Abstract
Exosomes are lipid bilayer vesicles in biological fluids, which can participate in biological processes by mediating intercellular communication and activating intracellular signaling pathways, especially cancerogenic processes, such as proliferation, metastasis, invasion, and immune regulation of cancer cells. Besides, cancer-derived exosomes are also involved in tumor diagnosis and therapy as biomarkers and nanotransport devices. This article reviews the latest research progress on the biological regulation and disease diagnosis of exosomes in tumors, with the aim of providing new ideas for the clinical treatment of cancers.
1. Introduction
In 1983,1 Rose M. Johnstone et al. discovered that sheep reticulocytes can release a vesicle, the secretion of which caused reticulocytes to lose most of their active membrane proteins, thereby transforming them into mature erythrocytes. This vesicle was then considered as a way for cells to expel excess membrane proteins. With the deepening of research, he defined this vesicle as the “exosome” in 1987.2 Today, it has been shown that exosomes are significant mediators of intercellular communication, and play important roles in transmitting genetic information, activating intracellular signaling pathways, regulating cell growth, and modulating body immunity.
Exosomes are defined as microvesicles of 30–100 nm in diameter formed by the invagination of the plasma membrane.3 And exosomes of different cellular origins are highly variable in number and content. The signal transmission function of exosomes depends on the specific cargo they transport, while the cargo of exosomes depends on the microenvironment in which they are located as well as on the cellular origin. Under normal conditions, maintaining the stability of the human internal environment requires delicate cell-to-cell communication. Exosomes travel in body fluids and cross biological barriers to regulate the function and biological behavior of cells.4 Due to the unrestricted growth and invasion traits of tumors, exosomes are higher in patients stimulated by the tumor microenvironment. Most cargos are able to promote tumor proliferation and metastasis, for example, activating proliferative signals, inducing epithelial-mesenchymal transition, promoting vascular growth, establishing premetastatic ecological niches and evading immune effects. However, further studies are needed to determine whether tumor exosomes have their unique functions. It is worth discussing that cargo in some exosomes is downregulated in tumor cells, whereas artificially overexpressing these genes suppresses tumor growth and invasion.5−7
Exosomes are highly valuable for research in cancer growth, diagnosis, treatment, and prognosis with their richness in function and expression, and it is worthy of in-depth discussion and summary. This article reviews the latest progress in the biological regulation, diagnosis, and treatment of exosomes in tumors8 (Figure 1).
Figure 1.
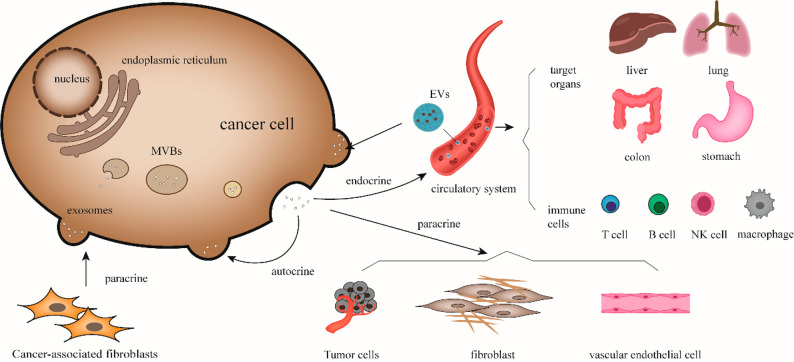
Biological regulation of exosomes on cancer cell. Exosomes released from cancer cells can act on neighboring tumor cells, vascular endothelial cells, and fibroblasts through paracrine secretion. They can also act on themselves through autocrine release of exosomes carrying signal molecules or enter the systemic circulation to target organs, such as liver, lung, colon, and stomach. They affect various immune cells and regulate the tumor microenvironment and the immune response of the body. Correspondingly, exosomes from external sources can act on cancer cells and promote their progression.
2. Literature Research
We searched the PubMed database for recently published original articles related to the topic. We used the PubMed Advanced Search Builder and Medical Subject Headings (MeSH) to add search terms, including exosomes, cancer or tumor, biological regulation, immunoregulation, cancer diagnosis, and exosome biomarkers. We retrieved a total of 8796 works related to tumors and exosomes. The number has increased exponentially especially in the past decade. Of these, 2456 articles were related to bioregulation and 4047 were related to diagnosis. Subsequently, we investigated prospective clinical studies conducted in the ClinicalTrials.gov registry.
3. The Regulation of Exosomes on Tumor Growth
3.1. Autocrine Regulation of Exosomes
Autocrine regulation refers to the ability of cells to release signaling factors in the form of exosomes, which act on themselves or on the same type of cells as themselves to exert regulatory effects. Cancer cells have a certain autonomy, which can actively proliferate, migrate and invade. This autonomy depends on their autocrine regulation to some extent. For instance, hypoxia-inducible factor HIF-1 can regulate a variety of genes under hypoxic conditions to enable cells to survive under hypoxia. Meshach Asare-Werehene et al.9 first discovered that circulating plasma gelsolin (pGSN) can be secreted and transported through exosomes, and it can activate α5β1 integrin-FAK-Akt-HIF1α signaling pathway and upregulate self-expression in an autocrine manner, thereby promoting the survival of ovarian cancer cells and inducing a drug-resistant phenotype in chemosensitive cells. In addition, ubiquitin specific peptidase 22 (USP22) is a ubiquitin hydrolase that can deubiquitinate substrate proteins and participate in gene transcription in various malignancies. Xian et al.10 also found that colorectal cancer cell-derived exosomes encapsulated Lnc RNA-KCNQ1OT1, mediated the miR-30a-5p/USP22 pathway in an autocrine manner, and regulated PD-L1 ubiquitination, thus inhibiting CD8+ cells and promoting tumor immune escape.
There have been many reports on the synthesis and release of growth factors by cancer cells to accelerate their growth; however, there are few related studies on the role of exosomes in it. Therefore, further research is needed.
3.2. Paracrine Regulation of Exosomes
In fact, as a humoral factor, most of the effects of exosomes are achieved through paracrine action, which can transmit signaling factors in the form of local diffusion between adjacent cells, affecting local cancer cells, mesenchymal cells and endothelial cells, providing a boost to the growth and metastasis of cancer cells.
Tumor cell-derived exosomes activate adjacent cancer cells and enhance their metastatic and invasive abilities. Lysyl oxidase-like 4 (LOXL4), which belongs to the lysyl oxidase protein family, is upregulated in various cancers. Rongkun Li et al.11 studied exosomes extracted from hepatocarcinoma cell lines and found that hepatoma cell-derived exosomes were able to transfer LOXL4 between hepatoma cells, change the expression level of LOXL4 in adjacent cancer cells, and promote invasion and progression of hepatocarcinoma cells by the upregulated LOXL4 through the hydrogen peroxide-mediated FAK/Src pathway.
Tumor cell-derived exosomes transfer to mesenchymal cells to form a cancer-promoting microenvironment. Li et al.12 demonstrated that breast cancer-derived exosomes carried the apoptosis inhibitory protein Survivin and transferred it to the mesenchymal. And Nunzia Novizio et al.13 also found that pancreatic cancer cells release Annexin A1 (ANXA1) through exosomes. These proteins activated fibroblasts and converted them into myofibroblasts, thus promoting cancer proliferation, epithelial-mesenchymal transition and stem cell differentiation.
Tumor cell-derived exosomes transfer to endothelial cells to promote angiogenesis and contribute to cancer growth. Chen et al.14 found that noncoding RNA-X26nt was significantly elevated in gastric cancer cell-derived exosomes. Subsequent experiments in vitro and in vivo showed that X26nt could bind to the 3′ noncoding region of vascular endothelial cell cadherin (VE-cadherin) mRNA and downregulate the expression of cadherin. Sajjad Masoumi-Dehghi et al.15 similarly found that in ovarian cancer, exosomes encapsulating miR-141-3p upregulating JAK-STAT3 pathway in endothelial cells. These pathways increasing vascular permeability and promoting endothelial cell proliferation, migration and neovascularization.
Mesenchymal-derived exosomes act on adjacent cancer cells to promote their proliferation and metastasis. A study by Li et al.5 found that miR-34a-5p-deficient exosomes derived from cancer-associated fibroblasts (CAFs) could act on oral squamous carcinoma cells in a paracrine manner, enhance tumor cell motility and migration to acquire a more aggressive phenotype in the tumor microenvironment, and, due to miR-34a-5p overexpression, can inhibit the invasion and development of cancer. Yan et al.16 found that miR-18b was upregulated in CAFs-derived exosomes. It activates NF-κB by binding specifically to the 3′UTR of Transcription Elongation Factor A Like 7 (TCEAL7) to induce epithelial mesenchymal transition and promote the migration and metastasis of breast cancer cells.
Indeed, the alteration in the ability to control cell proliferation is one of the main phenotypic characteristics of malignant cell populations. Cargoes in exosomes mediate the activation of signaling pathways and affect gene modification in a paracrine manner, thus promoting cell proliferation and the formation of the tumor microenvironment.17
3.3. Exosomes Regulate Tumor Growth through Systemic Fluid Circulation
In addition to the two local circulation modes of autocrine and paracrine, exosomes can also be released by the original cells and transported to the target organs or cells in distant sites to play their roles, since they can exist in the systemic fluid circulation.
3.3.1. Tumor Cell-Derived Exosomes Transfer to Target Organs to Form a Premetastatic Niche
The premetastatic niche refers to the formation of an environment conducive to cancer cell growth at the metastatic site before cancer cells reach their target organs, which is one of the important steps in cancer metastasis.
Yuan et al.18 conducted a study on SCP28 cells, a highly metastatic subline of human breast cancer cells. They found that miR-21-rich exosomes secreted by SCP28 cells could target PDCD4, downregulate its expression, and then accelerate bone damage and rebuild the premetastatic niche in bone. Breast cancer cell-derived exosomes encapsulating miR-200b-3p are taken up by alveolar type II epithelial cells, which bind to the target PTEN and activate the AKT/NF-kBp65/CLL2 cascade response, upregulating CCL2 expression, which in turn recruits myeloid-derived suppressor cells (MDSCs), resulting in an immunosuppressive microenvironment in the lung.19 Morrissey SM et al.20 also found that cancer-derived exosomes can increase PD-L1 expression, which polarized macrophages toward an immunosuppressive phenotype and contributed to the formation of premetastatic niches. In addition, it has also been found that exosomes derived from colorectal cancer21 and nonsmall cell lung cancer22 are involved in the formation of premetastatic niches by promoting cell migration and increasing vascular permeability, respectively.
Notably, although exosomes can enter the systemic blood circulation, exosomes derived from different types of tumors do not randomly select premetastatic niches and they have affinity for specific target organs. Ayuko Hoshino ’s team extracted and labeled exosomes from breast and pancreatic cancer cell lines, respectively, and injected them into nude mice. The results showed that breast cancer-derived exosomes were more efficiently taken up in the lungs, while exosomes of pancreatic cancer origin had higher uptake in the liver. This illustrates that organ specificity of exosome biodistribution may match the propensity for tumor metastasis. And this finding helps to predict metastatic propensity and identify organ locations for future metastases.23
3.3.2. Exosomes Carry Signaling Molecules That Directly Regulate Cancer Growth
Jiang et al.6 found that Angiopoietin-like protein 1(ANGPTL1)24 expression was lower in exosome derived from colorectal cancer tissues than in controls. The ANGPTL1-rich exosomes were taken up by hepatic Kupffer cells and inhibited JAK2-STAT3 signaling pathway, downregulated MMP9 levels and suppressed liver metastasis of colorectal cancer.
It has been shown that let-7f promotes tumor invasion and proliferation, and that hypoxic conditions can induce massive release of exosomes from tumor cells, which are involved in the progression of many cancers.25,26 However, Virginia Egea et al.27 found that HIF-1α in human bone marrow mesenchymal stem cells can upregulate let-7f expression under hypoxic conditions, resulting in the production of let-7f-rich exosomes. The uptake of exosomes by 4T1 breast cancer cells triggers the autophagic mechanism, which inhibits the proliferation and invasion of tumor cells. In fact, changes in the microenvironment have a huge impact on exosomes, pH and temperature can also affect exosome function. It is worthy of in-depth study and discussion.28
It was also found in patients with metastatic prostate cancer that exo-miR-424 was significantly elevated in their blood circulation. Exosomes cultured low tumorigenic cells at local and distant metastatic sites through the systemic circulation, which enabled normal prostate cancer epithelial cells to acquire tumorigenicity.29 In addition, the same regulation was found for exo-SOX2-OT in the blood circulation of ovarian cancer patients.30
Intercellular signaling mediated by exosomes can change the survival and metastasis of cancer cells in multiple ways. The regulation mechanism of exosomes on cancer cells also helps to further reveal the process of cancer progression, which shows promising development in tumor diagnosis and treatment.
3.4. Exosome-Mediated Cancer Immune Regulation
In 1996,31 it was first reported that B cells released exosomes containing MHC class II molecules through plasma membrane fusion. And then in 1998, Laurence Zrrvogel et al.32 first reported the exosomes secreted by dendritic cells and their application in tumor immunotherapy. These studies have laid the foundation for exosome-based tumor immunology research (Figure 2).
Figure 2.
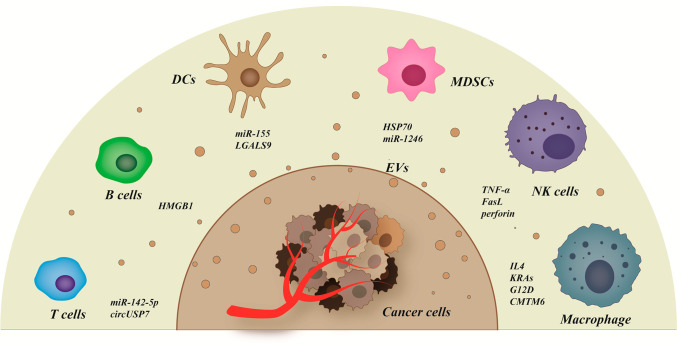
Exosome-mediated tumor immune regulation. Exosomes encapsulate a rich variety of signaling molecules, such as IL-4, miR-155, hsp70, TNF-α, etc., to transmit information between tumor cells and immune cells. The activation of these signals can affect the immune response through different pathways and play a role in promoting or suppressing tumors.
3.4.1. Exosome-Mediated Anticancer Immunity
In the current results, exosomes can promote tumor growth through a variety of pathways, while most studies on tumor inhibition have focused on the regulation of the immune system by exosomes. Interleukin-4 is mostly expressed on the surface of cancer cells. Gunassekaran GR et al.33 used M1 macrophage-derived exosomes as nanocarriers to inhibit tumor growth by reprogramming tumor-associated macrophages (TAMs) into M1-like macrophages and targeting IL4R. NK cells exert their killing effects mainly by releasing of perforin-granzyme and inducing apoptosis through Fas/FasL pathway. Zhu et al.34 found that NK cell-derived exosomes have cytotoxic effects on melanoma cells and other cancer cell lines, but have minimal side effects on normal cells. It is promising for clinical immunotherapy. Other researchers have also found that canine natural killer cell-derived exosomes can act on mouse breast cancer cell lines to play an anticancer effect by downregulating cancer stem cell activity to regulate apoptotic protein expression and enhancing the inhibitory function of p53.35 Ali Asadirad et al.36 modified and engineered dendritic cells (DCs) using miR-155-enriched exosomes and found that this construct had antitumor effects in mouse models of colorectal cancer. However, the roles of different genes play in it and the mechanism of antitumor remained to be further investigated.
3.4.2. Exosomes Promote Cancer Immune Escape
Exosomes can also suppress the body’s antitumor immunity and help tumor immune escape through different pathways. Zhou et al.37 found that miR-142–5p wrapped with exosomes from cervical squamous carcinoma transfers to lymphatic vessel endothelial cells, and downregulates AT-rich interactive domain-containing protein 2 (ARID2). Then it inhibits the recruitment of DNA methyltransferase 1 (DNMT1) to the interferon-γ promoter, thereby increasing the activity of indoleamine 2,3-dioxygenase (IDO) and depleting CD8+ T cells. Nonsmall cell lung cancer-derived exosomes are also capable of encapsulating circUSP7 and promoting CD8+ T cell dysfunction. In addition, dysfunction of CD8+ T cells can also be induced by circUSP7 encapsulated by exosomes derived from nonsmall cell lung cancer.38 Dai et al.39 also confirmed that oxidative stress-induced autophagy-dependent KRASG12D protein can be released from pancreatic ductal adenocarcinoma cells in the form of exosomes, and then taken up by macrophages through the AGER/RAGE mechanism. By inducing fatty acid oxidation, this protein polarizes macrophages toward M2 to form a pro-cancer microenvironment. Glioblastoma multiforme (GBM) is a malignancy with a high recurrence rate. Ming Wang et al.40 demonstrated that GBM-Exos in cerebrospinal fluid (CSF) contains a unique LGALS9 ligand that binds DC receptors and inhibits antigen recognition and presentation of DCs. Besides, exosomes derived from renal cancer cells and glioma cells encapsulated HSP70 and miR-1246, respectively, driving the expansion and activation of MDSCs and inhibiting the effects of cytotoxic T lymphocytes (CTL).41,42 All these processes prevented tumor from being killed by the immune system. Linsen Ye et al.43 also found that hepatocellular carcinoma cells were able to release exosomes expressing HMGB1, which promoted the aggregation of TIM-1+ Breg cells. Subsequently, Breg cells secreted IL-10 and suppressed the function of CD8+ Tcell, creating an immunosuppressive microenvironment. Nevertheless, B cell-related findings are not abundant. This might be a direction worth exploring.
3.4.3. PD-L1 Related Immune Regulation
Programmed cell death 1 ligand 1 (PD-L1) is an important component of various immune response mechanisms. PD-L1 expressed in tumor tissues inhibits lymphocyte proliferation and reduces CD8+ T cell toxicity after binding to immune cell surface receptors.44 Pang et al.45 found that exosomes from oral squamous carcinoma origin deliver CMTM6 to macrophages, induce macrophage polarization toward M2 by activating the ERK1/2 signaling pathway, and upregulate PD-L1 expression. The tumor-derived exosomes were also able to be directly taken up by CD8+ T cells and upregulate PD-1 on the surface, thus maintaining the self-renewal and immune escape properties of immune cells.46 LOXL4 mentioned previously11 was also able to shape immunosuppressive macrophages by inducing the presentation of PD-L1.47
In addition, anti-PD1 antibodies have been widely used in cancer immunotherapy, which can block the PD1/PD-L1 axis and inhibit the depletion of effector immune cells. Studies have confirmed that targeting macrophage CD39 is a potential therapeutic strategy to reverse the anti-PD1 resistance of hepatocarcinoma.48
It is not difficult to find that exosomes can affect various immune cells including CD8+ T cells, macrophages, NK cells, DCs, Breg cells, etc., and exert powerful immunomodulatory effects. The antigen presentation of exosomes can also serve as a good vehicle for cancer vaccines, providing a new direction for cancer immunotherapy.
4. Exosomes in Cancer Diagnosis
It is well-known that tissue biopsy has been the gold standard for clinical cancer diagnosis, but tissue biopsy as an invasive and invasive test still has certain risks. Therefore, liquid biopsy has a better prospect as a more convenient noninvasive test. With the continuous development of medical technology, the diagnostic efficacy of many classical tumor markers is no longer sufficient,49 and scholars have been searching for biomarkers with higher specificity and sensitivity. Exosomes can be found in blood, urine, cerebrospinal fluid, and even saliva, making it easier to obtain samples for testing. Nucleic acids inside are protected by phospholipid membranes and are not easily degraded by proteases or interfered by other free miRNA. Proteins are also able to avoid failure to function as intended due to changes in native conformation.50 Exosomes contain a variety of active molecules,51 of which miRNA is the most widely studied biomarker (Table 1), a small single-stranded RNA molecule that is involved in post-transcriptional regulation of gene expression. The protein fractions of exosomes can reflect the proteomic profile of their parental tumor cells and thus serve as a warning for tumorigenesis (Table 2).52 LncRNAs53 can also be encapsulated by exosomes to regulate a variety of cells (Table 3), and we summarize the latest findings in this regard. In addition, circRNAs,54 DNAs,55 and other active substances in exosomes can also be used as potential biomarkers. In the summarized experimental data, we selected the AUC as the main indicator of diagnostic efficacy.56 From the summarized literature, we can find that most genes or proteins in exosomes have higher diagnostic sensitivity than traditional biomarkers, and the combination of multiple genes or exosomal genes with traditional biomarkers tends to have higher diagnostic sensitivity than individual tests, which lays the foundation for further experiments with large samples in the future.
Table 1. Diagnostic Potential of Exosome-Related miRNAs as Biomarkers.
| cancer | miRNA | expression levels | AUC | sensitivity (%) | specificity (%) | sample size | source | application direction | quote |
|---|---|---|---|---|---|---|---|---|---|
| pancreatic cancer | miR-19b | down | 0.942 | 85.48 | 90.57 | 115 (62PCa/53HC) | blood | cancer diagnosis | (62) |
| gastric cancer | miR-590-5p | down | 0.81 | 63.70 | 86.00 | 218(168GC/50HC) | blood | early cancer diagnosis and prognosis | (63) |
| breast cancer | hsa-miR-423-5p | up | 0.68 | 66 | 68 | 337 (224BC/113HC) | blood | early cancer diagnosis | (64) |
| miR-370-3p | up | 0.6797 | 55.56 | 74.07 | 56 (28BC/28HC) | blood | early cancer diagnosis | (65) | |
| hsa-miR-21-5p | up | 0.961 | 86.70 | 93.30 | 60 (30BC/30HC) | blood | cancer diagnosis | (66) | |
| miR-1246 | up | 0.9828 | 93.94 | 97.30 | 70(33BC/37HC) | blood | cancer diagnosis | (67) | |
| prostate cancer | miR-19b-3p | up | 0.8661 | 81(23PA/58HC) | blood | cancer diagnosis | (68) | ||
| miR-101-3p | up | 0.759 | 38 (19 transferred/19 non) | blood | metastatic prediction | ||||
| miR-375 | down | 0.806 | 47 (PCa/25HC) | urine | identify localized and metastatic | (69) | |||
| miR-486-5p | up | 0.796 | cancer diagnosis | ||||||
| colon cancer | miR-1539 | up | 0.673 | 92.20 | 40.80 | 100(51CRC/49HC) | blood | early screening and prognosis | (70) |
| miR-15b | up | 0.86 | 81.33 | 91.80 | 171(81CRC/90HC) | blood | cancer diagnosis | (71) | |
| cervical cancer | miR-125a-5p | down | 0.7129 | 59.10 | 84.20 | 60 (38CC/22HC) | blood | cancer diagnosis | (72) |
| lung cancer | miR-96 | up | 0.9735 | 97 (52NSCLC/45HC) | blood | cancer diagnosis | (73) | ||
| 0.7496 | 52 (27 radiation resistant/25 radiation sensitive) | blood | radioresistance and prognostic assessment | ||||||
| papillary thyroid carcinoma | miR-29a | down | 0.884 | 78.99 | 85.71 | 219 (119PTC/100HC) | blood | cancer diagnosis, early and late identification and recurrence prediction | (74) |
| ovarian cancer | miR-4732-5p | up | 0.889 | 85.70 | 82.40 | 55 (34EOC/21HC) | blood | cancer diagnosis | (75) |
| bladder cancer | miR-96-5p | up | 0.85 | 80.40 | 91.80 | 100 (51BC/49 non-BC) | urine | cancer stage prediction and prognosis | (76) |
| miR-183-5p | up | 0.83 | 78.40 | 81.60 | |||||
| miR-93-5p | up | 0.838 | 74.10 | 90.20 | 104 (53BC/51HC) | urine | cancer stage and prediction of invasion degree | (77) | |
| oral squamous cell carcinoma | miR-130a | up | 0.812 | 98.50 | 45.70 | 380 (184OSCC/196HC) | blood | early diagnosis, cancer staging and prognostic assessment | (78) |
| liver cancer | miR-101 | down | 0.894 | 40 (20HCC/20HC) | blood | cancer diagnosis | (79) | ||
| miR-125b | down | 0.812 | |||||||
| endometrial cancer | miR-15a-5p | up | 0.819 | 56 (EC/31HC) | blood | early cancer diagnosis | (80) |
Table 2. Diagnostic Potential of Exosome-Related lncRNAs as Biomarkers.
| cancer | LncRNA | expression levels | AUC | sensitivity (%) | specificity (%) | sample size | source | application direction | quote |
|---|---|---|---|---|---|---|---|---|---|
| gastric cancer | FRLnc1 | up | 0.863 | 80.60 | 76.90 | 82 (52GC/30HC) | blood | cancer diagnosis | (81) |
| triple negative breast cancer | exo-XIST | up | 0.888 | 84.20 | 92.30 | 141 (91TNBC/50HC) | blood | recurrence and progression prediction | (82) |
| colon cancer | FOXD2-AS1 | up | 0.728 | 72.60 | 62.30 | 404 (203CRC/201HC) | blood | early cancer diagnosis | (83) |
| 0.743 | 70.60 | 59.40 | 281 (80 early CRC/201HC) | ||||||
| cervical cancer | DLX6-AS1 | up | 0.892 | 78.10 | 88.20 | 224(114CC/110HC) | blood | cancer diagnosis | (84) |
| 0.831 | 75.40 | 71.80 | 174 (114CC/60CIN) | benign and malignant identification | |||||
| lung cancer | SNHG15 | up | 0.856 | 198 (118NSCLC/80HC) | blood | diagnosis and prognosis | (85) | ||
| liver cancer | CRNDE | up | 0.839 | 69.30 | 85.00 | 266 (166HCC/100HC) | blood | cancer diagnosis | (86) |
Table 3. Diagnostic Potential of Exosome-Related Proteins As Biomarkers.
| cancer | proteins | expression levels | AUC | sensitivity (%) | specificity (%) | sample size | source | application direction | quote |
|---|---|---|---|---|---|---|---|---|---|
| pancreatic cancer | alix protein | up | 0.73 | 53.10 | 83.90 | 94 (62PC/32other) | blood | differentiate between benign and malignant | (87) |
| gastric cancer | AGT + SERPINH1 + MMP7 | up | 0.7734 | 72.73 | 71.6 | 218(132GC/86HC) | blood | early cancer diagnosis and prognosis | (88) |
| breast cancer | BATF2 | down | 0.8869 | 82.76 | 80.00 | 116(60BC/56HC) | blood | diagnosis and prognostic evaluation | (89) |
| colon cancer | MMP 9+ | up | 0.925 | 88 | 80 | 75 (15 polyp/60 cancer) | blood | prognosis and recurrence | (90) |
| 20S protease | up | 0.85 | 92 | 80 | |||||
| FGB | up | 0.871 | 68.35 | 86.27 | 50(30CRC/20HC) | blood | early cancer diagnosis | (91) | |
| β2-GP1 | up | 0.834 | 71.55 | 85.51 | |||||
| lung cancer | FHL1 (NSCLC) | down | 1 | 100 | 100 | blood | cancer diagnosis | (92) | |
| CD5L protein | up | 0.943 | 92.90 | 94.10 | 80(60LC/20HC) | blood | cancer diagnosis | (93) | |
| oral squamous cell carcinoma | alix protein | up | 0.685 | 34.50 | 1 | 40(29OSCC/21HC) | blood | cancer diagnosed | (94) |
| nasopharyngeal carcinoma | BATF2 | down | 0.8983 | 81 | 82 | 180 (130NPC/50HC) | blood | diagnosis and assessment of recurrence | (95) |
| perihilar cholangiocarcinoma | Cripto-1 | up | 0.874 | 79.10 | 87.50 | 227 (115PHCCA/112 non) | blood | diagnosis and prognosis | (96) |
James McKiernan’s team has successfully validated the ExoDx Prostate (IntelliScore) (EPI) urine exosome gene expression assay for more sensitive differentiation of different risk levels of prostate cancer in two prospective trials with over 1000 patients. It significantly reduced unnecessary biopsies compared to existing conventional methods. This assay had significant implications for exosomes to move toward clinical application.57,58 Two years later, another prospective study on thyroid cancer also successfully validated urinary exosomal thyroglobulin UEx-TG as an important indicator for predicting thyroid cancer recurrence. It can be used for accurate follow-up of patients after thyroid cancer surgery, reducing the patient’s trauma.59
In addition, there are several other completed prospective clinical trials in the ClinicalTrials.gov registry (Table 4) in order to investigate the diagnostic potential of exosomes and their cargoes as biomarkers in various cancers. However, in reality, the published reliable research results are still insufficient. We expect that more excellent findings will be generated in the future to bring the clinical application of exosomes to a whole new stage of development.60,61
Table 4. Completed Prospective Studies of Exosomes as Biomarkers for Various Types of Cancer Registered on ClinicalTrials.gov.
| ClinicalTrials.gov ID | cancer type and setting | objectives | primary outcome measures |
|---|---|---|---|
| NCT02702856 | prostate cancer | determine the association of an exosome urine test score with the presence of high Gleason grade/score (GS >/= 7) prostate cancer on a prostate needle biopsy | correlate an exosome gene expression signature with the presence or absence of high grade prostate cancer in the prostate needle biopsy |
| NCT03830619 | lung cancer | investigate the sensitivity and specificity of serum exosome noncoding RNA as a biomarker for the diagnosis of lung cancer | the expression levels of serum exosome long noncoding RNA |
| the expression levels of tumor biomarkers | |||
| the CT scans of the lung for the patients | |||
| NCT03895216 | bone metastases | obtain a panel of biomarkers predictive of bone metastasis | changes in miRNAs content of circulating tumor exosomes |
| changes in protein content of circulating tumor exosomes | |||
| NCT03032913 | pancreatic cancer | determining whether liquid biopsy approaches are valid in the diagnosis of pancreatic cancer | sensitivity of CTC detection with 3 methods in cell spiking experiments |
| diagnostic accuracy of the best CTC detection method and onco-exosome quantification for pancreatic adenocarcinoma diagnosis | |||
| NCT04720599 | urologic cancer | confirm the performance of the ExoDx prostate gene expression assay in patients presenting for an initial prostate biopsy | correlation of the ExoDx prostate test results with the outcome of prostate biopsies in an initial biopsy patient cohort |
| NCT03911999 | prostate cancer | investigate the relationship of urinary exosome and the aggressiveness of prostate cancer | differences in microRNA expression between nonprostate cancer subjects, pathologically insignificant, and significant prostate cancer patients |
| the accuracy of selected microRNAs for the differentiation of patients with pathologically insignificant and significant prostate cancer after radical prostatectomy | |||
| NCT03031418 | prostate cancer | a new and validated urine test which predicts the likelihood of high grade prostate cancer on an initial prostate biopsy | confirm performance of the ExoDx Prostate IntelliScore |
| NCT02862470 | thyroid cancer | analyze the urine exosomal proteins and probable biological markers, then find the prognostic biological markers | prognostic biological markers via this prospective study. |
| NCT05101655 | lung metastasis of osteosarcoma | use exosome microfluidic chips to establish a combination of exosome subgroup level (exosome barcode) markers for the early diagnosis of osteosarcoma lung recurrence | the association of disease recurrence with plasma levels of exosome and its subgroups |
| NCT05334849 | advanced gastric carcinoma | verify the function of circulating exosomal lncRNA-GC1 on predicting and monitoring immunotherapeutic outcomes of GC | levels of circulating exosomal lncRNA-GC1 |
5. Challenges and Opportunities for Exosomes
Discoveries in the field of exosome biology have greatly expanded our understanding of the major steps in cancer development. However, along with the rapid development of exosomes, a number of questions need to be clarified. For example, how do exosomes select their cargo in the tumor microenvironment? How do they select their target cells? These have important implications for the next step of exosome modification and modification. The currently recognized mechanism of exosome sorting is mainly related to the ESRCT-III pathway.97 An important role of the RAB family in the mechanism of exosome genesis has also been reported recently in the literature.98,99 In addition, it has been suggested that the mechanism controlling RNA-specific loading into exosomes may be related to the hnRNPA2B1 pathway.26,100 And ubiquitin-like protein 3 (UBL3)/membrane anchored Ub folding protein (MUB) is able to act as a post-translational modification (PTM) factor to regulate protein to exosome sorting.101 However, studies related to the sorting of other signaling molecules are still scarce, and the heterogeneity of exosomes dictates that many unknown findings remained to be explored.
Second, despite the tremendous efforts of scientists to demonstrate the value of exosomes in liquid biopsies, exosome isolation, mass production, loading, modification, and storage all face significant challenges before entering the clinic. The gold standard technique for exosome isolation remains ultracentrifugation, along with gradient centrifugation, ultrafiltration, hydrostatic filtration dialysis, size-exclusion chromatography, etc.102 However, none of these separation methods escapes low purity, low recovery, low yield, high cost, and inability to perform large-scale sample preparation. Endogenous loading often does not allow accurate detection of loading, and exogenous loading is accompanied by low efficiency. The technology for exosome production and quality control is also flawed.103 Although reports have illustrated the feasibility of developing high-scale and efficient clinical-grade exosomes using Good Manufacturing Practice (GMP) standards,104 there are still many pitfalls in large-scale production. The accuracy assessment of these microvesicles is often questioned because each step in exosome testing can be a source of heterogeneity. In recent studies, microfluidic chips-based separation platforms is rapidly developed,105,106 which can accurately control and manipulate the fluid in the microscale channel and improve the detection sensitivity and accuracy. However, whether this complex and sophisticated technology can meet the requirements of fast and convenient applications in the clinic, still needs to be further explored. Indeed, these practical problems faced require us to find a balance between maximizing the recovery of exosomes and minimizing impurities.
Not only is there no consensus on technical standards for exosome production and isolation, but also the storage and stability of exosomes have not been clearly studied. An interesting study concluded that the cargo loaded in exosomes is lost under unsuitable storage conditions.107 They recommend storing plasma at −80 °C, avoiding repeated freeze–thaw cycles, and thawing at 37 °C.
Finally, even if we achieve the extraction and preparation of exosomes, their long-term safety and efficacy in clinical treatment need to be verified by exhaustive clinical trials, which means we still have a long way to go.
6. Conclusion
We seek to review the regulatory mechanisms of exosomes in tumors and the recent progress of exosomes in tumor diagnosis. Exosomes are important mediators of intercellular information and are able to wrap signaling molecules to regulate many different signaling pathways affecting tumor development in autocrine, paracrine, fluid circulation and immunoregulatory ways. Moreover, exosomes, as promising novel biomarkers, have brought us new noninvasive diagnostic methods and reduced the damage to patients. Based on recent exosome-related studies, miRNAs, lncRNAs, proteins and other small molecules as tumor markers may provide a new direction for tumor diagnosis. However, circRNA, piRNA, and other related signaling molecules are still less studied. Due to their natural properties as biological carriers, processed and modified exosomes become new tools for targeted therapy.108 Many studies in recent years have developed tumor vaccines with exosomes as carriers.109 Exosomes that have been directly modified to kill tumor cells110,111 and used in synergistic chemotherapy112,113 for cancer have also made progress in these areas. The research prospect of exosomes is very promising, while the biological function of microvesicles, another type of exosomes, may also become a new hot direction.
Despite many challenges, we believe that as research progresses, scholars will provide more valuable results to broaden the application of exosomes.
Data Availability Statement
Data sharing not applicable to this article as no data sets were generated or analyzed during the current study.
Author Contributions
† These authors contributed equally to this work. Tong Wu and Ying Liu wrote the main manuscript text. Nasra Mohamoud Ali and Bin Zhang undertook the language polishing and revision of the article. Xiaonan Cui provided professional guidance and reviewed the content for accuracy. All authors read and approved the final manuscript.
This work was supported by Xiaonan Cui, the host of the Capacity Construction Project of Major Clinical (specialized) Departments of Traditional Chinese Medicine of Liaoning Province(No. LNZYXZK201909), and the host of the Distinguished Professor Program of Liaoning Province, and Ying Liu, the host of the Dalian Medical Science Research Program (No. 2011043).
The authors declare no competing financial interest.
References
- Pan B.; Johnstone R. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 1983, 33 (3), 967–978. 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- Johnstone R. M.; Adam M.; Hammond J. R.; Orr L.; Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262 (19), 9412–9420. 10.1016/S0021-9258(18)48095-7. [DOI] [PubMed] [Google Scholar]
- Mashouri L.; Yousefi H.; Aref A. R.; Ahadi A. m.; Molaei F.; Alahari S. K. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Molecular Cancer 2019, 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R.; LeBleu V. S. The biology, function, and biomedical applications of exosomes. Science 2020, 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.-y.; Tao Y.-w.; Gao S.; Li P.; Zheng J.-m.; Zhang S.-e.; Liang J.; Zhang Y. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine 2018, 36, 209–220. 10.1016/j.ebiom.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K.; Chen H.; Fang Y.; Chen L.; Zhong C.; Bu T.; Dai S.; Pan X.; Fu D.; Qian Y.; et al. Exosomal ANGPTL1 attenuates colorectal cancer liver metastasis by regulating Kupffer cell secretion pattern and impeding MMP9 induced vascular leakiness. J. Exp Clin Cancer Res. 2021, 40 (1), 21. 10.1186/s13046-020-01816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L.; Wang Z.; Geng X.; Zhang Y.; Xue Z. in vitroExosomal miRNA-34 from cancer-associated fibroblasts inhibits growth and invasion of gastric cancer cells and. Aging 2020, 12 (9), 8549–8564. 10.18632/aging.103157. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhu L.; Sun H. T.; Wang S.; Huang S. L.; Zheng Y.; Wang C. Q.; Hu B. Y.; Qin W.; Zou T. T.; Fu Y.; et al. Isolation and characterization of exosomes for cancer research. J. Hematol Oncol 2020, 13 (1), 152. 10.1186/s13045-020-00987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asare-Werehene M.; Nakka K.; Reunov A.; Chiu C.; Lee W.; Abedini M.; Wang P.; Shieh D.; Dilworth F.; Carmona E.; et al. The exosome-mediated autocrine and paracrine actions of plasma gelsolin in ovarian cancer chemoresistance. Oncogene 2020, 39 (7), 1600–1616. 10.1038/s41388-019-1087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian D.; Niu L.; Zeng J.; Wang L. LncRNA KCNQ1OT1 Secreted by Tumor Cell-Derived Exosomes Mediates Immune Escape in Colorectal Cancer by Regulating PD-L1 Ubiquitination via MiR-30a-5p/USP22. Front Cell Dev Biol. 2021, 9, 653808. 10.3389/fcell.2021.653808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R.; Wang Y.; Zhang X.; Feng M.; Ma J.; Li J.; Yang X.; Fang F.; Xia Q.; Zhang Z.; et al. Exosome-mediated secretion of LOXL4 promotes hepatocellular carcinoma cell invasion and metastasis. Molecular cancer 2019, 18 (1), 18. 10.1186/s12943-019-0948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K.; Liu T.; Chen J.; Ni H.; Li W. Survivin in breast cancer–derived exosomes activates fibroblasts by up-regulating SOD1, whose feedback promotes cancer proliferation and metastasis. J. Biol. Chem. 2020, 295 (40), 13737–13752. 10.1074/jbc.RA120.013805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novizio N.; Belvedere R.; Pessolano E.; Tosco A.; Porta A.; Perretti M.; Campiglia P.; Filippelli A.; Petrella A. Annexin A1 Released in Extracellular Vesicles by Pancreatic Cancer Cells Activates Components of the Tumor Microenvironment, through Interaction with the Formyl-Peptide Receptors. Cells 2020, 9 (12), 2719. 10.3390/cells9122719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Zhang S.; Du K.; Zheng N.; Liu Y.; Chen H.; Xie G.; Ma Y.; Zhou Y.; Zheng Y.; et al. Gastric cancer-secreted exosomal X26nt increases angiogenesis and vascular permeability by targeting VE-cadherin. Cancer Sci. 2021, 112 (5), 1839–1852. 10.1111/cas.14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoumi-Dehghi S.; Babashah S.; Sadeghizadeh M. microRNA-141–3p-containing small extracellular vesicles derived from epithelial ovarian cancer cells promote endothelial cell angiogenesis through activating the JAK/STAT3 and NF-kappaB signaling pathways. J. Cell Commun. Signal 2020, 14 (2), 233–244. 10.1007/s12079-020-00548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z.; Sheng Z.; Zheng Y.; Feng R.; Xiao Q.; Shi L.; Li H.; Yin C.; Luo H.; Hao C.; et al. Cancer-associated fibroblast-derived exosomal miR-18b promotes breast cancer invasion and metastasis by regulating TCEAL7. Cell Death Dis 2021, 12 (12), 1120. 10.1038/s41419-021-04409-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L.; Wang D.; Han Y.; Huang T.; He X.; Wang J.; Ou C. Emerging Role of Cancer-Associated Fibroblasts-Derived Exosomes in Tumorigenesis. Front Immunol 2022, 12, 795372. 10.3389/fimmu.2021.795372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X.; Qian N.; Ling S.; Li Y.; Sun W.; Li J.; Du R.; Zhong G.; Liu C.; Yu G.; et al. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics 2021, 11 (3), 1429–1445. 10.7150/thno.45351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu P.; Sun M.; Li L.; Yang Y.; Jiang Z.; Ge Y.; Wang W.; Mu W.; Wang H. Breast Tumor-Derived Exosomal MicroRNA-200b-3p Promotes Specific Organ Metastasis Through Regulating CCL2 Expression in Lung Epithelial Cells. Front. Cell. Dev. Biol. 2021, 9, 657158. 10.3389/fcell.2021.657158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey S.; Zhang F.; Ding C.; Montoya-Durango D.; Hu X.; Yang C.; Wang Z.; Yuan F.; Fox M.; Zhang H.; et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell metabolism 2021, 33, 2040. 10.1016/j.cmet.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.; Lu Z.; Fu W.; Lu K.; Gu X.; Xu F.; Dai J.; Yang Y.; Jiang J. Exosome-Derived ADAM17 Promotes Liver Metastasis in Colorectal Cancer. Front Pharmacol 2021, 12, 734351. 10.3389/fphar.2021.734351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z.; Wei K.; Yang F.; Guo Z.; Pan C.; He Y.; Wang J.; Li Z.; Chen L.; Chen Y.; et al. Tumor-derived exosomal miR-3157–3p promotes angiogenesis, vascular permeability and metastasis by targeting TIMP/KLF2 in non-small cell lung cancer. Cell Death Dis 2021, 12 (9), 840. 10.1038/s41419-021-04037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A.; Costa-Silva B.; Shen T. L.; Rodrigues G.; Hashimoto A.; Tesic Mark M.; Molina H.; Kohsaka S.; Di Giannatale A.; Ceder S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527 (7578), 329–335. 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli G. Angiopoietin-like proteins: a comprehensive look. Front Endocrinol (Lausanne) 2014, 5, 4. 10.3389/fendo.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W.; Hao Y.; He C.; Li L.; Zhu G. Exosome-orchestrated hypoxic tumor microenvironment. Mol. Cancer 2019, 18 (1), 57. 10.1186/s12943-019-0982-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Sai B.; Wang F.; Wang L.; Wang Y.; Zheng L.; Li G.; Tang J.; Xiang J. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol. Cancer 2019, 18 (1), 40. 10.1186/s12943-019-0959-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea V.; Kessenbrock K.; Lawson D.; Bartelt A.; Weber C.; Ries C. Let-7f miRNA regulates SDF-1alpha- and hypoxia-promoted migration of mesenchymal stem cells and attenuates mammary tumor growth upon exosomal release. Cell Death Dis 2021, 12 (6), 516. 10.1038/s41419-021-03789-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C.; Witwer K. W.; Aikawa E.; Alcaraz M. J.; Anderson J. D.; Andriantsitohaina R.; Antoniou A.; Arab T.; Archer F.; Atkin-Smith G. K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 2018, 7 (1), 1535750. 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albino D.; Falcione M.; Uboldi V.; Temilola D. O.; Sandrini G.; Merulla J.; Civenni G.; Kokanovic A.; Sturchler A.; Shinde D.; et al. Circulating extracellular vesicles release oncogenic miR-424 in experimental models and patients with aggressive prostate cancer. Commun. Biol. 2021, 4 (1), 119. 10.1038/s42003-020-01642-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y.; Dong L.; Jin H.; Li H.; Sun M.; Li J. Exosome long non-coding RNA SOX2-OT contributes to ovarian cancer malignant progression by miR-181b-5p/SCD1 signaling. Aging 2021, 13, 23726. 10.18632/aging.203645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G.; Nijman H.; Stoorvogel W.; Liejendekker R.; Harding C.; Melief C.; Geuze H. B lymphocytes secrete antigen-presenting vesicles. Journal of experimental medicine 1996, 183 (3), 1161–1172. 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L.; Regnault A.; Lozier A.; Wolfers J.; Flament C.; Tenza D.; Ricciardi-Castagnoli P.; Raposo G.; Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nature medicine 1998, 4 (5), 594–600. 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- Gunassekaran G.; Poongkavithai Vadevoo S.; Baek M.; Lee B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials 2021, 278, 121137. 10.1016/j.biomaterials.2021.121137. [DOI] [PubMed] [Google Scholar]
- Zhu L.; Kalimuthu S.; Gangadaran P.; Oh J.; Lee H.; Baek S.; Jeong S.; Lee S.; Lee J.; Ahn B. Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics 2017, 7 (10), 2732–2745. 10.7150/thno.18752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Lee S. A.; Gu N. Y.; Jeong S. Y.; Byeon J. S.; Jeong D. U.; Ouh I. O.; Lee Y. H.; Hyun B. H. Canine Natural Killer Cell-Derived Exosomes Exhibit Antitumor Activity in a Mouse Model of Canine Mammary Tumor. BioMed. Research International 2021, 6690704. 10.1155/2021/6690704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadirad A.; Baghaei K.; Hashemi S.; Dehnavi S.; Ghanbarian H.; Mortaz E.; Anissian A.; Asadzadeh Aghdaei H.; Amani D. Dendritic cell immunotherapy with miR-155 enriched tumor-derived exosome suppressed cancer growth and induced antitumor immune responses in murine model of colorectal cancer induced by CT26 cell line. International immunopharmacology 2022, 104, 108493. 10.1016/j.intimp.2021.108493. [DOI] [PubMed] [Google Scholar]
- Zhou C.; Zhang Y.; Yan R.; Huang L.; Mellor A. L.; Yang Y.; Chen X.; Wei W.; Wu X.; Yu L.; et al. Exosome-derived miR-142–5p remodels lymphatic vessels and induces IDO to promote immune privilege in the tumour microenvironment. Cell Death Differ. 2021, 28 (2), 715–729. 10.1038/s41418-020-00618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. W.; Zhu S. Q.; Pei X.; Qiu B. Q.; Xiong D.; Long X.; Lin K.; Lu F.; Xu J. J.; Wu Y. B. Cancer cell-derived exosomal circUSP7 induces CD8(+) T cell dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC. Mol. Cancer 2021, 20 (1), 144. 10.1186/s12943-021-01448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai E.; Han L.; Liu J.; Xie Y.; Kroemer G.; Klionsky D. J.; Zeh H. J.; Kang R.; Wang J.; Tang D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy 2020, 16 (11), 2069–2083. 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Cai Y.; Peng Y.; Xu B.; Hui W.; Jiang Y. Exosomal LGALS9 in the cerebrospinal fluid of glioblastoma patients suppressed dendritic cell antigen presentation and cytotoxic T-cell immunity. Cell Death Dis 2020, 11 (10), 896. 10.1038/s41419-020-03042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y.; Xu H.; Li N.; Wang H.; Ma L.; Chen S.; Liu J.; Zheng Y.; Zhang Y. Renal cancer-derived exosomes induce tumor immune tolerance by MDSCs-mediated antigen-specific immunosuppression. Cell Communication and Signaling 2020, 10.1186/s12964-020-00611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W.; Guo X.; Li B.; Wang J.; Qi Y.; Chen Z.; Zhao R.; Deng L.; Qian M.; Wang S.; et al. Exosomal miR-1246 from glioma patient body fluids drives the differentiation and activation of myeloid-derived suppressor cells. Mol. Ther 2021, 29 (12), 3449–3464. 10.1016/j.ymthe.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L.; Zhang Q.; Cheng Y.; Chen X.; Wang G.; Shi M.; Zhang T.; Cao Y.; Pan H.; Zhang L.; et al. Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1(+) regulatory B cell expansion. J. Immunother Cancer 2018, 6 (1), 145. 10.1186/s40425-018-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.; Huang A. C.; Zhang W.; Zhang G.; Wu M.; Xu W.; Yu Z.; Yang J.; Wang B.; Sun H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560 (7718), 382–386. 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X.; Wang S.-s.; Zhang M.; Jiang J.; Fan H.-y.; Wu J.-s.; Wang H.-f.; Liang X.-h.; Tang Y.-l. OSCC cell-secreted exosomal CMTM6 induced M2-like macrophages polarization via ERK1/2 signaling pathway. Cancer Immunology, Immunotherapy 2021, 70 (4), 1015–1029. 10.1007/s00262-020-02741-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.; Wang L.; Ge D.; Tan L.; Cao B.; Fan H.; Xue L. Exosomal O-GlcNAc transferase from esophageal carcinoma stem cell promotes cancer immunosuppression through up-regulation of PD-1 in CD8(+) T cells. Cancer Lett. 2021, 500, 98–106. 10.1016/j.canlet.2020.12.012. [DOI] [PubMed] [Google Scholar]
- Tan H. Y.; Wang N.; Zhang C.; Chan Y. T.; Yuen M. F.; Feng Y. Lysyl Oxidase-Like 4 Fosters an Immunosuppressive Microenvironment During Hepatocarcinogenesis. Hepatology 2021, 73 (6), 2326–2341. 10.1002/hep.31600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J. C.; Zhang P. F.; Huang X. Y.; Guo X. J.; Gao C.; Zeng H. Y.; Zheng Y. M.; Wang S. W.; Cai J. B.; Sun Q. M.; et al. Amplification of spatially isolated adenosine pathway by tumor-macrophage interaction induces anti-PD1 resistance in hepatocellular carcinoma. J. Hematol Oncol 2021, 14 (1), 200. 10.1186/s13045-021-01207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomka A.; Wang B.; Mocan T.; Horhat A.; Willms A. G.; Schmidt-Wolf I. G. H.; Strassburg C. P.; Gonzalez-Carmona M. A.; Lukacs-Kornek V.; Kornek M. T. Extracellular Vesicles and Circulating Tumour Cells - complementary liquid biopsies or standalone concepts. Theranostics 2022, 12 (13), 5836–5855. 10.7150/thno.73400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.; Wu D.; Ma X.; Wang J.; Hou W.; Zhang W. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. Biomed Pharmacother 2020, 128, 110237. 10.1016/j.biopha.2020.110237. [DOI] [PubMed] [Google Scholar]
- Zhu J. W.; Charkhchi P.; Akbari M. R. Potential clinical utility of liquid biopsies in ovarian cancer. Mol. Cancer 2022, 21 (1), 114. 10.1186/s12943-022-01588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C.; Jiang W.; Lv M.; Fan S.; Lu Y.; Wu Q.; Pi J. Potentiality of Exosomal Proteins as Novel Cancer Biomarkers for Liquid Biopsy. Front Immunol 2022, 13, 792046. 10.3389/fimmu.2022.792046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.; Chen Y.; Ma L.; Chen Y.; Liu J.; Guo Y.; Yu T.; Zhang L.; Zhu L.; Shu Y. Role of exosomal non-coding RNAs from tumor cells and tumor-associated macrophages in the tumor microenvironment. Mol. Ther 2022, 30, 3133. 10.1016/j.ymthe.2022.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Peng X.; Liu Y.; Hao R.; Zhao R.; Zhang L.; Zhao F.; Liu Q.; Liu Y.; Qi Y. The Diagnostic Value of Serum Exosomal Has_circ_0000615 for Breast Cancer Patients. Int. J. Gen Med. 2021, 14, 4545–4554. 10.2147/IJGM.S319801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier L.; Stachelek K.; Triska M.; Jubran R.; Huang M.; Li W.; Zhang J.; Li J.; Cobrinik D. Extracellular vesicle-associated repetitive element DNAs as candidate osteosarcoma biomarkers. Sci. Rep 2021, 11 (1), 94. 10.1038/s41598-020-77398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar J. Receiver operating characteristic curve in diagnostic test assessment. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer 2010, 5 (9), 1315–1316. 10.1097/JTO.0b013e3181ec173d. [DOI] [PubMed] [Google Scholar]
- McKiernan J.; Donovan M. J.; O’Neill V.; Bentink S.; Noerholm M.; Belzer S.; Skog J.; Kattan M. W.; Partin A.; Andriole G.; et al. A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy. JAMA Oncol 2016, 2 (7), 882–889. 10.1001/jamaoncol.2016.0097. [DOI] [PubMed] [Google Scholar]
- McKiernan J.; Donovan M. J.; Margolis E.; Partin A.; Carter B.; Brown G.; Torkler P.; Noerholm M.; Skog J.; Shore N.; et al. A Prospective Adaptive Utility Trial to Validate Performance of a Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer in Patients with Prostate-specific Antigen 2–10ng/mL at Initial Biopsy. Eur. Urol 2018, 74 (6), 731–738. 10.1016/j.eururo.2018.08.019. [DOI] [PubMed] [Google Scholar]
- Huang T. Y.; Wang C. Y.; Chen K. Y.; Huang L. T. Urinary Exosomal Thyroglobulin in Thyroid Cancer Patients With Post-ablative Therapy: A New Biomarker in Thyroid Cancer. Front Endocrinol (Lausanne) 2020, 11, 382. 10.3389/fendo.2020.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok V. C.; Yu C. C. Cancer-Derived Exosomes: Their Role in Cancer Biology and Biomarker Development. Int. J. Nanomedicine 2020, 15, 8019–8036. 10.2147/IJN.S272378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martellucci S.; Orefice N. S.; Angelucci A.; Luce A.; Caraglia M.; Zappavigna S. Extracellular Vesicles: New Endogenous Shuttles for miRNAs in Cancer Diagnosis and Therapy?. Int. J. Mol. Sci. 2020, 21 (18), 6486. 10.3390/ijms21186486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Wu J.; Ye N.; Li F.; Zhan H.; Chen S.; Xu J. Plasma-Derived Exosome MiR-19b Acts as a Diagnostic Marker for Pancreatic Cancer. Frontiers in Oncology 2021, 10.3389/fonc.2021.739111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G. D.; Xu Z. Y.; Hu C.; Lv H.; Xie H. X.; Huang T.; Zhang Y. Q.; Chen G. P.; Fu Y. F.; Cheng X. D. Exosomal miR-590–5p in Serum as a Biomarker for the Diagnosis and Prognosis of Gastric Cancer. Front Mol. Biosci 2021, 8, 636566. 10.3389/fmolb.2021.636566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D.; Li B.; Shi X.; Zhang J.; Chen A.; Xu J.; Wang W.; Huang K.; Gao J.; Zheng Z.; et al. Cross-platform genomic identification and clinical validation of breast cancer diagnostic biomarkers. Aging 2021, 13 (3), 4258–4273. 10.18632/aging.202388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J.; Wang L.; Wu J.; Wang Y.; Wen H.; Zhu X.; Wang B.; Yang H. miR-370–3p as a Novel Biomarker Promotes Breast Cancer Progression by Targeting FBLN5. Stem Cells Int. 2021, 4649890. 10.1155/2021/4649890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.; Mo F.; Song X.; He Y.; Yuan Y.; Yan J.; Yang Y.; Huang J.; Zhang S. Exosomal hsa-miR-21-5p is a biomarker for breast cancer diagnosis. Peer J. 2021, 9, e12147. 10.7717/peerj.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Zhai L. Y.; Zhang L. M.; Ma X. S.; Liu Z.; Li M. M.; Chen J. X.; Duan W. J. Breast cancer plasma biopsy by in situ determination of exosomal microRNA-1246 with a molecular beacon. Analyst 2021, 146 (7), 2264–2276. 10.1039/D0AN02224A. [DOI] [PubMed] [Google Scholar]
- Duca R.; Massillo C.; Dalton G.; Farré P.; Graña K.; Gardner K.; De Siervi A. MiR-19b-3p and miR-101-3p as potential biomarkers for prostate cancer diagnosis and prognosis. American Journal of Cancer Research 2021, 11 (6), 2802–2820. [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Li L. X.; Diao Y. J.; Wang J.; Ye Y.; Hao X. K. Identification of Urinary Exosomal miRNAs for the Non-Invasive Diagnosis of Prostate Cancer. Cancer Manag Res. 2021, 13, 25–35. 10.2147/CMAR.S272140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X.; Lv Z.; Ding H.; Xing C.; Yuan Y. MiR-1539 and Its Potential Role as a Novel Biomarker for Colorectal Cancer. Front Oncol 2021, 10, 531244. 10.3389/fonc.2020.531244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L.; Shi W. J.; Xie Y. B.; Zhang Z. G. Diagnostic value of four serum exosome microRNAs panel for the detection of colorectal cancer. World J. Gastrointest Oncol 2021, 13 (8), 970–979. 10.4251/wjgo.v13.i8.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv A.; Tu Z.; Huang Y.; Lu W.; Xie B. Circulating exosomal miR-125a-5p as a novel biomarker for cervical cancer. Oncol Lett. 2020, 21 (1), 54. 10.3892/ol.2020.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q.; Ding H.; Wang L.; Yan Y.; Wan Y.; Yi Y.; Tao L.; Zhu C. Circulating Exosomal miR-96 as a Novel Biomarker for Radioresistant Non-Small-Cell Lung Cancer. J. Oncol 2021, 5893981. 10.1155/2021/5893981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Q.; Wang Y.; Li X.; Jin X.; Wang G. Decreased serum exosomal miR-29a expression and its clinical significance in papillary thyroid carcinoma. J. Clin Lab Anal 2021, 35 (1), e23560. 10.1002/jcla.23560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Yoo J.; Ho J. Y.; Jung Y.; Lee S.; Hur S. Y.; Choi Y. J. Plasma-derived exosomal miR-4732–5p is a promising noninvasive diagnostic biomarker for epithelial ovarian cancer. J. Ovarian Res. 2021, 14 (1), 59. 10.1186/s13048-021-00814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shal A. S.; Shalaby S. M.; Abouhashem S. E.; Elbary E. H. A.; Azazy S.; Rashad N. M.; Sarhan W. Urinary exosomal microRNA-96–5p and microRNA-183–5p expression as potential biomarkers of bladder cancer. Mol. Biol. Rep 2021, 48 (5), 4361–4371. 10.1007/s11033-021-06451-5. [DOI] [PubMed] [Google Scholar]
- Lin H.; Shi X.; Li H.; Hui J.; Liu R.; Chen Z.; Lu Y.; Tan W. Urinary Exosomal miRNAs as biomarkers of bladder Cancer and experimental verification of mechanism of miR-93–5p in bladder Cancer. BMC Cancer 2021, 21 (1), 1293. 10.1186/s12885-021-08926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T.; Guo X.; Li X.; Liao C.; Wang X.; He K. Plasma-Derived Exosomal microRNA-130a Serves as a Noninvasive Biomarker for Diagnosis and Prognosis of Oral Squamous Cell Carcinoma. J. Oncol. 2021, 5547911. 10.1155/2021/5547911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.; Xu M.; Zhang G.; Dong L.; Wu J.; Wei C.; Xu K.; Zhang L. Identification of Circulating Exosomal miR-101 and miR-125b Panel Act as a Potential Biomarker for Hepatocellular Carcinoma. International Journal of Genomics 2021, 1326463. 10.1155/2021/1326463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L.; Wang W.; Wang F.; Yang S.; Hu J.; Lu B.; Pan Z.; Ma Y.; Zheng M.; Zhou L.; et al. Plasma-derived exosomal miR-15a-5p as a promising diagnostic biomarker for early detection of endometrial carcinoma. Mol. Cancer 2021, 20 (1), 57. 10.1186/s12943-021-01352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Chen L.; Ye X.; Wu Z.; Zhang Z.; Sun B.; Fu H.; Fu C.; Liang X.; Jiang H. Expression and mechanism of exosome-mediated A FOXM1 related long noncoding RNA in gastric cancer. J. Nanobiotechnology 2021, 19 (1), 133. 10.1186/s12951-021-00873-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan F.; Zhang X.; Li H.; Yue X.; Sun Q. Serum exosomal lncRNA XIST is a potential non-invasive biomarker to diagnose recurrence of triple-negative breast cancer. J. Cell Mol. Med. 2021, 25 (16), 7602–7607. 10.1111/jcmm.16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M.; Song X. G.; Zhao Y. J.; Dong X. H.; Niu L. M.; Zhang Z. J.; Shang X. L.; Tang Y. Y.; Song X. R.; Xie L. Circulating Serum Exosomal Long Non-Coding RNAs FOXD2-AS1, NRIR, and XLOC_009459 as Diagnostic Biomarkers for Colorectal Cancer. Front Oncol 2021, 11, 618967. 10.3389/fonc.2021.618967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X. Z.; Zhang S. Q.; Deng X. L.; Qiang J. H. Serum Exosomal lncRNA DLX6-AS1 Is a Promising Biomarker for Prognosis Prediction of Cervical Cancer. Technol. Cancer Res. Treat 2021, 20, 1533033821990060. 10.1177/1533033821990060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P.; Zhao J.; Gao L. Increased serum exosomal long non-coding RNA SNHG15 expression predicts poor prognosis in non-small cell lung cancer. Journal of Clinical Laboratory Analysis 2021, 10.1002/jcla.23979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.; Zhu H.; Xiao M.; Zhou S. Serum exosomal long noncoding RNA CRNDE as a prognostic biomarker for hepatocellular carcinoma. Journal of Clinical Laboratory Analysis 2021, 10.1002/jcla.23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.; Zhang Y.; Gao X.; Yuan Y.; Zhao J.; Zhou S.; Wang H.; Wang L.; Xu G.; Li X. Plasma-Derived Exosomal ALIX as a Novel Biomarker for Diagnosis and Classification of Pancreatic Cancer. Frontiers in Oncology 2021, 10.3389/fonc.2021.628346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.; Pang H.; He Q.; Pan B.; Sun X.; Shan J.; Wu L.; Wu K.; Yao X.; Guo Y. A novel strategy to identify candidate diagnostic and prognostic biomarkers for gastric cancer. Cancer Cell Int. 2021, 21 (1), 335. 10.1186/s12935-021-02007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.; Zhou X.; Peng W.; Wu J.; Wu X.; Chen Y.; Cui Z. Expression and clinical implications of basic leucine zipper ATF-like transcription factor 2 in breast cancer. BMC Cancer 2021, 21 (1), 1062. 10.1186/s12885-021-08785-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunusova N. V.; Zambalova E. A.; Patysheva M. R.; Kolegova E. S.; Afanas’ev S. G.; Cheremisina O. V.; Grigor’eva A. E.; Tamkovich S. N.; Kondakova I. V. Exosomal Protease Cargo as Prognostic Biomarker in Colorectal Cancer. Asian Pac J. Cancer Prev 2021, 22 (3), 861–869. 10.31557/APJCP.2021.22.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z.; Ji S.; Wu J.; Tian J.; Quan W.; Shang A.; Ji P.; Xiao W.; Liu D.; Wang X.; et al. Proteomics-Based Identification of Candidate Exosomal Glycoprotein Biomarkers and Their Value for Diagnosing Colorectal Cancer. Front Oncol 2021, 11, 725211. 10.3389/fonc.2021.725211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Fu B.; Li M.; Mi S. Secretome of Activated Fibroblasts Induced by Exosomes for the Discovery of Biomarkers in Non-Small Cell Lung Cancer. Small 2021, 17 (4), e2004750. 10.1002/smll.202004750. [DOI] [PubMed] [Google Scholar]
- Choi E. S.; Faruque H. A.; Kim J. H.; Kim K. J.; Choi J. E.; Kim B. A.; Kim B.; Kim Y. J.; Woo M. H.; Park J. Y. CD5L as an Extracellular Vesicle-Derived Biomarker for Liquid Biopsy of Lung Cancer. Diagnostics (Basel) 2021, 11 (4), 620. 10.3390/diagnostics11040620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamichi E.; Sakakura H.; Mii S.; Yamamoto N.; Hibi H.; Asai M.; Takahashi M. Detection of serum/salivary exosomal Alix in patients with oral squamous cell carcinoma. Oral Dis 2021, 27 (3), 439–447. 10.1111/odi.13565. [DOI] [PubMed] [Google Scholar]
- Cui Z.; Lin Y.; Hu D.; Wu J.; Peng W.; Chen Y. Diagnostic and Prognostic Potential of Circulating and Tissue BATF2 in Nasopharyngeal Carcinoma. Frontiers in molecular biosciences 2021, 8, 724373. 10.3389/fmolb.2021.724373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C.; Zhang Y.; Zhang M.; Li T.; Zheng X.; Guo Q.; Zhang X. Exosomal Cripto-1 Serves as a Potential Biomarker for Perihilar Cholangiocarcinoma. Front Oncol 2021, 11, 730615. 10.3389/fonc.2021.730615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel G.; D’Angelo G.; Raposo G. Shedding light on the cell biology of extracellular vesicles. Nature reviews. Molecular cell biology 2018, 19 (4), 213–228. 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- Wei D.; Zhan W.; Gao Y.; Huang L.; Gong R.; Wang W.; Zhang R.; Wu Y.; Gao S.; Kang T. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell research 2021, 31 (2), 157–177. 10.1038/s41422-020-00409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenific C.; Zhang H.; Lyden D. An exosome pathway without an ESCRT. Cell research 2021, 31 (2), 105–106. 10.1038/s41422-020-00418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya-Beltri C.; Gutierrez-Vazquez C.; Sanchez-Cabo F.; Perez-Hernandez D.; Vazquez J.; Martin-Cofreces N.; Martinez-Herrera D. J.; Pascual-Montano A.; Mittelbrunn M.; Sanchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013, 4, 2980. 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ageta H.; Ageta-Ishihara N.; Hitachi K.; Karayel O.; Onouchi T.; Yamaguchi H.; Kahyo T.; Hatanaka K.; Ikegami K.; Yoshioka Y.; et al. UBL3 modification influences protein sorting to small extracellular vesicles. Nat. Commun. 2018, 9 (1), 3936. 10.1038/s41467-018-06197-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M.; Zargartalebi H.; Salahandish R.; Aburashed R.; Wey Yong K.; Sanati-Nezhad A. Emerging technologies and commercial products in exosome-based cancer diagnosis and prognosis. Biosens Bioelectron 2021, 183, 113176. 10.1016/j.bios.2021.113176. [DOI] [PubMed] [Google Scholar]
- Tellez-Gabriel M.; Knutsen E.; Perander M. Current Status of Circulating Tumor Cells, Circulating Tumor DNA, and Exosomes in Breast Cancer Liquid Biopsies. Int. J. Mol. Sci. 2020, 21 (24), 9457. 10.3390/ijms21249457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendt M.; Kamerkar S.; Sugimoto H.; McAndrews K. M.; Wu C. C.; Gagea M.; Yang S.; Blanko E. V. R.; Peng Q.; Ma X. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 10.1172/jci.insight.99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B.; Lei Y.; Wang J.; Zhu L.; Wu Y.; Zhang H.; Wu L.; Zhang P.; Yang C. Microfluidic-Based Exosome Analysis for Liquid Biopsy. Small Methods 2021, 5 (3), e2001131. 10.1002/smtd.202001131. [DOI] [PubMed] [Google Scholar]
- Chen J.; Li P.; Zhang T.; Xu Z.; Huang X.; Wang R.; Du L. Review on Strategies and Technologies for Exosome Isolation and Purification. Front Bioeng Biotechnol 2022, 9, 811971. 10.3389/fbioe.2021.811971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thietart S.; Rautou P. E. Extracellular vesicles as biomarkers in liver diseases: A clinician’s point of view. J. Hepatol 2020, 73 (6), 1507–1525. 10.1016/j.jhep.2020.07.014. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Yu D. Exosomes in cancer development, metastasis, and immunity. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 2019, 1871 (2), 455–468. 10.1016/j.bbcan.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseri M.; Zoller M.; Hadjati J.; Ghods R.; Ranaei Pirmardan E.; Kiani J.; Eini L.; Bozorgmehr M.; Madjd Z. Dendritic cells loaded with exosomes derived from cancer stem cell-enriched spheroids as a potential immunotherapeutic option. J. Cell Mol. Med. 2021, 25 (7), 3312–3326. 10.1111/jcmm.16401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque S.; Vaiselbuh S. R. CD19 Chimeric Antigen Receptor-Exosome Targets CD19 Positive B-lineage Acute Lymphocytic Leukemia and Induces Cytotoxicity. Cancers (Basel) 2021, 13 (6), 1401. 10.3390/cancers13061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y.; Kim H.; Yoon S.; Lee H.; Hwang J.; Jung J.; Chang J. H.; Choi J.; Kim H. Exosome-based photoacoustic imaging guided photodynamic and immunotherapy for the treatment of pancreatic cancer. J. Controlled Release 2021, 330, 293–304. 10.1016/j.jconrel.2020.12.039. [DOI] [PubMed] [Google Scholar]
- Zhu D.; Liu Z.; Li Y.; Huang Q.; Xia L.; Li K. Delivery of manganese carbonyl to the tumor microenvironment using Tumor-Derived exosomes for cancer gas therapy and low dose radiotherapy. Biomaterials 2021, 274, 120894. 10.1016/j.biomaterials.2021.120894. [DOI] [PubMed] [Google Scholar]
- Pan S.; Zhang Y.; Huang M.; Deng Z.; Zhang A.; Pei L.; Wang L.; Zhao W.; Ma L.; Zhang Q.; et al. Urinary exosomes-based Engineered Nanovectors for Homologously Targeted Chemo-Chemodynamic Prostate Cancer Therapy via abrogating EGFR/AKT/NF-kB/IkB signaling. Biomaterials 2021, 275, 120946. 10.1016/j.biomaterials.2021.120946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no data sets were generated or analyzed during the current study.