Abstract
Objective
To verify whether children with possible sleep bruxism (PSB) had alterations in biological rhythm and to explore the possible factors involved, such as sleep characteristics, screen time, breathing, sugary food consumption, and clenching teeth during wakefulness reported by parents/guardians.
Methodology
Data were collected through online interviews with 178 parents/guardians of students aged 6 to14 years from Piracicaba, SP, BR, when the BRIAN-K scale was answered, which is composed of four domains (1) sleep; (2) daily routine activities; (3) social behavior; (4) eating; questions about predominant rhythms (willingness, concentration, and change day to night). Three groups were formed: (1) without PSB (WPSB), (2) with PSB sometimes (PSBS), and (3) with PSB frequently (PSBF).
Results
Sociodemographic variables were similar between groups (P > 0.05); the total value of the BRIAN-K was significantly higher for the PSBF group (P < 0.05); the first domain (sleep) presented significantly higher values for the PSB groups (P < 0.05); no significant difference for other domains and predominant rhythms occurred (P > 0.05). The involved factor that differed between groups was clenching teeth, as the number of children with PSBS was significantly higher (χ2, P = 0.005). The first domain of the BRIAN-K (P = 0.003; OR = 1.20), and clenching teeth (P = 0.048; OR = 2.04) were positively associated with PSB.
Conclusion
Difficulties in maintaining sleep rhythm and clenching teeth during wakefulness reported by parents/guardians may determine a greater chance to increase the frequency of PSB.
Clinical relevance
Good sleep seems to be important to maintain a regular biological rhythm and may reduce the frequency of PSB in the 6–14 age group.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00784-023-04900-y.
Keywords: Sleep bruxism, Circadian rhythm, Children, Bruxism, Pediatric dentistry
Introduction
Bruxism consists of masticatory muscle activities that occur during sleep or wakefulness, qualifying it as sleep and awake bruxism, respectively, which in turn are different behaviors [1]. The muscle activity in sleep bruxism is characterized as rhythmic (phasic) or nonrhythmic (tonic) and in awake bruxism as repetitive or sustained tooth contact and/or by bracing or thrusting of the mandible); both are not a movement disorder in otherwise healthy individuals. Moreover, sleep bruxism is not considered a sleep disorder [1]. Currently, it is conceptualized that bruxism is regulated centrally, not peripherally, that is, anatomical factors, such as characteristics of occlusion and dental articulation, are not causal factors [1, 2]. Thus, the etiology is multifactorial and involves complex processes that are not necessarily related to specific sleep correlates or to teeth contact [1–3]. Awake bruxism can be associated with psychosocial factors and psychopathology symptoms [3]. As the central nervous system plays a role in the pathogenesis of sleep bruxism, a genetic contribution of brain neurotransmitters such as dopamine and serotonin has been suggested in the etiology of sleep bruxism [4]. In fact, the involvement of the serotonergic pathway in the pathogenesis of sleep bruxism was recently evidenced [5].
The prevalence of sleep bruxism in children and adolescents varies widely between 3.5% and 40.6%, as defined by different diagnostic methods (interviews, parent reports, clinical assessment, objective polysomnography, and electromyographic assessment) [6]. The prevalence of awake bruxism due to difficult diagnosis is estimated only in adults, ranging from 5.0 to 31.0% [7].
Due to the limitation of diagnostic methods, sleep bruxism and awake bruxism are classified as possible, probable and definite [2]. Possible bruxism is based on positive self-report, through a questionnaire and/or anamnesis. In the meantime, the probable bruxism should be based on a positive clinical examination (such as tooth wear, muscle pain or fatigue, indentations in the mucosa of the tongue or cheek) with or without positive self-report. For definite sleep bruxism, a positive polysomnographic record is required; definite awake bruxism should be based on the graphic record of the electromyogram, preferably combined with the so-called momentary ecological assessment methodology, which allows obtaining a true estimate, among others, of the frequency of dental contacts during wakefulness; these indicators may or may not be associated with self-report or positive clinical examination [1, 3].
In fact, self-reported of possible sleep bruxism is considered reliable, although theoretically difficult as the individual is asleep while performing the activity, but informants can be interrogated, and for children, their parents/guardians are considered eligible and confident proxies.
According to Kuhn and Türp [8], behavioral problems, sleep conditions, excessive use of digital media and inappropriate eating habits can be considered risk factors for bruxism in children and adolescents. In fact, children with sleep bruxism can have poor sleep quality, sleeping less than 8 h a night [9]. Light and sound stimuli, direct and indirect, can be considered predisposing factors to bruxism in children [10]. The association of inappropriate eating habits mainly added sugar, with excessive use of media may trigger the onset of sleep bruxism [11], since they can alter dopamine neurotransmission, interfering with the rewarding effects of food [12] and videogame playing [13].
The COVID-19 pandemic, which started in 2020, determined a new factor possibly influencing sleep due to mandatory social isolation at home, as a public health measure to mitigate the population's infection [14, 15]. Such a measure could change the lifestyle of individuals [16] with possible negative consequences for well-being, therefore influencing the sleep/wake cycle and circadian rhythms [17, 18]. Furthermore, in general, the psycho-emotional state worsened with the COVID-19 pandemic, intensifying, or triggering adverse stomatognathic conditions, such as orofacial pain due to increased bruxism and TMD symptoms [19, 20]. Specifically in children, a significant increase in possible sleep bruxism and sleep disorders was observed during COVID-19 pandemic compared to time before pandemic, influenced by the mothers’ lower education, greater access to electronic devices, and the occurrence of sleep disorders [21].
In this context, biological rhythm could play a role in the etiology of bruxism [9, 22]. Biological rhythm consists of the physiological and behavioral expression that contains a regular periodicity, for example, the secretion of hormones, the sleep–wake cycles, and the regularity of feeding [23, 24]. Disruptions in the biological rhythm may cause emotional and behavioral changes and negative consequences on sleep–wake rhythm [24, 25].
Considering the central regulation of sleep bruxism [1, 2], the variables responsible for the biological rhythm disturbance in children [22, 26], the social, economic, and psycho-emotional factors affected by COVID-19 pandemic [16–18, 21], it is noteworthy to explore the multifactorial aspects involved in sleep bruxism, to provide new insights for clinical and research approaches.
Based on those premises, this study aimed to verify whether children with sleep bruxism presented changes in their biological rhythm related to sleep, daily routine activities, social behavior and eating, and to explore the possible factors involved.
Methodology
This was a cross-sectional observational study carried out with a convenience sample composed of 178 parent/guardian-child dyads. It was conducted during the period of the COVID-19 pandemic, from October 2020 to November 2021.
Ethical aspects
The project was submitted to the Research Ethics Committee (CEP) of the Piracicaba Dental School, University of Campinas (FOP-UNICAMP) and approved with CAAE opinion number 3618619.6.0000.5418. The parents/guardians signed the Free and Informed Consent Term on the Google forms digital platform, as detailed below.
Study design
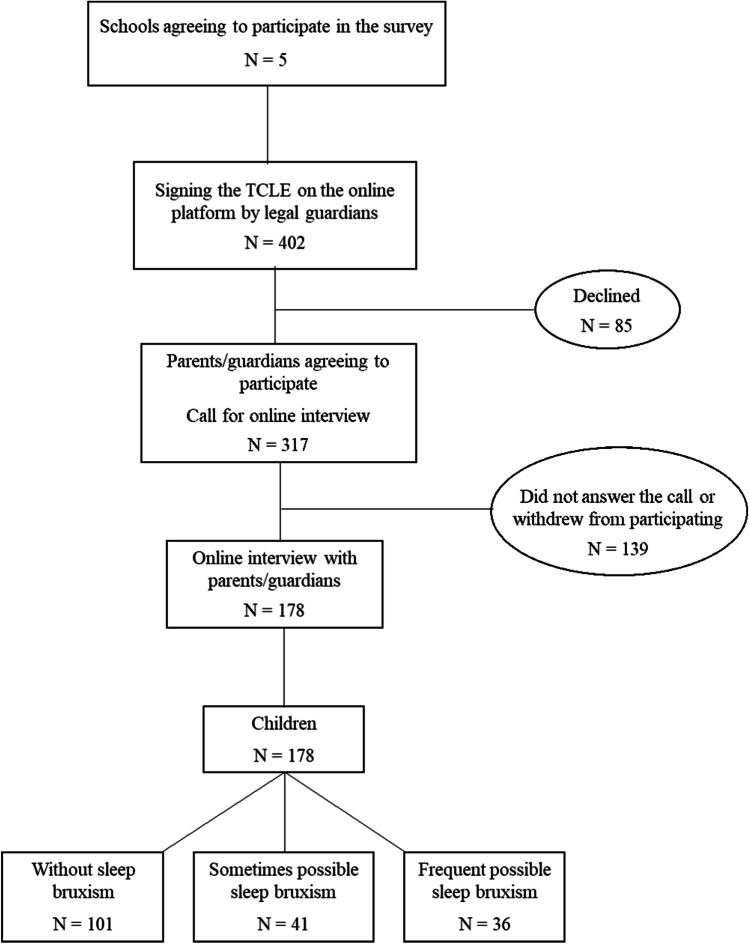
First, the São Paulo State Department of Education of Piracicaba City, SP, Brazil, was contacted to obtain authorization for access the state schools. After this step, the directors and coordinators were contacted by telephone to obtain authorization to enter the schools and send the documents related to the research. Ultimately, a total of 5 participating schools were obtained. Then, the respective directors were asked to provide the contacts of children/adolescents of both sexes, aged 6 to 14 years, and their parents/guardians; the accesses of the WhatsApp groups of the classrooms were obtained. Following, parents/guardians of children received an invitation to participate in the study and a link to access the TCLE on the Google forms digital platform, to indicate whether they agreed to participate (Fig. 1).
Fig. 1.
Flowchart of experimental design
Those who agreed to participate signed the consent form digitally and provided personal contacts, as asked in the invitation. Thus, a 20-min online interview with parents/guardians was asked and the day and time choose by them were informed to the examiner. During this interview, the inclusion and exclusion criteria were considered, with the characteristics of the children obtained only from the parents' reports, since a medical report was not required.
As inclusion criteria, children should be healthy, with normal cognitive development, as they should be enrolled at school in age-appropriate classes. Parents should be able to use computers or smartphone to participate the online interview. Exclusion criteria were children with mental retardation or psychiatric disorders, high complexity systemic problems (diabetes, hypertension, kidney problems, and/or heart problems) and parents who would not understand or could not answer the questions.
The online interview was characterized as semi-structured and divided into three moments: (1) anamnesis; (2) biological rhythm assessment, and (3) food diary reminder. The interview was recorded, with the respective agreement by the parents/guardian. The answers were verbally informed, and the video was saved on Google Drive.
-
Anamnesis
Parents/guardians verbally replied to the following information: personal data of the child and legal guardian, socioeconomic information (parents' education, marital status, professional status, family income), child’s medical history, presence or absence of menarche in girls, dental history (report of dental problems, information on oral hygiene, history of dental trauma and history of early loss of a tooth; type of dentition), child's weight and height to determine body mass index (BMI = weight/height2), and frequency of physical activities.
In addition, the following information was obtained:
Routine: child’s activities along the day, from the moment she/he woke up until bedtime during the 5 days of the week and during the weekends.
Assessment of the place where the child rests: presence or absence of lights on in the child’s room during sleep, absence or presence of noise, with whom the child slept, the distance from the parent’s room, and whether the door was open or closed during sleep.
Assessment of the use of technological means: device types to which the child had access (cell phones, tablets, computers, televisions, and video games) and the frequency of use during the total day (hours) were informed.
- Assessment of possible sleep bruxism [1]: three questions were addressed:
-
Does your child grind his/her teeth during sleep?Answer: Never, sometimes, or always.
-
How many times a week in the last month? (If answer 1 was positive)Answer: 1 time a week, 2 times a week, 3 times a week, or more than 4 times a week.
-
When waking up in the morning or at night, your son/daughter has a locked jaw and/or pain in the jaw?Answer: yes or no
-
- Assessment of clenching:
-
Has your child been clenching his teeth while awake?Answer: yes or no
-
Sleep bruxism was classified as “possible” when there was a positive report from parents/guardians [1]. The frequency of bruxism was considered as “sometimes” when the child clenched teeth 1–2 times a week and “frequent” when the child clenched three or more times a week.
-
(2)
Biological rhythm
The scale Biological Rhythms Interview for Assessment in Neuropsychiatry for Kids (BRIAN-K), translated into Portuguese by Berny et al. [26], has been used to measure biological rhythm disruption in Brazilian children and adolescents (Annex 2). This scale consists of 20 items, which were answered verbally by the parent/guardians. Items 1 to 17 correspond to children's difficulties in maintaining the biological rhythm in the last 15 days, in four domains: sleep, routine activities, social rhythm and eating pattern. Responses were categorized on the Likert scale from 0 to 3, with 0 = no difficulty; 1 = little difficulty; 2 = quite difficult; 3 = a lot of difficulty. Items 18, 19 and 20 assess whether the child displays a predominantly diurnal or nocturnal rhythm. Items 18 and 19 report, respectively, the part of the day parents/guardians feel that the child is more willing/active and more concentrated/productive to perform daily activities: 1 = morning; 2 = afternoon; 3 = at night; 4 = no specific shift. Item 20 informs whether the child switched from daytime to nighttime and the responses were categorized on a Likert scale from 1 to 4: 1 = never; 2 = rarely; 3 = almost always; 4 = always. In all domains, higher scores indicate greater difficulty in maintaining the biological rhythm.
In case of doubts from parents/guardians, the researcher helped them in a standardized way, clarifying the item.
-
(3)
Food diary reminder
Parents/guardians were asked to verbally inform the food record, which included a list of all food and drinks consumed within 24 h, that is, the day before the interview, describing the times and amounts, until the moment of the interview. For the present study, the frequency of sugary foods consumed per day was considered.
Statistical analysis
In the statistical analysis, the categorical variables were demonstrated by absolute and relative frequencies and numerical variables by means, standard deviations and medians, and intermediate quartiles. Normality was checked by using the Shapiro–Wilk test, homoscedasticity with the Levene’s test, and quantile–quantile-plot graphs (QQ-plot).
Considering the presence or absence of possible bruxism, three groups were formed: (1) group without possible sleep bruxism; (2) group sometimes possible sleep bruxism; (3) group frequent possible sleep bruxism. One way ANOVA and Tukey test (α = 0.05) were used to assess the differences between the three groups of children with and without bruxism; for data without normal distribution, the Kruskal–Wallis test was used. Moreover, the Mann–Whitney test was applied for comparisons between two variables when indicated. The scores of the 17 questions of the BRIAN-K scale were summed, as well as the items of each domain, to obtain continuous scores.
To verify the association between sociodemographic factors and those related to sleep characteristics, ordinal logistic regression models were constructed, with the dependent variable being the order of sleep bruxism, without, sometimes and frequent. First, the independent categorical variables whose P values in the bivariate analysis were less than 0.25 were selected, considering the inter- and intra-group comparisons. For continuous variables, a univariate ordinal logistic regression was constructed and those with P values less than 0.25 were also selected. After, the selected independent variables were included in two models of multivariate ordinal logistic regression. The first model included the sociodemographic characteristics as the independent variables, and the second model included those related to sleep characteristics, breathing, screen time, and clenching teeth during wakefulness reported by the parents/guardians as the independent variables. For this, the assumption of proportional odds was verified using the test of parallel lines, considering P values greater than 0.05, because the null hypothesis states that the slope coefficients in the model are the same across response categories (and lines of the same slope are parallel). Moreover, the assumption of multicollinearity was also verified, considering the variance inflation factor (VIF) less than 10 and tolerance around or less than 1, meaning that the independent variables were not correlated. Independent variables with a P value < 0.05 in the final models were found to be significantly associated with the order of possible sleep bruxism. The statistical software JAMOVI (version 2.3.9, retrieved from https://www.jamovi.org) was used, considering the significance level as alpha equal to 0.05.
Results
Table 1 shows the descriptive statistics for the sociodemographic variables. It was observed that the number of boys and girls was similar in the total sample, but the number of girls without possible sleep bruxism was significantly higher than boys (chi-square, P = 0.154). Moreover, the number of boys and girls who presented sometimes possible sleep bruxism or frequent possible sleep bruxism was similar (chi-square, P > 0.05). Height and weight did not show significant differences between groups. Only about 7% of the sample was underweight, differing significantly from the other weight status, while half of the sample was overweight or with obesity (49.44%). However, there was no significant difference in weight status between the children with or without possible sleep bruxism (chi-square, P > 0.05). Regarding the marital status and education of the mothers, there was a great variability, but most were married and had approximately 11 years of schooling (high school).
Table 1.
Sociodemographic characteristics of children and mothers and respective intra- and inter-group comparison
| Overall (N = 178) |
Without possible sleep bruxism (N = 101) |
Sometimes possible sleep bruxism (N = 41) |
Frequent possible sleep bruxism (N = 36) |
Between groups Test; P-values |
||
|---|---|---|---|---|---|---|
| Children | ||||||
| Sex [n (%)] | Girls | 99 (55.62) | 64 (63.37) | 19 (46.34) | 16 (44.44) |
χ2 P = 0.153 |
| Boys | 79 (44.38) | 37 (36.63) | 22 (53.66) | 20 (55.56) | ||
| χ2 | P = 0.134 | P = 0.007 | P = 0.639 | P=0.505 | ||
| Age (years) | Mean (SD) | 10.14 (1.97) | 10.24 (1.98) | 10.22 (2.04) | 9.78 (1.88) |
ANOVA one way P = 0.524 |
| Median (25%−75) | 10.21 (8.42−11.92) | 10.42 (8.42−11.83) | 9.83 (8.50−12.08) | 9.54 (8.33−11.43) | ||
| Range | 6.17−14.42 | 6.33−14.42 | 7.00−13.58 | 6.17−13.33 | ||
| Weight (kg) | Mean (SD) | 41.53 (14.30) | 41.44 (13.54) | 43.12 (16.13) | 39.97 (14.39) |
ANOVA one way P = 0.634 |
| Median (25−75%) | 38.95 (30.00−49.00) | 39.00 (30.00−50.00) | 39.00 (32.00−48.40) | 38.45 (30.00−46.25) | ||
| Range | 19.00−84.00 | 21.00−82.00 | 19.00−80.00 | 21.00−84.00 | ||
| Height (cm) | Mean (SD) | 1.43 (0.16) | 1.43 (0.17) | 1.43 (0.15) | 1.40 (0.16) |
ANOVA one way P = 0.656 |
| Median (25−75%) | 1.42 (1.30−154) | 1.43 (1.30−1.55) | 1.40 (1.30−1.54) | 1.40 (1.30−1.47) | ||
| Range | 1.05−1.82 | 1.05−1.82 | 1.17−1.80 | 1.15−1.75 | ||
| BMI | Mean (SD) | 20.11 (4.75) | 19.91 (4.22) | 20.71 (5.75) | 19.97 (5.00) |
Kruskal-Wallis P = 0.920 |
| Median (25−75%) | 19.45 (16.52−22.81) | 19.29 (16.85−22.23) | 19.51 (15.62−24.87) | 19.66 (17.19−21.38) | ||
| Range | 11.72−37.10 | 12.49−33.14 | 12.43−34.63 | 11.72−37.10 | ||
| Weight Status [n (%)] | Underweight | 12 (6.74) | 6 (5.94) | 2 (4.88) | 4 (11.11) |
Fisher’s exact test P = 0.780 |
| Normal weight | 78 (43.82) | 48 (47.52) | 16 (39.02) | 14 (38.89) | ||
| Overweight | 43 (24.16) | 23 (22.77) | 10 (24.39) | 10 (27.78) | ||
| Obesity | 45 (25.28) | 24 (23.76) | 13 (31.71) | 8 (22.22) | ||
| χ2 | P < 0.001 | P < 0.001 | P = 0.014 | P = 0.014 | ||
| Mothers | ||||||
| Marital Status [n (%)] | Single | 42 (23.60) | 23 (22.77) | 9 (21.95) | 10 (27.78) |
Fisher’s exact test P = 0.594 |
| Married | 87 (48.88) | 55 (54.46) | 16 (39.02) | 16 (44.44) | ||
| Divorced | 21 (11.80) | 10 (9.90) | 7 (17.07) | 4 (11.11) | ||
| Widow | 5 (2.81) | 3 (2.97) | 1 (2.44) | 1 (2.78) | ||
| Others | 23 (12.92) | 10 (9.90) | 8 (19.51) | 5 (13.89) | ||
| χ2 | P <0.0001 | P <0.001 | P=0.007 | P <0.001 | ||
| Educational Status [n (%)] | 1° Degree | 38 (21.30) | 23 (22.77) | 9 (22.00) | 6 (16.67) |
χ2 P = 0.937 |
| 2° Degree | 102 (57.30) | 57 (56.44) | 24 (58.54) | 21 (58.33) | ||
| Graduated | 38 (21.30) | 21 (20.79) | 8 (19.51) | 9 (25.00) | ||
| P <0.001 | P <0.001 | P = 0.003 | P = 0.005 | |||
| Family Income1 [n (%)] | < 1 | 21 (11.80) | 14 (13.86) | 3 (7.32) | 4 (11.11) | Fisher’s exact test P = 0.461 |
| Between 1 to 2 | 68 (38.20) | 41 (40.59) | 17 (41.46) | 10 (27.78) | ||
| Between 3 to 4 | 51 (28.65) | 27 (26.73) | 14 (34.15) | 10 (27.78) | ||
| > 4 | 38 (21.35) | 19 (18.81) | 7 (17.07) | 12 (33.33) | ||
| P <0.001 | P <0.001 | P = 0.003 | P = 0.261 | |||
SD standard deviation
1Wage per month–one wage = R$ 1100.00, approximately US$ 220.00 (during 2021, 1 US$ was approximately R$ 5.00)
χ2 chi square corresponding P values in each column mean intra-group comparisons for proportions (χ2 adherence)
The values for the BRIAN-K scale are shown in Table 2. For the overall sample, the difference occurred between children with frequent possible sleep bruxism and without possible sleep bruxism, the first ones with higher values. In the first domain, children with sometimes and frequent possible sleep bruxism showed significantly higher values than children without bruxism, and those did not differ from each other. For the other domains, no significant differences were found.
Table 2.
Descriptive data for BRIAN-K scores for the overall sample and groups and the respective comparisons
| BRIAN-K | Overall (N = 178) |
Without possible sleep bruxism (N = 101) |
Sometimes possible sleep bruxism (N = 41) |
Frequent possible sleep bruxism (N = 36) |
Between groups Test; P values |
|
|---|---|---|---|---|---|---|
|
Total scale Questions 1–17 |
Mean (SD) | 15.46 (9.32) | 13.91 (8.25) A | 16.76 (10.32) AB | 18.38 (10.34) B |
ANOVA one way P = 0.0305 Tukey test P < 0.05 |
| Median (25–75%) | 13.00 (8.00–22.75) | 11.00 (8.00–20.00) | 14.00 (8.00–25.00) | 16.50 (10.25–28.00) | ||
| Range | 1.00–43.00 | 1.00–42.00 | 1.00–35.00 | 2.00–43.00 | ||
|
First domain Sleep |
Mean (SD) | 4.44 (3.50) | 3.67 (3.21) A | 5.39 (3.72) B | 5.53 (3.61) B |
ANOVA one way P = 0.0035 Tukey test P < 0.05 |
| Median (25–75%) | 4.00 (2.00–6.00) | 3.00 (1.00–6.00) | 5.00 (3.00–8.00) | 5.50 (3.00–8.00) | ||
| Range | 0.00–14.00 | 0.00–14.00 | 0.00–12.00 | 0.00–13.00 | ||
|
Second domain Routine activities |
Mean (SD) | 4.49 (3.03) | 4.37 (2.78) | 4.15 (3.00) | 5.25 (3.63) | ANOVA one way P = 0.2269 |
| Median (25–75%) | 4.00 (2.00–7.00) | 4.00 (2.00–6.00) | 4.00 (2.00–6.00) | 4.50 (2.00–8.00) | ||
| Range | 0.00–12.00 | 0.00–11.00 | 0.00–11.00 | 0.00–12.00 | ||
|
Third domain Social rhythm |
Mean (SD) | 3.07 (2.64) | 2.74 (2.28) | 3.61 (3.11) | 3.94 (2.93) |
Kruskal–Wallis P = 0.3173 |
| Median (25–75%) | 3.00 (1.00–5.00) | 3.00 (1.00–4.00) | 3.00 (1.00–5.00) | 3.50 (1.00–5.25) | ||
| Range | 0.00–12.00 | 0.00–9.00 | 0.00–12.00 | 0.00–11.00 | ||
|
Fourth domain Eating pattern |
Mean (SD) | 3.44 (3.05) | 3.13 (2.82) | 3.61 (3.05) | 4.08 (3.59) | ANOVA one way P = 0.2480 |
| Median (25–75%) | 3.00 (1.00–5.00) | 2.00 (1.00–4.00) | 3.00 (1.00–6.00) | 3.50 (1.00–6.25) | ||
| Range | 0.00–12.00 | 0.00–12.00 | 0.00–10.00 | 0.00–12.00 |
Different superscript capital letters in columns mean significant differences between groups (respective lines in bold font)
For predominant rhythm (Table 3), it was observed that for overall children, the most willing/active period was the afternoon, like the no specific shift. Inter- and intra-groups comparisons showed no significant differences in periods in which children were more willing/active. Regarding the periods in which children were more concentrated/productive, for the overall sample and children without possible sleep bruxism, the morning and afternoon periods were the most frequent, whereas for those with possible sleep bruxism, there was no specific period. The distribution of children between the groups was similar. The intragroup comparisons showed that the proportion of children who never changed day to night was significantly higher for the overall sample and for the groups without or with frequent possible sleep bruxism, whereas for the group with sometimes bruxism, to change never or seldom were similar. The proportion of children who change day to night often or always was significantly lower than those who change never or seldom, except for children with frequent bruxism.
Table 3.
Sample distribution in accordance with the predominant rhythm of the BRIAN-K scale (questions 18, 19, and 20) and the respective comparisons for the overall sample and between groups
| BRIAN-K | Overall (N = 178) |
Without possible sleep bruxism (N = 101) |
Sometimes possible sleep bruxism (N = 41) |
Frequent possible sleep bruxism (N = 36) |
Between groups Test; P values |
|
|---|---|---|---|---|---|---|
|
Question 18 More willing/active [n (%)] |
Morning | 33 (18.54) | 16 (15.84) | 8 (19.51) | 9 (25.00) |
χ2 P = 0.812 |
| Afternoon | 51 (28.65) | 31 (30.69) | 13 (31.71) | 7 (19.44) | ||
| Night | 37 (20.79) | 22 (21.78) | 8 (19.51) | 7 (19.44) | ||
| No specific shift | 57 (32.02) | 32 (31.68) | 12 (29.27) | 13 (36.11) | ||
| χ2 (P values) | P = 0.0336 | P > 0.05 | P > 0.05 | P > 0.05 | ||
|
Question 19 More concentrated/ productive [n (%)] |
Morning | 62 (34.83) | 37 (36.63) | 12 (29.27) | 13 (36.11) |
Fisher’s exact test P = 0.685 |
| Afternoon | 65 (36.52) | 40 (39.60) | 13 (31.71) | 12 (33.33) | ||
| Night | 22 (12.36) | 11 (10.89) | 7 (17.07) | 4 (11.11) | ||
| No specific shift | 29 (16.29) | 13 (12.87) | 9 (21.95) | 7 (19.44) | ||
| χ2 (P values) | P < 0.0001 | P < 0.0001 | P > 0.05 | P > 0.05 | ||
|
Question 20 Change day to night [n (%)] |
Never | 122 (68.54) | 75 (74.26) | 23 (56.10) | 24 (66,67) |
Fisher’s exact test P = 0.130 |
| Seldom | 39 (21.91) | 18 (17.82) | 15 (36.59) | 6 (16.67) | ||
| Often | 10 (5.62) | 4 (3.96) | 2 (4.88) | 4 (11.11) | ||
| Always | 7 (3.93) | 4 (3.96) | 1 (2.44) | 2 (5.56) | ||
| χ2 (P values) | P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.001 |
χ2 chi square; corresponding P values in each column mean intra-group comparison for proportions (χ2 adherence)
The variables related to sleep, breathing, screen time, sugary food consumption, and teeth clenching are described in Table 4. It is possible to observe that no difference between groups occurred, except for clenching teeth since the group with sometimes possible sleep bruxism presented a higher proportion of children whose parents/guardian reported clenching teeth during wakefulness. On the other hand, the intragroup comparisons showed that for overall sample and children without possible sleep bruxism, the proportion of children with nasal breathing informed by parents/guardians was higher than oral breathing. The same happened for lights on in children’s bedroom and presence of noises near the children’s house. Although the respective absolute values for children with sometimes and frequent sleep bruxism were higher, they did not reach statistical significance. Intragroup comparisons showed that the number of children with or without reported clenching during wakefulness was similar only for those with sometimes sleep bruxism.
Table 4.
Descriptive data for variables related to sleep, breathing, screen time, sugary food consumption, and teeth clenching for the overall sample and groups and the respective comparisons
| Overall (N = 178) |
Without possible sleep bruxism (N = 101) |
Sometimes possible sleep bruxism (N = 41) |
Frequent possible sleep bruxism (N = 36) |
Between groups Test; P values |
||
|---|---|---|---|---|---|---|
|
Breathing [n (%)] |
Nasal | 115 (64.61) | 68 (67.33) | 25 (60.98) | 22 (61.11) |
χ2 P = 0.685 |
| Oral | 62 (34.83) | 32 (31.68) | 16 (39.02) | 14 (38.89) | ||
| χ2 | P < 0.001 | P < 0.001 | P = 0.160 | P = 0.182 | ||
| Children’s bedroom lights [n (%)] | Lights on | 108 (60.67) | 64 (63.37) | 25 (60.98) | 19 (52.78) |
χ2 P = 0.535 |
| Lights off | 70 (39.33) | 37 (36.63) | 16 (39.02) | 17 (47.22) | ||
| χ2 | P = 0.004 | P = 0.007 | P = 0.160 | P = 0.739 | ||
| Noisy places near the children’s house [n (%)] | Yes | 64 (35.96) | 34 (33.66) | 15 (36.59) | 15 (41.67) |
χ2 P = 0.668 |
| No | 114 (64.04) | 67 (66.34) | 26 (63.41) | 21 (58.33) | ||
| χ2 | P < 0.001 | P = 0.001 | P = 0.086 | P = 0.317 | ||
|
Screen time Working days (h) |
Mean (SD) | 26.33 (15.76) | 27.53 (15.79) | 24.72 (13.54) | 24.76 (18.02) |
ANOVA one way P = 0.511 |
| Median (25–75%) | 25 (15–36.83) | 26.83 (15–40) | 24 (16.83–35) | 20.50 (11.37–35) | ||
| Range | 0–60 | 0–55 | 0–80 | 0–80 | ||
|
Screen time Weekend (h) |
Mean (SD) | 7.54 (6.82) | 8.11 (7.14) | 8.04 (6.20) | 5.38 (6.29) |
ANOVA one way P = 0.101 |
| Median (25–75%) | 6.75 (0–13) | 7 (1–14) | 8 (2–12) | 2 (0–9.38) | ||
| Range | 0–24 | 0–24 | 0–19 | 0–21 | ||
| Screen time: working days × weekend | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | Mann–Whitney | |
| Hours of sleep | Mean (SD) | 9.66 (1.90) | 9.74 (1.89) | 9.42 (2.02) | 9.69 (1.83) |
ANOVA one way P = 0.658 |
| Median (25–75%) | 9.50 (8.50–11) | 9.50 (8.50–11) | 9.50 (8.00–11) | 10 (8.38–11.04) | ||
| Range | 3.50–16 | 3.5–15 | 5.5–16 | 5.5–13 | ||
|
Sugary food consumption (times a day) [n (%)] |
Never | 5 (2.8) | 2 (2.0) | 0 | 3 (8.3) |
χ2 P = 0.567 |
| 1 | 17 (9.6) | 9 (8.9) | 6 (14.6) | 2 (5.6) | ||
| 2 | 35 (19.7) | 20 (19.8) | 6 (14.6) | 9 (25.0) | ||
| 3 | 34 (19.1) | 21 (20.8) | 8 (19.5) | 5 (13.9) | ||
| 4 | 39 (21.9) | 21 (20.8) | 8 (19.5) | 10 (27.8) | ||
| 5 | 33 (18.5) | 20 (19.8) | 7 (17.1) | 6 (16.7) | ||
| 6 | 7 (3.9) | 4 (4.0) | 3 (7.3) | 0 | ||
| 7 | 6 (3.4) | 2 (2.0) | 3 (7.3) | 1 (2.8) | ||
| 8 | 1 (0.6) | 1 (1.0) | 0 | 0 | ||
| 9 | 1 (0.6) | 1 (1.0) | 0 | 0 | ||
| χ2 | P < 0.001 | P < 0.001 | P = 0.994 | P = 0.598 | ||
|
Sugary food consumption before bedtime [n (%)] |
Yes | 106 (59.6) | 61 (60.4) | 24 (58.5) | 21 (58.3) |
χ2 P = 0.966 |
| No | 72 (40.4) | 40 (39.6) | 17 (41.5) | 15 (41.7) | ||
| χ2 | P = 0.001 | P = 0.037 | P = 0.274 | P = 0.317 | ||
| Clenching teeth during wakefulness | Yes | 43 (24.2) | 16 (15.8) | 17 (41.5) * | 10 (27.8) |
χ2 * P = 0.005 |
| No | 135 (75.8) | 85 (84.2) | 24 (58.5) | 26 (72.2) | ||
| χ2 | P < 0.001 | P < 0.001 | P = 0.274 | P = 0.008 |
χ2 chi square corresponding P values in each column mean intra-group comparison for proportions (χ2 adherence)
*Means statistical significance between groups in the respective line
Interesting to note, that the screen time was similar between groups both on working days and weekends, with significantly higher values for working days.
The times a day that children consumed sugary foods were similar between the groups, but the number of children who consumed 3 to 5 times a day was significantly higher than at other times. In addition, the intra- and inter-group comparisons for hours of sleep and sugary food consumption before bedtime were not significantly different.
Tables 5 and 6 include the results of multivariate ordinal logistic regressions, considering the order for possible sleep bruxism as the dependent variable. Categorical variables with P values less than 0.25 for inter- and intra-groups comparisons (Tables 1, 3, and 4) were included into the final models. The same criterion was applied for continuous variables according to univariate models (Online Resource 1). The second domain and question 18 of the BRIAN-K scale were forced into the final model. In addition, variables that did not meet the assumptions of ordinal logistic regression were not included in the models, since they presented multicollinearity into the models.
Table 5.
Multivariate ordinal logistic regression analysis for possible sleep bruxism, as the dependent variable and sociodemographic variables, as the independent variables
| Model coefficients—possible sleep bruxism (order: without, sometimes, frequent) | |||||||
|---|---|---|---|---|---|---|---|
| 95% confidence interval | |||||||
| Predictor | Estimate | SE | Z | P | OR | Lower | Upper |
| Sex (children): | |||||||
| Masculine–feminine | 0.429 | 0.307 | 1.399 | 0.162 | 1.54 | 0.84 | 2.81 |
| Weight status (children) | |||||||
| Underweight–normal | 0.539 | 0.640 | 0.842 | 0.400 | 1.71 | 0.47 | 5.99 |
| Overweight–normal | 0.351 | 0.380 | 0.921 | 0.357 | 1.42 | 0.67 | 2.99 |
| Obesity–normal | 0.289 | 0.391 | 0.739 | 0.460 | 1.34 | 0.62 | 2.88 |
| Mother educational status | |||||||
| 1 degree–2 degree | − 0.088 | 0.389 | − 0.225 | 0.822 | 0.92 | 0.42 | 1.95 |
| Graduated–2 degree | 0.029 | 0.408 | 0.072 | 0.943 | 1.03 | 0.46 | 2.28 |
| Mother marital status: | |||||||
| Single–married | 0.608 | 0.427 | 1.422 | 0.155 | 1.84 | 0.79 | 4.27 |
| Divorced–married | 0.735 | 0.477 | 1.543 | 0.123 | 2.09 | 0.81 | 5.31 |
| Others–married | 0.459 | 0.440 | 1.042 | 0.297 | 1.58 | 0.66 | 3.75 |
| Family income (wage per month): | |||||||
| Less than 1–between 1 and 2 | 0.045 | 0.548 | 0.082 | 0.935 | 1.05 | 0.34 | 3.01 |
| Between 3 and 4–between 1 and 2 | 0.513 | 0.396 | 1.295 | 0.195 | 1.67 | 0.77 | 3.66 |
| More than 4–between 1 and 2 | 0.764 | 0.462 | 1.654 | 0.098 | 2.15 | 0.87 | 5.36 |
Nagelkerke’s (R2N) = 0.040, VIF = 1.03–1.08, Tolerance = 0.923–0.967, Proportional odds P > 0.05
Variables not included–did not meet ordinal logistic regression assumptions
Table 6.
Multivariate ordinal logistic regression analysis for possible sleep bruxism as the dependent variable, and variables related to sleep, screen time, sugary food consumption, and clenching teeth as the independent variables
| Model coefficients—possible sleep bruxism (order: without, sometimes, frequent) | |||||||
|---|---|---|---|---|---|---|---|
| 95% confidence interval | |||||||
| Predictor | Estimate | SE | Z | P | OR | Lower | Upper |
| First domain–sleep | 0.184 | 0.062 | 2.969 | 0.003 | 1.20 | 1.07 | 1.36 |
| Second domain–routine activities | 0.024 | 0.060 | 0.404 | 0.686 | 1.03 | 0.91 | 1.15 |
| Third domain–social rhythm | − 0.035 | 0.081 | − 0.429 | 0.668 | 0.97 | 0.82 | 1.13 |
| Fourth domain–eating pattern | − 0.005 | 0.067 | − 0.075 | 0.940 | 1.00 | 0.87 | 1.13 |
| Q 18: More willing/active | |||||||
| Afternoon–morning | − 0.749 | 0.491 | − 1.527 | 0.127 | 0.47 | 0.18 | 1.24 |
| Night–morning | − 0.979 | 0.571 | − 1.716 | 0.086 | 0.38 | 0.12 | 1.14 |
| No specific shift–morning | − 0.413 | 0.458 | − 0.903 | 0.366 | 0.66 | 0.27 | 1.63 |
| Q 19: More concentrated/productive | |||||||
| Afternoon–morning | − 0.375 | 0.422 | − 0.888 | 0.375 | 0.69 | 0.30 | 1.57 |
| Night–morning | − 0.759 | 0.609 | − 1.247 | 0.212 | 0.47 | 0.14 | 1.52 |
| No specific shift–morning | 0.375 | 0.470 | 0.798 | 0.425 | 1.46 | 0.58 | 3.67 |
| Q 20: Change day to night | |||||||
| Seldom–never | 0.209 | 0.412 | 0.506 | 0.613 | 1.23 | 0.55 | 2.77 |
| Often–never | 0.915 | 0.752 | 1.217 | 0.224 | 2.50 | 0.56 | 11.06 |
| Always–never | 0.222 | 0.846 | 0.263 | 0.793 | 1.25 | 0.21 | 6.37 |
| Breathing: | |||||||
| Mouth–nose | 0.122 | 0.332 | 0.366 | 0.714 | 1.13 | 0.59 | 2.16 |
| Children’s bedroom lights: | |||||||
| No–yes | 0.340 | 0.343 | 0.990 | 0.322 | 1.41 | 0.72 | 2.76 |
| Noisy places near the children’s house | |||||||
| No–yes | − 0.084 | 0.354 | − 0.237 | 0.813 | 0.92 | 0.46 | 1.85 |
| Screen time–working days (hs) | − 0.007 | 0.011 | − 0.637 | 0.524 | 0.99 | 0.97 | 1.01 |
| Screen time–weekend (hs) | − 0.036 | 0.026 | − 1.372 | 0.170 | 0.97 | 0.92 | 1.02 |
| Sugary food consumption before bedtime | |||||||
| No–yes | − 0135 | 0.342 | − 0.396 | 0.692 | 0.87 | 0.44 | 1.7 |
| Clenching teeth during wakefulness | |||||||
| Yes–no | 0.710 | 0.360 | 1.975 | 0.048 | 2.04 | 1.00 | 4.13 |
Nagelkerke’s (R2N) = 0.114, VIF 1.04–1.37, Tolerance 0.73–0.96, Proportional odds P > 0.05, SE standard error, OR odds ratio
Significant P-values are in bold fonts.
Sociodemographic variables were not associated with possible sleep bruxism, considering the P values greater than 0.05 and confidence intervals containing the value 1 in the model (Table 5).
The second model of multivariate ordinal analysis (Table 6) showed that the first domain of the BRIAN-K scale, which is related to sleep difficulties, was significantly associated with possible sleep bruxism. In this context, as the values of the first domain increase, i.e., more sleep difficulties, the chance of a child having possible sleep bruxism more frequently increases (OR = 1.20). Furthermore, the chance of children to increase the frequency of possible sleep bruxism if they clench their teeth during wakefulness was about twice as high (OR = 2.04). Unexpectedly, other variables related to sleep and sugary food consumption showed no association with possible sleep bruxism.
Discussion
The present study aimed to verify whether children with sleep bruxism present changes in their biological rhythm, regarding sleep, daily routine activities, social behavior and feeding and the possible associated factors, such as sociodemographic variables, weight status, sleep characteristics, and clenching teeth during wakefulness reported by parents/guardians. The possible association between sleep bruxism with the variables responsible for the interruption of the biological rhythm in children could help to clarify the multifactorial picture of bruxism, in addition to being a tool for possible diagnoses [22].
It was observed that the number of boys with possible sleep bruxism was the same as that of girls, corroborating previous studies [22], and disagreeing with Restrepo et al. [11]. Nevertheless, sleep bruxism prevalence in children has presented a greater variability, with a commonly described decrease with age and no gender differences, due to different methodologies concerning the age groups studied, as well as the different frequencies of self-reported sleep bruxism [6]. Geographical variation among studies exists in the epidemiology of parental-reported sleep bruxism in children, and cultural rules and standards could explain the variability in prevalence [27]. Moreover, in a recent systematic review [28], it was also considered that the prevalence does not vary between sexes, presumably due to individual factors rather than regional or collective ones.
The frequency of children with possible bruxism was almost half of the total sample, (43.26%), which can be considered high compared with other studies, as stated in the systematic review by Soares et al. [29]. This high frequency could be attributed to routine change due to the confinement determined by the COVID 19 pandemic, influencing sleep, schedules and feeding. According to Sohrab [30], the increase in cases of bruxism may be related to reduced contact with other children, increased pressure arising from the new context of online classes and the absence of team sports in this period of social confinement, which have contributed to the greater screen time and frequency during this pandemic period. In addition, uncertainty regarding factors such as the origin of SARS-COV-2, the government’s ability to prevent the spread of the disease, and the severity of the risks cause considerable family stress, with emotional and financial consequences that could influenced children behavior [31, 32]. However, we cannot yet confirm such an association, as no study with a longitudinal design has addressed aspects related to sleep in children, or the association between the period of social isolation and clinical factors related to dentistry [21].
Regarding the anthropometric assessment (weight and height), half of the sample was overweight or obese. This finding is in line with the high prevalence of overweight/obesity children in Brazil, agreeing with a projection made by the Ministry of Health in 2018 indicating that in the 2022, the number of obese children in Brazil would be around 46.5% (https://www.gov.br/saude). The COVID-19 pandemic, the period in which the present data were collected, can also aggravate the situation, and had an important impact on the diet of children and adolescents, in addition to the increase in sedentary lifestyle [33].
Considering the sleep-obesity relationship and the biopsychosocial characteristics involved [34] plus the sleep bruxism, which is an activity of the masticatory muscles that obviously occurs during sleep, the weight status could be an important factor in this context. However, no significant association was found between the children’s BMI and weight status with possible sleep bruxism, agreeing with Juliatte et al. [35] who observed no association in adults assisted by the public health system. The lack of association in the present study could be attributed to the fact that despite the high frequency of overweight and obesity in the sample, the children did not present comorbidities. In fact, sleep bruxism may be indirectly associated with obesity, as found by Holanda et al. [36] who observed in adults a significant relationship between sleep bruxism, BMI, and alcohol consumption. Moreover, obesity can increase the risk of snoring and obstructive sleep apnea in children [37, 38]. In this context, snoring has been linked to sleep bruxism [39], and an association between obstructive sleep apnea (OSA) and sleep bruxism may be possible in children, although supportive evidence is still required [40]. Thus, it is important that snoring and OSA are taken into account in studies of sleep bruxism, with accurate diagnostic methods, to increase the evidence [40]. Although the relationship between sleep bruxism and the presence of comorbidities has been emerging [41], it must be remembered that sleep bruxism is considered a sleep behavior rather than sleep disorder [1, 2].
The results showed that sociodemographic variables were not different among groups, or associated with possible sleep bruxism, probably due the homogeneity of the sample in those aspects, agreeing with Sampaio et al. [42]. The evaluation of different social strata could point out the influence of sociodemographic factors on sleep bruxism, as done by Manfredini et al. [43].
On the BRIAN-K total scale, children with sleep bruxism had higher scores on biological rhythm variables in general, meaning that they were having more difficulties in maintaining the biological rhythm than children without sleep bruxism. For identify the factors that were determining high scores for the total BRIAN-K scale, the respective domains were compared among groups, and only the first domain, sleep, showed higher significant values for children with possible sleep bruxism, as found previously [22]. Difficulty in maintaining sleep rhythm may be related, for example, to trouble waking up, feeling unrefreshed with the number of hours of sleep and having no regular bedtime, as assessed in the first domain of the BRIAN-K scale. In fact, it has been observed that sleep bruxism can determine the cited sleep problems [44] and can be associated with reduced sleep time [9]. These changes may be associated with the fact that sleep bruxism occurs in conjunction with sleep disturbances, body movements, breathing problems, increased muscle activity and heart rate disturbances, which can directly affect a child’s ability to have a good night sleep [45]. It must be considered that sleep disturbances can affect physical, behavioral, and cognitive functioning, and children are vulnerable to the effects of an inadequate amount or poor quality of sleep [46].
Although, in general, children with sleep bruxism had high scores in the other domains (activity, food and social), there were no significant differences between groups. These findings disagree with Bach et al. [22] who state that children with sleep bruxism had greater difficulty in maintaining rhythm in all domains of BRIAN-K scale. Most likely, the divergent results could be due to the pandemic period of COVID-19, since the confinement could have influenced or restricted the daily activities, socialization, and feeding, which are accessed on the BRIAN-K scale by second to fourth domains. Consequently, the predominant rhythms were similar between children with and without possible sleep bruxism. Most children were more active in the afternoon or did not have a specific shift, corroborating the findings of the first domain, related to sleep maintenance and its influence on feeling refreshed in the morning. Furthermore, most were more concentrated/productive during the day. Regarding the change day to night, most children never changed, but a higher proportion of those with sometime possible sleep bruxism who changed seldom was observed, perhaps due to poor sleep, which influenced factors must be determined [47].
The sample distribution related to sleep, such as lights on or off, sleep hours, and noises near the house did not differ between groups. These were unexpected results, first because noises, light stimulus, and sleep time less than 8 h have been considered influencing factors in children with sleep bruxism [9]; second to the fact that BRIAN-K scores for sleep domain was higher for groups with possible sleep bruxism. Nevertheless, this is still a controversial issue since environmental factors did not influence triggering sleep bruxism [48], as found in the present study. Perhaps the different designs between studies could explain the divergent results. Additionally, screen use, sugar consumption, and breathing were not different between groups. Interestingly, average screen time on weekdays was significantly higher than on weekends, which may be due to the period of the COVID-19 pandemic, when children had a break from routine and the inclusion of online classes. Additionally, screen use, sugary consumption, and breathing were not different between groups. Interestingly, the mean screen time during workdays was significantly higher that on weekends, which could be due to the online classrooms when the data were collected. High sugar consumption has been identified as an influencing factor on sleep bruxism [11], but in the present study, the general need to use technology to meet the needs during the pandemic, could justify the findings. It has also been stated that sugar consumption can be associated with sleeping and behavior disorders, which in turn can be associated with possible sleep bruxism [11] altering the circadian rhythm [49]. Dopamine is involved in the etiology of sleep bruxism and sugar and screen time can affect its neurotransmission [50]. Nevertheless, the frequency of sugar consumption and consumption before bedtime were similar, diverging from Restrepo et al. [11] who observed a positive association between possible sleep bruxism and increase-to-increase sugar-consumption. In the study by Restrepo et al. [11] the Health Behaviour in School-Aged Children Food-Frequency Questionnaire was applied, which could obtain more details about the foods consumed, whereas in the present study only the frequency was considered.
In the bivariate analysis, reported clenching teeth during wakefulness by children with sometimes possible sleep bruxism was proportionally higher than the other children. This is an interesting result, as clenching teeth during wakefulness is a sign of awake bruxism, but this behavior requires a more detailed and long-term assessment for a precise diagnosis. This is in line with the present result related to the association of clenching during wakefulness with possible sleeping bruxism, since the odds ratio showed that children who presented clenching during wakefulness are 2.04 times more likely to have sleep bruxism. This result agrees with Duarte et al. [51], who found that schoolchildren with awake bruxism presented the greater chance to have possible sleep bruxism about three times. Clenching teeth is a sign of awake bruxism, but it was not considered awake bruxism per se in the present methodology, since the respective diagnosis requires a more precise assessment, as commented above, such as reports of clenching for 1–2 weeks and the frequency of the behavior [1, 3, 52].
In a systematic review, Castroflorio et al. [53] sought to identify the risk factors associated with sleep bruxism in children, concluding that sleep disorders showed stronger association with sleep bruxism, that is children with sleep disturbances were more likely to have sleep bruxism. This is in line with the present result related to the association of the first domain of the BRIAN-K scale with possible sleeping bruxism, meaning that how much difficulty is required to maintain sleep rhythm, increases the probability of triggering possible sleep bruxism with more frequency. Previous studies report that possible sleep bruxism is more common in children with sleep deprivation and microarousals [54, 55]. This can be explained by the fact that the absence of restful sleep can predispose children to increased anxiety and stress [54], consequently increasing the likelihood of sleep bruxism. In this context, it must be remembered that the period of pandemic could be contributed to sleep disturbances.
The present study had limitations related to the lack of a definite sleep bruxism diagnosis by polysomnography. However, the assessment of sleep bruxism using reports by parents/guardians, including frequency, was in accordance with the latest International Consensus on Bruxism [1], thus ensuring the reasonableness of the data obtained. Although the present results on overweight and obesity were not linked to sleep bruxism, the lack of questions related to snoring and OSA can be considered as limitation and should be addressed in further studies. Moreover, the period in which the research was carried out (COVID-19 pandemic) resulted in some loss of data and participants, but the interviews with parents/guardians were carried out carefully on a digital platform, getting confident information. Another limitation was related to the fact that this is a cross-sectional study, consequently, it was not possible to establish causality. The period of the COVID-19 pandemic may have had influence on the variables studied. Therefore, post pandemic longitudinal studies are required to establish the cause-effect of factors related to sleep bruxism in children, considering the definitive diagnosis.
In conclusion, children with possible sleep bruxism had greater difficulty in maintaining biological rhythm, specifically in maintaining sleep, than children without sleep bruxism. The increase in the scores of the first domain of the Brian-K scale, related to sleep characteristics, plus the report of parents/guardians that their children clenched their teeth during wakefulness increased the chances of them having a possible sleep bruxism more frequently.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico, (CNPq, Brazil) for the scholarships for the first author.
The authors thank the directors and teachers from the Public Schools of Piracicaba, SP, Brazil, for collaboration and participating in the study and all parents/guardians for their adherence to the online interviews.
Authors’ contributions
C.R.V. Marceliano contributed to the study protocol, ethics application, performed the recruitment of the participants, collected and analyzed the data, contributed to the interpretation of the reported results and the production of figures, and wrote the first draft of the manuscript. This author gave final approval for submission.
M.B.D. Gavião developed the study concept, wrote the study protocol and ethics application, analyzed the data, performed statistical analysis, contributed to the interpretation of the reported results and the production of figures, and wrote the first draft of the manuscript. This author critically reviewed the manuscript, commented on the text and gave final approval for submission. All authors have read and approved the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES)—Finance Code 001 and Conselho Nacional de Desenvolvimento Científico e Tecnológico, Process 134016/2019–0 (CNPq, Brazil).
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval
Approval was obtained from the ethics committee of the Piracicaba Dental School, University of Campinas (FOP-UNICAMP) and approved under CAAE opinion number 3618619.6.0000.5418. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate
All participants signed the Free and Informed Consent Term.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lobbezoo F, Ahlberg J, Raphael KG, Wetselaar P, Glaros AG, Kato T, Santiago V, et al. International consensus on the assessment of bruxism: report of a work in progress. J Oral Rehabil. 2018;45:837–844. doi: 10.1111/joor.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lobbezoo F, Ahlberg J, Glaros AG, Kato T, Koyano K, Lavigne GJ, de Leeuw R, Manfredini D, Svensson P, Winocur E. Bruxism defined and graded: an international consensus. J Oral Rehabil. 2013;40:2–4. doi: 10.1111/joor.12011. [DOI] [PubMed] [Google Scholar]
- 3.Bracci A, Lobbezoo F, Häggman-Henrikson B, Colonna A, Nykänen L, Pollis M, Ahlberg J, Manfredini D, International network for orofacial pain and related disorders methodology INfORM Current Knowledge and Future Perspectives on Awake Bruxism Assessment Expert Consensus Recommendations. J Clin Med. 2022;11:5083. doi: 10.3390/jcm11175083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wieckiewicz M, Bogunia-Kubik K, Mazur G, Danel D, Smardz J, Wojakowska A, et al. Genetic basis of sleep bruxism and sleep apnea-response to a medical puzzle. Sci Rep. 2020;10:7497. doi: 10.1038/s41598-020-64615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smardz J, Martynowicz H, Wojakowska A, Wezgowiec J, Danel D, Mazur G, Wieckiewicz M. Lower serotonin levels in severe sleep bruxism and its association with sleep, heart rate, and body mass index. J Oral Rehabil. 2022;49:422–429. doi: 10.1111/joor.13295. [DOI] [PubMed] [Google Scholar]
- 6.Manfredini D, Restrepo C, Diaz-Serrano K, Winocur E, Lobbezoo F. Prevalence of sleep bruxism in children: a systematic review of the literature. J Oral Rehabil. 2013;40:6316–6342. doi: 10.1111/joor.12069. [DOI] [PubMed] [Google Scholar]
- 7.Wetselaar P, Vermaire EJH, Lobbezoo F, Schuller AA. The prevalence of awake bruxism and sleep bruxism in the Dutch adult population. J Oral Rehabil. 2019;46:617–623. doi: 10.1111/joor.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhn M, Türp JC. Risk factors for bruxism. Swiss Dent J. 2018;128:118–124. doi: 10.61872/sdj-2018-02-369. [DOI] [PubMed] [Google Scholar]
- 9.Serra-Negra JM, Lobbezoo F, Martins CC, Stlinni E, Manfredini D. Prevalence of sleep bruxism and awake bruxism in different chronotype profiles: hypothesis of an association. Medical Hypotheses. 2017;101:55–58. doi: 10.1016/j.mehy.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Guo H, Wang T, Niu X, Wang H, Yang W, Qiu J, Yang L. The risk factors related to bruxism in children: a systematic review and meta-analysis. Arch Oral Biol. 2018;86:18–34. doi: 10.1016/j.archoralbio.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Restrepo C, Santamaría A, Manrique R. Sleep bruxism in children: relationship with screen-time and sugar consumption. Sleep Med X. 2021;3:100035. doi: 10.1016/j.sleepx.2021.100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos-Lopez O, Panduro A, Rivera-Iñiguez I, Roman S. Dopamine D2 receptor polymorphism (C957T) is associated with sugar consumption and triglyceride levels in West Mexicans. Physiol Behav. 2018;1(194):532–537. doi: 10.1016/j.physbeh.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein A, Livny A, Weizman A. New developments in brain research of internet and gaming disorder. Neurosci Biobehav Rev. 2017;75:314–330. doi: 10.1016/j.neubiorev.2017.01.040. [DOI] [PubMed] [Google Scholar]
- 14.Blume C, Schmidt MH, Cajochen C. Effects of the COVID-19 lockdown on human sleep and rest-activity rhythms. Curr Biol. 2020;20(30):R795–R797. doi: 10.1016/j.cub.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korman M, Tkachev V, Reis C, Komada Y, Kitamura S, Gubin D, Kumar V, Roenneberg T. COVID-19-mandated social restrictions unveil the impact of social time pressure on sleep and body clock. Sci Rep. 2020;10(1):22225. doi: 10.1038/s41598-020-79299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilder-Smith A, Freedman DO. Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J Travel Med. 2020;27(2):taaa020. doi: 10.1093/jtm/taaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, Rubin GJ. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajkumar RP. COVID-19 and mental health: A review of the existing literature. Asian J Psychiatr. 2020;52:102066. doi: 10.1016/j.ajp.2020.102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emodi-Perlman A, Eli I, Smardz J, Uziel N, Wieckiewicz G, Gilon E, Grychowska N, Wieckiewicz M. Temporomandibular disorders and bruxism outbreak as a possible factor of orofacial pain worsening during the COVID-19 pandemic-concomitant research in two countries. J Clin Med. 2020;9(10):3250. doi: 10.3390/jcm9103250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winocur-Arias O, Winocur E, Shalev-Antsel T, Reiter S, Levartovsky S, Emodi-Perlman A, Friedman-Rubin P. Painful temporomandibular disorders, bruxism and oral parafunctions before and during the COVID-19 pandemic era: a sex comparison among dental patients. J Clin Med. 2022;11(3):589. doi: 10.3390/jcm11030589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lima LCM, Leal TR, Araújo LJS, Sousa MLC, Silva SED, Serra-Negra JMC, et al. Impact of the COVID-19 pandemic on sleep quality and sleep bruxism in children eight to ten years of age. Braz Oral Res. 2022;36:e046. doi: 10.1590/1807-3107bor-2022.vol36.0046. [DOI] [PubMed] [Google Scholar]
- 22.Bach SL, Moreira FP, Goettems ML, Brancher LC, Oses JP, da Silva RA, et al. Salivary cortisol levels and biological rhythm in schoolchildren with sleep bruxism. Sleep Med. 2019;54:48–52. doi: 10.1016/j.sleep.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 23.Schimitt RL, Zanetti T, Mayer M, Koplin C, Guarienti F, Hidalgo MP. Psychometric properties of Social Rhythm Metric in regular shift employees. Braz J Psychiatry. 2010;32:47–55. doi: 10.1590/s1516-44462010000100010. [DOI] [PubMed] [Google Scholar]
- 24.Mondin TC, Cardoso TA, Souza LDM, Jansen K, Da Silva Magalhães PV, Kapczinski F, et al. Mood disorders and biological rhythms in young adults: a large population-based study. J Psychiatr. 2017;84:98–104. doi: 10.1016/j.jpsychires.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 25.Barnard AR, Nolan PM. When clocks go bad: neurobehavioural consequences of disrupted circadian timing. PLoS Genet. 2008;4(5):e1000040. doi: 10.1371/journal.pgen.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berny T, Jansen K, Cardoso TA, Mondin TC, Silva RAD, Souza LDM, et al. Construction of a biological rhythm assessment scale for children. Trends Psychiatry Psychother. 2018;40:53–60. doi: 10.1590/2237-6089-2017-0081. [DOI] [PubMed] [Google Scholar]
- 27.Van Selms MKA, Marpaung C, Pogosian A, Lobbezoo F. Geographical variation of parental-reported sleep bruxism among children: comparison between the Netherlands, Armenia and Indonesia. Int Dent. 2019;J69:237–243. doi: 10.1111/idj.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrari-Piloni C, Barros LAN, Evangelista K, Serra-Negra JM, Silva MAG, Valladares-Neto J. Prevalence of bruxism in Brazilian children: a systematic review and meta-analysis. Pediatr Dent. 2022;15(44):8–20. [PubMed] [Google Scholar]
- 29.Soares JP, Giacomin A, Cardoso M, Serra-Negra JM, Bolan M. Association of gender, oral habits, and poor sleep quality with possible sleep bruxism in schoolchildren. Braz Oral Res. 2020;34:e019. doi: 10.1590/1807-3107bor-2020.vol34.0019. [DOI] [PubMed] [Google Scholar]
- 30.Sohrab C, Alsafi Z, O’Neill N, Khan M, Kerwan A, Al-Jabir A, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira VR, Jardim TV, Póvoa TIR, Viana RB, Sousa ALL, Jardim PCV. Physical inactivity during leisure and school time is associated with the presence of common mental disorders in adolescence. Rev Saude Publica. 2020;54:128. doi: 10.11606/s1518-8787.2020054001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization (2020) Coronavirus disease 2019 (COVID-19): situation report, 51. World Health Organization. https://apps.who.int/iris/handle/10665/331475
- 33.Zabetakis I, Lordan R, Norton C, Tsoupras A. COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients. 2020;12(5):146. doi: 10.3390/nu12051466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barragán R, Zuraikat FM, Tam V, Scaccia S, Cochran J, Li S, Cheng B, St-Onge M-P. Actigraphy-derived sleep is associated with eating behavior characteristics. Nutrients. 2021;13(3):852. doi: 10.3390/nu13030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juliatte TPR, Costa PD, Canaan JDR, Fonseca DC, Serra-Negra JM, Andrade EF, Castelo PM, Pereira LJ. Circadian preference and its relationship with possible sleep and awake bruxism in adults assisted by the public health system. Chronobiol Int. 2022;39:68–76. doi: 10.1080/07420528.2021.1973487. [DOI] [PubMed] [Google Scholar]
- 36.de Holanda TA, Castagno CD, Barbon FJ, Costa YM, Goettems ML, Boscato N. Sleep architecture and factors associated with sleep bruxism diagnosis scored by polysomnography recordings: A case-control study. Arch Oral Biol. 2020;112:104685. doi: 10.1016/j.archoralbio.2020.104685. [DOI] [PubMed] [Google Scholar]
- 37.Jehan S, Zizi F, Pandi-Perumal SR, Wall S, Auguste E, Myers AK, et al. Obstructive sleep apnea and obesity: implications for public health. Sleep Med Disord. 2017;1(4):00019. [PMC free article] [PubMed] [Google Scholar]
- 38.Tan YH, How CH, Chan YH, Teoh OH. Approach to the snoring child. Singapore Med J. 2020;61:170–175. doi: 10.11622/smedj.2020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michalek-Zrabkowska M, Wieckiewicz M, Macek P, Gac P, Smardz J, Wojakowska A, Poreba R, Mazur G, Martynowicz H. The relationship between simple snoring and sleep bruxism: a polysomnographic study. Int J Environ Res Public Health. 2020;17:8960. doi: 10.3390/ijerph17238960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pauletto P, Polmann H, Conti Réus J, Massignan C, de Souza BDM, Gozal D, et al. Sleep bruxism and obstructive sleep apnea: association, causality or spurious finding A scoping review. Sleep. 2022;45:zsac073. doi: 10.1093/sleep/zsac073. [DOI] [PubMed] [Google Scholar]
- 41.Segù M, Pollis M, Santagostini A, Meola F, Manfredini D. Correlation between Parental-Reported Tooth Grinding and Sleep Disorders: Investigation in a Cohort of 741 Consecutive Children. Pain Res Manag. 2020;2020:3408928. doi: 10.1155/2020/3408928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sampaio NM, Oliveira MC, Andrade AC, Santos LB, Sampaio M, Ortega A. Relationship between stress and sleep bruxism in children and their mothers: a case control study. Sleep Sci. 2018;11:239–244. doi: 10.5935/1984-0063.20180038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manfredini D, Lobbezoo F, Giancristofaro RA, Restrepo C. Association between proxy-reported sleep bruxism and quality of life aspects in Colombian children of different social layers. Clin Oral Investig. 2017;21:1351–1358. doi: 10.1007/s00784-016-1901-5. [DOI] [PubMed] [Google Scholar]
- 44.Carra MC, Rompré PH, Kato T, Parrino L, Terzano MG, Lavigne GJ, Macaluso GM. Sleep bruxism and sleep arousal: an experimental challenge to assess the role of cyclic alternating pattern. J Oral Rehabil. 2011;38:635–642. doi: 10.1111/j.1365-2842.2011.02203.x. [DOI] [PubMed] [Google Scholar]
- 45.Oliveira L, Gomes C, Bacelar Nicolau L, Ferreira L, Ferreira R. Environment in pediatric wards: light, sound, and temperature. Sleep Med. 2015;16:1041–1048. doi: 10.1016/j.sleep.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 46.Beebe DW. Cognitive, behavioral, and functional consequences of inadequate sleep-in children and adolescents. Pediatr Clin North Am. 2011;58:649–665. doi: 10.1016/j.pcl.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lages NC, Debbeler LJ, Blumenschein M, Kollmann J, Szymczak H, Keim DA, et al. Dynamic risk perceptions in times of avian and seasonal influenza epidemics: a repeated cross-sectional design. Risk Ana. 2021;41(11):2016–2030. doi: 10.1111/risa.13706. [DOI] [PubMed] [Google Scholar]
- 48.Serra-Negra JM, Ribeiro MB, Prado IM, Paiva SM, Pordeus IA. Association between possible sleep bruxism and sleep characteristics in children. Cranio. 2017;35:315–320. doi: 10.1080/08869634.2016.1239894. [DOI] [PubMed] [Google Scholar]
- 49.Becker DB, Schwartz AI. Let There Be Light. J Mass Dent Soc. 2015;64:7. [PubMed] [Google Scholar]
- 50.Scariot R, Brunet L, Olsson B, Palinkas M, Regalo SCH, Rebellato NLB, et al. Single nucleotide polymorphisms in dopamine receptor D2 are associated with bruxism and its circadian phenotypes in children. Cranio. 2022;40:152–159. doi: 10.1080/08869634.2019.1705629. [DOI] [PubMed] [Google Scholar]
- 51.Duarte J, Souza JF, Cavalcante-Leão B, Todero SRB, Ferreira FM, Fraiz FC. Association of possible sleep bruxism with daytime oral habits and sleep behavior in schoolchildren. Cranio. 2021;39:372–378. doi: 10.1080/08869634.2019.1661113. [DOI] [PubMed] [Google Scholar]
- 52.Dias R, Vaz R, Rodrigues MJ, Serra-Negra JM, Bracci A, Manfredini D. Utility of Smartphone-based real-time report (Ecological Momentary Assessment) in the assessment and monitoring of awake bruxism: a multiple-week interval study in a Portuguese population of university students. J Oral Rehabil. 2021;48:1307–1313. doi: 10.1111/joor.13259. [DOI] [PubMed] [Google Scholar]
- 53.Castroflorio T, Bargellini A, Rossini G, Cugliari G, Rainoldi A, Deregibus A. Risk factors related to sleep bruxism in children: a systematic literature review. Arch Oral Biol. 2015;60(11):1618–1624. doi: 10.1016/j.archoralbio.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 54.Bortoletto CC, Salgueiro MDCC, Valio R, Fragoso YD, Motta PB, Motta LJ, et al. The relationship between bruxism, sleep quality, and headaches in schoolchildren. J Phys Ther Sci. 2017;29:1889–1892. doi: 10.1589/jpts.29.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo L, Luo M, Wang WX, Huang GL, Xu Y, Gao X, et al. Association between problematic Internet use, sleep disturbance, and suicidal behavior in Chinese adolescents. J Behav Addict. 2018;1(7):965–975. doi: 10.1556/2006.7.2018.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.