Abstract
Background
Pneumocystis jirovecii pneumonia (PCP) is a serious, emerging complication of coronavirus disease 2019 (COVID-19).
Methods
We performed a systematic review of published cases. We describe 6 new cases of PCP/COVID-19 coinfection. Among our cases (n = 6) and those in the literature (n = 69) with available data, the median age (interquartile range [IQR]) was 59 (44–77) years (n = 38), 72% (47/65) were male, and the mortality rate was 30.9% (21/68).
Results
Long-term corticosteroid use was noted in 45.1% (23/51), advanced HIV infection (defined as a CD4 count <200 cells/μL) in 17.6% (9/51), and antineoplastic chemotherapy in 13.7% (7/51), consistent with known PCP risk factors. Notably, 56.7% (38/47) had verifiable risk factors for PCP (high-dose corticosteroids, immunosuppressive therapy, and HIV infection) before COVID-19 infection. A median absolute lymphocyte count (IQR) of 0.61 (0.28–0.92) ×103 cells/mm3 (n = 23) and CD4 count (IQR) of 66 (33–291.5) cells/mm3 (n = 20) were also discovered among the study population.
Conclusions
These findings suggest a need for greater attention to PCP risk factors among COVID-19 patients and consideration of PCP prophylaxis in these high-risk populations.
Keywords: COVID-19, PCP, coinfection, immunocompromised, invasive fungal infections, pneumocystis, Pneumocystis jirovecii
Anecdotal reports of invasive fungal coinfections in patients with coronavirus disease 2019 (COVID-19) began emerging during the earliest days of the COVID-19 pandemic. Subsequently, numerous reports have emerged noting that those with severe COVID-19 have greater incidences of pulmonary aspergillosis [1] and mucormycosis [2] than those with mild COVID-19. Importantly, invasive fungal coinfections are associated with worse clinical outcomes, with mortality rates between 40% and 50% if appropriate antifungal treatment is delayed or absent [3]. There have also been case reports [4–24] of Pneumocystis jirovecii pneumonia (PCP) among patients with severe COVID-19 [25].
P. jirovecii is a ubiquitous environmental fungus found globally that can cause pneumonia following inhalation and colonization within the host's lungs. PCP is predominately seen in immunocompromised hosts and is typically characterized by progressive dyspnea, fever, ground glass opacities on radiographic imaging, and hypoxemia [26]. The similar clinical and radiographic appearances of PCP and COVID-19 have made discernment between the 2 infections challenging [27].
Despite its prominent correlation with advanced HIV infection, PCP also occurs in other immunocompromised populations, such as transplant recipients, patients with cancer receiving antineoplastic chemotherapy, and patients on long-term glucocorticoids [28]. Notably, non-HIV PCP populations may have a higher mortality rate (30.6%) [29] when compared with persons with HIV infection (9.7%–16.9%), which may be relevant to COVID-19 and PCP coinfection [30]. Herein we present 6 cases of PCP after or during COVID-19 infection and systematically review the current literature to better characterize potential risk factors and the clinical outcomes of PCP in the setting of COVID-19.
METHODS
Case Series
Following institutional review board approval, cases were retrospectively identified using International Classification of Diseases, 10th Revision, codes for PCP and COVID-19 between the dates of March 1, 2020, and June 1, 2022. De-identified data were collected and stored on REDCap (Vanderbilt University, Nashville, TN, USA). Persons 18 years of age and older with PCP concurrent with or within 1 year after COVID-19 infection were eligible. COVID-19 infection was defined as a positive polymerase chain reaction (PCR) or antigen test in the context of symptoms attributable to COVID-19. PCP was defined by Mycoses Study Group Education and Research Consortium criteria (Table 1).
Table 1.
| Proven P. jirovecii | |
|---|---|
| Direct detection of the organism |
|
| Probable P. jiroveciib | |
| Host factors |
|
|
|
|
|
|
|
| Clinical features |
|
|
|
| Mycologic evidence |
|
|
|
Abbreviations: BAL, bronchoalveolar lavage; PCR, polymerase chain reaction.
Table adapted from Donnelly et al. for the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium.
Must meet at least 1 criterion from each of the 3 categories.
Information collected for each patient included age at diagnosis, sex, hospital unit type, length of stay, risk factors for PCP and COVID-19, therapeutics used for PCP and COVID-19, mechanical ventilation requirement, lowest pulse oximetry measurement, renal replacement therapy requirement, days between COVID-19 and PCP diagnoses, mortality at hospital discharge, and laboratory testing including lowest PaO2, lactate dehydrogenase (LDH), 1,3-β-D-glucan (BDG), CD4 and absolute lymphocyte counts at the time of PCP diagnosis, and diagnostic tests for PCP and COVID-19.
Literature Review
Systematic searches of Embase and Medline (January 2020–June 2022) were conducted to identify cases of PCP during or within 1 year of COVID-19. Duplicate results were removed, and the remaining papers were screened for inclusion criteria. Case reports, case series, case–control studies, and cohort studies with cases of PCP during or after COVID-19 were included. Reviews and opinion articles were excluded. Articles were only included if available in English or Spanish. Data collection was identical to our own reported cases as available.
Data from published reports and from the current report were combined as available for descriptive purposes.
RESULTS
Case Reports
Between March 1, 2020, and June 1, 2022, 6 patients were identified as having PCP among the 2324 patients hospitalized for COVID-19 pneumonia at our institution (incidence = 0.26% cases of PCP per COVID-19 hospitalization). Two of the cases are presented in narrative form below. All 6 cases are described below (Table 2).
Table 2.
Summary of Presented Cases at Time of PCP diagnosis
| Patients | 1 | 2 | 3 | 4 | 5 | 6 | Median [IQR] or No. (%) | |
|---|---|---|---|---|---|---|---|---|
| Demographics | Age, y | 84 | 49 | 58 | 61 | 69 | 66 | 63.5 [58–69] |
| … | Sex | Female | Female | Male | Female | Male | Male | 3/6 (50) female |
| … | Mortality at hospital dischargea | Living | Deceased | Living | Living | Living | Living | 1/6 (16.7) deceased |
| COVID-19 | Risk factors at PCP diagnosis | HTN, CAD, asthma, IgM deficiency | DM2, HTN, CAD, obese, S/P SOT (tacrolimus, corticosteroids, mycophenolate) | MM II, S/P ASCT long-term corticosteroids | CKD, DLBL IV | Obese, methotrexate for RA | HTN | 5/6 (83.3) had PCP risk factors before COVID-19 |
| … | Treatment | Remdesivir, dexamethasone | Remdesivir, dexamethasone | Remdesivir, dexamethasone, bamlanivimab-etesevimab | Dexamethasone, baricitinib, casirivimab- imdevimab | Remdesivir, CP, dexamethasone | Remdesivir, dexamethasone | … |
| … | Prednisone-equivalent steroid administration,b mg | 400 | 660 + 5 daily for SOT | 240 + 130 weekly for MM/ASCT | 1310 | 400 | 930 | 530 [400–930] |
| Pneumocystis | Status | Probable | Probable | Probable | Probable | Probable | Probable | 6/6 (100) |
| … | Diagnosis method | BAL PCR, β−D-Glucan | β−D-Glucan, LDH, clinical picture | BAL PCR, β−D-Glucan | BAL PCR, β−D-Glucan | Sputum PCR, clinical picture | BAL PCR, β−D-Glucan | … |
| … | Risk factors at PCP diagnosis | Corticosteroids | Immune compromised, corticosteroids | Immune compromised, corticosteroids, | Immune compromised, corticosteroids | Immune compromised, corticosteroids | Corticosteroids | 6/6 (100) corticosteroids |
| … | Treatment | TMP-SMX, then atovaquone | TMP-SMX | TMP-SMX, then atovaquone, prednisone | TMP-SMX, prednisone | TMP-SMX, then atovaquone | TMP-SMX, then atovaquone, prednisone | … |
| … | Time of PCP diagnosis, d from COVID-19 diagnosis | 39 | 19 | 58 | 199 | 16 | 43 | 41 [19–58] |
| Laboratory findings | β−D-Glucan [<80], pg/mL | 191 | 137 | >500 | >500 | 31 | 292 | 241.5 [137–>500] |
| … | LDH [91–180], units/L | 303 | 536 | 204 | 320 | 398 | 428 | 359 [303–428] |
| … | ALC [1.0–4.8], K/mm3 | … | 0.54 | 1.56 | 0.61 | 0.64 | 0.97 | 0.64 [0.58–1.27] |
| … | CD4 count [>200], cells/mm3 | 34 | … | 243 | 382 | 56 | … | 200 [56–382] |
| … | Lowest PaO2, mmHg | 78 | 45 | 72 | 52 | 58 | 45 | 55 [45–72] |
| Imagining findings | CT chest | Bilateral infiltrates and diffuse ground glass opacitiesc | Bilateral nodules and diffuse ground glass opacities | Diffuse ground glass opacities with new, focal nodular opacities | Diffuse ground glass opacities | New peripheral infiltrates | Diffuse ground glass opacities and prominent mediastinal lymph nodes | … |
| Hospital stay | ICU stay | Yes | Yes | No | Yes | Yes | No | 4/6 (66.7) ICU admission |
| … | ICU days | 10 | 13 | N/A | 27 | 7 | N/A | 10 [4.5–20] |
| … | Mechanical ventilation | Yes | Yes | No | Yes | No | No | 3/6 (50) MV |
Roman numerals indicate stage of malignancy.
Abbreviations: ALC, absolute lymphocyte count; ASCT, autologous hematopoietic stem cell transplant; BAL, bronchoalveolar lavage; CAD, coronary artery disease; CKD, chronic kidney disease; CP, convalescent plasma; CT, computed tomography; DLBL, diffuse large B-cell lymphoma; DM2, type 2 diabetes mellitus; HM, hematogenic malignancy; HSCT, nonautologous hematopoietic stem cell transplant; HTN, hypertension; ICU, intensive care unit; IgM, immunoglobulin M; IQR, interquartile range; LDH, lactate dehydrogenase; MDS, myelodysplastic syndrome; MM, multiple myeloma; PaO2, partial pressure of arterial oxygen; PCP, Pneumocystis jirovecii pneumonia; PCR, polymerase chain reaction; RA, rheumatoid arthritis; S/P, status post; SOT, solid organ transplant; TMP-SMX, trimethoprim-sulfamethoxazole.
From hospitalization with PCP diagnosis.
Prednisone-equivalent dosing from time of COVID-19 diagnosis until PCP diagnosis. Daily or weekly administration of steroids for existing conditions is in addition to administration during hospital admittance for at least 6 months before hospitalization.
Imaging findings at time of diagnosis only available on PA and lateral chest x-ray, but improving and comparable findings were found on CT chest at 1-month follow up.
Patient 1
An 84-year-old woman was hospitalized for COVID-19 pneumonia and pulmonary embolism and treated with remdesivir, dexamethasone, and apixaban. She was discharged after 15 days but readmitted a week later with worsening respiratory symptoms. Upon readmission, she was diagnosed with probable pulmonary histoplasmosis by detection of a positive urinary Histoplasma antigen and treated with itraconazole. BDG was also elevated at 191 pg/mL (reference range, <80 pg/mL).
Her hypoxemia increased, and she underwent bronchoalveolar lavage (BAL). PCR testing for P. jirovecii in BAL fluid was positive 39 days after her initial COVID-19 diagnosis, and she was started on trimethoprim-sulfamethoxazole (TMP-SMX). HIV testing was negative, but her CD4 count was 34 cells/mm3. Therapy for PCP was changed to atovaquone due to thrombocytopenia. She improved and was discharged on itraconazole, atovaquone, and a prednisone taper. She was readmitted 81 days after her initial COVID-19 diagnosis with decompensated congestive heart failure, and voriconazole was substituted for itraconazole. She improved and was discharged home on day 101.
Patient 2
A 49-year-old woman with numerous comorbidities (Table 2) including a deceased donor kidney transplant a year before presentation (on prednisone, mycophenolate, and tacrolimus) was admitted for COVID-19. She was treated with remdesivir and dexamethasone, as well as 2 units of anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) convalescent plasma. Tacrolimus and mycophenolate doses were reduced. She was discharged 6 days after admission to complete a course of dexamethasone for COVID-19, after which she would resume her prior prednisone.
The patient was readmitted to the intensive care unit (ICU) 11 days from initial presentation due to acute hypoxemic respiratory failure. Despite empiric antibiotic administration, her condition worsened, and she was intubated on day 15. On day 19 from the initial presentation, her BDG was elevated at 137 pg/mL and serum LDH was 627 unit/L (reference range, 91–180 unit/L). Computed tomography (CT) of the chest revealed pulmonary nodules and diffuse ground glass opacities. Given these findings, she was started on TMP-SMX for presumed PCP. Confirmation of PCP via BAL was unobtainable due to the patient's tenuous clinical status. Unfortunately, the patient's status continued to worsen, and she died 23 days after her initial presentation.
Systematic Review Results
A systematic review of the literature (Figure 1) was conducted, resulting in 29 articles (Table 3) describing 69 cases of PCP and COVID-19 coinfections. Cohort studies from France [25, 33] and India [34] contained the largest collection of cases reported but had limited patient information. Alanio et al. reported 10 cases of PCP among 108 COVID-19 French ICU patients during a 1.5-month period (9.25%) [25]. Bretagne et al. reported PCP in 17 of 244 patients with fungal infections and COVID-19 during a 5-month period at 36 centers in France, but the number of total COVID-19 patients was not available, so an incidence of PCP/COVID-19 coinfection could not be calculated [33]. Smaller studies in India (n = 5 PCP infections), Jordan (n = 3 PCP infections), and France (n = 5 PCP infection), respectively, reported incidences of 2.6% (ICU patients only) [34], 9.7% (among hospitalized asthmatics with COVID-19) [4], and 7% (among patients receiving a BAL PCP within a month of COVID-19 diagnosis) [35].
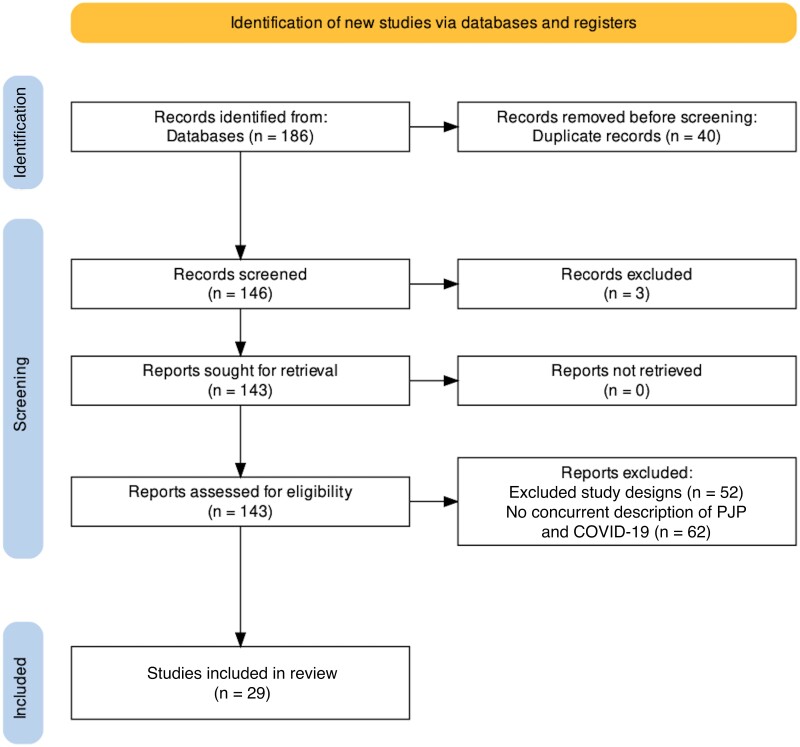
Figure 1.
Study inclusion procedures. Excluded Medline articles: Out of 95 articles, 33 articles were excluded because they were review or opinion articles, 3 articles were not in English or Spanish, and 32 articles were excluded because there was no description of PCP/COVID-19 co- or postinfections. Excluded Embase articles: Out of 91 articles, 40 were excluded due to duplication with the Medline database, 19 were excluded because they were review or opinion articles, and 28 were excluded because they contained no description of PCP/COVID-19 co- or postinfections. Included studies: Cohort, cross-sectional, case series, and case report studies that described concurrent or post-COVID-19 infections with PCP were included in the original screening [32]. Abbreviations: COVID-19, coronavirus disease 2019; PCP, Pneumocystis jirovecii pneumonia.
Table 3.
Literature Summary
| Study Design | Studies | Cases | % of Total Cases |
|---|---|---|---|
| Case series | 3 | 14 | 18.7 |
| Case report | 21 | 22 | 29.3 |
| Prospective cohort study | 4 | 35 | 46.7 |
| Retrospective cohort study | 1 | 4 | 5.3 |
| Totals | 29 | 75 | 100 |
Demographic, diagnostic, risk factor, and laboratory information is summarized in Table 4 for cases from our report and the literature review (n = 75). The median age (interquartile range [IQR]) was 59 (44–70) years (n = 38), and 72% were male (47/65) [4–12, 14–23, 35–38]. According to the previously described criteria by Donnelly et al., 8 cases were proven PCP and the remainder were probable [31]. Most patients had significant risk factors for PCP during their index COVID-19 illness, and 70% had risk factors for PCP before SARS-CoV-2 infection. Additionally, 82% (32/39) of patients received corticosteroids during their hospitalization for COVID-19. Mortality rates varied widely between studies, but overall, ∼31% of patients died in the hospital, either during their index admission or during readmission for PCP. This mortality rate is consistent with previously reported mortality rates of PCP in patients without HIV (30.6%) [29].
Table 4.
Summary of all Cases
| Characteristic | Patients With Available Data | Median [IQR] | (No.) % | Citations | |
|---|---|---|---|---|---|
| Demographics | Age, y | n = 48 | 59 [44–70] | … | [5–12, 14, 16, 17, 19–23, 25, 34–37] |
| … | Sex: male | n = 65 | … | (47/65) 72.3 | [5–12, 14–23, 25, 33, 35–38] |
| COVID-19 | Status: PCR-positive | n = 75 | … | (74/75) 98.7 | [4–12, 14-25, 33–38] |
| … | Treatment: corticosteroids | n = 39 | … | (32/39) 82.1 | [5, 7–12, 15–20, 23, 25, 36, 38] |
| Risk factorsa | HIV | n = 51 | … | (9/51) 17.6 | [4–12, 14-25, 33–38] |
| … | Long-term corticosteroid use before COVID-19 | n = 51 | … | (23/51) 45.1 | [4–12, 14–25, 33–38] |
| … | Prednisone-equivalent steroid administration,b mg | n = 13 | 630 [400–946] | … | [10, 19, 36] |
| … | Antineoplastic chemotherapy | n = 51 | … | (7/51) 13.7 | [4–12, 14–25, 33–38] |
| … | Transplant-related immunosuppression | n = 51 | … | (3/51) 5.9 | [4–12, 14–25, 33–38] |
| … | CKD, ESRD, or RRT | n = 51 | … | (10/52) 19.2 | [4–12, 14–25, 33–38] |
| … | Patients with verified PCP risk factors before COVID-19c | n = 67 | … | (38/67) 56.7 | [4–12, 14–23, 25, 33, 35–38] |
| Pneumocystis | Status: proven | n = 75 | … | (8/75) 10.7 | [4–12, 14–25, 33–38] |
| … | Status: probable | n = 75 | … | (67/75) 89.3 | [4–12, 14–25, 33–38] |
| … | PCP among severe COVID-19 | n = 501 | … | (12/279) 4.3 | [25, 34, 35] |
| … | PCP among all COVID-19 | n = 2324d | … | (6/2324) 0.26 | … |
| … | Days from COVID-19 diagnosis | n = 29 | 25 [4.5–42.5] | … | [5–8, 10–12, 14–17, 19–22, 35, 36] |
| Labs | Elevated β−D-Glucan [<80], pg/mL | n = 39 | … | (26/39) 66.7 | [6, 8, 11, 14, 16, 18, 19, 25, 33, 35, 36] |
| … | LDH [91–180], unit/L | n = 31 | 508 [346–641] | … | [6–8, 10, 11, 16, 17, 19, 21, 25, 35, 36] |
| … | ALC [1.0–4.8], ×103 cells/mm3 | n = 23 | 0.60 [0.26–0.86] | … | [5, 7, 8, 10, 11, 14, 16, 17, 21, 35, 36] |
| … | CD4 count, cells/mm3 | n = 20 | 64 [33–267] | … | [6–11, 15–18, 22, 36] |
| … | Lowest PaO2, mmHg | n = 16 | 64 [55–66.5] | … | [5–8, 19, 36] |
| Outcomes | ICU admission | n = 58 | … | (46/58) 79.3 | [4–6, 8–12, 15–17, 19, 23, 25, 33, 35–37] |
| … | ICU days admitted | n = 58 | 8.5 [3.5–16.0] | … | [4–6, 8–12, 15–17, 19, 23, 25, 33, 35–37] |
| … | Mechanical ventilation | n = 31 | … | (19/30) 63.3 | [4–6, 8–12, 15–17, 19, 23, 25, 35–37] |
| … | Mortality | n = 68 | … | (21/68) 30.9 | [4–12, 14–23, 25, 33, 35–38] |
Abbreviations: ALC, absolute lymphocyte count; CKD, chronic kidney disease; COVID-19, coronavirus disease of 2019; ESRD, end-stage renal disease; ICU, intensive care unit; IQR, interquartile range; LDH, lactose dehydrogenase; PaO2, partial pressure of arterial blood oxygen; PCP, Pneumocystis jirovecii pneumonia; PCR, polymerase chain reaction; RRT, renal replacement therapy.
Risk factors were identified as those happening both during treatment for COVID-19 and before diagnosis.
Prednisone equivalent determined using MedCalc(c).
Risk factors for PCP before COVID-19 infection identified from described past medical histories and defined as immunosuppression from long-term corticosteroids, antineoplastic chemotherapy, untreated HIV, immunosuppressive therapies accompanying transplants, and other immunosuppressed states in accordance with Donnelly et al. [31].
Based on the number of patients admitted to our institution between March 1, 2020, and June 1, 2022, for COVID-19.
DISCUSSION
The literature review identified demographic trends, with a median age of 59 years and a male predominance (72.3%). Patients in our case series were generally older (median age, 66 years), and the gender distribution was more equal, although we had only 6 cases. Variance between PCP prevalence among patient populations in our case series (0.26%) and the literature review (4.2%) [25, 34, 35] was impacted by 2 factors. First, our evaluation of patients at our institution was by no means exhaustive. Second, our prevalence was among all COVID-19 patients at our institution, and the literature review reflects a prevalence among COVID-19 patients admitted to the ICU [25, 34, 35].
While the causative relationship between moderate to severe COVID-19 and PCP still requires further investigation, the review revealed several potential risk factors. A previous review by Chong et al. identified 91.7% (11/12) of patients as having acquired immunodeficiencies through HIV infection, long-term glucocorticoid use, and other forms of immunosuppression [39]. Our larger review found that 56.7% (38/67, including 5 of 6 new cases we reported) of patients had PCP risk factors (ie, lymphopenia, high-dose glucocorticoids, immunosuppression, and HIV) before COVID-19 diagnosis as defined by Donnelly et al. [31].
Long-term glucocorticoid use before and, notably, during COVID-19 infection may be one of the more significant contributing risk factors. Glucocorticoid administration was described in 45.1% (17/51) of patients before COVID-19 infection, but only 1 could be verified as receiving high-dose glucocorticoids, defined as >20 mg prednisone equivalent per day for >4 weeks, before COVID-19 infection [21]. Additionally, 82% (32/39) of PCP cases reported COVID-19 treatment regimens including dexamethasone, prednisone, or methylprednisolone. Only 3 cases could be verified as receiving high-dose glucocorticoid administration for treatment of COVID-19 [10, 36]. Steroid administration for COVID-19 was described for 13 patients, including our presented cohort, between their COVID-19 and PCP diagnoses. The median total prednisone equivalent dose (IQR) was 630 (400–946) mg before PCP diagnosis, illustrating a potentially significant risk factor for PCP [10, 19, 36]. Conversely, most cases did not specify steroid administration, and an additional 10 cases received glucocorticoids for a co-presentation of PCP and COVID-19. Similarly, the literature review demonstrated HIV infection as a risk factor in 17.6% (9/51, none of our 6 cases) of patients. Of the 9 cases identified with HIV, 8 were newly diagnosed HIV infections at original presentation who were not on antiretroviral therapy (ART), and the 1 chronic case was receiving TMP-SMX PCP prophylaxis, suggesting a known CD4 count of <200 cells/mm3.
Lymphopenia was also identified as a significant risk factor for those with PCP following COVID-19 infection. The median absolute lymphocyte count from the literature review including our cases (IQR) was 0.6 (0.26–0.86) ×103 cells/mm3. However, we could only verify described lymphopenia at the time of COVID-19 diagnosis in 7 patients compared with the 25 cases identified with lymphopenia at the time of PCP diagnosis [5, 7, 8, 10, 11, 14, 16, 17, 21, 35, 36]. Our case series findings support lymphopenia as a possible risk factor for PCP in the setting of COVID-19 infection.
Of additional interest, BDG was only positive in 66.7% (26/39) of patients tested. In all cases where a BDG was collected, patients were verified to have PCP infection through clinical symptoms and a diagnostic PCP PCR testing. Discordance between BDG and PCP PCR is difficult to reconcile. It could indicate a false-negative BDG or a false-positive PCP PCR or represent PCP colonization rather than infection.
Lastly, outcomes were generally poor, with nearly 80% requiring ICU admission (46/58) and a >30% mortality rate (21/68). These outcomes are likely biased by both indication for testing (eg, testing for PCP is not done in those improving) and reporting (some reports only summarized ICU cases). A probable contributor to these poor outcomes, however, might be the lack of PCP prophylaxis administration in 3 patients with preexisting PCP risks before COVID-19 [14, 35].
Limitations
This study has several limitations. The most obvious is its small sample size and its retrospective design. Another limitation was lack of detail regarding immunomodulators used for the treatment of moderate to severe COVID-19. The literature we reviewed did not comment on the use of interleukin-6 or Janus kinase inhibitors. Additionally, the lack of a control population in our case series and literature review limits the strength of the association between identified patient risk factors and PCP in COVID-19 patients. However, this systematic review of 75 cases provides the most comprehensive look to date at PCP/COVID-19 coinfection.
CONCLUSIONS
This study adds to the growing body of evidence supporting an increased risk of invasive fungal infections in the context of COVID-19 and its treatment. This review highlights the importance of maintaining PCP as a diagnostic consideration in patients with severe COVID-19, especially when extended glucocorticoid courses are used to treat COVID-19 in the context of ongoing or worsening respiratory symptoms. While the exact mechanism that predisposes patients with severe COVID-19 to PCP infection is an area in need of further research, descriptive studies such as this one may raise awareness of this underreported phenomenon. We identified among the literature and our own cases a lack of PCP prophylaxis administration among patients meeting the high-risk categories described by the Mycoses Study Group Education and Research Consortium [31]. We recommend a low threshold for evaluation of PCP in the setting of COVID-19 patients with the following risk factors: high-dose corticosteroids within the past 60 days, advanced HIV infection, particularly those not on ART, immunosuppressive and antineoplastic treatment regimens, and lymphopenia [31]. With increased global collaboration, it is possible that greater insight might be gained into this deadly fungal coinfection and its prevention.
Acknowledgments
Financial support. Dr. Bahr receives support from the National Institutes of Health, National Institute of Neurological Disorders and Stroke (K23 NS110470). Paul Amstutz received funding from the Curtis Fitzsimmons Infectious Disease Research Fellowship at KUMC.
Patient consent. The design of the work has been approved by local ethical committees; the study conforms to standards currently applied in the country of origin and includes the name of the authorizing body, which should be stated in the paper.
Contributor Information
Paul Amstutz, School of Medicine, University of Kansas Medical Center, Kansas City, Kansas, USA.
Nathan C Bahr, Division of Infectious Diseases, Department of Medicine, University of Kansas Medical Center, Kansas City, Kansas, USA.
Karen Snyder, Division of Infectious Diseases, Department of Medicine, University of Kansas Medical Center, Kansas City, Kansas, USA.
D Matthew Shoemaker, Division of Infectious Diseases, Department of Medicine, University of Kansas Medical Center, Kansas City, Kansas, USA.
References
- 1. Chong WH, Neu KP. Incidence, diagnosis and outcomes of COVID-19-associated pulmonary aspergillosis (CAPA): a systematic review. J Hosp Infect 2021; 113:115–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al-Tawfiq JA, Alhumaid S, Alshukairi AN, et al. COVID-19 and mucormycosis superinfection: the perfect storm. Infection 2021; 49:833–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silva DL, Lima CM, Magalhães VCR, et al. Fungal and bacterial coinfections increase mortality of severely ill COVID-19 patients. J Hosp Infect 2021; 113:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alsayed AR, Talib W, Al-Dulaimi A, Daoud S, Al Maqbali M. The first detection of Pneumocystis jirovecii in asthmatic patients post COVID-19 in Jordan. Bosn J Basic Med Sci 2022; 22:784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jeican II, Inișca P, Gheban D, et al. COVID-19 and Pneumocystis jirovecii pulmonary coinfection—the first case confirmed through autopsy. Medicina (Kaunas) 2021; 57(302):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Menon AA, Berg DD, Brea EJ, et al. A case of COVID-19 and Pneumocystis jirovecii coinfection. Am J Respir Crit Care Med 2020; 202:136–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anggraeni AT, Soedarsono S, Soeprijanto B. Concurrent COVID-19 and Pneumocystis jirovecii pneumonia: the importance of radiological diagnostic and HIV testing. Radiol Case Rep 2021; 16:3685–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Broadhurst AGB, Lalla U, Taljaard JJ, Louw EH, Koegelenberg CFN, Allwood BW. The diagnostic challenge of Pneumocystis pneumonia and COVID-19 co-infection in HIV. Respirol Case Rep 2021; 9:e00725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Larzábal FJ, Vilela A, Brusca S, Saluzzi I, Ghergo GE, Angiono MA. Simultaneous diagnosis and favorable evolution of infection with Pneumocystis jirovecii, SARS-CoV-2 and advanced HIV. Medicina (B Aires) 2020; 80:554–6. [PubMed] [Google Scholar]
- 10. De Francesco MA, Alberici F, Bossini N, et al. Pneumocystis jirevocii and SARS-CoV-2 co-infection: a common feature in transplant recipients? Vaccines (Basel) 2020; 8:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peng J, Ni M, Du D, et al. Successful treatment of a kidney transplant patient with COVID-19 and late-onset Pneumocystis jirovecii pneumonia. Ann Clin Microbiol Antimicrob 2021; 20:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mang S, Kaddu-Mulindwa D, Metz C, et al. Pneumocystis jirovecii pneumonia and severe acute respiratory syndrome coronavirus 2 coinfection in a patient with newly diagnosed HIV-1 infection. Clin Infect Dis 2021; 72:1487–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo W, Wang M, Ming F, Tang W, Liang K. The diagnostic trap occurred in two COVID-19 cases combined Pneumocystis pneumonia in patient with AIDS. Res Sq. 2020; rs.3(rs-53350):1–5. [Google Scholar]
- 14. Mouren D, Goyard C, Catherinot E, et al. COVID-19 and Pneumocystis jirovecii pneumonia: back to the basics. Respir Med Res 2021; 79:100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhat P, Noval M, Doub JB, Heil E. Concurrent COVID-19 and Pneumocystis jirovecii pneumonia in a severely immunocompromised 25-year-old patient. Int J Infect Dis 2020; 99:119–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rubiano C, Tompkins K, Sellers SA, et al. Pneumocystis and severe acute respiratory syndrome coronavirus 2 coinfection: a case report and review of an emerging diagnostic dilemma. Open Forum Infect Dis 2021; 8:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Merchant EA, Flint K, Barouch DH, Blair BM. Co-infection with coronavirus disease 2019, previously undiagnosed human immunodeficiency virus, Pneumocystis jirovecii pneumonia and cytomegalovirus pneumonitis, with possible immune reconstitution inflammatory syndrome. IDCases 2021; 24:e01153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pillay S, Magula N. A trio of infectious diseases and pulmonary embolism: a developing world's reality. South Afr J HIV Med 2021; 22:1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chakravarthy KK, Cherukuri B, Anne S, Shankar TU, Mohan Reddy GM, Guttikonda N. An unusual case of severe Pneumocystis jiroveci pneumonia (PJP) presenting as “recurrent cytokine storm” following COVID-19 infection.” J Assoc Physicians India 2021; 69:78. [PubMed] [Google Scholar]
- 20. Nguyen H, Salkeld J, Agarwal S, Goodman A. Compassionate use of REGN-COV2 in the treatment of COVID-19 in a patient with impaired humoral immunity. Clin Infect Pract 2021; 12:100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quintana-Ortega C, Remesal A, de Valbuena MR, et al. Fatal outcome of anti-MDA5 juvenile dermatomyositis in a paediatric COVID-19 patient: a case report. Mod Rheumatol Case Rep 2021; 5:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wenlock RD, Brown CS, Iwuji C, Vera JH. Can I go back to work? A case of persistent SARS-CoV-2 with advanced untreated HIV infection. Int J STD AIDS 2022; 33:209–11. [DOI] [PubMed] [Google Scholar]
- 23. Ramdhanie L, Gafoor K, Chauhan S, Cervellione K. A case of COVID19 followed by PCP pneumonia in an immunocompromised host. Am J Respir Crit Care Med 2021; 203:A2465. [Google Scholar]
- 24. Saling C, Kasule SN, Vikram HR. COVID-19 and Pneumocystis jiroveci pneumonia. Open Forum Infect Dis 2021; 8(Suppl 1):S272–3. [Google Scholar]
- 25. Alanio A, Dellière S, Voicu S, Bretagne S, Mégarbane B. The presence of Pneumocystis jirovecii in critically ill patients with COVID-19. J Infect 2021; 82:114–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sokulska M, Kicia M, Wesołowska M, Hendrich AB. Pneumocystis jirovecii—from a commensal to pathogen: clinical and diagnostic review. Parasitol Res 2015; 114:3577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sajjad MM, Yousaf S, Ullah S. A diagnostic challenge in COVID-19 pandemic: PCP or COVID-19. J Coll Physicians Surg Pak 2021; 30:144. [DOI] [PubMed] [Google Scholar]
- 28. Avino LJ, Naylor SM, Roecker AM. Pneumocystis jirovecii pneumonia in the non–HIV-infected population. Ann Pharmacother 2016; 50:673–9. [DOI] [PubMed] [Google Scholar]
- 29. Liu Y, Su L, Jiang SJ, Qu H. Risk factors for mortality from Pneumocystis carinii pneumonia (PCP) in non-HIV patients: a meta-analysis. Oncotarget 2017; 8:59729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walzer PD, Evans HER, Copas AJ, Edwards SG, Grant AD, Miller RF. Early predictors of mortality from Pneumocystis jirovecii pneumonia in HIV-infected patients: 1985–2006. Clin Infect Dis 2008; 46:625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2020; 71:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: an R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev 2022; 18:e1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bretagne S, Sitbon K, Botterel F, et al. COVID-19-associated pulmonary Aspergillosis, fungemia, and pneumocystosis in the intensive care unit: a retrospective multicenter observational cohort during the first French pandemic wave. Microbiol Spectr 2021; 9:e0113821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sreenath K, Batra P, Vinayaraj EV, et al. Coinfections with other respiratory pathogens among patients with COVID-19. Microbiol Spectr 2021; 9:e0016321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gerber V, Ruch Y, Chamaraux-Tran TN, et al. Detection of Pneumocystis jirovecii in patients with severe COVID-19: diagnostic and therapeutic challenges. J Fungi (Basel) 2021; 7:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gentile I, Viceconte G, Lanzardo A, et al. Pneumocystis jirovecii pneumonia in non-HIV patients recovering from COVID-19: a single-center experience. Int J Environ Res Public Health 2021; 18:11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ayub M, bin Ali Zubairi M, Zubairi A. Case series of Pneumocystis jirovecii pneumonia in post-COVID-19 disease. Chest 2021; 160:A407. [Google Scholar]
- 38. Skonieczny P, Heleniak Z, Szostakiewicz M, Kuziemski K, Dębska-Ślizień A. Coinfection of COVID-19 and pneumocystosis in a patient after kidney transplantation. Pol Arch Intern Med 2021; 131:566–7. [DOI] [PubMed] [Google Scholar]
- 39. Chong WH, Saha BK, Chopra A. Narrative review of the relationship between COVID-19 and PJP: does it represent coinfection or colonization? Infection 2021; 49:1079–90. [DOI] [PMC free article] [PubMed] [Google Scholar]