Highlights
-
•
CKD is a global health crisis.
-
•
Nanomedicine is a promising tool in theranostics of CKD.
-
•
Targeting senescence at pre-diabetic stage could prevent end-stage complications.
Keywords: Nanoparticles, Diabetic kidney disease, Treatment, Diagnosis, Theranostics
Abstract
In the diabetic kidneys, morbidities such as accelerated ageing, hypertension and hyperglycaemia create a pro-inflammatory microenvironment characterised by extensive fibrogenesis. Radiological techniques are not yet optimised generating inconsistent and non-reproducible data. The gold standard procedure to assess renal fibrosis is kidney biopsy, followed by histopathological assessment. However, this method is risky, invasive, subjective and examines less than 0.01% of kidney tissue resulting in diagnostic errors. As such, less than 10% of patients undergo kidney biopsy, limiting the accuracy of the current diabetic kidney disease (DKD) staging method. Standard treatments suppress the renin-angiotensin system to control hypertension and use of pharmaceuticals aimed at controlling diabetes have shown promise but can cause hypoglycaemia, diuresis and malnutrition as a result of low caloric intake. New approaches to both diagnosis and treatment are required. Nanoparticles (NPs) are an attractive candidate for managing DKD due to their ability to act as theranostic tools that can carry drugs and enhance image contrast. NP-based point-of-care systems can provide physiological information previously considered unattainable and provide control over the rate and location of drug release. Here we discuss the use of nanotechnology in renal disease, its application to both the treatment and diagnosis of DKD. Finally, we propose a new method of NP-based DKD classification that overcomes the current systems limitations.
Introduction
Diabetes mellitus (DM) is a major public health concern with an increasing prevalence in several developing countries [1,2]. It is strongly associated with both micro and macrovascular complications and approximately 33 to 50% of diabetic patients suffer from organ and tissue damage within their lifetime [3]. Regular glucose control can help to prevent microvascular complications (4–6 mmol/l when fasting and <7.8 mmol/l within 2 h of a meal) but this is not easily achieved and can negatively influence mortality. As such, the prevalence of microvascular complications in patients with DM is high [4,5]. This can lead to the onset of chronic kidney disease (CKD) which is referred to as diabetic kidney disease (DKD) in the presence of DM [6,7].
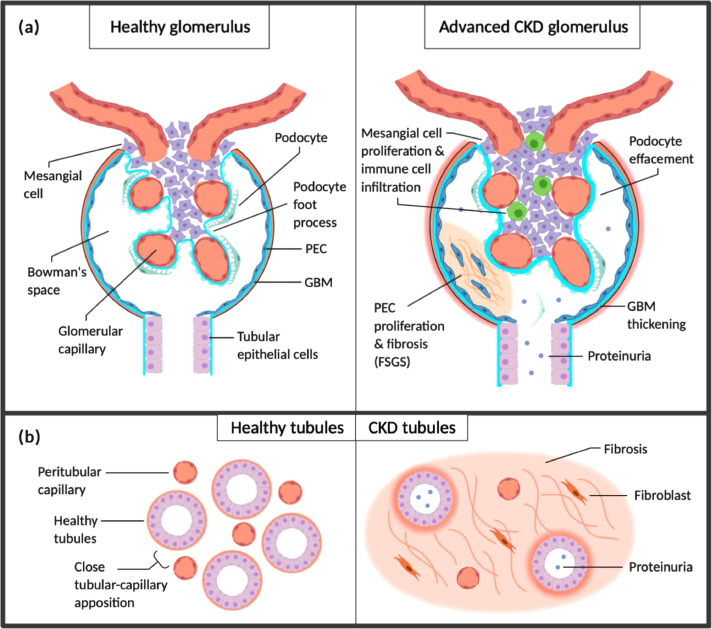
Thus, accelerated ageing, hypertension and hyperglycaemia are the core factors involved in the pathogenesis of DKD, creating a pro-inflammatory environment that promotes fibrogenesis [8]. Kidney fibrosis is characterised by increased synthesis and deposition of extracellular matrix components within the tubulointerstitial space (interstitial fibrosis) and glomeruli (glomerulosclerosis) [9], [10], [11]. The emergence of renal fibrosis is a consequence of maladaptive wound healing following tissue insult [11]. Interstitial fibrosis remains the main factor contributing to renal structural deterioration and loss of function prior to the need for dialysis (Fig. 1) [11]. Thus, early diagnosis and prevention of fibrosis could potentially save millions of lives.
Fig. 1.
Chronic kidney disease. (a) Diseases including hypertension, DM and glomerulopathies lead to loss of functional nephrons and glomerular hypertension. Resultant RAS activation and release of pro-inflammatory, pro-fibrotic factors including TGF-α and EGFR contribute to nephron and podocyte hypertrophy. Podocytes are eventually lost due to shear stress, increasing the permeability of the glomerular basement membrane (GBM) and contributing to the development of proteinuria. Loss of podocytes also impairs capillary endothelial cell integrity due to loss of supportive factors including VEGF produced by podocytes. The pro-inflammatory environment promotes parietal epithelial cell (PEC) proliferation with fibrosis, forming ‘crescents’ crossing Bowman's space and contributing to further podocyte loss (focal segmental glomerulosclerosis; FSGS). (b) Albuminuria and infiltrating immune cells cause tubular epithelial cell stress and activation, creating a pro-inflammatory interstitial environment. This promotes interstitial fibrosis with loss of peritubular capillaries which further stresses tubular cells by reducing oxygen and nutrient transfer from capillaries, contributing to tubular atrophy. Fibrosis and atrophy accelerate the progression of CKD by further increasing demand on remaining functional nephrons.
In the last decade there has been a surge in the use of nanotechnology for the diagnosis and treatment of human diseases, including DM [12]. Nanoparticles (NPs) are at the forefront of this field due to their ability to act as theranostic tools that can carry a therapeutic load and/or enhance image contrast in diagnostics [13]. The application of NPs for the diagnosis, treatment and prevention of disease is termed nanomedicine. Nanomedicine has been widely implemented in cancer diagnosis and treatment owing to its ability to improve drug delivery to tumours by means of the enhanced permeability and retention effect [14]. More than twenty NPs have received FDA approval for treating a range of cancers but their potential for diagnosing and treating DKD remains unfulfilled [15].
A variety of NPs can be generated with control over size, shape, morphology, surface to volume ratio and the capacity for functionalisation/bioconjugation with target moieties (e.g. antibodies, RNA, DNA and other biomolecules) [16,17]. Shapes of NPs include zero-dimensional (0D) structures (e.g., quantum dots, nanospheres), one-dimensional (1D) structures (e.g., nanowires, nanorods), two-dimensional (2D) structures (e.g., graphene and related materials) and three-dimensional (3D) structures (e.g., foam, aerogels, hydrogels) [16,17]. They have been heralded as efficient drug carriers due to their ability to selectively direct pharmaceutical loads to diseased tissue and can enhance drug efficacy locally whilst remaining non-toxic towards healthy tissues [18,19]. In this review we evaluate the current and potential uses of nanotechnology for the diagnosis and treatment of DKD. Unlike existing diagnostics, these theranostic agents could provide insight into DKD progression before, during and after therapeutic intervention.
Nanomedicine and the kidney
DKD is the leading global cause of end stage renal disease and mortality in DM patients [20], affecting approximately 30% of type 1 (T1DM) and 40% of type 2 (T2DM) patients. It has been estimated that by 2040 the global population with DM will rise from 415 million (2015) to 642 million [21], leading to a concurrent rise in DKD. In the traditional five-point model of DKD progression, the first stage is defined by small traces of albumin in the urine (microalbuminuria) [22,23]. Healthy kidneys normally inhibit albumin entry into the urine making it an ideal marker to denote abnormal kidney function. As the disease and subsequent kidney damage intensifies urinary albumin concentrations increase (macroalbuminuria) and a decrease In glomerular filtration rate (GFR) is observed [24]. For this reason, the diagnosis and screening of DKD is based on detection of persistent albuminuria in two out of three morning urine collections over a six month period [25]. In type 1 DM patients albuminuria screens can be performed up to five years post diagnosis but immediate kidney assessment is required following type 2 diagnosis [26]. This is mostly attributed to type 1 subjects being younger and carrying fewer co-morbidities than those with type 2 DM. This method is strongly contested amongst clinicians, however, as many, patients with DM do not fit a classical pattern of kidney disease. In fact, there is now a growing body of evidence suggesting that patients with either type of DM can exhibit an albuminuria independent reduction in renal function even after receiving reno-protective agents [[27], [28], [29]]. Furthermore, diabetic patients can also develop CKD independent of their DM status, and this should be considered when discussing treatment.
Current therapeutic approaches are only moderately efficacious at treating DKD and no curative measures are currently available. The standard treatment options include angiotensin converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) that suppress the renin-angiotensin system (RAS) to control hypertension and ameliorate proteinuria [30]. Intensive treatment using anti-DM pharmaceuticals can, however, cause hypoglycaemia, diuresis and malnutrition as a result of low caloric intake [31]. Cessation of smoking and diet control including lipid and protein reduction are also recommended to slow progression of nephropathy, but many patients still develop end stage kidney disease (ESKD), requiring renal transplant and or dialysis [[32], [33], [34]]. In several countries renal replacement therapies are not widely available with an estimated 2.3–7.1 million adults dying prematurely due to a lack of treatment access [35]. Thus, preventative measures and early-stage curative treatments are desirable.
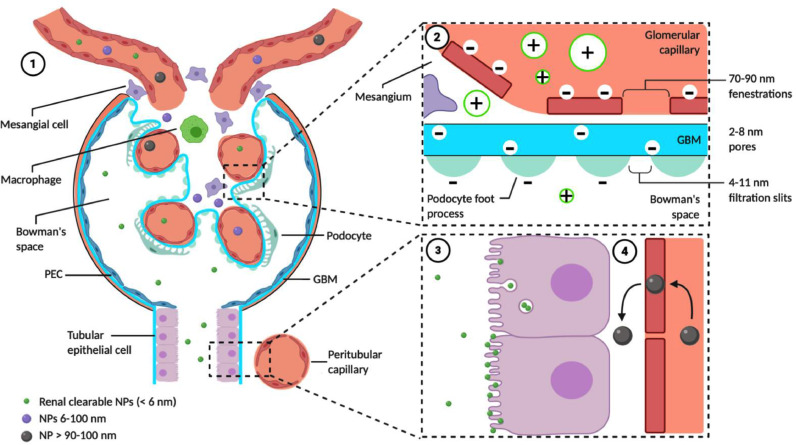
The study of NPs has advanced the treatment of several cancers but so far none have received clinical approval for the treatment of renal disease [36,37]. Several studies have noted poor in vivo stability and insufficient renal targeting when implementing this technology in practice whilst issues surrounding biodistribution and metabolism have also been reported [38]. Despite these challenges, a plethora of pre-clinical studies have outlined the potential applications of NPs in renal tissue targeting where an understanding of renal anatomy is critical. As shown in Fig. 2, each renal glomerulus comprises a compact collection of blood capillaries lined with fenestrated endothelium that is encased by either a thick basement membrane (the GBM) or mesangium that acts as supporting tissue [39]. The basement membrane is in turn lined with podocyte foot processes sized 4 to 11 nm to create a further filtration layer. Post entry into the glomerulus NPs may be deposited in different locations (e.g., endothelial cells, GBM etc.) according to their physiological properties including size, shape, surface charge and bioconjugates [[40], [41], [42]].
Fig. 2.
Renal filtration and accumulation of nanoparticles. (1) NPs are typically administered IV. Alternative routes include intraperitoneal administration and phagocytosis by macrophages, which subsequently migrate to foci of inflammation [43] and administration of NP labelled mesenchymal stem cells for theranostics [44,45]. (2) Renal clearable NPs must pass through the glomerular filtration membrane (GFM), comprising the capillary endothelial layer with 70–90 nm fenestrations, the thick GBM with 2–8 nm pores and the podocyte layer with 4–11 nm filtration slits. Each layer of the GFM is negatively charged; cationic NPs are therefore much more efficiently cleared by the kidney than anionic and neutral NPs. Filtration is strongly size dependant: large NPs > 90–100 nm do not pass through the GFM significantly whilst small NPs < 6 nm are freely filtered. Very small (< 1 nm) NPs exhibit reduced renal clearance due to interactions with the endothelial glycocalyx [46]. Some intermediate size NPs (e.g. PEG-coated AuNPs < 100 nm) pass through the endothelial layer and accumulate in the mesangium, with potential for glomerular targeting [47]. The dependency of filtration on size and charge is further complicated by their interaction with NP shape and flexibility and the potential for some NPs to partially or completely disassemble to cross the GFM [48]. Renal disease causes increased GFM permeability (due to various factors including podocyte injury and reduced endothelial integrity) potentially allowing increased NP filtration and renal accumulation. (3) Renal-clearable NPs accumulate in urine in the tubules. Their precise interactions are not fully understood but several pathways for their endocytosis and accumulation within tubular epithelial cells have been identified, including megalin-, caveolae- and clathrin-mediated endocytosis [49-51]. Renal-clearable NPs may also be captured by the microvilli (e.g. glutathione-coated AuNPs), saturating the brush border before being eliminated in the urine [52]. (4) Non-renal-clearable NPs rarely reach the tubules; however, endocytosis of organic NPs 400 nm in diameter by the peritubular capillary endothelium has been studied, allowing selective accumulation in the proximal tubules for up to 7 days [53].
NP shape
NP shape influences their glomerular behaviour. A specific example can be found in the difference in sieving coefficient between uncharged dextran and uncharged Ficoll. Ficoll displays a significantly lower coefficient than that of dextran due to the molecule's shape [54]. Ficoll is a highly crosslinked copolymer formed from sucrose and epichlorohydrin whilst dextran displays a more branched glucopyranose structure giving each macromolecule a spherical and prolate ellipsoid shape respectively [54]. Furthermore, elongated dextran is excreted from the kidneys at a greater rate than spherical horseradish peroxidase of identical size and charge [55] indicating macromolecular shape also plays a key role in renal uptake and filtration. Interestingly, a study by Ohlson et al. investigated the effect of molecular shape on transglomerular passage using elongated hikunin, spherical albumin and linear hyaluronan that all possess a similar Stokes-Einstein radius (3.4–3.6 nm) and net charge but different shape [56]. Fractional clearance of linear hyaluronan (0.4–0.7) and elongated bikunin (0.1–0.25) proved to be much greater than that of spherical albumin (<0.01) [56] suggesting that elongated macromolecules are more easily excreted from the kidney due in part to their frictional ratio. Such information is vital to the future development of NPs given that rate of clearance influences their ability to administer drugs to the intended target [47,57].
NP size
To reach the luminal surface of the proximal tubule NPs need to be sized <7 nm and positively charged as these characteristics correlate with passage through the glomerular filtration barrier [58]. Whilst larger NPs (400 nm) have been successfully used to target proximal epithelial cells little is known about how these NPs facilitate tissue entry [53]. Recently, mesoscale NPs (MNPs) which are sized within this “large” category (∼400 nm) were used to selectively target the proximal tubules [59]. Despite their dimensions exceeding the circumference of the GBM fenestrations MNPs were 26–92-fold more selective to the kidney than any other organ. The authors hypothesised MNPs do not undergo glomerular filtration and instead transcytose across the thin (<500 nm) endothelial layer of the capillaries, where they are then deposited between these capillaries and the proximal tubules. Here MNP uptake is mediated by the tubular epithelial cells via receptor-mediated endocytosis as proposed by Carteria et al. [60]. Should further study support this hypothesis, it would likely increase the utility of MNPS for drug delivery to the proximal tubules.
NP charge
The GFM plays a fundamental role in kidney filtration and is supported by cells of the mesangium [[61], [62], [63]]. Whilst responsible for size selectivity, the GFM also filters molecules according to their charge. Negatively charged heparan sulphate proteoglycans present in the glomerular endothelium, the GBM (also negatively charged) and glycocalyx of the podocytes selectively uptake cationic substances [64,65]. For this reason, positively charged NPs have been found to pass through the glomerular filtration barrier (GFB) more readily than their negatively charged counterparts [66,67].
NP charge has been used as an independent variable for the selective targeting of renal cells in many studies and has thus far produced inconsistent results. For example, Wang and colleagues previously developed small, organic NPs named peptide amphiphile micelles (PAM) functionalised with the zwitterionic peptide ligand, (KKEEE)3 K [68]. Whilst PAM NPs are able to pass through the GFB for kidney accumulation, they showed almost identical non-specific accumulation in the liver. More recently in an attempt to optimise the physiochemical properties of PAM NPs the same authors developed a library of PAMs according to size, charge, and peptide repeats [69]. Interestingly, whilst all PAMs showed kidney accumulation, positively charged NPs, of similar size to the renal filtration cut‐off (8–10 nm), conjugated to a zwitterionic peptide sequence showed greater renal accumulation. Given that kidney accumulation of larger NPs with size far greater than the GFB cut off has previously been reported (e.g., polycation‐siRNA NPs 60–100 nm, [70]), these results suggest an NP's charge is just as influential on filtration as size.
NP focused renal targeting
Achieving active targeting of NPs to renal destinations requires the conjugation of ligands such as antibodies, peptides and small molecules. These targeted NPs are often designed to specifically bind to receptors that are uniquely or heavily expressed in renal tissue. For example, using 10 nm bovine albumin-based NPs (ABNPs) Wu et al. selectively targeted the neonatal Fc receptor (FcRn) which is also an albumin receptor on the surface of human podocytes [71]. ABNPs delivered methylprednisolone, a glucocorticoid, to in vitro human podocytes which displayed a 36-fold higher NP uptake compared to vascular smooth muscle cells that lack FcRn [71]. Additional in vivo assays using female BALB/c mice revealed strong kidney targeting of the nanoconjugates but with slightly favourable liver accumulation. Similarly, Pollinger and colleagues developed cyclomodified quantum dots capable of binding to the αvβ3 integrin on the podocyte surface, however, the therapeutic application of these nanocarriers was not tested [72].
As DKD progresses the accumulation of pathogenic insults results in an inflammatory environment that promotes further nephrotic damage. Upon pro-inflammatory stimulation, the kidney displays a specific pattern of cell adhesion molecules including E-selectin and vascular cell adhesion protein 1 (VCAM-1) making them an ideal target for NP renal homing [[73], [74], [75], [76]]. Targeting of either of these peptides has mostly been achieved via antibody conjugation to the surface of NPs carrying a therapeutic load. For example, VCAM-1 conjugated lipid nanocarriers termed “SAINT-O-Somes” have been used to target inflamed podocytes pre-treated with tumour necrosis factor-alpha (TNF-α) [77]. Anti-VCAM-1 conjugated SAINT-O-Somes were used as a delivery platform for rapamycin. Rapamycin is an immunosuppressant drug prescribed to renal transplant patients but prolonged use results in proteinuria and progression of CKD [78]. Delivery of rapamycin to the kidney at controlled concentrations may reduce the risk of side effects. Delivery of anti-VCAM-1-rapamycin-SAINT-O-Somes to human AB8/13 podocytes had little effect on cellular viability compared to treatment with free rapamycin (tested at 8 μM and 32 μM rapamycin) [77]. However, anti-VCAM-1-rapamycin-SAINT-O-Some treated cells did display 3-fold greater inhibition of wound healing after 24 h exposure compared to control indicating a more effective anti-inflammatory effect with the targeted approach [77].
E-selectin has also been used as antibody target for NP renal targeting owing to its overexpression in highly inflammatory renal environments. In light of this, Asgeirsdottir et al. developed IgG conjugated E-selectin targeting liposomes (AbEsel) encapsulating the corticosteroid dexamethasone [79]. In glomeruli, the AbEsel liposomes (114 nm) colocalised with the endothelial marker CD31 and showed minimal accumulation in other non-target organs such as the liver, spleen, heart and lungs [79]. Similar studies have since been conducted using sialic acid (SA) conjugated 20 nm dexamethasone loaded micelles (SA-PEG-DXM), a known E-selectin ligand [80]. SA-PEG-DXM NPs showed greater cellular uptake and accumulation in the kidney of an acute kidney injury (AKI) murine model than non-SA bound NPs [80].
Nanomedicines for the diagnosis of kidney diseases
The prognosis of DKD is dependant on the disease's stage at the time of diagnosis. For this reason, a large proportion of research has focused on the development of new non-invasive diagnostic tools that may help detect DKD at an earlier, more treatable stage. Advancements in medical imaging technologies, such as magnetic resonance imaging (MRI) and positron emission tomography (PET)/ computerised tomography (CT) show promise as methods of achieving early diagnosis. Improved biomarker technology can now be paired with imaging techniques to give a more specific assessment of the disease [38]. For example, nephrons can now be examined individually to assess the affected area thus reducing the time a patient spends in diagnostic limbo [38]. Currently these NP-based tools are limited to a preclinical setting; however, their continued advancement highlights their potential for future clinical diagnosis (Table 1).
Table 1.
Promising nanoparticles for the diagnosis of CKD and DKD. A number of studies published within the last decade have shown promising pre-clinical results for the diagnosis of both DKD and CKD. Since both diseases show similar renal damage the use of NPs for therapeutic delivery have been interchangeably considered for both CKD and DKD within this table.
| Class | Nanoparticle | Size (nm) | Model | Summary | Refs. |
|---|---|---|---|---|---|
| Gold | Anti-collagen-I antibody-conjugated AuNPs (bare or PEG-coated) | 19 / 45 | In vitro (collagen coated plate; murine kidney sections) | Co-I-AuNPs bind specifically to with increased retention to mouse fibrotic kidneys. They can then be detected by micro-CT. | [81] |
| PEG-modified, highly stabilised core-satellite Au nano-assemblies with N-acetylation chitosan modification | Core: 18.7Satellite: 3.9 | BALB/c mice | Fluorescence imaging indicates NACS-PEG-CSAuNAs effectively target renal tubular cells with greater retention than standard NACS-PEG-AuNPs. | [82] | |
| Renal clearable glutathione coated AuNPs | 2.5 | CD-1 mouse UUO model | Non-invasive x-ray imaging shows increased retention and cellular uptake in UUO kidneys, with anatomical localisation precisely correlated with local pathology. Contrast enhancement is 6x higher than using diatrizoate meglumine (a clinically used agent). | [83] | |
| Renal clearable luminescent glutathione coated AuNPs | < 5.5 | Mouse UUO model | Fluorescence imaging using renal clearable near-infrared emitting AuNPs is a low cost, non-invasive measure of kidney dysfunction progression with greater contrast than small-molecule based contrast agents and greater sensitivity than typical blood markers. | [84] | |
| Iron oxide | SPION-labelled MSCs | 60 | Ischaemic AKI in rabbitsCKD in Sprague-Dawley rats | SPIONs are an effective, non-toxic agent for targeted MRI when combined with the use of MSCs to treat renal injury and CKD. | [44,45] |
| PEG-coated iron oxide NPs | 20 | BALB/cJRj mice | NPs accumulate in PTECs and generate clear negative contrast for renal MRI, with reduced RES retention and increased renal clearance. | [85] | |
| SPIONs | 5.8 | MPI is a new alternative to MRI which uses SPIONs. These are potentially a safer alternative to traditional contrast agents in CKD patients. | [[86], [87], [88]] | ||
| Ferumoxytol | 30 | Licensed in humans for treatment of anaemia.Human case study. | Ferumoxytol is an effective novel MRI contrast agent with an excellent safety profile in CKD patients. | [89,[90], [91], [92]] |
Nanotechnology to improve kidney imaging
CT and MRI are the standard imaging techniques used for the detection and diagnosis of DKD but require a high contrast ratio to distinguish between varying structures. NPs show great promise as image enhancement tools and can reduce the use of accepted contrast agents that display nephrotoxic side effects [93,94]. Compared with PET and CT, MRI has a much higher spatial resolution, especially for soft tissue, making it the favoured technique for characterising indeterminate renal diseases [95]. CT, however, offers prominent benefits in terms of speed of image acquisition and relative availability. Gold NPs (AuNPs) are considered an attractive candidate to enhance CT imaging due to their ability to heighten X-ray attenuation [96]. At both low (40–60 kVp) and high (100–140 kVp) tube potentials AuNPs have been found to significantly improve the contrast to noise ratio (89% and 114%, respectively) of CT in an imaging phantom compared to the clinically used contrast agent– iodine [97]. Similarly, anti-collagen-I antibody conjugated AuNPs have been used to visualise kidney fibrosis in vitro and do not induce renal damage in mice, suggesting strong clinical potential [81]. However, the acquisition of CT images using NP contrast agents has thus far been tested at variable voltages. Given the energy dependence of X-ray attenuation for a given substance, image production at oscillating voltages likely results in different estimations of contrast even when using a reference agent. Thus, without a standardisation protocol the accurate comparison of contrast agents for CT scanning remains difficult to obtain. NP based initiatives have therefore placed more focus on MR imaging for renal diagnostics.
MR imaging is the result of intermittent low energy nuclear rotations. To achieve this, pulses of radio frequency are emitted into a constant magnetic field. The rotation-relaxation process is then measured to form the image. The resolution of these images can be significantly enhanced through the use of contrast agents which shorten either the longitudinal (T1) or transverse (T2) relaxation periods of aqueous protons [98,99]. Paramagnetic compounds can enhance the contrast display of MRI by promoting the relaxation of the water molecules that surround it [100]. Whilst T1 contrast agents produce brighter images that are more distinguishable from artefacts than T2 [100] they are also quickly excreted by the kidneys due to their small molecular size [101]. In addition, gadolinium, the traditional agent used for T1 contrast, has a short blood circulation time so acquiring high resolution images proves challenging [102]. As such, new technologies that can safely increase the resolution of MRI are desirable.
Iron oxide NPs (IONPs) are the most widely researched nanoparticle for enhancing clinical imaging due to their intrinsic magnetic and biodegradable attributes [[103], [104], [105]]. The majority of studies have investigated IONPs as T2 contrast agents due to their ability to efficiently shorten transverse relaxation times. In addition, IONPs display desirable attributes such as adaptable surface chemistry and long blood half-lives with minimal toxicity [104,106]. In the kidney, IONPs have been used to quantitatively assess glomerular morphology. Using superparamagnetic ferritin-based NPs several studies have been able to acquire 3-dimensional images of the diseased tissue [107]. Ferritin NPs are superparamagnetic due to ferritin's ability to oxidise and incorporate iron in a crystalline form. Incorporating these NPs into MR imaging has since been used to create whole-kidney maps of glomerular number and estimate distribution of glomerular volumes [105,[107], [108], [109]].
Superparamagnetic iron oxide NPs (SPIONs), when used as a contrast agent, generate a local disturbance in the applied magnetic field which reduces transverse relaxation times (T2 and T2*), resulting in a local darkening of the image [110]. SPION accumulation at the target site is detected using T2 and T2* weighted MRI, with each glomerulus visualised as a black ‘dot’ [105,[107], [108], [109]]. Significantly, this technology has been used to track changes in renal function during the development of CKD. In this study, cationised ferritin (CF) was shown to selectively localise to the GBM [108]. In a preclinical model of glomerulosclerosis, male Sprague-Dawley rats were treated with CF and were subsequently examined by MRI [108]. Rats with glomerulosclerosis showed reduced glomerular accumulation of CF, but exhibited diffuse accumulation of CF in the renal tubules due to leakage through the damaged GBM) [108]. These NPs allowed visualisation via MRI of the renal damage associated with glomerulosclerosis [108]. Non-invasive methods such as this may help to distinguish between DKD and NDKD, thus informing more accurate clinical decision making.
Nanotechnology for detecting kidney inflammation
Kidney inflammation is a significant factor in the development of DKD. Kidney damage caused by the pre-requisite insults associated with disease onset (hyperglycaemia and hypertension) leads to proinflammatory cytokine production by tissue resident macrophages [111]. Cytokines are involved in further leucocyte recruitment to advance the wound healing process but can also exacerbate tissue damage by initiating an autoimmune reaction. Continued renal damage results in collagen deposition as part of a secondary mechanism of wound healing leading to fibrogenesis and the irreversible stages of DKD [111,112]. In fact, macrophage accumulation in the kidney interstitium correlates with a decline in renal function, increased proteinuria and advanced interstitial fibrosis [113]. For this reason, non-invasive imaging and tracking of macrophage activity within DM patients has been proposed as an effective method of early DKD detection.
SPIONs are amongst the array of technology previously used to detect renal inflammation. Following intravenous injection SPIONs are phagocytosed by macrophages and display prolonged T2 and T2* effects on MR images of macrophage-infiltrated tissues including the kidney [[114], [115], [116]]. Using either SPIONs or C3d conjugated NPs, Serkova et al. tested their ability to enhance MR imaging in a mouse model of lupus nephritis [116]. C3d conjugated NPs showed decreased water T2 in the selected model but not in wildtype mice whilst nontargeted SPIONs had no effect on contrast. It is worth noting that SPIONs have also been used to monitor macrophage infiltration of kidney tissue by acting as MRI contrast agents in patients receiving renal transplantation and suffering from transplant rejection [114]. SPIONs can therefore be used to examine longitudinal changes in inflamed kidneys. This data is not only of use in the clinic to inform therapeutic decisions but also in research to improve pharmaceutical development.
Other methods of inflammation assessment have focused on the proinflammatory cytokine TNF-α. Urinary TNF-α concentrations increase with progression of nephropathy [117]. Similarly, DKD patients have elevated levels of both serum and urinary TNF-α; these concentrations rise concomitantly with disease progression [118,119]. As such, TNF-α is a plausible marker of DKD onset and progression. Currently, the enzyme-linked immunosorbent assay (ELISA) test is the most commonly used method for detecting TNF-α in a given sample with a detection limit of 10 pg/ mL [120]. However, TNF-α plasma levels above 4.17 pg/mL are indicative of cardiovascular disease, the most common cause of mortality amongst DKD patients [121]. In addition, results can take between 2 and 4 weeks to obtain delaying diagnosis [122]. Thus, new approaches are required to use this cytokine as an accurate prognostic marker.
Recently Lai et al. used surface enhanced Ramen scattering (SERS) active small clusters of AuNPs conjugated to antibodies in a magnetic bead pull-down assay for TNF-α detection [123]. SERS is a surface-sensitive optical technique that enhances Ramen scattering through use of nanostructures to detect single molecules [124,125]. The Raman spectrum showed specificity and selectivity for TNF-α detection (limit of detection 1 pg/ mL), with minor signal display from negative controls including 0.05% bovine serum albumin, interleukin (IL)−1 and IL-8 at a concentration of 10 ng/ mL. More recently, unconjugated SERS have been reported to produce a limit of detection value of 0.125 pg/ mL [122]. Whilst the authors identified improved detection of TNF-α, this label-free approach is limited in its clinical application due to difficulties discerning different proteins within a given sample. Collectively, these data indicate that SERS based NPs are capable of detecting TNF-α at physiologically relevant concentrations, but further work is required to improve their label-free specificity.
Nanotechnology for detecting kidney biomarkers
Despite the limitations surrounding albumin as a marker of renal disease, this renally filtered peptide is still used to inform key clinical decisions. In healthy individuals, albumin is detectable at low levels, but diseases such as DM that result in kidney injury can initiate long-term adverse effects such as glomerular leakage and tubular dysfunction [126]. Such events can lead to albumin entry into the urine; this is termed albuminuria and currently acts as an accessible, non-invasive biomarker of DKD. At low concentrations, (30–300 mg/L of albumin in the urine) this is termed microalbuminuria and is often observed at the earlier stages of disease progression [127]. As such, microalbuminuria has become a significant area of interest for the early detection of kidney damage.
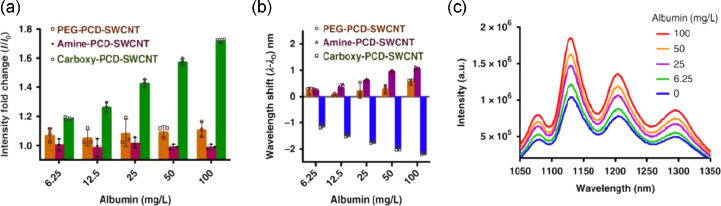
The current clinical method of microalbuminuria detection is an immune-turbidimetry assay using polyclonal antibodies against human albumin [127]. By combining this approach with AuNPs, Shaikh et al. developed a point-of-care system that could be used in almost any setting, from home to bedside [128]. AuNPs were used to enhance the biocompatibility and conductivity of this electrochemical immunosensor allowing for the near immediate detection of kidney damage. However, whilst antibody mediated detection shows high sensitivity these methods are expensive and require careful handling. To overcome these limitations, Budhathoki-Uprety et al. used polycarbodiimide polymers encapsulating single-walled carbon nanotubes (PCD-SWCNT) capable of binding to free albumin [129]. Upon the binding of albumin, at a range of concentrations (6.25 to 100 mg/L), to the nanotube a hypsochromic (blue) shift in photoluminescence was observed indicating the presence of quantifiable microalbuminuria (Fig. 3a–c). This point-of-care platform could easily be used in clinical situations and applied to other resource limited settings to determine early DKD onset.
Fig. 3.
Optical nanosensor for albumin detection. In an attempt to develop a nanotube probe to detect urinary albumin the authors synthesised a hydrophobic carboxylated chain to mimic fatty acids as these are known to bind to albumin [130]. Polymers with amine and polyethylene glycol (PEG) groups that could interact via Coulombic and hydrophobic interactions, respectively were also developed as controls. A. Emission intensity response of the PCD-SWCNT complexes ((9, 4) chirality) to albumin. B. Emission wavelength response of the PCD-SWCNT complexes to albumin ((9, 4) chirality). C. Photoluminescence emission spectra of carboxy-PCD-SWCNT complexes upon addition of albumin (concentration increasing from bottom to top). Reproduced with permission from reference [129].
The most established predicter of ESRD is the current GFR and past GFR trajectory, which relies on examination of serum creatinine to produce an estimated value (eGFR) [131,132]. However, creatinine is not directly proportional to GFR due to the influence of muscle mass on its serum concentration and its tendency to rise only when kidney function is already significantly impaired [133,134]. Serum cystatin C-based eGFR has been proposed as an alternative marker of kidney damage as it exhibits better diagnostic accuracy [135]. Cystatin C is a 13 kDa nuclear protein that is freely filtered and almost completely metabolised by the cells of the proximal tubules [136]. With this in mind, Sun et al. developed a sandwich chemiluminescence immunodetection method for cystatin C labelled with amino-functionalised mesoporous silica NPs encapsulating dye [137]. Compared with ELISA this NP based approach demonstrated considerably greater sensitivity (limit of detection 0.0333 ng/mL vs 0.0029 ng/mL, respectively). The authors attributed these results to the superior ability of mesoporous silica NP to carry a dye load than antibodies. Similarly, Lopes et al. utilised AuNPs as part of an electrochemical immunosensor to detect levels of cystatin C in CKD serum samples [138]. This diagnostic method provided precise results (relative standard deviation ≤ 6.2%) and quantified cystatin C in a manner that agreed with the values obtained by a particle-enhanced nephelometric immunoassay. To this end, the use of NPs to advance immunosensor methods shows great clinical promise.
Nanomedicines for the treatment of DKD
The overwhelming disadvantage of current methods of DKD treatment is their inability to reverse renal fibrosis: the pathological result of ongoing microvascular damage, metabolic changes and oxidative stress. Over a decade of research aimed at identifying therapeutic strategies for treating DKD and CKD has focused on fibrogenesis. Thus far little progress has been made in achieving fibrotic reversal. Whilst RAS blockade has been used to regress glomerulosclerosis in rat kidneys this process does not translate to humans [139]. In DKD patients, pancreatic transplantation has been shown to reverse some lesions of kidney fibrosis but this takes at least a decade to achieve [140]. Whilst new initiatives are constantly being considered, limitations surrounding their bioavailability, half-life and efficacy have thus far hindered any clinical impact. Nanotechnology represents an opportunity to overcome such issues (Table 2).
Table 2.
Promising nanoparticles for the treatment of CKD and DKD. A number of studies published within the last decade have shown promising pre-clinical results for the treatment of both DKD and CKD. Since both diseases can progress to ESRD the use of NPs for therapeutic delivery have been interchangeably considered for both CKD and DKD within this table.
| Class | Nanoparticle | Size (nm) | Model | Summary | Refs. |
|---|---|---|---|---|---|
| Gold | AuNPs | 50 | STZ-induced diabetic hyperglycaemia in rats | AuNPs prevent STZ-induced diabetic hyperglycaemia and have positive effects on renal function and oxidative stress. They downregulate TGF-β1, fibronectin, collagen IV, TNF-α and VEGF-A expression and ameliorate podocyte injury. | [141,142] |
| Pomegranate peel extract stabilised AuNPs | 20–120 | STZ-induced diabetic hyperglycaemia in mice | PPE-AuNPs normalise STZ-induced pancreatic beta-cell dysfunction and reduce glomerular sclerosis and renal fibrosis. They also reduce pro-inflammatory cytokines by modulating the MAPK/NF-kB/STAT3/cytokine axis. | [143] | |
| Organic | Chitosan (non water-soluble, LMW) polyplex (Ch/siRNA) | 200–250 | BALB/cJBomTac female mice | Ch/siRNA NPs accumulate specifically in PTECs, achieving knockdown of aquaporin 1 by up to 50%. | [144] |
| Chitosan siRNA | Mouse UUO model | Intraperitoneal Ch/siRNA targeting COX-2 in macrophages attenuates UUO-induced kidney injury. | [145] | ||
| Catechol-derived LMW chitosan with zinc + emodin | Mouse UUO model | Chi-Zn-emodin complexes attenuate fibrosis in ureter-obstructed mice. | [146] | ||
| 1-Serine-modified PAMAM dendrimer with captopril | 2–5 | Male ddY and Hos:HR-1 mice | Serine-modified PAMAM shows highly selective renal accumulation (vs unmodified), with effective renal delivery of captopril (ACE inhibitor). | [50] | |
| Polycationic cyclodextrin NPs containing siRNA | Female BALB/c mice and C57BL/6-Tg(CAG-EGFP)1Osb/J mice | siRNA/CDP-NPs localise to the glomerular mesangium where they are rapidly internalised and cause knockdown. | [147] | ||
| PEG-PCL-PPI triblock amphiphilic polymer(PPP NPs) with rhein | 75± 25 | STZ-induced murine DN | PPP-RH—NPs showed kidney-targeted distribution and improved efficacy of rhein against DN. | [148] | |
| Liposomal | Liposome-encapsulated clodronate (LEC) | Ang-II induced hypertensive C57BL/6 mice | LEC treatment reduces blood pressure and protects against renal injury and fibrosis in hypertensive mice by reducing macrophage-driven inflammation and oxidative stress. | [149] | |
| CoQ10-loaded liposomes | 180 | STZ-induced diabetic male Sprague-Dawley rats | CoQ10-loaded liposomes combined with UTMD may reverse early DN. | [150,151] | |
| bFGF-loaded liposome | 170 | STZ-induced diabetic male Sprague-Dawley rats | bFGF-loaded liposomes combined with UTMD may reverse early DN by inhibiting inflammation. | [152] | |
| CREKA-coupled celastrol-loaded liposomes | 110 | UUO model in C57BL/6 J male mice | CREKA-coupled liposomes enable anti-fibrotic drug (celastrol) targeting via fibronectin in fibrotic kidneys. | [153] | |
| Quercetin-loaded liposomes | 130 | STZ-induced DN in male Sprague-Dawley rats | Quercetin-loaded liposomes exhibit greater efficacy than standard quercetin. | [154] |
NPs for drug delivery
NPs are an attractive tool for the loaded delivery of drugs to the kidney. Due to their unique ability to improve drug efficiency and enhance controlled drug release several studies have been conducted in this field. The adjustable surface properties of NPs, such as the conjugation of poly (ethylene glycol) (PEG), has proven revolutionary in preventing opsonisation and systemic clearance of NP encapsulated chemotherapeutic agents [155]. Whilst the study of metallic NPs has seen a meteoric rise in recent years, owing to their ability to enhance MRI contrast, other materials have shown promise as drug nanocarriers. Various polymers, for example, have been used in colloidal drug delivery research to increase therapeutic value and decrease associated side effects [156]. Among these are the poly (D,L- lactic-co-glycolic acid) (PLGA) NPs which have shown great promise in the diagnosis and therapy of several cancers [157,158]. In the kidney, PEG conjugated PLGA NPs have been used to deliver dexamethasone acetate (A-DEX) to the glomerular mesangium [51]. A-DEX is an FDA approved immunosuppressive corticosteroid used for the treatment of various inflammatory diseases [159]. Although corticosteroids can be given systemically various side effects such as hypertension, hyperglycaemia, peptic ulcers and glucosuria can be prevented through local administration [160]. In this study, 90 nm sized NPs showed significant accumulation in rat kidneys and were rapidly (60 s) endocytosed by the mesangial cell line HBZY-1 [51]. These findings demonstrated the ability of PEG-PLGA NPs to act as therapeutic carriers to the kidney. The authors did not, however, compare the efficacy of this nanocarrier against free A-DEX, leaving questions over its therapeutic value.
In an attempt to target the inflammatory environment within the diabetic kidney Bruni and colleagues developed an ultrasmall colloidal nanomaterial of tuneable size (5–30 nm) with or without a hydrophobic poly-ε-caprolactone core and a brush-like PEG corona [161]. Using these NPs, they delivered A-DEX to a 3-dimensional in vitro co-cultured system of endothelial cells and podocytes previously treated with Adriamycin (doxorubicin hydrochloride) to replicate cellular damage [162]. Damaged podocytes displayed shortened cell processes and substantial remodelling of the actin cytoskeleton, with loss of filament bundles and rounding of the cell shape [161]. Cells treated with A-DEX loaded NPs recovered the normal orientation of actin stress fibres within 24 h. Unfortunately, the lack of in vivo data provided by this study raises questions regarding the biodistribution of A-DEX loaded NPs. Whilst a safe nanotoxicity profile was reported in the target cell line the authors failed to consider side effects that may occur in the surrounding organs (e.g., liver, spleen, lungs).
Renal fibrosis is the hallmark final stage of CKD and DKD regardless of aetiology. Fibrosis is characterised by the excessive deposition of fibrous connective tissue, including collagen and fibronectin, in the damaged environment [163]. Patients that develop renal fibrosis advance to ESRD requiring renal transplant as there is currently no approved treatment for this pathogenic process. For this reason, a lot of research has been focused on alternative therapeutics such as traditional Chinese medicines and transition metals as a method of reversing fibrosis. Preconditioning with the transition metal cobalt (Co2+), for example, has been shown to inactivate the collagen synthesis enzyme prolyl 4-hydroxylase suggesting an anti-fibrotic effect, although this remains to be seen in vivo [164]. Whilst treatment with CoCl2 has previously been effective in the attenuation of fibrosis [165], specific renal targeting of this therapy has proven challenging. To improve Co delivery to the kidney Tan et al. developed a drug-releasable self-assembly nanoplatform using glutathione (GSH)-modified AuNPs and Co2+(GLAuNPs-Co) [166]. Using an obstructed nephropathy mouse model, they observed a 2-fold increase in therapeutic efficacy with GLAuNPs-Co compared to free CoCl2. Subsequent polymerase chain reaction (PCR) analysis revealed upregulation of the anti-fibrotic microRNA, miR-29a, following GLAuNPs-Co treatment [166]. Mice injected with GLAuNPs-Co displayed significant attenuation of interstitial fibrosis, indicating that it is a promising therapy for treating the fibrotic kidney.
Curcumin, the active ingredient in traditional medicines and dietary spice turmeric has long been considered a therapeutic prospect for treating fibrosis. As an economically viable alternative to more expensive pharmacological agents, curcumin is now the subject of several studies for the treatment of stage 3 CKD [[167], [168], [169]]. In a randomised, double-blind study of patients with DKD, daily treatment with turmeric capsules for 2 months (containing 22.1 mg curcumin/per capsule) significantly reduced plasma TGF-β concentrations [170]. In fact, curcumin therapy can reduce glomerular hypertension, hyperfiltration, glomerular sclerosis and interstitial fibrosis in rats but its uses in healthcare have been limited by its poor bioavailability and metabolic stability [[171], [172], [173]]. In addition, curcumin targeting to the kidneys proves challenging due to its low tissue absorption, immediate metabolism and rapid elimination [174,175]. Conjugating the polyphenolic compound to NPs has allowed for specific kidney targeting with some now reaching clinical trial for the treatment of CKD [176]. From these studies it can be speculated that the increased bioavailability of nanocurcumin compared to free curcumin may promote methods of DKD treatment.
NPs for RNA delivery
Over the years accumulating evidence has revealed an epigenetic influence on the pathogenesis of DKD culminating in the identification of a number of therapeutic targets. Among these are the non-coding RNAs (ncRNAs) which have attracted a great deal of interest due to their pivotal role in several physiological and pathological processes [177]. The ncRNAs can be divided into distinct subgroups according to their length ranging from the long ncRNAs (>200 nucleotides), to the short interfering RNA (20–25 nucleotides) to the micro (mi)RNAs (18–22 nucleotides) [177]. Many miRNAs are associated with DKD progression and renal fibrosis making them ideal targets for nanomedicine interventions.
Recently, Wang et al. used exosome-encapsulated miR-29, which displays anti-fibrosis activity, to counteract muscular wastage and renal fibrosis in a ureteral obstruction (UUO) mouse model [178]. Exosomes (91 nm) were injected intramuscularly and contained the exosomal membrane protein gene Lamp2b that was fused with the targeting peptide rabies viral glycoprotein (RVG). The RVG peptide directs the transfected exome to organs, such as the kidney, that express the acetylcholine receptor [179]. Intramuscular injection of exosome-encapsulated miR-29 partially depressed renal fibrosis (confirmed by decreased TGF-β, α-smooth muscle actin, fibronectin, and collagen 1A1) in UUO mouse kidneys. Overexpression of miR-29 also correlated with a significant reduction in the protein levels of pro-fibrotic peptides TGF-β3 and Yin Yang 1 (YY1) [178]. YY1 plays a crucial role in the development of renal fibrosis by upregulating α-smooth muscle actin expression and inducing epithelial-mesenchymal transition [43]. The use of exosomes as a therapeutic delivery system has recently received approval to undergo clinical trial testing [180]. As such, exosome based targeted approaches such as this display high clinical potential.
Other methods of therapeutic intervention have utilised siRNAs to silence pathogenic genes. Using polycationic cyclodextrin NPs Zuckerman et al. successfully delivered siRNA to the glomerular mesangium of lpr mice (mouse lupus nephritis model) [147]. Importantly, NP encapsulated siRNA (siRNA/CDP-NPs) was detectable by real-time PCR but this finding was not shared with freely administered siRNA [147]. Higher uptake of siRNA/CDP-NPs was observed in vitro compared to free siRNA [147]. This method of siRNA transport may prove effective in DKD treatment especially given the fact that several pre-clinical studies have observed successful gene silencing by siRNA for renal fibrosis in vivo [[181], [182], [183], [184], [185]]. The need for repeated siRNA administration in chronic conditions such as DKD must be considered before these studies can reach clinical trial. Such necessity leads to concerns over the implementation of siRNA therapy, including healthcare costs and potential long-term side effects (e.g. inactivation of a multifunctional gene).
NPs for stem cell delivery
In recent years, the use of stem cell based regenerative therapies has gained attention as a method of reversing fibrosis in renal tissue. Mesenchymal stem cells (MSCs) have been considered a promising strategy to cure DKD but challenges surrounding targeting them to specific organs in vivo has limited their clinical utility [186]. For this reason, combining the targeting characteristics of NPs with MSCs’ “healing” properties has recently gained considerable interest.
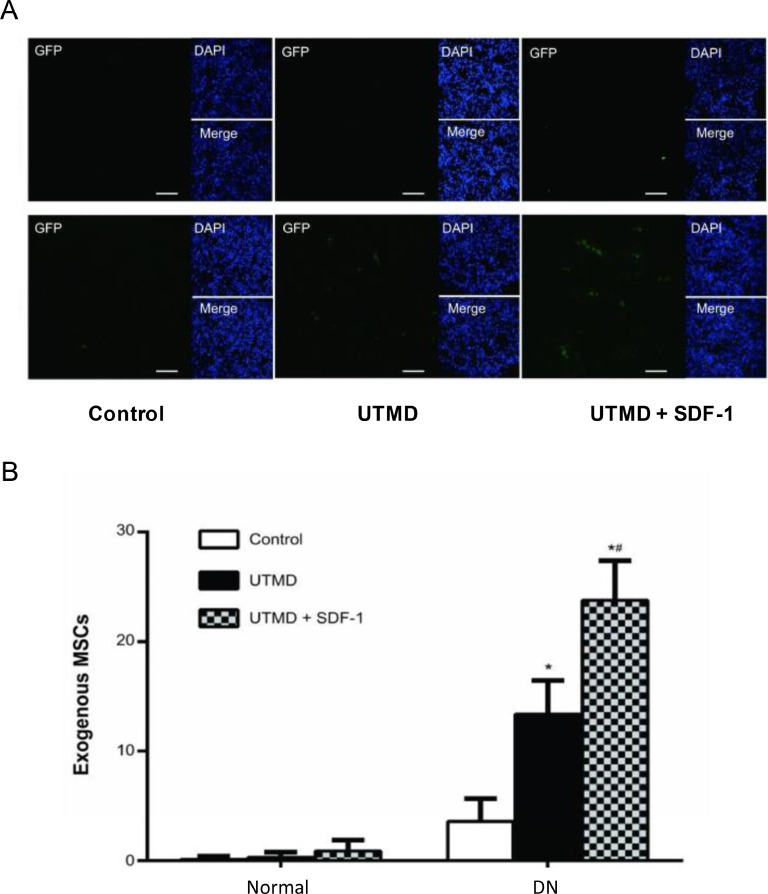
To improve MSC homing to DKD afflicted renal tissue Wu et al. delivered the MSC chemotactic peptide, SDF-1 (stromal cell-derived factor-1) loaded in microbubbles (MBSDF-1) to the kidneys of streptozotocin treated rats [187]. Streptozotocin is a toxin that negatively impacts the viability of pancreatic β-cells inducing hyperglycaemia in selected animal models [188]. Using ultrasound-targeted microbubble destruction (UTMD) the authors successfully released SDF-1 into the targeted kidneys with subsequent in vitro experiments revealing a loading efficacy of 79% and a loading content of 15.8 µg/mL. Implanted MSCs were scarcely observed in a control group of healthy rats (number of 3.6 ± 2.1) [187]. Importantly, UTMD delivered MSCs (number of 13.4 ± 3.1) were significantly increased when rats were also treated with SDF-1 (number of 23.8 ± 3.6, Fig. 4) [187]. MSCs were transfected with green fluorescent protein (GFP) for visualisation by confocal laser scanning microscopy [187]. The majority of MSCs clustered around the small blood vessels and in the peritubular interstitium [187]. Few MSCs were detected in the glomeruli suggesting this method of delivery may be more suited to the treatment of tubulointerstitial fibrosis than glomerulosclerosis.
Fig. 4.
In vivo detection of implanted exogenous MSCs Differences in implanted MSCs among groups under a confocal laser scanning microscope. Exogenous MSCs were labelled with GFP and displayed green signals. In normal rats, GFP-labelled MSCs were only occasionally detected in all three groups. In DN rats, GFP-labelled MSCs were rare in control group. UTMD increased the exogenous MSCs and UTMD + SDF-1 greatly improved the homing of exogenous MSCs. The white bar indicates 50 μm. B. Histogram comparison of the GFP-labelled MSCs among groups, *P < 0.05 (P-value of 4.93 × 10−6, DN + UTMD versus DN + control; P-value of 4.15 × 10−9, DN + UTMD + SDF-1 versus DN + control) and #P < 0.05 (P-value of 3.02 × 10−5, DN + UTMD + SDF-1 versus DN + UTMD). This data provided by Wu et al. shows a significant increase in MSC homing to the diabetic kidney using UTMD based transport, especially when paired with SDF-1 homing. Reproduced with permission from reference [187].
Recently, Li et al. labelled MSCs with fabricated polydopamine (PDA)-capped Fe3O4 (Fe3O4@PDA) NPs in an attempt to promote MSC migration to the site of injury [189]. MSC-loaded NPs exhibited improved homing to the sight of injury and increased expression of anti-inflammatory cytokines TGF-β and IL-10 in vivo. Interestingly, MSC-loaded Fe3O4@PDA NPs increased expression of the SDF-1 receptor C-X-C chemokine receptor 4 which has previously been implicated in the recruitment of MSCs to sites of inflammation/injury [190]. It is also important to note that the authors reported no adverse effects on MSC characteristics following Fe3O4@PDA NP encapsulation suggesting this technique of MSC homing may act as an effective method for treating inflammatory diseases such as DKD [190]. Given the significant evidence supporting Fe3O4 based NPs as effective contrast agents in MR imaging (see section 4.1), these findings highlight the possibility of NPs as theranostic tools of use in both DKD detection and treatment.
Challenges in nanomedicine – renal toxicity
This review has highlighted a wide variety of NPs for therapeutic and diagnostic purposes to overcome clinical obstacles found in different studies. As with any therapeutic intervention, NPs show off-target interactions that may ultimately damage the surrounding tissue causing undesirable side effects or progression of the diseased state. At present our understanding of the precise toxic effects of NPs on healthy tissue are under considerable debate owing to the variety of preparation methods used to assess NP function. The large range of toxicology assays, cell culture, animal models, dosing parameters and toxicity evaluations undertaken throughout the literature have only added to this conflict [191,192]. Studies show that the bulk of toxicity is observed when using inorganic NPs owing to their unique physio-chemical properties such as susceptibility to oxidation and small dimensions [38,193]. To this end organic NPs may show more clinical promise. Despite advancements in our understanding of NP activity in vivo their potential indirect toxicity toward healthy tissues requires considerable validation before being applied in a clinical setting.
Oxidative stress
Nanomaterials formed from a wide variety of compounds such as carbon nanotubes, fullerenes and metal oxides have proven to be effective inducers of oxidative stress [194,195]. Several NP properties have been attributed to their ability to induce reactive oxygen species (ROS) production such as immune cell activation, reactive particle surfaces and mitochondrial interaction [[196], [197], [198]]. The majority of studies investigating the use of NPs for therapeutic benefit in the kidney have used metallic NPs [199], as such this section will discuss the ability of metal NPs to induce oxidative stress.
Oxidative stress is considered one of the primary causes of NP induced nephrotoxicity and is characterised by an increase in ROS within the selected tissue/cell [200,201]. ROS are produced during normal physiological processes such as cellular respiration and metabolism. In the diabetic kidney, however, there is evidence that hyperglycaemia and hypertension associated with diabetes cause pathological renal changes associated with ROS overproduction [202,203]. To this end, ROS production mediated by NP activity represents a major limitation to their implementation. Studies have thus far shown that several metallic based NPs can cause significant ROS production. Silver NPs (AgNPs), for example, have previously been observed to inhibit electron transport in the mitochondrial respiratory chain resulting in dysfunctional activity of the organelle and ROS generation. In addition, AgNPs can limit production of the antioxidant glutathione by inhibiting its enzymatic synthesis causing an imbalance in the antioxidant to ROS ratio with subsequent ROS accumulation [204].
This ROS mediated renal damage is not limited to AgNPS. Zinc oxide NPs (ZnO NPs) have been found to cause excessive ROS accumulation in human embryonic kidney 293 (HEK 293) cells in vitro causing reduced viability and resultant apoptosis due to destruction of fundamental cellular components such as peptides, lipids and DNA [205]. In the same cell type copper adsorbed chitosan NPs generated sufficient ROS to cause poly-ADP ribose polymerase (PARP) depletion, preventing the DNA repair process and eventually leading to cell death [206]. It is important to note that the authors of both studies did not confirm these findings in an in vivo setting, reducing their translatable value. Shrivastava et al., however, observed significant elevation of ROS and the DNA damage marker, 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG), in the kidney of male Swiss albino mice when exposed to either Au or Ag NPs in vivo, suggesting NP toxicity is not limited to an in vitro setting [207]. Given that ROS levels significantly contribute to renal pathology in diabetes, these studies collectively suggest further research is required before metallic NP based DKD treatments can be transferred to a clinical setting.
Inflammation
Inflammation is a key component of the early immune mediated response to pathogenic infection or tissue insult. Initially formed as part of the innate response to external stimuli, inflammation is caused by the release of pro-inflammatory cytokines by an array of immune cells such as macrophages, neutrophils, dendritic cells and CD4+ T cells [208]. Excessive inflammation can result in over stimulation of the immune system and can lead to chronic renal damage and the development of fibrotic tissue [163].
NPs can be identified by cells of the immune system activating a cascade of pro-inflammatory signalling mechanisms in an attempt to destroy the foreign body. Mediators of these signals, cytokines, have been found to impair renal structure and function [[209], [210], [211]]. Reddy et al. for example, observed a significant time-dependant increase in IL-8 release from HEK293 cells following 48 h exposure to multi wall carbon nanotubes (MWCNT), a result that correlated with a significant decrease in cellular viability as recorded using 3-(4,5- dimethylthiazol-z-yl)−2,5-dyphenyl-tetrazolium bromide (MTT) assay [212]. Alternatively, titanium dioxide nanoparticles (TiO2 NPs) proved highly toxic when administered to CD-1 female mice [213]. TiO2 NPs accumulated in the kidney causing nephric inflammation leading to necrosis and dysfunction. TiO2 exposure also activated nucleic factor- κB (NF- κB) promoting expression of TNF-α, macrophage migration inhibitory factor, IL-2, IL-4, IL-6, IL-8, IL-10, IL- 18, IL-1β, cross-reaction protein, transforming growth factor- β (TGF- β), interferon- γ (IFN- γ) and CYP1A1. This suggests that inflammation is the main effect of TiO2-induced acute renal toxicity. Similarly, gold nanoparticles (AuNPs) have been reported to increase immune cell infiltration (largely neutrophils and mononuclear cells) in the hepatocytes of male Wistar-Kyoto rats resulting in cellular necrosis and loss of normal hepatic architecture [214].
Several studies have shown that NP properties such as size, charge hydrophobicity/hydrophilicity and external coating can influence their interaction with the host immune system [[215], [216], [217]]. To alleviate recognition by inflammatory cells NPs can be bound to poly(ethylene glycol) (PEG) which forms an immune tolerant hydrophilic environment [218]. Whilst this initiative has proven effective in some pre-clinical models, studies have found PEG-specific antibodies in the blood of Wistar rats resulting in accelerated PEG-liposome clearance [219,220]. As such, there is currently only one pegylated NP that has received FDA approval (Doxil) and this is not licensed for treatment of the diseased kidney [221]. As such the use of PEG to avoid NP immune destruction is considered a promising but incomplete initiative.
DNA damage
The prominent cause of DNA damage within the diabetic kidney is oxidative stress. Whilst most cells of the body are well equipped to prevent such events from escalating to serious ailments continued or untreated damage leads to elevated risk of proliferative diseases such as cancer. Markers of genomic damage are used to monitor a patient's probability of developing such ailments or assessing their risk of disease progression. In conditions such as CKD, markers include micronuclei and strand breaks in peripheral blood lymphocytes (PBLs) and the quantity of 8-oxodG in serum or urine [222]. As previously stated, metallic NPs are the greater producers of ROS than their non-metal counterparts thus there is wider literary understanding of their mechanisms of DNA impairment.
Numerous studies have evidenced the ability of metallic NPs to cause DNA damage in the kidney. Using the NRK-52E kidney epithelial cell line exposed to nickel oxide (NiO) NPs Abudayyak et al. observed dose-dependant DNA damage and oxidative damage evidenced by increasing levels of MDA, 8-OHdG, PC and depletion of the antioxidant, GSH. Comet assay studies used to determine genotoxic potential revealed a 1.4–5.6-fold increase in DNA damage in direct proportion to increased NiO NP concentration [223]. Similarly, Ranjbar et al. identified a significant increase in 8-OHdG in homogenised kidney samples derived from male Wistar rats treated with AgNPs at variable concentrations (0–250 ppm) for 24 h [224]. It is worth noting that the authors observed lower levels of 8-OHdG in rats treated with 5 ppm AgNP than the control population (0 ppm). This would suggest AgNPs display a potentially anti-genotoxic effect at low concentrations (∼5 ppm) but further data supporting this observation are required to validate such a claim.
The fibrotic kidney represents the latter stages of DKD progression and requires consistent dialysis or renal transplant to prevent fatality. In multiple models of renal injury, studies have revealed that epithelial cells can be arrested in the G2/M phase of the cell cycle which leads to the adoption of a profibrotic secretory phenotype [[225], [226], [227]]. In HEK2 cells AgNPs have been found to induce G2/M cell cycle arrest in depleted conditions of GSH [228]. Similarly, in HEK293 cells treated with single wall carbon nanotubes (SWCNTs) cell cycle arrest was observed in a time and dose dependant manner. Cell cycle analysis showed that 25 µg/ml SWCNTs in medium induced G1 arrest through downregulation of cell cycle genes cdk2, cdk4, cdk6 and cyclin D3. Interestingly, the authors also note that SWCNTs reduced HEK293 cellular adhesion by downregulating adhesion-associated proteins such as laminin, fibronectin, cadherin, FAK and collagen IV [229]. As a fundamental component to the development of renal fibrosis, collagen IV represents a highly promising target for the treatment of DKD [163]. Thus, SWCNTs may represent a promising tool for DKD treatment, however, additional research is required to alleviate their potentially genotoxic effects.
Future perspectives
Targeting senescent kidney cells
At present, the National Kidney Foundation recommends any patient diagnosed with either type 1 or type 2 DM to be screened for kidney damage within five years of their diagnosis. The current methods for DKD screening (eGFR and albuminuria), however, are time consuming and lack accuracy [230,231]. Identifying reliable biomarkers for the early detection of DKD could help identify vulnerable subjects before invasive procedures are required.
In recent years, senescent cells within the diseased kidney have shown promise as a target for DKD interventions. Both hyperglycaemia and hypertension have been found to induce cellular senescence in the renal microvascular endothelium and mesangium providing a causal link between DKD and onset of senescence [232]. Upon the cessation of cellular proliferation, senescent cells maintain their high metabolic activity and adopt a pro-inflammatory phenotype known as the senescence associated secretory phenotype (SASP) [233,234]. The SASP has been postulated to be one of the major contributors to renal inflammation, through modulation of the tissue environment and disruption of cellular functions [235,236] by secreting cytokines including IL-1, IL-6, IL-8 and TNF-α [237]. The release of TGF-β by senescence cells in turn promotes tubulointerstitial fibrosis, mesangial cell fibrogenesis and apoptosis of podocytes [238,239]. High mobility group box protein 1 (HMGB1) acts as a damage-associated molecular pattern (DAMP) following senescence induced stress and activates immunostimulatory molecules such as RAGE receptors and toll like receptor 4 [240], [241], [242]. HMGB1 has been found to promote several DKD associated phenotypes including renal inflammation, albuminuria, thickening of the mesangial matrix and tubulointerstitial fibrosis in streptozotocin-induced diabetic nude mice [243].
Adoption of a senescent phenotype precedes histological changes associated with DKD onset in murine models [244,245]. In patients with type 2 DM related DKD, elevated levels of the senescent marker SA-β-Gal in the tubular compartment correlate with an increase in blood glucose [246]. Similarly, upregulation of the senescent marker P16INK4A has been noted in diabetic tubular cells and podocytes and this is associated with proteinuria when found in the glomeruli [246]. However, it is the SASPs that cause extensive damage to local tissues and therefore therapeutic interventions should be identified to prevent this process. For instance, the therapeutic approaches used to treat senescence are termed senolytics and have shown great promise for the treatment of DKD [247]. However, many of the pharmaceuticals that are encompassed by this umbrella term show damaging off-target side effects that must be addressed [248,249]. Given the malleable properties of NPs described in this review nanotechnology presents as an attractive method for the safe delivery of senolytics. The combination of these approaches has been well summarised by other authors [250], [251], [252] and we suggest that with further research this will prove to be a viable method for the treatment of DKD.
Classifying the diabetic kidney
The clinical manifestations of DKD are the results of microvascular deterioration within the kidney glomeruli and analysis of this process can be used as a diagnostic measure [253]. Renal damage in DM patients is also associated with non-diabetic contributions, termed non-diabetic kidney disease (NDKD). One-third of patients with diabetes have NDKD [254], [255], [256], [257], [258]. The clinical features include a decline in kidney function and proteinuria in the absence of diabetic retinopathy [254]. Due to the complex nature and overlapping clinical entities between DKD and NDKD, patient differentiation remains a challenge; less than 10% of DKD patients receiving dialysis have their condition confirmed by biopsy [254,259]. Distinction between these conditions requires kidney biopsy, followed by histopathological assessment. Generating quantifiable data from these procedures is possible, but only moderately reproducible, due to subjective scores assigned by pathologists [260]. In fact, the prevalence of both DKD and NDKD shows significant variation amongst DM patients biopsied at different global institutions [261]. This warrants the use of new non-invasive approaches that can be used universally by clinicians to easily distinguish between DKD and NDKD.
In 2010 the renal pathological society introduced a new classification system for DKD, applicable to patients with both T1D and T2D (Table 3) [262]. This system defines 4 distinct categories of DKD based on glomerular findings observed under light and electron microscopy. Despite its reported success in several studies, this system has been heavily criticised for its arbitrary category distinctions and limited applicability to clinical practice [263], [264], [265]. Kidney biopsy is not a routine procedure undertaken for the diagnosis of DKD meaning the use of a histological classification system is limited. Gheith et al. have since proposed a 5-stage structure based on clinical observations but this system utilises a largely subjective method of clinical assessment [266]. Thus, there remains a significant need to stratify the risk of DKD progression to help inform clinical decisions.
Table 3.
Current criteria for DKD diagnosis Replica table of the 5 stages of DKD as originally depicted by Tervaert et al. [262].
| Class | Description | Inclusion criteria |
|---|---|---|
| I | Mild or nonspecific light microscopy changes and electron-microscope proven GBM thickening | Biopsy does not meet any of the criteria mentioned for class II, III or IV GBM > 395 nm in female and > 430 in male individuals 9 years of age or older |
| IIa | Mild mesangial expansion | Biopsy does not meet criteria for class III or IV |
| IIb | Severe mesangial expansion | Biopsy does not meet criteria for class III or IV |
| III | Nodular sclerosis (Kimmelstiel-Wilson lesion) | Biopsy does not meet criteria for class III or IVAt least one convincing Kimmelstiel-Wilson lesion |
| IV | Advanced diabetic glomerulosclerosis | Global glomerular sclerosis in > 50% glomeruliLesions from class I to III |
Throughout this review we have discussed the capacity of NPs to improve both diagnosis and therapy for DKD and argue that this same technology can be used to overcome the limitations associated with the current DKD staging system. Traditionally, assessment of GBM thickening has required biopsy for light and electron microscopy. In contrast, NPs can now achieve this by enhancing MRI contrast without the need for invasive tissue sampling [267]. Advanced stages require evidence of glomerulosclerosis such as the presence of Kimmelstiel-Wilson lesions but the data obtained from histopathological assessment is highly subjective [262]. To overcome these issues, we suggest the quantification of biomarkers using NPs as a tool of identification. This approach can provide a more detailed insight into disease pathology at an earlier stage, helping to inform clinical decisions.
The inflammatory marker oxidised low-density lipoprotein receptor 1 (LOX-1) has shown great promise as a candidate for this purpose. Expressed in macrophages, vascular endothelial cells, fibroblasts, and platelets, binding of LOX-1 to its respective ligand on leucocytes initiates an inflammatory response [268,269]. In the renal capillaries and tubules of diabetic rats, LOX-1 expression correlates with intense oxidative stress, leucocyte infiltration, depressed mitochondrial enzyme level and function and peritubular fibrosis [270]. Glucose has been found to enhance endothelial LOX-1 expression making it an ideal candidate for identifying the diabetic kidney [271]. For these reasons, we argue LOX-1 is a suitable marker of DKD progression that can be assessed using nanotechnology.
Anti-LOX-1 conjugated SPIONs have shown promise in their ability to obtain images of LOX-1 enriched DKD kidney lesions in vivo [272]. The ability of these NPs to safely target the glomerulus and enhance MRI contrast makes them a suitable tool for achieving this goal. As with all NP based approaches in the kidney this hypothesis requires additional study and human trials before being implemented in the clinic. Comparative measures of NP biomarker approaches against current diagnostic methods must also be conducted. However, the plethora of literature available regarding NP use in diagnostics supports our claim. Using SPIONs targeting LOX-1 could provide an affordable, non-invasive method of implementing the current DKD staging system [273].
NPs show numerous advantages including control over shape and size, tuneable physiochemical features, modifiable surface charge and responsiveness to external and internal stimuli (pH, reactive oxygen species, magnetic field, light) to achieve controlled delivery of drugs/agents. These features can be utilised to address unmet clinical needs in the field of kidney disease. Investigations evaluating the therapeutic and diagnostic roles of NPs in detecting and treating DKD still need to be undertaken. Currently there is no clinical data available on using nanomedicines for the diagnosis and treatment of DKD. A lack of animal models that accurately replicate the disease's main features have also hindered attempts to stratify risk of DKD among individuals with DM [274]. Numerous aspects require further exploration, including the effect of enhanced permeability retention effect on the use of NPs for therapeutic purposes, the ability of NPs to recognise disease biomarkers at correct concentrations and ratios of ligands (which can make them attracted towards site of interest), the identification of biomarkers for the monitoring of therapies and the potential roles of exosomes as biomarkers (they may contain crucial information related to disease progression and therapeutic responses). The lack of data in this field is likely because of the broad use of NPs in cancer and infectious diseases whilst DKD has been overlooked in this regard. The design and use of NPs may allow improved tracking of diseased cells/tissues at cellular and subcellular levels with minimal toxic effects towards surrounding healthy tissues, improved accumulation at the target site, on-demand drug-release and optimal therapeutic outcomes. We have presented studies in this review which show the importance of smart NP platforms in treating kidney disease. We believe that further research into NPs for the diagnosis and treatment of DKD may accelerate clinical progress in this field and eventually greatly improve patient outcomes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Md Zahidul I. Pranjol, Email: z.pranjol@sussex.ac.uk.
Muhammad M. Yaqoob, Email: m.m.yaqoob@qmul.ac.uk.
References
- 1.Dwyer-Lindgren L., et al. Diagnosed and undiagnosed diabetes prevalence by County in the U.S., 1999-2012. Diabetes Care. 2016;39(9):1556–1562. doi: 10.2337/dc16-0678. [DOI] [PubMed] [Google Scholar]
- 2.Patterson C.C., et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989-2013: a multicentre prospective registration study. Diabetologia. 2019;62(3):408–417. doi: 10.1007/s00125-018-4763-3. [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study Group. UK prospective diabetes study (UKPDS). VIII. Study design, progress and performance. Diabetologia. 1991;34(12):877–890. [PubMed] [Google Scholar]
- 4.Group A.S., et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoungas S., et al. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(6):431–437. doi: 10.1016/S2213-8587(17)30104-3. [DOI] [PubMed] [Google Scholar]
- 6.Muthuppalaniappan V.M., et al. Identification and management of diabetic nephropathy. Syst Dis Kidney. 2019;47(10):654–660. [Google Scholar]
- 7.Thomas M.C., et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1:15018. doi: 10.1038/nrdp.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroth J., Thiemermann C., Henson S.M. Senescence and the aging immune system as major drivers of chronic kidney disease. Front Cell Dev Biol. 2020;8 doi: 10.3389/fcell.2020.564461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrikopoulos P., et al. The MEK inhibitor trametinib ameliorates kidney fibrosis by suppressing ERK1/2 and mTORC1 signaling. J Am Soc Nephrol. 2019;30(1):33–49. doi: 10.1681/ASN.2018020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson F.L., et al. Inhibition of IkappaB kinase at 24 H after acute kidney injury improves recovery of renal function and attenuates fibrosis. J Am Heart Assoc. 2017;6(7) doi: 10.1161/JAHA.116.005092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho M.H. Renal fibrosis. Korean J Pediatr. 2010;53(7):735–740. doi: 10.3345/kjp.2010.53.7.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veiseh O., et al. Managing diabetes with nanomedicine: challenges and opportunities. Nat Rev Drug Discov. 2015;14(1):45–57. doi: 10.1038/nrd4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang C., Zhang M. Nanoparticle-based theragnostics: integrating diagnostic and therapeutic potentials in nanomedicine. J Control Release. 2010;146(1):2–5. doi: 10.1016/j.jconrel.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran S., et al. Cancer nanomedicine: a review of recent success in drug delivery. Clin Transl Med. 2017;6(1):44. doi: 10.1186/s40169-017-0175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis M.E., Chen Z.G., Shin D.M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 16.Conde J., et al. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front Chem. 2014;2:48. doi: 10.3389/fchem.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahato K., et al. Gold nanoparticle surface engineering strategies and their applications in biomedicine and diagnostics. 3 Biotech. 2019;9(2):57. doi: 10.1007/s13205-019-1577-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patra J.K., et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell M.J., et al. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miranda-Diaz A.G., et al. Oxidative stress in diabetic nephropathy with early chronic kidney disease. J Diabetes Res. 2016 doi: 10.1155/2016/7047238. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Diabetes Federation . 7th ed. International Diabetes Federation; 2015. IDF diabetes atlas. [Google Scholar]
- 22.National Kidney F. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Tuttle K.R., et al. Diabetic kidney disease: a report from an ADA consensus conference. Am J Kidney Dis. 2014;64(4):510–533. doi: 10.1053/j.ajkd.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Mogensen C.E., Christensen C.K., Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32(Suppl 2):64–78. doi: 10.2337/diab.32.2.s64. [DOI] [PubMed] [Google Scholar]
- 25.Parving H.H., et al. Early detection of patients at risk of developing diabetic nephropathy. A longitudinal study of urinary albumin excretion. Acta Endocrinol (Copenh) 1982;100(4):550–555. doi: 10.1530/acta.0.1000550. [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes, A., 16. Diabetes advocacy: standards of medical care in diabetes-2019. Diabetes Care. 2020;43(Suppl 1):S203–S204. doi: 10.2337/dc20-S016. [DOI] [PubMed] [Google Scholar]
- 27.Afghahi H., et al. Ongoing treatment with renin-angiotensin-aldosterone-blocking agents does not predict normoalbuminuric renal impairment in a general type 2 diabetes population. J Diabetes Complicat. 2013;27(3):229–234. doi: 10.1016/j.jdiacomp.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Macisaac R.J., Jerums G. Diabetic kidney disease with and without albuminuria. Curr Opin Nephrol Hypertens. 2011;20(3):246–257. doi: 10.1097/MNH.0b013e3283456546. [DOI] [PubMed] [Google Scholar]
- 29.Molitch M.E., et al. Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care. 2010;33(7):1536–1543. doi: 10.2337/dc09-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez-Fernandez B., et al. Therapeutic approaches to diabetic nephropathy–beyond the RAS. Nat Rev Nephrol. 2014;10(6):325–346. doi: 10.1038/nrneph.2014.74. [DOI] [PubMed] [Google Scholar]
- 31.Leehey DJ, Moinuddin I. Diabetic kidney disease. BMJ best practice. Available from: https://bestpractice.bmj.com/topics/en-gb/530.
- 32.KDOQI KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(Suppl 2):S12–154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Phisitkul K., et al. Continued smoking exacerbates but cessation ameliorates progression of early type 2 diabetic nephropathy. Am J Med Sci. 2008;335(4):284–291. doi: 10.1097/MAJ.0b013e318156b799. [DOI] [PubMed] [Google Scholar]
- 34.Friedman A.N., et al. Short-term changes after a weight reduction intervention in advanced diabetic nephropathy. Clin J Am Soc Nephrol. 2013;8(11):1892–1898. doi: 10.2215/CJN.04010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liyanage T., et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385(9981):1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 36.Aghebati-Maleki A., et al. Nanoparticles and cancer therapy: perspectives for application of nanoparticles in the treatment of cancers. J Cell Physiol. 2020;235(3):1962–1972. doi: 10.1002/jcp.29126. [DOI] [PubMed] [Google Scholar]
- 37.Awasthi R., et al. Nanoparticles in cancer treatment: opportunities and obstacles. Curr Drug Targets. 2018;19(14):1696–1709. doi: 10.2174/1389450119666180326122831. [DOI] [PubMed] [Google Scholar]
- 38.Ma Y., et al. A review of the application of nanoparticles in the diagnosis and treatment of chronic kidney disease. Bioact Mater. 2020;5(3):732–743. doi: 10.1016/j.bioactmat.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sraer J.D., et al. Species-specific properties of the glomerular mesangium. J Am Soc Nephrol. 1993;3(7):1342–1350. doi: 10.1681/ASN.V371342. [DOI] [PubMed] [Google Scholar]
- 40.Longmire M., Choyke P.L., Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (Lond) 2008;3(5):703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du B., Yu M., Zheng J. Transport and interactions of nanoparticles in the kidneys. Nat Rev Mater. 2018;3(10):358–374. [Google Scholar]
- 42.Desai N., et al. Nanomedicine in the treatment of diabetic nephropathy. Future Med Chem. 2021;13(7):663–686. doi: 10.4155/fmc-2020-0335. [DOI] [PubMed] [Google Scholar]
- 43.Yang T., et al. YY1: a novel therapeutic target for diabetic nephropathy orchestrated renal fibrosis. Metabolism. 2019;96:33–45. doi: 10.1016/j.metabol.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Zhang R., et al. In vivo magnetic resonance imaging of iron oxide-labeled, intravenous-injected mesenchymal stem cells in kidneys of rabbits with acute ischemic kidney injury: detection and monitoring at 1.5 T. Ren Fail. 2015;37(8):1363–1369. doi: 10.3109/0886022x.2015.1073542. [DOI] [PubMed] [Google Scholar]
- 45.Sheu J.J., et al. Intravenous administration of iPS-MSC(SPIONs) mobilized into CKD parenchyma and effectively preserved residual renal function in CKD rat. J Cell Mol Med. 2020;24(6):3593–3610. doi: 10.1111/jcmm.15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du B., et al. Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime. Nat Nanotechnol. 2017;12(11):1096–1102. doi: 10.1038/nnano.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi H.S., et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25(10):1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuckerman J.E., et al. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc Natl Acad Sci. 2012;109(8):3137–3142. doi: 10.1073/pnas.1200718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao S., et al. Megalin-mediated specific uptake of chitosan/siRNA nanoparticles in mouse kidney proximal tubule epithelial cells enables AQP1 gene silencing. Theranostics. 2014;4(10):1039–1051. doi: 10.7150/thno.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuura S., et al. L-Serine-modified polyamidoamine dendrimer as a highly potent renal targeting drug carrier. Proc Natl Acad Sci U S A. 2018;115(41):10511–10516. doi: 10.1073/pnas.1808168115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li S., et al. Design and evaluation of glomerulus mesangium-targeted PEG-PLGA nanoparticles loaded with dexamethasone acetate. Acta Pharmacol Sin. 2019;40(1):143–150. doi: 10.1038/s41401-018-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lawrence M.G., et al. Permeation of macromolecules into the renal glomerular basement membrane and capture by the tubules. Proc Natl Acad Sci U S A, 2017;114(11):2958–2963. doi: 10.1073/pnas.1616457114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams R.M., et al. Mesoscale nanoparticles selectively target the renal proximal tubule epithelium. Nano Lett. 2015;15(4):2358–2364. doi: 10.1021/nl504610d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bohrer M.P., et al. Influence of molecular configuration on the passage of macromolecules across the glomerular capillary wall. J Gen Physiol. 1979;74(5):583–593. doi: 10.1085/jgp.74.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rennke H.G., Venkatachalam M.A. Glomerular permeability of macromolecules. Effect of molecular configuration on the fractional clearance of uncharged dextran and neutral horseradish peroxidase in the rat. J Clin Invest. 1979;63(4):713–717. doi: 10.1172/JCI109354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohlson M., et al. Effects of filtration rate on the glomerular barrier and clearance of four differently shaped molecules. Am J Physiol Renal Physiol. 2001;281(1):F103–F113. doi: 10.1152/ajprenal.2001.281.1.F103. [DOI] [PubMed] [Google Scholar]
- 57.Arvizo R.R., et al. Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles. PLoS ONE. 2011;6(9):e24374. doi: 10.1371/journal.pone.0024374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamaly N., et al. Nanomedicines for renal disease: current status and future applications. Nat Rev Nephrol. 2016;12(12):738–753. doi: 10.1038/nrneph.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams R.M., et al. Selective nanoparticle targeting of the renal tubules. Hypertension. 2018;71(1):87–94. doi: 10.1161/HYPERTENSIONAHA.117.09843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cartiera M.S., et al. The uptake and intracellular fate of PLGA nanoparticles in epithelial cells. Biomaterials. 2009;30(14):2790–2798. doi: 10.1016/j.biomaterials.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farquhar M.G. Editorial: the primary glomerular filtration barrier-basement membrane or epithelial slits? Kidney Int. 1975;8(4):197–211. doi: 10.1038/ki.1975.103. [DOI] [PubMed] [Google Scholar]
- 62.Menon M.C., Chuang P.Y., He C.J. The glomerular filtration barrier: components and crosstalk. Int J Nephrol. 2012;2012 doi: 10.1155/2012/749010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh A., et al. Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J Am Soc Nephrol. 2007;18(11):2885–2893. doi: 10.1681/ASN.2007010119. [DOI] [PubMed] [Google Scholar]
- 64.Liu G.W., et al. Glomerular disease augments kidney accumulation of synthetic anionic polymers. Biomaterials. 2018;178:317–325. doi: 10.1016/j.biomaterials.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Miner J.H. Glomerular basement membrane composition and the filtration barrier. Pediatr Nephrol. 2011;26(9):1413–1417. doi: 10.1007/s00467-011-1785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chew C., Lennon R. Basement membrane defects in genetic kidney diseases. Front Pediatr. 2018;6:11. doi: 10.3389/fped.2018.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miner J.H. The glomerular basement membrane. Exp Cell Res. 2012;318(9):973–978. doi: 10.1016/j.yexcr.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang J., et al. Design and in vivo characterization of kidney-targeting multimodal micelles for renal drug delivery. Nano Res. 2018;11(10):5584–5595. [Google Scholar]
- 69.Huang Y., et al. The effect of size, charge, and peptide ligand length on kidney targeting by small, organic nanoparticles. Bioeng Transl Med. 2020;5(3):e10173. doi: 10.1002/btm2.10173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pombo Garcia K., et al. Zwitterionic-coated "stealth" nanoparticles for biomedical applications: recent advances in countering biomolecular corona formation and uptake by the mononuclear phagocyte system. Small. 2014;10(13):2516–2529. doi: 10.1002/smll.201303540. [DOI] [PubMed] [Google Scholar]
- 71.Wu L., et al. Albumin-based nanoparticles as methylprednisolone carriers for targeted delivery towards the neonatal Fc receptor in glomerular podocytes. Int J Mol Med. 2017;39(4):851–860. doi: 10.3892/ijmm.2017.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pollinger K., et al. Kidney podocytes as specific targets for cyclo(RGDfC)-modified nanoparticles. Small. 2012;8(21):3368–3375. doi: 10.1002/smll.201200733. [DOI] [PubMed] [Google Scholar]
- 73.Narumi S., et al. Tissue-specific induction of E-selectin in glomeruli is augmented following diabetes mellitus. Nephron. 2001;89(2):161–171. doi: 10.1159/000046063. [DOI] [PubMed] [Google Scholar]
- 74.Kurts C., et al. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13(10):738–753. doi: 10.1038/nri3523. [DOI] [PubMed] [Google Scholar]
- 75.Kato N., et al. The E-selectin ligand basigin/CD147 is responsible for neutrophil recruitment in renal ischemia/reperfusion. J Am Soc Nephrol. 2009;20(7):1565–1576. doi: 10.1681/ASN.2008090957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lange-Sperandio B., et al. Selectins mediate macrophage infiltration in obstructive nephropathy in newborn mice. Kidney Int. 2002;61(2):516–524. doi: 10.1046/j.1523-1755.2002.00162.x. [DOI] [PubMed] [Google Scholar]
- 77.Visweswaran G.R., et al. Targeting rapamycin to podocytes using a vascular cell adhesion molecule-1 (VCAM-1)-Harnessed SAINT-based lipid carrier system. PLoS ONE. 2015;10(9) doi: 10.1371/journal.pone.0138870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Canaud G., et al. AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nat Med. 2013;19(10):1288–1296. doi: 10.1038/nm.3313. [DOI] [PubMed] [Google Scholar]
- 79.Asgeirsdottir S.A., et al. Inhibition of proinflammatory genes in anti-GBM glomerulonephritis by targeted dexamethasone-loaded AbEsel liposomes. Am J Physiol Renal Physiol. 2008;294(3):F554–F561. doi: 10.1152/ajprenal.00391.2007. [DOI] [PubMed] [Google Scholar]
- 80.Hu J.B., et al. E-selectin-targeted Sialic Acid-PEG-dexamethasone micelles for enhanced anti-inflammatory efficacy for acute kidney injury. Theranostics. 2017;7(8):2204–2219. doi: 10.7150/thno.19571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu X.Y., et al. Targeted imaging of renal fibrosis using antibody-conjugated gold nanoparticles in renal artery stenosis. Invest Radiol. 2018;53(10):623–628. doi: 10.1097/RLI.0000000000000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang L., et al. Highly stabilized core-satellite gold nanoassemblies in vivo: DNA-directed self-assembly, PEG modification and cell imaging. Sci Rep. 2017;7(1):8553. doi: 10.1038/s41598-017-08903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu J., et al. In vivo X-ray imaging of transport of renal clearable gold nanoparticles in the kidneys. Angew Chem Int Ed Engl. 2017;56(43):13356–13360. doi: 10.1002/anie.201707819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu M., et al. Noninvasive staging of kidney dysfunction enabled by renal-clearable luminescent gold nanoparticles. Angew Chem Int Ed Engl. 2016;55(8):2787–2791. doi: 10.1002/anie.201511148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gomez-Vallejo V., et al. PEG-copolymer-coated iron oxide nanoparticles that avoid the reticuloendothelial system and act as kidney MRI contrast agents. Nanoscale. 2018;10(29):14153–14164. doi: 10.1039/c8nr03084g. [DOI] [PubMed] [Google Scholar]
- 86.Goodwill P.W., et al. X-space MPI: magnetic nanoparticles for safe medical imaging. Adv Mater. 2012;24(28):3870–3877. doi: 10.1002/adma.201200221. [DOI] [PubMed] [Google Scholar]
- 87.Saritas E.U., et al. Magnetic particle imaging (MPI) for NMR and MRI researchers. J Magn Reson. 2013;229:116–126. doi: 10.1016/j.jmr.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chandrasekharan P., et al. A perspective on a rapid and radiation-free tracer imaging modality, magnetic particle imaging, with promise for clinical translation. Br J Radiol. 2018;91(1091) doi: 10.1259/bjr.20180326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Toth G.B., et al. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int. 2017;92(1):47–66. doi: 10.1016/j.kint.2016.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mukundan S., et al. Ferumoxytol-enhanced magnetic resonance imaging in late-stage CKD. Am J Kidney Dis. 2016;67(6):984–988. doi: 10.1053/j.ajkd.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stoumpos S., et al. Ferumoxytol magnetic resonance angiography: a dose-finding study in patients with chronic kidney disease. Eur Radiol. 2019;29(7):3543–3552. doi: 10.1007/s00330-019-06137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Macdougall I.C., et al. Ferumoxytol for iron deficiency anemia in patients undergoing hemodialysis. The FACT randomized controlled trial. Clin Nephrol. 2019;91(4):237–245. doi: 10.5414/CN109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thomsen H.S. Nephrogenic systemic fibrosis: a serious late adverse reaction to gadodiamide. Eur Radiol. 2006;16(12):2619–2621. doi: 10.1007/s00330-006-0495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Katzberg R.W., Barrett B.J. Risk of iodinated contrast material–induced nephropathy with intravenous administration. Radiology. 2007;243(3):622–628. doi: 10.1148/radiol.2433061411. [DOI] [PubMed] [Google Scholar]
- 95.van Oostenbrugge T.J., Futterer J.J., Mulders P.F.A. Diagnostic imaging for solid renal tumors: a pictorial review. Kidney Cancer. 2018;2(2):79–93. doi: 10.3233/KCA-180028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Künzel R., et al. Evaluation of the X-ray absorption by gold nanoparticles solutions. ISRN Nanotechnol. 2013;2013 [Google Scholar]
- 97.Jackson P.A., et al. Potential dependent superiority of gold nanoparticles in comparison to iodinated contrast agents. Eur J Radiol. 2010;75(1):104–109. doi: 10.1016/j.ejrad.2009.03.057. [DOI] [PubMed] [Google Scholar]
- 98.Legacz M., et al. Contrast agents and cell labeling strategies for in vivo imaging. Adv Nanopart. 2014;03(02):13. Vol.No. [Google Scholar]
- 99.Peng Y.K., Tsang S.C.E., Chou P.T. Chemical design of nanoprobes for T1-weighted magnetic resonance imaging. Mater Today. 2016;19(6):336–348. [Google Scholar]
- 100.Wei H., et al. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proc Natl Acad Sci U S A, 2017;114(9):2325–2330. doi: 10.1073/pnas.1620145114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Terreno E., et al. Challenges for molecular magnetic resonance imaging. Chem Rev. 2010;110(5):3019–3042. doi: 10.1021/cr100025t. [DOI] [PubMed] [Google Scholar]
- 102.Ren L., et al. MRI-guided liposomes for targeted tandem chemotherapy and therapeutic response prediction. Acta Biomater. 2016;35:260–268. doi: 10.1016/j.actbio.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 103.Lee N., et al. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem Rev. 2015;115(19):10637–10689. doi: 10.1021/acs.chemrev.5b00112. [DOI] [PubMed] [Google Scholar]
- 104.Revia R.A., Zhang M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advances. Mater Today. 2016;19(3):157–168. doi: 10.1016/j.mattod.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stephen Z.R., Kievit F.M., Zhang M. Magnetite nanoparticles for medical MR imaging. Mater Today. 2011;14(7–8):330–338. doi: 10.1016/S1369-7021(11)70163-8. Kidlington. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chiarelli P.A., et al. Nanoparticle biokinetics in mice and nonhuman primates. ACS Nano. 2017;11(9):9514–9524. doi: 10.1021/acsnano.7b05377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Beeman S.C., et al. Measuring glomerular number and size in perfused kidneys using MRI. Am J Physiol Renal Physiol. 2011;300(6):F1454–F1457. doi: 10.1152/ajprenal.00044.2011. [DOI] [PubMed] [Google Scholar]
- 108.Bennett K.M., et al. MRI of the basement membrane using charged nanoparticles as contrast agents. Magn Reson Med. 2008;60(3):564–574. doi: 10.1002/mrm.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Qian C., et al. Wireless amplified nuclear MR detector (WAND) for high-spatial-resolution MR imaging of internal organs: preclinical demonstration in a rodent model. Radiology. 2013;268(1):228–236. doi: 10.1148/radiol.13121352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shan L. Molecular Imaging and Contrast Agent Database (MICAD) [Internet] Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013; 2009. Superparamagnetic iron oxide nanoparticles (SPION) stabilized by alginate. Oct 13 [Updated 2009 Nov 30] [PubMed] [Google Scholar]; Available from: https://www.ncbi.nlm.nih.gov/books/NBK23636/.
- 111.Mihai S., et al. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res. 2018;2018 doi: 10.1155/2018/2180373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tang S.C.W., Yiu W.H. Innate immunity in diabetic kidney disease. Nat Rev Nephrol. 2020;16(4):206–222. doi: 10.1038/s41581-019-0234-4. [DOI] [PubMed] [Google Scholar]
- 113.Nguyen D., et al. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology (Carlton) 2006;11(3):226–231. doi: 10.1111/j.1440-1797.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- 114.Hauger O., et al. USPIO-enhanced MR imaging of macrophage infiltration in native and transplanted kidneys: initial results in humans. Eur Radiol. 2007;17(11):2898–2907. doi: 10.1007/s00330-007-0660-8. [DOI] [PubMed] [Google Scholar]
- 115.Lutz A.M., et al. Imaging of macrophages in soft-tissue infection in rats: relationship between ultrasmall superparamagnetic iron oxide dose and MR signal characteristics. Radiology. 2005;234(3):765–775. doi: 10.1148/radiol.2343031172. [DOI] [PubMed] [Google Scholar]
- 116.Serkova N.J., et al. Renal inflammation: targeted iron oxide nanoparticles for molecular MR imaging in mice. Radiology. 2010;255(2):517–526. doi: 10.1148/radiol.09091134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kalantarinia K., Awad A.S., Siragy H.M. Urinary and renal interstitial concentrations of TNF-alpha increase prior to the rise in albuminuria in diabetic rats. Kidney Int. 2003;64(4):1208–1213. doi: 10.1046/j.1523-1755.2003.00237.x. [DOI] [PubMed] [Google Scholar]
- 118.Chen Y.L., et al. Serum TNF-alpha concentrations in type 2 diabetes mellitus patients and diabetic nephropathy patients: a systematic review and meta-analysis. Immunol Lett. 2017;186:52–58. doi: 10.1016/j.imlet.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 119.Navarro J.F., et al. Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. Am J Kidney Dis. 2003;42(1):53–61. doi: 10.1016/s0272-6386(03)00408-6. [DOI] [PubMed] [Google Scholar]
- 120.Farney J.K., et al. Technical note: validation of an ELISA for measurement of tumor necrosis factor alpha in bovine plasma. J Dairy Sci. 2011;94(7):3504–3509. doi: 10.3168/jds.2010-4082. [DOI] [PubMed] [Google Scholar]
- 121.Ridker P.M., et al. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101(18):2149–2153. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 122.Loredo-García E., et al. TNF-α detection using gold nanoparticles as a surface-enhanced Raman spectroscopy substrate. Nanomedicine (Lond) 2021;16(1):51–61. doi: 10.2217/nnm-2020-0307. [DOI] [PubMed] [Google Scholar]
- 123.Lai Y., Schlucker S., Wang Y. Rapid and sensitive SERS detection of the cytokine tumor necrosis factor alpha (TNF-alpha) in a magnetic bead pull-down assay with purified and highly Raman-active gold nanoparticle clusters. Anal Bioanal Chem. 2018;410(23):5993–6000. doi: 10.1007/s00216-018-1218-0. [DOI] [PubMed] [Google Scholar]
- 124.Nie S., Emory S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science. 1997;275(5303):1102–1106. doi: 10.1126/science.275.5303.1102. [DOI] [PubMed] [Google Scholar]
- 125.Xu X., et al. Near-field enhanced plasmonic-magnetic bifunctional nanotubes for single cell bioanalysis. Adv Funct Mater. 2013;23(35):4332–4338. [Google Scholar]
- 126.Gross J.L., et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28(1):164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 127.Busby D.E., Bakris G.L. Comparison of commonly used assays for the detection of microalbuminuria. J Clin Hypertens (Greenwich) 2004;6(11 Suppl 3):8–12. doi: 10.1111/j.1524-6175.2004.04237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shaikh M.O., et al. Electrochemical immunosensor utilizing electrodeposited Au nanocrystals and dielectrophoretically trapped PS/Ag/ab-HSA nanoprobes for detection of microalbuminuria at point of care. Biosens Bioelectron. 2019;126:572–580. doi: 10.1016/j.bios.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 129.Budhathoki-Uprety J., et al. Synthetic molecular recognition nanosensor paint for microalbuminuria. Nat Commun. 2019;10(1):3605. doi: 10.1038/s41467-019-11583-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Curry S., et al. Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites. Nat Struct Biol. 1998;5(9):827–835. doi: 10.1038/1869. [DOI] [PubMed] [Google Scholar]
- 131.Mula-Abed W.A.S., Al Rasadi K., Al-Riyami D. Estimated glomerular filtration rate (eGFR): a serum creatinine-based test for the detection of chronic kidney disease and its impact on clinical practice. Oman Med J. 2012;27(2):108–113. doi: 10.5001/omj.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jones R.H., et al. Progression of diabetic nephropathy. Lancet. 1979;1(8126):1105–1106. doi: 10.1016/s0140-6736(79)91788-4. [DOI] [PubMed] [Google Scholar]
- 133.Herget-Rosenthal S., et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66(3):1115–1122. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 134.Kar S., Paglialunga S., Islam R. Cystatin C Is a more reliable biomarker for determining eGFR to support drug development studies. J Clin Pharmacol. 2018;58(10):1239–1247. doi: 10.1002/jcph.1132. [DOI] [PubMed] [Google Scholar]
- 135.Mussap M., Plebani M. Biochemistry and clinical role of human cystatin C. Crit Rev Clin Lab Sci. 2004;41(5–6):467–550. doi: 10.1080/10408360490504934. [DOI] [PubMed] [Google Scholar]
- 136.Fassett R.G., et al. Biomarkers in chronic kidney disease: a review. Kidney Int. 2011;80(8):806–821. doi: 10.1038/ki.2011.198. [DOI] [PubMed] [Google Scholar]
- 137.Sun Y., et al. Development of an approach of high sensitive chemiluminescent assay for cystatin C using a nanoparticle carrier. Front Chem. 2020;8(802) doi: 10.3389/fchem.2020.00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lopes P., et al. Disposable electrochemical immunosensor for analysis of cystatin C, a CKD biomarker. Talanta. 2019;201:211–216. doi: 10.1016/j.talanta.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 139.Remuzzi A., et al. Regression of renal disease by angiotensin II antagonism is caused by regeneration of kidney vasculature. J Am Soc Nephrol. 2016;27(3):699–705. doi: 10.1681/ASN.2014100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fioretto P., et al. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339(2):69–75. doi: 10.1056/NEJM199807093390202. [DOI] [PubMed] [Google Scholar]
- 141.Alomari G., et al. Gold nanoparticles attenuate albuminuria by inhibiting podocyte injury in a rat model of diabetic nephropathy. Drug Deliv Transl Res. 2020;10(1):216–226. doi: 10.1007/s13346-019-00675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barathmanikanth S., et al. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J Nanobiotechnol. 2010;8:16. doi: 10.1186/1477-3155-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Manna K., et al. Amelioration of diabetic nephropathy using pomegranate peel extract-stabilized gold nanoparticles: assessment of NF-kappaB and Nrf2 signaling system. Int J Nanomed. 2019;14:1753–1777. doi: 10.2147/IJN.S176013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gao S., et al. Megalin-mediated specific uptake of chitosan/siRNA nanoparticles in mouse kidney proximal tubule epithelial cells enables AQP1 gene silencing. Theranostics. 2014;4(10):1039–1051. doi: 10.7150/thno.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang C., et al. Chitosan/siRNA nanoparticles targeting cyclooxygenase type 2 attenuate unilateral ureteral obstruction-induced kidney injury in mice. Theranostics. 2015;5(2):110–123. doi: 10.7150/thno.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Qiao H., et al. Kidney-specific drug delivery system for renal fibrosis based on coordination-driven assembly of catechol-derived chitosan. Biomaterials. 2014;35(25):7157–7171. doi: 10.1016/j.biomaterials.2014.04.106. [DOI] [PubMed] [Google Scholar]
- 147.Zuckerman J.E., et al. siRNA delivery to the glomerular mesangium using polycationic cyclodextrin nanoparticles containing siRNA. Nucleic Acid Ther. 2015;25(2):53–64. doi: 10.1089/nat.2014.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen D., et al. Kidney-targeted drug delivery via rhein-loaded polyethyleneglycol-co-polycaprolactone-co-polyethylenimine nanoparticles for diabetic nephropathy therapy. Int J Nanomedicine. 2018;13:3507–3527. doi: 10.2147/IJN.S166445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huang X., et al. Lower Circulating miR-122 level in patients with HNF1A variant-induced diabetes compared with type 2 diabetes. J Diabetes Res. 2018;2018 doi: 10.1155/2018/7842064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen P.P., et al. CoQ10-loaded liposomes combined with UTMD prevented early nephropathy of diabetic rats. Oncotarget. 2018;9(14):11767–11782. doi: 10.18632/oncotarget.24363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yue T., et al. Combination of coenzyme Q10-loaded liposomes with ultrasound targeted microbubbles destruction (UTMD) for early theranostics of diabetic nephropathy. Int J Pharm. 2017;528(1–2):664–674. doi: 10.1016/j.ijpharm.2017.06.070. [DOI] [PubMed] [Google Scholar]
- 152.Sheng W.S., et al. Intrarenal delivery of bFGF-loaded liposome under guiding of ultrasound-targeted microbubble destruction prevent diabetic nephropathy through inhibition of inflammation. Artif Cells Nanomed Biotechnol. 2018;46(sup2):373–385. doi: 10.1080/21691401.2018.1457538. [DOI] [PubMed] [Google Scholar]
- 153.Li R., et al. Targeted delivery of celastrol to renal interstitial myofibroblasts using fibronectin-binding liposomes attenuates renal fibrosis and reduces systemic toxicity. J Control Release. 2020;320:32–44. doi: 10.1016/j.jconrel.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 154.Tang L., et al. Quercetin liposomes ameliorate streptozotocin-induced diabetic nephropathy in diabetic rats. Sci Rep. 2020;10(1):2440. doi: 10.1038/s41598-020-59411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guo J., et al. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32(31):8010–8020. doi: 10.1016/j.biomaterials.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 156.Kreuter J. Drug targeting with nanoparticles. Eur J Drug Metab Pharmacokinet. 1994;19(3):253–256. doi: 10.1007/BF03188928. [DOI] [PubMed] [Google Scholar]
- 157.Kim S.H., et al. Induction of antigen-specific immune tolerance using biodegradable nanoparticles containing antigen and dexamethasone. Int J Nanomed. 2019;14:5229–5242. doi: 10.2147/IJN.S210546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nieto K., et al. In vivo controlled release of fenretinide from long-acting release depots for chemoprevention of oral squamous cell carcinoma recurrence. Int J Pharm. 2018;538(1–2):48–56. doi: 10.1016/j.ijpharm.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Krakauer T., Buckley M. Efficacy of two FDA-approved drug combination in a mouse model of staphylococcal enterotoxin B-induced shock. Mil Med. 2013;178(9):1024–1028. doi: 10.7205/MILMED-D-13-00129. [DOI] [PubMed] [Google Scholar]
- 160.Mehdipour M,Z.A. IntechOpen; 2012. Role of corticosteroids in oral lesions. in: state of the art of therapeutic endocrinology. [Google Scholar]
- 161.Bruni R., et al. Ultrasmall polymeric nanocarriers for drug delivery to podocytes in kidney glomerulus. J Control Release. 2017;255:94–107. doi: 10.1016/j.jconrel.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 162.Li M., et al. Three-dimensional podocyte-endothelial cell co-cultures: assembly, validation, and application to drug testing and intercellular signaling studies. Eur J Pharm Sci. 2016;86:1–12. doi: 10.1016/j.ejps.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 163.Humphreys B.D. Mechanisms of Renal Fibrosis. Annu Rev Physiol. 2018;80:309–326. doi: 10.1146/annurev-physiol-022516-034227. [DOI] [PubMed] [Google Scholar]
- 164.Yuan Y., et al. Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von hippel-lindau protein by direct binding to hypoxia-inducible factor-alpha. J Biol Chem. 2003;278(18):15911–15916. doi: 10.1074/jbc.M300463200. [DOI] [PubMed] [Google Scholar]
- 165.Matsuura H., et al. Inhibition of prolyl hydroxylase domain-containing protein downregulates vascular angiotensin II type 1 receptor. Hypertension. 2011;58(3):386–393. doi: 10.1161/HYPERTENSIONAHA.110.167106. [DOI] [PubMed] [Google Scholar]
- 166.Tan L., et al. A stimuli-responsive drug release nanoplatform for kidney-specific anti-fibrosis treatment. Biomater Sci. 2019;7(4):1554–1564. doi: 10.1039/c8bm01297k. [DOI] [PubMed] [Google Scholar]
- 167.Ali B.H., et al. Curcumin ameliorates kidney function and oxidative stress in experimental chronic kidney disease. Basic Clin Pharmacol Toxicol. 2018;122(1):65–73. doi: 10.1111/bcpt.12817. [DOI] [PubMed] [Google Scholar]
- 168.Ghosh S.S., Gehr T.W., Ghosh S. Curcumin and chronic kidney disease (CKD): major mode of action through stimulating endogenous intestinal alkaline phosphatase. Molecules. 2014;19(12):20139–20156. doi: 10.3390/molecules191220139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Weir M.A., et al. Micro-particle curcumin for the treatment of chronic kidney disease-1: study protocol for a multicenter clinical trial. Can J Kidney Health Dis. 2018;5 doi: 10.1177/2054358118813088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Khajehdehi P., et al. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-beta and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled study. Scand J Urol Nephrol. 2011;45(5):365–370. doi: 10.3109/00365599.2011.585622. [DOI] [PubMed] [Google Scholar]
- 171.Tapia E., et al. Curcumin induces Nrf2 nuclear translocation and prevents glomerular hypertension, hyperfiltration, oxidant stress, and the decrease in antioxidant enzymes in 5/6 nephrectomized rats. Oxid Med Cell Longev. 2012;2012 doi: 10.1155/2012/269039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Guo C., et al. Intranasal delivery of nanomicelle curcumin promotes corneal epithelial wound healing in streptozotocin-induced diabetic mice. Sci Rep. 2016;6:29753. doi: 10.1038/srep29753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Anand P., et al. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 174.Holder G.M., Plummer J.L., Ryan A.J. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica. 1978;8(12):761–768. doi: 10.3109/00498257809069589. [DOI] [PubMed] [Google Scholar]
- 175.Wahlstrom B., Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol (Copenh) 1978;43(2):86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 176.Clinicaltrials.gov. Micro-particle curcumin for the treatment of chronic kidney disease (MPAC-CKD). 2022 Available from: https://clinicaltrials.gov/ct2/show/NCT02369549.
- 177.Palazzo A.F., Lee E.S. Non-coding RNA: what is functional and what is junk? Front Genet. 2015;6:2. doi: 10.3389/fgene.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang H., et al. Exosome-mediated miR-29 transfer reduces muscle atrophy and kidney fibrosis in mice. Mol Ther. 2019;27(3):571–583. doi: 10.1016/j.ymthe.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Phoolcharoen W., et al. In vitro and in vivo evaluation of a single chain antibody fragment generated in planta with potent rabies neutralisation activity. Vaccine. 2019;37(33):4673–4680. doi: 10.1016/j.vaccine.2018.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cully M. Exosome-based candidates move into the clinic. Nat Rev Drug Discov. 2021;20(1):6–7. doi: 10.1038/d41573-020-00220-y. [DOI] [PubMed] [Google Scholar]
- 181.Zhou X., et al. Enhancer of zeste homolog 2 inhibition attenuates renal fibrosis by maintaining smad7 and phosphatase and tensin homolog expression. J Am Soc Nephrol. 2016;27(7):2092–2108. doi: 10.1681/ASN.2015040457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Luo G.H., et al. Inhibition of connective tissue growth factor by small interfering RNA prevents renal fibrosis in rats undergoing chronic allograft nephropathy. Transplant Proc. 2008;40(7):2365–2369. doi: 10.1016/j.transproceed.2008.07.100. [DOI] [PubMed] [Google Scholar]
- 183.Wei X., et al. Kindlin-2 mediates activation of TGF-beta/SMAD signaling and renal fibrosis. J Am Soc Nephrol. 2013;24(9):1387–1398. doi: 10.1681/ASN.2012101041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li Z., et al. Pro)renin receptor is an amplifier of WNT/beta-catenin signaling in kidney injury and fibrosis. J Am Soc Nephrol. 2017;28(8):2393–2408. doi: 10.1681/ASN.2016070811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Morishita Y., et al. siRNAs targeted to Smad4 prevent renal fibrosis in vivo. Sci Rep. 2014;4:6424. doi: 10.1038/srep06424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Han Y., et al. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8(8) doi: 10.3390/cells8080886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wu S., et al. Ultrasound-targeted stromal cell-derived factor-1-loaded microbubble destruction promotes mesenchymal stem cell homing to kidneys in diabetic nephropathy rats. Int J Nanomed. 2014;9:5639–5651. doi: 10.2147/IJN.S73950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50(6):537–546. [PubMed] [Google Scholar]
- 189.Li X., et al. Iron oxide nanoparticles promote the migration of mesenchymal stem cells to injury sites. Int J Nanomed. 2019;14:573–589. doi: 10.2147/IJN.S184920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wu Y., Zhao R.C. The role of chemokines in mesenchymal stem cell homing to myocardium. Stem Cell Rev Rep. 2012;8(1):243–250. doi: 10.1007/s12015-011-9293-z. [DOI] [PubMed] [Google Scholar]
- 191.Sakhtianchi R., et al. Exocytosis of nanoparticles from cells: role in cellular retention and toxicity. Adv Colloid Interface Sci. 2013:18–29. doi: 10.1016/j.cis.2013.10.013. 201-202. [DOI] [PubMed] [Google Scholar]
- 192.Teixeira P.R., et al. Photochemically-assisted synthesis of non-toxic and biocompatible gold nanoparticles. Colloids Surf B Biointerfaces. 2016;148:317–323. doi: 10.1016/j.colsurfb.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 193.De Matteis V. Exposure to inorganic nanoparticles: routes of entry, immune response, biodistribution and in vitro/in vivo toxicity evaluation. Toxics. 2017;5(4) doi: 10.3390/toxics5040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bonner J.C. Lung fibrotic responses to particle exposure. Toxicol Pathol. 2007;35(1):148–153. doi: 10.1080/01926230601060009. [DOI] [PubMed] [Google Scholar]
- 195.Vallyathan V., Shi X. The role of oxygen free radicals in occupational and environmental lung diseases. Environ Health Perspect. 1997;105(Suppl 1):165–177. doi: 10.1289/ehp.97105s1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Risom L., Moller P., Loft S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res. 2005;592(1–2):119–137. doi: 10.1016/j.mrfmmm.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 197.Sioutas C., Delfino R.J., Singh M. Exposure assessment for atmospheric ultrafine particles (UFPs) and implications in epidemiologic research. Environ Health Perspect. 2005;113(8):947–955. doi: 10.1289/ehp.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wilson M.R., et al. Interactions between ultrafine particles and transition metals in vivo and in vitro. Toxicol Appl Pharmacol. 2002;184(3):172–179. doi: 10.1006/taap.2002.9501. [DOI] [PubMed] [Google Scholar]
- 199.Williams R.M., Jaimes E.A., Heller D.A. Nanomedicines for kidney diseases. Kidney Int. 2016;90(4):740–745. doi: 10.1016/j.kint.2016.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Marano F., et al. Nanoparticles: molecular targets and cell signalling. Arch Toxicol. 2011;85(7):733–741. doi: 10.1007/s00204-010-0546-4. [DOI] [PubMed] [Google Scholar]
- 201.Passagne I., et al. Implication of oxidative stress in size-dependent toxicity of silica nanoparticles in kidney cells. Toxicology. 2012;299(2–3):112–124. doi: 10.1016/j.tox.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 202.Gill P.S., Wilcox C.S. NADPH oxidases in the kidney. Antioxid Redox Signal. 2006;8(9–10):1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 203.Kaneto H., et al. Involvement of oxidative stress in the pathogenesis of diabetes. Antioxid Redox Signal. 2007;9(3):355–366. doi: 10.1089/ars.2006.1465. [DOI] [PubMed] [Google Scholar]
- 204.Hussain S.M., et al. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In Vitro. 2005;19(7):975–983. doi: 10.1016/j.tiv.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 205.V G.R., V.M P. Cellular interactions of zinc oxide nanoparticles with human embryonic kidney (HEK 293) cells. Colloids Surf B Biointerfaces. 2017;157:182–190. doi: 10.1016/j.colsurfb.2017.05.069. [DOI] [PubMed] [Google Scholar]
- 206.Arora D., et al. Preparation, characterization and toxicological investigation of copper loaded chitosan nanoparticles in human embryonic kidney HEK-293 cells. Mater Sci Eng C Mater Biol Appl. 2016;61:227–234. doi: 10.1016/j.msec.2015.12.035. [DOI] [PubMed] [Google Scholar]
- 207.Shrivastava R., et al. Oxidative stress following exposure to silver and gold nanoparticles in mice. Toxicol Ind Health. 2016;32(8):1391–1404. doi: 10.1177/0748233714562623. [DOI] [PubMed] [Google Scholar]
- 208.Chen L., et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Coleman D.L., Ruef C. Interleukin-6: an autocrine regulator of mesangial cell growth. Kidney Int. 1992;41(3):604–606. doi: 10.1038/ki.1992.91. [DOI] [PubMed] [Google Scholar]
- 210.Koike N., Takamura T., Kaneko S. Induction of reactive oxygen species from isolated rat glomeruli by protein kinase C activation and TNF-alpha stimulation, and effects of a phosphodiesterase inhibitor. Life Sci. 2007;80(18):1721–1728. doi: 10.1016/j.lfs.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 211.Sassy-Prigent C., et al. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes. 2000;49(3):466–475. doi: 10.2337/diabetes.49.3.466. [DOI] [PubMed] [Google Scholar]
- 212.Reddy A.R., et al. Multi wall carbon nanotubes induce oxidative stress and cytotoxicity in human embryonic kidney (HEK293) cells. Toxicology. 2010;272(1–3):11–16. doi: 10.1016/j.tox.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 213.Gui S., et al. Molecular mechanism of kidney injury of mice caused by exposure to titanium dioxide nanoparticles. J Hazard Mater. 2011;195:365–370. doi: 10.1016/j.jhazmat.2011.08.055. [DOI] [PubMed] [Google Scholar]
- 214.Abdelhalim M.A., Jarrar B.M. Gold nanoparticles administration induced prominent inflammatory, central vein intima disruption, fatty change and Kupffer cells hyperplasia. Lipids Health Dis. 2011;10:133. doi: 10.1186/1476-511X-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Aggarwal P., et al. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61(6):428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dobrovolskaia M.A., et al. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm. 2008;5(4):487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Dobrovolskaia M.A., McNeil S.E. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2(8):469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 218.Moghimi S.M. Chemical camouflage of nanospheres with a poorly reactive surface: towards development of stealth and target-specific nanocarriers. Biochim Biophys Acta. 2002;1590(1–3):131–139. doi: 10.1016/s0167-4889(02)00204-5. [DOI] [PubMed] [Google Scholar]
- 219.Ishida T., et al. PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner. J Control Release. 2007;122(3):349–355. doi: 10.1016/j.jconrel.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 220.Wang X., Ishida T., Kiwada H. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J Control Release. 2007;119(2):236–244. doi: 10.1016/j.jconrel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 221.Barenholz Y. Doxil(R)–the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 222.Schupp N., Stopper H., Heidland A. DNA damage in chronic kidney disease: evaluation of clinical biomarkers. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/3592042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Abudayyak M., Guzel E., Ozhan G. Nickel oxide nanoparticles induce oxidative DNA damage and apoptosis in kidney cell line (NRK-52E) Biol Trace Elem Res. 2017;178(1):98–104. doi: 10.1007/s12011-016-0892-z. [DOI] [PubMed] [Google Scholar]
- 224.Ranjbar A., et al. Nitrosative DNA damage after sub-chronic exposure to silver nanoparticle induces stress nephrotoxicity in rat kidney. Toxin Rev. 2018;37(4):327–333. [Google Scholar]
- 225.DiRocco D.P., et al. CDK4/6 inhibition induces epithelial cell cycle arrest and ameliorates acute kidney injury. Am J Physiol Renal Physiol. 2014;306(4):F379–F388. doi: 10.1152/ajprenal.00475.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ferenbach D.A., Bonventre J.V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11(5):264–276. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yang L., et al. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16(5):535–543. doi: 10.1038/nm.2144. 1p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kang S.J., et al. Silver nanoparticles-mediated G2/M cycle arrest of renal epithelial cells is associated with NRF2-GSH signaling. Toxicol Lett. 2012;211(3):334–341. doi: 10.1016/j.toxlet.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 229.Cui D., et al. Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol Lett. 2005;155(1):73–85. doi: 10.1016/j.toxlet.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 230.Miller W.G., et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009;55(1):24–38. doi: 10.1373/clinchem.2008.106567. [DOI] [PubMed] [Google Scholar]
- 231.Perkins B.A., et al. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int. 2010;77(1):57–64. doi: 10.1038/ki.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Palmer A.K., et al. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes. 2015;64(7):2289–2298. doi: 10.2337/db14-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 234.Malaquin N., Martinez A., Rodier F. Keeping the senescence secretome under control: molecular reins on the senescence-associated secretory phenotype. Exp Gerontol. 2016;82:39–49. doi: 10.1016/j.exger.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 235.Campisi J., d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 236.Coppe J.P., et al. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kuilman T., et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133(6):1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 238.Loeffler I., Wolf G. Transforming growth factor-beta and the progression of renal disease. Nephrol Dial Transplant. 2014;29(Suppl 1):i37–i45. doi: 10.1093/ndt/gft267. [DOI] [PubMed] [Google Scholar]
- 239.Schnaper H.W., et al. TGF-beta signal transduction and mesangial cell fibrogenesis. Am J Physiol Renal Physiol. 2003;284(2):F243–F252. doi: 10.1152/ajprenal.00300.2002. [DOI] [PubMed] [Google Scholar]
- 240.Davalos A.R., et al. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol. 2013;201(4):613–629. doi: 10.1083/jcb.201206006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lin M., et al. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol. 2012;23(1):86–102. doi: 10.1681/ASN.2010111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Shi H., et al. High mobility group box 1 in diabetic nephropathy. Exp Ther Med. 2017;14(3):2431–2433. doi: 10.3892/etm.2017.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chen X., et al. Blockade of HMGB1 attenuates diabetic nephropathy in mice. Sci Rep. 2018;8(1):8319. doi: 10.1038/s41598-018-26637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Brosius F.C., et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2009;20(12):2503–2512. doi: 10.1681/ASN.2009070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Satriano J., et al. Transition of kidney tubule cells to a senescent phenotype in early experimental diabetes. Am J Physiol Cell Physiol. 2010;299(2):C374–C380. doi: 10.1152/ajpcell.00096.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Verzola D., et al. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2008;295(5):F1563–F1573. doi: 10.1152/ajprenal.90302.2008. [DOI] [PubMed] [Google Scholar]
- 247.Hickson L.J., et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–456. doi: 10.1016/j.ebiom.2019.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lamming D.W., et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335(6076):1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Roos C.M., et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15(5):973–977. doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Adamczyk-Grochala J., Lewinska A. Nano-based theranostic tools for the detection and elimination of senescent cells. Cells. 2020;9(12) doi: 10.3390/cells9122659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Dolgin E. Send in the senolytics. Nat Biotechnol. 2020;38(12):1371–1377. doi: 10.1038/s41587-020-00750-1. [DOI] [PubMed] [Google Scholar]
- 252.Muñoz-Espín D. Nanocarriers targeting senescent cells. Transl Med Aging. 2019;3:1–5. [Google Scholar]
- 253.Papadopoulou-Marketou N., Chrousos G.P., Kanaka-Gantenbein C. Diabetic nephropathy in type 1 diabetes: a review of early natural history, pathogenesis, and diagnosis. Diabetes Metab Res Rev. 2017;33(2) doi: 10.1002/dmrr.2841. [DOI] [PubMed] [Google Scholar]
- 254.Kritmetapak K., et al. Clinical and pathological characteristics of non-diabetic renal disease in type 2 diabetes patients. Clin Kidney J. 2018;11(3):342–347. doi: 10.1093/ckj/sfx111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Huang F., et al. Renal pathological change in patients with type 2 diabetes is not always diabetic nephropathy: a report of 52 cases. Clin Nephrol. 2007;67(5):293–297. doi: 10.5414/cnp67293. [DOI] [PubMed] [Google Scholar]
- 256.Soni S.S., et al. Non diabetic renal disease in type 2 diabetes mellitus. Nephrology (Carlton) 2006;11(6):533–537. doi: 10.1111/j.1440-1797.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 257.Tone A., et al. Clinical features of non-diabetic renal diseases in patients with type 2 diabetes. Diabetes Res Clin Pract. 2005;69(3):237–242. doi: 10.1016/j.diabres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 258.Zhou J., et al. A differential diagnostic model of diabetic nephropathy and non-diabetic renal diseases. Nephrol Dial Transplant. 2008;23(6):1940–1945. doi: 10.1093/ndt/gfm897. [DOI] [PubMed] [Google Scholar]
- 259.Espinel E., et al. Renal biopsy in type 2 diabetic patients. J Clin Med. 2015;4(5):998–1009. doi: 10.3390/jcm4050998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hogan J.J., Mocanu M., Berns J.S. The native kidney biopsy: update and evidence for best practice. Clin J Am Soc Nephrol. 2016;11(2):354–362. doi: 10.2215/CJN.05750515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Anders H.J., et al. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. 2018;14(6):361–377. doi: 10.1038/s41581-018-0001-y. [DOI] [PubMed] [Google Scholar]
- 262.Tervaert T.W., et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21(4):556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 263.An Y., et al. Renal histologic changes and the outcome in patients with diabetic nephropathy. Nephrol Dial Transplant. 2015;30(2):257–266. doi: 10.1093/ndt/gfu250. [DOI] [PubMed] [Google Scholar]
- 264.Oh S.W., et al. Clinical implications of pathologic diagnosis and classification for diabetic nephropathy. Diabetes Res Clin Pract. 2012;97(3):418–424. doi: 10.1016/j.diabres.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 265.Zhu X., et al. [New pathologic classification of diabetic nephropathy (retrospective study of 37 cases)] Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2012;37(2):185–189. doi: 10.3969/j.issn.1672-7347.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 266.Gheith O., et al. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol. 2016;5(1):49–56. [PMC free article] [PubMed] [Google Scholar]
- 267.Nosrati H., et al. New insight about biocompatibility and biodegradability of iron oxide magnetic nanoparticles: stereological and in vivo MRI monitor. Sci Rep. 2019;9(1):7173. doi: 10.1038/s41598-019-43650-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sawamura T., et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386(6620):73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 269.Honjo M., et al. Lectin-like oxidized LDL receptor-1 is a cell-adhesion molecule involved in endotoxin-induced inflammation. Proc Natl Acad Sci U S A, 2003;100(3):1274–1279. doi: 10.1073/pnas.0337528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Dominguez J.H., et al. Anti-LOX-1 therapy in rats with diabetes and dyslipidemia: ablation of renal vascular and epithelial manifestations. Am J Physiol Renal Physiol. 2008;294(1):F110–F119. doi: 10.1152/ajprenal.00013.2007. [DOI] [PubMed] [Google Scholar]
- 271.Li L., Sawamura T., Renier G. Glucose enhances endothelial LOX-1 expression: role for LOX-1 in glucose-induced human monocyte adhesion to endothelium. Diabetes. 2003;52(7):1843–1850. doi: 10.2337/diabetes.52.7.1843. [DOI] [PubMed] [Google Scholar]
- 272.Luo B., et al. LOX-1-targeted iron oxide nanoparticles detect early diabetic nephropathy in db/db mice. Mol Imaging Biol. 2015;17(5):652–660. doi: 10.1007/s11307-015-0829-5. [DOI] [PubMed] [Google Scholar]
- 273.Lin M.M., et al. Development of superparamagnetic iron oxide nanoparticles (SPIONS) for translation to clinical applications. IEEE Trans Nanobioscience. 2008;7(4):298–305. doi: 10.1109/TNB.2008.2011864. [DOI] [PubMed] [Google Scholar]
- 274.Azushima K., Gurley S.B., Coffman T.M. Modelling diabetic nephropathy in mice. Nat Rev Nephrol. 2018;14(1):48–56. doi: 10.1038/nrneph.2017.142. [DOI] [PubMed] [Google Scholar]