Highlights
-
•
Mitochondrial dysfunction is a hallmark of human cancers and is a major obstacle that limits the efficacy of targeted therapy.
-
•
Mitochondria is an emerging pharmacological target for treating cancer.
-
•
Nanoparticles could function as safe carriers to deliver anticancer drugs to mitochondria.
-
•
The use of nanotechnology to target tumour offers a considerable reduction in adverse side effects through a combination of cytotoxic chemotherapy and other light-mediated therapies such as photodynamic therapy (PDT) and photothermal therapy (PTT).
-
•
The selective delivery of nanoparticles to mitochondria is an elegant shortcut to more selective, targeted, and safer cancer treatment.
Abstract
The early understanding of mitochondria posited that they were ‘innocent organelles’ solely devoted to energy production and utilisation. Intriguingly, recent findings have outlined in detail the ‘modern-day’ view that mitochondria are an important but underappreciated drug target. Mitochondria have been implicated in the pathophysiology of many human diseases, ranging from neurodegenerative disorders and cardiovascular diseases to infections and cancer. It is now clear that normal mitochondrial function involves the building blocks of a cell to generate lipids, proteins and nucleic acids thereby facilitating cell growth. On the other hand, mitochondrial dysfunction reprograms crucial cellular functions into pathological pathways, and is considered as an integral hallmark of cancer. Therefore, strategies to target mitochondria can provide a wealth of new therapeutic approaches in the fight against cancer, by overcoming a number of problems associated with conventional pharmaceutical drugs, including low solubility, poor bioavailability and non-selective biodistribution. The combination of nanoparticles with ‘classical’ chemotherapeutic drugs to create biocompatible, multifunctional mitochondria-targeted nanoplatforms has been recently studied. This approach is now rapidly expanding for targeted drug delivery systems, and for hybrid nanostructures that can be activated with light (photodynamic and/or photothermal therapy). The selective delivery of nanoparticles to mitochondria is an elegant shortcut to more selective, targeted, and safer cancer treatment. We propose that the use of nanoparticles to target mitochondria be termed “mitoNANO”. The present minireview sheds light on the design and application of mitoNANO as advanced cancer therapeutics, that may overcome drug resistance and show fewer side effects.
Graphical abstract
Nanotechnology: tiny particles against big problems
Nanotechnology is a multidisciplinary field, which has provided many technological breakthroughs, most notably in electronics, energy storage, photovoltaics, pharmaceutics, and diagnostics. Nanomedicine uses nanotechnology to create advanced therapeutic approaches, particularly in the fight against cancer. Nanomedicine is driven by the synergy between nanoparticles (NPs) and their unique tunable features (size, shape, surface properties, optical properties, etc.) compared to conventional pharmaceutical compounds. The use of nanotechnology to detect biomarkers for more precise diagnosis of cancer at a curable stage, as well its use to treat cancer in a timely fashion has been employed for nearly 50 years [1]. NPs offer a wide variety of advantages for diagnosis and therapy, such as control over shape and size, large surface area to carry both therapeutic and imaging payloads, extremely high surface-to-volume ratio, ability to solubilise hydrophobic drugs, and the ease of surface functionalisation. NPs can be selectively accumulated in tumours using either active or passive targeting strategies. In passive tumour targeting, the enhanced permeability and retention (EPR) effect leads to preferential extravasation of NPs through the leaky blood vessels. In active targeting, ligands are attached to the NPs that can recognise molecules that are over-expressed on tumour cells. A key overall advantage of NPs over standard chemotherapy drugs is their ability to be selectively delivered to the desired target [2]. Active targeting approaches include covalent linkage of antibodies, aptamers, or peptides, or ligands, which bind to receptors. The use of NPs can extend the blood circulation time, reduce the rate of excretion, and protect the drugs from biodegradation [3]. NPs can be constructed with different morphologies, such as zero-dimensional (0D) structures (e.g., quantum dots), one-dimensional (1D) structures (e.g., nanowires, nanotubes), two-dimensional (2D) structures (e.g., single-layered graphene and other molecular sheets), or three-dimensional (3D) structures (e.g., liposomes, micelles, hydrogels) [4].
Drug delivery is considered one of the most important and promising aspects of cancer treatment. The application of NPs in drug delivery systems include, controlled, sustained and predictable release of drugs or biomolecules, external/internal stimulus-responsive site-targeted delivery, and the sensing of specific biomolecules that can provide therapeutic feedback. Drug delivery platforms can precisely be controlled via varying their size, shape, surface chemistry, and chemical composition in order to achieve optimal cellular and subcellular delivery. Liposomes loaded with doxorubicin were approved 25 years ago for the treatment of Kaposi's sarcoma, and have recently been approved to treat breast and ovarian cancer [5]. For diagnostic purposes, other NPs have been used clinically both to enhance imaging contrast and to improve targeting at molecular and cellular levels. For instance, gadolinium [6] and iron oxide-based [7] NPs have been clinically approved to improve MRI imaging contrast, while lipid NPs are used to improve ultrasound imaging [5], which highlight the ability of NPs to solve real-world clinical problems.
Until now more than 20 different NPs have been FDA approved for use in clinical settings [8]. The synthetic methods for NPs continue to be refined for more targeted therapies. Examples of extensively employed NPs for cancer nanomedicine are, liposomes [9], polymeric and inorganic NPs [10], metallic NPs (gold or silver) [11], and carbon nanotubes [12]. Given the therapeutic benefits of nanotechnology for the targeted delivery of anticancer agents, nanomedicine may address unmet clinical problems, reduce side-effects, and improve the bioavailability of drugs. The present perspective aims to summarise the use of nanoplatforms to target mitochondria for cancer therapy, and to discuss the future possibilities and problems that may occur.
Mitochondria: function and dysfunction
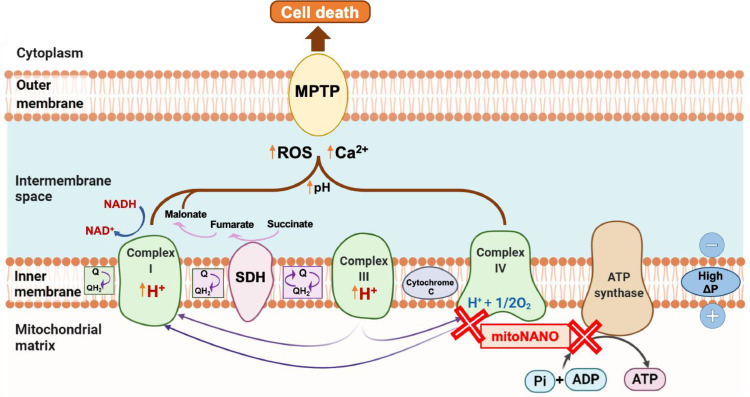
Mitochondria are subcellular organelles with a double membrane located within the cytoplasm of eukaryotic cells. Mitochondria are composed of four components (Fig. 1): the outer mitochondrial membrane, the inner mitochondrial membrane, the intermembrane space, and the matrix. The inner mitochondrial membrane preserves the negative membrane potential which is needed to initiate the electron transport chain (ETC) and adenosine triphosphate (ATP) production. Moreover, the inner membrane also carries several proteins involved in the respiratory chain [13]. Mitochondria play multiple crucial roles in the cellular function of normal cells, including energy supply, oxidative phosphorylation, ATP production, production of reactive oxygen species (ROS), cell cycle control, cell differentiation, and programmed cell death. Mitochondria are responsible for mediating cell apoptosis by controlling the formation of the mitochondrial permeability transition pore (MPTP), and play a central role in cell proliferation, growth and survival. Mitochondria function as an enzymatic factory for ATP production either via oxidative phosphorylation, or via aerobic glycolysis in cancer (known as the Warburg effect) [13]. ATP production is carried out on the inner membrane of mitochondria via the ATP synthase enzyme. During ATP production, protons are pumped out of the mitochondrial matrix into the intermembrane space via complexes I, III and IV [14]. Fig. 1 shows the involvement of complexes (I, III, and IV) in the additional processes of ROS generation and movement of calcium ions, which in turn open the mitochondrial transition pores and induce cell death. Electrons transferred from reduced coenzymes via the ETC are used to pump out protons from the inner membrane, thereby resulting in maintenance of the normal mitochondrial membrane potential (∼140 mV).
Fig. 1.
Schematic showing mitochondria as a key driver and important therapeutic target of cancer. This figure shows the chaotic progression of events linked to cell death. Proton translocation derived by respiratory actions of complexes I, III and IV across the inner mitochondrial membrane mediates the generation of adenosine triphosphate (ATP) from adenosine diphosphate (ADP) and Pi. Oxidation of mitochondrial electron transfer chain by quinone oxidoreductase results in the reduction of coenzyme quinone (Q). Reduced Q generates cytochrome c (via complex III) which catalyses the reduction of molecular oxygen to water (via complex IV). Reactive oxygen species (ROS) generation derived from complex I along with accumulation of calcium ions facilitates the opening of mitochondrial permeability transition pore (MPTP) thereby resulting in programmed cell death.
On the other hand, prolonged oxidative stress or genetic mutations in genes encoding mitochondrial proteins induce mitochondrial dysfunction, which in turn contributes to a number of diseases, such as cancer, Alzheimer's disease, Parkinson's disease, and diabetes. Mitochondrial dysfunction is closely associated with cell apoptosis, autophagy, and necrosis. In cancer, mitochondrial dysfunction is involved in more rapid tumour cell proliferation, cell survival, tumour progression, and recurrence. Cancer cells show an increased membrane potential (∼ 220 mV) compared to normal cells (∼140 mV) [15]. Additionally, the mitochondrial dysfunction found in tumours is associated with impaired oxidative phosphorylation, overproduction of ROS, accumulation of calcium, and elevated inflammation. These key aspects of mitochondrial dysfunction mean that tumours are more sensitive to reduced energy metabolism in comparison to normal cells and tissues. It has widely been reported that mitochondrial dysfunction also promotes hypoxia, an acidic microenvironment, and cancer drug resistance. Owing to these distinctive features of mitochondrial function and dysfunction, targeting mitochondria has been proposed and evaluated as a promising therapeutic strategy. However, the complex nature, negative membrane potential and highly dense double membrane of mitochondria make it challenging to target them selectively and safely. Therefore, the conjugation of mitochondria-targeting moieties/ligands with drugs or NPs have received much attention over recent years. NPs which have the ability to selectively probe and perturb mitochondrial function, represent a new approach for cancer therapy. These NPs can act as drug-carriers, photosensitisers, radiosensitisers, or theranostic agents, and may be an alternative anticancer approach by targeting the energy machinery of tumours. Some specific examples of these ligands and mitochondria-targeting strategies will be discussed in the following sections.
Mitochondria-targeting strategies
The current mainstream cancer therapies include, surgery, chemotherapy, immunotherapy, radiation therapy, along with photothermal and photodynamic therapy In general, only surgical removal of early-stage cancer is curative, while the other modalities only aim to extend survival and can also cause considerable long-term side-effects. Despite the key advances made in cancer treatment over the past several decades, the development of resistance to chemotherapy, radiation therapy, and immunotherapy is a major failure in cancer management. Chemotherapy drugs can eradicate drug-responsive tumour cells, whilst the surviving drug-resistant tumour cells allow tumour recurrence and ultimately metastasis [16]. Drug resistance occurs by either intrinsic or acquired mechanisms [17]. Intrinsic drug resistance exists in some tumour types prior to treatment, while acquired drug resistance emerges during the course of treatment. Drug resistance may result from a wide range of mechanisms including, genetic mutations, up-regulation of drug efflux pumps, dysregulation of cell death mechanisms, alterations within the tumour microenvironment, and activation of alternative survival pathways. The tumour heterogeneity and the propensity for steadily increasing cellular invasion and metastasis, make the emergence of drug resistance even more lethal. Drug resistance accounts for 90% of cancer related deaths [18], thus ensuring it has received a great deal of attention in oncology over recent decades.
Intriguingly, the targeting of subcellular organelles has emerged as an important strategy in order to overcome drug resistance and associated problems, including limited penetration of drugs into the site of action, targeted and safe delivery of drugs to the target site etc. NPs can be used to specifically target a range of different intracellular organelles, such as mitochondria [19], lysosomes [20, 21], endoplasmic reticulum [22] and Golgi apparatus [23, 24]. This targeting ability could be achieved with rationally designed engineered NPs, depending on the desired organelle.
Over the past two decades, a wide variety of strategies have been devised and evaluated for mitochondria-targeted therapeutics. Mitochondria-targeted therapy primarily relies on the selective and targeted delivery of drugs at correct doses and concentrations to the mitochondria in order to achieve optimal therapeutic outcomes while avoiding off-target toxicity to healthy cells and tissues. Typical examples of mitochondria-targeted anticancer drugs are mito-lonidamine [25] and cisplatin [26], which promote mitochondrial-dependent ROS generation, and induce programmed cell death. One of the mechanisms of action of cisplatin involves its targeting to mitochondrial DNA. Liposomes co-loaded with either lonidamine or epirubicin for improved mitochondrial targeting showed the highest cytotoxic effect on A549/cDDP cells in vitro and significant antitumour activity against A549/cDDP xenografts in nude mice, when compared with free lonidamine or epirubicin used alone [27]. Lonidamine has also been tested in Phase II and Phase III trials targeting lung cancer and was shown to be safe, but with only limited anticancer effects [28]. These drugs trigger programmed cell death via activating the BAX and BAK proteins. BAX and BAK are key facilitators of mitochondrial outer membrane permeability, which typically governs the fate between cell survival and cell death. Mitochondria-targeted NPs (mitoNANO) co-loaded with lonidamine showed 10-fold higher antitumour effects compared to pure lonidamine. Therefore, selective targeting of mitochondria using NPs (either as a drug carrier or as drug-free light-responsive materials) can be an alternative approach to develop innovative cancer treatments. mitoNANO may also lead to mitochondrial impairment via photodynamic therapy or photothermal therapy.
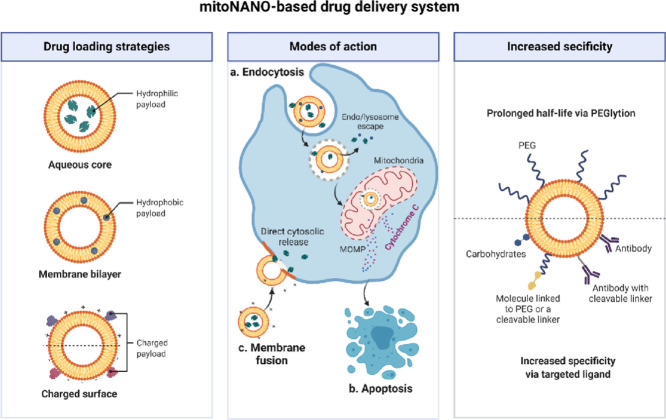
The complex structure of the mitochondrial membrane along with its high negative potential prevents the entry of anionic drugs and molecules into the mitochondrial matrix [29], [30]. Therefore, the design and development of mitochondria ligand conjugated NPs is a promising strategy to target mitochondria. There are two approaches available to achieve the mitochondrial targeting of NPs; active and passive targeting. Passive targeting involves the pH-dependent surface cationic charge of NPs (which could help them bind to the negative potential of the mitochondrial membrane), while active targeting involves the surface functionalisation of NPs with lipophilic cations (mitochondrial specific ligands). Owing to the hydrophobic and highly negative mitochondria membrane potential, the passive targeting approach of preparing mitoNANO offers a number of advantages such as a simple and cost-effective synthetic procedure. Despite these advantages, these passively targeted NPs can also undergo aggregation leading to rapid clearance from the living system. For active targeting of mitoNANO, there are a wide variety of mitochondrial-specific moieties that can be attached either to drug-loaded or free NPs. These ligands include triphenylphosphonium (TPP+) [31], tetramethylrhodamine-5-isothiocyanate [32], mitochondrial peptides [33], dequalinium [34], various cationic heterocycles [35], vitamin E analogue α-tocopheryl [36, 37], 7-amino coumarin [38, 39, 40] and some natural products (hypericin and glycyrrhetinic acid) [41]. There are some specific issues associated with these ligands, such as immunogenicity, high costs, complex synthesis routes, off-target toxicity, long blood circulation time, and delayed clearance/excretion from the living system.
TPP+ is a widely studied mitochondrial ligand used in the preparation of nontoxic NPs that can be selectively internalised into mitochondria. The conjugation of TPP+ to NPs increases the lipophilicity and enables them to freely pass through the membrane bilayers of the mitochondria. The more negative plasma membrane potential of the mitochondria in cancer cells facilitates the transport of the TPP+ tagged NPs from the cytoplasm into mitochondria up to one hundred times compared to non-functionalised NPs. Hence, TPP+ is a frequently employed approach owing to its lipophilic nature, stable cationic charge, and ability to escape from lysosomal compartments. TPP+ functionalised NPs such as polymeric, gold, silver, silica, iron, core-shell nanostructures, carbon nanotubes, graphene oxide, graphene quantum dots, loaded with a variety of drugs, and with varying sizes and shapes have shown promise for mitochondrial targeting [42]. Despite these advantages, the delayed delivery of TPP+ conjugated NPs to tumour mitochondria can reduce the targeting ability of NPs to the site of action. In addition to mitochondria ligands, short mitochondria-penetrating or targeting signal peptides have also been used for the development of mitochondria targeted formulations. However, these peptide-conjugated NPs show some limitations, such as limited storage, lack of controlled release of drugs from NPs, and limited penetration of the formulations into diseased mitochondria [40].
In order to achieve the optimal therapeutic effects and targeting of mitochondria, an ideal design of mitoNANO formulations should meet the following requirements. (i) A suitable surface charge, which can facilitate the escape of particles from the endolysosomes. One promising strategy is to coat NPs with PEG, which can also reduce the likelihood of adsorption of proteins onto NPs. Moreover, wrapping of NPs with PEG can facilitate the passive targeting of NPs into the tumour via the EPR effect. (ii) Stimulus responsive release of drugs triggered by pH, redox reactions, light irradiation, magnetic field, etc. (iii) A sufficient drug loading abilities of the formulation. (iv) Controlled, predictable and slow release of drugs in the optimum amount at the desired time and place. In addition to these requirements, further studies are needed to design mitoNANO, which can actively interact with respiratory chains and complexes to produce ROS, thereby resulting the opening of transition pores and triggering apoptosis.
Mitochondria-targeted nanoparticles (mitoNANO) exerts anticancer effects
Recent reports have shown that mitoNANO can exert toxic effects in cancer cells but not in surrounding healthy cells. NP-based therapies may act directly on the cancer cell mitochondria to accelerate mitochondrial blockades such as, electron transport chain inhibition, respiratory inhibition, modulation of the MPTP, inhibition of anti-apoptotic protein family members, suppression of phenotypes linked with mutated DNA, and the promotion of mitochondrial regulated cancer cell death.
Chen et al. [43] described gold nanostars that were functionalised with cationic octaarginine peptide and with TPP+. These were loaded with doxorubicin and tested for combined photothermal therapy and chemotherapy. This formulation was taken up into squamous cell carcinoma (SCC-7) and doxorubicin-resistant breast cancer cells (MCF-7) via interaction with CD44 receptors. Not only did this formulation selectively deliver doxorubicin to mitochondria, but also produced hyperthermia when triggered by NIR laser irradiation. TPP+ anchored drug-free NPs have been investigated as photosensitisers (for use in photodynamic or photothermal therapy). Gold nanostructures such as nanostars, nanorods, or nanohybrids have also shown excellent therapeutic effects owing to their size-dependant optical properties, high yield of ROS generation, and surface plasmon resonance phenomena. For instance, Guo et al. [44] reported that TPP+ covalently conjugated to nitrogen-doped graphene quantum dots could selectively deliver nitric oxide to tumours. This platform efficiently targeted the cancer cell mitochondria. Gold-platinum bimetallic NPs have also been shown to enhance the catalytic conversion of H2O2 into O2 for improving the oxygen availability in photodynamic therapy [45]. Recently Hu et al. [46] reported the synthesis and photodynamic effects of mitoDNA-targeted mesoporous silica shell coated upconversion NPs, demonstrating their rapid accumulation within cancer cell's mitochondria with light activated generation of H2O2 and hydroxyl radicals. NIR mediated photothermal therapy has ability to overcome some issues of photodynamic therapy including the dependence on oxygen levels and the low penetration of ultraviolet/visible light. In another study, Xu et al. [47] reported the design of polypyrrole-silica-based (Py@Si) hybrid NPs for targeting cancer cell mitochondria via conjugation of NPs with TPP+. This hybrid showed good internalisation of NPs into mitochondria. The combination of chemotherapy and PTT using NIR (808 nm) laser irradiation, reduced the tumour weight in the Py@Si-TH-DOX treatment group to 8.5 times lower than that in the Py@Si-H-DOX group. These results further confirm that targeting mitochondria is a valid approach for targeted therapy. Clearly, TPP+ functionalised NPs have become an attractive therapeutic platform to achieve better targeting with minimal aggregation. Nevertheless, the synthesis methods for the functionalisation of NPs with TPP+ are expensive and time consuming, and can have a low yield with relatively poor control over the size of the final product. As such there are a wide variety of experimental studies available on in vitro and in vivo utility of mitoNANO for cancer targeting, in the current minireview, we have therefore, focussed on the latest strategies, potentials and challenges of this emerging technology in selectively targeting mitochondria with minimal side-effects for cancer therapy.
Despite some promising developments regarding the preparation and therapeutic evaluation of mitoNANO, the progress of this technology towards clinical trials is slow. This delay might be attributed to several issues associated with the tumour microenvironment and extracellular matrix where NPs can form a protein corona as a result of their interaction with extracellular proteins. The protein corona may interfere with the circulation of NPs in the biological environment as well as the clearance of NPs from living systems. Therefore, the protein corona tends to inhibit the entry of NPs into cancer cells thereby leading to poor tumour accumulation. In summary, an in-depth investigation into the innovative design of targeted NPs to improve the biopharmaceutical properties of NPs and their molecular and cellular fate within the tumour mitochondria is necessary to achieve the maximum on-target cytotoxic effects.
Problems and future possibilities
Over the past two decades, it has become clear that mitochondria play a central role in the processes of both cell survival and cell death. More recent findings have suggested that mitochondria are a major therapeutic target in common diseases such as cancer, infections, neurodegenerative, and cardiovascular diseases. The pivotal role of mitochondria in many facets of the malignant phenotype that control cancer progression, invasion and metastasis has led to the development of innovative small molecules and drugs. Nevertheless, only a few mitochondria-targeted drugs have been approved by the FDA so far. To date, there have been no drug-loaded or drug-free NPs that have received clinical approval for the controlled delivery of anticancer drugs to mitochondria. Targeted drug delivery relies on the ability of drugs to reach the target site, with controlled release of the correct amounts of drug at the correct time. mitoNANO platforms constitute a novel class of agents capable of delivering drugs to the mitochondria where they are most needed. Based on the hydrophobicity of the mitochondrial membrane and its hyperpolarised potential, mitoNANO have ability to be accumulated in tumour mitochondria. The interplay between size and shape as well as the hydrophobicity of the NPs affect their transport rate across the mitochondrial membrane mediated by passive diffusion or active translocation. The encapsulation of drugs inside mitochondrial ligand-coated NPs provides controlled, sustained, and predictable release of drugs at the right site inside the cell. This mitoNANO approach has been proven to be efficacious in laboratory models of cancer, such as lung, breast, ovarian, pancreatic, and melanoma, and has stimulated scientists across multiple disciplines to pursue advanced designs of these nanodrugs.
Personalised medicine involves a combination of addressing individual clinical needs, image-guided therapy, and monitoring of treatment responses. The translation of personalised targeted therapies from the laboratory to the clinic is urgently required to determine when and why these therapies suits each individual. The rich tapestry of variability in cancer patients has not yet been fully taken into account. Some severe side effects associated with chemotherapy include cardiotoxicity, neurotoxicity, and hepatotoxicity. It has yet to be determined whether the anticancer activity of mitoNANO is sufficient to trigger mitochondrial dependent cell death pathways with fewer side effects. A further concern is the underlying mechanism of mitoNANO that can trigger lipid peroxidation within the mitochondrial inner membrane, whose implications remain to be resolved. However, exosomes and other biomarkers of oxidative damage in the mitochondria could be examined to confirm how mitoNANO triggers oxidative stress and associated damage. This also applies to mitochondria-targeted drugs as well as NPs. The ultimate goal of cancer therapy is to destroy tumours, which some studies have shown that mitoNANO can accomplish efficiently, but the specific mechanisms involved in the cell death need to be cogently evaluated. Another important issue is that if the mitoNANO drugs are recycled by the electron transport respiratory chain, what will be their cellular fate and could they result in unwanted cell death? A wide variety of imaging tools, including fluorescence lifetime imaging and coherent Raman scattering microscopy could be used to investigate these questions at a single cell level. In drug discovery, the Lipinski rule of five (RO5) describes the desirable physiochemical features of an ideal drug, and is generally used to determine whether a candidate pharmacological compound will suffer from pharmacokinetic complications. This rule specifies the desirable parameters for drug-like molecules, including the molecular mass (<500 Da), water-octanol partition coefficient (log P <5), number of hydrogen bonding donors (<5), and number of hydrogen bonding acceptors (<10). It is yet to be ascertained whether mitoNANO follow these rules, as most of the mitoNANO compounds do not show the water-solubility and dispersibility characteristics of small molecule drugs. Given that nanotechnology-based mitochondria-targeted therapy is still in its infancy, we have tried to rekindle interest in these mitoNANO formulations, in order to improve the therapeutic responses and to further investigate the mechanisms of action. mitoNANO have the potential to fight against cancer without causing any collateral damage to healthy tissues, while in parallel, allowing the monitoring of the treatment response in real time.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Mitchell M.J., Jain R.K., Langer R. Engineering and physical sciences in oncology: challenges and opportunities. Nat Rev Cancer. 2017;17(11):659. doi: 10.1038/nrc.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamaly N., Xiao Z., Valencia P.M., Radovic-Moreno A.F., Farokhzad O.C. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41(7):2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabish T.A., Hamblin M.R. Multivalent nanomedicines to treat COVID-19: a slow train coming. Nano Today. 2020:35. doi: 10.1016/j.nantod.2020.100962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabish T.A., Abbas A., Narayan R.J. Graphene nanocomposites for transdermal biosensing. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2021;13(4):e1699. doi: 10.1002/wnan.1699. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 6.Lu R., Zhang Y., Tao H., Zhou L., Li H., Chen T., et al. Gadolinium-hyaluronic acid nanoparticles as an efficient and safe magnetic resonance imaging contrast agent for articular cartilage injury detection. Bioactive Mater. 2020;5(4):758–767. doi: 10.1016/j.bioactmat.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong Y., Hwang H.S., Na K. Theranostics and contrast agents for magnetic resonance imaging. Biomater Res. 2018;22(1):20. doi: 10.1186/s40824-018-0130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabish T.A., Narayan R.J. Mitochondria-targeted graphene for advanced cancer therapeutics. Acta Biomater. 2021 doi: 10.1016/j.actbio.2021.04.054. [DOI] [PubMed] [Google Scholar]
- 9.Shi J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfram J., Ferrari M. Clinical cancer nanomedicine. Nano Today. 2019;25:85–98. doi: 10.1016/j.nantod.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabish T.A., Dey P., Mosca S., Salimi M., Palombo F., Matousek P., et al. Smart gold nanostructures for light mediated cancer theranostics: combining optical diagnostics with photothermal therapy. Adv Sci. 2020;7(15) doi: 10.1002/advs.201903441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabish T.A. Graphene-based materials: the missing piece in nanomedicine? Biochem Biophys Res Commun. 2018;504(4):686–689. doi: 10.1016/j.bbrc.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 13.Hou X.S., Wang H.S., Mugaka B.P., Yang G.J., Ding Y. Mitochondria: promising organelle targets for cancer diagnosis and treatment. Biomater Sci. 2018;6(11):2786–2797. doi: 10.1039/c8bm00673c. [DOI] [PubMed] [Google Scholar]
- 14.Smith R.A., Hartley R.C., Cocheme H.M., Murphy M.P. Mitochondrial pharmacology. Trends Pharmacol. Sci. 2012;33(6):341–352. doi: 10.1016/j.tips.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Fu X., Shi Y., Qi T., Qiu S., Huang Y., Zhao X., et al. Precise design strategies of nanomedicine for improving cancer therapeutic efficacy using subcellular targeting. Signal Transduction Targeted Therapy. 2020;5(1):1–15. doi: 10.1038/s41392-020-00342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H., Gao Z., Liu X., Agarwal P., Zhao S., Conroy D.W., ..., He X. Targeted production of reactive oxygen species in mitochondria to overcome cancer drug resistance. Nat Commun. 2018;9(1):1–16. doi: 10.1038/s41467-018-02915-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z., Zhou L., Xie N., Nice E.C., Zhang T., Cui Y., et al. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduction Targeted Therapy. 2020;5(1):1–25. doi: 10.1038/s41392-020-00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan X. Cancer metastases: challenges and opportunities. Acta pharmaceutica sinica B. 2015;5(5):402–418. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porporato P.E., Filigheddu N., Pedro Bravo-San, J. M., Kroemer G., Galluzzi L. Mitochondrial metabolism and cancer. Cell Res. 2018;28(3):265–280. doi: 10.1038/cr.2017.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonam S.R., Wang F., Muller S. Lysosomes as a therapeutic target. Nat Rev Drug Discovery. 2019:1–26. doi: 10.1038/s41573-019-0036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashrafizadeh M., Ahmadi Z., Farkhondeh T., Samarghandian S. Back to nucleus: combating with cadmium toxicity using Nrf2 signaling pathway as a promising therapeutic target. Biol Trace Elem Res. 2020;197(1):52–62. doi: 10.1007/s12011-019-01980-4. [DOI] [PubMed] [Google Scholar]
- 22.Pandey S., Nandi A., Basu S., Ballav N. Inducing endoplasmic reticulum stress in cancer cells using graphene oxide-based nanoparticles. Nanoscale Adv. 2020;2(10):4887–4894. doi: 10.1039/d0na00338g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H., Zhang P., Luo J., Hu D., Huang Y., Zhang Z.R., Gong T. Chondroitin sulfate-linked prodrug nanoparticles target the golgi apparatus for cancer metastasis treatment. ACS Nano. 2019;13(8):9386–9396. doi: 10.1021/acsnano.9b04166. [DOI] [PubMed] [Google Scholar]
- 24.Arvizo R.R., Miranda O.R., Thompson M.A., Pabelick C.M., Bhattacharya R., Robertson J.D., et al. Effect of nanoparticle surface charge at the plasma membrane and beyond. Nano Lett. 2010;10(7):2543–2548. doi: 10.1021/nl101140t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang B.F., Xing L., Cui P.F., Wang F.Z., Xie R.L., Zhang J.L., et al. Mitochondria apoptosis pathway synergistically activated by hierarchical targeted nanoparticles co-delivering siRNA and lonidamine. Biomaterials. 2015;61:178–189. doi: 10.1016/j.biomaterials.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 26.Yang S.K., Han Y.C., He J.R., Yang M., Zhang W., Zhan M., et al. Mitochondria targeted peptide SS-31 prevent on cisplatin-induced acute kidney injury via regulating mitochondrial ROS-NLRP3 pathway. Biomed Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110521. [DOI] [PubMed] [Google Scholar]
- 27.Li N., Zhang C.X., Wang X.X., et al. Development of targeting lonidamine liposomes that circumvent drug-resistant cancer by acting on mitochondrial signalling pathways. Biomaterials. 2013;34(13):3366–3380. doi: 10.1016/j.biomaterials.2013.01.055. [DOI] [PubMed] [Google Scholar]
- 28.Cheng G., Zhang Q., Pan J., Lee Y., Ouari O., Hardy M., You M. Targeting lonidamine to mitochondria mitigates lung tumourigenesis and brain metastasis. Nat Commun. 2019;10(1):1–14. doi: 10.1038/s41467-019-10042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J.H., Li R.S., Yuan B., Wang J., Li Y.F., Huang C.Z. Mitochondria-targeting single-layered graphene quantum dots with dual recognition sites for ATP imaging in living cells. Nanoscale. 2018;10(36):17402–17408. doi: 10.1039/c8nr06061d. [DOI] [PubMed] [Google Scholar]
- 30.Mallick A., Nandi A., Basu S. Polyethylenimine coated graphene oxide nanoparticles for targeting mitochondria in cancer cells. ACS Appl Bio Mater. 2018;2(1):14–19. doi: 10.1021/acsabm.8b00519. [DOI] [PubMed] [Google Scholar]
- 31.Dong L., Gopalan V., Holland O., Neuzil J. Mitocans revisited: mitochondrial targeting as efficient anti-cancer therapy. Int J Mol Sci. 2020;21(21):7941. doi: 10.3390/ijms21217941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bauer C.A., Chi G., Likens O.Q., Brown S.M. A convenient, bio-inspired approach to the synthesis of multi-functional, stable fluorescent silica nanoparticles using poly (ethylene-imine) Nanoscale. 2017;9(19):6509–6520. doi: 10.1039/c7nr00462a. [DOI] [PubMed] [Google Scholar]
- 33.Chen W.H., Chen J.X., Cheng H., Chen C.S., Yang J., Xu X.D., et al. A new anti-cancer strategy of damaging mitochondria by pro-apoptotic peptide functionalized gold nanoparticles. Chem Commun. 2013;49(57):6403–6405. doi: 10.1039/c3cc43283a. [DOI] [PubMed] [Google Scholar]
- 34.Mallick S., Song S.J., Bae Y., Choi J.S. Self-assembled nanoparticles composed of glycol chitosan-dequalinium for mitochondria-targeted drug delivery. Int J Biol Macromol. 2019;132:451–460. doi: 10.1016/j.ijbiomac.2019.03.215. [DOI] [PubMed] [Google Scholar]
- 35.Hickey J.L., Ruhayel R.A., Barnard P.J., Baker M.V., Berners-Price S.J., Filipovska A. Mitochondria-targeted chemotherapeutics: the rational design of gold (I) N-heterocyclic carbene complexes that are selectively toxic to cancer cells and target protein selenols in preference to thiols. J Am Chem Soc. 2008;130(38):12570–12571. doi: 10.1021/ja804027j. [DOI] [PubMed] [Google Scholar]
- 36.Wen R., Dhar S. Turn up the cellular power generator with vitamin E analogue formulation. Chem Sci. 2016;7(8):5559–5567. doi: 10.1039/c6sc00481d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng G., Zielonka J., McAllister D.M., Mackinnon A.C., Joseph J., Dwinell M.B., et al. Mitochondria-targeted vitamin E analogs inhibit breast cancer cell energy metabolism and promote cell death. BMC Cancer. 2013;13(1):1–14. doi: 10.1186/1471-2407-13-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J.H., Kim K.Y., Jin H., Baek Y.E., Choi Y., Jung S.H., et al. Self-assembled coumarin nanoparticle in aqueous solution as selective mitochondrial-targeting drug delivery system. ACS Appl Mater Interfaces. 2018;10(4):3380–3391. doi: 10.1021/acsami.7b17711. [DOI] [PubMed] [Google Scholar]
- 39.Chen Z., Zhang L., Song Y., He J., Wu L., Zhao C., et al. Hierarchical targeted hepatocyte mitochondrial multifunctional chitosan nanoparticles for anticancer drug delivery. Biomaterials. 2015;52:240–250. doi: 10.1016/j.biomaterials.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Liew S.S., Qin X., Zhou J., Li L., Huang W., Yao S.Q. Smart Design of Nanomaterials for Mitochondria-Targeted Nanotherapeutics. Angew Chem Int Ed. 2021;60(5):2232–2256. doi: 10.1002/anie.201915826. [DOI] [PubMed] [Google Scholar]
- 41.Han C., Zhang C., Ma T., Zhang C., Luo J., Xu X., Kong L. Hypericin-functionalized graphene oxide for enhanced mitochondria-targeting and synergistic anticancer effect. Acta Biomater. 2018;77:268–281. doi: 10.1016/j.actbio.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 42.Huang J.G., Leshuk T., Gu F.X. Emerging nanomaterials for targeting subcellular organelles. Nano Today. 2011;6(5):478–492. [Google Scholar]
- 43.Chen S., Lei Q., Qiu W.X., Liu L.H., Zheng D.W., Fan J.X., et al. Mitochondria-targeting “Nanoheater” for enhanced photothermal/chemo-therapy. Biomaterials. 2017;117:92–104. doi: 10.1016/j.biomaterials.2016.11.056. [DOI] [PubMed] [Google Scholar]
- 44.Guo M., Xiang H.J., Wang Y., Zhang Q.L., An L., Yang S.P., et al. Ruthenium nitrosyl functionalized graphene quantum dots as an efficient nanoplatform for NIR-light-controlled and mitochondria-targeted delivery of nitric oxide combined with photothermal therapy. Chem Commun. 2017;53(22):3253–3256. doi: 10.1039/c7cc00670e. [DOI] [PubMed] [Google Scholar]
- 45.Song Y., Shi Q., Zhu C., Luo Y., Lu Q., Li H., Lin Y. Mitochondrial-targeted multifunctional mesoporous Au@ Pt nanoparticles for dual-mode photodynamic and photothermal therapy of cancers. Nanoscale. 2017;9(41):15813–15824. doi: 10.1039/c7nr04881e. [DOI] [PubMed] [Google Scholar]
- 46.Hu P., Wu T., Fan W., Chen L., Liu Y., Ni D., et al. Near infrared-assisted Fenton reaction for tumour-specific and mitochondrial DNA-targeted photochemotherapy. Biomaterials. 2017;141:86–95. doi: 10.1016/j.biomaterials.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 47.Xu J., Shamul J.G., Wang H., Lin J., Agarwal P., Sun M., ..., He X. Targeted Heating of Mitochondria Greatly Augments Nanoparticle-Mediated Cancer Chemotherapy. Adv Healthc Mater. 2020;9(14) doi: 10.1002/adhm.202000181. [DOI] [PMC free article] [PubMed] [Google Scholar]