Abstract
Leishmaniases, a group of vector-borne diseases, are caused by the protozoan intracellular parasite Leishmania (L.) and are transmitted by the phlebotomine sandflies. A wide range of clinical manifestations in L- infection is observed. The clinical outcome ranges from asymptomatic, cutaneous leishmaniasis (CL) to severe mucosal leishmaniasis (ML) or visceral leishmaniasis (VL), depending on the L. species. Interestingly, only a fraction of L.-infected individuals progress to disease development, suggesting a key role of host genetics in the clinical outcome. NOD2 plays a critical role in the control of host defense and inflammation. The NOD2-RIK2 pathway is involved in developing a Th1- type response in patients with VL and C57BL/6 mice infected with L. infantum. We investigated whether variants in the NOD2 gene (R702W rs2066844, G908R rs2066845, and L1007fsinsC rs2066847) are associated with susceptibility to CL caused by L. guyanensis (Lg) in 837 patients with Lg-Cl and 797 healthy controls (HC) with no history of leishmaniasis. Both patients and HC are from the same endemic area of the Amazonas state of Brazil. The variants R702W and G908R were genotyped by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), and L1007fsinsC was by direct nucleotide sequencing. The minor allele frequency (MAF) of L1007fsinsC was 0.5% among the patients with Lg-CL and 0.6% in the healthy controls group. R702W genotypes frequencies were similar in both groups. Only 1% and 1.6% were heterozygous for G908R among the patients with Lg-CL and HC, respectively. None of the variants revealed any association with susceptibility to the development of Lg-CL. Correlations of genotypes with the level of plasma cytokines revealed that individuals with the mutant alleles of R702W tend to have low levels of IFN-γ. G908R heterozygotes also tend to have low IFN-γ, TNF-α, IL-17, and IL-8. Variants of NOD2 are not involved in the pathogenesis of Lg-CL.
Introduction
Leishmaniases are vector-borne parasitic diseases caused by the protozoan Leishmania (L.), are transmitted by phlebotomine sandflies. Leishmaniases manifest into visceral and tegumentary leishmaniasis. Tegumentary leishmaniasis may manifest as localized cutaneous leishmaniasis (CL), diffuse CL, disseminated CL, and mucocutaneous leishmaniasis (ML). Symptoms of visceral leishmaniasis (VL), a life-threatening disease and fatal in 95% of untreated cases, manifest by irregular bouts of fever, weight loss, enlargement of the spleen and liver, and anemia. Nearly one billion individuals are at risk of developing leishmaniases, which are present in more than 98 countries [1].
Leishmaniases are endemic in 97 countries and affect around 12 million people worldwide, especially in the tropic and subtropical countries. According to the world health organization, 253,435 cases of CL were notified in 2018 [1]. In Brazil, 16,813 new cases of CL were reported [2]. In the Amazonas, 1692 new cases of CL were registered [2].
VL is caused by L. infantum in America, Central Asia, and the Middle East, and by L. donovani in Asia and Africa. In Brazil, L. braziliensis, L. guyanensis, L. lainsoni, L. amazonensis, L. shawi, L. naiffi, and L. lindenberghi are the major species causing American tegumentary leishmaniasis. L. guyanensis is the main etiological agent of CL in the Amazonas [3].
NOD-like receptors (NLR) induce host protective immunity against intracellular parasitic infections [4, 5]. NOD2, also known as CARD15, triggers host-innate immunity [6]. NOD2 recognizes conserved muramyl dipeptides of bacterial peptidoglycan [7, 8] and activates nuclear factor-kappaB (NF-ĸB) through the RIP2 kinase pathway [9, 10], to trigger the transcription of proinflammatory cytokines such as IL-12 and IL-1β [11, 12]. Th1 cell immune response protects against L.-infection by releasing proinflammatory cytokines, IL-12, IFN-γ, and TNF-α [13].
Peripheral blood mononuclear cells (PBMCs) of individuals bearing the loss of function genetic variants of NOD2 displayed lower levels of proinflammatory cytokines than individuals with the functional variants when exposed to L. amazonensis or L. braziliensis in vitro [14]. The NOD2-RIK2 pathway is involved in the development of a Th1- type response in patients with VL and C57BL/6 mice infected with L. infantum [15]. Leishmania possesses lipophosphoglycans (LPG), glycoinositolphospholipids (GIPLs), and glycoprotein 63 (gp63) in its membrane which are recognized by pathogen recognition receptors [16, 17].
LPG can activate the NOD-like receptor NLPR3 in a non-canonical pathway [18]. RIPK2 is a critical kinase for the NOD2 signaling cascade and production of inflammatory cytokines. Inhibition of RIPK2 phosphorylation in PBMCs, exposed to LPG of L. braziliensis or L. amazonensis, leads to a reduction of IL-32, IL-6, and IL-1β [19].
NOD2 is on chromosome 16q21. Loss of function variants in the NOD2 gene (R702W, G908R, and L1007fsinsC) leads to a hypoimmune response and impairs NOD2 activation [7, 20]. The missense mutation rs2066844 C/T (R702W), located in exon 4, substitutes arginine at codon position 702 with tryptophan. The rs2066845 C/G (G908R) in exon 8 leads to the substitution of glycine at position 908 with arginine, while the rs2066847 (L1007fsinsC), located in exon 11, inserts a cytosine (C) at nucleotide position 3020 leading to a frameshift and premature stop codon at codon 1007. All these variants are present in the leucine-rich region of the protein that is involved in ligand recognition [21].
Interestingly, the clinical manifestations of infection with Leishmania depend on the host-genetic background, the vector, the site of infection, the skin microbiota, and the Leishmania spp [22, 23]. In light of the importance of NOD2 in immune response, this study evaluated whether the genetic variants (R702W, G908R, and L1007fsinsC) of NOD2 may be associated with susceptibility to CL caused by L. guyanensis and influenced plasma circulating proinflammatory cytokines.
Materials and methods
This case-control study was performed according to the guidelines strengthening the reporting of genetic association studies (STREGA). The study was carried out in the state of Amazonas, Brazil. Patients with CL as well as healthy controls with no scar or history of leishmaniasis came from the peripheral regions of Manaus, the capital city of Amazonas. Briefly, the areas surrounding BR-174 and AM-010 in the peripheral regions of Manaus became endemicity for Lg-infection due to human invasion (i.e., communities of Pau-Rosa, Cooperativa, Agua-Branca, Leão, and Brasileirinho). Patients with CL were attended at the Fundação de Medicina Tropical Doutor Heitor Vieira Dourado (FMT-HVD), the referral centre for treatment of leishmaniasis.
Ethical approval
This study was carried out under the Helsinki Declaration and was approved by the Research Ethics Committee of the FMT-HVD/2012 (CAAE—Certificado de Apresentacão para Apreciacão Etica: 09995212.0.0000.0005). All volunteers provided written informed consent for sample collection and subsequent analysis. For patients younger than 18 years of age, the parents or responsible party consented to the child’s participation and provided the written Informed Consent Form.
Identification of Leishmania spp.
Patients with CL provided a biopsy specimen of the skin lesion to identify the Leishmania species. DNA was extracted from the biopsies and submitted to Leishmania viannia subgenus-specific PCR according to the established protocols [24, 25]. Leishmania spp. was identified by direct nucleotide sequencing of a fragment of HSP 70 and mini-exon genes as described elsewhere [26].
DNA extraction from whole blood for alleles discrimination of variants of NOD2
All the participants gave five mL of peripheral blood by venipuncture in a vacutainer tube containing ethylenediaminetetraacetatic acid (Becton Dickinson). Plasma was separated and kept frozen in a freezer at -80°C until usage. Genomic DNA was extracted by the salting out method [27].
The variant L1007fsinsC was genotyped by direct nucleotide sequencing. The following pair of primers, forward 5`-GGATGTGTCTAAGGGACAGGTG-3`and reverse 5`-CTGAGGTTCGGAGAGCTA-3`were designed to amplify a fragment of 251bp. Nucleotide sequencing was performed using the forward primer with the kit BigDyes from Applied Biosystem (Thermofisher, MA USA) following the protocols suggested by the manufacturer.
The variants R702W (rs2066844 C/T) and G908R (rs2066845 G/C) of the NOD2 gene were typed by PCR-RFLP using the restriction enzymes Hpa II for R702W and Hha I for G908R (New England Biolabs). The generated PCR fragment was 171bp for R702W, using the following pair of primers, forward 5`- GCACAACCTTCAGATCACAGCA-3`and reverse 5`- GCTGGCGGGATGGAGTGGAAG-3`. 10uL of the PCR products was digested with the restriction enzymes Hpa II. The PCR product (171bp) is cleaved into three fragments, 71bp, 54bp, and 46bp in the presence of allele C and in two fragments (125bp and 46bp) when the T allele is present. A fragment of 249 bp was amplified by PCR using the following pair of primers, forward 5`-CAGTGAGGCCACTCTGGGATTG-3`and reverse 5`-AAAACTGCAGGATAGACTCT-3`for G908R. If the allele G is present, the 245bp fragment remains uncleaved by the restriction enzyme Hha I, while in the presence of allele C, the fragment is cleaved into 145 and 104 bp. PCR restriction fragments were size separated by electrophoresis in 3% agarose gel.
Cytokine assay by Luminex
5 mL of blood from patients with Lg-CL before antimonial treatment and from healthy controls were collected. Plasma was separated and kept at -80°C until plasma cytokines assay.
The levels of IFNγ, IL-1β, IL-6, IL-8, IL-12 (p70), IL-13, IL-17A, RANTES, and TNFα were determined using the multiplex cytokine commercial kit Bio-PlexPro-Human Cytokine GrpI Panel 27-Plex (Bio-Rad) according to the manufacturer’s instructions in the Bio-Plex 200 Protein Array System (Luminex Corporation).
Statistical analysis
Genotypes and allele frequencies were calculated by direct counting. HWE (Hardy-Weinberg Equilibrium) and logistic regression analysis with a confidence interval (CI) of 95% and χ2 test to compare patients with Lg-Cl and healthy control groups (HC) were performed using the website https://ihg.helmholtz-muenchen.de/cgi-bin/hw/hwa1.pl. Quantitative trait analysis was used to correlate the NOD2 genotypes with circulating plasma cytokines in R software (version 4.2.1) using package SNPassoc (version 2.0–11) and ggplot2 package for visualization. P values of cytokines correlations with genotypes were corrected by the false discovery rate (FDR) of Benjamini-Hochberg.
Results
Population of the study
The study population was previously described [28, 29]. This study includes 850 patients with Lg-CL and 891 healthy controls (HC). The HC has the same socio-epidemiology characteristics as the patients and were from the same endemic area. The patients with Lg-CL were first-time diagnosed, treatment-naïve, and had fewer or equal to six lesions. The HC was not stratified as asymptomatic as delayed- test of hypersensitivity to Leishmania-antigens was not performed. All the participants were HIV-negative and devoid of cardiac, renal, or diabetes disease. Most of the participants are agricultural or farms-workers. The basic characteristics of the study population are shown in (Table 1).
Table 1. Basic characteristics of the patients with Leishmania guyanensis-cutaneous leishmaniasis (Lg-CL) and healthy controls (HC).
| Patients with Lg-CL | HC | ||||
|---|---|---|---|---|---|
| N = 850 | N = 891 | p-value 1 | |||
| Males | Females | Males | Females | ||
| Sex | 639 (75%) | 211 (25%) | 608 (68%) | 283 (32%) | 0.001 |
| Age (mean ± SEM 2) years | 34.4 ± 13.7 | 37.5 ± 15.7 | 42 ± 17.5 | 40 ± 17.4 | |
Abbreviations:
1 p-value <0.05 is significant,
2 SEM: Standard error of mean.
The HC is slightly older than the patients with Lg-CL (P<0.0001). The age of male participants was higher in the HC group to male patients with Lg-CL (P<0.0001). There was no age difference among female participants (P<0.077).
The variants R702W, G908R, and L1007fsinsC were genotyped in 821, 837, and 762 patients with Lg-CL, respectively. Similarly, the R702W, G908R, and L1007fsinsC were genotyped in 777, 797, and 746 healthy controls, respectively. The distribution of the genotypes of the three variants was in Hardy-Weinberg equilibrium in both groups. The frequencies of the genotypes and alleles are shown in (Table 2).
Table 2. Genotypes and alleles frequencies of the variants R702W, G908R, and L1007fsinsC of the NOD2 gene among patients with Lg-CL (cases) and healthy controls (HC).
| Genotypes | Cases (%) | HC (%) | Comparisons | P-value 1 | OR 2(95% CI3) | |
|---|---|---|---|---|---|---|
| n = 821 | n = 777 | |||||
| R702W | C/C | 789 (96,1) | 748 (96,3) | CC vs. TT | 0.96 | 0.94 [0.06–15] |
| rs2066844 | C/T | 31 (3,8) | 28 (3,6) | CC vs. CT | 0.85 | 1.0 [0.6–1.7] |
| T/T | 1 (0,1) | 1 (0,1) | CC vs CT+TT | 0.86 | 1.0 [0.6–1.7] | |
| Alleles | C | 1609 (98) | 1524 (98) | C vs. T | 0.87 | 1.0 [0.6–1.7] |
| T | 33 (2) | 30 (2) | ||||
| n = 837 | n = 797 | |||||
| G908R rs2066845 | G/G | 828 (98,9) | 784 (98,4) | GG vs CC | 0.33 | 2.8 [0.1–69] |
| G/C | 8 (1) | 13 (1,6) | GG vs. GC | 0.22 | 0.58 [0.2–1.4] | |
| C/C | 1 (0,1) | 0 | GG vs GC+CC | 0.32 | 0.65 [0.2–1.5] | |
| Alleles | G | 1664 (99,4) | 1581 (99,2) | C vs. G | 0.45 | 0.73 [0.3–1.6] |
| C | 10 (0,6) | 13 (0,8) | ||||
| n = 762 | n = 746 | |||||
| L1007fsinsC rs2066847 | -/- | 754 (99) | 737 (99) | |||
| -/C | 8 (1) | 9 (1) | -/- vs. -/C | 0.77 | 0.86 [0.3–2.2] | |
| C/C | 0 | 0 | - vs. C | 0.77 | 0.87 [0.33–2.2] | |
| Alleles | - | 1516 (99,5) | 1483 (99,4) | |||
| C | 8 (0,5) | 9 (0,6) | ||||
Abbreviations:
1 p-value <0.05 is significant,
2 OR: Odds ratio.
3 CI: confidence interval.
The minus sign–of the L1007fsinsC denotes the absence of the insertion of the nucleotide cytosine C.
For the frameshift variant L1007fs, the minor allele frequency (MAF) was 0.5% among the patients with Lg-CL and 0.6% in the HC group. Only eight individuals (1%) among the patients with Lg-CL and nine (1%) among the HC were heterozygotes for the L1007fs variant. For the R702W, 31 (3.8%) and 28(3.6%) were heterozygotes in the patients and HC groups, respectively. Similarly, only eight (1%) and 13 (1.6%) individuals were heterozygotes for G908R among the patients with Lg-CL and HC, respectively. None of the variants revealed any association with either susceptibility or protection to Lg-CL. (Table 3) showed the statistical comparisons between the patients with Lg-CL and HC according to inheritance models and revealed no association with susceptibility to Lg-CL.
Table 3. Statistical comparisons between patients with Lg-CL and healthy controls based on inheritance models with p-values and odd ratios adjusted for age and sex.
| Inheritance models | p-value | Padj 4 | ||
|---|---|---|---|---|
| [OR (95% CI] | [OR 5 adj (95% CI 6] | |||
| R702W rs2066844 | 1 | C/C vs C/T+T/T | 0.86 [1.05 (0.63–1.75)] | 0.96 [1.01 (0.59–1.72)] |
| 2 | C/C + C/T vs T/T | 0.96 [0.95 (0.06–15.16)] | 0.77 [0.66 (0.04–11.54)] | |
| 3 | C/C+T/T vs CT | 0.85 [1.05 (0.62–1.77)] | 0.92 [1.03 (0.60–1.77)] | |
| G908R | 1 | C/C vs C/G+G/G | 0.32 [0.66 (0.28–1.54)] | 0.40 [1.45(0.61–3.46)] |
| 2 | C/C+C/G vs G/G | 1.00 [0.00 (0.00–0.00)] | 0.33 [1.59 (0.00–0.00)] | |
| rs2066845 | 3 | C/C+G/G vs C/G | 0.22 [0.58 (0.24–1.41)] | 0.30 [1.60(0.65–3.93)] |
| L1007fsinsC rs2066847 | -/C vs C/C | 0.77 [0.87 (0.33–2.26)] | 0.6 [1.31 (0.48–3.56)] |
Abbreviations:
1: dominant,
2: recessive, and
3: overdominant.
4 Padj: p-value adjusted by sex and age,
5 OR: odds ratio,
6 CI: confidence interval.
The minus sign–of the L1007fsinsC denotes the absence of the insertion of the nucleotide cytosine C.
None of the participants had more than one of the three variants. The minor alleles of the three variants (R702W, G908R, and L1007fsinsC) termed allele O and wild-type alleles as A were statistically compared between the patients with Lg-CL and HC (Table 4). Heterozygotes A/O frequency was 6.3% and 5.6% among the healthy controls and patients with Lg-CL, respectively. The frequency of the minor allele O was 3.3% among the HC and 3.1% in the patients with Lg-CL. Comparisons of genotype or allele frequencies between patients with Lg-CL and healthy controls did not reveal any association. The minor alleles were not associated with either susceptibility or protection to Lg-CL.
Table 4. Pooled combination of the genotypes and alleles of the three variants R702W, G908R, and L1007fsinsC among patients with Lg-CL (cases) and healthy controls (HC).
| Genotypes | Cases (%) | HC (%) | Comparisons | p-value 1 | OR 2(95% CI3) | |
|---|---|---|---|---|---|---|
| n = 837 | n = 797 | |||||
| NOD2 | A/A | 788 (94,1) | 746 (93,6) | A/A vs. O/O | 0.60 | 1.8 [0.1–21] |
| A/O | 47 (5,6) | 50 (6,3) | A/A vs. A/O | 0.57 | 0.9 [0.6–1.3] | |
| O/O | 2 (0,3) | 1 (0,1) | A/A vs. A/O + O/O | 0.65 | 0.9 [0.6–13] | |
| Alleles | O | 1623 (96,9) | 1542 (96,7) | A vs. O | 0.72 | 0.9 [0.6–1.4] |
| A | 51 (3,1) | 52 (3,3) | ||||
Abbreviations: A represents the pooled wild-type alleles and O the pooled mutant alleles of R702W, G908R, and L1007fsinsC.
1 p-value <0.05 is significant;
2 OR: odds ratio;
3 CI: confidence interval.
Plasma cytokines levels by NOD2 genotypes
Assay of plasma cytokines levels was in 354 patients with Lg-CL (264 males and 90 females) and 376 (269 males and 107 females) HC. The mean age (mean ± standard error of the mean) of the male patients with Lg-CL and HC were 39.8±1.57 and 45.2±1.58 years old, respectively. Similarly, female patients with Lg-CL and HC were 34.6±0.80 and 43.7±1.80 years old, respectively.
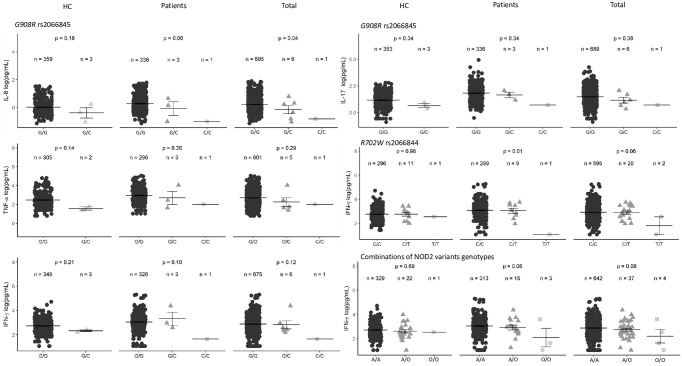
Monocytes and lymphocytes from individuals harboring the L1007fs, infected with either L. braziliensis or L. amazonensis, exhibit lower levels of TNFα, IL-1β, IL-6, IL-8, IFN-γ, and IL-17 [14]. Genotypes of the three variants were evaluated with levels of plasma cytokines to investigate whether they may influence the levels of proinflammatory cytokines, as shown in (S1–S3 Figs). A pooled analysis of all genotypes of the three variants is shown in S4 Fig. G908R genotypes tended to correlate with plasma cytokine TNF-α, IFN-γ, IL-17, and the chemokine IL-8, but did not reach statistical significance (Fig 1).
Fig 1. Evaluation of the NOD2 variants genotypes on circulating IL-8, IL-17, IFN-γ, and TNF-α plasma levels in HC, patients with CL, and total (HC + patients with Lg- CL) adjusted for age and sex.
The combination for all genotypes, common homozygous (A/A), heterozygous (A/O) and rare homozygous (O/O) are also shown in HC, Patients, and Total (A represent wild-type alleles and O mutant alleles of the three variants). The crossbars (black) represent the mean concentrations in picogram per milliliter log-scale transformed [log(pg/mL)] and the error bars represent the standard error (SE) of means. P values < 0.05 are considered significant. P values were corrected for False Discovery Rates using Benjamini Hochberg procedures.
The mean levels, expressed in log (pg/mL), of plasma circulating IL-8, IL-17, IFN-γ, and TNF-α by genotypes of the three variants, are shown in (S1 Table). Individuals heterozygous for the variant G908R tended to correlate with low levels of IL-8 among the HC (p = 0.18) and in patients with Lg-CL (p = 0.06). Combining patients and HC, heterozygous individuals G/C have a mean of plasma IL-8 of G/C = -0.14±0.29 log(pg/mL) compared to individuals G/G = 0.21±0.02 log(pg/mL) and C/C = -0.82±0.0 log(pg/mL), p = 0.04). Of note, after P values correction according to False Discovery Rates following Benjamini and Hochberg, the statistical significance is abolished. Similarly, heterozygous individuals tended to have low plasma TNF-α, IL-17, and IFN-γ mean levels.
The genotypes of NOD2 R702W seem to influence the plasma level of IFN-γ in both patients with Lg-CL and HC. Carriers of the C/C genotypes among patients with Lg-CL have higher mean levels than bearers of the C/T and T/T genotypes (p = 0.01). After corrections of P values by FDR, the statistical significance disappeared.
We also compared all individuals harboring at least one of the variants (heterozygotes or homozygotes for R702W, G908R, and L1007fsinsC mutant alleles) with the wild-type genotypes. Individuals with the mutant alleles seem to have lower levels of IFN-γ.
Discussion
In endemic areas of leishmaniasis, only a fraction of the endemic population develops the disease. Discovering the exact cause that leads some individuals to progress to disease development in the endemic area while others sharing the same area of endemicity remain asymptomatic is challenging. Assessing the impact of genetic variation on immune gene expression and function may provide clues to the underlying mechanism of why some individuals are prone to develop the disease.
Monocytes isolated from patients with Crohn`s disease and bearing the R702W variant showed impaired activation of NF-kB signaling [30]. The variant R702W abrogates NF-kB activation when introduced into the HEK293 cell line, exposed to Helicobacter pylori [31]. In contrast, mice carrying a variant corresponding to the human L1007fsinsC displayed elevated NF-kB activation [32].
The NOD signaling pathway is involved in the pathogenesis of Crohn`s disease [20, 33]. Patients, carriers of deleterious variants of the NOD2 gene, have low expression of NOD2 and increased expression of nuclear factor kappa B inhibitor alfa (NFKBIA) [34]. Furthermore, the same group of researchers compared patients bearing at least one of the variants R702W, G908R, or 1007fs to non-bearers and observed a significantly reduced NOD2 transcript count among the bearers of variants [34], allowing the researchers to suggest that genetic variation in the NOD2 pathway signaling culminates to a decreased proinflammatory response [34]. The frameshift variant L1007fs truncates most of the terminal of the leucine-rich region of NOD2 that is responsible for the recognition of LPS and leads to decreased NF-kB-activation [30].
Another study also showed that R702W, G908R, and L1007fs lead to decreased activity of NF-kB [7]. NOD2 and RIP2 knockout mice infected with L. infantum showed a higher parasite load and low IFNγ than wild-type mice [15]. Furthermore, RNAseq of peripheral blood from patients with VL revealed an upregulation of TH1 genes. A NOD2-driven Th1 response protects against L. infantum pathogenesis [15], Trypanosoma cruzi [35], and Toxoplasma gondii [36]. NOD2 variants are associated with a high level of IL-17 in PBMC from patients with multiple sclerosis or with ocular toxoplasmosis stimulated with myelin-basic protein and soluble Toxoplasma antigen, respectively [37, 38].
Monocytes and lymphocytes from individuals harboring the L1007fs, infected with either L. braziliensis or L. amazonensis, exhibit lower levels of TNFα, IL-1β, IL-6, IL-8, IFN-γ, and IL-17 [14]. The genotypes of the three variants with levels of plasma cytokine were evaluated. NOD2 G908R genotypes tended to correlate with plasma cytokine TNF-α, IFN-γ, IL-17, and the chemokine IL-8 was observed but did not reach statistical significance. The NOD2 R702W genotypes showed a tendency of correlation with IFN-γ level. We compared all individuals harboring at least one of the variants with the wild-type genotypes. Carriers of the loss of function alleles tend to correlate with lower levels of IFN-γ compared to individuals homozygous for wild-type alleles. It is noteworthy to highlight that it is difficult in vivo to associate the exact impact of these variants on plasmatic cytokine levels as other genetic variants downstream in the NOD2 pathway may also contribute to the cytokines level compared to study in vitro by transfecting cell lines with the variant of interest.
Variants of the NOD2 gene were initially associated with an increased risk of Crohn`s disease [21]. Since then, various studies have shown that the NOD2 variants are associated with other diseases, including Blau syndrome [39], bipolar disorder [40], leprosy [41], and different types of cancer [42, 43]. NOD2 involvement in host defense has been shown extensively in cellular-system in vitro, animal models and genetic susceptibility studies in humans [44, 45]. We tested the three loss of function variants located in the leucine-rich region of the protein of NOD2 in a large sample of patients with Lg-Cl. None of the variants are associated with either susceptibility or protection to the development of CL.
In this study, the minor allele frequency (MAF) of the variant G908R was 0.8% and 0.6% among the healthy controls and patients with Lg-CL, respectively. For the R702W, similar frequencies were observed among healthy controls and patients. The MAF was 2%. Similarly, the MAF of 3020insC was 0.6% and 0.5% among healthy controls and patients, respectively. In the Caucasian population, the MAF of R702W is 4–5%, G908R 1–2%, and L1007fs 2–3% [46]. According to the SNP database of the NCBI (ncbi.nlm.nih.gov/snp), the MAF of G908R in European is 1.5%, African 0.3%, and Latin American 1.6%. The MAF for R702W in European is 4.6%, African 0.8%, and Latin American 2.5%. Similarly, the MAF for L1007fs in European is 9.4%, African 0.5%, and Latin American 0.6%. Notably, the population of this study is an admixture of Native American (50–60%), European (40–60%), and African ancestry (10%) [47].
R702W, G908R, and L1007fsinsC are genetically associated with Crohn`s disease in Europe and America, albeit not all individuals harboring the variants develop the disease [21, 48, 49]. In this study, none of the participants had Crohn`s disease. Furthermore, none of the participants was homozygote for the protein-truncating L100fsinsC.
To the best of our knowledge, this is the first study investigating the variants of NOD2 among patients with Lg-CL. Despite elegant experiments showing that NOD2 may recognize Leishmania components [14] and influence the expression of proinflammatory cytokines in vitro, there is no clear evidence that variants of NOD2 participate in the immunopathogenesis of Lg-CL. It is noteworthy to specify that various factors are involved in the development of CL. Any immune response genes also may play a role. The contribution of any gene may be small and sometimes difficult to detect when the frequencies of MAF are very low and may lack the power to observe any association.
These data demonstrate that variants of NOD2 associated with decreased proinflammatory gene transcription are neither associated with protection nor susceptibility to Lg-CL. However, further studies in geographically distinct population groups of patients with Lg-CL or with CL caused by other species of Leishmania are needed before excluding genetic variants of NOD2 in the involvement of CL.
Supporting information
The crossbars (black) represent the mean concentrations in picogram per milliliter log-scale transformed [log(pg/mL)] and the error bars represent the standard error (SE) of means. P values < 0.05 are considered significant.
(PDF)
The crossbars (black) represent the mean concentrations in picogram per milliliter log-scale transformed [log(pg/mL)] and the error bars represent the standard error (SE) of means. P values < 0.05 are considered significant.
(PDF)
The crossbars (black) represent the mean concentrations in picogram per milliliter log-scale transformed [log(pg/mL)] and the error bars represent the standard error (SE) of means. P values < 0.05 are considered significant.
(PDF)
The crossbars (black) represent the mean concentrations in picogram per milliliter log-scale transformed [log(pg/mL)] and the error bars represent the standard error (SE) of means. P values < 0.05 are considered significant. The combination for all genotypes (G908R, R702W, and Lf1007ins C), common homozygous (A/A), heterozygous (A/O) and rare homozygous (O/O) are also shown in HC, Patients, and Total (A represent wild-type alleles and O mutant alleles of the three variants.
(PDF)
(DOCX)
Acknowledgments
The authors would like to thank all the participants in this work. We also thank Krys Layane Guimarães Duarte, Queiroz Cláudio Marcello da Silveira Júnior and Thais Carneiro de Lacerda for performing technical work.
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
This research was funded by the Brazilian Council for Scientific and Technological Development (CNPq), grant number 404181/2012-0 to Rajendranath Ramasawmy, Fundação de Amparo e Pesquisa do Estado do Amazonas (FAPEAM), grant number 06200151/2020 and 01.02.016301.03393/2021-80 to Rajendranath Ramasawmy and FAPEAM RESOLUÇÃO N. 002/2008, 007|2018 e 005|2019 – PRÓ-ESTADO. The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. https://www.who.int/publications/i/item/who-wer9525 (accessed on 20 June 2022).
- 2.Ministério da Saúde do Brasil. Indicadores de morbidade e fatores de risco. Fundação Nacional de Saúde. http://tabnet.datasus.gov.br (accessed on 20 Dec 2022).
- 3.Benicio Ede A, Gadelha EP, Talhari A, et al. Combining diagnostic procedures for the management of leishmaniasis in areas with high prevalence of Leishmania guyanensis. An Bras Dermatol. 2011;86(6):1141–1144. doi: 10.1590/s0365-05962011000600012 [DOI] [PubMed] [Google Scholar]
- 4.Lee JY, Hwang EH, Kim DJ, Oh SM, Lee KB, Shin SJ, et al. The role of nucleotide-binding oligomerization domain 1 during cytokine production by macrophages in response to Mycobacterium tuberculosis infection. Immunobiology. 2016. Jan;221(1):70–5. doi: 10.1016/j.imbio.2015.07.020 [DOI] [PubMed] [Google Scholar]
- 5.Kim TH, Chae GT, Kang TJ. Expression of Nucleotide-oligomerization Domain (NOD) and Related Genes in Mouse Tissues Infected with Mycobacterium leprae. Immune Netw. 2015. Dec;15(6):319–24. doi: 10.4110/in.2015.15.6.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caruso R, Núñez G. Innate Immunity: ER Stress Recruits NOD1 and NOD2 for Delivery of Inflammation. Curr Biol. 2016. Jun 20;26(12):R508–R511. doi: 10.1016/j.cub.2016.05.021 [DOI] [PubMed] [Google Scholar]
- 7.Bonen DK, Ogura Y, Nicolae DL, Inohara N, Saab L, Tanabe T, et al. Crohn’s disease-associated NOD2 variants share a signaling defect in response to lipopolysaccharide and peptidoglycan. Gastroenterology. 2003. Jan;124(1):140–6. doi: 10.1053/gast.2003.50019 [DOI] [PubMed] [Google Scholar]
- 8.Girardin S E, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003. Mar 14;278(11):8869–72. doi: 10.1074/jbc.C200651200 [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005. Feb 4;307(5710):731–4. doi: 10.1126/science.1104911 [DOI] [PubMed] [Google Scholar]
- 10.Lécine P, Esmiol S, Métais JY, Nicoletti C, Nourry C, McDonald C, et al. The NOD2-RICK complex signals from the plasma membrane. J Biol Chem. 2007. May 18;282(20):15197–207. doi: 10.1074/jbc.M606242200 [DOI] [PubMed] [Google Scholar]
- 11.Li J, Moran T, Swanson E, Julian C, Harris J, Bonen DK, et al. Regulation of IL-8 and IL-1beta expression in Crohn’s disease associated NOD2/CARD15 mutations. Hum Mol Genet. 2004. Aug 15;13(16):1715–25. doi: 10.1093/hmg/ddh182 [DOI] [PubMed] [Google Scholar]
- 12.Tada H, Aiba S, Shibata K, Ohteki T, Takada H. Synergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect Immun. 2005. Dec;73(12):7967–76. doi: 10.1128/IAI.73.12.7967-7976.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabriel Á, Valério-Bolas A, Palma-Marques J, Mourata-Gonçalves P, Ruas P, Dias-Guerreiro T, et al. Cutaneous Leishmaniasis: The Complexity of Host’s Effective Immune Response against a Polymorphic Parasitic Disease. J Immunol Res. 2019. Dec 1;2019:2603730. doi: 10.1155/2019/2603730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dos Santos JC, Damen M, Oosting M, de Jong DJ, Heinhuis B, Gomes RS, et al. The NOD2 receptor is crucial for immune responses towards New World Leishmania species. Sci Rep. 2017. Nov 9;7(1):15219. doi: 10.1038/s41598-017-15412-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nascimento MS, Ferreira MD, Quirino GF, Maruyama SR, Krishnaswamy JK, Liu D, et al. NOD2-RIP2-Mediated Signaling Helps Shape Adaptive Immunity in Visceral Leishmaniasis. J Infect Dis. 2016. Dec 1;214(11):1647–1657. doi: 10.1093/infdis/jiw446 [DOI] [PubMed] [Google Scholar]
- 16.Turco SJ, Sacks DL. Control of Leishmania-sand fly interactions by polymorphisms in lipophosphoglycan structure. Methods Enzymol. 2003;363:377–81. doi: 10.1016/S0076-6879(03)01066-8 [DOI] [PubMed] [Google Scholar]
- 17.McConville MJ, Ferguson MA. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993. Sep 1;294 (Pt 2)(Pt 2):305–24. doi: 10.1042/bj2940305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Carvalho R, Andrade WA, Lima-Junior DS, Dilucca M, de Oliveira CV, Wang K, et al. Leishmania Lipophosphoglycan Triggers Caspase-11 and the Non-canonical Activation of the NLRP3 Inflammasome. Cell Rep. 2019. Jan 8;26(2):429–437.e5. doi: 10.1016/j.celrep.2018.12.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silveira MB, Gomes RS, Shio MT, Rugani JN, Paranaiba LF, Soares RP, et al. Lipophosphoglycan From Dermotropic New World Leishmania Upregulates Interleukin-32 and Proinflammatory Cytokines Through TLR4 and NOD2 Receptors. Frontiers in cellular and infection microbiology, 12, 805720. doi: 10.3389/fcimb.2022.805720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caruso R, Warner N, Inohara N, Núñez G. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity. 2014. Dec 18;41(6):898–908. doi: 10.1016/j.immuni.2014.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001. May 31;411(6837):599–603. doi: 10.1038/35079107 [DOI] [PubMed] [Google Scholar]
- 22.Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. 2011. Jul 11;9(8):604–15. doi: 10.1038/nrmicro2608 [DOI] [PubMed] [Google Scholar]
- 23.Stamper LW, Patrick RL, Fay MP, Lawyer PG, Elnaiem DE, Secundino N, et al. Infection parameters in the sand fly vector that predict transmission of Leishmania major. PLoS Negl Trop Dis. 2011. Aug;5(8):e1288. doi: 10.1371/journal.pntd.0001288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marfurt J, Nasereddin A, Niederwieser I, Jaffe CL, Beck HP, Felger I. Identification and differentiation of Leishmania species in clinical samples by PCR amplification of the miniexon sequence and subsequentrestriction fragment length polymorphism analysis. J Clin Microbiol. 2003; 41: 3147–3153. doi: 10.1128/JCM.41.7.3147-3153.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia L, Kindt A, Bermudez H, Llanos-Cuentas A, De Doncker S, Arevalo J, et al. Culture-independent species typing of neotropical Leishmania for clinical validation of a PCR-based assay targeting heatshock protein 70 genes. J Clin Microbiol. 2004; 42: 2294–2297. doi: 10.1128/JCM.42.5.2294-2297.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Silva G, de Mesquita T, de Souza Encarnação HV, do Espírito Santo J Junior, da Costa Sabino K, de Aguiar Neres I, et al. A polymorphism in the IL1B gene (rs16944 T/C) is associated with cutaneous leishmaniasis caused by Leishmania guyanensis and plasma cytokine interleukin receptor antagonist. Cytokine. 2019. Nov;123:154788. doi: 10.1016/j.cyto.2019.154788 [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. In: Cold Spring, editors. New York: 1989. p. 9.16–9.23. [Google Scholar]
- 28.de Mesquita T, Junior J, de Lacerda TC, Queiroz K, Júnior C, Neto J, et al. Variants of MIRNA146A rs2910164 and MIRNA499 rs3746444 are associated with the development of cutaneous leishmaniasis caused by Leishmania guyanensis and with plasma chemokine IL-8. PLoS Negl Trop Dis. 2021. Sep 20;15(9):e0009795. doi: 10.1371/journal.pntd.0009795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Júnior JES, de Mesquita TGR, da Silva LDO, de Araújo FJ, Souza JL, de Lacerda TC, et al. TREM1 rs2234237 (Thr25Ser) Polymorphism in Patients with Cutaneous Leishmaniasis Caused by Leishmania guyanensis: A Case-Control Study in the State of Amazonas, Brazil. Pathogens. 2021. Apr 20;10(4):498. doi: 10.3390/pathogens10040498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001. May 31;411(6837):603–6. doi: 10.1038/35079114 [DOI] [PubMed] [Google Scholar]
- 31.Rosenstiel P, Hellmig S, Hampe J, Ott S, Till A, Fischbach W, et al. Influence of polymorphisms in the NOD1/CARD4 and NOD2/CARD15 genes on the clinical outcome of Helicobacter pylori infection. Cell Microbiol. 2006. Jul;8(7):1188–98. doi: 10.1111/j.1462-5822.2006.00701.x [DOI] [PubMed] [Google Scholar]
- 32.Maeda S, Hsu LC, Liu H, Bankston LA, Iimura M, Kagnoff MF, et al. Nod2 mutation in Crohn’s disease potentiates NF-kappaB activity and IL-1beta processing. Science 2011. Jul 15; 307: 734–738, 2005. doi: 10.1126/science.1103685 [DOI] [PubMed] [Google Scholar]
- 33.Andreoletti G, Shakhnovich V, Christenson K, Coelho T, Haggarty R, Afzal NA, et al. Exome Analysis of Rare and Common Variants within the NOD Signaling Pathway. Sci Rep. 2017. Apr 19;7:46454. doi: 10.1038/srep46454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashton JJ, Boukas K, Stafford IS, Cheng G, Haggarty R, Coelho T, et al. Deleterious Genetic Variation Across the NOD Signaling Pathway Is Associated With Reduced NFKB Signaling Transcription and Upregulation of Alternative Inflammatory Transcripts in Pediatric Inflammatory Bowel Disease. Inflamm Bowel Dis. 2022. Jun 3;28(6):912–922. doi: 10.1093/ibd/izab318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva GK, Gutierrez FR, Guedes PM, Horta CV, Cunha LD, Mineo TW, et al. Cutting edge: nucleotide-binding oligomerization domain 1-dependent responses account for murine resistance against Trypanosoma cruzi infection. J Immunol. 2010. Feb 1;184(3):1148–52. doi: 10.4049/jimmunol.0902254 [DOI] [PubMed] [Google Scholar]
- 36.Shaw MH, Reimer T, Sánchez-Valdepeñas C, Warner N, Kim YG, Fresno M, et al. T cell-intrinsic role of Nod2 in promoting type 1 immunity to Toxoplasma gondii. Nat Immunol. 2009. Dec;10(12):1267–74. doi: 10.1038/ni.1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedegaard CJ, Enevold C, Sellebjerg F, Bendtzen K, Nielsen CH. Variation in NOD2 augments Th2- and Th17 responses to myelin basic protein in multiple sclerosis. PLoS One. 2011;6(5):e20253. Epub 2011 May 20. doi: 10.1371/journal.pone.0020253 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dutra MS, Béla SR, Peixoto-Rangel AL, Fakiola M, Cruz AG, Gazzinelli A, et al. Association of a NOD2 gene polymorphism and T-helper 17 cells with presumed ocular toxoplasmosis. J Infect Dis. 2013. Jan 1;207(1):152–63. doi: 10.1093/infdis/jis640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miceli-Richard C, Lesage S, Rybojad M, Prieur AM, Manouvrier-Hanu S, Hafner R, et al. CARD15 mutations in Blau syndrome. Nat Genet. 2001. Sep;29(1):19–20. doi: 10.1038/ng720 [DOI] [PubMed] [Google Scholar]
- 40.Oliveira J, Hamdani N, Etain B, Bennabi M, Boukouaci W, Amokrane K, et al. Genetic association between a ’standing’ variant of NOD2 and bipolar disorder. Immunobiology. 2014. Oct;219(10):766–71. doi: 10.1016/j.imbio.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 41.Sales-Marques C, Cardoso CC, Alvarado-Arnez LE, Illaramendi X, Sales AM, Hacker MA, et al. Genetic polymorphisms of the IL6 and NOD2 genes are risk factors for inflammatory reactions in leprosy. PLoS Negl Trop Dis. 2017. Jul 17;11(7):e0005754. doi: 10.1371/journal.pntd.0005754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurzawski G, Suchy J, Kladny J, Grabowska E, Mierzejewski M, Jakubowska A, et al. The NOD2 3020insC mutation and the risk of colorectal cancer. Cancer Res. 2004. Mar 1;64(5):1604–6. doi: 10.1158/0008-5472.can-03-3791 [DOI] [PubMed] [Google Scholar]
- 43.Liu J, He C, Xu Q, Xing C, Yuan Y. NOD2 polymorphisms associated with cancer risk: a meta-analysis. PLoS One. 2014. Feb 20;9(2):e89340. doi: 10.1371/journal.pone.0089340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Philpott DJ, Sorbara MT, Robertson SJ, Croitor K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 2014. Jan;14(1):9–23. doi: 10.1038/nri3565 [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee T, Hovingh ES, Foerster EG, Abdel-Nour M, Philpott DJ, Girardin SE. NOD1 and NOD2 in inflammation, immunity and disease, Arch Biochem Biophys. 2019. Jul 30;670:69–81. doi: 10.1016/j.abb.2018.12.022 [DOI] [PubMed] [Google Scholar]
- 46.Hugot JP, Zaccaria I, Cavanaugh J, Yang H, Vermeire S, Lappalainen M, et al. Prevalence of CARD15/NOD2 mutations in Caucasian healthy people. Am J Gastroenterol. 2007. Jun;102(6):1259–67. doi: 10.1111/j.1572-0241.2007.01149.x [DOI] [PubMed] [Google Scholar]
- 47.Ruiz-Linares A, Adhikari K, Acuña-Alonzo V, Quinto-Sanchez M, Jaramillo C, Arias W, et al. Admixture in Latin America: geographic structure, phenotypic diversity and self-perception of ancestry based on 7,342 individuals. PLoS genetics 2014, 10(9), e1004572. doi: 10.1371/journal.pgen.1004572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hampe J, Cuthbert A, Croucher PJ, Mirza MM, Mascheretti S, Fisher S, et al. Association between insertion mutation in NOD2 gene and Crohn’s disease in German and British populations. Lancet. 2001. Jun 16;357(9272):1925–8. doi: 10.1016/S0140-6736(00)05063-7 [DOI] [PubMed] [Google Scholar]
- 49.Cuthbert AP, Fisher SA, Mirza MM, King K, Hampe J, Croucher PJ, et al. The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology. 2002. Apr;122(4):867–74. doi: 10.1053/gast.2002.32415 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The crossbars (black) represent the mean concentrations in picogram per milliliter log-scale transformed [log(pg/mL)] and the error bars represent the standard error (SE) of means. P values < 0.05 are considered significant.
(PDF)
The crossbars (black) represent the mean concentrations in picogram per milliliter log-scale transformed [log(pg/mL)] and the error bars represent the standard error (SE) of means. P values < 0.05 are considered significant.
(PDF)
The crossbars (black) represent the mean concentrations in picogram per milliliter log-scale transformed [log(pg/mL)] and the error bars represent the standard error (SE) of means. P values < 0.05 are considered significant.
(PDF)
The crossbars (black) represent the mean concentrations in picogram per milliliter log-scale transformed [log(pg/mL)] and the error bars represent the standard error (SE) of means. P values < 0.05 are considered significant. The combination for all genotypes (G908R, R702W, and Lf1007ins C), common homozygous (A/A), heterozygous (A/O) and rare homozygous (O/O) are also shown in HC, Patients, and Total (A represent wild-type alleles and O mutant alleles of the three variants.
(PDF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.