Abstract
Vaccine hesitancy is a serious threat to global health; however, significant COVID-19 vaccine hesitancy exists throughout the United States. The 5C model, which postulates five person–level determinants for vaccine hesitancy – confidence, complacency, constraints, risk calculation, and collective responsibility – provides one theoretical way of understanding COVID-19 vaccine hesitancy. The present study examined the effects of these 5C drivers of vaccine behavior on early vaccine adoption and vaccine intentions above and beyond theoretically salient demographic characteristics and compared these associations across a National sample (n = 1634) and a statewide sample from South Carolina (n = 784) – a state with documented low levels of COVID-19 vaccination uptake. This study used quantitative and qualitative data collected in October 2020 to January 2021 from the MFour-Mobile Research Panel, a large, representative non-probability sample of adult smartphone users. Overall, the South Carolina sample reported lower COVID-19 vaccine intentions and higher levels of 5C barriers to vaccine uptake compared to the National sample. Findings further indicated that both demographic characteristics (race) and certain drivers of vaccine behavior (confidence and collective responsibility) are associated with vaccine trust and intentions across samples above and beyond other variables. Qualitative data indicated that COVID-19 vaccine hesitancy was driven by fears about the quick vaccine development, limited research, and potential side effects. Although there are some limitations to the cross-sectional survey data, the present study offers valuable insight into factors associated with early COVID-19 vaccine hesitancy across the United States.
Keywords: Vaccine hesitancy, COVID-19 vaccination, COVID-19, Vaccine acceptance
Historically, vaccines have provided relief from a variety of life-threatening diseases (LaForce and Okwo-Bele, 2011; Okwo-Bele and Cherain, 2011; Wiysonge et al., 2022). Although public vaccine uptake has been largely successful, many individuals refuse or are hesitant to receive recommended vaccinations, with the rate of vaccine hesitancy significantly increasing over the past few decades (Betsch et al., 2020; MacDonald and Dubé, 2015; Wilson and Wiysonge, 2020; Wiyeh et al., 2019). Vaccine hesitancy is a serious threat to global health due to the ongoing threat and resurgence of life-threatening diseases (Phadke et al., 2016; Benecke and DeYoung, 2019; Wong et al., 2020).
Since the development of vaccines that effectively reduce SARS-CoV-2 infections and illness severity, global efforts have focused on COVID-19 vaccine administration; however, significant COVID-19 vaccine hesitancy emerged throughout the world (Sallam, 2021; Troiano and Nardi, 2021). Globally there are significant differences in COVID-19 vaccine acceptance, with the highest vaccination rates above 90% in some countries (Chile, Singapore, China, and Cuba), and only around 50–60% in other countries (Russia, Poland, and Honduras) (Our World in Data, 2022). The United States (US) fell among the highest in COVID-19 vaccine hesitancy, with vaccination rates around 68% (Our World in Data, 2022). As of May 2022, significant differences remain within the US; certain states have achieved vaccination rates above 80% while other states continue to report rates below 60% (COVID Act Now, 2022). Understanding COVID-19 vaccine hesitancy within the US is crucial given global efforts to control the spread of the disease (Hamadani et al., 2020).
Recent reviews have identified demographic characteristics associated with COVID-19 vaccine hesitancy. Specifically, Black, female, younger, and lower income individuals were found to report higher levels vaccine hesitancy compared to White, male, older, and higher income individuals (Troiano and Nardi, 2021). However, only a small number of these studies focused solely on vaccine hesitancy in the US (Troiano and Nardi, 2021). Beyond demographic characteristics, vaccine hesitancy has been associated with psychological barriers including safety concerns, views that COVID-19 is not a threat, doubts about vaccine efficacy, and beliefs of pre-existing immunity (Troiano and Nardi, 2021). To effectively address the public health need for COVID-19 vaccine administration it is critical to understand how such beliefs may be contributing to vaccine hesitancy above and beyond demographic characteristics.
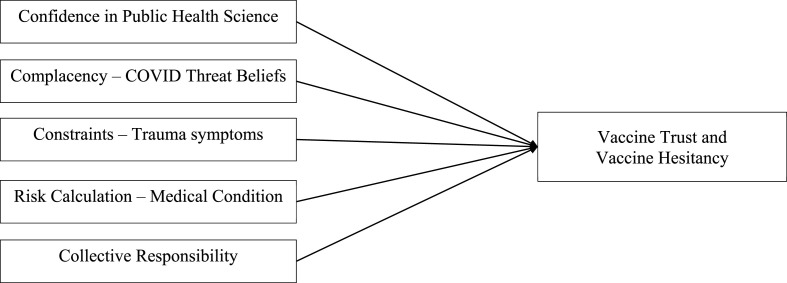
The 5C model describing the drivers of vaccine behavior provides one theoretical way of understanding COVID-19 vaccine hesitancy. This model postulates five person–level determinants of vaccine hesitancy: confidence, complacency, constraints, risk calculation, and collective responsibility (Betsch et al., 2018) (See Fig. 1 ). Confidence refers to trust in public health science and the safety and effectiveness of the vaccine (MacDonald and Dubé, 2015). Complacency refers to perceptions of the disease as a threat and whether vaccination is necessary (MacDonald and Dubé, 2015). Constraints include structural and psychological barriers related to vaccination intention and uptake, including political and sociocultural barriers and psychological distress (Wismans et al., 2021). Risk calculation refers to the comparison of personal health risks of infection versus vaccination (Betsch et al., 2018). Finally, collective responsibility involves the desire and willingness to become vaccinated to protect others or to generate population or herd immunity (Betsch et al., 2020). One recent study of the 5C model among university students from the Netherlands, Belgium, and Portugal found higher levels of confidence in public health science and collective responsibility were associated with lower levels of COVID-19 vaccine hesitancy (Wismans et al., 2021). Similarly, a recent survey including two of the 5C drivers found that confidence in public health agencies and personal risk calculation predicted COVID-19 vaccination behaviors among adults in the US (Boyle et al., 2022).
Fig. 1.
Conceptual model of the 5C drivers of vaccine behavior.
Note. The 5C model of the drivers of vaccine behavior provides five main individual person–level determinants for vaccine hesitancy: confidence, complacency, constraints, risk calculation, and collective responsibility.
The current study seeks to enhance scientific understanding of early COVID-19 vaccine hesitancy in the US. This present study contributes to existing research by 1) analyzing the effects of the 5C drivers of vaccine behavior above and beyond theoretically salient demographic characteristics, 2) contextualizes COVID-19 vaccine hesitancy with mixed methods data, and 3) compares these associations across a US National sample and a statewide sample from South Carolina – a state with documented low levels of COVID-19 vaccination uptake. Results from this study provide insight into COVID-19 vaccine hesitancy, which can inform public health efforts to improve vaccine uptake in the current and future pandemics that require vaccines to address life-threatening diseases.
1. Methods
1.1. Participants and procedures
Data were collected as part of a larger study designed to assess the behavioral, economic, social, and emotional outcomes related to the COVID-19 pandemic and to test the feasibility of completing a COVID-19 antibody home test. The broader study included a self-administered, online survey with items selected from the research literature or contemporary surveys (e.g., Behavioral Risk Factor Surveillance Survey) where appropriate. Two samples, a South Carolina (SC) statewide sample and a National sample (excluding SC residents), were recruited from the MFour-Mobile Research panel. The panel requires access to a smartphone and registration to receive survey opportunities using MFour's Surveys On The Go® app. At the time of the study, the MFour panel included approximately 2 million people representative of all 50 states and the District of Columbia. Although the MFour panel does not provide a comprehensive population frame or enable probability sampling, it is designed to provide non-probability samples of adults that are reflective of the US population. Panelists' zip code, age, gender, race/ethnicity, and education are collected so that samples can be weighted against Census estimates. The National sample included an oversample of Black adults (25% of the total sample), affording a large enough subsample to detect differences by race. This oversampling was unnecessary for the SC sample due to statewide populations of Black adults. Panelists were invited to complete the survey, although the topic was not disclosed in the initial invitation to avoid self-selection bias based on topic. Panelists provided consent for their responses to be used for research purposes. This study was approved by the Institutional Review Board at the first author's institution.
SC Statewide sample. The survey was conducted with the SC sample between October 9 and November 10, 2020. A total of 895 SC MFour panelists accessed the study, and 784 (87.5%) provided consent and completed the survey.
National sample. The survey was conducted with the National sample between December 11, 2020 and January 4, 2021. A total of 1634 National panelists, excluding panelists located in SC, accessed the study and 1450 (88.7%) provided consent and completed the survey.
1.2. Measures
Vaccine trust. Participants completed 11 items measuring their trust in vaccines (e.g., “Vaccines are important for my health”) on a 4-point scale (1 = Strongly Disagree, 2 = Somewhat disagree, 3 = Somewhat agree, 4 = Strongly agree). Items were adapted from a national assessment of parent beliefs regarding child immunization (Boyle et al., 2020). Items were summed such that higher scores indicate higher levels of vaccine trust. Coefficient alpha was .91 for both the SC and National samples.
Vaccine intentions. Participants reported on their expected vaccine behavior by answering the question, “How likely are you to try and get the coronavirus (COVID-19) vaccine as soon as an FDA approved one becomes available?” Responses were coded such that 0 = not likely to get vaccine, 1 = likely to get vaccine.
Confidence. Participants responded to a single item assessing their confidence in public health science: “How much confidence, if any, do you have in public health scientists to act in the best interests of the public?” Responses were made on a 4-point scale (0 = No confidence at all, 1 = Not too much, 2 = A fair amount, 3 = A great deal).
Complacency. Participants completed 5 items assessing their belief that the COVID-19 pandemic is a threat (e.g., “How much of a threat is the coronavirus (COVID-19) outbreak for the health of US population as a whole?”). Responses were made on a 3-point scale (0 = Not a threat, 1 = Minor threat, 2 = Major threat). Items were reverse scored and summed such that higher scores indicate lower beliefs that COVID-19 is a threat (i.e., higher complacency). Coefficient alpha was .77 for the SC sample and .73 for the National sample.
Constraints. Participants completed 8 items assessing their Posttraumatic Stress Disorder (PTSD) symptoms in the last 30 days (e.g., “Trying to avoid thoughts, feelings, or physical sensations that reminded you of an extremely stressful experience”). Responses were made on a 4-point scale (1 = Not at all, 2 = A little bit, 3 = Moderately, 4 = Extremely). Items were summed such that higher scores indicate higher levels of PTSD symptoms. Items were based on a network analysis examining the core symptoms of PTSD (Cero and Kilpatrick, 2020). Confirmatory factor analyses indicated these items demonstrated adequate factor loadings 0.70 to 0.88. Coefficient alpha was .77 for the SC sample and .93 for the National sample.
Risk calculation. Participant objective risk calculation was assessed across 3 items assessing prior diagnosis of diseases known to confer higher risk for severe COVID-19 outcomes (diabetes, high blood pressure, and auto-immune disease). Responses were combined and coded dichotomously (0 = No history of disease, 1 = History of one or more illnesses). Participant subjective risk calculation was assessed on a single item assessing their perception of health risk related to COVID-19: “Do you have an underlying health condition that would increase your risk of dying from coronavirus (COVID-19) if you were infected?” Responses were made on a dichotomous scale (0 = No, 1 = Yes).
Collective responsibility. Participants completed 11 items assessing their beliefs in the collective responsibility to prevent COVID-19 (e.g., “How important do you think it is for people like you to stay home with a cough or fever in order to stop the spread of COVID-19?“). Responses were made on a 4-point scale (1 = It should not be done, 2 = Not too important, 3 = Somewhat important, 4 = Very important). Items were summed such that higher scores indicate higher levels of collective responsibility. Coefficient alpha was .89 in both the SC and National samples.
Qualitative data. Participants who indicated they were unlikely to receive the COVID-19 vaccine (n = 1355) provided qualitative responses to a single open-ended question, “Why are you unlikely to get an FDA approved coronavirus (COVID-19) vaccine as soon as it becomes available?”
1.3. Statistical analyses
The SC and National data were weighted to match the SC and US populations respectively based on age, sex, race/ethnicity, and educational attainment based on the 2015–2019 American Community Survey. Weights greater than three times the median, were trimmed to be equal to three times the median. Descriptive statistics explored any differences between the SC and National samples across the outcome variables (vaccine trust and vaccine intentions), the 5C predictor variables (confidence, complacency, constraints, risk calculation, and collective responsibility), and the demographic variables (age, race, income, gender). We conducted linear regression analyses with vaccine trust and logistic regression analyses with vaccine intentions. The 5C predictor variables and demographic variables were entered simultaneously into each model. We conducted separate analyses for the SC and National samples. We report the semi-partial correlations squared (sr2) and the Exp(b) for the linear and logistic regression analyses, respectively, to represent the unique variance explained by each variable, over and above other variables in the analysis.
2. Results
2.1. Descriptive statistics
Demographic characteristics of both samples are presented in Table 1 . There were no statistically significant differences between the weighted samples (ps > .05). Means, standard deviations, and correlations among study variables are presented in Table 2 . The National sample reported higher confidence, less complacency, lower constraints, and higher collective responsibility compared to the SC sample, ps < .001. There were no differences across samples in vaccine trust, t(2201) = 0.75, p = .45. Participants in the SC sample were less likely to report vaccine intentions (43.6%) compared to the National sample (58.3%), X 2 (1, 2163) = 42.12, p < .001.
Table 1.
Demographic characteristics.
| Age |
National (1,450) |
South Carolina (784) |
||||
|---|---|---|---|---|---|---|
| M (SD) |
M (SD) |
|||||
| 46.54 (17.23) |
43.09 (13.03) |
|||||
| N | Unweighted % | Weighted % | N | Unweighted % | Weighted % | |
| Race | ||||||
| White | 938 | 62.6% | 70.8% | 510 | 63.4% | 68.9% |
| Black or African American | 334 | 23.0% | 14.7% | 226 | 28.8% | 25.5% |
| American Indian or Alaskan Native | 33 | 2.2% | 2.3% | 17 | 2.1% | 1.5% |
| Asian | 70 | 4.7% | 5.3% | 23 | 2.9% | 1.5% |
| Pacific Islander | 8 | 0.5% | 0.5% | 3 | 0.4% | 0.2% |
| Other | 115 | 7.7% | 6.4% | 26 | 3.2% | 2.4% |
| Gender | ||||||
| Male | 776 | 53.6% | 48.7% | 227 | 29.0% | 47.8% |
| Female | 672 | 46.4% | 51.3% | 555 | 70.8% | 52.1% |
| Annual Household Income | ||||||
| Less than $25,000 | 292 | 20.5% | 20.7% | 170 | 22.8% | 20.6% |
| $25,000 to $34,000 | 220 | 15.5% | 15.9% | 131 | 17.9% | 17.9% |
| $35,000 to $49,000 | 227 | 16.0% | 15.4% | 121 | 16.3% | 15.7% |
| $50,000 to $74,999 | 244 | 17.2% | 18.0% | 138 | 18.5% | 17.9% |
| $75,000 to $99,999 | 203 | 14.3% | 14.1% | 86 | 11.6% | 12.2% |
| $100,000 or more | 219 | 15.3% | 14.8% | 74 | 9.9% | 13.5% |
| Don't not/not sure | 17 | 1.2% | 1.1% | 24 | 3.2% | 2.1% |
Note. According to chi-square analyses there were no differences between the weighted samples (ps > .05). Differences did emerge in unweighted comparisons across sample age, t(2232) = 394.62, p < .001, race, X2 (1, 2234) = 9.09, p = .003, gender, X2 (1, 2230) = 123.79, p < .001, and income, X2 (1, 2125) = 16.51, p = .006.
Table 2.
Means, standard deviations, and correlations among study variables.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| South Carolina | Mean (SD) | 32.08 (7.58) | – | 2.02 (0.89) | 3.07 (2.45) | 16.80 (6.42) | 0.28 (0.45) | 0.26 (0.44) | 36.89 (6.32) | 43.09 (13.03) | – | 3.08 (1.65) | – |
| National | Mean (SD) | 32.34 (7.48) | – | 2.21 (0.87) | 2.46 (2.29) | 15.54 (6.49) | 0.36 (0.48) | 0.35 (0.48) | 38.00 (5.96) | 46.54 (17.23) | – | 3.36 (1.74) | – |
| 1. Vaccine trust | – | .34*** | .45*** | −.14*** | −.11** | .05 | .13** | .21*** | .09* | −.11** | .19*** | .02 | |
| 2. Vaccine intentions | .54*** | – | .36*** | −.22*** | .10* | .00 | .07 | .26*** | .06 | −.05 | .00 | −.11** | |
| 3. Confidence | .51*** | .44*** | – | −.24*** | −.01 | .06 | .08* | .32*** | .07* | .03 | .10* | .03 | |
| 4. Complacency | −.20*** | −.21*** | −.28*** | – | −.23*** | −.15*** | −.23*** | −.55*** | −.10** | −.20** | .03 | −.06 | |
| 5. Constraints | −.12*** | −.03 | −.03 | −.18*** | – | .08* | .15*** | .15*** | −.15*** | .05 | −.22*** | .12** | |
| 6. Risk calculation – Objective | .01 | .03 | .03 | −.09** | .10*** | – | .41*** | .07* | .33*** | .03 | .02 | −.03 | |
| 7. Risk calculation – Subjective | .08** | .08** | .03 | −.18*** | .17*** | .41*** | – | .17*** | .24*** | −.04 | −.03 | .04 | |
| 8. Collective responsibility | .27*** | .25*** | .37*** | −.59*** | .10*** | .05* | .10*** | – | .08* | .29*** | −.07 | .15*** | |
| 9. Age | .17*** | .11*** | .08** | −.05 | −.23*** | .30*** | .18*** | .05 | – | −.15*** | .19*** | .02 | |
| 10 Race (Black = 1, Non-Black = 0) | −.20*** | −.11*** | −.01 | −.14*** | .06* | −.01 | −.04 | .13*** | −.16 | – | −.16*** | .02 | |
| 11. Income | .17*** | .07** | .06 | .08** | −.15*** | .03 | −.08** | −.08** | .11*** | −.15*** | – | −.12** | |
| 12. Gender (Male = 1, Female = 2) | −.04 | −.14*** | −.08** | −.08** | .11*** | −.05 | .06* | .13*** | .03 | −.03 | −.13*** | – | |
Note. Vaccine intentions: 0 = not likely to get vaccine, 1 = likely to get vaccine; South Carolina sample appears above the diagonal; National sample appears below the diagonal. Table reports unweighted correlation coefficients. *p < .05, **p < .01, ***p < .001.
2.2. Relations among 5C predictors and vaccine trust
Results of the regression analyses are presented in Table 3 . Across both samples, among the 5C predictors, higher confidence, lower constraints, higher subjective risk calculation and higher collective responsibility were associated with higher levels of vaccine trust. In the National sample, lower levels of objective risk calculation were also associated with higher levels of vaccine trust. Across both samples, Black participants and those with lower income reported lower levels of vaccine trust.
Table 3.
Linear regression analyses predicting vaccine trust.
| Variable |
Vaccine Trust |
|||||
|---|---|---|---|---|---|---|
|
South Carolina |
National |
|||||
| Βeta | B (SE) | sr2 | Beta | B (SE) | sr2 | |
| Confidence | 0.40*** | 3.56 (0.33) | .16 | 0.45*** | 3.86 (0.22) | .20 |
| Complacency | −0.01 | −0.04 (0.13) | .00 | −0.05 | −0.19 (0.10) | .00 |
| Constraints | −0.09* | −0.11 (0.04) | .01 | −0.08** | −0.10 (0.03) | .01 |
| Risk Calculation – Objective | −0.03 | −0.45 (0.66) | .00 | −0.07* | −1.05 (0.41) | .01 |
| Risk Calculation – Subjective | 0.10* | 1.75 (0.67) | .01 | 0.08** | 1.30 (0.40) | .01 |
| Collective Responsibility | 0.10* | 0.13 (0.06) | .01 | 0.11*** | 0.14 (0.04) | .01 |
| Age | −0.01 | −0.01 (0.03) | .00 | 0.07** | 0.03 (0.01) | .01 |
| Race (Black = 1, Non-Black = 0) | −0.14*** | −2.39 (0.62) | .02 | −0.19*** | −3.37 (0.42) | .05 |
| Income | 0.12** | 0.55 (0.17) | .02 | 0.11*** | 0.49 (0.10) | .02 |
| Gender (Male = 1, Female = 2) | 0.01 | 0.12 (0.58) | .00 | −0.02 | −0.25 (0.35) | .00 |
Note. South Carolina: F (10, 636) = 22.94, p < .001, R2 = 0.27. National: F (10, 1259) = 70.52, p < .001, R2 = 0.36. *p < .05, **p < .01, ***p < .001.
2.3. Relations among 5C predictors and vaccine intentions
Results of the regression analyses are presented in Table 4 . Across both samples, among the 5C predictors, higher confidence and collective responsibility were associated with higher levels of vaccine intentions. In the National sample, lower complacency and higher subjective risk calculation were associated with higher levels of vaccine intentions. In the SC sample, higher levels of constraints were associated with higher levels of vaccine intentions. Across both samples, Black participants and female participants reported lower levels of vaccine intentions.
Table 4.
Logistic regression analyses predicting vaccine intentions.
| Variable |
Vaccine Intentions (0 = not likely to get vaccine, 1 = likely to get vaccine) |
|||||
|---|---|---|---|---|---|---|
|
South Carolina |
National |
|||||
| Wald | B (SE) | Exp(B) | Wald | B (SE) | Exp(B) | |
| Confidence | 47.68*** | 0.86 (0.13) | 2.37 | 141.64*** | 1.12 (0.09) | 3.05 |
| Complacency | −1.85 | −0.07 (0.05) | 1.07 | −8.85** | −0.12 (0.04) | 1.13 |
| Constraints | 5.09* | 0.04 (0.02) | 1.04 | −1.43 | −0.01 (0.01) | 0.99 |
| Risk Calculation – Objective | −1.09 | −0.24 (0.23) | .79 | −2.65 | −0.27 (0.16) | 0.77 |
| Risk Calculation – Subjective | −0.02 | −0.03 (0.23) | .97 | 3.79* | 0.31 (0.16) | 1.37 |
| Collective Responsibility | 15.14*** | 0.08 (0.02) | 1.09 | 13.15*** | 0.06 (0.02) | 1.06 |
| Age | 2.33 | 0.01 (0.01) | 1.01 | 3.54 | 0.01 (0.01) | 1.01 |
| Race (Black = 1, Non-Black = 0) | −11.52** | −0.74 (0.22) | 0.48 | −32.70*** | −0.96 (0.17) | 0.39 |
| Income | −0.42 | −0.04 (0.06) | 0.96 | 0.76 | 0.04 (0.04) | 1.04 |
| Gender (Male = 1, Female = 2) | −19.95*** | −0.93 (0.21) | 0.40 | −27.27*** | −0.74 (0.14) | 0.48 |
Note. South Carolina: X2 (10) = 143.13, p < .001, Nagelkerke R2 = 0.27, correct classification of 69% of cases. National: X2 (10) = 393.99, p < .001, Nagelkerke R2 = 0.36, correct classification of 74% of cases. *p < .05, **p < .01, ***p < .001.
2.4. Qualitative data
Participants who indicated they were unlikely to receive any COVID-19 vaccine (n = 1355) provided qualitative responses. Content analyses yielded 8 primary themes: (1) concern about vaccine safety (54.5%); (2) lack of trust (16.7%); (3) COVID-19 disbelief (9.2%); (4) reserving vaccine for high-risk or front-line workers (6.3%); (5) broad vaccine refusal (5.6%); (6) perceived barriers to vaccination (3.4%); (7) medical contraindications (3.0%); and (8) vaccine ingredient concerns (1.2%).
Concern about safety. Participants (54.5%) reported concerns about the quick timing of developing the vaccine, limited research, potential short- and long-term side effects, and wanting to see how others react to the vaccine prior to committing to vaccination. One participant mentioned, “I am nervous about how quickly the vaccine was approved. I feel like there might be long term health consequences from receiving the vaccine,” and another stated, “I want to make sure enough people have gotten it and it's shown to be safe.” Another participant reported, “Looking at the past previous diseases like the Spanish flu and the black plague. It took years to perfect the vaccines some people died because of those vaccines.”
Lack of trust. Many participants (16.7%) discussed lack of trust in the vaccine, producers of the vaccine, or the government. Most participants within this theme discussed lack of trust in the government or political concerns. Participants made comments such as, “The US is largely an anti-black racist country. I don't trust them at all,” “Worried it is just being rushed for political reasons,” and “Because I feel like it has a lot to do with the government tracking people and stealing information from people.”
COVID-19 disbelief. Several participants (9.2%) reported that the vaccine is unnecessary due to skepticism about the severity, or reality, of COVID-19 as an infectious disease. One participant stated, “It's about control, not your health. This is all blown way out of proportion.” Another person mentioned, “I don't believe that the coronavirus is a legitimate virus.” When discussing disbelief that he/she could be diagnosed with COVID, one participant stated, “Research says blood type O is less likely to get COVID, so I'm safe since I'm that blood type.” Some other participants reported lack of concern about COVID-19, including statements such as, “I am not likely to get the vaccine because I am still young and won't likely die from the coronavirus,” and “My chances of catching the virus are low.” Another participant stated, “My immune system is so highly evolved that coronavirus is a snack to my white blood cells.”
Reserving vaccine for high-risk or front-line workers. Many participants (6.3%) discussed that they would like to reserve the vaccine for high-risk individuals or front-line workers. Some participants stated, “There's a lot of people with higher risk that should probably get it first,” “I do not have a serious medical condition,” or “I'm young and healthy, there are people who are more at risk and need it first.” Other participants discussed wanting to reserve the vaccine for front-line workers, making statements such as, “Essential and healthcare workers should go first.”
Broad vaccine refusal. Some participants (5.6%) reported that they are unwilling to take any vaccine, so their refusal is not limited to the COVID-19 vaccine. Reasons for being unwilling to take any vaccines included that they never receive the flu shot so there is no reason to receive the COVID-19 vaccine, have never taken any vaccines, and are afraid of taking shots. For example, one participant stated, “I don't get the flu shot, why would I get the ‘rona vaccine,” while another participant said, “I am against all vaccines.” Another individual stated, “Vaccines have had an extremely negative impact in my family.”
Perceived barriers to vaccination. Participants (3.4%) also discussed perceived inability to receive the vaccine due to waiting lists and/or cost of the vaccine. Specifically related to waiting lists, individuals stated comments such as, “It will be awhile before I am able to get one,” “won't be in my area for awhile,” and “want to see if I am eligible.” Regarding the cost or ability to pay for the vaccine, one participant stated, “I'm not sure I will be able to afford it,” while another participant mentioned, “I do not have insurance.”
Medical contraindications. A few participants (3.0%) described that they would not be likely to receive the vaccine due to medical reasons, including pre-existing medical conditions, being afraid of needles, history of adverse reactions to vaccines, or being pregnant. For example, in discussing prior medical conditions, one participant stated, “I have allergies and I refuse to take the vaccine,” while another discussed, “Since I'm diabetic, I'm scared I can die from the vaccine.” Regarding concern due to pregnancy, one individual reported, “Because I am pregnant and I am concerned about the effects in my unborn child,” and another said, “I'm 16 weeks pregnant so I am not sure if my doctor would want me to get the vaccine.”
Vaccine ingredient concerns. Finally, a few participants (1.2%) reported refusal associated with what they believed to be ingredients in the vaccine or procedure to make the vaccine. Several participants noted that the vaccine was developed using cells from aborted fetuses, including comments such as, “I know they put aborted babies in the vaccine and I don't want that.” Other participants discussed general disagreement with vaccine ingredients. Including “I think there may be ingredients in the vaccine that may not be necessary and could be harmful.”
3. Discussion
The present study found evidence that both demographic characteristics (race) and certain drivers of vaccine behavior (confidence and collective responsibility) are associated with vaccine trust and intentions across samples above and beyond other variables. Qualitative analyses suggest it is imperative to find ways to build trust in the scientific and medical community. These findings hold important implications to support public health efforts to increase COVID-19 vaccine uptake.
It is important to consider the historical timing of this study in relation to the availability of COVID-19 vaccines. Both surveys were collected between October 2020 and January 2021 – just before COVID-19 vaccines were widely available. Therefore, these data provide a good “baseline” assessment of early vaccine hesitancy and an opportunity to compare how COVID-19 vaccine intentions relate to actual vaccine uptake. We found 44% of SC and 58% of National samples reported they were likely to get the COVID-19 vaccine. As of May 2022, 13 months after vaccines were widely available, approximately 78% of the US population had received at least one COVID-19 vaccine dose and 66% were fully vaccinated, while in SC, only 67% had received one dose and 57% were fully vaccinated (USAFacts, 2022). This suggests our self-report survey items reflect estimates of actual vaccine uptake across the US.
Results indicated that Black individuals reported lower levels of COVID-19 vaccine intentions, which is consistent with research on vaccine hesitancy (Freimuth et al., 2017; Fu et al., 2017) and general medical research (Mainous et al., 2006). This is congruent with long-standing distrust in the US healthcare system, with origins in racial discrimination in medical research and clinical settings (Assari, 2018; Kennedy et al., 2007). Past research on vaccine trust among Black adults has found that individuals who trust their provider are more likely to receive vaccines (Freimuth et al., 2017; Fu et al., 2017). This finding is critical to public health efforts as it suggests building provider trust throughout the Black community is imperative in decreasing COVID-19 vaccine hesitancy. Previous research has identified that the most effective ways to build trust between Black individuals and healthcare providers is through communication, perceived commitment, quality relationships, and shared control of healthcare decisions (Hansen et al., 2016).
The present findings contribute novel comparisons between National and SC samples. Overall, the SC sample reported lower COVID-19 vaccine intentions and higher levels of 5C barriers compared to the National sample. These differences highlight the need for heterogenous strategies to combat COVID-19 vaccine hesitancy within the US. For example, our findings suggest building confidence in science may be more effective among individuals in SC and addressing personal risk calculation may be more effective for the broader National population. Additionally, higher constraints (operationalized as psychological distress) were associated with lower vaccine trust across both samples, but higher constraints were associated with higher vaccine intentions for SC. Of note, vaccine trust was weakly correlated with vaccine intentions in SC (r = 0.34), but moderately correlated with vaccine intentions in the National sample (r = 0.54). It may be that among individuals in SC psychological distress is a driving factor to seek safety in COVID-19 vaccination, irrespective of vaccine trust. Although more research is needed in this area, the present findings offer some insight into the different drivers of vaccine uptake observed across the US.
The qualitative responses offer greater contextualization of COVID-19 vaccine hesitancy. Several themes emerged; however, the most common theme, reported by nearly 55% of participants involved the safety of the COVID-19 vaccines. Participants reported they were unlikely to receive the vaccine due to fears about the quick vaccine development, limited research, and potential side effects. This concern for vaccine safety and potential side effects is not unique to the COVID-19 vaccine. Fears that the measles mumps rubella (MMR) vaccine increased the risk of autism caused an influx of public concern, vaccine hesitancy, and lower MMR vaccination rates (Miyamoto et al., 1995). Although rigorous research showed no evidence of a link between the MMR vaccine and any medical disorders, the miscommunication about vaccine safety caused an ongoing public health crisis due to outbreak MMR cases in unvaccinated children (Jefferson, 2000). The present qualitative data, and historical salience of public concern for vaccine safety, highlights the importance of addressing safety concerns in public health efforts to promote vaccine uptake. These results call for accurate messaging and relaying of information from the medical community to the general public to dissuade fears driven by misinformation.
3.1. Limitations and future research
This study has several limitations. First, we measured COVID-19 vaccine intentions – not vaccination behavior. There is some evidence of an intention-behavior gap and longitudinal research is needed to establish temporal relations between vaccine intentions and behavior. Second, we used non-probability sampling, potentially limiting generalizability of our findings; however, the MFour panel is designed to provide national sampling of adults that are reflective of the US population. Further, as noted by Boyle (Boyle et al., 2022) the MFour research panel allows for quick and timely sampling when equivalent probability samples are unavailable. Third, many of our measures involved single items, although this is common in research using the 5C model, multi-item measures would increase confidence in the psychometric rigor of our findings. Finally, we examined vaccine hesitancy in late 2020 to early 2021 and there was a gap of approximately two months between the SC and National sample surveys; attitudes and intentions towards vaccination may have changed over time as more information about vaccine safety and efficacy emerged.
Despite these limitations, the present study offers valuable insight into factors associated with early COVID-19 vaccine hesitancy across the US. Given the recent stagnation in COVID-19 vaccine uptake, it remains relevant to understand the barriers to vaccination to help end this pandemic and plan for future public health efforts that require population vaccine uptake.
Credit roles
Caitlin Rancher: Formal analysis, Writing – original draft; Angela D. Moreland: Conceptualization, Methodology, Supervision, Writing – original draft; Daniel W. Smith: Conceptualization, Writing – review & editing; Vickey Cornelison: Conceptualization, Project Administration, Writing – review & editing; Mike Schmidt: Conceptualization, Methodology, Writing – review & editing; John Boyle: Methodology, Project administration, Data curation, Writing – review & editing; James Dayton: Methodology, Investigation, Data curation; Dean G. Kilpatrick: Conceptualization, Methodology, Resources, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare no conflict of interest.
Footnotes
Funding was provided internally by the National Crime Victims Research and Treatment Center, Medical University of South Carolina.
References
- Assari S. Health disparities due to diminished return among black Americans: public policy solutions. Soc. Iss. Policy Rev. 2018;12(1):112–145. [Google Scholar]
- Benecke O., DeYoung S.E. Anti-vaccine decision-making and measles resurgence in the United States. Global Ped. Health. 2019;6:1–5. doi: 10.1177/2333794X19862949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsch C., Schmid P., Heinemeier D., Korn L., Holtmann C., Böhm R. Beyond confidence: development of a measure assessing the 5C psychological antecedents of vaccination. PLoS One. 2018;13(12) doi: 10.1371/journal.pone.0208601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsch C., Korn L., Sprengholz P., Felgendreff L., Eitze S., Schmid P., Böhm R. Social and behavioral consequences of mask policies during the COVID-19 pandemic. Proc. Natl. Acad. Sci. USA. 2020;117(36):21851–21853. doi: 10.1073/pnas.2011674117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J., Berman L., Nowak G.J., Iachan R., Middleton D., Deng Y. An assessment of parents' childhood immunization beliefs, intentions, and behaviors using a smartphone panel. Vaccine. 2020;38(10):2416–2423. doi: 10.1016/j.vaccine.2020.01.032. [DOI] [PubMed] [Google Scholar]
- Boyle J., Nowak G., Kinder R., Iachan R., Dayton J. Better understanding adult COVID-19 vaccination hesitancy and refusal: the influence of broader beliefs about vaccines. Int. J. Environ. Res. Publ. Health. 2022;19(11):1–16. doi: 10.3390/ijerph19116838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cero I., Kilpatrick D.G. Network analysis of posttraumatic stress disorder symptoms in a national sample of US Adults: implications for the phenotype and the ICD‐11 model of PTSD. J. Trauma. Stress. 2020;33(1):52–63. doi: 10.1002/jts.22481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID Act Now . 2022. U.S. COVID Tracker. COVID Act Now.https://covidactnow.org/?s=39912739 May. [Google Scholar]
- Freimuth V.S., Jamison A.M., An J., Hancock G.R., Quinn S.C. Determinants of trust in the flu vaccine for African Americans and Whites. Soc. Sci. Med. 2017;193:70–79. doi: 10.1016/j.socscimed.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L.Y., Zimet G.D., Latkin C.A., Joseph J.G. Associations of trust and healthcare provider advice with HPV vaccine acceptance among African American parents. Vaccine. 2017;35(5):802–807. doi: 10.1016/j.vaccine.2016.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamadani J.D., Hasan M.I., Baldi A.J., Hossain S.J., Shiraji S., Bhuiyan M.S.A., Mehrin S.F., Fisher J., Tofail F., Tipu S.M.U. Immediate impact of stay-at-home orders to control COVID-19 transmission on socioeconomic conditions, food insecurity, mental health, and intimate partner violence in Bangladeshi women and their families: an interrupted time series. Lancet Global Health. 2020;8:e1380–e1389. doi: 10.1016/S2214-109X(20)30366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen B.R., Hodgson N.A., Gitlin L.N. It's a matter of trust: older African Americans speak about their health care encounters. J. Appl. Gerontol. 2016;35(10):1058–1076. doi: 10.1177/0733464815570662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson T.O.M. Real or perceived adverse effects of vaccines and the media—a tale of our times. J. Epidemiol. Community. 2000;54(6):402–403. doi: 10.1136/jech.54.6.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy B.R., Mathis C.C., Woods A.K. African Americans and their distrust of the health care system: healthcare for diverse populations. J. Cult. Divers. 2007;14(2):56–60. [PubMed] [Google Scholar]
- LaForce F.M., Okwo-Bele J.M. Eliminating epidemic group A meningococcal meningitis in Africa through a new vaccine. Health Aff. 2011;30(6):1049–1057. doi: 10.1377/hlthaff.2011.0328. [DOI] [PubMed] [Google Scholar]
- MacDonald N.E., Dubé E. Unpacking vaccine hesitancy among healthcare providers. EBioMedicine. 2015;2(8):792–793. doi: 10.1016/j.ebiom.2015.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainous A.G., Smith D.W., Geesey M.E., Tilley B.C. Development of a measure to assess patient trust in medical researchers. Ann. Fam. Med. 2006;4:247–252. doi: 10.1370/afm.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto H., Tanaka T., Kitamoto N., et al. Detection of immunereactive antigen with monoclonal antibody to measles virus in tissue from patients with Crohn's disease. J. Gastreont. 1995;30:28–33. doi: 10.1007/BF01211371. [DOI] [PubMed] [Google Scholar]
- Okwo-Bele J.M., Cherian T. The expanded programme on immunization: a lasting legacy of smallpox education. Vaccine. 2011;29:D74–D79. doi: 10.1016/j.vaccine.2012.01.080. [DOI] [PubMed] [Google Scholar]
- Our World in Data . 2022. Coronavirus (COVID-19) vaccinations.https://ourworldindata.org/covid-vaccinations September 19. [Google Scholar]
- Phadke V.K., Bednarczyk R.A., Salmon D.A., Omer S.B. Association between vaccine refusal and vaccine-preventable diseases in the United States: a review of measles and pertussis. JAMA. 2016;315:1149–1158. doi: 10.1001/jama.2016.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines. 2021;9:1–15. doi: 10.3390/vaccines9020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano G., Nardi A. Vaccine hesitancy in the era of COVID-19. Publ. Health. 2021;194:245–251. doi: 10.1016/j.puhe.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USAFacts . 2022. https://usafacts.org/visualizations/covid-vaccine-tracker/states/ Retrieved from. [Google Scholar]
- Wilson S.L., Wiysonge C. Social media and vaccine hesitancy. BMJ Global Health. 2020;5(10) doi: 10.1136/bmjgh-2020-004206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wismans A., Thurik R., Baptista R., Dejardin M., Janssen F., Franken I. Psychological characteristics and the mediating role of the 5C Model in explaining students' COVID-19 vaccination intention. PLoS One. 2021;16(8) doi: 10.1371/journal.pone.0255382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L.P., Wong P.F., AbuBakar S. Vaccine hesitancy and the resurgence of vaccine preventable diseases: the way forward for Malaysia, a Southeast Asian country. Hum. Vaccines Immunother. 2020;16:1511–1520. doi: 10.1080/21645515.2019.1706935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiyeh A.B., Cooper S., Jaca A., Mavundza E., Ndwandwe D., Wiysonge C.S. Social media and HPV vaccination: unsolicited public comments on a Facebook post by the Western Cape Department of Health provide insights into determinants of vaccine hesitancy in South Africa. Vaccine. 2019;37(43):6317–6323. doi: 10.1016/j.vaccine.2019.09.019. [DOI] [PubMed] [Google Scholar]
- Wiysonge C.S., Ndwandwe D., Ryan J., Jaca A., Batouré O., Anya B.P.M., Cooper S. Vaccine hesitancy in the era of COVID-19: could lessons from the past help in divining the future? Hum. Vaccines Immunother. 2022;18(1):1–3. doi: 10.1080/21645515.2021.1893062. [DOI] [PMC free article] [PubMed] [Google Scholar]