Abstract
Background
The emergence of immune-escape variants of severe acute respiratory syndrome coronavirus 2 warrants the use of sequence-adapted vaccines to provide protection against coronavirus disease 2019.
Methods
In an ongoing phase 3 trial, adults older than 55 years who had previously received three 30-μg doses of the BNT162b2 vaccine were randomly assigned to receive 30 μg or 60 μg of BNT162b2, 30 μg or 60 μg of monovalent B.1.1.529 (omicron) BA.1–adapted BNT162b2 (monovalent BA.1), or 30 μg (15 μg of BNT162b2+15 μg of monovalent BA.1) or 60 μg (30 μg of BNT162b2+30 μg of monovalent BA.1) of BA.1–adapted BNT162b2 (bivalent BA.1). Primary objectives were to determine superiority (with respect to 50% neutralizing titer [NT50] against BA.1) and noninferiority (with respect to seroresponse) of the BA.1-adapted vaccines to BNT162b2 (30 μg). A secondary objective was to determine noninferiority of bivalent BA.1 to BNT162b2 (30 μg) with respect to neutralizing activity against the ancestral strain. Exploratory analyses assessed immune responses against omicron BA.4, BA.5, and BA.2.75 subvariants.
Results
A total of 1846 participants underwent randomization. At 1 month after vaccination, bivalent BA.1 (30 μg and 60 μg) and monovalent BA.1 (60 μg) showed neutralizing activity against BA.1 superior to that of BNT162b2 (30 μg), with NT50 geometric mean ratios (GMRs) of 1.56 (95% confidence interval [CI], 1.17 to 2.08), 1.97 (95% CI, 1.45 to 2.68), and 3.15 (95% CI, 2.38 to 4.16), respectively. Bivalent BA.1 (both doses) and monovalent BA.1 (60 μg) were also noninferior to BNT162b2 (30 μg) with respect to seroresponse against BA.1; between-group differences ranged from 10.9 to 29.1 percentage points. Bivalent BA.1 (either dose) was noninferior to BNT162b2 (30 μg) with respect to neutralizing activity against the ancestral strain, with NT50 GMRs of 0.99 (95% CI, 0.82 to 1.20) and 1.30 (95% CI, 1.07 to 1.58), respectively. BA.4–BA.5 and BA.2.75 neutralizing titers were numerically higher with 30-μg bivalent BA.1 than with 30-μg BNT162b2. The safety profile of either dose of monovalent or bivalent BA.1 was similar to that of BNT162b2 (30 μg). Adverse events were more common in the 30-μg monovalent-BA.1 (8.5%) and 60-μg bivalent-BA.1 (10.4%) groups than in the other groups (3.6 to 6.6%).
Conclusions
The candidate monovalent or bivalent omicron BA.1–adapted vaccines had a safety profile similar to that of BNT162b2 (30 μg), induced substantial neutralizing responses against ancestral and omicron BA.1 strains, and, to a lesser extent, neutralized BA.4, BA.5, and BA.2.75 strains. (Funded by BioNTech and Pfizer; ClinicalTrials.gov number, NCT04955626.)
Vaccination remains a critical mitigation tool in the ongoing coronavirus disease 2019 (Covid-19) pandemic. The 30-μg dose of the BNT162b2 messenger RNA (mRNA) vaccine encoding the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein is licensed as a two-dose primary series for persons 12 years of age or older; a phase 2–3 trial conducted early on in the pandemic showed an efficacy of 95 to 100% with this primary series.1-3
BNT162b2 has provided broad protection across previously dominant SARS-CoV-2 variants of concern, which had relatively low potential to escape vaccine-elicited immunity.4-7 Conversely, effectiveness against mild disease caused by the more antigenically distinct B.1.1.529 (omicron) variant has been markedly decreased.8-10 Although immune escape could be mitigated by a third or fourth vaccine dose, effectiveness against the omicron BA.1 subvariant, including effectiveness in preventing severe disease, waned faster than that observed for previous variants of concern.9-13 Because it was desirable to engineer a sequence-adapted vaccine matching the circulating variant of concern, an approach supported by regulatory agencies,14,15 this trial sought to evaluate boosting strategies with different omicron BA.1–adapted BNT162b2 vaccines and dose levels. During the conduct of the trial and because of the high transmissibility of omicron BA.1, further mutations of omicron BA.1 led to the emergence and subsequent dominance of the omicron BA.4 and BA.5 subvariants and the recently identified omicron BA.2.75 subvariant, which are comparatively even more antigenically distinct from the previous SARS-CoV-2 variants of concern than the BA.1 subvariant.16-18 Therefore, we also evaluated the ability of monovalent and bivalent omicron BA.1–adapted BNT162b2 vaccines to neutralize BA.4 and BA.5, which encode the same spike sequence,17 and BA.2.75.
Methods
Objectives, Participants, and Oversight
In this ongoing phase 3 randomized trial assessing BNT162b2 booster doses,19 which was conducted at 36 sites in the United States, we evaluated the safety profile and immunogenicity of the following vaccine formulations and dose levels: 30 μg (the original dose) and 60 μg of BNT162b2; 30 μg and 60 μg of a monovalent omicron BA.1–adapted BNT162b2 vaccine (monovalent BA.1); and 30 μg and 60 μg of a bivalent omicron BA.1–adapted BNT162b2 vaccine (30-μg bivalent BA.1 [15 μg of BNT162b2+15 μg of monovalent BA.1] or 60-μg bivalent BA.1 [30 μg of BNT162b2+30 μg of monovalent BA.1]). BNT162b2 is a lipid nanoparticle–formulated vaccine containing nucleoside-modified mRNA (with noncoding elements enhanced for translational performance) that encodes the conformationally stabilized spike glycoprotein of the SARS-CoV-2 ancestral strain USA-WA1/202020; in the monovalent BA.1 vaccine, the coding sequence is substituted by the omicron BA.1 spike glycoprotein sequence. The bivalent vaccine contains equal amounts of ancestral spike mRNA and omicron BA.1 spike mRNA coformulated into lipid nanoparticles.
The participants were older than 55 years of age and had previously received three 30-μg doses of BNT162b2, with the last dose administered 5 to 12 months before randomization. Adults 18 to 55 years of age were enrolled separately, and the results among these participants are not reported here. Further details regarding eligibility criteria, the ethical conduct of the trial, and blinding of the vaccine-group assignments are provided in the Supplementary Appendix and trial protocol, both available with the full text of this article at NEJM.org.
Representatives of the financial sponsor (Pfizer) were responsible for the conduct of the trial; the collection, analysis, and interpretation of the data; and the writing and review of the manuscript. Pfizer and BioNTech were responsible for the design of the trial and for the manufacture of the vaccine. BioNTech was the regulatory sponsor and the company representatives contributed to the interpretation of the data and the writing and review of the manuscript. All data were available to the authors, who vouch for the accuracy and completeness of the data and for the adherence of the trial to the protocol. All the authors approved the final version of the manuscript for submission.
Procedures
With the use of interactive response technology, participants were randomly assigned in a 1:1:1:1:1:1 ratio to receive the original 30-μg dose or a 60-μg dose of BNT162b2, a 30-μg or 60-μg dose of monovalent BA.1, or a 30-μg or 60-μg dose of bivalent BA.1. Twenty participants per group were initially enrolled (sentinel cohort). An additional 300 participants per group (expanded cohort) were scheduled to be enrolled after an independent review committee had confirmed the acceptable safety profile of the vaccines from the data collected from the sentinel cohort data through day 7 after vaccination. The results of the analyses in the sentinel cohort are provided in the Supplementary Appendix.
Immunogenicity
Primary and secondary immunogenicity analyses of the vaccines against omicron subvariant BA.121-23 and the ancestral strain were based on 50% neutralizing titers (the interpolated reciprocal of the dilutions yielding 50% in fluorescent viral foci) against SARS-CoV-2 that were measured with the use of a validated recombinant SARS-CoV-2 neutralization assay in a 384-well format before and 1 month after administration of the vaccine. The results were reported as geometric mean titers (GMTs), geometric mean ratios (GMRs; calculated by exponentiating the mean of the difference of logarithmically transformed results), geometric mean fold rise, and percentages and differences in percentages of participants with a seroresponse (defined as an increase by a factor of ≥4 from baseline [before injection on the day of the fourth dose] in 50% neutralizing titer against SARS-CoV-2). Additional details regarding the calculations are provided in the Supplementary Appendix.
Immunogenicity objectives were in accordance with guidance from the Food and Drug Administration (FDA) for emergency use authorization of adapted Covid-19 vaccines.14 The primary immunogenicity objectives were to evaluate superiority (with respect to the 50% neutralizing titers against SARS-CoV-2) and noninferiority (with respect to seroresponse) of the immune response against omicron BA.1 induced by monovalent BA.1 or bivalent BA.1 (given as a fourth dose at a level of either 30 μg or 60 μg) to that induced by a fourth dose of 30 μg of BNT162b2 (the original dose). Secondary immunogenicity objectives were to evaluate noninferiority of 30-μg or 60-μg bivalent BA.1 to 30-μg BNT162b2 with respect to the 50% neutralizing titers against the ancestral strain.
An exploratory objective was to describe the immune response to emerging variants of concern. Because omicron BA.4, BA.5, and BA.2.75 subvariants have several unique mutations,16,17 it was important to characterize the immune response induced by 30-μg bivalent BA.1 against these omicron strains, as compared with the 30-μg dose of BNT162b2. A fluorescent focus reduction neutralization test was used to determine neutralizing titers against omicron BA.4, BA.5, and BA.2.75 subvariants.22-25
Safety
Reactogenicity events and antipyretic use were recorded by the participants in electronic diaries for 7 days after vaccination. Data on adverse events occurring within 1 month after vaccination were collected. Data on serious adverse events occurring within 6 months after vaccination are being collected. Potential events of myocarditis or pericarditis are being monitored as adverse events of special interest.
Covid-19 Surveillance
Potential Covid-19 cases are being monitored as an exploratory end point (see the Supplementary Appendix). In the current analysis, the results for these events are reported up to the data-cutoff date (May 16, 2022).
Statistical Analysis
We determined the sample size in consideration of providing adequate safety data for a variant-adapted Covid-19 vaccine. Primary and secondary immunogenicity objectives were evaluated in a planned random sample of 230 participants selected from each group in the expanded cohort. Sample-size considerations for the immunogenicity analyses are described in the Supplementary Appendix, and the trial populations are defined in Table S1 in the Supplementary Appendix. The primary immunogenicity analysis included participants without serologic or virologic evidence of previous SARS-CoV-2 infection (i.e., no medical history of Covid-19 or no positive nucleic acid amplification tests for SARS-CoV-2 nucleoprotein−binding antibody) up to 1 month after vaccination.
According to FDA guidance, “simple” superiority of immune response with the 30-μg or 60-μg dose of monovalent BA.1 or bivalent BA.1 over the 30-μg dose of BNT162b2 with respect to the 50% neutralizing titers against omicron BA.1 was declared if the lower limit of the two-sided 95% confidence interval of the GMR was greater than 1 after adjustment for multiplicity; “super” superiority was declared if the corresponding lower limit was greater than 1.5 after adjustment for multiplicity (details on the prespecified sequential hypothesis testing are provided in the Supplementary Appendix).14 Noninferiority of 30-μg or 60-μg monovalent BA.1 or bivalent BA.1 to the 30-μg dose of BNT162b2 with respect to seroresponse against omicron BA.1 was declared if the lower limit of the two-sided 95% confidence interval for the difference in the percentage of participants with a seroresponse exceeded –5 percentage points after adjustment for multiplicity. Noninferiority of immune response with respect to the 50% neutralizing titers against the ancestral strain was declared if the lower limit of the two-sided 95% confidence interval of the GMR was greater than 0.67 and the point estimate of the GMR was 0.8 or greater after adjustment for multiplicity.
Immunogenicity data regarding omicron BA.4, BA.5, and BA.2.75 subvariants are presented descriptively. Safety end points are presented descriptively as counts, percentages, and associated Clopper−Pearson two-sided 95% confidence intervals. Adverse events are categorized according to preferred terms in the Medical Dictionary for Regulatory Activities (version 25.0).
Results
Participants
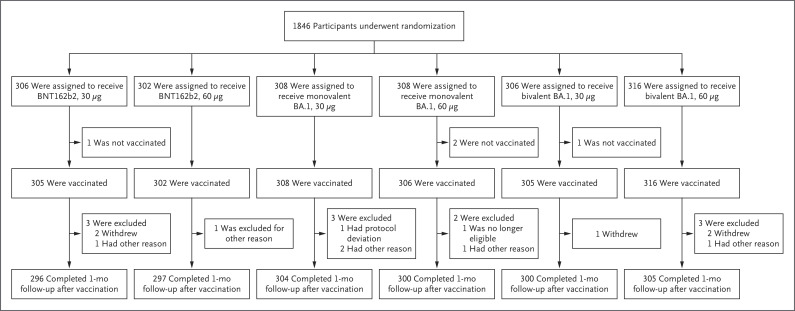
From March 21 to April 15, 2022, a total of 1846 expanded-cohort participants underwent randomization (Figure 1). The demographic characteristics of the participants were well-balanced across the treatment groups (Table 1), and the participants were generally representative of the expected population with respect to age, sex, race, and ethnic group (Table S2). The median age of the participants was 67 years, and 49.5% were male, 86.6% White, 6.3% Black, 5.5% Asian, and 14.9% Hispanic or Latinx. More than one third of the participants had obesity, and 12.6% had serologic or virologic evidence of previous SARS-CoV-2 infection. The median interval between the third and fourth dose was 6.3 months.
Figure 1. Randomization and Follow-up.
Shown is the randomization and follow-up of participants in the expanded cohort as of the database cutoff date of May 16, 2022. Adults older than 55 years of age who had previously received three 30-μg doses of the BNT162b2 vaccine were randomly assigned in a 1:1:1:1:1:1 ratio to receive the original 30-μg dose or a 60-μg dose of BNT162b2, a 30-μg or 60-μg dose of monovalent B.1.1.529 (omicron) BA.1–adapted BNT162b2 vaccine (monovalent BA.1), or bivalent combinations thereof (30-μg bivalent BA.1 [15 μg of BNT162b2+15 μg of monovalent BA.1] or 60-μg bivalent BA.1 [30 μg of BNT162b2+30 μg of monovalent BA.1]). At the data-cutoff date, some participants had not reached the 1-month postvaccination visit. Participants were recruited from 36 sites in the United States. Randomization and follow-up in the sentinel cohort are shown in Figure S1 in the Supplementary Appendix.
Table 1. Demographic and Clinical Characteristics of the Participants at Baseline (before Trial Vaccination).*.
| Characteristic | BNT162b2, 30 μg (N=305) |
BNT162b2, 60 μg (N=302) |
Monovalent BA.1, 30 μg (N=307) |
Monovalent BA.1, 60 μg (N=306) |
Bivalent BA.1, 30 μg (N=305) |
Bivalent BA.1, 60 μg (N=316) |
Total (N=1841) |
|---|---|---|---|---|---|---|---|
| Median age (range) — yr | 66.0 (56 to 87) | 67.0 (56 to 86) | 67.0 (56 to 84) | 67.0 (56 to 87) | 67.0 (56 to 85) | 67.0 (56 to 87) | 67.0 (56 to 87) |
| Male sex — no. (%) | 145 (47.5) | 145 (48.0) | 154 (50.2) | 153 (50.0) | 162 (53.1) | 153 (48.4) | 912 (49.5) |
| Race — no. (%)† | |||||||
| White | 268 (87.9) | 254 (84.1) | 261 (85.0) | 262 (85.6) | 274 (89.8) | 275 (87.0) | 1594 (86.6) |
| Black | 19 (6.2) | 22 (7.3) | 23 (7.5) | 20 (6.5) | 13 (4.3) | 19 (6.0) | 116 (6.3) |
| Asian | 13 (4.3) | 20 (6.6) | 16 (5.2) | 19 (6.2) | 16 (5.2) | 17 (5.4) | 101 (5.5) |
| Other‡ | 5 (1.6) | 6 (2.0) | 7 (2.3) | 5 (1.6) | 2 (0.7) | 5 (1.6) | 30 (1.6) |
| Ethnic group — no. (%)† | |||||||
| Hispanic or Latinx | 57 (18.7) | 38 (12.6) | 44 (14.3) | 46 (15.0) | 45 (14.8) | 44 (13.9) | 274 (14.9) |
| Not Hispanic or Latinx | 248 (81.3) | 264 (87.4) | 263 (85.7) | 260 (85.0) | 260 (85.2) | 272 (86.1) | 1567 (85.1) |
| SARS-CoV-2 status — no. (%) | |||||||
| Positive§ | 41 (13.4) | 28 (9.3) | 45 (14.7) | 41 (13.4) | 38 (12.5) | 39 (12.3) | 232 (12.6) |
| Negative¶ | 262 (85.9) | 274 (90.7) | 261 (85.0) | 265 (86.6) | 267 (87.5) | 277 (87.7) | 1606 (87.2) |
| Missing data | 2 (0.7) | 0 | 1 (0.3) | 0 | 0 | 0 | 3 (0.2) |
| Interval between third dose and trial vaccination — mo | |||||||
| Mean | 6.8±1.44 | 6.8±1.42 | 6.8±1.37 | 6.9±1.49 | 6.8±1.39 | 6.9±1.45 | 6.8±1.43 |
| Median (range) | 6.3 (5.3 to 13.1) | 6.3 (5.3 to 12.9) | 6.3 (5.1 to 11.4) | 6.3 (5.4 to 12.8) | 6.3 (4.7 to 11.5) | 6.3 (5.3 to 11.2) | 6.3 (4.7 to 13.1) |
| Distribution — no. (%) | |||||||
| <5 | 0 | 0 | 0 | 0 | 1 (0.3) | 0 | 1 (<0.1) |
| 5 to <7 | 234 (76.7) | 235 (77.8) | 233 (75.9) | 223 (72.9) | 230 (75.4) | 229 (72.5) | 1384 (75.2) |
| 7 to <9 | 40 (13.1) | 39 (12.9) | 47 (15.3) | 48 (15.7) | 43 (14.1) | 51 (16.1) | 268 (14.6) |
| 9 to <11 | 28 (9.2) | 22 (7.3) | 23 (7.5) | 31 (10.1) | 26 (8.5) | 30 (9.5) | 160 (8.7) |
| ≥11 | 3 (1.0) | 6 (2.0) | 4 (1.3) | 4 (1.3) | 5 (1.6) | 6 (1.9) | 28 (1.5) |
| Body-mass index — no. (%)‖ | |||||||
| <18.5 | 4 (1.3) | 4 (1.3) | 0 | 3 (1.0) | 1 (0.3) | 3 (0.9) | 15 (0.8) |
| ≥18.5 to 24.9 | 85 (27.9) | 91 (30.1) | 79 (25.7) | 78 (25.5) | 71 (23.3) | 84 (26.6) | 488 (26.5) |
| ≥25.0 to 29.9 | 108 (35.4) | 90 (29.8) | 120 (39.1) | 121 (39.5) | 129 (42.3) | 113 (35.8) | 681 (37.0) |
| ≥30.0 | 108 (35.4) | 117 (38.7) | 108 (35.2) | 104 (34.0) | 104 (34.1) | 115 (36.4) | 656 (35.6) |
| Missing data | 0 | 0 | 0 | 0 | 0 | 1 (0.3) | 1 (<0.1) |
Plus–minus values are means ±SD. Data are for the safety population (all participants who had undergone randomization and received a trial vaccine). Demographic characteristics of the sentinel cohort are shown in Table S3. The participants were randomly assigned to receive the original 30-μg dose or a 60-μg dose of BNT162b2, a 30-μg or 60-μg dose of monovalent B.1.1.529 (omicron) BA.1–adapted BNT162b2 vaccine (monovalent BA.1), or bivalent combinations thereof (30-μg bivalent BA.1 [15 μg of BNT162b2+15 μg of monovalent BA.1] or 60-μg bivalent BA.1 [30 μg of BNT162b2+30-μg of monovalent BA.1]).
Race and ethnic group were reported by the participant.
“Other” includes the following subgroups: multiracial (21 participants [1.1%]); Native Hawaiian or Pacific Islander (3 participants [0.2%]); American Indian or Alaska Native (3 participants [0.2%]); and not reported (3 participants [0.2%]).
Positive status was defined as a positive SARS-CoV-2 nucleoprotein–binding (N-binding) antibody test at baseline, a positive nucleic acid amplification test (NAAT) at baseline, or a medical history of Covid-19.
Negative status was defined as a negative N-binding antibody test at baseline, a negative NAAT at baseline, and no medical history of Covid-19.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Immunogenicity
Omicron BA.1 Strain
Among 230 participants without evidence of previous SARS-CoV-2 infection (up to 1 month after vaccination) who were included in the immunogenicity analysis, the GMRs of 50% neutralizing titers against omicron BA.1 in the 30-μg and 60-μg monovalent-BA.1 groups, as compared with the 30-μg BNT162b2 group, were 2.23 (95% confidence interval [CI], 1.65 to 3.00) and 3.15 (95% CI, 2.38 to 4.16), respectively, 1 month after vaccination (Table 2); the corresponding values in the 30-μg and 60-μg bivalent-BA.1 groups were 1.56 (95% CI, 1.17 to 2.08) and 1.97 (95% CI, 1.45 to 2.68). The 60-μg dose of monovalent BA.1 and the 30-μg and 60-μg doses of bivalent BA.1 thus showed simple superiority to the 30-μg dose of BNT162b2; the 60-μg dose of monovalent BA.1 also showed “super” superiority according to the FDA requirements for emergency use authorization of adapted Covid-19 vaccines.14 The lack of “super” superiority of the bivalent vaccines precluded formal hypothesis testing for the 30-μg dose of monovalent BA.1 owing to prespecified sequential hypothesis testing; however, the lower limit of the 95% confidence interval of the GMR in this group was consistent with the “super” superiority criterion.
Table 2. Geometric Mean Ratios and Seroresponse against Omicron BA.1 and Ancestral USA-WA1/2020 Strains.*.
| End Point | BNT162b2, 30 μg | BNT162b2, 60 μg | Monovalent BA.1, 30 μg | Monovalent BA.1, 60 μg | Bivalent BA.1, 30 μg | Bivalent BA.1, 60 μg |
|---|---|---|---|---|---|---|
| GMR against omicron BA.1 | ||||||
| Participants — no.† | 163 | NE | 169 | 174 | 178 | 175 |
| GMR vs. 30-μg BNT162b2 (95% CI)‡ | Reference | NE | 2.23 (1.65–3.00) | 3.15 (2.38–4.16) | 1.56 (1.17–2.08) | 1.97 (1.45–2.68) |
| Seroresponse against omicron BA.1 | ||||||
| Participants — no.§ | 149 | 175 | 163 | 166 | 169 | 162 |
| Participants with seroresponse¶ | ||||||
| Number | 85 | 108 | 125 | 143 | 121 | 110 |
| Percent (95% CI) | 57.0 (48.7–65.1) | 61.7 (54.1–68.9) | 76.7 (69.4–82.9) | 86.1 (79.9–91.0) | 71.6 (64.2–78.3) | 67.9 (60.1–75.0) |
| Difference vs. 30-μg BNT162b2 — percentage points (95% CI) | Reference | NE | 19.6 (9.3–29.7) | 29.1 (19.4–38.5) | 14.6 (4.0–24.9) | 10.9 (0.1–21.4) |
| GMR against ancestral strain | ||||||
| Participants — no.‡ | 182 | NE | NE | NE | 186 | 180 |
| GMR vs. 30-μg BNT162b2 (95% CI)‡ | Reference | NE | NE | NE | 0.99 (0.82–1.20) | 1.30 (1.07–1.58) |
| Seroresponse against ancestral strain | ||||||
| Participants — no.§ | 179 | 197 | 176 | 181 | 186 | 178 |
| Participants with seroresponse¶ | ||||||
| Number | 88 | 115 | 98 | 104 | 93 | 110 |
| Percent (95% CI) | 49.2 (41.6–56.7) | 58.4 (51.2–65.3) | 55.7 (48.0–63.2) | 57.5 (49.9–64.8) | 50.0 (42.6–57.4) | 61.8 (54.2–69.0) |
Immunogenicity analyses were performed in the immunogenicity subset of 230 participants who had been randomly selected from each treatment group in the expanded cohort to evaluate primary and secondary immunogenicity objectives, and the results are shown for those who had immunogenicity data that could be evaluated. Participants had no serologic or virologic evidence (before the 1-month postvaccination blood sample collection) of previous SARS-CoV-2 infection (i.e., a negative SARS-CoV-2 nucleoprotein–binding antibody test at the trial vaccination visit and the 1-month postvaccination visit and a negative nucleic acid amplification test at the trial vaccination visit and any unscheduled visit before the 1-month postvaccination blood sample collection) and no medical history of Covid-19. GMR denotes geometric mean ratio, and NE not evaluated.
The participants who were included in the analysis had valid and determinate assay results at 1 month after vaccination.
The GMRs for the 50% neutralizing titers and two-sided 95% confidence intervals were calculated by exponentiating the mean difference of the logarithms of the titers (the value in the candidate vaccine group minus the value in the 30-μg BNT162b2 group) and the corresponding 95% confidence interval on the basis of the Student’s t distribution.
The participants who were included in the analysis had valid and determinate assay results at both baseline and 1 month after vaccination.
Seroresponse was defined as an increase from baseline in the 50% neutralizing titer against SARS-CoV-2 by a factor of at least 4. If the baseline measurement was below the lower limit of quantitation (LLOQ), then seroresponse was defined as a postvaccination measure of ≥4×LLOQ. The 95% confidence intervals for the percentage of participants with a seroresponse were calculated by means of the Clopper–Pearson method, whereas the 95% confidence intervals for difference in the percentage of participants with a seroresponse were calculated by means of the Miettinen–Nurminen method.
Among the participants without evidence of previous SARS-CoV-2 infection, the percentage of those with a seroresponse against omicron BA.1 in the 30-μg and 60-μg monovalent-BA.1 groups and the 30-μg and 60-μg bivalent-BA.1 groups ranged from 67.9 to 86.1%, and the differences in these percentages from the percentage of participants with a seroresponse in the 30-μg BNT162b2 group ranged from 10.9 to 29.1 percentage points (Table 2). Noninferiority according to the FDA requirements14 was shown for the 60-μg dose of monovalent BA.1 and the 30-μg and 60-μg doses of bivalent BA.1. The result for the 30-μg dose of monovalent BA.1, although not formally evaluated owing to the hierarchical plan to adjust for multiplicity, was also consistent with the noninferiority criterion.
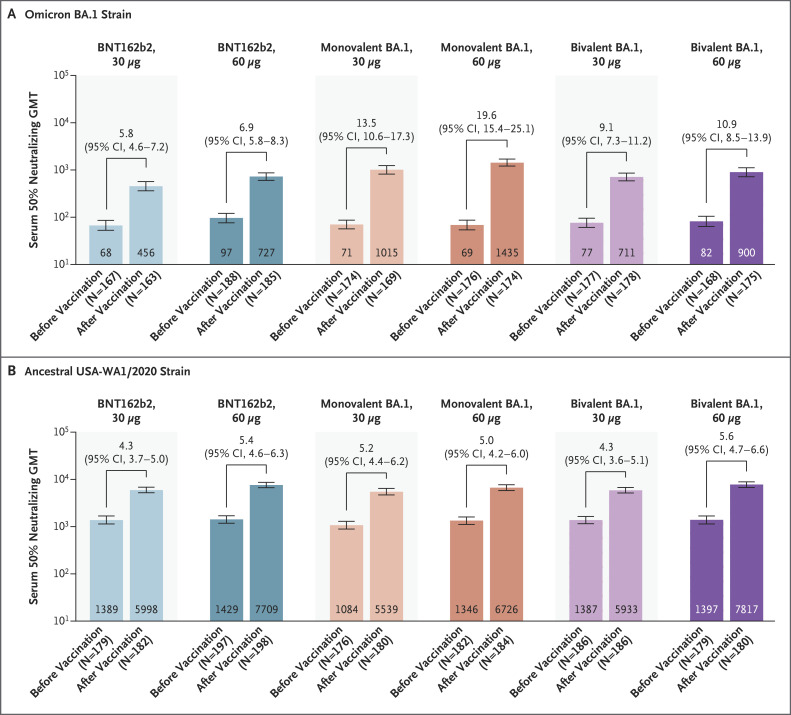
The GMTs of neutralizing antibodies against the omicron BA.1 strain had increased substantially from baseline to 1 month after vaccination among the participants who had no evidence of previous SARS-CoV-2 infection in all six treatment groups (Figure 2). Responses were highest in the 30-μg and 60-μg monovalent-BA.1 groups and the 30-μg and 60-μg bivalent-BA.1 groups, with geometric mean fold rises of 13.5, 19.6, 9.1, and 10.9, respectively. Among the participants regardless of SARS-CoV-2 infection, the pattern of the findings regarding omicron BA.1 neutralizing antibody response across the treatment groups was similar to that observed among the participants who had no evidence of previous SARS-CoV-2 infection, although titers were generally higher among those with previous SARS-CoV-2 infection than among those without previous infection (Table S4 and Fig. S2A).
Figure 2. SARS-CoV-2 Neutralization Assay Results at 1 Month after Vaccination among Participants without Evidence of Previous SARS-CoV-2 Infection.
Shown are the geometric mean titers (GMTs) of neutralizing antibodies against the omicron BA.1 strain (Panel A) and the ancestral USA-WA1/2020 strain (Panel B) before and 1 month after vaccination and the associated geometric mean fold rises from baseline. The analyses were performed in the immunogenicity subset of 230 participants who had been randomly selected from each treatment group in the expanded cohort to evaluate primary and secondary immunogenicity objectives, and the results are shown for those who had immunogenicity data that could be evaluated (according to the definitions in Table S1). Before the 1-month postvaccination blood sample collection, participants had no serologic or virologic evidence of previous severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (i.e., a negative SARS-CoV-2 nucleoprotein–binding antibody test at the trial vaccination visit and the 1-month postvaccination visit and a negative nucleic acid amplification test at the trial vaccination visit and any unscheduled visit before the 1-month postvaccination blood sample collection) and no medical history of Covid-19. The GMTs are shown within the bars (𝙸 bars indicate the 95% confidence interval), and the geometric mean fold rises from before to 1 month after vaccination are shown above the bars. The GMTs, geometric mean fold rises, and associated 95% confidence intervals were calculated by exponentiating the mean logarithm of the 50% neutralizing titers or fold rises and the corresponding 95% confidence intervals on the basis of the Student’s t-test distribution; assay results below the lower limit of quantitation (LLOQ) were imputed as 0.5×LLOQ (the LLOQ is 32 for the neutralizing titer against BA.1 and 87 for the neutralizing titer against the ancestral strain). The 30-μg dose of BNT162b2 was the original dose the participants had received at three previous vaccinations. The 30-μg dose of bivalent BA.1 contained 15 μg of BNT162b2plus15 μg of monovalent BA.1, and the 60-μg dose contained 30 μg of BNT162b2 plus 30 μg of monovalent BA.1.
Ancestral Strain
Among the participants without evidence of previous SARS-CoV-2 infection, GMRs of the 50% neutralizing titers against the ancestral strain in the 30-μg and 60-μg bivalent-BA.1 groups, as compared with the 30-μg BNT162b2 group, were 0.99 (95% CI, 0.82 to 1.20) and 1.30 (95% CI, 1.07 to 1.58), respectively (Table 2). Thus, noninferiority was shown for both doses of the bivalent vaccine, with lower limits of the 95% confidence interval that were greater than 0.67.
Among participants without evidence of previous SARS-CoV-2 infection, the percentage of those with a seroresponse against the ancestral strain and the GMTs at 1 month after vaccination were generally greater in the groups that received the 60-μg dose of BNT162b2, monovalent BA.1, or bivalent BA.1 than in the respective 30-μg groups (Table 2 and Figure 2). The geometric mean fold rise in neutralizing antibodies against the ancestral strain from baseline to 1 month after vaccination was similar across the six treatment groups. Among the participants regardless of SARS-CoV-2 infection status, the pattern of findings regarding response against the ancestral strain across the treatment groups was similar to that observed among the participants who had no evidence of previous SARS-CoV-2 infection (Table S4 and Fig. S2B).
Additional Omicron Subvariants
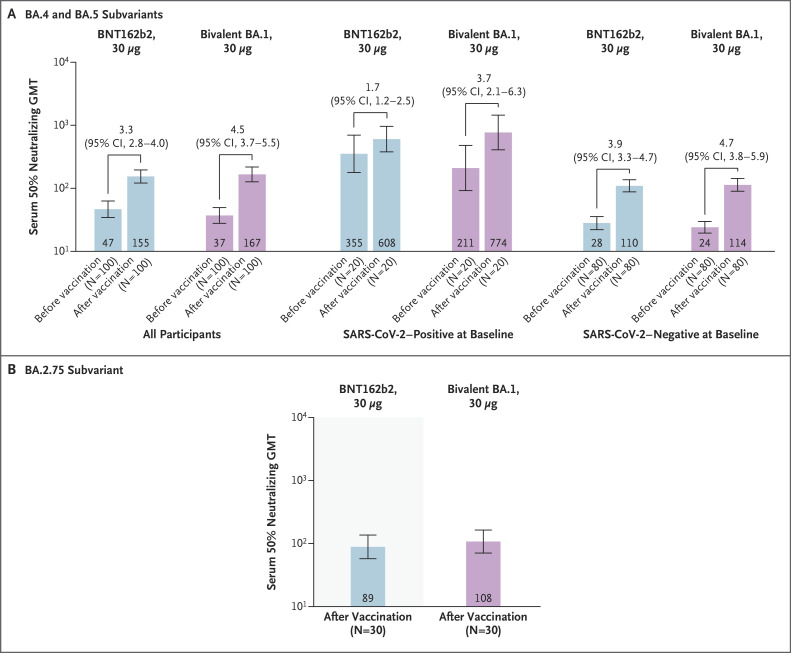
The 50% neutralizing GMTs against omicron BA.4 and BA.5 and the geometric mean fold rise from baseline to 1 month after vaccination were numerically higher in the 30-μg bivalent-BA.1 group than in the 30-μg BNT162b2 group in the overall sample and in subgroups defined according to SARS-CoV-2 infection status. Higher GMTs but lower geometric mean fold rises were observed in the subgroup with evidence of previous SARS-CoV-2 infection than in the subgroup without such evidence (Figure 3A). The percentage of participants with a seroresponse was also higher in the 30-μg bivalent-BA.1 group than in the 30-μg BNT162b2 group (56.0% vs. 42.0%) in the overall sample and in the subgroups defined according to SARS-CoV-2 infection status; the percentages were lower in the subgroup with evidence of previous SARS-CoV-2 infection than in the subgroup without such evidence (Table S5).
Figure 3. SARS-CoV-2 Fluorescent Focus Reduction Neutralization Test Results for Omicron Subvariants.
Panel A shows the GMTs against omicron BA.4 and BA.5 subvariants and the geometric mean fold rises from before to 1 month after vaccination among participants in the omicron BA.4–BA.5 neutralization assay subset, which included 100 participants (20 with SARS-CoV-2–positive status at baseline and 80 with SARS-CoV-2–negative status up to 1 month after vaccination) who had been randomly selected from each of the 30-μg BNT162b2 and 30-μg bivalent-BA.1 groups in the expanded cohort. Panel B shows GMTs against the omicron BA.2.75 subvariant 1 month after vaccination among participants in the omicron BA.2.75 neutralization assay subset, which included 30 participants in each of the 30-μg BNT162b2 and 30-μg bivalent-BA.1 groups who had been randomly selected from the omicron BA.4–BA.5 neutralization assay subset, all of whom did not have serologic or virologic evidence of previous SARS-CoV-2 infection up to 1 month after vaccination. SARS-CoV-2–positive status was defined as a positive SARS-CoV-2 nucleoprotein–binding antibody test at baseline, a positive nucleic acid amplification test at baseline, or a medical history of Covid-19. The GMTs are shown within the bars in Panels A and B (𝙸 bars indicate the 95% confidence interval), and the geometric mean fold rises from before to 1 month after vaccination are shown above the bars in Panel A. The GMTs, geometric mean fold rises, and associated 95% confidence intervals were calculated by exponentiating the mean logarithm of the 50% neutralizing titers or fold rises and the corresponding 95% confidence intervals on the basis of the Student’s t distribution. Assay results below the LLOQ were imputed as 0.5×LLOQ (the LLOQ for the neutralizing titers against BA.4, BA.5, and BA.2.75 by the fluorescent focus reduction neutralization test is 20).
In the subgroup of participants in whom an omicron BA.2.75 neutralization assay was performed, the 50% neutralizing GMT at 1 month after vaccination was numerically higher in the 30-μg bivalent-BA.1 group than in the 30-μg BNT162b2 group (Figure 3B). Thus, the direction of the findings regarding BA.2.75 neutralization in the two treatment groups was similar to that observed for omicron BA.4 and BA.5 neutralization.
Safety
Injection-site pain was the most common local reaction, and fatigue was the most common systemic event (Fig. S3). In general, the percentage of participants with a reactogenicity event was higher in the 60-μg dose groups than in the 30-μg dose groups. Most reactogenicity events were mild to moderate. Severe reactogenicity events were infrequent; across the six treatment groups, severe fatigue was reported in 2.2% of the participants, and all other severe reactogenicity events were reported in less than 1% of the participants. No grade 4 (life-threatening) reactogenicity events were reported. The median onset of reactogenicity events among the participants was 2 to 3 days, and the median duration of such events was 1 to 2 days. One participant in the 60-μg BNT162b2 group reported a fever higher than 40.0°C, as well as other mild-to-severe reactogenicity events, on day 3 after vaccination; antipyretics and analgesics were taken on days 1 to 3 and day 5, and the fever resolved on day 5.
The percentage of participants with adverse events was higher in the 30-μg monovalent-BA.1 group (8.5%) and the 60-μg bivalent-BA.1 group (10.4%) than in the other treatment groups (3.6 to 6.6%) (Table S6). The percentages of participants with treatment-related adverse events (as assessed by the investigator) were generally similar across the treatment groups, with the highest percentages in the 60-μg BNT162b2 group (4.3%) and the 60-μg bivalent-BA.1 group (5.1%); the pattern of these findings was mostly consistent with that for reactogenicity events. All events of lymphadenopathy (eight events across the six treatment groups [0.4%]) were considered to be related to treatment; these events (including one occurrence of axillary pain) were mild to moderate, occurred generally 4 days or less after vaccination, and resolved within 2 to 8 days after vaccination. Severe adverse events included one report of dehydration in the 30-μg monovalent-BA.1 group, which was considered to be a treatment-related serious adverse event; one report of gastroesophageal reflux disease in the 30-μg bivalent-BA.1 group, which was considered to be a serious adverse event unrelated to treatment; and one report each of injection-site swelling, headache, and muscle weakness in the 60-μg bivalent-BA.1 group. A life-threatening adverse event of atrial fibrillation was reported on day 1 after vaccination by one participant in the 60-μg bivalent-BA.1 group who had a medical history of diabetes, hyperlipidemia, hypercholesterolemia, and coronary artery disease; the event resolved within 4 days and was not considered to be related to treatment. As of the data-cutoff date (representing a median follow-up of at least 1.7 months after vaccination), no deaths or cases of myocarditis or pericarditis, Bell’s palsy (or facial paralysis or paresis), appendicitis, or vaccine-related anaphylaxis were reported.
Covid-19 Surveillance
At data cutoff, 30 cases of Covid-19 were reported in the six treatment groups (7 cases in the 30-μg BNT162b2 group, 6 cases in the 60-μg BNT162b2 group, 7 cases in the 30-μg monovalent-BA.1 group, 3 cases in the 60-μg monovalent-BA.1 group, 1 case in the 30-μg bivalent-BA.1 group, and 6 cases in the 60-μg bivalent-BA.1 group). No severe cases of Covid-19, as defined by the FDA and Centers for Disease Control and Prevention, were reported.
Discussion
In the current analysis, monovalent and bivalent omicron BA.1–adapted BNT162b2 vaccines were shown to induce higher variant-matched neutralization titers than the original 30-μg dose of BNT162b2 when given as a fourth dose. Among adults older than 55 years of age who had previously received three 30-μg doses of BNT162b2, the increase in immunogenicity against omicron BA.1 was substantially greater with the omicron BA.1–adapted BNT162b2 vaccines than with the original 30-μg dose of BNT162b2. The monovalent and bivalent omicron BA.1–adapted BNT162b2 versions also maintained robust neutralization titers against the ancestral strain, an observation that was also noted for another bivalent mRNA omicron BA.1–adapted vaccine26 and that was in line with the finding that exposure to the omicron BA.1 spike strongly activates memory B cells directed against epitopes conserved between the ancestral and BA.1 strains.27 Responses against omicron BA.1 after vaccination with an omicron BA.1–adapted BNT162b2 booster trended upward in favor of the monovalent versions over the bivalent versions; however, whether this upward trend would confer a differentiating clinical benefit is unclear. Given that the aim of adapted vaccines is to provide a larger breadth of immunity against SARS-CoV-2 variants and because future evolutionary steps for SARS-CoV-2 are uncertain, bivalent vaccines incorporating an omicron descendent lineage and ancestral virus are preferred.28
Although this clinical trial is ongoing, as of September 2022, omicron BA.1 has been displaced by newly emerging omicron subvariants; omicron BA.5 accounted for 88% of infections in the United States and was the predominant omicron subvariant worldwide.29,30 Recently, the omicron BA.2.75 subvariant emerged in several countries and has additional unique and potentially immune-escaping mutations in the N-terminal and receptor-binding domains of the spike protein.16 Therefore, we undertook descriptive exploratory immunogenicity analyses to characterize cross-neutralization responses against the contemporary omicron BA.4, BA.5, and BA.2.75 subvariants after boosting with 30 μg of bivalent BA.1 or with 30 μg of BNT162b2 (the original dose). Neutralizing titers were not substantially increased from baseline in either group, although they were numerically higher with 30-μg bivalent BA.1 than with 30-μg BNT162b2. These findings emphasize that the evolution of omicron and future variants of concern with increasing potential for immune escape warrants the use of adaptive vaccine strategies to maximize protection by providing greater and broader immune responses.26
The 30-μg dose bivalent BA.1 used in this trial was recently recommended for conditional marketing authorization by the Committee for Medicinal Products for Human Use at the European Medicines Agency for persons 12 years of age or older.31 In addition, because of the emergence of the BA.4 and BA.5 subvariants and the recognized need for agility in the face of the ongoing pandemic, the FDA and European Medicines Agency recently authorized bivalent omicron BA.4– and BA.5–adapted BNT162b2 booster doses for persons 12 years of age or older.32,33 The data showing a superior neutralizing titer response with the omicron BA.1–adapted BNT162b2 vaccines against the matching omicron BA.1 subvariant (as compared with the 30-μg dose of BNT162b2), alongside preclinical data showing improved neutralizing responses with an omicron BA.4– and BA.5–adapted vaccine (as compared with the 30-μg dose of BNT162b2 and the omicron BA.1–adapted BNT162b2 vaccines)34 support the use of preclinical data for authorization now and in the future. Extrapolation of preclinical data in the absence of clinical trial data represents a model already established for annual updates of influenza vaccines35 and may signal a similar path for future updates of Covid-19 vaccines, if required on account of changing circulating strains.
In the current trial, the observed safety profiles of the omicron BA.1–adapted BNT162b2 vaccines did not indicate any concern over the administration of a fourth dose and were consistent with the known safety profile of the original 30-μg dose of BNT162b2.2,19,36 Although adapted vaccines have updated mRNA sequences, the lipid nanoparticle and mRNA dose remain the same as those in the 30-μg dose of BNT162b2, which has already been administered safely to millions of people.37-39 The ability to adapt an effective vaccine with a known safety profile to an emerging variant of concern is crucial, given the antigenic differences between the currently circulating strains of SARS-CoV-2 and the ancestral strain, as well as the uncertainties in the evolution of SARS-CoV-2 and characteristics of future variants (e.g., virulence and transmissibility).40
Limitations of this trial include the lack of longer-term follow-up to assess the duration of immune response and safety, an older and predominantly White trial population, no data in immunocompromised persons, and recruitment only from the United States. Case numbers were too few across the treatment groups to compare the different versions and dose levels of the omicron BA.1–adapted BNT162b2 vaccines with respect to vaccine efficacy; effectiveness data will emerge from countries where the bivalent BA.1 vaccine is in use.
It is paramount to sustain Covid-19 vaccination to protect health, social, and economic systems against potential new waves and new SARS-CoV-2 variants of concern, while ensuring that surveillance mechanisms are in place to determine if and when a variant-adapted vaccine is warranted. Booster doses with bivalent variant-adapted BNT162b2 vaccines aim to provide broad protection against circulating and emerging variants. In the current trial, boosting with the candidate monovalent or bivalent omicron BA.1–adapted vaccines had a safety profile similar to that of the original 30-μg dose of BNT162b2, induced substantial neutralizing responses against ancestral and omicron BA.1 strains, and neutralized BA.4, BA.5, and BA.2.75 strains to a lesser extent than the BA.1 strain.
Acknowledgments
We thank all the participants who volunteered for this trial and the trial site personnel for their contributions; the members of the C4591031 data and safety monitoring board, including Jonathan Zenilman, Kathryn Edwards, Robert Belshe, Lawrence Stanberry, Steve Self, R. Phillips Heine, and Heather S. Lipkind for their review of the data; our colleagues at Pfizer (Greg Adams, Ayman Ayoub, Ruth Bailey, Mark Boaz, Salim Bouguermouh, Christopher Bowen, Donna Boyce, Sarah Burden, Adriana Cahill, Patrick Caubel, Andrea Cawein, Anissa Cheikh, Darren Cowan, Yvette Crawley, Kimberly A. Cristall, Sirisha Davuluri, Fanayea Dejen-Crooks, Carmel Devlin, Daniel Flannery, Emily Graham, Jose Guerra, Tina Guina, Cynthia Ingerick-Holt, Sarah Jarvis, Luis Jodar, Chuck Kolluru, Sherri Kuss, M. Maddalena Lino, Maria Lorenzini, Robert Maroko, Susan Mather, Jason McKinley, Veronica Murphy, Elie Needle, Tracy Pandina, Elizabeth Paulukonis, Santhosh R. Jala, Kellie L. Richardson, Holly Ross, Carol Ryan, Helen Smith, Elisa Harkins Tull, Sarah Tweedy, Venerando Umali, Chris Webber, Jenah West, Caroline Westgarth, David Whritenour, Helen Yang, and Gabriel Zegrean and the Pfizer Vaccines Clinical Assay Team, the Pfizer Vaccines Assay Development Team, and all of the Pfizer colleagues not named here who contributed to the success of this trial); our colleagues at BioNTech (Ruben Rizzi, Zakaria Khondker, Alexander Muik, Nadine Salisch, Kimberly Krüger, Svetlana Shpyro, and Anna Sokolowska and all of the BioNTech colleagues not named here who contributed to the success of this trial); and Sheena Hunt, Judith Kandel, and Tricia Newell of ICON, who wrote the first draft of the manuscript under the authors’ direction.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by BioNTech and Pfizer.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Frenck RW Jr, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med 2021;385:239-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfizer–BioNTech. Highlights of prescribing information: comirnaty (COVID-19 vaccine, mRNA) (https://www.fda.gov/media/151707/download).
- 4.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021;397:1819-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer SR, Angulo FJ, Swerdlow DL, et al. Effectiveness of BNT162b2 mRNA COVID-19 vaccine against SARS-CoV-2 variant beta (B.1.351) among persons identified through contact tracing in Israel: a prospective cohort study. EClinicalMedicine 2021;42:101190-101190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med 2021;385:585-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruell H, Vanshylla K, Tober-Lau P, et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 omicron variant. Nat Med 2022;28:477-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Cheng G. Sequence analysis of the emerging SARS-CoV-2 variant omicron in South Africa. J Med Virol 2022;94:1728-1733. [DOI] [PubMed] [Google Scholar]
- 9.Tartof SY, Slezak JM, Puzniak L, et al. Durability of BNT162b2 vaccine against hospital and emergency department admissions due to the omicron and delta variants in a large health system in the USA: a test-negative case-control study. Lancet Respir Med 2022;10:689-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med 2022;386:1532-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collie S, Nayager J, Bamford L, Bekker L-G, Zylstra M, Gray G. Effectiveness and durability of the BNT162b2 vaccine against omicron sublineages in South Africa. N Engl J Med 2022;387:1332-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gazit S, Saciuk Y, Perez G, Peretz A, Pitzer VE, Patalon T. Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative, case-control study. BMJ 2022;377:e071113-e071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bar-On YM, Goldberg Y, Mandel M, et al. Protection by a fourth dose of BNT162b2 against omicron in Israel. N Engl J Med 2022;386:1712-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Food and Drug Administration. Emergency use authorization for vaccines to prevent COVID-19. Guidance for industry. March 31, 2022. (https://www.fda.gov/media/142749/download).
- 15.European Medicines Agency. Procedural guidance for variant strain(s) update to vaccines intended for protection against human coronavirus. June 8, 2022. (https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/procedural-guidance-variant-strains-update-vaccines-intended-protection-against-human-coronavirus_en.pdf).
- 16.Wang Q, Iketani S, Li Z, et al. Antigenic characterization of the SARS-CoV-2 omicron subvariant BA.2.75. Cell Host Microbe 2022; 30(11):1512-1517.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tegally H, Moir M, Everatt J, et al. Emergence of SARS-CoV-2 omicron lineages BA.4 and BA.5 in South Africa. Nat Med 2022;28:1785-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. WHO coronavirus (COVID-19) dashboard. September 15, 2022. (https://covid19.who.int/).
- 19.Moreira ED Jr, Kitchin N, Xu X, et al. Safety and efficacy of a third dose of BNT162b2 Covid-19 vaccine. N Engl J Med 2022;386:1910-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogel AB, Kanevsky I, Che Y, et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 2021;592:283-289. [DOI] [PubMed] [Google Scholar]
- 21.Xie X, Zou J, Kurhade C, Liu M, Ren P, Shi P-Y. Neutralization of SARS-CoV-2 omicron sublineages by 4 doses of mRNA vaccine. August 8, 2022. (https://www.biorxiv.org/content/10.1101/2022.07.29.502055v2). preprint. [DOI] [PMC free article] [PubMed]
- 22.Kurhade C, Zou J, Xia H, et al. Neutralization of omicron sublineages and deltacron SARS-CoV-2 by three doses of BNT162b2 vaccine or BA.1 infection. Emerg Microbes Infect 2022;11:1828-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie X, Lokugamage KG, Zhang X, et al. Engineering SARS-CoV-2 using a reverse genetic system. Nat Protoc 2021;16:1761-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurhade C, Zou J, Xia H, et al. Neutralization of omicron BA.1, BA.2, and BA.3 SARS-CoV-2 by 3 doses of BNT162b2 vaccine. Nat Commun 2022;13:3602-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou J, Xia H, Xie X, et al. Neutralization against omicron SARS-CoV-2 from previous non-omicron infection. Nat Commun 2022;13:852-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chalkias S, Harper C, Vrbicky K, et al. A bivalent omicron-containing booster vaccine against Covid-19. N Engl J Med 2022;387:1279-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quandt J, Muik A, Salisch N, et al. Omicron BA.1 breakthrough infection drives cross-variant neutralization and memory B cell formation against conserved epitopes. Sci Immunol 2022;7(75):eabq2427-eabq2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.International Coalition of Medicines Regulatory Authorities. SARS-CoV-2 variant workshop. June 30, 2022. (https://icmra.info/drupal/covid-19/30june2022).
- 29.Centers for Disease Control and Prevention. Covid data tracker. Variant proportions. September 15, 2022. (https://covid.cdc.gov/covid-data-tracker/#variant-proportions).
- 30.World Health Organization. COVID-19 weekly epidemiological update, edition 109. September 14, 2022. (https://apps.who.int/iris/handle/10665/362877).
- 31.Pfizer. Pfizer and BioNTech receive positive CHMP opinion for omicron BA.1-adapted bivalent COVID-19 vaccine booster in European Union. September 1, 2022. (https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-receive-positive-chmp-opinion-omicron).
- 32.Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes Moderna, Pfizer-BioNTech bivalent COVID-19 vaccines for use as a booster dose. August 31, 2022. (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use).
- 33.European Medicines Agency. Adapted vaccine targeting BA.4 and BA.5 omicron variants and original SARS-CoV-2 recommended for approval. September 12, 2022. (https://www.ema.europa.eu/en/news/adapted-vaccine-targeting-ba4-ba5-omicron-variants-original-sars-cov-2-recommended-approval).
- 34.Centers for Disease Control and Prevention. Pfizer/BioNTech COVID-19 Omicron-modified bivalent vaccine. September 1, 2022. (https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-09-01/07-covid-swanson-508.pdf).
- 35.Weir JP, Gruber MF. An overview of the regulation of influenza vaccines in the United States. Influenza Other Respir Viruses 2016;10:354-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas SJ, Moreira ED Jr, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med 2021;385:1761-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hause AM, Baggs J, Marquez P, et al. Safety monitoring of COVID-19 mRNA vaccine second booster doses among adults aged ≥50 years — United States, March 29, 2022–July 10, 2022. MMWR Morb Mortal Wkly Rep 2022;71:971-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hause AM, Baggs J, Marquez P, et al. Safety monitoring of COVID-19 vaccine booster doses among adults — United States, September 22, 2021–February 6, 2022. MMWR Morb Mortal Wkly Rep 2022;71:249-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hause AM, Baggs J, Marquez P, et al. Safety monitoring of COVID-19 vaccine booster doses among persons aged 12–17 years — United States, December 9, 2021–February 20, 2022. MMWR Morb Mortal Wkly Rep 2022;71:347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markov PV, Katzourakis A, Stilianakis NI. Antigenic evolution will lead to new SARS-CoV-2 variants with unpredictable severity. Nat Rev Microbiol 2022;20:251-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.