ABSTRACT
Chlamydia is an obligate intracellular pathogen with a highly reduced genome devoid of major stress response genes like relA and spoT, which mediate the stringent response. Interestingly, as an intracellular bacterium dependent on its host for nutrients and as a tryptophan (Trp) auxotroph, Chlamydia is very sensitive to Trp starvation, which is induced in vivo by the host cytokine interferon-γ. In response to Trp starvation, Chlamydia enters a viable but nonreplicating state called persistence. A major characteristic of chlamydial persistence is a block in cell division. We hypothesized that cell division is blocked during persistence by the inability to translate Trp-rich cell division proteins. To test this, we first investigated the translation of various cell division proteins under Trp starvation conditions using inducible expression strains. We observed that the Trp-poor protein MurG and the Trp-neutral protein FtsL were still expressed during persistence, while the expression of the Trp-rich proteins Pbp2, RodA, FtsI/Pbp3, and MraY was significantly reduced. As proof of concept for our hypothesis, we compared expression of a wild-type and mutant isoform of RodZ in which its four Trp codons were mutated. These experiments demonstrated that decreased expression of RodZ during persistence was reversed when no Trp was present in the protein, thus directly linking its expression to its Trp content. Together, these experiments indicate that specific cell division proteins are not produced during persistence. For the first time, our data provide a mechanism that explains the inhibition of cell division during chlamydial persistence mediated by Trp starvation.
KEYWORDS: Chlamydia, tryptophan, persistence, persister, cell division, peptidoglycan
INTRODUCTION
To survive adverse conditions, a common bacterial response is to enter an alternative growth mode or survival state called persistence. Persistence has been historically defined as the phenotypic resistance of a subset of the population to a bactericidal drug concentration (1). Persistence was initially characterized for free-living bacteria exposed to antibiotics but is now appreciated as a common stress response to a variety of environmental stressors. During persistence, a pathogen is viable, but quiescent, where it can survive for long periods of time until the inducing stress is removed. In parallel to the growing appreciation for understanding persister populations of bacteria in model systems, there has been an ongoing interest in understanding how intracellular pathogenic bacteria persist within their host cells. In this regard, the ability of the obligate intracellular bacterium Chlamydia to persist within its host cell has been described and studied for almost 30 years, yet a mechanistic understanding of how Chlamydia persists is lacking.
The Gram-negative bacteria from the Chlamydiaceae family are pathogens for humans and animals that undergo a biphasic developmental cycle within a host cell. During its developmental cycle, Chlamydia alternates between two distinct morphological forms: a small extracellular, metabolically quiescent, infectious form called the elementary body (EB), and a larger intracellular replicative form called the reticulate body (RB) (2–5). Briefly, following initial attachment, EBs are internalized into a vesicle of the host cell through endocytosis or phagocytosis where they undergo primary differentiation into replicative RBs. The RBs reside in a Chlamydia-containing vacuole called the inclusion whose maturation is necessary for chlamydial growth. Inclusion maturation comprises blocking lysosome or autophagosome fusion with the inclusion and redirecting host nutrients to the inclusion. Inside the inclusion, Chlamydia is protected from osmotic stress and the host immune system via the incorporation in the inclusion membrane of host lipids and secreted bacterial effector proteins that mimic host proteins (6). Chlamydia then proceeds through several cycles of division by a unique MreB-dependent polarized budding mechanism wherein a peptidoglycan (PG) ring is synthesized exclusively at the division septum localized initially on one side of the mother cell (7–11). Subsequently, RBs undergo secondary differentiation to form new EBs, which egress via host cell lysis or by an inclusion extrusion mechanism (5, 12).
The host immune response to Chlamydia infection results in the production of the cytokine interferon-γ (IFN-γ). After binding to its receptor, IFN-γ activates multiple responses, including the production of indoleamine 2,3-dioxygenase (IDO) by the host cell (13). IDO then catabolizes tryptophan (Trp) to the N′-formylkynurenine metabolite resulting in Trp starvation (13, 14). Because Chlamydia is auxotrophic for Trp, the response to the low Trp environment is the development of noninfectious, nondividing atypical chlamydial forms within smaller inclusions (15, 16). Hence, IFN-γ is commonly used to trigger persistence of Chlamydia in cell culture models (15, 17–23). Most eubacteria experiencing amino acid starvation will enact the stringent response, which can signal entry into a persistent state (24, 25). The stringent response is a global regulatory response to amino acid starvation. When an uncharged tRNA binds in the A site of the ribosome, the ribosome-associated protein RelA is activated to synthesize the guanosine pentaphosphate or guanosine tetraphosphate signaling molecule (abbreviated [p]ppGpp). The accumulation of ppGpp in the bacterial cytoplasm alters the expression of genes involved in amino acid biosynthesis and proteolysis. Because of its adaptation to the intracellular niche, Chlamydia has reduced its genome size and eliminated stress response elements such as toxin-antitoxin systems and the stringent response (19, 26).
In Chlamydia, persistence is defined as a viable but noninfectious reversible state of chlamydial development that is characterized by morphologically aberrant, enlarged RBs (aRB, size of ≈2 to 10 μm), lack of cell division, and severe reduction/elimination in progeny production (27). However, the mechanisms underlying chlamydial entry into persistence and resulting morphological forms are still poorly understood. It was demonstrated that Chlamydia responds to IFN-γ-mediated Trp starvation by a global increase of transcription and a decrease in translation (19). Specifically, the transcription of genes containing Trp codons was generally increased in response to IFN-γ treatment whereas those transcripts containing no or few Trp codons were generally unchanged (21). We further demonstrated that this may be a conserved mechanism among Trp auxotrophs in response to Trp starvation (21, 23). In addition, we observed a measurable decrease in transcript abundance at the 3′ ends of larger transcripts, creating a skew between the 5′ and 3′ ends, and this was linked to Trp codon content and Rho-mediated transcriptional polarity (22). Overall, these findings raise the question of how chlamydial growth is blocked in response to Trp starvation. One possible explanation is the inefficient translation of critical components within distinct pathways that are necessary for normal developmental cycle progression.
Given the enlarged size of persistent chlamydial forms associated with a block in cell division, we wanted to understand how division is inhibited during persistence elicited by Trp starvation. A recent study demonstrated the lack of PG in persistent chlamydial forms (28), which was expected given its function in division specifically (8, 9), but it did not reveal why PG is not made. In the C. trachomatis serovar L2 genome, 34 genes encode proteins with multiple Trp codons (WW or WWW; see Table S1 in the supplemental material) that are likely to stall translation complexes during Trp starvation, as has been characterized for the Trp leader peptide in E. coli (29). These proteins belong to multiple functional groups, including chlamydial cell division and PG synthesis, with specific proteins enriched in Trp compared to the overall proteome of Chlamydia, as noted in a recent bioinformatics study (30). We recently demonstrated that the prokaryotic tryptophanyl-tRNA synthetase inhibitor called indolmycin (31), which acts as a Trp analog, is an alternative way to deplete Trp and induce chlamydial persistence when combined with a Trp-depleted media, such as IFN-γ-conditioned medium (ICM) (32). The use of indolmycin, combined with the development of genetic tools for Chlamydia, has opened new avenues to mechanistically study chlamydial persistence. For example, we and colleagues recently demonstrated that Trp starvation prevents the translation of the YtgCR iron-dependent transcriptional regulator, which encodes a WWW motif (29).
We hypothesize that chlamydial cell division is blocked during Trp starvation-mediated persistence by the inability to translate Trp-rich cell division and PG synthesis proteins. To examine this, we performed a series of experiments to evaluate the expression of selected genes during normal growth and during Trp starvation conditions. We measured transcripts for genes encoding the large Trp-rich proteins FtsI/Pbp3, Pbp2, and MraY as well as Trp-poor proteins like MreB and MurG during Trp starvation but found that transcriptional changes could not explain why cell division is blocked during Trp starvation. By immunofluorescence, we examined the expression of various proteins during indolmycin-mediated persistence using a collection of inducible expression strains. During these experiments, we found that the Trp-poor protein MurG and the Trp-neutral protein FtsL were still expressed during persistence while the expression of the Trp-enriched proteins Pbp2, RodA, FtsI/Pbp3, and MraY was significantly reduced. To determine whether the Trp codon content of a target gene leads to these effects, we compared the expression of wild-type RodZ and the hypothetical CTL0293/Ct038 proteins containing WW motifs to isoforms lacking Trp codons and revealed that their Trp content significantly affected their expression during persistence induced by Trp starvation. Finally, we leveraged our recent observation that the cardiolipin synthase, Cls, demarcates the polar site of division to determine whether Trp starvation negatively impacts the overall polarity of chlamydial aberrant forms (33). We show that the Cls protein maintained a polarized or punctate distribution during persistence. This suggests that the organization of the different cellular components of Chlamydia is not completely lost during persistence, which could allow a rapid reactivation of chlamydial growth when Trp is again available to synthesize cell division proteins. Overall, our data provide a mechanistic explanation for how cell division is inhibited in Chlamydia during Trp starvation conditions.
RESULTS
Trp content of C. trachomatis L2 division and PG synthesis proteins.
We hypothesize that cell division is blocked during persistence by the inability to translate Trp-rich cell division proteins. To begin testing our hypothesis, we bioinformatically examined the Trp content of chlamydial cell division and peptidoglycan (PG)-related proteins in comparison to model Gram-positive and Gram-negative organisms. In Chlamydia trachomatis serovar L2, the proteome average of Trp is 0.94% (23), and about 20% of the proteins lack Trp. The number of Trp codons for cell division and PG synthesis proteins, percentage of Trp, and the presence of a Trp motif for C. trachomatis L2, Escherichia coli K-12, and Bacillus subtilis 168 are shown in Table 1. Two chlamydial proteins lack Trp codons, the rod-shape-determining protein MreB and the UDP-N-acetylenolpyruvoylglucosamine reductase MurB. Eight proteins have a lower percentage of Trp than the C. trachomatis L2 proteome, including the N-acetylmuramoyl alanine amidase AmiA, the PG transferase MurG, and the PG ligase MurE, which have only one Trp codon (Table 1). On the contrary, the cytoskeletal protein RodZ, the PG transferase MraY, and the putative Pbp2 chaperone and transglycosylase RodA have a higher percentage of Trp than the C. trachomatis L2 average proteome (2.8%, 2.4%, and 2.1%, respectively), and their sequence is further enriched in Trp by the presence of a WW motif (or WAW for RodA) (Table 1). Because of its short sequence of 95 amino acids, the percentage of Trp of the cell division protein FtsL is higher than the average proteome while having only one Trp codon. For the PG synthetase FtsI/Pbp3, its percentage of Trp is 0.9%, but the FtsI/Pbp3 sequence contains a WW motif. The percentage of Trp in some cell division proteins is higher than the average chlamydial proteome, and some of them are further enriched in Trp with a WW or a WWW motif (Table S1 and Table 1). Therefore, Trp residues are significantly more abundant in some cell division proteins than the rest of the C. trachomatis L2 proteome. Homologues of these proteins are present in the model organisms E. coli and B. subtilis. Of note, the Trp codon is encoded by the GC-rich sequence UGG, suggesting that genomes with a higher G+C percentage should have a higher probability of Trp codons. The genome of E. coli K-12 has a higher G+C percentage than the C. trachomatis L2 genome with an average Trp content of 1.52%, but only the proteins Pbp2 and RodZ contain a WW motif. The genome of B. subtilis 168 has a G+C percentage similar to the Chlamydia genome with an average Trp content of 1.02%, but the percentage of Trp in the B. subtilis homologues of chlamydial cell division and PG synthesis proteins is, in general, lower than C. trachomatis homologues. Furthermore, none of the B. subtilis homologues contain a WW motif (Table 1). This suggests that some C. trachomatis L2 cell division genes encode proteins enriched in Trp residues.
TABLE 1.
Tryptophan content in division and peptidoglycan synthesis proteins in Chlamydia trachomatis L2, Escherichia coli K-12, and Bacillus subtilis 168a
| Functional group | Common protein name | Chlamydia trachomatis L2/434/Bu | Presence in an operonb | Tryptophan codons | Tryptophan motif | Percentage of tryptophans | Percentage of G+C |
Escherichia coli K-12 |
Bacillus subtilis 168c |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentage of Tryptophans | Percentage of G+C | Percentage of tryptophans | Percentage of G+C | ||||||||
| Division proteins | |||||||||||
| FtsI/Pbp3 | CTL0522 | Yes, end of the operon | 6 | WW | 0.9% | 40.1% | 1.0% | 54.0% | 1.6% | 42.1% | |
| FtsK | CTL0108 | Yes, start of the operon | 7 | No | 0.9% | 41.9% | 1.4% | 54.0% | 0.8% | 47.3% | |
| FtsL | CTL0523 | Yes, start of the operon | 1 | No | 1.1% | 36.1% | 0.8% | 50.3% | 0% | 42.1% | |
| FtsQ | CTL0133 | No | 2 | No | 0.7% | 40.9% | 2.5% | 53.3% | 0.8% | 37.4% | |
| FtsW | CTL0129 | Yes, middle of the operon | 4 | No | 1% | 40.0% | 2.4% | 53.0% | 0.2% | 42.8% | |
| MreB | CTL0078 | Yes, middle of the operon | 0 | No | 0% | 45.1% | 0.0% | 54.3% | 0.3% | 47.1% | |
| MreC | CTL0006 | Yes, end of the operon | 6 | No | 1.8% | 41.6% | 0.3% | 56.0% | 0.7% | 42.4% | |
| Pbp2 | CTL0051 | Yes, start of the operon | 13 | No | 1.2% | 42.6% | WW, 1.6% | 52.5% | 0.7% | 39.9% | |
| RodA | CTL0095 | No | 8 | WAW | 2.1% | 42.4% | 2.2% | 52.0% | 1.3% | 37.7% | |
| RodZ | CTL0264 | No | 4 | WW | 2.8% | 40.0% | WWW, 1.8% | 54.5% | 0.3% | 39.9% | |
| Peptidoglycan synthesis proteins | AmiA | CTL0520 | Yes, end of the operon | 1 | No | 0.4% | 40.1% | 0.3% | 49.8% | 0.7% | 39.2% |
| MraY | CTL0126 | Yes, start of the operon | 8 | WW | 2.4% | 39.2% | 2.8% | 53.4% | 1.9% | 42.8% | |
| MraW | CTL0524 | Yes, start of the operon | 3 | No | 1% | 41.1% | 1.0% | 55.2% | 0.3% | 45.7% | |
| MurA | CTL0715 | No | 5 | No | 1.1% | 43.8% | 0.5% | 53.3% | 0% | 50.2%d | |
| MurB | CTL0203 | No | 0 | No | 0% | 42.0% | 2.0% | 46.0% | 0.7% | 50.2%d | |
| MurC/DdlA | CTL0131 | Yes, end of the operon | 7 | No | 0.9% | 39.9% | 2.0% | 54.7% | 0% | 44.0% | |
| MurD | CTL0127 | Yes, midddle of the operon | 2 | No | 0.5% | 42.1% | 0.7% | 54.9% | 0.7% | 45.1% | |
| MurE | CTL0521 | Yes, end of the operon | 1 | No | 0.2% | 39.6% | 1.4% | 56.6% | 0% | 46.1% | |
| MurF | CTL0125 | No | 4 | No | 0.9% | 43.5% | 1.1% | 55.1% | 0.2% | 47.0% | |
| MurG | CTL0130 | Yes, midddle of the operon | 1 | No | 0.3% | 40.9% | 2.5% | 56.1% | 0% | 44.0% | |
| MurJ | CTL0888 | No | 9 | No | 1.7% | 40.8% | 1.8% | 53.7% | 0.6% | 45.6% | |
Tryptophan motifs are indicated in boldface.
Based on genomic context.
No B. subtilis proteins contain the WW motif.
murA and murB are within the murAB operon in Bacillus subtilis 168.
Among several chlamydial species, the Trp number can vary for cell division and PG synthesis proteins (Table S2). The WW motif (WAW for RodA) is conserved among species for FtsI/Pbp3, RodA, and RodZ. RodZ contains two WW motifs in C. pneumoniae CWL029, C. psittaci 6BC, and C. caviae GPIC. Interestingly, the WW motif of MraY seems to be restricted to C. trachomatis species (Table S2). In summary, FtsI/Pbp3, RodA, RodZ, and MraY contain a WW motif (WAW for RodA) and, except for MraY, that motif is conserved among chlamydial species. Among the analyzed species, these observations suggest that, in some instances, Chlamydia spp. may have specifically selected for an enriched Trp content in selected division-associated genes.
Indolmycin induces morphological and transcriptional changes in C. trachomatis L2 consistent with persistence.
Trp limitation mediated by IFN-γ or the tryptophanyl-tRNA synthetase inhibitor indolmycin was demonstrated to trigger chlamydial persistence (15, 32). To validate that indolmycin induces changes consistent with persistence in our studies, HEp-2 cells were infected with C. trachomatis L2 at a multiplicity of infection (MOI) of 2. At 10 h postinfection (hpi), the cells were starved for Trp by the addition of indolmycin in interferon-γ conditioned medium (ICM; filtered culture medium harvested from HEp-2 cells treated with 2 ng/mL IFN-γ). A previous study from our lab has established that the combination of indolmycin and a Trp-depleted medium like ICM is the most robust and consistent method to mimic IFN-γ-induced persistence (32). Indolmycin or Trp-depleted medium alone is not sufficient to effectively induce persistence in Chlamydia. To verify our treatment conditions, coverslips were fixed to monitor chlamydial morphology at 10 hpi and at 24 hpi with or without indolmycin. As expected, prolonged indolmycin treatment results in morphological changes characteristic of chlamydial persistence: aberrant organisms in smaller inclusions (Fig. 1).
FIG 1.

Indolmycin induces morphological changes in C. trachomatis L2. Treatment with indolmycin in IFN-γ-conditioned medium (ICM) results in C. trachomatis L2 persistence. Representative images of HEp-2 cells infected with C. trachomatis L2 and treated or not as indicated and outlined by the diagram are shown. Cells were fixed at 10 hpi or 24 hpi and stained using primary antibodies to the major outer membrane protein (MOMP; green) followed by the appropriate secondary antibody. Nuclear DNA was stained with DAPI (blue). All images were acquired on a Zeiss AxioImager.Z2 equipped with an Apotome2 using a 100× lens objective. Scale bar = 2 μm Treatment of 120 μM indolmycin given at 10 hpi resulted in smaller inclusions and morphologically aberrant organisms.
One hypothesis for why cell division is blocked during persistence is that division-associated genes are transcriptionally downregulated. Therefore, we next examined the transcript levels of selected cell division and PG synthesis genes using RT-qPCR. We also measured transcript levels at the 5′ and 3′ ends of larger genes (and 5′ or 3′ to WW motifs where possible) since we had previously observed that Trp starvation can destabilize the 3′ ends of larger transcripts due to Rho-mediated transcriptional polarity (22). We collected RNA and DNA samples from four independent experiments at two time points (10 hpi and 24 hpi). As chlamydial persistent forms arise from RBs, the most accurate comparison is between 10 hpi untreated/Trp-replete cultures (RB-only) to 24 hpi Trp-starved cultures (aRB-only), which is consistent with the methodology established in previous studies (21–23) (Table S3). At 24 hpi, Chlamydia undergoing normal development will exhibit a mix of EB and RB morphology. Therefore, a direct comparison between untreated and Trp-starved cultures at 24 hpi is inexact and not informative.
We observed that the Trp codon-rich genes ftsI/pbp3, pbp2, and mraY displayed a destabilization at their 3′ ends in the presence of indolmycin, whereas the Trp-codon poor genes murG and mreB did not, consistent with our prior observations (Fig. S1 and 2) (21). Interestingly, ftsK (with 7W codons) did not display any destabilization, but a prior study has characterized an antisense small RNA at the 3′ end of this gene (34), which may affect our analysis. Transcripts for rodA were highly variable, but we note the presence of a convergently transcribed gene that may contribute to this (Fig. S2). The gene birA is oriented tail-to-tail with rodA on the opposite strand, and we measured birA 3′ transcripts increased with some variability during persistence, which could explain the rodA transcript effects (Fig. S2). Consistent with previous studies that induced persistence with IFN-γ (21), we observed that rodZ transcript levels (of note, using a primer set 5′ to a WW motif) were increased during indolmycin-mediated Trp starvation (Fig. S2). The transcript level of ftsL, a Trp codon-neutral gene in an operon 5′ to ftsI/pbp3, remained constant during indolmycin-mediated persistence (Fig. S2). Importantly, no cell division or PG synthesis gene studied displayed a significant decrease in transcription during persistence compared to the RB control (10 hpi) (Table S3). These transcript data indicate that, contrary to the hypothesis that division-associated genes are transcriptionally downregulated, a specific regulon to reduce transcription of division genes to abrogate cell division is not enacted in Chlamydia. Therefore, transcriptional changes cannot explain why cell division is blocked during persistence.
Tryptophan limitation inhibits expression of Pbp2_6×H, RodA_6×H, FtsI/Pbp3_6×H, and MraY_6×H but not expression of FtsL_flag and MurG_6×H.
As changes in transcription of cell division and PG synthesis genes did not explain why cell division is blocked, we next leveraged genetic tools in Chlamydia to further investigate the effect of Trp starvation on the expression of chlamydial cell division and PG synthesis proteins. We generated C. trachomatis L2 transformants that ectopically expressed recombinant C-terminally 6×His- or flag-tagged Pbp2, RodA, FtsL, FtsI/Pbp3, MraY, and MurG from anhydrotetracycline (aTc)-inducible vectors. To determine whether the overexpression of Pbp2_6×H, RodA_6×H, FtsL_flag, FtsI/Pbp3_6×H, MraY_6×H, and MurG_6×H impacted chlamydial growth, we measured inclusion-forming units (IFUs) in the presence and absence of aTc. We chose to perform induction with aTc at the same time point (12 hpi) and doses (1 or 2 nM aTc) as in the persistence experiments in 24-well plates for subsequent immunofluorescence analysis. Under these conditions, the overexpression of Pbp2_6×H, RodA_6×H, FtsL_flag, FtsI/Pbp3_6×H, MraY_6×H, and MurG_6×H did not significantly reduce the generation of IFUs (Fig. S3).
Pbp2 (13 W), RodA (8 W), and MraY (8 W) are enriched for Trp codons relative to the chlamydial proteome (1.2, 2.1, and 2.4%, respectively, versus 0.94%), while FtsL is a Trp-neutral and MurG is a Trp-poor protein with 1 Trp. FtsI/Pbp3 and MraY encode a WW motif, and RodA encodes a WAW motif. We hypothesized that the Trp content of these cell division and PG synthesis proteins may affect their expression during persistence mediated by Trp starvation induced by indolmycin treatment. To test the Trp dependency of their expression, we used immunofluorescence microscopy, where we could easily detect a specific 6×His or flag signal after a period of induction with aTc, in comparison to the control signal for the major outer membrane protein (MOMP). A previous study has determined that immunofluorescence microscopy is more suitable than Western blot to study chlamydial persistence given the low biomass of Chlamydia in the indolmycin-treated samples, which can lead to the failure of protein detection via Western blot during indolmycin-mediated persistence, even if the protein is tagged (29). Immunofluorescence analysis of replicate experiments and quantification of the mean fluorescence intensity (MFI) per area of inclusion for 150 inclusions per condition revealed distinct effects on gene expression upon Trp starvation (Fig. 2 to 4). HEp-2 cells infected with each transformant were starved or not for Trp prior to an aTc induction period. Individual measurements for 6×His or flag signals as well as the MOMP signal were quantified and graphed from each condition. We noted that for all samples induced with aTc in the absence of indolmycin treatment (i.e., untreated (UTD)), expression of Pbp2_6×H, RodA_6×H, FtsL_flag, FtsI/Pbp3_6×H, MraY_6×H, and MurG_6×H were easily detected (Fig. 2 to 4A and C). As for wild-type C. trachomatis L2 (Fig. 1), we observed that prolonged treatment with indolmycin in ICM is effective at inducing persistent Chlamydia in the transformant strains, observed as smaller inclusions containing aberrant organisms (Fig. 2 to 4A and C). We observed that Trp depletion significantly and dramatically reduced the expression of Pbp2_6×H and RodA_6×H (Fig. 2B and D) as well as FtsI/Pbp3_6×H (Fig. 3B) and MraY_6×H expression (Fig. 4B) under inducing conditions. FtsL_Flag expression was slightly decreased, and no change in relative intensity for MurG_6×H expression was detected. The MOMP signal was generally unaffected by Trp starvation for most of the transformants (Fig. 2 to 4B and D). Exceptions to this included the Pbp2_6×H strain, which showed slightly elevated MOMP intensities (Fig. 2B), and the FtsL_Flag strain, which showed slightly decreased MOMP intensities (Fig. 3B). Although both were statistically significant, they represented less than a 25% change from the control. To further assess the target protein, the signal intensity from the MOMP channel was used as a control to normalize the MFI per area of inclusion values quantified for the 6×His or flag channel. After normalization, we still observed a significant inhibition of expression of the Trp codon-rich proteins Pbp2_6×H and RodA_6×H (Fig. 3B and D), FtsI/Pbp3_6×H (Fig. 4D), and MraY_6×H (Fig. 5B) during persistence mediated by indolmycin treatment. However, for FtsL_flag (Fig. 4B) and MurG_6×H (Fig. 5D), which have 1 Trp, expression is unaffected during indolmycin-mediated persistence. Combined, these data demonstrate that during persistence, expression of cell division and PG proteins correlates with the amount of Trp within the protein sequence.
FIG 2.

Pbp2_6×H and RodA_6×H expression is inhibited by persistence mediated by indolmycin in ICM. HEp-2 cells were infected at an MOI of 1 and treated or not as indicated in the diagram. 2 nM aTc was added to induce Pbp2_6×H and RodA_6×H expression. (A and C) Samples were fixed at 20 hpi and processed for immunofluorescence to detect 6×His (red), MOMP (green), and DNA (blue). Scale bar = 2 μm. (B and D) Immunofluorescence images were quantified using Fiji/ImageJ software to determine mean fluorescence intensity (MFI) of MOMP or epitope-tagged protein per area of inclusion and results were graphed and statistically analyzed using GraphPad Prism. Horizontal lines indicate mean and vertical lines indicate standard deviation. Statistical significance was determined by unpaired Student's t test. ns, nonsignificant; ****, P < 0.0001. Data are also expressed as average 6×His/MOMP ratios for both conditions with standard deviation between three independent experiments. Statistical significance was determined by unpaired Student's t test. ****, P < 0.0001.
FIG 4.

Expression of the WW-containing protein MraY_6×H is inhibited by indolmycin-mediated persistence while the expression of the Trp-poor protein MurG_6×H is maintained. A diagram representing the experimental design and highlighting important time points is shown. HEp-2 cells were infected at an MOI of 1. Two or 1 nM aTc was added to induce MraY_6×H and MurG_6×H expression, respectively. (A and C) Samples were fixed at 20 hpi and processed for immunofluorescence to detect 6×His (red), MOMP (green), and DNA (blue). Scale bar = 2 μm. (B and D) Immunofluorescent images were quantified using Fiji/ImageJ software to determine MFI of MOMP or epitope-tagged protein per area of inclusion and results were graphed and statistically analyzed using GraphPad Prism. Horizontal lines indicate mean and vertical lines indicate standard deviation. Statistical significance was determined by unpaired Student's t test. ns, nonsignificant; ****, P < 0.0001. Data are also expressed as average 6×His/MOMP ratios for both conditions with standard deviation between three independent experiments. Statistical significance was determined by unpaired Student's t test. ns, nonsignificant; ****, P < 0.0001.
FIG 3.
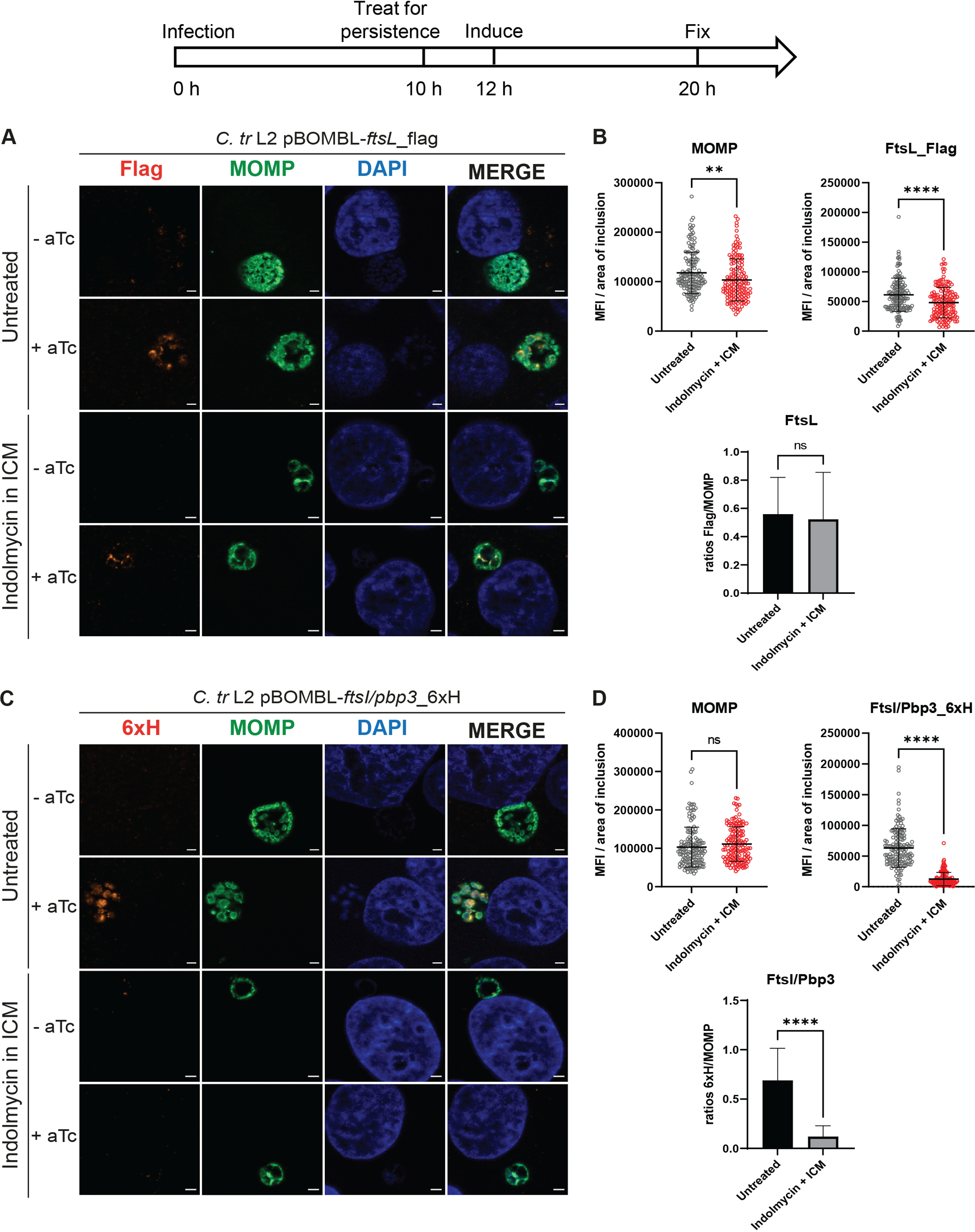
FtsL_flag expression is unaffected by persistence mediated by indolmycin in ICM while FtsI/Pbp3_6×H expression is inhibited. A diagram representing the experimental design and highlighting important time points is shown. HEp-2 cells were infected at an MOI of 1. Two or 1 nM aTc was added to induce FtsL_flag and FtsI/Pbp3_6×H expression, respectively. (A and C) Samples were fixed at 20 hpi and processed for immunofluorescence to detect flag (FtsL construct; red), 6×His (FtsI/Pbp3 construct, red), MOMP (green), and DNA (blue). Scalebar = 2 μm. (B and D) Immunofluorescence images were quantified using Fiji/ImageJ software to determine MFI of MOMP or epitope-tagged protein per area of inclusion and results were graphed and statistically analyzed using GraphPad Prism. Horizontal lines indicate mean and vertical lines indicate standard deviation. Statistical significance was determined by unpaired Student's t test. ns, nonsignificant; **, P < 0.01; ****, P < 0.0001. Data are also expressed as average flag/MOMP and 6×His/MOMP ratios for both conditions with standard deviation between three independent experiments. Statistical significance was determined by unpaired Student's t test. ns, nonsignificant; ****, P < 0.0001.
FIG 5.
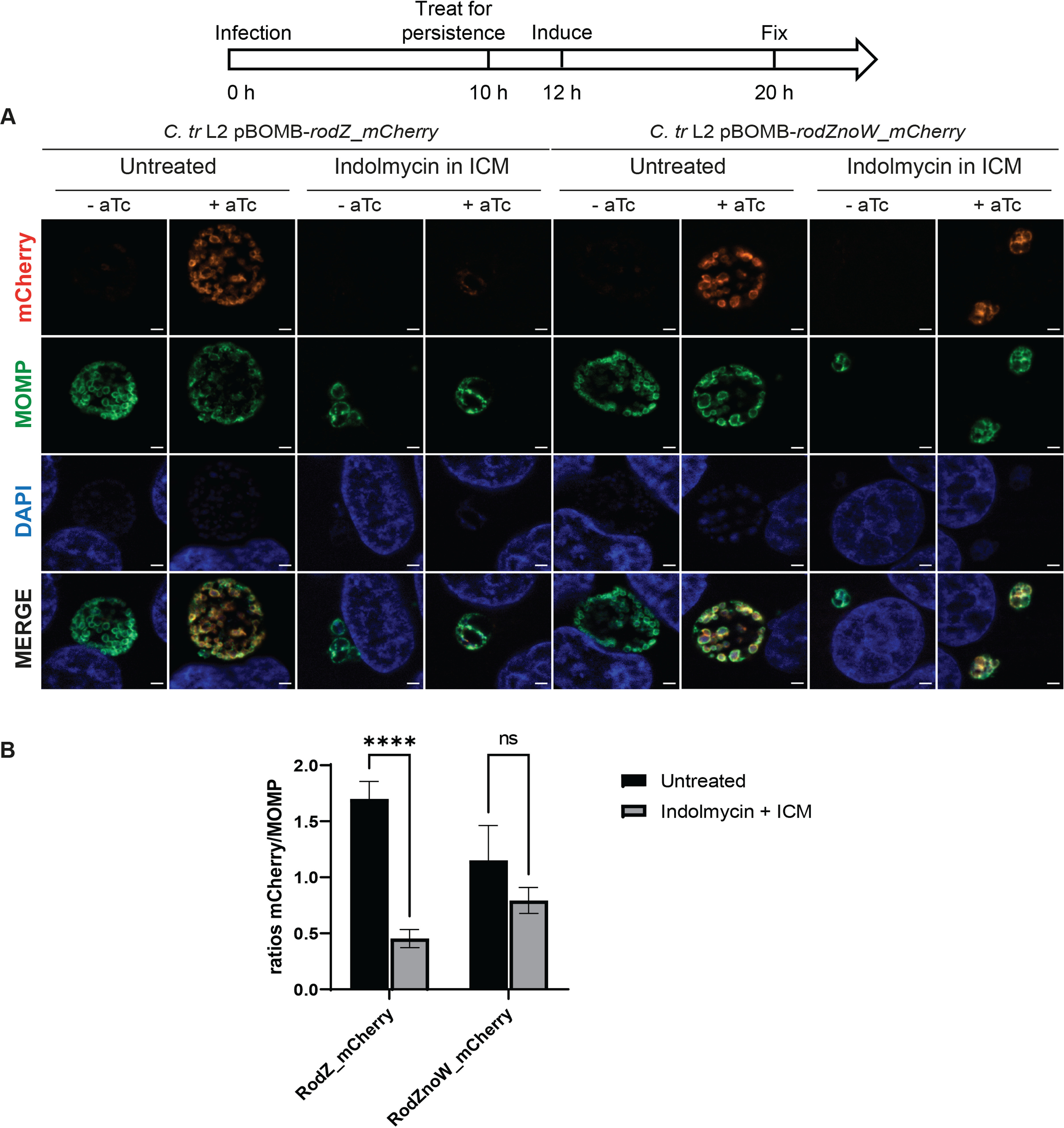
Expression of RodZ upon Trp starvation mediated by indolmycin is dependent on the Trp content of RodZ. A diagram representing the experimental design and highlighting important time points is shown. HEp-2 cells were infected at an MOI of 1. A total of 0.5 nM aTc was added to induce RodZ_mCherry and RodZnoW_mCherry expression. (A) Samples were fixed at 20 hpi and processed for immunofluorescence to detect mCherry (red), MOMP (green), and DNA (blue). Scale bar = 2 μm. (B) Immunofluorescent images were quantified using Fiji/ImageJ software to determine MFI of MOMP or mCherry fused protein per area of inclusion and results were graphed and statistically analyzed using GraphPad Prism. Data are expressed as average mCherry/MOMP ratios for both conditions with standard deviation between three independent experiments. Statistical significance was determined by two-way ANOVA test with multiple comparisons. ns, nonsignificant; ****, P < 0.0001.
Eliminating the Trp content of a target protein restores its expression during chlamydial persistence.
Given its Trp codon content, including a WW motif, RodZ is highly enriched for Trp codons relative to the chlamydial proteome (2.8% versus 0.94%). We sought to determine whether Trp limitation effectively blocked RodZ expression and whether eliminating the Trp residues would restore its expression. We assessed the expression of the wild-type RodZ protein from C. trachomatis L2 or a RodZnoW mutant, wherein each Trp was substituted with a phenylalanine (Phe) to preserve the overall hydrophobicity of the protein. We cloned each RodZ variant with a C-terminal red fluorescent protein mCherry into an aTc-inducible expression vector. Of note, the mCherry protein contains three W residues scattered throughout its sequence. We transformed these constructs into C. trachomatis L2 without plasmid (–pL2) and used the mCherry level to assess the expression of RodZ and RodZnoW during untreated and persistence conditions by immunofluorescence analysis, as described above. Importantly, RodZ_mCherry and RodZnoW_mCherry overexpression did not significantly affect chlamydial growth (Fig. S3), and both were membrane-localized as expected and easily visualized (Fig. 5A, top row, untreated, +aTc). To assess the effect of Trp availability on RodZ_mCherry and RodZnoW_mCherry expression, samples were infected at an MOI of 1 and starved for Trp at 10 hpi using indolmycin in ICM or left untreated. RodZ_mCherry and RodZnoW_mCherry expression was induced or not at 12 hpi. All samples were fixed at 20 hpi and stained for immunofluorescence assay (Fig. 5A). Upon indolmycin treatment, induction of RodZ_mCherry expression was inhibited while RodZnoW_mCherry expression was maintained. mCherry signal was normalized to the area of the inclusion and then to the MOMP signal. We observed that indolmycin-mediated persistence results in a significantly decreased induction of RodZ_mCherry expression while the expression of RodZnoW_mCherry was not significantly affected (Fig. 5B). Therefore, we conclude that the Trp content of wild-type RodZ negatively impacts its expression during Trp starvation.
As a related proof-of-concept that the Trp-codon richness of a transcript prevents its efficient translation during Trp starvation, we employed the same strategy as described above for RodZ with the hypothetical protein CTL0293/Ct038, which is also highly enriched in Trp codons compared to the chlamydial proteome (3.5% versus 0.94%) and has a WW motif. For CTL0293, the WW motif is near the 5′ end. We quantified transcript levels of CTL0293 and compared the 5′/3′ ratios between the 10 hpi samples (RBs) and the indolmycin-treated samples at 24 hpi (aRBs). For ctl0293, we observed no change in transcript level between 10 hpi and 24 hpi during indolmycin treatment (Fig. S4A). To test expression during persistence, we created fusion constructs with the wild-type CTL0293 protein from C. trachomatis L2 or the CTL0293noW mutant with an N-terminal green fluorescent protein (GFP) (35). Of note, GFP includes a single W residue. We transformed these constructs into C. trachomatis L2 –pL2 and used the GFP fluorescence to determine the level of expression of CTL0293 or CTL0293noW during untreated and persistence conditions by immunofluorescence analysis. Overexpression of CTL0293 and CTL0293noW had no impact on C. trachomatis L2 growth (Fig. S4B). To assess the effect of Trp availability on GFP_CTL0293 and GFP_CTL0293noW expression, HEp-2 cells were infected at an MOI of 1 and starved for Trp or not at 10 hpi using indolmycin in ICM. GFP_CTL0293 and GFP_CTL0293noW expression was induced or not at 12 hpi. All samples were fixed at 20 hpi and stained for immunofluorescence, imaged, and quantified by normalizing the GFP signal to the area of the inclusion and MOMP expression. Upon indolmycin treatment, GFP_CTL0293 signal was inhibited while GFP_CTL0293noW signal was not (Fig. S4C). By quantifying and normalizing the data, we observed that indolmycin-mediated persistence results in significantly decreased expression of GFP_CTL0293, while the expression of GFP_CTL0293noW was unaffected (Fig. S4D). Therefore, we conclude that the Trp content of CTL0293 negatively impacts its expression during Trp starvation and that this may be a general feature of chlamydial persistence.
Cls polarity is not drastically affected by indolmycin-mediated persistence.
Chlamydia divides by an MreB-dependent, asymmetric polarized division process (10, 36). The RB itself is highly polarized with MOMP enriched on one side of the mother cell. We recently described that the cardiolipin synthase, Cls, is also highly polarized and localizes to the MOMP-enriched side of the mother cell (33). We further demonstrated an important function of Cls in directing MreB to the division site. Cls is thus a marker for the polarity of chlamydial RBs. We wanted to determine whether the polarization state of Chlamydia was maintained during persistence. HEp-2 cells were infected with the C. trachomatis L2 pBOMB-cls_6×H strain and treated or not with indolmycin in ICM at 10 hpi. Of note, Cls contains 7 Trp residues (equaling 1.48% Trp content) interspersed throughout its sequence. The induction of Cls_6×H expression was performed by the addition of 5 nM aTc at 4 hpi or 12 hpi. The 4 hpi induction time point allowed us to have good Cls_6×H expression before the indolmycin treatment to determine whether persistence alters its localization, whereas the 12 hpi induction time allowed us to determine whether Cls_6×H was expressed during Trp starvation and, if so, properly localized to a distinct site on the membrane. At 16 hpi, cells were fixed for immunofluorescence analysis. Immunofluorescence microscopy revealed that, in the untreated condition, the Cls_6×H protein was present at the chlamydial membrane and, more specifically, concentrated at one side of the membrane for both induction times (Fig. 6). This is consistent with our previous observations, showing that Cls localization is polarized in C. trachomatis L2 (33). When Cls_6×H was induced either before or after indolmycin treatment, we still observed a polarized localization of the Cls_6×H protein, suggesting that chlamydial polarity is likely maintained during persistence and that Cls expression is not affected by Trp starvation (Fig. 6).
FIG 6.

Polarity of Cls localization is maintained during persistence. The diagram represents the experimental design, including important time points at which Cls_6×H expression was induced relative to treatment with indolmycin. Representative images are shown of HEp-2 cells infected with C. trachomatis L2 transformed with pBOMB-cls_6×H and treated or not as indicated. Cls_6×H expression was induced at 4 hpi (t1) or 12 hpi (t2) with 5 nM aTc. Cells were fixed at 16 hpi and processed for immunofluorescence to detect 6×His (red), MOMP (green), and DNA (blue). Scale bar = 2 μm.
DISCUSSION
Chlamydia trachomatis, specifically serovars D through K, L1, L2, and L3, is the leading cause of bacterial sexually transmitted infections (STIs) worldwide (https://www.cdc.gov/std/statistics/2020/overview.htm#Chlamydia). In 2020, the U.S. Centers for Disease Control and Prevention reported over 1.5 million cases of C. trachomatis STIs (https://www.cdc.gov/std/statistics/2020/overview.htm#Chlamydia). However, as approximately 70% of C. trachomatis infections are asymptomatic, the number of reported cases is significantly underdiagnosed each year (37, 38). Hence, undiagnosed asymptomatic infections are likely responsible for prolonged urogenital infections, which can result in chronic inflammation in the upper genital tract leading to tissue damage and scarring and long-term sequelae, including pelvic inflammatory disease, ectopic pregnancy, and infertility in women and epididymitis or urethritis in men (39, 40). Many of these asymptomatic cases of Chlamydia may be caused by a persistent infection. A hallmark of chlamydial persistence in cell culture is the presence of the aRB morphological form, and C. trachomatis aRBs have been observed in human endocervix samples, suggesting the presence of persistent Chlamydia in patients (41). In addition, chlamydial antigens, as well as DNA or RNA, can be detected in clinical samples, even after antibiotic treatment and culture negativity. Further, C. trachomatis DNA was found in fallopian tube tissue biopsy specimens from a woman culture negative for Chlamydia but suffering from postinfectious infertility, a sequelae associated with persistent infection (42).
The clinical relevance of chlamydial persistence highlights the importance of understanding how Chlamydia enters, remains in, and exits the persistence state. The most physiologically relevant model of chlamydial persistence is that induced by Trp limitation. When a human epithelial cell is activated by the immune cytokine IFN-γ, the production of the Trp catabolizing enzyme IDO is induced. Prior work in cell culture models of IFN-γ-mediated persistence have established that depriving C. pneumoniae of Trp by IFN-γ treatment does not induce the expression of a specific regulon. Rather, C. pneumoniae transcription and translation are disconnected with transcription globally increased but translation globally decreased (19). The translational effects are intuitive given the amino acid starvation conditions, but the transcriptional effects are an unusual finding. Transcript levels were shown to be altered differently relative to their Trp codon content where the transcripts of Trp codon-rich genes were observed to increase whereas the transcripts of Trp codon-free or Trp codon-poor genes were decreased or unchanged during IFN-γ-mediated Trp starvation (21). We also noted the destabilizing effect of Trp codons on larger transcripts such that the 3′ transcript levels are typically reduced compared to the 5′ transcript levels (22), and this effect is mediated by Rho-dependent polarity in at least some cases (22). More recently, similar Trp codon-dependent transcriptional effects were measured for C. trachomatis and another Trp auxotrophic pathogen, Streptococcus pyogenes (23), indicating that transcriptional control may be negatively impacted by Trp starvation in these bacteria. Regardless, these data indicate that, to understand the phenotypic effects of Trp starvation on Chlamydia, we need a better understanding of how translation of specific proteins is impacted.
At the protein level, prior work demonstrated a serovar-specific response to IFN-γ-mediated Trp starvation, with reduced levels of MOMP in C. trachomatis serovar A, but not serovar L2, during persistence (15, 43). A common response of C. trachomatis serovars A and L2 was the upregulation of the two subunits of the Trp synthase (43). This first observation was then confirmed by Østergaard and colleagues who demonstrated that, during IFN-γ-mediated persistence of C. trachomatis serovar D, the proteomes of RBs and aRBs, were broadly similar, except for the two subunits of the Trp synthase TrpA and TrpB that were present at very high levels in aRBs (44, 45). This suggests an attempt by C. trachomatis to synthesize Trp from indole, the substrate of the Trp synthase. Furthermore, aRBs were found to express lower levels of proteins with high Trp content (45), which is consistent with the hypothesis proposed by previous work from our lab where ribosomes stalling at Trp codons decrease global translation to trigger persistence (21).
Recently, Brockett and Liechti (28) assessed the ability of C. trachomatis L2 to synthesize PG upon treatment with various persistence-inducing stressors, including IFN-γ. They were unable to detect PG during IFN-γ-mediated Trp starvation, which is consistent with the inhibition of cell division during IFN-γ-mediated persistence since a PG ring is produced only at the time of division in Chlamydia (8, 28). However, the lack of PG does not explain why PG is not made. One possible explanation is that division-associated genes are transcriptionally downregulated, but, as noted above, we did not measure any statistically significant decrease in any cell division or PG-related gene we assessed (Fig. S2). Therefore, transcriptional changes do not explain the block in cell division.
We wanted to mechanistically address how cell division is blocked in Chlamydia during persistence induced by Trp limitation. To do this, we must leverage genetic tools that have been recently developed for C. trachomatis. However, these tools are best developed for the fast-growing serovar L2, which can outgrow IFN-γ-mediated Trp starvation unless the experimental conditions are carefully considered. For example, treating with IFN-γ too soon can completely block differentiation to the RB (46), whereas treating too late (i.e., at the time of infection) does not sufficiently block developmental cycle progression (32, 47). To overcome these challenges of experimental design, we leveraged the bacterial tryptophanyl-tRNA synthetase inhibitor, indolmycin (32). This allowed us to establish an infection and then to induce persistence at an appropriate time postinfection while inducing or not the expression of a specific protein of interest. We used this strategy recently to explore the function of YtgR, a repressor of the trpRBA and ytg operons encoded within the ytgCR open reading frame (29). We and colleagues demonstrated that the translation of YtgR during Trp starvation is decreased by the presence of a rare WWW motif in the sequence of the YtgC domain of the ytgCR open reading frame and that mutating the sequence to YYF restored its expression (29).
We hypothesized that, during persistence elicited by Trp starvation, the inability to translate critical cell division proteins is causally linked to the phenotypic block in cell division. More specifically, our work aimed to determine if the expression of cell division proteins during Trp starvation is affected by their Trp content and/or the presence of a tandem Trp motif (i.e., WW). We noted that, among our list of cell division proteins in Chlamydia, four proteins are enriched in Trp codons, with RodA and RodZ having an additional Trp motif, and that four proteins have a neutral Trp content with FtsI/Pbp3 having a WW motif (Table 1). Interestingly, this is not the case for the PG synthesis proteins because their percentage of Trp is generally neutral or lower than the chlamydial proteome average, with only MurJ and MraY being the exceptions, with MurJ having 1.7% Trp and MraY having a WW motif (Table 1). We show here that the expression of the Trp-rich 6×His-fused proteins Pbp2, RodA, FtsI/Pbp3, and MraY is significantly decreased during Trp limitation induced by indolmycin. Under the same conditions, the expression of the Trp-neutral construct FtsL_flag and the Trp-poor construct MurG_6×H was unaffected (Fig. 2 to 4). These data demonstrate that the translation of critical Trp-rich cell division-associated proteins, like YtgR (29), is negatively impacted by a lack of Trp.
Leveraging our ability to create transformants in Chlamydia, we mechanistically assessed the direct contribution of Trp codons in the inability to translate Trp-rich proteins during Trp starvation. To do this, we created mutant isoforms of RodZ and the Trp-rich hypothetical protein CTL0293 wherein each Trp residue was replaced by a Phe residue (i.e., noW). Both wild-type proteins contain four Trp residues. Using overexpression strains of RodZ_mCherry, RodZnoW_mCherry, GFP_CTL0293, and GFP_CTL0293noW, our data indicate that the Trp content of the wild-type proteins negatively impacted their expression during indolmycin-mediated persistence, which was not the case for the noW mutant isoforms (Fig. 5 and Fig. S4C to D). Given the Trp content of the fluorescent proteins (1 for GFP, 3 for mCherry), it further indicates that an isolated W codon does not disrupt translation of the encoded protein during Trp starvation. A similar result was noted for Cls, which has a higher Trp content interspersed throughout its sequence. Rather, it appears that the presence of a WW(W) motif and/or a high Trp codon density is required to disrupt translation. Importantly, organisms expressing the noW variants were still persistent. Thus, these data also show that restoring the expression of a single division protein by eliminating its Trp content is incapable of allowing normal division to resume. This has important ramifications for the ability of the organism to become resistant to Trp starvation, since it would require extensive changes to the Trp content of multiple proteins, and why these bacteria are so sensitive to conditions of limited Trp. Overall, these data directly link the Trp content of a protein to its ability to be efficiently translated during Trp starvation.
Our data allow us to suggest a model for chlamydial persistence in response to Trp starvation. When Trp is available, all the proteins necessary for chlamydial division are effectively translated, and active cell division with PG ring formation allows chlamydial growth (Fig. 7, left). In contrast, Trp deprivation (drug- or IFN-γ-induced) limits the expression of key chlamydial cell division and PG synthesis proteins, likely by ribosome stalling at the WW motifs resulting in a reduction of their translation (Fig. 7, right). Depending on the Trp amount in their protein sequence, expression of cell division and PG synthesis proteins is maintained (e.g., FtsL and MurG) or significantly reduced (e.g., Pbp2, RodA, FtsI/Pbp3, MraY, and RodZ) during persistence. Furthermore, proteins such as FtsQ, FtsW, FtsK, and MreB, with a neutral percentage of Trp, are expected to be expressed. On the contrary, we hypothesize that the expression of MurJ and MreC might be decreased given their percentage of Trp (1.7% and 1.8%, respectively). PG synthesis is then likely to be highly impacted by Trp starvation if MraY and MurJ are not translated. Therefore, the inability to produce multiple cell division and PG synthesis proteins may decrease the ability of Chlamydia to construct a divisome and synthesize PG at the septum (Fig. 7). One interesting prediction from this model is that peptidoglycan precursors are expected to accumulate. This, along with the continued expression of Cls, may facilitate the rapid reactivation of growth once Trp becomes available again. We are currently exploring this.
FIG 7.

Model of Trp-dependent chlamydial division inhibition during persistence mediated by Trp starvation. (Left) Under normal growth, when Trp is available in the environment, ribosomes are able to translate the chlamydial divisome components (53) and the PG synthesis proteins necessary to chlamydial division and growth. (Right) Under Trp starvation mediated by indolmycin, the ribosome stalling at the WW (or WAW) motifs and the reduced translation affect the translation of the chlamydial cell division and PG synthesis proteins depending on their overall Trp content. In this scenario, only MurG and FtsL are made. Further based on our supporting data, we hypothesize that FtsB (putative), MurJ, and MreC expression would also be decreased during persistence. Similarly, we predict that the low Trp content of FtsQ, FtsW, FtsK, and MreB proteins suggest that these proteins would be expressed during persistence. Overall, the inability to produce key divisome components and PG synthesis proteins leads to a block of chlamydial division during Trp starvation-induced persistence. Some elements in this figure were adapted from (53).
From a clinical point of view, the burden of persistent chlamydial infections derives from the development of long-term sequelae by the patients, especially women, and on the relapse of infections. Infection relapse implies the reactivation of persistent bacteria to active growth. Future studies will be aimed at monitoring the expression of our target proteins and at determining how chlamydial division is reactivated when the Trp pool is restored (48). Overall, our data have provided the first mechanistic description for how cell division is blocked during persistence induced by Trp starvation, and our findings can be extrapolated to other physiological systems to explain why Chlamydia becomes persistent under low Trp conditions.
MATERIALS AND METHODS
Organisms and cell culture.
The human epithelial cell line HEp-2 was used for the persistence assays, inclusion-forming unit assays, and gDNA and RNA extractions. McCoy mouse fibroblasts were used for transforming C. trachomatis serovar L2. The HEp-2 and McCoy cells were a kind gift of Harlan Caldwell (NIH/NIAID). All these cell lines were routinely cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Waltham, MA) containing 10% fetal bovine serum (FBS; HyClone, Logan, UT) and 10 μg/mL gentamicin (Gibco, Waltham, MA) at 37°C and 5% CO2. The HEp-2 and McCoy cells were verified to be mycoplasma negative using the LookOut mycoplasma PCR detection kit (Sigma, St. Louis, MO). Density gradient-purified C. trachomatis L2/434/Bu EBs were used for the transcription studies. C. trachomatis serovar L2 EBs naturally lacking the endogenous plasmid were prepared and used for transformation (49). HEp-2 cells were infected with C. trachomatis serovar L2 EBs or chlamydial transformants in DMEM with 10% FBS and 10 μg/mL gentamicin. For infections, cells were first washed with Hanks Buffered Saline Solution (HBSS; Gibco) and ice-cold inoculum prepared in HBSS at the indicated multiplicity of infection (MOI) was added to the cell monolayer. To synchronize the infection, inoculated cells were then centrifuged for 15 min at 400 g, 4°C. The inoculum was then aspirated and prewarmed DMEM was added to the cells. Infected cultures were then returned to the tissue culture incubator until the indicated time postinfection.
Treatment conditions.
For persistence mediated by Trp starvation, we used indolmycin (Cayman Chemical, Ann Arbor, MI) prepared in dimethyl sulfoxide (DMSO; Sigma) and stored at −80°C. In brief, Trp starvation was performed by first washing cells twice with HBSS at 10 h postinfection (hpi) and then replacing DMEM with IFN-γ-conditioned medium (ICM) supplemented with indolmycin at a working concentration of 120 μM (32). The treatment was allowed to proceed for 10 h and ICM was used to enhance the inhibitory effect of indolmycin. Recombinant human IFN-γ was purchased from Cell Sciences (Canton, MA), resuspended to 100 μg/mL in 0.1% bovine serum albumin (BSA; Sigma) diluted in water. Aliquots were kept at −80°C and used only once to avoid freeze-thawing. ICM was prepared by adding 2 ng/mL IFN-γ to uninfected HEp-2 cells for approximately 54 h prior to collection and filtration of the medium. For longer storage, ICM was kept at −20°C.
Bioinformatics analysis.
Sequences of the division and PG synthesis proteins of interest for Chlamydia trachomatis L2/434/Bu, C. trachomatis D/UW-3CX, C. pneumoniae CWL029, C. psittaci 6BC, C. muridarum Nigg, C. caviae GPIC, C. suis MD56, E. coli K-12, and Bacillus subtilis 168 were obtained from the NCBI database (https://www.ncbi.nlm.nih.gov/).
Cloning.
The plasmids and primers used in this study are described in Table S4. Constructs for chlamydial transformation were created using the HiFi DNA assembly cloning kit (New Englands Biolabs, Ipswich, MA). Primers were designed to add a polyhistidine tag (6×His) or a flag tag (only for pBOMB-ftsL_flag) to the gene of interest with the overlap to insert into the vector. The backbones used were pBOMB4-Tet (ftsL construct only; pBOMB4-Tet is a kind gift from Ted Hackstadt) (50) and its derivative pBOMBL containing a modified ribosome binding site to reduce leaky expression (51). The genes ftsI/pbp3, pbp2, rodA, mraY, murG, and ftsL were amplified by PCR with Phusion DNA polymerase (NEB) using C. trachomatis serovar L2 434/Bu chromosome as a template. The PCR products were purified using a PCR purification kit (Qiagen, Hilden, Germany). The HiFi Assembly reaction master mix was used according to the manufacturer’s instructions in conjunction with the pBOMBL or pBOMB4-Tet plasmids cut with FastDigest EagI and KpnI enzymes, and dephosphorylated with FastAP (Thermo Fisher, Waltham, MA).
mCherry or GFP fusion constructs were also created for this study by amplifying rodZ, mutant rodZ without Trp codons (rodZnoW), ctl0293, and mutant ctl0293 without Trp codons (ctl0293noW) by PCR with Phusion DNA polymerase using gBlock fragments (Integrated DNA Technologies, Coralville, IA) as a template and primers with overlapping regions with mCherry or GFP depending on the construct being made. gBlocks were designed based on C. trachomatis serovar L2 434/Bu genes, and ctl0293noW and rodZnoW mutants were obtained by exchanging the TGG codons with TTT or TTC codons encoding Phe residues. GFP and mCherry genes were amplified by PCR from the pBOMB4-Tet plasmid, with primers overlapping the gene of interest fused with GFP or mCherry. rodZ_mCherry and rodZnoW_mCherry were cloned onto the EagI/KpnI sites of the plasmid pBOMB4-Tet using the HiFi DNA assembly cloning kit (NEB). Similarly, gfp_ctl0293 and gfp_ctl0293noW fusions were inserted into pBOMB-G (no GFP on backbone).
In all cases, the products of the% glucose. Plasmids were subsequently isolated from individual colonies grown overnight in HiFi reaction were transformed into DH10β E. coli competent cells (NEB) and plated on LB plates containing 100 μg/mL ampicillin and 0.4 LB by using a miniprep kit (Qiagen) and confirmed by restriction digest and sequencing.
Transformation of Chlamydia trachomatis.
For transformation, 106 C. trachomatis serovar L2 EBs without plasmid (–pL2) were incubated with 2 μg of plasmid in a volume of 50 μL Tris-CaCl2 buffer (10 mM Tris, 50 mM CaCl2, pH 7,4) for 30 min at room temperature. McCoy cells were washed with 2 mL HBSS and 1 mL HBSS was added back into each well. The transformation inoculum, mixed to 1 mL HBSS, was then used to infect a confluent monolayer of McCoy cells by centrifugation at 400 g for 15 min at room temperature followed by incubation at 37°C for 15 min. The inoculum was then aspirated, and DMEM containing 10% FBS and 10 μg/mL gentamicin was added to the well. At 8 hpi, the medium was replaced with DMEM containing 1 or 2 U/mL penicillin G and 1 μg/mL cycloheximide. The plate was incubated at 37°C for 48 h, then the transformants were harvested (T1) to infect a new McCoy cell monolayer. Transformants were passaged and subcultured every 48 hpi until a population of penicillin-resistant bacteria was observed. EBs were harvested and frozen in sucrose-phosphate (2SP) (49) solution and stored at −80°C. To verify each transformant, plasmid DNA was isolated from infected cells for subsequent restriction digest and sequencing.
Effect of Trp starvation mediated by indolmycin on chlamydial transcription of cell division and PG synthesis genes.
HEp-2 cells were plated in triplicate wells of 6-well tissue culture plates with coverslips in one well per test condition. For each tested condition, samples were simultaneously collected for RNA (to monitor gene expression by RT-qPCR), gDNA (to normalize RT-qPCR data), and immunofluorescence microscopy (to verify that culture conditions induced persistence). HEp-2 cells were infected with C. trachomatis L2 wild-type at an MOI of 2. To collect RNA samples, infected cell monolayers were harvested with 500 μL TRIzol (Invitrogen/Thermo Fisher). To remove DNA contamination, samples were treated with Turbo DNAfree (Ambion/Thermo Fisher). cDNA was synthesized from DNA-free RNA using random nonamers (NEB) and SuperScript III reverse transcriptase (RT; Invitrogen/Thermo Fisher) following the manufacturers’ instructions. Reaction end products were diluted 10-fold with molecular biology-grade water and stored at −80°C. Equal volumes of each reaction mixture were used in 25 μL quantitative PCR (qPCR) mixtures with SYBR green master mix (Applied Biosystems) and quantified on a QuantStudio 3 (Applied Biosystems/Thermo Fisher) using the standard amplification cycle with a melting curve analysis. Results were compared to a standard curve generated against purified C. trachomatis L2 genomic DNA as appropriate.
DNA samples were collected using the DNeasy blood and tissue kit (Qiagen). Equal total DNA quantities were used in qPCR with an incA primer set to quantify chlamydial genomes. Genome values were used to normalize respective transcript data. Table S4 provides all qPCR primer sequences. RT-qPCR results were normalized for efficiency, with typical results demonstrating an r2 of >0.995 and efficiencies greater than 90%. Student’s t tests were used to compare the 5′ value to that for the 3′ value for each gene of interest. Student’s t tests were also used to compared each untreated (UTD) 24 hpi value to that at 10 hpi, each persistence 24 hpi value to that at 10 hpi, and each persistence 24 hpi value to that at UTD 24 hpi. Table S3 provides a summary of the P values.
To verify our conditions induced chlamydial persistence, cells grown on coverslips were fixed in 100% methanol for 5 min. Organisms were stained using a primary goat antibody specific to C. trachomatis major outer membrane protein (MOMP; Meridian, Memphis, TN) and a donkey antigoat secondary antibody conjugated to Alexa Fluor 488 (Invitrogen, Carlsbad, CA). DAPI (4′,6-diamidino-2-phenylindole) (Invitrogen) was added to visualize DNA. Images of C. trachomatis L2 infected cells at 10 hpi and 24 hpi, treated or not with indolmycin, were acquired at 100× magnification on a Zeiss AxioImager.Z2 microscope equipped with an Apotome2 and an Axiocam 506 6 MP digital monochrome camera, essentially as in Fig. 1.
Determining the effect of overexpression of wild-type and mutant genes of interest via inclusion-forming unit analysis.
Infectious progeny (number of EBs) was determined from a primary infection based on inclusions formed in a secondary infection. C. trachomatis L2 transformants containing plasmids encoding the 6×His-tagged, flag-tagged, GFP-fused, or mCherry-fused genes of interest were used to infect a confluent monolayer of HEp-2 cells. At 12 hpi, samples were induced or not with the appropriate anhydrotetracycline (aTc) concentration for each construct (see below). At 24 hpi, primary infection samples were harvested by scraping cells in 2SP buffer. Samples were lysed via a single freeze-thaw cycle, serially diluted, and used to infect a fresh cell monolayer. At the same time, a coverslip was fixed in 3.2% formaldehyde and 0.022% glutaraldehyde for 2 min, followed by permeabilization with 90% methanol for 1 min. Coverslips were observed under the microscope to confirm construct expression (data not shown). The secondary infection was allowed to progress for 24 h prior to fixation with methanol and labeling for immunofluorescence microscopy using antibodies directed against MOMP. Titers were enumerated by calculating the total number of inclusions per field based on counts from 15 fields of view.
Analysis of constructs expression during persistence mediated by indolmycin in ICM.
The 24-well plates of HEp-2 cells were infected at an MOI of 1 with either pBOMBL-pbp2_6×H, pBOMBL-rodA_6×H, pBOMB-ftsL_flag, pBOMBL-ftsI/pbp3_6×H, pBOMBL-mraY_6×H, pBOMBL-murG_6×H, pBOMB-rodZ_mCherry, pBOMB-rodZnoW_mCherry, pBOMB-G-gfp_ctl0293, or pBOMB-G-gfp_ctl0293noW transformed into C. trachomatis L2 –pL2. Persistence was induced or not with 120 μM indolmycin following the procedures described above. Induction of Pbp2_6×H, RodA_6×H, FtsL_flag, MraY_6×H, GFP_CTL0293, and GFP_CTL0293noW expression was performed by the addition of 2 nM aTc at 12 hpi and allowed to proceed for 8 h. The expression of FtsI/Pbp3_6×H and MurG_6×H was induced by adding 1 nM aTc, and the expression of RodZ_mCherry and RodZnoW_mCherry was induced by adding 0.5 nM aTc at 12 hpi. At 20 hpi, coverslips were fixed in 3.2% formaldehyde and 0.022% glutaraldehyde for 2 min, followed by a permeabilization step with 90% methanol for 1 min. Organisms were detected using goat anti-MOMP antibodies (Meridian). Epitope-tagged proteins were detected using monoclonal rabbit anti-6×His antibody (Abcam, Cambridge, MA) or mouse antiflag antibody (Sigma). All primary antibodies were followed with the appropriate secondary antibody made in donkey and conjugated to Alexa Fluor 488 or 594 (Invitrogen). Immunofluorescent micrographs of Pbp2_6×H, RodA_6×H, FtsL_flag, MraY_6×H, FtsI/Pbp3_6×H, MurG_6×H, RodZ_mCherry, RodZnoW_mCherry, GFP_CTL0293, and GFP_CTL0293noW expression from C. trachomatis L2 transformants treated or not with indolmycin in ICM were acquired on a Zeiss AxioImager.Z2 microscope as above and using identical exposure between conditions. Fiji/ImageJ software (52) was used to quantify MOMP, 6×His/flag, mCherry, and GFP fluorescence intensity. Then, 50 inclusions per condition for each replicate were measured. Regions of interest (ROI) were defined in merged fluorescent images and the mean fluorescent intensity per pixel from the defined ROI in the images of the individual channels: MOMP-488 (or MOMP-594 for the GFP fusion constructs), 6×His-594, flag-594, mCherry-594, and GFP-488 channels. In total, the mean fluorescent intensity (MFI) was measured for 150 inclusions and normalized to the area of the inclusion and/or normalized to the MOMP values and expressed as 6×His/MOMP, flag/MOMP, mCherry/MOMP, and GFP/MOMP ratios. Data were plotted, and statistically analyzed by unpaired Student's t test or two-way ANOVA test with multiple comparisons as appropriate, using GraphPad PRISM software.
Localization analyses.
To study Cls localization within Chlamydia, 24-well plates of HEp-2 cells were infected at an MOI of 1 with pBOMB-cls_6×H transformed into C. trachomatis L2 –pL2. Persistence was induced or not with 120 μM indolmycin at 10 hpi. Induction of Cls_6×H expression was performed by the addition of 5 nM aTc at 4 hpi or at 12 hpi. At 16 hpi, coverslips were fixed in 3.2% formaldehyde and 0.022% glutaraldehyde for 2 min, followed by a permeabilization step with 90% methanol for 1 min. Coverslips were labeled with primary rabbit anti-6×His antibodies (Abcam), primary goat anti-MOMP antibodies, followed by appropriate secondary antibodies, and DAPI. Images of Cls_6×H localization were acquired on a Zeiss AxioImager.Z2 microscope essentially as described above.
ACKNOWLEDGMENTS
We acknowledge H. Caldwell (NIH) for eukaryotic cell lines and T. Hackstadt (RML/NIAID) for providing the pBOMB4-Tet plasmid. We thank Laura Fisher-Marvin for technical assistance and Jeonghoon Lee for advice and technical help. The authors are thankful to the members of the Rucks/Ouellette lab for helpful comments and suggestions regarding this work.
C.M.R., S.P.O., and E.A.R. designed and performed the experiments. S.P.O. and E.A.R. supervised research. The data were analyzed by C.M.R., S.P.O., and E.A.R. C.M.R. prepared the figures and wrote the manuscript, which was edited by E.A.R. and S.P.O.
This work was supported by funding from the U.S. National Institutes of Health grant 1R01AI132406 to S.P.O. and E.A.R.
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Scot P. Ouellette, Email: scot.ouellette@unmc.edu.
Craig R. Roy, Yale University School of Medicine
REFERENCES
- 1.Balaban NQ, Helaine S, Lewis K, Ackermann M, Aldridge B, Andersson DI, Brynildsen MP, Bumann D, Camilli A, Collins JJ, Dehio C, Fortune S, Ghigo J-M, Hardt W-D, Harms A, Heinemann M, Hung DT, Jenal U, Levin BR, Michiels J, Storz G, Tan M-W, Tenson T, Van Melderen L, Zinkernagel A. 2019. Definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol 17:441–448. 10.1038/s41579-019-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward ME. 1983. Chlamydial classification, development and structure. Br Med Bull 39:109–115. 10.1093/oxfordjournals.bmb.a071800. [DOI] [PubMed] [Google Scholar]
- 3.Hatch TP, Allan I, Pearce JH. 1984. Structural and polypeptide differences between envelopes of infective and reproductive life cycle forms of Chlamydia spp. J Bacteriol 157:13–20. 10.1128/jb.157.1.13-20.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hackstadt T, Baehr W, Ying Y. 1991. Chlamydia trachomatis developmentally regulated protein is homologous to eukaryotic histone H1. Proc Natl Acad Sci USA 88:3937–3941. 10.1073/pnas.88.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AbdelRahman YM, Belland RJ. 2005. The chlamydial developmental cycle. FEMS Microbiol Rev 29:949–959. 10.1016/j.femsre.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Moore ER, Ouellette SP. 2014. Reconceptualizing the chlamydial inclusion as a pathogen-specified parasitic organelle: an expanded role for Inc proteins. Front Cell Infect Microbiol 4:157. 10.3389/fcimb.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouellette SP, Karimova G, Subtil A, Ladant D. 2012. Chlamydia co-opts the rod shape-determining proteins MreB and Pbp2 for cell division. Mol Microbiol 85:164–178. 10.1111/j.1365-2958.2012.08100.x. [DOI] [PubMed] [Google Scholar]
- 8.Liechti GW, Kuru E, Hall E, Kalinda A, Brun YV, VanNieuwenhze M, Maurelli AT. 2014. A new metabolic cell-wall labelling method reveals peptidoglycan in Chlamydia trachomatis. Nature 506:507–510. 10.1038/nature12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liechti G, Kuru E, Packiam M, Hsu YP, Tekkam S, Hall E, Rittichier JT, VanNieuwenhze M, Brun YV, Maurelli AT. 2016. Pathogenic Chlamydia Lack a Classical Sacculus but Synthesize a Narrow, Mid-cell Peptidoglycan Ring, Regulated by MreB, for Cell Division. PLoS Pathog 12:e1005590. 10.1371/journal.ppat.1005590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J, Cox JV, Ouellette SP. 2020. Critical role for the extended n terminus of Chlamydial mreb in directing its membrane association and potential interaction with divisome proteins. J Bacteriol 202. 10.1128/JB.00034-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox JV, Abdelrahman YM, Ouellette SP. 2020. Penicillin-binding proteins regulate multiple steps in the polarized cell division process of Chlamydia. Sci Rep 10:12588. 10.1038/s41598-020-69397-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hybiske K, Stephens RS. 2007. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc Natl Acad Sci USA 104:11430–11435. 10.1073/pnas.0703218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfefferkorn ER. 1984. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA 81:908–912. 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrne GI, Lehmann LK, Landry GJ. 1986. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun 53:347–351. 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beatty WL, Byrne GI, Morrison RP. 1993. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci USA 90:3998–4002. 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beatty WL, Belanger TA, Desai AA, Morrison RP, Byrne GI. 1994. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect Immun 62:3705–3711. 10.1128/iai.62.9.3705-3711.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perry LL, Feilzer K, Caldwell HD. 1997. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol 158:3344–3352. 10.4049/jimmunol.158.7.3344. [DOI] [PubMed] [Google Scholar]
- 18.Kane CD, Vena RM, Ouellette SP, Byrne GI. 1999. Intracellular tryptophan pool sizes may account for differences in gamma interferon-mediated inhibition and persistence of chlamydial growth in polarized and nonpolarized cells. Infect Immun 67:1666–1671. 10.1128/IAI.67.4.1666-1671.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouellette SP, Hatch TP, AbdelRahman YM, Rose LA, Belland RJ, Byrne GI. 2006. Global transcriptional upregulation in the absence of increased translation in Chlamydia during IFNgamma-mediated host cell tryptophan starvation. Mol Microbiol 62:1387–1401. 10.1111/j.1365-2958.2006.05465.x. [DOI] [PubMed] [Google Scholar]
- 20.Aiyar A, Quayle AJ, Buckner LR, Sherchand SP, Chang TL, Zea AH, Martin DH, Belland RJ. 2014. Influence of the tryptophan-indole-IFNgamma axis on human genital Chlamydia trachomatis infection: role of vaginal co-infections. Front Cell Infect Microbiol 4:72. 10.3389/fcimb.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouellette SP, Rueden KJ, Rucks EA. 2016. Tryptophan Codon-Dependent Transcription in Chlamydia pneumoniae during Gamma Interferon-Mediated Tryptophan Limitation. Infect Immun 84:2703–2713. 10.1128/IAI.00377-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouellette SP, Messerli PR, Wood NA, Hajovsky H. 2018. Characterization of Chlamydial Rho and the role of Rho-mediated transcriptional polarity during interferon gamma-mediated tryptophan limitation. Infect Immun 86. 10.1128/IAI.00240-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouellette SP, Hatch ND, Wood NA, Herrera AL, Chaussee MS. 2021. Codon-dependent transcriptional changes in response to tryptophan limitation in the tryptophan auxotrophic pathogens Chlamydia trachomatis and Streptococcus pyogenes. mSystems 6:e0126921. 10.1128/mSystems.01269-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hauryliuk V, Atkinson GC, Murakami KS, Tenson T, Gerdes K. 2015. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat Rev Microbiol 13:298–309. 10.1038/nrmicro3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gourse RL, Chen AY, Gopalkrishnan S, Sanchez-Vazquez P, Myers A, Ross W. 2018. Transcriptional responses to ppGpp and DksA. Annu Rev Microbiol 72:163–184. 10.1146/annurev-micro-090817-062444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephens RS, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, Mitchell W, Olinger L, Tatusov RL, Zhao Q, Koonin EV, Davis RW. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754–759. 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 27.Wyrick PB. 2010. Chlamydia trachomatis persistence in vitro: an overview. J Infect Dis 201 Suppl 2:S88–95. 10.1086/652394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brockett MR, Liechti GW. 2021. Persistence alters the interaction between Chlamydia trachomatis and Its Host Cell. Infect Immun 89:e0068520. 10.1128/IAI.00685-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pokorzynski ND, Hatch ND, Ouellette SP, Carabeo RA. 2020. The iron-dependent repressor YtgR is a tryptophan-dependent attenuator of the trpRBA operon in Chlamydia trachomatis. Nat Commun 11:6430. 10.1038/s41467-020-20181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonner CA, Byrne GI, Jensen RA. 2014. Chlamydia exploit the mammalian tryptophan-depletion defense strategy as a counter-defensive cue to trigger a survival state of persistence. Front Cell Infect Microbiol 4:17. 10.3389/fcimb.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werner RG, Thorpe LF, Reuter W, Nierhaus KH. 1976. Indolmycin inhibits prokaryotic tryptophanyl-tRNA ligase. Eur J Biochem 68:1–3. 10.1111/j.1432-1033.1976.tb10758.x. [DOI] [PubMed] [Google Scholar]
- 32.Hatch ND, Ouellette SP. 2020. Inhibition of tRNA Synthetases Induces Persistence in Chlamydia. Infect Immun 88. 10.1128/IAI.00943-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouellette SP, Fisher-Marvin LA, Harpring M, Lee J, Rucks EA, Cox JV. 2022. Localized cardiolipin synthesis is required for the assembly of MreB during the polarized cell division of Chlamydia trachomatis. PLoS Pathog 18:e1010836. 10.1371/journal.ppat.1010836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdelrahman YM, Rose LA, Belland RJ. 2011. Developmental expression of non-coding RNAs in Chlamydia trachomatis during normal and persistent growth. Nucleic Acids Res 39:1843–1854. 10.1093/nar/gkq1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood NA, Blocker AM, Seleem MA, Conda-Sheridan M, Fisher DJ, Ouellette SP. 2020. The ClpX and ClpP2 Orthologs of Chlamydia trachomatis perform discrete and essential functions in organism growth and development. mBio 11. 10.1128/mBio.02016-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.AbdelRahman Y, Ouellette SP, Belland RJ, Cox JV. 2016. Polarized cell division of Chlamydia trachomatis. PLoS Pathog 12:e1005822. 10.1371/journal.ppat.1005822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stamm WE. 1999. Chlamydia trachomatis infections: progress and problems. J Infect Dis 179 Suppl 2:S380–383. 10.1086/513844. [DOI] [PubMed] [Google Scholar]
- 38.Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MC, Su J, Xu F, Weinstock H. 2013. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis 40:187–193. 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 39.Brunham RC, Rey-Ladino J. 2005. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol 5:149–161. 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 40.Darville T, Hiltke TJ. 2010. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J Infect Dis 201 Suppl 2:S114–125. 10.1086/652397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis ME, Belland RJ, AbdelRahman YM, Beatty WL, Aiyar AA, Zea AH, Greene SJ, Marrero L, Buckner LR, Tate DJ, McGowin CL, Kozlowski PA, O'Brien M, Lillis RA, Martin DH, Quayle AJ. 2014. Morphologic and molecular evaluation of Chlamydia trachomatis growth in human endocervix reveals distinct growth patterns. Front Cell Infect Microbiol 4:71. 10.3389/fcimb.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patton DL, Askienazy-Elbhar M, Henry-Suchet J, Campbell LA, Cappuccio A, Tannous W, Wang SP, Kuo CC. 1994. Detection of Chlamydia trachomatis in fallopian tube tissue in women with postinfectious tubal infertility. Am J Obstet Gynecol 171:95–101. 10.1016/s0002-9378(94)70084-2. [DOI] [PubMed] [Google Scholar]
- 43.Shaw AC, Christiansen G, Birkelund S. 1999. Effects of interferon gamma on Chlamydia trachomatis serovar A and L2 protein expression investigated by two-dimensional gel electrophoresis. Electrophoresis 20:775–780. . [DOI] [PubMed] [Google Scholar]
- 44.Wood H, Fehlner-Gardner C, Berry J, Fischer E, Graham B, Hackstadt T, Roshick C, McClarty G. 2003. Regulation of tryptophan synthase gene expression in Chlamydia trachomatis. Mol Microbiol 49:1347–1359. 10.1046/j.1365-2958.2003.03638.x. [DOI] [PubMed] [Google Scholar]
- 45.Østergaard O, Follmann F, Olsen AW, Heegaard NH, Andersen P, Rosenkrands I. 2016. Quantitative protein profiling of Chlamydia trachomatis growth forms reveals defense strategies against tryptophan starvation. Mol Cell Proteomics 15:3540–3550. 10.1074/mcp.M116.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leonhardt RM, Lee SJ, Kavathas PB, Cresswell P. 2007. Severe tryptophan starvation blocks onset of conventional persistence and reduces reactivation of Chlamydia trachomatis. Infect Immun 75:5105–5117. 10.1128/IAI.00668-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrison RP. 2000. Differential sensitivities of Chlamydia trachomatis strains to inhibitory effects of gamma interferon. Infect Immun 68:6038–6040. 10.1128/IAI.68.10.6038-6040.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beatty WL, Morrison RP, Byrne GI. 1995. Reactivation of persistent Chlamydia trachomatis infection in cell culture. Infect Immun 63:199–205. 10.1128/iai.63.1.199-205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Kahane S, Cutcliffe LT, Skilton RJ, Lambden PR, Clarke IN. 2011. Development of a transformation system for Chlamydia trachomatis: restoration of glycogen biosynthesis by acquisition of a plasmid shuttle vector. PLoS Pathog 7:e1002258. 10.1371/journal.ppat.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bauler LD, Hackstadt T. 2014. Expression and targeting of secreted proteins from Chlamydia trachomatis. J Bacteriol 196:1325–1334. 10.1128/JB.01290-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ouellette SP, Blay EA, Hatch ND, Fisher-Marvin LA. 2021. CRISPR interference to inducibly repress gene expression in Chlamydia trachomatis. Infect Immun 89:e0010821. 10.1128/IAI.00108-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ouellette SP, Lee J, Cox JV. 2020. Division without binary fission: cell division in the FtsZ-less Chlamydia. J Bacteriol 202. 10.1128/JB.00252-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S4 and Fig. S1 to S4. Download iai.00513-22-s0001.pdf, PDF file, 6.4 MB (6.4MB, pdf)


