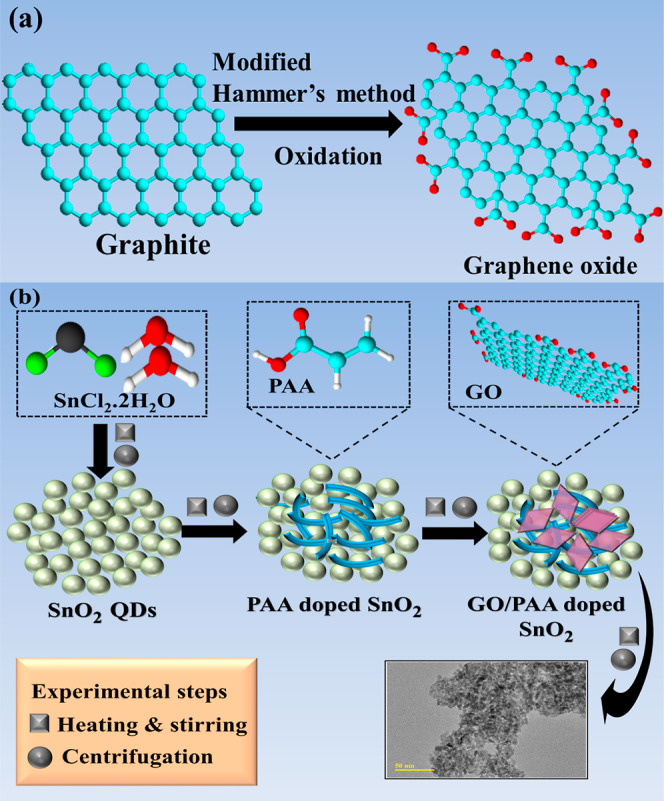
(a) Schematic illustration of GO and (b) synthesis of pristine and doped SnO2.
2.3. Synthesis of GO/PAA-Doped SnO2
To synthesize SnO2 quantum dots (QDs), 0.5 M of SnCl2·2H2O was prepared under constant magnetic stirring at 80 °C by the co-precipitation method. 1 M solution of precipitating agent NaOH was added in the above solution drop-by-drop to retain pH ∼10, and the precipitate formed. Subsequently, the colloidal solution was centrifuged at 7000 rpm repeatedly for 8 min to collect the precipitates. The sediments were heated overnight at 90 °C to obtain nanopowders and later crushed into a fine powder. Similarly, various concentrations of GO (50 and 100 mg) and a fixed amount of PAA (6 wt %)-doped SnO2 QDs were prepared, as demonstrated in Figure 1b.
2.4. Isolation and Identification of E. coli
2.4.1. Sample Collection
Samples from the selected breastfeeding cows are sucked up directly into sterile glassware and supplied at various markets, veterinary centers, and farms in Punjab, Pakistan. After being collected at 4 °C, raw milk was immediately brought to the lab. On MacConkey agar, pathogens in raw milk were collected. For 48 h, all plates were cultured at 37 °C.
2.4.2. Identification and Characterization of Bacterial Isolates
According to Bergey’s Manual of Determinative Bacteriology, the primary detection of E. coli is based on the Gram stain of colonial morphology and different biochemical tests.33
2.4.3. Antibiotic Susceptibility
Mueller Hinton agar (MHA) utilized for the disc diffusion method of Bauer et al.(34) to perform the antimicrobial sensitivity test. The test was performed to find out whether E. coli was resistant to the following antibiotics (classes): Ceftriaxone (Cro) 30 μg (Cephalosporins), Gentamicin (Gm) 10 μg (Aminoglycosides), Ciprofloxacin (Cip) 5 μg (Quinolones), Tetracycline (Te) 30 μg (Tetracyclines), Imipenem (Imi) 10 μg (Carbapenem), Amoxycillin (A) 30 μg (Penicillins), and Azithromycin (Azm) 15 μg (Macrolides).35 From these antibiotics, Ciprofloxacin (Cip) 5 μg (Quinolones) was adopted for the antibacterial activity of synthesized GO/PAA-SnO2. E. coli-purified cultures developed and balanced 0.5 MacFarland turbidity. After that, it was spread-plated on Muller Hinton Agar (MHA) (Oxoid Limited, Basingstoke, UK). The antibiotic discs were kept from the infected plate to prevent the intersection of inhibition zones. The results were interpreted following the Clinical and Laboratory Standard Institute36 when plates were incubated at 37 °C for 24 h. A bacterium is an MDR if it is robust to at least three antibiotics.37
2.4.4. Antimicrobial Activity
The antimicrobial sensitivity of pure and doped SnO2 nanoparticles was analyzed by the agar well diffusion method for 10 characteristic isolates of E. coli taken from mastitic milk. E. coli at 1.5 108 CFU/mL (0.5 McFarland standard) was swabbed onto MacConkey agar in Petri plates. Employing a sterile cork borer, 6 mm diameter wells were created. Various concentrations of GO/PAA-SnO2 incorporated with the prepared well as maximum and minimum doses. A positive control of ciprofloxacin and a negative control of DIW were utilized.38
2.4.5. Statistical Analysis
The inhibition region diameters were statistically evaluated using one-way analysis of variance (ANOVA) in SPSS 20 to access the antibacterial activity measured for the inhibition zone size (mm).39
2.5. Molecular Docking Studies
The role of molecular docking studies in revealing mysteries behind numerous biological activities has gained considerable attention over the last few decades. Using computational tools to predict the possible part of a given molecule has made novel drug discovery more feasible and cost effective. Inhibitors of enzymes essential for bacterial growth lead to the discovery of new drug candidates, specifically those belonging to cell wall synthesis and nucleic acid syntheses like beta-lactamase and DNA gyrase.40−42 Here, we evaluated the binding capacity of the given nanocomposites, i.e., SnO2, PAA-SnO2, and GO/PAA-SnO2, against these enzyme targets. Molecular docking calculations were conducted using ICM Molsoft software.39 3D structural coordinates of enzymes selected as possible targets were obtained from the protein data bank. The PDB IDs used for beta-lactamase and DNA gyrase were 4KZ9 (Res: 1.7 Å)43 and 5MMN (Res: 1.9 Å),44 respectively. The energy minimization of enzyme structures was done using the MMFF94x force field and gradient: 0.05 while the binding pocket was specified (within 5 Å of native ligand) to obtain a detailed view of the interaction pattern of the given nanocomposites inside the active pocket.
The standard protocol of ICM Molsoft was used for protein structure preparation involving the elimination of water molecules and native ligands, accumulation of gastegier charges, and polar H-atoms. The best-docked complexes were generated in each case for an in-depth analysis (i.e. 10 top-ranked docked conformations). Finally, the 3D-viewer of ICM Molsoft was used for the graphical representation of interaction patterns. The ligand structures were prepared using the Chemdraw and ligedit tool of ICM and the most stable conformation was produced for every ligand using the conformational analysis tool.
2.6. Catalytic Activity
The catalytic activity (CA) of GO/PAA-SnO2 was determined in the presence of NaBH4 to decolorize the MB. First, 3 mL aqueous solution of MB was mixed with a synthesized (400 μL) solution of NaBH4 and after regular intervals, the absorption rate was calculated. Subsequently, 400 μL of pure and GOPAA-SnO2 was added as a catalyst under vigorous stirring, and the decolorization of dye occurred as a result of the redox reaction. By a UV–vis spectrophotometer, degradation efficiency was examined, and maximum wavelength (λmax) for MB was achieved at 665 nm for all samples. The CA was calculated by using the % degradation formula given as
where Co represents the initial value of dye and Ct shows the time-dependent concentration.
2.6.1. Catalytic Mechanism
NaBH4 acts as a reducing agent for the catalytic reduction and donates an electron to the redox reduction. MB is a positive dye that receives an electron as an oxidizing agent. Redox reaction occurs during CA when an electron transfers from a reducing agent to an oxidizing agent. The electron is absorbed by MB and causes the degradation of synthetic dye. Eventually, MB dye was evaluated in the presence of NaBH4, which was time consuming and slow. To solve this issue, the addition of the nanocatalyst (GO/PAA-SnO2) into the oxidation–reduction reaction provides electrons and transferred electrons from BH–4 to MB dye and as a result, MB was reduced into LMB (Figure 2).
Figure 2.
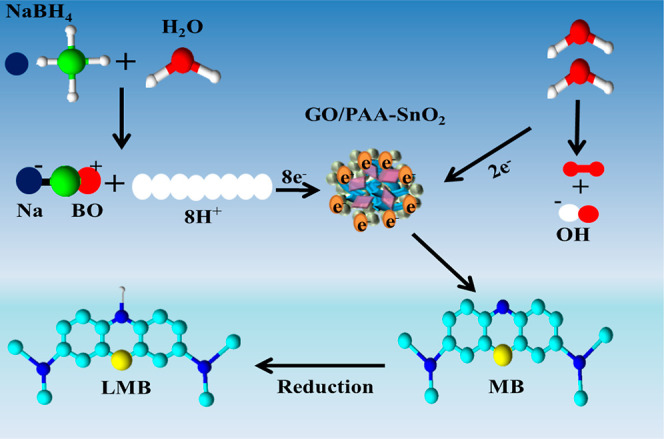
Schematic illustration of catalysis of doped SnO2.
2.7. Radical Scavenging Assay
To analyze free radical active species and anti-oxidant activity of nanocomposites, a modified version of the DPPH scavenging experiment was adopted. GO/PAA-doped SnO2 nanoparticles (50–250 μg/mL) were mixed with an equal volume of (0.1 mM) DPPH solution. This mixture was vortexed and incubated for 30 min at room temperature in the dark. A standard solution of ascorbic acid was employed as a reference sample. The degradation of DPPH solution (λ = 517 nm) was employed to calculate the scavenging rate (%) of each sample by eq 1
| 1 |
A0 and A1 = control absorbance and standard absorbance.
3. Results and Discussion
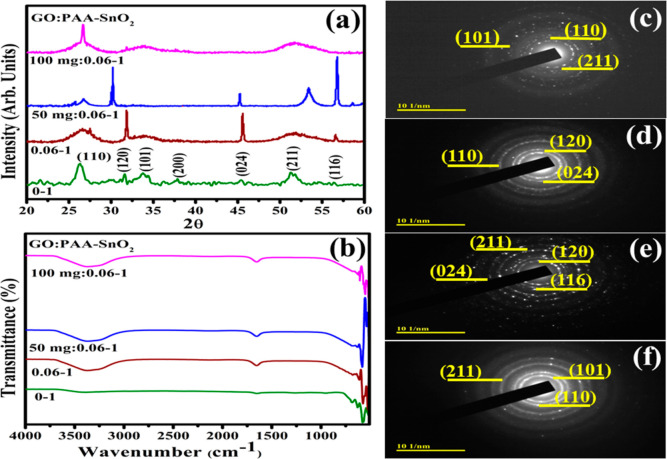
X-ray diffraction (XRD) analysis was employed in the 2θ range from 20 to 60° to investigate the crystal structure, phase constitution, and crystallinity of pristine and GO/PAA-SnO2, as illustrated in Figure 3a. The observed diffraction peaks sited at 26.4° (110), 33.8° (101), 37.7° (200), 51.36° (211), and 56.4° (116) were attributed to the tetragonal structure of SnO2 (JCPDS card 00-041-1445) along with the P42/mm space group.45 Additional peaks detected at 30.1° (020) and 45.4° (024) correspond to the anorthic structure of SnO (JCPDS no. 01-085-0423).46 The broadness of peaks observed upon the incorporation of PAA might be associated with its amorphous nature.26 It is noteworthy that peak intensity increased with a low concentration of GO to PAA/SnO2 relative to a higher concentration of GO attributed to the anchoring of QDs on GO sheets.47 The shifting of peaks toward lower angles upon doping represented the incorporation of functionalized GO on SnO2 QDs.48 The trend was reversed upon the higher concentration of GO due to the formation of oxygen-containing functional groups, including hydroxyl, epoxy, and carboxyl, that might have enhanced the interlayer distance.49
Figure 3.
XRD patterns (a), Fourier transform infrared (FTIR) spectra (b), and SAED patterns (c–f) of SnO2 and GO/PAA-SnO2.
To elucidate the existence of a functional group present in the product as pristine and doped SnO2, the Fourier transform infrared (FTIR) technique was employed, as elaborated in Figure 3b. Transmittance bands at 3662 and 1650 cm–1 can be attributed to O–H vibrations.50 The band at 625 cm–1 is due to SnO2 framework vibrations in the doped SnO2,51 and peaks at 681 and 572 cm–1 are assigned to Sn–O–Sn stretching and bending modes of SnO2, respectively.52,53 Upon the addition of PAA, the bands shifted toward a higher wave number of ∼3700–3000 cm–1, ascribed to the occurrence of absorbed water molecule and O–H group.53 There was no notable shift in spectra by the incorporation of GO. Selected area electron diffraction (SAED) analysis indicates the bright circular rings of pristine and GO/PAA-SnO2 exhibited the high crystalline nature of samples. The corresponding planes (110), (211), (101), and (120) are well correlated with XRD results (Figure 3c–f).
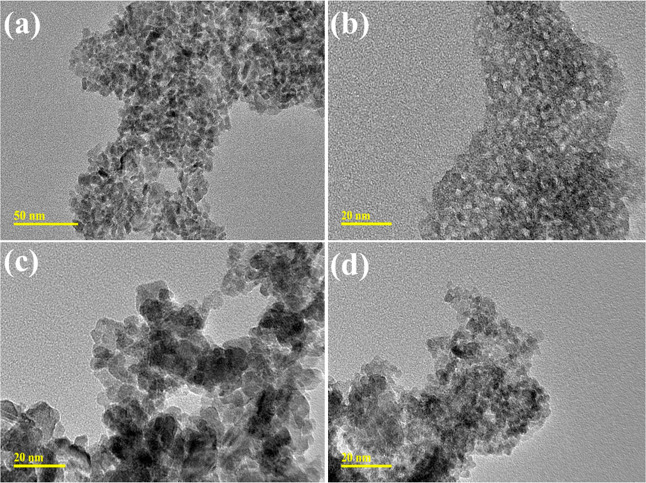
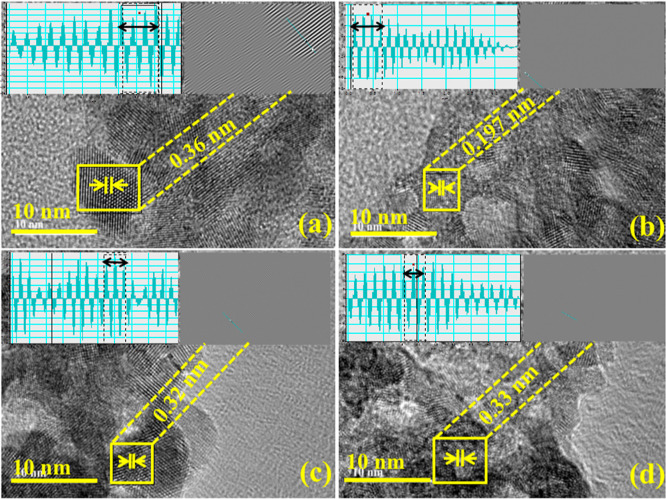
The morphologies of SnO2 and doped SnO2 were examined by transmission electron microscopy (TEM) analysis (Figure 4a–d). TEM micrographs confirmed the formation of agglomerated QDs of SnO2;54 this agglomeration could be due to hydrogen bonding of DI water during the synthesizing process (Figure 4a). Upon doping of the capping agent (PAA), a higher degree of agglomerations was observed with a reduction in the particle size confirmed through image J software (Figure 4b). The incorporation of GO (50 mg) into PAA/SnO2 showed overlapping of GO nanosheets around QDs (Figure 4c) and the same trend was noted with a higher concentration of GO (100 mg), as illustrated in Figure 5d. The particle sizes of 8 and 6 nm were calculated through image J software of SnO2 and PAA-SnO2, respectively (Figure 5c,d). The measured d-spacing values of SnO2 and doped SnO2 were 0.27, 0.17, 0.19, and 0.26 nm, which are well harmonized with XRD analysis (Figure 5a–d).
Figure 4.
(a–d) Transmission electron microscopy (TEM) morphology of synthesized SnO2 and GO/PAA-SnO2.
Figure 5.
(a–d): d-spacing of SnO2 and PAA-SnO2, 50 and 100 mg GO/PAA-SnO2, respectively.
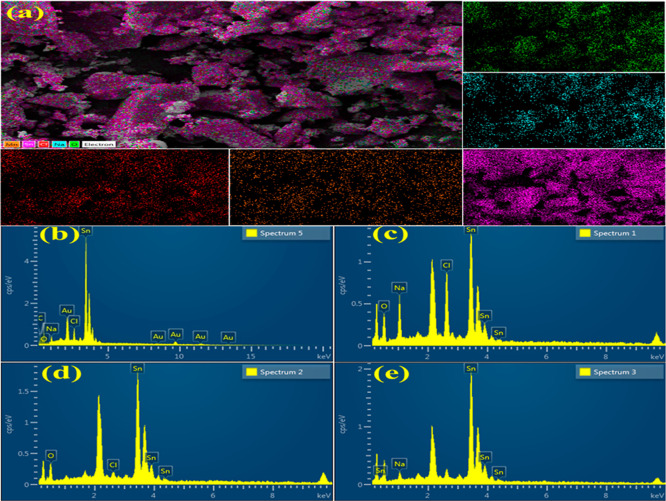
Energy-dispersive spectroscopy (EDS) analysis was performed to identify the elemental composition of SnO2 and doped SnO2 (Figure 6b–e). Sn and O peaks were observed that confirm the formation of SnO2. The Na and Cl peaks were detected due to precipitating agent NaOH during the synthesis of samples for pH control. The Au peak originated during the coating sputtered on the samples to lessen the charging effect. The elemental mapping of the prepared samples (Figure 6a) confirms the presence of Sn and O.
Figure 6.
(a) Mapping of all components distribution, EDS image of SnO2 (b), PAA-SnO2 (c), 50 mg GO/PAA-SnO2 (d), and 100 mg GO/PAA-SnO2 (e).
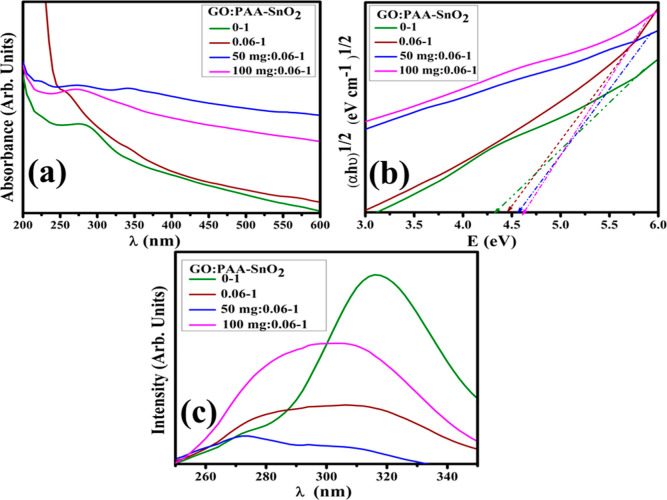
To examine the optical properties of synthesized QDs, electronic spectroscopy was utilized in a wavelength range of 200–600 nm (Figure 7a). Spectra revealed a strong absorption peak at 284 nm for SnO255 corresponding to the π–π*electronic transition.56 Tauc’s equation determined the band gap energy (Eg), and indirect Eg was calculated at 4.4 eV for SnO2 (Figure 7b). After doping, Eg increased from 4.4 to 4.75 eV, indicating the functionalization of GO nanosheets on smaller QDs.57 This increase in Eg upon doping confirmed the reduction in the crystallite size.
Figure 7.
(a) Absorption spectra, (b) band gap energy plot, and (c) photoluminescence spectra.
PL spectroscopy was employed to determine the electron–hole recombination rate and charge-transfer capability of bare and doped SnO2 (Figure 7c). The emission peak of SnO2 was noted at 314 nm,58 which is attributed to fluorescence phenomena. Peak intensity was reduced by adding PAA to SnO2, indicating a low recombination rate and introducing a blue shift. The PL intensity of PAA-SnO2 is significantly lower than that of SnO2 QDs, indicating that charge transfer occurs efficiently in the PAA-SnO2. The PL results showed that SnO2 dispersed uniformly and that PAA conjugation effectively suppressed electron–hole pair recombination in the prepared sample.59 The addition of GO to PAA/SnO2 decreased the peak intensity gradually, with higher concentrations ascribed to phosphorescence phenomena.60
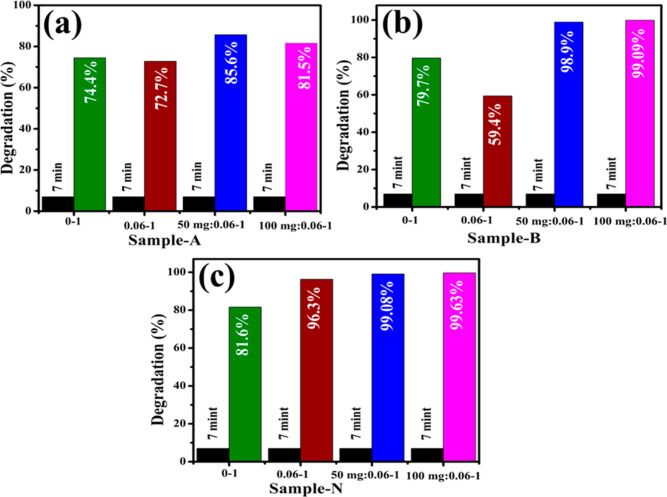
A UV–vis spectrophotometer investigated the CA of control and GO/PAA-SnO2. Degradation efficiency for MB was calculated in the presence of reducing agent NaBH4 and prepared samples that acted as a catalyst in a redox reaction. An electrochemical mechanism can explain the catalytic process, in which GO/PAA-SnO2 acts as an electron relay for the oxidant and reductant and electron transfer occurs via the metal nanoparticles.61 Catalytic activity is dependent on the catalyst concentration because it enhances dye degradation by lowering the activation energy of a chemical reaction. In general, larger surface area catalysts have demonstrated a higher catalytic performance because they contain more active sites and the pH of the solution has a significant impact on the degradation efficiency.54 Due to the possibility of a large redox potential difference between the electron donors and acceptor species, which can inhibit electron transfer, the electron-transfer step plays a dynamic role in dye degradation.62 The bond dissociation energy is important during chemical reactions as it breaks and/or generates new bonds. Electron transfer occurs during the reaction in which NaBH4 acts as a donor and the dye as an acceptor.63 The addition of SnO2 nanocatalysts to the reaction mixture acted as a potential intermediate between MB dye and BH4 ions. Dye degradation for 7 min was measured to be 74.4, 72.7, 85.6, and 81.5% in an acidic medium; 79.9, 59.4, 98.9, and 99.09% in a basic medium; and 81.6, 96.3, 99.0, and 99.63% in a neutral medium (Figure 8a–c). The absorption spectra of GO/PAA-SnO2 in the neutral medium have been provided in the supplementary file (Figure S1). The highest catalytic activity was exhibited by 100 mg (GO) doped PAA/SnO2 in basic and neutral media, while in an acidic medium, 50 mg (GO) showed the maximum effect of degradation. Incorporation of PAA provides carboxylic groups, and more carboxyl groups can provide more active absorption sites to remove the dyes.64 CA performance is substantially affected by the crystallite size, surface area, and shape of nanocatalysts and combination of 2D materials and quantum dots. This elevated efficiency was attributed to more active sites offered by the large surface area of the nanocatalyst. Moreover, a tiny variation between neutral and basic media is ascribed to the enhanced electrostatic attraction between MB+, a positively charged dye, and the negatively charged catalyst. The nanocatalyst surface in the basic medium tends to acquire a negative charge, while the absorption of cationic adsorbate species in acidic media is hindered by the positively charged surfaces of catalysts.65 The charge on the catalyst surface developed gradually became negative as pH was raised, boosting the adsorption ability of cationic dyes on SnO2 and GO/PAA-SnO2 nanocatalysts.
Figure 8.
Catalytic dye degradation (%) GO/PAA-SnO2 in (a) acidic, (b) basic, and (c) neutral media.
The bactericidal potential of pure and doped SnO2 was evaluated through an agar well diffusion approach. E. coli bacteria extracted from caprine or bovine mastitic milk. For synthesized samples, inhibition regions of E. coli (Gram −ve bacteria) were revealed to be 0.35–2.95 mm and 1.75–3.45 mm for the corresponding lower (0.5 mg/50 μL) and higher (1.0 mg/50 μL) doses. Positive control ciprofloxacin indicated 5.35 mm inhibitory domains against E. coli compared to the negative control DI water 0 mm, as listed in Table 1.
Table 1. Measurement of Inhibition Zone (mm).
| E. coli inhibition zone (mm) | ||
|---|---|---|
| GO/PAA-SnO2 | 0.5 mg/50 μL | 1.0 mg/50 μL |
| 0–1 | 0.35 | 1.75 |
| 0.06–1 | 1.90 | 2.75 |
| 50 mg: 0.06–1 | 2.45 | 3.10 |
| 100 mg: 0.06–1 | 2.95 | 3.45 |
| ciprofloxacin | 5.35 | 5.35 |
| DI water | 0 | 0 |
The antibacterial activity has an inverse relation with the size of the material. Small-sized NPs produced reactive oxygen species (ROS) that are effectively influenced by Gram-negative bacteria with an extra outer membrane. Nanomaterials destroy the micro-organisms by attacking the cell wall/membrane, destroy DNA to stop the production of cells, and produce ROS, such as hydrogen peroxide (H2O2), radicals (OH–), and anions (O2–), which ultimately results in cell death. Electrostatic interface, membrane deformation, cytoplasmic material leakage, DNA breakage, and protein denaturation induced by the positively charged NP surface acting as an antibacterial agent toward the highly reactive negatively charged bacterial cell membrane are66 shown in Figure 9.
Figure 9.
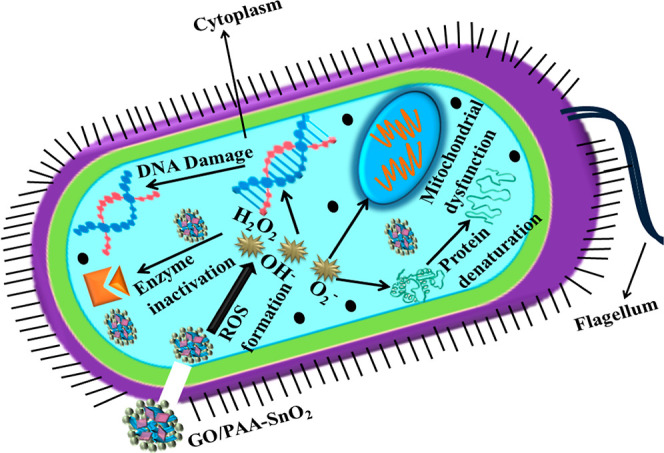
Bactericidal mechanism of prepared samples.
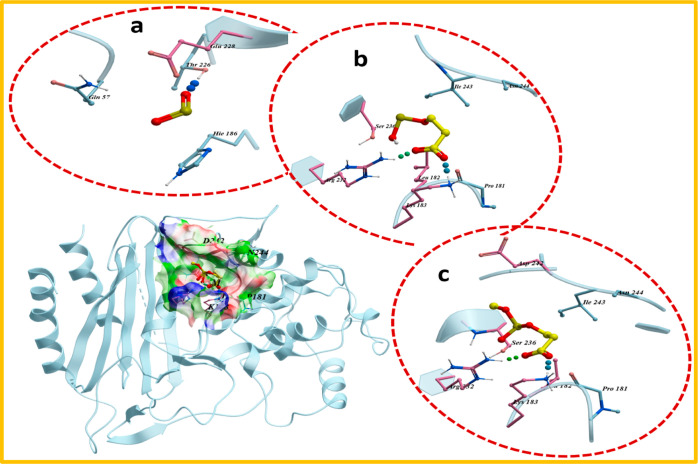
Molecular docking studies of synthesized nanomaterials revealed their possible interactions with active site residues of given enzyme targets. These nanocomposites demonstrated moderate binding energies against beta-lactamaseE. coli showing their critical interaction with key amino acids. Docked complexes obtained for PAA-SnO2 and GO/PAA-SnO2 showed H-bonds with Arg232 (2.8 Å; 2.5 Å) and Lys183 (2.1 Å; 2.0 Å) having binding scores −6.928 and −7.223 kcal/mol, respectively, as depicted in Figure 10b,c. Similarly, pristine SnO2 also formed a stable docked complex (binding score −4.675 kcal/mol) with beta-lactamaseE. coli showing H-bond interactions with Thr226 (2.2 Å), suggesting its possible role as an inhibitor for beta-lactamase (Figure 10a).
Figure 10.
3D view of binding interaction of nanocomposites within active sites of beta-lactamaseE. coli (a) SnO2, (b) PAA-SnO2, and (c) GO/PAA-SnO2.
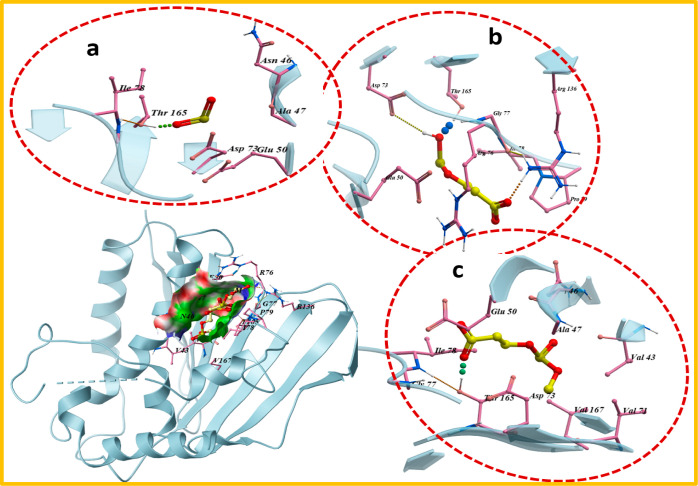
In the case of DNA gyraseE. coli, almost similar binding trends were shown by synthesized nanocomposites where pristine SnO2 showed single H-bonds (2.1 Å) with Thr165 amino acid of active pocket alongside metal-contact interactions with Asp73 with an overall binding score of −5.771 kcal/mol, as shown in Figure 11a. On the other hand, PAA-SnO2 showed a more stable docked complex having three H-bonds within the active site, i.e., Asp73 (2.9 Å), Arg136 (2.4 Å), and Thr165 (2.0 Å) with a binding score of −8.552 kcal/mol. Similarly, the GO/PAA-SnO2 docked complex involved two amino acid residues of active pocket, i.e., Thr165 (H-bond: 2.2 Å) and Asp73 (metal-contact interaction) having a binding score of −4.461 kcal/mol, as depicted in Figure 11b,c.
Figure 11.
3D view of binding interaction of nanocomposites within the active site of DNA gyraseE. coli (a) SnO2, (b) PAA-SnO2, and (c) GO/PAA-SnO2.
In silico molecular docking studies are comparable to in vitro bactericidal activity against E. coli and suggested pristine SnO2 and its composites with PAA and GO/PAA as potential inhibitors of beta-lactamase and DNA gyrase that needs to be explored further.
DPPH scavenging assay was developed to investigate and quantify anti-oxidant effects of active radical species (Figure 12). Antioxidant properties of compounds are inter-related with their tendency to transfer hydrogen or electron atoms to DPPH free radicals, resulting in stable diamagnetic compounds. All prepared samples displayed a dose-dependent anti-oxidant potential. GO/PAA-SnO2 (100 mg: 0.06–1) exhibited highest scavenging performance of up to 53.45% at a 250 μg/mL concentration and scavenged DPPH radicals. The formation of highly reactive •OH and •O2 radical species can interact with DPPH free radicals and results in its degradation,67 which is highly correlated with the standard (ascorbic acid).
Figure 12.
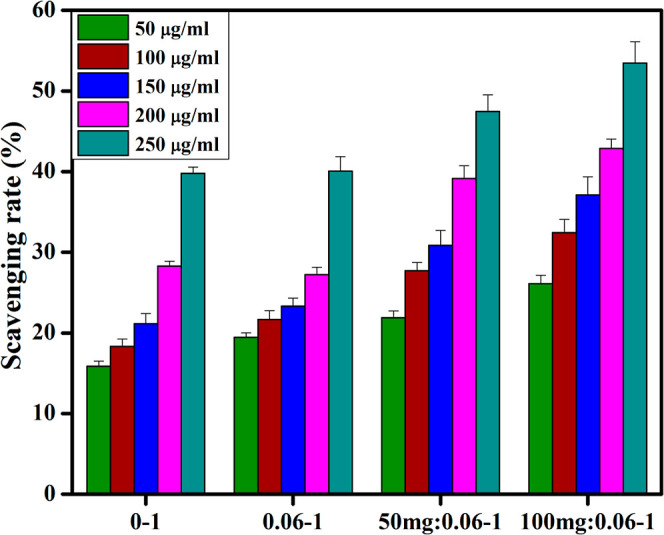
Scavenging potential of synthesized nanocomposites.
In order to facilitate electron transport, bacteria used a class of chemicals called redox mediators. Similarly to oxidation–reduction reactions, degradation of dyes proceeds. The greatest deterioration was seen when a redox mediator was used.68 The rate-limiting phase in the anaerobic dye reduction process has been proven to involve the transfer of reducing equivalents from the main electron donor (like co-substrate) to the terminal electron acceptor.69 In order to hasten the electron-transfer phase while decreasing steric hindrance from dye molecules, a little amount of redox mediator supplementation is necessary.19,70
4. Conclusions
In this study, we successfully synthesized the QDs of SnO2 doped with varying GO concentrations (50 and 100 mg) with a fixed amount of PAA (6 wt %) for bactericidal and catalytic efficiencies via the co-precipitation technique. XRD analysis confirmed the tetragonal structure of SnO2. The decrease in absorption was observed upon doping accompanied by a blue shift, and band gap energy was increased from 4.3 to 4.75 nm. The formation of SnO2 QDs was confirmed by HR-TEM analysis. The FTIR peak at 6250 cm–1 confirmed the presence of SnO2 through Sn–O stretching vibrations. The pristine and co-doped SnO2 showed excellent catalytic activity against MB dyes in all media (81.5, 99.09, and 99.63%). Pure SnO2 and GO/PAA-SnO2 demonstrated good catalytic and antibacterial potency for removing dyes and pathogens from wastewater, respectively. In silico predictions agreed with antibacterial activities against E. coli and suggested the given nanocomposites as possible inhibitors of beta-lactamase and DNA gyrase.
Acknowledgments
The authors thank higher education commission (HEC), Pakistan, for financial support through NRPU 20-17615 (Dr. Muhammad Ikram).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c07460.
Absorption graph of GO/PAA-SnO2 for degradation of MB dye (PDF)
The authors declare no competing financial interest.
Notes
Availability of data: data available on demand.
Supplementary Material
References
- Wang Z.; Wu A.; Colombi Ciacchi L. C.; Wei G. Recent Advances in Nanoporous Membranes for Water Purification. Nanomaterials 2018, 8, 65. 10.3390/nano8020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen S.; Iqbal A.; Ikram M.; Ul-Ain K.; Naz S.; Ul-Hamid A.; Shahzadi A.; Haider A.; Nabgan W.; Haider J. Effective Disposal of Methylene Blue and Bactericidal Benefits of Using GO-Doped MnO2Nanorods Synthesized through One-Pot Synthesis. ACS Omega 2021, 6, 24866–24878. 10.1021/acsomega.1c03723. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang J. The Impact of Water Quality on Health: Evidence from the Drinking Water Infrastructure Program in Rural China. J. Health Econ. 2012, 31, 122–134. 10.1016/j.jhealeco.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Shaban M.; Ashraf A. M.; Abukhadra M. R. TiO2 Nanoribbons/Carbon Nanotubes Composite with Enhanced Photocatalytic Activity; Fabrication, Characterization, and Application. Sci. Rep. 2018, 8, 781. 10.1038/s41598-018-19172-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiq A.; Imran M.; Aqeel M.; Naz M.; Ikram M.; Ali S. Study of Transition Metal Ion Doped CdS Nanoparticles for Removal of Dye from Textile Wastewater. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1915–1923. 10.1007/s10904-019-01343-5. [DOI] [Google Scholar]
- Kausar A.; Iqbal M.; Javed A.; Aftab K.; Nazli Z. i. H.; Bhatti H. N.; Nouren S. Dyes Adsorption Using Clay and Modified Clay: A Review. J. Mol. Liq. 2018, 256, 395–407. 10.1016/j.molliq.2018.02.034. [DOI] [Google Scholar]
- Mahanthappa M.; Kottam N.; Yellappa S. Enhanced Photocatalytic Degradation of Methylene Blue Dye Using CuS–CdS Nanocomposite under Visible Light Irradiation. Appl. Surf. Sci. 2019, 475, 828–838. 10.1016/j.apsusc.2018.12.178. [DOI] [Google Scholar]
- Lops C.; Ancona A.; Di Cesare K.; Dumontel B.; Garino N.; Canavese G.; Hérnandez S.; Cauda V. Sonophotocatalytic Degradation Mechanisms of Rhodamine B Dye via Radicals Generation by Micro- and Nano-Particles of ZnO. Appl. Catal. B Environ. 2019, 243, 629–640. 10.1016/j.apcatb.2018.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neena D.; Kondamareddy K. K.; Bin H.; Lu D.; Kumar P.; Dwivedi R. K.; Pelenovich V. O.; Zhao X. Z.; Gao W.; Fu D. Enhanced Visible Light Photodegradation Activity of RhB/MB from Aqueous Solution Using Nanosized Novel Fe-Cd Co-Modified ZnO. Sci. Rep. 2018, 8, 10691. 10.1038/s41598-018-29025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama Aziz K. H.; Miessner H.; Mueller S.; Mahyar A.; Kalass D.; Moeller D.; Khorshid I.; Rashid M. A. M. Comparative Study on 2,4-Dichlorophenoxyacetic Acid and 2,4-Dichlorophenol Removal from Aqueous Solutions via Ozonation, Photocatalysis and Non-Thermal Plasma Using a Planar Falling Film Reactor. J. Hazard. Mater. 2018, 343, 107–115. 10.1016/j.jhazmat.2017.09.025. [DOI] [PubMed] [Google Scholar]
- Fu W.; Zhang W. Microwave-Enhanced Membrane Filtration for Water Treatment. J. Memb. Sci. 2018, 568, 97–104. 10.1016/j.memsci.2018.09.064. [DOI] [Google Scholar]
- Salimi F.; Emami S. S.; Karami C. Removal of Methylene Blue from Water Solution by Modified Nano-Boehmite with Bismuth. Inorg. Nano-Metal Chem. 2018, 48, 31–40. 10.1080/24701556.2017.1357628. [DOI] [Google Scholar]
- Nasrollahzadeh M.; Issaabadi Z.; Sajadi S. M. Green Synthesis of a Cu/MgO Nanocomposite by: Cassytha Filiformis L. Extract and Investigation of Its Catalytic Activity in the Reduction of Methylene Blue, Congo Red and Nitro Compounds in Aqueous Media. RSC Adv. 2018, 8, 3723–3735. 10.1039/c7ra13491f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrollahzadeh M.; Sajjadi M.; Sajadi S. Biosynthesis of Copper Nanoparticles Supported on Manganese Dioxide Nanoparticles Using Centella Asiatica L. Leaf Extract for the Efficient Catalytic Reduction of Organic Dyes and Nitroarenes. Cuihua Xuebao/Chinese J. Catal. 2018, 39, 109–117. 10.1016/S1872-2067(17)62915-2. [DOI] [Google Scholar]
- Zhang Y.; Zhai Y. Preparation of Y-Doped ZrO2 Coatings on MnO2 Electrodes and Their Effect on Electrochemical Performance for MnO2 Electrochemical Supercapacitors. RSC Adv. 2016, 6, 1750–1759. 10.1039/c5ra20543c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jildeh Z. B.; Oberländer J.; Kirchner P.; Wagner P. H.; Schöning M. J. Thermocatalytic Behavior of Manganese (IV) Oxide as Nanoporous Material on the Dissociation of a Gas Mixture Containing Hydrogen Peroxide. Nanomaterials 2018, 8, 262. 10.3390/nano8040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J.; Ge Y.; Tan C.; Wang H.; Li Q.; Zhou S.; Zhang K. Degradation of Ciprofloxacin Using Α-MnO2 Activated Peroxymonosulfate Process: Effect of Water Constituents, Degradation Intermediates and Toxicity Evaluation. Chem. Eng. J. 2017, 330, 1390–1400. 10.1016/j.cej.2017.07.137. [DOI] [Google Scholar]
- Mustajab M.; Ikram M.; Haider A.; Ul-Hamid A.; Nabgan W.; Haider J.; Ghaffar R.; Shahzadi A.; Ghaffar A.; Saeed A. Promising Performance of Polyvinylpyrrolidone-Doped Bismuth Oxyiodide Quantum Dots for Antibacterial and Catalytic Applications. Appl. Nanosci. 2022, 12, 2621–2633. 10.1007/s13204-022-02547-x. [DOI] [Google Scholar]
- Atta A. H.; El-Shenawy A. I.; Koura F. A.; Refat M. S. Synthesis and Characterization of Some Selenium Nanometric Compounds: Spectroscopic, Biological and Antioxidant Assessments. World J. Nano Sci. Eng. 2014, 04, 58–69. 10.4236/wjnse.2014.42009. [DOI] [Google Scholar]
- Alharbi N. K.; Alsaloom A. N. Characterization of Lactic Bacteria Isolated from Raw Milk and Their Antibacterial Activity against Bacteria as the Cause of Clinical Bovine Mastitis. J. Food Qual. 2021, 2021, 1. 10.1155/2021/6466645. [DOI] [Google Scholar]
- Jensen K.; Günther J.; Talbot R.; Petzl W.; Zerbe H.; Schuberth H. J.; Seyfert H. M.; Glass E. J. Escherichia Coli- and Staphylococcus Aureus-Induced Mastitis Differentially Modulate Transcriptional Responses in Neighbouring Uninfected Bovine Mammary Gland Quarters. BMC Genom. 2013, 14, 36. 10.1186/1471-2164-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Liu H.; Gao F.; Tan X.; Cai Y.; Hu B.; Huang Q.; Fang M.; Wang X. Application of MOFs and COFs for Photocatalysis in CO2 Reduction, H2 Generation, and Environmental Treatment. EnergyChem 2022, 4, 100078. 10.1016/j.enchem.2022.100078. [DOI] [Google Scholar]
- Liu X.; Verma G.; Chen Z.; Hu B.; Huang Q.; Yang H.; Ma S.; Wang X.. Metal-Organic Framework Nanocrystal-Derived Hollow Porous Materials: Synthetic Strategies and Emerging Applications; The Innovation, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S.; Tang H.; Zhang D.; Wang S.; Qiu M.; Song G.; Fu D.; Hu B.; Wang X.. MXenes as Emerging Nanomaterials in Water Purification and Environmental Remediation; Science of the Total Environment, 2022. [DOI] [PubMed] [Google Scholar]
- Sberveglieri G.; Concina I.; Comini E.; Falasconi M.; Ferroni M.; Sberveglieri V. Synthesis and Integration of Tin Oxide Nanowires into an Electronic Nose. Vacuum 2012, 86, 532–535. 10.1016/j.vacuum.2011.10.004. [DOI] [Google Scholar]
- Jamal F.; Ikram M.; Haider A.; Ul-Hamid A.; Ijaz M.; Nabgan W.; Haider J.; Shahzadi I. Facile Synthesis of Silver and Polyacrylic Acid Doped Magnesium Oxide Nanostructure for Photocatalytic Dye Degradation and Bactericidal Behavior. Appl. Nanosci. 2022, 12, 2409–2419. 10.1007/s13204-022-02504-8. [DOI] [Google Scholar]
- He X.; Dong W.; Zheng F.; Fang L.; Shen M. Effect of Tartaric Acid on the Microstructure and Photoluminescence of SrTiO3:Pr3+ Phosphors Prepared by a Sol-Gel Method. Mater. Chem. Phys. 2010, 123, 284–288. 10.1016/j.matchemphys.2010.04.012. [DOI] [Google Scholar]
- Bhattacharya S.; Saha I.; Mukhopadhyay A.; Chattopadhyay D.; Chand U. Role of Nanotechnology in Water Treatment and Purification: Potential Applications and Implications. Int. J. Chem. Sci. Technol. 2013, 3, 59–64. [Google Scholar]
- Gnecchi M.; Zhang Z.; Ni A.; Dzau V. J. Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy. Circ. Res. 2008, 103, 1204–1219. 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F.; Cheng T.; Zong J.; Zhao M.; Yang S.; Song X. SnO2/Graphene Nanocomposite Coated by Carbonized Polyacrylic Acid Hydrogel as a High-Performance Anode for Lithium-Ion Batteries. ChemistrySelect 2019, 4, 8082–8088. 10.1002/slct.201901479. [DOI] [Google Scholar]
- Wang Y.; Djerdj I.; Smarsly B.; Antonietti M. Antimony-Doped Sno2 Nanopowders with High Crystallinity for Lithium-Ion Battery Electrode. Chem. Mater. 2009, 21, 3202–3209. 10.1021/cm9007014. [DOI] [Google Scholar]
- Qumar U.; Hassan J.; Naz S.; Haider A.; Raza A.; Ul-Hamid A.; Haider J.; Shahzadi I.; Ahmad I.; Ikram M. Silver Decorated 2D Nanosheets of GO and MoS2serve as Nanocatalyst for Water Treatment and Antimicrobial Applications as Ascertained with Molecular Docking Evaluation. Nanotechnology 2021, 32, 255704. 10.1088/1361-6528/abe43c. [DOI] [PubMed] [Google Scholar]
- Sinclair C. G. Bergey’s Manual of Determinative Bacteriology. Am. J. Trop. Med. Hyg. 1939, s1-19, 605–606. 10.4269/ajtmh.1939.s1-19.605. [DOI] [Google Scholar]
- Bauer A. W.; Kirby W. M.; Sherris J. C.; Turck M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- Adzitey F.; Yussif S.; Ayamga R.; Zuberu S.; Addy F.; Adu-Bonsu G.; Huda N.; Kobun R. Antimicrobial Susceptibility and Molecular Characterization of Escherichia Coli Recovered from Milk and Related Samples. Microorganisms 2022, 10, 1335. 10.3390/microorganisms10071335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter S. S.; Ferraro M. J.. Susceptibility Testing Instrumentation and Computerized Expert Systems for Data Analysis and Interpretation. In Manual of Clinical Microbiology; Wiley, 2011; pp 1144–1154. [Google Scholar]
- Iwalokun B. A.; Ogunledun A.; Ogbolu D. O.; Bamiro S. B.; Jimi-Omojola J. In Vitro Antimicrobial Properties of Aqueous Garlic Extract against Multidrug-Resistant Bacteria and Candida Species from Nigeria. J. Med. Food 2004, 7, 327–333. 10.1089/jmf.2004.7.327. [DOI] [PubMed] [Google Scholar]
- Haider A.; Ijaz M.; Imran M.; Naz M.; Majeed H.; Khan J. A.; Ali M. M.; Ikram M. Enhanced Bactericidal Action and Dye Degradation of Spicy Roots’ Extract-Incorporated Fine-Tuned Metal Oxide Nanoparticles. Appl. Nanosci. 2020, 10, 1095–1104. 10.1007/s13204-019-01188-x. [DOI] [Google Scholar]
- Abagyan R.; Totrov M. Biased Probability Monte Carlo Conformational Searches and Electrostatic Calculations for Peptides and Proteins. J. Mol. Biol. 1994, 235, 983–1002. 10.1006/jmbi.1994.1052. [DOI] [PubMed] [Google Scholar]
- Lee N. L. S.; Yuen K. Y.; Kumana C. R. β-Lactam Antibiotic and β-Lactamase Inhibitor Combinations. JAMA 2001, 285, 386–388. 10.1001/jama.285.4.386. [DOI] [PubMed] [Google Scholar]
- Drawz S. M.; Bonomo R. A. Three Decades of β-Lactamase Inhibitors. Clinical Microbiology Reviews 2010, 23, 160–201. 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiyama F.; Amada H.; Takeuchi T.; Tanaka-Yamamoto N.; Kanazawa H.; Nakano K.; Mima M.; Masuko A.; Takata I.; Hitaka K.; Iwamoto K.; Sugiyama H.; Ohtake N. Lead Identification of 8-(Methylamino)-2-Oxo-1,2-Dihydroquinoline Derivatives as DNA Gyrase Inhibitors: Hit-to-Lead Generation Involving Thermodynamic Evaluation. ACS Omega 2020, 5, 10145–10159. 10.1021/acsomega.0c00865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barelier S.; Eidam O.; Fish I.; Hollander J.; Figaroa F.; Nachane R.; Irwin J. J.; Shoichet B. K.; Siegal G. Increasing Chemical Space Coverage by Combining Empirical and Computational Fragment Screens. ACS Chem. Biol. 2014, 9, 1528–1535. 10.1021/cb5001636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchaud P.; Bruyère T.; Blumstein A. C.; Bur D.; Chambovey A.; Ertel E. A.; Gude M.; Hubschwerlen C.; Jacob L.; Kimmerlin T.; Pfeifer T.; Prade L.; Seiler P.; Ritz D.; Rueedi G. Discovery and Optimization of Isoquinoline Ethyl Ureas as Antibacterial Agents. J. Med. Chem. 2017, 60, 3755–3775. 10.1021/acs.jmedchem.6b01834. [DOI] [PubMed] [Google Scholar]
- Paramarta V.; Taufik A.; Munisa L.; Saleh R.. Sono-and Photocatalytic Activities of SnO2 Nanoparticles for Degradation of Cationic and Anionic Dyes. AIP Conference Proceedings; American Institute of Physics Inc., 2017; Vol. 1788.
- Kaizra S.; Bellal B.; Louafi Y.; Trari M. Improved Activity of SnO for the Photocatalytic Oxygen Evolution. J. Saudi Chem. Soc. 2018, 22, 76–83. 10.1016/j.jscs.2017.07.005. [DOI] [Google Scholar]
- Munawar T.; Nadeem M. S.; Mukhtar F.; Rehman M. N. U.; Riaz M.; Batool S.; Hasan M.; Iqbal F. Transition Metal-Doped SnO2 and Graphene Oxide (GO) Supported Nanocomposites as Efficient Photocatalysts and Antibacterial Agents. Environ. Sci. Pollut. Res. 2022, 29, 90995. 10.1007/s11356-022-22144-3. [DOI] [PubMed] [Google Scholar]
- Reddy Channu V. S.; Ravichandran D.; Rambabu B.; Holze R. Carbon and Functionalized Graphene Oxide Coated Vanadium Oxide Electrodes for Lithium Ion Batteries. Appl. Surf. Sci. 2014, 305, 596–602. 10.1016/j.apsusc.2014.03.140. [DOI] [Google Scholar]
- Fu C.; Zhao G.; Zhang H.; Li S. Evaluation and Characterization of Reduced Graphene Oxide Nanosheets as Anode Materials for Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2013, 8, 6269–6280. [Google Scholar]
- Zhan S.; Li D.; Liang S.; Chen X.; Li X. A Novel Flexible Room Temperature Ethanol Gas Sensor Based on SnO2 Doped Poly-Diallyldimethylammonium Chloride. Sensors (Switzerland) 2013, 13, 4378–4389. 10.3390/s130404378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashad M. M.; Ismail A. A.; Osama I.; Ibrahim I. A.; Kandil A. H. T. Photocatalytic Decomposition of Dyes Using ZnO Doped SnO2 Nanoparticles Prepared by Solvothermal Method. Arab. J. Chem. 2014, 7, 71–77. 10.1016/j.arabjc.2013.08.016. [DOI] [Google Scholar]
- Adjimi A.; Zeggar M. L.; Attaf N.; Aida M. S. Fluorine-Doped Tin Oxide Thin Films Deposition by Sol-Gel Technique. J. Cryst. Process Technol. 2018, 08, 89–106. 10.4236/jcpt.2018.84006. [DOI] [Google Scholar]
- Wang Q.; Zhang J.; Wang A. Preparation and Characterization of a Novel PH-Sensitive Chitosan-g-Poly (Acrylic Acid)/Attapulgite/Sodium Alginate Composite Hydrogel Bead for Controlled Release of Diclofenac Sodium. Carbohydr. Polym. 2009, 78, 731–737. 10.1016/j.carbpol.2009.06.010. [DOI] [Google Scholar]
- Ikram M.; Shahzadi I.; Haider A.; Hayat S.; Haider J.; Ul-Hamid A.; Shahzadi A.; Nabgan W.; Dilpazir S.; Ali S. Improved Catalytic Activity and Bactericidal Behavior of Novel Chitosan/V2O5 Co-Doped in Tin-Oxide Quantum Dots. RSC Adv. 2022, 12, 23129–23142. 10.1039/d2ra03975c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran V. H.; Ambade R. B.; Ambade S. B.; Lee S. H.; Lee I. H. Low-Temperature Solution-Processed SnO2 Nanoparticles as a Cathode Buffer Layer for Inverted Organic Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 1645–1653. 10.1021/acsami.6b10857. [DOI] [PubMed] [Google Scholar]
- Skrabania K.; Miasnikova A.; Bivigou-Koumba A. M.; Zehm D.; Laschewsky A. Examining the UV-Vis Absorption of RAFT Chain Transfer Agents and Their Use for Polymer Analysis. Polym. Chem. 2011, 2, 2074–2083. 10.1039/c1py00173f. [DOI] [Google Scholar]
- Lozovskis P.; Jankauskaitė V.; Guobienė A.; Kareivienė V.; Vitkauskienė A. Effect of Graphene Oxide and Silver Nanoparticles Hybrid Composite on p. Aeruginosa Strains with Acquired Resistance Genes. Int. J. Nanomedicine 2020, 15, 5147–5163. 10.2147/IJN.S235748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhu G.; Biju V. Effect of Ni2+ and O2- Vacancies on the Electrical and Optical Properties of Nanostructured Nickel Oxide Synthesized through a Facile Chemical Route. Phys. E Low-dimens. Syst. Nanostruct. 2014, 60, 200–205. 10.1016/j.physe.2014.02.011. [DOI] [Google Scholar]
- Iqbal S.; Javed M.; Bahadur A.; Qamar M. A.; Ahmad M.; Shoaib M.; Raheel M.; Ahmad N.; Akbar M. B.; Li H. Controlled Synthesis of Ag-Doped CuO Nanoparticles as a Core with Poly(Acrylic Acid) Microgel Shell for Efficient Removal of Methylene Blue under Visible Light. J. Mater. Sci. Mater. Electron. 2020, 31, 8423–8435. 10.1007/s10854-020-03377-9. [DOI] [Google Scholar]
- Ahmed A. S.; Shafeeq M. M.; Singla M. L.; Tabassum S.; Naqvi A. H.; Azam A. Band gap narrowing and fluorescence properties of nickel doped SnO2 nanoparticles. J. Lumin. 2011, 131, 1–6. 10.1016/j.jlumin.2010.07.017. [DOI] [Google Scholar]
- Jiang Z. J.; Liu C. Y.; Sun L. W. Catalytic Properties of Silver Nanoparticles Supported on Silica Spheres. J. Phys. Chem. B 2005, 109, 1730–1735. 10.1021/jp046032g. [DOI] [PubMed] [Google Scholar]
- Khan M. M.; Lee J.; Cho M. H. Au@TiO2 Nanocomposites for the Catalytic Degradation of Methyl Orange and Methylene Blue: An Electron Relay Effect. J. Ind. Eng. Chem. 2014, 20, 1584–1590. 10.1016/j.jiec.2013.08.002. [DOI] [Google Scholar]
- Saha J.; Begum A.; Mukherjee A.; Kumar S. A Novel Green Synthesis of Silver Nanoparticles and Their Catalytic Action in Reduction of Methylene Blue Dye. Sustain. Environ. Res. 2017, 27, 245–250. 10.1016/j.serj.2017.04.003. [DOI] [Google Scholar]
- Guan Y.; Yu H. Y.; Abdalkarim S. Y. H.; Wang C.; Tang F.; Marek J.; Chen W. L.; Militky J.; Yao J. M. Green One-Step Synthesis of ZnO/Cellulose Nanocrystal Hybrids with Modulated Morphologies and Superfast Absorption of Cationic Dyes. Int. J. Biol. Macromol. 2019, 132, 51–62. 10.1016/j.ijbiomac.2019.03.104. [DOI] [PubMed] [Google Scholar]
- Bari A.; Ikram M.; Haider A.; Ul-Hamid A.; Haider J.; Shahzadi I.; Nazir G.; Shahzadi A.; Imran M.; Ghaffar A. Evaluation of Bactericidal Potential and Catalytic Dye Degradation of Multiple Morphology Based Chitosan/Polyvinylpyrrolidone-Doped Bismuth Oxide Nanostructures. Nanoscale Adv. 2022, 4, 2713–2728. 10.1039/d2na00105e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Feng Y.; Li H.; Zhang Q. Effect of Anionic Groups on the Antibacterial Activity of Magnesium Oxide Nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 635, 127978. 10.1016/j.colsurfa.2021.127978. [DOI] [Google Scholar]
- Shahzadi I.; Islam M.; Saeed H.; Haider A.; Shahzadi A.; Haider J.; Ahmed N.; Ul-Hamid A.; Nabgan W.; Ikram M.; Rathore H. A. Formation of Biocompatible MgO/Cellulose Grafted Hydrogel for Efficient Bactericidal and Controlled Release of Doxorubicin. Int. J. Biol. Macromol. 2022, 220, 1277–1286. 10.1016/j.ijbiomac.2022.08.142. [DOI] [PubMed] [Google Scholar]
- Ikram M.; Naeem M.; Zahoor M.; Hanafiah M. M.; Oyekanmi A. A.; Ullah R.; Farraj D. A. A.; Elshikh M. S.; Zekker I.; Gulfam N. Biological Degradation of the Azo Dye Basic Orange 2 by Escherichia Coli: A Sustainable and Ecofriendly Approach for the Treatment of Textile Wastewater. Water 2022, 14, 2063. 10.3390/w14132063. [DOI] [Google Scholar]
- van der Zee F. P.; Bouwman R. H. M.; Strik D. P. B. T. B.; Lettinga G.; Field J. A. Application of Redox Mediators to Accelerate the Transformation of Reactive Azo Dyes in Anaerobic Bioreactors. Biotechnol. Bioeng. 2001, 75, 691–701. 10.1002/bit.10073. [DOI] [PubMed] [Google Scholar]
- Moir D.; Masson S.; Chu I. Structure-Activity Relationship Study on the Bioreduction of Azo Dyes by Clostridium Paraputrificum. Environ. Toxicol. Chem. 2001, 20, 479–484. 10.1002/etc.5620200304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.