Abstract
Background:
Drug resistant epilepsy (DRE) patients not amenable to epilepsy surgery can benefit from neurostimulation. Few data compare different neuromodulation strategies.
Objective:
Compare five invasive neuromodulation strategies for treatment of DRE: anterior thalamic nuclei deep brain stimulation (ANT-DBS), centromedian thalamic nuclei DBS (CM-DBS), responsive neurostimulation (RNS), chronic subthreshold stimulation (CSS), and vagus nerve stimulation (VNS).
Methods:
Single center retrospective review and phone survey for patients implanted with invasive neuromodulation for 2004–2021.
Results:
N=159 (ANT-DBS=38, CM-DBS=19, RNS=30, CSS=32, VNS=40). Total median seizure reduction (MSR) was 61% for the entire cohort (IQR 5–90) and in descending order: CSS (85%), CM-DBS (63%), ANT-DBS (52%), RNS (50%), and VNS (50%); p=0.07. Responder rate was 60% after median follow-up time of 26 months. Seizure severity, life satisfaction and quality of sleep were improved. Cortical stimulation (RNS and CSS) was associated with improved seizure reduction compared to subcortical stimulation (ANT-DBS, CM-DBS, and VNS) (67% vs. 52%). Effectiveness was similar for focal epilepsy vs generalized epilepsy, closed loop vs open loop stimulation, pediatric vs. adult cases, and high frequency (>100 Hz) vs low frequency (<100 Hz) stimulation settings. Delivered charge per hour varied widely across approaches but was not correlated with improved seizure reduction.
Conclusions:
Multiple invasive neuromodulation approaches are available for treatment of DRE but little evidence compares the approaches. This study used a uniform approach for single center results and represents an effort to compare neuromodulation approaches.
Keywords: Neurostimulation, deep brain stimulation, responsive neurostimulation, chronic subthreshold stimulation, vagus nerve stimulation, low frequency stimulation
Introduction
Epilepsy is a highly prevalent disease that affects up to 1.2% of the United States population [1]. In about one third of cases, two or more appropriately dosed and tolerated anti-seizure drugs (ASD) fail to attain seizure freedom putting patients at risk of the multitude of comorbidities associated with drug-resistant epilepsy (DRE) [2, 3]. In these cases, ablative/resective epilepsy surgery when feasible is the next best step to reach seizure freedom [4]. However, this might not be an option for individuals with generalized epilepsies, diffuse and/or multifocal seizure onset, eloquent cortex involvement, or per patient preference. Additionally, some individuals continue to have seizures despite epilepsy surgery.
Neuromodulation provides a palliative approach to DRE cases. The FDA has approved three neuromodulation strategies for focal DRE in adults. Vagus nerve stimulation (VNS) provides intermittent delivery of an electrical stimulus through a bipolar lead connecting the left vagus nerve to an implantable pulse generator (IPG). VNS may exert its effect by modulating the activity of brainstem nuclei [5, 6]. Responsive neurostimulation (RNS) is a closed loop system that detects intracranial epileptiform activity arising from one or two seizure foci and stimulates in response to detected events according to physician-programed settings [7]. RNS was designed to interrupt ictal discharges as its main mechanism of action; however, long term indirect neuromodulatory effects likely account for a large proportion of its benefit [8, 9]. Anterior thalamic nuclei deep brain stimulation (ANT-DBS) involves open loop stimulation that provides seizure lowering benefits by disrupting epileptogenic networks. The network theory of DBS suggests this effect is mediated through seizure network connections with the Papez circuit [10–12].
Other approaches not approved by the FDA may also be helpful. Generalized and posterior onset epilepsies have distinct connectivity and may benefit more from different thalamic nuclei stimulation such as centromedian and pulvinar nuclei [13–15]. Only VNS is FDA approval for the pediatric population, which limits access [16]. Larger epileptogenic zones may benefit from broader electrode coverage than allowed by two lead systems[17], such as via Chronic Subthreshold Stimulation (CSS) [17]. CSS involves open loop stimulation of cortical areas, at times in conjunction with subcortical targets, with up to 4 leads and 16 electrode contacts; stimulation is provided using FDA-approved hardware on an off-label basis. The optimal area of stimulation is delimited by invasive EEG, including stereo electroencephalography (sEEG) or subdural EEG. If the patient is not a surgical resection candidate, once the seizure onset zone is delineated via the standard of care process of capturing seizures, the patient undergoes a trial of stimulation using the temporary invasive EEG leads to help determine the suitability of long-term electrical stimulation [18–20]. This is usually due to eloquent cortex involvement. Response to the stimulation trial is determined by changes in seizure frequency, any patient-reported symptoms, and changes in interictal epileptiform activity (IEA). If successful, permanent hardware is subsequently implanted [21]. CSS has been associated with responder rates as high as 89% [19, 20].
One challenge is the number of neuromodulation treatment options without clear data to guide decision-making. Our goal is to compare multiple invasive neuromodulation approaches for pediatrics and adults from our center.
Methods
Study methodology
After Institutional Review Board approval, we performed a retrospective review and telephone survey of patients implanted with ANT-DBS, centromedian thalamic nuclei deep brain stimulation (CM-DBS), CSS, RNS, and VNS at Mayo Clinic Rochester from August 2004 to July 2021. For the VNS patient cohort, we included a random sample of 40 patients from all first-time implants at our center. All patients were diagnosed with DRE epilepsy according to ILAE criteria prior to implantation [22]. The multiple stimulation approaches presented from the past 16 years reflect an effort to individualize therapy for patients and improve outcomes. De-identified data is available upon request.
Variables
Through a standardized telephone questionnaire, we assessed seizure frequency, patient-perceived outcomes, and side effects from stimulation or from the device as has been used by our group previously [15, 20]. For current seizure frequency and pre-implantation seizure frequency, patients were instructed to consider only disabling seizures defined as “seizures that interrupt day-to-day activities” over the past three months and the past three months prior to implantation. If patients expressed uncertainty about the meaning of disabling seizures, all seizures were considered. When seizure freedom was reported, its duration beyond three months was not assessed. Convulsive seizures were defined as “seizures where both arms and legs stiffen up and shake rhythmically while unconscious”. The seizure frequency data obtained from the questionnaire was compared to the medical record to ensure consistency and decrease recall bias. If there was significant discordance, the case was reviewed (JLAZ, BNL). For patients that did not answer the standardized questionnaire, seizure frequency data was obtained from medical records. For one patient (CSS) there was no disabling seizure frequency data in the medical record and the patient could not be contacted by telephone. Patient perceived outcomes including seizure severity, life satisfaction, and quality of sleep were obtained through numeric analog scales ranging from 1–10. Stimulation related side effects and device related side effects were subclassified by type and were ascertained by medical record review and standardized questionnaire for maximal sensitivity. In the case of pediatric patients, documented cognitive impairment, epileptic encephalopathy, or unreliable tracking of seizure frequency, parents or family members answered the questionnaire. Deceased patients, patients living internationally, and those requiring an interpreter were not included in the questionnaire call list. Follow up time was defined as time from implantation until questionnaire assessment if the device remained active, or until the last documentation of the device being active in case of explantation, deactivation or lack of standardized questionnaire data. When costimulation with VNS was present, the questionnaire focused on the intracranial device. Thalamic DBS patients implanted with both CM and ANT leads (4-lead system) were grouped with patients implanted exclusively with CM (2-lead system).
The reported neurostimulation parameters are the last documented set of parameters in the medical record prior to the cross-sectional assessment. We calculated the charge per hour per lead reported as millicoulombs per hour (mC/h) to compare between neuromodulation groups. Stimulation parameter details are available in the supplementary material.
To assess the possible role of ASDs in the perceived benefit we reported ASD change, in which positive numbers reflect an increase in the total number of prescribed ASDs and negative numbers correspond to fewer ASDs compared to baseline.
Statistics
SPSS Version 27.0 (Armonk, NY) and GraphPad Prism V 9.3.1, GraphPad Software (San Diego, CA) were used for statistical analysis, logistic regression and chart generation. Continuous and categorical variables are described as median with interquartile range (IQR) or range and percentages, respectively. The Chi-squared test was used for comparison of frequencies while Mann-Whitney U, Wilcoxon signed ranks, or Kruskal-Wallis tests were used for median comparison between groups as appropriate. We used Mann-Whitney U tests and Chi-Square /Fisher exact tests for pairwise comparisons as a pre-planned secondary step when comparing across neuromodulation groups or seizure onset groups. The Bonferroni correction method was used post-hoc to adjust for multiple comparisons regarding seizure reduction and responder rate and is reported after the unadjusted pairwise comparisons. Spearman Rho was used for correlation analysis. We explored the relation between clinically relevant variables and clinical response (≥50% seizure frequency reduction) with univariate logistic regression. Variables with a p value <0.25 were selected to perform a multivariate logistic regression. The p-value cut off has been previously used as the initial step for purposeful covariate selection [23]. The area under the receiving operating curve (AUC) was used as a measure of discrimination power. P values ≤0.05 were considered statistically significant.
Results
Baseline characteristics
A total of 164 patients were initially reviewed for the study and included: ANT-DBS (n=38), CM-DBS (n=19), CSS (n=32), RNS (n=35) and VNS (n=40). Seven RNS patients had their initial implant elsewhere, five of whom were excluded and two of whom were included as they had implant revision with lead repositioning surgery at our center and continued to follow for programming changes. For our analysis, 159 patients were included (Table 1).
Table 1:
Baseline Characteristics
| Variable | Total, n=159 |
ANT-DBS n=38 |
CM-DBS n=19 |
RNS n=30 |
CSS n=32 |
VNS N=40 |
P value |
|---|---|---|---|---|---|---|---|
| Age, y; median (Range) | 25 (6–71) | 30 (15–71) | 19 (6–51) | 39 (14–65) | 22 (6–57) | 27 (6–69) | <0.001 |
| Female sex; % (n) | 54 (86) | 50 (19) | 63 (12) | 63 (19) | 50 (16) | 48 (19) | 0.59 |
| Epilepsy duration, y; median (Range) | 15 (1–53) | 18 (2–53) | 15 (5–50) | 18 (5–51) | 12 (1–50) | 12 (5–24) | 0.07 |
| Median disabling seizure frequency, sz/mo; median (IQR) | 10 (4–38) | 8.5 (4.5–28.5) | 30 (6–105) | 7.3 (3.9–12.4) | 20 (4–131.3) | 16 (2.6–42.3) | 0.15 |
| Median convulsive seizure frequency, sz/y; median (IQR)* | n=95 4 (0–36) |
n=24 0 (0–1.5) |
n=14 20.3 (1.5–102) |
n=18 2.3 (0–34.5) |
n=20 21 (0.5–34.5) |
n=19 18 (0.5–52) |
0.004 |
| Median ASDs tried prior to implant; median (Range)** | 5 (0–22) | 6 (1–16) | 8 (2–16) | 4 (1–9) | 4 (0–11) | 4 (0–22) | <0.001 |
| Median ASDs taken at time of implant; median (Range) | 3 (1–7) | 3 (1–5) | 3 (1–7) | 2 (1–4) | 3 (1–6) | 3 (1–5) | 0.06 |
| Prior epilepsy surgery; % (n) | |||||||
| Anterior temporal lobectomy | 8 (13) | 11 (4) | 11 (2) | 13 (4) | 0 (0) | 13 (5) | 0.69 |
| Neocortical resection | 9 (15) | 16 (6) | 0 (0) | 0 (0) | 9 (3) | 15 (6) | 0.08 |
| Corpus callosotomy | 3 (5) | 0 (0) | 21 (4) | 0 (0) | 0 (0) | 3 (1) | <0.001 |
| Subpial transection | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (1) | 0.56 |
| Other | 1 (2) | 0 (0) | 0 (0) | 7 (2) | 0 (0) | 0 (0) | 0.07 |
| Epilepsy etiology; % (n) | |||||||
| Structural | 46 (76) | 58 (22) | 16 (3) | 51 (18) | 50 (16) | 43 (17) | 0.04 |
| Genetic | 2 (4) | 0 (0) | 16 (3) | 0 (0) | 0 (0) | 3 (1) | 0.003 |
| Infectious | 6 (9) | 11 (4) | 11 (2) | 3 (1) | 3 (1) | 3 (1) | 0.41 |
| Immune | 4 (6) | 3 (1) | 0 (0) | 6 (2) | 3 (1) | 5 (2) | 0.89 |
| Mixed | 4 (7) | 0 (0) | 0 (0) | 3 (1) | 6 (2) | 10 (4) | 0.08 |
| Unknown | 38 (62) | 29 (11) | 58 (11) | 37 (13) | 38 (12) | 38 (15) | 0.34 |
| Localization of seizure onset; % (n) | |||||||
| Frontal | 9 (15) | 5 (2) | 0 (0) | 10 (3) | 22 (7) | 8 (3) | 0.07 |
| Temporal | 27 (43) | 18 (7) | 5 (1) | 78 (23) | 9 (3) | 23 (9) | <0.001 |
| Paracentral | 13 (20) | 3 (1) | 0 (0) | 6 (2) | 50 (16) | 2 (1) | <0.001 |
| Posterior | 4 (6) | 8 (3) | 5 (1) | 0 (0) | 3 (1) | 2 (1) | 0.51 |
| Diffuse/Multifocal | 23 (37) | 55 (21) | 5 (1) | 6 (2) | 16 (5) | 20 (8) | <0.001 |
| Generalized | 9 (15) | 0 (0) | 42 (8) | 0 (0) | 0 (0) | 18 (7) | <0.001 |
| Mixed† | 15 (23) | 11 (4) | 42 (8) | 0 (0) | 0 (0) | 27 (11) | <0.001 |
| Invasive EEG; % (n) | 52 (82) | 55 (21) | 16 (3) | 63 (19) | 100 (32) | 18 (7) | <0.001 |
ANT-DBS, anterior thalamic nuclei deep brain stimulation; ASDs, anti-seizure drugs; CM-DBS, centromedian thalamic nuclei deep brain stimulation; CSS, chronic subthreshold stimulation; IQR, interquartile range; RNS, responsive neurostimulation; sz/mo, seizures per month; sz/y, seizures per year; VNS, vagus nerve stimulation; y, years.
Information not available/applicable for all patients
Excluding medications already taken by patient at time of implant
Generalized onset with another onset
There were significant baseline differences across the different neuromodulation groups. RNS patients were older at time of implant while CM-DBS and CSS patients were younger at implant (H(4)=22.27, p<0.001). ANT-DBS and CM-DBS had tried more ASDs prior to implant than RNS, CSS, and VNS (H(4)=21.04, p<0.001). Thirty-five patients had previous epilepsy surgery (22%). Of those, corpus callosotomy, which was present only in CM-DBS and VNS patients, was significantly more common amongst CM-DBS patients (Χ2(4)=24.18, p<0.001). When an epilepsy etiology was identified, structural abnormalities were the most common cause and were approximately evenly distributed between ANT-DBS, CSS, RNS and VNS (Χ2(4)=10.16, p=0.04) while a genetic etiology was significantly more common in the CM-DBS group (Χ2(4)=16.23, p=0.003). Seizure onset varied significantly across neuromodulation groups as well. Temporal onset was significantly more prevalent in RNS patients (Χ2(4)=48.92, p<0.001) while paracentral onset was more common in the CSS group (Χ2(4)=51.55, p<0.001). ANT-DBS had the highest prevalence of multifocal/diffuse seizure onset (Χ2(4)=31.15, p<0.001). As expected, patients with generalized epilepsy were only present in the CM-DBS and VNS groups, with CM-DBS having significantly more (Χ2(4)=37.20, p<0.001). Patients with combined focal and generalized epilepsy were present in the ANT-DBS, CM-DBS, and VNS groups with CM-DBS having significantly more (Χ2(4)=28.19, p<0.001). Figure 1 provides a visualization of the different approaches and the distribution of seizure onset according to neuromodulation strategy.
Figure 1: Different Modalities of Invasive Brain Stimulation and Distribution of Patients by Seizure Onset across Neurostimulation Modalities –
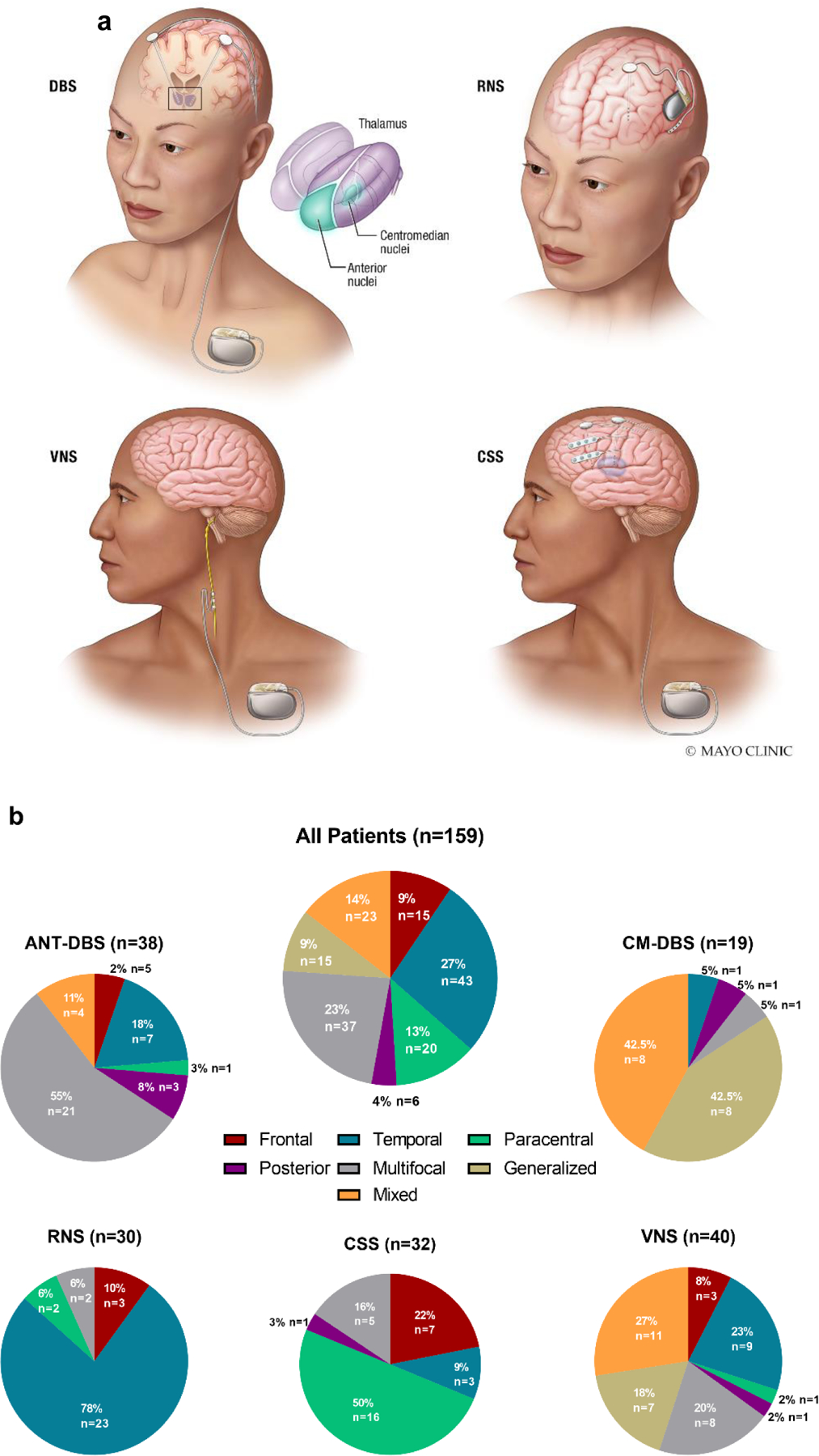
(a) Visual representation of the invasive neuromodulation strategies included in the present study. The top left panel represents deep brain stimulation (DBS) of either the anterior thalamic nuclei, centromedian nuclei or both. The top right panel represents responsive neurostimulation (RNS) with a depth lead and a subcortical lead (8 contacts total). The bottom left panel represents vagus nerve stimulation (VNS). The bottom right panel represents chronic subthreshold stimulation (CSS) with two depth electrodes and two subdural strips (16 contacts total). DBS, VNS and CSS are connected to an implantable pulse generator (IPG) placed in the chest while RNS has cranial-mounted IPG. (b) Paracentral epilepsy reflects primary sensory and/or primary motor seizure onset. Posterior epilepsy reflects patients with parietal/occipital onset. Mixed epilepsy signifies generalized epilepsy in combination with focal/multifocal epilepsy.
ANT-DBS, anterior thalamic nuclei deep brain stimulation; CM-DBS, centromedian thalamic nuclei deep brain stimulation; CSS, chronic subthreshold stimulation; RNS, responsive neurostimulation; VNS, vagus nerve stimulation.
Seizure frequency reduction, responder rate, logistic regression analyses, and patient perceived outcomes
Table 2 and Figure 2 summarize overall outcomes, including median seizure reduction (MSR), responder rates (at least 50% seizure reduction), and reported seizure freedom. Median follow-up time was 26 months (IQR 13–52) and was significantly different across groups (H(4)=21.97, p<0.001). Patients with VNS had significantly longer follow-up times than ANT-DBS (p<0.001), RNS (p<0.001), and CSS (p=0.01). Neuromodulation was associated with a 61% MSR (IQR 5–90) with no significant differences at the group level (H(4)=8.6, p=0.07). In pairwise comparisons, CSS was associated with higher seizure reduction than ANT-DBS (52%, IQR 15–86, p=0.01), CM-DBS (63%, IQR 0–85; p=0.04), RNS (50%, IQR 31–89; p=0.04) and VNS (50%, IQR 0–90; p=0.02). These findings were not significant after correcting for multiple comparisons (p-values > 0.005, Bonferroni correction). Responder rate was 60% for the whole cohort and not significantly different between approaches (Χ2(4)=6.98, p=0.14). In pairwise comparisons CSS had a higher frequency of responders (81%, n=25), compared to ANT (55%, n=21; p=0.03), RNS (56%, n=17; p=0.04), and VNS (53%, n=21; p=0.01). These findings were not significant after correcting for multiple comparisons (p-values > 0.005, Bonferroni correction). Twenty-two patients (14%) reported seizure freedom at last follow up for at least the three months prior; this was more prevalent in the CSS group (9 patients, 29%; Χ2(4)=10.05, p=0.04). Neuromodulation was associated with a 70% overall reduction in convulsive seizures (p<0.001). CM-DBS and VNS were not associated with a significant improvement in convulsive seizure frequency when analyzed on an individual group basis (p=0.09 and p=0.06, respectively). Perceived seizure severity and life satisfaction were significantly improved for the whole cohort (p<0.001) and for all neuromodulation modalities individually (p<0.03). Quality of sleep was improved for the whole cohort (p<0.001); but on individual group analysis ANT-DBS, RNS and VNS did not show significant improvement (p=0.17, p=0.11, p=0.05; respectively). The median ASD did not change at last follow up (Range −3 to 7), and there were no significant differences across neurostimulation groups (H(4)=1.23, p=0.87).
Table 2:
Summary of Results
| Result | Total | ANT-DBS | CM-DBS | RNS | CSS | VNS | P value |
|---|---|---|---|---|---|---|---|
| Median Seizure Frequency Reduction % (IQR) | 61 (IQR 5–90)§ | 52 (IQR 15–86) | 63 (IQR 0–85) | 50 (IQR 31–89) | 85 (IQR 57–100)† | 50 (IQR 0–90) | 0.07 |
| Responder Rate % (n) | 60 (95) | 55 (21) | 58 (11) | 56 (17) | 81 (25) | 53 (21) | 0.14 |
| Seizure freedom for at least 3 mo at last FU % (n) | 14 (22) | 11 (4) | 17 (5) | 29 (9) | 10 (4) | 0.04 | |
| Median Convulsive Seizure Frequency Reduction % (IQR) | 70 (0–97)§ | 95 (25–100) | 63 (0–95)‡ | 63 (40–100) | 75 (30–100) | 54 (0–94)‡ | 0.57 |
| Seizure Severity Improvement (Range)* | 2 (−4 to 9)§ | 2 (−3 to 7) | 2 (−3 to 7) | 2 (−4 to 7) | 3 (−3 to 8) | 3 (−3 to 9) | 0.51 |
| Life Satisfaction Improvement (IQR)** | 2 (−7 to 9) § | 1 (−7 to 6) | 2 (−6 to 6) | 2 (−6 to 6) | 3 (−5 to 8) | 2 (−6 to 9) | 0.58 |
| Quality of Sleep Improvement (IQR)** | 1 (−6 to 9)§ | 1 (−4 to 5) ‡ | 0 (−1 to 9) | 1 (−5 to 8) ‡ | 2 (−6 to 8) | 1 (−3 to 7) ‡ | 0.14 |
| Follow-up Time mo (IQR) | 26 (13–52) | 23 (11–28) | 27 (17– 81) | 19 (10–30)‡ | 32 (9–59) | 50 (26–73) | <0.001 |
ANT-DBS, anterior thalamic nuclei deep brain stimulation; CM-DBS, centromedian thalamic nuclei deep brain stimulation; CSS, chronic subthreshold stimulation; IQR, interquartile range; mo, months; RNS, responsive neurostimulation; VNS, vagus nerve stimulation.
Pre-implantation value minus post-implantation value, higher numbers indicate a decrease in seizure severity, expressed as mean value with range.
Post-implantation value minus pre-implantation value, higher numbers indicate an increase in life satisfaction and quality of sleep, expressed as mean values with ranges.
p<0.001 Wilcoxon signed rank test compared to a median of 0
Significantly different in unadjusted pairwise comparisons (Mann-Whitney U) with ANT-DBS (p=0.01), CM-DBS (p=0.04), RNS (0.04) and VNS (p=0.02)
Not statistically significant on individual group test between pre and post implantation values (Wilcoxon signed-rank test)
Figure 2: Individual Seizure Frequency Reduction and Responder Rate –
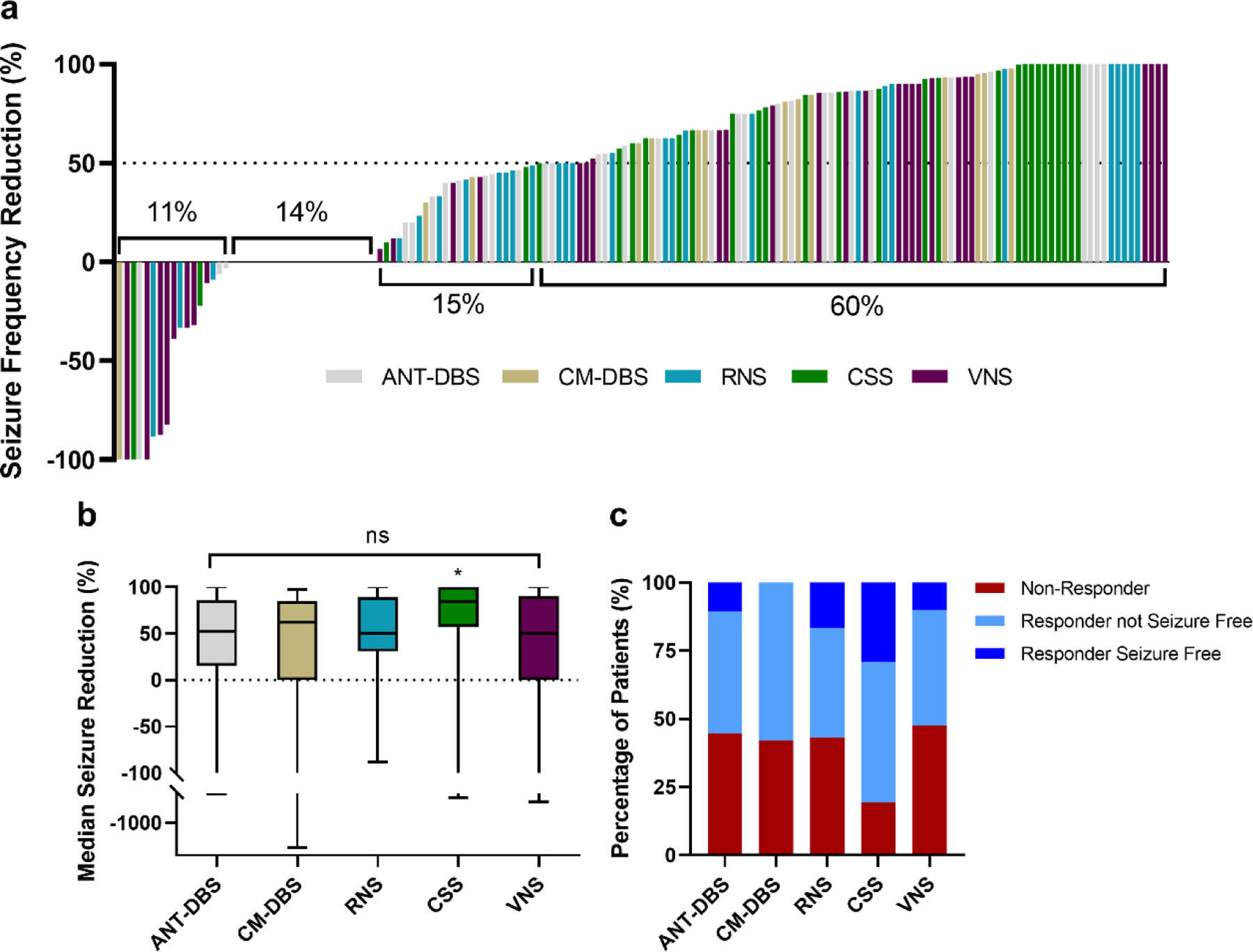
(a) Individual seizure frequency reduction (n = 159) from worse (upper left) to best (upper right). Each bar is color coded by neuromodulation strategy. The segment with no bars represents patients who had no change in seizure frequency (6 ANT-DBS patients, 5 CM-DBS patients, 2 RNS patients, 2 CSS patients and 7 VNS patients). (b) MSR was not different across neuromodulation strategies at the group level, although CSS was associated with an improved MSR compared to ANT-DBS, CM-DBS, RNS, and VNS in pairwise comparisons (* indicates p values < 0.05, unadjusted). (c) Responder rates and seizure freedom over past 3 months.
ANT-DBS, anterior thalamic nuclei deep brain stimulation; CM-DBS, centromedian thalamic nuclei deep brain stimulation; CSS, chronic subthreshold stimulation; RNS, responsive neurostimulation; VNS, vagus nerve stimulation
Multiple simultaneous stimulation approaches
Eleven CM-DBS patients were also implanted with ANT leads (four-lead system). There was no difference in MSR for patients with only CM stimulation (65%, IQR 0–82) and simultaneous CM and ANT stimulation (60%, IQR 0–95; p=0.59). Out of the 119 patients with an intracranial stimulation modality, 58(49%) had VNS implanted previously and 34(28%) of them continued to have active VNS stimulation at the time of implantation of the intracranial device. Most were ANT-DBS (n=20, 53%) with the remainder in CM-DBS (n=5, 26%), RNS (n=7, 23%) and CSS (n=2, 6%).
Further analyses
In general, MSR was similar across location of seizure onset (Figure 3). Seizure onset was divided into frontal, temporal, paracentral (primary sensory and/or primary motor seizure onset), posterior onset (parietal/occipital onset), multifocal or diffuse, generalized, and mixed (implying generalized onset in addition to focal/multifocal onset). MSR was not significantly different across groups in the one-way comparison (H(6)=0.17, p=0.17). In pairwise comparisons, frontal onset epilepsies (90%, IQR 57–100) showed an improved MSR compared to posterior (45%, IQR 0–68; p=0.049) and multifocal onsets (50%, IQR 0–26, p=0.02) but not temporal (59%, IQR 33–87; p=0.1), paracentral (85%, IQR 0–99%, p=0.29), generalized (50%, IQR 0–98; p=0.05), or mixed onsets (67%, IQR 0–93; p=0.09). Pairwise comparisons were not significant after correcting for multiple comparisons (p-values > 0.0024, Bonferroni correction).
Figure 3: Seizure Reduction by Seizure Onset Location and Other Characteristics –
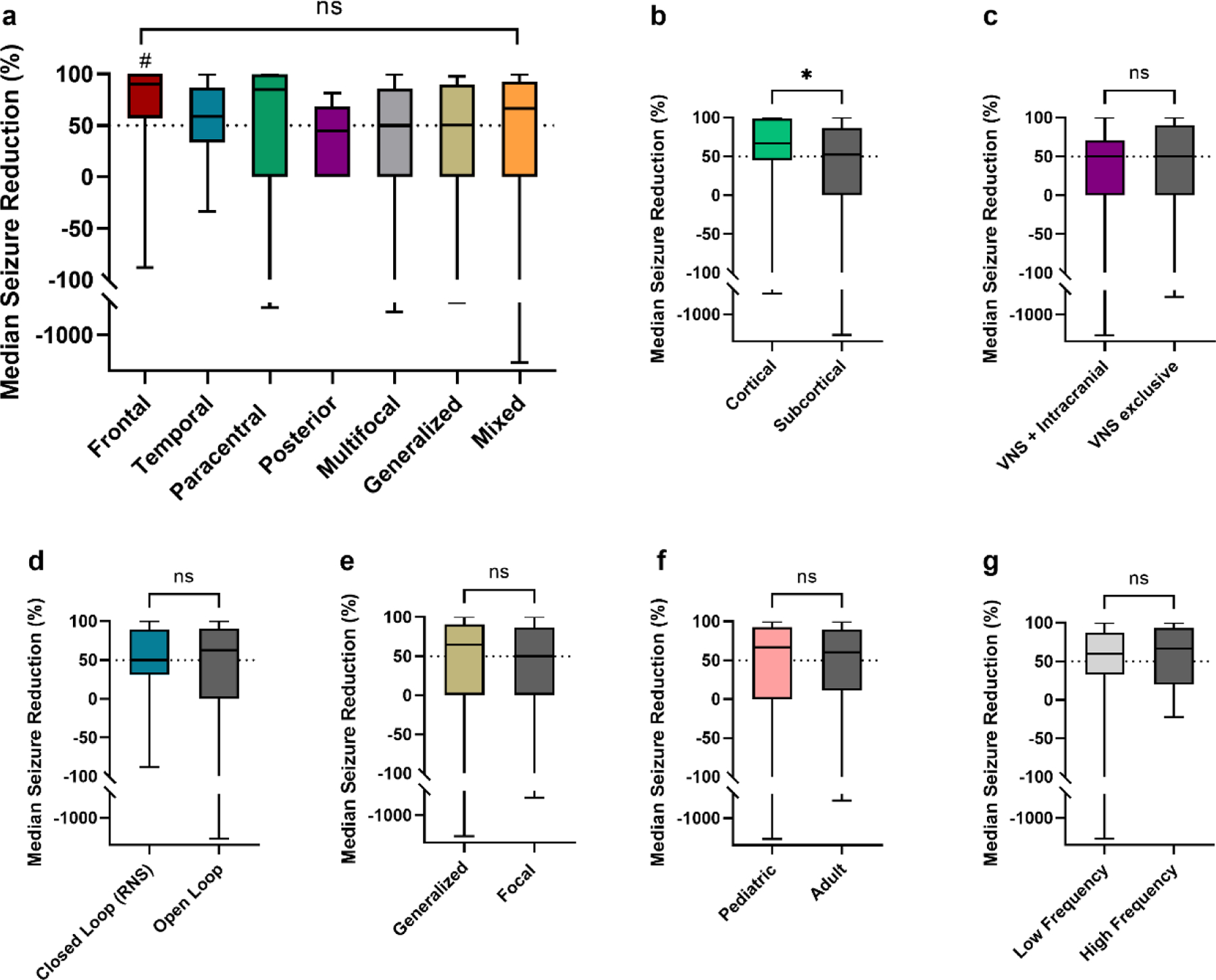
(a) MSR was not different across seizure onset groups. In pairwise comparions, frontal onset epilepsy patients (n=15) showed improved MSR compared to posterior (n=6) and multifocal patients (n=37) (# = p values < 0.05, unadjusted); but not compared to temporal (n=43), paracentral (n=20), generalized (n=15) and mixed (n=23) onsets. (b) Cortical stimulation (RNS and CSS, n=62) compared to subcortical stimulation (DBS and VNS, n=97) showed improved outcomes (* p = 0.03, unadjusted). (c) There was no difference in outcomes for active VNS device at the time of implantation with intracranial neurostimulation (DBS, RNS, CSS, n=34) vs. patients with only VNS stimulation (n=40). (d) There was no difference between closed loop stimulation (RNS) and open loop stimulation (DBS, CSS, VNS). (e) In patients implanted with subcortical stimulation devices (ANT-DBS, CM-DBS and VNS) seizure reduction was non significantly different in patients with generalized and mixed epilepsies (n=38) compared to focal and multifocal epilepsies (n=59). (f) There was no difference in MSR for pediatric patients (<18 years old at the time of implantation, n=37) vs. adults (≥18 years old at the time of implantation, n=122). (g) DBS, RNS and CSS patients with low frequency stimulation (<100 Hz) at last follow up (n=72) had comparable MSR than those with high frequency stimulation at last follow-up (n=47).
ANT-DBS, anterior thalamic nuclei deep brain stimulation; CM-DBS, centromedian thalamic nuclei deep brain stimulation; CSS, chronic subthreshold stimulation; RNS, responsive neurostimulation; VNS, vagus nerve stimulation.
For patients with temporal epilepsy (n=43, 27%), neocortical temporal patients (n=9, 6%) were distributed relatively evenly across the different neuromodulation groups except for CM-DBS, which had only one temporal epilepsy patient with mesial onset (Χ2(4)=2.07, p=0.723). Patients with mesial temporal epilepsy (n=34, 21%) were typically implanted with RNS (n=21, 70% of RNS patients) when compared to other modalities (Χ2(4)=55.71, p<0.001). MSR was comparable in the neocortical group vs. the mesial group (59%, IQR 31–88, vs. 59%, IQR 33–87; p=0.92).
Primarily cortical neurostimulation (CSS, RNS) (67%; IQR 45–99) was associated with improved seizure frequency than subcortical stimulation (ANT-DBS, CM-DBS, VNS) (52%; IQR 0–87, p=0.03). MSR of closed loop stimulation (RNS) (50%, IQR 31–89) was not significantly different than open loop stimulation (ANT-DBS, CM-DBS, CSS, and VNS) (63%, IQR 0–90; p=0.91). Neurostimulation with only VNS had a similar MSR as patients with intracranial neurostimulation and ongoing active VNS stimulation (50%, IQR 0–90 vs. 50%, IQR 0–71; p=0.77). For patients treated with subcortical stimulation devices (DBS and VNS) MSR was not significantly different between those with generalized and mixed epilepsy (65%, IQR 0–91) vs. focal and multifocal epilepsy (50%, IQR 0–87; p=0.81). Neurostimulation in pediatric patients (n=37, 23%) was associated with comparable MSR to adults (n=121, 77%), (67%; IQR 0–93 vs. 60%; IQR 11–89; p=0.96).
Adverse effects and deaths
A total of 127 AEs were reported by 77 patients and were classified as stimulation related (76 AEs in 51 patients), device related (49 AEs in 37 patients) and implantation surgery related side effects (6 AEs in 6 patients) (Supplementary table 3). The most common stimulation related side effect was dysphonia/hoarseness and/or throat discomfort in 14% of patients and was only reported by VNS patients; VNS was associated with more sided effects (Χ2(4)=27.19, p<0.001) than other modalities. The most common device related side effect was discomfort from leads and was present in 10% of patients. Four patients had neuropsychiatric AEs and all were part of the ANT-DBS subcohort (11%); three individuals reported word finding difficulties, and one individual had new onset severe depression that resolved with change in stimulation frequency.
A total of 16 patients required surgery due to AEs. The overall identified infection rate for the cohort was 3% (5 patients total). Six patients had immediate postprocedural complications none of which had lasting deficits. A total of 4 deaths were noted during the follow-up period. One ANT patient passed away due to SUDEP. None of the deaths were thought secondary to neurostimulation. A narrative description of the individual cases requiring surgery due to AEs, implantation related side effects, postprocedural complications and deaths can be found in the supplementary material.
Stimulation parameters
CM-DBS (63.5 mC/h, Range 1.1–144.7) and ANT-DBS (28.4 mC/h, Range 2.6–468) delivered more charge per hour than CSS, VNS, and RNS (6.5 mC/h, Range 0.1–835.9; 8.0 mC/h, Range 0.7–33.8; and 0.9 mC/h, Range 0–12.8, respectively)(H(4)=78.44, p<0.05) (Table 3). In unadjusted pairwise comparisons the only two neuromodulation strategies that did not show differences between their charge per hour were CSS with VNS (p=0.98). There was no correlation between MSR and charge per hour (r= −0.08, 95%CI −0.25 to 0.07; p = 0.27). Although DBS and RNS are typically programmed using high frequencies (e.g. 145 Hz), we have tended to trial both low (e.g. 7 Hz) as well as high frequencies in at least some patients. CSS typically employed lower stimulation frequencies than other approaches, with a median frequency of 2 Hz. Low frequency stimulation (<100 Hz) was being used at last follow up in 55% of ANT-DBS patients (n=21), 58% of CM-DBS patients (n=11), 41% of RNS patients (n=12), and 84% of CSS patients (n=27). Patients with VNS were only programed with frequencies 15 and 30 Hz. MSR was not significantly different for those patients with intracranial stimulation on low frequency stimulation (n=71) vs. high frequency stimulation (n=47) at last follow up (60%, IQR 33–88 vs. 67%, IQR 20–93; p=0.62)(Figure 3).
Table 3:
Stimulation Parameters§
| Stimulation | Lead Configuration | Amplitude | Pulse Width, μS |
Frequency, Hz |
Cycling | Stimulation Specific Parameters | Charge delivered per hour per lead mC/h (Range)†* | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bipolar % (n) | Referential % (n) | Voltage-Based IPG % (n), V (Range) | Current-Based IPG % (n), mA (Range) | Continuous % (n) |
Intermittent (Duty Cycle) % (n), s (Range) | |||||||
| ANT n=38 | 53 (20) | 47 (18) | 61 (23) 3.8 (Range 0–7.5) |
39 (15) 5 (Range 2.5–8) |
120 (Range 20–250) | 40 (Range 7–145) | 58 (22) | 42 (16) On: 60 (Range 30–60) Off: 300 (Range 180–600) |
NA | NA | NA | 28.4 (Range 2.6–468) ** |
| CM n=19 | 84 (16) | 16 (3) | 84 (16) 4 (Range 1–6) |
16 (3) 3 (Range 1–4) |
90 (Range 60–210) | 60 (Range 2–100) | 84 (16) | 16 (3) On: 0.1 (Range 0.1–15) Off: 0.1 (Range 0.1–15) |
NA | NA | NA | 63.5 (Range 1.1–144.7) ** |
| RNS n=30 | 83 (25) | 17 (5) | NA | 83 (100) 2.8 (Range 0–12) |
160 (Range 0–160 | 100 (Range 0–200) | NA | NA | 2 (Range 0–10.1) | 200 (Range 0–5000) | 1000 (Range 0–3400) | 0.9 (Range 0–12.8) ** |
| CSS n=32 | 100 (32) | 62 (20) 3 (Range 0–6.8) |
38 (12) 1.5 (Range 0.4–32) |
210 (Range 90–450) | 2 (Range 2–100) | 90 (29) | 10 (3) On: 10 (Range 0.2–60) Off: 50 (Range 0.2–3000) |
NA | NA | NA | 6.5 (Range 0.1–835.9) *** | |
| VNS n=40 | NA | NA | NA | 40 (100) 1.8 (Range 0.5–8) |
250 (Range 130–500) | 20 (Range 15–30) | NA | On: 30 (Range 7–60) Off: 66 (Range 30–300) |
32.5 (Range 10–51) | NA | NA | 8.0 (Range 0.7–33.8) *** ‡ |
ANT, anterior thalamic nucleus deep brain stimulation; CM, centromedian thalamic nucleus deep brain stimulation; CSS, chronic subthreshold stimulation; Hz, Hertz; IPG, implantable pulse generator; mA, milliamperes; μC/cm2, microcoulombs per centimeter square; μS, microseconds; msec, milliseconds; RNS, responsive neurostimulation; V, Volts; VNS, vagus nerve stimulation
All values are expressed as median
For converting voltage to current in patients with voltage based IPG, a resistance of 1000 Ω was assumed (A=V/Ω)
p<0.001 in Kruskal-Wallis comparing across neuromodulation groups
p<0.05 in two-tailed pairwise comparisons (Mann-Whitney U) with all groups
p<0.05 in two-tailed pairwise comparisons (Mann-Whitney U) with all groups except with VNS and CSS, respectively
Magnet swipes and automatic stimulations are not include
Discussion
Our study demonstrates a 61% median seizure reduction and 60% responder rate across neuromodulation modalities in this single center review. In general, neither the type of neuromodulation nor the type of seizure onset were associated with clearly improved seizure reduction or responder rates. In unadjusted pairwise comparisons MSR was improved for CSS compared to the other devices, while other approaches (ANT-DBS, CM-DBS, RNS, and VNS) were not statistically different from each other. Similarly, relatively improved MSR was reported for frontal onset seizures compared to posterior and multifocal onset seizures, while other locations were not significantly different in unadjusted pairwise comparisons. Cortical stimulation (RNS and CSS) was associated with an improved MSR compared to subcortical stimulation (ANT-DBS, CM, and VNS). The amount of charge delivered per hour varied widely between approaches but was not correlated with seizure reduction. Pediatric patients had a similar MSR to adults. Adverse events are consistent with previous literature. Seizure onset clustered with specific neuromodulation strategies: the RNS group was mostly comprised of patients with temporal epilepsy (78%) and most of them had mesial onset (70%); CSS had the highest number of paracentral epilepsy patients (50%); ANT-DBS was mostly comprised of patient with multifocal/diffuse onset (55%); generalized and mixed epilepsy patients were mostly distributed amongst CM-DBS and VNS.
When considering individual approaches, ANT-DBS in our cohort had a MSR of 52% at 23 months that is comparable with the 56% MSR at 2 years in the open label phase of the SANTE trial [24]. CM-DBS patients reported a MSR of 63% over a median of 27 months. The literature on CM-DBS efficacy is more heterogeneous. A recent meta-analysis concluded that CM-DBS was associated with a 73% mean seizure frequency reduction ranging from 44% to 80% [25], comparable with our findings. Another important point is that patients with CM-DBS had the largest disabling seizure burden and largest number of ASDs trialed prior to implant highlighting the severity of the epilepsies treated by this modality at our center. Regarding RNS, the 50% MSR over a median of 19 months was similar to the two-year 53% MSR reported by the open label extension phase from the pivotal RNS trial [26]. CSS was the stimulation modality with the most patients reporting at least 3 months of seizure freedom at the time of the assessment. Potential reasons are the highly selective process for CSS patient implantation that includes trial stimulation during invasive monitoring (intracranial EEG) for epilepsy surgery with temporarily implanted hardware prior to permanent hardware placement [21]. Additionally, CSS was used for the majority of paracentral epilepsy cases, which could respond particularly well to focal stimulation[27][28, 29]. Finally, the broader stimulation fields allowed by 4 leads and up to 16 electrodes, may impart increased benefits, especially for larger epileptogenic foci such as in frontal lobe epilepsies. Our VNS subcohort reported 50% MSR after a median follow up period of 50 months. In comparison to other approaches in our cohort, the 50-month median follow-up time is significantly longer. This may artificially improve VNS outcomes in comparison to the other approaches as the efficacy of neurostimulation in general improves over time [9, 12, 30].
Additional analyses showed that closed loop stimulation (RNS) was not associated with better MSR when compared to open loop stimulation, and cortical stimulation had significantly better MSR when compared to subcortical stimulation. This supports the notion that it is perhaps not the intermittent interruption of ictal discharges that provides benefit but rather the long-term plasticity of epileptogenic brain and the networks generating seizures [8, 20, 31]. Patients with intracranial neuromodulation devices in addition to active VNS therapy had similar rates of MSR as those with only VNS neurostimulation. In a previous study, our group did not see any difference in seizure reduction across ANT-DBS patients who also had been previously implanted with VNS that was inactive, those who had active VNS stimulation at the time of ANT-DBS implant, and patients who had ANT-DBS without previously having VNS [32]. This suggests that patients can benefit from a second device if there is unsatisfactory seizure control with VNS alone. VNS and DBS devices were implanted for patients with generalized epilepsy as well as focal epilepsy; there was not a significant difference in seizure frequency reduction between these two groups. VNS is considered effective for generalized seizures [5, 33]. The largest double blinded randomized trial for CM-DBS in generalized epilepsy was restricted to Lennox-Gastaut patients and did not demonstrate a significant clinical seizure frequency reduction between treatment and control groups after 3 months. However, there was a significant difference in electrographic seizures, and a 47% MSR after a subsequent 3-month unblinded period [34]. Similarly, our data suggest generalized epilepsy can respond well to neuromodulation. Despite the absence of FDA approved neuromodulation strategies for pediatric patients, several studies have demonstrated its benefit int this population [16]. In our cohort, children and adolescents treated with invasive neurostimulation seem to benefit equally from neuromodulation when compared to adults further supporting this notion.
Overall, the incidence of serious AEs (leading to a repeat surgery) in our cohort was 10% with only 16 patients requiring an unexpected surgical procedure due to AEs. Specifically pertaining to infection, four patients (3%) required hardware removal or lavage surgery for system related infection. This is comparable to the 4.5% of patients requiring explant surgery in the SANTE trial [24], the 4.1% infection rate per device implant noted for the RNS system [9] and the 2.6% infection rate reported for VNS [35]. All neuropsychiatric AEs noted in our cohort were restricted to ANT-DBS; although we report a smaller prevalence of 11% in our cohort vs. 30–40% in the long-term arm of SANTE [12]. This may be related to shorter follow-up times or differences in programming [36]. Although VNS had the most side effects when compared to other strategies, most were hoarseness and dysphonia, which are mild and well-known to be common[37].
Regarding the overall amount of charge per lead delivered per unit time, modalities with open loop stimulation programmed with higher frequencies delivered more current than modalities programmed with lower frequencies and/or duty cycle stimulation, i.e., CM>ANT>CSS>VNS. As expected, RNS had the smallest charge per hour given the intermittent nature of stimulation. For example, DBS delivered approximately 30 times more charge per hour than RNS our cohort. Regardless, there was no correlation between MSR and the amount of delivered current, which suggests a complex interplay between delivered current and benefit. Finally, low frequency stimulation was used in approximately half of our patients with ANT-DBS, CM-DBS and RNS patients and in 85% of CSS patients at last follow up. The MRS for these patients was not significantly different to those with high frequency stimulation. Despite concerns about its safety[38] and possible seizure-inducing properties[39]; our data indicates that low frequency stimulation can be effective across multiple neurostimulation devices without significant risk. Low frequency stimulation can be an alternative approach when high frequency stimulation is not as effective as desired.
Aligned with our results, other groups have retrospectively compared different neuromodulation strategies. No differences in efficacy have been reported when comparing VNS vs. RNS in temporal lobe epilepsy [40], VNS vs. RNS in patients with focal impaired awareness seizures [41], or ANT-DBS vs. RNS in temporal lobe epilepsy [42]. No prospective comparisons between neuromodulation strategies have been reported.
Despite our efforts to assess these different modalities uniformly, this retrospective review is limited by inconsistencies related to health record documentation, lack of control data and matched cohorts, and selection biases driven by individual patient characteristics and physicians’ preferences. Any comparison between approaches is therefore limited. Our study used a pragmatic approach of assessing seizure severity, life satisfaction, and quality of sleep through 1–10 point scales with the goal of decreasing survey fatigue. We acknowledge that these evaluations are limited in comparison to comprehensive formal evaluations for such outcomes like the Liverpool Seizure Severity Scale (LSSS), or the Quality of Life in Epilepsy Inventories (QOLIE). Our cohort is representative of the clinical preferences and biases of one tertiary epilepsy center.
Conclusions
This study represents an effort to directly compare five invasive neuromodulation approaches while acknowledging that individual patient characteristics may drive the decision of a specific strategy versus the other. Median seizure reduction and responder rates were overall similar across groups. When feasible, cortical stimulation of the epileptogenic zone, especially for frontal and paracentral onset epilepsies, may be preferable relative to subcortical stimulation. Closed loop stimulation performed similarly to open loop stimulation. Patients implanted with DBS or VNS with generalized epilepsy had comparable benefits to those with focal epilepsy. Pediatric patients derived similar benefit from neuromodulation as adults. Low frequency stimulation was used in approximately half of patients with DBS, RNS or CSS and had comparable results to high frequency stimulation, potentially representing an effective alternative approach.
Supplementary Material
Acknowledgments:
Special thanks to Cindy Nelson for her support in patient clinical and neurophysiology follow up and Melinda Marthaler (NeuroPace Field Engineer) for support and advice related to RNS engineering and programing details. We also acknowledge the important contributions of Dr. Matt Stead (Dark Horse Neuro, Inc.) to our neuromodulation patients.
Funding:
The research was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke: K23NS112339 (B.N.L.)
Footnotes
Declaration of interests
GW, JJVG and BNL are named inventors for intellectual property licensed to Cadence Neuroscience Inc, which is co-owned by Mayo Clinic. BNL waived contractual rights to royalties.
GW, JJVG, NMG, and BNL are investigators for the Medtronic EPAS trial and Medtronic-supported NIH grants (UH3-NS95495 and UH3-NS112826).
BNL is an investigator for Neuropace RNS System Responsive Stimulation for Adolescents with Epilepsy (RESPONSE) Study and Neuroelectrics tDCS for Patients with Epilepsy Study. Mayo Clinic has received consulting fees on behalf of BNL from Epiminder, Medtronic, Neuropace and Philips Neuro.
Mayo Clinic has received research support and consulting fees on behalf of GW from UNEEG, NeuroOne Inc., and Medtronic.
GW has licensed intellectual property developed at Mayo Clinic to NeuroOne, Inc.
References:
- [1].Zack MM, Kobau R. National and State Estimates of the Numbers of Adults and Children with Active Epilepsy - United States, 2015. MMWR Morb Mortal Wkly Rep 2017;66: 821–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sultana B, Panzini MA, Veilleux Carpentier A, Comtois J, Rioux B, Gore G, Bauer PR, Kwon CS, Jette N, Josephson CB, Keezer MR. Incidence and Prevalence of Drug-Resistant Epilepsy: A Systematic Review and Meta-analysis. Neurology 2021;96: 805–817. [DOI] [PubMed] [Google Scholar]
- [3].Laxer KD, Trinka E, Hirsch LJ, Cendes F, Langfitt J, Delanty N, Resnick T, Benbadis SR. The consequences of refractory epilepsy and its treatment. Epilepsy Behav 2014;37: 59–70. [DOI] [PubMed] [Google Scholar]
- [4].Vakharia VN, Duncan JS, Witt JA, Elger CE, Staba R, Engel J Jr., Getting the best outcomes from epilepsy surgery. Ann Neurol 2018;83: 676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Englot DJ, Rolston JD, Wright CW, Hassnain KH, Chang EF. Rates and Predictors of Seizure Freedom With Vagus Nerve Stimulation for Intractable Epilepsy. Neurosurgery 2016;79: 345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Krahl SE, Clark KB. Vagus nerve stimulation for epilepsy: A review of central mechanisms. Surg Neurol Int 2012;3: S255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Morrell MJ, Group RNSSiES. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011;77: 1295–304. [DOI] [PubMed] [Google Scholar]
- [8].Kokkinos V, Sisterson ND, Wozny TA, Richardson RM. Association of Closed-Loop Brain Stimulation Neurophysiological Features With Seizure Control Among Patients With Focal Epilepsy. JAMA Neurol 2019;76: 800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nair DR, Laxer KD, Weber PB, Murro AM, Park YD, Barkley GL, Smith BJ, Gwinn RP, Doherty MJ, Noe KH, Zimmerman RS, Bergey GK, Anderson WS, Heck C, Liu CY, Lee RW, Sadler T, Duckrow RB, Hirsch LJ, Wharen RE Jr., Tatum W, Srinivasan S, McKhann GM, Agostini MA, Alexopoulos AV, Jobst BC, Roberts DW, Salanova V, Witt TC, Cash SS, Cole AJ, Worrell GA, Lundstrom BN, Edwards JC, Halford JJ, Spencer DC, Ernst L, Skidmore CT, Sperling MR, Miller I, Geller EB, Berg MJ, Fessler AJ, Rutecki P, Goldman AM, Mizrahi EM, Gross RE, Shields DC, Schwartz TH, Labar DR, Fountain NB, Elias WJ, Olejniczak PW, Villemarette-Pittman NR, Eisenschenk S, Roper SN, Boggs JG, Courtney TA, Sun FT, Seale CG, Miller KL, Skarpaas TL, Morrell MJ, Study RNSSL. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology 2020;95: e1244–e1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Middlebrooks EH, Lin C, Okromelidze L, Lu CQ, Tatum WO, Wharen RE Jr., Grewal SS. Functional Activation Patterns of Deep Brain Stimulation of the Anterior Nucleus of the Thalamus. World Neurosurg 2020;136: 357–363 e2. [DOI] [PubMed] [Google Scholar]
- [11].Papez JW. A proposed mechanism of emotion. 1937. J Neuropsychiatry Clin Neurosci 1995;7: 103–12. [DOI] [PubMed] [Google Scholar]
- [12].Salanova V, Sperling MR, Gross RE, Irwin CP, Vollhaber JA, Giftakis JE, Fisher RS, Group SS. The SANTE study at 10 years of follow-up: Effectiveness, safety, and sudden unexpected death in epilepsy. Epilepsia 2021;62: 1306–1317. [DOI] [PubMed] [Google Scholar]
- [13].Valentin A, Garcia Navarrete E, Chelvarajah R, Torres C, Navas M, Vico L, Torres N, Pastor J, Selway R, Sola RG, Alarcon G. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia 2013;54: 1823–33. [DOI] [PubMed] [Google Scholar]
- [14].Burdette D, Mirro EA, Lawrence M, Patra SE. Brain-responsive corticothalamic stimulation in the pulvinar nucleus for the treatment of regional neocortical epilepsy: A case series. Epilepsia Open 2021;6: 611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Alcala-Zermeno JL, Gregg NM, Wirrell EC, Stead M, Worrell GA, Van Gompel JJ, Lundstrom BN. Centromedian thalamic nucleus with or without anterior thalamic nucleus deep brain stimulation for epilepsy in children and adults: A retrospective case series. Seizure 2021;84: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Starnes K, Miller K, Wong-Kisiel L, Lundstrom BN. A Review of Neurostimulation for Epilepsy in Pediatrics. Brain Sci 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Child ND, Stead M, Wirrell EC, Nickels KC, Wetjen NM, Lee KH, Klassen BT. Chronic subthreshold subdural cortical stimulation for the treatment of focal epilepsy originating from eloquent cortex. Epilepsia 2014;55: e18–21. [DOI] [PubMed] [Google Scholar]
- [18].Lundstrom BN, Worrell GA, Stead M, Van Gompel JJ. Chronic subthreshold cortical stimulation: a therapeutic and potentially restorative therapy for focal epilepsy. Expert Rev Neurother 2017;17: 661–666. [DOI] [PubMed] [Google Scholar]
- [19].Lundstrom BN, Van Gompel J, Britton J, Nickels K, Wetjen N, Worrell G, Stead M. Chronic Subthreshold Cortical Stimulation to Treat Focal Epilepsy. JAMA Neurol 2016;73: 1370–1372. [DOI] [PubMed] [Google Scholar]
- [20].Lundstrom BN, Gompel JV, Khadjevand F, Worrell G, Stead M. Chronic subthreshold cortical stimulation and stimulation-related EEG biomarkers for focal epilepsy. Brain Commun 2019;1: fcz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kerezoudis P, Grewal SS, Stead M, Lundstrom BN, Britton JW, Shin C, Cascino GD, Brinkmann BH, Worrell GA, Van Gompel JJ. Chronic subthreshold cortical stimulation for adult drug-resistant focal epilepsy: safety, feasibility, and technique. J Neurosurg 2018;129: 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshe SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51: 1069–77. [DOI] [PubMed] [Google Scholar]
- [23].Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med 2008;3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Salanova V, Witt T, Worth R, Henry TR, Gross RE, Nazzaro JM, Labar D, Sperling MR, Sharan A, Sandok E, Handforth A, Stern JM, Chung S, Henderson JM, French J, Baltuch G, Rosenfeld WE, Garcia P, Barbaro NM, Fountain NB, Elias WJ, Goodman RR, Pollard JR, Troster AI, Irwin CP, Lambrecht K, Graves N, Fisher R. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology 2015;84: 1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vetkas A, Fomenko A, Germann J, Sarica C, Iorio-Morin C, Samuel N, Yamamoto K, Milano V, Cheyuo C, Zemmar A, Elias G, Boutet A, Loh A, Santyr B, Gwun D, Tasserie J, Kalia SK, Lozano AM. Deep brain stimulation targets in epilepsy: Systematic review and meta-analysis of anterior and centromedian thalamic nuclei and hippocampus. Epilepsia 2022;63: 513–524. [DOI] [PubMed] [Google Scholar]
- [26].Heck CN, King-Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, Salanova V, Cole AJ, Smith MC, Gwinn RP, Skidmore C, Van Ness PC, Bergey GK, Park YD, Miller I, Geller E, Rutecki PA, Zimmerman R, Spencer DC, Goldman A, Edwards JC, Leiphart JW, Wharen RE, Fessler J, Fountain NB, Worrell GA, Gross RE, Eisenschenk S, Duckrow RB, Hirsch LJ, Bazil C, O’Donovan CA, Sun FT, Courtney TA, Seale CG, Morrell MJ. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia 2014;55: 432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jobst BC, Kapur R, Barkley GL, Bazil CW, Berg MJ, Bergey GK, Boggs JG, Cash SS, Cole AJ, Duchowny MS, Duckrow RB, Edwards JC, Eisenschenk S, Fessler AJ, Fountain NB, Geller EB, Goldman AM, Goodman RR, Gross RE, Gwinn RP, Heck C, Herekar AA, Hirsch LJ, King-Stephens D, Labar DR, Marsh WR, Meador KJ, Miller I, Mizrahi EM, Murro AM, Nair DR, Noe KH, Olejniczak PW, Park YD, Rutecki P, Salanova V, Sheth RD, Skidmore C, Smith MC, Spencer DC, Srinivasan S, Tatum W, Van Ness P, Vossler DG, Wharen RE Jr., Worrell GA, Yoshor D, Zimmerman RS, Skarpaas TL, Morrell MJ. Brain-responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas. Epilepsia 2017;58: 1005–1014. [DOI] [PubMed] [Google Scholar]
- [28].Kerezoudis P, Lundstrom BN, Meyer FB, Worrell GA, Van Gompel JJ. Surgical approaches to refractory central lobule epilepsy: a systematic review on the role of resection, ablation, and stimulation in the contemporary era. J Neurosurg 2022: 1–12. [DOI] [PubMed] [Google Scholar]
- [29].Alcala-Zermeno JL, Gregg NM, Van Gompel JJ, Stead M, Worrell GA, Lundstrom BN. Cortical and thalamic electrode implant followed by temporary continuous subthreshold stimulation yields long-term seizure freedom: A case report. Epilepsy Behav Rep 2020;14: 100390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhu J, Wang X, Xu C, Zhang X, Qiao L, Zhang X, Yan X, Ni D, Yu T, Zhang G, Li Y. Comparison of efficiency between VNS and ANT-DBS therapy in drug-resistant epilepsy: A one year follow up study. J Clin Neurosci 2021;90: 112–117. [DOI] [PubMed] [Google Scholar]
- [31].Jarosiewicz B, Morrell M. The RNS System: brain-responsive neurostimulation for the treatment of epilepsy. Expert Rev Med Devices 2021;18: 129–138. [DOI] [PubMed] [Google Scholar]
- [32].Parisi V, Lundstrom BN, Kerezoudis P, Alcala Zermeno JL, Worrell GA, Van Gompel JJ. Anterior Nucleus of the Thalamus Deep Brain Stimulation with Concomitant Vagus Nerve Stimulation for Drug-Resistant Epilepsy. Neurosurgery 2021;89: 686–694. [DOI] [PubMed] [Google Scholar]
- [33].Morris GL 3rd, Gloss D, Buchhalter J, Mack KJ, Nickels K, Harden C. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013;81: 1453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dalic LJ, Warren AEL, Bulluss KJ, Thevathasan W, Roten A, Churilov L, Archer JS. DBS of Thalamic Centromedian Nucleus for Lennox-Gastaut Syndrome (ESTEL Trial). Ann Neurol 2022;91: 253–267. [DOI] [PubMed] [Google Scholar]
- [35].Revesz D, Rydenhag B, Ben-Menachem E. Complications and safety of vagus nerve stimulation: 25 years of experience at a single center. J Neurosurg Pediatr 2016;18: 97–104. [DOI] [PubMed] [Google Scholar]
- [36].Fasano A, Eliashiv D, Herman ST, Lundstrom BN, Polnerow D, Henderson JM, Fisher RS. Experience and consensus on stimulation of the anterior nucleus of thalamus for epilepsy. Epilepsia 2021;62: 2883–2898. [DOI] [PubMed] [Google Scholar]
- [37].Toffa DH, Touma L, El Meskine T, Bouthillier A, Nguyen DK. Learnings from 30 years of reported efficacy and safety of vagus nerve stimulation (VNS) for epilepsy treatment: A critical review. Seizure 2020;83: 104–123. [DOI] [PubMed] [Google Scholar]
- [38].Yu T, Wang X, Li Y, Zhang G, Worrell G, Chauvel P, Ni D, Qiao L, Liu C, Li L, Ren L, Wang Y. High-frequency stimulation of anterior nucleus of thalamus desynchronizes epileptic network in humans. Brain 2018;141: 2631–2643. [DOI] [PubMed] [Google Scholar]
- [39].Velasco F, Velasco M, Marquez I, Velasco G. Role of the centromedian thalamic nucleus in the genesis, propagation and arrest of epileptic activity. An electrophysiological study in man. Acta Neurochir Suppl (Wien) 1993;58: 201–4. [DOI] [PubMed] [Google Scholar]
- [40].Wang AJ, Bick SK, Williams ZM. Vagus Nerve Stimulation versus Responsive Neurostimulator System in Patients with Temporal Lobe Epilepsy. Stereotact Funct Neurosurg 2020;98: 21–29. [DOI] [PubMed] [Google Scholar]
- [41].Ellens NR, Elisevich K, Burdette DE, Patra SE. A Comparison of Vagal Nerve Stimulation and Responsive Neurostimulation for the Treatment of Medically Refractory Complex Partial Epilepsy. Stereotact Funct Neurosurg 2018;96: 259–263. [DOI] [PubMed] [Google Scholar]
- [42].Yang JC, Bullinger KL, Dickey AS, Karakis I, Alwaki A, Cabaniss BT, Winkel D, Rodriguez-Ruiz A, Willie JT, Gross RE. Anterior nucleus of the thalamus deep brain stimulation vs temporal lobe responsive neurostimulation for temporal lobe epilepsy. Epilepsia 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


