Abstract
Patients with congenital unilateral absence of the vas deferens (CUAVD) manifest diverse symptoms from normospermia to azoospermia. Treatment for CUAVD patients with obstructive azoospermia (OA) is complicated, and there is a lack of relevant reports. In this study, we describe the clinical features and evaluate the treatments and outcomes of CUAVD patients with OA. From December 2015 to December 2020, 33 patients were diagnosed as CUAVD with OA in Shanghai General Hospital (Shanghai, China). Patient information, ultrasound findings, semen analysis, hormone profiles, and treatment information were collected, and the clinical outcomes were evaluated. Of 33 patients, 29 patients were retrospectively analyzed. Vasoepididymostomy (VE) or cross VE was performed in 12 patients, the patency rate was 41.7% (5/12), and natural pregnancy was achieved in one of the patients. The other 17 patients underwent testicular sperm extraction as the distal vas deferens (contralateral side) was obstructed. These findings showed that VE or cross VE remains an alternative treatment for CUAVD patients with OA, even with a relatively low rate of patency and natural pregnancy.
Keywords: congenital unilateral absence of the vas deferens, natural pregnancy, obstructive azoospermia, patency, vasoepididymostomy
INTRODUCTION
Congenital absence of the vas deferens (CAVD) is a rare clinical entity related to a complete or partial defect of Wolffian ducts, with an incidence of 0.1% in men.1 According to the absence of the vas deferens, CAVD is classified into three subtypes: congenital bilateral absence of the vas deferens (CBAVD), congenital unilateral absence of the vas deferens (CUAVD), and congenital bilateral partial aplasia of the vas deferens (CPAVD).2 CBAVD is the most common subtype causing obstructive azoospermia (OA), and accounts for 1%–2% of the cases of male infertility.3 CUAVD manifests from normospermia to azoospermia, depending on the function of the contralateral vas deferens, epididymis, and testicle. CUAVD is usually diagnosed in men during the examination of infertility or the vasectomy procedure; thus, the incidence might be underestimated.4
It is well known that CAVD could lead to OA; however, these patients commonly have normal spermatogenesis, which suggests that they have the ability to produce and develop mature spermatozoa and can have their own offspring. For patients with CBAVD, assisted reproductive technology (ART) after sperm extraction is the main treatment. However, patients with CUAVD may present with azoospermia, oligozoospermia, or normospermia, so the treatment is more complicated, especially in CUAVD patients with OA.
Thus far, previous studies have mostly focused on the genetics of CUAVD, such as mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene.5–7 A recent meta-analysis reported that 46% of patients with CUAVD had at least one CFTR variant.5 However, there is little evidence about the treatments and outcomes of CUAVD with OA, especially after microsurgical reconstruction. In the present study, we retrospectively described the clinical features and analyzed the surgical treatments and outcomes of these patients in Shanghai General Hospital (Shanghai, China).
PATIENTS AND METHODS
Patients and diagnosis of CUAVD
This study was approved by the Ethics Review Board of Shanghai General Hospital (approval No. 2017KY020-2), and written informed consent was obtained from all the patients. Thirty-three males who presented with CUAVD with OA and were admitted to Shanghai General Hospital between December 2015 and December 2020 were included in this study. All the patients had normal karyotypes, and no Y chromosomal microdeletions were found. Based on scrotal and transrectal ultrasounds, CUAVD was defined as a complete or partial absence of the vas deferens on the one side, while the other side was completely present.8
Semen and hormone analyses
Semen analysis was carried out, and azoospermia was defined as at least 3 ejaculates without spermatozoa after centrifugation. The semen volume, pH, and fructose test were recorded. Hormone profiles, including follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone (T), were detected through chemiluminescence immunoassay.
Treatment and follow-up
To verify the unilateral absence of the vas deferens and whether the distal vas deferens (contralateral side) was obstructed, exploratory surgery was performed. For patients with distal vas deferens (contralateral side) obstruction who could not undergo vasoepididymostomy, testicular sperm extractions were performed as described previously.9 If the distal vas deferens (contralateral side) was not obstructed, modified single-armed 2-suture longitudinal intussusception vasoepididymostomy (SA-LIVE) or cross SA-LIVE10 was then performed according to whether the sperm or sperm fragments were present in the exuded epididymal fluid. Patients who underwent vasoepididymostomy (VE) or cross VE were followed up every 3–6 months, with a range of 6–36 months and a mean follow-up of 18 months. Semen analysis was performed and natural pregnancy was evaluated at each follow-up.
Statistical analyses
The statistical analyses were performed using SPSS software, version 19.0 (SPSS Inc., Chicago, IL, USA). Parameters are described as the mean ± standard deviation (s.d.). The patency and natural pregnancy rates were calculated.
RESULTS
Demographics and comorbidity
Among 33 patients, 29 patients were finally included in this study (4 were lost to follow-up), of which, 11 (37.9%) with left absence and 18 (62.1%) with right absence. The ages (mean ± s.d.) of the male patients and their female partners in the current cohort were 29.2 ± 4.4 years and 27.1 ± 3.1 years, respectively. The mean duration of infertility was 24 (range: 2–180) months (Table 1).
Table 1.
Characteristics of congenital unilateral absence of the vas deferens patients with obstructive azoospermia
| Characteristic | Value |
|---|---|
| Patients (n) | 29 |
| Age (year), mean±s.d. (range) | |
| Patients | 29.2±4.4 (24–46) |
| Female partners | 27.1±3.1 (24–38) |
| Infertility duration (month), mean±s.d. (range) | 33.5±35.1 (2–180) |
| Laterality, n (%) | |
| Left | 11 (37.9) |
| Right | 18 (62.1) |
| Testicular size, n (%) | |
| Left ≥15 ml | 13 (44.8) |
| Left <15 ml | 16 (55.2) |
| Right ≥15 ml | 14 (48.3) |
| Right <15 ml | 15 (51.7) |
| Comorbidity, n (%) | |
| Absence of unilateral renal | 2 (6.9) |
| Epididymal agenesis | 9 (31.0) |
| Seminal vesicle agenesis/absence | 11 (37.9) |
| Absence of ejaculatory ducts | 1 (3.4) |
| Inguinal hernia | 2 (6.9) |
| Undescended testis | 4 (13.8) |
s.d.: standard deviation
Based on scrotal palpation, ultrasound examination, and surgical exploration, comorbidities were found in patients with CUAVD. The absence of unilateral renal was observed in 2 (6.9%) patients, epididymal agenesis in 9 (31.0%) patients, seminal vesicle agenesis/absence in 11 (37.9%) patients, and absence of ejaculatory ducts in 1 (3.4%) patient. In addition, 2 (6.9%) patients had inguinal hernia and 4 (13.8%) patients had undescended testes on the unilateral side.
Semen and hormone analyses
As shown in Table 2, a low ejaculate volume of less than 1.5 ml was found in 18 (62.1%) participants, including 10 (34.5%) with less than 0.5 ml. Sixteen (55.2%) participants presented a low ejaculate pH value of less than 7.2, and 13 (44.8%) had a negative fructose test. In the analyses of hormone levels, the parameters (mean ± s.d.) were basically in the normal range (FSH: 5.60 ± 3.46 IU l−1, LH: 4.71 ± 1.41 IU l−1, and T: 3.85 ± 1.77 μg l−1).
Table 2.
Semen parameters and hormone levels in congenital unilateral absence of the vas deferens patients with obstructive azoospermia
| Parameter | Value |
|---|---|
| Semen volume, n (%) | |
| ≥1.5 ml | 11 (37.9) |
| <1.5 ml | 18 (62.1) |
| Semen pH, n (%) | |
| ≥7.2 | 13 (44.8) |
| <7.2 | 16 (55.2) |
| Semen fructose, n (%) | |
| Negative | 13 (44.8) |
| Positive | 16 (55.2) |
| Hormone levels, mean±s.d. (range) | |
| FSH (IU l−1) | 5.60±3.46 (1.58–20.41) |
| LH (IU l−1) | 4.71±1.41 (2.68–7.98) |
| T (µg l−1) | 3.85±1.77 (1.85–8.80) |
FSH normal range: 1.27–19.26 IU l−1; LH normal range: 1.24–8.62 IU l−1; T normal range: 1.75–7.81 μg l−1. s.d.: standard deviation; FSH: follicle-stimulating hormone; LH: luteinizing hormone; T: testosterone
Treatments
Among 29 patients, 17 (58.6%) had distal vas deferens obstruction (contralateral side) and could not undergo VE. All 17 patients underwent testicular sperm extraction instead. For the other 12 patients, the distal vas deferens (contralateral side) was not obstructed. Three of 12 (25.0%) patients underwent cross VE, while 9 of 12 (75.0%) underwent VE (Figure 1).
Figure 1.
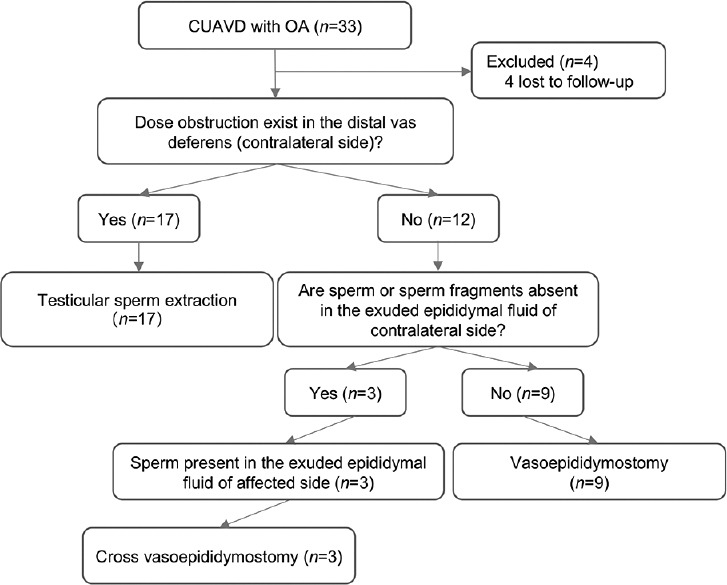
Flow diagram of the treatments for CUAVD patients with obstructive azoospermia. CUAVD: congenital unilateral absence of the vas deferens; OA: obstructive azoospermia.
Patency and natural pregnancy
For these 17 patients who underwent testicular sperm extraction, all patients successfully underwent sperm retrieval. ART was successfully performed in 92.3% (12/13) of patients.
The patency rate after VE or cross VE was 41.7% (5/12). Among the 5 couples, 1 couple achieved natural pregnancy, 1 couple achieved pregnancy through intracytoplasmic sperm injection (ICSI) using the sperm in the fresh ejaculate, and the other 3 couples were still trying to have a baby in a natural way (Table 3).
Table 3.
Treatments and outcomes for congenital unilateral absence of the vas deferens patients with obstructive azoospermia
| Patient number | Intraoperative finding | Intraoperative treatment | Postoperative treatment | Patency | Pregnancy |
|---|---|---|---|---|---|
| 1 | Obstruction in distal vas deferens (contralateral side) | TESE | ART | - | Yes |
| 2 | Obstruction in distal vas deferens (contralateral side) | TESE | ART | - | Yes |
| 3 | Obstruction in distal vas deferens (contralateral side) | TESE | ART | - | Yes |
| 4 | Obstruction in distal vas deferens (contralateral side) | TESE | None | - | - |
| 5 | Obstruction in distal vas deferens (contralateral side) | TESE | None | - | - |
| 6 | Obstruction in distal vas deferens (contralateral side) | TESE | ART | - | Yes |
| 7 | Obstruction in distal vas deferens (contralateral side) | TESE | ART | - | Yes |
| 8 | Obstruction in distal vas deferens (contralateral side) | TESE | ART | - | Yes |
| 9 | Obstruction in distal vas deferens (contralateral side) | TESE | ART | - | Yes |
| 10 | Obstruction in distal vas deferens (contralateral side) | TESE | ART | - | Yes |
| 11 | Obstruction in distal vas deferens (contralateral side) | TESE | ART | - | No |
| 12 | Obstruction in distal vas deferens (contralateral side) | TESE | ART | - | Yes |
| 13 | Obstruction in distal vas deferens (contralateral side) | TESE | ART | - | Yes |
| 14 | Obstruction in distal vas deferens (contralateral side) | TESE | None | - | - |
| 15 | Obstruction in distal vas deferens (contralateral side) | TESE | None | - | - |
| 16 | Obstruction in distal vas deferens (contralateral side) | TESE | ART | - | Yes |
| 17 | Obstruction in distal vas deferens (contralateral side) | TESE | ART | - | Yes |
| 18 | Sperm present in the exuded fluid of contralateral corpus epididymis | VE | ART | No | Yes |
| 19 | Sperm present in the exuded fluid of contralateral corpus epididymis | VE | None | No | No |
| 20 | Sperm present in the exuded fluid of contralateral cauda epididymis | VE | ART | No | Yes |
| 21 | Sperm absent in the exuded fluid of contralateral caput epididymis but present in the caput epididymis of affected side | Cross VE | None | No | No |
| 22 | Sperm present in the exuded fluid of contralateral cauda epididymis | VE | None | Yes | No |
| 23 | Sperm present in the exuded fluid of contralateral corpus epididymis | VE | ART | No | Yes |
| 24 | Sperm present in the exuded fluid of contralateral cauda epididymis | VE | None | Yes | Yesa |
| 25 | Sperm present in the exuded fluid of contralateral cauda epididymis | VE | ART | Yes | Yesb |
| 26 | Sperm absent in the exuded fluid of contralateral caput epididymis but present in the caput epididymis of affected side | Cross VE | None | Yes | No |
| 27 | Sperm absent in the exuded fluid of contralateral caput epididymis but present in the caput epididymis of affected side | Cross VE | None | No | No |
| 28 | Sperm present in the exuded fluid of contralateral cauda epididymis | VE | None | Yes | No |
| 29 | Sperm present in the exuded fluid of contralateral cauda epididymis | VE | None | No | No |
aNatural pregnancy; bachieved pregnancy by ART using sperm in the fresh ejaculate. ART: assisted reproductive technology; TESE: testicular sperm extraction; VE: vasoepididymostomy; -: unable to evaluate
DISCUSSION
First noted in 1755, CAVD has gradually been considered to be related to male infertility.11 CUAVD is one subtype of CAVD, with a prevalence of 0.5%–1.0% in men. Unlike patients with CBAVD who always present with OA, patients with CUAVD may present with azoospermia, oligozoospermia, or normospermia. In a recent study involving 63 patients with CUAVD, 25 (39.7%) patients demonstrated azoospermia, 17 (27.0%) oligozoospermia, and 21 (33.3%) normozoospermia.8 Thus, a large number of men with CUAVD can be fertile. However, most CUAVD cases are commonly confirmed when men come to the hospital with complaints of infertility, and this percentage could be as high as 93.75% (135/144).12
During embryonic development, the vas deferens, seminal vesicle, distal two-thirds of the epididymis, and ureteral-renal system share the same Wolffian origin. As a result, CAVD presents some other genotypic or phenotypic disorders in addition to the absence of the vas deferens.12,13 One of the most concerning comorbidities is renal abnormalities, which are always found in renal ultrasound examinations. Kidney anomalies include renal agenesis, ectopic kidney, renal absence, multicystic kidney, horseshoe kidney, and so on.5,8,14,15 Several studies have shown a higher prevalence of renal abnormalities in CUAVD patients than in CBAVD patients.5,12 However, the incidence of renal absence in CUAVD patients is uncertain. Luo et al.7 reported that none of the CUAVD patients had renal agenesis. Renal absence was observed in 29% (17/59) of CUAVD patients, as reported by Mieusset et al.8 In the present study, 6.9% (2/29) of patients presented with renal absence. The genetic link between CFTR variants and renal abnormalities in CUAVD patients has been widely discussed, and a recent meta-analysis showed that the higher risk of renal abnormalities in CUAVD patients was not related to CFTR variants. Thus, a large sample survey is needed to elucidate the association among CFTR variants, CUAVD, and the risk of renal abnormality.
To date, the diagnosis of CUAVD is based on preoperative scrotal palpation and ultrasound examination.8,16 For CUAVD patients with oligozoospermia or normospermia, the diagnosis of CUAVD is correct. However, this is not a guarantee in patients who present with azoospermia, since the integrity of the contralateral vas deferens is not certain. Some researchers suggested that CUAVD should be regarded as “incomplete CBAVD” or “developing CBAVD”.7 In the current cohort, obstruction of the distal vas deferens (contralateral side) was found in 17 patients during surgical exploration and these patients could not undergo microsurgical reconstruction. We believe that there are some potential CBAVD cases, rather than CUAVD cases. Hence, challenges remain for the diagnosis of CUAVD.
Microsurgical reconstruction and ART are alternative treatments for CUAVD patients with OA. The patency of the distal vas deferens (contralateral side) and the presence or absence of sperm or sperm fragments in the exuded epididymal fluid were key points for further surgical decision-making. In this cohort, testicular sperm extraction was performed in 17 (58.6%) patients, as the distal vas deferens was obstructed. For the other 12 patients, VE or cross VE was performed according to the presence or absence of sperm or sperm fragments in the exuded epididymal fluid. The patency rate was 41.7% (5/12), and the natural pregnancy rate was 20.0% (1/5). With the development of ART, ICSI can be a remedy for CUAVD patients with OA. In the present study, ICSI was successfully performed in 12 couples who could not undergo VE and in 1 couple who failed to achieve natural pregnancy after VE.
CUAVD with OA was attributed to 0.4% of infertility cases in men.17 In the past two decades, most studies have focused on the genetics of CUAVD. Studies have elucidated the link between several mutations and CUAVD, especially CFTR variants.6,12 However, the treatments and outcomes of CUAVD with OA have seldom been investigated. To the best of our knowledge, this is the first study on the treatments and outcomes of CUAVD with OA. Based on fertility status, CUAVD patients can be divided into two categories: fertile patients with a high incidence of renal dysplasia and infertile patients with partial obstruction of the contralateral vas deference.17 Thus, it is possible for CUAVD patients with OA to achieve natural pregnancy through reconstruction microsurgery. VE is an effective approach to achieve high patency and natural pregnancy in epididymal OA (EOA).10,18 The patency rate and pregnancy rate could reach 52%–92% and 11%–56%, respectively.19,20 Our results showed lower rates of patency and natural pregnancy than those in the above-published articles, with a patency rate of 41.7% (5/12) and a natural pregnancy rate of 20.0% (1/5). The reasons for the low rate may be as follows. First, bilateral VE obtains an increased recanalization rate compared with unilateral VE. It has been reported that 81.6% of EOA patients with bilateral VE achieved patency, while the rate of patients with unilateral VE decreased to 60.8%.18 Similarly, a recent review showed that the patency rate of patients who underwent bilateral VE exhibited a higher risk ratio of 1.38 compared to unilateral VE.21 For CUAVD patients with OA, only unilateral VE can be performed. Second, the comorbidities in CUAVD, such as epididymal agenesis and seminal vesicle agenesis/absence, may decrease the patency rate and natural pregnancy rate.
There are certain limitations in the present study. First, since the incidence of CUAVD is rare, our study was retrospectively designed and included a small number of patients. To provide a more convincing result, prospective, multicenter, and large sample cohort studies need to be performed in future. In addition, genetic factors were not examined in the present study. An increasing number of studies have demonstrated the role of genetic factors in CUAVD, and a better understanding of the correlation between genetic factors and outcomes of CUAVD will be helpful for preoperative and intraoperative decision-making.
CONCLUSIONS
In summary, CUAVD with OA is a rare clinical entity and co-exists with some comorbidities, such as renal absence. CUAVD patients with OA may manifest a low semen volume and pH value but normal hormone levels. VE or cross VE is still an alternative treatment for CUAVD patients with OA, although with relatively low rates of patency and natural pregnancy.
AUTHOR CONTRIBUTIONS
PL, ZL, and YHZ designed the study. YHZ, JJD, and NCL collected the data. YHZ, JJD, and PL analyzed the data and drafted the manuscript. ELZ, CCY, YHH, RHT, HRC, and HXC participated in its design and helped draft the manuscript. YBD, YXT, and FJZ helped draft and revise the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by grants from the Shanghai Science and Technology Innovation Action Plan Project (20Y11907600), National Natural Science Foundation of China (82001530), Shanghai Key Laboratory of Molecular Andrology (SLMA-014), and Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16020701).
REFERENCES
- 1.Bieth E, Hamdi SM, Mieusset R. Genetics of the congenital absence of the vas deferens. Hum Genet. 2021;140:59–76. doi: 10.1007/s00439-020-02122-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagenknecht LV, Lotzin CF, Sommer HJ, Schirren C. Vas deferens aplasia: clinical and anatomical features of 90 cases. Andrologia. 1983;15:605–13. doi: 10.1111/j.1439-0272.1983.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 3.Cioppi F, Rosta V, Krausz C. Genetics of azoospermia. Int J Mol Sci. 2021;22:3264. doi: 10.3390/ijms22063264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller S, Couture S, James G, Plourde S, Rioux J, et al. Unilateral absence of vas deferens: prevalence among 23,013 men seeking vasectomy. Int Braz J Urol. 2016;42:1010–7. doi: 10.1590/S1677-5538.IBJU.2015.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai H, Qing X, Niringiyumukiza JD, Zhan X, Mo D, et al. CFTR variants and renal abnormalities in males with congenital unilateral absence of the vas deferens (CUAVD): a systematic review and meta-analysis of observational studies. Genet Med. 2019;21:826–36. doi: 10.1038/s41436-018-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan P, Liang ZK, Liang H, Zheng LY, Li D, et al. Expanding the phenotypic and genetic spectrum of Chinese patients with congenital absence of vas deferens bearing CFTR and ADGRG2 alleles. Andrology. 2019;7:329–40. doi: 10.1111/andr.12592. [DOI] [PubMed] [Google Scholar]
- 7.Luo S, Feng J, Zhang Y, Yang X, Ma G, et al. Mutation analysis of the cystic fibrosis transmembrane conductance regulator gene in Chinese congenital absence of vas deferens patients. Gene. 2021;765:145045. doi: 10.1016/j.gene.2020.145045. [DOI] [PubMed] [Google Scholar]
- 8.Mieusset R, Bieth E, Daudin M, Isus F, Delaunay B, et al. Male partners of infertile couples with congenital unilateral absence of the vas deferens are mainly non-azoospermic. Andrology. 2020;8:645–53. doi: 10.1111/andr.12749. [DOI] [PubMed] [Google Scholar]
- 9.Li P, Yao CC, Zhi EL, Xu Y, Wan Z, et al. Modified stepwise mini-incision microdissection testicular sperm extraction: a useful technique for patients with a history of orchidopexy affected by non-obstructive azoospermia. J Zhejiang Univ Sci B. 2020;21:87–92. doi: 10.1631/jzus.B1900232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu N, Li P, Zhi E, Yao C, Yang C, et al. A modified single-armed microsurgical vasoepididymostomy for epididymal obstructive azoospermia: intraoperative choice and postoperative consideration. BMC Urol. 2020;20:121. doi: 10.1186/s12894-020-00692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson RE. Congenital absence of the vas deferens;a review of the literature and report of three cases. J Urol. 1950;63:176–82. doi: 10.1016/S0022-5347(17)68750-0. [DOI] [PubMed] [Google Scholar]
- 12.Akinsal EC, Baydilli N, Dogan ME, Ekmekcioglu O. Comorbidity of the congenital absence of the vas deferens. Andrologia. 2018;50:e12994. doi: 10.1111/and.12994. [DOI] [PubMed] [Google Scholar]
- 13.Gibbons MD, Cromie WJ, Duckett JW., Jr Ectopic vas deferens. J Urol. 1978;120:597–604. doi: 10.1016/s0022-5347(17)57294-8. [DOI] [PubMed] [Google Scholar]
- 14.Fedder J, Jørgensen MW, Engvad B. Prevalence of CBAVD in azoospermic men carrying pathogenic CFTR mutations –evaluated in a cohort of 639 non-vasectomized azoospermic men. Andrology. 2021;9:588–98. doi: 10.1111/andr.12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salwan A, Abdelrahman A. Congenital absence of vas deferens and ectopic kidney. Int J Surg Case Rep. 2017;34:90–2. doi: 10.1016/j.ijscr.2017.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolettis PN, Sandlow JI. Clinical and genetic features of patients with congenital unilateral absence of the vas deferens. Urology. 2002;60:1073–6. doi: 10.1016/s0090-4295(02)01973-8. [DOI] [PubMed] [Google Scholar]
- 17.Weiske WH, Sälzler N, Schroeder-Printzen I, Weidner W. Clinical findings in congenital absence of the vasa deferentia. Andrologia. 2000;32:13–8. [PubMed] [Google Scholar]
- 18.Peng J, Zhang Z, Yuan Y, Cui W, Song W. Pregnancy and live birth rates after microsurgical vasoepididymostomy for azoospermic patients with epididymal obstruction. Hum Reprod. 2017;32:284–9. doi: 10.1093/humrep/dew331. [DOI] [PubMed] [Google Scholar]
- 19.Zhang GX, Bai WJ, Xu KX, Wang XF, Zhu JC. Clinical observation of loupe-assisted intussusception vasoepididymostomy in the treatment of obstructive azoospermia (analysis of 49 case reports) Asian J Androl. 2009;11:193–9. doi: 10.1038/aja.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan PT. The evolution and refinement of vasoepididymostomy techniques. Asian J Androl. 2013;15:49–55. doi: 10.1038/aja.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon YE, Lee HH, Park SY, Moon HS, Kim DS, et al. The role of vasoepididymostomy for treatment of obstructive azoospermia in the era of in vitro fertilization: a systematic review and meta-analysis. Asian J Androl. 2018;21:67–73. doi: 10.4103/aja.aja_59_18. [DOI] [PMC free article] [PubMed] [Google Scholar]


