Abstract
Context
Hyponatremia often reflects a free water excess. Sodium/glucose cotransporter 2 (SGLT2) inhibitors increase free water excretion through glucose-induced osmotic diuresis. In 2 randomized double-blind, placebo-controlled trials in patients with the syndrome of inappropriate antidiuresis (SIAD), we showed that empagliflozin increased plasma sodium concentration more effectively than placebo.
Objective
We hypothesized that long-term therapy with SGLT2 inhibitors might reduce the prevalence of hyponatremia on hospital admission.
Methods
In this retrospective analysis, we extracted data from adult patients with type 2 diabetes (T2DM) hospitalized at the University Hospital Basel between 2015 and 2020. Patients with an SGLT2 inhibitor on admission were matched 1:1 according to age, gender, diagnosis of heart failure, and principal diagnosis to patients without an SGLT2 inhibitor on admission. The primary outcome was the prevalence of hyponatremia (plasma sodium concentration corrected for glycemia <135 mmol/L) on admission.
Results
We analyzed 821 patients with T2DM treated with and 821 patients with T2DM without an SGLT2 inhibitor on admission. Hyponatremia prevalence on admission was 9.9% in the treated group, and 8.9% in the matched control group (P = .554), in other words, the risk for hyponatremia did not differ (multivariable adjusted odds ratio 1.08, 95% CI 0.72-1.44, P = .666). There was no difference in the median (interquartile range) plasma sodium concentration between the groups (treated 140 mmol/L [138-142], controls 140 mmol/L [138-142]; P = .1017).
Conclusion
Based on these retrospective findings, treatment with SGLT2 inhibitors does not prevent hyponatremia. However, prospective randomized data suggest their efficacy at a higher dosage in overt SIAD.
Keywords: sodium/glucose cotransporter 2 inhibitor, type 2 diabetes, hyponatremia, dysnatremia
Hyponatremia, defined as plasma sodium concentration <135 mmol/L, is the most common electrolyte disturbance in hospitalized patients [1]. Its prevalence on hospital admission ranges from 3% to 38%, depending on the severity of hyponatremia [1-4]. Hyponatremia on hospital admission is associated with increased in-hospital [1, 2, 4], 1-year [2, 5], and 5-year mortality [2]. Furthermore, it is associated with an increased risk for intensive care unit admission and mechanical ventilation [3], longer hospital stays [3, 4], higher hospital costs [3], and discharge to care facilities [4].
In prevailing guidelines, treatments for hypovolemic hyponatremia and acute severely symptomatic hyponatremia are well established [6-8]. By contrast, patients with chronic euvolemic or hypervolemic hyponatremia are often discharged still hyponatremic because of the limited efficacy of the available therapeutic options [9].
Both euvolemic and hypervolemic hyponatremia result primarily from arginine vasopressin (AVP)–mediated free water retention [10]. Accordingly, fluid restriction is usually the first-line therapy but has limited efficacy [11]. AVP antagonists (vaptans) lead to increased aquaresis and can be used as a second-line treatment [6, 8, 12, 13]. However, they are costly and carry the risk for overly rapid plasma sodium correction, requiring close plasma sodium monitoring on treatment initiation [14-16].
The sodium/glucose cotransporter 2 (SGLT2) is expressed in the proximal tubules of the kidneys and reabsorbs approximately 90% of the filtered glucose [17]. Blockade of SGLT2 with SGLT2 inhibitors (oral antidiabetic drugs) results in renal excretion of glucose [18] and subsequent osmotic diuresis [19]. The SGLT2 inhibitor empagliflozin reduces the risk of major adverse cardiovascular events [20] and heart failure [21] and slows progression of kidney disease in patients with diabetes with high cardiovascular risk [22]. Empagliflozin has also cardiovascular and renal benefits regardless of diabetes mellitus in patients with heart failure and reduced ejection fraction (HFrEF) [23] or heart failure and preserved ejection fraction [24]. Furthermore, we showed in a randomized, double-blind, placebo-controlled trial in 87 hospitalized euvolemic hyponatremic patients with the syndrome of inappropriate antidiuresis (SIAD) that a 4-day treatment with empagliflozin combined with fluid restriction led to a higher plasma sodium increase than fluid restriction alone [25]. We recently confirmed these findings in a randomized, double-blind, placebo-controlled, crossover trial in 14 outpatients with chronic SIAD [26].
To our knowledge, the effect of long-term treatment with SGLT2 inhibitors on hyponatremia prevalence in hospitalized patients has never been investigated. We aimed to compare hyponatremia prevalence on admission in patients with type 2 diabetes (T2DM) treated with an SGLT2 inhibitor with that in control patients with T2DM but without SGLT2 inhibitors. We hypothesized that hyponatremia prevalence is lower in patients treated with an SGLT2 inhibitor. This would support their use as a prophylaxis for hyponatremia recurrence in patients with chronic hyponatremia or as a prophylaxis for hyponatremia in general in at-risk patients.
Materials and Methods
Patients Selection and Extraction
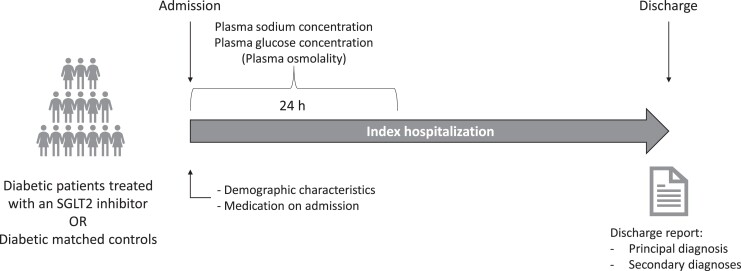
This retrospective, cross-sectional study selected all patients with (T2DM) hospitalized at the University Hospital of Basel, Switzerland, between 2015 and 2020 with available plasma sodium measurement within the first 24 hours following admission (Fig. 1). Demographic characteristics, medication on admission, plasma glucose and osmolality (if available at the timepoint of sodium measurement), and comorbidities were extracted from the electronic health records at the same time by the Information and Communication Technologies Department of the University Hospital of Basel and transmitted to the first author for statistical analysis. Diagnoses were coded with the International Statistical Classification of Diseases and Related Health Problems (ICD) 10-GM (version 2014, 2016, and 2018) [27-29] and taken from discharge reports. The extraction of the health-related data from the electronic health records of the University Hospital of Basel required for this study was approved by the local ethics committee (Ethikkommision Nordwest- und Zentralschweiz, EKNZ 2021-00649).
Figure 1.
Study diagram. Study diagram showing patients selection. All patients with type 2 diabetes hospitalized between 2015 and 2020 and with a plasma sodium and a glucose measurement within the first 24 hours following admission were selected.
Laboratory
Plasma concentrations of sodium, glucose, and osmolality were from the same timepoint and measured by the central laboratory of the University Hospital Basel. Plasma sodium concentration was measured by the indirect ion selective electrode (ISE) method (cobas 8000 modular analyzer, Roche Diagnostics) in centrifuged lithium–heparin plasma. Plasma osmolality levels were measured using the freezing point depression osmometric method.
At higher concentrations, glucose can cause translocational isotonic or hypertonic hyponatremia [30]. Because all selected patients were diabetic, we corrected plasma sodium values for glycemia according to the linear model of Hillier et al [30], as recommended by European guidelines [6]. For each patient with glycemia above 5.5 mmol/L, sodium levels were corrected by adding 2.4 mmol/L per 5.5 mmol/L glucose using the following equation:
Hyponatremia was defined as a plasma sodium concentration <135 mmol/L and further subclassified according to biochemical severity (mild, plasma sodium concentration 130-134 mmol/L; moderate, plasma sodium concentration 125-129 mmol/L; profound, plasma sodium concentration <125 mmol/L) [6]. Hypernatremia was defined as a plasma sodium concentration >145 mmol/L.
Study Outcomes
The primary outcome was the prevalence of hyponatremia on hospital admission in patients with T2DM treated with an SGLT2 inhibitor vs matched control patients with T2DM without an SGLT2 inhibitor.
The secondary outcomes included the difference in plasma sodium concentration, the prevalence of hyponatremia severities, and hypernatremia in patients with T2DM treated with an SGLT2 inhibitor vs matched control patients with T2DM who were not treated with an SGLT2 inhibitor. We additionally computed the prevalence of hyponatremia, hyponatremia severities, and hypernatremia in a subset excluding patients with an ICD10 code for hypovolemia or hypotension and in a subset containing only patients with hypervolemia with heart failure, chronic kidney disease, or liver cirrhosis as a comorbidity. Furthermore, we investigated the association between SGLT2 inhibitors and hyponatremia on admission adjusted for medication and comorbidities, as well as whether SGLT2 inhibitors have an influence on plasma sodium levels when adjusted for medication and comorbidities.
Statistical Analysis
Baseline characteristics are summarized using descriptive statistics. Discrete variables are expressed as frequencies (% and number of patients, n). Continuous variables are expressed as median and interquartile range (IQR, 25th to 75th percentiles).
Eight hundred twenty-one patients treated with an SGLT2 inhibitor, and 15 999 control patients met the selection criteria. We performed 1:1 propensity score matching using the package MatchIt [31]. The average treatment effect on the treated (ATT) was estimated by fitting a generalized linear model with the variable SGLT2 inhibitor as the dependent variable and the covariates gender, age (±5 years), heart failure diagnosis, and main diagnosis (as ICD10 chapter) as independent variables. Patients were matched 1:1 using the nearest neighbor matching (ie, “greedy matching”) method without replacement; therefore, each treated patient was matched to 1 control patient and 15 178 controls were discarded. Further details on matching specification and covariate balance can be found elsewhere [32].
Prevalence in each group was compared using the chi-squared test. Plasma sodium concentration in each group was compared using the Wilcoxon–Mann–Whitney test. The independent effect of SGLT2 inhibitors on hyponatremia occurrence on admission was investigated by fitting a univariable and a multivariable logistic regression model. The model with the lowest Akaike information criterion was selected in a stepwise way using the step function, with SGLT2 inhibitors as a fixed predictor [33]. The association between plasma sodium concentration and SGLT2 inhibitors was investigated in the same way, in other words, with a univariable and multivariable linear model, with additional verification of assumptions and multicollinearity. Detailed covariables fitting and outputs can be found elsewhere [32].
All analyses were performed using the statistical program R (version 4.0.5 or higher). A 2-sided significance level of .05 was set for every analyses.
Results
Baseline Characteristics
Eight hundred and twenty-one patients treated with an SGLT2 inhibitor were matched to 821 control patients. Covariate balance was achieved as emphasized by the final matching specification including a standardized mean difference of −0.0001 (standardized mean difference before matching = 0.3751), a variance ratio of 0.9994 (variance ratio before matching = 0.9869) and a mean empirical cumulative density function (eCDF) of 0.0001 (mean eCDF before matching = 0.0954). Twenty-nine percent of patients (n = 238) were female, and the median (IQR) age was 70 years (61; 78) in each group. Detailed baseline characteristics including comorbidities, medications, and laboratory parameters of each group were well balanced and are shown in Tables 1, 2, and 3.
Table 1.
Demographic characteristics
| No SGLT2 inhibitor on admission (n = 821) | SGLT2 inhibitor on admission (n = 821) | |
|---|---|---|
| Female, n (%) | 238 (29) | 238 (29) |
| Admission year, n (%) | ||
| 2015 | 567 (69.1) | 18 (2.2) |
| 2016 | 129 (15.7) | 56 (6.8) |
| 2017 | 55 (6.7) | 124 (15.1) |
| 2018 | 30 (3.7) | 144 (17.5) |
| 2019 | 24 (2.9) | 198 (24.1) |
| 2020 | 16 (1.9) | 281 (34.2) |
| Age, y, median (IQR) | 70.00 (61.00, 78.00) | 70.00 (61.00, 78.00) |
Demographic characteristics in patients with T2DM treated with an SGLT2 inhibitor and in matched controls with T2DM.
Table 2.
Admission diagnoses and comorbidities
| No SGLT2 inhibitor on admission (n = 821) | SGLT2 inhibitor on admission (n = 821) | |
|---|---|---|
| ICD10 chapter of admission diagnosis, n (%) | ||
| I: Certain infectious and parasitic diseases | 52 (6.3) | 53 (6.5) |
| II: Neoplasms | 47 (5.7) | 48 (5.8) |
| III: Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism | 3 (0.4) | 2 (0.2) |
| IV: Endocrine, nutritional and metabolic diseases | 50 (6.1) | 47 (5.7) |
| V: Mental and behavioral disorders | 6 (0.7) | 7 (0.9) |
| VI: Diseases of the nervous system | 29 (3.5) | 32 (3.9) |
| VII: Diseases of the eye and adnexa | 3 (0.4) | 4 (0.5) |
| VIII: Diseases of the ear and mastoid process | 3 (0.4) | 5 (0.6) |
| IX: Diseases of the circulatory system | 258 (31.4) | 256 (31.2) |
| X: Diseases of the respiratory system | 95 (11.6) | 86 (10.5) |
| XI: Diseases of the digestive system | 57 (6.9) | 55 (6.7) |
| XII: Diseases of the skin and subcutaneous tissue | 12 (1.5) | 15 (1.8) |
| XIII: Diseases of the musculoskeletal system and connective tissue | 40 (4.9) | 40 (4.9) |
| XIV: Diseases of the genitourinary system | 42 (5.1) | 44 (5.4) |
| XVII: Congenital malformations, deformations and chromosomal abnormalities | 0 (0.0) | 0 (0.0) |
| XVIII: Symptoms, signs and abnormal clinical and laboratory findings, not elsewhere classified | 1 (0.1) | 1 (0.1) |
| XIX: Injury, poisoning and certain other consequences of external causes | 42 (5.1) | 43 (5.2) |
| XXI: Factors influencing health status and contact with health services | 81 (9.9) | 82 (10.0) |
| All diagnoses, n (%) | ||
| Acute kidney injury | 45 (5.5) | 107 (13.0) |
| Coronary heart disease | 308 (37.5) | 399 (48.6) |
| Acute coronary syndrome | 69 (8.4) | 91 (11.1) |
| Chronic kidney disease | 262 (31.9) | 252 (30.7) |
| Heart failure | 149 (18.1) | 149 (18.1) |
| Hypertension | 429 (52.3) | 470 (57.2) |
| Hyponatremia diagnosis | 25 (3.0) | 29 (3.5) |
| Hypotension | 11 (1.3) | 23 (2.8) |
| Hypovolemia | 19 (2.3) | 32 (3.9) |
| Liver cirrhosis | 13 (1.6) | 16 (1.9) |
| Lung cancer | 16 (1.9) | 16 (1.9) |
| Pneumonia | 86 (10.5) | 86 (10.5) |
| Seizure | 42 (5.1) | 26 (3.2) |
| Stroke | 47 (5.7) | 56 (6.8) |
| Ischemic stroke | 43 (5.2) | 54 (6.6) |
| Subarachnoid hemorrhage | 1 (0.1) | 0 (0.0) |
| Syndrome of inappropriate antidiuresis (SIAD) | 3 (0.4) | 2 (0.2) |
| Tuberculosis | 4 (0.5) | 3 (0.4) |
Admission principal diagnosis as ICD10 chapter and comorbidities in patients with T2DM treated with an SGLT2 inhibitor and in matched controls with T2DM.
Table 3.
Medication and laboratory values on admission
| No SGLT2 inhibitor on admission (n = 821) | SGLT2 inhibitor on admission (n = 821) | P value | |
|---|---|---|---|
| Medication on admission, n (%) | |||
| Antihypertensive agents and diuretics | |||
| ACE inhibitor | 191 (23.3) | 268 (32.6) | |
| ARB | 172 (21.0) | 236 (28.7) | |
| ARNI | 1 (0.1) | 32 (3.9) | |
| Beta blocker | 286 (34.8) | 406 (49.5) | |
| Calcium channel antagonists | 198 (24.1) | 221 (26.9) | |
| Loop diuretic | 222 (27.0) | 244 (29.7) | |
| Mineralocorticoid receptor antagonist | 74 (9.0) | 110 (13.4) | |
| Renin inhibitor | 6 (0.7) | 0 (0.0) | |
| Thiazide or thiazide-like diuretic | 145 (17.7) | 183 (22.3) | |
| Antidiabetic drugs | |||
| Acarbose | 0 (0.0) | 0 (0.0) | |
| Biguanide | 329 (40.1) | 514 (62.6) | |
| DDP4 inhibitor | 189 (23.0) | 217 (26.4) | |
| Glinide | 5 (0.6) | 4 (0.5) | |
| GLP-1 receptor agonist | 31 (3.8) | 100 (12.2) | |
| Insulin or insulin analog | 226 (27.5) | 325 (39.6) | |
| SGLT2 inhibitor | 0 (0.0) | 821 (100.0) | |
| Canagliflozin | 0 (0.0) | 39 (4.8) | |
| Dapagliflozin | 0 (0.0) | 184 (22.4) | |
| Empagliflozin | 0 (0.0) | 599 (73.0) | |
| Ertugliflozin | 0 (0.0) | 0 (0.0) | |
| Sulfonylurea | 77 (9.4) | 82 (10.0) | |
| Thiazolidinedione | 5 (0.6) | 5 (0.6) | |
| Antidepressants | |||
| Other antidepressant | 5 (0.6) | 8 (1.0) | |
| SNRI | 11 (1.3) | 13 (1.6) | |
| SSRI | 62 (7.6) | 97 (11.8) | |
| Tetracyclic antidepressant | 28 (3.4) | 17 (2.1) | |
| Tricyclic antidepressant | 17 (2.1) | 15 (1.8) | |
| Psycholeptics | |||
| Atypical neuroleptics | 61 (7.4) | 51 (6.2) | |
| Lithium | 8 (1.0) | 0 (0.0) | |
| Typical neuroleptics | 16 (1.9) | 5 (0.6) | |
| Anticonvulsants | 86 (10.5) | 93 (11.3) | |
| Laboratory values on admission, median (IQR) | |||
| Plasma sodium corrected for glucose (mmol/L) | 140 (138, 142), (n = 821) | 140 (138, 142), (n = 821) | .1017 |
| Raw plasma sodium (mmol/L) | 138 (136, 140), (n = 821) | 138 (136, 141), (n = 821) | .2473 |
| Plasma osmolality (mOsm/kg) | 300 (290, 317), (n = 23) | 296 (287, 314) (n = 26) | .4056 |
| Plasma glucose (mmol/L) | 8.8 (6.9, 11.9), (n = 821) | 9.1 (7, 12.1), (n = 821) | .2555 |
Medication and laboratory values on admission in patients with T2DM treated with an SGLT2 inhibitor and in matched controls with T2DM. Raw plasma sodium values and plasma sodium values corrected for glucose are displayed. Plasma sodium values are the first available in the 24 hours following admission. Plasma osmolality and glucose values are from the same samples as plasma sodium values. The 2 groups were compared with a Wilcoxon–Mann–Whitney test.
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor/neprilysin inhibitor; GLP, glucagon-like peptide; SGLT2, sodium/glucose cotransporter 2; SNRI, serotonin–norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitors.
Prevalence of Hyponatremia on Admission and Association With SGLT2 Inhibitors
Patients treated with SGLT2 inhibitors showed no difference in hyponatremia prevalence on admission compared with the matched control group (9.9%, n = 81 vs 8.9%, n = 73, P = .554) (Table 4).
Table 4.
Prevalence of dysnatremia on admission
| No SGLT2 inhibitor on admission (n = 821) | SGLT2 inhibitor on admission (n = 821) | P value | |
|---|---|---|---|
| Hypernatremia (plasma sodium >145 mmol/L), n (%) |
46 (5.6) | 33 (4.0) | .166 |
| Normonatremia (plasma sodium 135–145 mmol/L), n (%) |
702 (85.5) | 707 (86.1) | .777 |
| Hyponatremia (corrected plasma sodium <135 mmol/L), n (%) | 73 (8.9) | 81 (9.9) | .554 |
| Mild hyponatremia (plasma sodium 130–134 mmol/L), n (%) |
57 (6.9) | 65 (7.9) | .510 |
| Moderate hyponatremia (plasma sodium 125–129 mmol/L), n (%) |
11 (1.3) | 10 (1.2) | 1 |
| Profound hyponatremia (plasma sodium <125 mmol/L), n (%) |
5 (0.6) | 6 (0.7) | 1 |
Prevalence of dysnatremia on admission in patients with T2DM treated with an SGLT2 inhibitor and in matched controls with T2DM (plasma sodium corrected for glucose). The 2 groups were compared with a chi-squared test.
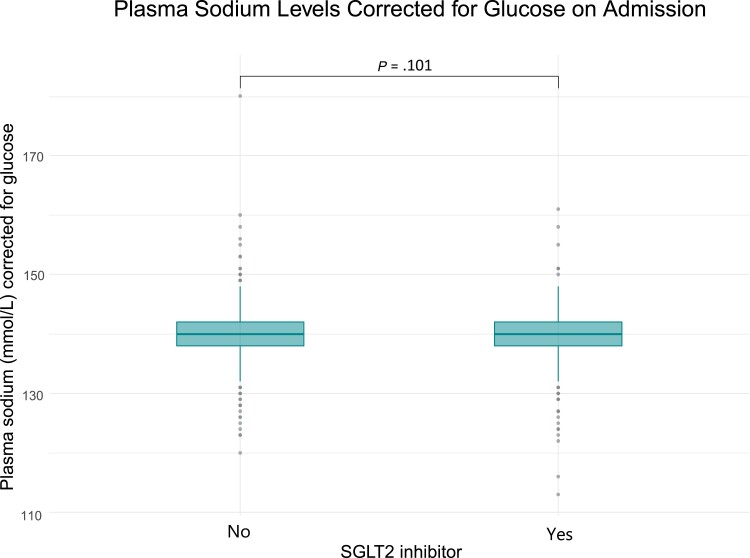
There was no difference in the different hyponatremia severities, specifically, mild (7.9%, n = 65 vs 6.9%, n = 57, P = .510), moderate (1.2%, n = 10 vs 1.3%, n = 11, P = 1.0), and profound (0.7%, n = 6 vs 0.6%, n = 5, P = 1.0), and in hypernatremia prevalence (4.0%, n = 33 vs 5.6%, n = 46, P = .116) (Table 4). SGLT2 inhibitors were not associated with hyponatremia (unadjusted odds ratio [OR] 1.12, 95% CI 0.79-1.45, P = .499; multivariable adjusted OR 1.08, 95% CI 0.72-1.44, P = .666). Furthermore, there was no difference in the median [IQR] plasma sodium concentration between the groups (treated, 140 mmol/L [138-142]; controls, 140 mmol/L [138-142], P = .1017) (Fig. 2 and Table 3). SGLT2 inhibitors were not associated with a significant change in plasma sodium levels (unadjusted β = −.08, 95% CI −0.35-0.51, P = .712; multivariable adjusted β = −.24, 95% CI −0.20-0.68, P = .280). Detailed statistical models can be found elsewhere [32].
Figure 2.
Plasma sodium levels on admission. Plasma sodium levels in mmol/L for each group: patients with diabetes treated with an SGLT2 inhibitor (n = 821) and matched controls (n = 774). Boxes span the interquartile range (IQR); the thick horizontal line is the median. Whiskers are the most extreme values lying within the box edge and 1.5 times the IQR. All other values are considered to be outliers and plotted as individual points. A Wilcoxon–Mann–Whitney test was performed to compare hyponatremia levels in both groups.
After excluding patients with an ICD10 code for hypovolemia or hypotension, there was still no difference in hyponatremia prevalence on admission between patients treated with SGLT2 inhibitors and their matched control patients (9.2%, n = 71 vs 9.0%, n = 71, P = .936). There was no difference in the different hyponatremia severities, specifically, mild (7.4%, n = 57 vs 7.0%, n = 55, P = .806), moderate (1.0%, n = 8 vs 1.4%, n = 11, P = .687), and profound (0.8%, n = 6 vs 0.6%, n = 5, P = .964), and in hypernatremia prevalence (3.4%, n = 26 vs 5.1%, n = 40, P = .128) [32].
Similarly, hyponatremia prevalence was similar in hypervolemic patients treated with an SGLT2 inhibitor and without an SGLT2 inhibitor (13.3%, n = 43 vs 10.7%, n = 37, P = .363). There was no difference in the different hyponatremia severities, specifically, mild (10.8%, n = 35 vs 7.8%, n = 27, P = .228), moderate (1.0%, n = 8 vs 1.4%, n = 11, P = .860), and profound (0.9%, n = 3 vs 0.9%, n = 3, P = 1.0), and in hypernatremia prevalence (5.2%, n = 17 vs 5.8%, n = 20, P = .894) [32].
Discussion
The main finding of this cross-sectional study is that hyponatremia prevalence and plasma sodium concentration were the same in patients with T2DM treated with and without SGLT2 inhibitors, irrespective of comorbidities and comedications.
To our knowledge, this is the first study providing data on hyponatremia prevalence in patients with T2DM treated with an SGLT2 inhibitor. Falhammar et al investigated the association between hyponatremia and glucose-lowering drugs; however, the number of patients treated with an SGLT2 inhibitor (n = 2) in their study was too low to investigate their effect on plasma sodium levels [34]. In the current analysis, we chose hyponatremia on admission to investigate the effect of SGLT2 inhibitors because these drugs are commonly paused during hospitalization. Contrary to our hypothesis, we found no difference in the hyponatremia prevalence and plasma sodium levels on admission between patients with T2DM treated with SGLT2 inhibitors and control patients with T2DM without SGLT2 inhibitors. The prevalence of the different hyponatremia severities and of hypernatremia did not differ either.
A first plausible explanation is that we, unfortunately, could not truly differentiate hyponatremia subtypes; in particular, we could not precisely identify hypovolemic hyponatremia, which is one of the most common causes for hyponatremia [35]. A recent meta-analysis by Rong et al suggested that SGLT2 inhibitors are not associated with orthostatic hypotension [36]; however, they reduce blood pressure [37] and induce volume depletion [19]. In hypovolemic hyponatremia, SGLT2 inhibitors might therefore show no effects or even lower plasma sodium levels through hemodynamic AVP stimulation, and thus counterbalance the benefit of SGLT2 inhibitors on plasma sodium levels in euvolemic and hypervolemic patients in the full dataset. The subgroup analysis we performed was inconclusive: we only extracted ICD10 codes for hypovolemia and hypotension but were not able to account for the other diverse etiologies for hypovolemic hyponatremia (eg, bleeding, third spacing, or gastrointestinal fluid loss) and, therefore, a reliable subset analysis was not possible. In addition, there was no difference in the subgroup of patients with heart failure, liver cirrhosis, or chronic kidney disease as comorbidities. Because we did not perform chart review, we were not able to recognize patients with decompensated aforementioned conditions that might have been causative for hyponatremia.
Second, the inhibition of SGLT2 increases glucosuria and natriuresis [38]. One could argue that it would increase urinary sodium clearance and worsen hyponatremia. However, hyponatremia is not a side effect of SGLT2 inhibitors, mainly because the pathophysiology of hyponatremia relies more on a relative water excess than an absolute sodium deficit [39]. Interestingly, our data showed no difference in urine sodium concentration and fractional excretion of sodium between patients with SIAD treated with empagliflozin or a placebo [25, 26]. In patients with T2DM, natriuresis seems to be transient as well [40].
Of note, all patients in this study have T2DM. Even though benefits from SGLT2 inhibitors in heart failure [24, 41] and CKD [42] are irrespective of T2DM, the current findings cannot be extended to patients without T2DM. Glucosuria is more prominent in T2DM [18, 43]; therefore, osmotic diuresis might be greater and favor hypovolemic hyponatremia. Furthermore, we were not able to record treatment duration. Patients with T2DM treated with an SGLT2 inhibitor initially show a reduction of extracellular fluid and an activation of the renin angiotensin aldosterone system, both of which do not persist after 6 months of treatment [44], whereas reduction of extracellular volume persists after 12 weeks in patients with heart failure independently of diabetes [45], which support the hypothesis that the effect of SGLT2 inhibitors might differ in patients with a relative water excess (eg, heart failure, SIAD). In support of this, a recent post hoc analysis of the DAPA-HF placebo-controlled trial investigating the effect of dapagliflozin 10 mg in patients with HFrEF showed a higher prevalence of hyponatremia after 14 days (11.3% vs 9.4%; P = .04) but a reduced prevalence of hyponatremia after 12 months (4.6% vs 6.7%; P = .003) in the dapagliflozin group [46].
Two of our randomized, double-blind, placebo-controlled trials provided evidence that empagliflozin is an effective treatment: first, in hospitalized patients with SIAD [25], and, second, in outpatients with chronic SIAD [26]. Furthermore, a post hoc analysis in patients with HFrEF [46] and case reports in patients with liver cirrhosis [47] suggests that SGLT2 inhibitors might represent an effective option for these hyponatremic subgroups. Long-term SGLT2 inhibitor treatment might only influence plasma sodium levels in patients with overt euvolemic or hypervolemic hyponatremia, in other words, with a relative body water excess. The effect of SGLT2 inhibitors in hypervolemic hyponatremic patients with heart failure or liver cirrhosis is currently being investigated in a multicentric, randomized, double-blind, placebo-controlled trial (NCT04447911).
Finally, cross-sectional studies provide helpful insight into associations but yield poor information about causal relationships. Therefore, findings should be cautiously interpreted [48]. The incongruence between our retrospective observational results and our prospective randomized data [25, 26] underlines this limitation.
Conclusion
Based on this cross-sectional retrospective study, SGLT2 inhibitors do not prevent hyponatremia development. These findings do not support their use as hyponatremia prophylaxis in at-risk patients. Prospective randomized data suggest their efficacy at a higher dosage in overt SIAD [25, 26], but their efficacy in other hyponatremia subtypes remains to be demonstrated. An ongoing randomized, placebo-controlled study will help better define the role of empagliflozin in overt euvolemic and hypervolemic hyponatremia (NCT04447911).
Acknowledgments
The authors thank the Department of Clinical Research of the University of Basel and the Information and Communication Technologies Department of the University Hospital of Basel for the data extraction from the patients management system.
Abbreviations
- AVP
arginine vasopressin
- HFrEF
heart failure and reduced ejection fraction
- IQR
interquartile range
- SGLT2
sodium/glucose cotransporter 2
- SIAD
syndrome of inappropriate antidiuresis
- T2DM
type 2 diabetes
Contributor Information
Sophie Monnerat, Email: sophie.monnerat@usb.ch, Department of Endocrinology, Diabetology and Metabolism, University Hospital Basel, Basel 4031, Switzerland; Department of Clinical Research, University of Basel, Basel 4031, Switzerland.
Cihan Atila, Department of Endocrinology, Diabetology and Metabolism, University Hospital Basel, Basel 4031, Switzerland; Department of Clinical Research, University of Basel, Basel 4031, Switzerland.
Julie Refardt, Department of Endocrinology, Diabetology and Metabolism, University Hospital Basel, Basel 4031, Switzerland; Department of Clinical Research, University of Basel, Basel 4031, Switzerland.
Mirjam Christ-Crain, Department of Endocrinology, Diabetology and Metabolism, University Hospital Basel, Basel 4031, Switzerland; Department of Clinical Research, University of Basel, Basel 4031, Switzerland.
Funding
S.M. and M.C.C. are supported by a grant from the Swiss National Science Foundation (S.M., SNF-199391; M.C.C., SNF-162608). C.A. received the Young Talents in Clinical Research grant from the Swiss Academy of Medical Sciences and Gottfried und Julia Bangerter-Rhyner-Stiftung.
Author Contributions
S.M. conceived, designed, and performed the analysis and wrote the first draft of the manuscript. C.A. and J.R. reviewed the manuscript. M.C.C. revised the manuscript and supervised all steps of the work.
Disclosures
S.M., C.A., J.R., and M.C.C. have nothing to disclose.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Hawkins RC. Age and gender as risk factors for hyponatremia and hypernatremia. Clin Chim Acta. 2003;337(1-2):169–172. [DOI] [PubMed] [Google Scholar]
- 2. Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009;122(9):857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zilberberg MD, Exuzides A, Spalding J, et al. Epidemiology, clinical and economic outcomes of admission hyponatremia among hospitalized patients. Curr Med Res Opin. 2008;24(6):1601–1608. [DOI] [PubMed] [Google Scholar]
- 4. Wald R. Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med. 2010;170(3):294. [DOI] [PubMed] [Google Scholar]
- 5. Winzeler B, Jeanloz N, Nigro N, et al. Long-term outcome of profound hyponatremia: a prospective 12 months follow-up study. Eur J Endocrinol. 2016;175(6):499–507. [DOI] [PubMed] [Google Scholar]
- 6. Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol. 2014;170(3):G1–47. [DOI] [PubMed] [Google Scholar]
- 7. Hoorn EJ, Spasovski G. Recent developments in the management of acute and chronic hyponatremia. Curr Opin Nephrol Hypertens. 2019;28(5):424–432. [DOI] [PubMed] [Google Scholar]
- 8. Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10):S1–S42. [DOI] [PubMed] [Google Scholar]
- 9. Greenberg A, Verbalis JG, Amin AN, et al. Current treatment practice and outcomes. Report of the hyponatremia registry. Kidney Int. 2015;88(1):167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rondon-Berrios H, Berl T. Physiology and Pathophysiology of Water Homeostasis - Disorders of Fluid and Electrolyte Metabolism. Focus on Hyponatremia. Front Horm Res. Basel Karger, 2019. [DOI] [PubMed] [Google Scholar]
- 11. Garrahy A, Galloway I, Hannon AM, et al. Fluid restriction therapy for chronic SIAD; results of a prospective randomized controlled trial. J Clin Endocrinol Metab. 2020;105(12):e4360–e4369. [DOI] [PubMed] [Google Scholar]
- 12. Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099–2112. [DOI] [PubMed] [Google Scholar]
- 13. Pose-Reino A, Runkle de la Vega I, de Jong-Laird A, Kabra M, Lindner U. Real-World, non-interventional, retrospective study (SAMPLE) of tolvaptan in patients with hyponatraemia secondary to the syndrome of inappropriate antidiuretic hormone secretion. Adv Ther. 2021;38(2):1055–1067. [DOI] [PubMed] [Google Scholar]
- 14. Rozen-Zvi B, Yahav D, Gheorghiade M, Korzets A, Leibovici L, Gafter U. Vasopressin receptor antagonists for the treatment of hyponatremia: systematic review and meta-analysis. Am J Kidney Dis. 2010;56(2):325–337. [DOI] [PubMed] [Google Scholar]
- 15. Jaber BL, Almarzouqi L, Borgi L, Seabra VF, Balk EM, Madias NE. Short-term efficacy and safety of vasopressin receptor antagonists for treatment of hyponatremia. Am J Med. 2011;124(10):977.e1–977.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou Y, Yang W, Liu G, Gao W. Risks of vaptans in hypernatremia and serum sodium overcorrection: a systematic review and meta-analysis of randomised controlled trials. Int J Clin Pract. 2020; 75(6):e13939. [DOI] [PubMed] [Google Scholar]
- 17. Scheen AJ. Pharmacodynamics, efficacy and safety of sodium–glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75(1):33–59. [DOI] [PubMed] [Google Scholar]
- 18. Seman L, Macha S, Nehmiz G, et al. Empagliflozin (BI 10773), a potent and selective SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Drug Dev. 2013;2(2):152–161. [DOI] [PubMed] [Google Scholar]
- 19. Refardt J, Winzeler B, Meienberg F, Vogt DR, Christ-Crain M. Empagliflozin increases short-term urinary volume output in artificially induced syndrome of inappropriate antidiuresis. Int J Endocrinol. 2017;2017(vol. 2017, Article ID 7815690):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. [DOI] [PubMed] [Google Scholar]
- 21. Fitchett D, Inzucchi SE, Cannon CP, et al. Empagliflozin reduced mortality and hospitalization for heart failure across the Spectrum of cardiovascular risk in the EMPA-REG OUTCOME trial. Circulation. 2019;139(11):1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. [DOI] [PubMed] [Google Scholar]
- 23. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–1424. [DOI] [PubMed] [Google Scholar]
- 24. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–1461. [DOI] [PubMed] [Google Scholar]
- 25. Refardt J, Imber C, Sailer CO, et al. A randomized trial of empagliflozin to increase plasma sodium levels in patients with the syndrome of inappropriate antidiuresis. J Am Soc Nephrol. 2020;31(3):615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Refardt J, Imber C, Nobbenhuis R, et al. Treatment effect of the SGLT2 inhibitor empagliflozin on chronic syndrome of inappropriate antidiuresis: results of a randomized, double-blind, placebo-controlled, crossover trial. J Am Soc Nephrol. 2022;34(2). DOI: 10.1681/ASN.2022050623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deutsches Institut für Medizinische Dokumentation und Information (DIMDI) . Internationale statistische Klassifikation der Krankheiten und verwandter Gesundheitsprobleme 10. Revision German Modification Version 2016. Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM). Updated September 25, 2015. 2021. https://www.dimdi.de/static/de/klassifikationen/icd/icd-10-gm/kode-suche/htmlgm2016/zusatz-03-anleitung-zur-verschluesselung.htm [PubMed]
- 28. Deutsches Institut für Medizinische Dokumentation und Information (DIMDI) . Internationale statistische Klassifikation der Krankheiten und verwandter Gesundheitsprobleme 10. Revision German Modification Version 2018. Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM). Updated September 22, 2017. 2021. https://www.dimdi.de/static/de/klassifikationen/icd/icd-10-gm/kode-suche/htmlgm2018/ [PubMed]
- 29. Deutsches Institut für Medizinische Dokumentation und Information (DIMDI) . Internationale statistische Klassifikation der Krankheiten und verwandter Gesundheitsprobleme 10. Revision German Modification Version 2014. Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM). Updated September 20, 2013. https://www.dimdi.de/static/de/klassifikationen/icd/icd-10-gm/kode-suche/htmlgm2014/
- 30. Hillier TA, Abbott RD, Barrett EJ. Hyponatremia: evaluating the correction factor for hyperglycemia. Am J Med. 1999;106(4):399–403. [DOI] [PubMed] [Google Scholar]
- 31. Ho D, Imai K, King G, Stuart EA. Matchit: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42(8):1–28. [Google Scholar]
- 32. Monnerat S, Atila C, Refardt J, Christ-Crain M. Supplementary Material - Prevalence of admission hyponatremia in diabetic patients treated with and without an SGLT2-inhibitor - a cross-sectional study. J Endocr Soc. (Version 1). Zenodo.2023. DOI: 10.5281/zenodo.7612286 [DOI] [PMC free article] [PubMed]
- 33. Venables WN, Ripley BD. Modern Applied Statistics with S. 4th ed. Springer, 2002. [Google Scholar]
- 34. Falhammar H, Skov J, Calissendorff J, Lindh JD, Mannheimer B. Inverse association between glucose-lowering medications and severe hyponatremia: a Swedish population-based case-control study. Endocrine. 2020;67(3):579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cuesta M, Garrahy A, Slattery D, et al. Mortality rates are lower in SIAD, than in hypervolaemic or hypovolaemic hyponatraemia: results of a prospective observational study. Clin Endocrinol (Oxf). 2017;87(4):400–406. [DOI] [PubMed] [Google Scholar]
- 36. Rong X, Li X, Gou Q, Liu K, Chen X. Risk of orthostatic hypotension associated with sodium-glucose cotransporter-2 inhibitor treatment: a meta-analysis of randomized controlled trials. Diab Vasc Dis Res. 2020;17(5):1479164120953625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vardeny O, Vaduganathan M. Practical guide to prescribing sodium-glucose cotransporter 2 inhibitors for cardiologists. JACC Heart Fail. 2019;7(2):169–172. [DOI] [PubMed] [Google Scholar]
- 38. Ansary TM, Nakano D, Nishiyama A. Diuretic effects of sodium glucose cotransporter 2 inhibitors and their influence on the renin-angiotensin system. Int J Mol Sci. 2019;20(3):629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rondon-Berrios H, Agaba EI, Tzamaloukas AH. Hyponatremia: pathophysiology, classification, manifestations and management. Int Urol Nephrol. 2014;46(11):2153–2165. [DOI] [PubMed] [Google Scholar]
- 40. Tanaka H, Takano K, Iijima H, et al. Factors affecting canagliflozin-induced transient urine volume increase in patients with type 2 diabetes Mellitus. Adv Ther. 2017;34(2):436–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. [DOI] [PubMed] [Google Scholar]
- 42. The EMPA-KIDNEY Collaborative Group . Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2022;388(2):117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(7):613–621. [DOI] [PubMed] [Google Scholar]
- 44. Schork A, Saynisch J, Vosseler A, et al. Effect of SGLT2 inhibitors on body composition, fluid status and renin–angiotensin–aldosterone system in type 2 diabetes: a prospective study using bioimpedance spectroscopy. Cardiovasc Diabetol. 2019;18(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jensen J, Omar M, Kistorp C, et al. Effects of empagliflozin on estimated extracellular volume, estimated plasma volume, and measured glomerular filtration rate in patients with heart failure (Empire HF Renal): a prespecified substudy of a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2020;9(2):106–116. [DOI] [PubMed] [Google Scholar]
- 46. Yeoh SE, Docherty KF, Jhund PS, et al. Relationship of dapagliflozin with serum sodium: findings from the DAPA-HF trial. JACC Heart Fail. 2022;10(5):306–318. [DOI] [PubMed] [Google Scholar]
- 47. Montalvo-Gordon I, Chi-Cervera LA, García-Tsao G. Sodium-glucose cotransporter 2 inhibitors ameliorate ascites and peripheral edema in patients with cirrhosis and diabetes. Hepatology. 2020;72(5):1880–1882. [DOI] [PubMed] [Google Scholar]
- 48. Hulley SB. Designing Clinical Research. Lippincott Williams & Wilkins, 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.