Abstract

Zn(II), Pd(II), and Cd(II) complexes, [LTHMCl2] (M = Zn, Pd; X = Br, Cl) and [LTHCd(μ-X)X]n (X = Cl, Br; n = n, 2), supported by the (E)-N1,N1-dimethyl-N2-(thiophen-2-ylmethylene)ethane-1,2-diamine (LTH) ligand are synthesized and structurally characterized. Density functional theory (DFT) electronic structure calculations and variable-temperature NMR support the presence of two conformers and a dynamic interconversion process of the minor conformer to the major one in solution. It is found that the existence of two relevant complex conformers and their respective ratios in solution depend on the central metal ions and counter ions, either Cl– or Br–. Among the two relevant conformers, a single conformer is crystallized and X-ray diffraction analysis revealed a distorted tetrahedral geometry for Zn(II) complexes, and a distorted square planar and square pyramidal geometry for Pd(II) and Cd(II) complexes, respectively. It is shown that [LTHMCl2]/LiOiPr (M = Zn, Pd) and [LTHCd(μ-Cl)Cl]n/LiOiPr can effectively catalyze the ring-opening polymerization (ROP) reaction of rac-lactide (rac-LA) with 94% conversion within 30 s with [LTHZnCl2]/LiOiPr at 0 °C. Overall, hetero-enriched poly(lactic acid)s (PLAs) were provided by these catalytic systems with [LTHZnCl2]/LiOiPr producing PLA with higher heterotactic bias (Pr up to 0.74 at 0 °C).
1. Introduction
Schiff base ligands have gained superior attention owing to their extraordinary synthetic and structural properties, fine tunability, and chemical selectivity of the central metal atom.1,2 Schiff bases are considered the most promising ligands, due to their exceptional chelating properties with diverse geometries, ranging from N,N′-bidentate to N,N′,X-tridentate and N,N′,N,X′-tetradentate.3−6 The azomethine group in these Schiff bases controls the central ion performance in a wide range of promising catalytic reactions like olefin hydrogenation, cyclic olefin ring-opening polymerization (ROP), photochromic properties, pharmacological and biological applications.5−11 Several transition metal complexes supported by Schiff base ligands with thiophene as a structural fragment were studied using a combined experimental and theoretical approach12,13 and it was found that ab initio density functional theory (DFT) calculations reproduce the atomic and electronic structure and spectra of the complexes with high accuracy.
In this study, the C1-symmetric thiophene-derived Schiff base ligand (E)-N1,N1-dimethyl-N2-(thiophen-2-ylmethylene)ethane-1,2-diamine (LTH)14 was administered and studied using a joint experimental and theoretical approach. In particular, the coordination of the Schiff base to Zn(II), Cd(II), and Pd(II) metal ions was determined. Depending on the metal center in the chelate, diversity in coordination geometries resulted in the studied complexes. Detailed variable-temperature NMR studies revealed a dynamic interconversion process of a minor conformer to a major one in solution, probably arising from the orientation of the thiophene moiety with respect to the coordination plane. These experiments were verified using density functional quantum chemical electronic structure calculations.
Since the discovery of green polymers as substituents of petroleum-based analogues, biodegradable synthetic polymers are widely used for commodity, biomedical, and pharmaceutical applications,15,16 in particular for cyclic esters’ ring-opening polymerization (ROP) reactions. Frequently, it involves application of an alkoxide metal-derived catalyst or other species that can generate an alkoxide in-situ.17−20 The choice of the ligands determines the catalytic behavior of central metals in the complexes. The main goal of the study is to develop a promising catalyst to promote ROP reactions using Schiff base metal complexes as an effective ROP of lactide (LA) for controllable polymerization and to achieve polymers with narrow-molecular-weight distributions.19,21−30 In the current study, the preliminary polymerization data revealed that the in situ-generated [LTHZnCl2]/[LiOiPr] system resulted in high polymerization activity with heterotactic enchainment in the resultant poly(lactic acid)s (PLAs) compared to its Cd(II) and Pd(II) complexes.
2. Experimental Section
2.1. Materials and Methods
The protocols for synthesis of the ligand (LTH) and the corresponding zinc(II) palladium(II) and cadmium(II) complexes followed the bench-top techniques. The starting materials, including N,N-dimethylethylenediamine, 2-thiophenecarboxaldehyde, zinc bromide (ZnBr2), cadmium bromide tetrahydrate (CdBr2·4H2O), and magnesium sulfate (MgSO4), were obtained from TCI and Aldrich. Zinc chloride (ZnCl2), palladium chloride (PdCl2), palladium bromide (PdBr2), and cadmium chloride (CdCl2) were obtained from Aldrich and stored under controllable conditions in a glovebox. Solvents like methylene chloride (CH2Cl2), acetonitrile (MeCN), ethanol (EtOH), diethyl ether (Et2O), n-hexane (n-Hex), N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), acetone, and molecular sieves (0.4 nm) were purchased from Merck. The studied ligand31 and [Pd(MeCN)2]Cl2(32) were resynthesized following previously reported protocols. The melting points of all of the complexes were determined using an IA9100 (Electrothermal) instrument. The 500 MHz 1H and 125 MHz 13C NMR spectra of the corresponding Zn(II), Pd(II), and Cd(II) complexes (Figures S2–S13) were recorded using a Bruker Avance Digital 500-NMR spectrometer (Bruker, Billerica, MA), with chemical shifts in ppm units (δ) using SiMe4 as an internal standard and coupling constants (J) measured in hertz (Hz). The Fourier-transform infrared (FTIR) spectra (cm–1) recorded using Bruker FT/IR-α (neat) are presented in Figures S14–S19. Using elemental analysis (EA) with EA 1108-elemental analyzer (Carlo Erba, Milan, Italy), the empirical formulas of the synthesized Zn(II), Cd(II), and Pd(II) complexes were determined and are presented in Figure S20. The variable-temperature NMR (operating at 500 MHz) was recorded using a Bruker BBFO plus 500 MHz SmartProbe NMR spectrometer (Figures S22–S27).
2.2. Synthesis Procedures
2.2.1. (E)-N1,N1-dimethyl-N2-(thiophen-2-ylmethylene)ethane-1,2-diamine(dichloro)zinc(II), [LTHZnCl2]
The mixture of 0.182 g with 1.00 mmol concentration of LTH and 0.136 g, 1.00 mmol ZnCl2 in 20.0 mL of EtOH was stirred at ambient temperature for 24 h. The precipitated white solid was collected and washed with cold 20.0 mL × 2 EtOH and 20.0 mL × 3 Et2O. The solid (0.272 g, 85%) was dried under vacuum and stored. M.P.: 221 °C. Anal. calcd for C9H14Cl2N2SZn (%): C, 33.9; H, 4.43, N; 8.79. Found: C, 33.9; H, 4.40; N, 8.69. The [LTHZnCl2] single crystals appropriate for an X-ray crystallographic analysis were obtained from the CH2Cl2/n-Hex solvent system. 1H NMR (500 MHz; DMSO-d6): Conformer a δ 8.66 (1H, s, −N=CH-Thiophene), 7.85 (1H, d, J = 4.74 Hz, Thiophene-H), 7.67 (1H, d, J = 3.41 Hz, Thiophene-H), 7.21 (1H, t, J = 3.61 Hz, Thiophene-H), 3.75 (2H, t, J = 5.58 Hz, =N–CH2–CH2–N–(CH3)2), 2.66 (2H, t, J = 6.38 Hz, =N–CH2–CH2–N–(CH3)2), 2.35 (6H, s, =N–CH2–CH2–N–(CH3)2). Conformer b (5%) δ 8.79 (1H, s, −N=CH-Thiophene), 8.35 (1H, d, J = 5.01 Hz, Thiophene-H), 8.11 (1H, d, J = 3.41 Hz, Thiophene-H), 7.44 (1H, t, J = 4.01 Hz, Thiophene-H), 3.82 (2H, t, J = 5.81 Hz, =N–CH2–CH2–N–(CH3)2), 2.84 (2H, t, J = 5.85 Hz, =N–CH2–CH2–N–(CH3)2), 2.42 (6H, s, =N–CH2–CH2–N–(CH3)2). 13C NMR (125 MHz; DMSO-d6): Conformer a δ 158.51 (1C, Thiophene-C), 143.51 (1C, Thiophene-C), 133.56 (1C, Thiophene-C), 131.94 (1C, −N=CH-Thiophene), 128.02 (1C, Thiophene-C), 58.90 (1C, =N–CH2–CH2–N–(CH3)2), 56.11 (1C, =N–CH2–CH2–N–(CH3)2), 45.61 (2C, CH2–N–(CH3)2). Conformer b (5%) δ 159.75 (1C, Thiophene-C), 139.72 (1C, Thiophene-C), 137.95 (1C, Thiophene-C), 136.11 (1C, −N=CH-Thiophene), 128.96 (1C, Thiophene-C), 59.05 (1C, =N–CH2–CH2–N–(CH3)2), 45.14 (1C, =N–CH2–CH2–N–(CH3)2), 45.98 (2C, CH2–N–(CH3)2). FTIR (solid (neat); cm–1): ν(sp3 C–H) 2894 w; ν(C=N)imine 1626 s; ν(C=C)thiophene 1455 m; ν(C–N) 1258 m; δ(sp2 C–H) 725 s; ν(M–N) 583 m.
2.2.2. (E)-N1,N1-dimethyl-N2-(thiophen-2-ylmethylene)ethane-1,2-diamine(dibromo)zinc(II), [LTHZnBr2]
[LTHZnBr2] was synthesized following the procedure used for [LTHZnCl2] with 0.365 g, 2.00 mmol LTH and 0.450 g, 2.00 mmol ZnBr2 used to get a 0.775 g, 95% white substance. M.P.: 245 °C. Anal. calcd for C9H14Br2N2SZn (%): C, 26.5; H, 3.46, N; 6.87. Found: C, 26.7; H, 3.46; N, 6.96. [LTHZnBr2] monocrystals for the X-ray crystallographic analysis were extracted from the CH2Cl2/n-Hex solvent system. 1H NMR (500 MHz; DMSO-d6): Conformer a δ 8.64 (1H, s, −N=CH-Thiophene), 7.83 (1H, d, J = 4.86 Hz, Thiophene-H), 7.65 (1H, d, J = 3.07 Hz, Thiophene-H), 7.20 (1H, t, J = 3.71 Hz, Thiophene-H), 3.73 (2H, t, J = 6.40 Hz, =N–CH2–CH2–N–(CH3)2), 2.63 (2H, t, J = 6.40 Hz, =N–CH2–CH2–N–(CH3)2), 2.32 (6H, s, =N–CH2–CH2–N–(CH3)2). Conformer b (7%) δ 8.78 (1H, s, −N=CH-Thiophene), 8.36 (1H, d, J = 4.99 Hz, Thiophene-H), 8.13 (1H, d, J = 3.58 Hz, Thiophene-H), 7.45 (1H, t, J = 3.84 Hz, Thiophene-H), 3.84 (2H, t, J = 6.01 Hz, =N–CH2–CH2–N–(CH3)2), 2.86 (2H, t, J = 6.14 Hz, =N–CH2–CH2–N–(CH3)2), 2.44 (6H, s, =N–CH2–CH2–N–(CH3)2). 13C NMR (125 MHz; DMSO-d6): Conformer a δ 158.65 (1C, Thiophene-C), 143.50 (1C, Thiophene-C), 133.84 (1C, Thiophene-C), 132.13 (1C, −N=CH-Thiophene), 127.97 (1C, Thiophene-C), 58.76 (1C, =N–CH2–CH2–N–(CH3)2), 56.08 (1C, =N–CH2–CH2–N–(CH3)2), 45.76 (2C, CH2–N–(CH3)2). Conformer b (7%) δ 159.72 (1C, Thiophene-C), 139.52 (1C, Thiophene-C), 137.95 (1C, Thiophene-C), 136.10 (1C, −N=CH-Thiophene), 128.95 (1C, Thiophene-C), 58.92 (1C, =N–CH2–CH2–N–(CH3)2), 45.37 (1C, =N–CH2–CH2–N–(CH3)2), 46.39 (2C, CH2–N–(CH3)2). Solid neat FTIR (in cm–1): ν(sp3 C–H) 2876 w; ν(C=N)imine 1627 s; ν(C=C)thiophene 1458 m; ν(C–N) 1257 m; δ(sp2 C–H) 727 s; ν(M–N) 589 m.
2.2.3. (E)-N1,N1-dimethyl-N2-(thiophen-2-ylmethylene)ethane-1,2-diamine(dichloro)palladium(II), [LTHPdCl2]
[LTHPdCl2] was synthesized in accordance with the method used to prepare [LAZnCl2]. The 0.182 g, 1.00 mmol LTH and 0.259 g, 1.00 mmol [Pd(MeCN)2Cl2] were used to obtain the final 0.242 g, 67% yellow powder substance. M.P.: 198 °C. Anal. calcd for C9H14Cl2N2PdS (%): C, 30.1; H, 3.92, N; 7.79. Determined: C, 30.1; H, 4.06; N, 7.77. Monocrystalline samples of [LTHPdCl2] for X-ray crystallographic analysis were crystallized from the CH2Cl2/n-Hex solvent. 1H NMR (500 MHz; DMSO-d6): δ 8.86 (1H, s, −N=CH-Thiophene), 8.34 (1H, d, J = 5.02 Hz, Thiophene-H), 7.99 (1H, d, J = 3.56 Hz, Thiophene-H), 7.40 (1H, t, J = 3.98 Hz, Thiophene-H), 3.97 (2H, t, J = 6.07 Hz, =N–CH2–CH2–N–(CH3)2), 2.86 (2H, t, J = 6.07 Hz, =N–CH2–CH2–N–(CH3)2), 2.70 (6H, s, =N–CH2–CH2–N–(CH3)2). 13C NMR (125 MHz; DMSO-d6): δ 159.84 (1C, Thiophene-C), 139.81 (1C, Thiophene-C), 137.64 (1C, Thiophene-C), 132.56 (1C, −N=CH–Thiophene), 128.83 (1C, Thiophene-C), 64.88 (1C, =N–CH2–CH2–N–(CH3)2), 54.68 (1C, =N–CH2–CH2–N–(CH3)2), 50.68 (2C, CH2–N–(CH3)2). FTIR (solid (neat); cm–1): ν(sp3 C–H) 2900 w; ν(C=N)imine 1615 s; ν(C=C)thiophene 1472 m; ν(C–N) 1327 m; δ(sp2 C–H) 763 s; ν(M–N) 605 m.
2.2.4. (E)-N1,N1-dimethyl-N2-(thiophen-2-ylmethylene)ethane-1,2-diamine(dibromo)palladium(II), [LTHPdBr2]
0.182 g, 1.00 mmol LTH and 0.266 g, 1.00 mmol PdBr2 were mixed in MeCN (20.0 mL) and stirred and refluxed for 24 h. The resultant dark yellow substance was collected and washed using cold 20.0 mL × 2 EtOH and 20.0 mL × 3 Et2O. The crystalline solid (0.255 g, 57%) was dried in vacuum conditions and stored. M.P.: 182 °C. Anal. calcd for C9H14Br2N2PdS (%): C, 24.1; H, 3.15, N; 6.25. Found: C, 23.8; H, 3.08; N, 6.56. Monocrystalline [LTHPdBr2] samples for X-ray crystallographic analysis were collected from the CH2Cl2/n-Hex solvent. 1H NMR (500 MHz; DMSO-d6): δ 9.09 (1H, s, −N=CH-Thiophene), 8.35 (1H, d, J = 4.91 Hz, Thiophene-H), 7.98 (1H, d, J = 3.13 Hz, Thiophene-H), 7.40 (1H, t, J = 4.03 Hz, Thiophene-H), 3.99 (2H, t, J = 5.84 Hz, =N–CH2–CH2–N–(CH3)2), 2.87 (2H, t, J = 5.84 Hz, =N–CH2–CH2–N–(CH3)2), 2.79 (6H, s, =N–CH2–CH2–N–(CH3)2). 13C NMR (125 MHz; DMSO-d6): δ 161.27 (1C, Thiophene-C), 139.72 (1C, Thiophene-C), 137.67 (1C, Thiophene-C), 133.00 (1C, −N=CH-Thiophene), 128.87 (1C, Thiophene-C), 64.73 (1C, =N–CH2–CH2–N–(CH3)2), 54.64 (1C, =N–CH2–CH2–N–(CH3)2), 51.33 (2C, CH2–N–(CH3)2). FTIR (solid (neat); cm–1): ν(sp3 C–H) 2909 w; ν(C=N)imine 1609 s; ν(C=C)thiophene 1469 m; ν(C–N) 1324 m; δ(sp2 C–H) 723 s; ν(M–N) 603 m.
2.2.5. (E)-N1,N1-dimethyl-N2-(thiophen-2-ylmethylene)ethane-1,2-diamine(dichloro)cadmium(II), [LTHCd(μ-Cl)Cl]n
[LTHCd(μ-Cl)Cl]n was synthesized by following the procedure used to prepare [LTHZnCl2], for which 0.182 g, 1.00 mmol LTH and 0.183 g, 1.00 mmol CdCl2 were used to collect the 0.319 g, 87% final white polycrystalline product. M.P.: 202 °C. Anal. calcd for C18H28Cd2Cl4N4S2 (%): C, 29.6; H, 3.86, N; 7.66. Determined: C, 29.6; H, 3.89; N, 7.69. Monocrystals of [LTHCd(μ-Cl)Cl]n for the X-ray crystallographic analysis were collected from CH2Cl2/n-Hex solvent. 1H NMR (500 MHz; DMSO-d6): Conformer a δ 8.46 (1H, s, −N=CH-Thiophene), 7.65 (1H, d, J = 5.16 Hz, Thiophene-H), 7.45 (1H, d, J = 3.53 Hz, Thiophene-H), 7.13 (1H, t, J = 3.66 Hz, Thiophene-H) 3.60 (2H, t, J = 6.78 Hz, =N–CH2–CH2–N–(CH3)2), 2.47 (2H, t, J = 6.78 Hz, =N–CH2–CH2–N–(CH3)2), 2.17 (6H, s, =N–CH2–CH2–N–(CH3)2). Conformer b (8%) δ 8.69 (1H, s, −N=CH-Thiophene), 8.25 (1H, d, J = 5.02 Hz, Thiophene-H), 7.96 (1H, d, J = 3.66 Hz, Thiophene-H), 7.40 (1H, t, J = 3.80 Hz, Thiophene-H), 3.70 (2H, t, J = 6.28 Hz, =N–CH2–CH2–N–(CH3)2), 2.71 (2H, t, J = 5.87 Hz, =N–CH2–CH2–N–(CH3)2), 2.33 (6H, s, =N–CH2–CH2–N–(CH3)2). 13C NMR (125 MHz; DMSO-d6): Conformer a δ 155.46 (1C, Thiophene-C), 141.87 (1C, Thiophene-C), 131.32 (1C, Thiophene-C), 129.60 (1C, −N=CH-Thiophene), 127.70 (1C, Thiophene-C), 59.61 (1C, =N–CH2–CH2–N–(CH3)2), 57.80 (1C, =N–CH2–CH2–N–(CH3)2), 45.51 (2C, CH2–N–(CH3)2). Conformer b (8%) δ 157.63 (1C, Thiophene-C), 138.26 (1C, Thiophene-C), 136.08 (1C, Thiophene-C), 134.31 (1C, −N=CH-Thiophene), 128.48 (1C, Thiophene-C), 57.33 (1C, =N–CH2–CH2–N–(CH3)2), 46.98 (1C, =N–CH2–CH2–N–(CH3)2), 45.40 (2C, CH2–N–(CH3)2). Solid neat FTIR (in cm–1): ν(sp3 C–H) 2900 w; ν(C=N)imine 1625 s; ν(C=C)thiophene 1467 m; ν(C–N) 1330 m; δ(sp2 C–H) 741 s; ν(M–N) 595 m.
2.2.6. (E)-N1,N1-dimethyl-N2-(thiophen-2-ylmethylene)ethane-1,2-diamine(dibromo)cadmium(II), [LTHCd(μ-Br)Br]2
[LTHCd(μ-Br)Br]2 was synthesized using the mixture of 0.365 g, 2.00 mmol LTH and 0.689 g, 2.00 mmol CdBr2·4H2O, and the 0.817 g, 90% white powder product was obtained. M.P.: 203 °C. Anal. calcd for C18H28Br2CdN2S2 (%): C, 23.8; H, 3.10, N; 6.16. Discovered: C, 24.0; H, 3.10; N, 6.21. For the X-ray crystallographic analysis, monocrystals of [LTHCd(μ-Br)Br]2 isolated from the CH2Cl2/n-Hex solvent were used. 1H NMR (500 MHz; DMSO-d6): Conformer a δ 8.46 (1H, s, −N=CH-Thiophene), 7.65 (1H, d, J = 4.55 Hz, Thiophene-H), 7.46 (1H, d, J = 3.76 Hz, Thiophene-H), 7.13 (1H, t, J = 3.49 Hz, Thiophene-H), 3.60 (2H, t, J = 6.64 Hz, =N–CH2–CH2–N–(CH3)2), 2.47 (2H, t, J = 6.69 Hz, =N–CH2–CH2–N–(CH3)2), 2.17 (6H, s, =N–CH2–CH2–N–(CH3)2). Conformer b (11%) δ 8.70 (1H, s, −N=CH-Thiophene), 8.25 (1H, d, J = 5.11 Hz, Thiophene-H), 7.96 (1H, d, J = 3.75 Hz, Thiophene-H), 7.41 (1H, t, J = 3.66 Hz, Thiophene-H), 3.70 (2H, t, J = 5.91 Hz, =N–CH2–CH2–N–(CH3)2), 2.72 (2H, t, J = 5.79 Hz, =N–CH2–CH2–N–(CH3)2), 2.33 (6H, s, =N–CH2–CH2–N–(CH3)2). 13C NMR (125 MHz; DMSO-d6): Conformer a δ 155.69 (1C, Thiophene-C), 141.64 (1C, Thiophene-C), 131.53 (1C, Thiophene-C), 129.78 (1C, −N=CH-Thiophene), 127.71 (1C, Thiophene-C), 59.62 (1C, =N–CH2–CH2–N–(CH3)2), 57.62 (1C, =N–CH2–CH2–N–(CH3)2), 45.59 (2C, CH2–N–(CH3)2). Conformer b (11%) δ 157.64 (1C, Thiophene-C), 138.34 (1C, Thiophene-C), 136.19 (1C, Thiophene-C), 134.19 (1C, −N=CH-Thiophene), 128.50 (1C, Thiophene-C), 57.20 (1C, =N–CH2–CH2–N–(CH3)2), 46.96 (1C, =N–CH2–CH2–N–(CH3)2), 45.45 (2C, CH2–N–(CH3)2). Solid neat FTIR (in cm–1): ν(sp3 C–H) 2878 w; ν(C=N)imine 1617 s; ν(C=C)thiophene 1466 m; ν(C–N) 1330 m; δ(sp2 C–H) 746 s; ν(M–N) 577 m.
2.3. X-ray Crystallographic Studies
For X-ray study, the sample crystals were layered with Paratone-N oil, and the diffraction data were measured under different synchrotron radiation wavelengths λ, namely (i) for the colorless crystal, [LTHZnCl2], λ = 0.700 Å at 293(2) K; (ii) for [LTHZnBr2], λ = 0.630 Å at 293(2) K; (iii) for [LTHCd(μ-Cl)Cl]n, λ = 0.650 Å at 100(2) K; (iv) for [LTHCd(μ-Br)Br]2, λ = 0.610 Å at 220(2) K; (v) for the yellow crystal, [LTHPdCl2], λ = 0.630 Å at 100(2) K; and (vi) for [LTHPdBr2], λ = 0.630 Å at 293(2) K. The crystals were studied at Pohang Accelerator Laboratory (South Korea) using the ADSC Quantum-210 detector at 2D-SMC with a silicon(111) double-crystal monochromator (DCM) with detector distance 63 mm, omega scan, Δω = 3°, and exposure time 1 s per frame. The PAL BL2D-SMDC program33 was employed for data collection. HKL3000sm (Ver. 703r)34,35 was utilized for cell refinement, reduction, and absorption correction. The intrinsic phasing approach implemented in SHELXT36 code was used for structure resolution. Structural data were refined by full-matrix least-squares refinement by means of the SHELXL-2018 code. In particular, the atomic coordinates of all nonhydrogen atoms were refined by the anisotropic displacement factors, and the atomic coordinates of hydrogen atoms with respect to their parent atoms were restored using the riding model with the HFIX command in SHELXL-2014. Crystallographic refrainment is summarized in Table S1.
2.4. Polymerization Procedures
For ROP reactions, all procedures were performed under argon atmosphere in the high-vacuum Schlenk line and glovebox. The Zn(II), Pd(II), and Cd(II) complexes (0.250 mmol) (which are in fact very reactive catalytic species) and LiOiPr as the initiating reagent were used. Under argon atmosphere, 0.0796 g of [LTHZnCl2], 0.0899 g of [LTHPdCl2], and 0.0914 g of [LTHCd(μ-Cl)Cl]2 were added to a 50.0 mL Schlenk flask. To produce the diisopropoxide catalytic species, 3.75 mL of tetrahydrofuran (THF) was added to dissolve the complexes, followed by addition of 0.250 mL of 2.0 M LiOiPr in 0.500 mmol THF at 0 °C. The resultant solution was stirred for 5 min and employed as a catalyst toward the ROP of rac-LA. To proceed the polymerization reaction, 0.901 g, 6.25 mmol rac-LA was deposited into a Schlenk flask, followed by the addition of dried 5.00 mL of CH2Cl2 and a 0.085 mL, 0.625 mmol standard reference tetralin with subsequent addition of the 1.00 mL, 0.0625 mmol catalyst solution dropwise. For the allotted time, the flask was sealed and stirred at 25 and 0 °C. To quench the reaction, 1.00 mL of H2O was added to the polymerization mixture at a specific time, followed by addition of 2.00 mL of hexane to precipitate the polymer. Subsequently, the solvent was directly removed, affording the crude polymer as a sticky material. 1H NMR spectroscopy was used to study the monomer conversion to polymer using tetralin (δ 2.76) as a reference material in the NMR spectrum. Decantation of the solvent yielded white solids, which were vacuum-dried for 12 h at room temperature to a constant weight with 1H NMR (CDCl3) to obtain the PLA: δ 5.13–5.20 (m, 1H), 1.51–1.63 (m, 3H).
3. Results and Discussion
3.1. Synthesis and Characterization
The Schiff base ligand LTH is easily synthesized using the one-pot condensation reaction, as previously described (Scheme 1).14 The M(II) complexes were formed by direct ligation of LTH in the appropriate molar ratio to metal precursors and furnished the corresponding complexes in appreciable yields (57–95%). Additionally, the elemental analysis of the synthesized M(II) complexes, [LTHMX2] (M = Zn, Pd; X = Cl, Br) and [LTHCd(μ-X)X]2 (X = Cl, Br), also verifies the % composition of C, H, and N constituents, and agreed with the proposed structures. The characteristic azomethine ν(C=N)imine peak appeared at 1632 cm–1 in LTH, which shifted to a lower frequency in the corresponding M(III) complexes, 1609–1627 cm–1 range, confirming the involvement of imine nitrogen in coordination with metal ions (Figures S14–S19).37,38 Chelation leads to a weakening of the C=N bond owing to the inductive effect of the lone electron pair on imine nitrogen being shared with the M(II) center.39−42 In the 2900–3100 cm–1 region, the medium-to-weak bands are assigned to aliphatic and aromatic υ(C–H) stretching vibrations.43
Scheme 1. Synthesis of LTH and the Corresponding Complexes [LTHMX2] (M = Zn, Pd; X = Cl, Br) and [LTHCd(μ-X)X]n (X = Cl, Br; n = n, 2).
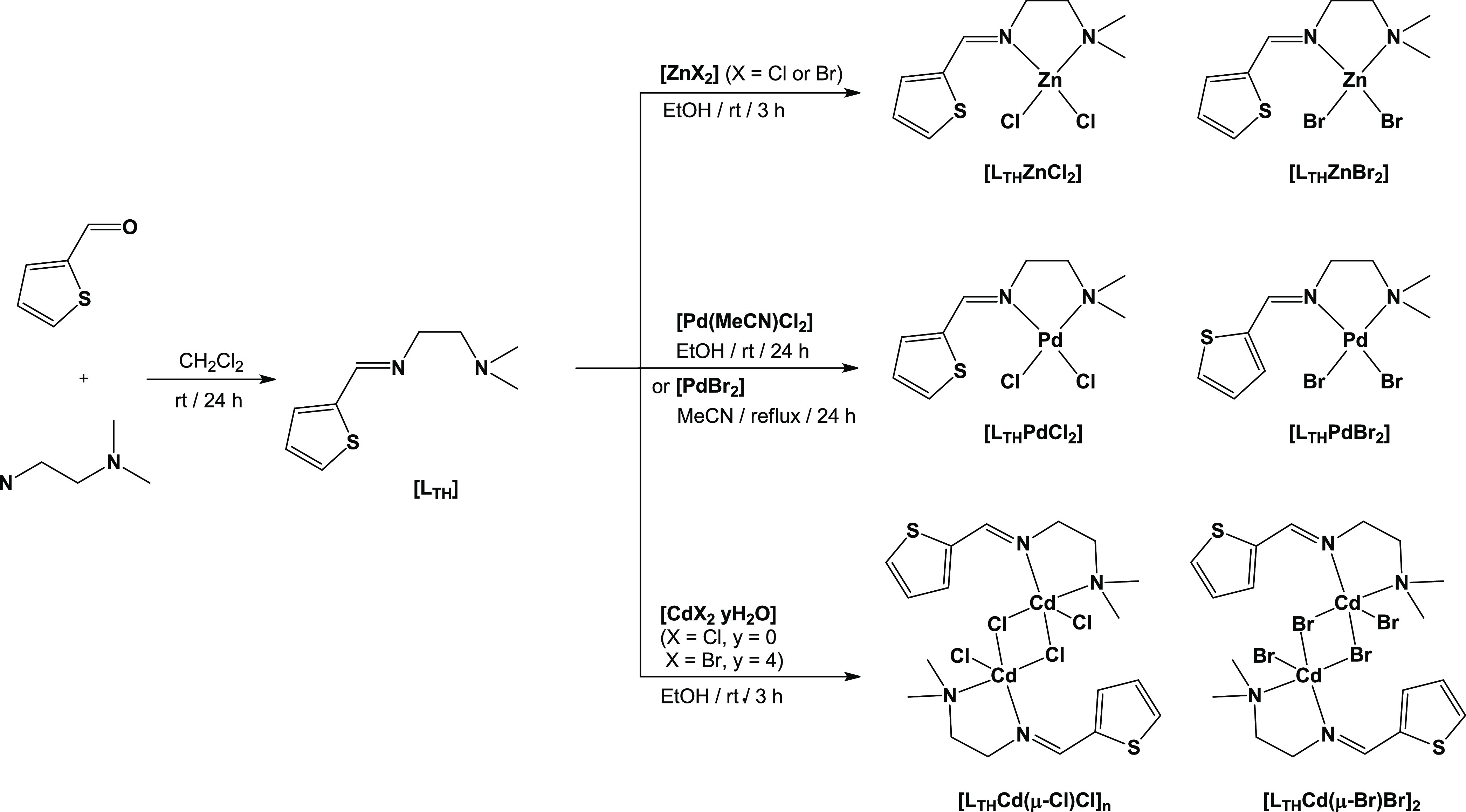
The FTIR bands at 1472–1416 cm–1 are assigned to the symmetrical and asymmetrical ν(C=C) bond stretch of the thiophene moiety. The C–C in the ring stretching of the thiophene moiety appeared at 1340–1327 cm–1.44,45 Notably, the medium intensity in the 746–731 cm–1 region is assigned to ν(C–S) vibrations, which remained unaltered, demonstrating the noninvolvement of thiophene sulfur in the metal coordination.46,47 Also, the sp3 and sp2v(C–H) stretching bands appeared at the expected frequencies.39−43 The FTIR spectra of all of the M(II) complexes showed a prominent band around the 578–564 cm–1 range, which can be assigned to ν(M–N) bond stretching vibrations.38,43 In the 1H NMR spectra, the characteristic signal of the imine proton of LTH appeared at 8.43 ppm, which shifted to a higher ppm in the corresponding M(II) complexes.43 The complexes’ chemical shifts were just slightly changed with δ = 0.03–0.66 in respect to the associated ligands. The 13C NMR spectrum revealed representative peaks as well and was consistent with a complex format.
The NMR spectra of the [LTHMX2] (M = Zn, Cd; X = Cl, Br) complexes showed two sets of resonances, with different intensities, corresponding to two different coordination environments for the synthesized complexes, in contrast to Pd(II) complexes, for which only one set of signals were observed in 1H and 13C NMR, with the existence of just one possible conformation in solution for [LTHPdCl2] and [LTHPdBr2] (Figures S2–S13). The pendant thiophene moiety in these complexes is observed to rotate to a certain extent in Zn(II) and Cd(II) complexes. The metal center variation evidently facilitates such a rotation, for instance, the rotation barrier of the thiophene ring was calculated through DFT calculation, and it was confirmed computationally that Zn(II) and Cd(II) complexes allow rotation of the thiophene moiety but no such rotation is evident for Pd(II). The relative abundance of each of the two conformers of the [LTH-MX2] complexes also changes with metal center variation, one of them being more stable for the larger cations, Cd(II), while the other predominates for the smaller ions, Zn(II) ion.
The VT-NMR study performed for the [LTHZnCl2] complex showed that the major peak (a) and minor peak (b) are well separated, and the integral value of the minor peak increases from 6 to 12% as the temperature decreases from 323 to 243 K since at lower temperature, the lower the kinetic energy and the slower the rotational speed of the thiophene ring, the better the separation of each state (Figure 1). However, the VT-NMR results for Pd(II) complexes showed only one spectral proton of the ligand frame, i.e., no minor and major peaks appeared, confirming that the thiophene ring flipping/rotation does not exist in the Pd(II) complexes (Figure S23). The spectrum of [LTHCd(μ-Cl)Cl]n showed minor and major peaks, with minor peaks being 45% at 243 K, which further increased up to 60% at 323 K (Figures S25 and S26), representing the thiophene ring rotations. However, the coalescence temperature (the temperature at which the spectrum changes from two separate peaks to a single flat-topped peak) was not observed for the complexes.
Figure 1.
VT-NMR spectrum of [LTHZnCl2] at five different temperatures.
3.2. DFT Calculations
The thiophene ring rotations’ potential energy surfaces (PES) were calculated through extensive DFT calculations. The potential energy surfaces were calculated at the intermediate states designed by rotation of the ligands around the M-L bond at 30° to get 6 points on the potential energy surfaces with the ligands rotated at 30, 60, 90, 120, 150, and 180°, respectively (Figure 2). In particular, the PESes for Zn and Cd complexes resemble the Gaussian type curves with the transition states (TS) located just in the middle of the rotation pathway (∼90°), which separate almost equivalent minima except for [LTHCd(μ-Cl)Cl]n for 0 and 180°, with relative energies of 180° minima equal to 0.52, 0.8, and 0.5 eV for [LTHCd(μ-Br)Br]2, [LTHZnCl2], and [LTHZnBr2], respectively. For [LTHCd(μ-Cl)Cl]n, [LTHPdCl2], and [LTHPdBr2], the energies of the second minimum (180° rotation) are much higher and equal to 4.28, 9.15, and 5.85 eV, making the isomers with 180° rotated thiophene ring very energetically unfavorable. Compared with Cd and Zn complexes, Pd-derived ones, namely [LTHPdCl2] and [LTHPdBr2], have remarkedly different potential energy surfaces with TSes between global and local minima around 120°. The high potential barriers and high energies of the second minima make almost impossible the synthesis of the Pd-derived complexes with rotated ligands almost impossible most time; the unsurmountable values of the energy barriers (Figure 2) of all Zn, Pd, and Cd complexes make the ground-state configurations fixed with rotation angles equal to 0°. Summarizing the theoretical data, it was confirmed that each Zn(II) and Cd(II) complex may form two different isomers with rotated thiophene moiety, but no such rotation is evident for Pd(II).
Figure 2.

Potential energy surfaces for the [LTH] ligand rotation of each transition metal complex. The structures of the complexes in the ground states are depicted for each potential energy surface with the following energy barrier values (in eV).
3.3. X-ray Crystallography
Unlike recent studies,48 during the single-crystal X-ray diffraction procedure, the major conformers crystallize out at room temperature. The Oak Ridge thermal ellipsoid plot (ORTEP) drawings and structural parameters (selected bond lengths and angles) of the complexes are presented in Figures 3–5 and Table 1. The tetracoordinate Zn(II) and Pd(II) centers in [LTHZnCl2], [LTHZnBr2], [LTHPdCl2], and [LTHPdBr2] can be described as tetrahedral and square planes, respectively, since the τ4 value is equal to unity for a perfect tetrahedron and zero for a perfect planar square.49 Dimeric structures with a pentacoordinate mode for the Cd(II) center have been observed for [LTHCd(μ-Cl)Cl]n and [LTHCd(μ-Br)Br]2. The geometry around the Cd(II) center is considered to be that of a distorted square pyramid through coordination with two nitrogen atoms of the bidentate chelating ligand, two halogen atoms, and one halogen atom from the adjacent molecule. The τ5 parameters of the bipyramidal Cd(II) complexes are equal to unity for a perfect trigonal bipyramidal geometry and equal to zero for a perfect square pyramid (Table 2).50,51 The M–Nimine and M–Namine bond lengths of the synthesized M(II) complexes lie between 2.010(4)–2.330(5) and 2.0857(2)–2.415(5) Å, respectively, and agree well with the M–N parameters for the reported complexes.52,53 Analyzing the bond lengths, M–Namine distances are slightly longer than the M–Nimine distance because of the differences in hybridization. Additionally, the M–Cl and M–Br bond lengths lie in the expected range, as found in similar complexes.26,54
Figure 3.
[LTHZnCl2] (a) and [LTHZnBr2] (b) structures presented using ORTEP with thermal ellipsoids at 50% probability. The hydrogen atoms are omitted for the sake of clarity.
Figure 5.
[LTHCd(μ-Cl)Cl]n (a) and [LTHCd(μ-Br)Br]2 (b) complexes drawn using ORTEP with thermal ellipsoids at 30 and 50% probability, respectively. The hydrogen atoms are omitted for the sake of clarity.
Table 1. Selected Bond Lengths (Å) and Angles (deg) of the Zn(II) Complexes.
| [LTHZnCl2] | [LTHZnBr2] | [LTHPdCl2] | [LTHPdBr2] | [LTHCd(μ-Cl)Cl]n | [LTHCd(μ-Br)Br]2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bond Lengths (Å) | |||||||||||
| Zn(1)–N(1) | 2.0544(2) | Zn(1)–N(1) | 2.0566(2) | Pd(1)–N(1) | 2.010(4) | Pd(1)–N(1) | 2.043(3) | Cd(1)–N(1) | 2.330(5) | Cd(1)–N(1) | 2.321(4) |
| Zn(1)–N(2) | 2.0997(2) | Zn(1)–N(2) | 2.0857(2) | Pd(1)–N(2) | 2.097(4) | Pd(1)–N(2) | 2.093(3) | Cd(1)–N(2) | 2.415(5) | Cd(1)–N(2) | 2.406(4) |
| Zn(1)–Cl(2) | 2.2196(8) | Zn(1)–Br(1) | 2.3709(4) | Pd(1)–Cl(1) | 2.3107(1) | Pd(1)–Br(2) | 2.4197(7) | Cd(1)–Cl(2) | 2.5822(1) | Cd(1)–Br(2) | 2.5782(1) |
| Zn(1)–Cl(1) | 2.2200(7) | Zn(1)–Br(2) | 2.3543(7) | Pd(1)–Cl(2) | 2.3112(1) | Pd(1)–Br(1) | 2.4395(7) | Cd(1)–Cl(1) | 2.6221(1) | Cd(1)–Br(1) | 2.7297(8) |
| N(1)–C(5) | 1.283(2) | N(1)–C(5) | 1.288(2) | N(1)–C(5) | 1.292(7) | N(1)–C(5) | 1.285(5) | N(1)–C(5) | 1.274(7) | N(1)–C(5) | 1.280(6) |
| N(1)–C(6) | 1.474(2) | N(1)–C(6) | 1.477(3) | N(1)–C(6) | 1.470(7) | N(1)–C(6) | 1.449(5) | N(1)–C(6) | 1.442(7) | N(1)–C(6) | 1.469(6) |
| Bond Angles (deg) | |||||||||||
| N(1)–Zn(1)–N(2) | 86.22(6) | N(1)–Zn(1)–N(2) | 84.98(6) | N(1)–Pd(1)–N(2) | 83.02(2) | N(1)–Pd(1)–N(2) | 83.83(1) | N(1)–Cd(1)–N(2) | 73.34(2) | N(1)–Cd(1)–N(2) | 73.43(1) |
| N(1)–Zn(1)–Cl(2) | 112.31(5) | N(1)–Zn(1)–Br(2) | 117.63(5) | N(1)–Pd(1)–Cl(1) | 93.96(1) | N(1)–Pd(1)–Br(2) | 175.65(9) | N(1)–Cd(1)–Cl(2) | 164.66(1) | N(1)–Cd(1)–Br(2) | 145.73(1) |
| N(2)–Zn(1)–Cl(2) | 111.45(5) | N(2)–Zn(1)–Br(2) | 112.58(5) | N(2)–Pd(1)–Cl(1) | 169.20(1) | N(2)–Pd(1)–Br(2) | 91.86(9) | N(2)–Cd(1)–Cl(2) | 91.39(1) | N(2)–Cd(1)–Br(2) | 93.48(1) |
| N(1)–Zn(1)–Cl(1) | 113.76(5) | N(1)–Zn(1)–Br(1) | 112.15(5) | N(1)–Pd(1)–Cl(2) | 175.45(1) | N(1)–Pd(1)–Br(1) | 93.88(9) | N(1)–Cd(1)–Cl(1) | 91.39(1) | N(1)–Cd(1)–Br(1) | 102.91(1) |
| N(2)–Zn(1)–Cl(1) | 108.28(5) | N(2)–Zn(1)–Br(1) | 107.02(5) | N(2)–Pd(1)–Cl(2) | 92.93(1) | N(2)–Pd(1)–Br(1) | 173.85(9) | N(2)–Cd(1)–Cl(1) | 164.64(1) | N(2)–Cd(1)–Br(1) | 92.40(1) |
| Cl(2)–Zn(1)–Cl(1) | 119.73(3) | Br(2)–Zn(1)–Br(1) | 117.432(2) | Cl(1)–Pd(1)–Cl(2) | 90.40(5) | Br(2)–Pd(1)–Br(1) | 90.35(2) | Cl(2)–Cd(1)–Cl(1) | 103.92(4) | Br(2)–Cd(1)–Br(1) | 109.29(3) |
Table 2. Four-Coordinate and Five-Coordinate Geometry Indices for the Synthesized Complexes and Representative Examples from the Literature.
| complexes | geometry | τ4 | THCDA/100 | FCGP/100 | τ5 | reference |
|---|---|---|---|---|---|---|
| square planar (D4h) | square planar | 0.000 | –1.43 | –0.400 | (49) | |
| trigonal pyramidal (C3v) | trigonal pyramidal | 0.850 | 0.000 | 1.00 | (49) | |
| [LTHZnCl2] | tetrahedral | 0.897 | 0.514 | 0.402 | this work | |
| [LTHZnBr2] | tetrahedral | 0.886 | 0.458 | 0.427 | this work | |
| [LTHPdCl2] | square planar | 0.109 | –1.26 | –0.170 | this work | |
| [LTHPdBr2] | square planar | 0.0745 | –1.32 | –0.249 | this work | |
| [(L-b)2ZnCl2]a | tetrahedral | 0.885 | 0.432 | 0.452 | (43) | |
| [(L-b)2PdCl2]a | square planar | 0.0626 | –1.34 | –0.265 | (43) | |
| [LCZnCl2]b | tetrahedral | 0.887 | 0.370 | 0.422 | (29) | |
| [LDPdCl2]c | tetrahedral | 0.870 | 0.337 | 0.415 | (29) | |
| [LBPdCl2]d | square planar | 0.0774 | –1.31 | –0.231 | (56) | |
| [LCZnCl2]e | square planar | 0.0754 | –1.31 | –0.243 | (56) | |
| tetrahedral (Td) | tetrahedral | 1.00 | 1.00 | 0.000 | (49) | |
| square pyramidal (C4v) | square pyramidal | 0.000 | (50, 51) | |||
| [LTHCd(μ-Cl)Cl]n | square pyramidal | 0.0033 | this work | |||
| [LTHCd(μ-Br)Br]2 | square pyramidal | 0.324 | this work | |||
| [(bpma)Cd(μ-Br)Br]2f | trigonal bipyramidal | 0.606 | (57) | |||
| [LACdBr2]g | square pyramidal | 0.071 | (26) | |||
| [LECdBr2]h | square pyramidal | 0.003 | (26) | |||
| trigonal bipyramidal (D3h) | trigonal bipyramidal | 1.00 | (50, 51) |
L-b = 4-methoxy-N-methyl-N-(pyridin-2-ylmethyl) aniline.
LC = N-cyclohexyl-1-(pyridin-2-yl)methanimine.
LD = 2,6-diethyl-N-(pyridin-2-ylmethylene)aniline.
LB = 2,4,6-trimethyl-N-((pyridin-2-yl)methylene)aniline.
LC = 4-nitro-N-((pyridin-2-yl)methylene)aniline.
bmpa = 4-bromo-N-((pyridin-2-yl)methylene) benzenamine.
LA = N1,N1-dimethyl-N2-(pyridin-2-ylmethylene)ethane-1,2-diamine.
LE = N1,N1-dimethyl-N3-(pyridin-2-ylmethylene) propane-1,3-diamine.
Figure 4.
[LTHPdCl2] (a) and [LTHPdBr2] (b) structures presented using ORTEP with thermal ellipsoids at 50% probability. The hydrogen atoms are omitted for the sake of clarity.
The buried volumes using the SambVca program55 were calculated and the total steric hindrance provided by the ligand framework considering the M(II) (M = Zn, Pd, and Cd) center can be quantified by comparing the topographic steric maps of these M(II) complexes (Figure 6). In the case of Zn(II) complexes, there is no significant difference in the buried volume because the thiophene ring is oriented toward the Zn(II) center in both complexes. However, in the case of Pd(II) complexes, the different orientations of the thiophene rings in [LTHPdCl2] and [LTHPdBr2] (as evident from X-ray structures) resulted in an appreciable difference in the buried volumes; the thiophene ring is located away from the Pd(II) center in [LTHPdBr2], which resulted in a much smaller %Vbur. In the case of Cd(II) complexes, the difference in steric hindrance provided by the ligand in [LTHCd(μ-Cl)Cl]n and [LTHCd(μ-Br)Br]2 is negligible, with the thiophene ring in both complexes residing away from the Cd(II) center; the pentacoordinate mode of each Cd(II) center in these dimeric complexes provided smaller buried volumes.
Figure 6.
Topographic steric maps of the synthesized complexes.
3.4. Polymerization Studies
The polymerization capabilities of the synthesized complexes have also been tested. The polymerization experiment was carried out using the [LTHMCl2]/LiOiPr system in CH2Cl2 at 0 and 25 °C, with the results presented in Table 3. It was shown that the complexes are effective initiators with 96% conversion achieved in 20 min. M = Zn, Cd, Pd was assessed as a potential catalyst for rac-LA in CH2Cl2 ROP at room temperature at a [rac-LA]/[catalyst] ratio of 100. No conversion to PLA was detected under the experimental conditions, which could be attributed to the strong M–Cl bonds. To activate the dichloro complexes for ROP, an in situ approach was used with dichloro M(II) complexes pretreated by LiOiPr to form the metal-isopropoxide derivatives. Earlier, the preactivation strategy was reported as an effective means to generate highly efficient initiators.58,59 The Mn values of PLA obtained with gel permeation chromatography (GPC) indicated a low-molecular-weight polymer; almost half of the theoretical Mn values suggested the growth of two polymer chains per metal center. The low-molecular-weight PLA was attained because of two reasons. First, transesterification can lead to either intra- or intermolecular results by way of a lower Mn or broader polydispersity index (PDI), respectively. Inefficient initiation of the catalytic system, which is the second reason, can result in several polymer chains growing faster than expected due to a much higher rate of initiation compared to the propagation step.60 However, variations of Mn values with increasing conversion can be seen as polymerization proceeds (Figure 7). For instance, the value of Mn was 1.43 × 103 g mol–1 for 30 s, 2.06 × 103 g mol–1 for 1 min, 2.57 × 103 g mol–1 for 2 min, 3.18 × 103 g mol–1 for 5 min, and 3.59 × 103 g mol–1 for 10 min polymerization time. Similarly, a rise in the molecular weight of PLA was observed with an increase in the ratio of monomer load, suggesting the somewhat living characteristics of polymerization. For instance, 98% conversion was obtained with the [LTHZnCl2]/LiOiPr system up to a [rac-LA]0/[catalyst] ratio of 500:1. However, further increase in the [rac-LA]0/[catalyst] ratio up to 700:1 resulted in only 31% conversion under identical experimental protocols, and a decrease in the molecular weight of resultant PLA was also observed with this decrease in conversion (Figure 8). Depending on the metal center variation, [LTHZnCl2]/LiOiPr resulted in a high heterotactic enchainment (Pr = 0.74 at 0 °C)61 compared to its Cd(II) and Pd(II) complexes, consistent with the findings of previous studies.62,63 Decrease in polymerization temperature did not show any significant enhancement of the heterotactic bias, particularly for the Cd(II)-based initiator. The geometry of the M(II) complex might have steered the hetereotacticity of PLA in the studied complexes. However, the final heterotacticities are comparable to the ones previously reported for diisopropoxide-Zn(II) complexes.5 Additionally, the PDIs of the observed PLA was also not affected by the metal center variation.
Table 3. Polymerization of rac-LA with, [LTH-M(OiPr)2] Initiators, Generated In Situ from the Reaction of [LTH-MCl2] Complexes and [LiOiPr].
| runa | catalyst | T (°C) | conv.b (%) | Mnc (g mol–1) × 103 (calcd) | Mnd (g mol–1) × 103 (GPC) | PDId | Pre |
|---|---|---|---|---|---|---|---|
| 1 | LiOiPr | 0 | 97.2 | 14.0 | 13.1 | 1.25 | 0.56 |
| 2 | LiOiPr | 25 | 96.4 | 13.9 | 8.94 | 1.25 | 0.54 |
| 3 | [LTHZn(OiPr)2] | 0 | 96.6 | 13.9 | 6.273 | 1.25 | 0.74 |
| 4 | [LTHPd(OiPr)2] | 0 | 96.3 | 13.9 | 8.564 | 1.25 | 0.61 |
| 5 | [LTHCd(OiPr)2] | 0 | 95.7 | 13.8 | 5.347 | 1.25 | 0.54 |
| 6 | [LTHZn(OiPr)2] | 25 | 96.2 | 13.9 | 5.400 | 1.25 | 0.58 |
| 7 | [LTHPd(OiPr)2] | 25 | 96.5 | 13.9 | 6.409 | 1.25 | 0.54 |
| 8 | [LTHCd(OiPr)2] | 25 | 95.4 | 13.8 | 4.358 | 1.25 | 0.54 |
Specifications: [catalyst] 0.0625 mmol; [rac-LA]/[LiOiPr]/[catalyst] 100:2:1; solvent (CH2Cl2) 5.00 mL; polymerization time 20 min.
Conversion rate (%) was determined by 1H NMR spectroscopy in the presence of tetralin.
Calculated using [{molecular weight of rac-LA} × {(mol concentration of used rac-LA)/(mol concentration of catalyst)}] × [conversion].
Determined by gel permeation chromatography (GPC) in chloroform, relative to the poly(methyl methacrylate) standard.
Figure 7.
Plot representing conversion and Mn values with the increasing time of polymerization.
Figure 8.
Plot representing conversion and Mn values with the increasing load of the monomer.
Considering the stability of the catalytic system, the [LTHZnCl2]/LiOiPr system is durable, as is evident from the conversion values, even after adding the monomer externally after every 5 min interval for five cycles. For instance, in the [LTHZnCl2]/LiOiPr system the conversion reduced from 97 to 47% after the fifth cycle, i.e., rac-LA/catalyst ratio of 500:1 (Figures S51 and S52). This behavior is pertinent to our previously studied Zn(II) system supported by the aminomethylquinoline ligand, where 58% conversion was achieved after the fifth cycle.61 In comparison to our studied Zn(II) system, the current [LTHZnCl2]/LiOiPr system is faster, as evidenced by the 94% conversion within 30 s compared to the 44% conversion obtained with 4-(quinolin-2-ylmethyl)morpholine-derived Zn(II)-diisopropoxide initiators under identical experimental conditions. This might be due to the rigidity of the 4-(quinolin-2-ylmethyl)morpholine ligand framework compared to LTH. As it was shown earlier,62,64−66 the rac-LA ROP proceeds through a monomer-activated mechanism initiated by diispropoxide-based Zn(II) species. It was shown67 that for rac-LA polymerization, the studied catalytic system outperforms the Zn(II) complexes of tridentate Schiff bases with morpholine/piperidine/pyridine/quinoline functionalities for rac-LA polymerization with better conversion and polymerization control of PLA production. The developed Zn(II) spicy demonstrates high catalytic activities and heterotacticities with 97% conversion/20 min rate: Pr = 0.74 at 0 °C (Table 3, entry 3) in comparison with the previously reported68 Zn(II) systems with the azobenzene Schiff base ligand with 78% conversion rate within 4 h at 80 °C, and Pr up to 0.40. The proposed and developed system exhibited higher activities but yielded lower heterotacticities compared to the N,N″,N-bis((1H-pyrazol-1-yl)methyl)amines-derived Zn(II) catalytic system (Pr = 0.95 at −50 °C with 90% conversion).69 Polymerization of rac-LA using amine bis(phenolate) zinc complexes with 98% conversion rate within 2 h at 25 °C and almost atactic bias (Pr = 0.53) has lower activity and heteroselectivity with higher PDIs in comparison with previously developed complexes.70 A detailed polymerization study for deepening the structure–activity relationship and reactivity of the complexes, their activity, and stereoselectivity is underway.
4. Conclusions
In this study, novel Zn(II), Pd(II), and Cd(II) complexes, namely [LTHMX2] (M = Zn, Pd; X = Cl, Br) and [LTHCd(μ-X)X]n (X = Cl, Br; n = n, 2) with the iminomethylthiophene-derived ligand (LTH), were synthesized and characterized. X-ray crystallography revealed diverse coordination geometries of the resultant M(II) complexes. DFT calculations showed that the rotation of the thiophene moiety of the ligand (LTH) can be observed for Zn(II) and Cd(II) centers, confirmed by VT-NMR, but no such rotation is evident for Pd(II). The preliminary polymerization studies carried out by isopropoxide derivatives of the studied complexes resulted in high activities with moderate to high heterotacticities and low molecular weights of PLA. The complexes’ geometry might help steer the catalytic performance and stereoselectivity of these complexes.
Acknowledgments
This research was supported by the National Research Foundation (NRF) of the Republic of Korea, funded by the Ministry of Education, Science, and Technology (MEST) (Grant No. 2019R1A2C1088654). This work also was supported by the Technology Innovation Program (TIP # 20011123, Development of Cyclic Olefin Polymer (COP) with High Heat Resistance and High Transmittance) funded by the Korea Evaluation Institute of Industrial Technology (KEIT) and the Ministry of Trade, Industry & Energy (MOTIE, Republic of Korea). X-ray crystallography with PLS-II 2D-SMC beamline was supported by MSIP and POSTECH. P.A. acknowledges NRF grant 2021R1A2C1010455.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c08001.
Experimental details, 1H NMR, 13C NMR, FTIR, elemental analysis, X-ray crystal data, and structure refinements, ball and stick models, VT-NMR spectra, GPC data, and polymerization results (PDF)
Accession Codes
CCDC 221691, 2221692, 2221693, 2221694, 2221695, 2221696 contain the supporting crystallographic data for [LTHZnCl2], [LTHZnBr2], [LTHPdCl2], [LTHPdBr2], [LTHCd(μ-Cl)Cl]n, and [LTHCd(μ-Br)Br]n, respectively. These data can be obtained free of charge viahttp://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, U.K.; fax: (+44) 1223-336-033; or email: deposit@ccdc.cam.ac.uk.
Author Contributions
CRediT: J.L.: methodology, analysis; I.M.: data curation; S.N.: writing the original draft—supporting; K.K.: supporting; Y.H.K.: supporting; M.Y.: supporting; P.A.: supervision-lead, conceptualization, H.L.: writing—review and editing.
The authors declare no competing financial interest.
Supplementary Material
References
- Vigato P. A.; Tamburini S. The challenge of cyclic and acyclic Schiff bases and related derivatives. Coord. Chem. Rev. 2004, 248, 1717–2128. 10.1016/j.cct.2003.09.003. [DOI] [Google Scholar]
- Gupta K. C.; Sutar A. K. Catalytic activities of Schiff base transition metal complexes. Coord. Chem. Rev. 2008, 252, 1420–1450. 10.1016/j.ccr.2007.09.005. [DOI] [Google Scholar]
- Cozzi P. G. Metal–Salen Schiff base complexes in catalysis: practical aspects. Chem. Soc. Rev. 2004, 33, 410–421. 10.1039/B307853C. [DOI] [PubMed] [Google Scholar]
- Nithya P.; Simpson J.; Govindarajan S. Syntheses, structural diversity and thermal behavior of first row transition metal complexes containing potential multidentate ligands based on 2,6-diacetylpyridine and benzyl carbazate. Polyhedron 2018, 141, 5–16. 10.1016/j.poly.2017.11.009. [DOI] [Google Scholar]
- Cho J.; Chun M. K.; Nayab S.; Jeong J. H. Synthesis, characterisation, and X-ray structures of zinc(II) complexes bearing camphor-based ethyleneamineimines as pre-catalysts for heterotactic-enriched polylactide from rac-lactide. Transition Met. Chem. 2019, 44, 175–185. 10.1007/s11243-018-0282-9. [DOI] [Google Scholar]
- Kim K.; Nayab S.; Jeong A. R.; Cho Y.; Yeo H.; Lee H. Vinyl-addition polymerizations of norbornene and methyl methacrylate by the palladium(II) complexes ligated by 2-iminomethylquinoline and 2-iminomethylpyridine derivatives. Inorg. Chim. Acta 2022, 539, 121025 10.1016/j.ica.2022.121025. [DOI] [Google Scholar]
- Singh R. K.; Kukrety A.; Saxena R. C.; Thakre G. D.; Atray N.; Ray S. S. Use of an Acylated Chitosan Schiff Base as an Ecofriendly Multifunctional Biolubricant Additive. Ind. Eng. Chem. Res. 2016, 55, 2520–2526. 10.1021/acs.iecr.5b04242. [DOI] [Google Scholar]
- Segura J. L.; Mancheno M. J.; Zamora F. Covalent organic frameworks based on Schiff-base chemistry: synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. 10.1039/C5CS00878F. [DOI] [PubMed] [Google Scholar]
- More M. S.; Joshi P. G.; Mishra Y. K.; Khanna P. K. Metal complexes driven from Schiff bases and semicarbazones for biomedical and allied applications: a review. Mater. Today Chem. 2019, 14, 100195 10.1016/j.mtchem.2019.100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary N. K.; Mishra P. Metal Complexes of a Novel Schiff Base Based on Penicillin: Characterization, Molecular Modeling, and Antibacterial Activity Study. Bioinorg. Chem. Appl. 2017, 2017, 6927675 10.1155/2017/6927675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.; Brookhart M. Exploring Ethylene/Polar Vinyl Monomer Copolymerizations Using Ni and Pd α-Diimine Catalysts. Acc. Chem. Res. 2018, 51, 1831–1839. 10.1021/acs.accounts.8b00225. [DOI] [PubMed] [Google Scholar]
- Kundu S.; Pramanik A. K.; Mondal A. S.; Mondal T. K. Ni(II) and Pd(II) complexes with new N,O donor thiophene appended Schiff base ligand: Synthesis, electrochemistry, X-ray structure and DFT calculation. J. Mol. Struct. 2016, 1116, 1–8. 10.1016/j.molstruc.2016.03.013. [DOI] [Google Scholar]
- John L.; Dasan A.; Joseyphus R. S.; Joe I. H. Molecular docking, structural characterization, DFT and cytotoxicity studies of metal(II) Schiff base complexes derived from thiophene-2-carboxaldehyde and l-histidine. J. Mol. Struct. 2019, 1198, 126934 10.1016/j.molstruc.2019.126934. [DOI] [Google Scholar]
- Anderson C.; Crespo M.; Font-Bardía M.; Klein A.; Solans X. Cyclometallated platinum complexes with thienyl imines. X-ray crystal structure of [PtMe{3-(PhCH2NCH)C4H2S}PPh3]. J. Organomet. Chem. 2000, 601, 22–33. 10.1016/S0022-328X(00)00017-6. [DOI] [Google Scholar]
- Tian H.; Tang Z.; Zhuang X.; Chen X.; Jing X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. 10.1016/j.progpolymsci.2011.06.004. [DOI] [Google Scholar]
- Zhang X.; Fevre M.; Jones G. O.; Waymouth R. M. Catalysis as an Enabling Science for Sustainable Polymers. Chem. Rev. 2018, 118, 839–885. 10.1021/acs.chemrev.7b00329. [DOI] [PubMed] [Google Scholar]
- Nifant’ev I.; Ivchenko P. Coordination Ring-Opening Polymerization of Cyclic Esters: A Critical Overview of DFT Modeling and Visualization of the Reaction Mechanisms. Molecules 2019, 24, 4117 10.3390/molecules24224117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubov D. M.; Tolpygin A. O.; Trifoner A. A. Rare-earth metal complexes as catalysts for ring-opening polymerization of cyclic esters. Coord. Chem. Rev. 2019, 392, 83–145. 10.1016/j.ccr.2019.04.013. [DOI] [Google Scholar]
- Santoro O.; Zhang X.; Redshaw C. Synthesis of Biodegradable Polymers: A Review on the Use of Schiff-Base Metal Complexes as Catalysts for the Ring Opening Polymerization (ROP) of Cyclic Esters. Catalysts 2020, 10, 800 10.3390/catal10070800. [DOI] [Google Scholar]
- Gruszka W.; Garden J. A. Advances in heterometallic ring-opening (co)polymerisation catalysis. Nat. Commun. 2021, 12, 3252 10.1038/s41467-021-23192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro O.; Elsegood M. R. J.; Bedwell E. V.; Pryce J. A.; Redshaw C. INSIGHTS into the structures adopted by titanocalix[6 and 8]arenes and their use in the ring opening polymerization of cyclic esters. Dalton Trans. 2020, 49, 11978–11996. 10.1039/D0DT02130J. [DOI] [PubMed] [Google Scholar]
- Xing T.; Prior T. J.; Elsegood M. R. J.; Semikolenova N. V.; Soshnikov I. E.; Bryliakov K.; Chen K.; Redshaw C. Vanadium complexes derived from oxacalix[6]arenes: structural studies and use in the ring opening homo-/co-polymerization of ε-caprolactone/δ-valerolactone and ethylene polymerization. Catal.: Sci. Technol. 2021, 11, 624–636. 10.1039/D0CY01979H. [DOI] [Google Scholar]
- Santoro O.; Redshaw C. Metallocalix[n]arenes in catalysis: A 13-year update. Coord. Chem. Rev. 2021, 448, 214173 10.1016/j.ccr.2021.214173. [DOI] [Google Scholar]
- Pongpanit T.; Saeteaw T.; Chumsaeng P.; Chasing O.; Phomphrai K. Highly Active Homoleptic Zinc and Magnesium Complexes Supported by Constrained Reduced Schiff Base Ligands for the Ring-Opening Polymerization of Lactide. Inorg. Chem. 2021, 60, 17114–17122. 10.1021/acs.inorgchem.1c02382. [DOI] [PubMed] [Google Scholar]
- Roymuhury S. K.; Mandal M.; Chakraborty D.; Ramkumar V. Homoleptic titanium and zirconium complexes exhibiting unusual Oiminol–metal coordination: application in stereoselective ring-opening polymerization of lactide. Polym. Chem. 2021, 12, 3953–3967. 10.1039/D1PY00237F. [DOI] [Google Scholar]
- Lee J.; Lee H.; Nayab S.; Yoon K. B. Synthesis, characterization and polymerisation studies of cadmium(II) complexes containing N,N0,X-tridentate X-substituted (X = N, O) 2- iminomethylpyridines. Polyhedron 2019, 158, 432–440. 10.1016/j.poly.2018.11.033. [DOI] [Google Scholar]
- Lee J.; Kim K.; Lee H.; Nayab S. Cobalt(II) complexes supported by iminomethylpyridine derived ligands: Synthesis, characterization and catalytic application towards methyl methacrylate and rac-lactide polymerisations. Polyhedron 2021, 196, 115003 10.1016/j.poly.2020.115003. [DOI] [Google Scholar]
- Lee J.; Yoon M.; Lee H.; Nayab S. Stereoselective polymerization of methyl methacrylate and rac-lactide mediated by iminomethylpyridine based Cu(II) complexes. RSC Adv. 2020, 10, 16209–16220. 10.1039/D0RA00805B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J.; Lee H.; Nayab S. Polymerizations of methyl methacrylate and rac-lactide by zinc(II) precatalyst containing N-substituted 2-iminomethylpyridine and 2-iminomethylquinoline. J. Coord. Chem. 2017, 70, 3837–3858. 10.1080/00958972.2017.1416106. [DOI] [Google Scholar]
- Fuchs M.; Schmitz S.; Schäfer P. M.; Secker T.; Metz A.; Ksiazkiewicz A. N.; Pich A.; Kögerler P.; Monakhov K. Y.; Herres-Pawlis S. Herres-Pawlis, Mononuclear zinc(II) Schiff base complexes as catalysts for the ring-opening polymerization of lactide. Eur. Polym. J. 2020, 122, 109302 10.1016/j.eurpolymj.2019.109302. [DOI] [Google Scholar]
- Goh C.; Remillard Z. D.; Martinez A. P.; Keeley A. C.; Jasinski J. P. Di-μ-bromido-bis{[N,N-dimethyl-N0-(thiophen-2-ylmethylidene)ethane-1,2-diamine]copper(II)}. Acta Crystallogr., Sect. E: Struct. Rep. Online 2012, 68, m691–m692. 10.1107/S1600536812017989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews C. J.; Smith P. J.; Welton T. Novel palladium imidazole catalysts for Suzuki cross-coupling reaction. J. Mol. Catal. A: Chem. 2003, 206, 77–82. 10.1016/S1381-1169(03)00447-3. [DOI] [Google Scholar]
- Otwinowski Z.; Minor W.. Processing of X-ray diffraction data collected in oscillation mode. Methods in Enzymology; Elsevier B.V., 1997; pp 307–326. [DOI] [PubMed] [Google Scholar]
- Shin J. W.; Eom K.; Moon D. BL2D-SMC, the supramolecular crystallography beamline at the Pohang Light Source II, Korea. J. Synchrotron Radiat. 2016, 23, 369–373. 10.1107/S1600577515021633. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z.; Borek D.; Majewski W.; Minor W. Multiparametric scaling of diffraction intensities. Acta Crystallogr., Sect. A: Found. Crystallogr. 2003, 59, 228–234. 10.1107/S0108767303005488. [DOI] [PubMed] [Google Scholar]
- Sheldrick G. M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8. 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzeiwa W. A.; Nyamoria V. O.; Omondi B. N,O-Amino-phenolate Mg(II) and Zn(II) Schiff base complexes: Synthesis and application in ring-opening polymerization of ε-caprolactone and lactides. Inorg. Chim. Acta 2019, 487, 264–274. 10.1016/j.ica.2018.12.028. [DOI] [Google Scholar]
- Mohamed G. G.; Omar M. M.; Hindy A. M. M. Synthesis, characterization and biological activity of some transition metals with Schiff base derived from 2-thiophene carboxaldehyde and aminobenzoic acid. Spectrochim. Acta, Part A 2005, 62, 1140–1150. 10.1016/j.saa.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Nakamoto K.Infrared and Raman Spectra of Inorganic and Coordination Compounds Part B, 6th ed.; John Wiley and Sons, Inc.: New Jersey, 2009. [Google Scholar]
- Alturiqi A. S.; Alaghaz A. N. M. A.; Ammar R. A.; Zayed M. E. Synthesis, Spectral Characterization, and Thermal and Cytotoxicity Studies of Cr(III), Ru(III), Mn(II), Co(II), Ni(II), Cu(II), and Zn(II) Complexes of Schiff Base Derived from 5-Hydroxymethylfuran-2-carbaldehyde. J. Chem. 2018, 2018, 5816906 10.1155/2018/5816906. [DOI] [Google Scholar]
- Kaya I.; Sandal B. S.; Karaer H. Synthesis, characterization and electrochemical properties of poly(phenoxy-imine)s containing peril and tert-butyl units. J. King Saud Univ., Sci. 2019, 31, 75–82. 10.1016/j.jksus.2017.06.008. [DOI] [Google Scholar]
- Al-Saif F. A.; Al-Humaidi J. Y.; Binjawhar D. N.; Refat M. S. Six new palladium(II) mixed ligand complexes of 2-, 3-, 4- monosubstituted derivative of pyridine ring with caffeine moiety: Synthesis, spectroscopic, morphological structures, thermal, antimicrobial and anticancer properties. J. Mol. Struct. 2020, 1218, 128547 10.1016/j.molstruc.2020.128547. [DOI] [Google Scholar]
- Park S.; Lee J. K.; Lee H.; Nayab S.; Shin J. W. Zinc (II), palladium (II) and cadmium (II) complexes containing 4-methoxy-N-(pyridin-2-ylmethylene) aniline derivatives: Synthesis, characterization and methyl methacrylate polymerization. Appl. Organomet. Chem. 2019, 33, e4797 10.1002/aoc.4797. [DOI] [Google Scholar]
- Mei-Rong Y.; Yu S.; Jin X. Y. Vibrational spectroscopic, NMR parameters and electronic properties of three 3-phenylthiophene derivatives via density functional theory. SpringerPlus 2014, 3, 701 10.1186/2193-1801-3-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulandaivalu S.; Zainal Z.; Sulaiman Y. Influence of Monomer Concentration on the Morphologies and Electrochemical Properties of PEDOT, PANI, and PPy Prepared from Aqueous Solution. Int. J. Polym. Sci. 2016, 2016, 8518293 10.1155/2016/8518293. [DOI] [Google Scholar]
- Vairalakshmi M.; Princess R.; Rani B. K.; Raja S. J. Synthesis, structural elucidation, catalytic, antibacterial and antioxidant activity of thiophene derived mixed ligand metal complexes. J. Chil. Chem. Soc. 2018, 63, 3844. 10.4067/s0717-97072018000103844. [DOI] [Google Scholar]
- Chandra S.; Kumar R. Synthesis and spectral studies on mononuclear complexes of chromium(III) and manganese(II) with 12-membered tetradentate N2O2, N2S2 and N4 donor macrocyclic ligands. Transition Met. Chem. 2004, 29, 269–275. 10.1023/B:TMCH.0000020359.84853.72. [DOI] [Google Scholar]
- Goldoni F.; Antolini L.; Pourtois G.; Schenning A. P. H. J.; Janssen R. A. J.; Lazzaroni R.; Brédas J.-L.; Meijer E. W. Effect of Ion Coordination on the Conformational and Electronic Structure of 3,4-Bis(alkylthio)thiophenes. Eur. J. Inorg. Chem. 2001, 821–828. . [DOI] [Google Scholar]
- Yang L.; Powell D. R.; Houser R. P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 955–964. 10.1039/B617136B. [DOI] [PubMed] [Google Scholar]
- Crans D. C.; Tarlton M. L.; McLauchlan C. C. Trigonal Bipyramidal or Square Pyramidal Coordination Geometry? Investigating the Most Potent Geometry for Vanadium Phosphatase Inhibitors. Eur. J. Inorg. Chem. 2014, 2014, 4450–4468. 10.1002/ejic.201402306. [DOI] [Google Scholar]
- Addison A. W.; Rao T. N.; Reedijk J.; van Rijn J.; Verschoor G. C. Synthesis, Structure, and Spectroscopic Properties of Copper(11) Compounds containing Nitrogen-Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua[l,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) Perchlorate. J. Chem. Soc., Dalton Trans. 1984, 1349–1356. 10.1039/DT9840001349. [DOI] [Google Scholar]
- Tyagi P.; Chandra S.; Saraswat B. S. Ni(II) and Zn(II) complexes of 2-((thiophen-2- ylmethylene)amino)benzamide: Synthesis, spectroscopic characterization, thermal, DFT and anticancer activities. Spectrochim. Acta, Part A 2015, 134, 200–209. 10.1016/j.saa.2014.06.112. [DOI] [PubMed] [Google Scholar]
- Obuah C.; Ainooson M. K.; Boltina S.; Guzei I. A.; Nozaki K.; Darkwa J. Ethylene and Styrene Carbon Monoxide Copolymerization Catalyzed by Pyrazolyl Palladium(II) Complexes. Organometallics 2013, 32, 980–988. 10.1021/om300798y. [DOI] [Google Scholar]
- Sun Y.-X. Dichloro[N,N-dimethyl-N′-(pyridin-2-ylmethylidene)-ethane-1,2-diamine] zinc(II). Acta Crystallogr., Sect. E: Struct. Rep. Online 2005, 61, m373–m374. 10.1107/S1600536805002278. [DOI] [Google Scholar]
- Falivene L.; Credendino R.; Poater A.; Petta A.; Serra L.; Oliva R.; Scarano V.; Cavallo L. A Web Tool for Analyzing Catalytic Pockets with Topographic Steric Maps. Organometallics 2016, 35, 2286–2293. 10.1021/acs.organomet.6b00371. [DOI] [Google Scholar]
- Park S.; Lee J.; Jeong J. H.; Lee H.; Nayab S. Palladium(II) complexes containing N,N′-bidentate imine ligands derived from picolinaldehyde and substituted anilines: Synthesis, structure and polymerisation of methyl methacrylate. Polyhedron 2018, 151, 82–89. 10.1016/j.poly.2018.05.031. [DOI] [Google Scholar]
- Park S.; Lee J.; Lee H.; Jeong A. R.; Min K. S.; Nayab S. Five-coordinate dinuclear cobalt (II), copper (II), zinc (II) and cadmium (II) complexes with 4-bromo-N-(2-pyridinylmethylene)benzenamine: Synthesis, characterisation and methyl methacrylate polymerization. Appl. Organometal. Chem. 2019, 33, e4766 10.1002/aoc.4766. [DOI] [Google Scholar]
- Kang M.-S.; Cho J.; Nayab S.; Jeong J. H. Synthesis and characterization of Zn(II) and Cu(II) complexes bearing (chiral substituent)(diethyl)-ethanediamine derivatives as precatalysts for rac-lactide polymerisation. Polyhedron 2019, 158, 135–143. 10.1016/j.poly.2018.10.068. [DOI] [Google Scholar]
- Kwon K. S.; Nayab S.; Lee H.; Jeong J. H. Synthesis and structural characterisation of zinc complexes bearing furanylmethyl and thiophenylmethyl derivatives of (R,R)-1,2-diaminocyclohexanes for stereoselective polymerisation of poly(rac-lactide). Polyhedron 2014, 77, 32–38. 10.1016/j.poly.2014.03.055. [DOI] [Google Scholar]
- Appavoo D.; Spencer L. C.; Guzei I. A.; Gomez-García C. J.; van Wyk J. L.; Darkwa J. Ring opening polymerization of D,L-lactide and ε-caprolactone catalysed by (pyrazol-1-yl)copper(II) carboxylate complexes. RSC Adv. 2021, 11, 13475–13485. 10.1039/D1RA00339A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Kim D.; Lee H.; Nayab S.; Han J. H. Effect of initiator on the catalytic performance of zinc(II) complexes supported by aminomethylquinoline and aminomethylpyridine derived ligands in stereoselective ring opening polymerization of rac-lactide. Polyhedron 2022, 216, 115696 10.1016/j.poly.2022.115696. [DOI] [Google Scholar]
- Choe S.; Lee H.; Nayab S. Synthesis, structures, and catalytic efficiency in ring opening polymerization of rac-lactide with tridentate vs. bidentate cobalt(II), zinc(II), and cadmium(II) complexes containing N-substituted N,N-bis((3,5-dimethyl-1H-pyrazol-1-yl)methyl)amine ligands. RSC Adv. 2021, 11, 18840–18851. 10.1039/D1RA02365A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S.; Lee H.; Nayab S. Diverse coordination geometry of cobalt (II), zinc (II), and cadmium (II) complexes comprising N,N-bis(1H-pyrazol-1-yl)methyl)amines derivatives: Synthesis, structures, and ring opening polymerization of rac-lactide. Appl. Organomet. Chem. 2021, 35, e6204 10.1002/aoc.6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown P. S.; McCormick N.; Mahon M. F.; Jones M. D. Highly active Mg(II) and Zn(II) complexes for the ring opening polymerisation of lactide. Polym. Chem. 2018, 9, 5339–5347. 10.1039/C8PY01369A. [DOI] [Google Scholar]
- Wojtaszak J.; Mierzwicki K.; Szafert S.; Gulia N.; Ejfler J. Homoleptic aminophenolates of Zn, Mg and Ca. Synthesis, structure, DFT studies and polymerization activity in ROP of lactides. Dalton Trans. 2014, 43, 2424–2436. 10.1039/C3DT52868E. [DOI] [PubMed] [Google Scholar]
- Ajellal N.; Lyubov D. M.; Sinenkov M. A.; Fukin G. K.; Cherkasov A. V.; Thomas C. M.; Carpentier J.-F.; Trifonov A. A. Bis(guanidinate) Alkoxide Complexes of Lanthanides: Synthesis, Structures and Use in Immortal and Stereoselective Ring-Opening Polymerization of Cyclic Esters. Chem.—Eur. J. 2008, 14, 5440–5448. 10.1002/chem.200800288. [DOI] [PubMed] [Google Scholar]
- Gallaway J. B. L.; McRae J. R. K.; Decken A.; Shaver M. P. Ring-opening polymerization of rac-lactide and ε-caprolactone using zinc and calcium salicylaldiminato complexes. Can. J. Chem. 2012, 90, 419–426. 10.1139/v2012-012. [DOI] [Google Scholar]
- Kaler S.; McKeown P.; Ward B. D.; Jones M. D. Aluminium(III) and zinc(II) complexes of azobenzene-containing ligands for ring-opening polymerisation of ε-caprolactone and rac-lactide. Inorg. Chem. Front. 2021, 8, 711–719. 10.1039/D0QI01303J. [DOI] [Google Scholar]
- Shin S.; Cho H.; Lee H.; Nayab S.; Kim Y. Zinc(II) complexes containing N′-aromatic group substituted N,N′,N-bis((1H-pyrazol-1- yl)methyl)amines: Synthesis, characterization, and polymerizations of methyl methacrylate and rac-lactide. J. Coord. Chem. 2018, 71, 556–584. 10.1080/00958972.2018.1437266. [DOI] [Google Scholar]
- Quilter H. C.; Drewitt R. H.; Mahon M. F.; Kociok-Kohn G.; Jones M. D. Synthesis of Li(I), Zn(II) and Mg(II) complexes of amine bis(phenolates) and their exploitation for the ring opening polymerisation of rac-lactide. J. Organomet. Chem. 2017, 848, 325–331. 10.1016/j.jorganchem.2017.08.014. [DOI] [Google Scholar]
- Chamberlain B. M.; Cheng M.; Moore D. R.; Ovitt T. M.; Lobkovsky E. B.; Coates G. W. Polymerization of Lactide with Zinc and Magnesium β-Diiminate Complexes: Stereocontrol and Mechanism. J. Am. Chem. Soc. 2001, 123, 3229–3238. 10.1021/ja003851f. [DOI] [PubMed] [Google Scholar]
- Zell M. T.; Padden B. E.; Paterick A. J.; Thakur K. A. M.; Kean R. T.; Hillmyer M. A.; Munson E. J. Unambiguous Determination of the 13C and 1H NMR Stereosequence Assignments of Polylactide Using High-Resolution Solution NMR Spectroscopy. Macromolecules 2002, 35, 7700–7707. 10.1021/ma0204148. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









