Abstract
Background
Autoimmune hepatitis (AIH) can result in end-stage liver disease that requires inpatient treatment of the hepatic complications. Given this phenomenon, it is important to analyse the impact of gender and race on the outcomes of patients who are admitted with AIH using a national hospital registry.
Methods
The 2012–2017 National Inpatient Sample database was used to select patients with AIH, who were stratified using gender and race (Hispanics and blacks as cases and whites as reference). Propensity score matching was employed to match the controls with cases and compare mortality, length of stay and hepatic complications.
Results
After matching, there were 4609 females and 4609 males, as well as 3688 blacks and 3173 Hispanics with equal numbers of whites, respectively. In multivariate analysis, females were less likely to develop complications, with lower rates of cirrhosis, ascites, variceal bleeding, hepatorenal syndrome, encephalopathy and acute liver failure (ALF); they also exhibited lower length of stay (adjusted OR, aOR 0.96 95% CI 0.94 to 0.97). When comparing races, blacks (compared with whites) had higher rates of ALF and hepatorenal syndrome related to ALF, but had lower rates of cirrhosis-related encephalopathy; in multivariate analysis, blacks had longer length of stay (aOR 1.071, 95% CI 1.050 to 1.092). Hispanics also exhibited higher rates of hepatic complications, including ascites, varices, variceal bleeding, spontaneous bacterial peritonitis and encephalopathy.
Conclusion
Males and minorities are at a greater risk of developing hepatic complications and having increased hospital costs when admitted with AIH.
Keywords: liver, ascites, encephalopathy, liver failure, autoimmune hepatitis
WHAT IS ALREADY KNOWN ON THIS TOPIC
While prior studies suggest a differential pattern of disease progression among autoimmune hepatitis (AIH) patients depending on gender and race, less is known about the gender and race-specified patterns of hepatic decompensation and liver failure that are observed in admitted AIH patients.
WHAT THIS STUDY ADDS
In this study, we stratify the admitted AIH population using gender and race in order to define the patterns of liver failure and hepatic decompensation among the susceptible cohorts. Furthermore, we use a propensity-score matched analysis in order to control for various medical confounders when assessing the relationships.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
By delineating the patterns of hepatic decompensation that are observed in gender and race-stratified strata of AIH patients, we are able to better understand disparities in outcomes observed in AIH cohorts, and furthermore improve the prognostication of risks in these vulnerable patients on their hospital admission.
Introduction
In patients with autoimmune hepatitis (AIH), failed immune tolerance leads to T-cell-mediated destruction of the hepatic parenchyma and stellate cellular production of matrices in the interstitial space.1 2 These matrices culminate in fibrosis and lead to cirrhosis,3 4 which can cause complications such as portal hypertension, ascites, variceal bleeding, encephalopathy and coagulopathy,5 6 affecting mortality and morbidity.7–9 In conjunction with the natural course of untreated or advanced AIH, studies have investigated the roles of predisposing genetic and racial components in the prognostics of AIH-associated liver disease. These studies have noted signature differences in disease phenotypes stratified per race and genetic composition.10–12 However, since the studies are primarily based on institutional data collection, further validation is required from a clinical perspective to understand differences in phenotype using race/gender while concurrently exploring the differences in hepatic and extrahepatic manifestations in hospital settings.
This study aims to evaluate the effects of race and gender in patients with AIH using national hospital data, specifically focusing on hepatic and extrahepatic comorbidities that result in hospital admission.
Methods
Database
Funded by the Agency for Healthcare Research and Quality through the Healthcare Cost and Utilisation Project, the National Inpatient Sample (NIS) aggregates data compiled from statewide inpatient databases (SID) that comprise hospital claims data collected from predesignated states.13 14 The database includes data from 2012 to 2017 sampled systematically across SID databases.15–17 The discharge diagnoses are encoded using ICD 9 or ICD 10.18 19 Variables were selected through a cross-referencing programme involving the General Equivalence Mappings base,20 21 which converts between the ICD 9 and 10 systems.22
Weighted analysis
The formal weighing method as delineated by the NIS was used to delegate appropriate hospital-level strata and year information for the weighting analysis.23 24 In addition to using the yearly estimates for each captured variable, trend analyses were performed for study variables and endpoints as stratified by predefined covariate terms. Best-fit regression analysis was performed to calculate the trend R2 and p values.
Missing information
For the missing data, multiple imputations with chained equations were used to populate missing data. This method has been verified per literature to be an effective tool for representing missing data in administrative/large-database-driven studies.25–27
Comparative statistics
From NIS, the cohort of interest was found by isolating the in-hospital population with the cohort diagnoses. From this, exclusion criteria were applied to subset the final population of interest. Those under 18 years of age were excluded. The exposure variable was the diagnosis of AIH as defined using corresponding ICD terms. The endpoints included primary outcomes: mortality, length of stay and discharge disposition; secondary outcomes included ascites, varices, variceal bleeding, spontaneous bacterial peritonitis, hepatorenal syndrome and encephalopathy. Each secondary outcome was analysed as a composite variable and as pertaining to the main underlying liver complication: cirrhosis or acute liver failure (ALF). The cohort was further stratified by gender and race.
To minimise covariate confounding, propensity scores were derived for each case using subselected covariate terms. The covariates were fitted into a multivariate prediction model to derive the propensity scores, and terms included: diabetes, hyperlipidaemia, hypertension, chronic obstructive pulmonary disease (COPD), coronary artery disease, chronic kidney disease, congestive heart failure, coagulopathies and smoking. Furthermore, depending on the strata of interest (ie, gender or racial category), the non-used variable was inserted into the propensity score-generating model. Once the model was generated, the nearest neighbour mode was utilised to create 1:1 matches between the cases and controls. For gender comparisons, males were used as the reference group; for race comparisons, Whites were used as the reference group.
To generate univariate comparisons, the Jarque-Bera test was used to analyse variable parametricity.28 29 χ2 or Fisher’s exact test was used for the analysis of nominal variables, and Student’s t-test or Whitney-Mann U test was used for the analysis of non-nominal variables. To generate the multivariate analysis, the variable terms corresponding to hospital admission characteristics were imputed in the regression equations as covariates. P values ≤0.05 were designated statistical significance. Crude and adjusted ORs (aOR) with 95% CIs were quantified for nominal comparisons.
Results
Patient selection
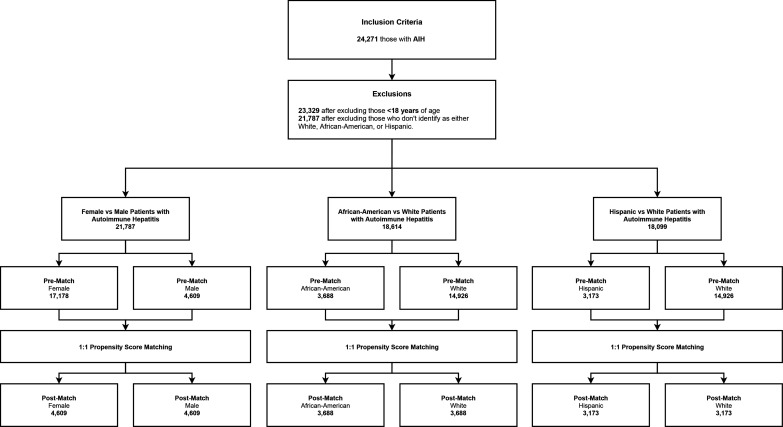
Figure 1 demonstrates the patient selection process. Patients with AIH were selected for this study and stratified either by gender or race. For the gender comparison cohort, there were a total of 21 787 patients including 17 178 females and 4609 males. After matching, there were 9218 patients, including 4609 female and 4609 male patients. For the race comparison cohort, there were a total of 14 926 white patients, 3688 black patients and 3173 Hispanic patients. After matching, 7376 patients were stratified into 3688 black patients and 3688 white patients, and 6346 patients were stratified into 3173 Hispanic patients and 3173 white patients for comparison.
Figure 1.
This figure shows the patient selection procedure of the study. AIH, autoimmune hepatitis.
Postmatch comparison of demographics and comorbidities
Table 1 compares prematch and postmatch demographics and medical comorbidities between female and male patients admitted with AIH. After matching, there were no differences in age and racial distribution. In terms of medical comorbidities, females persistently had lower rates of COPD (6.92% vs 8.03%, p=0.05) and congestive heart failure (8.53% vs 10.10%, p<0.01). Females also had lower rates of primary liver cancer (1.41% vs 2.26%, p<0.01), which includes hepatocellular carcinoma (1.30% vs 1.87%, p=0.04) and cholangiocarcinoma (0.11% vs 0.39%, p=0.01), alcoholic liver disease (2.02% vs 5.92%, p<0.001) and hepatitis B (0.09% vs 0.52%, p<0.001). Females also had a lower rate of postmatch malnutrition (9.16% vs 11.90%, p<0.001), which includes protein-calorie malnutrition, sarcopenia and cachexia.
Table 1.
Comparison of demographics and medical comorbidities in patients admitted with autoimmune hepatitis; male versus female
| Demographics | Prematch comparison | Postmatch comparison | ||||||||||
| Female | Male | P value | Univariate analysis | Female | Male | P value | Univariate analysis | |||||
| n=17 178 | 78.85% | n=4609 | 21.15% | OR (95% CI) | n=4609 | 50% | n=4609 | 50% | OR (95% CI) | |||
| Age (years) | 58.3 | ±17.60 years | 54.1 | ±18.30 years | <0.001*** | 54 | ±18.10 years | 54.1 | ±18.30 years | 0.7 | ||
| Race | <0.001*** | 0.65 | ||||||||||
| White (%) | 11 579 | 67.4 | 3347 | 72.6 | 3373 | 73.2 | 3347 | 72.6 | ||||
| Black (%) | 2981 | 17.4 | 707 | 15.3 | 675 | 14.6 | 707 | 15.3 | ||||
| Hispanic (%) | 2618 | 15.2 | 555 | 12 | 561 | 12.2 | 555 | 12 | ||||
| Comorbidities | ||||||||||||
| Diabetes (%) | 4678 | 27.2 | 1292 | 28 | 0.29 | 0.96 (0.89 to 1.03) | 1283 | 27.8 | 1292 | 28 | 0.85 | 0.99 (0.90 to 1.08) |
| Hyperlipidaemia (%) | 3750 | 21.8 | 994 | 21.6 | 0.72 | 1.02 (0.94 to 1.10) | 968 | 21 | 994 | 21.6 | 0.52 | 0.97 (0.88 to 1.07) |
| Hypertension (%) | 6397 | 37.2 | 1384 | 30 | <0.001*** | 1.38 (1.29 to 1.48) | 1380 | 29.9 | 1384 | 30 | 0.95 | 1 (0.91 to 1.09) |
| Chronic obstructive pulmonary disease (%) | 1726 | 10 | 370 | 8.03 | <0.001*** | 1.28 (1.14 to 1.44) | 319 | 6.92 | 370 | 8.03 | 0.05* | 0.85 (0.73 to 1.00) |
| Coronary artery disease (%) | 1908 | 11.1 | 719 | 15.6 | <0.001*** | 0.68 (0.62 to 0.74) | 670 | 14.5 | 719 | 15.6 | 0.16 | 0.92 (0.82 to 1.03) |
| Chronic kidney disease (%) | 2536 | 14.8 | 816 | 17.7 | <0.001*** | 0.81 (0.74 to 0.88) | 787 | 17.1 | 816 | 17.7 | 0.44 | 0.96 (0.86 to 1.07) |
| Congestive heart failure (%) | 2018 | 11.7 | 467 | 10.1 | 0.002** | 1.18 (1.06 to 1.31) | 393 | 8.53 | 467 | 10.1 | 0.009** | 0.83 (0.72 to 0.95) |
| Coagulopathies (%) | 769 | 4.48 | 227 | 4.93 | 0.21 | 0.9 (0.78 to 1.05) | 203 | 4.4 | 227 | 4.93 | 0.26 | 0.89 (0.73 to 1.08) |
| Smoking (%) | 4264 | 24.8 | 1387 | 30.1 | <0.001*** | 0.77 (0.71 to 0.82) | 1365 | 29.6 | 1387 | 30.1 | 0.63 | 0.98 (0.89 to 1.07) |
| Liver aetiologies | ||||||||||||
| Primary liver cancer (%) | 246 | 1.43 | 104 | 2.26 | <0.001*** | 0.63 (0.50 to 0.79) | 65 | 1.41 | 104 | 2.26 | 0.003** | 0.62 (0.45 to 0.85) |
| Hepatocellular carcinoma (%) | 225 | 1.31 | 86 | 1.87 | 0.006** | 0.7 (0.54 to 0.90) | 60 | 1.3 | 86 | 1.87 | 0.04* | 0.69 (0.50 to 0.97) |
| Cholangiocarcinoma (%) | 21 | 0.12 | 18 | 0.39 | <0.001*** | 0.31 (0.17 to 0.59) | 5 | 0.11 | 18 | 0.39 | 0.01* | 0.28 (0.10 to 0.75) |
| Alcoholic liver diseases (%) | 332 | 1.93 | 273 | 5.92 | <0.001*** | 0.31 (0.27 to 0.37) | 93 | 2.02 | 273 | 5.92 | <0.001*** | 0.33 (0.26 to 0.42) |
| Hepatitis B (%) | 39 | 0.23 | 24 | 0.52 | 0.002** | 0.43 (0.26 to 0.72) | 4 | 0.09 | 24 | 0.52 | <0.001*** | 0.17 (0.04 to 0.48) |
| Hepatitis C (%) | 171 | 1 | 73 | 1.58 | 0.001** | 0.62 (0.47 to 0.82) | 23 | 0.5 | 73 | 1.58 | <0.001*** | 0.31 (0.19 to 0.50) |
| Non-alcoholic fatty liver disease (%) | 710 | 4.13 | 199 | 4.32 | 0.61 | 0.96 (0.81 to 1.12) | 175 | 3.8 | 199 | 4.32 | 0.22 | 0.87 (0.71 to 1.08) |
| Nutrition | ||||||||||||
| Malnutrition (%) | 1745 | 10.2 | 548 | 11.9 | <0.001*** | 0.84 (0.76 to 0.93) | 422 | 9.16 | 548 | 11.9 | <0.001*** | 0.75 (0.65 to 0.85) |
*P<0.05, **p<0.01, ***p<0.001.
Table 2 compares prematch and postmatch demographics and medical comorbidities between racial groups. Comparing black and white patients after matching, there were no significant differences in age, gender or comorbidities, except for greater rate of coronary artery disease (8.57% vs 6.94%, p=0.01) in blacks. There were fewer discrepancies in the rate of medical comorbidities postmatch. In terms of postmatch liver aetiologies, black patients had increased hepatitis B (0.73% vs 0.11%, p<0.001), increased hepatitis C (1.82% vs 0.60%, p<0.001) and decreased non-alcoholic fatty liver disease (2.58% vs 4.26%, p<0.001). Blacks were found to have a higher rate of malnutrition (10.90% vs 9.06%, p=0.01).
Table 2.
Comparison of demographics and medical comorbidities in patients admitted with autoimmune hepatitis; stratified by race
| Demographics | Black versus white prematch comparison | Black versus white postmatch comparison | ||||||||||
| Black | White | P value | Univariate analysis | Black | White | P value | Univariate analysis | |||||
| n=3688 | 19.81% | n=14 926 | 80.19% | OR (95% CI) | n=3688 | 50% | n=3688 | 50% | OR (95% CI) | |||
| Age (years) | 49.5 | ±18.10 years | 60.3 | ±17.10 years | <0.001*** | 49.5 | ±18.10 years | 49.3 | ±18.50 years | 0.65 | ||
| Female (%) | 2981 | 80.8 | 11 579 | 77.6 | <0.001*** | 1.22 (1.11 to 1.33) | 2981 | 80.8 | 2970 | 80.5 | 0.77 | 1.02 (0.91 to 1.14) |
| Comorbidities | ||||||||||||
| Diabetes (%) | 1074 | 29.1 | 3885 | 26 | <0.001*** | 1.17 (1.08 to 1.26) | 1074 | 29.1 | 1049 | 28.4 | 0.54 | 1.03 (0.93 to 1.14) |
| Hyperlipidaemia (%) | 708 | 19.2 | 3500 | 23.4 | <0.001*** | 0.78 (0.71 to 0.85) | 708 | 19.2 | 645 | 17.5 | 0.06 | 1.12 (1.00 to 1.26) |
| Hypertension (%) | 1366 | 37 | 5487 | 36.8 | 0.77 | 1.01 (0.94 to 1.09) | 1366 | 37 | 1349 | 36.6 | 0.7 | 1.02 (0.93 to 1.12) |
| Chronic obstructive pulmonary disease (%) | 271 | 7.35 | 1698 | 11.4 | <0.001*** | 0.62 (0.54 to 0.71) | 271 | 7.35 | 240 | 6.51 | 0.17 | 1.14 (0.95 to 1.36) |
| Coronary artery disease (%) | 316 | 8.57 | 2065 | 13.8 | <0.001*** | 0.58 (0.52 to 0.66) | 316 | 8.57 | 256 | 6.94 | 0.01* | 1.26 (1.06 to 1.49) |
| Chronic kidney disease (%) | 703 | 19.1 | 2171 | 14.5 | <0.001*** | 1.38 (1.26 to 1.52) | 703 | 19.1 | 658 | 17.8 | 0.19 | 1.08 (0.96 to 1.22) |
| Congestive heart failure (%) | 418 | 11.3 | 1782 | 11.9 | 0.32 | 0.94 (0.84 to 1.06) | 418 | 11.3 | 376 | 10.2 | 0.12 | 1.13 (0.97 to 1.30) |
| Coagulopathies (%) | 210 | 5.69 | 578 | 3.87 | <0.001*** | 1.5 (1.27 to 1.76) | 210 | 5.69 | 205 | 5.56 | 0.84 | 1.03 (0.84 to 1.25) |
| Smoking (%) | 928 | 25.2 | 4195 | 28.1 | <0.001*** | 0.86 (0.79 to 0.93) | 928 | 25.2 | 883 | 23.9 | 0.23 | 1.07 (0.96 to 1.19) |
| Liver aetiologies | ||||||||||||
| Primary liver cancer (%) | 53 | 1.44 | 214 | 1.43 | 1 | 1 (0.74 to 1.36) | 53 | 1.44 | 39 | 1.06 | 0.17 | 1.36 (0.90 to 2.07) |
| Hepatocellular carcinoma (%) | 46 | 1.25 | 184 | 1.23 | 1 | 1.01 (0.73 to 1.40) | 46 | 1.25 | 34 | 0.92 | 0.22 | 1.36 (0.87 to 2.12) |
| Cholangiocarcinoma (%) | 7 | 0.19 | 30 | 0.2 | 1 | 0.94 (0.41 to 2.15) | 7 | 0.19 | 5 | 0.14 | 0.77 | 1.4 (0.44 to 4.42) |
| Alcoholic liver diseases (%) | 87 | 2.36 | 415 | 2.78 | 0.17 | 0.84 (0.67 to 1.07) | 87 | 2.36 | 106 | 2.87 | 0.19 | 0.82 (0.61 to 1.09) |
| Hepatitis B (%) | 27 | 0.73 | 28 | 0.19 | <0.001*** | 3.92 (2.31 to 6.67) | 27 | 0.73 | 4 | 0.11 | < 0.001†*** | 6.79 (2.36 to 26.73) |
| Hepatitis C (%) | 67 | 1.82 | 136 | 0.91 | <0.001*** | 2.01 (1.50 to 2.70) | 67 | 1.82 | 22 | 0.6 | < 0.001*** | 3.08 (1.90 to 5.00) |
| Non-alcoholic fatty liver disease (%) | 95 | 2.58 | 647 | 4.33 | <0.001*** | 0.58 (0.47 to 0.73) | 95 | 2.58 | 157 | 4.26 | < 0.001*** | 0.59 (0.46 to 0.77) |
| Nutrition | ||||||||||||
| Malnutrition (%) | 401 | 10.9 | 1528 | 10.2 | 0.27 | 1.07 (0.95 to 1.20) | 401 | 10.9 | 334 | 9.06 | 0.01* | 1.23 (1.05 to 1.43) |
| Demographics | Hispanic versus white prematch comparison | Hispanic versus white postmatch comparison | ||||||||||
| Hispanic | White | P value | Univariate analysis | Hispanic | White | P value | Univariate analysis | |||||
| n=3173 | 17.53 | n=14 926 | 82.47 | OR 95% CI | n=3173 | 50 | n=3173 | 50 | OR 95% CI | |||
| Age (years) | 52.9 | ±17.20 | 60.3 | ±17.10 | <0.001*** | 52.9 | ±17.20 | 52.7 | ±17.60 | 0.74 | ||
| Female (%) | 2618 | 82.5 | 11 579 | 77.6 | <0.001*** | 1.36 (1.23 to 1.51) | 2618 | 82.5 | 2625 | 82.7 | 0.84 | 0.98 (0.86 to 1.12) |
| Comorbidities | ||||||||||||
| Diabetes (%) | 1011 | 31.9 | 3885 | 26 | <0.001*** | 1.33 (1.22 to 1.44) | 1011 | 31.9 | 943 | 29.7 | 0.07 | 1.11 (0.99 to 1.23) |
| Hyperlipidaemia (%) | 536 | 16.9 | 3500 | 23.4 | <0.001*** | 0.66 (0.60 to 0.73) | 536 | 16.9 | 530 | 16.7 | 0.87 | 1.01 (0.89 to 1.16) |
| Hypertension (%) | 928 | 29.2 | 5487 | 36.8 | <0.001*** | 0.71 (0.65 to 0.77) | 928 | 29.2 | 947 | 29.8 | 0.62 | 0.97 (0.87 to 1.08) |
| Chronic obstructive pulmonary disease (%) | 127 | 4 | 1698 | 11.4 | <0.001*** | 0.32 (0.27 to 0.39) | 127 | 4 | 98 | 3.09 | 0.06 | 1.31 (1.00 to 1.71) |
| Coronary artery disease (%) | 246 | 7.75 | 2065 | 13.8 | <0.001*** | 0.52 (0.46 to 0.60) | 246 | 7.75 | 236 | 7.44 | 0.67 | 1.05 (0.87 to 1.26) |
| Chronic kidney disease (%) | 478 | 15.1 | 2171 | 14.5 | 0.47 | 1.04 (0.94 to 1.16) | 478 | 15.1 | 416 | 13.1 | 0.03* | 1.18 (1.02 to 1.35) |
| Congestive heart failure (%) | 285 | 8.98 | 1782 | 11.9 | <0.001*** | 0.73 (0.64 to 0.83) | 285 | 8.98 | 238 | 7.5 | 0.04* | 1.22 (1.02 to 1.46) |
| Coagulopathies (%) | 208 | 6.56 | 578 | 3.87 | <0.001*** | 1.74 (1.48 to 2.05) | 208 | 6.56 | 195 | 6.15 | 0.54 | 1.07 (0.88 to 1.31) |
| Smoking (%) | 528 | 16.6 | 4195 | 28.1 | <0.001*** | 0.51 (0.46 to 0.56) | 528 | 16.6 | 508 | 16 | 0.52 | 1.05 (0.92 to 1.20) |
| Liver aetiologies | ||||||||||||
| Primary liver cancer (%) | 83 | 2.62 | 214 | 1.43 | <0.001*** | 1.85 (1.43 to 2.39) | 83 | 2.62 | 42 | 1.32 | < 0.001*** | 2 (1.38 to 2.91) |
| Hepatocellular carcinoma (%) | 81 | 2.55 | 184 | 1.23 | <0.001*** | 2.1 (1.61 to 2.73) | 81 | 2.55 | 37 | 1.17 | < 0.001*** | 2.22 (1.50 to 3.29) |
| Cholangiocarcinoma (%) | 2 | 0.06 | 30 | 0.2 | 0.11 | 0.31 (0.04 to 1.24) | 2 | 0.06 | 5 | 0.16 | 0.45† | 0.4 (0.04 to 2.44) |
| Alcoholic liver diseases (%) | 103 | 3.25 | 415 | 2.78 | 0.17 | 1.17 (0.94 to 1.46) | 103 | 3.25 | 89 | 2.8 | 0.34 | 1.16 (0.87 to 1.55) |
| Hepatitis B (%) | 8 | 0.25 | 28 | 0.19 | 0.6 | 1.34 (0.61 to 2.95) | 8 | 0.25 | 1 | 0.03 | 0.04*† | 8.02 (1.07 to 355.31) |
| Hepatitis C (%) | 41 | 1.29 | 136 | 0.91 | 0.06 | 1.42 (1.00 to 2.02) | 41 | 1.29 | 17 | 0.54 | 0.002** | 2.43 (1.38 to 4.29) |
| Non-alcoholic fatty liver disease (%) | 167 | 5.26 | 647 | 4.33 | 0.03* | 1.23 (1.03 to 1.46) | 167 | 5.26 | 123 | 3.88 | 0.01** | 1.38 (1.09 to 1.75) |
| Nutrition | ||||||||||||
| Malnutrition (%) | 364 | 11.5 | 1528 | 10.2 | 0.04* | 1.14 (1.01 to 1.28) | 364 | 11.5 | 295 | 9.3 | 0.005** | 1.26 (1.08 to 1.49) |
*P<0.05, **p<0.01, ***p<0.001.
†
Comparing postmatch Hispanic and white patients, there were no significant differences in age, gender or comorbidities, except for greater rates of chronic kidney disease (15.10% vs 13.10%, p=0.03) and congestive heart failure (8.98% vs 7.50%, p=0.04) in Hispanic patients. Hispanics had greater rates of primary liver cancer (2.62% vs 1.32%, p<0.001), hepatocellular carcinoma (2.55% vs 1.17%, p<0.001), non-alcoholic fatty liver disease (5.26% vs 3.88%, p=0.01), hepatitis B (0.25% vs 0.03%, p=0.04) and hepatitis C (1.29% vs 0.54%, p=0.002) after matching.
Comparison of hospital outcomes and liver complications
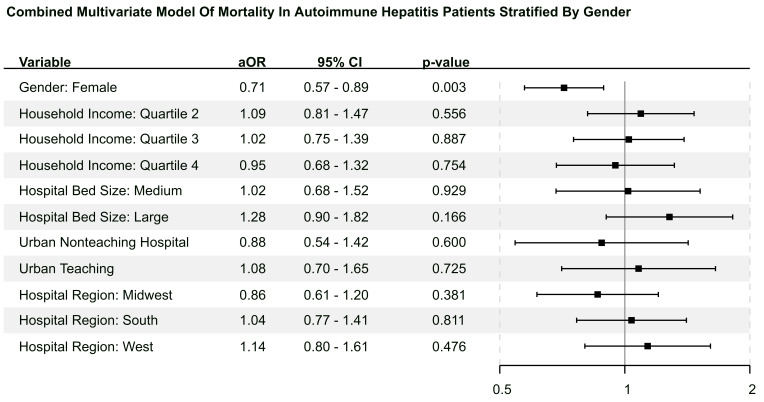
Table 3 compares postmatch hospital outcomes between female and male patients. Female patients had a lower rate of mortality (3.10% vs 4.36%, p=0.002; OR 0.70, 95% CI 0.56 to 0.87)) than male patients but no difference in the length of stay. Females were less likely to undergo routine discharge. In terms of liver complications, female patients had a lower rate of cirrhosis (34.20% vs 41.60%, p<0.001), ALF (4.21% vs 5.25%, p=0.02), ascites (12.50% vs 17.30%, p<0.001; OR 0.68 95% CI 0.61 to 0.77), varices (11.80% vs 15.10%, p<0.001; OR 0.75, 95% CI 0.66 to 0.84), cirrhosis-related variceal bleeding (1.91% vs 2.65%, p=0.02; OR 0.72, 95% CI 0.54 to 0.94), cirrhosis-related spontaneous bacterial peritonitis (1.32% vs 2.78%, p<0.001; OR 0.47, 95% CI 0.35 to 0.64), cirrhosis-related hepatorenal syndrome (1.13% vs 2.10%, p<0.001; OR 0.53, 95% CI 0.38 to 0.75) and cirrhosis-related encephalopathy (8.16% vs 11.00%, p<0.001; OR 0.72, 95% CI 0.63 to 0.83). In the multivariate analysis, female patients had lower length of stay (p<0.001; aOR 0.96, 95% CI 0.94 to 0.97) and lower mortality (p=0.003; aOR 0.71, 95% CI 0.57 to 0.89). Figure 2 shows the multivariate analysis using gender as exposure and mortality as the primary endpoint.
Table 3.
Postmatch comparison of hospital outcomes in patients admitted with autoimmune hepatitis; male versus female
| Hospital outcomes | Female | Male | P value | Univariate analysis | Multivariate analysis | P value | ||
| n=4609 | 50% | n=4609 | 50% | OR (95% CI) | aOR (95% CI) | |||
| Mortality (%) | 143 | 3.1 | 201 | 4.36 | 0.002** | 0.7 (0.56 to 0.87) | 0.71 (0.57 to 0.89) | 0.003** |
| Length of stay (days) | 5.57 | 5.9 | 0.77 | 0.96 (0.94 to 0.97) | <0.001†** | |||
| Hospitalisation cost ($) | 52 413 | 62 658 | <0.001*** | 0.86 (0.86 to 0.86) | <0.001†** | |||
| Disposition at discharge | <0.001*** | |||||||
| Routine (%) | 3039 | 65.9 | 3047 | 66.1 | ||||
| Short-term hospital (%) | 155 | 3.36 | 203 | 4.4 | ||||
| SNF or other facility (%) | 574 | 12.5 | 487 | 10.6 | ||||
| Home healthcare (%) | 659 | 14.3 | 615 | 13.3 | ||||
| Left AMA (%) | 39 | 0.85 | 55 | 1.19 | ||||
| Died (%) | 143 | 3.1 | 201 | 4.36 | ||||
| Unknown (%) | 0 | 0 | 1 | 0.02 | ||||
| Liver complications | ||||||||
| Acute liver failure (%) | 194 | 4.21 | 242 | 5.25 | 0.02* | 0.79 (0.65 to 0.96) | ||
| Cirrhosis (%) | 1578 | 34.2 | 1919 | 41.6 | <0.001*** | 0.73 (0.67 to 0.79) | ||
| Ascites (%)‡ | 577 | 12.5 | 799 | 17.3 | <0.001*** | 0.68 (0.61 to 0.77) | ||
| Cirrhosis related (%) | 560 | 12.2 | 777 | 16.9 | <0.001*** | 0.68 (0.61 to 0.77) | ||
| ALF related (%) | 46 | 1 | 82 | 1.78 | 0.002** | 0.56 (0.39 to 0.80) | ||
| Varices (%)‡ | 400 | 8.68 | 576 | 12.5 | <0.001*** | 0.67 (0.58 to 0.76) | ||
| Cirrhosis related (%) | 398 | 8.64 | 568 | 12.3 | <0.001*** | 0.67 (0.59 to 0.77) | ||
| ALF related (%) | 15 | 0.33 | 32 | 0.69 | 0.02* | 0.47 (0.25 to 0.86) | ||
| Variceal bleeding (%)‡ | 11 | 1.56 | 18 | 2.55 | 0.26 | 0.6 (0.28 to 1.29) | ||
| Cirrhosis related (%) | 88 | 1.91 | 122 | 2.65 | 0.02* | 0.72 (0.54 to 0.94) | ||
| ALF related (%) | 1 | 0.02 | 6 | 0.13 | 0.12§ | 0.17 (0.00 to 1.37) | ||
| Spontaneous bacterial peritonitis (%)‡ | 65 | 1.41 | 131 | 2.84 | <0.001*** | 0.49 (0.36 to 0.66) | ||
| Cirrhosis related (%) | 61 | 1.32 | 128 | 2.78 | <0.001*** | 0.47 (0.35 to 0.64) | ||
| ALF related (%) | 10 | 0.22 | 17 | 0.37 | 0.25 | 0.59 (0.27 to 1.28) | ||
| Hepatorenal syndrome (%)‡ | 61 | 1.32 | 110 | 2.39 | <0.001*** | 0.55 (0.40 to 0.75) | ||
| Cirrhosis related (%) | 52 | 1.13 | 97 | 2.1 | <0.001*** | 0.53 (0.38 to 0.75) | ||
| ALF related (%) | 18 | 0.39 | 28 | 0.61 | 0.18 | 0.64 (0.35 to 1.16) | ||
| Encephalopathy (%)‡ | 399 | 8.66 | 527 | 11.4 | <0.001*** | 0.73 (0.64 to 0.84) | ||
| Cirrhosis related (%) | 376 | 8.16 | 506 | 11 | <0.001*** | 0.72 (0.63 to 0.83) | ||
| ALF related (%) | 48 | 1.04 | 44 | 0.96 | 0.75 | 1.09 (0.72 to 1.65) | ||
*P<0.05, **p<0.01, ***p<0.001.
†Used Poisson regression analysis.
‡These variables include both cirrhosis and ALF-related events counting overlapping incidences.
§Fisher’s exact test.
ALF, acute liver failure; AMA, against medical advice; aOR, adjusted OR; SNF, skilled nursing facility.
Figure 2.
This figure is the multivariate forest plot representation using patient gender as exposure term and mortality as the endpoint.
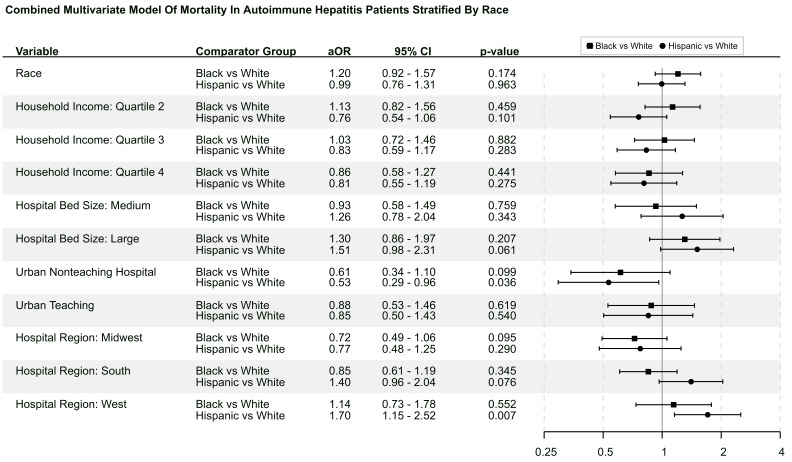
Table 4 compares postmatch hospital outcomes between racial groups. In univariate comparison, black patients had a longer length of stay (6.4 vs 5.60 days, p<0.001) compared with white patients; there was no significant difference in mortality or disposition after discharge. In terms of liver complications, blacks had a greater rate of ALF (6.64% vs 4.37%, p<0.001), in the setting of a lower rate of encephalopathy (8.19% vs 9.76%, p=0.02). When further analysed by underlying liver complication, blacks had lower rates of cirrhosis-related encephalopathy (7.40% vs 9.33%, p=0.003; OR 0.78, 95% CI 0.66 to 0.92), while no difference was observed in ALF-related encephalopathy. Blacks also had greater rates of ALF-related hepatorenal syndrome (0.90% vs 0.43%, p=0.02; OR 2.07, 95% CI 1.14 to 3.77). In the multivariate analysis, blacks had a longer length of stay (p<0.001; aOR 1.071, 95% CI 1.050 to 1.092) but no difference in mortality compared with whites.
Table 4.
Postmatch comparison of hospital outcomes in Patients admitted with autoimmune hepatitis; stratified by race
| Hospital outcomes | Black | White | P value | Univariate analysis | Multivariate analysis | P value | ||
| n=3688 | 50% | n=3688 | 50% | OR (95% CI) | aOR (95% CI) | |||
| Mortality (%) | 142 | 3.85 | 116 | 3.15% | 0.11 | 1.23 (0.96 to 1.58) | 1.2 (0.92 to 1.57) | 0.17 |
| Length of stay (days) | 6.4 | ±7.41 days | 5.6 | ±6.28 days | <0.001*** | 1.07 (1.05 to 1.09) | <0.001†** | |
| Hospital charges ($) | 62 220 | ±111 728 | 54 457 | ±101 707 | <0.001*** | 1.06 (1.06 to 1.07) | <0.001†** | |
| Disposition at discharge | 0.51 | |||||||
| Routine (%) | 2456 | 66.6 | 2486 | 67.4 | ||||
| Short-term Hospital (%) | 142 | 3.85 | 144 | 3.9 | ||||
| SNF or other facility (%) | 395 | 10.7 | 401 | 10.9 | ||||
| Home healthcare (%) | 514 | 13.9 | 492 | 13.3 | ||||
| Left AMA (%) | 39 | 1.06 | 48 | 1.3 | ||||
| Died (%) | 142 | 3.85 | 116 | 3.15 | ||||
| Unknown (%) | 0 | 0 | 1 | 0.03 | ||||
| Liver complications | ||||||||
| Acute liver failure (%) | 245 | 6.64 | 161 | 4.37 | <0.001*** | 1.56 (1.27 to 1.91) | ||
| Cirrhosis (%) | 1338 | 36.3 | 1310 | 35.5 | 0.51 | 1.03 (0.94 to 1.14) | ||
| Ascites (%)‡ | 470 | 12.7 | 490 | 13.3 | 0.51 | 0.95 (0.83 to 1.09) | ||
| Cirrhosis related (%) | 444 | 12 | 474 | 12.9 | 0.31 | 0.93 (0.81 to 1.07) | ||
| ALF related (%) | 61 | 1.65 | 51 | 1.38 | 0.39 | 1.2 (0.82 to 1.74) | ||
| Varices (%)‡ | 348 | 9.44 | 315 | 8.54 | 0.19 | 1.12 (0.95 to 1.31) | ||
| Cirrhosis related (%) | 338 | 9.16 | 310 | 8.41 | 0.27 | 1.1 (0.94 to 1.29) | ||
| ALF related (%) | 28 | 0.76 | 19 | 0.52 | 0.24 | 1.48 (0.82 to 2.65) | ||
| Variceal bleeding (%)‡ | 348 | 9.44 | 315 | 8.54 | 0.19 | 1.12 (0.95 to 1.31) | ||
| Cirrhosis related (%) | 57 | 1.55 | 59 | 1.6 | 0.93 | 0.97 (0.67 to 1.39) | ||
| ALF related (%) | 5 | 0.14 | 2 | 0.05 | 0.45§ | 2.5 (0.41 to 26.30) | ||
| Spontaneous bacterial peritonitis (%)‡ | 67 | 1.82 | 69 | 1.87 | 0.93 | 0.97 (0.69 to 1.36) | ||
| Cirrhosis related (%) | 61 | 1.65 | 66 | 1.79 | 0.72 | 0.92 (0.65 to 1.31) | ||
| ALF related (%) | 10 | 0.27 | 8 | 0.22 | 0.81 | 1.25 (0.49 to 3.17) | ||
| Hepatorenal syndrome (%)‡ | 75 | 2.03 | 67 | 1.82 | 0.55 | 1.12 (0.80 to 1.56) | ||
| Cirrhosis related (%) | 57 | 1.55 | 61 | 1.65 | 0.78 | 0.93 (0.65 to 1.34) | ||
| ALF related (%) | 33 | 0.9 | 16 | 0.43 | 0.02* | 2.07 (1.14 to 3.77) | ||
| Encephalopathy (%)‡ | 302 | 8.19 | 360 | 9.76 | 0.02* | 0.82 (0.70 to 0.97) | ||
| Cirrhosis related (%) | 273 | 7.4 | 344 | 9.33 | 0.003** | 0.78 (0.66 to 0.92) | ||
| ALF related (%) | 48 | 1.3 | 40 | 1.08 | 0.45 | 1.2 (0.79 to 1.83) | ||
| Hospital outcomes | Hispanic | White | P value | Univariate analysis | Multivariate analysis | P value | ||
| n=3173 | 50 | n=3173 | 50 | OR 95% CI | aOR 95% CI | |||
| Mortality (%) | 142 | 4.48 | 116 | 3.66% | 0.11 | 1.23 (0.96 to 1.59) | 0.99 (0.76 to 1.31) | 0.96 |
| Length of stay (days) | 6 | ±7.35 days | 5.7 | ±6.75 days | 0.03* | 1 (0.98 to 1.02) | 0.98† | |
| Hospital charges ($) | 72 233 | ± 123 440 | 58 070 | ±119 450 | <0.001*** | 1.06 (1.06 to 1.06) | <0.001†** | |
| Disposition at discharge | <0.001*** | |||||||
| Routine (%) | 2210 | 69.7 | 2087 | 65.8 | ||||
| Short-term hospital (%) | 88 | 2.77 | 139 | 4.38 | ||||
| SNF or other facility (%) | 274 | 8.64 | 382 | 12 | ||||
| Home healthcare (%) | 430 | 13.6 | 423 | 13.3 | ||||
| Left AMA (%) | 28 | 0.88 | 26 | 0.82 | ||||
| Died (%) | 142 | 4.48 | 116 | 3.66 | ||||
| Unknown (%) | 1 | 0.03 | 0 | 0 | ||||
| Liver complications | ||||||||
| Acute liver failure (%) | 144 | 4.54 | 138 | 4.35 | 0.76 | 1.05 (0.82 to 1.33) | ||
| Cirrhosis (%) | 1569 | 49.4 | 1182 | 37.3 | <0.001*** | 1.65 (1.49 to 1.82) | ||
| Ascites (%)‡ | 604 | 19 | 469 | 14.8 | <0.001*** | 1.36 (1.19 to 1.55) | ||
| Cirrhosis related (%) | 592 | 18.7 | 456 | 14.4 | <0.001*** | 1.37 (1.20 to 1.56) | ||
| ALF related (%) | 52 | 1.64 | 39 | 1.23 | 0.21 | 1.34 (0.88 to 2.03) | ||
| Varices (%)‡ | 463 | 14.6 | 312 | 9.83 | <0.001*** | 1.57 (1.34 to 1.83) | ||
| Cirrhosis related (%) | 460 | 14.5 | 309 | 9.74 | <0.001*** | 1.57 (1.35 to 1.83) | ||
| ALF related (%) | 16 | 0.5 | 13 | 0.41 | 0.71 | 1.23 (0.59 to 2.57) | ||
| Variceal bleeding (%)‡ | 463 | 14.6 | 312 | 9.83 | <0.001*** | 1.57 (1.34 to 1.83) | ||
| Cirrhosis related (%) | 118 | 3.72 | 63 | 1.99 | <0.001*** | 1.91 (1.40 to 2.60) | ||
| ALF related (%) | 1 | 0.03 | 0 | 0 | 1§ | Inf (0.03 to Inf) | ||
| Spontaneous bacterial peritonitis (%)‡ | 89 | 2.8 | 47 | 1.48 | <0.001*** | 1.92 (1.34 to 2.74) | ||
| Cirrhosis related (%) | 87 | 2.74 | 45 | 1.42 | <0.001*** | 1.96 (1.36 to 2.82) | ||
| ALF related (%) | 7 | 0.22 | 7 | 0.22 | 1 | 1 (0.35 to 2.85) | ||
| Hepatorenal syndrome (%)‡ | 80 | 2.52 | 60 | 1.89 | 0.1 | 1.34 (0.96 to 1.88) | ||
| Cirrhosis related (%) | 77 | 2.43 | 54 | 1.7 | 0.05 | 1.44 (1.01 to 2.04) | ||
| ALF related (%) | 12 | 0.38 | 14 | 0.44 | 0.84 | 0.86 (0.40 to 1.85) | ||
| Encephalopathy (%)‡ | 455 | 14.3 | 364 | 11.5 | <0.001*** | 1.29 (1.11 to 1.50) | ||
| Cirrhosis related (%) | 444 | 14 | 342 | 10.8 | <0.001*** | 1.35 (1.16 to 1.57) | ||
| ALF related (%) | 29 | 0.91 | 40 | 1.26 | 0.23 | 0.72 (0.45 to 1.17) | ||
*P<0.05, **p<0.01, ***p<0.001.
†Used Poisson regression analysis.
‡These variables include both cirrhosis and ALF-related events counting overlapping incidences.
§Fisher’s exact test.
ALF, acute liver failure; AMA, against medical advice; aOR, adjusted OR; SNF, skilled nursing facility.
Hispanic patients had a longer length of stay (6.00 vs 5.68 days, p=0.033) and higher rate of routine discharge compared with white patients. There was no difference in mortality between Hispanic and white patients. In terms of liver complications, Hispanics had greater rates of cirrhosis (49.40% vs 37.30%, p<0.001), cirrhosis-related ascites (18.70% vs 14.40%, p<0.001, OR 1.37, 95% CI 1.20 to 1.56), cirrhosis-related varices (14.50% vs 9.74%, p<0.001; OR 1.57, 95% CI 1.35 to 1.83), cirrhosis-related variceal bleeding (3.72% vs 1.99%, p<0.001; OR 1.57, 95% CI 1.34 to 1.83), cirrhosis-related spontaneous bacterial peritonitis (2.74% vs 1.42%, p<0.001; OR 1.96, 95% CI 1.36 to 2.82) and cirrhosis-related encephalopathy (14.00% vs 10.80%, p<0.001; OR 1.35, 95% CI 1.16 to 1.57). There were no significant differences in ALF-related liver complications between the two cohorts. In the multivariate analysis, Hispanics were shown to have no difference in mortality or length of stay compared with white patients. Figure 3 represents the multivariate model using race as exposure and mortality as the primary endpoint.
Figure 3.
This figure shows the combinational multivariate forest plot using race as exposure and mortality as the endpoint.
Supplementary tables
Online supplemental table 1 shows the ICD codes used in the study. Online supplemental table 2 shows the comparison of socioeconomic and hospital characteristics stratified by gender and race. Online supplemental table 3 shows the prematch comparisons of clinical outcomes stratified by gender and race. Online supplemental tables 4–6 show the comparisons of clinical outcomes between males and females within each racial group (white, black and Hispanic). Online supplemental table 7 shows the comparison of clinical outcomes between blacks and Hispanics.
flgastro-2022-102113supp001.pdf (65.7KB, pdf)
flgastro-2022-102113supp002.pdf (115.4KB, pdf)
flgastro-2022-102113supp003.pdf (130.4KB, pdf)
flgastro-2022-102113supp004.pdf (47.1KB, pdf)
flgastro-2022-102113supp005.pdf (47.9KB, pdf)
flgastro-2022-102113supp006.pdf (46.7KB, pdf)
flgastro-2022-102113supp007.pdf (47.2KB, pdf)
Discussion
This study examines the effects of race and gender in patients with AIH using weighted analysis with propensity-matched comparisons. The results demonstrate that female patients have a lower tendency to experience hepatic complications, mortality and other AIH-related adverse events, including ascites, varices, variceal bleeding, spontaneous bacterial peritonitis, hepatorenal syndrome and encephalopathy in comparison to their male counterparts.
A nationwide cohort study in Denmark regarding AIH corroborates the current findings of a significantly higher rate of AIH-related deaths in male subjects.30 31 The higher risk of diagnosing AIH-related hepatocellular carcinoma in males may indicate that further gender-related comorbidities are playing a role in the mortality rate of male patients with AIH. While further evidence is required to explain the gender-specific differences in AIH outcomes, sex-related dissimilarities in immunogenetics, hypothalamic–pituitary–gonadal system, and sex hormones are postulated to interplay with autoimmune liver diseases.32 33 These factors can influence disease activity and progression.33 34 Additionally, physician biases in treatment plans may play a role in disease prognosis. For instance, female patients are generally more likely to be prescribed medications than male patients.35 As evident in our current study, these biased treatment strategies may potentially attenuate the risk of liver failure and other fulminant diseases in female AIH patients. Furthermore, female patients are more likely to attend designated appointments,36 37 which may also contribute to better disease control and reduce hepatic complications and liver failure.
When examining racial differences in AIH outcomes, we found that black and Hispanic patients suffer higher rates of hepatic complications, consistent with prior studies that suggest that minority populations with AIH experience more severe liver manifestations.38–40 This phenomenon can also be explained by differences in treatment response. Prior studies have demonstrated that minority populations experience variable treatment outcomes during pharmacological interventions due to racial differences in genetics and pharmacokinetics. Thus, physicians must consider these factors when making treatment plans to control AIH-induced hepatic inflammation.41 42 As observed in our study, other studies have shown that other variables may be related to prolonged hospitalisation including severity of disease at time of presentation and challenges with placement at the time of discharge.43
Besides causes related to disease progression, socioeconomic factors and general medical accessibility may have also contributed to racial health disparities.44 For instance, black patients experience limitations in insurance options and access to healthcare services compared with white patients. Furthermore, Hispanic patients and other minority racial groups may experience barriers due to health literacy and communication, which renders navigating through the hospital systems difficult.45 Additionally, social and cultural biases may undermine effective patient–provider rapport, resulting in delays in diagnosis and treatment.46–49 These inequalities in healthcare will likely lead to adverse outcomes in disease progression in both outpatient and inpatient settings.44 50
The current findings can be traced to symmetric disparities in outpatient approach and diagnostic workup. For instance, minority patients without access to primary care are more likely to have adverse AIH outcomes.44 To overcome this, accessibility to medical care needs to be improved among minority populations via increasing AIH awareness and disease recognition, as well as implementing adjunctive measures (ie, health coordinators, case managers) to promote health literacy for vulnerable patients in navigating medical systems.51 The burden of disease imposed on both patients and hospital systems can be curtailed by preventing the development of fulminant AIH disease through preventive or screening procedures. Given the differences in disease activity and progression from an inpatient perspective, diagnosis should not be delayed and there should be a lower threshold for starting immunosuppressive and other interventional therapies for AIH in minority patients. In particular, given the higher likelihood of dire complications observed in males and minority patients, there should be an earlier involvement of multidisciplinary services that can render various risk-appropriate levels of care.
Conclusion
Male patients experienced worse hospital outcomes, had higher rates of disease complications and higher hospital costs compared with female patients. Black and Hispanic patients experienced worse hospital outcomes, had higher rates of disease complications and higher hospital costs compared with white patients.
Footnotes
Contributors: DL: roles: conceptualisation, data curation, formal analysis, investigation, methodology, supervision, validation, writing-original draft, writing-review and editing, manuscript submission; JK: roles: methodology, writing-original draft, visualisation, writing-review and editing; CK, JH, GHF, DJ, EAA and KC: roles: writing-original draft, investigation; NHU: roles: supervision, writing-review and editing. DUL acted as the guarantor.
Funding: DL was supported by Grant Number 5 T32 DK 067872-17 from the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Li D, Friedman SL. Liver fibrogenesis and the role of hepatic stellate cells: new insights and prospects for therapy. J Gastroenterol Hepatol 1999;14:618–33. 10.1046/j.1440-1746.1999.01928.x [DOI] [PubMed] [Google Scholar]
- 2. Nakatani K, Seki S, Kawada N, et al. Expression of SPARC by activated hepatic stellate cells and its correlation with the stages of fibrogenesis in human chronic hepatitis. Virchows Arch 2002;441:466–74. 10.1007/s00428-002-0631-z [DOI] [PubMed] [Google Scholar]
- 3. Manns MP, Strassburg CP. Autoimmune hepatitis: clinical challenges. Gastroenterology 2001;120:1502–17. 10.1053/gast.2001.24227 [DOI] [PubMed] [Google Scholar]
- 4. Werner M, Prytz H, Ohlsson B, et al. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: a nationwide study. Scand J Gastroenterol 2008;43:1232–40. 10.1080/00365520802130183 [DOI] [PubMed] [Google Scholar]
- 5. Bosch J, García-Pagán JC. Complications of cirrhosis. I. portal hypertension. J Hepatol 2000;32:141–56. 10.1016/S0168-8278(00)80422-5 [DOI] [PubMed] [Google Scholar]
- 6. Heidelbaugh JJ, Sherbondy M. Cirrhosis and chronic liver failure: Part II. complications and treatment. Am Fam Physician 2006;74:767–76. [PubMed] [Google Scholar]
- 7. Nusrat S, Khan MS, Fazili J, et al. Cirrhosis and its complications: evidence based treatment. World J Gastroenterol 2014;20:5442. 10.3748/wjg.v20.i18.5442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benvegnù L, Gios M, Boccato S, et al. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut 2004;53:744–9. 10.1136/gut.2003.020263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Butterworth RF. Complications of cirrhosis III. hepatic encephalopathy. J Hepatol 2000;32:171–80. 10.1016/S0168-8278(00)80424-9 [DOI] [PubMed] [Google Scholar]
- 10. de Boer YS, Gerussi A, van den Brand FF, et al. Association Between Black Race and Presentation and Liver-Related Outcomes of Patients With Autoimmune Hepatitis. Clin Gastroenterol Hepatol 2019;17:1616–24. 10.1016/j.cgh.2018.11.028 [DOI] [PubMed] [Google Scholar]
- 11. Lim KN, Casanova RL, Boyer TD, et al. Autoimmune hepatitis in African Americans: presenting features and response to therapy. Am J Gastroenterol 2001;96:3390–4. 10.1111/j.1572-0241.2001.05272.x [DOI] [PubMed] [Google Scholar]
- 12. Verma S, Torbenson M, Thuluvath PJ. The impact of ethnicity on the natural history of autoimmune hepatitis. Hepatology 2007;46:1828–35. 10.1002/hep.21884 [DOI] [PubMed] [Google Scholar]
- 13. Lee DU, Fan GH, Ahern RR, et al. The effect of malnutrition on the infectious outcomes of hospitalized patients with cirrhosis: analysis of the 2011–2017 hospital data. Eur J Gastroenterol Hepatol 2021;32:269–78. 10.1097/MEG.0000000000001991 [DOI] [PubMed] [Google Scholar]
- 14. Lee DU, Fan GH, Hastie DJ, et al. The clinical impact of cirrhosis on the hospital outcomes of patients admitted with influenza infection: propensity score matched analysis of 2011-2017 us hospital data. J Clin Exp Hepatol 2021;11:531–43. 10.1016/j.jceh.2021.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Healthcare Cost and Utilization Project (HCUP) . 2017 introduction to the HCUP national inpatient sample (NIS. Rockville, MD: U.S. Agency for Healthcare Research and Quality, 2019. https://www.hcup-us.ahrq.gov/db/nation/nis/NISIntroduction2017.pdf [Google Scholar]
- 16. Healthcare Cost and Utilization Project (HCUP) . HCUP nationwide inpatient sample (NIS. Rockville: Agency for Healthcare Research and Quality, 2011. [PubMed] [Google Scholar]
- 17. Healthcare Cost and Utilization Project (HCUP) . Agency for healthcare research and qualityHCUP national inpatient sample (NIS) 2012-2017. Rockville, 2019. [Google Scholar]
- 18. Houchens R, Ross D, Elixhauser A. Nationwide inpatient sample (NIS) redesign final report. Rockville MD: U.S. Agency for Healthcare Research and Quality, 2014. https://www.hcup-us.ahrq.gov/reports/methods/2014-04.pdf [Google Scholar]
- 19. Elixhauser A, Heslin KC, Owens PL. Healthcare cost and utilization project (HCUP) recommendations for reporting trends using ICD-9-CM and ICD-10-CM/PCS data. U.S. Agency for Healthcare Research and Quality, 2017. https://www.hcup-us.ahrq.gov/datainnovations/HCUP_RecomForReportingTrends_070517.pdf [Google Scholar]
- 20. Centers for Medicare and Medicaid Services (CMS) . 2017 ICD-10 PCS General equivalence mappings (GEMs) – procedure codes, 2016. Available: https://www.cms.gov/Medicare/Coding/ICD10/Downloads/2017-GEM-PCS.zip [Accessed 17 Aug 2020].
- 21. Centers for Medicare and Medicaid Services (CMS) . 2017 ICD-10-CM General equivalence mappings (GEMs) – diagnosis codes, 2016. Available: https://www.cms.gov/Medicare/Coding/ICD10/Downloads/2017-GEM-DC.zip [Accessed 17 Aug 2020].
- 22. Fung KW, Richesson R, Smerek M, et al. Preparing for the ICD-10-CM transition: automated methods for translating ICD codes in clinical phenotype definitions. EGEMS 2016;4:4. 10.13063/2327-9214.1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agency for Healthcare Research and Quality, Rockville . HCUP NIS trend weights. healthcare cost and utilization project (HCUP), 2021. Available: www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp
- 24. Agency for Healthcare Research and Quality, Rockville . Producing National HCUP Estimates - Accessible Version. Healthcare Cost and Utilization Project (HCUP). Available: www.hcup-us.ahrq.gov/tech_assist/nationalestimates/508_course/508course_2018.jsp
- 25. Zhang Z. Multiple imputation with multivariate imputation by chained equation (mice) package. Ann Transl Med 2016;4:30. 10.3978/j.issn.2305-5839.2015.12.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu Y, Han H, Shah G, et al. Significant nationwide variability in the costs and hospital mortality rates of autologous stem cell transplantation for multiple myeloma: an analysis of the nationwide inpatient sample database. Biol Blood Marrow Transplant 2019;25:41–6. 10.1016/j.bbmt.2018.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Houchens R. Missing data methods for the NIS and the SID. HCUP methods series, 2015. Available: http://www.hcup-us.ahrq.gov/reports/methods/methods.jsp
- 28. Thadewald T, Büning H. Jarque–Bera test and its competitors for testing normality – a power comparison. J Appl Stat 2007;34:87–105. 10.1080/02664760600994539 [DOI] [Google Scholar]
- 29. Jarque CM, Bera AK. A test for normality of observations and regression residuals. Int Stat Rev 1987;55:163–72. 10.2307/1403192 [DOI] [Google Scholar]
- 30. Yang F, Wang Q, Bian Z, et al. Autoimmune hepatitis: East meets West. J Gastroenterol Hepatol 2015;30:1230–6. 10.1111/jgh.12952 [DOI] [PubMed] [Google Scholar]
- 31. Grønbæk L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol 2014;60:612–7. 10.1016/j.jhep.2013.10.020 [DOI] [PubMed] [Google Scholar]
- 32. Guy J, Peters MG. Liver disease in women: the influence of gender on epidemiology, natural history, and patient outcomes. Gastroenterol Hepatol 2013;9:633–9. [PMC free article] [PubMed] [Google Scholar]
- 33. Al-Chalabi T, Underhill JA, Portmann BC, et al. Impact of gender on the long-term outcome and survival of patients with autoimmune hepatitis. J Hepatol 2008;48:140–7. 10.1016/j.jhep.2007.08.013 [DOI] [PubMed] [Google Scholar]
- 34. Berczi I. The influence of pituitary-adrenal axis on the immune system. Pituitary Function and Immunity 2019:49–132. [Google Scholar]
- 35. Manteuffel M, Williams S, Chen W, et al. Influence of patient sex and gender on medication use, adherence, and prescribing alignment with guidelines. J Womens Health 2014;23:112–9. 10.1089/jwh.2012.3972 [DOI] [PubMed] [Google Scholar]
- 36. Davies M, Goffman R, May J, et al. Large-Scale No-Show patterns and distributions for clinic operational research. Health Care 2016;4:15. 10.3390/healthcare4010015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sninsky BC, Nakada SY, Penniston KL. Does socioeconomic status, age, or gender influence appointment attendance and completion of 24-hour urine collections? Urology 2015;85:568–73. 10.1016/j.urology.2014.10.043 [DOI] [PubMed] [Google Scholar]
- 38. Nguyen GC, Thuluvath PJ. Racial disparity in liver disease: biological, cultural, or socioeconomic factors. Hepatology 2008;47:1058–66. 10.1002/hep.22223 [DOI] [PubMed] [Google Scholar]
- 39. Czaja AJ. Autoimmune hepatitis in diverse ethnic populations and geographical regions. Expert Rev Gastroenterol Hepatol 2013;7:365–85. 10.1586/egh.13.21 [DOI] [PubMed] [Google Scholar]
- 40. Wong RJ, Gish R, Frederick T, et al. The impact of race/ethnicity on the clinical epidemiology of autoimmune hepatitis. J Clin Gastroenterol 2012;46:155–61. 10.1097/MCG.0b013e318228b781 [DOI] [PubMed] [Google Scholar]
- 41. Czaja AJ, Bianchi FB, Carpenter HA, et al. Treatment challenges and investigational opportunities in autoimmune hepatitis. Hepatology 2005;41:207–15. 10.1002/hep.20539 [DOI] [PubMed] [Google Scholar]
- 42. Burroughs VJ, Maxey RW, Levy RA. Racial and ethnic differences in response to medicines: towards individualized pharmaceutical treatment. J Natl Med Assoc 2002;94:1–26. [PMC free article] [PubMed] [Google Scholar]
- 43. Ghosh AK, Geisler BP, Ibrahim S. Racial/Ethnic and socioeconomic variations in hospital length of stay: a state-based analysis. Medicine 2021;100:e25976. 10.1097/MD.0000000000025976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim D, Eshtiaghpour D, Alpern J, et al. Access to primary care is associated with better autoimmune hepatitis outcomes in an urban County Hospital. BMC Gastroenterol 2015;15:91. 10.1186/s12876-015-0318-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. White S, Bennett I, Cordell T. Health Literacy of America’s Adults: Results from the 2003 National Assessment of Adult Literacy. PsycEXTRA Dataset, 2007. [Google Scholar]
- 46. Weinick RM, Zuvekas SH, Cohen JW. Racial and ethnic differences in access to and use of health care services, 1977 to 1996. Med Care Res Rev 2000;57 Suppl 1:36–54. 10.1177/1077558700057001S03 [DOI] [PubMed] [Google Scholar]
- 47. Hammond WP, Matthews D, Mohottige D, et al. Masculinity, medical mistrust, and preventive health services delays among community-dwelling African-American men. J Gen Intern Med 2010;25:1300–8. 10.1007/s11606-010-1481-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zolfino T, Heneghan MA, Norris S, et al. Characteristics of autoimmune hepatitis in patients who are not of European Caucasoid ethnic origin. Gut 2002;50:713–7. 10.1136/gut.50.5.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mullins CD, Blatt L, Gbarayor CM, et al. Health disparities: a barrier to high-quality care. Am J Health Syst Pharm 2005;62:1873–82. 10.2146/ajhp050064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wen JW, Kohn MA, Wong R, et al. Hospitalizations for autoimmune hepatitis disproportionately affect black and Latino Americans. Am J Gastroenterol 2018;113:243–53. 10.1038/ajg.2017.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Isoda H, Eguchi Y, Takahashi H. Hepatitis medical care coordinators: comprehensive and seamless support for patients with hepatitis. Glob Health Med 2021;3:343–50. 10.35772/ghm.2021.01073 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
flgastro-2022-102113supp001.pdf (65.7KB, pdf)
flgastro-2022-102113supp002.pdf (115.4KB, pdf)
flgastro-2022-102113supp003.pdf (130.4KB, pdf)
flgastro-2022-102113supp004.pdf (47.1KB, pdf)
flgastro-2022-102113supp005.pdf (47.9KB, pdf)
flgastro-2022-102113supp006.pdf (46.7KB, pdf)
flgastro-2022-102113supp007.pdf (47.2KB, pdf)
Data Availability Statement
Data are available on reasonable request.