ABSTRACT
Dendritic cells (DCs) belong to the first line of innate defense and come into early contact with invading pathogens, including the zoonotic bacterium Coxiella burnetii, the causative agent of Q fever. However, the pathogen-host cell interactions in C. burnetii-infected DCs, particularly the role of mechanisms of immune subversion beyond virulent phase I lipopolysaccharide (LPS), as well as the contribution of cellular self-defense strategies, are not understood. Using phase II Coxiella-infected DCs, we show that impairment of DC maturation and MHC I downregulation is caused by autocrine release and action of immunosuppressive transforming growth factor-β (TGF-β). Our study demonstrates that IFN-γ reverses TGF-β impairment of maturation/MHC I presentation in infected DCs and activates bacterial elimination, predominantly by inducing iNOS/NO. Induced NO synthesis strongly affects bacterial growth and infectivity. Moreover, our studies hint that Coxiella-infected DCs might be able to protect themselves from mitotoxic NO by switching from oxidative phosphorylation to glycolysis, thus ensuring survival in self-defense against C. burnetii. Our results provide new insights into DC subversion by Coxiella and the IFN-γ-mediated targeting of C. burnetii during early steps in the innate immune response.
KEYWORDS: Coxiella burnetii, immune subversion, major histocompatibility complex
INTRODUCTION
Dendritic cells (DCs) are professional antigen-presenting cells (APCs), which respond to foreign antigens and mediate between innate and adaptive immune responses (1). They are crucial components of the first line of immunological defense against infection, and are critically involved in immunological activation/control (2). Pathogens taken up by immature DCs are processed for the surface presentation of antigens, which is accompanied by a multifaceted activation program leading to functional DC maturation. Matured DCs enhance the major histocompatibility complex (MHC) class I-mediated activation of cytotoxic CD8+ T cells (3), and are critical producers of pro-inflammatory cytokines as well as bactericidal effectors such as nitric oxide (NO) (4).
Different intracellular bacterial pathogens preferentially use these and other APCs as initial host cells during infection. This includes Coxiella burnetti, a zoonotic, Gram-negative bacterium (5). Coxiella exhibits a broad host range, including domestic ruminants, rodents, and humans (6). In livestock, coxiellosis usually causes abortions or birth of weak offspring, while a flu-like illness (Q fever) occurs in humans (7). Transmission occurs via inhalation of contaminated aerosols/dust (8), during which C. burnetii specifically infects monocytes, macrophages, and DCs (5). After entering host cells, the bacterium recruits compartments of the autophagy pathway to establish its characteristic Coxiella-containing vacuole (CCV) (9). C. burnetii undergoes a biphasic development cycle, forming large and small cell variants (LCVs and SCVs). During the intracellular growth cycle, which lasts several days, non-replicating SCVs differentiate into replicating LCVs, which in turn differentiate back into SCVs.
The existence of a characteristic lipopolysaccharide (LPS) phase (Ph) variation in C. burnetii (i.e., between virulent PhI and avirulent PhII) was first described by Stoker and Fiset (10) after serial passaging in vitro in embryonated eggs. LPS of PhII bacteria is referred to as “intermediate/truncated” due to the absence of terminal polysaccharides found in PhI LPS (10). Most interestingly, this appears to be not exclusively associated with loss of genomic information (11), and both phase variants show comparable growth and SCV/LCV development in CCVs of infected cells (12). PhII whole-cell vaccines (WCVs) (13) induce protective immunity (14, 15) and also confer immunity against subsequent infections with PhI C. burnetii (16). Pathogenic and physiological mechanisms controlling Coxiella’s in vivo phase variation are not clear. Nevertheless, previous studies describe an early/acute response against PhII and a late reaction against PhI bacteria (17), raising the question of whether this reflects a sequential switch in the occurrence of early PhII to late PhI Coxiella under in vivo conditions. In this context, recent working models discuss a bacterial subversion strategy in which PhII C. burnetii might act in vivo as a subversive decoy that specifically redirects the immune response in the early phase of infection, thereby delaying neutralization/elimination of PhI Coxiella that appear later (18).
Cellular immune responses against PhI/II Coxiella are critical for eliminating the pathogen (19, 20). Th1-based immunity leads to the release of interferon-γ (IFN-γ), which controls the intracellular establishment and replication of C. burnetii in both immune and non-immune cells (19, 21). As an effective subversive strategy, C. burnetii prevents the activation of infected immune cells to evade recognition/elimination by the cellular immune system. In infected macrophages, C. burnetii induces polarization toward the anti-inflammatory M2 phenotype, associated with enhanced bacterial growth (22). Moreover, one study provided evidence that PhI Coxiellainfects DCs without inducing their functional maturation (23). It was speculated that PhI, but not PhII, LPS acts as a “passive virulence factor” (23), which shields TLR2 on infected DCs. However, this idea raises several issues, as other studies suggest that there may not be such pronounced differences in the immune evasion/subversion properties of PhI and II Coxiella in infected APCs (24, 25). Indeed, different groups provide evidence that PhII C. burnetii also has the potential to attack different immune pathways of infected APCs (26–29). Taken together with the notion that PhII C. burnetii, which may occur in the acute phase of infection, plays a critical role in DC-mediated immune defense against Coxiella in vivo (30), more experimental work is required to understand the APC-based response against this bacterial phase variant and its subversion strategy in DCs.
Here, we discovered a new mechanism of immune subversion in PhII C. burnetii-infected DCs that are directly attributable to the autocrine release and action of immunosuppressive transforming growth factor-β (TGF-β). Moreover, we found that IFN-γ restores MHC I presentation as well as cytokine/chemokine secretion in Coxiella-infected DCs, and ensures efficient cellular self-defense via the induction of nitric oxide synthase (iNOS)/NO. This IFN-γ-induced NO synthesis eliminates C. burnetii by affecting the intracellular bacterial growth/development cycle. The potentially mitotoxic effects of iNOS-produced NO in infected DCs are countered by switching the cellular metabolism from oxidative phosphorylation (OXPHOS) to glycolysis, thus ensuring host cell survival. To our knowledge, this is the first cell biological evidence that TGF-β/IFN-γ antagonism may play a critical role during the early response to C. burnetii.
RESULTS
PhII C. burnetii infection affects MHC I presentation and maturation of DCs.
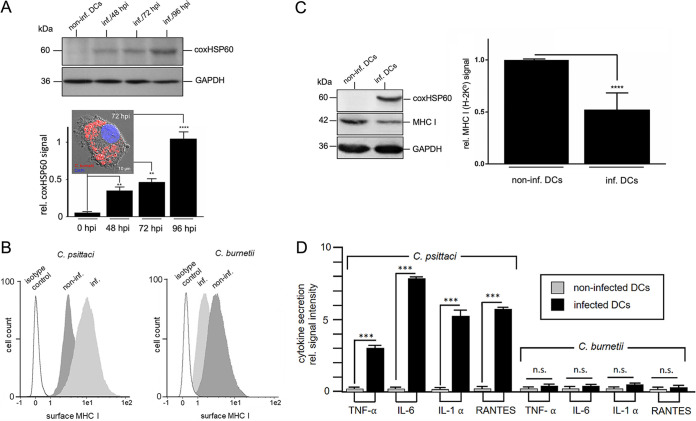
Several immunological factors controlling C. burnetii infection have been identified in murine systems (31). Therefore, we used the mouse DC line JAWS II (32) and the PhII C. burnetii strain RSA 439 NMII (33) as a model system to characterize the pathogen/host cell interaction of PhII Coxiella-infected DCs under BSL 2 conditions. We first analyzed the course of DC infection with PhII Coxiella (0 to 96 hours postinfection [hpi]) by Western blotting via detection of C. burnetii HSP60 (coxHSP60) as a proxy for bacterial growth, as previously described (34). In infected DCs, the expression of coxHSP60 correlated directly with the progression of infection (Fig. 1A). In line with this, quantitative real-time PCR (qRT-PCR) analysis revealed that PhII Coxiella grows in the host cells (Fig. S1). Up to 70% of the DCs in culture were infected by C. burnetii (MOI 10) at 72 hpi (Fig. S1, S2, and S4). Moreover, immunofluorescence studies showed accumulation of C. burnetii-positive vacuoles in DCs with diameters of 1 to 5 μm at 72 hpi (Fig. 1A, lower panel, inset). These intracellular bacterial structures display colocalization with lysosomal and autophagolysosomal vacuoles (Fig. S2), suggesting that in infected DCs, Coxiella resides in membrane-enclosed acidic compartments.
FIG 1.
PhII C. burnetii infection causes MHC I- and cytokine/chemokine downregulation in DCs. (A) Western blot of DC lysates (non-infected/infected JAWS II, MOI 10/48-96 hpi) stained for coxHSP60 (top). GAPDH was used as loading control. coxHSP60 signals were quantified by densitometric analysis (bottom). The background values in control cells at 0 hpi were set to 0.1 (**, P < 0.01; ****, P < 0.0001 versus 0 hpi cell lysate; n = 3 ± SD). Immunofluorescence of C. burnetii (red) infected JAWS II (72 hpi) is shown as an inset (DNA is stained via DAPI [blue]). (B) Flow cytometry of surface MHC I on C. psittaci- (left, MOI 10, 48 hpi) and C. burnetii-infected (right, MOI 10, 72 hpi) JAWS II cells. Isotype and non-infected controls were included. (C) Western blot of MHC I expression in non-infected and C. burnetii-infected JAWS II cells (MOI 10, 72 hpi) (left). GAPDH was used as loading control. MHC signals were quantified by densitometric analysis (right). The signal in non-infected cells was set to 1 (****, P < 0.0001 versus control [non-infected]; n = 6 ± SD). (D) Profile of secreted cytokines/chemokines during C. burnetii infection of DCs. JAWS II were infected for 72 h with C. psittaci (MOI 10, 48 hpi) or C. burnetii (MOI 10, 72 hpi). Non-infected cells served as control. Secretion of various cytokines/chemokines (i.e., TNF-α, IL-6, IL-1α, and RANTES) was analyzed by cytokine/chemokine multiplex array. The values obtained for non-infected cells were set to 0.1. (n.s.; ***, P < 0.001 versus control; n = 3 ± SD).
Next, we examined the surface expression of MHC I molecules, which usually are upregulated during the maturation of bacteria-infected DCs (35), 72 hpi with C. burnetii by flow cytometry (Fig. 1B). As controls, we used Chlamydia psittaci- (48 hpi) (Fig. 1B, left) and non-infected DCs. Consistent with our previous findings, Chlamydia-infected DCs exhibited a pronounced induction of surface MHC I expression (35), whereas C. burnetii infection resulted in reduced MHC I surface levels (Fig. 1B, right). This was also the case when increased numbers of bacteria were used to infect DCs (MOI 100) (Fig. S2). Consistent with this, corresponding Western blots revealed a decrease in total MHC I during Coxiella infection (Fig. 1C). The observed defect in DC maturation upon Coxiella infection was also evident by the lack of surface induction of MHC II and co-stimulatory molecules (CD40, CD80, and CD86) (Fig. S2). Moreover, in contrast to Chlamydia, Coxiella failed to trigger any substantial TNF-α, IL-6, IL-1β, or RANTES release (Fig. 1D), suggesting that PhII C. burnetii, unlike C. psittaci, does not activate the DCs it infects. In support of this, IL-12 was also not detected in the culture supernatant of Coxiella-infected DCs (Fig. S2).
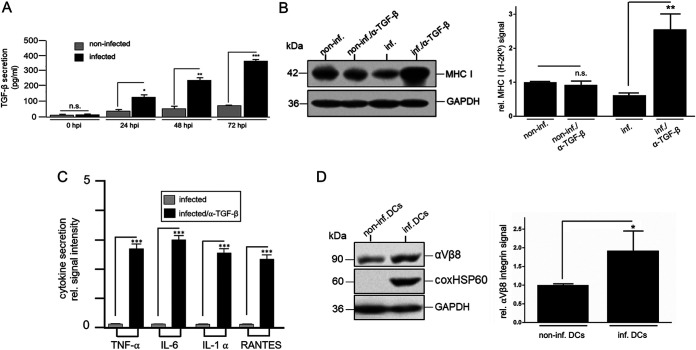
It is well known that autocrine/paracrine TGF-β signaling negatively influences the maturation, function, and antigen presentation of DCs (36). Consequently, TGF-β has supportive effects on the course of bacterial infections (37). Thus, we next looked at whether, and to what extent, TGF-β is involved in the observed suppression of MHC I presentation in Coxiella-infected DCs. Culture supernatants of infected DCs (0 to 72 hpi) were first analyzed by ELISA for elevated TGF-β levels. Compared with non-infected controls, culture supernatants from C. burnetii-infected DCs showed significantly higher TGF-β release, which increased over time (Fig. 2A). Based on this, we next analyzed the autocrine influence of TGF-β on MHC I expression using antibody neutralization experiments. For these experiments, C. burnetii- and non-infected DCs were cultured for 72 h in the presence or absence of a TGF-β-neutralizing antibody, and the corresponding cell extracts were stained for MHC I by Western blotting. Compared with corresponding controls, neutralization of TGF-β caused a significant increase in MHC I expression in C. burnetii-infected DCs (Fig. 2B). TGF-β neutralization also positively influenced the secretion of TNF-α, IL-6, IL-1β, and RANTES (Fig. 2C). TGF-β receptor binding occurs exclusively in the presence of membrane-anchored integrins, such as αVβ8-integrin on DCs (38). Therefore, we wanted to know whether the expression of TGF-β-activating αVβ8-integrin was also altered in infected DCs. Western blot analysis revealed increased steady-state levels of αVβ8-integrin in Coxiella-infected DCs compared with the corresponding controls (Fig. 2D), suggesting that the elevated TGF-β release is accompanied by an augmented expression of the activating integrin. Therefore, it is tempting to assume that the infection-induced suppression of MHC I and cytokine/chemokine release in DCs is due to enhanced functional interplay between TGF-β and αVβ8-integrin.
FIG 2.
TGF-β/αVβ8-mediated suppression of MHC I in PhII C. burnetii-infected DCs. (A) TGF-β secretion by non-infected and infected JAWS II (0-72 hpi, MOI 10) was measured by ELISA. Non-infected cells served as a negative control (n.s.; *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus control [0 to 72 h]; n = 3 ± SD). (B) Effect of TGF-β-neutralization on MHC-I expression. Western blots of C. burnetii- and non-infected JAWS II (MOI 10, 72 hpi) in the presence of TGF-β-neutralizing or isotype/control antibodies (left) were stained for MHC I. GAPDH was used as loading control. MHC I signals were quantified by densitometric analysis (right). MHC I signals obtained for non-infected cells treated with control antibodies were set to 1. (n.s.; **, P < 0.01 versus non-infected or infected controls; n = 3 ± SD). (C) Effect of TGF-β neutralization on cytokine/chemokine secretion by Coxiella-infected DCs. JAWS II were infected with C. burnetii (MOI 10, 72 hpi) in the presence of TGF-β-neutralizing or isotype/control antibodies. Secretion of TNF-α, IL-6, IL-1α, and RANTES was analyzed using a cytokine/chemokine multiplex array. The values for infected control cells were set to 0.1 (***, P < 0.001 versus control; n = 3 ± SD). (D) αVβ8-integrin expression in Coxiella-infected DCs. JAWS II were infected with C. burnetii (MOI 10, 72 hpi) or left uninfected (left). GAPDH served as loading control. αVβ8-signals were measured by densitometry (right). The αVβ8-signal in non-infected cells was set to 1 (*, P < 0.05 versus non-infected control; n = 3 ± SD).
IFN-γ rescues MHC I presentation in PhII C. burnetii-infected DCs.
Our studies revealed that the receptor for IFN-γ (CD119 [IFNGR1]) is not downregulated on the surface of DCs by Coxiella infection (Fig. 3A). As a major antagonist of TGF-β, which regulates Th1 immune responses in the opposite direction, IFN-γ enhances MHC I presentation by DCs (39), and plays a critical role during Q fever infection (19). Indeed, we observed a comparable induction of total MHC I in both Coxiella- and non-infected DCs in Western blots in the presence of IFN-γ (12 h) ante infection (hai)/7 hpi compared with corresponding controls (Fig. 3B). Further experiments demonstrated that pre- or post-treatment with IFN-γ also caused a marked reduction in αVβ8-integrin levels in infected DCs (Fig. 3C). Taken together, this suggests that IFN-γ abrogates the TGF-β/αVβ8-mediated suppression of MHC I presentation in Coxiella-infected DCs. Consistent with this, flow cytometry (Fig. 3D) and immunofluorescence (Fig. 3E) also showed augmented MHC I surface presentation in Coxiella-infected DCs treated with IFN-γ when compared to infected DCs without IFN-γ. No detectable difference was found between pre- and postinfection treatment with IFN-γ, suggesting that under both experimental conditions, IFN-γ is able to rescue MHC I presentation in infected DCs. Furthermore, mass spectrometry of the MHC I-bound peptide cargo (Fig. 3F) revealed IFN-γ-induced presentation of Coxiella-specific peptide antigens. Notably, two H-2Kb-presented Coxiella peptide antigens (LAGLINAI and LGANVIPVL, derived from the transport permease CBU_0933 and the multifunctional fusion protein CBU_1155, respectively) were specifically identified as prominent MHC I cargo in infected DC cells treated 7 hpi with IFN-γ, suggesting that the IFN-γ-mediated rescue of MHC I presentation in C. burnetii-infected DCs is also accompanied by efficient processing and surface presentation of bacterial peptide antigens. Since TNF-α is critical for the proper function of DCs (40), and thus also the control of Coxiella’s growth/replication in host cells (28), we also investigated whether IFN-γ restores the secretion of this anti-Coxiella cytokine in infected DCs. As shown in Fig. 3G, infected DCs indeed recovered their ability to secrete TNF-α in the presence of IFN-γ. This was also true for IL-6, IL-1α, and RANTES, whose secretion was also inhibited by C. burnetii infection (c.f. Fig. 1D), and are well-known targets of TGF-β suppression (41–43).
FIG 3.
IFN-γ-mediated rescue of MHC I presentation in PhII C. burnetii-infected DCs. (A) CD119 (IFNGR1) surface expression. C. burnetii-infected (MOI 10, 72 hpi) or uninfected JAWS II cells were analyzed by flow cytometry for CD119 (IFNGR1) surface expression. Isotype controls with non-infected cell were included. (B) MHC I expression. C. burnetii- (MOI 10) and non-infected JAWS II were cultured in the presence or absence of IFN-γ (12 hai/7 hpi). MHC I expression levels were analyzed at 72 hpi via Western blot (top). GAPDH was used as loading control. MHC I signals were quantified by densitometry (bottom). Untreated non-infected cells were set to 1 (**, P < 0.01; ***, P < 0.001 versus non-infected or infected untreated control; n = 3 ± SD). (C) αVβ8-integrin expression. C. burnetii- (MOI 10) and non-infected JAWS II in the presence or absence of IFN-γ (12 hai/7 hpi) 72 hpi were analyzed by Western blotting for αVβ8-integrin expression (left). GAPDH served as loading control. αVβ8-integrin signals were quantified by densitometry (right). The signal of untreated cells was set to 1 (****, P < 0.0001 versus infected control; n = 3 ± SD). (D) Surface MHC I expression. C. burnetii-infected (MOI 10, 72 hpi) or non-infected JAWS II (+/− IFN-γ, 12 hai/7 hpi) cells were analyzed by flow cytometry. Isotype controls were included. (E) Immunofluorescence analysis of surface MHC I expression (green) and intracellular C. burnetii antigen (red) in non-infected and infected (MOI 10, 72 hpi) JAWS II (+/− IFN-γ, 12 hai/7 hpi). DNA was stained via DAPI (blue). (F) Peptide spectra of eluted MHC I-bound antigens (sketched on the left) from C. burnetii-infected DCs in the presence of IFN-γ. The red boxes and insets of magnified spectrum regions show 2 identified MHCI-bound peptides of C. burnetii (LAGLINAI, CBU_0933, and LGANVIPVL, CBU_1155) detected at 784.501 and 895.565 m/z, respectively. (G) Cytokine/chemokine secretion. JAWS II cells were either infected with C. burnetii (MOI 10, 72 hpi) or left uninfected in the presence or absence (untreated) of IFN-γ (7 hpi). Secretion of TNF-α, IL-6, IL-1α, and RANTES was analyzed using a cytokine/chemokine multiplex array. The values for non-infected cells were set to 0.1 (***, P < 0.001 versus control; n = 3 ± SD).
IFN-γ promotes efficient bacterial elimination in PhII C. burnetii-infected DCs.
To investigate the influence of IFN-γ on host cell-pathogen interaction, we infected DCs with red-fluorescent C. burnetii in the presence or absence of this cytokine, and analyzed the cultures for 96 h by LCI (Fig. 4A). Interestingly, infected cells without IFN-γ showed 2 characteristic sequential phases during cultivation. Phagocytic uptake of C. burnetii was initially followed by high motility of infected DCs (i.e., at 24 to 60 h) (Fig. 4A, top). After unsuccessful attempts to combat the infection (i.e., 36 to 60 h post uptake), there was an abrupt cessation of cell movement (Fig. 4A, top), accompanied by an increase in the bacterial load (Fig. 4B). Quantification of the relative fluorescence intensity of C. burnetii per cell revealed a 3-fold increase in the bacterial signal in the infected DCs. A completely different scenario was seen in the presence of IFN-γ. Throughout the whole course of infection, IFN-γ-treated DCs maintained their morphological dynamics (Fig. 4A, bottom). Most importantly, they did not permit the growth of the engulfed bacteria (Fig. 4B), indicating a negative effect of IFN-γ on bacterial growth and development. To gain deeper insight into the intracellular fate of Coxiella in the presence and absence of IFN-γ, we next examined C. burnetii-infected DCs by electron microscopy. Infection experiments performed in the absence of IFN-γ showed the formation of intact Coxiella-containing vacuoles (CCVs) in DCs (Fig. 4C, left, inset). In contrast, in the presence of IFN-γ (added either 12 hai or 7 hpi), only a reduced number of bacteria without any proper CCV formation were observed (Fig. 4C, middle and left, inset). Consistent with this, corresponding qRT-PCR and Western blot experiments revealed that genome equivalents (GEs) (Fig. 4D, left) and coxHSP60 signals (Fig. 4D, right), respectively, were significantly reduced in infected DCs in the presence of IFN-γ (12 hai/7 hpi). As both pre- and post-treatment with IFN-γ yielded comparable results, the latter approach (with IFN-γ addition at 7 hpi) was chosen for subsequent re-infection studies with reporter cells. Here, we observed that bacterial extracts isolated from infected/IFN-γ-treated DCs had drastically reduced infectivity (≥75% reduction) compared with corresponding extracts from infected DCs not treated with IFN-γ (Fig. 4E). Re-infection experiments over 72 h revealed that only bacteria from untreated DCs multiply well in reporter cells (Fig. S3). In conclusion, our results demonstrate that IFN-γ enables PhII Coxiella-infected DCs to restore their MHC I presentation and control intracellular bacterial growth and subversion of the infected cell.
FIG 4.
IFN-γ-induced cellular self-defense in PhII C. burnetii-infected DCs. (A) Long-term time-lapse image series of C. burnetii infection in DCs. 1 × 104 DCs were infected with C. burnetii (MOI 50) in the absence (top) or presence (bottom) of IFN-γ. LCI was started 1 h post uptake, and continued for about 60 h at 37°C in a heated chamber. Time-lapse image series were acquired with a Leica THUNDER imager (10× magnification, imaging interval, 15 min; frame rate, 4 frames per hour; scale bar, 75 μm). Images show an overlay of fluorescence and phase-contrast images. Insets show enlargements of the fluorescence, corresponding to the analyzed bacterial structures (red, arrowheads) before/after cellular uptake. LCI movies of the depicted image sequences are available on request. (B) Quantification of the relative fluorescence intensity of intracellular C. burnetii antigen following infection in the presence or absence of IFN-γ during long-term time-lapse recording. ImageJ was used to measure the fluorescence intensity in the respective red fluorescence images. Fourteen infected host cells (7 each in the presence or absence of IFN-γ) were used to quantify Coxiella-fluorescence signals. The measurement was started after bacterial uptake by DCs, and continued in 12 h intervals up to 60 h post uptake. (*, P < 0.05; **, P < 0.01 versus IFN-γ-treated cells; n = 7 ± SD). (C) TEM of C. burnetii-infected JAWS II cells (MOI 10) after 72 hpi in the absence of IFN-γ (left) or with IFN-γ treatment either 12 hai (middle) or 7 hpi (right). Enlarged photographs of the white-framed sections show the morphology/structure of the intracellular bacteria (colored green). (D) Analysis of genome content and coxHSP60 expression. C. burnetii (MOI 10) infection was performed in JAWS II cells in the presence or absence of IFN-γ (added either 12 hai or 7 hpi, as indicated). Genome equivalents (GEs) were quantified following gDNA isolation and qPCR (based on C. burnetii dotA/T4SS) (left). coxHSP60 expression was densitometrically analyzed via Western blot (non-infected cells were included as a negative control, and samples were normalized to GAPDH) (left). The results are plotted as relative bacterial load (each n = 3 ± SD, **, P < 0.01 versus control [- IFN-γ]). A representative Western blot stained for coxHSP60 is shown (right). (E) Impact of IFN-γ on the infectivity of C. burnetii isolated from DCs. JAWS II were infected with C. burnetii (MOI 10, 72 hpi), and treated or not with IFN-γ (7 hpi). Subsequently, intracellular bacteria were prepared by host cell homogenization and used to infect L929 reporter cells (for a further 72 h). The analysis of re-infection was performed by flow cytometry. To detect/quantify C. burnetii-positive cells (green), the negative cell population (black) was identified and gated based on corresponding non-infected controls and then subtracted from the total cell population in infected samples (left). The corresponding quantification (right) shows the relative amount of re-infected reporter cells. Re-infected controls from untreated infected DCs were set to 1 (****, P < 0.0001 versus re-infected control; n = 9 ± SD).
NO plays a critical role in the cellular self-defense of PhII C. burnetii-infected DCs.
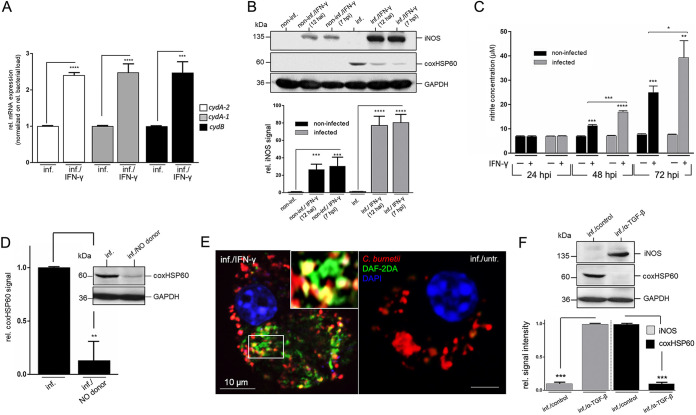
It is known that in infected APCs, IFN-γ substantially induces the production of reactive nitrogen species (RNS) by iNOS/NO (44), which attacks respiration, growth, and development of intracellular bacteria (45). Nevertheless, pathogens are able to respond to RNS by upregulating protective detoxification mechanisms mediated by cytochrome bd (46). Thus, we first investigated the effect of IFN-γ on Coxiella’s cytochrome bd expression in infected DCs. The qPCR experiments depicted in Fig. 5A demonstrated that mRNA levels (normalized to bacterial load and GEs) of the cytochrome bd subunits cydA-2 (CBU_0965), cydA-1 (CBU_0218), and cydB (CBU_0966) were significantly upregulated in the presence of IFN-γ. This suggests that, in the presence of IFN-γ, a bacterial counter-reaction is directed against the action of DC-produced RNS. Based on this, we next asked whether IFN-γ-induced iNOS/NO is functionally involved in the cellular self-defense of PhII C. burnetii-infected DCs. Western blot showed that iNOS expression in both non-infected and infected DCs increased significantly after IFN-γ-treatment and that this induction correlated with a marked reduction in bacterial load, as indicated by the decrease in coxHSP60 (Fig. 5B). As expected, iNOS induction was more pronounced in infected DCs than in uninfected DCs due to the additional presence of bacterial pathogen-associated molecular patterns (PAMPs). Consistent with these observations, Griess assays in the presence of IFN-γ showed enhanced NO production, which was higher in infected DCs than in non-infected DCs (Fig. 5C). To determine the extent to which NO is responsible for cellular self-defense of Coxiella-infected DCs, the corresponding cultures were treated with the NO donor DETA NONOate. Infected DCs showed a significantly reduced bacterial load in the presence of the NO donor with a cellular infection phenotype similar to IFN-γ-treated cultures (Fig. 5D). In addition, microscopic studies using DAF-2DA detected host cell-produced NO in spatial proximity to bacterial structures in Coxiella-infected DCs (Fig. 5E). Since pathogen-triggered iNOS expression is downregulated by TGF-β (47), we wanted to know whether TGF-β-suppression of C. burnetii-infected DCs (Fig. 2) is related to its effect on iNOS. Indeed, we observed in Western blots that antibody neutralization of the autocrine TGF-β action resulted in readily detectable iNOS levels in Coxiella-infected DCs, accompanied by a marked reduction in the bacterial load (Fig. 5F) and growth (Fig. S4). Thus, even in the absence of IFN-γ, infected DCs appear to increase iNOS expression and fight intracellular Coxiella once the suppression by TGF-β is abolished. This suggests that the opposing influences of TGF-β and IFN-γ on infected DCs also play a critical role in iNOS/NO-based cellular self-defense.
FIG 5.
IFN-γ-induced iNOS/NO in infected DCs counteracts bacterial growth and subversion of DC function. (A) PCR analysis of cytochrome bd (cydA-2, cydA-1, cydB) transcript levels. JAWS II cells were infected with C. burnetii (MOI 10) and treated or untreated with IFN-γ (added 7 hpi) for 72 hpi. mRNA expression levels for cytochrome bd (cydA-2, cydA-1, cydB) were normalized to the relative bacterial load (based on GEs and coxHSP60 levels), and untreated controls were set to 1 (***, P < 0.001; ****, P < 0.0001 versus infected control; n = 3 ± SD). (B) iNOS expression in DCs before/after IFN-γ stimulation. C. burnetii- (MOI 10) and non-infected JAWS II cells were cultured in the presence or absence of IFN-γ (12 hai/7 hpi). iNOS expression was analyzed at 72 hpi via Western blot (top). GAPDH and coxHSP60 were used as loading and infection controls, respectively. iNOS signals were quantified by densitometry (bottom). The background signals in untreated cells (non-infected/infected) were set to 1 (***, P < 0.001; ****, P < 0.0001 versus control [non-infected/infected]; n = 3 ± SD). (C) Quantification of nitrite during C. burnetii infection in DCs. C. burnetii-infected JAWS II cells (MOI 10) were incubated in the presence and absence of IFN-γ (7 hpi) for 24 to 72 h. At different time points, supernatants were analyzed by Griess assay. Non-infected cells served as a negative control (*, P < 0.05 versus control [non-infected/IFN-γ, 72 h]; ***, P < 0.001 versus control [non-infected/IFN-γ, 48 h]; **, P < 0.01 versus infected control [72 hpi]; ***, P < 0.001 versus control [non-infected, 48 h and 72 h]; ****, P < 0.0001 versus control [infected, 48 hpi]; n = 3 ± SD). (D) Effect of NO on C. burnetii infection in DCs. JAWS II cells were infected with C. burnetii (MOI 10) and cultured in the presence or absence of a NO producer (DETA NONOate, 100 μM, 7 hpi) for 72 h. A representative Western blot stained for coxHSP60 is shown (inset). GAPDH served as loading control. coxHSP60 signals were quantified by densitometry. The signal of untreated infected cells was set to 1 (**, P < 0.01 versus infected control; n = 3 ± SD). (E) Fluorescence analysis of endogenous NO production. JAWS II cells were infected with C. burnetii (MOI 10, 72 hpi) in the presence (left) or absence (right) of IFN-γ (7 hpi). Bacterial antigen (red) and NO production were stained (DAF-2 DA, green). DNA was stained via DAPI (blue). The insert in the left image shows a magnified subsection and highlights the co-localization between the NO marker and bacterial structures. (F) JAWS II cells were infected with C. burnetii (MOI 10, 72 hpi) in the presence or absence of a TGF-β neutralizing antibody. Cell lysates were analyzed by Western blots stained for iNOS and coxHSP60. GAPH was used as loading control. Signals were quantified by densitometry (iNOS left; coxHSP60 right) and normalized to the loading control (bottom). Signal values of iNOS (inf./α-TGF-β) and coxHSP60 (inf./control) were set to 1 (***, P < 0.001 versus infected controls; n = 3 ± SD).
Antibacterial defense of PhII C. burnetii-infected DCs relies primarily on IFN-γ-induced iNOS/NO.
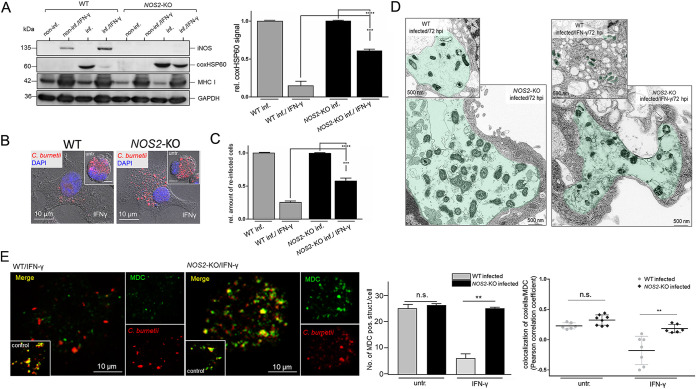
Interestingly, multiple bacterial inclusions with a diameter of ≥ 5 μm were often observed in the DC line (i.e., JAWS II) used in our experiments (i.e., Fig. 1A and Fig. 3E). This characteristic phenotypic marker of cell infection was also observed in PhII C. burnetii-infected bone marrow-derived DCs (BMDCs) isolated from C57BL/6 mice (Fig. 6A), suggesting, in agreement with previous findings (48), that immortalized and primary BMDCs exhibit comparable host cell-pathogen interactions. Indeed, further experiments with Coxiella-infected primary BMDCs (Fig. 6) confirmed our findings with immortalized DCs (Fig. 1 to 5), including (i) IFN-γ-mediated rescue of suppressed MHC I presentation and induction of iNOS (Fig. 6B), (ii) co-localization of bacteria and host cell-produced NO in the presence of IFN-γ (Fig. 6C), and (iii) reduced bacterial infectivity in the presence of IFN-γ and/or a NO donor (Fig. 6D). As in the case of JAWS II, re-infection studies over 72 h demonstrated that only Coxiella from untreated primary BMDCs grow well in reporter cells (Fig. S5). Therefore, we continued our studies with immortalized DCs, which provided a more tractable experimental system for a CRISPR/Cas9 approach to generate a loss-of-function mutation within the iNOS gene (NOS2) (GenBank: NC_000077.7), thereby offering direct insights into the significance of iNOS/NO in cellular self-defense against C. burnetii. Wildtype- (WT-) and NOS2 knockout- (NOS2-KO-) DCs were infected with C. burnetii in the presence and absence of IFN-γ, and analyzed by Western blotting (72 hpi). As shown by the comparison of WT- and NOS2-KO-DCs, the absence of iNOS/NO in IFN-γ-treated knockout-variants significantly impaired Coxiella’s elimination (Fig. 7A), suggesting that iNOS/NO plays a vital role in the cellular self-defense of DCs against C. burnetii. This was further confirmed by immunofluorescence studies of infected WT- and NOS2-KO-DCs in the presence or absence of IFN-γ (Fig. 7B). The increased survival of Coxiella in IFN-γ-treated NOS2-KO-DCs compared to WT-DCs was reflected in the different re-infection rates of the respective bacterial extracts (Fig. 7C), and the distinct pathogen distribution observed in TEM with the 2 DC variants (Fig. 7D). In the presence of IFN-γ, infectious bacteria and intact CCVs disappeared markedly in infected WT-DCs (Fig. 7D, top), whereas both were almost entirely preserved in infected NOS2-KO-DCs (Fig. 7D, bottom).
FIG 6.
Bacterial subversion and IFN-γ-based rescue of DC function in PhII C. burnetii-infected primary murine BMDCs. (A) Immunofluorescence analysis of the C. burnetii infection. JAWS II cells (left) or primary BMDCs were infected with C. burnetii (MOI 10, 72 hpi). Bacterial structures were stained in red. DNA was stained via DAPI in blue. (B) MHC I and iNOS expression in primary BMDCs. C. burnetii- (MOI 10) and non-infected primary BMDCs were treated or untreated (untr.) with IFN-γ (added 7 hpi). MHC I and iNOS expressions were analyzed at 72 hpi via Western blot (top). GAPDH was used as loading control. The signals were quantified by densitometry (MHC I, lower right; iNOS, lower left). Untreated cells (either non-infected or infected) were set to 1 (**, P < 0.01 versus controls without IFN-γ; n = 3 ± SD). (C) Fluorescence analysis of NO production. Primary BMDCs were infected with C. burnetii (MOI 10, 72 hpi). Bacterial structures (red) and NO (DAF-2 DA, green) were stained. DNA was stained via DAPI (blue). The separate channels for Coxiella, DAF-2 DA, and DAPI staining are shown at the sides of the respective merged image. (D) Impact of IFN-γ and NO on the infectivity of C. burnetii isolated from primary BMDCs. Primary BMDCs were infected with C. burnetii (MOI 10), treated with either IFN-γ (7 hpi) or an NO donor (DETA NONOate, 100 μM, 7 hpi), or were left untreated. At 72 hpi, intracellular bacteria were prepared by host cell homogenization and used to re-infect reporter L929 cells (for a further 72 h). The analysis of re-infection was performed by flow cytometry of Coxiella-stained reporter cells. To detect/quantify C. burnetii-positive cells (green), the negative cell population (black) was identified and gated in non-infected controls and then subtracted from the total cell population in infected samples (top). The corresponding plot (bottom) shows the relative amount of re-infected reporter cells. Cells re-infected with bacterial extracts lacking IFN-γ or NO donor were used as controls, and the number of Coxiella-positive re-infected cells was set to 1 (***, P < 0.001 versus control; n = 3 ± SD).
FIG 7.
iNOS/NO is key in the IFN-γ-mediated defense of PhII Coxiella-infected DCs. (A) coxHSP60 expression of infected after IFN-γ stimulation. Non-infected and C. burnetii-infected (MOI 10) WT- and NOS2-KO-DCs were cultured in the presence or absence of IFN-γ (7 hpi). coxHSP60 expression was analyzed at 72 hpi via Western blot (left). GAPDH was used as loading control. Signals were quantified by densitometry (right). Untreated and infected DCs (WT and NOS2-KO) were set to 1 (***, P < 0.001 versus control [WT infected/IFN-γ]; n = 3 ± SD). (B) Immunofluorescence analysis of C. burnetii infection in WT- and NOS2-KO-DCs. C. burnetii-infected (MOI 10, 72 hpi) or non-infected WT- and NOS2-KO-DCs in the presence or absence of IFN-γ were stained for bacterial structures (red). DNA was stained via DAPI (blue). The immunofluorescence images (and corresponding insets of untreated cells) show the impact of IFN-γ on C. burnetii isolated from WT- and NOS2-KO-DCs. (C) WT- and NOS2-KO-cells were infected with C. burnetii (MOI 10), treated or not with IFN-γ (7 hpi). At 72 hpi, intracellular bacteria were prepared by host cell homogenization and used to re-infect L929 reporter cells (72 h). The analysis was performed by flow cytometry. The bar plot shows the relative amount of re-infected reporter cells. Untreated re-infected controls (WT inf. and NOS2-KO inf.) were set to 1 (***, P < 0.001 versus control [WT inf./IFN-γ and NOS2-KO inf./FN-γ]; n = 3 ± SD). (D) TEM of C. burnetii-infected (MOI 10) WT- (top) and NOS2-KO-DCs (bottom) in the presence (right) or absence (left) of IFN-γ (7 hpi). Bacterial structures/vacuoles were colored in green. (E) Fluorescence analysis of autophagosomes in C. burnetii-infected WT- and NOS2-KO-DCs. C. burnetii-infected (MOI 10, 72 hpi) WT- and NOS2-KO-DCs were treated or untreated (untr.) with IFN-γ (added 7 hpi). Cells were stained for bacterial structures (red) as well as with monodansylcadaverine (MDC, green) for autophagosome detection (left). In addition to the merged images, the individual channels are displayed on the side. The lower left insets in the merged images show untreated control infections. The number of MDC-positive vacuoles in WT- and NOS2-KO-DCs was quantified using ImageJ for 30 individual cells per condition (middle). The Pearson correlation coefficient was determined using the AxioVision software (Zeiss) (right) to analyze the co-localization between bacteria and MDC staining (n.s.; **, P < 0.01 versus control [WT-DCs infected with or without IFN-γ]; n = 30 ± SD).
Because induced iNOS/NO impairs the formation of autophagosomes (49), which are known to fuze with CCVs and are critically involved in the biogenesis and development of these bacterial compartments (50), we next wanted to know whether IFN-γ-induction of iNOS/NO affects the interaction of Coxiella with autophagosomes. To this end, we specifically stained autophagic vacuoles in Coxiella-infected WT- and NOS2-KO-DCs with monodansylcadaverine (MDC), which preferentially accumulates in multilamellar autophagosomes (51). Fluorescence microscopy shows that in the presence of IFN-γ, WT-DCs have a reduced level of MDC-positive compartments, whereas this does not happen in the NOS2-KO-DCs (Fig. 7E, left and middle). In addition, in the presence of IFN-γ and NOS2-KO-DCs, but not WT-DCs, there was retained colocalization of MDC and C. burnetii (Pearson correlation coefficient [PCC] of 0.2 to 0.5) (Fig. 7E, right). This suggests that IFN-γ-induced iNOS/NO in WT-DCs, in addition to its destructive effects on bacteria themselves (52) (c.f. Fig. 4 and 5), has a profound influence on the provision of autophagosomes for fusion, biogenesis, and development of CCVs. Our results obtained for WT- and NOS2-KO-DCs show that the IFN-γ-induced iNOS/NO pathway plays a crucial role in the cellular self-defense of Coxiella-infected DCs by impairing bacterial compartment formation, viability, and infectivity. It can be concluded that the synthesis and action of NO are both necessary and sufficient to eliminate Coxiella in infected DCs.
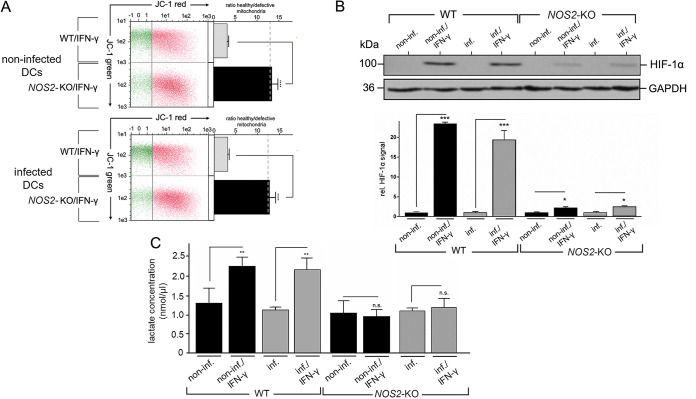
Mitotoxic effects of IFN-γ-induced NO might be counterbalanced by a metabolic switch in Coxiella- and non-infected DCs.
NO and its derivatives not only act on intracellular bacterial pathogens but also have detrimental effects on mitochondrial respiratory function (53). Indeed, a comparison of IFN-γ-treated WT- and NOS2-KO-DCs (either uninfected or infected) examining JC-1 staining revealed that NOS2-KO-DCs contained 4 to 5 times as many intact/healthy mitochondria as WT-DCs (Fig. 8A). Thus, compensatory mechanisms are required to protect IFN-γ-treated DCs from the side effects of their iNOS/NO-based self-defense against Coxiella. To ensure survival when mitochondria are functionally impaired, DCs are able to switch their metabolism from OXPHOS to glycolysis (54). An important regulator of this physiological process is hypoxia-inducible factor-1 alpha (HIF-1α) (55), which controls glycolytic gene expression and metabolism via its stabilization in cells (55). Therefore, we asked whether HIF-1α stabilization and the resulting metabolic switch might also occur in the presence of IFN-γ in Coxiella-infected and/or non-infected DCs. Western blot experiments showed a clear stabilization of HIF-1α in WT-DCs in the presence of IFN-γ, regardless of whether the cells were infected (Fig. 8B). A similar effect was observed in NOS2-KO-DCs, but it was greatly reduced compared to WT-DCs (Fig. 8B). In agreement with others (56), this suggests that induced NO production in cells plays a critical role in the stabilization of HIF-1α. Next, we analyzed whether a metabolic switch to glycolysis could explain the increased steady-state level of HIF-1α in IFN-γ-treated WT-DCs. To this end, we analyzed the cellular synthesis of the glycolytic product lactate. Non-infected and infected WT-DCs showed strongly elevated lactate production in the presence of IFN-γ, indicating increased glycolytic activity of these cells. In contrast, no such phenomenon was observed in IFN-γ-treated NOS2-KO-DCs (neither uninfected nor infected cells) (Fig. 8C). Thus, in the absence of the detrimental influence of NO on proper mitochondrial function (Fig. 8A) and a corresponding lack of HIF-1α stabilization (Fig. 8B), NOS2-KO-DCs seem to lack both the need and the ability to enhance glycolysis in the presence of IFN-γ (Fig. 8C). In summary, these results provide the first evidence that IFN-γ-induced iNOS/NO production facilitates efficient bacterial elimination in Coxiella-infected DCs, and possibly ensures, to some extent, the host cells' survival during this self-defense by balancing the mitotoxic effects of iNOS/NO by inducing a concomitant metabolic switch to glycolysis.
FIG 8.
Cellular self-protection by DCs against mitotoxic NO effects. (A) Intact and defective mitochondria. A JC-1-flow cytometry assay analyzed cellular mitochondrial activity/inactivity in IFN-γ-treated WT- and NOS2-KO-DCs (left part). JC-1 emits red fluorescence in cells with intact mitochondria, while the fluorescence is green when mitochondria are defective. The dashed line shows the average value of the mitochondrial JC-1 ratio (red/green) of control cells without infection and IFN-γ treatment. The corresponding plot (right) shows the ratio of healthy and defective mitochondria (****, P < 0.0001 versus control [WT/IFN-γ]; n = 3 ± SD). (B) HIF-1α expression. Non-infected and C. burnetii-infected WT- and NOS2-KO-DCs (MOI 10) were cultured in the presence or absence of IFN-γ (7 hpi). Protein expression for HIF-1α was analyzed at 72 hpi via Western blot (top panel). GAPDH served as loading control. HIF-1α signals were quantified by densitometry (WT and NOS2-KO, bottom panel). Untreated cells (infected WT and NOS2-KO) were set to 1 (*, P < 0.05; ***, P < 0.001 versus non-infected or infected control; n = 3 ± SD). (C) Quantification of lactate. Non-infected and C. burnetii-infected WT- and NOS2-KO-DCs (MOI 10, 72 hpi) in the presence or absence of IFN-γ (added 7 hpi) were analyzed for lactate content using ELISA (n.s.; **, P < 0.01 versus control [WT or NOS2-KO, non-infected or infected]; n = 3 ± SD).
DISCUSSION
Our findings indicate that the immunosuppressive IFN-γ antagonist TGF-β (57) acts as an effective tool in the subversion of DC function during Coxiella infection (Fig. 2). The positive effect of TGF-β on bacterial growth (22) (Fig. 5), as well as its negative influence on cellular self-defense, MHC I presentation, and cytokine/chemokine release by infected DCs (Fig. 2 and 5), could have a dramatic impact on infection control in vivo. In this context, it is interesting to note that TGF-β release has been associated with intracellular growth of Coxiella and pathogenesis in humans (58, 59). TGF-β is overproduced by APCs of patients with chronic Q fever (60). In line with this, Benoit et al. (22) observed for C. burnetii-infected macrophages upregulated expression of genes associated with M2 polarization, including TGF-β. Moreover, multiple genes involved in the TGF-β pathway seem to be associated with susceptibility to Coxiella (61). However, in light of these observations, as well as our own findings, the question arises as to why effects of TGF-β have not previously been observed in PhII Coxiella-infected human DCs (23). A possible explanation could be the use of the cytokine IL-4 to generate monocyte-derived DCs in vitro. TGF-β and IL-4 are known to act antagonistically (62). Moreover, IL-4 negatively affects TGF-β expression (and its receptor) (63), and generates human DCs with functional deficiencies (64). Since previous studies (23) performed their experiments in the presence of IL-4, it is reasonable to speculate that the infection-mediated effect of TGF-β was abolished.
The influence of TGF-β on PhII Coxiella-infected DCs (Fig. 2) also seems to be driven by the upregulation of TGF-β-activating αVβ8-integrin (Fig. 2). Previous studies suggested that the suppressive influence of TGF-β on DC function is mediated via increased αVβ8-integrin expression (65). TGF-β production/action is upregulated by bacterial infections (66). Consistent with our findings (Fig. 2), it was observed that the presence of bacterial PAMPs enhances both αVβ8-integrin and TGF-β activity in DCs (67). Autocrine TGF-β induction by bacterial infection is triggered via the TLR2/NF-κB and STAT3/Smad3 signaling pathways (68). It is interesting to note that PhII C. burnetii are sensed by TLR2 (69), which is involved in the immune response, but dispensable for bacterial clearance (69). Most interestingly, TLR2−/− mice are protected from re-infection by PhII C. burnetii, suggesting that under certain conditions, TLR2 favors Coxiella-infection (70). It will be of interest to gain more insight into the role of TLR2 signaling in the infection-mediated suppression of DC function. Interestingly, the T4SS-defective dotA mutant of PhII C. burnetii (which is engulfed by host cells, like DCs, but does not grow in them [71, 72]) (Fig. S1 and S6) showed a subversion phenotype in infected DCs similar to that of wildtype bacteria (Fig. S6), suggesting that functional DC impairment based on pathogen recognition begins early before C. burnetii establishes itself in the host cell by forming functional CCVs for its growth. This indicates that initial DC subversion might be independent of Coxiella’s effectors secreted via the T4SS (73). To date, more than 130 effector proteins of C. burnetii have been identified (73). It seems that they act on various pathways in host cells during infection. Thus, distinct effectors of Coxiella control the establishment of CCVs (74, 75), and influence cellular vesicle and protein transport (75, 76). In future studies, it will be interesting to find out whether similar processes also occur in infected DCs and affect their functional properties. Our observation that neither TGF-β-release nor its suppressive effects on MHC I presentation and cytokine/chemokine secretion were observed for chlamydial DC infections could be explained by the fact that TLR4 plays a crucial functional role in the activation of Chlamydia-infected APCs (77). As previously noted, a reciprocal/antagonistic relationship has been described between TLR4 and TGF-β, in which activated TLR4 inhibits the TGF-β pathway (78). This important cross-regulation might save Chlamydia-infected APCs from TGF-β suppression.
Downregulation of the IFN-γ receptor (IFNGR1/CD119), as described for other pathogens (79), was not observed in C. burnetii-infected DCs (Fig. 3), suggesting that Coxiella might not target the IFN-γ pathway at this step. Indeed, IFN-γ counters the TGF-β/αVβ8-induced immune subversion of PhII Coxiella-infected DCs (Fig. 3 to 7). This is facilitated by the antagonistic relationship between IFN-γ and TGF-β (80), in which the TGF-β/Smad and the IFN-γ/JAK-STAT pathways are in close exchange (81, 82). The negative influence of IFN-γ on TGF-β is further enhanced by parallel downregulation of αVβ8-integrin, which we also observed (Fig. 3) (38). IFN-γ contributes to the upregulation of iNOS/NO, MHC I, and cytokine/chemokine secretion via the JAK/STAT1 pathway (83–85). The primary innate source of IFN-γ is activated NK cells (86), which are functionally involved in the early fight against Coxiella infection (19). Most importantly, C. burnetii-infected NK cells are characterized by an increased release of IFN-γ (34), which, as our studies demonstrate (Fig. 3 to 7), appears to be essential for the abrogation of TGF-β suppression of infected DC function.
Our results support the notion that protective immunity against C. burnetii also involves the functional contribution of APCs (87) and IFN-γ (19). IFN-γ produced by various APC-interacting lymphocytes appears essential for controlling C. burnetii infection in vivo (19). In murine DCs, cellular self-defense (with a marked reduction in bacterial load) in the presence of IFN-γ (Fig. 5) and/or neutralization of TGF-β (Fig. 5, 6, and 7) is mainly due to opposing effects of these cytokines on iNOS/NO regulation (47, 88). In different species, iNOS/NO and its derivatives play a key role in combating various bacterial infections (52), including coxiellosis and Q fever (89, 90). Considering our results and those of Brennan et al. on PhI and II Coxiella (91), one can assume that iNOS/NO acts against Coxiella in a phase-independent manner. One possible explanation for why iNOS/NO plays such a dominant role in Coxiella-infected DCs is that reactive oxygen species (ROS) in these APCs are primarily used for antigen cross-presentation rather than actual pathogen killing (92). Importantly, the identification of TNF-α/iNOS-producing Tip-DCs suggests that iNOS/NO also plays a critical role in cellular self-defense in human APCs (93). Such Tip-DCs are also present in other mammals and display cellular and functional similarities to myeloid dendritic cells (mDCs) (94). Further, it is proposed that the differentiation of monocytes into Tip-DCs is mediated by IFN-γ (95). In particular, they seem to play a critical role in pathogen elimination and have an important impact on the polarization of the downstream T-cell response (95). Consequently, the absence of this DC subpopulation can lead to uncontrolled bacterial replication and, ultimately, host death (96). Since Tip-DCs are involved in fighting various bacterial infections (96, 97), it is reasonable to speculate that IFN-γ-activated Tip-DCs may also be involved in Coxiella infection in vivo.
Additional IFN-γ-induced processes, such as transferrin receptor (CD71) downregulation (98) or indoleamine 2,3-dioxygenase (IDO1)-mediated tryptophan degradation (99), may make further important contributions to cellular self-defense of infected DCs. Indeed, Coxiella seems to be dependent on CD71-mediated iron uptake in infected macrophages (100). In contrast, since iNOS/NO negatively modulates the expression/activity of IDO1 and vice versa in IFN-γ-primed murine and human APCs (101–103), one could assume that iNOS/NO and IDO1 are mutually exclusive in the defense against C. burnetii. However, in the absence of iNOS/NO (as in NOS2-KO-DCs) (Fig. 7 and 8), IDO1 might indeed exert a consequently increased effect on Coxiella infection.
Several bacterial PAMPs induce iNOS/NO in infected cells, even in the absence of IFN-γ (104, 105). Consistent with this, we observed that iNOS/NO is induced in DCs by Coxiella infection when the autocrine TGF-β blockage is neutralized (Fig. 5). TGF-β suppression of iNOS/NO expression/function at different cellular levels is a well-known phenomenon (47, 106, 107) that promotes intracellular growth of pathogens (108). However, since other cellular factors besides iNOS/NO are also controlled by TGF-β (109), future studies should focus on the precise molecular mechanisms of how exactly this immunosuppressive cytokine supports C. burnetii development and growth in infected APCs and other host cells.
Specifically, in the context of Coxiella infection, our studies suggest that IFN-γ-induced iNOS/NO affects bacterial growth in infected DCs by preventing the formation/establishment of CCVs (Fig. 8). The bactericidal action of NO results in large part from its attack on the electron transport chain (ETC) of the pathogen (110). In some cases, bacteria are able to counteract the action of NO by increased expression of the respiratory oxidoreductase cytochrome bd (111, 112), which has a high NO dissociation rate (113). Although Coxiella also has the potential to use this mechanism, it does not appear to be effective enough in protecting against NO, suggesting that iNOS/NO is also highly effective in attacking other critical targets of the pathogen (110) and its physiological needs besides the bacterial ETC. In particular, the host cell autophagosome (Fig. 7), which is essential for Coxiella’s survival and replication (114), is a known target of NO-based nitrosylation (115).
Coxiella-infected DCs seem to protect themselves from the mitotoxic effects of NO generation (116) by undergoing a switch from OXPHOS to glycolysis (Fig. 8). This metabolic process maintains ATP production despite the loss of mitochondrial function (40), and, thus, potentially ensures host cell survival during bacterial elimination. As a similar metabolic switch was observed in Chlamydia-infected DCs (40), we hypothesize that this reprogramming allows DCs to limit their infection by multiple pathogens by modulating the host environment while preserving their own metabolic processes. This is likely a critical feature of basic DC biology that enables the uptake of and subsequent fight against infectious pathogens while ensuring cellular survival and pathogen elimination.
In summary, we propose a working model based on our findings (Fig. 9) in which the Coxiella infection-related impairment of DC function can be attributed to the autocrine action of TGF-β. This leads to decreased MHC I presentation, as well as suppressed iNOS and cytokine responses. Effective cellular self-defense in infected DCs is mediated by IFN-γ-induced iNOS/NO production, which impairs Coxiella’s survival by inhibiting the formation/integrity of the CCVs. By this mechanism, the joint forces of IFN-γ-producing NK cells (34) and DCs are able to reduce Coxiella's growth at the immediate site of infection and prevent its subversion of DC function. Moreover, our results hint that the survival of infected DCs is ensured by a metabolic switch from OXPHOS to glycolysis. Clearly, further studies will be necessary to shed more light on this interesting first observation. At this early stage of defense against infection, impairment and/or (re)activation of DCs determines downstream immune responses and the outcome of C. burnetii infection. Future studies will reveal whether and how this NK cell/IFN-γ/DC cooperation scenario plays a functional role in immune defense during natural infections.
FIG 9.
Working model of subversion and cellular self-defense in C. burnetii-infected DCs. C. burnetii infection in DCs leads to a subversion of proper DC function via αVβ8-integrin upregulation and subsequent autocrine TGF-β release. Both iNOS/NO-mediated defense and MHC I presentation are impaired. This leads to conditions that support bacterial growth and CCV formation. However, the presence of IFN-γ released by activated NK cells reduces surface expression of αVβ8-integrin, which attenuates autocrine TGF-β action. Consequently, it allows efficient MHC I presentation, TNF-α release, and cellular self-defense against C. burnetii via iNOS/NO. This then prevents CCV formation and blocks bacterial growth. At the same time, Coxiella-infected DCs might protect themselves to some extent from the mitotoxic effects of NO production by switching from oxidative phosphorylation to glycolysis. Further studies are required to get more insight into this phenomenon.
MATERIALS AND METHODS
Cell culture.
The murine DC line JAWS II (ATCC, #CRL-11904) was cultivated at 37°C/7.5% CO2 in Iscove's Modified Dulbecco's Medium (IMDM) with 2 mM l-glutamine, 10% fetal bovine serum (FBS), and 100 μg/mL granulocyte-macrophage colony-stimulating factor (GM-CSF). Immortalized murine fibroblasts (L929, obtained from ATCC #CCL-1) were used as reporter cells for analyzing C. burnetii infectivity and were grown at 37°C/7.5% CO2 in IMDM/10% FBS. Isolation and cultivation of murine BMDCs were performed as previously described (40). All animal procedures were approved by the local District Government (State Office for Agriculture, Food Safety and Fishery in Mecklenburg-Western Pomerania - LALFF M-V, registration number 7221.3-2-042/17, FLI #: FLI 28/17), and were carried out in accordance with the relevant guidelines and regulations of the German law for the protection of animal life. Two different standard procedures for IFN-γ treatment were performed: IFN-γ (100 U/mL) was added either 12 h before/ante infection (hai) or 7 h after/postinfection (hpi). Autocrine TGF-β was neutralized with an anti-TGF-β (1–3) antibody (R&D Systems). Synthetic NO release was achieved by treatment with DETA NONOate (Enzo Life Science). DAF-2 DA (abcam) was used for intracellular NO detection.
Bacterial strains.
Infection studies were performed with C. burnetii NMII RSA 439 (phase II, low virulent) or a tdTomato-expressing C. burnetii NMII RSA 439, as already described (34), as well as a T4SS-/replication-defective dotA mutant (117). Preparation of C. burnetii stocks, determination of titers as infection forming units/mL (IFU/mL), and multiplicity of infection (MOI) were performed as previously described (34). Non-avian C. psittaci strain DC15 (118) was grown and cultivated following the protocol of Radomski et al. (40).
Antibodies and Western blot.
To verify C. burnetii infections, anti-Coxiella HSP60 (coxHSP60, Enzo Life Science) and an anti-C. burnetii antiserum (34) were used. Antibodies against MHC I (H-2Kb/H-2Db) (3.B10.7, kindly provided by P. Cresswell, Yale University, USA and Y-3, hybridoma cell line, ATCC: HB-176), αVβ8-integrin (ThermoFisher), iNOS (ThermoFisher), HIF-1α (Novus Biologicals), TGF-β (1–3) (R&D Systems), CD119/IFNRG1 (Miltenyi Biotec), and GAPDH (Millipore) were used. Corresponding secondary antibodies were purchased from Dianova, ThermoFisher, and BioLegend. Western blotting was performed as previously described (35). The SDS-PAGE protein markers were obtained from ThermoFisher. Fluorographs were scanned and quantified with ImageStudioLite 4.0.2.1 (LI-COR Biosciences).
Peptide isolation and mass spectrometry.
Peptides were isolated by mild acidic elution as previously described (119), with slight modifications. For the analysis, 1×107 C. burnetii-infected JAWS II (MOI 10) were cultured for 72 h in the presence or absence of IFN-γ. Peptides were released by treatment with citrate buffer (pH 3.3), and cellular debris was removed by centrifugation (20,000 × g, 10 min). After filtration through 3 kDa cutoff ultrafiltration units (Amicon), samples were desalted with Empore HP Extraction Disk Cartridges (C18-SD, 3 M), vacuum dried, and finally dissolved in 0.1% trifluoroacetic acid (TFA) for analysis on a nLC-MALDI-TOF-MS/MS platform (Ultimate nano Liquid Chromatography System, ThermoFisher; Proteineer fcII Sample Spotting Robot, Bruker; Ultraflextreme MALDI-TOF/TOF Instrument, Bruker). Here, 2 μg of the peptide samples were loaded on a trap column (Acclaim PepMap100 C18, ThermoFisher) and eluted over an analytical column (Acclaim PepMap100, 75 μm × 15 cm, C18, 3 μm, 100 Å, ThermoFisher) at a constant flow rate of 300 nl/min with a binary gradient of solvent A (0.05% TFA) and B (90% acetonitrile, 0.05% TFA). A linear gradient from 2% to 40% buffer B was applied in 32 min, and 192 fractions were spotted on an anchor chip target plate (Bruker) together with the MALDI matrix according to the standard protocol provided by the manufacturer. Mass spectrometry was performed on an Ultraflextreme MALDI-TOF/TOF instrument (Bruker). For peptide identification, spectra were searched with Mascot software (version 2.4.1, Matrix Science) using the Uniprot (120) sequence databases (C. burnetii NMII RSA 439/proteome ID: UP000002671 and Mus musculus/proteome ID: UP0000589), and results were evaluated with Proteinscape software (version 3.1, Bruker). Peptides with ion scores >25 were used to identify peptide antigens via the SYFPEITHI web tool (http://www.syfpeithi.de/) for binding to MHC I (H-2Kb and H-2Db).
Cytokine/chemokine analysis.
The mouse Proteome Profiler Mouse Cytokine Array Kit (Panel A from R&D Systems) was used to detect cytokines/chemokines. To prepare culture supernatants, 1×105 DCs were seeded in a 6-well plate, infected with C. burnetii (MOI 10), and/or treated with additional reagents, as indicated. After 72 h, supernatants were collected, centrifuged at 4°C/10,000 × g for 30 min, and analyzed immediately. Analysis/quantification of signals was performed according to the manufacturer's instructions.
RT-PCR and mRNA analysis.
For determination of GE by RT-PCR, genomic DNA (gDNA) was isolated from 1×105 infected DCs infected with C. burnetii at MOI 10, with or without IFN-γ treatment using the DNeasy blood and tissue kit (Qiagen). The PCR primers for amplification of C. burnetii dotA/T4SS were: 5‘-GCGCAATACGCTCAATCACA-3′, 5‘CCATGGCCCCAATTCT CTT-3′. A 190 bp amplicon of the C. burnetii-specific gene dotA (cloned into pcDNA3.1) served as a standard for calculating GEs (121). For semiquantitative analysis of mRNA, total RNA from 1×106 C. burnetii-infected (MOI 10) or non-infected DCs, with or without IFN-γ treatment, was isolated. Here, the respective PCR primers were: 5′-GCCCATTTCCGATGACCAGA-3′ and 5′-TCCCCACGAGGTCCCAATTA-3′ (C. burnetii catalase [CBU_0281]), 5′-TCAGGCGTGGCGCTAAAATA-3′ and 5′-CCAATTTGCGAGGCTTACCG-3′ (C. burnetii superoxide dismutase [SOD CU/ZN, CBU_1822]), 5′-ATCGAAGGCACCCCTTTTGA-3′ and 5′-TCTTTAACGAGCCAAGCCCA-3′ (C. burnetii superoxide dismutase [SOD FE, CBU_1708]), 5′-GTCGGGGCGCATTTTGATTT-3′ and 5′-ATAGCCACTGTCATCGAGCG-3′ (C. burnetii cytochrome bd [cydA-2, CBU_0965]), 5′-TGGCGGTTGCTTTTTATGGC-3′ and 5′-ACCGGGCACAACACCTTTTA-3′ (C. burnetii cytochrome bd [cydA-1, CBU_0218]), 5′-GCGGATCATGCCGCTTTTAG-3′ and 5′-AATCTTGGGAAGCGGCGTAT-3′‘(C. burnetii cytochrome bd [cydB, CBU_0966]). The MassRuler DNA ladder (ThermoFisher) was used in 1% agarose gels as a size marker. Digital images were densitometrically analyzed using ImageStudioLite 4.0.2.1 (LI-COR Biosciences).
CRISPR/Cas9 knockout.
Knockout (NOS2-KO) of the iNOS gene (NOS2) (GenBank: NC_000077.7) in DCs (JAWS II) was performed using the CRISPR/Cas9 system. Here, a modified version of the plasmid pX330-U6-Chimeric_BB-CBh-hSpCas9 (Addgene #42230, a gift from Feng Zhang [122]) was used. The plasmid encodes for a guide RNA (gRNA), trans-activating crRNA (tracrRNA), Cas9 nuclease, and an additional neomycin resistance cassette (pX330-neoR, kindly provided by Walter Fuchs, Friedrich-Loeffler-Institut, Germany) (123). Three different gRNAs for the iNOS gene were designed with CRISPOR, after which the corresponding oligonucleotides were annealed and cloned into pX330-neoR using BbsI. All constructs were validated by sequencing. Details of the cloning strategy are available upon request. Primers for gRNAs (EurofinsGenomics) were: 5′-CACCCGACCCGTCCGTCCGTCCAGTATGTGGTTT-3′ (mouse iNOS gRNA1), 5′-CACCCATGACTCTCTTCTTCACCAGTTT-3′ (mouse iNOS gRNA2), and 5′-CACCTGCAGCTTGTCCAGGGGATTCGTTT-3′ (mouse iNOS gRNA3) (BbsI compatible overhangs are underlined). DCs were co-transfected with all three pX330 neoR constructs (gRNA1, 2, and 3) using a nucleofector (Lonza). The transfected cells recovered for 2 days. Afterwards, the cells were cultured with IMDM, 10% FBS, penicillin/streptomycin (5000 U/mL), and 300 μg/mL Geneticin (G418). Each transfectant was then clonally seeded using a 96-well plate and further cultured before being examined for iNOS expression (in the presence and absence of IFN-γ) using Western blot. The genomic DNA of the clone used for subsequent infection experiments was sequenced (sequencing primers: 5′-GTAGAGGGGTGGCAGAGAGA-3′ and 5′-GGAAGCTTCCTCTGCCAGTT-3′), which confirmed a deletion of 2 nucleotides in exon 4 of the NOS2 DNA, resulting in a 2-base frameshift mutation and a premature stop codon at position 270 in the ORF.
Flow cytometry.
For intracellular bacterial staining and titer determination, 1×105 cells were fixed with 2% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 10 min at room temperature (RT). After permeabilization in washing buffer (0.5%) bovine serum albumin ([BSA], Carl Roth), 0.5% saponin (Sigma-Aldrich) in PBS at RT for 30 min, cells were incubated with primary antibodies. Staining of surface molecules occurred without fixation/permeabilization, and samples were gated on live cells. To analyze the mitochondrial membrane potential (ΔΨM), the JC-1 Flow Cytometry assay kit (Cayman Chemicals) was used. Also here, 1×105 DCs cultured in 6-well plates were either infected with C. burnetii (MOI 10) or left uninfected. Cell staining was performed after incubation for 72 h according to the manufacturer’s instructions using the MACSQuant Analyzer and MACSQuantify software (version 2.11) (Miltenyi Biotec).
Microscopy.
For fluorescence microscopy, 1×105 cells were grown on coverslips and fixed for 20 min in 2% PFA, blocked with 3% BSA (Carl Roth), permeabilized with 0.1% saponin (Sigma-Aldrich), and incubated with the indicated primary and fluorochrome-labeled secondary antibodies. Mounting and DNA staining were achieved using 10 μL ProLong Gold Antifade Mountant with DAPI (Invitrogen). Monodansylcadaverine ([MDC], 50 μM) was used to detect autophagosomes as previously described (51). Images were taken with an Axiovert 200M/ApoTome microscope using AxioVision and Zen 2009 software (Zeiss). For live-cell imaging (LCI) over longer time spans, 1×104 DCs were seeded in μ-dishes (ibidi, 35 mm, low wall), infected with C. burnetii expressing tdTomato (MOI 50), and either treated with or without IFN-γ. The culture medium was supplemented with 20 mM HEPES. In addition, a layer of 0.6% low melting point (LMP) agarose was applied to reduce high cell mobility. For this purpose, a double-concentrated culture medium was heated to 37°C and mixed 1:1 with sterile 1.2% LMP agarose. The time-lapse image series were acquired with a Leica THUNDER imager (objective: HC PL FLUOTAR 10×/0.32 DRY, camera: Leica K5) at 37°C using a heated chamber (HT200, ibidi). Images were acquired every 15 min for approximately 4 days. Analysis and visualization were done with arivis vision4D (v3.4.0), Fiji (124), and ImageJ (v 1.48). For transmission electron microscopy (TEM) analysis, 1×106 DCs were infected with C. burnetii (MOI 10) and cultured for 72 h in the presence or absence of IFN-γ (12 hai/7 hpi). Cells were removed from the culture flasks and centrifuged at 300 × g for 5 min at 4°C. The pellet was fixed (2.5% glutaraldehyde buffered in 0.1 M sodium cacodylate [pH 7.2], 300 mosmol, Merck), and embedded in 1.8% LMP agarose (Biozym). Small pieces were postfixed in 1.0% aqueous OsO4 and stained with 1% uranyl acetate. After stepwise dehydration in ethanol, cells were cleared in propylene oxide, embedded in Glycid Ether 100 (Serva), and polymerized at 60°C for 3 days. Ultrathin sections counterstained with uranyl acetate and lead salts were analyzed with a Tecnai-Spirit TEM (FEI).
Enzyme-linked immunosorbent assays.
To detect TGF-β in culture supernatants of C. burnetii-infected or non-infected DCs, the TGF beta-1 human/mouse enzyme-linked immunosorbent assays (ELISA) Kit (ThermoFisher) was used. For the analysis, 1×105 DCs were infected with C. burnetii (MOI 10) for 0 to 72 h. Culture supernatants were collected, centrifuged for 30 min at 21,000 × g (4°C), and analyzed via ELISA according to the manufacturer’s instructions. Griess assay (Sigma-Aldrich) was used to quantify the stable NO derivate nitrite (125) in culture supernatants from 1×105 C. burnetii- and non-infected DCs (MOI 10) cultured in the presence or absence of IFN-γ. The absorbance was measured at 540 nm in a microplate reader (Sunshine Remote ELISA reader, Tecan). To detect lactate in the culture supernatant of infected or uninfected DCs, the L-lactate assay from abcam was used. Here, 1×105 DCs were infected with C. burnetii (MOI 10) or left uninfected for 72 h. Culture supernatants were centrifuged for 30 min at 21,000 × g (4°C), and analyzed according to the manufacturer’s instructions.
Statistical analysis.
Data are shown as the means ± standard deviations (SD) of 3 individual experiments, and were calculated using GraphPad Prism 7 (GraphPad Software). Data were analyzed by t test, or one-way analysis of variance (ANOVA), followed by Dunnett’s and/or Tukey’s post hoc test (n.s., not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; and ****, P < 0.0001).
Data availability.
All data generated or analyzed during this study are included in this article (and its supplementary file).
ACKNOWLEDGMENTS
Anja Lührmann is acknowledged for providing tdTomato-expressing C. burnetii. Jonathan Pioch, Björn Corleis, Feng Zhang, and Walter Fuchs are recognized for providing the CRISPR/Cas9 vector system. Christine Luttermann, Elke Rufer, Annica Rebbig, Petra Meyer, Mandy Jörn, and Barbara Bettin are acknowledged for their technical assistance. We thank Allison Groseth for her helpful comments and for critically reading the manuscript. The BMBF is gratefully acknowledged for financial support under the project number 01KI1726C of “Q-GAPS” (MRK) as part of the research network zoonotic infectious diseases.
S.M., K.F., and M.R.K. directed and planned the studies. S.M., K.F., and M.R.K. designed and performed the experiments with the support of L.M.Z., S.F., K.F., B.C., A.K., R.J., and A.K. All authors contributed to the interpretation of the results, provided critical feedback, and helped shape the research, analysis, and manuscript. S.M. and M.R.K. wrote the final manuscript.
Footnotes
Supplemental material is available online only.
Contributor Information
Michael R. Knittler, Email: michael.knittler@fli.de.
Craig R. Roy, Yale University School of Medicine
REFERENCES
- 1.Kapsenberg ML. 2003. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol 3:984–993. 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Xiong X, Wu D, Wang X, Wen B. 2011. Efficient activation of T cells by human monocyte-derived dendritic cells (HMDCs) pulsed with Coxiella burnetii outer membrane protein Com1 but not by HspB-pulsed HMDCs. BMC Immunol 12:52. 10.1186/1471-2172-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rock KL. 2006. Exiting the outside world for cross-presentation. Immunity 25:523–525. 10.1016/j.immuni.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Bogdan C. 2001. Nitric oxide and the immune response. Nat Immunol 2:907–916. 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 5.Khavkin T, Tabibzadeh SS. 1988. Histologic, immunofluorescence, and electron microscopic study of infectious process in mouse lung after intranasal challenge with Coxiella burnetii. Infect Immun 56:1792–1799. 10.1128/iai.56.7.1792-1799.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eldin C, Melenotte C, Mediannikov O, Ghigo E, Million M, Edouard S, Mege JL, Maurin M, Raoult D. 2017. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev 30:115–190. 10.1128/CMR.00045-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurin M, Raoult D. 1999. Q fever. Clin Microbiol Rev 12:518–553. 10.1128/CMR.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McQuiston JH, Childs JE. 2002. Q fever in humans and animals in the United States. Vector Borne Zoonotic Dis 2:179–191. 10.1089/15303660260613747. [DOI] [PubMed] [Google Scholar]
- 9.Colombo MI, Gutierrez MG, Romano PS. 2006. The two faces of autophagy: Coxiella and Mycobacterium. Autophagy 2:162–164. 10.4161/auto.2827. [DOI] [PubMed] [Google Scholar]
- 10.Stoker MG, Fiset P. 1956. Phase variation of the Nine Mile and other strains of Rickettsia burneti. Can J Microbiol 2:310–321. 10.1139/m56-036. [DOI] [PubMed] [Google Scholar]
- 11.Vodkin MH, Williams JC. 1988. A heat shock operon in Coxiella burnetti produces a major antigen homologous to a protein in both Mycobacteria and Escherichia coli. J Bacteriol 170:1227–1234. 10.1128/jb.170.3.1227-1234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howe D, Shannon JG, Winfree S, Dorward DW, Heinzen RA. 2010. Coxiella burnetii phase I and II variants replicate with similar kinetics in degradative phagolysosome-like compartments of human macrophages. Infect Immun 78:3465–3474. 10.1128/IAI.00406-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vivona S, Lowenthal JP, Berman S, Benenson AS, Smadel JE. 1964. Report of a field study with Q fever vaccine. Am J Hyg 79:143–153. 10.1093/oxfordjournals.aje.a120370. [DOI] [PubMed] [Google Scholar]
- 14.Genig VA. 1965. Experience in mass immunization of humans with the M-44 live vaccine against Q-fever. 2. Skin and oral routes of immunization. Vopr Virusol 10:703–707. [PubMed] [Google Scholar]
- 15.Robinson DM, Hasty SE. 1974. Production of a potent vaccine from the attenuated M-44 strain of Coxiella burnetii. Appl Microbiol 27:777–783. 10.1128/am.27.4.777-783.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumaresan V, Alam S, Zhang Y, Zhang G. 2021. The feasibility of using Coxiella burnetii avirulent Nine Mile phase II viable bacteria as a live attenuated vaccine against Q fever. Front Immunol 12:754690. 10.3389/fimmu.2021.754690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dupuis G, Peter O, Peacock M, Burgdorfer W, Haller E. 1985. Immunoglobulin responses in acute Q fever. J Clin Microbiol 22:484–487. 10.1128/jcm.22.4.484-487.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuley R, Bossers-deVries R, Smith HE, Smits MA, Roest HI, Bossers A. 2015. Major differential gene regulation in Coxiella burnetii between in vivo and in vitro cultivation models. BMC Genomics 16:953. 10.1186/s12864-015-2143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andoh M, Zhang G, Russell-Lodrigue KE, Shive HR, Weeks BR, Samuel JE. 2007. T cells are essential for bacterial clearance, and gamma interferon, tumor necrosis factor alpha, and B cells are crucial for disease development in Coxiella burnetii infection in mice. Infect Immun 75:3245–3255. 10.1128/IAI.01767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang G, Russell-Lodrigue KE, Andoh M, Zhang Y, Hendrix LR, Samuel JE. 2007. Mechanisms of vaccine-induced protective immunity against Coxiella burnetii infection in BALB/c mice. J Immunol 179:8372–8380. 10.4049/jimmunol.179.12.8372. [DOI] [PubMed] [Google Scholar]
- 21.Turco J, Thompson HA, Winkler HH. 1984. Interferon-gamma inhibits growth of Coxiella burnetii in mouse fibroblasts. Infect Immun 45:781–783. 10.1128/iai.45.3.781-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benoit M, Barbarat B, Bernard A, Olive D, Mege JL. 2008. Coxiella burnetii, the agent of Q fever, stimulates an atypical M2 activation program in human macrophages. Eur J Immunol 38:1065–1070. 10.1002/eji.200738067. [DOI] [PubMed] [Google Scholar]
- 23.Shannon JG, Howe D, Heinzen RA. 2005. Virulent Coxiella burnetii does not activate human dendritic cells: role of lipopolysaccharide as a shielding molecule. Proc Natl Acad Sci USA 102:8722–8727. 10.1073/pnas.0501863102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zamboni DS, Campos MA, Torrecilhas AC, Kiss K, Samuel JE, Golenbock DT, Lauw FN, Roy CR, Almeida IC, Gazzinelli RT. 2004. Stimulation of toll-like receptor 2 by Coxiella burnetii is required for macrophage production of pro-inflammatory cytokines and resistance to infection. J Biol Chem 279:54405–54415. 10.1074/jbc.M410340200. [DOI] [PubMed] [Google Scholar]
- 25.Sobotta K, Hillarius K, Mager M, Kerner K, Heydel C, Menge C. 2016. Coxiella burnetii infects primary bovine macrophages and limits their host cell response. Infect Immun 84:1722–1734. 10.1128/IAI.01208-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norville IH, Hartley MG, Martinez E, Cantet F, Bonazzi M, Atkins TP. 2014. Galleria mellonella as an alternative model of Coxiella burnetii infection. Microbiology (Reading) 160:1175–1181. 10.1099/mic.0.077230-0. [DOI] [PubMed] [Google Scholar]
- 27.Cunha LD, Ribeiro JM, Fernandes TD, Massis LM, Khoo CA, Moffatt JH, Newton HJ, Roy CR, Zamboni DS. 2015. Inhibition of inflammasome activation by Coxiella burnetii type IV secretion system effector IcaA. Nat Commun 6:10205. 10.1038/ncomms10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley WP, Boyer MA, Nguyen HT, Birdwell LD, Yu J, Ribeiro JM, Weiss SR, Zamboni DS, Roy CR, Shin S. 2016. Primary role for toll-like receptor-driven tumor necrosis factor rather than cytosolic immune detection in restricting Coxiella burnetii phase II replication within mouse macrophages. Infect Immun 84:998–1015. 10.1128/IAI.01536-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clemente TM, Mulye M, Justis AV, Nallandhighal S, Tran TM, Gilk SD. 2018. Coxiella burnetii blocks intracellular interleukin-17 signaling in macrophages. Infect Immun 86:e00532-18. 10.1128/IAI.00532-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Y, Wang X, Xiong X, Wen B. 2011. Coxiella burnetii antigen-stimulated dendritic cells mediated protection against Coxiella burnetii in BALB/c mice. J Infect Dis 203:283–291. 10.1093/infdis/jiq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sireci G, Badami GD, Di Liberto D, Blanda V, Grippi F, Di Paola L, Guercio A, de la Fuente J, Torina A. 2021. Recent advances on the innate immune response to Coxiella burnetii. Front Cell Infect Microbiol 11:754455. 10.3389/fcimb.2021.754455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacKay VL, Moore EE. 15 July 1997. Immortalized dendritic cells. US Patent 5,648,219.
- 33.Amano K, Williams JC, Missler SR, Reinhold VN. 1987. Structure and biological relationships of Coxiella burnetii lipopolysaccharides. J Biol Chem 262:4740–4747. 10.1016/S0021-9258(18)61258-X. [DOI] [PubMed] [Google Scholar]
- 34.Matthiesen S, Zaeck L, Franzke K, Jahnke R, Fricke C, Mauermeir M, Finke S, Lührmann A, Knittler MR. 2020. Coxiella burnetii-infected NK cells release infectious bacteria by degranulation. Infect Immun 88:e00172-20. 10.1128/IAI.00172-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiegl D, Kägebein D, Liebler-Tenorio EM, Weisser T, Sens M, Gutjahr M, Knittler MR. 2013. Amphisomal route of MHC class I cross-presentation in bacteria-infected dendritic cells. J Immunol 190:2791–2806. 10.4049/jimmunol.1202741. [DOI] [PubMed] [Google Scholar]
- 36.Geissmann F, Revy P, Regnault A, Lepelletier Y, Dy M, Brousse N, Amigorena S, Hermine O, Durandy A. 1999. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol 162:4567–4575. 10.4049/jimmunol.162.8.4567. [DOI] [PubMed] [Google Scholar]
- 37.Reed SG. 1999. TGF-beta in infections and infectious diseases. Microbes Infect 1:1313–1325. 10.1016/s1286-4579(99)00252-x. [DOI] [PubMed] [Google Scholar]
- 38.Boucard-Jourdin M, Kugler D, Endale Ahanda ML, This S, De Calisto J, Zhang A, Mora JR, Stuart LM, Savill J, Lacy-Hulbert A, Paidassi H. 2016. Beta8 integrin expression and activation of TGF-beta by intestinal dendritic cells are determined by both tissue microenvironment and cell lineage. J Immunol 197:1968–1978. 10.4049/jimmunol.1600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sitkovsky M, Lukashev D. 2005. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol 5:712–721. 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 40.Radomski N, Kägebein D, Liebler-Tenorio E, Karger A, Rufer E, Tews BA, Nagel S, Einenkel R, Muller A, Rebbig A, Knittler MR. 2017. Mito-xenophagic killing of bacteria is coordinated by a metabolic switch in dendritic cells. Sci Rep 7:3923. 10.1038/s41598-017-04142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musso T, Espinoza-Delgado I, Pulkki K, Gusella GL, Longo DL, Varesio L. 1990. Transforming growth-factor-beta down-regulates interleukin-1 (IL-1)-induced IL-6 production by human monocytes. Blood 76:2466–2469. 10.1182/blood.V76.12.2466.2466. [DOI] [PubMed] [Google Scholar]
- 42.Dai CS, Wen XY, He WC, Liu YH. 2011. Inhibition of proinflammatory RANTES expression by TGF-beta 1 is mediated by glycogen synthase kinase-3 beta-dependent beta-catenin signaling. J Biol Chem 286:7052–7059. 10.1074/jbc.M110.174821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho ML, Min SY, Chang SH, Kim KW, Heo SB, Lee SH, Park SH, Cho CS, Kim HY. 2006. Transforming growth factor beta 1(TGF-beta 1) down-regulates TNF alpha-induced RANTES production in rheumatoid synovial fibroblasts through NF-kappa B-mediated transcriptional repression. Immunol Lett 105:159–166. 10.1016/j.imlet.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Xue QJ, Yan YC, Zhang RH, Xiong HB. 2018. Regulation of iNOS on immune cells and its role in diseases. Int J Mol Sci 19:3805. 10.3390/ijms19123805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bogdan CT, Rollinghoff M, Diefenbach A. 2000. The role of nitric oxide in innate immunity. Immunol Rev 173:17–26. 10.1034/j.1600-065X.2000.917307.x. [DOI] [PubMed] [Google Scholar]
- 46.Giuffre A, Borisov VB, Arese M, Sarti P, Forte E. 2014. Cytochrome bd oxidase and bacterial tolerance to oxidative and nitrosative stress. Biochim Biophys Acta 1837:1178–1187. 10.1016/j.bbabio.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 47.Berg DT, Gupta A, Richardson MA, O'Brien LA, Calnek D, Grinnell BW. 2007. Negative regulation of inducible nitric-oxide synthase expression mediated through transforming growth factor-beta-dependent modulation of transcription factor TCF11. J Biol Chem 282:36837–36844. 10.1074/jbc.M706909200. [DOI] [PubMed] [Google Scholar]
- 48.Jiang XZ, Shen CX, Rey-Ladino J, Yu H, Brunham RC. 2008. Characterization of murine dendritic cell line JAWS II and primary bone marrow-derived dendritic cells in Chlamydia muridarum antigen presentation and induction of protective immunity. Infect Immun 76:2392–2401. 10.1128/IAI.01584-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarkar S, Korolchuk VI, Renna M, Imarisio S, Fleming A, Williams A, Garcia-Arencibia M, Rose C, Luo SQ, Underwood BR, Kroemer G, O'Kane CJ, Rubinsztein DC. 2011. Complex inhibitory effects of nitric oxide on autophagy. Mol Cell 43:19–32. 10.1016/j.molcel.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berón W, Gutierrez MG, Rabinovitch M, Colombo MI. 2002. Coxiella burnetii localizes in a Rab7-labeled compartment with autophagic characteristics. Infect Immun 70:5816–5821. 10.1128/IAI.70.10.5816-5821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Biederbick A, Kern HF, Elsasser HP. 1995. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur J Cell Biol 66:3–14. [PubMed] [Google Scholar]
- 52.Chakravortty D, Hensel M. 2003. Inducible nitric oxide synthase and control of intracellular bacterial pathogens. Microbes Infect 5:621–627. 10.1016/s1286-4579(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 53.Brown GC. 1999. Nitric oxide and mitochondrial respiration. Biochim Biophys Acta 1411:351–369. 10.1016/s0005-2728(99)00025-0. [DOI] [PubMed] [Google Scholar]
- 54.Everts B, Amiel E, van der Windt GJ, Freitas TC, Chott R, Yarasheski KE, Pearce EL, Pearce EJ. 2012. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood 120:1422–1431. 10.1182/blood-2012-03-419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krzywinska E, Stockmann C. 2018. Hypoxia, metabolism and immune cell function. Biomedicines 6:56. 10.3390/biomedicines6020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quintero M, Brennan PA, Thomas GJ, Moncada S. 2006. Nitric oxide is a factor in the stabilization of hypoxia-inducible factor-1alpha in cancer: role of free radical formation. Cancer Res 66:770–774. 10.1158/0008-5472.CAN-05-0333. [DOI] [PubMed] [Google Scholar]
- 57.Sanjabi S, Oh SA, Li MO. 2017. Regulation of the immune response by TGF-beta: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol 9:a022236. 10.1101/cshperspect.a022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benoit M, Ghigo E, Capo C, Raoult D, Mege JL. 2008. The uptake of apoptotic cells drives Coxiella burnetii replication and macrophage polarization: a model for Q fever endocarditis. PLoS Pathog 4:e1000066. 10.1371/journal.ppat.1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pennings JL, Kremers MN, Hodemaekers HM, Hagenaars JC, Koning OH, Renders NH, Hermans MH, de Klerk A, Notermans DW, Wever PC, Janssen R. 2015. Dysregulation of serum gamma interferon levels in vascular chronic Q Fever patients provides insights into disease pathogenesis. Clin Vaccine Immunol 22:664–671. 10.1128/CVI.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Capo C, Zaffran Y, Zugun F, Houpikian P, Raoult D, Mege JL. 1996. Production of interleukin-10 and transforming growth factor beta by peripheral blood mononuclear cells in Q fever endocarditis. Infect Immun 64:4143–4147. 10.1128/iai.64.10.4143-4147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guzman RM, Howard ZP, Liu ZY, Oliveira RD, Massa AT, Omsland A, White SN, Goodman AG. 2021. Natural genetic variation in Drosophila melanogaster reveals genes associated with Coxiella burnetii infection. Genetics 217:iyab005. 10.1093/genetics/iyab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guironnet G, Dezutter-Dambuyant C, Vincent C, Bechetoille N, Schmitt D, Peguet-Navarro J. 2002. Antagonistic effects of IL-4 and TGF-beta1 on Langerhans cell-related antigen expression by human monocytes. J Leukoc Biol 71:845–853. 10.1189/jlb.71.5.845. [DOI] [PubMed] [Google Scholar]
- 63.Miller-Graziano CL, Zhu D, Kodys K. 1994. Differential induction of human monocyte transforming growth factor beta 1 production and its regulation by interleukin 4. J Clin Immunol 14:61–72. 10.1007/BF01541176. [DOI] [PubMed] [Google Scholar]
- 64.Thurnher M, Zelle-Rieser C, Ramoner R, Bartsch G, Holtl L. 2001. The disabled dendritic cell. FASEB J 15:1054–1061. 10.1096/fj.00-0508hyp. [DOI] [PubMed] [Google Scholar]
- 65.Munger JS, Sheppard D. 2011. Cross talk among TGF-beta signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol 3:a005017. 10.1101/cshperspect.a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kashiwagi I, Morita R, Schichita T, Komai K, Saeki K, Matsumoto M, Takeda K, Nomura M, Hayashi A, Kanai T, Yoshimura A. 2015. Smad2 and smad3 inversely regulate TGF-beta autoinduction in Clostridium butyricum-activated dendritic cells. Immunity 43:65–79. 10.1016/j.immuni.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 67.Fenton TM, Kelly A, Shuttleworth EE, Smedley C, Atakilit A, Powrie F, Campbell S, Nishimura SL, Sheppard D, Levison S, Worthington JJ, Lehtinen MJ, Travis MA. 2017. Inflammatory cues enhance TGFbeta activation by distinct subsets of human intestinal dendritic cells via integrin alphavbeta8. Mucosal Immunol 10:624–634. 10.1038/mi.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie WJ, Huang YF, Xie WL, Guo AM, Wu W. 2010. Bacteria peptidoglycan promoted breast cancer cell invasiveness and adhesiveness by targeting toll-like receptor 2 in the cancer cells. PLoS One 5:e10850. 10.1371/journal.pone.0010850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meghari S, Honstettre A, Lepidi H, Ryffel B, Raoult D, Mege JL. 2005. TLR2 is necessary to inflammatory response in Coxiella burnetii infection. Rickettsioses: from genome to proteome, pathobiology, and rickettsiae as an international threat. Ann N Y Acad Sci 1063:161–166. 10.1196/annals.1355.025. [DOI] [PubMed] [Google Scholar]
- 70.Ochoa-Reparaz J, Sentissi J, Trunkle T, Riccardi C, Pascual DW. 2007. Attenuated Coxiella burnetii phase II causes a febrile response in gamma interferon knockout and toll-like receptor 2 knockout mice and protects against reinfection. Infect Immun 75:5845–5858. 10.1128/IAI.00901-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Newton HJ, Kohler LJ, McDonough JA, Temoche-Diaz M, Crabill E, Hartland EL, Roy CR. 2014. A screen of Coxiella burnetii mutants reveals important roles for Dot/Icm effectors and host autophagy in vacuole biogenesis. PLoS Pathog 10:e1004286. 10.1371/journal.ppat.1004286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martinez E, Cantet F, Fava L, Norville I, Bonazzi M. 2014. Identification of OmpA, a Coxiella burnetii protein involved in host cell invasion, by multi-phenotypic high-content screening. PLoS Pathog 10:e1004013. 10.1371/journal.ppat.1004013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moffatt JH, Newton P, Newton HJ. 2015. Coxiella burnetii: turning hostility into a home. Cell Microbiol 17:621–631. 10.1111/cmi.12432. [DOI] [PubMed] [Google Scholar]
- 74.Beare PA, Gilk SD, Larson CL, Hill J, Stead CM, Omsland A, Cockrell DC, Howe D, Voth DE, Heinzen RA. 2011. Dot/Icm type IVB secretion system requirements for Coxiella burnetii growth in human macrophages. mBio 2:e00175-11. 10.1128/mBio.00175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Larson CL, Beare PA, Howe D, Heinzen RA. 2013. Coxiella burnetii effector protein subverts clathrin-mediated vesicular trafficking for pathogen vacuole biogenesis. Proc Natl Acad Sci USA 110:E4770–E4779. 10.1073/pnas.1309195110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Latomanski EA, Newton P, Khoo CA, Newton HJ. 2016. The effector Cig57 hijacks FCHO-mediated vesicular trafficking to facilitate intracellular replication of Coxiella burnetii. PLoS Pathog 12:e1006101. 10.1371/journal.ppat.1006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nosratababadi R, Bagheri V, Zare-Bidaki M, Hakimi H, Zainodini N, Arababadi MK. 2017. Toll like receptor 4: an important molecule in recognition and induction of appropriate immune responses against Chlamydia infection. Comp Immunol Microbiol Infect Dis 51:27–33. 10.1016/j.cimid.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 78.Chen CL, Tsukamoto H, Liu JC, Kashiwabara C, Feldman D, Sher L, Dooley S, French SW, Mishra L, Petrovic L, Jeong JH, Machida K. 2013. Reciprocal regulation by TLR4 and TGF-beta in tumor-initiating stem-like cells. J Clin Invest 123:2832–2849. 10.1172/JCI65859. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Kak G, Tiwari BK, Singh Y, Natarajan K. 2020. Regulation of Interferon-gamma receptor (IFN-gammaR) expression in macrophages during Mycobacterium tuberculosis infection. Biomol Concepts 11:76–85. 10.1515/bmc-2020-0006. [DOI] [PubMed] [Google Scholar]
- 80.Yoshimura A, Wakabayashi Y, Mori T. 2010. Cellular and molecular basis for the regulation of inflammation by TGF-beta. J Biochem 147:781–792. 10.1093/jb/mvq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eickelberg O, Pansky A, Koehler E, Bihl M, Tamm M, Hildebrand P, Perruchoud AP, Kashgarian M, Roth M. 2001. Molecular mechanisms of TGF-(beta) antagonism by interferon (gamma) and cyclosporine A in lung fibroblasts. FASEB J 15:797–806. 10.1096/fj.00-0233com. [DOI] [PubMed] [Google Scholar]
- 82.Ulloa L, Doody J, Massague J. 1999. Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature 397:710–713. 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- 83.Zhou F. 2009. Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int Rev Immunol 28:239–260. 10.1080/08830180902978120. [DOI] [PubMed] [Google Scholar]
- 84.Blanchette J, Jaramillo M, Olivier M. 2003. Signalling events involved in interferon-gamma-inducible macrophage nitric oxide generation. Immunology 108:513–522. 10.1046/j.1365-2567.2003.01620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vila-del Sol V, Punzon C, Fresno M. 2008. IFN-gamma-induced TNF-alpha expression is regulated by interferon regulatory factors 1 and 8 in mouse macrophages. J Immunol 181:4461–4470. 10.4049/jimmunol.181.7.4461. [DOI] [PubMed] [Google Scholar]
- 86.Tato C, Martins GA, High FA, DiCioccio CB, Reiner SL, Hunter CA. 2004. Cutting edge: Innate production of IFN-gamma by NK cells is independent of epigenetic modification of the IFN-gamma promoter. J Immunol 173:1514–1517. 10.4049/jimmunol.173.3.1514. [DOI] [PubMed] [Google Scholar]
- 87.Buttrum L, Ledbetter L, Cherla R, Zhang Y, Mitchell WJ, Zhang G. 2018. Both major histocompatibility complex class I (MHC-I) and MHC-II molecules are required, while MHC-I appears to play a critical role in host defense against primary Coxiella burnetii infection. Infect Immun 86:e00602-17. 10.1128/IAI.00602-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Remy MM, Sahin M, Flatz L, Regen T, Xu L, Kreutzfeldt M, Fallet B, Doras C, Rieger T, Bestmann L, Hanisch UK, Kaufmann BA, Merkler D, Pinschewer DD. 2017. Interferon-gamma-driven iNOS: a molecular pathway to terminal shock in arenavirus hemorrhagic fever. Cell Host Microbe 22:354–365. 10.1016/j.chom.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 89.Howe D, Barrows LF, Lindstrom NM, Heinzen RA. 2002. Nitric oxide inhibits Coxiella burnetii replication and parasitophorous vacuole maturation. Infect Immun 70:5140–5147. 10.1128/IAI.70.9.5140-5147.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zamboni DS, Rabinovitch M. 2003. Nitric oxide partially controls Coxiella burnetii phase II infection in mouse primary macrophages. Infect Immun 71:1225–1233. 10.1128/IAI.71.3.1225-1233.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brennan RE, Russell K, Zhang G, Samuel JE. 2004. Both inducible nitric oxide synthase and NADPH oxidase contribute to the control of virulent phase I Coxiella burnetii infections. Infect Immun 72:6666–6675. 10.1128/IAI.72.11.6666-6675.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Dumenil AM, Seabra MC, Raposo G, Amigorena S. 2006. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 126:205–218. 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 93.Wilsmann-Theis D, Koch S, Mindnich C, Bonness S, Schnautz S, von Bubnoff D, Bieber T. 2013. Generation and functional analysis of human TNF-alpha/iNOS-producing dendritic cells (Tip-DC). Allergy 68:890–898. 10.1111/all.12172. [DOI] [PubMed] [Google Scholar]
- 94.Buckland J. 2003. DCs 'Tip' off the innate immune system. Nat Rev Immunol 3:693. 10.1038/nri1186. [DOI] [Google Scholar]
- 95.Chong SZ, Wong KL, Lin G, Yang CM, Wong SC, Angeli V, Macary PA, Kemeny DM. 2011. Human CD8(+) T cells drive Th1 responses through the differentiation of TNF/iNOS-producing dendritic cells. Eur J Immunol 41:1639–1651. 10.1002/eji.201041022. [DOI] [PubMed] [Google Scholar]
- 96.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. 2003. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19:59–70. 10.1016/S1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 97.Engel D, Dobrindt U, Tittel A, Peters P, Maurer J, Gütgemann I, Kaissling B, Kuziel W, Jung S, Kurts C. 2006. Tumor necrosis factor alpha- and inducible nitric oxide synthase-producing dendritic cells are rapidly recruited to the bladder in urinary tract infection but are dispensable for bacterial clearance. Infect Immun 74:6100–6107. 10.1128/IAI.00881-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taetle R, Honeysett JM. 1988. Gamma-interferon modulates human monocyte/macrophage transferrin receptor expression. Blood 71:1590–1595. 10.1182/blood.V71.6.1590.1590. [DOI] [PubMed] [Google Scholar]
- 99.Ganesan S, Roy CR. 2019. Host cell depletion of tryptophan by IFNgamma-induced Indoleamine 2,3-dioxygenase 1 (IDO1) inhibits lysosomal replication of Coxiella burnetii. PLoS Pathog 15:e1007955. 10.1371/journal.ppat.1007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Howe D, Mallavia LP. 1999. Coxiella burnetii infection increases transferrin receptors on J774A. 1 cells. Infect Immun 67:3236–3241. 10.1128/IAI.67.7.3236-3241.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lopez AS, Alegre E, Diaz A, Mugueta C, Gonzalez A. 2006. Bimodal effect of nitric oxide in the enzymatic activity of indoleamine 2,3-dioxygenase in human monocytic cells. Immunol Lett 106:163–171. 10.1016/j.imlet.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 102.Oh GS, Pae HO, Choi BM, Chae SC, Lee HS, Ryu DG, Chung HT. 2004. 3-Hydroxyanthranilic acid, one of metabolites of tryptophan via indoleamine 2,3-dioxygenase pathway, suppresses inducible nitric oxide synthase expression by enhancing heme oxygenase-1 expression. Biochem Biophys Res Commun 320:1156–1162. 10.1016/j.bbrc.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 103.Samelson-Jones BJ, Yeh SR. 2006. Interactions between nitric oxide and indoleamine 2,3-dioxygenase. Biochemistry 45:8527–8538. 10.1021/bi060143j. [DOI] [PubMed] [Google Scholar]
- 104.Battisti JM, Hicks LD, Minnick MF. 2011. A unique Coxiella burnetii lipoprotein involved in metal binding (LimB). Microbiology (Reading) 157:966–976. 10.1099/mic.0.046649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De Kimpe SJ, Kengatharan M, Thiemermann C, Vane JR. 1995. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc Natl Acad Sci USA 92:10359–10363. 10.1073/pnas.92.22.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vodovotz Y, Bogdan C, Paik J, Xie QW, Nathan C. 1993. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J Exp Med 178:605–613. 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boutard V, Havouis R, Fouqueray B, Philippe C, Moulinoux JP, Baud L. 1995. Transforming growth factor-beta stimulates arginase activity in macrophages. Implications for the regulation of macrophage cytotoxicity. J Immunol 155:2077–2084. 10.4049/jimmunol.155.4.2077. [DOI] [PubMed] [Google Scholar]
- 108.Iniesta V, Gomez-Nieto LC, Molano I, Mohedano A, Carcelen J, Miron C, Alonso C, Corraliza I. 2002. Arginase I induction in macrophages, triggered by Th2-type cytokines, supports the growth of intracellular Leishmania parasites. Parasite Immunol 24:113–118. 10.1046/j.1365-3024.2002.00444.x. [DOI] [PubMed] [Google Scholar]
- 109.Ranganathan P, Agrawal A, Bhushan R, Chavalmane AK, Kalathur RK, Takahashi T, Kondaiah P. 2007. Expression profiling of genes regulated by TGF-beta: differential regulation in normal and tumour cells. BMC Genomics 8:98. 10.1186/1471-2164-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Henard CA, Vazquez-Torres A. 2011. Nitric oxide and Salmonella pathogenesis. Front Microbiol 2:84. 10.3389/fmicb.2011.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moore CM, Nakano MM, Wang T, Ye RW, Helmann JD. 2004. Response of Bacillus subtilis to nitric oxide and the nitrosating agent sodium nitroprusside. J Bacteriol 186:4655–4664. 10.1128/JB.186.14.4655-4664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Richardson AR, Dunman PM, Fang FC. 2006. The nitrosative stress response of Staphylococcus aureus is required for resistance to innate immunity. Mol Microbiol 61:927–939. 10.1111/j.1365-2958.2006.05290.x. [DOI] [PubMed] [Google Scholar]
- 113.Borisov VB, Gennis RB, Hemp J, Verkhovsky MI. 2011. The cytochrome bd respiratory oxygen reductases. Biochim Biophys Acta 1807:1398–1413. 10.1016/j.bbabio.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Romano PS, Gutierrez MG, Beron W, Rabinovitch M, Colombo MI. 2007. The autophagic pathway is actively modulated by phase II Coxiella burnetii to efficiently replicate in the host cell. Cell Microbiol 9:891–909. 10.1111/j.1462-5822.2006.00838.x. [DOI] [PubMed] [Google Scholar]
- 115.Zhang XG, Jin L, Tian Z, Wang J, Yang Y, Liu JF, Chen Y, Hu CH, Chen TY, Zhao YR, He YL. 2019. Nitric oxide inhibits autophagy and promotes apoptosis in hepatocellular carcinoma. Cancer Sci 110:1054–1063. 10.1111/cas.13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Caneba CA, Yang L, Baddour J, Curtis R, Win J, Hartig S, Marini J, Nagrath D. 2014. Nitric oxide is a positive regulator of the Warburg effect in ovarian cancer cells. Cell Death Dis 5:e1302. 10.1038/cddis.2014.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Beare PA, Larson CL, Gilk SD, Heinzen RA. 2012. Two systems for targeted gene deletion in Coxiella burnetii. Appl Environ Microbiol 78:4580–4589. 10.1128/AEM.00881-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Goellner S, Schubert E, Liebler-Tenorio E, Hotzel H, Saluz HP, Sachse K. 2006. Transcriptional response patterns of Chlamydophila psittaci in different in vitro models of persistent infection. Infect Immun 74:4801–4808. 10.1128/IAI.01487-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lanoix J, Durette C, Courcelles M, Cossette E, Comtois-Marotte S, Hardy MP, Cote C, Perreault C, Thibault P. 2018. Comparison of the MHC I immunopeptidome repertoire of B-cell lymphoblasts using two isolation methods. Proteomics 18:e1700251. 10.1002/pmic.201700251. [DOI] [PubMed] [Google Scholar]
- 120.The UniProt Consortium. 2021. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res 49:D480–D489. 10.1093/nar/gkaa1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schulze-Luehrmann J, Eckart RA, Olke M, Saftig P, Liebler-Tenorio E, Lührmann A. 2016. LAMP proteins account for the maturation delay during the establishment of the Coxiella burnetii-containing vacuole. Cell Microbiol 18:181–194. 10.1111/cmi.12494. [DOI] [PubMed] [Google Scholar]
- 122.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. 2013. Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823. 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hubner A, Petersen B, Keil GM, Niemann H, Mettenleiter TC, Fuchs W. 2018. Efficient inhibition of African swine fever virus replication by CRISPR/Cas9 targeting of the viral p30 gene (CP204L). Sci Rep 8:1449. 10.1038/s41598-018-19626-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ignarro LJ, Fukuto JM, Griscavage JM, Rogers NE, Byrns RE. 1993. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from L-arginine. Proc Natl Acad Sci USA 90:8103–8107. 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S6 and supplemental materials and methods. Download iai.00323-22-s0001.pdf, PDF file, 1.6 MB (1.6MB, pdf)
Data Availability Statement
All data generated or analyzed during this study are included in this article (and its supplementary file).