Abstract
Introduction
Psoriasis is a chronic inflammatory skin disease that negatively impacts the quality of life of patients and their families. However, the most commonly used decision-making tools in psoriasis, Psoriasis Area and Severity Index (PASI), Physician Global Assessment (PGA) and Dermatology Life Quality Index (DLQI), do not fully capture the impact of psoriasis on patients’ lives. In contrast, the well-established 5-item WHO Well-being Index (WHO-5) assesses the subjective psychological well-being of patients. Moreover, while drug innovations became available for psoriasis, data on the impact of these therapies on patients’ lives and their closest environment (family, physicians) are limited. This study will assess the effect of tildrakizumab, an interleukin-23p19 inhibitor, on the overall well-being of patients with moderate-to-severe psoriasis. Moreover, the long-term benefit of tildrakizumab on physicians' satisfaction and partners' lives of patients with psoriasis will be evaluated.
Methods and analysis
This non-interventional, prospective, observational, real-world evidence study will involve multiple sites in Europe and approximately 500 adults with moderate-to-severe psoriasis treated with tildrakizumab. Each patient will be followed for 24 months. The primary endpoint is well-being measured by the WHO-5 questionnaire. Key secondary endpoints include Physician’s Satisfaction and partner’s quality of life (FamilyPso). Other endpoints will evaluate skin-generic quality of life (DLQI-R), Treatment Satisfaction Questionnaire for Medication (TSQM-9), Treatment-related Patient Benefit Index ‘Standard’, 10 items (PBI-S-10) and work productivity and activity impairment due to psoriasis (WPAI:PSO). Statistical analyses will be based on observed cases. Multiple imputations will be performed as a sensitivity analysis, and adverse events will be reported.
Ethics and dissemination
The study will be conducted according to the protocol, which received ethics committee approval and applicable regulatory requirements of each participating country. The results will be disseminated through scientific publications and congress presentations.
Trail registration number
ClinicalTrials.gov Identifier: NCT04823247 (Pre-results)
Keywords: psoriasis, therapeutics, epidemiology
Strengths and limitations of this study.
First clinical study in dermatology using the patient-reported well-being as the primary endpoint in accordance with the holistic definition of health by the WHO.
Innovative secondary endpoints such as physician’s well-being, patient-defined benefit assessment and patients’ partner well-being, following a people-centred approach claimed by the WHO, for a better understanding of the impact of a chronic disease such as psoriasis both on patients and their close environment.
Relevant sample size.
Patients may experience a biased self-perception of well-being due to their inclusion in a clinical study.
Potential drop-outs or losses to follow-up due to the long observational period.
Introduction
Psoriasis is an immune-mediated, chronic relapsing inflammatory condition characterised by abnormal differentiation and proliferation of the epidermis affecting skin, nails and joints.1 The severity of psoriasis is profound as it includes physical and psychological components.2 Increasing evidence suggests that psoriasis considerably affects the overall well-being of patients and their families,3 as well as their personal, social and occupational life,4 often resulting in psychological distress and depression.5–7 An Europe-wide survey examining quality of life reported that almost 77% of patients believed psoriasis negatively affected normal daily activities and well-being.8 Moreover, a more recent survey showed that patients with psoriasis generally report poor quality of life and physicians are often also not satisfied with their patients’ treatment.9 These studies highlight the need for a well-being evaluation strategy besides the available psoriasis severity scoring assessments.10 Patient-reported outcomes (PROs) are being used with increasing frequency in clinical trials and provide valuable information on how psoriasis affects physical, social and psychological aspects of patients’ lives. However, the most commonly used decision-making tools in psoriasis, the clinician-reported Psoriasis Area Severity Index (PASI), Physician Global Assessment (PGA) and the patient-reported Dermatology Life Quality Index (DLQI), do not take into account the entire patient disease perspective, leading to misaligned perception between physicians and patients.11 While the PASI and PGA track changes in psoriasis severity from a clinician’s perspective,12 13 the skin-generic DLQI has been used to evaluate psoriasis symptoms and their impact on quality of life.14 However, the use of the DLQI has been associated with several measurement problems related to age, gender difference, inadequate power to differentiate patients with mild illness and underestimating patient’s emotional status.15 16 Furthermore, being a sum score and permitting to rate ‘not relevant’ in 8 out of 10 items, DLQI has been shown to provide marked bias and to underestimate the burden of very severely affected patients.16 Moreover, a systematic review on skin conditions has highlighted the critical need for psychiatric assessments in addition to the DLQI score for a full assessment of patient psychologic well-being.17 18
Unmet needs at the time of biologics
Studies have indicated that a patient-oriented evaluation is critical for overall symptom assessment as physicians’ indicators alone cannot capture the disease burden of psoriasis.19–21 The advent of biologics as monotherapy or in combination with other topical or systemic medications significantly changed the treatment approach for psoriasis representing an advancement over topical treatments or traditional immunosuppressive therapies.22–24 Biologics target specific components of the immune system and have shown a high benefit-to-risk ratio, high efficacy and acceptable safety profiles.25 Further, more advanced biologicals have been shown to be more cost-effective than first-generation drugs.26 27 Monoclonal antibodies, selective for the interleukin (IL)-23p19 subunit inhibition, represent a new standard of treatment currently available for the long-term management of psoriasis.28 Among these, tildrakizumab has demonstrated long-term efficacy and safety in the pivotal phase III studies (reSURFACE 1 and 2) comparing tildrakizumab versus placebo or etanercept for moderate-to-severe psoriasis.29 However, while biologics are becoming a more important treatment option for individuals with psoriasis, current therapy goals and PROs in dermatology do not fully capture the impact of this therapy on patients’ and physicians’ well-being.30 31 Moreover, although several studies have shown that psoriasis significantly affects mental health,32 33 standard severity scores have been found to be very weakly associated with the overall well-being of these patients.34 35 In practice, there is high need to translate patient burden to patients needs and then derive patient goals conjointly with the patient. Thus, understanding the needs and expectations of patients beyond measuring burden constitutes a fundamental part of treatment in psoriasis.36 This is particularly important in sensitive conditions such as nail and anogenital affections.37–39
Well-being in psoriasis: clinical goals
Insights on the well-being of the patients are based on the patient’s view of their condition, and this concept is increasingly being embraced as a more comprehensive outcome in line with a holistic view and people-centred perspective, but still undervalued in traditional PROs. Well-being is a holistic multifaceted concept that in a study context describes ‘to what extent patients will get their healthy lives back’, where mental, physical and social functioning are interdependent.40 According to the WHO, the ultimate goal of therapy in chronic diseases such as psoriasis is achieving well-being.41 Nevertheless, well-being has been rarely measured in dermatology so far. A recent study concluded that the DLQI primarily measures physical impairment associated with negative emotions and, thus, provides only a limited evaluation of well-being.42 Therefore, assessing well-being or specific dimensions of well-being can contribute to a holistic evaluation of patients’ health in accordance with the WHO’s holistic definition.
Innovative endpoints in this study
To assess patient well-being, this study will apply the 5-item WHO Well-being Index (WHO-5), a widely used questionnaire assessing psychological health-related well-being in a variety of chronic diseases such as diabetes, cardiovascular disease, cancer and depression.43 For the first time, WHO-5 will be used as a primary endpoint in patients with psoriasis to investigate the improvements that tildrakizumab can achieve on patients’ well-being in a real-world setting. Moreover, building a positive cross-talk environment between healthcare professionals and patients is critical. A meta-analysis study including 400 publications reported a higher quality of life and overall improved treatment outcome when patients had higher trust in their physician,21 underlining how physician’s well-being is essential as it can improve patients’ satisfaction and interpersonal aspects of care.22 Patient’s behaviour, in turn, can affect physicians’ well-being, including unrealistic expectations, aggression or by developing family-like relationships, resulting in emotional detachment and burnout.23 As there is no complete correlation between PRO and physician-reported outcome measures, both PROs and physician-reported scores should be considered when assessing treatment outcomes.24 In this study, the ‘Physician’s satisfaction’ score will be exploited as an innovative tool to investigate the impact of the psoriasis treatment process on physicians’ well-being. Furthermore, information on the impact of psoriasis on patients’ families, particularly partners, is also lacking, even though the impact of skin disease on family members or partners of patients should be measured while evaluating the burden of psoriasis.3 Thus, this study will include the questionnaire FamilyPso,44 enabling physicians to better understand the impact of psoriasis as a lifelong chronic disease on partners. This study will provide novel insights into the dimensions of patients' perspectives and their overall state of well-being using a holistic patient–partner and physicians-centred approach. The current study is designed to investigate patient-reported well-being using tildrakizumab in a real-world setting, as well as the impact on their closing environment, including partners and physicians, to provide a more comprehensive overview of people-centred healthcare and their overall state of well-being.
Methods and analysis
The primary objective is to assess the effect of tildrakizumab on the overall well-being in patients with moderate-to-severe psoriasis using the WHO-5 questionnaire. Secondary objectives are to evaluate the long-term benefit of tildrakizumab on physician’s satisfaction (Physician’s Satisfaction Questionnaire) and the impact of psoriasis on patients’ partners (FamilyPso questionnaire); to assess the long-term benefit of tildrakizumab reported by patients in terms of skin-generic quality of life (DLQI-R), treatment satisfaction (TSQM-9) and treatment-related benefits (PBI-S-10), work impairment due to psoriasis (WPAI:PSO) and extent of the skin manifestations on the entire body using a ‘heat map’ in a subgroup of patients; to correlate the change of WHO-5 with patients’ health-related quality of life (HRQoL) and patients’ treatment benefits; to assess the control of skin manifestations (absolute PASI) and its impact on patients, physician’s satisfaction and on partners of patients; to describe the sociodemographic and clinical profile of the patients being treated with tildrakizumab, according to the routine clinical practice; and to assess the safety and tolerability of tildrakizumab in a real-world setting. Exploratory objectives are to assess the impact of tildrakizumab on PGA of the skin manifestations and to assess the concordance between patients and physician’s satisfaction regarding response to the treatment with tildrakizumab.
Study design
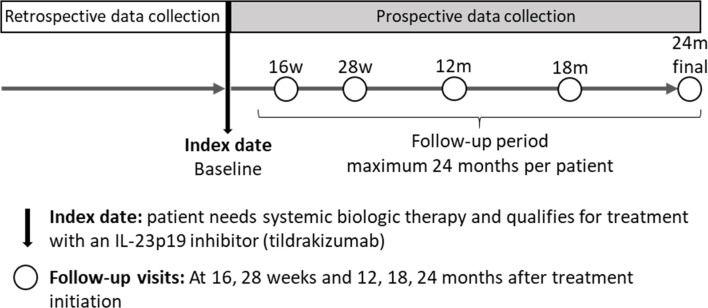
This is an ethics-approved, multinational, phase IV, 1-cohort prospective observational study planned to be conducted in several countries (Austria, Belgium, France, Germany, Italy, Spain, Switzerland, The Netherlands and UK) to assess patient-reported well-being using tildrakizumab in a live setting. The study design is summarised in figure 1.
Figure 1.
Study design of the POSITIVE study. IL, interleukin; m, month; w, week.
Study population
The study population will comprise a minimum of 338 patients with moderate-to-severe plaque psoriasis who need systemic biologic therapy and qualify for treatment with an IL-23p19 inhibitor in real-world clinical practice. Consecutive patients satisfying all the inclusion criteria and none of the exclusion criteria shown in table 1 to be eligible for participation will be offered participation. Recruitment will be competitive across all countries participating in the study (ie, no fixed number of patients per country).
Table 1.
Eligibility criteria
| Inclusion criteria | |
| 1 | Patient with diagnosis of moderate-to-severe chronic plaque psoriasis documented in the medical chart. |
| 2 | Patient who needs systemic biologic therapy and qualifies for treatment with an IL-23p19 inhibitor. Tildrakizumab must be the anti-IL-23p19 selected therapy before including the patient in the study. |
| 3 | Patient aged 18 years or older at the time of patient recruitment. |
| 4 | Patient who has provided written informed consent (if required by country regulations). |
| Exclusion criteria | |
| 1 | Patients unable to comply with the requirements of the study or who, in the opinion of the study physician, should not participate in the study. |
| 2 | Patients included in a clinical trial. |
IL, interleukin.
Endpoints
Study endpoints cover outcomes reported by patients, their partners and physicians. The primary endpoint is to assess the overall health-related well-being (evaluated through WHO-5 questionnaire) in patients treated with tildrakizumab according to the marketing authorisation and routine clinical practice at the long-term. The secondary endpoints are physicians’ satisfaction evaluation through a composite survey with 16 items (10 generic professional quality of life items and 6 items related to the biological treatment); partner well-being evaluation through the FamilyPso questionnaire; patient’s perspective on tildrakizumab long-term benefit evaluation through the (1) skin-generic quality of life questionnaire (DLQI-R), (2) Treatment Satisfaction Questionnaire for Medication (TSQM-9), (3) Treatment-related Patient Benefit Index ‘Standard’, 10 items (PBI-S-10), (4) Work Productivity and Activity Impairment Questionnaire: Psoriasis (WPAI:PSO), (5) skin manifestations distribution (heat map/patient’s grid in a subgroup of patients) according to location and total percentage of area affected and (6) the Numeric Rating Scale (NRS) for itch, pain, joint pain and fatigue assessments; disease control evaluated through the absolute PASI; description of the sociodemographic (age, gender, educational and working status, etc) and clinical (location of moderate-to-severe psoriasis, comorbid disease, laboratory tests, etc) characteristics; adverse events (AEs) reported during the use of tildrakizumab. The exploratory endpoints are the control of the skin manifestation evaluation through the PGA; patient’s and physician’s opinion about tildrakizumab treatment in relation to the therapeutic objectives’ evaluation through the Patient’s and physician’s questionnaire (table 2).
Table 2.
Endpoints of the study
| Patient-reported outcomes | Partners-reported outcomes | Physician-reported outcomes | Patient-reported and physician-reported outcomes | |
| Primary endpoint |
|
|||
| Secondary endpoint |
|
|
|
|
| Exploratory endpoints |
|
|
AEs, adverse events; DLQI-R, Dermatology Life Quality Index-Revised; NRS, Numeric Rating Scale; PASI, Psoriasis Area Severity Index; PBI-S-10, Patient Benefit Index 'Standard', 10 items short form; PGA, Physician Global Assessment; TSQM-9, Treatment Satisfaction Questionnaire for Medication; WHO-5, World Health Organization-Five Well-being Index; WPAI:PSO, Work Productivity and Activity Impairment Questionnaire: Psoriasis.
Data sources and collection
All data will be collected prospectively from the medical charts and entered directly into the electronic data capture system. The study will include both variables extracted from the medical chart and obtained directly from the patients. No visits or examinations, laboratory tests, or procedures are mandated or recommended as part of this study. The data collection schedule is shown in table 3.
Table 3.
Data collection schedule
| Baseline visit | Follow-up visit (at 16 and 28 weeks, 12 and 18 months after baseline visit) | Final visit (24 months from baseline) | Early termination visit | |
| Patient characteristics and demographics (including date of ICF signature) | X | X* | X* | |
| Disease characteristics (diagnosis date, type of psoriasis, severity of psoriasis) | X | |||
| Laboratory test (if it is available) | X | X | X | X |
| Comorbidities | X | X | X | X |
| Concomitant medication | X | X | X | X |
| Drug therapy history | X | |||
| Tildrakizumab drug therapy (dose) | X | X | X | X |
| PASI | X | X | X | X |
| Heat map/patient’s grid (subgroup of patients): location of skin manifestations | X | X | X | X |
| Adverse event reporting | X | X | X | X |
| Questionnaires | ||||
| WHO-5 | X | X | X | X |
| DLQI-R | X | X | X | X |
| PBI-S-10 | X | X | X | X |
| TSQM-9 | X | X | X | |
| WPAI:PSO | X | X | X | X |
| FamilyPso | X | X | X | X |
| NRS (itch, pain, joint pain, fatigue) | X | X | X | X |
| Patient’s and physician’s satisfaction of being part in the study (open field question) | X | X | X | |
| PGA | X | X | X | X |
| Physician’s satisfaction | X | X | X | X |
| Patient’s and physician’s questionnaire | X | X | X |
*Variables such as gender or height will not be collected at these visits.
DLQI-R, Dermatology Life Quality Index-Revised; ICF, informed consent form; NRS, Numeric Rating Scale; PASI, Psoriasis Area Severity Index; PBI-S-10, Patient Benefit Index 'Standard', 10 items short form; PGA, Physician Global Assessment; TSQM-9, Treatment Satisfaction Questionnaire for Medication; WHO-5, World Health Organization-Five Well-being Index; WPAI:PSO, Work Productivity and Activity Impairment Questionnaire: Psoriasis.
Questionnaires
All the questionnaires included in the study have validated translations according to international guidelines from the original language to the official languages in the participating countries.
Primary endpoint
The 5-item WHO Well-being Index (WHO-5): the WHO-5 is a generic global rating scale measuring subjective well-being containing positive statements. The respondent is asked to rate how well each of the five items applies to him or her when considering the last 14 days. Each of the five items is scored from 5 (all of the time) to 0 (none of the time). The raw score therefore theoretically ranges from 0 (absence of well-being) to 25 (maximal well-being). To standardise the score on a scale from 0 (absent) to 100 (maximal), it is recommended to multiply the raw score by 4.43
Secondary endpoints
Dermatology Life Quality Index-Relevant (DLQI-R): the DLQI-R is a recently developed scoring that adjusts the total score of the questionnaire for the number of 'not relevant' responses indicated by a patient. It is a self-administrated questionnaire validated as a dermatology-specific quality of life instrument. It includes 10 items to assess patient’s HRQoL during the previous week on a 4-point scale, indicating ‘not at all’, ‘a little’, ‘a lot’ and ‘very much’, respectively. For each patient, the DLQI-R score is estimated as a sum score of the original DLQI score replacing items rated ‘not relevant’ by the mean of the other items.45
Patient Benefit Index ‘Standard’, 10-item short form (PBI-S-10): 10 items describing treatment goals of patients with skin diseases. Before treatment, these goals are rated on a 5-point Likert-scale (0=‘not at all’, 4=‘very’) according to their perceived relevance (PNQ). Alternatively, patients can state that a goal ‘does not apply’ to them. In the course of treatment, patients are asked to express the achievement of these 10 treatment goals (treatment benefit) on the same 5-point Likert-scale (PBQ), again with the opportunity to state that the goal did not apply.46 An overall index of patient benefits (PBI) is calculated by adding the single weighted benefits to a sum score reaching from 0=no patient benefit to 4=very strong patient benefit.
Treatment Satisfaction Questionnaire for Medication (TSQM-9): it is a self-administrated questionnaire that measures patients’ drug therapy satisfaction considering the last 2 or 3 weeks or since the last time the patient took the medication. It consists of nine statements distributed in three domains: efficacy (three items), convenience (three items) and global satisfaction (three items). Responses are measured on a Likert-scale of 5 or 7 points.47 48
Work Productivity and Activity Impairment Questionnaire: Psoriasis (WPAI:PSO): it is a self-administrated questionnaire that measures the impact on the work productivity in patients with psoriasis considering the last 7 days. The questionnaire contains six items with dichotomous response options.49
Numeric Rating Scale (NRS) (itch, pain, joint pain, fatigue): it is a self-administrated questionnaire to assess patients’ itch severity, pain, joint pain and fatigue. The questionnaire has a single-item that describes the worst level of itching, joint paint and fatigue, respectively, due to psoriasis in the past 24 hours.50
FamilyPso: it is a self-administrated questionnaire to assess the burden on partners of patients with psoriasis. The questionnaire has 15 items divided into five factors: (1) perceived strain by social reactions to the partner’s psoriasis; (2) strain caused by cleaning; (3) acute emotional strain attributed directly to the psoriasis; (4) restrictions of social life; and (5) general emotional strain. The items are scaled in a 5-point Likert format: 0=‘not true’, 1=‘somewhat true’, 2=‘moderately true’, 3=‘quite true’, 4=‘very true’, with the supplementary option ‘does not apply to me’.44
Patient’s and physician’s questionnaire: survey of 10 questions elaborated for this project with the objective to assess the opinion of physicians and patients about tildrakizumab treatment in relation to the therapeutic objectives. The physician will respond according to his/her assessment in relation to the response to tildrakizumab treatment of each patient. Each item is scaled in a 5-points Likert format: ‘strongly agree’, ‘agree’, ‘uncertain’, ‘disagree’ and ‘strongly disagree’.
Psoriasis Area and Severity Index (PASI): measure of psoriasis severity average to quantify psoriasis grade,51 combining an assessment of each psoriasis lesion graded on a 0–4 scale (0=none and 4=maximum) based on three parameters of the lesions including, erythema (redness), induration (thickness), desquamation (scaliness) and on a weighted evaluation of the affected area (from 0=affecting 0% to 6=affecting 100%), dividing the body into four sections: head (10% of person’s skin), trunk (30%), upper extremities (20%) and lower extremities (40%). Each area is scored by itself.
-
Physician Global Assessment (PGA): a 5-point ordinal scale used to assess the severity of disease over the body including global, scalp, palmoplantar and nails. The scale ranges from 0 to 4 for global, scalp and palmoplantar assessment52:
0=clear: no signs of psoriasis
1=almost clear: only minimal plaque elevation, scaling and/or erythema
2=mild: sight plaque elevation, scaling and/or erythema
3=moderate: moderate plaque elevation, scaling and/or erythema
4=severe: very marked plaque elevation, scaling and/or erythema
For nails assessment, the scale ranges from 0 to 4:
0=none: normal nail appearance
1=mild: pitting and/or oil drops; mild disfigurement
2=moderate: prominent visible pitting and/or oil drops, distal onycholysis with/without hyperkeratosis; moderate disfigurement
3=severe: pronounced nail changes including pitting, oil drops, distal onycholysis with/without hyperkeratosis, onychodystrophy; substantial disfigurement
4=very severe: severe distal onycholysis with/without hyperkeratosis and/or severe onychodystrophy; prominent disfigurement
Physician’s satisfaction: a composite survey with 16 items, 10 of them are generic items and correspond to the compassion subscale of the Professional Quality of Life questionnaire53; and 6 items were specifically created for this study regarding biological treatment. There are 5-point scales that reflect how frequently the physician experienced this thing in the last 30 days.
Patient’s and physician’s satisfaction of being part of the study: open question to assess the satisfaction that the participation on a clinical study may provide to the patients and physicians.
Sample size calculation
A total sample size of 338 patients with baseline WHO-5 score ≤50 will provide a 95% power to detect a difference of 10 points43 in the absolute change from baseline in the WHO-5 total score at week 24, assuming 50.5 as the SD of the difference. This sample size of 338 patients produces a two-sided 95% CI with a precision of ±5.4 points. A conservative estimation assumes that 75% of the population with psoriasis may have a baseline WHO-5 score ≤50.54 Therefore, around 450 patients will have to be included in the study to ensure the 338 patients previously described. Also considering a 10% drop out rate, the final number of patients to be included will be approximately 500.
Data analyses and reporting
All data analyses will be performed using SAS V.9.2 or higher (SAS Institute, Cary, North Carolina, USA). Descriptive analyses will be performed. Statistical analyses will be based on observed cases. Multiple imputations will be performed as a sensitivity analysis. All AE verbatim terms will be coded using the Medical Dictionary for Regulatory Activities. Medication will be coded using WHO Drug Dictionary. Final analyses will be performed once the data from all patients have been collected in the database, cleaned, and database lock has occurred. Analyses will be performed overall and per country.
Changes in WHO-5 (to assess the primary objective) and changes in DLQI-R, PBI-S-10, TSQM-9, patient’s and physician’s and NRS scores will be calculated as the difference between baseline and follow-up visits (up to 24 months) and will be compared using paired t-test (or Wilcoxon signed-rank test). Furthermore, all answers will be described by number of patients who answer in each category or domain (if applicable). To assess the ‘correlation of the change of health-related well-being (WHO-5)’, a Pearson product–moment correlation coefficient (r) will be used and, for ordinal data, a Spearman’s rank-order correlation will be used. Work and activity impairment will be analysed using the responses to the WPAI:PSO questionnaire in patients actively working in the last 7 days. Activity impairment will be calculated in each study visit. Changes in WPAI:PSO scores will be calculated as the difference between baseline and follow-up visits (up to 24 months) and will be compared using paired t-test (or Wilcoxon signed-rank test). FamilyPso questionnaire will be described through each factor and for total score. For additional outcomes, PASI values will be correlated with the different clinical questionnaires through Pearson correlation (r) in order to assess positive impact of the control of skin manifestations.
Patient and public involvement statement
Patients or the public were not involved in the design of the study, and will not be involved in the conduct, reporting or dissemination plans of this research.
Ethics and dissemination
This non-interventional study will be conducted in compliance with the protocol, all applicable regulatory requirements of the countries where the study is being conducted and the ethical principles of the latest version of the Declaration of Helsinki that are consistent with Good Pharmacoepidemiology Practices. Data protection and privacy regulations will be strictly observed in capturing, forwarding, processing and storing patient data according to the Directive 95/46/EC on the protection of individuals and in compliance with the Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation). The protocol has been approved by the following ethics committees: (1) Medizinische Universität Graz - Ethikkommission (Austria, reference number: 33-555 ex 20/21), (2) Comité d’Ethique hospitalo-facultaire (CEHF) Cliniques universitaires Saint-Luc (Belgium, reference number BC-10914), (3) Comité de Protection des Personnes Nord-Ouest III (France, reference number: 2021-61), (4) Medizinishe Fakultät der Christian-Albrechts-Universität zu Kiel - Ethik Kommission (Germany, reference number: D 480/21), (5) Comitato Etico Università Federico II (Italy, reference number: 295/21), (6) CEIC Aragón (CEICA) (Spain, reference number: EPA21/016, Acta 11/2021), (7) Ethikkommission Nordwest- und Zentralschweiz (EKNZ) (Switzerland, reference number: 2021-01705), (8) DCRF/NWMO Advisory Commission UMC Groningen (The Netherlands, reference number: NWMO 21.09.035) and (9) London - Hampstead Research Ethics Committee (UK, reference number: 21/PR/0883).
The results of the POSITIVE study will be disseminated through one or more scientific manuscripts and may also be presented at medical congresses.
Discussion
The lack of PRO measures for assessing the overall well-being of patients with psoriasis is becoming a concern in dermatology clinical practice and research. Psoriasis heavily affects the physical, psychological and social well-being of patients and that of their family members and partners, thus, beside clinical assessments, the full impact of psoriasis treatment on the patient and people around should be recognised. To our knowledge, the POSITIVE study is the first study to apply the WHO-5 as a primary endpoint to estimate the improvements on patients’ well-being treated with tildrakizumab in a real-world setting. This study will measure the well-being of patients, their partners and of physicians, for a better understanding of the impact of psoriasis as a chronic disease on patients and their close environment. The results of this study could potentially be added to the available psoriasis evaluation methods and provide dermatologists with new tools to improve their own and patient’s well-being enhancing patient–clinician relationships. The need to provide results from the POSITIVE study becomes essential given the WHO claim for people-centred healthcare and patient commitment. The POSITIVE study will significantly promote patients' involvement and awareness as recommended by the WHO global report on psoriasis,5 as the most common root for patient dissatisfaction is being poorly engaged in the treatment decision-making process. Limitations of the study will include potential bias and missing information or possible drop-outs or lost to follow-up in the last follow-up visits of the study because of the long observational period. In addition, potential time-related maturation/history effects on the primary outcome measure of general well-being should be considered, especially post-pandemic, given the long observational period and the lack of a control group. Moreover, patients included in this study will have to be preselected to initiate tildrakizumab therapy before patient inclusion in the study. Finally, a limitation could be represented by patient-related bias on self-perception of well-being due to the inclusion in a clinical study.
In summary, this prospective observation study constitutes an innovative people-centric approach to investigate how intervention with tildrakizumab may improve the well-being of patients, their families, and healthcare professionals.
Supplementary Material
Acknowledgments
Editorial assistance and writing support were provided by Paula Casajust, MSc and Stefania Ippati, PhD of TFS HealthScience.
Footnotes
Contributors: MA, RS, ED, PL, EdJ, GF, LN, AAN, JL, ZR, SG, EM, TO, IK and UM were involved in conception and study design. MA, RS, ED, PL, EdJ, GF, LN, AAN, JL, ZR, SG, EM, TO, IK and UM provided edits, critiqued the manuscript for intellectual content and approved the final manuscript.
Funding: This work was supported by Almirall, Barcelona, Spain. Award/grant number: N/A.
Competing interests: MA has served as consultant for, or has been a paid speaker for clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including Abbvie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli-Lilly, GSK, Janssen-Cilag, Leo Pharma, Medac, Merck, MSD, Novartis, Pfizer, UCB and Xenoport. RS has been an advisor and/or received speaker honoraria and/or received grants from AbbVie, Almirall, Beiersdorf, Janssen, LEO Pharma and Novartis. ED has the following conflict of interests: Advisory Board member, consultant, grants, research support, participation in clinical trials, honorarium for speaking, research support, with the following pharmaceutical companies: Abbvie/Abbott, Almirall, Amgen, Janssen-Cilag, Leo Pharma, Novartis, Pfizer, MSD-Schering-Plough, Celgene, Lilly and UCB. PL received honoraria and/or grants as an investigator, speaker, and/or advisory board member for AbbVie, Almirall, Actelion, Celgene, Janssen, Lilly, Sanofi, Leo, UCB, and Novartis. EdJ has received research grants for the independent research fund of the department of dermatology of the Radboud University Medical Center Nijmegen, The Netherlands from AbbVie, Leo Pharma, Janssen Pharmaceuticals, Novartis, Pfizer, and UCB and has acted as consultant and/or paid speaker for and/or participated in research sponsored by companies that manufacture drugs used for the treatment of psoriasis including AbbVie, Almirall, Celgene, Galapagos, Janssen Pharmaceutica, Leo Pharma, Lilly, Novartis, Sanofi and UCB. All funding is not personal but goes to the independent research fund of the department of dermatology of Radboud University Medical Center Nijmegen (Radboudumc), The Netherlands. GF has acted as speaker and consultant for AbbVie, Almirall, Eli Lilly, Leo Pharma, Janssen, Novartis and UCB. LN received consultation fees from Abbvie, Amgen, Boehringer Inghelheim, Celgene, Eli Lilly, IBSA, Menarini, Janssen, Novartis, and Sanofi. AN declares being a consultant and advisor and/or receiving speaking fees and/or grants and/or served as an investigator in clinical trials for AbbVie, Almirall, Amgen, BMS, Boehringer Ingelheim, Celgene, Eli Lilly, Galderma, GSK, LEO Pharma, Janssen-Cilag, MSD, Novartis, Pfizer, Sandoz, Sanofi, Serono and UCB. JL has been a speaker/advisor for and her institution received grants from AbbVie, Almirall, Argenx, BMS, Celgene, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer and UCB. ZR has been an advisor, investigator, and/or speaker for Novartis, Janssen-Cilag, Lilly, eo-Pharma, Celgene, Pfizer, UCB, Amgen, Almirall, MSD, and MEDAC. SG has been an advisor and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials of the following companies: AbbVie, Affibody AB, Akari Therapeutics Plc, Almirall-Hermal, Amgen, Anaptys Bio, Argenx BV, AstraZeneca AB, Biogen Idec, Bioskin, Boehringer-Ingelheim, Celgene, Dermira, Eli Lilly, Forward Pharma, Galderma, Hexal AG, Incyte Inc, Janssen-Cilag, Johnson & Johnson, Kymab, Leo Pharma, Medac, MSD, Neubourg Skin Care GmbH, Novartis, Pfizer, Principia Biopharma, Regeneron Pharmaceutical, Sandoz Biopharmaceuticals, Sanofi-Aventis, Trevi Therapeutics and UCB Pharma. EM and IK are employees of Almirall. TO has served as medical advisor for Almirall. UM has been an advisor and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials of the following companies: AbbVie, Aditxt, Almirall, Amgen, Aristea, Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Dr Reddy’s, Eli Lilly, Foamix, Formycon, Immunic, Janssen, LEO Pharma, Medac, MetrioPharm, Novartis, Phi-Stone, Pierre Fabre, Sanofi-Aventis and UCB Pharma.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Mrowietz U, Reich K. Psoriasis -- new insights into pathogenesis and treatment. Dtsch Arztebl Int 2009;106:11–8, 10.3238/arztebl.2009.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhosle MJ, Kulkarni A, Feldman SR, et al. Quality of life in patients with psoriasis. Health Qual Life Outcomes 2006;4:35. 10.1186/1477-7525-4-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eghlileb AM, Davies EEG, Finlay AY. Psoriasis has a major secondary impact on the lives of family members and partners. Br J Dermatol 2007;156:1245–50. 10.1111/j.1365-2133.2007.07881.x [DOI] [PubMed] [Google Scholar]
- 4.Villacorta R, Teeple A, Lee S, et al. A multinational assessment of work-related productivity loss and indirect costs from a survey of patients with psoriasis. Br J Dermatol 2020;183:548–58. 10.1111/bjd.18798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global report on psoriasis. Available: https://www.who.int/publications-detail-redirect/global-report-on-psoriasis [Accessed 17 Nov 2021].
- 6.Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol 2017;31:1999–2009. 10.1111/jdv.14460 [DOI] [PubMed] [Google Scholar]
- 7.Sallemi R, Bouhamed M, Masmoudi R, et al. Anxiety and depressive symptoms in patients with psoriasis. Eur Psychiatr 2021;64:S183–4. 10.1192/j.eurpsy.2021.485 [DOI] [Google Scholar]
- 8.Dubertret L, Mrowietz U, Ranki A, et al. European patient perspectives on the impact of psoriasis: the EUROPSO patient membership survey. Br J Dermatol 2006;155:729–36. 10.1111/j.1365-2133.2006.07405.x [DOI] [PubMed] [Google Scholar]
- 9.Palota T, Szepietowski JC, Pec J, et al. A survey of disease severity, quality of life, and treatment patterns of biologically naive patients with psoriasis in central and eastern Europe. Acta Dermatovenerol Croat 2010;18:151–61. [PubMed] [Google Scholar]
- 10.Naldi L. Scoring and monitoring the severity of psoriasis. What is the preferred method? what is the ideal method? Is PASI passé? Facts and controversies. Clin Dermatol 2010;28:67–72. 10.1016/j.clindermatol.2009.03.001 [DOI] [PubMed] [Google Scholar]
- 11.Korman NJ, Zhao Y, Pike J, et al. Relationship between psoriasis severity, clinical symptoms, quality of life and work productivity among patients in the USA. Clin Exp Dermatol 2016;41:514–21. 10.1111/ced.12841 [DOI] [PubMed] [Google Scholar]
- 12.Berth-Jones J, Grotzinger K, Rainville C, et al. A study examining inter- and intrarater reliability of three scales for measuring severity of psoriasis: psoriasis area and severity index physician’s global assessment and lattice system physician’s global assessment. Br J Dermatol 2006;155:707–13. 10.1111/j.1365-2133.2006.07389.x [DOI] [PubMed] [Google Scholar]
- 13.Puzenat E, Bronsard V, Prey S, et al. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J Eur Acad Dermatol Venereol 2010;24 Suppl 2:10–6. 10.1111/j.1468-3083.2009.03562.x [DOI] [PubMed] [Google Scholar]
- 14.Silva M da, Fortes MRP, Miot LDB, et al. Psoriasis: correlation between severity index (PASI) and quality of life index (DLQI) in patients assessed before and after systemic treatment. An Bras Dermatol 2013;88:760–3. 10.1590/abd1806-4841.20132052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Twiss J, Meads DM, Preston EP, et al. Can we rely on the dermatology life quality index as a measure of the impact of psoriasis or atopic dermatitis? J Invest Dermatol 2012;132:76–84. 10.1038/jid.2011.238 [DOI] [PubMed] [Google Scholar]
- 16.Langenbruch A, Radtke MA, Gutknecht M, et al. Does the dermatology life quality index (DLQI) underestimate the disease-specific burden of psoriasis patients? J Eur Acad Dermatol Venereol 2019;33:123–7. 10.1111/jdv.15226 [DOI] [PubMed] [Google Scholar]
- 17.Ali FM, Johns N, Salek S, et al. Correlating the dermatology life quality index with psychiatric measures: a systematic review. Clin Dermatol 2018;36:691–7. 10.1016/j.clindermatol.2018.08.014 [DOI] [PubMed] [Google Scholar]
- 18.Pattinson RL, Trialonis-Suthakharan N, Gupta S, et al. Patient-reported outcome measures in dermatology: a systematic review. Acta Derm Venereol 2021;101:adv00559. 10.2340/00015555-3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman SR, Regnier SA, Chirilov A, et al. Patient-reported outcomes are important elements of psoriasis treatment decision making: a discrete choice experiment survey of dermatologists in the United States. J Am Acad Dermatol 2019;80:1650–7. 10.1016/j.jaad.2019.01.039 [DOI] [PubMed] [Google Scholar]
- 20.Montenegro H, Ramagem C, Kawar R, et al. Making progress in people-centred care: country experiences and lessons learnt. Int J Pers Cent Med 2012;2:64–72. 10.5750/ijpcm.v2i1.93 [DOI] [Google Scholar]
- 21.Augustin M, Alvaro-Gracia JM, Bagot M, et al. A framework for improving the quality of care for people with psoriasis. J Eur Acad Dermatol Venereol 2012;26 Suppl 4:1–16. 10.1111/j.1468-3083.2012.04576.x [DOI] [PubMed] [Google Scholar]
- 22.Masson E. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [EM-Consulte]. Available: https://www.em-consulte.com/article/1281151/joint-aad-npf-guidelines-of-care-for-the-managemen [Accessed 18 May 2021].
- 23.Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol 2020;156:258–69. 10.1001/jamadermatol.2019.4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langenbruch A, Mohr N, Kirsten N, et al. Quality of psoriasis care in Germany-results from the nationwide health care studies psohealth 2004-2017. J Eur Acad Dermatol Venereol 2021;35:1536–42. 10.1111/jdv.17220 [DOI] [PubMed] [Google Scholar]
- 25.Tada Y, Watanabe R, Noma H, et al. Short-term effectiveness of biologics in patients with moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis. J Dermatol Sci 2020;99:53–61. 10.1016/j.jdermsci.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 26.Augustin M, Wirth D, Mahlich J, et al. Cost per responder analysis of guselkumab versus targeted therapies in the treatment of moderate to severe plaque psoriasis in Germany. J Dermatolog Treat 2022;33:976–82. 10.1080/09546634.2020.1793891 [DOI] [PubMed] [Google Scholar]
- 27.Augustin M, McBride D, Gilloteau I, et al. Cost-effectiveness of secukinumab as first biologic treatment, compared with other biologics, for moderate to severe psoriasis in Germany. J Eur Acad Dermatol Venereol 2018;32:2191–9. 10.1111/jdv.15047 [DOI] [PubMed] [Google Scholar]
- 28.Crowley JJ, Warren RB, Cather JC. Safety of selective IL-23p19 inhibitors for the treatment of psoriasis. J Eur Acad Dermatol Venereol 2019;33:1676–84. 10.1111/jdv.15653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (resurface 1 and resurface 2): results from two randomised controlled, phase 3 trials. Lancet 2017;390:276–88. 10.1016/S0140-6736(17)31279-5 [DOI] [PubMed] [Google Scholar]
- 30.Imafuku S, Kanai Y, Murotani K, et al. Utility of the dermatology life quality index at initiation or switching of biologics in real-life japanese patients with plaque psoriasis: results from the prologue study. J Dermatol Sci 2021;101:185–93. 10.1016/j.jdermsci.2021.01.002 [DOI] [PubMed] [Google Scholar]
- 31.Strober BE, van der Walt JM, Armstrong AW, et al. Clinical goals and barriers to effective psoriasis care. Dermatol Ther (Heidelb) 2019;9:5–18. 10.1007/s13555-018-0279-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleming P, Bai JW, Pratt M, et al. The prevalence of anxiety in patients with psoriasis: a systematic review of observational studies and clinical trials. J Eur Acad Dermatol Venereol 2017;31:798–807. 10.1111/jdv.13891 [DOI] [PubMed] [Google Scholar]
- 33.Korman AM, Hill D, Alikhan A, et al. Impact and management of depression in psoriasis patients. Expert Opin Pharmacother 2016;17:147–52. 10.1517/14656566.2016.1128894 [DOI] [PubMed] [Google Scholar]
- 34.Rzeszutek M, Podkowa K, Pięta M, et al. Comparative study of life satisfaction among patients with psoriasis versus healthy comparison group: the explanatory role of body image and resource profiles. Qual Life Res 2021;30:181–91. 10.1007/s11136-020-02621-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fortune DG, Richards HL, Kirby B, et al. Successful treatment of psoriasis improves psoriasis-specific but not more general aspects of patients’ well-being. Br J Dermatol 2004;151:1219–26. 10.1111/j.1365-2133.2004.06222.x [DOI] [PubMed] [Google Scholar]
- 36.Blome C, Augustin M, Behechtnejad J, et al. Dimensions of patient needs in dermatology: subscales of the patient benefit index. Arch Dermatol Res 2011;303:11–7. 10.1007/s00403-010-1073-0 [DOI] [PubMed] [Google Scholar]
- 37.Blome C, Costanzo A, Dauden E, et al. Patient-relevant needs and treatment goals in nail psoriasis. Qual Life Res 2016;25:1179–88. 10.1007/s11136-015-1136-y [DOI] [PubMed] [Google Scholar]
- 38.da Silva N, Augustin M, Langenbruch A, et al. Disease burden and treatment needs of patients with psoriasis in sexually-sensitive and visible body areas: results from a large-scale survey in routine care. Eur J Dermatol 2020;30:267–78. 10.1684/ejd.2020.3768 [DOI] [PubMed] [Google Scholar]
- 39.da Silva N, Augustin M, Langenbruch A, et al. Sex-related impairment and patient needs/benefits in anogenital psoriasis: difficult-to-communicate topics and their impact on patient-centred care. PLoS One 2020;15:e0235091. 10.1371/journal.pone.0235091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The world Health organisation - five well-being index (WHO-5). Available: https://www.corc.uk.net/outcome-experience-measures/the-world-health-organisation-five-well-being-index-who-5/ [Accessed 18 May 2021].
- 41.Sustainable development goals 3 - the global action plan for healthy lives and well-being for all. Available: https://www.who.int/initiatives/sdg3-global-action-plan [Accessed 17 Nov 2021].
- 42.Schuster B, Ziehfreund S, Albrecht H, et al. Happiness in dermatology: a holistic evaluation of the mental burden of skin diseases. J Eur Acad Dermatol Venereol 2020;34:1331–9. 10.1111/jdv.16146 [DOI] [PubMed] [Google Scholar]
- 43.Topp CW, Østergaard SD, Søndergaard S, et al. The WHO-5 well-being index: a systematic review of the literature. Psychother Psychosom 2015;84:167–76. 10.1159/000376585 [DOI] [PubMed] [Google Scholar]
- 44.Mrowietz U, Hartmann A, Weißmann W, et al. FamilyPso-a new questionnaire to assess the impact of psoriasis on partners and family of patients. J Eur Acad Dermatol Venereol 2017;31:127–34. 10.1111/jdv.13872 [DOI] [PubMed] [Google Scholar]
- 45.Rencz F, Gulácsi L, Péntek M, et al. Proposal of a new scoring formula for the dermatology life quality index in psoriasis. Br J Dermatol 2018;179:1102–8. 10.1111/bjd.16927 [DOI] [PubMed] [Google Scholar]
- 46.Augustin M, Radtke MA, Zschocke I, et al. The patient benefit index: a novel approach in patient-defined outcomes measurement for skin diseases. Arch Dermatol Res 2009;301:561–71. 10.1007/s00403-009-0928-8 [DOI] [PubMed] [Google Scholar]
- 47.Bharmal M, Payne K, Atkinson MJ, et al. Validation of an abbreviated treatment satisfaction questionnaire for medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes 2009;7:36. 10.1186/1477-7525-7-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaarschmidt M-L, Kromer C, Herr R, et al. Treatment satisfaction of patients with psoriasis. Acta Derm Venereol 2015;95:572–8. 10.2340/00015555-2011 [DOI] [PubMed] [Google Scholar]
- 49.Wu JJ, Lin C, Sun L, et al. Minimal clinically important difference (MCID) for work productivity and activity impairment (WPAI) questionnaire in psoriasis patients. J Eur Acad Dermatol Venereol 2019;33:318–24. 10.1111/jdv.15098 [DOI] [PubMed] [Google Scholar]
- 50.Kimball AB, Naegeli AN, Edson-Heredia E, et al. Psychometric properties of the itch numeric rating scale in patients with moderate-to-severe plaque psoriasis. Br J Dermatol 2016;175:157–62. 10.1111/bjd.14464 [DOI] [PubMed] [Google Scholar]
- 51.Langley RG, Ellis CN. Evaluating psoriasis with psoriasis area and severity index, psoriasis global assessment, and lattice system physician’s global assessment. J Am Acad Dermatol 2004;51:563–9. 10.1016/j.jaad.2004.04.012 [DOI] [PubMed] [Google Scholar]
- 52.Langley RGB, Feldman SR, Nyirady J, et al. The 5-point investigator’s global assessment (IgA) scale: a modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatolog Treat 2015;26:23–31. 10.3109/09546634.2013.865009 [DOI] [PubMed] [Google Scholar]
- 53.The Professional Quality of Life Scale . NovoPsych. 2021. Available: https://novopsych.com.au/assessments/clinician-self-assessment/the-professional-quality-of-life-scale-5-proqol/ [Accessed 17 Nov 2021].
- 54.Liu L, Li S, Zhao Y, et al. Health state utilities and subjective well-being among psoriasis vulgaris patients in mainland China. Qual Life Res 2018;27:1323–33. 10.1007/s11136-018-1819-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.