Highlights
-
•
Pharmacokinetics (PK) and pharmacodynamics (PD) of cellular therapeutics are intimately associated with the cell viability, homing/retention, interactions, clearance and therapeutic outcomes.
-
•
Cell engineering strategies develop as novel cell formulation technologies to improve the pharmacology of therapeutic cells.
-
•
The major cell engineering approaches are genetic engineering, preconditioning, biomaterial encapsulation, cell surface modification and cell assembly.
-
•
Improved PK/PD framework of cellular therapeutics will hasten the progression of their clinical translation.
Keywords: Cell engineering, Cell formulation technologies, Cell pharmacology
Abstract
Despite the rapid growth of clinical trials for cellular therapy worldwide, their clinical success is still afflicted with formidable challenges demanding conceptual and technological overhaul. Pharmacology, which is conventionally divided into pharmacokinetics (PK) and pharmacodynamics (PD) in drug discovery have emerged as a prominent research direction to elucidate the cell fate and ensure the efficacy and safety of the therapeutic cells. Herein, we concisely present the dilemmas of cellular therapies, the concept of cell pharmacology, and the advances in cell engineering that leverage the cell formulation technologies to modulate cellular PK/PD for development of more cogent and versatile cell-based therapies.
Hurdles confronted by cellular therapeutics
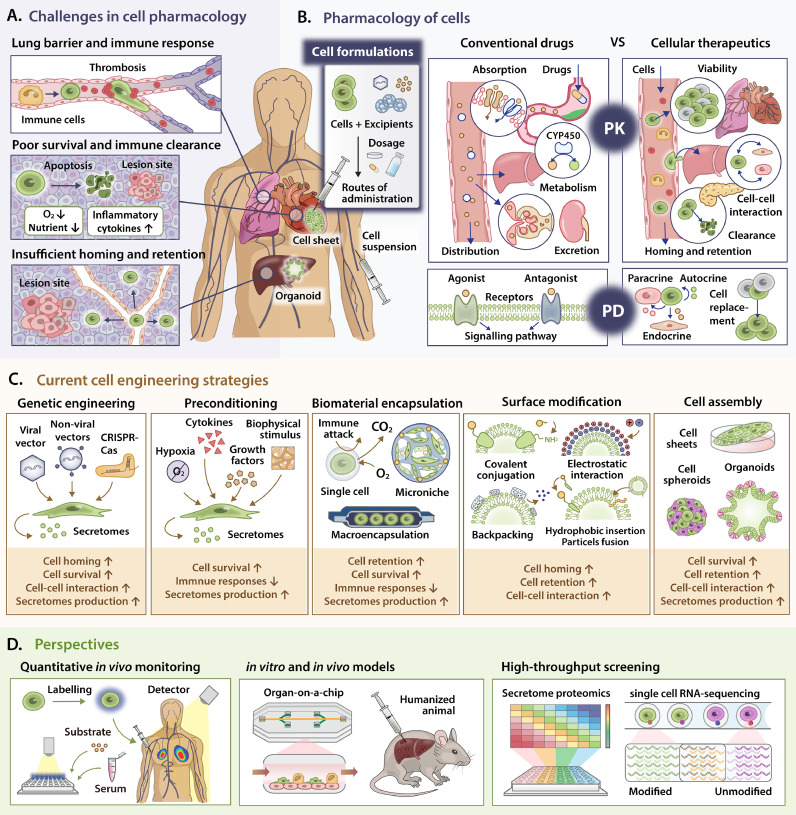
Cellular therapies, which involve a diverse cell types and therapeutic indications, have emerged as alternative modalities to tackle a plethora of refractory diseases, including neurological disorder, graft-versus-host disease, diabetes and cancer. Nevertheless, only limited cellular and gene therapy products have heretofore been approved by FDA. Albeit the therapeutic success substantiated by in vitro and animal studies, the living cellular therapeutics have been less-effective or safe in humans. These suboptimal clinical outcomes are immensely associated with the incompatible pharmacokinetics (PK) and pharmacodynamics (PD) of the therapeutic cells, which have been conventionally applied in drug development to delineate the fate of drugs and their therapeutic effects in vivo, respectively (Fig. 1A). Primarily, therapeutic cells derived from diverse sources and methods show varied gene and protein expression, causing inconsistent cellular interactions (PK) and therapeutic outcomes (PD). Besides, the administration methods primarily used in animal studies will cause impoverished cell survival and inferior therapeutic potency in vivo. During local administration, only 5% of free cells persist at the injection site within hours of post-transplantation and most cells lost their viability due to immune-mediated damage and the odds of hostility within pathological microenvironment [1]. During intravenous delivery, most cells are immediately trapped in the lung capillaries and phagocytosed by monocytes/macrophages within 24 h, denoting that the PK of therapeutic cells does not comply with the criteria of PD as cells are eliminated before exerting their therapeutic functions [1]. Unfortunately, increasing cell dose is disproportionately associated with the therapeutic benefits due to the lung barriers, exemplifying an unmet need for enhanced cell homing. Besides, elevated concentration of procoagulants-like tissue factors expressed by non-hematopoietic cells triggers the instant blood-mediated inflammatory reaction, along with the activation of coagulation and immune cascades, thereby increases the immune clearance of cells [1].
Fig. 1.
Pharmacology of therapeutic cells in vivo can be potentially modulated by various cell engineering techniques. A. Current hurdles confronted by cellular therapeutics after delivery into the human body, which are closely associated with the lung barrier, immune responses, poor cell retention and insufficient cell homing to the target sites. B. The concept of cell formulation and the comparisons of PK and PD between conventional drugs and cellular therapeutics. C. A myriad of cell engineering approaches, including genetic engineering, preconditioning modification, biomaterial encapsulation, surface modification and cell assembly have been established as cell formulation technologies to improve cellular PK/PD by modulating the constituents of cellular products, thus facilitate their therapeutic effects from the prospect of cell viability, retention, homing and interactions. D. Perspectives to further augment the cell function and accelerate clinical translation by taking advantages of quantitative in vivo cell tracking and detection, novel in vitro and in vivo models and high-throughput screening.
Pharmacology of cellular therapeutics
The concept of pharmacology has been widely used in traditional drug development to elucidate the drug efficacy and safety under diverse formulation and dosing regimens. Cell pharmacology can be adapted from conventional principles of molecular pharmaceuticals to quantitatively comprehend the biodistribution and rate-limiting constraints of administered cells in vivo (Fig. 1B). Pharmacology is divided into two broad divisions, namely PK and PD. Briefly, conventional PK delineates the time course of drug absorption, distribution, metabolism and excretion (ADME) in terms of drugs concentration, which are translated into the cell viability, homing/retention, cell-cell interaction and clearance in cellular therapies respectively. Conversely, classical PD describes the relationship between PK of a particular drug and the corresponding drug responses in terms of biochemical interaction, which is ideally translated to the measurable biomarkers representing the therapeutic effects of cellular therapies according to their mechanisms of actions. Indisputably, the bioactive substances to be incorporated in the PK/PD model are unrestricted to the cells themselves, as therapeutic benefits of cell-based medicine are highly subjected to the functions of cell secretomes. In particular, we propose the concept of ‘cell formulation’ which mirrors the drug formulation in pharmaceutical industry, describing the process in which therapeutic cells and other excipients (e.g., biomaterials or bioactive factors) are combined to produce final cellular products with different forms. Therefore, a well-characterized cell formulation in the prospect of pharmacology can facilitate the cell viability, homing/retention and cellular interactions by manipulating the cell properties, administration routes or other affiliated factors, thereby accelerate their clinical translation.
Cell engineering strategies as cell formulation technologies to improve cell pharmacology
In the quest to improve the pharmacology of therapeutic cells, novel cell engineering approaches have been developed as cell formulation technologies to facilitate their therapeutic efficacy (Fig. 1C).
Genetic engineering
Cells are genetically engineered to serve as producers and carriers of biologics by harnessing the gene-editing tools such as CRISPR technology and viral/non-viral vectors. Genetic engineering expands the therapeutic scope of cells by inducing the stable expression of poorly-expressed/non-native proteins with desired functions. Besides, controllable expression of cytotoxic therapeutics at the target site with minimal deleterious effects to normal tissue is achieved by employing an inducible promoter. Briefly, a mechano-responsive cell system was demonstrated under the control of YAP/TAZ promoter, which exogenous prodrugs could be activated by sensing aberrant tissue stiffness in cancer and fibrotic diseases [2]. Another indispensable benefit of genetic engineering includes the introduction of chimeric antigen receptor (CAR) into the autologous T-cells, which present a promising approach in treating malignancies. Recently, genetic modification of CAR or other therapeutic genes is expanded into other immune cells (e.g., natural killer cells, macrophages) to broaden their therapeutic abilities in combating various diseases beyond CAR T-cell-based treatment.
Preconditioning
Preconditioning of cells encompasses the ex vivo treatment with chemical and physical cues to augment the properties of cells against unamiable environments. Particularly, hypoxic priming up-regulates the expression of pro-survival factors HIF-1α in therapeutic cells to assist their adaption to the ischemia [1]. Likewise, treatment with inflammatory cytokines advocates the communication of mesenchymal stem cells (MSCs) with immune system, thereby escapes immune cell-mediated cytotoxicity and improves cellular survival. Adoptively transferred macrophages can be pre-educated with stimulating factors to convey appropriate phenotypes, concerning that macrophage polarization is highly pliable regarding their external cues. In addition, it is progressively appreciated that cells response to mechanics of microenvironment, indicating that the recapitulation of mechano-biology as a key biological cue to govern the cellular PK/PD. As differentiation of stem cells can be managed through myosin-II contractility and focal adhesion, ex vivo culturing of hematopoietic stem cells (HSCs) on soft matrices mimic the marrow elasticity inhibits the contractile force and significantly enriches functional HSCs, thus improving cell potency in vivo [3].
Biomaterial encapsulation
Biomaterial encapsulation provides an amenable armour to shield the cells from mechanical shears and immune attack with tailorable in situ mechanical properties (e.g., stiffness, pore size), thereby guarantee a prolonged cell survival in vivo [1]. Macroencapsulation device is a popular methodology in islet allograft transplantation with innovative designation focusing on biocompatibility, achieving immune escape, and maintaining sufficient nutrient availability. Besides, injectable 3D porous gelatine microcyogels was developed as cell carriers to increase the paracrine secretion and accumulation of the bioactive factors of the encapsulated-MSCs, thus creating a novel cell formulation in maintaining propitious microniche for improved cell survival and therapeutic efficacy [4]. Single-cell encapsulation with alginate microgels allowed systemic administration of cells with extended half-life and biologics secretion, owing to the protection from shears and immune clearance and the higher surface area-to-volume ratio, respectively [5]. Moreover, biomaterial-assisted immune cell delivery provides unprecedented synergies to maintain their long-term therapeutic potency. Macroporous scaffold functionalized with microparticles that release IL-15-IL-15Rα complex and present costimulatory antibodies improved T-cell expansion and egression into immunosuppressive tumour microenvironment, resulting in reduced rate of metastatic relapse [6].
Cell surface modification
Modification of cell membrane functionality through functional moiety impartment has been deciphered to enhance cell-cell interactions. Among them, covalent conjugation is the most forthright method by harnessing the naturally existed functional groups on cell surface. Concisely, functional peptides were bestowed onto the MSCs, inflammatory endothelial cells (ECs) or immune cells whose membrane expresses Transglutaminase 2 via enzyme-catalyzed crosslinking with the surface lysine residue, thus enhancing the cell targeting efficiency to injured tissues [7]. Meanwhile, electrostatic interactions between the negatively charged plasma membrane surfaces with cationic/anionic polymers is extensively employed to shield the cells from shears and immune responses. Tethering the functional groups through spontaneous liposome fusion or hydrophobic insertion rewires the cell surface functions through bio-orthogonal chemistry. Additionally, ‘cellular backpacks’ are designed to attach the cell surface and influence cell functions by tuning their physical properties and payloads. Briefly, IFN-γ-loaded backpacks were attached to macrophage surfaces, demonstrating a favourable release of cytokines to maintain M1 phenotype and potentiate their anti-tumour responses [8].
Cell assembly
Bioengineered tissues which empower the direct implantation of massive cells to the damaged organs has evolved to reconcile the major impediments of cell suspension injection. Cell sheet engineering harvests the monolayer cells (2D) as contiguous stratum along with their deposition of extracellular matrix (ECM) in temperature-responsive culture dishes, thus emulating cell-cell and cell-ECM connections to optimize cell integration in vivo. In vitro fabrication of EC co-cultured multilayer cardiac cells (3D) with perfusable blood vessels facilitated cell vascularization, which is a prerequisite for optimal cell survival [9]. Besides, cells which self-coalesced into a 3D spheroid/organoid recapitulate the intricate morphology and physiological task of nature tissues. Concisely, ex vivo transplantation of cholangiocyte organoids in human liver paves the way for the use of cell-based therapy to augment the graft function and regenerative capacity of bile duct [10].
Perspectives
Notwithstanding the foreknown strategies, innovative in vivo imaging and quantitative detection methods are imperative in determining the distribution, viability and functionalities of therapeutic cells, thus assessing their fate and therapeutic efficacy (Fig. 1D). Besides, characterization of the modified cells with high-throughput screening can provide substantial clues in selecting optimal cell formulations. Moreover, the employment of suitable in vitro and in vivo models can mimic human body response for systematic cellular PK/PD study in designated pathological conditions. Conclusively, improvement in PK/PD framework will enable the production of manageable cell-based therapeutics with desirable risk–benefit ratio, therefore hasten the progression of their clinical translation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to acknowledges the financial supported by Beijing Natural Science Foundation (grant no. JQ18022), the Beijing Municipal Science & Technology Commission (grant no. Z181100001818005).
References
- 1.Levy O., Kuai R., Siren E.M.J., Bhere D., Milton Y., Nissar N., et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6(30):eaba6884. doi: 10.1126/sciadv.aba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu L., Zhang S.X., Liao W., Farhoodi H.P., Wong C.W., Chen C.C., et al. Mechanoresponsive stem cells to target cancer metastases through biophysical cues. Sci Transl Med. 2017;9(400):eaan2966. doi: 10.1126/scitranslmed.aan2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin J.W., Buxboim A., Spinler K.R., Swift J., Christian D.A., Hunter C.A., Léon C., Gachet C., Dingal P.C., Ivanovska I.L., Rehfeldt F., Chasis J.A., Discher D.E. Contractile forces sustain and polarize hematopoiesis from stem and progenitor cells. Cell Stem Cell. 2014;14(1):81–93. doi: 10.1016/j.stem.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y., Liu W., Liu F., Zeng Y., Zuo S., Feng S., et al. Primed 3D injectable microniches enabling low-dosage cell therapy for critical limb ischemia. Proc Natl Acad Sci. 2014;111(37):13511–13516. doi: 10.1073/pnas.1411295111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao A.S., Shin J.-W., Utech S., Wang H., Uzun O., Li W., et al. Deterministic encapsulation of single cells in thin tunable microgels for niche modelling and therapeutic delivery. Nat Mater. 2017;16(2):236–243. doi: 10.1038/nmat4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephan S.B., Taber A.M., Jileaeva I., Pegues E.P., Sentman C.L., Stephan M.T. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat Biotechnol. 2015;33(1):97–101. doi: 10.1038/nbt.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi C., Jin Y., Chen Y., Li W., Li Y., Liang K., et al. TGase-mediated cell membrane modification and targeted cell delivery to inflammatory endothelium. Biomaterials. 2020 doi: 10.1016/j.biomaterials.2020.120276. [DOI] [PubMed] [Google Scholar]
- 8.Shields C.W., Evans M.A., Wang L.L.-W., Baugh N., Iyer S., Wu D., et al. Cellular backpacks for macrophage immunotherapy. Sci Adv. 2020;6(18):eaaz6579. doi: 10.1126/sciadv.aaz6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekine H., Shimizu T., Sakaguchi K., Dobashi I., Wada M., Yamato M., et al. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun. 2013;4(1):1399. doi: 10.1038/ncomms2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampaziotis F., Muraro D., Tysoe O.C., Sawiak S., Beach T.E., Godfrey E.M., et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science. 2021;371(6531):839. doi: 10.1126/science.aaz6964. [DOI] [PMC free article] [PubMed] [Google Scholar]