Abstract
Premise
Daffodils (Narcissus, Amaryllidaceae) are iconic ornamentals with a complex floral biology and many fragrant species; however, little is known about floral plant volatile organic compounds (pVOCs) across the genus and additional sampling is desirable. The present study investigates whether the floral scent of 20 species of Narcissus can be characterized using gas chromatography–coupled ion mobility spectrometry (GC–IMS), with the aim of building a comparative pVOC data set for ecological and evolutionary studies.
Methods
We used a commercial GC–IMS equipped with an integrated in‐line enrichment system for a fast, sensitive, and automated pVOC analysis. This facilitates qualitative and (semi)‐quantitative measurements without sample preparation.
Results
The GC–IMS provided detailed data on floral pVOCs in Narcissus with very short sampling times and without floral enclosure. A wide range of compounds was recorded and partially identified. The retrieved pVOC patterns showed a good agreement with published data, and five “chemotypes” were characterized as characteristic combinations of floral volatiles.
Discussion
The GC–IMS setup can be applied to rapidly generate large amounts of pVOC data with high sensitivity and selectivity. The preliminary data on Narcissus obtained here indicate both considerable pVOC variability and a good correspondence of the pVOC patterns with infrageneric classification, supporting the hypothesis that floral scent could represent a considerable phylogenetic signal.
Keywords: benzyl acetate, ion mobility spectrometry, linalool, Narcissus, ocimene, plant volatile organic compounds
Most angiosperms depend on the help of other organisms (mainly insects) for successful cross‐pollination. Flowers therefore show a range of traits relevant to these plant–animal interactions, including shape, color, reward system, nectar composition, flowering time, and fragrance, all of which are the result of complex evolutionary processes (Bouwmeester et al., 2019).
Pollination biology tends to focus on morphology, reward, and breeding systems, but the complex role of floral plant volatile organic compound (pVOC) emissions in plant–pollinator interactions remains comparatively poorly understood (Raguso, 2008; Burkle and Runyon, 2019). However, some researchers have closely investigated floral fragrance, and demonstrated its evolutionary importance in pollinator attraction or repulsion (e.g., Dötterl et al., 2005; Chess et al., 2008; Waelti et al., 2008; de Vega et al., 2014).
The analysis of pVOCs usually involves the use of appropriate adsorption techniques (i.e., solid‐phase microextraction) for sampling and subsequent gas chromatography–mass spectrometry (GC–MS) measurements (Materić et al., 2015; Tholl et al., 2020). However, GC–MS instruments are bulky and expensive, and sample collection and subsequent analyses tend to be both time consuming and spatially separated, rendering the detection and analysis of fragrances under natural conditions challenging (Tholl et al., 2006). For an improved understanding of the ecological role of pVOCs in general and floral scent in particular, a faster and highly time‐resolved technique for scent analyses would be highly desirable. The use of ion mobility spectrometry (IMS) is likely to address challenges such as small sample sizes, and we therefore investigated its potential for floral pVOC detection in the present study.
IMS is a gas‐phase analytical method for the fast (few seconds) and sensitive (at the level of a few parts per billion by volume [ppbv] down to parts per trillion by volume [pptv]) detection of VOCs. In stand‐by mode, a β‐radiation source is commonly used to ionize the drift gas flushing through the IMS, forming the so‐called reactant ions in the ionization region (protonated water clusters). If a sample with other gas‐phase compounds is introduced into the ionization region, the analyte molecules are ionized mainly by proton transfer. The ions are accelerated toward the detector in a weak electric field, but only clouds of ions are periodically introduced into the drift region by a Bradbury–Nilsson ion grid. During their drift from the grid to the detector, the ions collide with the molecules of the drift gas counterflow. The collision frequency depends on the size and shape of the ions, thus influencing the drift velocity. The drift velocity can be determined by measuring the drift time of the particular ions under a known drift length, and the ion mobility is obtained by normalization to the electric field strength (Eiceman et al., 2016; Gabelica and Marklund, 2018; Dodds and Baker, 2019). Further normalization to pressure and temperature obtains the reduced ion mobility, which is specific to a particular analyte and independent of the instrumentation. Coupling fast gas chromatographic pre‐separation to IMS provides a characteristic retention time as an additional parameter for the identification of the analyte, and furthermore avoids the clustering of different analytes in very complex mixtures (Vautz et al., 2018).
In order to investigate the applicability of GC–IMS for floral scent analyses, we selected the ornamental and fragrant daffodils (Narcissus L., Amaryllidaceae). Narcissus is a genus of 35 to 70 species of geophytes, widely distributed in the Mediterranean basin (Kubitzki, 2014). This genus has been formally subdivided into two subgenera, Hermione (Haw.) Spach and Narcissus, and a total of 10 sections (Marques et al., 2017), but details of the phylogenetic relationships are incompletely resolved (Marques et al., 2010). The flowers of many species are overwhelmingly fragrant and have been the source of perfumes for millennia, with N. tazetta L. and N. poeticus L. still widely used in the fragrance industry for the production of exclusive “absolutes” (Remy, 2002). The floral function and ecology of Narcissus have been widely studied over the past two decades (Barrett et al., 2004; Cesaro et al., 2004; Pérez et al., 2004; Barrett and Harder, 2005; Medrano et al., 2005; Hodgins and Barrett, 2006, 2008; Pérez‐Barrales et al., 2006; Pérez‐Barrales and Arroyo, 2010; Navarro et al., 2012; Simón‐Porcar et al., 2014), and the relevance of different pollinators and pollinator guilds has been studied for a range of species (Arroyo and Dafni, 1995; Marques et al., 2007, 2016, 2017; Marques and Draper, 2012; Santos‐Gally et al., 2013). As scent is likely to play a major role in floral ecology of Narcissus, it is surprising that there has been only one major study of fragrance covering nine species (Dobson et al., 1997), along with a few smaller studies of individual species and hybrids with a more commercial approach (Chen et al., 2013; Ruíz‐Ramón et al., 2014; Terry et al., 2020).
Overall, the chemical diversity of Narcissus floral scent remains poorly studied. A more comprehensive understanding of scent chemistry in this intriguing genus is highly relevant to its complex floral biology and associated evolutionary questions. As a first step, the present study aims to investigate the utility of GC–IMS for rapidly generating data sets on pVOC emissions and expanding our understanding of floral fragrance in Narcissus by investigating 20 different species (from both subgenera and a total of seven sections). We aim to address the following questions:
-
1.
Can GC–IMS be successfully applied for the analysis of floral volatiles in Narcissus?
-
2.
What are the advantages of using GC–IMS in comparison with classical analytical methods (GC–MS)?
-
3.
How do pVOC patterns differ between different species of Narcissus?
METHODS
Plant material
The plant material was provided by the Bonn University Botanic Gardens (University of Bonn, Germany). All individual plants of a species originate from the same accession. The plants were potted in clay pots and grown in an unheated greenhouse (alpine house) until flowering. The flowering period depends on the species, ranging from September (N. broussonetii Lag.) to April (N. pseudonarcissus L.). We investigated 20 species from seven sections, covering a wide range of phenotypic variations (Table 1, Appendix S1). Between two and five individual plants were investigated per species.
Table 1.
Overview of the examined species from the genus Narcissus as well as their division into the corresponding subgenera and sections. All of the species came from the collection of the Botanical Gardens in Bonn, Germany, and are listed with their corresponding accession numbers.
| Entry | Species | Section | Subgenus | N a | Accession |
|---|---|---|---|---|---|
| 1 | N. broussonetii | Aurelia | Hermione | 3 | 4379 |
| 2 | N. obsoletus | Serotini | Hermione | 3 | 15270 |
| 3 | N. serotinus | Serotini | Hermione | 4 | 22916 |
| 4 | N. elegans | Tazettae | Hermione | 3 | 15255 |
| 5 | N. papyraceus | Tazettae | Hermione | 4 | 15245 |
| 6 | N. tazetta | Tazettae | Hermione | 2 | 9150 |
| 7 | N. calcicola | Apodanthi | Narcissus | 4 | 31788 |
| 8 | N. cantabricus subsp. monophyllus | Bulbocodii | Narcissus | 4 | 5347 |
| 9 | N. cantabricus subsp. foliosus | Bulbocodii | Narcissus | 3 | 15175 |
| 10 | N. romieuxii | Bulbocodii | Narcissus | 4 | 9147 |
| 11 | N. hedraeanthus | Bulbocodii | Narcissus | 2 | 37898 |
| 12 | N. graellsii | Bulbocodii | Narcissus | 2 | 31791 |
| 13 | N. (bulbocodium var.) obesus | Bulbocodii | Narcissus | 5 | 36469 |
| 14 | N. assoanus | Jonquillae | Narcissus | 4 | 31784 |
| 15 | N. fernandesii | Jonquillae | Narcissus | 4 | 31790 |
| 16 | N. jonquilla | Jonquillae | Narcissus | 4 | 7798 |
| 17 | N. viridiflorus | Jonquillae | Narcissus | 4 | 35144 |
| 18 | N. asturiensis | Pseudonarcissi | Narcissus | 4 | 31786 |
| 19 | N. pseudonarcissus | Pseudonarcissi | Narcissus | 4 | 31409 |
| 20 | N. pseudonarcissus | Pseudonarcissi | Narcissus | 2 | 3099b |
No. of individual plants.
No accession number is available, so a herbarium voucher is provided.
Instruments
The measurements were performed using a mobile ppq‐tec GC–IMS (ION‐GAS, Dortmund, Germany) based on hardware provided by STEP (Pockau‐Lengefeld, Germany). For increased sensitivity, the commonly used sample loop (1 mL) was substituted with a micro‐electro‐mechanical systems–based in‐line pre‐concentration chip filled with Carbograph 4 as the adsorbent (CNR‐IMM, Bologna, Italy) (for detailed description, see Liedtke et al., 2019). For the GC pre‐separation, we used a MXT‐200 capillary column (30 m × 0.53 mm, 1.5 µm coating; Restek, Centre County, Pennsylvania, USA), which is operated isothermally at 80°C, with a carrier gas flow (filtered air from the internal gas circuit) of 21 mL min−1.
Ionization was performed using tritium as the source of β‐radiation (100 MBq). The drift length of the IMS was 5.61 cm. The drift tube was operated isothermally at 70°C at a field strength of 300 V cm−1.
The technical data and the experimental setup of the GC–IMS are summarized in Appendix S2.
pVOC sampling and analysis
The sampling (enrichment) and subsequent analysis were performed in‐line under daylight conditions (between 1000 and 1700 hours) in a ventilated room at ambient temperature. Measurements of ambient air were performed prior to each series of measurements to identify the possible presence of interfering substances in the background. To sample the pVOCs from individual plants, the sample inlet of the GC–IMS (PFA tubing; Bohlender, Grünsfeld, Germany) was positioned in proximity to the flower (~2 cm to the corona; no flower enclosure). A sample volume of 10 mL (few seconds) was pulled through the chip by the internal sample pump, where analyte molecules were adsorbed. The sample volume can be adjusted, but for the Narcissus species this small sample volume was sufficient. The analyte molecules are thermally desorbed (50°C for 5 s, after which the temperature was ramped to 290°C in 5 s and was held at 290°C for 7 s). The volatile analyte molecules were then transported into the GC–IMS for analysis.
Each individual plant was measured three times. After each measurement, the pre‐concentrator chip was automatically purged (290°C for 1 min) to eliminate carryover.
pVOC identification
In general, GC–IMS cannot identify unknown substances, but the combination of GC retention time and ion mobility renders identification possible via a comparison to an in‐house substance database. This database was generated by the analysis of pure reference compounds (purchased from Merck, Darmstadt, Germany, and Carl Roth, Karlsruhe, Germany) and by comparison of the Kovats retention indices (RI) to a series of n‐ketones, combined with complementary GC–MS (Appendix S3). The sampling for the GC–MS measurements was conducted using adsorption needles (NeedleTraps; PAS Technology, Magdala, Germany), which were filled with a combination of three different sorbents (Carbopack B, 1 cm; Carboxen 1016, 1 cm; and Carboxen 1000, 1 cm; Sigma‐Aldrich, St. Louis, Missouri, USA) to ensure the adsorption of the entire expected range of pVOCs.
Reference compounds of common floral volatiles in the genus Narcissus were selected based on data from the literature (Dobson et al., 1997; Marques et al., 2016) and the complementary GC–MS measurements.
Data evaluation and visualization
Data processing, evaluation, and visualization of the heatmaps were performed with IONysos, a custom software developed by ION‐GAS. Each heatmap consists of the GC retention time, the relative drift time, and a color‐coded signal intensity. The drift time (t D,rel) was normalized to the reactant ion peak, which is proportional to the inverse reduced ion mobility (Vautz et al., 2009). The recorded spectra of Narcissus were compared against background measurements. The signals from the samples were manually selected, and the signal intensity was calculated by peak integration (peak volume). In case of a superimposition over the background signals, the background measurements were subtracted from the sample measurements.
The mean signal intensities for a species (2–5 individual plants per species) were calculated from the median signal intensities of three technical replicates. The significance of differences in the number of signals across species was tested with the non‐parametric Kruskal–Wallis test.
A further statistical analysis was conducted using the open‐source software R (version 4.1.0; R Core Team, 2022). For the visualization of interspecific pVOC profiles, we calculated the ratio of each signal within a species in relation to the strongest signal. This facilitates a better visualization of the data without affecting the data integrity, as the ratios are maintained. The pVOC profiles were visualized with the “ComplexHeatmap” package.
The similarity of the intraspecific emission patterns was evaluated using Pearson's correlation for the pairwise comparison of plant individuals at a confidence level of 0.95 (cf. Kriegeskorte et al., 2008), using the Hmisc and corrplot packages. We used a similar approach to examine the interspecific similarities of the emission patterns; however, because the overall intensity and composition of signals differs considerably between species, we used a non‐parametric approach. Each signal of a respective species was therefore ranked according to its intensity. By performing pairwise comparisons of these ranks, we generated a correlation matrix (Spearman correlation) for the interspecific comparison of emission patterns.
The dissimilarity matrix computed from this (1 minus the correlation between species) served as the distance matrix in hierarchical clustering (cf. Hasan Ali et al., 2021). To perform the agglomeration, we employed the widely used Ward's error sum of squares algorithm (Ward.D2).
RESULTS
GC–IMS can be applied to the scent analysis of floral volatiles in Narcissus
We were able to record pVOC emission patterns for all species studied using GC–IMS. The use of the two separation levels (ion mobility and retention time) resulted in a full separation of the floral bouquet and, with the exception of benzaldehyde, no interferences with the background. By following the sampling protocol (sample volume: 10 mL, total analysis time: 10–30 min), we detected a total of 64 distinct signals across the species studied. Furthermore, several signals could be identified by a comparison of the characteristic ion mobility and retention time with our in‐house database. The complete list of detected signals and the corresponding parameters (relative drift time and retention time) and representative reference spectra for the studied species are provided in Appendix S4.
The measurements provided characteristic emission patterns for the studied species, and the intraspecific emission patterns were found to be highly consistent (Figure 1, Appendix S5), with no significant differences between almost all the individual plants of a species (Pearson correlation >0.8, P < 0.05). For N. asturiensis (Jord.) Pugsley, N. graellsii Webb ex Graels, and N. hedraeanthus Colmeiro, no significance test could be performed due to the low number of detected signals (<5 signals), but the emission patterns were highly congruent (Pearson correlation >0.95). Despite the high degree of similarity in terms of the emission patterns, we found considerable differences in the total signal intensity; in some cases, the standard deviations reached up to 70% of the mean value between individual plants of the same species (Appendix S6).
Figure 1.
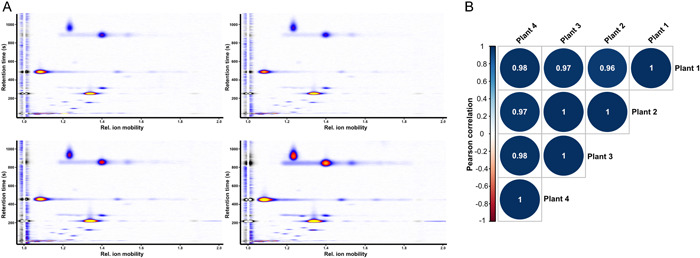
Comparison of the intraspecific pVOC profiles of four individual plants from the species Narcissus fernandesii (section Jonquillae). (A) Heatmap visualization of the detected signals obtained from the GC–IMS measurements, showing the general agreement of emission patterns. The retention time (y‐axis) and relative ion mobility (x‐axis) provide information about the identity of a substance. The signal intensity is color coded, with blue for low intensity, red/orange for medium intensity, and yellow for high intensity. The signal intensity is a semi‐quantitative measure for the abundance of a substance. (B) Correlation matrix (Pearson correlation) of the four individual plants. The similarity between the pVOC profiles is indicated by 1 (dark blue) for a perfect correlation, 0 (white) for no correlation, and −1 (dark red) for a perfect anticorrelation (P = 0.01).
For the interspecific pVOC compositions, we found striking diversity. The signal count and the complexity of the pVOC profiles varied significantly across the species (Kruskal–Wallis test, P < 0.001), with a mean signal count of 8.76 per species. In N. viridiflorus Schousb., 16 different substances were detected, including (E)‐β‐ocimene, eucalyptol, benzyl acetate, phenethyl acetate, p‐cresol, and dl‐α‐terpineol. Conversely, in N. asturiensis, only three substances could be detected, with 3,5‐dimethoxytoluene as the main component and lilac aldehydes A and B as trace compounds. In addition to the number of detected signals, there were also differences in the composition of the emission patterns of the different species (Figure 2). Overall, the emission patterns were species‐specific but sometimes differed in nuances of relative composition (e.g., species from the section Bulbocodii DC. had low emission rates overall). We also found unique compounds that were characteristic for particular species (N. jonquilla L. and N. viridiflorus). We identified three principal compounds in the pVOC profiles that were detected individually or in combinations in high abundance in almost all species: the terpenoids (E)‐β‐ocimene and linalool were detected in 90% and 80% of the species, respectively, while the aromatic compound benzyl acetate was detected in 45% of the species. This observation is consistent with previous studies by Dobson et al. (1997) and Marques et al. (2016), who found the same principal substances using much smaller species samples.
Figure 2.
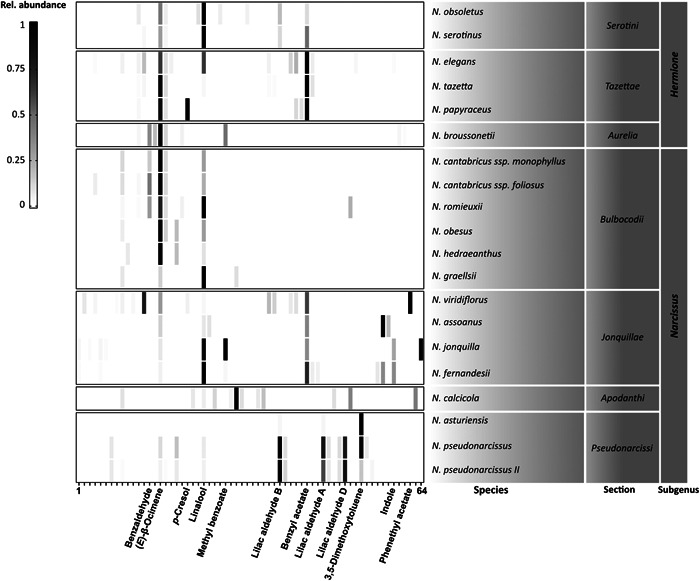
Distribution and relative abundance of the 64 detected signals in the 20 studied taxa of Narcissus. The x‐axis represents the detected signals (ordered by increasing retention time). The main identified compounds are named. The color code represents the abundance of the compound relative to the strongest signal detected in that species, where 1 (black) represents the strongest signal in a species and 0 (white) indicates the absence of a certain signal. The species were grouped based on previous phylogenetic divisions (Fernandes, 1975).
Relative contribution of the principal compounds
The relative contribution of the individual substances to the overall floral scent differed dramatically between the taxa studied. Usually, only a small number of compounds are responsible for the bulk of the emissions, while the majority of the signals detected are present in low concentrations. The composition of the principal compounds partially reflects infrageneric taxonomy (i.e., assignment to sections; Figure 3). (E)‐β‐ocimene, linalool, and benzyl acetate dominated the floral scent of five of the six species studied from the subgenus Hermione, often in a combined relative abundance of ≥70%. Narcissus broussonetii is the only exception in the subgenus Hermione, as it lacks both linalool and benzyl acetate. The subgenus Hermione shares the presence of the floral scent compound benzyl acetate with section Jonquillae DC. from the subgenus Narcissus. The section Jonquillae also has linalool and (E)‐β‐ocimene, although at lower proportions; however, other substances, some of which are still to be identified, contribute more to the bouquet as a whole.
Figure 3.
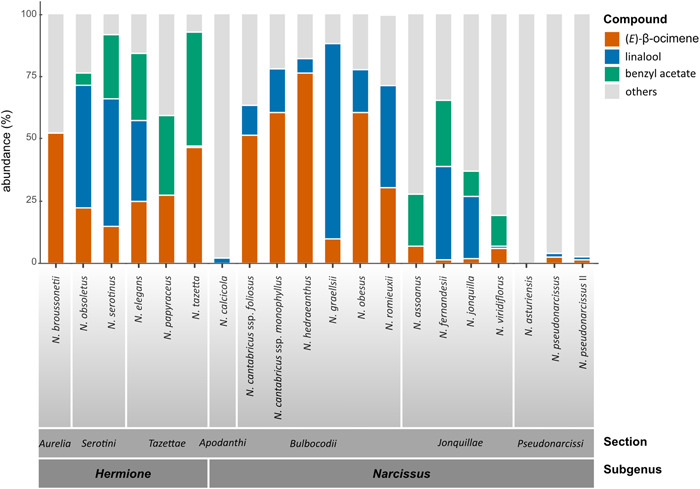
Stacked bar chart of the percentage distribution of the principal compounds among the studied species of Narcissus. The distribution is indicated by different colors for (E)‐β‐ocimene (orange), linalool (blue), benzyl acetate (green), and the sum of other compounds (gray).
The floral scent of the section Bulbocodii (“petticoat daffodils”) is dominated by linalool and (E)‐β‐ocimene, with these two compounds making up the bulk of the compounds detected (up to 80% in N. graellsii). Benzyl acetate was not detected in this section. In N. calcicola Mendonça (section Apodanthi) and N. pseudonarcissus (section Pseudonarcissi DC.), only traces of linalool and (E)‐β‐ocimene were detected; these substances were not detected at all in N. asturiensis (section Pseudonarcissi).
Comparison of pVOC profiles
The computed Spearman correlation matrix for the quantification of interspecific differences revealed remarkable differences between the pVOC patterns of the studied species (Figure 4). Some species showed significant similarity regarding their pVOC profiles (e.g., species from the sections Bulbocodii or Jonquillae, or from the subgenus Hermione). By contrast, the pVOC profiles of other species, such as N. calcicola, showed a low similarity with the other species studied. The same is applicable to the rather unique emission patterns of species from the section Pseudonarcissi (N. pseudonarcissus and N. asturiensis).
Figure 4.
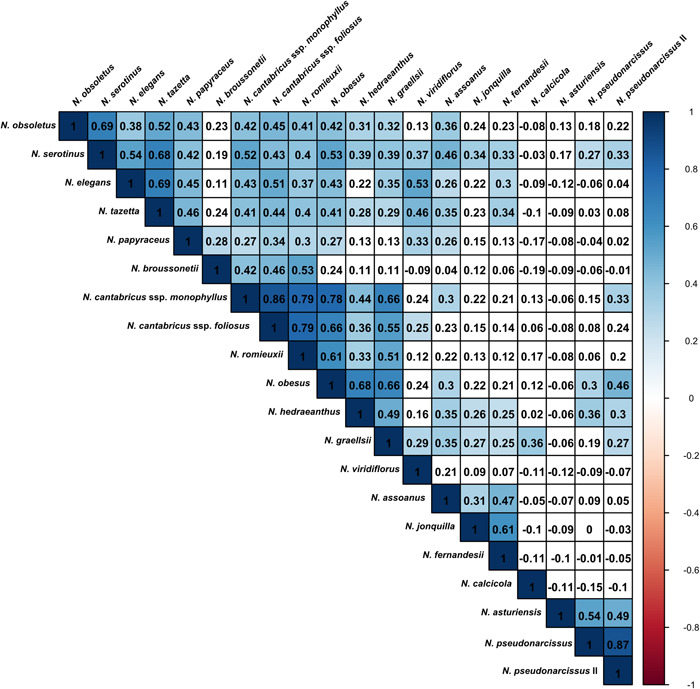
Comparison of the pVOC profiles for each pair of species in a correlation matrix, using Spearman's rank correlation. The similarity between the pVOC profiles is indicated by 1 for a perfect correlation, 0 for no correlation, and −1 for a perfect anticorrelation. The strength of the significant correlations (P = 0.05) is color coded, with blank cells representing no significant correlation. Each cell of the correlation matrix therefore compares the compositions of the detected pVOCs between two species.
To investigate whether particular scent compositions can be grouped according to similarity and if the resulting clusters in any way reflect the phylogenetic relatedness of the taxa involved, we applied a hierarchical clustering algorithm (Murtagh and Contreras, 2012) (Figure 5). Five clusters were retrieved, which largely coincide with the currently accepted infrageneric classification of the daffodils (Fernandes, 1975; Marques et al., 2017). Cluster 1, comprising three of the four species from the section Jonquillae, and Cluster 3, comprising the subgenus Hermione sections Tazettae + Serotini and N. viridiflorus from the section Jonquillae, were retrieved as distinct but similar clusters. Narcissus calcicola from section Apodanthi was retrieved as sister to Cluster 4, which comprises section Bulbocodii, here including N. broussonetii from subgenus Hermione section Aurelia as a sister group. The weakly scented section Pseudonarcissi formed Cluster 5, which was clearly differentiated from all other groups. The different taxonomic subgroups of Narcissus are here retrieved more or less unequivocally in the cluster analysis, indicating that there is some degree of conservation in the floral scents of the individual clades.
Figure 5.
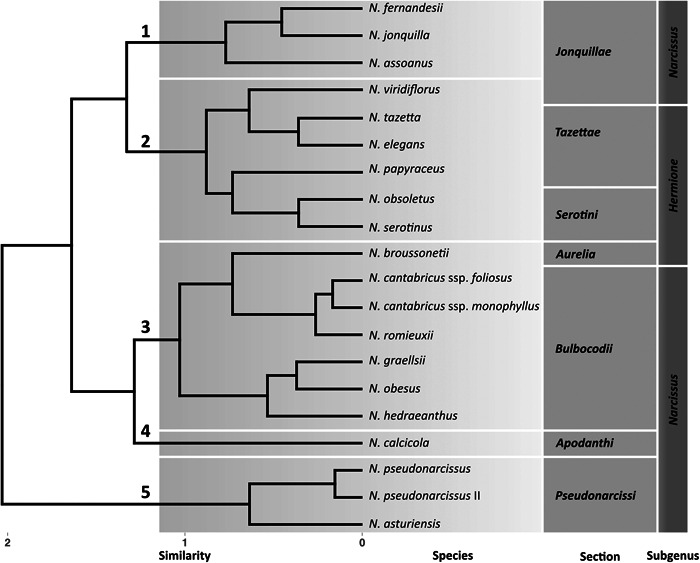
Hierarchical cluster dendrogram of the 20 investigated taxa of Narcissus based on the similarity of their pVOC profiles. The distance matrix is calculated as 1 minus the Spearman's rank correlation (dissimilarity matrix). For the agglomeration, the widely used Ward.D2 algorithm was applied. Five clusters (chemotypes) were retrieved, representing the likeness between the emission patterns.
DISCUSSION
Can GC–IMS be successfully applied to a scent analysis of floral volatiles in Narcissus?
In the present study, we demonstrated that GC–IMS is a rapid, sensitive, and selective, yet technically simple, method to use for the sampling and analysis of the pVOC emissions of daffodil flowers. Even at a small sample volume of only 10 mL, we were able to detect characteristic pVOC patterns in the investigated species, underscoring the high sensitivity of GC–IMS. In addition, the resolution was sufficient to fully separate the pVOCs in heavily scented daffodils. The identification of several substances demonstrates the possibility of identifying compounds based on their characteristic parameters (e.g., retention time, relative drift time). The main advantage, however, was the possibility for rapid measurements with relatively small effort (no sample preparation) and no additional material required. The in‐line sampling and analysis meant the data acquisition only took between 10 and 30 minutes (depending on the pVOC composition) from the initial enrichment to the emission pattern (heatmap) of an individual plant. Thus, GC–IMS enables the performance of high‐throughput measurements for cross‐species and within‐species comparisons of pVOC patterns.
For the majority of the species studied here, this is the first comprehensive data set of their pVOC compositions or pVOC profiles, and we can only make limited comparisons to data in the literature. Published scent data (identified with GC–MS) are available for N. assoanus Dufour, N. jonquilla, N. serotinus L., and N. papyraceus Ker Gawl. (Dobson et al., 1997), and for N. elegans Spach and N. serotinus (Marques et al., 2016). Our data are in good agreement with those of Dobson et al. (1997) regarding the principal compounds, such as (E)‐β‐ocimene and benzyl acetate. Also, the reported emissions of p‐cresol by N. papyraceus or methyl benzoate by N. jonquilla are consistent with our data (Dobson et al., 1997), even if the relative abundance of p‐cresol was comparatively higher in our case. Furthermore, we found good agreement with the data published by Marques et al. (2016) on the floral scents of N. serotinus and N. elegans, which also highlighted (E)‐β‐ocimene, linalool, and benzyl acetate as leading compounds. Dobson et al. (1997) and Marques et al. (2016) reported a number of minor components (<1% of the total emissions) that we have not yet been able to confirm. The differences between the published scent profiles recorded and our results may be partly explained by some of the minor peaks we detected but have not yet been able to identify. Also, there is a high likelihood that scent shows ecotypic variation in Narcissus, as has been demonstrated for many other plant groups (Delle‐Vedove et al., 2017). The studies of Dobson et al. (1997) and Marques et al. (2016) used different plant materials from those used in our study. Furthermore, we used a very small sample volume (10 mL over a few seconds for enrichment). Although GC–IMS is able to detect substances in the concentration range of ppbv to pptv, it is conceivable that some substances are below the limit of detection for such small sample volumes.
What are the advantages of GC–IMS compared with the classical analytical method (GC–MS)?
The classical GC–MS approach is a powerful technique for the comprehensive identification and quantification of volatile organic compounds; however, GC–MS is restricted to laboratory use and requires elaborate data analysis. This usually requires the spatial separation of the sampling and subsequent analysis, potentially compromising the time‐resolution and sample size. In‐line methods such as proton transfer reaction–mass spectrometry (PTR–MS) address this issue and are able to perform real‐time gas analysis with high mass resolution (Danner et al., 2012) at the cost of a limited selectivity: substances with an identical nominal mass (e.g., many monoterpenes) cannot be separated.
The GC–IMS technique used here combines sampling (enrichment) and subsequent analysis, thereby eliminating spatial separation and allowing automated in‐line measurements to be taken with short intervals, while the orthogonal separation principles provide the required selectivity. There is no need for time‐consuming sample preparation, and the integrated pre‐concentrator chip obviates the need for the spatial separation of sampling and analyses. Furthermore, the orthogonal separation principles distinguish this method from other mobile applications such as mobile GC or electronic noses. It is possible to build substance libraries, and once a compound (peak) is referenced in the GC–IMS substance library no additional analytical steps are required for identification, making it possible to automatically evaluate measurements.
Thus, GC–IMS can be used to obtain selective, highly sensitive, and highly time‐resolved pVOC profiles to investigate and compare the floral fragrances of different individuals, different populations, and different subspecies, or those of the same individual plant, dramatically improving the volume of data available for ecological, physiological, or systematic studies. This addresses one crucial shortcoming of most published pVOC analyses: the very low number of samples. In the present study, we were primarily limited by the number of open flowers available at any given time, not by technical constraints.
How do pVOC patterns differ between Narcissus species?
Our GC–IMS data for 20 species of Narcissus indicate that the bouquet is taxon‐specific. Most of the detected and identified pVOCs corresponded to terpenoids, especially (E)‐β‐ocimene and linalool, and phenylpropanoids/benzenoids, with benzyl acetate as the most abundantly prominent. These are rather typical components of floral scent across many plant groups. (E)‐β‐ocimene, for example, has been reported in approximately 60% of all plant families studied (Farré‐Armengol et al., 2017).
Nevertheless, cluster analysis retrieved five clusters of pVOC profiles that could be roughly translated into chemotypes. Cluster 1 (Figure 5) represents the most complex chemotype and comprises three of the four representatives from section Jonquillae. This cluster displays a large number of peaks with considerable interspecific variability. Large amounts of benzyl acetate were detected in all species, with linalool and (E)‐β‐ocimene as the other two principal compounds. These three compounds make up less than 50% of the total emissions (>50% only in N. fernandesii Pedro). Furthermore, considerable amounts of methyl benzoate could be detected in N. jonquilla, and indole could be detected in N. jonquilla and N. fernandesii. Species from the sections Tazettae and Serotini (subgenus Hermione), as well as N. viridiflorus from section Jonquillae, were contained in Cluster 2. Species in this cluster are characterized by the presence of (E)‐β‐ocimene, linalool, and benzyl acetate as the principal compounds (>50% of pVOCs). Small amounts of eucalyptol were also detected in N. serotinus, N. tazetta, and N. elegans. Narcissus papyraceus had high emissions of p‐cresol, a substance mainly associated with feces, but lacked linalool. The peculiar green‐flowered N. viridiflorus largely lacked linalool; instead, considerable amounts of eucalyptol and phenethyl acetate were detected together with traces of p‐cresol.
Cluster 3 is characterized by a dominance of (E)‐β‐ocimene and linalool in the rather weak floral scent. This cluster contains all species examined from the section Bulbocodii (“petticoat daffodils”), as well as N. broussonetii from the section Aurelia (subgenus Hermione). The pVOC patterns are relatively homogenous, differing primarily in the relative contribution of the two principal compounds. For N. romieuxii Braun‐Blanq. & Maire and N. cantabricus DC. (both subspecies), we also detected characteristic emissions of benzaldehyde, which these species share with N. broussonetii (section Aurelia) from the subgenus Hermione; however, benzyl acetate was not detected in this chemotype. Narcissus calcicola (section Apodanthi) is somewhat isolated in Cluster 4, with rather weak overall emissions. Linalool and (E)‐β‐ocimene were detected in trace amounts, and benzyl acetate was absent. The bulk of the pVOC profile in Cluster 4 is made up of unidentified compounds not found in the other species investigated here. Cluster 5 includes the three samples of section Pseudonarcissi. Lilac aldehydes and 3,5‐dimethoxytoluene were characteristic pVOCs for this cluster, while the principal substances found in the other clusters appear to be largely or completely absent.
Floral scent is a highly variable and complex mixture of organic molecules, which appears to be a rapidly evolving floral trait (Dudareva et al., 2013). Floral fragrance has been hypothesized to be a weak phylogenetic signal (Schiestl, 2010; Schiestl and Dötterl, 2012). The present study comprises less than 50% of the currently recognized species of the genus Narcissus, but we detected a considerable phylogenetic signal in the pVOC profiles of the taxa studied here. The patterns largely reflect the infrageneric classification of Narcissus, indicating some degree of phylogenetic constraint on the chemical signals in this plant group.
CONCLUSIONS
Our data show that GC–IMS is a rapid and reliable technology that can be used to obtain floral pVOC data. The study presented here was geared toward generating a reference data set for the genus Narcissus. Future studies should be directed toward expanding the pVOC analyses to the remaining species and integrating these data with floral function (i.e., morphology and reward) and pollinator data, in order to come to an integrated understanding of flower ecology and the evolution of flower biology in this iconic ornamental. At present, the identification of the signals detected (peaks) with GC–IMS remains a challenge. No public databases for GC–IMS are currently available, but this problem will gradually be solved as more reference libraries are made available. In the present study, the GC–MS reference measurements enabled the identification of (E)‐β‐ocimene, linalool, benzyl acetate, p‐cresol, methyl benzoate, phenethyl acetate, lilac aldehydes (A, B, and D), 3,5‐dimethoxytoluene, dl‐α‐terpineol, and indole. Furthermore, measurements of authentic standards were used to identify or confirm the presence of benzaldehyde, dl‐limonene, eucalyptol, β‐pinene, and α‐pinene. The identification of new signals requires either reference measurements with GC–MS or the measurement of authentic standards, as retention indices can only be used for reliable identification to a limited extent. To date, many signals have not yet been identified, particularly in the chemically complex section Jonquillae; thus, broadening the basis of the GC–MS analyses and reference measurements to address this issue will be the focus of future studies. Overall, short sampling and analysis times and the mobility of the GC–IMS itself are likely to lead to a quantum leap in pVOC analyses. Our experimental GC–IMS setup (no flower enclosure, ambient temperature, sample volume: 10 mL, sampling time: <10 s, analysis time: 10–30 min) was highly successful in capturing and documenting a wide range of different floral volatiles. Research challenges that have been very difficult to address in the past can now be addressed with relative ease, including emission time series, the comparison of a large number of individuals within and between populations, diurnal variability, and pVOC responses to various events, including temperature shifts or flower visitation.
AUTHOR CONTRIBUTIONS
F.L. and M.W. planned and designed the research. F.L. performed the experiments. F.L. and S.L. analyzed the data and identified the compounds. F.L. wrote the original manuscript. W.V. and M.W. reviewed and edited the manuscript and added essential content to the original draft. All authors approved the final version of the manuscript.
Supporting information
Appendix S1. Example photos of daffodils from different sections of Narcissus, representing the phenotypical differences and similarities among the species studied. (A) Narcissus pseudonarcissus (section Pseudonarcissi). (B) N. calcicola (section Apodanthi). (C) N. fernandesii (section Jonquillae) (D) N. cantabricus (section Bulbocodii). (E) N. papyraceus (section Tazettae). (F) N. tazetta (section Tazettae).
Appendix S2. Technical data and the experimental setup of the GC–IMS.
Appendix S3. Detailed GC–MS method for reference measurements and compound identification.
Appendix S4. List of substances detected in the floral scents of the genus Narcissus and representative heatmaps. Detected substances appear as colorized signals. The relative drift time and the retention time are substance specific and can be used for signal identification. The color‐coded signal intensity is a measure of quantity.
Appendix S5. Comparison of the intraspecific variation in Narcissus floral scent emissions among the studied Narcissus species.
Appendix S6. Number of unique compound signals detected for floral scents of each Narcissus species, and the average total signal intensity and standard deviation among the individual plants of the species studied.
ACKNOWLEDGMENTS
The authors thank Bonn University Botanic Gardens, particularly Michael Neumann, for providing the plant material; Tim Böhnert (Rheinische Friedrich‐Wilhelmsuniversität Bonn) for help with photography; and Martin Losch for support in designing the GC–IMS scheme. Funding for this project was provided by the German Bundesministerium für Bildung und Forschung in the context of the Automated Multisensor Station for Monitoring Species Diversity (AMMOD; FKZ 01LC1903B). Open Access funding enabled and organized by Projekt DEAL.
Losch, F. , Liedtke S., Vautz W., and Weigend M.. 2023. Evaluation of floral volatile patterns in the genus Narcissus using gas chromatography–coupled ion mobility spectrometry. Applications in Plant Sciences 11(1): e11506. 10.1002/aps3.11506
DATA AVAILABILITY STATEMENT
Raw and preprocessed data supporting this study have been deposited to Zenodo (https://doi.org/10.5281/zenodo.7463126). The processed data supporting the findings of this study are available in the Supporting Information.
REFERENCES
- Arroyo, J. , and Dafni A.. 1995. Variations in habitat, season, flower traits and pollinators in dimorphic Narcissus tazetta L. (Amaryllidaceae) in Israel. New Phytologist 129: 135–145. [DOI] [PubMed] [Google Scholar]
- Barrett, S. C. H. , Cole W. W., and Herrera C. M.. 2004. Mating patterns and genetic diversity in the wild daffodil Narcissus longispathus (Amaryllidaceae). Heredity 92(5): 459–465. 10.1038/sj.hdy.6800441 [DOI] [PubMed] [Google Scholar]
- Barrett, S. C. H. , and Harder L. D.. 2005. The evolution of polymorphic sexual systems in daffodils (Narcissus). New Phytologist 165(1): 45–53. 10.1111/j.1469-8137.2004.01183.x [DOI] [PubMed] [Google Scholar]
- Bouwmeester, H. , Schuurink R. C., Bleeker P. M., and Schiestl F.. 2019. The role of volatiles in plant communication. Plant Journal 100(5): 892–907. 10.1111/tpj.14496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkle, L. A. , and Runyon J. B.. 2019. Floral volatiles structure plant–pollinator interactions in a diverse community across the growing season. Functional Ecology 33(11): 2116–2129. 10.1111/1365-2435.13424 [DOI] [Google Scholar]
- Cesaro, A. C. , Barrett S. C. H., Maurice S., Vaissiere B. E., and Thompson J. D.. 2004. An experimental evaluation of self‐interference in Narcissus assoanus: Functional and evolutionary implications. Journal of Evolutionary Biology 17(6): 1367–1376. 10.1111/j.1420-9101.2004.00767.x [DOI] [PubMed] [Google Scholar]
- Chen, H.‐C. , Chi H.‐S., and Lin L.‐Y.. 2013. Headspace solid‐phase microextraction analysis of volatile components in Narcissus tazetta var. chinensis Roem. Molecules 18(11): 13723–13734. 10.3390/molecules181113723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess, S. K. , Raguso R. A., and LeBuhn G.. 2008. Geographic divergence in floral morphology and scent in Linanthus dichotomus (Polemoniaceae). American Journal of Botany 95(12): 1652–1959. [DOI] [PubMed] [Google Scholar]
- Danner, H. , Samudrala D., Cristescu S. M., and van Dam N. M.. 2012. Tracing hidden herbivores: Time‐resolved non‐invasive analysis of belowground volatiles by proton‐transfer‐reaction mass spectrometry (PTR‐MS). Journal of Chemical Ecology 38(6): 785–794. 10.1007/s10886-012-0129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delle‐Vedove, R. , Schatz B., and Dufay M.. 2017. Understanding intraspecific variation of floral scent in light of evolutionary ecology. Annals of Botany 120(1): 1–20. 10.1093/aob/mcx055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vega, C. , Herrera C. M., and Dötterl S.. 2014. Floral volatiles play a key role in specialized ant pollination. Perspectives in Plant Ecology, Evolution and Systematics 16(1): 32–42. 10.1016/j.ppees.2013.11.002 [DOI] [Google Scholar]
- Dobson, H. E. , Arroyo J., Bergström G., and Groth I.. 1997. Interspecific variation in floral fragrances within the genus Narcissus (Amaryllidaceae). Biochemical Systematics and Ecology 25(8): 685–706. 10.1016/S0305-1978(97)00059-8 [DOI] [Google Scholar]
- Dodds, J. N. , and Baker E. S.. 2019. Ion mobility spectrometry: Fundamental concepts, instrumentation, applications, and the road ahead. Journal of the American Society for Mass Spectrometry 30(11): 2185–2195. 10.1007/s13361-019-02288-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dötterl, S. , Wolfe L. M., and Jürgens A.. 2005. Qualitative and quantitative analyses of flower scent in Silene latifolia . Phytochemistry 66(2): 203–213. 10.1016/j.phytochem.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Dudareva, N. , Klempien A., Muhlemann J. K., and Kaplan I.. 2013. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytologist 198(1): 16–32. 10.1111/nph.12145 [DOI] [PubMed] [Google Scholar]
- Eiceman, G. A. , Karpas Z., and Hill H. H.. 2016. Ion mobility spectrometry, 3rd ed. CRC Press, Boca Raton, Florida, USA. [Google Scholar]
- Farré‐Armengol, G. , Filella I., Llusià J., and Peñuelas J.. 2017. β‐Ocimene, a key floral and foliar volatile involved in multiple interactions between plants and other organisms. Molecules 22(7): 1148. 10.3390/molecules22071148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes, A. 1975. L'evolution chez le genre Narcissus L. Anales del Instituto Botánico A. J. Cavanilles 32: 843–872. [Google Scholar]
- Gabelica, V. , and Marklund E.. 2018. Fundamentals of ion mobility spectrometry. Current Opinion in Chemical Biology 42: 51–59. 10.1016/j.cbpa.2017.10.022 [DOI] [PubMed] [Google Scholar]
- Hasan Ali, O. , Bomze D., Risch L., Brugger S. D., Paprotny M., Weber M., Thiel S., et al. 2021. Severe coronavirus disease 2019 (COVID‐19) is associated with elevated serum immunoglobulin (Ig) A and antiphospholipid IgA antibodies. Clinical Infectious Diseases 73(9): 2869–2874. 10.1093/cid/ciaa1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgins, K. A. , and Barrett S. C. H.. 2006. Female reproductive success and the evolution of mating‐type frequencies in tristylous populations. New Phytologist 171(3): 569–580. 10.1111/j.1469-8137.2006.01800.x [DOI] [PubMed] [Google Scholar]
- Hodgins, K. A. , and Barrett S. C. H.. 2008. Natural selection on floral traits through male and female function in wild populations of the heterostylous daffodil Narcissus triandrus . Evolution 62(7): 1751–1763. 10.1111/j.1558-5646.2008.00404.x [DOI] [PubMed] [Google Scholar]
- Kriegeskorte, N. , Mur M., and Bandettini P.. 2008. Representational similarity analysis: Connecting the branches of systems neuroscience. Frontiers in Systems Neuroscience 2: 4. 10.3389/neuro.06.004.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitzki, K. 2014. Flowering plants. Eudicots: The families and genera of vascular plants, vol. 11. Springer, Berlin, Germany. [Google Scholar]
- Liedtke, S. , Zampolli S., Elmi I., Masini L., Barboza T., Dalcanale E., Pinalli R., et al. 2019. Hyphenation of a MEMS based pre‐concentrator and GC‐IMS. Talanta 191: 141–148. 10.1016/j.talanta.2018.07.057 [DOI] [PubMed] [Google Scholar]
- Marques, I. , and Draper D.. 2012. Pollination activity affects selection on floral longevity in the autumnal‐flowering plant, Narcissus serotinus L. Botany 90(4): 283–291. 10.1139/b11-110 [DOI] [Google Scholar]
- Marques, I. , Rosselo‐Graell A., Draper D., and Iriondo J. M.. 2007. Pollination patterns limit hybridization between two sympatric species of Narcissus (Amaryllidaceae). American Journal of Botany 94(8): 1352–1359. [DOI] [PubMed] [Google Scholar]
- Marques, I. , Feliner G. N., Draper Munt D., Martins‐Loução M. A., and Aguilar J. F.. 2010. Unraveling cryptic reticulate relationships and the origin of orphan hybrid disjunct populations in Narcissus . Evolution 64(8): 2353–2368. 10.1111/j.1558-5646.2010.00983.x [DOI] [PubMed] [Google Scholar]
- Marques, I. , Jürgens A., Aguilar J. F., and Feliner G. N.. 2016. Convergent recruitment of new pollinators is triggered by independent hybridization events in Narcissus . New Phytologist 210(2): 731–742. [DOI] [PubMed] [Google Scholar]
- Marques, I. , Fuertes Aguilar J., Martins‐Louçao M. A., Moharrek F., and Nieto Feliner G.. 2017. A three‐genome five‐gene comprehensive phylogeny of the bulbous genus Narcissus (Amaryllidaceae) challenges current classifications and reveals multiple hybridization events. Taxon 66(4): 832–854. 10.12705/664.3 [DOI] [Google Scholar]
- Materić, D. , Bruhn D., Turner C., Morgan G., Mason N., and Gauci V.. 2015. Methods in plant foliar volatile organic compounds research. Applications in Plant Sciences 3(12): 1500044. 10.3732/apps.1500044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano, M. , Herrera C. M., and Barrett S. C. H.. 2005. Herkogamy and mating patterns in the self‐compatible daffodil Narcissus longispathus . Annals of Botany 95(7): 1105–1111. 10.1093/aob/mci129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtagh, F. , and Contreras P.. 2012. Algorithms for hierarchical clustering: An overview. WIREs Data Mining and Knowledge Discovery 2(1): 86–97. 10.1002/widm.53 [DOI] [Google Scholar]
- Navarro, L. , Ayensa G., Ferrero V., and Sánchez J. M.. 2012. The avoidance of self‐interference in the endemic daffodil Narcissus cyclamineus (Amaryllidaceae). Plant Ecology 213(11): 1813–1822. 10.1007/s11258-012-0137-y [DOI] [Google Scholar]
- Pérez, R. , Vargas P., and Arroyo J.. 2004. Convergent evolution of flower polymorphism in Narcissus (Amaryllidaceae). New Phytologist 161(1): 235–252. 10.1046/j.1469-8137.2003.00955.x [DOI] [Google Scholar]
- Pérez‐Barrales, R. , and Arroyo J.. 2010. Pollinator shifts and the loss of style polymorphism in Narcissus papyraceus (Amaryllidaceae). Journal of Evolutionary Biology 23(6): 1117–1128. 10.1111/j.1420-9101.2010.01988.x [DOI] [PubMed] [Google Scholar]
- Pérez‐Barrales, R. , Vargas P., and Arroyo J.. 2006. New evidence for the Darwinian hypothesis of heterostyly: Breeding systems and pollinators in Narcissus sect. Apodanthi . New Phytologist 171(3): 553–567. 10.1111/j.1469-8137.2006.01819.x [DOI] [PubMed] [Google Scholar]
- R Core Team . 2022. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Website: http://www.R-project.org/ [accessed 23 November 2022]. [Google Scholar]
- Raguso, R. A. 2008. Wake up and smell the roses: The ecology and evolution of floral scent. Annual Review of Ecology, Evolution and Systematics 39(1): 549–569. 10.1146/annurev.ecolsys.38.091206.095601 [DOI] [Google Scholar]
- Remy, C. 2002. Narcissus in perfumery. In Hanks G. R. [ed.], Narcissus and daffodil: The genus Narcissus, 392–398. Taylor & Francis, London, United Kingdom. [Google Scholar]
- Ruíz‐Ramón, F. , Águila D. J., Egea‐Cortines M., and Weiss J.. 2014. Optimization of fragrance extraction: Daytime and flower age affect scent emission in simple and double Narcissi . Industrial Crops and Products 52: 671–678. 10.1016/j.indcrop.2013.11.034 [DOI] [Google Scholar]
- Santos‐Gally, R. , Pérez‐Barrales R., Simón V. I., and Arroyo J.. 2013. The role of short‐tongued insects in floral variation across the range of a style‐dimorphic plant. Annals of Botany 111(2): 317–328. 10.1093/aob/mcs261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl, F. P. 2010. The evolution of floral scent and insect chemical communication. Ecology Letters 13(5): 643–656. 10.1111/j.1461-0248.2010.01451.x [DOI] [PubMed] [Google Scholar]
- Schiestl, F. P. , and Dötterl S.. 2012. The evolution of floral scent and olfactory preferences in pollinators: Coevolution or pre‐existing bias? Evolution 66(7): 2042–2055. 10.1111/j.1558-5646.2012.01593.x [DOI] [PubMed] [Google Scholar]
- Simón‐Porcar, V. I. , Santos‐Gally R., and Arroyo J.. 2014. Long‐tongued insects promote disassortative pollen transfer in style‐dimorphic Narcissus papyraceus (Amaryllidaceae). Journal of Ecology 102(1): 116–125. 10.1111/1365-2745.12179 [DOI] [Google Scholar]
- Terry, M. I. , Ruiz‐Hernández V., Águila D. J., Weiss J., and Egea‐Cortines M.. 2020. The effect of post‐harvest conditions in Narcissus sp. cut flowers scent profile. Frontiers in Plant Science 11: 540821. 10.3389/fpls.2020.540821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholl, D. , Boland W., Hansel A., Loreto F., Röse U. S. R., and Schnitzler J.‐P.. 2006. Practical approaches to plant volatile analysis. Plant Journal 45(4): 540–560. 10.1111/j.1365-313X.2005.02612.x [DOI] [PubMed] [Google Scholar]
- Tholl, D. , Weinhold A., and Röse U. S. R.. 2020. Practical approaches to plant volatile collection and analysis. In Pichersky E. and Dudareva N. [eds.], Biology of plant volatiles. CRC Press, Boca Raton, Florida, USA. [Google Scholar]
- Vautz, W. , Bödeker B., Baumbach J. I., Bader S., Westhoff M., and Perl T.. 2009. An implementable approach to obtain reproducible reduced ion mobility. International Journal for Ion Mobility Spectrometry 12(2): 47–57. 10.1007/s12127-009-0018-9 [DOI] [Google Scholar]
- Vautz, W. , Franzke J., Zampolli S., Elmi I., and Liedtke S.. 2018. On the potential of ion mobility spectrometry coupled to GC pre‐separation: A tutorial. Analytica Chimica Acta 1024: 52–64. 10.1016/j.aca.2018.02.052 [DOI] [PubMed] [Google Scholar]
- Waelti, M. O. , Muhlemann J. K., Widmer A., and Schiestl F. P.. 2008. Floral odour and reproductive isolation in two species of Silene . Journal of Evolutionary Biology 21(1): 111–121. 10.1111/j.1420-9101.2007.01461.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Example photos of daffodils from different sections of Narcissus, representing the phenotypical differences and similarities among the species studied. (A) Narcissus pseudonarcissus (section Pseudonarcissi). (B) N. calcicola (section Apodanthi). (C) N. fernandesii (section Jonquillae) (D) N. cantabricus (section Bulbocodii). (E) N. papyraceus (section Tazettae). (F) N. tazetta (section Tazettae).
Appendix S2. Technical data and the experimental setup of the GC–IMS.
Appendix S3. Detailed GC–MS method for reference measurements and compound identification.
Appendix S4. List of substances detected in the floral scents of the genus Narcissus and representative heatmaps. Detected substances appear as colorized signals. The relative drift time and the retention time are substance specific and can be used for signal identification. The color‐coded signal intensity is a measure of quantity.
Appendix S5. Comparison of the intraspecific variation in Narcissus floral scent emissions among the studied Narcissus species.
Appendix S6. Number of unique compound signals detected for floral scents of each Narcissus species, and the average total signal intensity and standard deviation among the individual plants of the species studied.
Data Availability Statement
Raw and preprocessed data supporting this study have been deposited to Zenodo (https://doi.org/10.5281/zenodo.7463126). The processed data supporting the findings of this study are available in the Supporting Information.


