Abstract
Introduction
Stroke survivors usually experience long-lasting functional, emotional and social consequences that might contribute to sedentary behaviour and participation restrictions, which are important targets to address during rehabilitation. However, the trajectory and inter-relationship between these factors are unknown.
Methods and analysis
Part&Sed is a research project based on an observational study design with 6 and 12 months of follow-ups in stroke survivors. In addition, a qualitative analysis of the impact of the stroke on the stroke survivor, validation of the Satisfaction with Daily Occupation-Occupational Balance assessment tool and analysis of the reliability of the Fitbit Inspire 2 activity tracker wristband will be carried out. Participants will be chronic stroke survivors with independent walking capacity. Sociodemographic and clinical data, physical activity, ambulation, sleep, quality of life, anxiety and depression, community participation, and occupational satisfaction and balance, as well as data provided by the activity tracker wristband, will be collected. In addition, if the participant has a primary caregiver, the caregiver will also be monitored. A minimum of 130 participants will be recruited to conduct a random-effects multiple regression model. Mixed models for repeated measures will assess the variation over time of the different variables associated with participation and sedentary behaviour. Psychometric properties (eg, internal consistency, construct validity, test–retest reliability) of the Satisfaction with Daily Occupation-Occupational Balance will be determined. Additionally, intraclass correlation coefficients and minimum detectable change will be calculated to assess intrasubject reliability of physical activity and sleep parameters recorded by the Fitbit Inspire 2. The qualitative analysis process will be carried out using the analysis proposed by Giorgi.
Ethics and dissemination
The study received ethical approval from the Spanish Regional Ethics Committee ‘Comité de Ética de la Investigación de la Comunidad de Aragón’ (PI21/333). The results will be made available via peer-reviewed publications, international conferences and official channels.
Keywords: stroke, stroke medicine, neurology, neurological injury
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This large prospective multicentre project examines the multifactorial interaction between physical activity and participation dimensions in Spanish stroke survivors.
Training sessions on the procedures and administration of the evaluation tools will be conducted to minimise inter-rater variability.
A novel method for assessing physical activity and sleep parameters in stroke survivors will be explored.
Stroke survivors with aphasia, no technological knowledge or those living in nursing homes and hospital settings will not be included.
Physical activity is being monitored by wrist-worn devices which are known to have limitations.
Introduction
Stroke has an annual global incidence of 12.2 million, being the second leading cause of death in developed countries.1 2 There is an estimated prevalence of 143 million people living with poststroke disability, thus becoming the third leading cause of disability worldwide and representing the main need for neurorehabilitation.3 In 2019, Spanish statistics revealed an annual incidence of 61 102 cases and a prevalence of 512 380 people with poststroke disability.1 Although the most visible poststroke consequences are physical, this population also presents long-lasting cognitive and emotional alterations,4 as well as restrictions in participation in self-care, work, leisure and free time, and social activities.5–8 Additionally, two-thirds show difficulty in walking independently, which is maintained beyond 3 months after stroke.9
Participation is defined by the International Classification of Functioning, Disability and Health (ICF) as ‘a person’s involvement in life situations’ and is the product of an interaction between the individual’s health condition and contextual factors.10 Therefore, participation comprehends meaningful activities performed by individuals in their real environment (eg, family or community) that occupy time and give meaning and purpose to their life.11 Thus, areas of occupation consist of the basic and instrumental activities of daily living, rest and sleep, education, work, playing, leisure and social participation.12 13
Physical inactivity and sedentary behaviour are one of the main lifestyle-related risk factors for stroke recurrence.14 Physical inactivity is defined as an insufficient physical activity level to meet the current recommendations established by WHO15 and endorsed by the American Heart Association Council.16 These recommendations consist of achieving 150 min of moderate to vigorous-intensity physical activity per week or 75 min of vigorous-intensity physical activity per week.17 On the other hand, sedentary behaviour is defined as any waking behaviour characterised by an energy expenditure ≤1.5 metabolic equivalents, while sitting, reclining or lying.18 If the sedentary behaviour is maintained throughout the day or week while being awake, it can be classified as a sedentary behaviour pattern.19 Adult populations who comply with WHO recommendations for physical activity show a lower risk of death and developing non-communicable diseases.20 However, stroke survivors often report sedentary behaviour most of the day, especially in the afternoon and evening.21 Additionally, the average number of sleep hours is much higher in stroke survivors than in the healthy population, which is associated with cardiovascular morbidity and mortality.22
Regarding the factors that contribute to sedentary lifestyles in the stroke population, it has been observed that inpatient stroke survivors in rehabilitation centres are more sedentary.23 24 However, those living in the community still do not meet a healthy level of physical activity.25 On the other hand, the fear of falling is associated with sedentary lifestyles,26 27 to the extent that stroke survivors reduce activities involving standing or walking by 20% on average.28 In turn, the intensity and frequency of rehabilitation treatment,23 29 the place of residence and characteristics of the community,25 social support,23 years after stroke,30 initial stroke severity,31 32 the type of hemiparesis, greater cognition,32–34 better endurance and gait speed32–35 are further examples of contributors to a sedentary lifestyle. Finally, psychosocial factors, such as anxiety, depression and self-efficacy, can play a role.36 37 This ensemble of factors warrants studies analysing multifactorial relationships and establishing a firm consensus on policies for the rehabilitation of stroke survivors.
Systematic reviews show that poststroke interventions do not impact community participation until several years after stroke38 and that identifying barriers is key.39 However, despite the limited evidence regarding the factors that influence participation,30 having an extensive social network and the ability to walk for a few hundred metres or drive are potential facilitators.8 Additionally, improvements in physical functioning do not necessarily translate into improved participation in work, domestic, social and leisure activities.40 41 This could be why stroke survivors feel unsatisfied about not recovering prestroke participation levels in relevant activities, which may be due to their environment, age, acceptance of the new situation or degree of affectation,42 as well as due to the presence of comorbidities related to cognitive impairment and depressive states.43
Therefore, aiming to emphasise social reintegration as a long-term goal,44 several studies highlight the need to consider other underinvestigated factors, such as the influence of the primary caregiver and the family environment36; environmental factors such as climate and daylight hours33; or the impact of treatments focusing on health education45 and the person-centred model.2
Part&Sed-Stroke project justification
The stroke population in Europe, Australia, Canada and the USA is known to have high levels of sedentary lifestyles.21 25 31 32 46–50 However, there is no solid information about the Spanish population, which would allow the development, as recommended by WHO, of a specific public health plan for the stroke survivors in Spain. For this reason, longitudinal studies are needed to determine the evolution of the level of physical activity after stroke.44 46
The assessment tools included in the present protocol have been selected based on the 2018 Cochrane review authored by Lynch et al,51 and by the recommendations of Kwakkel et al,52 and its updates in a 2020 Delphi study.53 Additionally, other reliable assessment tools are needed to ascertain the amount of sedentary time, assess its effect on health and identify significant predictors of physical activity levels.14 In this sense, devices with high potential have already been identified at the scientific level,54 55 and it is known that three or more days, for a minimum of 14 hours/day, are required for accurate monitoring.56 However, more studies are needed to analyse the potential of new wearable devices to record this physical activity and measure adherence or compliance with physical activity recommendations in the stroke population,57 as well as to assess their suitability and ease of use in the clinical setting by professionals and patients. Consequently, this protocol will evaluate the Fitbit Inspire 2 activity tracker wristband (Fitbit, San Francisco, California, USA). On the other hand, with the aim of promoting the recovery of participatory life after stroke, it is relevant that clinicians have validated assessment tools for the stroke population on occupational satisfaction.
The current protocol has also included the Activity Card Sort (ACS), recommended in systematic reviews for the ICF domains of activity and participation in the stroke population.58 59 Finally, following the recommendations of other studies, other factors such as personal, sociodemographic and psychological factors that may have an impact on mobility in the community have been considered.33
Considering that the current goal of neurorehabilitation is to design and personalise physical activity programmes according to the ability, goals and preferences of the stroke survivor, as well as to encourage long-term lifestyle habits,60 it is warranted to conduct longitudinal studies investigating the trajectory and the inter-relationship between sedentary behaviour and participation in stroke survivors.
Objectives
To multidimensionally explore the factors associated with participation and sedentary behaviour in Spanish stroke survivors.
To investigate the influence of sedentary behaviour and individual characteristics of the primary caregiver on the participation and sedentary lifestyle of Spanish stroke survivors.
To analyse the natural fluctuation of participation, sedentary behaviour and associated factors over 12 months in Spanish stroke survivors.
To validate and establish the psychometric properties of the Satisfaction with Daily Occupation-Occupational Balance (SDO-OB) assessment scale for Spanish stroke survivors.
To determine the intrasubject reliability values in the short term (1 week) and medium term (6 months) of the Fitbit Inspire 2 activity tracker wristband and evaluate the construct validity of monitoring physical activity and sleep quality using Fitbit Inspire 2 activity tracker wristband versus the scores obtained in the International Physical Activity Questionnaire Short Form (IPAQ-SF) and Sleep Scale from the Medical Outcomes Study (MOS-Sleep) self-reported questionnaires in Spanish stroke survivors.
To qualitatively analyse the lived experience of Spanish stroke survivors in terms of barriers and facilitators for the return to physical activity and participation in occupational living, along with the knowledge needed to achieve it.
Methods
Study design
The present protocol complies with the Strengthening the Reporting of Observational Studies in Epidemiology recommendations,61 the Consensus-based Standards for the Selection of Health Status Measurement Instruments,62 the Guidelines for Reporting Reliability and Agreement Studies,63 the Consolidated Criteria for Reporting Qualitative Research64 and the Standards for Reporting Qualitative Research.65
This study protocol will follow a prospective design with a follow-up of 12 months (figure 1), in which several centres distributed throughout Spain will participate (online supplemental material 1). Specifically, a cross-sectional design will be used to assess factors associated with sedentary behaviours and participation levels in stroke survivors, including the influence of the primary caregiver. The validation and establishment of the psychometric properties of the SDO-OB questionnaire in Spanish stroke survivors will be performed. Additionally, a prospective cohort design will be used to assess the evolution of sedentary and participation behaviours in stroke survivors over time, identifying those factors that may contribute to a modification of these behaviours. Follow-up assessments will be carried out at 6 and 12 months to provide a sufficient time frame in which to appreciate meaningful changes. Finally, the life experience of stroke survivors will be investigated through unstructured in-depth interviews and semistructured interviews, which will be analysed by means of a descriptive phenomenological approach.
Figure 1.
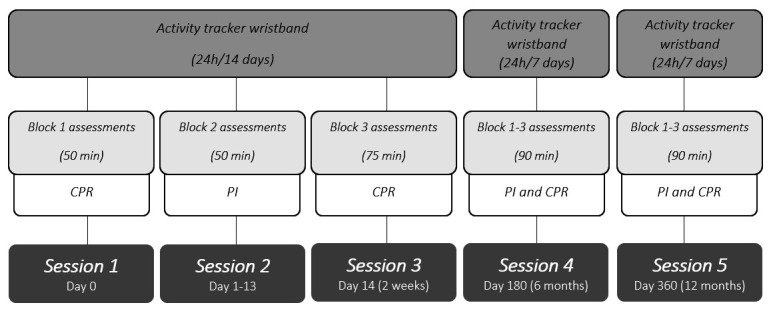
Study timeline design. CPR, collaborating professional researcher; PI, principal investigator.
bmjopen-2022-065628supp001.pdf (108.7KB, pdf)
Population
The study sample will consist of people of both sexes who have suffered a stroke and reside in Spain. The inclusion criteria will consist of (1) being over 18 years of age; (2) having a history of stroke with a medical diagnosis for more than 6 months, regardless of its aetiology; (3) outpatient living at home; (4) having cognitive and speech ability to perform and understand the tests to be administered and the purpose of the research project (ie, no aphasia and a Mini-Mental Cognitive Test score >2466); (5) being able to ambulate with or without aids, which represent an ambulation ability ≥3 in the Functional Ambulatory Category67 (not applicable for SDO-OB validation); and (6) availability of a mobile phone with Bluetooth and internet connection. The exclusion criteria will be (1) non-acceptance of participation in the research project by the primary caregiver; (2) not tolerating being monitored with an activity tracker wristband; (3) residing in institutions (eg, nursing homes); (4) no commitment to continuity; and (5) a history of more than one symptomatic stroke. Missing evaluation sessions, an existing personal or family situation that interferes with data collection and willingness to discontinue the study were considered withdrawal criteria.
If the stroke survivor has a primary caregiver, the primary caregiver will be invited to participate in the study. A primary caregiver is defined as a person, either a family member or an employee, who spends more than half of their daily time supporting or caring for the stroke survivor.
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Contact and recruitment
Centres and health professionals
The collaborators will be composed of health professionals (physiotherapists and occupational therapists) working in institutions, centres and associations, as well as self-employed professionals whose field of action is focused on the neurological rehabilitation of stroke survivors. Specifically, in the first phase, contact will be established via email or telephone with professionals who form part of the research team’s network. While in the second phase, general information about the research study will be disseminated through social networks (ie, Twitter and LinkedIn) to increase the number of potential organisations and professionals collaborating. These collaborators will meet the conditions of residing in different parts of Spain, having extensive experience in neurological rehabilitation and currently providing care to stroke survivors.
All those who will show interest in the study will receive a document that includes information on the objective of the study, criteria for the selection of the sample, functions to be carried out that would imply their commitment to participate and a timetable for data collection. If there is continued interest in participating, a first meeting will be held to provide further information and resolve doubts. After this meeting, the centres and professionals will express their willingness to participate. Each participating centre must sign the collaboration agreement, and each professional belonging to these centres must accept the commitment document to participate in the research. These documents will regulate the transfer of the anonymised data collected for research use, as well as the commitments and functions of the institutions and professionals involved.
Participating centres will be instructed in homogenising data collection through videoconference meetings and training sessions. With the aim of facilitating access to information on the study, a secure shared folder has been created in a virtual space containing documents on the study, information sheets for participants, consents, collaboration documents and manuals of procedures to be followed. In addition, the research team will provide and send a minimum of two Fitbit Inspire 2 monitoring wristbands to each collaborating centre.
Stroke survivor participants and primary caregivers
The recruitment process of participants (stroke survivors and their respective caregivers) will be carried out in two phases. First, each of the collaborating centres will be responsible for promoting the existence of the study through their usual internal channels of communication with their users, such as posters, information circulars and posts on social networks. Each potential participant will be given an information sheet about the research project, and any questions they may have regarding the development of the research project and their participation in it will be answered.
Second, those users who voluntarily agree to participate in the study will be assessed by healthcare professionals (occupational therapists and physiotherapists) with training and clinical experience in neurorehabilitation to corroborate compliance with the eligibility criteria. Those who meet the selection criteria will be selected as potential participants and will be asked to sign the informed consent for inclusion in the study. Once the informed consent is signed, they will be assigned an appointment for the first assessment session within approximately 1 week. The same assessors will conduct the assessment sessions at each centre.
Data collection and security measures
Each collaborator will be responsible for collecting part of the data for participants from their centres. The collection of all the data will be supervised and coordinated by the research team, and the principal investigator will be in direct contact with each collaborator to assist with solving any problems that may arise.
In order to maintain security in the process of collecting data from each participant: (1) each collaborator will be assigned an account and password to access the P4Work application where the data collected from each participant in the study are recorded68; and (2) each collaborator will be provided with accounts and passwords that must be assigned to each participant (stroke survivor and their main caregiver) to use the Fitbit application.
Only the collaborators at each centre will know the identity of each participant recruited at their centre. In such a way, the research team will have access to anonymised data in both the Fitbit and P4Work applications.
Outcome measures
Sociodemographic data
Sociodemographic data will include age, sex, smoking, educational level, economic level, employment status, height and weight, municipality, type of housing, presence of architectural barriers and home cohabitation.
Clinical data
Clinical data will include information such as age at the time of stroke, type of stroke, damaged cerebral hemisphere, time of evolution, pain experience, other pathologies (including the number of silent or subclinical strokes), current medication, current rehabilitation and hours per week, number of falls in the last 6 months, use of assistive devices, the Barthel Index,69 the Fall Efficacy Scale International (FES-I)70 and the modified Rankin Scale.71
Self-reported physical activity
Self-reported physical activity levels will be evaluated with the IPAQ-SF. The IPAQ-SF is a self-report scale that aims to determine the level of physical activity in the current period (last 7 days) and the time spent sitting down. The final score categorises physical activity levels into low, moderate or high physical activity. The IPAQ is a suitable questionnaire for population-based physical activity monitoring. The IPAQ-SF can be used for physical activity prevalence studies to monitor the population and has demonstrated adequate reliability and validity72 73 and has been used in previous studies with stroke population.74
Self-perceived quality and quantity of sleep
MOS-Sleep will be used to assess the most important dimensions of sleep quantitatively and qualitatively as well as to assess potential sleep disturbances. The tool consists of 12 items whose responses are based on a retrospective assessment over the last 4 weeks.75 The MOS-Sleep is a suitable and valid questionnaire to obtain information on multiple aspects of sleep quality. The scale has been shown to have good validity and reliability for the assessment of sleep disturbances. It is a sensitive assessment tool for detecting the impact of an illness or medication on the different dimensions of sleep.76
Current health status
Current health status will be evaluated with the self-assessed, health-related, quality of life questionnaire 5-Level version of EuroQol-5 Dimension (EQ-5D-5L). The EQ-5D-5L measures quality of life on a 5-component scale, including mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each of these dimensions has five possible answers or levels of severity. In the second part of the questionnaire, respondents are asked to rate their current health status on a scale, where 100 corresponds to ‘the best health status you can imagine’ and 0 to ‘the worst health status you can imagine’. The EQ-5D-5L is a valid and reliable tool for assessing the quality of life.77
State of anxiety and depression
The state of anxiety and depression will be evaluated with the Hospital Anxiety and Depression Scale (HADS). The HADS is a 14-item questionnaire consisting of a depression and anxiety subscale with interspersed items. The items are scored on a 4-point Likert scale (0–3) with a total score ranging from 0 to 21 for each subscale, where a higher score indicates greater symptom severity. The HADS is a reliable and valid tool for assessing levels of depression and anxiety78 and has been used with stroke survivors in previous studies.79
Degree of participation
The degree of participation will be assessed using the ACS for the Spanish population. The ACS is a scale that measures self-perceived participation using photographs reflecting everyday activities. The activities are categorised into four domains: instrumental activities, leisure activities with low physical demand, leisure activities with high physical demand and socioeducational activities. It covers eight of the nine domains of participation as defined by the ICF.10 The final score is the percentage of activities maintained after an illness, which is developed as a quotient between the number of activities carried out before the illness and those carried out at present. The ACS is a reliable and validated tool for measuring the perceived level of participation.80 81
Degree of occupational satisfaction and balance
The degree of occupational satisfaction and balance will be assessed with the SDO-OB. The SDO-OB is an interview-based instrument that assesses 13 different occupational items organised into four domains: productivity, leisure or free time, housework and self-care. The SDO-OB assesses the level of participation in an activity, the occupational satisfaction derived from participating in this activity and the perceived occupational balance within each domain. For each item, the interviewer will determine whether there is participation by the person and then indicate the degree of satisfaction from 1 to 7 (1 being extremely dissatisfied and 7 being extremely satisfied). The SDO-OB has good reliability and validity.82
Physical mobility tests
The 10-metre walk test (10MWT) will be used to assess walking speed in metres per second. The person will be asked to walk for 10 m while being timed. The comfortable speed for walking over 10 m will be taken three times. The average of the three recordings will be the value of the assessment. The 10MWT is a reliable and validated tool for measuring walking speed.83
The 6-minute walk test (6MWT) will be used to assess the distance walked by the participant in 6 min. Standardised guidelines will be followed during the test to give verbal indications concerning the time elapsed and positive reinforcement without any other indications not allowed by the test. The 6MWT is a reliable and valid tool for measuring distance run, endurance and aerobic capacity.84
Additionally, walking aids and a modified Borg Index (0–10) will be collected after each test.
Furthermore, physical mobility tests will be performed in all centres under similar standardised conditions. Before signing the collaborating agreement, each centre will ensure sufficient space on its premises to carry out the tests.
Activity tracker wristband
The Fitbit Inspire 2 activity tracker will be used to assess the level of activity throughout 14 days continuously. The device is based on a three-axis accelerometry system that monitors physical activity, number of steps, heart rate, sleep duration and sleep score. The Fitbit wristbands are considered a valid and reliable tool for monitoring physical activity and sleep hours,85 86 which are considered a valid procedure in stroke survivors.87 88
Evaluation of caregivers
Sociodemographic data and IPAQ-SF will be collected from primary caregivers during the first interview with study participants who have suffered a stroke. Caregivers will be monitored with the Fitbit activity wristband while the stroke survivor is monitored.
Assessments and temporality
The evaluations will be carried out similarly at three points in time over a 12-month period (ie, baseline, 6 months and 12 months). Each evaluation will be composed of different tests and questionnaires grouped by blocks to avoid saturation of the participants. Specifically, block 1 will be assessed by the collaborators approximately 1 week after recruitment, consisting of sociodemographic data, IPAQ-SF, FES-I, Barthel Index, HADS and EQ-5D-5L. After completing the assessment of block 1, the Fitbit Inspire 2 activity tracker wristband will be linked to the mobile device for 14 days of 24-hour monitoring. Block 2 will be assessed in the settings of collaborator centres approximately 2 weeks after completing block 1, coinciding with the return of the activity tracker wristband, and will consist of the ACS, the 6MWT and the 10MWT. Block 3 will be assessed homogenously in all participants by the principal investigator via teleconference within the period between the blocks 1 and 2, and will consist of clinical data, MOS-Sleep and SDO-OB (figure 1).
One to two weeks after completing the baseline evaluation, a minimum of 50 participants will complete the IPAQ-SF, MOS-Sleep and SDO-OB again. Additionally, during the follow-up period after baseline, a random sample of around 30 participants will participate in unstructured and semistructured interviews as part of the qualitative design.
Qualitative design
A qualitative phenomenological design will be followed based on Husserl’s framework.89 Data collection will be completed when the information obtained in the interviews becomes repetitive.90 First-person data collection instruments (unstructured and semistructured interviews) and the researcher’s field notes will be used simultaneously.90 Data collection will be conducted in two phases. The first phase of data collection will be conducted through unstructured in-depth interviews. The second phase will be conducted through semistructured interviews based on the analysis of participants’ responses from the first phase, together with questions grouped into three research areas: physical activity, participation and health education.
The analysis proposed by Giorgi will be used.91 The researcher’s field notes will complement the analysis of the interviews recorded and transcribed verbatim. MAXQDA 2022 software (Verbi Software, Berlin, Germany) will be used for data analysis.
Different strategies will be followed before and during the data collection process to ensure the methodological rigour and quality of this study (ie, establishing the positioning of the researchers, triangulation, auditing of the material obtained and participant verification).
Monitoring of the methodological quality
Given the multicentric nature of the research study, several mechanisms will be implemented to avoid interobserver bias and reduce random errors as much as possible. First, the assessment protocol comprises measurement instruments with reliable interobserver psychometric characteristics, allowing a standardised data collection. Second, meetings will be held to unify the protocol for the administration of the tools and data collection, establishing a unification of the evaluation criteria for each tool. Third, training sessions on the procedures and administration of the evaluation tools will be conducted both face to face and virtually. Additionally, all collaborators will have written manuals that include these standardised and unified procedures. Finally, the project research team will supervise all procedures and solve any potential issues, sharing the relevant information with all collaborators participating.
Sample size
The sample size has been calculated with G*Power (V.3.1.9.4; Heinrich Heine University, Dusseldorf, Germany) based on the requirements of the most demanding research objective in terms of the number of participants (ie, objective 1). Specifically, after running a priori analysis with an alpha value of 0.05, a power of 80% and expecting a coefficient of multiple determination (r²) between 0.30 and 0.50, a minimum of 130 participants will be required to perform a random-effects multiple regression model with up to 15 variables. Furthermore, a sample size higher than 73 participants during follow-ups will be sufficient to perform a mixed model for repeated measures with a power of 80% and an alpha error of 0.05 to detect a small to medium standardised mean difference (ie, f=0.15) and expecting at least a moderate correlation among repeated measures (ie, r=0.5).
Statistical analysis
The results will be presented in tables and graphs by presenting the mean and SD, or the median and IQR values, depending on the normality of the data.
Random-effects multiple regression models will be used to examine the factors associated with the stroke survivor’s participation and sedentary behaviour, including the sedentary behaviour and individual characteristics of the primary caregiver.
Mixed models for repeated measures will be used to assess the variation over time of the different variables associated with participation and sedentary behaviour.
In the validation process of the SDO-OB in the Spanish stroke survivors, Cronbach’s alpha statistic will be used to test internal consistency and intraclass correlation coefficients to assess test–retest reliability. In addition, Spearman’s rank correlation will be used to assess the construct validity of the SDO-OB compared with EQ-5D-5L and ACS.
Pearson’s correlations will be used to assess the construct validity of the activity tracker wearable compared with self-reported measures. Furthermore, intraclass correlation coefficients with a 95% CI, the SE of measurement and minimum detectable change will be calculated to assess intrasubject reliability of physical activity and sleep parameters recorded by the Fitbit Inspire 2 collected during two consecutive weeks.
Limitations
This project has some limitations. First, our population does not include stroke survivors with aphasia, an important group who are often excluded from research. Additionally, our population does not include those with limited technical knowledge or people living in nursing homes. Therefore, the generalisability of the results of this study will be limited. This study will not provide insights into physical activity and participation in the acute and subacute phases of stroke recovery, as it only commences in the chronic phase. Finally, physical activity is being monitored by wrist-worn devices which are known to have limitations.87
Ethics and confidentiality
The study protocol has been designed following the Helsinki statement and approved by the Spanish Regional Ethics Committee ‘Comité de Ética de la Investigación de la Comunidad de Aragón’ (PI21/333). All patients will receive an information sheet explaining the purpose of the study and the tests and assessments that will be performed if they agree to participate in the study. In addition, subjects will sign the informed consent form once they have agreed to participate in the study. There will be no financial compensation of any kind to the participants in this project.
Dissemination
Any deviation from the protocol will be presented and justified at the time of publication of the results. Regardless of the outcome, the results will be made available via peer-reviewed publications in open-source journals and relevant international conferences within the field of health and behavioural sciences or neurology, among others.
Supplementary Material
Acknowledgments
We thank all the collaborating centres involved in the recruitment of study participants: ADACECO (Asociación de Daño Cerebral de A Coruña) in A Coruña; AENO (Asociación de Enfermos Neurológicos Oscense) in Huesca; AGREDACE (Asociación de Daño Cerebral Adquirido de Granada) in Granada; AIDA (Asociación de Ictus de Aragón) in Zaragoza; ASPAYM (Asociación de personas con lesión medular y otras discapacidades física) in Burgos; CENNER (Centro de NeuroRehabilitación, Nutrición y Fisioterapia) in Pamplona; Centro de neurorrehabilitación (Clínica de Rehabilitación Neurológica Sant Cugat) in San Cugat del Valles; CIRON (Centro Integral de Rehabilitación ON) in Valladolid; freelance physiotherapist in Madrid and Sanlúcar de Barrameda; Fundación Pita López (Centro de Neurorehabilitación de Daño Cerebral Adquirido) in Madrid; Grupo 5 CIAN Navarra (Centro Integral de Atención Neurorehabilitadora) in Pamplona; Grupo 5 CIAN Zaragoza (Centro Integral de Atención Neurorehabilitadora) in Zaragoza; Hospital Provincial Sagrado Corazón de Jesús in Huesca; Hospital Universitario San Jorge in Huesca; INEURO (Neurorrehabilitación y Atención al Neurodesarrollo) in Sevilla; NEUFIS (Centro de Fisioterapia y Neurorrehabilitación) in Vitoria; NEURAXIS (Rehabilitación Neurológica y Desarrollo Infantil) in Ferrol; NEUROESPLUGUES (Centre de Rehabilitació Neurològica) in Esplugues de Llobregat; Rehabilitación El Carmen in Zaragoza.
Footnotes
Twitter: @CdeDiegoAlonso, @NatFiniPhysio, @_Victordomenech
Collaborators: ‘Part&Sed-Stroke collaborators’ list of names: Alicia, Tornero Navarro; Ana, Conte Lamenca; Andrea, Yerro Astrain; Beatriz, Martín Lamata; Carina, Salgueiro; Claudia, Marín Marín; Diana, Ruiz Ramos; Elena, Sanz Sanza; Enrique, Villa Berges; Fernando, Cuesta Ruiz; Inés, Cortés Cabeza; Javier, Harguindey; Juan Luis, Abeledo Alcón; Laura, Ares Barge; Leyre, Leceaga Gaztambide; Lilian, Le Roux; Lourdes, Martín Gros; Lucía, Díez Fuentes; María Carmen, Grácia Sen; María Pilar, Pardo Sanz; Paloma, Rodríguez Escudero; Paz Cristina, Sánchez Lecina; Rebeca, Yebra Vilarchao; Sara, Beltrán Roche; Verónica, Montoya Murillo.
Contributors: CdD-A and PB-L conceived and planned the project. CdD-A, PB-L, JA-A and VD-G, with the collaboration of the rest of the authors, contributed to the design of the study protocol. CdD-A, AB, JG-R and PR-P contributed to the qualitative study design. CdD-A, PB-L, JB-A, RG-N, MPL-R and VD-G contributed to the reliability study design. CdD-A led the recruitment of participating centres and coordination of the professional collaborators. ‘Part&Sed collaborators’ contributed to checking and testing the protocol design and will conduct the data collection. CdD-A, PB-L, AB, JB-A, JG-R, VD-G and NAF contributed to writing the article. All authors have read and approved the final manuscript.
Funding: This work was supported by ‘Ayudas a Proyectos Internos de Investigación Universidad San Jorge curso 2021–2022’ (grant number: 2122037) and ‘Ayudas a Proyectos Internos de Investigación Universidad San Jorge curso 2022–2023’ (grant number: 2122037). PB-L is supported by Grant FPU19/05237 and JB-A by Grant PIF 2022-2026 from ‘Gobierno de Aragón’.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: Part&Sed-Stroke Collaborators Group, Alicia Tornero Navarro, Ana Conte Lamenca, Andrea Yerro Astrain, Beatriz Martín Lamata, Carina Salgueiro, Claudia Marín Marín, Diana Ruiz Ramos, Elena Sanz Sanza, Enrique Villa Berges, Fernando Cuesta Ruiz, Inés Cortés Cabeza, Javier Harguindey, Juan Luis Abeledo Alcón, Laura Ares Barge, Leyre Leceaga Gaztambide, Lilian Le Roux, Lourdes Martín Gros, Lucía Díez Fuentes, María Carmen Grácia Sen, María Pilar Pardo Sanz, Paloma Rodríguez Escudero, Paz Cristina Sánchez Lecina, Rebeca Yebra Vilarchao, Sara Beltrán Roche, and Verónica Montoya Murillo
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.GBD 2019 Stroke Collaborators . Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol 2021;20:795–820. 10.1016/S1474-4422(21)00252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Framework on integrated, people-centred health services: report by the secretariat. Geneva: World Health Organization, 2016. [Google Scholar]
- 3.Cieza A, Causey K, Kamenov K, et al. Global estimates of the need for rehabilitation based on the global burden of disease study 2019: a systematic analysis for the global burden of disease study 2019. Lancet 2021;396:2006–17. 10.1016/S0140-6736(20)32340-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viktorisson A, Reinholdsson M, Danielsson A, et al. Pre-stroke physical activity in relation to post-stroke outcomes - linked to the international classification of functioning, disability and health (ICF): a scoping review. J Rehabil Med 2022;54:51. 10.2340/jrm.v53.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee Y, Nicholas ML, Connor LT. Identifying emotional contributors to participation post-stroke. Top Stroke Rehabil 2021;2021:1–13. 10.1080/10749357.2021.2008597 [DOI] [PubMed] [Google Scholar]
- 6.Svensson JS, Westerlind E, Persson HC, et al. Occupational gaps 5 years after stroke. Brain Behav 2019;9:e01234. 10.1002/brb3.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singam A, Ytterberg C, Tham K, et al. Participation in complex and social everyday activities six years after stroke: predictors for return to pre-stroke level. PLoS One 2015;10:e0144344. 10.1371/journal.pone.0144344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norlander A, Carlstedt E, Jönsson A-C, et al. Long-Term predictors of social and leisure activity 10 years after stroke. PLoS One 2016;11:e0149395. 10.1371/journal.pone.0149395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy C, Bernhardt J, Churilov L, et al. Factors associated with time to independent walking recovery post-stroke. J Neurol Neurosurg Psychiatry 2021;92:702–8. 10.1136/jnnp-2020-325125 [DOI] [PubMed] [Google Scholar]
- 10.World health Organization . International classification of functioning, disability and health (ICF). Geneva: World Health Organization, 2001. [Google Scholar]
- 11.Wu C, Lin K. Defining occupation: A comparative analysis. Journal of Occupational Science 1999;6:5–12. 10.1080/14427591.1999.9686446 [DOI] [Google Scholar]
- 12.Occupational therapy practice framework: domain and process-fourth edition. Am J Occup Ther 2020;74:7412410010p1–87. 10.5014/ajot.2020.74S2001 [DOI] [PubMed] [Google Scholar]
- 13.Roley SS, DeLany JV, Barrows CJ, et al. Occupational therapy practice framework: domain & practice, 2nd edition. Am J Occup Ther 2008;62:625–83. 10.5014/ajot.62.6.625 [DOI] [PubMed] [Google Scholar]
- 14.English C, Manns PJ, Tucak C, et al. Physical activity and sedentary behaviors in people with stroke living in the community: a systematic review. Phys Ther 2014;94:185–96. 10.2522/ptj.20130175 [DOI] [PubMed] [Google Scholar]
- 15.Bull FC, Al-Ansari SS, Biddle S, et al. World Health organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54:1451–62. 10.1136/bjsports-2020-102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon NF, Gulanick M, Costa F, et al. Physical activity and exercise recommendations for stroke survivors: an american heart association scientific statement from the council on clinical cardiology, subcommittee on exercise, cardiac rehabilitation, and prevention; the council on cardiovascular nursing; the council on nutrition, physical activity, and metabolism; and the stroke council. Circulation 2004;109:2031–41. 10.1161/01.CIR.0000126280.65777.A4 [DOI] [PubMed] [Google Scholar]
- 17.WHO . Physical activity strategy for the WHO european region 2016–2025. WHO-Europe, 2015. [Google Scholar]
- 18.Sedentary Behaviour Research Network . Letter to the editor: standardized use of the terms “ sedentary ” and “ sedentary behaviours. ” Appl Physiol Nutr Metab 2012;37:540–2. 10.1139/h2012-024 [DOI] [PubMed] [Google Scholar]
- 19.Chastin SFM, Granat MH. Methods for objective measure, quantification and analysis of sedentary behaviour and inactivity. Gait Posture 2010;31:82–6. 10.1016/j.gaitpost.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 20.Ekelund U, Tarp J, Steene-Johannessen J, et al. Dose-Response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ 2019;366:l4570. 10.1136/bmj.l4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.English C, Wondergem R, Hendrickx W, et al. People with stroke are most sedentary in the afternoon and evening. Cerebrovasc Dis 2022;51:511–6. 10.1159/000521209 [DOI] [PubMed] [Google Scholar]
- 22.Ezeugwu VE, Manns PJ. Sleep duration, sedentary behavior, physical activity, and quality of life after inpatient stroke rehabilitation. J Stroke Cerebrovasc Dis 2017;26:2004–12. 10.1016/j.jstrokecerebrovasdis.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 23.Barrett M, Snow JC, Kirkland MC, et al. Excessive sedentary time during in-patient stroke rehabilitation. Top Stroke Rehabil 2018;25:366–74. 10.1080/10749357.2018.1458461 [DOI] [PubMed] [Google Scholar]
- 24.Bernhardt J, Dewey H, Thrift A, et al. Inactive and alone: physical activity within the first 14 days of acute stroke unit care. Stroke 2004;35:1005–9. 10.1161/01.STR.0000120727.40792.40 [DOI] [PubMed] [Google Scholar]
- 25.Hassett L, Ada L, Hellweg S, et al. Active and sedentary bouts in people after stroke and healthy controls: an observational study. Physiother Res Int 2020;25:e1845. 10.1002/pri.1845 [DOI] [PubMed] [Google Scholar]
- 26.Hellström K, Lindmark B, Wahlberg B, et al. Self-Efficacy in relation to impairments and activities of daily living disability in elderly patients with stroke: a prospective investigation. J Rehabil Med 2003;35:202–7. 10.1080/16501970310000836 [DOI] [PubMed] [Google Scholar]
- 27.Salbach NM, Mayo NE, Robichaud-Ekstrand S, et al. Balance self-efficacy and its relevance to physical function and perceived health status after stroke. Arch Phys Med Rehabil 2006;87:364–70. 10.1016/j.apmr.2005.11.017 [DOI] [PubMed] [Google Scholar]
- 28.Hanna E, Janssen H, Crowfoot G, et al. Participation, fear of falling, and upper limb impairment are associated with high sitting time in people with stroke. Occup Ther Health Care 2019;33:181–96. 10.1080/07380577.2019.1587675 [DOI] [PubMed] [Google Scholar]
- 29.Hebert D, Lindsay MP, McIntyre A, et al. Canadian stroke best practice recommendations: stroke rehabilitation practice guidelines, update 2015. Int J Stroke 2016;11:459–84. 10.1177/1747493016643553 [DOI] [PubMed] [Google Scholar]
- 30.Thilarajah S, Mentiplay BF, Bower KJ, et al. Factors associated with post-stroke physical activity: a systematic review and meta-analysis. Arch Phys Med Rehabil 2018;99:1876–89. 10.1016/j.apmr.2017.09.117 [DOI] [PubMed] [Google Scholar]
- 31.English C, Healy GN, Coates A, et al. Sitting time and physical activity after stroke: physical ability is only part of the story. Top Stroke Rehabil 2016;23:36–42. 10.1179/1945511915Y.0000000009 [DOI] [PubMed] [Google Scholar]
- 32.English C, Healy GN, Coates A, et al. Sitting and activity time in people with stroke. Phys Ther 2016;96:193–201. 10.2522/ptj.20140522 [DOI] [PubMed] [Google Scholar]
- 33.Hendrickx W, Riveros C, Askim T, et al. Identifying factors associated with sedentary time after stroke. secondary analysis of pooled data from nine primary studies. Top Stroke Rehabil 2019;26:327–34. 10.1080/10749357.2019.1601419 [DOI] [PubMed] [Google Scholar]
- 34.Mountain A, Patrice Lindsay M, Teasell R, et al. Canadian stroke best practice recommendations: rehabilitation, recovery, and community participation following stroke. Part two: transitions and community participation following stroke. Int J Stroke 2020;15:789–806. 10.1177/1747493019897847 [DOI] [PubMed] [Google Scholar]
- 35.Fini NA, Bernhardt J, Holland AE. Low gait speed is associated with low physical activity and high sedentary time following stroke. Disabil Rehabil 2021;43:2001–8. 10.1080/09638288.2019.1691273 [DOI] [PubMed] [Google Scholar]
- 36.Hall J, Morton S, Fitzsimons CF, et al. Factors influencing sedentary behaviours after stroke: findings from qualitative observations and interviews with stroke survivors and their caregivers. BMC Public Health 2020;20:967. 10.1186/s12889-020-09113-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Espernberger KR, Fini NA, Peiris CL. Personal and social factors that influence physical activity levels in community-dwelling stroke survivors: a systematic review of qualitative literature. Clin Rehabil 2021;35:1044–55. 10.1177/0269215521993690 [DOI] [PubMed] [Google Scholar]
- 38.Becker I, Maleka MD, Stewart A, et al. Community reintegration post-stroke in New Zealand: understanding the experiences of stroke survivors in the lower South island. Disability and Rehabilitation 2022;44:2815–22. 10.1080/09638288.2020.1839792 [DOI] [PubMed] [Google Scholar]
- 39.Wesselhoff S, Hanke TA, Evans CC. Community mobility after stroke: a systematic review. Top Stroke Rehabil 2018;25:224–38. 10.1080/10749357.2017.1419617 [DOI] [PubMed] [Google Scholar]
- 40.O’Brien AN, Wolf TJ. Determining work outcomes in mild to moderate stroke survivors. Work 2010;36:441–7. 10.3233/WOR-2010-1047 [DOI] [PubMed] [Google Scholar]
- 41.Kaskutas V. Stroke rehabilitation. A function-based approach. St. Louis, Missouri, 2016: 224–36. [Google Scholar]
- 42.Engel-Yeger B, Tse T, Josman N, et al. Scoping review: the trajectory of recovery of participation outcomes following stroke. Behav Neurol 2018;2018:5472018. 10.1155/2018/5472018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kapoor A, Lanctôt KL, Bayley M, et al. “ good outcome ” i’ n't good enough: cognitive impairment, depressive symptoms, and social restrictions in physically recovered stroke patients. Stroke 2017;48:1688–90. 10.1161/STROKEAHA.117.016728 [DOI] [PubMed] [Google Scholar]
- 44.Norlander A, Iwarsson S, Jönsson A-C, et al. Participation in social and leisure activities while re-constructing the self: understanding strategies used by stroke survivors from a long-term perspective. Disabil Rehabil 2022;44:4284–92. 10.1080/09638288.2021.1900418 [DOI] [PubMed] [Google Scholar]
- 45.Stern BZ. Critical reflections on self-management support in chronic disease: the value of occupational therapy in health promotion. The Open Journal of Occupational Therapy 2018;6. 10.15453/2168-6408.1461 [DOI] [Google Scholar]
- 46.Fini NA, Holland AE, Keating J, et al. How physically active are people following stroke? systematic review and quantitative synthesis. Phys Ther 2017;97:707–17. 10.1093/ptj/pzx038 [DOI] [PubMed] [Google Scholar]
- 47.Mahendran N, Kuys SS, Brauer SG. Recovery of ambulation activity across the first six months post-stroke. Gait Posture 2016;49:271–6. 10.1016/j.gaitpost.2016.06.038 [DOI] [PubMed] [Google Scholar]
- 48.Hendrickx W, Riveros C, Askim T, et al. An exploration of sedentary behavior patterns in community-dwelling people with stroke: a cluster-based analysis. J Neurol Phys Ther 2021;45:221–7. 10.1097/NPT.0000000000000357 [DOI] [PubMed] [Google Scholar]
- 49.Tieges Z, Mead G, Allerhand M, et al. Sedentary behavior in the first year after stroke: a longitudinal cohort study with objective measures. Arch Phys Med Rehabil 2015;96:15–23. 10.1016/j.apmr.2014.08.015 [DOI] [PubMed] [Google Scholar]
- 50.Paul L, Brewster S, Wyke S, et al. Physical activity profiles and sedentary behaviour in people following stroke: a cross-sectional study. Disabil Rehabil 2016;38:362–7. 10.3109/09638288.2015.1041615 [DOI] [PubMed] [Google Scholar]
- 51.Lynch EA, Jones TM, Simpson DB, et al. Activity monitors for increasing physical activity in adult stroke survivors. Cochrane Database Syst Rev 2018;7:CD012543. 10.1002/14651858.CD012543.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwakkel G, Lannin NA, Borschmann K, et al. Standardized measurement of sensorimotor recovery in stroke trials: consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Int J Stroke 2017;12:451–61. 10.1177/1747493017711813 [DOI] [PubMed] [Google Scholar]
- 53.Pohl J, Held JPO, Verheyden G, et al. Consensus-Based core set of outcome measures for clinical motor rehabilitation after stroke-A Delphi study. Front Neurol 2020;11:875. 10.3389/fneur.2020.00875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fini NA, Holland AE, Keating J, et al. How is physical activity monitored in people following stroke? Disabil Rehabil 2015;37:1717–31. 10.3109/09638288.2014.978508 [DOI] [PubMed] [Google Scholar]
- 55.Kringle EA, Skidmore ER, Terhorst L, et al. Sedentary behavior patterns over 6 weeks among ambulatory people with stroke. Top Stroke Rehabil 2021;28:537–44. 10.1080/10749357.2020.1846934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fini NA, Holland AE, Bernhardt J, et al. How many hours of device wear time are required to accurately measure physical activity post stroke? Int J Environ Res Public Health 2022;19:1191. 10.3390/ijerph19031191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levy T, Laver K, Killington M, et al. A systematic review of measures of adherence to physical exercise recommendations in people with stroke. Clin Rehabil 2019;33:535–45. 10.1177/0269215518811903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kessler D, Egan M. A review of measures to evaluate participation outcomes post-stroke. British Journal of Occupational Therapy 2012;75:403–11. 10.4276/030802212X13470263980757 [DOI] [Google Scholar]
- 59.Tse T, Douglas J, Lentin P, et al. Measuring participation after stroke: a review of frequently used tools. Arch Phys Med Rehabil 2013;94:177–92. 10.1016/j.apmr.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 60.Fini NA, Bernhardt J, Said CM, et al. How to address physical activity participation after stroke in research and clinical practice. Stroke 2021;52:e274–7. 10.1161/STROKEAHA.121.034557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Elm E, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res 2010;19:539–49. 10.1007/s11136-010-9606-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kottner J, Audigé L, Brorson S, et al. Guidelines for reporting reliability and agreement studies (GRRAS) were proposed. J Clin Epidemiol 2011;64:96–106. 10.1016/j.jclinepi.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 64.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349–57. 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- 65.O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med 2014;89:1245–51. 10.1097/ACM.0000000000000388 [DOI] [PubMed] [Google Scholar]
- 66.Lobo A, Saz P, Marcos G, et al. Revalidation and standardization of the cognition mini-exam (first Spanish version of the Mini-Mental status examination) in the general geriatric population. Med Clin (Barc) 1999;112:767–74. [PubMed] [Google Scholar]
- 67.Van Bloemendaal M, Bout W, Bus SA, et al. Validity and reproducibility of the functional gait assessment in persons after stroke. Clin Rehabil 2019;33:94–103. 10.1177/0269215518791000 [DOI] [PubMed] [Google Scholar]
- 68.Bellosta-López P, Domenech-Garcia V, Palsson TS, et al. European knowledge alliance for innovative measures in prevention of work-related musculoskeletal pain disorders (prevent4work project): protocol for an international mixed-methods longitudinal study. BMJ Open 2021;11:e052602. 10.1136/bmjopen-2021-052602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao Y, Wang Y, Li D, et al. Disability assessment in stroke: relationship among the pictorial-based longshi scale, the barthel index, and the modified Rankin scale. Clin Rehabil 2021;35:606–13. 10.1177/0269215520975922 [DOI] [PubMed] [Google Scholar]
- 70.Lomas-Vega R, Hita-Contreras F, Mendoza N, et al. Cross-Cultural adaptation and validation of the falls efficacy scale international in Spanish postmenopausal women. Menopause 2012;19:904–8. 10.1097/gme.0b013e3182475f6e [DOI] [PubMed] [Google Scholar]
- 71.Quinn TJ, Dawson J, Walters MR, et al. Reliability of the modified Rankin scale. Stroke 2009;40:3393–5. 10.1161/STROKEAHA.109.557256 [DOI] [PubMed] [Google Scholar]
- 72.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 73.Mantilla Toloza SC, Gómez-Conesa A. El cuestionario Internacional de actividad Física. un instrumento adecuado en El seguimiento de la actividad Física poblacional. Revista Iberoamericana de Fisioterapia y Kinesiología 2007;10:48–52. 10.1016/S1138-6045(07)73665-1 [DOI] [Google Scholar]
- 74.Phusuttatam T, Saengsuwan J, Kittipanya-Ngam P. Development and preliminary validation of a stroke physical activity questionnaire. Stroke Res Treat 2019;2019:6764834. 10.1155/2019/6764834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rejas J, Ribera MV, Ruiz M, et al. Psychometric properties of the mos (medical outcomes study) sleep scale in patients with neuropathic pain. Eur J Pain 2007;11:329–40. 10.1016/j.ejpain.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 76.Hays RD, Martin SA, Sesti AM, et al. Psychometric properties of the medical outcomes study sleep measure. Sleep Medicine 2005;6:41–4. 10.1016/j.sleep.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 77.Hernandez G, Garin O, Pardo Y, et al. Validity of the EQ–5D–5L and reference norms for the Spanish population. Qual Life Res 2018;27:2337–48. 10.1007/s11136-018-1877-5 [DOI] [PubMed] [Google Scholar]
- 78.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 79.Ayis SA, Ayerbe L, Ashworth M, et al. Evaluation of the hospital anxiety and depression scale (HADS) in screening stroke patients for symptoms: item response theory (irt) analysis. Journal of Affective Disorders 2018;228:33–40. 10.1016/j.jad.2017.11.037 [DOI] [PubMed] [Google Scholar]
- 80.Alegre-Muelas C, Alegre-Ayala J, Huertas-Hoyas E, et al. Spanish transcultural adaptation of the activity card sort. Occup Ther Int 2019;2019:4175184. 10.1155/2019/4175184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hartman-Maeir A, Eliad Y, Kizoni R, et al. Evaluation of a long-term community based rehabilitation program for adult stroke survivors. NeuroRehabilitation 2007;22:295–301. 10.3233/NRE-2007-22407 [DOI] [PubMed] [Google Scholar]
- 82.Vidaña-Moya L, Eklund M, Merchán-Baeza JA, et al. Cross-cultural adaptation, validation and reliability of the spanish satisfaction with daily occupations-occupational balance (SDO-OB): an evaluation tool for people with mental disorders. Int J Environ Res Public Health 2020;17:8906. 10.3390/ijerph17238906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tyson S, Connell L. The psychometric properties and clinical utility of measures of walking and mobility in neurological conditions: a systematic review. Clin Rehabil 2009;23:1018–33. 10.1177/0269215509339004 [DOI] [PubMed] [Google Scholar]
- 84.Macchiavelli A, Giffone A, Ferrarello F, et al. Reliability of the six-minute walk test in individuals with stroke: systematic review and meta-analysis. Neurol Sci 2021;42:81–7. 10.1007/s10072-020-04829-0 [DOI] [PubMed] [Google Scholar]
- 85.Ringeval M, Wagner G, Denford J, et al. Fitbit-based interventions for healthy lifestyle outcomes: systematic review and meta-analysis. J Med Internet Res 2020;22:e23954. 10.2196/23954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feehan LM, Geldman J, Sayre EC, et al. Accuracy of fitbit devices: systematic review and narrative syntheses of quantitative data. JMIR Mhealth Uhealth 2018;6:e10527. 10.2196/10527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klassen TD, Simpson LA, Lim SB, et al. “ stepping up ” activity poststroke: ankle-positioned accelerometer can accurately record steps during slow walking. Phys Ther 2016;96:355–60. 10.2522/ptj.20140611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Klassen TD, Semrau JA, Dukelow SP, et al. Consumer-based physical activity monitor as a practical way to measure walking intensity during inpatient stroke rehabilitation. Stroke 2017;48:2614–7. 10.1161/STROKEAHA.117.018175 [DOI] [PubMed] [Google Scholar]
- 89.Kim H-K, Jun M, Rhee S, et al. Husserlian phenomenology in korean nursing research: analysis, problems, and suggestions. J Educ Eval Health Prof 2020;17:13. 10.3352/jeehp.2020.17.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moser A, Korstjens I. Series: practical guidance to qualitative research. Part 3: sampling, data collection and analysis. Eur J Gen Pract 2018;24:9–18. 10.1080/13814788.2017.1375091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giorgi A. The theory, practice, and evaluation of the phenomenological method as a qualitative research procedure. J Phenomenol Psychol 1997;28:235–60. 10.1163/156916297X00103 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-065628supp001.pdf (108.7KB, pdf)


