Highlights
-
•
There is a need for new paradigms in biomaterials that can push the current boundaries that guide, yet also limit, their design.
-
•
Embracing complexity in biomaterials design will require an holistic approach that considers a systems-level design.
-
•
Embracing complexity in biomaterials design will enable a new generation of biomaterials with life-like properties and will extend their capabilities to unleash new paradigms in medical treatment.
Keywords: Biomaterials, Complexity, Human biology, Variability, Reproducibility, Order/disorder
Abstract
Animate materials, man-made materials behaving like living systems, are attracting enormous interest across a range of sectors, from construction and transport industry to medicine. In this leading opinion article, we propose that embracing complexity in biomaterials design offers untapped opportunities to create biomaterials with innovative life-like properties that extend their capabilities and unleash new paradigms in medical treatment.
Complexity in biology
Biology is full of beautiful examples of remarkable structures and complex phenomena made and regulated by multiple molecular and cellular building blocks interacting cooperatively through controlled mechanisms that give rise to sophisticated properties and materials [1, 2]. From the toughness and resilience of silk or resilin to the effective catalytic functions of enzymes at the molecular scale; from the locomotive properties of kinesin and dynein to the dynamic disorder-to-order transitions of membraneless organelles at the nanoscale scale; from the responsive coordinated migration of and communication between cells during development to the way organisms such as the salamander are able to regrow lost limbs at the micro and macroscale. Independently of the size scale at which one looks, biological systems inspire us. In doing so, it is easy to mistake their remarkable reproducibility and efficiency for simplicity. In fact, biology is anything but simple, and it is its inherent complexity in the way biological systems grow, respond, diversify, and optimize that give rise to their outstanding properties.
Progresses in biomaterials design
Biomaterials have improved healthcare in many ways and continuous developments in the field are expected to lead to breakthrough solutions for many clinical problems. While initially designed to be passive (i.e. biologically inert to minimize immune responses) and restore the basic functions of the tissues being replaced by matching their mechanical properties [3], increasing control over chemical functionality and resulting properties have led to an explosion in diversity and potential applications of biomaterials [3, 4]. Furthermore, as our understanding of how biological systems function has increased, so has the pursuit for biomaterials that can mimic their properties. A common thread in this extensive biomaterial landscape has been the search of reductionist approaches, which have been largely imposed by practical, economic and regulatory constraints to afford reproducible manufacturing and facilitate clinical translation. For example, testing biomaterials by undertaking cell culture in 2D, rather than 3D, benefits higher throughput but fails to recreate the in vivo environment, removes critical system interactions, and introduces dimensionality artefacts. However, simplicity has also enabled the biomaterials community to dissect the inherent complexity of biological systems [5] and lay down underlying principles and design rules [6] to attempt to recreate them. Pioneering studies have generated examples of biomaterial systems capable of growing, dividing and multiplying, self-healing, and exhibiting hierarchical structures. Nonetheless, these approaches have mainly used single components or enabled the recreation of single properties (e.g. composition, stiffness, shape, topography, degradability, ligand density) [4].
In spite of remarkable advances, we are far from developing practical materials that can emulate the vast majority of complex functions exhibited by living systems [6]. In fact, there is a growing frustration that materials science has not delivered the kinds of practical breakthroughs that have been expected [6]. There is a need for new paradigms that can push the current boundaries that guide, yet also limit, materials design. In doing so, it is helpful to realize that we have evolved to pursue order and symmetry and to avoid chaos and disorder. Our natural proclivity for simplification has enabled remarkable advances in materials science but can also constrain creativity and access to mechanisms that make biology so rich, efficient, and functional. Here, we argue that embracing complexity, rather than avoiding it, can lead to new ways of thinking about biomaterials design. This approach does not have to replace minimalistic strategies but rather offers opportunities to complement and enhance them. The need for embracing and engineering complexity is increasingly being recognized in fields expanding from precision medicine [7] to biofabrication [8].
Open challenges and opportunities in biomaterials design
In this perspective, we argue that new ways of thinking about materials design could offer solutions to major unmet needs. For example, the fields of tissue engineering and regenerative medicine continue to face gruelling challenges because of the difficulty of recreating the inherent complexity of the regenerative milieu. Biology is complex and minimalistic approaches to recreate it can only go so far. However, given the increasing understanding of underlying biological principles and capacity to manipulate molecules, new opportunities are emerging. How far these go may depend on our capacity to think outside conventional conceptual boundaries and operate at the interface of traditionally unrelated fields such as for example structural biology and biofabrication or supramolecular chemistry and mechanobiology.
Materials design through biological organization principles
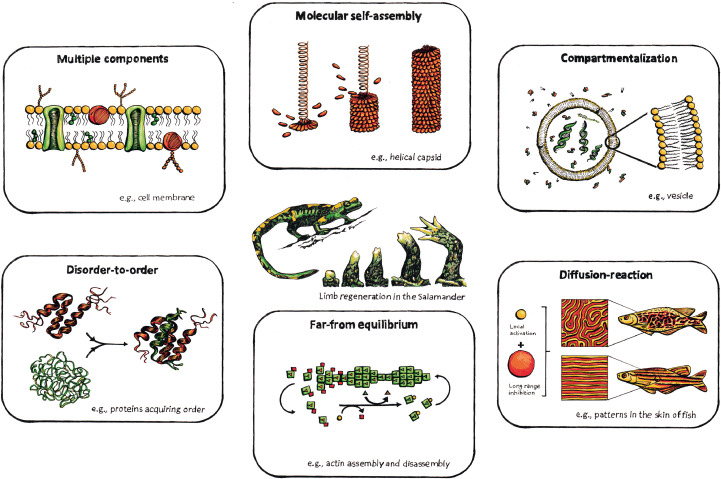
Like biological systems, chemical systems can exhibit complex behaviour that emerges from cooperative interactions and chemical networks formed by multiple components, not observed in isolated molecules or reactions [9]. These interactions enable both functionality of individual components (e.g. protein disorder-order transitions) as well as the emergence of mechanisms of assembly that nature uses to diversify, respond, and ultimately optimize. Embracing complexity in biomaterials will require a similar holistic approach that considers a systems-level design and integrates interdependent parts (Fig. 1), briefly discussed in the following sub-sections.
Fig. 1.
Proposed holistic approach to integrate complexity in biomaterials design harnessing supramolecular biological organization principles that nature has evolved as assembling rules for materials design. Adapted from [8] with permission.
Self-assembly and multicomponent self-assembly
Biological systems rely on self-assembling processes that have high-level of organization across scales, and which are specific, selective, and adaptable. Reductionist approaches have enabled the generation of remarkable materials such as those based on self-assembling peptides. However, self-assembling systems that integrate multiple types of building-blocks can amplify interactions and facilitate the generation of emergent and innovative properties that go beyond those of individual components. In fact, biology builds by integrating and coordinating multiple molecular building-blocks. The use of multiple molecular components can significantly enhance the diversity of the resulting structures thus avoiding limits on the emergence of complexity that homogeneity imposes.
Disorder-to-order transitions
We have evolved to pursuit order and avoid disorder. However, living systems operate within a balance of order and disorder that enables both stability and growth. This is particularly clear when looking at proteins. It is becoming increasingly recognised that proteins function as a network and that their interactions with one another determine their activity. There is growing consensus that the disordered regions of proteins play a key structural and signalling role in protein function, through interactions with other molecules, facilitated by their conformational flexibility [10].
Compartmentalization and diffusion-reaction processes
As multiple components interact, supramolecular structures emerge. In biological systems, interactions between different molecules and disorder-to-order transitions can lead to the emergence of distinct compartments that trigger downstream events such as diffusion or the generation of gradients, which lead to dynamic behaviours such as growth and healing. Complex patterns, such as animal skin patterns and skeletal shapes in vertebrate limbs, are widely observed in biology. Pattern formation is driven by concentration gradients regulated by reaction-diffusion mechanisms. Morphogenesis and hierarchical organization have been achieved in synthetic chemical systems through reaction-diffusion systems (e.g. compartmentalization via a semi-permeable barrier/membrane) that produce concentration differences between different chemical environments which evolve into spatio-temporal patterns.
Far-from equilibrium systems and emergence of properties
In living systems, molecular assemblies are regulated by competitive chemical (anabolic or catabolic) reaction networks that control the activation and deactivation of their precursors. For assemblies to emerge and breakdown over time, the anabolic and catabolic reactions must be regulated independently. Initially, the rate of the anabolic reaction needs to be higher than the catabolic reaction to favour temporary accumulation of precursors to promote their assembly, but then self-assembly can be maintained in a steady state when the rate constants of assembly and disassembly are equal. The microenvironment affects the kinetics of the reaction cycle, influencing the assembly propensity. Furthermore, the interplay between assembly and reaction cycles can generate oscillations in the assembling and disassembling processes, triggering transient order and resulting in pattern formation and assemblies with dynamic behaviour. Dynamic self-assembling systems require continuous energy supply to sustain their maintenance so assembly will stop when the fuel is completely used or when the rate of the catabolic reaction takes control over the reaction cycle.
Living systems exhibit properties that go beyond those of the individual building-blocks that compose them. Independently of size scale, biological structures display properties such as adaptation, replication, augmentation, or the capacity to grow and heal that emerge upon interactions between the components that form them. By emulating or incorporating biological organization principles within materials design, it is possible to move away from limits that homogeneity imposes and, instead, trigger and control emergent properties.
Beyond bioinspiration – biocooperative materials
Interest in bioinspired materials has grown for decades because of the remarkable advantages that structures and properties of biological materials could offer. Advances in fields such as structural and synthetic biology, supramolecular chemistry, and biofabrication continue to grow our toolkit to engineer synthetic materials that emulate features of biological ones. However, given the inherent complexity of biology, it is important to also explore other avenues that can facilitate access to the properties of natural materials. For example, the possibility to work with endogenous molecules and assembling mechanisms of living systems could offer an attractive alternative to reach this goal.
Final remarks
The realization of biomaterials that function as those in living systems requires an inclusion of complexity that exploits heterogenous building blocks and emerging phenomena. This approach enables the incorporation of collective interactions and assembly of cooperative structures, as well as exogenous interventions for kinetic control and dynamic behaviour. This new generation of biomaterials has the potential to push current boundaries in materials performance and drive major changes in the way we deliver medical treatments that can be more precise and adapted to each patient.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
A. M. acknowledges the support by the ERC Proof-of-Concept Grant MINGRAFT (790240) and the Medical Research Council (UK Regenerative Medicine Platform Hub Acellular Smart Materials 3D Architecture, MR/R015651/1).
Contributor Information
Helena S. Azevedo, Email: h.azevedo@qmul.ac.uk.
Alvaro Mata, Email: A.Mata@nottingham.ac.uk.
References
- 1.Fratzl P., Speck T., Gorb S. Function by internal structure–preface to the special issue on bioinspired hierarchical materials. Bioinspirat Biomimetics. 2016;11(6) doi: 10.1088/1748-3190/11/6/060301. [DOI] [PubMed] [Google Scholar]
- 2.Wegst U.G.K., Bai H., Saiz E., Tomsia A.P., Ritchie R.O. Bioinspired structural materials. Nat Mater. 2015;14(1):23–36. doi: 10.1038/nmat4089. [DOI] [PubMed] [Google Scholar]
- 3.Hench L.L., Polak J.M. Third-generation biomedical materials. Science. 2002;295(5557):1014–1017. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 4.Tibbitt M.W., Rodell C.B., Burdick J.A., Anseth K.S. Progress in material design for biomedical applications. Proc Natl Acad Sci. 2015;112(47):14444–14451. doi: 10.1073/pnas.1516247112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darnell M., Mooney D.J. Leveraging advances in biology to design biomaterials. Nat Mater. 2017;16(12):1178–1185. doi: 10.1038/nmat4991. [DOI] [PubMed] [Google Scholar]
- 6.Grzybowski B.A., Huck W.T.S. The nanotechnology of life-inspired systems. Nat Nanotechnol. 2016;11(7):585–592. doi: 10.1038/nnano.2016.116. [DOI] [PubMed] [Google Scholar]
- 7.Chin M.H.W., Gentleman E., Coppens M.O., Day R.M. Rethinking cancer immunotherapy by embracing and engineering complexity. Trends Biotechnol. 2020;38(10):1054–1065. doi: 10.1016/j.tibtech.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Hill J., Wildman R., Mata A. Exploiting the fundamentals of biological organization for the advancement of biofabrication. Curr Opin Biotechnol. 2022;74:42–54. doi: 10.1016/j.copbio.2021.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Azevedo H.S., Perry S.L., Korevaar P.A., Das D. Complexity emerges from chemistry. Nat Chem. 2020;12(9):793–794. doi: 10.1038/s41557-020-0537-x. [DOI] [PubMed] [Google Scholar]
- 10.van der Lee R., Buljan M., Lang B., Weatheritt R.J., Daughdrill G.W., Dunker A.K., Fuxreiter M., Gough J., Gsponer J., Jones D.T., Kim P.M., Kriwacki R.W., Oldfield C.J., Pappu R.V., Tompa P., Uversky V.N., Wright P.E., Babu M.M. Classification of intrinsically disordered regions and proteins. Chem Rev. 2014;114(13):6589–6631. doi: 10.1021/cr400525m. [DOI] [PMC free article] [PubMed] [Google Scholar]