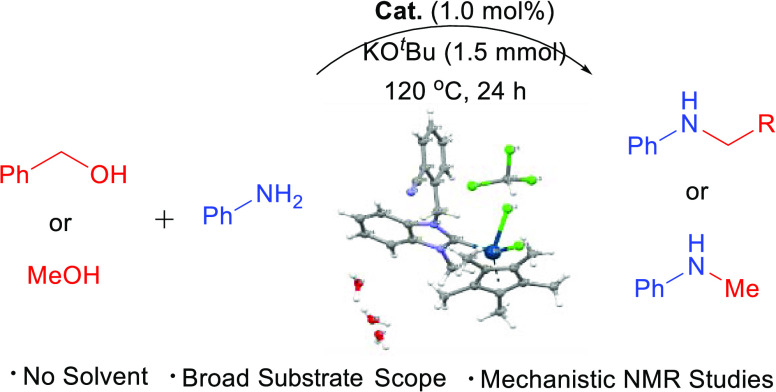
Abstract
A series of nitrile-modified N-heterocyclic carbene (NHC) complexes of Ir(III) (2a–e) and Ru(II) (3a–d) have been prepared by transmetallation of [IrCp*Cl2]2 and [RuCl2(p-cymene)]2 forming an in situ NHC–Ag complex. The structures of all complexes were characterized by 1H NMR, 13C NMR, and Fourier transform infrared (FT-IR) spectroscopies. And the structures were clearly elucidated by performing X-ray diffraction studies on 2b, 3a, and 3c single crystals. The complexes of NHC–Ir(III) (2a–e) and NHC–Ru(II) (3a–d) were investigated in the N-alkylation reaction of aniline derivatives with benzyl alcohols to form N-benzyl amines and in the N-methylation reaction of aniline derivatives with methanol. Both reactions were performed in solvent-free media. The Ir(III) complexes (2a–e) were found to perform essentially better than similar Ru(II) complexes (3a–d) in the N-alkylation and N-methylation reactions. Among the Ir(III) complexes (2a–e), the best results were obtained with 2b. The catalytic mechanisms of both reactions were revealed by 1H NMR study. Formation of Ir-hydride species was observed for both reactions. This new report provides useful information to evaluate the activity of complexes and the differences in sensitivity between the NHCs.
Introduction
In recent years, N-heterocyclic carbenes (NHCs) have been increasingly used as alternatives to phosphine ligands due to their easy preparation in organometallic chemistry for homogeneous catalyst, their enhanced stability resulting from strong metal–NHC bonds, and high tunability of steric and electronic properties.1 In addition, NHC ligands, unlike phosphine ligands, have good σ-donating properties and low toxicity, which is one of the main reasons why they are so preferred.2 It was determined that NHC complexes are versatile tools in homogeneous catalysis thanks to these properties. To date, NHCs have been involved in many reactions in the field of catalysis because they form stable complexes with many transition metals regardless of their oxidation state.3 Examples of these reactions are transfer hydrogenation,4 C–C coupling,5 olefin metathesis,6 hydrosilylation,7 and N-alkylation of amines.8−10
The transition-metal-catalyzed N-alkylation reaction was first performed by Grigg et al.9 and Watanable et al.11 Grigg’s rhodium and Watanable’s ruthenium catalysts performed the alkylation reaction of different amines with alcohols. To date, many catalytic reactions have been reported for the N-alkylation reaction. Many catalysts with different metal complexes including Ru, Ir, Fe, Co, Mn, Cu, Pd, Ni, and Cr have been synthesized for the N-alkylation of amines with alcohol.12 Especially, Ir and Ru complexes with NHC ligands have emerged as one of the most effective catalysts in the N-alkylation reactions.
The synthesis of target products from inexpensive, accessible, and nontoxic starting materials using transition-metal catalysts with a green chemistry approach is the focus of both inorganic and organic chemists.13 Amine-containing compounds are widely used in the manufacture of many materials as pharmaceutical, agrochemical, and synthetic intermediates.14−16 Therefore, the synthesis of C–N bond formation is of great interest to researchers. Many synthetic methods have been developed.17,18 Although most of the developed methods are realized with high efficiency, they produce high amounts of waste. In addition to these, since the materials used are highly toxic, they pose a significant problem for the environment.19 Therefore, the development of efficient and environmentally friendly catalytic strategies in the methods designed by researchers has become an important goal in synthesis chemistry. In recent years, methods based on borrowing hydrogen (BH) or hydrogen auto transfer (HA) methodology for transition-metal-catalyzed N-alkylation of amine with alcohol have attracted much attention due to their more sustainable and environmentally friendly nature.20−23 The use of alcohols as inexpensive and greener alkylating agents is one of the main reasons why the reaction is preferred. The BH methodology is also a powerful atom-economical catalytic strategy.24
Our previous results obtained by CN-modified Pd(II) catalysts in Suzuki–Miyaura cross-coupling reactions25 encouraged us to investigate the catalytic activities of Ir(III) and Ru(II) complexes of similar NHC ligands. The previous results prompted us to synthesize Ir(III) (2a–e) and Ru(II) (3a–d) complexes of similar NHC ligands and to explore the catalytic activities in the N-alkylation and N-methylation reactions. Our objective was to investigate the catalytic properties of Ir(III) and Ru(II) complexes bearing CN-modified NHC ligand in the different catalytic reactions. So, we reported NHC–Ir(III) (2a–e) and NHC–Ru(II) (3a–d) complexes of different azole skeletons (imidazole, benzimidazole, and benzothiazole) with CN substituent. The catalytic activity of complexes was investigated in the N-alkylation of aniline derivatives with benzyl alcohols. And we also report here that instead of reactive and toxic methyl halides that require their use in traditional N-methylation methods, it can be applied with good yields in direct N-methylation of amines with methanol by means of synthesized catalysts.
The strategies have been reported for both N-alkylation and N-methylation reactions with Ir and Ru catalysts. Huang et al. have reported the N-alkylation of amines with alcohols by the hetero-bidentate NHC-phosphine Ru catalyst with low catalyst loading (0.25 mol %).8a Williams et al. developed an in situ catalytic system using [Ru(p-cymene)Cl2]2 with the bidentate phosphines dppf or DPEphos ligands.8b The authors reported the preparation of some simple pharmaceutical drugs by means of N-alkylation reactions. Valerga et al. reported the use of Ru(II) arene complexes with picolyl-functionalized NHC ligands to the N-alkylation of amines with alcohol reaction.8c There are many reported studies by Ozdemir et al. using NHC–Ru complexes including aromatic substituents for the N-alkylation reaction.8d−8f Also, Ozdemir et al. reported acetal-functionalized NHC–Ru complexes to evaluate the N-alkylation of pyrrolidine and morpholine with benzyl alcohol.8g Seayed et al. prepared NHC–Ru complexes with benzannulated ligands, and they used N-alkylation reaction for the synthesis of pharmaceutically important amines.8h Bruneau et al. developed in situ catalytic conditions with benzimidazolium sulfanate salts and [RuCl2(p-cymene)]2 for the N-alkylation reaction.8i Although studies of Ir(III) complexes are not as famous as Ru(II) complexes, some studies have been reported with NHC–Ir(III) complexes. Hou et al. produced an efficient and recyclable catalyst for N-alkylation reaction with silica-supported NHC–Ir complexes.9a Royo et al. reported water-soluble NHC–Ir complexes bearing ester and amide groups for the N-alkylation reaction in water.9b
N-Alkylation of amines with methanol using NHC complexes is limited in the literature. Fujita et al. reported NHC–Ir(III)Cp* complexes as catalysts for the N-alkylation of primary amines with methanol.10a They developed N,C(carbene)-chelated Ir(III) complexes with different electronic effects and steric hindrances.10b Other researchers reported Ir-bis(NHC) catalysts for the transformation of amine monoalkylation.10c These literature reports demonstrate the ability of iridium NHC complexes in N-alkylation reactions. Our study also provides good results that can compete with the literature reports and include in-depth mechanism studies in both reactions.
Results and Discussion
Synthesis of Azolium Salts, and Ir(III)–NHC and Ru(II)–NHC complexes
Azolium salts (1a–d) were synthesized in a one-pot reaction according to the previously developed procedure.25 Ligands were characterized by 1H-, 13C-NMR, and Fourier transform infrared (FT-IR) spectroscopic studies, and the spectroscopic data of the compounds obtained agree with the previous report. The characteristic signal of the −C≡N– group of azolium salts around 117 ppm confirmed the presence of −C≡N– in 13C NMR spectra. Also, the down-field signal at 10.75, 9.84, and 10.22 ppm proved the formation of benzothiazole, benzimidazole, and imidazole salts, respectively. [IrCl2(Cp*)(NHC)] and [RuCl2(p-cymene)(NHC)] complexes (2a–e and 3a–d) were obtained in 75–86% yields by transmetalation from Ag–NHC derivatives using a two-step procedure (Scheme 1). The complexes (2d,e and 3d) were synthesized to better understand the importance of the nitrile group. Column chromatography was used for the purification of the complexes. NHC iridium and ruthenium complexes (2a–e and 3a–d) were isolated as orange-brown and air-stable solids with good yields. All of the resulting complexes were found to be well soluble in chlorinated solvents such as CH2Cl2 and CHCl3.
Scheme 1. Synthesis of Ir(III)–/Ru(II)–NHC Complexes 2a–e and 3a–d.

The examination of the structures of complexes (2a–e and 3a–d) was provided by 1H and 13C NMR spectra. The absence of characteristic down-field signals of NCHN protons for the corresponding ligands (1a–e) in the 1H NMR spectra of the complexes (2a–e and 3a–d) proved their formation. Meanwhile, Ir(III) (2a–e) and Ru(II) (3a–d) complexes showed characteristic Ru–Ccarbene and Ir–Ccarbene signals at δ = 203.3, 172.2, 157.9, 156.6, 170.9, 229.2, 191.5, 230.7, and 174.2 ppm, respectively. In the 1H NMR spectrum, the benzylic CH2 protons of 3a showed a single peak, while the benzylic CH2 protons of 2b and 3c were not observed in the spectrum. This difference in 2b and 3c may be due to the intermolecular interaction of hydrogen. The structures of the complexes (2b and 3c) were confirmed by X-ray diffraction studies (Figures 1–3) and the datas were represented in Table 2. The analytical data of the synthesized complexes (2a–e and 3a–d) are summarized in Table 1. The FT-IR spectra of the complexes (2a–e and 3a–d) were almost the same with similar structures (Figures S30–S38). The stretching vibrations of the nitrile functionality (C≡N) involved in the structure were demonstrated by observing a sharp band of medium intensity at 2219–2222 cm–1. The presence of the −C=N– group in the complexes was confirmed by the presence of ν(C=N) bands in the spectrum between 1656 and 1510 cm–1.
Figure 1.
Molecular structure of 2b showing the atom numbering scheme.
Figure 3.
Molecular structure of 3c showing the atom numbering scheme.
Table 2. Selected Bond Distances (Å) and Angles (°) for Complexes 2b, 3a, and 3c.
| complex 3a | |||
| C11–Ru1 | 2.059(10) | C36–Ru2 | 2.038(10) |
| Cl1–Ru1 | 2.417(3) | Cl2–Ru1 | 2.418(3) |
| Cl1–Ru1–Cl2 | 87.27(10) | C11–Ru1–Cl1 | 89.0(3) |
| Cl1–Ru1–Cl2 | 87.27(10) | C36–Ru2–Cl3 | 90.3(3) |
| C36–Ru2–Cl4 | 86.3(3) | Cl4–Ru2–Cl3 | 86.01(11) |
| complex 2b | |||
| C11–Ir1 | 2.050(15) | Cl1–Ir1 | 2.450(5) |
| Cl2–Ir1 | 2.424(5) | ||
| C11–Ir1–Cl1 | 93.7(5) | Cl2–Ir1–Cl1 | 84.25(19) |
| complex 3c | |||
| C1–Ru1 | 2.082(3) | Cl1–Ru1 | 2.4350(11) |
| Cl2–Ru1 | 2.4287(10) | ||
| C1–Ru1–Cl1 | 88.56(10) | C1–Ru1–Cl2 | 90.08(10) |
Table 1. Melting Points, Yields, and Selected 13C NMR Data of 2a–e and 3a–d.
| entry | complex | yield (%) | m.p. (°C) | υ (C≡N) (cm)−1 | 13C NMR |
|---|---|---|---|---|---|
| 1 | 2a | 80 | 290.3 | 2221 | 203.3 |
| 2 | 2b | 75 | 275.1 | 2221 | 172.2 |
| 3 | 2c | 78 | 281.1 | 2219 | 157.9 |
| 4 | 2d | 61 | 239.4 | 156.6 | |
| 5 | 2e | 52 | 229.8 | 170.9 | |
| 6 | 3a | 85 | 243.0 | 2221 | 229.2 |
| 7 | 3b | 83 | 248.4 | 2221 | 191.5 |
| 8 | 3c | 86 | 230.4 | 2222 | 230.7 |
| 9 | 3d | 57 | 211.3 | 174.3 |
Figure 2.
Molecular structure of 3a showing the atom numbering scheme.
Catalytic N-Alkylation of Amines with Alcohols
N-Alkylation of amines with alcohols has become of interest because of its relevance to the atom-economic synthesis of pharmaceutically important molecules.26 Initially, in our study, N-alkylation reaction of aniline (10a) and benzyl alcohol (11a) was chosen as a model reaction to determine the catalytic potential of NHC–Ir(III) and NHC–Ru(II) complexes (2a–e and 3a–d). The results of the relevant studies are listed in Table 3. The reaction was carried out in the presence of complex (2a–e and 3a–d) as a catalyst and KOtBu (1.5 mmol) in a solvent-free setting at 120 °C open to air for 24 h (Table 3, entries 1–6). In general, NHC–Ir(III) complexes (2a–e) exhibited higher catalytic activity than NHC–Ru(II) complexes (3a–d). Among the NHC–Ir(III) complexes, complex 2b containing benzimidazole skeleton was the most effective catalyst and the product (12aa) was obtained in 80% yield after 20 h. A significant decrease in the catalytic activity was observed with the Ir(III) complex (2a), which has different heteroatoms in the structure of the catalyst. Here, due to the inability of the methyl group of the sulfur atom in the structure, the electronic and steric effects change and affect the charge density on the metal, leading to a decrease in the catalytic activity (Table 3, entry 1). Here again, the reason why 2b was more active than 2c is that it has a benzene ring fused in the 4,5-position of the imidazole ring in the structure. This was a result of electron density originating from the benzene ring (Table 3, entries 2 and 3). In the next step, the effect of base was investigated. Replacing KOtBu with KOH, Cs2CO3, or K2CO3 did not positively affect yield. In fact, the use of K2CO3 significantly reduced the yield (Table 3, entries 7–9). When the catalyst loading was reduced from 1 to 0.5 mol %, an unsatisfactory effect on the yield was observed and the yield decreased to 48% (Table 3, entry 11). Thus, the optimum catalyst loading in this reaction was determined as 1.0 mol %. [IrCl2Cp*]2 did not catalyze the reaction (Table 3, entry 12). However, in situ experiment did not display good efficiency in the catalytic cycle (Table 3, entry 13). There was no yield in the absence of catalyst (Table 3, entry 14). Also, an air atmosphere was required for the reaction (Table 3, entry 15). As a result, the reaction conditions were determined (Table 3, entry 10). The catalytic results of 2d, 2e, and 3d showed the importance of the nitrile group (Table 3, entries 16–18). To better understand the effect of the nitrile group, 2b was heated in the presence of NaBF4. The newly formed cationic nitrile coordinated complex could not be isolated and it may be unstable. FT-IR analyses of the samples taken from the solution medium were performed at the beginning and end of the experiment. According to the FT-IR spectra, the changes in the fingerprint region and the newly formed metal–nitrogen bond around 630 cm–1 support the formation of the cationic complex (Figures S43 and S44). This shows that the nitrile can coordinate to the metal in the catalytic process. Literature reports indicate that the presence of the nitrile group in the side chain provides stability to the complex.41
Table 3. Optimization of Reaction Conditions for N-Alkylation of Aniline with Benzyl Alcohola.
| entry | cat. | base | yield (%) |
|---|---|---|---|
| 1 | 2a | tBuOK | 55 |
| 2 | 2b | tBuOK | 80 |
| 3 | 2c | tBuOK | 72 |
| 4 | 3a | tBuOK | 40 |
| 5 | 3b | tBuOK | 67 |
| 6 | 3c | tBuOK | 67 |
| 7 | 2b | Cs2CO3 | 62 |
| 8 | 2b | K2CO3 | 33 |
| 9 | 2b | KOH | 58 |
| 10b | 2b | tBuOK | 93 |
| 11c | 2b | tBuOK | 48 |
| 12d | [IrCl2Cp*]2 | tBuOK | 22 |
| 13e | 1b/[IrCl2Cp*]2 | tBuOK | 42 |
| 14 | tBuOK | trace | |
| 15f | 2b | tBuOK | 69 |
| 16 | 2d | tBuOK | 62 |
| 17 | 3d | tBuOK | 58 |
| 18 | 2e | tBuOK | 64 |
Reaction conditions: Aniline (1.0 mmol), benzyl alcohol (1.5 mmol), tBuOK (1.5 mmol), cat. (1.0% mol), 20 h, 120 °C, air atmosphere.
24 h.
(0.5% mol).
Only [IrCl2Cp*]2 complex was used as the catalyst.
In situ generated catalytic system with 1b carbene precursor and [IrCl2Cp*]2 was used.
Under argon atmosphere. Yields were determined using 1H NMR spectroscopy.
Many different methods can be applied in the synthesis of secondary amines. However, conventional methods use environmentally harmful organic solvents and alkyl halides or stoichiometric amounts of reducing agents.27−29 In recent studies, the use of cheap and low-toxic alcohols instead of environmentally harmful organic solvents has attracted a lot of attention. N-Alkylation of amines with alcohols is a green method in the synthesis of substituted amines, which are of great importance in synthetic applications. Ru- and Ir-catalyzed amine alkylation has previously attracted the attention of several groups and has been reported.8,9 The extent of Ir-catalyzed N-alkylation of amines with benzyl alcohols under optimal reaction conditions was investigated (Table 4). A wide variety of substrates bearing electron-donating or electron-withdrawing substituents on the aryl ring of aniline were successfully completed with benzyl alcohol in high yields at 120 °C (Table 4). N-Alkylation of 4-methylaniline and 2-methylaniline with benzyl alcohol was investigated using 2b as the catalyst to obtain N-benzyl-4-methylaniline (12b) and N-benzyl-2-methylaniline (12c) with 72 and 65% yields, respectively. For the di-alkylated substituent, such as 2,4-dimethylaniline, N-benzyl-2,4-dimethylaniline (12d) was obtained with relatively low conversion compared to mono-alkylated substrate. Moreover, 64% catalytic yield was achieved by 2-nitroaniline and benzyl alcohol, resulting in product N-benzyl-2-nitroaniline (12e). It was observed that the methoxy substituent in the para-position of aniline had a better formation rate of the corresponding product than in the ortho-position substituent. And the desired products N-benzyl-4-methoxyaniline (12f) and N-benzyl-2-methoxyaniline (12g) were converted in 86 and 71% yields, respectively. The products of halogen-substituted anilines, N-benzyl-4-bromoaniline (12h) and N-benzyl-4-chloroaniline (12i), were obtained in yields of 82 and 80%, respectively. In addition, aliphatic amine such as N-hexylamine was converted to the desired product, N-benzylhexan-1-amine (12j), in a yield of 45%. Aniline derivatives containing sterically hindered heterocyclics also resulted in good yields (89 and 79%) in N-alkylation reactions with the products of N-benzylnaphthalen-2-amine (12k) and N-benzyl-[1,1′-biphenyl]-4-amine (12l).
Table 4. N-Alkylation of Amines Using Benzyl Alcohola.
Reaction conditions: 10 (1.0 mmol), 11a (1.5 mmol), KOtBu (1.5 mmol), 2b (1.0% mol), 24 h, 120 °C. Isolated yields.
To determine the broader applicability of catalytic conversions, we investigated the N-alkylation of aniline with benzyl alcohol derivatives (Table 5). As shown in Table 5, the reaction of both electron-rich and electron-deficient benzyl alcohols was investigated, and the reactions proceeded smoothly. The desired products were obtained in 60–93% yields. First, benzyl alcohol-containing electron-donating group at the para-position, namely, CH3 was tested, and good yields were obtained with N-(4-methylbenzyl)aniline (13a). The reaction of aniline with benzyl alcohol derivatives bearing 4-bromo and 2-bromo yielded corresponding products N-(4-bromobenzyl)aniline (13b) and N-(2-bromobenzyl)aniline (13c) in 73–65% yields, respectively. When using alcohols consisting of 4-Cl, 4-OCH3, and 2,4,6-CH3 groups, the corresponding products N-(4-chlorobenzyl)aniline (13d), N-(4-methoxybenzyl)aniline (13e), and N-(2,4,6-trimethylbenzyl)aniline (13f) were obtained in good yields of 81, 93, and 60%, respectively.
Table 5. N-Alkylation of Aniline Using Benzyl Alcohol Derivativesa.
Reaction conditions: 10a (1.0 mmol), 11 (1.5 mmol), KOtBu (1.5 mmol), 2b (1.0% mol), 24 h, 120 °C. Isolated yields.
The control experiments were conducted to understand the progress of the reaction (Scheme 2). The mercury test was performed for precatalyst 2b using one drop of Hg. Observation of 91% efficiency supported homogeneous catalysis. The formation of amine from the imine was controlled, and the amine was successfully formed in 93% yield. The catalyst (2b) was proven to successfully convert benzyl alcohol to benzaldehyde.
Scheme 2. Mechanism and Control Experiments for N-Alkylation of Aniline with Benzyl Alcohol.
We performed NMR experiment to investigate the mechanism of N-alkylation of aniline with benzyl alcohol (Figure 4). The NMR experiment was carried out in CD3OD (1 mL) at 120 °C for 24 h in the presence of 2b as the catalyst and KOtBu (0.15 mmol). The possible mechanism of the N-alkylation of amines with primary alcohols is presented in Scheme 3. The mechanism starts with the leaving of proton of benzyl alcohol with help of KOtBu. One of the metal-coordinated chlorine anions is separated to form KCl precipitate. Deprotonated alkoxy derivative coordinates to the metal from the oxygen atom (I). It loses one of its benzyl protons and leaves as an aldehyde. This step is essential for the formation of metal hydride species (II). The imine species is formed by the reaction of aldehyde and amine. The imine coordinates to the metal center and is reduced to amine (III). Imine formation and imine reduction to amine are rate-determining steps for this reaction. Imine and Ir-H formation were observed as soon as the reaction started and persisted throughout the reaction. A main hydride peak appeared in the hydridic region of the 1H NMR spectrum (δ = −16.408 ppm), which suggested that the catalytically active species were related to Ir-H formation (Figure 4). The imine peak was observed at 8.54 ppm in the low area of the 1H NMR spectra (Figure S39). Since the imine was formed as soon as the reaction starts, no aldehyde peak was observed. All 1H NMR spectra of the reaction mechanisms are presented in the Supporting Information. The reaction mechanism is completely dependent on the formation of imine. Under our reaction conditions, double-alkylated products were not observed.
Figure 4.

1H NMR monitoring of the hydride signal of Ir-hydride (located at −16.40 ppm and zoomed in): aniline (0.1 mmol), benzyl alcohol (0.15 mmol), KOtBu (0.15 mmol), 2b (5 mmol %), CD3OD (1 mL), 120 °C.
Scheme 3. Proposed Reaction Pathway for the Iridium(III)–NHC-Catalyzed N-Alkylation.
N-Methylation of Anilines with Methanol
N-Methylamines are widely used as basic intermediates and building blocks for the synthesis of many bulk and fine chemicals. The direct N-methylation of amines has attracted the attention of many researchers due to its significant selectivity and increased overall product yields, without using reactive and toxic methyl halides as methylating agents30 used in conventional N-methylation methods.31,32 In our study, N-methylation of amines with methanol was also reported. As shown in Table 6, N-methylation of anilines with methanol was successfully performed at 120 °C in the presence of 1.0 mol % 2b and 1.5 mmol of base. The products were obtained in a yield of above 80%. The reaction of methanol with 4-methyl-, 2-methyl-, 2,4-methyl-, and 2,4,6-methyl-bearing aniline derivatives gave products 4-methyl-N-methylaniline (14b), 2-methyl-N- methylaniline (14c), 2,4-methyl-N-methylaniline (14d), and 2,4,6-methyl-N-methylaniline (14e), respectively, with yields in the range of 68–80%. In addition, N-methylation of 2-NO2-, 2-OCH3-5-CH3-, 4-OCH3-, 2-OCH3-, 4-Br-, and 4-Cl-substituted anilines gave the desired products 2-nitro-N-methylaniline (14f), 2-methoxy-5-methyl-N-methylaniline (14g), 4-methoxy-N-methylaniline (14h), 2-methoxy-N-methylaniline (14i), 4-bromo-N-methylaniline (14j), and 4-chloro-N-methylaniline (14k), respectively, in 60–91% yields. Anilines composed of different substituents, 4-Br-2,6-CH3, 2-CF3, naphthalene, and biphenyl, gave products 4-bromo-2,6-methyl-N-methylaniline (14l), 2-trifluoromethyl-N-methylaniline (14m), N-methylnaphthalen-2-amine (14n), and N-methyl-[1,1′-biphenyl]-4-amine (14o) in good yields of 72, 59, 88, and 85%, respectively. Although we increased the amount of methanol, double N-methylation did not occur, which led to a mixture of dimethylated amines. However, we performed the reaction with aliphatic amine, which is generally more nucleophilic than aromatic amines, such as N-octadecylamine. In our study, attempts for N-methylation of N-octadecylamine did not result in positive results and only trace yields were achieved.
Table 6. N-Alkylation of Amines with Methanola.

Reaction conditions: 10a (1.0 mmol), MeOH (0.4 mL), KOtBu (1.5 mmol), 2b (1.0% mol), 24 h, 120 °C. Isolated yields.
We also performed NMR experiment to investigate the mechanism of N-methylation of aniline with methanol. The NMR experiment was carried out in excess CD3OD (1 mL) at 120 °C for 24 h in the presence of 2b as the catalyst and KOtBu (0.15 mmol) (Figure 5). The possible mechanism of the N-alkylation of amines with methanol is presented in Scheme 4. The mechanism is quite like the N-alkylation of amines with primary alcohols. The mechanism starts with the leaving of a proton of methanol with help of KOtBu. One of the metal-coordinated chlorine anions is separated to form KCl precipitate. Deprotonated methanol derivative coordinates to the metal from the oxygen atom (I). It loses one of its protons and leaves as a formaldehyde. This step is essential for the formation of metal hydride species (II). There is a high probability of double proton bonding to the metal center by dissociating the other chloride ion. Other unstable peaks seen in the hydride region of the 1H NMR spectra support this. The aldehyde peak was observed in the first 1H NMR spectrum as soon as the reaction was prepared (Figure S40). But from the second spectrum, it quickly disappeared, and the imine was formed. The imine species is formed by the reaction of formaldehyde and amine. The imine coordinates to the metal center and is reduced to amine (III). Imine formation and imine reduction to amine are rate-determining steps for this reaction. Ir-H formation was observed as soon as the reaction started and persisted throughout the reaction. A main hydride peak appeared in the hydridic region of the 1H NMR spectrum (δ = −16.413 ppm), which suggested that the catalytically active species were related to Ir-H formation (Figure 5). The imine peak was observed at 8.15 ppm in the low area of the 1H NMR spectra (Figure S40).
Figure 5.

1H NMR monitoring of the hydride signal of Ir-hydride (located at −16.41 ppm and zoomed in): aniline (0.1 mmol), KOtBu (0.15 mmol), 2b (5 mmol %), CD3OD (1 mL), 120 °C.
Scheme 4. Proposed Reaction Pathway for the Iridium(III)–NHC-Catalyzed N-Methylation.
N-Methylation of aniline was performed using deuterated methanol to investigate the further mechanism (Scheme 5). Deuterated N-methylaniline was obtained with 71% isolated yield. 1H NMR spectra of deuterated and protonated N-methylaniline support the successful formation and isolation of deuterated N-methylaniline (Figures S41 and S42).35
Scheme 5. N-Methylation of Aniline with Methanol-d4.
Conclusions
In conclusion, a series of NHC–Ir(III) (2a–e) and NHC–Ru(II) (3a–d) complexes bearing nitrile moiety were prepared. The complexes (2a–e and 3a–d) were fully characterized via1H, 13C NMR and FT-IR spectroscopies. The crystal structures confirmed that complexes 2b, 3a, and 3c exhibited piano-stool geometry. The complexes (2a–e and 3a–d) were evaluated as catalysts in the N-alkylation of amines with primary alcohols and N-methylation of aniline with methanol. High conversions were obtained under mild conditions in both reactions. Mechanistic studies of both reactions were performed by NMR and their catalytic cycles were established. The Ir(III) complex (2b) was the most effective catalyst in both reactions. For both reactions, the mechanism proceeds through the metal hydride mechanism. The process of imine formation and reduction of the imine to the amine determines the rate of these reactions. The Ir-hydride species of 2b were detected at −16.408 and −16.413 ppm.
Experimental Section
X-ray Crystallography
Suitable crystals of 2b, 3a, and 3c were selected for data collection, which was performed on a D8-QUEST diffractometer equipped with a graphite-monochromatic Mo Kα radiation. The structure was solved by direct methods using SHELXS-201336 and refined by full-matrix least-squares methods on F2 using SHELXL-2013.36,37 All nonhydrogen atoms were refined with anisotropic parameters. The water H atoms were refined freely. The other H atoms were located from different maps and then treated as riding atoms with C–H distances of 0.93–0.97 Å. The following procedures were implemented in our analysis: data collection: Bruker APEX2;38 program used for molecular graphics: MERCURY program;39 software used to prepare material for publication: WinGX.40 Details of data collection and crystal structure determinations are given in Table 2.
Materials
Unless otherwise noted, all operations were performed without taking precautions to exclude air and moisture. The glass equipment was heated under vacuum to remove oxygen and moisture, and then they were filled with argon. Starting compounds and reagents were obtained from Merck, Fluka, Alfa Aesar, and Acros Organics; Ruthenium(III) chloride hydrate, iridium(III) chloride hydrate, and α-terpinene were obtained from Alfa Aesar, and dichloromethane, diethyl ether, and toluene were obtained from Merck and Ridel de Haen. [IrCl2Cp*]2 was synthesized according to the published procedures by a reaction of iridium(III) chloride and pentamethylcyclopentadiene.33 [RuCl2(p-cymene)]2 was prepared according to the method reported by Bennett and Smith through the reaction of ruthenium(III) chloride with α-terpinene.34 Catalytic reactions were carried out under the inert atmosphere on Carousel 12 Plus Reaction Station system. 1H and 13C NMR spectra were recorded on a Varian AS 400 Mercury instrument. Melting points were measured on Gallenkamp electrothermal melting point apparatus without correction. FT-IR spectra were recorded on a PerkinElmer Spectrum 100 series.
Synthesis of Compound 1e
1-Methylbenzimidazole (250 mg, 1.89 mmol) was dissolved in 5 mL of toluene, and benzyl bromide (324 mg, 1.89 mmol) was added to boil under reflux for 24 h. The precipitate was then filtered off, washed with diethyl ether, and dried under vacuum to yield. Yield = 82%, 470 mg. 1H NMR (400 MHz, CDCl3): δ 11.15 (s, 1 H, NCHN), 7.69 (d, J = 8.4 Hz, 1 H, Ar–H), 7.59 (d, J = 8.4 Hz, 1 H, Ar–H), 7.49 (t, J = 8.4 Hz,1 H, Ar–H), 7.43 (m, 3 H, Ar–H), 7.19 (m, 3 H, Ar–H), 5.78 (s, 2H, N–CH2), 4.16 (s, 3H, N–CH3). 13C NMR (100 MHz, CDCl3): δ 142.6, 132.7, 132.0, 130.8, 129.3, 129.2, 129.0, 128.4, 128.3, 127.3, 113.8, 113.1, 53.5, 51.2, 34,1. IR, νmax (cm–1) (CH2Cl2): 3434, 3141, 3033, 2055, 1811, 1705, 1612, 1569, 1490, 1456, 1427, 1358, 1349, 1274, 1265, 1209, 1195, 1166, 1132, 1093, 1020, 855, 821, 791, 750, 705, 660, 605, 569, 560, 532, 467, 423.
Synthesis of Complex 2a
In a balloon, 1a (250 mg, 0.75 mmol) under argon gas was suspended in dichloromethane. Ag2O (175 mg, 0.75 mmol) and [IrCl2Cp*]2 (301 mg, 0.38 mmol) were added over and stirred at 39 °C for 24 h protected from light. The remaining solid residue was washed with diethyl ether and then dried under vacuum. Yield = 80%, 400 mg. Elemental analyses for C25H26Cl2IrN2S (648.67): C, 44.9310; H, 4.2528; N, 3.9534; S, 2.7159. 1H NMR (400 MHz, CDCl3): δ 7.79 (d, J = 8.0 Hz, 1 H, Ar–H), 7.70 (d, J = 7.2 Hz, 1 H, Ar–H), 7.39 (d, J = 7.6 Hz, 1 H, Ar–H), 7.35 (m, 3 H, Ar–H), 7.30 (m, 1 H, Ar–H), 7.12 (t, J = 8.0 Hz, 2 H, N–CH2), 1.68 (s, 15 H, C5(CH3)5). 13C NMR (100 MHz, CDCl3): δ 203.3, 143.6, 139.1, 136.6, 133.4, 132.3, 128.8, 128.3, 126.6, 125.3, 121.9, 117.3, 114.5, 109.9, 91.1, 55.3, 8.6. IR, νmax (cm–1) (CH2Cl2): 3418, 3060, 2965, 2910, 2221, 1599, 1510, 1483, 1454, 1379, 1357, 1314, 1284, 1270, 1207, 1141, 1116, 1053, 1029, 982, 913, 793, 762, 718, 674, 604, 552, 450, 432.
Synthesis of Complex 2b
In a balloon, 1b (250 mg, 0.76 mmol) and Ag2O (177 mg, 0.76 mmol) under argon gas were suspended in dichloromethane (5 mL) and stirred at 39 °C for 6 h protected from light. Then, [IrCl2Cp*]2 (303 mg, 0.38 mmol) was added and refluxed for 6 h. The remaining solid residue was washed with diethyl ether and then dried under vacuum. Yield = 75%, 380 mg. Elemental analyses for C26H28Cl2IrN3 (645,13): C, 46.4660; H, 4.8401; N, 5.8835. 1H NMR (400 MHz, CDCl3): δ 7.69 (d, J = 7.2 Hz, 1 H, Ar–H), 7.41 (d, J = 8.0 Hz, 1 H, Ar–H), 7.38 (d, J = 6.4 Hz, 1 H, Ar–H), 7.33 (t, J = 7.2 Hz, 1 H, Ar–H), 7.27 (t, J = 8.8 Hz, 2 H, Ar–H), 7.11 (t, J = 8.0 Hz, 1 H, Ar–H), 6.79 (d, J = 8.0 Hz, 1 H, Ar–H), 4.26 (s, 3 H, N–CH3), 1.69 (s, 15 H, C5(CH3)5). 13C NMR (100 MHz, CDCl3): δ 172.2, 140.5, 136.1, 134.3, 133.2, 132.2, 129.2, 127.9, 123.7, 123.5, 117.6, 111.3, 110.6, 110.0, 89.9, 51.3, 35.7, 9.2. IR, νmax (cm–1) (CH2Cl2): 3485, 3059, 2970, 2915, 2221, 1943, 1601, 1483, 1452, 1435, 1381, 1359, 1343, 1287, 1266, 1241, 1194, 1141, 1129, 1092, 1028, 976, 828, 747, 662, 615, 589, 559, 546, 444.
Synthesis of Complex 2c
Prepared according to procedure 2b using 1c (250 mg, 0.89 mmol), Ag2O (208 mg, 0.89 mmol), and [IrCl2Cp*]2 (358 mg, 0.45 mmol). Yield = 78%, 430 mg. Elemental analyses for C22H26Cl2IrN3 (595,11): C, 46.4570; H, 5.2603; N, 6.2050. 1H NMR (400 MHz, CDCl3): δ 7.80 (d, J = 8.0 Hz, 1 H, Ar–H), 7.66 (d, J = 7.6 Hz, 1 H, Ar–H), 7.55 (t, J = 7.6 Hz, 1 H, Ar–H), 7.39 (t, J = 7.6 Hz, 1 H, Ar–H), 6.94 (d, J = 2.0 Hz, 1 H, N–CHCH–N), 6.65 (d, J = 2.0 Hz, 1 H, N–CHCH–N), 5.07 (d, J = 14.4 Hz, 1 H, N–CH2), 4.02 (s, 3 H, N–CH3), 1.66 (s, 15 H, C5(CH3)5). 13C NMR (100 MHz, CDCl3): δ 157.9, 140.3, 133.6, 132.1, 130.8, 129.5, 123.9, 121.5, 117.6, 111.6, 90.6, 89.1, 51.8, 38.8, 9.2. IR, νmax (cm–1) (CH2Cl2): 3414, 2955, 2912, 2219, 1656, 1603, 1453, 1403, 1379, 1301, 1241, 1191, 1116, 1077, 1030, 824, 763, 729, 692, 617, 415.
Synthesis of Complex 2d
Prepared according to procedure 2b using 1d (250 mg, 0.99 mmol), Ag2O (228 mg, 0.99 mmol), and [IrCl2Cp*]2 (394 mg, 0.49 mmol). Yield = 61%, 430 mg. Elemental analyses for C25H29Cl2IrN2 (620,13): C, 45.0320; H, 4.9800; N, 4.7528. 1H NMR (400 MHz, CDCl3): δ 7.29 (m, 4 H, Ar–H), 7.25 (m, 1 H, Ar–H), 6.88 (d, J = 2.0 Hz, 1 H, N–CHCH–N), 6.63 (d, J = 2.0 Hz, 1 H, N–CHCH–N), 5.97 (d, J = 14.8 Hz, 1 H, N–CH2), 5.15 (d, J = 14.8 Hz, 1 H, N–CH2), 3.95 (s, 3 H, N-CH3), 1.58 (s, 15 H, C5(CH3)5). 13C NMR (100 MHz, CDCl3): δ 156.6, 136.8, 128.6, 128.5, 127.9, 123.4, 121.8, 88.8, 54.4, 38.7, 9.2. IR, νmax (cm–1) (CH2Cl2): 3167, 3051, 3107, 2994, 2941, 2917, 1578, 1510, 1496, 1458, 1403, 1385, 1370, 1354, 1290, 1219, 1181, 1160, 1109, 1082, 1028, 993, 928, 837, 785, 739, 701, 689, 607, 578, 470, 462, 447.
Synthesis of Complex 2e
Prepared according to procedure 2b using 1e (250 mg, 0.83 mmol), Ag2O (193 mg, 0.83 mmol), and [IrCl2Cp*]2 (398 mg, 0.41 mmol). Yield = 52%, 266 mg. 1H NMR (400 MHz, CDCl3): δ 7.62 (d, J = 7.6 Hz, 1 H, Ar–H), 7.45 (d, J = 7.6 Hz, 1 H, Ar–H), 7.34 (d, J = 7.6 Hz, 1 H, Ar–H), 7.23 (m, 3 H, Ar–H), 7.08 (d, J = 7.2 Hz, 1 H, Ar–H), 7.00 (t, J = 7.2 Hz, 1 H, Ar–H), 6.84 (t, J = 7.2 Hz, 1 H, Ar–H), 5.13 (d, J = 14.0 Hz, 1 H, N–CH2), 4.88 (d, J = 14.0 Hz, 1 H, N–CH2), 4.12 (s, 3 H, N–CH3), 1.71 (s, 15 H, C5(CH3)5). 13C NMR (100 MHz, CDCl3): δ 170.9, 143.3, 141.2, 138.0, 135.6, 134.0, 127.8, 124.5, 122.6, 122.3, 122.1, 109.9, 109.3, 91.2, 52.7, 33.9, 9.4. IR, νmax (cm–1) (CH2Cl2): 3436, 3049, 2970, 2914, 1733, 1575, 1558, 1484, 1454, 1430, 1406, 1390, 1334, 1258, 1211, 1190, 1155, 1092, 1029, 853, 821, 756, 748, 575, 545, 435.
Synthesis of Complex 3a
Prepared according to procedure 2a using 1a (250 mg, 0.75 mmol), Ag2O (175 mg, 0.75 mmol), and [RuCl2(p-cymene)]2 (231 mg, 0.38 mmol). Yield = 85%, 365 mg. Elemental analyze for C25H24Cl2N2RuS (556.51): C, 52.5850; H, 4.8610; N, 4.4582; S, 4.1602. 1H NMR (400 MHz, CDCl3): δ 7.78 (d, J = 8.4 Hz, 1 H, Ar–H), 7.74 (m, 1 H, Ar–H), 7.37 (m, 2 H, Ar–H), 7.32 (d, J = 7.6 Hz, 1 H, Ar–H), 7.28 (d, J = 7.6 Hz, 1 H, Ar–H), 7.13 (d, J = 8.0 Hz, 1 H, Ar–H), 6.98 (m, 1 H, Ar–H), 6.51 (s, 2 H, N–CH2), 5.46 (d, J = 6.0 Hz, 2 H, p-cymene-Ar–H), 5.31 (d, J = 5.6 Hz, 2 H, p-cymene-Ar–H), 2.87 (m, 1 H, p-cymene-CH), 2.16 (s, 3 H, p-cymene-CH3), 1.26 (d, J = 6.8 Hz, 6 H, p-cymene-(CH3)2). 13C NMR (100 MHz, CDCl3): δ 229.2, 143.8, 139.3, 136.4, 133.4, 132.9, 128.5, 128.2, 126.4, 124.9, 121.6, 117.1, 114.2, 110.1, 107.5, 100.8, 87.0, 86.9, 65.8, 55.8, 30.7, 22.3, 18.3, 15.2. IR, νmax (cm–1) (CH2Cl2): 3447, 3038, 2963, 2221, 1629, 1598, 1457, 1377, 1315, 1202, 1162, 1141, 1113, 1081, 1055, 980, 908, 762, 752, 673, 551, 512, 448.
Synthesis of Complex 3b
Prepared according to procedure 2b using 1b (250 mg, 0.76 mmol), Ag2O (177 mg, 0.76 mmol), and [RuCl2(p-cymene)]2 (233 mg, 0.38 mmol). Yield = 83%, 360 mg. Elemental analyses for C26H27Cl2N3Ru (553.06): C, 54.7640; H, 5.3754; N, 7.2315. 1H NMR (400 MHz, CDCl3): δ 7.68 (d, J = 7.2 Hz, 1 H, Ar–H), 7.41 (d, J = 8.0 Hz, 1 H, Ar–H), 7.35 (m, 2 H, Ar–H), 7.30 (m, 1 H, Ar–H), 7.15 (d, J = 8.0 Hz, 1 H, Ar–H), 7.12 (m, 1 H, Ar–H), 6.79 (d, J = 8.0 Hz, 1 H, N–CH2), 5.53 (d, J = 2.8 Hz, 2 H, p-cymene-Ar–H), 5.23 (d, J = 6.0 Hz, 2 H, p-cymene-Ar–H), 4.29 (s, 3 H, N-CH3), 3.01 (m, 1 H, p-cymene-CH), 2.09 (s, 3 H, p-cymene-CH3), 1.28 (d, J = 7.2 Hz, 6 H, p-cymene-(CH3)2). 13C NMR (100 MHz, CDCl3): δ 191.5, 140.5, 136.2, 134.5, 133.1, 132.3, 129.4, 127.9, 123.5, 123.3, 117.6, 110.9, 110.4, 110.2, 110.0, 99.2, 86.5, 83.5, 51.7, 36.8, 30.9, 29.7, 22.5, 18.8. IR, νmax (cm–1) (CH2Cl2): 3446, 3379, 3039, 2958, 2221, 1887, 1600, 1461, 1378, 1350, 1265, 1193, 1127, 1089, 1030, 973, 925, 842, 752, 664, 558, 442.
Synthesis of Complex 3c
Prepared according to procedure 2b using 1c (250 mg, 0.89 mmol), Ag2O (208 mg, 0.89 mmol), and [RuCl2(p-cymene)]2 (275 mg, 0.45 mmol). Yield = 86%, 403 mg. Elemental analyses for C22H25Cl2N3Ru (503.05): C, 49.1370; H, 5.2781; N, 8.0532. 1H NMR (400 MHz, CDCl3): δ 7.65 (t, J = 8.0 Hz, 2 H, Ar–H), 7.51 (t, J = 8.0 Hz, 1 H, Ar–H), 7.38 (t, J = 7.6 Hz, 1 H, Ar–H), 6.99 (d, J = 2.0 Hz, 1 H, N–CHCH–N), 7.66 (d, J = 2.0 Hz, 1 H, N–CHCH–N), 5.47 (d, J = 4.8 Hz, 2 H, p-cymene-Ar–H), 5.20 (d, J = 6.0 Hz, 2 H, p-cymene-Ar–H), 4.05 (s, 3 H, N–CH3), 2.95 (m, 1 H, p-cymene–CH), 2.10 (s, 3 H, p-cymene–CH3), 1.27 (d, J = 6.8 Hz, 6 H, p-cymene–(CH3)2). 13C NMR (100 MHz, CDCl3): δ 230.7, 178.1, 133.5, 132.3, 131.0, 128.6, 124.4, 121.9, 111.7, 109.0, 99.0, 83.1, 52.5, 39.8, 30.9, 18.7. IR, νmax (cm–1) (CH2Cl2): 3414, 3090, 2959, 2222, 1617, 1549, 1450, 1384, 1232, 1084, 865, 769, 689, 618, 468.
Synthesis of Complex 3d
Prepared according to procedure 2b using 1d (250 mg, 0.99 mmol), Ag2O (228 mg, 0.99 mmol), and [RuCl2(p-cymene)]2 (302 mg, 0.49 mmol). Yield = 57%, 403 mg. Elemental analyses for C21H26Cl2N2Ru (478.05): C, 48.8320; H, 5.8370; N, 5.6175. 1H NMR (400 MHz, CDCl3): δ 7.32 (m, 3 H, Ar–H), 7.25 (m, 2 H, Ar–H), 6.97 (d, J = 2.0 Hz, 1 H, N–CHCH–N), 7.81 (d, J = 2.0 Hz, 1 H, N–CHCH–N), 5.65 (s, 2 H, N–CH2), 5.30 (s, 2 H, p-cymene–Ar–H), 4.97 (s, 2 H, p-cymene–Ar–H), 3.99 (s, 3 H, N-CH3), 2.88 (m, 1 H, p-cymene–CH), 2.01 (s, 3 H, p-cymene–CH3), 1.21 (d, J = 6.8 Hz, 6 H, p-cymene–(CH3)2). 13C NMR (100 MHz, CDCl3): δ 174.3, 137.6, 128.8, 127.9, 127.6, 123.9, 122.9, 108.5, 98.8, 54.7, 39.7, 30.7, 18.7. IR, νmax (cm–1) (CH2Cl2): 3527, 3180, 3140, 3126, 3103, 3090, 3057, 3028, 2964, 2922, 2872, 1965, 1869, 1825, 1664, 1628, 1602, 1582, 1558, 1495, 1454, 1403, 1386, 1372, 1352, 1331, 1276, 1225, 1138, 1114, 1082, 1056, 1008, 990, 888, 836, 733, 726, 702, 682, 635, 621, 608, 470.
General Procedure for the N-Alkylation of Aniline with Alcohols
A Radley’s tube was charged with aniline (1.0 mmol), alcohols (1.0 mmol), KOtBu (1.5 mmol), and catalyst (1.0 mol %). The mixture was stirred to 120 °C for 24 h under air atmosphere. The reaction mixture was cooled to room temperature and filtered.
General Method for the N-Methylation of Anilines with Methanol
A Radley’s tube was charged with aniline (1.0 mmol), KOtBu (1.5 mmol), and catalyst (1.0 mol %) in methanol (0.4 mL). The mixture was stirred to 120 °C for 24 h under air atmosphere. The reaction mixture was cooled to room temperature and filtered.
N-Alkylation of Aniline with Alcohol Products42−51
N-Benzylaniline (12a)
1H NMR (400 MHz, CDCl3): δ 7.38 (m, 4 H, Ar–H), 7.30 (m, 1 H, Ar–H), 7.20 (m, 2 H, Ar–H), 6.74 (t, J = 7.2 Hz, 1 H, Ar–H), 6.67 (m, 2 H, Ar–H), 4.35 (s, 2 H, CH2), 4.04 (s, 1 H, N–H). 13C NMR (100 MHz, CDCl3): δ 148.2, 139.4, 129.3, 128.6, 127.5, 127.2, 117.6, 112.8, 48.3.
N-Benzyl-4-toluidine (12b)
1H NMR (400 MHz, CDCl3): δ 7.36 (m, 4 H, Ar–H), 7.27 (m, 1 H, Ar–H), 6.99 (d, J = 8.4 Hz, 2 H, Ar–H), 6.58 (m, 2 H, Ar–H), 4.32 (s, 2 H, CH2), 3.91 (s, 1 H, N–H), 2.25 (s, 3 H, CH3). 13C NMR (100 MHz, CDCl3): δ 145.9, 139.6, 129.7, 129.6, 128.6, 128.5, 127.5, 127.4, 127.1, 126.7, 112.9, 48.6, 20.4.
N-Benzyl-2-toluidine (12c)
1H NMR (400 MHz, CDCl3): δ 7.56 (m, 3 H, Ar–H), 7.52 (m, 2 H, Ar–H), 7.46 (m, 1 H, Ar–H), 7.29 (m, 1 H, Ar–H), 6.88 (t, J = 7.2 Hz, 1 H, Ar–H), 6.80 (d, J = 8.0 Hz, 1 H, Ar–H), 4.53 (s, 2 H, CH2), 4.02 (s, 1 H, N–H), 2.34 (s, 3 H, CH3). 13C NMR (100 MHz, CDCl3): δ 146.2, 139.7, 130.3, 128.8, 127.9, 127.7, 127.4, 127.3, 122.1, 117.4, 110.2, 48.4, 17.7.
N-Benzyl-2,4-dimethylaniline (12d)
1H NMR (400 MHz, CDCl3): δ 7.51 (m, 1 H, Ar–H), 7.35 (m, 3 H, Ar–H), 7.04 (m, 3 H, Ar–H), 6.86 (t, J = 7.6 Hz, 1 H, Ar–H), 4.12 (s, 2 H, CH2), 2.29 (s, 6 H, (CH3)2). 13C NMR (100 MHz, CDCl3): δ 144.7, 140.8, 135.6, 128.5, 127.6, 126.9, 126.2, 118.8, 116.9, 65.3, 52.8.
N-Benzyl-2-nitroaniline (12e)
1H NMR (400 MHz, CDCl3): δ 8.10 (d, J = 8.4 Hz, 1 H, Ar–H), 7.35 (m, 4 H, Ar–H), 6.80 (d, J = 8.4 Hz, 1 H, Ar–H), 6.69 (m, 1 H, Ar–H), 3.33 (s, 2 H, CH2).13C NMR (100 MHz, CDCl3): δ 144.7, 135.6, 128.5, 128.4, 128.2, 127.6, 126.9, 126.7, 126.1, 118.8, 116.9, 52.8.
N-Benzyl-4-methoxyaniline (12f)
1H NMR (400 MHz, CDCl3): δ 7.42 (m, 4 H, Ar–H), 7.33 (m, 1 H, Ar–H), 6.88 (m, 2 H, Ar–H), 6.74 (t, J = 7.2 Hz, 1 H, Ar–H), 6.66 (d, J = 7.6 Hz, 1 H, Ar–H), 4.69 (s, 1 H, N–H), 4.41 (s, 2 H, CH2), 3.90 (s, 3 H, CH3). 13C NMR (100 MHz, CDCl3): δ 146.8, 139.6, 138.2, 128.6, 127.5, 127.1, 121.3, 116.7, 110.1, 109.4, 55.4, 48.1.
N-Benzyl-2-methoxyaniline (12g)
1H NMR (400 MHz, CDCl3): δ 7.39 (m, 4 H, Ar–H), 7.31 (m, 1 H, Ar–H), 6.82 (m, 2 H, Ar–H), 6.64 (m, 2 H, Ar–H), 4.31 (s, 2 H, CH2), 3.77 (s, 3 H, CH3).13C NMR (100 MHz, CDCl3): δ 152.2, 142.5, 139.7, 128.6, 127.6, 127.2, 114.9, 114.1, 55.8, 49.3.
N-Benzyl-4-bromoaniline (12h)
1H NMR (400 MHz, CDCl3): δ 7.36 (d, J = 4.8 Hz, 4 H, Ar–H), 7.27 (m, 3 H, Ar–H), 6.51 (m, 2 H, Ar–H), 4.31 (s, 2 H, CH2), 4.08 (s, 1 H, N–H). 13C NMR (100 MHz, CDCl3): δ 147.1, 138.9, 131.9, 128.7, 127.4, 127.3, 114.4, 109.1, 48.2.
N-Benzyl-4-chloroaniline (12i)
1H NMR (400 MHz, CDCl3): δ 7.36 (m, 5 H, Ar–H), 7.16 (m, 2 H, Ar–H), 6.58 (m, 2 H, Ar–H), 4.33 (s, 2 H, CH2), 4.08 (s, 1 H, N–H). 13C NMR (100 MHz, CDCl3): δ 146.7, 138.9, 129.1, 128.7, 127.5, 127.4, 122.1, 113.9, 48.4.
N-benzyl-hexylamine (12j)
1H NMR (400 MHz, CDCl3): δ 7.32 (d, J = 4.4 Hz, 4 H, Ar–H), 7.25 (m, 1 H, Ar–H), 3.79 (s, 2 H, CH2), 2.63 (t, J = 7.2 Hz, 2 H, NH–CH2), 1.53 (m, 2 H, NHCH2CH2), 1.33 (m, 6 H, NHCH2CH2(CH2)3), 0.90 (t, J = 6.8 Hz, 3 H, NHCH2CH2(CH2)3–CH3). 13C NMR (100 MHz, CDCl3): δ 140.6, 128.3, 128.1, 126.8, 54.1, 49.5, 31.8, 30.1, 27.1, 22.6, 14.1.
N-Benzyl-2-naphthylamine (12k)
1H NMR (400 MHz, CDCl3): δ 7.81 (d, J = 8.0 Hz, 1 H, Ar–H), 7.74 (t, J = 9.2 Hz, 2 H, Ar–H), 7.48 (m, 5 H, Ar–H), 7.41 (m, 1 H, Ar–H), 7.33 (m, 1 H, Ar–H), 6.96 (m, 2 H, Ar–H), 4.49 (s, 2 H, CH2), 4.21 (s, 1 H, N–H). 13C NMR (100 MHz, CDCl3): δ 145.9, 139.3, 135.3, 129.1, 128.8, 127.8, 127.7, 127.4, 126.5, 126.1, 122.2, 117.9, 104.8, 48.4.
N-Benzyl-4-biphenylamine (12l)
1H NMR (400 MHz, CDCl3): δ 7.46 (m, 4 H, Ar–H), 7.35 (m, 5 H, Ar–H), 7.22 (m, 2 H, Ar–H), 7.13 (d, J = 7.6 Hz, 1 H, Ar–H), 6.79 (t, J = 7.6 Hz, 1 H, Ar–H), 6.67 (d, J = 8.4 Hz, 1 H, Ar–H), 4.41 (s, 1 H, N–H), 4.34 (s, 2 H, CH2). 13C NMR (100 MHz, CDCl3): δ 144.9, 139.5, 139.4, 130.2, 129.4, 128.9, 128.7, 128.6, 127.7, 127.2, 127.0, 117.1, 110.7, 48.1.
N-(4-Methylbenzyl)aniline (13a)
1H NMR (400 MHz, CDCl3): δ 7.32 (d, J = 8.0 Hz, 2 H, Ar–H), 7.24 (m, 4 H, Ar–H), 6.78 (m, 1 H, Ar–H), 6.69 (m, 2 H, Ar–H), 4.33 (s, 2 H, CH2), 4.02 (s, 1 H, N–H), 2.41 (s, 3 H, CH3). 13C NMR (100 MHz, CDCl3): δ 148.3, 136.9, 136.4, 129.4, 129.3, 127.6, 117.5, 112.9, 48.1, 21.2.
N-(4-Bromobenzyl)aniline (13b)
1H NMR (400 MHz, CDCl3): δ 7.47 (m, 2 H, Ar–H), 7.26 (m, 2 H, Ar–H), 7.18 (m, 2 H, Ar–H), 6.74 (m, 1 H, Ar–H), 6.62 (d, J = 8.8 Hz, 2 H, Ar–H), 4.30 (s, 2 H, CH2), 4.06 (s, 1 H, N–H). 13C NMR (100 MHz, CDCl3): δ 147.8, 138.5, 131.7, 129.3, 129.0, 120.9, 117.8, 112.9, 47.7.
N-(2-Bromobenzyl)aniline (13c)
1H NMR (400 MHz, CDCl3): δ 7.44 (m, 2 H, Ar–H), 7.23 (m, 4 H, Ar–H), 6.77 (m, 1 H, Ar–H), 6.66 (m, 2 H, Ar–H), 4.47 (s, 2 H, CH2), 4.17 (s, 1 H, N–H). 13C NMR (100 MHz, CDCl3): δ 147.9, 136.9, 133.3, 132.3, 129.6, 129.4, 129.1, 128.5, 121.3, 117.9, 113.0, 45.9.
N-(4-Chlorobenzyl)aniline (13d)
1H NMR (400 MHz, CDCl3): δ 7.39 (m, 4 H, Ar–H), 7.29 (m, 2 H, Ar–H), 6.85 (m, 1 H, Ar–H), 6.70 (m, 2 H, Ar–H), 4.36 (s, 2 H, CH2), 4.10 (s, 1 H, N–H). 13C NMR (100 MHz, CDCl3): δ 147.9, 138.2, 132.9, 129.4, 129.1, 128.9, 128.8, 117.9, 113.0, 47.6.
N-(4-Methoxybenzyl)aniline (13e)
1H NMR (400 MHz, CDCl3): δ 7.37 (d, J = 8.4 Hz, 2 H, Ar–H), 7.26 (m, 2 H, Ar–H), 6.97 (m, 2 H, Ar–H), 6.81 (m, 1 H, Ar–H), 6.71 (m, 2 H, Ar–H), 4.32 (s, 2 H, CH2), 4.02 (s, 1 H, N–H), 3.87 (s, 3 H, CH3). 13C NMR (100 MHz, CDCl3): δ 158.9, 148.3, 131.5, 129.3, 128.9, 117.5, 114.1, 112.9, 55.3, 47.8.
N-(2,4,6-trimethylbenzyl)aniline (13f)
1H NMR (400 MHz, CDCl3): δ 7.37 (m, 2 H, Ar–H), 7.06 (s, 2 H, Ar–H), 6.89 (t, J = 6.8 Hz, 1 H, Ar–H), 6.80 (d, J = 8.0 Hz, 2 H, Ar–H), 4.34 (s, 2 H, CH2), 3.54 (s, 1 H, N–H), 2.51 (s, 6 H, (CH3)2), 2.46 (s, 3 H, CH3). 13C NMR (100 MHz, CDCl3): δ 148.8, 137.6, 137.4, 132.4, 129.4, 129.2, 117.4, 112.6, 42.5, 21.1, 19.6.
Acknowledgments
Financial support from Ege University (Project FYL-2018-20197) is gratefully acknowledged. The authors acknowledge Scientific and Technological Research Application and Research Center, Sinop University, Turkey, for the use of the Bruker D8 QUEST diffractometer. They are grateful to Ege University Planning and Monitoring Coordination of Organizational Development and Directorate of Library and Documentation for their support in editing and proofreading service of this study.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c06341.
1H and 13C NMR spectra; IR spectrum; 1H NMR monitoring of N-methylation of aniline with methanol; elemental analysis results of the complexes 2a–d and 3a–d; and crystal data and structure refinement parameters for complexes 2b, 3a, and 3c (PDF)
Accession Codes
Crystallographic data for the structural analysis have been deposited with the Cambridge Crystallographic Data Centre, CCDC Nos. 1935381, 2165787, and 2165788. Copies of this information may be obtained free of charge from the Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, U.K. (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk or www:http://www.ccdc.cam.ac.uk).
The authors declare no competing financial interest.
Supplementary Material
References
- a Herrmann W. A.; A W. N-Heterocyclic Carbenes: A New Concept in Organometallic Catalysis. Angew. Chem., Int. Ed. 2002, 41, 1290–1309. . [DOI] [PubMed] [Google Scholar]; b Bourissou D.; Guerret O.; Gabbai F. P.; Bertrand G. Stable Carbenes. Chem. Rev. 2000, 100, 39–92. 10.1021/cr940472u. [DOI] [PubMed] [Google Scholar]; c Scott N. M.; Nolan S. P. Stabilization of Organometallic Species Achieved by the Use of N-Heterocyclic Carbene (NHC) Ligands. Eur. J. Org. Chem. 2005, 2005, 1815–1828. 10.1002/ejic.200500030. [DOI] [Google Scholar]
- a Crabtree R. H. J. NHC ligands versus cyclopentadienyls and phosphines as spectator ligands in organometallic catalysis. J. Organomet. Chem. 2005, 690, 5451–5457. 10.1016/j.jorganchem.2005.07.099. [DOI] [Google Scholar]; b Díez-González S.; Nolan S. P. Stereoelectronic parameters associated with N-heterocyclic carbene (NHC) ligands: A quest for understanding. Coord. Chem. Rev. 2007, 251, 874–883. 10.1016/j.ccr.2006.10.004. [DOI] [Google Scholar]
- a Crudden C. M.; Allen D. P. Stability and reactivity of N-heterocyclic carbene complexes. Coord. Chem. Rev. 2004, 248, 2247–2273. 10.1016/j.ccr.2004.05.013. [DOI] [Google Scholar]; b Normand A. T.; Cavell K. J. Donor-Functionalised N-Heterocyclic Carbene Complexes of Group 9 and 10 Metals in Catalysis: Trends and Directions. Eur. J. Inorg. Chem. 2008, 2008, 2781–2800. 10.1002/ejic.200800323. [DOI] [Google Scholar]
- a Corberan R.; Peris E. Highly Stable Cp*–Ir(III) Complexes with N-Heterocyclic Carbene Ligands as C–H Activation Catalysts for the Deuteration of Organic Molecules. Organometallics 2008, 27, 1954–1958. 10.1021/om800095t. [DOI] [PubMed] [Google Scholar]; b Gnanamgari D.; Moores A.; Rajaseelan E.; Crabtree R. H. Transfer Hydrogenation of Imines and Alkenes and Direct Reductive Amination of Aldehydes Catalyzed by Triazole-Derived Iridium(I) Carbene Complexes. Organometallics 2007, 26, 1226–1230. 10.1021/om060938m. [DOI] [Google Scholar]
- a Liu Z.; Zhang T.; Shi M. Cyclometalated cis-Chelated Bidentate N-Heterocyclic Carbene Palladium Complexes: Synthetic, Structural, and Catalytic Studies. Organometallics 2008, 27, 2668–2671. 10.1021/om800069p. [DOI] [Google Scholar]; b Xi Z.; Liu B.; Chen W. Room-Temperature Kumada Cross-Coupling of Unactivated Aryl Chlorides Catalyzed by N-Heterocylic Carbene-Based Nickel((II)) Complexes. J. Org. Chem. 2008, 73, 3954–3957. 10.1021/jo800197u. [DOI] [PubMed] [Google Scholar]
- Sanford M. S.; Love J. A.; Grubbs R. H. A Versatile Precursor for the Synthesis of New Ruthenium Olefin Metathesis Catalysts. Organometallics 2001, 20, 5314–5318. 10.1021/om010599r. [DOI] [Google Scholar]
- Poyatos M.; Maisse-Francois A.; Bellemin Laponnaz S.; Gade L. H. oordination Chemistry of a Modular N,C-Chelating Oxazole-Carbene Ligand and Its Applications in Hydrosilylation Catalysis. Organometallics 2006, 25, 2634–2641. 10.1021/om060166u. [DOI] [Google Scholar]
- a Huang M.; Li Y.; Lan X.-B.; Liu J.; Zhao C.; Liu Y.; Ke Z. Ruthenium((II)) complexes with N-heterocyclic carbene–phosphine ligands for the N-alkylation of amines with alcohols. Org. Biomol. Chem. 2021, 19, 3451–3461. 10.1039/D1OB00362C. [DOI] [PubMed] [Google Scholar]; b Hamid M. H. S. A.; Allen C. L.; Lamb G. W.; Maxwell A. C.; Maytum H. C.; Watson A. J. A.; Williams J. M. J. Ruthenium-Catalyzed N-Alkylation of Amines and Sulfonamides Using Borrowing Hydrogen Methodology. J. Am. Chem. Soc. 2009, 131, 1766–1774. 10.1021/ja807323a. [DOI] [PubMed] [Google Scholar]; c Fernández F. E.; Puerta M. C.; Valerga P. Ruthenium((II)) Picolyl-NHC Complexes: Synthesis, Characterization, and Catalytic Activity in Amine N-alkylation and Transfer Hydrogenation Reactions. Organometallics 2012, 31, 6868–6879. 10.1021/om300692a. [DOI] [Google Scholar]; d Karaca E. Ö.; Dehimat Z. I.; Yaşar S.; Gürbüz N.; Tebbani D.; Çetinkaya B.; Özdemir İ. Ru(II)-NHC catalysed N-Alkylation of amines with alcohols under solvent-free conditions. Inorg. Chim. Acta. 2021, 520, 120294 10.1016/j.ica.2021.120294. [DOI] [Google Scholar]; e Yiğit B.; Karaca E. Ö.; Yiğit M.; Gürbüz N.; Arslan H.; Özdemir İ. Active ruthenium((II))-NHC complexes for alkylation of amines with alcohols using solvent-free conditions. Polyhedron 2020, 175, 114234 10.1016/j.poly.2019.114234. [DOI] [Google Scholar]; f Kaloğlu M.; Gürbüz N.; Sémeril D.; Özdemir İ. Ruthenium((II))-(p-cymene)-N-Heterocyclic Carbene Complexes for the N-Alkylation of Amine Using the Green Hydrogen Borrowing Methodology. Eur. J. Inorg. Chem. 2018, 10, 1236–1243. 10.1002/ejic.201701479. [DOI] [Google Scholar]; g Chakraborty S.; Piszel P. E.; Brennessel W. W.; Jones W. D. A Single Nickel Catalyst for the Acceptorless Dehydrogenation of Alcohols and Hydrogenation of Carbonyl Compounds. Organometallics 2015, 34, 5203–5206. 10.1021/acs.organomet.5b00824. [DOI] [Google Scholar]; h Shan S. P.; Xiaoke X.; Gnanaprakasam B.; Dang T. T.; Ramalingam B.; Huynh H. V.; Seayad A. M. Benzimidazolin-2-ylidene N-heterocyclic carbene complexes of ruthenium as a simple catalyst for the N-alkylation of amines using alcohols and diols. RSC Adv. 2015, 5, 4434–4442. 10.1039/C4RA15398G. [DOI] [Google Scholar]; i Kaloglu N.; Özdemir İ.; Gürbüz N.; Achard M.; Bruneau C. Benzimidazolium sulfonate ligand precursors and application in ruthenium-catalyzed aromatic amine alkylation with alcohols. Catal. Commun. 2016, 74, 33–38. 10.1016/j.catcom.2015.10.028. [DOI] [Google Scholar]
- a Wang D.; Guo X.-Q.; Wang C.-X.; Wang Y.-N.; Zhong R.; Zhu X.-H.; Cai L.-H.; Gao Z.-W.; Hou X.-F. An Efficient and Recyclable Catalyst for N-Alkylation of Amines and β-Alkylation of Secondary Alcohols with Primary Alcohols: SBA-15 Supported N-Heterocyclic Carbene Iridium Complex. Adv. Synth. Catal. 2013, 355, 1117–1125. 10.1002/adsc.201200732. [DOI] [Google Scholar]; b Fernandes A.; Royo B. Water-Soluble Iridium N-Heterocyclic Carbene Complexes for the Alkylation of Amines with Alcohols. ChemCatChem 2017, 9, 3912–3917. 10.1002/cctc.201700678. [DOI] [Google Scholar]
- a Toyooka G.; Tuji A.; Fujita K. Efficient and Versatile Catalytic Systems for the N-Methylation of Primary Amines with Methanol Catalyzed by N-Heterocyclic Carbene Complexes of Iridium. Synthesis 2018, 50, 4617–4626. 10.1055/s-0037-1610252. [DOI] [Google Scholar]; b Huang S.; Hong X.; Cui H.-Z.; Zhou Q.; Lin Y.-J.; Ho X.-F. N-Methylation of ortho-substituted aromatic amines with methanol catalyzed by 2-arylbenzo[d]oxazole NHC–Ir(III) complexes. Dalton Trans. 2019, 48, 5072–5082. 10.1039/C9DT00218A. [DOI] [PubMed] [Google Scholar]; c Campos J.; Sharninghausen L. S.; Manas M. G.; Crabtree R. H. Methanol Dehydrogenation by Iridium N-Heterocyclic Carbene Complexes. Inorg. Chem. 2015, 54, 5079–5084. 10.1021/ic502521c. [DOI] [PubMed] [Google Scholar]
- Watanabe T. Y.; Tsuji Y.; Oshugi Y. The Ruthenium Catalyzed N-Alkylation and N-Heterocyclization of Aniline Using Alcohols and Aldehydes. Tetrahedron Lett. 1981, 22, 2667–2770. 10.1016/S0040-4039(01)92965-X. [DOI] [Google Scholar]
- a Singh A.; Maji A.; Joshi M.; Choudhury A. R.; Ghosh K. Designed pincer ligand supported Co(II)-based catalysts for dehydrogenative activation of alcohols: Studies on N-alkylation of amines, α-alkylation of ketones and synthesis of quinolines. Dalton Trans. 2021, 50, 8567–8587. 10.1039/D0DT03748F. [DOI] [PubMed] [Google Scholar]; b Huang M.; Li Y.; Li Y.; Liu J.; Shu S.; Liu Y.; Ke Z. Room temperature N-heterocyclic carbene manganese catalyzed selective N-alkylation of anilines with alcohols. Chem. Commun. 2019, 55, 6213–6216. 10.1039/C9CC02989C. [DOI] [PubMed] [Google Scholar]; c Fernandes A.; Royo B. Water-Soluble Iridium N-Heterocyclic Carbene Complexes for the Alkylation of Amines with Alcohols. ChemCatChem 2017, 9, 3912–3917. 10.1002/cctc.201700678. [DOI] [Google Scholar]; d Ramachandran R.; Prakash G.; Viswanathamurthi P.; Malecki J. G. Ruthenium(II) complexes containing phosphino hydrazone/thiosemicarbazone ligand: An efficient catalyst for regioselective N-alkylation of amine via borrowing hydrogen methodology. Inorg. Chim. Acta 2018, 477, 122–129. 10.1016/j.ica.2018.03.007. [DOI] [Google Scholar]; e Prakash G.; Nirmala M.; Ramachandran R.; Viswanathamurthi P.; Malecki J. G.; Sanmartin J. Heteroleptic binuclear copper(I) complexes bearing bis(salicylidene)hydrazone ligands: Synthesis, crystal structure and application in catalytic N-alkylation of amines. Polyhedron 2015, 89, 62–69. 10.1016/j.poly.2014.12.015. [DOI] [Google Scholar]; f Mamidala R.; Mukundam V.; Dhanunjayarao K.; Venkatasubbaiah K. Cyclometalated palladium pre-catalyst for N-alkylation of amines using alcohols and regioselective alkylation of sulfanilamide using aryl alcohol. Tetrahedron 2017, 73, 2225–2233. 10.1016/j.tet.2017.03.001. [DOI] [Google Scholar]; g Winans C. F.; Adkins H. The Alkylation of Amines as Catalyzed by Nickel. J. Am. Chem. Soc. 1932, 54, 306. 10.1021/ja01340a046. [DOI] [Google Scholar]; h Yan T.; Feringa B. L. T.; Barta K. Iron catalysed direct alkylation of amines with alcohols. Nat. Commun. 2014, 5, 5602 10.1038/ncomms6602. [DOI] [PubMed] [Google Scholar]
- Butters M.; Catterick D.; Craig A.; Curzons A.; Dale D.; Gillmore A.; Green S. P.; Marziano I.; Sherlock J.-P.; White W. Critical Assessment of Pharmaceutical ProcessesA Rationale for Changing the Synthetic Route. Chem. Rev. 2006, 106, 3002–3027. 10.1021/cr050982w. [DOI] [PubMed] [Google Scholar]
- Lawrence S. A.Amines: Synthesis, Properties and Applications, Cambridge University: Cambridge, 2004. [Google Scholar]
- a Nugent S. T. C.; El-Shazlya M. Chiral Amine Synthesis – Recent Developments and Trends for Enamide Reduction, Reductive Amination, and Imine Reduction. Adv. Synth. Catal. 2010, 352, 753–819. 10.1002/adsc.200900719. [DOI] [Google Scholar]; b Gomez S.; Peters J. A.; Maschmeyer T. The Reductive Amination of Aldehydes and Ketones and the Hydrogenation of Nitriles: Mechanistic Aspects and Selectivity Control. Adv. Synth. Catal. 2002, 344, 1037–1057. . [DOI] [Google Scholar]
- Salvatore R. N.; Yoon C. H.; Jung K. W. Synthesis of secondary amines. Tetrahedron 2001, 57, 7785–7811. 10.1016/S0040-4020(01)00722-0. [DOI] [Google Scholar]
- Kolesnikov P. N.; Yagafarov N. Z.; Usanov D. L.; Maleev V. I.; Chusov D. Ruthenium-Catalyzed Reductive Amination without an External Hydrogen Source. Org. Lett. 2015, 17, 173–175. 10.1021/ol503595m. [DOI] [PubMed] [Google Scholar]
- Salomé C.; Schmitt M.; Bourguignon J.-J. Novel access to 1,4-benzodiazepin-2-ones via the Buchwald reaction and application to the synthesis of novel heterocyclics. Tetrahedron Lett. 2012, 53, 1033–1035. 10.1016/j.tetlet.2011.12.045. [DOI] [Google Scholar]
- Cantrell G. K.; Meyer T. Y. Catalytic CN Bond Formation by Metal-Imide-Mediated Imine Metathesis. J. Am. Chem. Soc. 1998, 120, 8035–8042. 10.1021/ja981272t. [DOI] [Google Scholar]
- Huang M.; Li Y.; Liu J.; Lan X.-B.; Liu Y.; Zhaoa C.; Ke Z. A bifunctional strategy for N-heterocyclic carbene-stabilized iridium complex-catalyzed N-alkylation of amines with alcohols in aqueous media. Green Chem. 2019, 21, 219–224. 10.1039/C8GC02298D. [DOI] [Google Scholar]
- Yiğit B.; Karaca E. Ö.; Yiğit M.; Gürbüz N.; Arslan H.; Özdemir İ. Active ruthenium(II)-NHC complexes for alkylation of amines with alcohols using solvent-free conditions. Polyhedron 2019, 175, 114234 10.1016/j.poly.2019.114234. [DOI] [Google Scholar]
- Huang M.; Li Y.; Lan X.-B.; Liu J.; Zhao C.; Liu Y.; Ke Z. Ruthenium((II)) complexes with N-heterocyclic carbene–phosphine ligands for the N-alkylation of amines with alcohols. Org. Biomol. Chem. 2021, 19, 3451–3461. 10.1039/D1OB00362C. [DOI] [PubMed] [Google Scholar]
- a Corma A.; Navas J.; Sabater M. J. Advances in One-Pot Synthesis through Borrowing Hydrogen Catalysis. Chem. Rev. 2018, 118, 1410–1459. 10.1021/acs.chemrev.7b00340. [DOI] [PubMed] [Google Scholar]; b Rodríguez-Bárzano A.; Fonseca J. D. A.; Blacker A. J.; McGowan P. C. Ruthenium Halide Complexes as N-Alkylation Catalysts. Eur. J. Inorg. Chem. 2014, 11, 1974–1983. 10.1002/ejic.201400117. [DOI] [Google Scholar]; c Yang F. L.; Wang Y. H.; Ni Y. F.; Gao X.; Song B.; Zhu X.; Hao X. Q. An Efficient Homogenized Ruthenium(II) Pincer Complex for N-Monoalkylation of Amines with Alcohols. Eur. J. Org. Chem. 2017, 2017, 3481–3486. 10.1002/ejoc.201700486. [DOI] [Google Scholar]
- Prades A.; Corberán R.; Poyatos M.; Peris E. [IrCl2Cp*(NHC)] Complexes as Highly Versatile Efficient Catalysts for the Cross-Coupling of Alcohols and Amines. Chem. - Eur. J. 2008, 14, 11474–11479. 10.1002/chem.200801580. [DOI] [PubMed] [Google Scholar]
- Çakır S.; Kavukcu S. B.; Karabıyık H.; Rethinam S.; Türkmen H. C(acyl)–C(sp2) and C(sp2)–C(sp2) Suzuki–Miyaura cross-coupling reactions using nitrile-functionalized NHC palladium complexes. RSC Adv. 2021, 11, 37684–37699. 10.1039/D1RA07231E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S.; Ishitani H. Catalytic Enantioselective Addition to Imines. Chem. Rev. 1999, 99, 1069–1094. 10.1021/cr980414z. [DOI] [PubMed] [Google Scholar]
- Driver M. S.; Hartwig J. F. A Second-Generation Catalyst for Aryl Halide Amination: Mixed Secondary Amines from Aryl Halides and Primary Amines Catalyzed by (DPPF)PdCl2. J. Am. Chem. Soc. 1996, 118, 7217–7218. 10.1021/ja960937t. [DOI] [Google Scholar]
- Saidi O.; Blacker A. J.; Farah M. M.; Marsden S. P.; Williams J. M. J. Selective amine cross-coupling using iridium-catalyzed ″borrowing hydrogen″ methodology.. Angew. Chem., Int. Ed. 2009, 48, 7375–7378. 10.1002/anie.200904028. [DOI] [PubMed] [Google Scholar]
- Guo D. L.; Huang H.; Xu J. Y.; Jiang H. L.; Liu H. Efficient Iron-Catalyzed N-Arylation of Aryl Halides with Amines. Org. Lett. 2008, 10, 4513–4516. 10.1021/ol801784a. [DOI] [PubMed] [Google Scholar]
- Gomez S.; Peters J. A.; Maschmeyer T. The Reductive Amination of Aldehydes and Ketones and the Hydrogenation of Nitriles: Mechanistic Aspects and Selectivity Control. Adv. Synth. Catal. 2002, 344, 1037–1057. . [DOI] [Google Scholar]
- Dang T. T.; Ramalingam B.; Seayad A. M. Efficient Ruthenium-Catalyzed N-Methylation of Amines Using Methanol. ACS Catal. 2015, 5, 4082–4088. 10.1021/acscatal.5b00606. [DOI] [Google Scholar]
- Liu Z.; Yang Z.; Yu X.; Zhang H.; Yu B.; Zhao Y.; Liu Z. Efficient Cobalt-Catalyzed Methylation of Amines Using Methanol. Adv. Synth. Catal. 2017, 359, 4278–4283. 10.1002/adsc.201701044. [DOI] [Google Scholar]
- White C.; Yates A.; Maitlis P. M.; Heinekey D. M.. (η5-Pentamethylcyclopentadienyl)Rhodium and −Iridium Compounds. In Inorganic Syntheses; John Wiley & Sons, 1992; Vol. 29, pp 228–234. [Google Scholar]
- Bennett M. A.; Smith A. K. Arene ruthenium(II) complexes formed by dehydrogenation of cyclohexadienes with ruthenium(III) trichloride. J. Chem. Soc. Dalton Trans. 1974, 2, 233–241. 10.1039/dt9740000233. [DOI] [Google Scholar]
- Roy B. C.; Debnath S.; Chakrabarti K.; Paul B.; Maji M.; Kundu S. ortho-Amino group functionalized 2,2′-bipyridine based Ru(II) complex catalysed alkylation of secondary alcohols, nitriles and amines using alcohols. Org. Chem. Front. 2018, 5, 1008–1018. 10.1039/C7QO01061C. [DOI] [Google Scholar]
- Sheldrick G. M. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, 64, 112–122. 10.1107/S0108767307043930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. M. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. 10.1107/s2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APEX2; Bruker AXS Inc.: Madison Wisconsin USA, 2013.
- Macrae C. F.; Bruno I. J.; Chisholm J. A.; Edgington P. R.; McCabe P.; Pidcock E.; Rodriguez-Monge L.; Taylor R.; van de Streek J.; Wood P. A. Mercury CSD 2.0 – new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. 10.1107/S0021889807067908. [DOI] [Google Scholar]
- Farrugia L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854. 10.1107/S0021889812029111. [DOI] [Google Scholar]
- a Zhao D.; Fei Z.; Geldbach T. J.; Scopelliti R.; Dyson P. J. Nitrile-Functionalized Pyridinium Ionic Liquids: Synthesis, Characterization, and Their Application in Carbon–Carbon Coupling Reactions. J. Am. Chem. Soc. 2004, 126, 15876–15882. 10.1021/ja0463482. [DOI] [PubMed] [Google Scholar]; b Taskin M.; Cognigni A.; Zirbs R.; Reimhult E.; Bica K. Surface-active ionic liquids for palladium-catalysed cross coupling in water: effect of ionic liquid concentration on the catalytically active species. RSC Adv. 2017, 7, 41144–41151. 10.1039/C7RA07757B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösler S.; Ertl M.; Irrgang T.; Kempe R. Cobalt-Catalyzed Alkylation of Aromatic Amines by Alcohols. Angew. Chem., Int. Ed. 2015, 54, 15046–15050. 10.1002/anie.201507955. [DOI] [PubMed] [Google Scholar]
- Ohta H.; Yuyama Y.; Uozumi Y.; Yamada Y. M. In-water dehydrative alkylation of ammonia and amines with alcohols by a polymeric bimetallic catalyst. Org. Lett. 2011, 13, 3892–3895. 10.1021/ol201422s. [DOI] [PubMed] [Google Scholar]
- Lee C. C.; Liu S. T. Preparation of secondary and tertiary amines from nitroarenes and alcohols. Chem. Commun. 2011, 47, 6981–6983. 10.1039/c1cc11609f. [DOI] [PubMed] [Google Scholar]
- Elangovan S.; Neumann J.; Sortais J. B.; Junge K.; Darcel C.; Beller M. Efficient and selective N-alkylation of amines with alcohols catalysed by manganese pincer complexes. Nat. Commun. 2016, 7, 12641 10.1038/ncomms12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastalir M.; Tomsu G.; Pittenauer E.; Allmaier G.; Kirchner K. Co(II) PCP Pincer Complexes as Catalysts for the Alkylation of Aromatic Amines with Primary Alcohols. Org. Lett. 2016, 18, 3462–3465. 10.1021/acs.orglett.6b01647. [DOI] [PubMed] [Google Scholar]
- Corre Y.; Iali W.; Hamdaoui M.; Trivelli X.; Djukic J. P.; Agbossou-Niedercorn F.; Michon C. Efficient hydrosilylation of imines using catalysts based on iridium(III) metallacycles. Catal. Sci. Technol. 2015, 5, 1452–1458. 10.1039/C4CY01233J. [DOI] [Google Scholar]
- Homberg L.; Roller A.; Hultzsch K. C. A Highly Active PN3 Manganese Pincer Complex Performing N-Alkylation of Amines under Mild Conditions. Org. Lett. 2019, 21, 3142–3147. 10.1021/acs.orglett.9b00832. [DOI] [PubMed] [Google Scholar]
- Wetzel A.; Wöckel S.; Schelwies M.; Brinks M. K.; Rominger F.; Hofmann P.; Limbach M. Selective alkylation of amines with alcohols by Cp*–Iridium(III) halfsandwich complexes. Org. Lett. 2013, 15, 266–269. 10.1021/ol303075h. [DOI] [PubMed] [Google Scholar]
- Cui X.; Deng Y.; Shi F. Reductive N-alkylation of nitro compounds to N-Alkyl and N,Ndialkyl amines with glycerol as the hydrogen source. ACS Catal. 2013, 3, 808–811. 10.1021/cs400049b. [DOI] [Google Scholar]
- Yang H.; Cui X.; Dai X.; Deng Y.; Shi F. Carbon-catalysed reductive hydrogen atom transfer reactions. Nat. Commun. 2015, 6, 6478 10.1038/ncomms7478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.