Abstract
The taxonomic status of the sergestid shrimp, Acetes americanus, has been questioned for several decades. No specific study has been performed thus far to resolve the incongruences. This species has a wide geographical range in the western Atlantic and is represented by two formally accepted subspecies: Acetes americanus carolinae, distributed in North America, and Acetes americanus americanus, present in South America. However, there are regions where the coexistence of both subspecies has been reported, such as Central America. This study aimed to genetically compare specimens of A. a. americanus collected in South America with A. a. carolinae sampled in North America to check for possible differences and the existence of more than one subspecies of A. americanus on the Brazilian coast. Based on the sequences of two informative markers, the cytochrome oxidase I region (COI) and 16S rRNA, phylogenetic reconstruction demonstrated well-defined clades with high support values, reinforcing the idea that A. a. americanus is genetically different from A. a. carolinae. Our hypothesis was corroborated as the specimens collected in Brazil were divided into two distinct lineages: the first composed of A. a. americanus sensu stricto (Brazil 1) and the second by Acetes americanus (Brazil 2). The three groups evidenced in the haplotype network were the same as those observed in the phylogenetic tree. The morphometric character (height/length of the thelycum) was effective in distinguishing A. a. Brazil 1 from A. a. carolinae. However, more detailed and conclusive studies comprising other characteristics to propose and describe a possible new entity are necessary. To the best of our knowledge, for the first time, the results of this study provide some insights into the taxonomic status of the sergestid shrimp A. americanus in the western Atlantic.
Keywords: Crustacea, Molecular phylogeny, Western Atlantic, 16S rDNA, Sergestidae, Cytochrome Oxidase I (COI), Sergestoidea, Phylogenetics
Introduction
Genus Acetes H. Milne Edwards, 1830 comprises small planktonic shrimps (Wong, 2013), which are essential components of marine systems (Xiao & Greenwood, 1993). For a long time, the genus remained poorly understood among decapods concerning phylogeny. Recently, species have been contextualized in a global phylogeny (Vereshchaka, Lunina & Olesen, 2016a; Vereshchaka, 2017). Fourteen species of Acetes are recognized worldwide (De Grave & Fransen, 2011; WoRMS, 2022). Three species occur in the western Atlantic: Acetes americanus Ortmann, 1893; Acetes marinus (Omori, 1975); and Acetes paraguayensis Hansen, 1919.
Historically, four subspecies of A. americanus have been identified: A. a. carolinae Hansen, 1933 (type locality: Cove Beaufort, South Carolina, USA), A. a. louisianensis (Burkenroad, 1934) (type locality: Louisiana coast, from the west of the Mississippi River to Timbalier Island, Gulf of Mexico, USA), A. a. limonensis (Burkenroad, 1934) (type locality: Sweetwater River mouth, Panama), and A. a. americanus (Ortmann, 1893) (type locality: Tocantins River mouth, Brazil) (Burkenroad, 1934).
However, the subspecies A. a. louisianensis and A. a. limonensis presented intermediate characteristics of the other two subspecies. Therefore, they were traditionally considered clinal variants, which are not considered valid (Holthuis, 1948). Currently, A. a. louisianensis and A. a. limonensis are accepted as synonyms for A. a. americanus (WoRMS, 2022).
The existing taxonomy considers only A. a. carolinae and A. a. americanus as valid subspecies (Holthuis, 1948). Although these two subspecies are very similar, careful examination reveals minute morphological differences, as the body and cornea lengths of the southern representatives (A. a. americanus) are slightly larger than those of the northern representatives (A. a. carolinae) (Omori, 1975). Taxonomic inconsistencies in the subspecies of A. americanus have been reported since the 1970s (Omori, 1975). However, this remains unsolved.
Acetes americanus carolinae is distributed from North Carolina, Florida to the Gulf of Mexico, Panama, Suriname, and French Guiana (Omori, 1975); A. a. americanus Ortmann, 1893 occurs in Puerto Rico, Panama, Venezuela, Suriname, French Guiana, and Brazil (D’Incao & Martins, 2000; Mantelatto et al., 2022) (Fig. 1).
Figure 1. Distribution of studied species of Acetes in the Western Atlantic.
Geographic distribution of the Acetes americanus carolinae (*) (Omori, 1975) and Acetes americanus americanus (+) (D’Incao and Martins, 2000). The highlighted circles indicate the sites studied by molecular analysis, Acetes americanus USA (pink), Acetes americanus Brazil 1 (yellow), Acetes americanus Brazil 2 (blue), Acetes petrunkevitchi (orange) and Acetes paraguayensis (black). Map created by authors using Qgis 3.24.3 (https://www.qgis.org/en/site/index.html).
The uncertainty regarding the difference in the extant subspecies results from: (a) insufficient information about the subspecies’ habitats, especially where the two subspecies co-occur, (b) the small magnitude of morphological divergence among species of the genus Acetes and (c) the distribution.
Molecular tools were used to elucidate the taxonomic status of several marine shrimp species, whose morphology is insufficient to clarify their species identity (Carvalho, Magalhães & Mantelatto, 2014; Tavares & Gusmão, 2016; Carvalho, Magalhães & Mantelatto, 2020). Combined with morphological data, genetic data allowed the interpretation of variability patterns along the distribution of some taxa (Silva, Mesquita & Paula, 2010; Terossi & Mantelatto, 2012; Rossi & Mantelatto, 2013; Teodoro et al., 2016; Carvalho-Batista et al., 2019; França et al., 2019; Terossi & Mantelatto, 2020; França et al., 2021). The mitochondrial genes cytochrome c oxidase I (COI) and 16S rRNA have been considered powerful markers for molecular analysis in several studies of decapod shrimp at species and population levels (Gusmão, Lazoski & Solé-Cava, 2000; Maggioni et al., 2001; Lavery et al., 2004; Vergamini, Pileggi & Mantelatto, 2011; Terossi & Mantelatto, 2012; Teodoro et al., 2016; Carvalho-Batista et al., 2018; França et al., 2019).
In this study, we compared the A. a. americanus specimens collected from South America with those of A. a. carolinae sampled in North America using COI and 16S rRNA markers to test the genetic validity of both subspecies and the possible existence of other entities distributed along the western Atlantic.
Materials & Methods
Sampling of the biological material
The specimens used in this study were obtained through samplings and loans. All individuals of A. americanus from Penha/Santa Catarina, Ubatuba, Cananéia, São Vicente/São Paulo, Macaé/Rio de Janeiro, Baía Formosa/Rio Grande do Norte, and A. petrunkevitchi from Ubatuba/São Paulo were sampled using a fishing boat equipped with an otter trawl net with 2 m opening and 3 m in length. The mesh diameter (interknot distance) was five mm in the first half of the net and two mm in the final part. The obtained specimens were collected under field permit approval by Instituto Chico Mendes de Biodiversidade/ICMBio, number 23008-1 and Permanent License to FLM 11777-2) and deposited in the Crustacean Collection of the Department of Biology of the Faculty of Philosophy, Sciences, and Letters of Ribeirão Preto of the University of São Paulo, Brazil (CCDB/FFCLRP/USP) and in the Crustacean Collection of the Laboratory of Biology of Marine and Freshwater Shrimp, UNESP, Bauru, Brazil (CCLC/FC/UNESP) (Table 1 and Table S1).
Table 1. Size dimension relationship of females Acetes americanus.
Sample size and ratio between height and length of the third thoracic sternite of the females of Acetes americanus Ortmann, 1893.
| Subspecies | Number of specimens | Locality (Latitud) | Catalog number | Average ± Standard Deviation |
|---|---|---|---|---|
| Acetes americanus USA | 28 | Off Beaufort Inlet, North Carolina, United States (34 °N) | USNM74550 | 0.64 ± 0.08 |
| Acetes americanus USA | 2 | Cape Lookout, North Carolina, United States (34°N) | USNM258701 | 0.65 ± 0.07 |
| Acetes americanus USA | 6 | Beaufort, North Carolina, United States (34°N) | YPM005386 | 0.48 ± 0.08 |
| Acetes americanus USA | 5 | Off mouth of North Edisto River, South Carolina, United States (32°N) | USNM258707 | 0.60 ± 0.11 |
| Acetes americanus USA | 9 | Louisiana, Gulf of Mexico, United States (29°N) | YPM005387 | 0.57 ± 0.08 |
| Acetes americanus USA | 17 | Laguna de Términos, Frente el Faro de Xicalango, México (19°N) | CNCR2402 | 0.56 ± 0.11 |
| Acetes americanus Brazil 1 | 6 | Channel at mouth of Bay, Puerto Rico (18°N) | USNM134695 to USNM134697 | 0.24 ± 0.04 |
| Acetes americanus Brazil 1 | 17 | Playa de Guayanes, Puerto Yabucoa, Puerto Rico (18°N) | USNM186645 to USNM186647 | 0.22 ± 0.06 |
| Acetes americanus Brazil 1 | 20 | Off Surinam Coast, Suriname (6°N) | USNM103101 to USNM103106 | 0.29 ± 0.08 |
| Acetes americanus Brazil 1 | 26 | Maceió,AL, Brazil (09°S) |
MZUSP21210 | 0.38 ± 0.08 |
| Acetes americanus Brazil 1 | 35 | Macaé, RJ, Brazil (22°S) | CCLC0254 | 0.36 ± 0.07 |
| Acetes americanus Brazil 1 | 28 | Ubatuba, SP, Brazil (23°S) | CCLC0253 | 0.40 ± 0.34 |
| Acetes americanus Brazil 1 | 13 | Rio Grande, RS, Brazil (32°S) | MZUSP 9079 | 0.33 ± 0.07 |
| Acetes americanus Brazil 2 | 35 | Macaé, RJ, Brazil (22°S) | CCLC0261 | 0.79 ± 0.19 |
| Acetes americanus Brazil 2 | 34 | Cananéia, SP, Brazil (25°S) | CCLC0262 | 1.03 ± 0.29 |
Notes.
- CCLC
- Crustacean Collection of the Laboratory of Biology of Marine and Freshwater Shrimp, UNESP, Bauru, Brazil
- MZUSP
- Zoology Museum of the University of São Paulo, São Paulo, Brazil
- USNM
- National Museum of Natural History, Smithsonian Institution, United States
- CNCR
- National Crustacean Collection, UNAM, Mexico
- YPM
- Peabody Museum of Natural History Yale University, United States
All other specimens used in this study were loaned from scientific collections: Zoology Museum of the University of São Paulo, São Paulo, Brazil (MZUSP); National Museum of Natural History, Smithsonian Institution, United States (USNM); National Museum of the Federal University of Rio de Janeiro (MNRJ); Oceanographic Museum of the University of Pernambuco, Brazil (MOUFPE); Federal University of Rio Grande (FURG); Crustacean Collection from the Department of Biology of the Faculty of Philosophy, Sciences and Letters of Ribeirão Preto, University of São Paulo (CCDB); Collection of Crustaceans of the Federal University of Sergipe (UFS); Collection of Crustaceans from the Federal University of Espírito Santo (UFES); Collection of Crustaceans from the PUCRS Museum of Science and Technology (MCP); Carcinological Collection of the Institute of Scientific and Technological Research of the State of Amapá, Macapá, AP (IEPA); National Crustacean Collection, UNAM, Mexico (CNCR); Peabody Museum of Natural History Yale University, United States (YPM); University of Louisiana at Lafayette, USA (ULLZ); and Zoological Museum Kiel, Germany (ZMK) (Table 1 and Table S1).
Specimens were morphologically identified following the identification key proposed by Omori (1975), D’Incao & Martins (2000), Vereshchaka, Lunina & Olesen (2016a), and Vereshchaka, Olesen & Lunina (2016b), using the shape of the third thoracic sternite (thelycum) in females and the petasma shape in males.
The names currently used for extant taxa follow the most recent literature (Vereshchaka, Lunina & Olesen, 2016a; Vereshchaka, Olesen & Lunina, 2016b) and are currently adopted by WoRMS (2022). Genus Peisos is dealt with differently. A review of the shrimp genera Acetes, Peisos, and Sicyonella (Vereshchaka, Lunina & Olesen, 2016a) proposed to include Peisos within the genus Acetes using morphological and phylogenetic information. Later, in a global phylogeny, Vereshchaka (2017) proposed new families, including Acetidae, to accommodate all species of Acetes. WoRMS (2022) does not adopt the proposed changes, that is, Peisos is an accepted genus, and Acetidae does not exist. We can conjecture a lack of taxonomic reassignment with a proper nomenclatural act in the previous paper. Considering that we were not able to find a clear justification and to avoid taxonomic instability, we maintained the proposed taxonomic status of Peisos as part of Acetes following Vereshchaka, Lunina & Olesen (2016a).
DNA extraction and amplification
DNA extraction from the muscular abdominal tissue was performed following Mantelatto, Robles & Felder (2007); Mantelatto et al. (2009a); Mantelatto et al. (2009b) and Pileggi & Mantelatto (2010), with specific modifications (Carvalho-Batista et al., 2014). The regions of interest were amplified by the polymerase chain reactions (PCR) using the primers described in Table S2. Primers specific for this region were designed due to the difficulty in the amplification of COI (see Mantelatto et al., 2016 for details) (Table S2).
PCR reactions were performed with a total volume of 25 µL, containing 5 µL of betaine (5M) (Acros Organics), 4 µL of dNTPs (5 mM), 3 µl of MgCl2 (25 mM), 3 µl of 10X Taq buffer with KCl (Thermo Scientific), 2 µl of 1% bovine albumin (Sigma), 1 µl of each primer (10 µM), 1 µl of resuspended DNA (50 ng/ ml), and 0.5 µl of recombinant Taq DNA Polymerase (Thermo Scientific). The missing volume (25 µl) was filled with ultrapure water. PCR was performed using an Applied Biosystems© Veriti 96 well thermal cycler. PCR steps comprised an initial denaturation period of 3 min at 95 °C, followed by 40 thermal cycles [30 s of denaturation at 95 °C, 45 s for annealing at variable temperature (42–44 °C for 16S; 44–50 °C for COI), 1 min for the extension at 72 °C, and final extension for 10 min at 72 °C. The obtained results were observed by 1.5% agarose gel electrophoresis and photographed with Olympus© C-7070 digital camera in a UV M20 UV transilluminator.
The PCR products were purified using the Sureclean purification kit following the manufacturer’s protocol. The PCR-purified products were sequenced bidirectionally in automated sequencers (ABI 3100 Genetic Analyzer) at the Department of Technology of the Faculty of Sciences Agricultural and Veterinary Sciences of Jaboticabal, São Paulo State University.
The generated sequences were confirmed and edited (sequence consensus obtained from sense and antisense) in BioEdit 7.0.7.1 software (Hall, 1999) and aligned using CLUSTAL W (Thompson, Higging & Gibson, 1994).
Each genetic sample obtained for the analyses was deposited in the scientific collection of origin (Table S1).
Molecular analyses
A total of 118 sequences used in this study were generated for this project. For the 16S rRNA region, 52 sequences of 518 bp were obtained, of which 29 were A. a. Brazil 1, nine were A. a. Brazil 2, five were A. a. carolinae, five were A. paraguayensis, and four were A. petrunkevitchi. For the COI region, 66 sequences of 589 bp were generated of which 36 were A. a. Brazil 1, 15 were A. a. Brazil 2, six were A. a. carolinae, six were A. paraguayensis, and three were A. petrunkevitchi.
However, an additional 20 sequences (COI) of A. sibogae, A. japonicus, A. serrulatus, and A. indicus, two sequences (16S and COI) of A. petrunkevitchi, and two sequences (16S and COI) of B. faxoni (outgroup) were retrieved from the GenBank database and used for phylogenetic and genetic distance analyses to complement this study (Table S1). As there are COI sequences for more Acetes species than 16S sequences available on GenBank, phylogenetic analysis with the COI gene generated a larger number of clades.
The mean nucleotide composition and genetic distances were estimated using MEGA 5.0 software (Tamura et al., 2011) and the Neighbor-Joining dendrogram based on the Kimura 2-parameter substitution model (Kimura, 1980).
The appropriate models of nucleotide evolution HKY + G for 16S and TPM1uf + G for COI were selected by Bayesian information criterion (BIC) in jModeltest 2.1.4 (Darriba et al., 2012). Selected models and estimated parameters (Table S3) were implemented in the Bayesian inferences and considered for the choice of the closest models in the maximum likelihood analyses. Belzebub faxoni (Borradaile, 1915) (Superfamily Sergestoidea, Family Luciferidae) was included as an outgroup following the most recent global phylogeny (Vereshchaka, Lunina & Olesen, 2016a; Vereshchaka, 2017). Bayesian inference was used to reconstruct the phylogenetic relationships of the species analyzed, with the two genes as distinct partitions in MrBayes v. 3.2.2 (Ronquist et al., 2012). Bayesian inference was performed with 30 million generations in two independent analyses, with five parallel chains each, one “cold” and four “hot”. The parameters were saved every 1,000 simulations. The analysis was completed on attaining stationarity (mean standard deviation <0.01) after the stipulated number of generations. The first quarter of the parameters and trees were discarded as burn-in (Ronquist, Van Der Mark & Huelsenbeck, 2009). The support values of the branches were obtained using the a posteriori probability method.
This analysis was performed for the 16S and COI genes separately and a concatenated matrix of both the trees was generated and edited in the program Figtree v.1.3.1 (Rambaut, 2007).
Population analyses
Only the COI gene was used for population analyses. The use of this gene has been shown to be efficient in decapod population studies (Gusmão, Lazoski & Solé-Cava, 2005; Laurenzano, Mantelatto & Schubart, 2013; Rossi & Mantelatto, 2013; Carvalho-Batista et al., 2014; Teodoro et al., 2015) because it is considered to be more variable (Schubart & Huber, 2006).
The number of haplotypes was calculated in the DnaSP 4.10.9 software (Rozas & Rozas, 1999). Haplotype networks were constructed using the median-joining method, using the network program (Bandelt, Forster & Röhl, 1999), based on the data prepared in the DnaSP. Haplotype and nucleotide diversities, analysis of molecular variance (AMOVA), and pairwise fixation indices (Fst) (Excoffier, Smouse & Quattro, 1992) were calculated using Arlequin 3.11 (Excoffier, Laval & Schneider, 2005).
Morphometric analyses
We used only females (281 individuals) of A. americanus for morphometric analyses. Previous observations focused on the difference in size and shape of the female thelycum between the subspecies, motivating us to obtain potential information to complement our study.
Individuals were sexed based on the presence of petasma (first pleopod) in males and the thelycum (third thoracic sternite) in females (Xiao & Greenwood, 1993).
Morphometric measurements were obtained using a stereo microscope Zeiss© Stemi 2000C connected to an imaging system Zeiss© AxioVision, with an error of up to 0.01 mm. We measured the height (HT) and length (LT) of the thelycum and carapace length (CL).
Following the methodology proposed by Marramà & Kriwet (2017), data were standardized by calculating the ratio between each measure and carapace length (CL), removing the effect of size. The log transformation of data (Log (X+1)) was used to overcome the issue of the non-normal distribution of data by unstretching large-scale values. Moreover, log transformation is useful to considerably reduce variation due to ontogeny (allometric effect) since we assumed that specimens of different developmental stages were subjected to our analyses. A Euclidean distance matrix was constructed using the log-transformed data. Multivariate analysis of variance (PERMANOVA) was applied to test similarities within subspecies and localities (P < 0.005) (Anderson, 2001) using PRIMER software (version 6; Clarke & Gorley, 2006). A Permanova pairwise post-hoc test was performed to further investigate the differences between the subspecies (P < 0.005). Principal component analysis (PCA) was performed to characterize the differences between groups using the measured parameters (HT/CL and LT/CL ratios). Similarity percentage tests (SIMPER) were used to evaluate which measure contributed more to the differentiation between subspecies.
Results
Morphological identification of taxonomic entities
All specimens loaned from the United States of America presented characteristics of the subspecies A. a. carolinae (hereafter named A. a. USA) (Fig. 2C). However, Brazilian samples fell into two categories: (i) samples exhibiting characteristics of the subspecies A. a. americanus senso stricto (hereafter named “A. a. Brazil 1”; Fig. 2A) and (ii) samples that exhibited morphological divergence from the nominal subspecies (hereafter named “A. a. Brazil 2” (Fig. 2B)). The sampling localities of A. a. Brazil 1 and A. a. Brazil 2 are shown in Fig. 1.
Figure 2. Color photos of estudied species of Acetes.
Lateral view of Acetes americanus Ortmann, 1893. (A) Female of the Acetes americanus Brazil 1 (CCLC 0260); (B) female of the Acetes americanus Brazil 2 (CCLC 0255); (C) male of the Acetes americanus USA (CCLC 0268). CCLC: Crustacean Collection of the Laboratory of Biology of Marine and Freshwater Shrimp. Photo credit: Régis Augusto Pescinelli.
Genetic distance
16S rRNA gene: The intraspecific distances varied from 0% (A. a. USA, A. a. Brazil 2, and A. petrunkevitchi) to 0.20% (A. paraguayensis) (Table 2). The interspecific distances between congeneric species varied from 0.99 to 11.8%. The distance to the outgroup was 25.9 to 28.5% (Table 3). Regarding the A. americanus subspecies, the lowest interspecific distance was between A. a. Brazil 2 and A. a. USA (0.99%). The highest was A. a. Brazil 1 and A. a. USA (2.26%) (Table 3).
Table 2. Intraspecific genetic distance from 16S and COI genes.
Average intraspecific genetic distance (%) from 16S and Cytochrome Oxidase 1 (COI) gene ± standard deviation.
| Subspecies and species | 16S | COI |
|---|---|---|
|
Genetic distance
(± standard deviation) |
Genetic distance
(± standard deviation) |
|
| Acetes americanus USA | 0.00 (± 0.000) | 0.18 (± 0.001) |
| Acetes americanus Brazil 1 | 0.01 (± 0.001) | 0.02 (± 0.000) |
| Acetes americanus Brazil 2 | 0.00 (± 0.000) | 0.26 (± 0.001) |
| Acetes paraguayensis | 0.20 (± 0.0012) | 0.97 (± 0.003) |
| Acetes petrunkevitchi | 0.00 (± 0.000) | 0.26 (± 0.001) |
Table 3. Interspecific distance from 16S and COI genes between species of Acetes.
Matrix of average interspecific distance (%) from 16S (below) and Cytochrome Oxidase 1 (COI) gene (above) between species of Acetes (numbers on top) ± standard deviation (values on bottom).
| Acetes americanus Brazil 1 |
Acetes americanus Brazil 2 |
Acetes americanus USA |
Acetes paraguayensis | Acetes petrunkevitchi | Belzebub faxoni | |
|---|---|---|---|---|---|---|
| Acetes americanus Brazil 1 | 6.44 (± 0.011) |
4.86 (± 0.010) |
22.1 (± 0.023) |
19.0 (± 0.019) |
21.8 (± 0.021) |
|
|
Acetes americanus Brazil 2 |
1.97 (± 0.006) |
8.08 (± 0.013) |
22.0 (± 0.023) |
20.2 (± 0.020) |
22.9 (± 0.021) |
|
|
Acetes americanus USA |
2.26 (± 0.007) |
0.99 (± 0.004) |
23.3 (± 0.023) |
21.6 (± 0.022) |
23.7 (± 0.022) |
|
| Acetes paraguayensis | 7.93 (± 0.012) |
8.38 (± 0.013) |
8.04 (± 0.013) |
21.4 (± 0.021) |
25.5 (± 0.021) |
|
| Acetes petrunkevitchi | 11.8 (± 0.016) |
11.1 (± 0.015) |
11.4 (± 0.015) |
10.0 (± 0.015) |
25.4 (± 0.019) |
|
| Belzebub faxoni | 26.6 (± 0.026) |
25.9 (± 0.026) |
26.3 (± 0.027) |
28.5 (± 0.028) |
27.5 (± 0.026) |
COI gene: The intraspecific distances varied from 0.02% (A. a. Brazil 1) to 0.97% (A. paraguayensis) (Table 2). The interspecific distances between the congeneric species varied from 4.86 to 22.3%. The distance to the outgroup was 21.8 to 25.5% (Table 3). Regarding the A. americanus subspecies, the lowest interspecific distance was between A. a. Brazil 1 and A. a. USA (4.86%). The highest was A. a. Brazil 2 and A. a. USA (8.08%) (Table 3).
Phylogenetic analyses
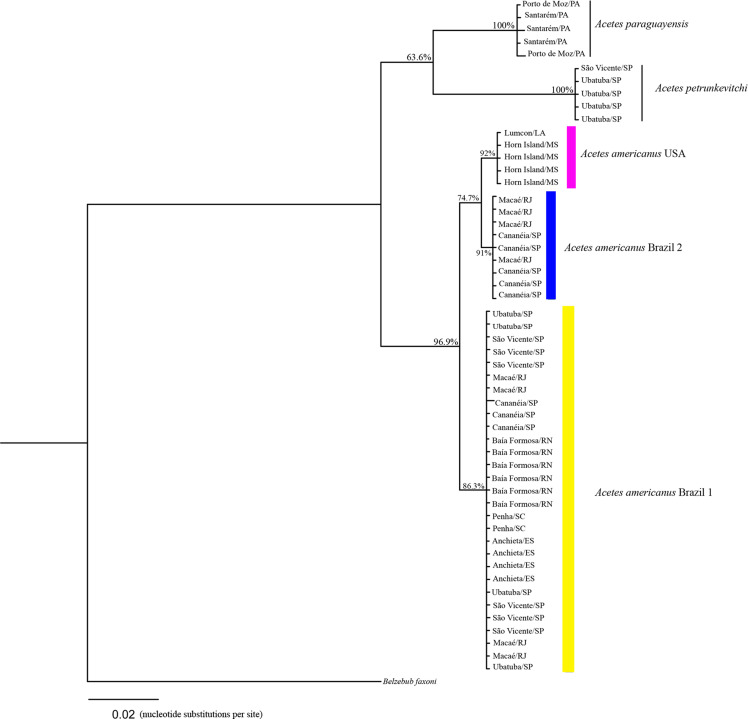
The 16S rRNA phylogenetic analyses showed the following clades (Fig. 3): Clade 1, formed by A. paraguayensis sampled in north Brazil (Santarém and Porto de Moz/Pará); Clade 2, formed by A. petrunkevitchi sampled in southeast Brazil (São Vicente and Ubatuba/São Paulo); Clade 3, formed by A. a. USA sampled in the United States (Lumcon/LA and Horn Island/MS); Clade 4, formed by A. a. Brazil 2 sampled in southeast Brazil (Cananéia/São Paulo and Macaé/Rio de Janeiro); Clade 5, formed by A. a. Brazil 1 sampled in the south (Penha/Santa Catarina), southeast (Ubatuba, São Vicente and Cananéia/São Paulo, Macaé/ Rio de Janeiro, Anchieta/Espirito Santo) and northeast Brazil (Baía Formosa/Rio Grande do Norte).
Figure 3. Phylogenetic reconstruction of Acetes based on 16mt marker.
Phylogenetic tree of Bayesian inference for the Acetes species based on the 16S region with Bayesian posterior probabilities indicated (only posterior probabilities > 50% are shown).
Based on the COI sequences, the phylogenetic analysis revealed the following clades (Fig. 4): Clade 1, formed by A. a. Brazil 1 sampled in the south (Penha/Santa Catarina), southeast (Ubatuba and Cananéia/São Paulo, Macaé/Rio de Janeiro, Anchieta/Espirito Santo) and northeast Brazil (Baía Formosa/Rio Grande do Norte, Maceió/Alagoas); Clade 2, formed by A. a. USA sampled in the United States (Lumcon/LA and Horn Island/MS); Clade 3, formed by A. a. Brazil 2 sampled in southeast Brazil (Cananéia/SP and Macaé/RJ); Clade 4, formed by A. sibogae sampled in Peninsula Malaysia; Clade 5, formed by A. japonicus sampled in Peninsula Malaysia; Clade 6, formed by A. serrulatus sampled in Peninsula Malaysia; Clade 7, formed by A. paraguayensis sampled in north Brazil (Santarém and Porto de Moz/Pará); Clade 8, formed by A. petrunkevitchi sampled in southeast Brazil (Ubatuba/SP); Clade 9, formed by A. indicus sampled in Peninsula Malaysia.
Figure 4. Phylogenetic reconstruction of Acetes based on COI marker.
Phylogenetic tree of Bayesian inference for the Acetes species based on the COI region with Bayesian posterior probabilities indicated (only posterior probabilities > 50% are shown).
The phylogenetic tree constructed based on the concatenated data (16S rRNA and COI) generated the same clades observed in the phylogenetic tree constructed with 16S rRNA and COI separately. In addition, A. a. USA and A. a. Brazil 2 were sister clades (Fig. 5), whereas COI, A. a. USA was a sister clade of A. a. Brazil 1 (Fig. 4).
Figure 5. Pylogenetic reconstruction of Acetes based on concatenated markers.
Phylogenetic tree of Bayesian inference for the Acetes species based on 16S and COI concatenated data with Bayesian posterior probabilities indicated (only posterior probabilities > 50% are shown).
The phylogenetic trees constructed (16S rRNA, COI, and concatenated data) resulted in the formation of two distinct clades of A. americanus sampled in Brazil with a high support value, in contrast to the clade formed by A. a. carolinae sampled in the United States. Furthermore, the A. americanus group appears to be a sister taxon to all other Acetes (Fig. 4).
Population analyses
The haplotype network exhibited a genetic structure in the three groups, corresponding to those observed in the phylogenetic trees (Fig. 6). Available COI sequences of A. americanus (N = 57) resulted in 12 haplotypes of which six were unique, that is, represented by a single individual. “Brazil 1″presented the lowest haplotype (h = 0.11) and nucleotide (π = 0.00022) diversities. This group included 34 individuals sharing one haplotype and two individuals with unique haplotypes. “USA” and “Brazil 2” presented similar high haplotype (h = 0.73 and h = 0.76, respectively) and nucleotide diversities (π = 0.00181 and π = 0.00277, respectively).
Figure 6. Haplotype network (COI region) showing the groups of Acetes.
Haplotype network (COI region) according to a median-joining analysis, indicating the distribution of 12 haplotypes of Acetes americanus Ortmann, 1893. The size of the circle of each haplotype is proportionate to its frequency in the sample. Each small dash represents a mutational step.
Analysis of molecular variance (AMOVA) did no detect genetic structuring of any subspecies among the location studied (p > 0.05) (Table 4).
Table 4. Molecular variance with specimens of Acetes americanus.
Analysis of molecular variance (AMOVA) performed with specimens of Acetes americanus.
| Acetes americanus Brazil 1 | Acetes americanus Brazil 2 | Acetes americanus USA | ||
|---|---|---|---|---|
| Variation source (%) | Among locations | −14.82 | 8.65 | −12.50 |
| Within locations | 114.82 | 91.35 | 112.50 | |
| FST (P) | −0.1482 (0.967) | 0.0865 (0.152) | −0.125 (1.000) | |
Notes.
Significant values, P < 0.05.
Morphometric analysis
The ratio between the height and length of the thelycum was determined for several western Atlantic locations (Table 1). The ratio values were higher in A. a. USA and A. a. Brazil 2 than in A. a. Brazil 1 (Table 1). The studied A. americanus groups (BR1, BR2, and the USA) were morphologically different (P = 0.0002) (Table 5). Permanova Pairwise tests indicated that at least one of the taxonomic entities is different from the others (P = 0.0001) (Table 6). However, when comparing A. americanus groups with each other, Pairwise tests indicated that there was a statistically significant difference only between Brazil 1 and Brazil 2 (t = 3.1563; p = 0.0018).
Table 5. Permanova analysis of Acetes americanus groups.
Results of Permanova analysis considering Acetes americanus groups (BR1, BR2 and USA).
| Source | Df | SS | MS | Pseudo-F | P(perm) | perms |
|---|---|---|---|---|---|---|
| Subspecies | 1 | 1.3898E−2 | 1.3898E−2 | 7.1277 | 0.0058 | 9927 |
| Locality | 12 | 13.679 | 1.1399 | 584.6 | 0.0001 | 9909 |
| Res | 266 | 0.51867 | 1.9499E−3 |
Notes.
- D.f.
- degrees of freedom
- SS
- sum of squares
- MS
- mean square
- Pseudo-F
- statistic
- P(perm)
- probability
- Perms
- permutations performed
Table 6. Permanova Pairwise test of Acetes americanus groups.
Results of the Permanova Pairwise test considering Acetes americanus groups (BR1, BR2 and USA).
| Source | df | SS | MS | Pseudo-F | P(perm) | Unique perms |
|---|---|---|---|---|---|---|
| Subspecies | 2 | 7.843 | 3.9215 | 76.786 | 0.0001 | 9949 |
| Res | 278 | 14.198 | 5,11E+02 | |||
| Total | 280 | 22.041 |
Notes.
- D.f.
- degrees of freedom
- SS
- sum of squares
- MS
- mean square
- Pseudo-F
- statistic
- P(perm)
- probability
- Perms
- permutations performed
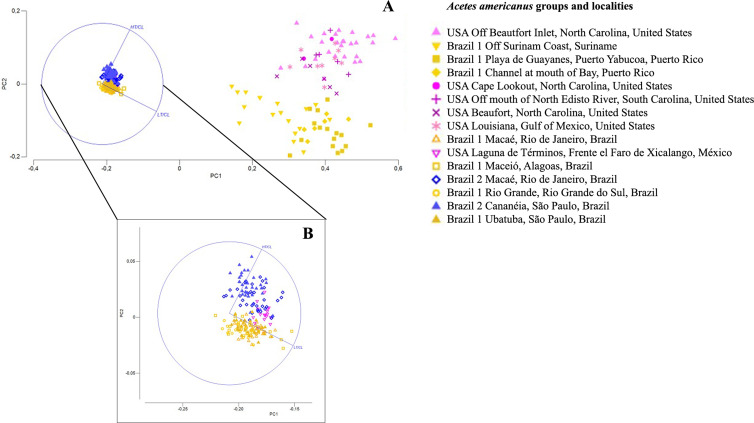
The PCA visualization plot shows that A. a. Brazil 1 is more similar to A. a. Brazil 2 than to A. a. USA (Fig. 7). A simplified Simper test showed that the LT/CL RATIO was responsible for the differences between subspecies, contributing more than 73% for Brazil 1, more than 53% for Brazil 2 and more than 61% for the USA (Table 7; Fig. 7).
Figure 7. Principal component analysis depicting the morphometric groups of Acetes.
(A) Principal Component Analysis (PCA) of the variation in thelycum morphology of the Acetes americanus groups (A. americanus USA, A. americanus Brazil 1 and A. americanus Brazil 2); (B) An enlarged area of the image “A”.
Table 7. Results of similarity percentages analysis of body dimensions.
Results of SIMPER (Similarity Percentages) analysis.
| Species | Average Abundance | Average Similarity | Sim/SD | Contrib% | Cum.% |
|---|---|---|---|---|---|
| Group Acetes americanus carolinae (USA) | |||||
| Average similarity: 67.12 | |||||
| LT/CL ratio | 0.46 | 41.37 | 2.03 | 61.64 | 61.64 |
| HT/CL ratio | 0.30 | 25.75 | 1.89 | 38.36 | 100.00 |
| Group Acetes americanus ”BRAZIL 1” | |||||
| Average similarity: 63.52 | |||||
| LT/CL ratio | 0.24 | 46.81 | 1.91 | 73.69 | 73.69 |
| HT/CL ratio | 0.08 | 16.71 | 1.89 | 26.31 | 100.00 |
| Group Acetes americanus ”BRAZIL 2” | |||||
| Average similarity: 89.83 | |||||
| LT/CL ratio | 0.07 | 47.63 | 7.97 | 53.03 | 53.03 |
| HT/CL ratio | 0.06 | 42.20 | 7.71 | 46.97 | 100.00 |
Notes.
- CL
- Carapace Length
- HT
- height of the third thoracic sternite
- LT
- length of the third thoracic sternite
- SD
- Standard Deviation
Discussion
Our analyses revealed the existence of three lineages of A. americanus: A. a. Brazil 1 sensu stricto, A. a. Brazil 2, and A. a. USA. Through molecular analysis, we were also able to identify and contextualize another species of Acetes that occurs in Brazil, A. paraguayensis. Therefore, mitochondrial DNA can be considered an efficient tool for solving taxonomic identification of the genus Acetes at species level.
One of the species concepts widely accepted in systematics (Tsoi, Wang & Chu, 2005) is defined as a group of mating individuals or having the potential for it differing from other groups because they are reproductively isolated (Mayr, 1942). However, there are several alternative concepts of species (De Queiroz, 2007). The haplotype network results suggest that there are three possible lineages for A. americanus: A. a. Brazil 1 sensu stricto, A. a. Brazil 2, and A. a. USA. Population-based analyses of mitochondrial DNA indicate that entities are reproductively isolated when gene flow is low (Tsoi, Wang & Chu, 2005). The low level of gene flow is essential evidence for speciation (Futuyma, 1998). Therefore, the non-sharing of haplotypes found in this study indicates that A. a. Brazil 1, A. a. Brazil 2, and A. a. USA are genetically distinct, with a low gene flow between them.
The A. a. Brazil 1 individuals sampled in several Brazilian regions (northeast: RN, AL, SE; southeast: ES, RJ, SP; south: SC) shared haplotypes between them, indicating the existence of gene flow among these populations. Recent studies on other decapod crustaceans have also shown genetic homogeneity among populations sampled along the western Atlantic, along the Brazilian coast (Laurenzano, Farias & Schubart, 2012; Terossi & Mantelatto, 2012; Rossi & Mantelatto, 2013; Wieman et al., 2013; Laurenzano, Mantelatto & Schubart, 2013; Carvalho-Batista et al., 2014; Teodoro et al., 2015; Nishikawa, Negri & Mantelatto, 2021). A potential reason for population homogeneity can be ascribed to the high dispersion capacity of planktonic larvae and the absence of barriers to gene flow. Although nothing is known about the larval dispersal of A. americanus, the dispersal power is high for species of the genus Acetes, as they exhibit long planktonic larval stages (∼6 weeks) before becoming juveniles and adults (Rao, 1968). This premise has also been proposed for the other decapods tested (see references above).
The levels of genetic divergence (COI) among congeneric species of the crustaceans may vary up to 17%, a high value compared to other animal groups (Costa et al., 2007). Lepidopteran insects have a genetic divergence among congeneric species of only 6.1% (Hebert, Ratnasingham & Waard, 2003). Bird species show a variation of 7.93% (Hebert et al., 2004a), and fish have a 9.93% divergence (Ward et al., 2005). For sergestid shrimp, the rate of genetic divergence (COI) was also high. The genetic divergence found between A. indicus, A. serrulatus, A. japonicus, and A. sibogae ranged from 14.6 to 20.47% (Wong, 2013).
Our results indicated genetic divergence values (COI) from 4.86 to 8.08% between the A. americanus subspecies, which are low when compared with the studies mentioned above. However, if we compare these results to those of studies of cryptic or closely related shrimp species, these values are similar. Species morphologically similar displayed genetic divergences of 2.4 to 7% and were considered different (Gusmão, Lazoski & Solé-Cava, 2000; Lavery et al., 2004; Tsoi, Wang & Chu, 2005; Lagrue et al., 2014). Carvalho-Batista et al. (2019) found higher genetic divergence values (up to 13.5%) among the genus Seabob Xiphopenaeus. However, among some closely related Xiphopenaeus species, the variation ranged from 2.7–3.3%.
The intraspecific distance (COI) (0.02–0.97%) was lower than the interspecific (4.86–22.3%) for Acetes americanus subspecies. This difference, known as the gap, is used by the DNA barcode technique to differentiate species (Hebert et al., 2004b; Ward, 2009; Carvalho-Batista et al., 2019), which reinforces that the three lineages analyzed can potentially be considered as different taxonomic entities, pending future morphological characterization. This study showed variation between A. americanus subspecies (0.99–2.26%) similar to the ones previously reported for penaeid shrimps (Costa et al., 2007; Silva, Mesquita & Paula, 2010).
Comparing only the measures of the height/length ratio of the female thelycum (Table 1), our results corroborated Omori (1975), which also found that this measurement differed between the two subspecies (A. a. americanus e A. a. carolinae). The values found by Omori (1975) for A. a. carolinae were 0.56–0.80 (mean 0.68) for North Carolina, 0.50–0.83 (0.66) for Louisiana and Texas, 0.70 and 0.53 for Panama and Suriname specimens, respectively, and values of 0.21–0.31 for specimens of A. a. americanus collected in Santos/SP. In our study, A. a. USA also presented higher values of height/length of the thelycum when compared to A. a. Brazil 1. However, we revealed the presence of A. a. Brazil 2 on the Brazilian coast showing high values of the height/length of the thelycum (Table 1). Therefore, if only this character is analyzed, the individual could be misidentified with A. a. USA.
PCA analysis showed a separation of the three subspecies groups (Brazil 1, Brazil 2 and USA) when considering thelycum measurements and the carapace length. In the transition areas where the subspecies occur, however, individuals from Suriname and Puerto Rico identified as A.a. Brazil 1 were close to A. a. USA, and individuals from Mexico identified as A. a. USA were close A. a. Brazil 1 and A. a. Brazil 2. These results point to an interesting geographic pattern, with the separation between individuals collected in the south (Terminos Mexico) and the north of the Gulf of Mexico - GOM (Louisiana). The two portions of the GOM have different water temperatures and have been separated into distinct biogeographic provinces by different authors (Boschi, 2000; Briggs & Bowen, 2012). Additionally, within the GOM, different cyclonic and anti-ciclonic flows occur separating the circulation in each locality (Schmitz Jr et al., 2005) which may be responsible for maintaining the isolation within each one of them. Therefore, this result can be associated with responses to the environmental conditions of the region, as phenotypic variations can be caused by both genetic information and environmental variations (Templeton, 2006). Considering these results, the molecular identification of the subspecies from these transition areas, using the protocols of this study, would be recommended to clarify this issue.
Acetes americanus Brazil 1 is genetically different from A. a. USA. In addition, the specimens sampled in Brazil formed two distinct clades: the first was composed of A. a. Brazil 1 and the second of A. a. Brazil 2. As stated earlier, A. a. Brazil 1 exhibits the diagnostic characteristics of A. americanus americanus, whereas A. a. Brazil 2 exhibits characteristics similar to those of A. amecicanus carolinae. Phylogenetic and population analyses pointed to the divergence of A. a. Brazil 2 from A. a. USA. Usually, a single characteristic to be fixed after reproductive isolation is sufficient for the diagnosis of a species (Mink & Sites Jr, 1996). However, no morphological characteristics that can discriminate these subspecies have been detected thus far. Several studies have shown that many species with few or no morphological characteristics are distinguished by genetic differences (Reuschel, Cuesta & Schubart, 2010; Puillandre et al., 2011; Carvalho, Magalhães & Mantelatto, 2014; Delić et al., 2017; Mandai et al., 2018). A study focusing on the morphological characteristics used to discriminate A. americanus in Brazil should clarify and provide more information about this new possible taxonomic entity. Furthermore, the hypothesis that A. a. Brazil 2 is a different entity, previously described (A. a. louisianensis or A. a. limonensis), cannot be disregarded.
General phylogeny
A previous phylogenetic study of Acetes proposed that the Acetes clade without A. petrunkevitchi (former Peisos petrunkevitchi) never gained robust support, thus considering Peisos as a junior synonym of Acetes (Vereshchaka, Lunina & Olesen, 2016a). Although we carried out an analysis with a robust but limited number of samples, our results confirmed the phylogenetic positioning recovered by both 16S rRNA and COI genes and indicated that A. petrunkevitchi is part of the Acetes group, but in all analyses forming a single clade of “Acetes paraguayensis + A. petrunkevitchi”, with a high support value for 16S rRNA and concatenated data. As only mitochondrial genes were used, further studies adding nuclear markers should be carried out to test the topology recovered herein.
However, recent studies about the morphology of the male reproductive system and spermatophore of A. petrunkevitchi differed from those of A. americanus, A. marinus and A. paraguayensis, which remains open the discussion about the inclusion of Peisos in the Acetes group (Salti, 2020).
The global morphological phylogeny proposed for the superfamily Sergestoidea showed significant changes in taxonomy, with the description of three new families, particularly because the family Sergestidae was not considered monophyletic (Vereshchaka, 2017). As a result, A. americanus was classified in the new family Acetidae, as proposed by Vereshchaka (2017). The author proposed that a single clade of A. marinus and A. paraguayensis within Acetidae received high bootstrap support. Our concatenated molecular phylogeny indicated a close relationship between A. paraguayensis and A. petrunkevitchi in a separate clade, as mentioned above. Additional samples of these species, including A. marinus, confirm this hypothesis. This molecular reconstruction sheds light on the unsolved evolutionary relations between the species of the genus Acetes, which should be investigated using more comprehensive integrated studies and the addition of nuclear markers.
Supplemental Information
Specimens of Acetes and outgroup species used in phylogenetic analyses, sampling locality, catalog number, primers and GenBank accession number.
Information on the primers used in the present study.
Models of nucleotide evolution selected in jModeltest for 16S rRNA and cytochrome c oxidase subunit I (COI) genes based on the Bayesian information criterion.
Acknowledgments
We thank the LABCAM and LBSC members for their help during the fieldwork, molecular and morphometric analysis, and their valuable suggestions and contributions. The authors are thankful to the curators of the following crustacean collections for providing valuable helping with samplings, loans and donations samples for this work: Adriane A. Braga (UFES), Cristiana S. Serejo (MNRJ), Darryl L. Felder (ULLZ), Dirk Brandis (ZMK), Gustavo L. Hirose (UFS), Glaucia Pontes (MCP), Inácia M. Vieira (IEPA), Jesser F. de Souza-Filho (MOUFPE), Jose L. Villalobos (CNCR), Luis F. Dumont (FURG), Lourdes Rojas (YPM), Marcos S. Tavares (MZUSP), Rafael Lemaitre (USNM), and Raymond Bauer (ULL). We thank the anonymous reviewers for their suggestions to improve the quality of the manuscript.
Funding Statement
Financial support for this project was provided by research grants from São Paulo Research Foundation - FAPESP (Temáticos Biota 2010/50188-8 and INTERCRUSTA 2018/13685-5; Post-doctoral fellowship 2014/01632-3 to SSM). Additional support was provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES - Código de Financiamento 001 (Ciências do Mar II Proc. 2005/2014 - 23038.004308/201414). Fernando L. Mantelatto, Rogério Caetano Costa, and Fabricio Lopes Carvalho received support from Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (Research Scholarships PQ 302253/2019-0, 306672/2018-9, and 315997/2021-4, respectively). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Sabrina Morilhas Simões conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Rogério Caetano Costa conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, grant support coordinator, and approved the final draft.
Fabricio Lopes Carvalho performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Abner Carvalho-Batista performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Sarah de Souza Alves Teodoro performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Fernando L. Mantelatto conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, grant support coordinator, and approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
All specimens used in our analysis were collected under Federal autorization from the Instituto Chico Mendes de Biodiversidade/ICMBio (#11777-2, #23008-1).
DNA Deposition
Data Availability
The following information was supplied regarding data availability:
The raw data is available in the Supplemental Files.
References
- Anderson (2001).Anderson M. A new method for non-parametric multivariate analysis of variance. Austral Ecology. 2001;26:32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x. [DOI] [Google Scholar]
- Bandelt, Forster & Röhl (1999).Bandelt HJ, Forster P, Röhl A. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- Boschi (2000).Boschi EE. Species of decapod crustaceans and their distribution in the American marine zoogeographic provinces. Revista de Investigacion y Desarrollo Pesquero. 2000;13:7–64. [Google Scholar]
- Briggs & Bowen (2012).Briggs JC, Bowen BW. A realignment of marine biogeographic provinces with particular reference to fish distributions. Journal of Biogeography. 2012;39(1):12–30. doi: 10.1111/j.1365-2699.2011.02613.x. [DOI] [Google Scholar]
- Burkenroad (1934).Burkenroad MD. Littoral Penaeidae chiefly from the Bingham Oceanographic Collection. Bulletin of the Bingham Oceanographic Collection. 1934;4:1–109. [Google Scholar]
- Carvalho, Magalhães & Mantelatto (2014).Carvalho FL, Magalhães C, Mantelatto FL. Molecular and morphological differentiation between two Miocene-divergent lineages of Amazonian shrimps, with the description of a new species (Decapoda, Palaemonidae, Palaemon) ZooKeys. 2014;457:79–108. doi: 10.3897/zookeys.457.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho, Magalhães & Mantelatto (2020).Carvalho FL, Magalhães C, Mantelatto FL. A molecular and morphological approach on the taxonomic status of the Brazilian species of Palaemon (Decapoda, Palaemonidae) Zoologica Scripta. 2020;49:101–116. doi: 10.1111/zsc.12394. [DOI] [Google Scholar]
- Carvalho-Batista et al. (2014).Carvalho-Batista A, Negri M, Pileggi LG, Castilho AL, Costa RC, Mantelatto FL. Inferring population connectivity across the range of distribution of the stiletto shrimp Artemesia longinaris Spence Bate, 1888 (Decapoda, Penaeidae) from DNA barcoding: implications for fishery management. ZooKeys. 2014;457:271–288. doi: 10.3897/zookeys.457.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Batista et al. (2018).Carvalho-Batista A, Negri M, Tamburus AF, Costa RC, Castilho AL, Zara FJ, Mantelatto FL. Genetic comparison of the red shrimp Pleoticus muelleri (Decapoda:Solenoceridae) using the barcode gene reveals the absence of cryptic speciation along its distribution. Regional Studies in Marine Science. 2018;24:392–399. doi: 10.1016/j.rsma.2018.10.003. [DOI] [Google Scholar]
- Carvalho-Batista et al. (2019).Carvalho-Batista A, Terossi M, Zara FJ, Mantelatto FL, Costa RC. A multigene and morphological analysis expands the diversity of the seabod shrimp Xiphopenaeus Smith, 1869 (Decapoda: Penaeidae), with descriptions of two new species. Scientific Reports. 2019;9:15281. doi: 10.1038/s41598-019-51484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke & Gorley (2006).Clarke KR, Gorley RN. PRIMER-e. Vol. 866. Plymouth: 2006. Primer. [Google Scholar]
- Costa et al. (2007).Costa FO, de Waard JR, Boutillier J, Ratnasingham S, Dooh RT, Hajibabaei M, Hebert DN. Biological identification through DNA barcodes: the case of the Crustacea. Canadian Journal of Fisheries and Aquatic Sciences. 2007;64:272–295. doi: 10.1139/f07-008. [DOI] [Google Scholar]
- Darriba et al. (2012).Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods. 2012;9(8):772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Grave & Fransen (2011).De Grave S, Fransen CHJM. Carideorum catalogus: the recent species of the dendrobranchiate, stenopodidean, procarididean and caridean shrimps (Crustacea: Decapoda) Zoologische Mededeelingen. 2011;85(9):195–589. [Google Scholar]
- De Queiroz (2007).De Queiroz K. Species concepts and species delimitation. Systematic Biology. 2007;56(6):879–886. doi: 10.1080/10635150701701083. [DOI] [PubMed] [Google Scholar]
- Delić et al. (2017).Delić T, Trontelj P, Rendoš M, Fišer C. The importance of naming cryptic species and the conservation of endemic subterranean amphipods. Scientific Reports. 2017;7:3391. doi: 10.1038/s41598-017-02938-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Incao & Martins (2000).D’Incao F, Martins STS. Brazilian species of the genera Acetes H. Milne Edwards, 1830 and Peisos Burkenroad, 1945 (Decapoda: Sergestidae) Journal of Crustacean Biology. 2000;20(2):78–86. doi: 10.1163/1937240X-90000010. [DOI] [Google Scholar]
- Excoffier, Laval & Schneider (2005).Excoffier L, Laval G, Schneider S. Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- Excoffier, Smouse & Quattro (1992).Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 1992;131(2):479–491. doi: 10.1093/genetics/131.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França et al. (2019).França NFC, de Moraes AB, Carvalho-Batista A, de Melo MCRB, López-Greco L, Mantelatto FL, de Morais Freire FA. Farfantepenaeus subtilis (Pérez-Farfante, 1967) and F. brasiliensis (Latreille, 1817) (Decapoda, Penaeidae): Ontogenetic comparison using the combined analysis of secondary sexual characters and molecular markers. Fisheries Research. 2019;216:89–95. doi: 10.1016/j.fishres.2019.03.024. [DOI] [Google Scholar]
- França et al. (2021).França NFC, Moraes AB, Carvalho-Batista A, Melo MCRB, Zara FJ, Mantelatto FL, Freire FAM. An integrative approach using DNA barcode and scanning electron microscopy for the effective identification of sympatric species of the genus Farfantepenaeus Burukovsky, 1997. Regional Studies in Marine Science. 2021;43:101670. doi: 10.1016/j.rsma.2021.101670. [DOI] [Google Scholar]
- Futuyma (1998).Futuyma DJ. Evolutionary biology. 3rd ed Sinauer; Sunderland: 1998. [Google Scholar]
- Gusmão, Lazoski & Solé-Cava (2000).Gusmão J, Lazoski C, Solé-Cava AM. A new species of Penaeus (Crustacea: Penaeidae) revealed by allozyme and cytochrome oxidase I analysis. Marine Biology. 2000;137:435–446. doi: 10.1007/s002270000365. [DOI] [Google Scholar]
- Gusmão, Lazoski & Solé-Cava (2005).Gusmão J, Lazoski C, Solé-Cava AM. Population genetic structure of Brazilian shrimp species (Farfantepenaeus sp., F. brasiliensis, F. paulensis and Litopenaeus schmitti: Decapoda: Penaeidae) Genetics and Molecular Biology. 2005;28(1):165–171. doi: 10.1590/S1415-47572005000100029. [DOI] [Google Scholar]
- Hall (1999).Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. http://www.mbio.ncsu.edu/JWB/papers/1999Hall1.pdf Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Hebert et al. (2004b).Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proceedings of the National Academy of Sciences of the United States of America. 2004b;101(41):14812–14817. doi: 10.1073/pnas.0406166101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert, Ratnasingham & Waard (2003).Hebert PDN, Ratnasingham S, Waard JR. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proceedings of the Royal Society B: Biological Sciences. 2003;270:S96–S99. doi: 10.1098/rsbl.2003.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert et al. (2004a).Hebert PDN, Stoeckle MY, Zemlak TS, Francis CM. Identification of birds through DNA barcodes. PLOS Biology. 2004a;2(10):e312. doi: 10.1371/journal.pbio.0020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis (1948).Holthuis LB. Notes on some Crustacea Decapoda Natantia from Surinam. Proceedings Koninklijke Nederlandsche Akademie van Wetenschappen. 1948;51:1104–1113. [Google Scholar]
- Kimura (1980).Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Lagrue et al. (2014).Lagrue C, Wattier R, Galipaud M, Gauthey Z, Rullmann J-P, Dubreuil C, Rigaud T, Bollache L. Confrontation of cryptic diversity and mate discrimination within Gammarus pulex and Gammarus fossarum species complexes. Freshwater Biology. 2014;59:2555–2570. doi: 10.1111/fwb.12453. [DOI] [Google Scholar]
- Laurenzano, Farias & Schubart (2012).Laurenzano C, Farias NE, Schubart CD. Mitochondrial genetic structure of two populations of Uca uruguayensis fails to reveal an impact of the Rio de la Plata on gene flow. Nauplius. 2012;20:15–25. doi: 10.1590/S0104-64972012000100003. [DOI] [Google Scholar]
- Laurenzano, Mantelatto & Schubart (2013).Laurenzano C, Mantelatto FL, Schubart CD. South American homogeneity versus Caribbean heterogeneity: population genetic structure of the western Atlantic fiddler crab Uca rapax (Brachyura, Ocypodidae) Journal of Experimental Marine Biology and Ecology. 2013;449:22–27. doi: 10.1016/j.jembe.2013.08.007. [DOI] [Google Scholar]
- Lavery et al. (2004).Lavery S, Chan TY, Chan YK, Tam YK, Chu HK. Phylogenetic relationships and evolutionary history of the shrimp genus Penaeus s.l. derived from mitochondrial DNA. Molecular Phylogenetics and Evolution. 2004;31:39–49. doi: 10.1016/j.ympev.2003.07.015. [DOI] [PubMed] [Google Scholar]
- Maggioni et al. (2001).Maggioni R, Rogers AD, Maclean N, D’Incao F. Molecular phylogeny of Western Atlantic Farfantepenaeus and Litopenaeus shrimp based on mitochondrial 16S partial sequences. Molecular Phylogenetics and Evolution. 2001;18(1):66–73. doi: 10.1006/mpev.2000.0866. [DOI] [PubMed] [Google Scholar]
- Mandai et al. (2018).Mandai SS, Buranelli RC, Schubart CD, Mantelatto FL. Phylogenetic and phylogeographic inferences based on two DNA markers reveal geographic structure of the orange claw hermit crab Calcinus tibicen (Anomura: Diogenidae) in the western Atlantic. Marine Biology Research. 2018;14(6):565–580. doi: 10.1080/17451000.2018.1497184. [DOI] [Google Scholar]
- Mantelatto et al. (2016).Mantelatto FL, Carvalho FL, Simões SM, Negri M, Souza-Carvalho EA, Terossi M. New primers for amplification of cytochrome c oxidase subunit I barcode region designed for species of Decapoda (Crustacea) Nauplius. 2016;24:e2016030. doi: 10.1590/2358-2936e2016030. [DOI] [Google Scholar]
- Mantelatto et al. (2009a).Mantelatto FL, Pardo LM, Pileggi LG, Felder DL. Taxonomic reexamination of the hermit crab species Pagurus forceps and Pagurus comptus (Decapoda, Paguridae) by molecular analysis. Zootaxa. 2009a;2133:20–32. doi: 10.11646/zootaxa.2133.1.2. [DOI] [Google Scholar]
- Mantelatto, Robles & Felder (2007).Mantelatto FL, Robles R, Felder DL. Molecular phylogeny of the western Atlantic species of the genus Portunus (Crustacea: Brachyura, Portunidae) Zoological Journal of the Linnean Society. 2007;150(1):211–220. doi: 10.1111/j.1096-3642.2007.00298.x. [DOI] [Google Scholar]
- Mantelatto et al. (2009b).Mantelatto FL, Robles R, Schubart CD, Felder DL. Molecular phylogeny of the genus Cronius Stimpson 1860, with reassignment of C. tumidulus and several American species of Portunus to the genus Achelous de Haan, 1833 (Brachyura: Portunidae) In: Martin JW, Crandall KA, Felder DL, editors. Crustacean issues: decapod crustacean phylogenetics. CRC Press; Boca Raton: 2009b. pp. 567–579. [Google Scholar]
- Mantelatto et al. (2022).Mantelatto FL, Tamburus AF, Carvalho-Batista A, Rossi N, Buranelli RC, Pantaleão JAF, Teles JN, Zara FJ, Carvalho FL, Bochini GL, Terossi M, Robles R, Castilho AL, Costa RC. Checklist of decapod crustaceans from the coast of the São Paulo state (Brazil) supported by integrative molecular and morphological data: V. Dendrobranchiata and Pleocyemata [Achelata, Astacidea, Axiidea, Caridea (Alpheoidea and Processoidea excluded), Gebiidea, Stenopodidea. Zootaxa. 2022;5121(1):1–074. doi: 10.11646/zootaxa.5121.1.1. [DOI] [PubMed] [Google Scholar]
- Marramà & Kriwet (2017).Marramà G, Kriwet J. Principal component and discriminant analyses as powerful tools to support taxonomic identification and their use for functional and phylogenetic signal detection of isolated fossil shark teeth. PLOS ONE. 2017;12(11):e0188806. doi: 10.1371/journal.pone.0188806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr (1942).Mayr E. Systematics and the origin of species from the viewpoint of a zoologist. Columbia University Press; New York: 1942. [Google Scholar]
- Mink & Sites Jr (1996).Mink DG, Sites Jr JW. Species limits, phylogenetic relationships, and origins of viviparity in the Scalaris complex of the lizard genus Sceloporus (Phrynosomatidae) Herpetologica. 1996;52:551–557. [Google Scholar]
- Nishikawa, Negri & Mantelatto (2021).Nishikawa KS, Negri M, Mantelatto FL. Unexpected absence of population structure and high genetic diversity of the western Atlantic hermit crab Clibanarius antillensis Stimpson, 1859 (Decapoda: Diogenidae) based on mitochondrial markers and morphological data. Diversity. 2021;13(2):56. doi: 10.3390/d13020056. [DOI] [Google Scholar]
- Omori (1975).Omori M. The systematics, biogeography, and fishery of epipelagic shrimps of the genus Acetes (Crustacea, Decapoda, Sergestidae) Bulletin of the Ocean Research Institute, University of Tokyo. 1975;7:1–91. [Google Scholar]
- Pileggi & Mantelatto (2010).Pileggi LG, Mantelatto FL. Molecular phylogeny of the freshwater prawn genus Macrobrachium (Decapoda, Palaemonidae), with emphasis on the relationships among selected American species. Invertebrate Systematics. 2010;24(1):194–208. doi: 10.1071/IS09043. [DOI] [Google Scholar]
- Puillandre et al. (2011).Puillandre N, Macpherson E, Lambourdière J, Cruaud C, Boisselierdubayle MC, Samadi S. Barcoding type specimens helps to identify synonyms and an unnamed new species in Eumunida Smith. 1883 (Decapoda: Eumunididae). Invertebrate Systematics. 2011;25(4):322–333. doi: 10.1071/IS11022. [DOI] [Google Scholar]
- Rambaut (2007).Rambaut A. FigTree. http://tree.bio.ed.ac.uk/software/figtree/ 2007
- Rao (1968).Rao PV. A new species of shrimp, Acetes cochinensis (Crustacea: Decapoda, Sergestidae) from southwest coast of India with an account of its larval development. Journal of the Marine Biological Association of India. 1968;10(2):298–320. [Google Scholar]
- Reuschel, Cuesta & Schubart (2010).Reuschel S, Cuesta JA, Schubart CD. Marine biogeographic boundaries and human introduction along the European coast revealed by phytogeography of the prawn Palaemon elegans. Molecular Phylogenetics and Evolution. 2010;55(3):765–775. doi: 10.1016/j.ympev.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Ronquist et al. (2012).Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist, Van Der Mark & Huelsenbeck (2009).Ronquist F, Van Der Mark P, Huelsenbeck JP. Bayesian phylogenetic analysis using MrBays. In: Lemey P, Sameli M, Vandamme A-M, editors. The phylogenetic handbook. Cambridge University Press; Cambridge: 2009. pp. 210–266. [Google Scholar]
- Rossi & Mantelatto (2013).Rossi N, Mantelatto FL. Molecular analysis of the freshwater prawn Macrobrachium olfersii (Decapoda, Palaemonidae) supports the existence of a single species throughout its distribution. PLOS ONE. 2013;8(1):e54698. doi: 10.1371/journal.pone.0054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozas & Rozas (1999).Rozas J, Rozas R. DnaSP version 3.0: an integrated program for molecular population genetic and molecular evolution analysis. Bioinformatics. 1999;15:174–175. doi: 10.1093/bioinformatics/15.2.174. [DOI] [PubMed] [Google Scholar]
- Salti (2020).Salti FC. Master Dissertation. 2020. Sistema reprodutor masculino de camarões Dendrobranchiata sob aspectos evolutivos: padrões morfo-histológicos e reconstrução de estado ancestral de caráter; p. 139. [Google Scholar]
- Schmitz Jr et al. (2005).Schmitz Jr WJ, Biggs DC, Lugo-Fernandez A, Oey L-Y, Sturges W. A synopsis of the circulation in the Gulf of Mexico and on its continental margins. Washington DC American Geophysical Union Geophysical Monograph Series. 2005;161:11–29. doi: 10.1029/161GM03. [DOI] [Google Scholar]
- Schubart & Huber (2006).Schubart CD, Huber MGJ. Genetic comparisons of german populations of the stone crayfish. Austropotamo biustorrentium (Crustacea: Astacidae). Bulletin Francais de la Pêche et de la Pisciculturȩ. 2006;380:1019–1028. [Google Scholar]
- Silva, Mesquita & Paula (2010).Silva IC, Mesquita N, Paula J. Genetic and morphological differentiation of the mangrove crab Perisesarma guttatum (Brachyura: Sesarmidae) along an East African latitudinal gradient. Biological Journal of the Linnean Society. 2010;99:28–46. doi: 10.1111/j.1095-8312.2009.01338.x. [DOI] [Google Scholar]
- Tamura et al. (2011).Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA 5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares & Gusmão (2016).Tavares C, Gusmão J. Description of a new Penaeidae (Decapoda: Dendrobranchiata) species, Farfantepenaeus isabelae sp. nov. Zootaxa. 2016;4171(3):505–516. doi: 10.11646/zootaxa.4171.3.6. [DOI] [PubMed] [Google Scholar]
- Templeton (2006).Templeton AR. Population genetics and microevolutionary theory. John Wiley & Sons; Hoboken: 2006. p. 705. [DOI] [Google Scholar]
- Teodoro et al. (2015).Teodoro SSA, Terossi M, Costa RC, Mantelatto FL. Genetic homogeneity in the commercial pink shrimp Farfantepenaeus paulensis revealed by COI barcoding gene. Estuarine, Coastal and Shelf Science. 2015;166:124–130. doi: 10.1016/j.ecss.2015.07.009. [DOI] [Google Scholar]
- Teodoro et al. (2016).Teodoro SSA, Terossi M, Mantelatto FL, Costa RC. Discordance in the identification of juvenile pink shrimp (Farfantepenaeus brasiliensis and F. paulensis: family Penaeidae): an integrative approach using morphology, morphometry and barcoding. Fisheries Research. 2016;183:244–253. doi: 10.1016/j.fishres.2016.06.009. [DOI] [Google Scholar]
- Terossi & Mantelatto (2012).Terossi M, Mantelatto FL. Morphological and genetic variability in Hippolyte obliquimanus Dana, 1852 (Decapoda, Caridea, Hippolytidae) from Brazil and the Caribbean Sea. Crustaceana. 2012;85:685–712. doi: 10.1163/156854012X643762. [DOI] [Google Scholar]
- Terossi & Mantelatto (2020).Terossi M, Mantelatto FL. Revalidation of Ogyrides occidentalis (Ortmann, 1893) (Decapoda: Caridea: Ogyrididae) from Brazil based on morphological and mtDNA evidences. Journal of Crustacean Biology. 2020;40:627–633. doi: 10.1093/jcbiol/ruaa054. [DOI] [Google Scholar]
- Thompson, Higging & Gibson (1994).Thompson JD, Higging DG, Gibson TJ. CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22:4673e4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi, Wang & Chu (2005).Tsoi KH, Wang ZY, Chu KH. Genetic divergence between two morphologically similar varieties of the kuruma shrimp Penaeus japonicus. Marine Biology. 2005;147:367–379. doi: 10.1007/s00227-005-1585-x. [DOI] [Google Scholar]
- Vereshchaka (2017).Vereshchaka AL. The shrimp superfamily Sergestoidea: a global phylogeny with definition of new families and an assessment of the pathways into principal biotopes. Royal Society Open Science. 2017;4(9):170221. doi: 10.1098/rsos.170221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereshchaka, Lunina & Olesen (2016a).Vereshchaka AL, Lunina AA, Olesen J. Phylogeny and classification of the shrimp genera Acetes, Peisos, and Sicyonella (Sergestidae: Crustacea: Decapoda) Zoological Journal of the Linnean Society. 2016a;177(2):353–377. doi: 10.1111/zoj.12371. [DOI] [Google Scholar]
- Vereshchaka, Olesen & Lunina (2016b).Vereshchaka AL, Olesen J, Lunina AA. A phylogeny-based revision of the family Luciferidae (Crustacea: Decapoda) Zoological Journal of the Linnean Society. 2016b;178:15–32. doi: 10.1111/zoj.12398. [DOI] [Google Scholar]
- Vergamini, Pileggi & Mantelatto (2011).Vergamini FG, Pileggi LG, Mantelatto FL. Genetic variability of the Amazon River prawn Macrobrachium amazonicum (Decapoda, Caridea, Palaemonidae) Contributions to Zoology. 2011;80(1):67–83. doi: 10.1163/18759866-08001003. [DOI] [Google Scholar]
- Ward (2009).Ward RD. DNA barcode divergence among species and genera of birds and fishes. Molecular Ecology Resources. 2009;9:1077–1085. doi: 10.1111/j.1755-0998.2009.02541.x. [DOI] [PubMed] [Google Scholar]
- Ward et al. (2005).Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PD. DNA barcoding Australia’s fish species. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005;360(1462):1847–1857. doi: 10.1098/rstb.2005.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieman et al. (2013).Wieman AC, Berendzen PB, Hampton KR, Jang J, Hopkins MJ, Jurgenson J, McNamara JC, Thurman CL. A panmictic fiddler crab from the coast of Brazil? Impact of divergent ocean currents and larval dispersal potential on genetic and morphological variation in Uca maracoani. Marine Biology. 2013;161:173–185. doi: 10.1007/s00227-013-2327-0. [DOI] [Google Scholar]
- Wong (2013).Wong BW. Ph D Thesis, Faculty to the Engineering and Science. 2013. Genetic diversity and morphometric characterization of Acetes (Decapoda: Sergestidae) species collected from the West Coast of Peninsular Malaysia; p. 204. [Google Scholar]
- WoRMS (2022).WoRMS Acetes H. Milne Edwards, 1830. 2022. https://www.marinespecies.org/aphia.php?p=taxdetailsid=158343 https://www.marinespecies.org/aphia.php?p=taxdetailsid=158343
- Xiao & Greenwood (1993).Xiao Y, Greenwood JG. The biology of Acetes (Crustacea, Sergestidae) Oceanography and Marine Biology: An Annual Review. 1993;31:259–444. [Google Scholar]
Further Reading
- Crandall & Fitzpatrick Jr (1996).Crandall KA, Fitzpatrick Jr JF. Crayfish molecular systematics: using a combination of procedures to estimate phylogeny. Systematic Biology. 1996;45:1–26. doi: 10.1093/sysbio/45.1.1. [DOI] [Google Scholar]
- Schubart, Cuesta & Felder (2002).Schubart CD, Cuesta JA, Felder DL. Glyptograpsidae, a new brachyuran family from Central America: larval and adult morphology, and a molecular phylogeny of the Grapsoidea. Journal of Crustacean Biology. 2002;22(1):28–44. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Specimens of Acetes and outgroup species used in phylogenetic analyses, sampling locality, catalog number, primers and GenBank accession number.
Information on the primers used in the present study.
Models of nucleotide evolution selected in jModeltest for 16S rRNA and cytochrome c oxidase subunit I (COI) genes based on the Bayesian information criterion.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is available in the Supplemental Files.