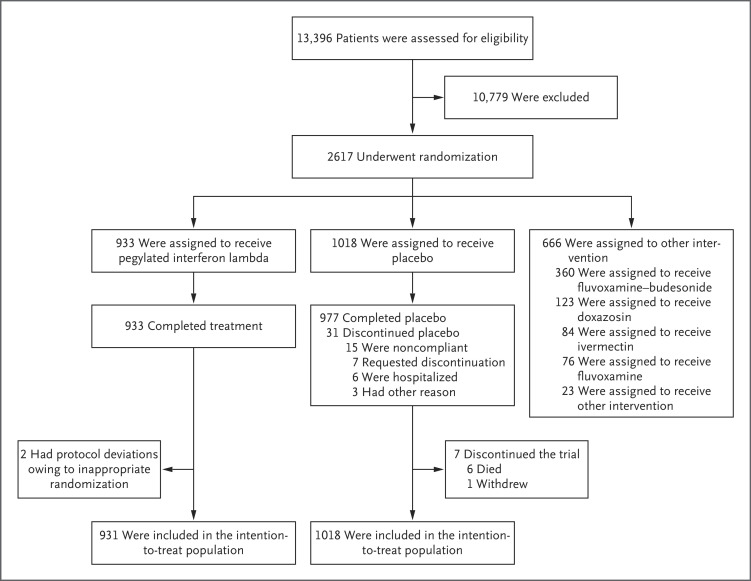
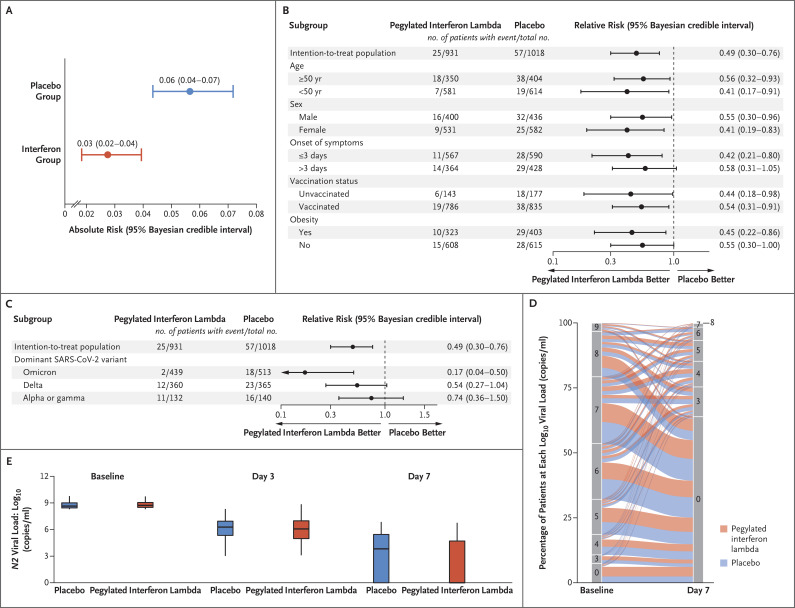
Gilmar Reis
Gilmar Reis, M.D., Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Eduardo AS Moreira Silva
Eduardo AS Moreira Silva, M.D., Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Daniela C Medeiros Silva
Daniela C Medeiros Silva, M.D., Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Lehana Thabane
Lehana Thabane, Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Vitoria HS Campos
Vitoria HS Campos
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Thiago S Ferreira
Thiago S Ferreira, M.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Castilho VQ Santos
Castilho VQ Santos
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Ana MR Nogueira
Ana MR Nogueira, M.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Ana PFG Almeida
Ana PFG Almeida, M.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Leonardo CM Savassi
Leonardo CM Savassi, M.D., Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Adhemar D Figueiredo-Neto
Adhemar D Figueiredo-Neto, M.D., Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Ana CF Dias
Ana CF Dias, Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Adelino M Freire Júnior
Adelino M Freire Júnior, M.D., Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Carina Bitarães
Carina Bitarães, R.N.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Aline C Milagres
Aline C Milagres, R.N.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Eduardo D Callegari
Eduardo D Callegari, M.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Maria IC Simplicio
Maria IC Simplicio, B.Sc.Pharm.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Luciene B Ribeiro
Luciene B Ribeiro, R.N., M.P.H.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Rosemary Oliveira
Rosemary Oliveira
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Ofir Harari
Ofir Harari, Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Lindsay A Wilson
Lindsay A Wilson, M.Sc.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Jamie I Forrest
Jamie I Forrest, Ph.D., M.P.H.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Hinda Ruton
Hinda Ruton, M.Sc.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Sheila Sprague
Sheila Sprague, Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Paula McKay
Paula McKay, M.Sc.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Christina M Guo
Christina M Guo, B.Com.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Eve H Limbrick-Oldfield
Eve H Limbrick-Oldfield, Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Steve Kanters
Steve Kanters, Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Gordon H Guyatt
Gordon H Guyatt, M.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Craig R Rayner
Craig R Rayner, Pharm.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Christopher Kandel
Christopher Kandel, M.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Mia J Biondi
Mia J Biondi, Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Robert Kozak
Robert Kozak, Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Bettina Hansen
Bettina Hansen, Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
M Atif Zahoor
M Atif Zahoor, Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Paul Arora
Paul Arora, Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Colin Hislop
Colin Hislop, M.B., B.S.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Ingrid Choong
Ingrid Choong, Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Jordan J Feld
Jordan J Feld, M.D., M.P.H.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Edward J Mills
Edward J Mills, Ph.D., F.R.C.P.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,
Jeffrey S Glenn
Jeffrey S Glenn, M.D., Ph.D.
1From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., T.S.F., C.V.Q.S., M.I.C.S., L.B.R., R.O.), the Department of Medicine, Pontifícia Universidade Católica de Minas Gerais (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C., C.V.Q.S.), and Target Medicina de Precisão (A.C.F.D., A.M.F.J.), Belo Horizonte, the Department of Public Health and Mental and Family Medicine, Ouro Preto Federal University, Ouro Preto (L.C.M.S., C.B., A.C.M.), the Public Health Care Division, Ibirité (C.B., A.C.M.), the Department of Public Health at UNIFIPMoc and Family Medicine Fellowship Program, Montes Claros (A.M.R.N., A.P.F.G.A.), the Public Health Fellowship Program, Governador Valadares Public Health Authority, Governador Valadares (A.D.F.-N.), and the Public Health Care Division, Brumadinho (E.D.C., B.H.) — all in Brazil; the Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON (G.R., L.T., S.S., P.M., G.H.G., E.J.M.), Cytel (O.H., H.R., P.A., E.J.M.), Platform Life Sciences (L.A.W., J.I.F., C.M.G., E.J.M.), and RainCity Analytics (E.H.L.-O., S.K.), Vancouver, BC, Michael Garron Hospital (C.K.) and the Toronto Centre for Liver Disease, University Health Network (M.A.Z., J.J.F.), University of Toronto, the School of Nursing, York University (M.J.B.), and Sunnybrook Health Sciences Centre (R.K.), Toronto — all in Canada; Certara, Princeton, NJ (C.R.R.); Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, VIC, Australia (C.R.R.); and Erasmus University Rotterdam, Rotterdam, the Netherlands (B.H.).
1,✉,
the TOGETHER Investigators