Abstract
Sequence-specific transcription factors often function as components of large regulatory complexes. LIM-domain binding protein (LDB) and single-stranded DNA-binding protein (SSDP) function as core scaffolds of transcriptional complexes in animals and plants. Little is known about potential partners and functions for LDB/SSDP complexes in the context of tissue regeneration. In this work, we find that planarian LDB1 and SSDP2 promote tissue regeneration, with a particular function in mediolateral polarity reestablishment. We find that LDB1 and SSDP2 interact with one another and with characterized planarian LIM-HD proteins Arrowhead, Islet1, and Lhx1/5–1. SSDP2 and LDB1 also function with islet1 in polarity reestablishment and with lhx1/5–1 in serotonergic neuron maturation. Finally, we show new roles for LDB1 and SSDP2 in regulating gene expression in the planarian intestine and parenchyma; these functions may be LIM-HD-independent. Together, our work provides insight into LDB/SSDP complexes in a highly regenerative organism. Further, our work provides a strong starting point for identifying and characterizing potential binding partners of LDB1 and SSDP2 and for exploring roles for these proteins in diverse aspects of planarian physiology.
Introduction
Dynamic gene expression drives consequential changes in cellular behavior during development and regeneration. Though DNA-binding transcription factors are sometimes considered in isolation, most such factors function in vivo in modular transcriptional complexes that bring together numerous gene regulatory proteins to elicit changes in chromatin architecture and transcriptional activity. LIM-domain binding protein (LDB)—also known as CLIM, NLI, and CHIP—scaffolds transcriptional complexes with the help of Single-stranded DNA-binding protein (SSDP) (Chen et al., 2002; van Meyel et al., 2003). LDB and SSDP scaffolded complexes are ancient, present in diverse animals, and are related to similar scaffolding proteins (SEUSS/LEUNIG) that work together to organize gene regulatory complexes in plants (Franks et al., 2002; van Meyel et al., 2003; Sridhar et al., 2004).
As core transcriptional regulatory factors in gene expression across broad cell types, LDB and SSDP orthologs play diverse roles in development, particularly in formation of the head and brain (Matthews and Visvader, 2003). Drosophila Chip was identified in a genetic screen as a regulator of wing morphology (Morcillo et al., 1996; Morcillo et al., 1997), and Drosophila SSDP is essential, with mutants lacking maternal and zygotic SSDP dying during larval development (Chen et al., 2002). C. elegans SAM-10 (ortholog of SSDP) and ldb-1 promote normal gene expression in neurons during maturation, modulating synapse and neurite formation (Cassata et al., 2000; Zheng et al., 2011). Zebrafish SSDP and CLIM/LDB paralogs function in neural patterning, eye formation, and sensory axon formation (Becker et al., 2002; Zhong et al., 2011). Mice lacking ssdp1 develop with aberrant head morphogenesis; the initial mouse mutant in ssdp1 was named headshrinker (Nishioka et al., 2005). A proline-rich region within SSDP1 was also found to be essential for proper head development (Enkhmandakh et al., 2006). Mice lacking LDB1 also develop with severe anterior truncations and defects in head development, as well as other pleiotropic phenotypes including heart defects and patterning problems (Mukhopadhyay et al., 2003), while murine LDB2 plays overlapping and unique roles in neural development (Gueta et al., 2016; Leone et al., 2017). Finally, LDB proteins can function in chromatin looping and recruitment of complexes to modify chromatin (Krivega et al., 2014; Caputo et al., 2015; Lee et al., 2016; Magli et al., 2019).
In animals, LDB/SSDP complexes often include LIM-homeodomain proteins and other sequence-specific DNA-binding transcription factors that direct the complex to specific regions of the genome (Matthews and Visvader, 2003) (Fig. 1A). LDB itself can function as a dimer or trimer, resulting in the potential bridging of two transcription factors together to form homo- or hetero-multimeric DNA-binding structures (Jurata et al., 1998; Cross et al., 2010; Wang et al., 2020). Though perhaps best known for binding LIM-HD proteins (Agulnick et al., 1996; Morcillo et al., 1997; Hobert and Westphal, 2000; van Meyel et al., 2003; Yasuoka and Taira, 2021), LDB/SSDP complexes can also include transcriptional regulators that do not possess LIM domains. Additional LDB/SSDP-associated transcription factor proteins have been thoroughly documented in Drosophila, where they include Pygopus, Bicoid, Pannier, Groucho, and Ftz (Torigoi et al., 2000; Bronstein et al., 2010; Fiedler et al., 2015). Cooperation between LDB, SSDP, and transcription factors leads to varied outcomes. For example, murine Lim1 plays a critical role in head organization in development (Shawlot and Behringer, 1995), similar to functions of mouse LDB1 and SSDP. And murine Lhx2 cooperates with LDB1 for a narrower role during several neurodevelopmental processes, including regionalization of the hippocampus, development of olfactory neurons, and regulation of neurogenic vs. gliogenic choices in the retina (de Melo et al., 2018; Monahan et al., 2019; Kinare et al., 2020).
1). Planarian SSDP and LDB homologs promote regeneration.
A) Diagram showing domain architecture in canonical LDB transcriptional complexes (Enkhmandakh et al., 2006; Bronstein et al., 2010). LDB=LIM Domain Binding protein; SSDP=Single-Stranded DNA Binding Protein; and LIM-HD=LIM-Homeodomain, where LIM is a region of homology first identified between Lin-11, Isl-1 and Mec-3. LDB proteins have a long dimerization domain (blue) and a LIM-binding domain (green). SSDP proteins have a conserved domain (red) and a long, unstructured region. LIM-HD proteins have a pair of LIM domains (yellow) and a homeodomain (purple). Complexes form to bind DNA and regulate gene expression. B) in situ hybridization showing expression patterns of planarian LDB1, LDB2, SSDP1, and SSDP2. All four genes are broadly expressed with some enrichment, particularly for LDB1 and SSDP2, in the brain. C) Images of live planarians after RNAi, head amputation, and 6 days of regeneration. control(RNAi) animals have a rounded blastema with two eyespots visible (n=10). LDB1(RNAi) and SSDP2(RNAi) regenerates have pointed blastemas with zero or one eyespot (6/6 and 9/10, respectively). D). in situ hybridization to mark the broadly expressed transcript ChAT (choline acetyltransferase; (Nishimura et al., 2010)) in animals after RNAi, head amputation, and 6 days of regeneration. LDB1(RNAi) and SSDP2(RNAi) animals have smaller brains which are fused at the midline. E) Quantification of animals from (D) showing significantly smaller brains. (n=5–9; Kruskal-Wallis with Dunn’s correction for multiple comparisons.) P is an adjusted value, * indicates P≤0.05 and ** indicates P≤0.005. Scale=500 μm.
SSDP and LDB homologs regulate head organization and neural development during embryogenesis, but few studies have explored potential roles for these core regulators during regeneration. We opted to explore potential functions of LDB, SSDP, and associated transcription factors in regeneration using planarian flatworms of the species Schmidtea mediterranea. Planarians complete whole-body regeneration from tiny fragments of starting tissue using adult, pluripotent stem cells (for review, see (Ivankovic et al., 2019)). During regeneration, planarians reestablish diverse cell types in organs that include muscle, a brain, protonephridia, and a muscular feeding organ called the pharynx (Roberts-Galbraith and Newmark, 2015). Due to their unique biology, planarians are outstanding model organisms with which to discover genetic mechanisms that regulate regeneration and stem cell biology. Planarian SSDP and LDB homologs have not been characterized, but a handful of LIM-homeodomain proteins have been studied. Planarian islet1 plays roles in reestablishment of anterior, posterior, and midline polarity (Hayashi et al., 2011; Marz et al., 2013). lhx1/5–1 directs maturation of serotonergic neurons in the central and peripheral nervous system of the planarian (Currie and Pearson, 2013). And an arrowhead homolog is required for reestablishment of medial brain structures, including the anterior commissure, through creation of neurons that serve as guidepost cells (Roberts-Galbraith et al., 2016; Scimone et al., 2020).
Here, we identify and characterize roles for planarian LDB and SSDP homologs in the context of regeneration. We find that LDB1 and SSDP2 are required for proper regeneration, playing important roles in reestablishment of polarity. Using yeast two-hybrid assays, we show that LDB1 interacts with itself, with SSDP2, and with LIM-HD proteins Islet1, Lhx1/5–1, and Arrowhead. We further show that LDB1 and SSDP2 likely cooperate with binding partners Islet1 and Lhx1/5–1 in polarity reestablishment and serotonergic maturation, respectively. Broadening our study, we describe the complement of genes that encode LIM-homeodomain and LIM-only domain proteins in planarians. Finally, to begin the process of identifying potential targets of SSDP2-containing complexes, we performed RNAi and RNA-sequencing and identified dozens of genes that depend on SSDP2 for proper expression. We conclude that planarian SSDP2 and LDB1 play key roles in regulating gene expression during planarian regeneration both by cooperating with known LIM-HD partners and likely other transcriptional regulators. Our work provides an important foundation for exploring the shared and unique roles of conserved LDB/SSDP complexes in the context of robust tissue regeneration.
Results and Discussion
Planarian LDB1 and SSDP2 are required for blastema size and organization
To identify planarian LDB and SSDP homologs, we searched available transcriptomes (Brandl et al., 2016; Rozanski et al., 2019) and found two homologs each of LDB and SSDP (Supp. Tables 1, 2). We examined the expression patterns of planarian homologs and determined that LDB1, LDB2, SSDP1, and SSDP2 have nearly ubiquitous expression with some potential enrichment in the planarian brain (Fig. 1B). Wide-ranging expression was also indicated in published single-cell sequencing atlases, with some specific enrichment of LDB1 in subsets of cathepsin+ cell types (Supp. Tables 1, 2, (Fincher et al., 2018; Plass et al., 2018)). We also note that both LDB1 and SSDP2 were significantly upregulated at 36 hours and 72 hours post-amputation in our prior gene expression analysis (Roberts-Galbraith et al., 2016).
Next, we investigated whether planarian LDB and SSDP homologs play roles in regeneration. We knocked down LDB1, LDB2, SSDP1, or SSDP2, amputated prepharyngeally, and allowed heads to regenerate for 6 days. We did not note any regeneration defects after LDB2(RNAi) or SSDP1(RNAi). However, LDB1(RNAi) and SSDP2(RNAi) animals exhibited slower movement and regenerated with pointy head blastemas and cyclopia (Fig. 1C). We also repeated RNAi of each homolog, amputated heads, and examined brain organization at 6 days post-amputation (dpa) via in situ hybridization (ISH) with a choline acetyltransferase (ChAT) riboprobe (Nishimura et al., 2010). We found that brain regeneration proceeded normally after LDB2(RNAi) or SSDP1(RNAi). However, LDB1(RNAi) and SSDP2(RNAi) animals regenerated with significantly smaller brains that were fused at the midline (Fig. 1D–E). We conclude that planarian LDB1 and SSDP2 are important for head regeneration, just as SSDP and LDB homologs are important for head development in mice (Mukhopadhyay et al., 2003; Nishioka et al., 2005). We opted to focus on LDB1 and SSDP2 for the remainder of our studies.
Smed-LDB1 interacts with SSDP2 and LIM-HD binding partners
In other animals, LDB homologs scaffold protein complexes and bind to SSDP and LIM-HD proteins to regulate transcription (Fig. 1A, (Matthews and Visvader, 2003)). To test whether planarian LDB1 binds similarly to SSDP and LIM-HD proteins, we turned to yeast two-hybrid analysis. We decided to focus on characterized planarian LIM-HD proteins Lhx1/5–1, Islet1, and Arrowhead (Hayashi et al., 2011; Currie and Pearson, 2013; Marz et al., 2013; Roberts-Galbraith et al., 2016; Scimone et al., 2020). We cloned full-length (FL) versions of each gene into yeast two-hybrid vectors to create fusions of planarian proteins with either the DNA-binding domain (BD, Bait) or the activation domain of GAL4 (AD, Prey) (Chien et al., 1991). We also cloned short (Sh) versions of each gene that included only the domains relevant for known protein-protein interactions (Fig. 2A). For LDB1, we cloned the dimerization domain and LIM-binding domain. For SSDP2, we cloned the conserved N-terminal domain. We cloned the N-termini of Islet1, Lhx1/5–1, and Arrowhead (Ah), including both LIM domains for each LIM-HD protein.
2). Planarian LDB1 interacts with SSDP2, as well as the LIM domains of Arrowhead, Islet, and Lhx1/5–1.
A) Diagram showing domain architecture for LDB1, SSDP2, Arrowhead, Islet, and Lhx1/5–1. “FL” indicates full length protein and “Sh” indicates a shorter truncation used in subsequent analyses. The color scheme used is the same as in Fig. 1A. B) Yeast two-hybrid results showing the interaction of full-length LDB1 with full-length SSDP2, Arrowhead, Islet, and Lhx1/5–1. Full-length Ssdp2, Islet, and Lhx1/5–1 exhibited transactivation when used as the Bait (dark grey), so these pairings were not used for the final assessment. Full length planarian LDB1 did not show interaction with itself, despite known homodimerization in other models. (− indicates no interaction; +/− indicates weak growth; +, ++, or +++ indicates growth on restrictive medium). C) Yeast two-hybrid results showing that the core domains of LDB1 (LDB1-Sh) interact with the conserved domain of SSDP1 and the LIM domains of Arrowhead, Islet, and Lhx1/5–1. In this experiment, we did note a weak self-interaction between the core domains of LDB1, which could support some dimerization of the protein. S. pombe Mob1 and Sid2 were used as controls (Balasubramanian et al., 1998; Hou et al., 2004). Cell growth for B and C is shown in Supp. Fig. 1A–B.
In this yeast two-hybrid system, pairs of plasmids are transformed into an auxotrophic strain of Saccharomyces cerevisiae. Interaction between bait and prey plasmids enables activation of gene expression to allow growth on drop-out media lacking histidine and adenine supplements. With this assay, we determined that full-length planarian LDB1 interacted with planarian SSDP2, Arrowhead, Lhx1/5–1, and Islet1 (Fig. 2B, Supp. Fig. 1A). We did not see evidence of LDB1 self-interaction using full-length protein, contrary to studies of LDB in other organisms. We were only able to establish binding of LDB1 to partners using LDB1 as bait, due to transactivation of planarian SSDP2, Islet1, and Lhx1/5–1 when fused with the DNA-binding domain (BD, bait) (Fig. 2B, Supp. Fig. 1A). This experimental complication may indicate that full-length SSDP2, Islet1, and Lhx1/5–1 can directly recruit S. cerevisiae proteins to promote transcription.
We confirmed interactions and narrowed interaction domains using our “Sh” constructs. We found that the conserved interior of planarian LDB1 interacts with the conserved N-terminus of SSDP2 (Fig. 2C, Supp. Fig. 1B). We also found that conserved LDB1 domains interact with the LIM domains of Arrowhead, Islet1, and Lhx1/5–1 (Fig. 2C, Supp. Fig. 1B). We additionally detected some interaction of the short construct of LDB1 with itself in this assay (Fig. 2C, Supp. Fig. 1B), which may indicate that planarian LDB1 does indeed self-interact. We did not observe transactivation of any short construct, which indicates that the excluded regions of SSDP2 and LIM-HD proteins are responsible for transactivation in this system.
We conclude that planarian LDB1 and SSDP2 interact similarly to their homologs in other animals (Fig. 1A). Further, LDB1 can interact with the LIM domains of planarian Islet1, Lhx1/5–1, and Arrowhead. Smed-LDB1 also self-interacts, albeit weakly in this assay. These protein-protein interactions demonstrate potentially conserved organization within planarian LDB1/SSDP2 complexes, though studies in planarian cells or extract will be required to demonstrate in vivo interactions and study complex composition in more detail.
LDB1 and SSDP2 cooperate with LIM-HD proteins Islet1 and Lhx1/5–1
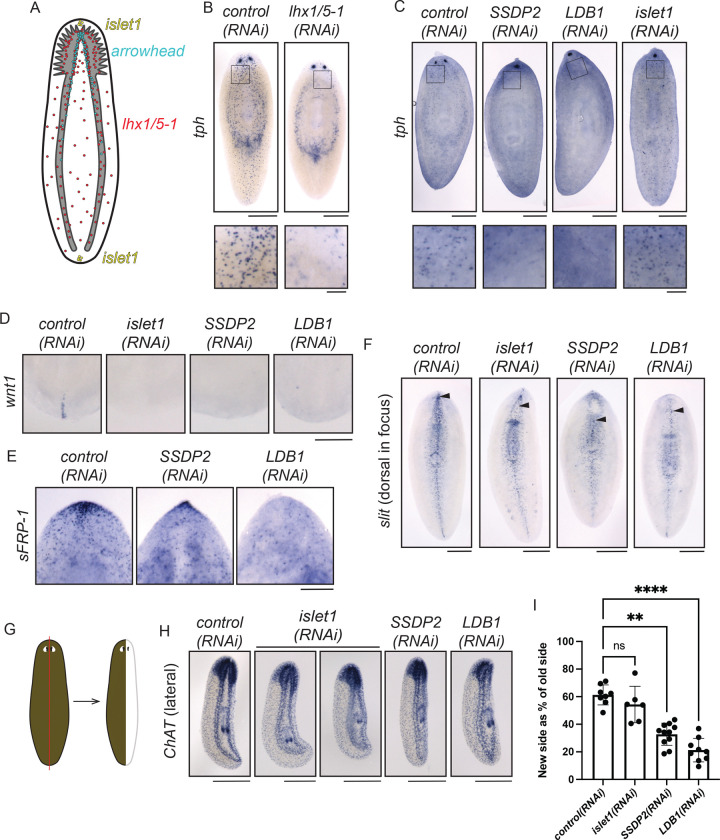
Based on our determination that planarian LDB1 can bind to Arrowhead, Islet1, and Lhx1/5–1, we next sought to understand whether planarian LDB1 and SSDP2 cooperate with these LIM-HD proteins. We first investigated whether planarian LDB1 and SSDP2 cooperate with Lhx1/5–1. Lhx1/5–1 is expressed in cells throughout the central and peripheral nervous system and promotes maturation of serotonergic neurons (Fig. 3A, (Currie and Pearson, 2013)). Knockdown of lhx1/5–1 reduces expression of tryptophan hydroxylase (TPH), a marker of peripheral serotonergic neurons, without affecting TPH+ cells in the eyes (Fig. 3B, inset, (Nishimura et al., 2007; Currie and Pearson, 2013)). To assess whether LDB1 or SSDP2 is required for the function of lhx1/5–1 in serotonergic neurons, we performed RNAi and examined TPH expression (Fig. 3C). We found that expression of TPH in the peripheral nervous system was reduced after LDB1(RNAi) or SSDP2(RNAi) (Fig. 3C, insets). We also detected cyclopia using the TPH marker that was not present after lhx1/5–1(RNAi) but was present after islet1(RNAi), as has been previously reported (Fig. 3B–C, (Marz et al., 2013)). We conclude that LDB1 and SSDP2 overlap functionally with Lhx1/5–1 in promoting gene expression in peripheral serotonergic neurons, supporting the idea that these proteins work in a transcriptional complex in this process.
3). SSDP2(RNAi) and LDB1(RNAi) phenotypes indicate functional overlap with Islet1 and Lhx1/5–1.
A) Diagram depicting published expression patterns of planarian LIM-HD-encoding genes islet1 (Hayashi et al., 2011; Marz et al., 2013), arrowhead (Roberts-Galbraith et al., 2016), and lhx1/5–1 (Currie and Pearson, 2013). B) control(RNAi) and lhx1/5–1(RNAi) animals underwent in situ hybridization with TPH (tryptophan hydroxylase; (Nishimura et al., 2007)) 6 days after head amputation. Fewer peripheral TPH+ neurons are seen after lhx1/5–1(RNAi) (see inset at bottom), as seen in a prior study (Currie and Pearson, 2013). C) control(RNAi), SSDP2(RNAi), LDB1(RNAi), and islet1(RNAi) animals stained for TPH+ via in situ hybridization 6 days after head amputation. Fused eyespots are visible after SSDP2(RNAi), LDB1(RNAi), or islet1(RNAi). Decreased peripheral TPH+ puncta were noted after knockdown of SSDP2 or LDB1, but not islet1 (see insets). D) control(RNAi), islet1(RNAi), SSDP2(RNAi) and LDB1(RNAi) animals underwent tail amputation, 6 days of regeneration, and in situ hybridization with the Wnt1 probe (Petersen and Reddien, 2008; Adell et al., 2009). Only posterior ends are shown. E) control(RNAi), islet1(RNAi), SSDP2(RNAi) and LDB1(RNAi) animals underwent head amputation, 7 days of regeneration, and in situ hybridization with the sFRP-1 probe (Gurley et al., 2008; Petersen and Reddien, 2008). Only anterior ends are shown. F) control(RNAi), islet1(RNAi), SSDP2(RNAi) and LDB1(RNAi) animals underwent head amputation, 6 days of regeneration, and in situ hybridization with the slit1 probe (Cebrià et al., 2007). Animals were imaged with the dorsal side facing up; arrowheads indicate the anterior-most dorsal slit signal. G) Diagrams of the amputation plane used for experiments in (H). H) control(RNAi), islet1(RNAi), SSDP2(RNAi) and LDB1(RNAi) animals underwent sagittal amputation, 9 days of regeneration, and in situ hybridization with the ChAT probe (Nishimura et al., 2010). Animals are shown with the regenerating side to the right. I) We measured the area of both the newly regenerated brain lobe (right) and the existing brain lobe (left) and created a ratio for each animal in (H). SSDP2(RNAi) and LDB1(RNAi) animals regenerated significantly less new brain tissue than control(RNAi) animals. (n=6–11; Kruskal-Wallis with Dunn’s correction for multiple comparisons. P is an adjusted value, ** indicates P≤0.005 and **** indicates P≤0.0001). Scale=500 μm (B, C, F, H), 100 μm (B, C insets), 200 μm (D, E).
Previous research indicated that planarian islet1 is expressed in anterior and posterior poles during regeneration (Fig. 3A, (Hayashi et al., 2011; Marz et al., 2013)), and we find that both SSDP2 and islet are expressed broadly in regenerating tissue (Supp. Fig. 2A). Knockdown of islet1 leads to eye fusion at the midline, failure to reestablish anterior and posterior poles, and defective gene expression at the midline (Hayashi et al., 2011; Marz et al., 2013). Due to the superficial similarities of the islet1(RNAi), SSDP2(RNAi), and LDB1(RNAi) phenotypes, we next investigated whether SSDP2 and LDB1 are required for reestablishment of polarity. islet1(RNAi) leads to reduced wnt1 expression in tails after posterior amputation (Fig. 3D, (Hayashi et al., 2011; Marz et al., 2013)). Similarly, SSDP2(RNAi) or LDB1(RNAi) decreases wnt1 expression in regenerating tails (Fig. 3D). We also found that SSDP2(RNAi) or LDB1(RNAi) reduces sFRP-1 expression in regenerating heads (Fig. 3E). We conclude that SSDP2 and LDB1 likely cooperate with Islet1 in regeneration of anterior and posterior pole structures.
RNAi targeting planarian islet-1 also leads to mediolateral patterning defects, which are reflected in regeneration of cyclopic heads and reduced expression of slit (Fig. 3F, (Marz et al., 2013)). We confirmed that islet1(RNAi) specifically reduced the dorsal expression domain of slit, with a posterior shift in dorsal slit expression (Fig. 3F, arrowhead). We next investigated whether knockdown of SSDP2 or LDB1 phenocopied islet1(RNAi) impacts on slit expression. Indeed, SSDP2(RNAi) or LDB1(RNAi) led to reduced dorsal slit expression domains (Fig. 3F, arrowheads), though ventral slit expression was less affected. Based on these results, we expected that islet1, SSDP2, and LDB1 might be important for lateral regeneration as well as anterior regeneration. We performed RNAi targeting islet1, SSDP2, or LDB1 and completed sagittal amputations along the midline of the planarian body (Fig. 3G). We killed and fixed animals 9 days post-amputation and completed ISH with our ChAT riboprobe to investigate regeneration of internal structures (Fig. 3H). After lateral regeneration, SSDP2(RNAi) or LDB1(RNAi) led to diminished lateral regeneration, significantly smaller regenerated brain lobes, and frequently undetectable regenerated ventral nerve cords (Fig. 3H–I, Supp. Fig. 2B–C). We detected a small decrease in brain size after islet1(RNAi) that was not significant (Fig. 3H–I). Taken together, we conclude that planarian LDB1 and SSDP2 play roles in midline reestablishment and lateral regeneration. Although Islet1 is also a logical partner for the SSDP2/LDB1 transcriptional complex in the context of lateral regeneration, the severity of LDB1(RNAi) and SSDP2(RNAi) phenotypes indicate that other transcriptional complex partners might also be involved.
Identification of additional planarian LIM-HD- and LMO-encoding genes
To identify other partners in LDB1/SSDP2 complexes, we first explored LIM-HD proteins. In addition to prior characterization of islet1, arrowhead, and lhx1/5–1 (Hayashi et al., 2011; Currie and Pearson, 2013; Marz et al., 2013; Roberts-Galbraith et al., 2016; Scimone et al., 2020), a few additional LIM-HD-encoding proteins have been identified. A second homolog of Islet, islet2, is expressed in the anterior pole within the planarian head (Li et al., 2019). lhx2/9–1 (previously called lhx2b) is expressed in the planarian intestine and RNAi causes a mild reduction in goblet cells (Forsthoefel et al., 2020). lhx2/9–3 (previously lhx2/9-like) and lhx3/4 (previously lhx3/4-like) were both identified as transcription factor-encoding genes expressed in planarian stem cells (Scimone et al., 2014).
We searched available transcriptomes (Brandl et al., 2016; Rozanski et al., 2019) to reveal the full complement of LIM-HD- and LMO-encoding genes in S. mediterranea. We found that planarians express 13 genes that encode proteins with a pair of LIM domains and a homeodomain (i.e. LIM-HD proteins) (Supp. Table 3, supported by data in Molina and Cebrià, personal communication and forthcoming preprint). We also found that planarians express 3 genes that have LIM domains only and that also have homology to LIM-only (LMO) genes like beadex, rhombotin, and lmo4 (Supp. Table 3, Supp. Fig. 3). We also identified 41 transcripts that encode proteins with LIM domains but that do not clearly belong within LIM-HD or LMO families (Supp. Table 3).
Next, we sought to characterize additional LIM-HD- and LMO-encoding transcripts by determining their expression patterns. Our ISH for published transcripts largely confirmed previous expression patterns, with the additional finding that islet1 is strongly expressed in the planarian brain and is broadly expressed outside the poles (Fig. 4A). We found that two LIM-HD-encoding genes, lhx2/9–1 (previously called lhx2b) and lhx1/5–2, are expressed in the planarian intestine (Fig. 4A). We noted one gene, lmx1a/b-4, expressed in the parenchyma, and we saw enriched expression of the majority of LIM-HD genes in subsets of cells in the planarian brain (Fig. 4A). We found that the three LMO-encoding genes had enriched expression in neurons and, for lmo1/3–2, in additional cell types (Fig. 4B). We saw broad concordance between our ISH results and results from single-cell sequencing analyses (Supp. Table 3 (Fincher et al., 2018; Plass et al., 2018)), though our ISH experiments also revealed region-specific expression patterns for several genes that were not significantly enriched in single-cell analyses. Taken together, the tissue-specific expression patterns of LIM-HD and LMO genes offer hints to potential functions of these transcription factors and additional LDB/SSDP complexes in planarian neurobiology and regeneration. Further, neural expression of islet1 indicates that there could be additional roles still to be discovered for known LIM-HD transcription factors.
4). Expression of planarian LIM-homeodomain and LIM-only-encoding genes.
A) Domain architecture of a typical LIM-homeodomain (LIM-HD) protein. 13 planarian genes encode LIM-HD proteins. Expression of these genes is shown in untreated planarians and genes are grouped by family. B) LIM-only (LMO) proteins have a typical domain architecture with two LIM domains each. 3 planarian genes encode LMO proteins. in situ hybridization for each LMO gene is shown. Scale=500 μm.
Impacts of SSDP2(RNAi) on gene expression in planarians
Finally, we sought to perturb elements of the conserved LDB1/SSDP2 transcriptional complex for several reasons: 1) to uncover downstream impacts of their perturbation, 2) to discover new functions for the complex, and 3) to explore whether novel functions were accomplished through LIM-HD-dependent or independent mechanisms. We performed SSDP2(RNAi) in triplicate (Fig. 5A) and used bulk RNA-sequencing to identify genes differentially expressed after knockdown. We confirmed that SSDP2 itself was 3.5x downregulated in the samples (Supp. Table 4–5). We identified 64 upregulated transcripts and 53 downregulated transcripts after SSDP2(RNAi) (fold change ≥1.5x, corrected P≤0.05; Fig. 5B, Supp. Table 4–5). A large percentage of genes downregulated after SSDP2(RNAi) are expressed in the intestine, in parenchymal cell types, or in cathepsin+ cell types (Supp. Table 4–5, examples in Fig. 5C).
5). Identification of genes differentially expressed after SSDP2(RNAi).
A) RNAi paradigm used for bulk RNA-seq experiments. Animals underwent head amputation and 6 days of regeneration. B) We identified 64 genes that were upregulated ≥1.5x with a P value <0.05 after false discovery rate correction. We also identified 53 genes that were downregulated ≥1.5x with a P value <0.05 after false discovery rate correction. C) In situ hybridization showing normal expression of four genes significantly downregulated after SSDP2(RNAi). Two parenchymal- and two intestine-enriched genes are shown. D) One transcript downregulated after SSDP2(RNAi) in our RNA-Seq data is dd_215. Using in situ hybridization, we show that dd_215 is absent from the blastema and is downregulated in both the existing intestine and pharynx after either LDB1(RNAi) or SSDP2(RNAi), but not after knockdown of the gut-enriched LIM-HD-encoding genes lhx1/5–2 or lhx2/9–1. E-H) dd_120 and dd_240 are decreased in expression after either LDB1(RNAi) or SSDP2(RNAi), but not after knockdown of the parenchymal-enriched lmx1a/b-4. Ventral (E-F) and dorsal (G-H) views of animals are shown. For dd_120, we noted expression in two distinct cell types with strong (arrowhead) and weak (arrow) expression. Cells with weak expression are nearly absent after LDB1(RNAi) or SSDP2(RNAi). Animals are 6 days post-head amputation and both ventral (top) and dorsal (bottom) views are shown. Scale=200 μm.
We cloned a subset of differentially expressed genes and examined their expression after SSDP2(RNAi), LDB1(RNAi), or RNAi targeting candidate LIM-HD genes. We confirmed that prosaposin (dd_215) is downregulated in both the intestine and the pharynx after SSDP2(RNAi) or LDB1(RNAi), supporting roles for both complex members in prosaposin expression (Fig. 5D). Surprisingly, knockdown of lhx1/5–2 or lhx2/9–1—the two intestine-expressed LIM-HD genes—did not alter prosaposin expression (Fig. 5D). We conclude that SSDP2 and LIM1 play roles in regulating intestinal gene expression, in both existing and regenerating tissue. We also examined the expression of two parenchymal-expressed genes, dd_120 and dd_240, and found that their expression was decreased in SSDP2(RNAi) and LDB1(RNAi) animals (Fig. 5E–H). We hypothesized that parenchymal roles for LDB1/SSDP2 could be accomplished through the function of a parenchyma-expressed LIM-HD-encoding gene, lmx1a/b-4. However, neither dd_120 nor dd_240 exhibited altered expression after lmx1a/b-4(RNAi) (Fig. 5E–H).
Taken together, our work shows that SSDP2 impacts gene expression in the intestine and parenchyma, in addition to roles completed through previously described LIM-HD-encoding genes. We further find that LDB1(RNAi) phenocopies SSDP2(RNAi) impacts on intestinal and parenchymal gene expression, indicating that the two proteins likely cooperate in gene regulation in these contexts. However, we were not able to assign a LIM-HD-encoding protein partner for either intestinal or parenchymal roles, suggesting that additional LDB1/SSDP2 complex members may be important. One caveat of the RNA-Seq experiment is that we used the entire regenerating animals for our analysis; this could bias our results toward highly expressed genes as well as genes like dd_215, dd_120, and dd_240 that showed global changes in gene expression after SSDP2(RNAi), even in non-regenerating tissues.
Conclusions and future directions
In this work, we characterized planarian homologs of LDB and SSDP and discovered that LDB1 and SSDP2 play roles in organization and polarity of new tissue during regeneration. Our results provide evidence that core transcriptional scaffolds can be targeted during regeneration to identify both new and conserved gene functions and to prioritize transcriptional regulators for further study. We show that LDB1 binds to SSDP2 and to LIM-HD proteins Arrowhead, Lhx1/5–1, and Islet1. We further demonstrate that LDB1 and SSDP2 are required for functions of Lhx1/5–1 and Islet1 in serotonergic neuron maturation and polarity, respectively. We identified the full complement of LIM-HD- and LMO-encoding proteins in planarians, though further work will be required to discover and characterize planarian LIM-HD proteins, LMO proteins, and other non-LIM-domain transcriptional partners of the SSDP2/LDB1 complex. Due to the expression of many LIM-HD and LMO genes in the planarian nervous system, we are particularly eager to discover roles for LDB1/SSDP2 and related factors in neuronal regeneration and function. Finally, we identify genes differentially regulated after SSDP2(RNAi) and discover novel roles for the transcriptional core proteins in gene expression in the intestine and parenchyma of the planarian body. We expect that future work will reveal specific DNA target sequences for LDB1/SSDP2 complexes. Taken together, our work has uncovered conserved roles for LDB/SSDP complexes in head formation and provides a starting point for further exploration of these ancient transcriptional scaffolds in animal regeneration.
Materials and Methods
Planarian care
We used asexually reproducing S. mediterranea (strain CIW4 (Newmark and Sánchez Alvarado, 2000)) for all experiments. Planarians were housed in Montjuïc salts (Cebrià and Newmark, 2005) at 18°C in the dark. We fed animals organic beef liver puree (White Oak Pastures, Bluffton, GA) approximately once per week. Animals for in situ hybridization or RNA interference (RNAi) were starved at least one week prior to experiments. We also used gentamicin sulfate (50 μg/mL final concentration, Gemini Bio-Products) to treat animals as needed and during all RNAi experiments.
Sequence identification and domain analysis
We identified homologs of SSDP and LDB families using TBLASTN searches of S. mediterranea transcriptomes (Brandl et al., 2016; Rozanski et al., 2019). We used both BLAST and domain search resources at Planmine (Brandl et al., 2016; Rozanski et al., 2019) to identify genes that encode LIM-domain proteins (LIM domain = IPR001781, PF00412). LIM-HD-encoding genes were classified as per (Molina and Cebrià, personal communication).
Translated LMO proteins were subjected to phylogenetic analysis alongside homologs of LMO proteins from Drosophila melanogaster, Homo sapiens, Mus musculus, Gallus gallus, Danio rerio, and Trichoplax adhaerens (sequences in Supp. Text 1). Human Pax1 was used as an outgroup. We completed phylogenetic analysis using the a la carte option on www.phylogeny.fr (Dereeper et al., 2008). MUSCLE was used for alignment (Edgar, 2004), Gblocks was used for curation (Castresana, 2000), and PhyML was used for tree construction. 100 bootstrap replicates were performed (PhyML) and we used the WAG model of amino acid substitution (specific parameters: 4 substitution rate categories; estimates used for invariable site proportion and transition ratios).
Molecular biology methods
For in situ hybridization and RNA interference experiments, we cloned 500–800 bp fragments of each gene into pJC53.2 (Collins et al., 2010). Briefly, cDNA was prepared from planarians using an iSCRIPT kit (Bio-Rad) and primers shown in Supp. Table 6 were used to amplify fragments of each gene using Platinum Taq (Invitrogen). PCR amplicons were ligated into pJC53.2, which had been digested with Eam1105I, using standard ligation methods. Kanamycin was used to select transformants and sequencing was used to verify insert sequences and orientation (Azenta).
For yeast two-hybrid analysis, full length and short fragments of LDB1, SSDP2, islet1, lhx1/5–1, and arrowhead were amplified from cDNA with Phusion polymerase (New England Biosciences). Primers were designed to include restriction sites and are described in Supplemental Table 6. Amplicons were digested with restriction enzymes as follows: BamHI and PstI (LDB1, arrowhead, islet1); EcoRI/PstI (SSDP2); or SmaI and BamHI (lhx1/5–1). Digested fragments were inserted into digested pGAD424 and pGBT9 through standard molecular methods. Inserts were sequenced using primers described in Supp. Table 7.
To generate dsRNA and riboprobes, we amplified pJC53.2 inserts using a T7 primer (sequence in Supp. Table 7). PCR products were purified with a DNA clean and concentrator kit (Zymo). We synthesized dsRNA as previously described (Chong et al., 2013; Rouhana et al., 2013). Riboprobes were generated from PCR products at 30°C overnight using either Sp6 or T3 polymerase, depending on the orientation of the insert. We included digoxigenin-11-UTP (Roche) during riboprobe synthesis. Riboprobes were treated with DNase (Promega) and purified by ammonium acetate precipitation. Riboprobes and dsRNA concentrations were determined through band intensity after gel electrophoresis, which we have found to be more reliable than a spectrophotometer as only full-length products are quantified.
Yeast two-hybrid analysis
Saccharomyces cerevisiae strain PJ69–4A was used for yeast two-hybrid analysis as previously described (James et al., 1996). Transformants were selected on agar plates made from dropout media (-Trp, -Leu). Interactions were evaluated on agar that was - His or -His -Ade (Clontech, now Takara Bio). Growth in the absence of either supplement indicates transcription from reconstituted GAL4 proteins. Empty vectors were used as negative controls. A pair of plasmids producing Schizosaccharomyces pombe Mob1 and Sid2 was used as a positive control (Salimova et al., 2000; Hou et al., 2004).
In situ hybridization
Whole and regenerating planarians were processed for in situ hybridization as described in (King and Newmark, 2013). Riboprobes produced as described above were detected using alkaline-phosphatase-conjugated anti-digoxigenin Fab fragments (1:2000, [Roche]). Colorimetric signals were generated using a development solution containing 5-Bromo-4-chloro-3-indolyl phosphate (BCIP, [Roche]) and nitro blue tetrazolium chloride (NBT, [Roche]).
RNA interference
2–5 μg dsRNA generated as described above was combined with 30 μL of 4:1 liver:salts mix for each feeding. RNA feeding paradigms consisted of 3 feedings per experiment, with the first feeding on day 1, the second feeding on day 6, and the third feeding between days 10–12. Amputation occurred 5–7 days after the final feeding along prepharyngeal (anterior to the pharynx), postpharyngeal (posterior to the pharynx), or sagittal (down the midline) amputation planes. Animals were fixed and killed (or processed for RNA purification) 6 days post-amputation, with the exception being 9 days post-amputation for lateral regeneration experiments (Fig. 3G–H). dsRNA matching Aequorea green fluorescent protein (GFP) was used as a negative control in RNAi experiments.
Microscopy
Animals that had been subjected to in situ hybridization were mounted in 80% glycerol or 1xPBS prior to imaging. Stained and live animals were imaged with either an Axiocam color camera mounted on a Zeiss Axio Zoom.V16 microscope or with a Leica DFC420 camera mounted on a Leica M205A stereomicroscope. Images of yeast strains (Supp. Fig. 1) were captured on an Apple iPhone 5.
RNA sequencing and analysis
For each control and SSDP2(RNAi) samples, 4 plates of 10 worms were fed dsRNA as per the paradigm above. Animals were amputated prepharyngeally 5 days after the final feeding. 6 days post-amputation, animals for each sample were transferred to a 1.5 mL tube and salts were replaced with 250 μL TRIzol reagent (Invitrogen) before freezing on dry ice for storage at −80°C. RNAs were purified from each sample as per the manufacturer’s protocol, were DNAse treated (Promega), and were further cleaned with an RNA clean and concentrator kit (Zymo Research). RNAs were eluted 2x in 8 μL RNAse-free water. Three samples for each RNAi condition were submitted for quality analysis and library preparation with Illumina’s TruSeq Stranded mRNAseq kit. Libraries were sequenced on a HiSeq 4000 platform (100 nt sequencing, using sequencing kit v.1). Fastq files were demultiplexed and adaptors were trimmed. We received between 37 million and 45 million reads per sample. RNA-seq reads have been deposited as BioProject PRJNA907760 (Accession: SRX18465766-SRX18465771).
Sequences were imported into CLC Genomics Workbench 8 (QIAgen) and were mapped to the dd_Smed_v6 transcriptome (Brandl et al., 2016; Rozanski et al., 2019) using “batch” mode and standard parameters. We then completed gene expression comparison (EDGE) and statistical analysis, with a filter cutoff of 3 counts and with FDR correction. We present data in Supp. Table 4–5 with common and tagwise dispersion. For further analysis, we prioritized genes with P<0.05 after FDR correction and with the highest fold changes.
Supplementary Material
Acknowledgements
The authors thank Dr. Phillip Newmark and past members of the Newmark laboratory for their feedback on this project in its earliest stages. We thank current and past members of the Roberts-Galbraith laboratory for feedback and support during this project. We are grateful to Drs. M. Delores (Loli) Molina Jimenez and Francesc Cebrià for helpful discussion and for sharing their work on planarian LIM-HD genes. We are grateful to Dr. Alejandro Sánchez Alvarado and Shane Merryman, of the Stowers Institute for Medical Research, for providing S. mediterranea during our laboratory start-up and for sharing advice on planarian aquaculture. We thank Dr. Alvaro Hernandez and the staff of the Roy J. Carver Biotechnology Center (University of Illinois, Urbana-Champaign) for sequencing and experimental design advice for our RNA-sequencing experiments. We thank Dr. Kathleen Gould (Vanderbilt University) for sharing yeast strains and plasmids used in yeast two-hybrid analysis. We also thank the farmers and staff of White Oak Pastures for raising healthy cattle and providing beef liver we use to feed our planarians (Bluffton, GA). This work was supported by funding from the McKnight Foundation (McKnight Scholars Award to RRG) and the National Institute for Neurological Disease and Stroke at the National Institutes of Health (1R01NS128096–01). KBC was supported by the National Institute of General Medical Sciences of the National Institute of Health under award number 1T32GM142623 and the National Institute of Neurological Disorders and Stroke of the National Institute of Health under project number 5R25NS107179–02. KBC was also supported by the University of Georgia Research Foundation and the ARCS Foundation.
Abbreviations:
- CLIM
co-factor of LIM domains
- DNA
deoxyribonucleic acid
- dpa
days post-amputation
- ISH
in situ hybridization
- LDB
LIM domain binding protein
- LIM
LIN-11, Isl-1, and Mec3
- LIM-HD
LIM homeodomain
- LHX
LIM homeobox
- LMO
LIM only
- RNA
ribonucleic acid
- SSDP
single-stranded DNA-binding protein
References
- Adell T., Saló E., Boutros M., and Bartscherer K. (2009). Smed-Evi/Wntless is required for beta-catenin-dependent and -independent processes during planarian regeneration. Development 136, 905–910. [DOI] [PubMed] [Google Scholar]
- Agulnick A.D., Taira M., Breen J.J., Tanaka T., Dawid I.B., and Westphal H. (1996). Interactions of the LIM-domain-binding factor Ldb1 with LIM homeodomain proteins. Nature 384, 270–272. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M.K., McCollum D., Chang L., Wong K.C., Naqvi N.I., He X., Sazer S., and Gould K.L. (1998). Isolation and characterization of new fission yeast cytokinesis mutants. Genetics 149, 1265–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T., Ostendorff H.P., Bossenz M., Schluter A., Becker C.G., Peirano R.I., and Bach I. (2002). Multiple functions of LIM domain-binding CLIM/NLI/Ldb cofactors during zebrafish development. Mech Dev 117, 75–85. [DOI] [PubMed] [Google Scholar]
- Brandl H., Moon H., Vila-Farre M., Liu S.Y., Henry I., and Rink J.C. (2016). PlanMine - a mineable resource of planarian biology and biodiversity. Nucleic Acids Res 44, D764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein R., Levkovitz L., Yosef N., Yanku M., Ruppin E., Sharan R., Westphal H., Oliver B., and Segal D. (2010). Transcriptional regulation by CHIP/LDB complexes. PLoS Genet 6, e1001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo L., Witzel H.R., Kolovos P., Cheedipudi S., Looso M., Mylona A., van I.W.F., Laugwitz K.L., Evans S.M., Braun T., Soler E., Grosveld F., and Dobreva G. (2015). The Isl1/Ldb1 Complex Orchestrates Genome-wide Chromatin Organization to Instruct Differentiation of Multipotent Cardiac Progenitors. Cell Stem Cell 17, 287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassata G., Rohrig S., Kuhn F., Hauri H.P., Baumeister R., and Burglin T.R. (2000). The Caenorhabditis elegans Ldb/NLI/Clim orthologue ldb-1 is required for neuronal function. Dev Biol 226, 45–56. [DOI] [PubMed] [Google Scholar]
- Castresana J. (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17, 540–552. [DOI] [PubMed] [Google Scholar]
- Cebrià F., Guo T., Jopek J., and Newmark P.A. (2007). Regeneration and maintenance of the planarian midline is regulated by a slit orthologue. Dev Biol 307, 394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebrià F., and Newmark P.A. (2005). Planarian homologs of netrin and netrin receptor are required for proper regeneration of the central nervous system and the maintenance of nervous system architecture. Development 132, 3691–3703. [DOI] [PubMed] [Google Scholar]
- Chen L., Segal D., Hukriede N.A., Podtelejnikov A.V., Bayarsaihan D., Kennison J.A., Ogryzko V.V., Dawid I.B., and Westphal H. (2002). Ssdp proteins interact with the LIM-domain-binding protein Ldb1 to regulate development. Proc Natl Acad Sci U S A 99, 14320–14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien C.T., Bartel P.L., Sternglanz R., and Fields S. (1991). The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci U S A 88, 9578–9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong T., Collins J.J. 3rd, Brubacher J.L., Zarkower D., and Newmark P.A. (2013). A sex-specific transcription factor controls male identity in a simultaneous hermaphrodite. Nature communications 4, 1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J.J. 3rd, Hou X., Romanova E.V., Lambrus B.G., Miller C.M., Saberi A., Sweedler J.V., and Newmark P.A. (2010). Genome-wide analyses reveal a role for peptide hormones in planarian germline development. PLoS Biol 8, e1000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A.J., Jeffries C.M., Trewhella J., and Matthews J.M. (2010). LIM domain binding proteins 1 and 2 have different oligomeric states. Journal of molecular biology 399, 133–144. [DOI] [PubMed] [Google Scholar]
- Currie K.W., and Pearson B.J. (2013). Transcription factors lhx1/5–1 and pitx are required for the maintenance and regeneration of serotonergic neurons in planarians. Development 140, 3577–3588. [DOI] [PubMed] [Google Scholar]
- de Melo J., Clark B.S., Venkataraman A., Shiau F., Zibetti C., and Blackshaw S. (2018). Ldb1- and Rnf12-dependent regulation of Lhx2 controls the relative balance between neurogenesis and gliogenesis in the retina. Development 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., Chevenet F., Dufayard J.F., Guindon S., Lefort V., Lescot M., Claverie J.M., and Gascuel O. (2008). Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36, W465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32, 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkhmandakh B., Makeyev A.V., and Bayarsaihan D. (2006). The role of the proline-rich domain of Ssdp1 in the modular architecture of the vertebrate head organizer. Proc Natl Acad Sci U S A 103, 11631–11636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler M., Graeb M., Mieszczanek J., Rutherford T.J., Johnson C.M., and Bienz M. (2015). An ancient Pygo-dependent Wnt enhanceosome integrated by Chip/LDB-SSDP. eLife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincher C.T., Wurtzel O., de Hoog T., Kravarik K.M., and Reddien P.W. (2018). Cell type transcriptome atlas for the planarian Schmidtea mediterranea. Science 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsthoefel D.J., Cejda N.I., Khan U.W., and Newmark P.A. (2020). Cell-type diversity and regionalized gene expression in the planarian intestine. eLife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks R.G., Wang C., Levin J.Z., and Liu Z. (2002). SEUSS, a member of a novel family of plant regulatory proteins, represses floral homeotic gene expression with LEUNIG. Development 129, 253–263. [DOI] [PubMed] [Google Scholar]
- Gueta K., David A., Cohen T., Menuchin-Lasowski Y., Nobel H., Narkis G., Li L., Love P., de Melo J., Blackshaw S., Westphal H., and Ashery-Padan R. (2016). The stage-dependent roles of Ldb1 and functional redundancy with Ldb2 in mammalian retinogenesis. Development 143, 4182–4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley K.A., Rink J.C., and Sánchez Alvarado A. (2008). Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science 319, 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Motoishi M., Yazawa S., Itomi K., Tanegashima C., Nishimura O., Agata K., and Tarui H. (2011). A LIM-homeobox gene is required for differentiation of Wnt-expressing cells at the posterior end of the planarian body. Development 138, 3679–3688. [DOI] [PubMed] [Google Scholar]
- Hobert O., and Westphal H. (2000). Functions of LIM-homeobox genes. Trends in genetics : TIG 16, 75–83. [DOI] [PubMed] [Google Scholar]
- Hou M.C., Guertin D.A., and McCollum D. (2004). Initiation of cytokinesis is controlled through multiple modes of regulation of the Sid2p-Mob1p kinase complex. Mol Cell Biol 24, 3262–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivankovic M., Haneckova R., Thommen A., Grohme M.A., Vila-Farre M., Werner S., and Rink J.C. (2019). Model systems for regeneration: planarians. Development 146. [DOI] [PubMed] [Google Scholar]
- James P., Halladay J., and Craig E.A. (1996). Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144, 1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurata L.W., Pfaff S.L., and Gill G.N. (1998). The nuclear LIM domain interactor NLI mediates homo-and heterodimerization of LIM domain transcription factors. J Biol Chem 273, 3152–3157. [DOI] [PubMed] [Google Scholar]
- Kinare V., Iyer A., Padmanabhan H., Godbole G., Khan T., Khatri Z., Maheshwari U., Muralidharan B., and Tole S. (2020). An evolutionarily conserved Lhx2-Ldb1 interaction regulates the acquisition of hippocampal cell fate and regional identity. Development 147. [DOI] [PubMed] [Google Scholar]
- King R.S., and Newmark P.A. (2013). in situ hybridization protocol for enhanced detection of gene expression in the planarian Schmidtea mediterranea. BMC developmental biology 13, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivega I., Dale R.K., and Dean A. (2014). Role of LDB1 in the transition from chromatin looping to transcription activation. Genes & development 28, 1278–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Lee S., Agulnick A.D., Lee J.W., and Lee S.K. (2016). Single-stranded DNA binding proteins are required for LIM complexes to induce transcriptionally active chromatin and specify spinal neuronal identities. Development 143, 1721–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone D.P., Panagiotakos G., Heavner W.E., Joshi P., Zhao Y., Westphal H., and McConnell S.K. (2017). Compensatory Actions of Ldb Adaptor Proteins During Corticospinal Motor Neuron Differentiation. Cereb Cortex 27, 1686–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.J., McMann C.L., and Reddien P.W. (2019). Nuclear receptor NR4A is required for patterning at the ends of the planarian anterior-posterior axis. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magli A., Baik J., Pota P., Cordero C.O., Kwak I.Y., Garry D.J., Love P.E., Dynlacht B.D., and Perlingeiro R.C.R. (2019). Pax3 cooperates with Ldb1 to direct local chromosome architecture during myogenic lineage specification. Nature communications 10, 2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marz M., Seebeck F., and Bartscherer K. (2013). A Pitx transcription factor controls the establishment and maintenance of the serotonergic lineage in planarians. Development 140, 4499–4509. [DOI] [PubMed] [Google Scholar]
- Matthews J.M., and Visvader J.E. (2003). LIM-domain-binding protein 1: a multifunctional cofactor that interacts with diverse proteins. EMBO reports 4, 1132–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan K., Horta A., and Lomvardas S. (2019). LHX2- and LDB1-mediated trans interactions regulate olfactory receptor choice. Nature 565, 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcillo P., Rosen C., Baylies M.K., and Dorsett D. (1997). Chip, a widely expressed chromosomal protein required for segmentation and activity of a remote wing margin enhancer in Drosophila. Genes & development 11, 2729–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcillo P., Rosen C., and Dorsett D. (1996). Genes regulating the remote wing margin enhancer in the Drosophila cut locus. Genetics 144, 1143–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay M., Teufel A., Yamashita T., Agulnick A.D., Chen L., Downs K.M., Schindler A., Grinberg A., Huang S.P., Dorward D., and Westphal H. (2003). Functional ablation of the mouse Ldb1 gene results in severe patterning defects during gastrulation. Development 130, 495–505. [DOI] [PubMed] [Google Scholar]
- Newmark P.A., and Sánchez Alvarado A. (2000). Bromodeoxyuridine specifically labels the regenerative stem cells of planarians. Dev Biol 220, 142–153. [DOI] [PubMed] [Google Scholar]
- Nishimura K., Kitamura Y., Inoue T., Umesono Y., Yoshimoto K., Takeuchi K., Taniguchi T., and Agata K. (2007). Identification and distribution of tryptophan hydroxylase (TPH)-positive neurons in the planarian Dugesia japonica. Neurosci Res 59, 101–106. [DOI] [PubMed] [Google Scholar]
- Nishimura K., Kitamura Y., Taniguchi T., and Agata K. (2010). Analysis of motor function modulated by cholinergic neurons in planarian Dugesia japonica. Neuroscience 168, 18–30. [DOI] [PubMed] [Google Scholar]
- Nishioka N., Nagano S., Nakayama R., Kiyonari H., Ijiri T., Taniguchi K., Shawlot W., Hayashizaki Y., Westphal H., Behringer R.R., Matsuda Y., Sakoda S., Kondoh H., and Sasaki H. (2005). Ssdp1 regulates head morphogenesis of mouse embryos by activating the Lim1-Ldb1 complex. Development 132, 2535–2546. [DOI] [PubMed] [Google Scholar]
- Petersen C.P., and Reddien P.W. (2008). Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science 319, 327–330. [DOI] [PubMed] [Google Scholar]
- Plass M., Solana J., Wolf F.A., Ayoub S., Misios A., Glazar P., Obermayer B., Theis F.J., Kocks C., and Rajewsky N. (2018). Cell type atlas and lineage tree of a whole complex animal by single-cell transcriptomics. Science 360. [DOI] [PubMed] [Google Scholar]
- Roberts-Galbraith R.H., Brubacher J.L., and Newmark P.A. (2016). A functional genomics screen in planarians reveals regulators of whole-brain regeneration. eLife 5, pii: e17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Galbraith R.H., and Newmark P.A. (2015). On the organ trail: insights into organ regeneration in the planarian. Curr Opin Genet Dev 32, 37–46. [DOI] [PubMed] [Google Scholar]
- Rouhana L., Weiss J.A., Forsthoefel D.J., Lee H., King R.S., Inoue T., Shibata N., Agata K., and Newmark P.A. (2013). RNA interference by feeding in vitro-synthesized double-stranded RNA to planarians: Methodology and dynamics. Dev Dyn 242, C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanski A., Moon H., Brandl H., Martin-Duran J.M., Grohme M.A., Huttner K., Bartscherer K., Henry I., and Rink J.C. (2019). PlanMine 3.0-improvements to a mineable resource of flatworm biology and biodiversity. Nucleic Acids Res 47, D812–D820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimova E., Sohrmann M., Fournier N., and Simanis V. (2000). The S. pombe orthologue of the S. cerevisiae mob1 gene is essential and functions in signalling the onset of septum formation. J Cell Sci 113 ( Pt 10), 1695–1704. [DOI] [PubMed] [Google Scholar]
- Scimone M.L., Atabay K.D., Fincher C.T., Bonneau A.R., Li D.J., and Reddien P.W. (2020). Muscle and neuronal guidepost-like cells facilitate planarian visual system regeneration. Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimone M.L., Kravarik K.M., Lapan S.W., and Reddien P.W. (2014). Neoblast specialization in regeneration of the planarian Schmidtea mediterranea. Stem cell reports 3, 339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawlot W., and Behringer R.R. (1995). Requirement for Lim1 in head-organizer function. Nature 374, 425–430. [DOI] [PubMed] [Google Scholar]
- Sridhar V.V., Surendrarao A., Gonzalez D., Conlan R.S., and Liu Z. (2004). Transcriptional repression of target genes by LEUNIG and SEUSS, two interacting regulatory proteins for Arabidopsis flower development. Proc Natl Acad Sci U S A 101, 11494–11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torigoi E., Bennani-Baiti I.M., Rosen C., Gonzalez K., Morcillo P., Ptashne M., and Dorsett D. (2000). Chip interacts with diverse homeodomain proteins and potentiates bicoid activity in vivo. Proc Natl Acad Sci U S A 97, 2686–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meyel D.J., Thomas J.B., and Agulnick A.D. (2003). Ssdp proteins bind to LIM-interacting co-factors and regulate the activity of LIM-homeodomain protein complexes in vivo. Development 130, 1915–1925. [DOI] [PubMed] [Google Scholar]
- Wang H., Kim J., Wang Z., Yan X.X., Dean A., and Xu W. (2020). Crystal structure of human LDB1 in complex with SSBP2. Proc Natl Acad Sci U S A 117, 1042–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuoka Y., and Taira M. (2021). LIM homeodomain proteins and associated partners: Then and now. Current topics in developmental biology 145, 113–166. [DOI] [PubMed] [Google Scholar]
- Zheng Q., Schaefer A.M., and Nonet M.L. (2011). Regulation of C. elegans presynaptic differentiation and neurite branching via a novel signaling pathway initiated by SAM-10. Development 138, 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z., Ma H., Taniguchi-Ishigaki N., Nagarajan L., Becker C.G., Bach I., and Becker T. (2011). SSDP cofactors regulate neural patterning and differentiation of specific axonal projections. Dev Biol 349, 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.