Abstract
Neuroticism or negative emotionality (N/NE)—the propensity to experience and express more intense, frequent, or persistent negative affect—is a fundamental dimension of mammalian temperament. Elevated levels of N/NE are associated with a wide range of practically important outcomes, from marital stability and socioeconomic attainment to mental illness and premature death. Despite this, our understanding of the brain bases of N/NE remains far from complete. Converging lines of mechanistic and neuroimaging evidence suggest that N/NE reflects heightened reactivity to genuinely threatening stimuli in the extended amygdala, a circuit encompassing the dorsal amygdala in the region of the central nucleus (Ce) and the lateral division of the neighboring bed nucleus of the stria terminalis (BST), and that this association may be more evident when threat is uncertain. Here, we used a combination of approaches—including a multi-trait, multi-occasion composite of N/NE and neuroimaging assays of threat anticipation and perception—to demonstrate that individuals with a more negative disposition show heightened BST activation during uncertain-threat anticipation. A series of cross-validated, robust regression analyses revealed that N/NE is uniquely predicted by BST reactivity to uncertain-threat anticipation. In contrast, N/NE was unrelated to Ce activation during the anticipation of threat or to extended amygdala activation during the presentation of ‘threatening’ faces. Follow-up analyses revealed inconsistent evidence of between-task convergence in the BST and Ce, suggesting that threat-anticipation and emotional-face paradigms are not interchangeable probes of extended amygdala function. Collectively, these observations lay the foundation for the kinds of prospective-longitudinal and mechanistic studies that will be necessary to determine causation and, ultimately, to develop improved interventions for extreme N/NE.
INTRODUCTION
Neuroticism/Negative Emotionality (N/NE)—the tendency to experience heightened negative affect—is a fundamental dimension of childhood temperament and adult personality with profound consequences for health, wealth, and wellbeing (1, 2). Individuals with a more negative disposition show lower levels of socioeconomic attainment (3–5). They are more likely to experience interpersonal conflict, unemployment, and divorce; to have difficulty adjusting to major life transitions; and to feel lonely, dissatisfied, and burned out (3, 4, 6, 7). They are more likely to engage in unhealthy behaviors, to develop chronic disease, and to die prematurely (8–12). The deleterious consequences of N/NE are especially robust in the sphere of mental health. Individuals with a negative disposition are more likely to develop anxiety disorders and depression—and, among those who do, to experience more severe, recurrent, and treatment-resistant symptoms (12–23). Despite this burden, the neural systems underlying variation in the risk-conferring N/NE phenotype remain incompletely understood, impeding the development of more effective or tolerable biological interventions.
It is widely believed that N/NE reflects a neurobiological tendency to overreact to novelty, threat, and other ‘trait-relevant’ challenges, increasing the likelihood or intensity of negative emotions when aversive stimuli are encountered (24–27). While a number of brain regions have been implicated, the central extended amygdala (EAc) has received the most empirical scrutiny and occupies a privileged position in theoretical models of N/NE (1). The EAc is an anatomical macrocircuit encompassing the dorsal amygdala in the region of the central nucleus (Ce) and the neighboring bed nucleus of the stria terminalis (BST) (28). Perturbation studies in animals demonstrate that the Ce and BST are critical for orchestrating adaptive defensive responses to a wide variety of threats (29, 30). Large-scale neuroimaging studies (N=592) in monkeys show that Ce and BST reactivity to uncertain threat covaries with stable individual differences in anxious temperament, a core facet of N/NE (31). But the relevance of these discoveries to the complexities of the human brain and human temperament remains unclear. Only a handful of human neuroimaging studies have used genuinely distress-eliciting threats to examine relations between N/NE and EAc function and nearly all have focused exclusively on the amygdala (Supplementary Table S1). Studies using aversive photographs and Pavlovian threat cues have yielded inconsistent results. Much less is known about the BST. Only one small-scale (N=50) study has directly addressed this question, providing preliminary evidence that individuals with a more negative disposition show heightened BST activation during uncertain-shock anticipation (32). Although modest sample sizes, limited neuroanatomical resolution, and a lack of attention to the BST preclude decisive inferences, these observations motivate the hypothesis that N/NE reflects heightened recruitment of the BST, and possibly the dorsal amygdala, during aversive anticipation and that these associations may be more evident when threat is uncertain in its timing, likelihood, or intensity.
Here we used fMRI to quantify individual differences in EAc reactivity to a well-established anxiety-provocation (certain/uncertain threat-anticipation) paradigm in 220 young adults (Fig. 1). A multiband fMRI sequence, best-practices data processing pipeline, and spatially unsmoothed data enhanced our ability to resolve the Ce and BST. Subjects were selectively recruited from a pool of 6,594 pre-screened individuals, ensuring a broad spectrum of N/NE and addressing another key limitation of prior work in this area (33). We focused on ‘emerging adulthood’ because it is a time of profound, often stressful transitions (34). In fact, more than half of undergraduate students report moderate-to-severe symptoms of anxiety and depression, with many experiencing frank psychopathology during this turbulent developmental chapter (35, 36). Prior neuroimaging studies of N/NE have relied on optimistically biased analytic approaches (37). To address this bias, we focused on a priori anatomically defined regions-of-interest (ROIs) and cross-validated robust estimates of brain-trait associations. To enhance power and ensure crisp inferences, we used a composite measure of N/NE that was aggregated across repeated measurements, minimizing state-like fluctuations in responding that can attenuate brain-trait associations (Supplementary Fig. S1; 38, 39). Study hypotheses and approach were pre-registered.
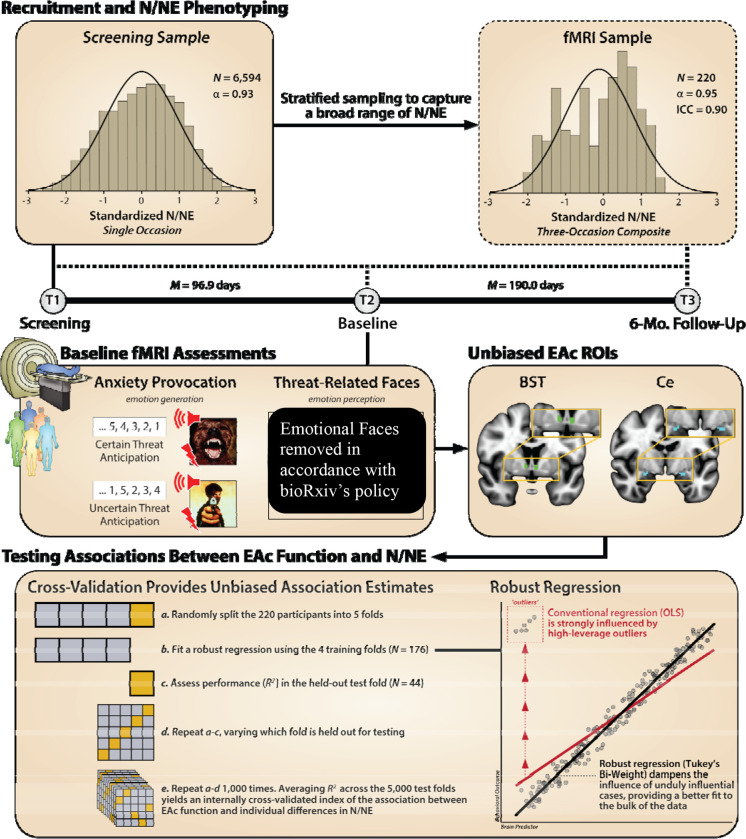
Figure 1. Threat anticipation increases anxious distress and arousal, and both threat anticipation and ‘threatening’ faces recruit the EAc.
Recruitment and N/NE Phenotyping. As depicted in the ‘Screening Sample’ histogram (top left), subjects were selectively recruited from a pool of over 6,000 previously phenotyped individuals, enabling us to examine a broad spectrum of N/NE. Stratified sampling was then used to ensure that this broad range of N/NE was maintained in the ‘fMRI Sample’ (N = 220; top right) was quantified using a well-established, 18-item measure that shows excellent psychometric characteristics. To minimize occasion-specific fluctuations, N/NE was quantified using the average of 3 timepoints – a screening, baseline, and 6-month follow-up visit (black timeline depicted above). Baseline fMRI Assessments. The present study used two fMRI paradigms: an fMRI-optimized threat-anticipation (anxiety-provocation/’threat-of-shock’) task (left), and an oft-used emotional-faces perception task (right). For further details about each of the two tasks, see Suppl. Fig. S2 and S3, respectively. Unbiased EAc ROIs. To test whether individual differences in N/NE are positively associated with extended amygdala reactivity in an unbiased manner, we extracted regression coefficients from anatomically-defined, spatially-unsmoothed BST and Ce ROIs (middle right; insets enlarged to highlight regions). Cross-Validation Provides Unbiased Association Estimates. To provide an unbiased estimate of model performance, present analyses used a repeated cross-validation approach (5-folds, 1,000 repetitions) (43). Specifically, we utilized the caret package (44) in R Version 4.0.2 (45), which includes functions to streamline the model training and evaluation process for complex regression and classification problems. The dataset was randomly subdivided into 5 ‘folds’ of approximately equal size. Then, the regression model was trained using 4 folds of the data (80%) and tested on the ‘held-out’ fold (20%). This was iteratively repeated 4 times using a different fold for testing each time. This method was then repeated 1,000 times, randomly re-allocating the data to a new set of 5 folds on each repetition. Final model coefficients we trained/fit using all of the data. Thus, in the present case, repeated cross-validation provides an unbiased test of Tukey’s bi-weight robust regression performance in data not used for training/fitting, not used to choose a family of models or tune hyper-parameters. Relative to other common machine learning resampling strategies (e.g., holdout approaches), repeated k-folds cross-validation reduces bias by increasing the size of the training sets and reduces the variance by aggregating several test set performances. Robust Regression. Models utilized a Tukey’s bi-weight robust regression to dampen the influence of high-leverage outliers.
To provide a more direct link with on-going research, we performed parallel analyses using data from a subset of 213 subjects who also completed an emotional-faces fMRI paradigm. Variants of the emotional-faces paradigm are widely used as probes of amygdala function—often in the guise of quantifying variation in Research Domain Criteria (RDoC) ‘Negative Valence Systems’ (40)—and have been incorporated into many prominent biobank studies (e.g. ABCD, UK Biobank). Although photographs of models posing ‘threat-related’ (fearful/angry) facial expressions strongly activate the amygdala (41), they do not elicit distress in typical adults and, as such, are better conceptualized as a probe of threat perception, rather than the experience or expression of distress (3). Here, we tested whether EAc reactivity to threat-related faces is associated with variation in N/NE.
The inclusion of the two neuroimaging tasks also afforded the opportunity to determine whether they are statistically interchangeable. It is often implicitly assumed that different experimental tasks that target a common function—such as ‘threat’—are quasi-equivalent probes of individual differences in circuit function. Yet this has rarely been examined empirically, never in a large sample, and never in the BST. Here, we formally quantified the degree to which the threat anticipation and perception tasks show statistical evidence of interchangeability (often termed ‘convergent validity’) in the EAc.
Identifying the neural systems sensitive to different kinds of threat and understanding their relevance to N/NE is important, and has the potential to refine basic scientific models of temperament and personality, guide the use and interpretation of biobank data, and inform the development of improved biological interventions for maladaptive N/NE (42).
RESULTS
Threat anticipation increases anxious distress and arousal
As a precursor to hypothesis testing, we used a series of repeated-measures generalized linear models to confirm that the threat-anticipation task had the intended effects on behavior (Fig. 2a, b). To inform interpretation of predicted brain-behavior associations, N/NE was included in the model.
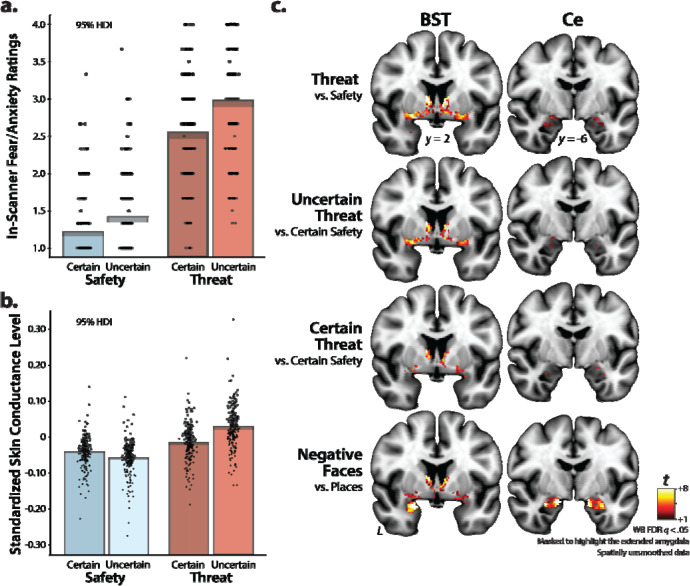
Figure 2. Threat anticipation increases anxious distress and arousal, and both threat anticipation and ‘threatening’ faces recruit the EAc.
As shown in panels a and b, threat anticipation robustly increased subjective symptoms (in-scanner ratings; top left) and objective signs (skin conductance; bottom left) of anxiety, and this was particularly evident when the timing of aversive stimulation was uncertain (Threat > Safety, ps < 0.001; Uncertain Threat > Certain Threat, ps < 0.001). Skin conductance results also revealed a Valence x Certainty interaction, such that the difference in skin conductance levels during Threat and Safety conditions was significantly greater when timing was uncertain, p < 0.001. Data (black points; individual participants), Bayesian 95% highest density interval (gray bands), and mean (bars) for each condition. Highest density intervals permit population-generalizable visual inferences about mean differences and were estimated using 1000 samples from a posterior Gaussian distribution. The coronal slices shown in panel c (right) depict voxels showing significantly increased activity within the BST (left column) and the dorsal amygdala/Ce (right column) for various contrasts of interest. All images are masked to highlight significant voxels in the extended amygdala. Together, these observations suggest that these regions are sensitive to both temporally certain and uncertain threat, as well as to threat-related face stimuli. For additional details, see Supplementary Tables 2–5; FDR, false discovery rate; WB, whole-brain-corrected.
Results revealed that subjects experienced significantly greater distress when anticipating aversive outcomes (Valence: t = 56.65, p < 0.001), and when anticipating outcomes with uncertain timing (Certainty: t = 11.54, p < 0.001). Furthermore, individuals with higher levels of N/NE showed indiscriminately elevated distress across conditions (N/NE: t = 4.12, p < 0.001). None of the interactions were significant, ps > 0.126.
A similar pattern was evident for anxious arousal. Skin conductance levels were significantly elevated when anticipating aversive outcomes (Valence: t = 38.00, p < 0.001), and when anticipating outcomes with uncertain timing (Certainty: t = 8.84, p < 0.001). The impact of threat on skin conductance was potentiated by temporal uncertainty (Valence x Certainty: t = 20.54, p < 0.001), such that the difference in skin conductance levels during Threat and Safety conditions was significantly greater when timing was uncertain (β = 0.09, t = 41.24, p < 0.001) than when it was predictable (β = 0.03, t = 12.30, p < 0.001).
Taken together, these observations confirm that the threat-anticipation task elicits robust anxiety across multiple response channels.
Threat anticipation and ‘threatening’ faces recruit the extended amygdala
We used a series of whole-brain voxelwise GLMs to confirm that the threat-anticipation and the emotional-faces tasks both engaged the extended amygdala. Results revealed significant BST and dorsal amygdala activation during threat anticipation (FDR q < 0.05, whole-brain corrected; Fig. 2c and Supplementary Table 2). The same general pattern was evident for the anticipation of Uncertain Threat and Certain Threat, relative to the implicit baseline (Fig. 2c and Supplementary Tables 3–4). Analyses focused on the presentation of ‘threatening’ faces also revealed significant activation in the BST and the dorsal amygdala (Fig. 2c and Supplementary Table 5).
Collectively, these findings demonstrate that both threat anticipation and emotional-face presentation are valid probes of extended amygdala function.
Individuals with higher levels of N/NE show increased BST reactivity to Uncertain Threat
We leveraged unbiased anatomical ROIs and spatially unsmoothed fMRI data to rigorously test the hypothesis that N/NE is associated with exaggerated recruitment of the EAc (BST and Ce) during aversive anticipation and to explore the possibility that these associations would be more evident when threat is uncertain (Fig. 1). As a precursor to hypothesis testing, we used a series of t-tests to confirm that the BST and Ce ROIs exhibit significant activation during the anticipation of Certain and Uncertain Threat, relative to their respective control conditions (Supplementary Fig. S7). Consistent with the voxelwise results (Fig. 2), both ROIs were significantly recruited by both kinds of Threat, ts(219)>3.10, ps<0.002 (Supplementary Table S7). We then used a cross-validated robust regression framework for hypothesis testing.
Results revealed that neither BST nor Ce activation during threat anticipation was significantly related to individual differences in N/NE (BST: β = 0.12, t(218) = 1.57, p = 0.117, R2CV: 0.037; Ce: β = 0.02, t(218) = 0.32, p = 0.749, R2CV = 0.019; Fig. 3).
Figure 3. Individuals higher in N/NE show increased BST reactivity to Uncertain Threat.
Individuals with a more negative disposition show increased BST reactivity to Uncertain Threat. Figure depicts standardized, cross-validated robust regression coefficients for threat-anticipation and emotional-faces contrasts of interest. The left side of the bar graph show findings for the BST. The right side of the bar graph show findings for the Ce. Error bars indicate the SE. Inset depicts the scatterplot corresponding to the key significant finding—that BST reactivity to Uncertain Threat is associated with heightened dispositional negativity when controlling for Certain Threat.
Prior work raises the possibility that relations between N/NE and extended amygdala function will be magnified when threat is uncertain. To test this, we computed robust regressions between N/NE and extended amygdala reactivity to temporally uncertain threat, separately for each ROI. To clarify specificity, models controlled for activation during the anticipation of certain threat. Results revealed that individuals with a more negative disposition showed significantly greater activation in the BST during Uncertain-Threat anticipation, controlling for Certain Threat (β = 0.24, t(217) = 2.61, p = 0.010, R2CV = 0.041; Fig. 3). This association remained significant in models that included Ce reactivity to threat (β = 0.26, t(215) = 2.80, p = 0.006, R2CV = 0.035), included Ce reactivity to just Uncertain Threat (β = 0.26, t(216) = 2.78, p = 0.006, R2CV = 0.040), included BST reactivity to emotional faces (β = 0.25, t(205) = 2.60, p = 0.010, R2CV = 0.033), or excluded BST reactivity to Certain Threat (β = 0.19, t(218) = 2.59, p = 0.010, R2CV: 0.051, 95% CI[0.10, 0.35]). Individual differences in N/NE were not significantly related to BST reactivity to Certain Threat (β = −0.09, t(217) = −1.00, p = 0.321, R2CV = 0.041; Fig. 3). N/NE was also unrelated to Ce reactivity for both types of Threat (Uncertain: β = −0.10, t(217) = −0.99, p = 0.323, R2CV = 0.016; Certain: β = 0.04, t(217) = 0.45, p = 0.650, R2CV = 0.016; Fig. 3). In short, relations between N/NE and extended amygdala reactivity are only evident for the BST and unique to Uncertain-Threat anticipation.
BST reactivity to Uncertain Threat was related to anxious arousal, but not subjective distress
To inform interpretation of the observed association between N/NE and BST function, we performed a series of follow-up analyses. The first examined relations between BST reactivity to Uncertain-Threat anticipation and concurrent measures of anxious distress and arousal. BST activation was associated with elevated levels of physiological arousal during Uncertain-Threat anticipation (β = 0.16, t(216) = 2.64, p = 0.009, R2CV = 0.045), but was unrelated to the intensity of subjective anxiety (β = 0.07, t(218) = 0.99, p = 0.323, R2CV = 0.028). A second set of analyses examined relations between N/NE and threat-elicited distress and arousal. Results mirrored the first set. Here, higher levels of N/NE were associated with more intense anxiety (β = 0.35, t(218) = 5.05, p < 0.001, R2CV = 0.124), but were unrelated to the degree of physiological arousal elicited by Uncertain-Threat anticipation (β = 0.00, t(216)=0.05, p = 0.959, R2CV = 0.020). Individual differences in anxious distress and arousal were not significantly associated, β = 0.10, t(216) = 1.31, p = 0.192, R2CV = 0.033. Together, these results suggest that relations between N/NE—the propensity to experience heightened negative affect—and BST reactivity to Uncertain Threat are indirect. BST reactivity to Uncertain Threat is associated with heightened physiological arousal, consistent with prior work, but not increased feelings of distress.
Individuals with higher levels of specific facets of N/NE (i.e., anxiety, depression) show increased BST reactivity to Uncertain Threat
Rather than being regarded as a unitary construct, recent psychometric work makes it clear that N/NE can be fractionated into more specific facets (e.g., anxious, depressive, emotionally volatile) (46). While our multi-occasion, multi-measure N/NE composite has many psychometric strengths, it cannot help us address which facets are most relevant to BST function, therefore precluding our ability to determine which facet trait(s) may be driving the association between high levels of N/NE and increased BST reactivity to Uncertain Threat. To address this, we turned to the BFI-2, which provides optimized subscales for the three major facets of N/NE; anxiety, depression, and emotional volatility (47).
Analogous to analyses with our primary outcome (N/NE), we computed robust regressions between BFI-2 facet subscales and extended amygdala reactivity to temporally uncertain threat, separately for each ROI. To clarify specificity, models controlled for activation during the anticipation of certain threat. Results revealed that individuals with higher levels of anxiety showed significantly greater activation in the BST during Uncertain-Threat anticipation, controlling for Certain Threat (β = 0.20, t(217) = 2.19, p = 0.029, R2CV = 0.032). These results are not particularly surprising given the strong positive correlation between our 3-occasion N/NE composite and the 2-occasion BFI-2 anxiety facet trait composite (r = .92; Supplementary Table 1), and the fact that most of the items on our N/NE scale are anxiety-related. Despite this difference in item content, higher levels of depression facet trait was also significantly related to increased BST reactivity to Uncertain Threat, β = 0.22, t(217) = 2.45, p = 0.015, R2CV: 0.034). Emotional volatility facet traits, however, were found to be unrelated to BST reactivity to Uncertain Threat, (β = 0.10, t(217) = 1.10, p = 0.272, R2CV = 0.018).
Collectively, these findings demonstrate that the significant association between increased BST reactivity to Uncertain-Threat and high N/NE does not hinge on our idiosyncratic N/NE measure, nor is it driven solely by anxiety. Rather, greater anxiety and depression were both significantly related to increased BST reactivity to Uncertain-Threat, suggesting that significant BST-N/NE relations may be reflective of a set of general ‘internalizing’ tendencies.
To date, the vast majority of human neuroimaging studies of N/NE have relied on emotional-face paradigms. While emotional faces are widely used and evoke robust extended amygdala activation, they do not elicit meaningful distress or arousal in typical populations. Here, we leveraged the same ROI-based analytic approach used to interrogate relations with threat anticipation to test relations between N/NE and extended amygdala reactivity to ‘threatening’ faces (Angry and Fearful Faces vs. Places). Results failed to reveal significant relations with either the BST (β = 0.03, t(211) = 0.37, p = 0.715, R2CV = 0.020) or Ce (β = 0.03, t(211) = 0.38, p = 0.702, R2CV = 0.022). Likewise, a series of exploratory voxelwise analyses did not detect significant relations between (mean-centered) N/NE and extended amygdala reactivity to either the threat-anticipation or the emotional-faces tasks (FDR q < .05, whole-brain corrected).
Individual differences in extended amygdala reactivity to the threat-anticipation and emotional-faces tasks show inconsistent evidence of convergent validity
Implicit in much of the literature is the assumption that different fMRI paradigms targeting a common function (e.g., ‘emotion’) are exchangeable probes of individual differences in brain function (e.g., amygdala). Yet, this assumption of ‘convergent validity’ has rarely been examined. Here, we used robust regressions with repeated cross-validation to test whether individual differences in BST and Ce reactivity to the anticipation of threat and the presentation of ‘threatening’ faces co-vary. Given our particular interest in temporally uncertain threat, we also examined the degree of convergent validity between: (1) uncertain-threat anticipation and emotional-face perception and (2) certain-threat anticipation and emotional-face perception.
Consistent with prior work (48), robust regression showed no evidence of convergence in Ce reactivity between threat anticipation and emotional-face perception,β = −0.01, t(207) = −0.19, p = 0.850, R2CV = 0.020, 95% CI[−0.15, 0.13] (Supplementary Table S6). In contrast to the Ce, robust regression yielded marginally significant evidence of between-task convergence in the BST, β = 0.11, t(207) = 1.82, p = 0.070, R2CV = 0.067, 95% CI[−0.03, 0.24].
Examination of relations between uncertain-threat anticipation and emotional-face perception showed similar non-significance, with no evidence of convergence in Ce reactivity (β = −0.10, t(207) = −1.47, p = 0.144, R2CV = 0.028, 95% CI[−0.23, 0.04]) or BST reactivity (β = 0.03, t(207) = 0.48, p = 0.633, R2CV = 0.045, 95% CI[−0.11, 0.16]) between tasks.
Lastly, robust regression showed no evidence of convergence in Ce reactivity between certain-threat anticipation and emotional-face perception,β = −0.11 , t(207) = −1.55, p = 0.122, R2CV = 0.030 , 95% CI[−0.24, 0.03]. In contrast to the Ce, robust regression yielded marginally significant evidence of between-task convergence in the BST, β = 0.12, t(207) = 1.72, p = 0.088, R2CV = 0.038, 95% CI[−0.02, 0.25].
Together, these observations raise important questions as to the exchangeability of ‘threat’ fMRI tasks, and caution against relying on a single task to understand individual differences in extended amygdala reactivity.
DISCUSSION
Elevated levels of N/NE confer increased risk for anxiety disorders, depression, and a variety of other adverse outcomes, but the underlying neurobiology has remained incompletely understood (1, 3, 49, 50). The present results demonstrate that individuals with a more negative disposition show heightened BST activation during temporally uncertain—but not certain—threat anticipation. In fact, BST reactivity to Uncertain Threat remained predictive of N/NE after controlling for either BST reactivity to Certain Threat or Ce reactivity to Uncertain Threat. Our results further suggest that relations between N/NE—the propensity to experience heightened negative affect—and BST function are indirect. BST reactivity to Uncertain Threat was associated with heightened signs of threat-elicited arousal, but not increased feelings of distress. N/NE was unrelated to Ce activation during threat anticipation and to extended amygdala (BST/Ce) activation during ‘threatening’ face presentation. While it is tempting to treat different ‘threat’ paradigms—from viewing photographs of ‘threatening’ faces to anticipating the delivery of aversive stimulation—as interchangeable probes of individual differences in extended amygdala function, the underlying assumption of convergent validity has rarely been examined. The present results provide no evidence of between-task convergence in the Ce, consistent with prior work (48), and only marginal evidence in the BST.
The present study provides new evidence that individual differences in N/NE are associated with heightened BST activation during Uncertain-Threat anticipation. This is consistent with anatomical evidence that the BST sends dense projections to the subcortical and brainstem regions that proximally mediate behavioral and physiological signs of negative affect (3). While the mechanistic relevance of the BST to N/NE remains under-explored, perturbation studies in rodents suggest that it is crucial for some forms of anxiety (51, 52). For example, excitotoxic lesions of the BST attenuate defensive responses (freezing) to diffuse, uncertain threat (elevated-plus maze; 53).
These mechanistic observations are consistent with neuroimaging evidence that the BST is sensitive to a range of noxious and threatening stimuli, including aversive photographs (54, 55), horror film clips (56; https://neurovault.org/collections/6237), and the uncertain anticipation of aversive stimuli (57, 58). With regard to N/NE, PET studies in monkeys demonstrate that BST metabolism is phenotypically and genetically correlated with trait anxiety and behavioral inhibition (31, 59). Likewise, fMRI work in humans shows that individuals with a more negative disposition are characterized by heightened BST engagement during the anticipation of temporally uncertain shock (32). The present results reinforce and extend this work by showing that BST reactivity to Uncertain-Threat anticipation is uniquely associated with individual differences in N/NE, over and above variation in BST reactivity to Certain Threat, and dorsal amygdala (Ce) reactivity to Uncertain Threat. Together, these observations reinforce the hypothesis that the BST is a central component of the distributed neural system governing N/NE. A key challenge for the future will be to clarify causation. There is compelling evidence that N/NE can be dampened through both psychological and pharmacological interventions (60–63). It would be fruitful to test whether these effects reflect attenuated BST reactivity to uncertain threat.
The present results have implications for understanding how N/NE confers risk for anxiety disorders and depression. Our findings show that individuals who, by virtue of their more negative disposition, are at risk for developing internalizing disorders are marked by heightened BST reactivity to Uncertain Threat. Moreover, we found that when N/NE was fractionated into more specific facets (e.g., anxious, depressive, emotionally volatile; 47), the significant association between increased BST reactivity to Uncertain-Threat and high N/NE was not contingent on our idiosyncratic N/NE measure, nor was it driven solely by anxiety. Rather, greater anxiety and depression were both significantly related to increased BST reactivity to Uncertain-Threat, suggesting that significant BST-N/NE relations may be reflective of a set of general internalizing risk. Taken together, these observations are consistent with conceptual models that emphasize the central role of threat uncertainty to the development and maintenance of pathological anxiety (1, 64, 65). It is also consistent with recent meta-analytic evidence that individuals with anxiety disorders show exaggerated BST reactivity to threat (66, 67). Collectively, this work motivates the hypothesis that exaggerated BST reactivity to uncertain threat is an active ingredient (i.e., diathesis) that helps mediate the association between N/NE and internalizing illnesses. Prospective-longitudinal studies in more nationally representative, diverse populations will be a key step to addressing this hypothesis.
Our findings also demonstrate that BST reactivity to Uncertain-Threat anticipation is associated with elevated physiological arousal, but not the intensity of threat-elicited anxiety. This result is broadly consistent with the theoretical model articulated by LeDoux and colleagues, who argue that the BST is primarily responsible for orchestrating behavioral and physiological responses to uncertain threat, and that it only indirectly contributes to anxious feelings (68–70). The present results reinforce the possibility that relations between BST function and N/NE—the tendency to experience heightened negative emotions—are implicit and indirect.
Our findings also have implications for the interpretation and design of neuroimaging studies of psychiatric risk and disease. Much of this work relies on emotional-faces tasks as the sole probe of negative valence systems. Yet, the present results demonstrate that extended amygdala reactivity to emotional faces is unrelated to the risk-conferring N/NE phenotype, consistent with three large-scale studies (71, 72). Moreover, analyses of convergent validity in the present study revealed modest between-task convergence in the BST, and negligible convergence in the dorsal amygdala (Ce), in broad accord with prior work (48). These observations caution against relying on a single task to understand the role of individual differences in extended amygdala function in internalizing illness (73). They also caution against muddling ‘threat-related’ faces (emotion perception) and genuinely distress-eliciting stimuli (emotion generation), a practice which is routine in experimental studies of novel psychiatric therapeutics (74–77). To the extent that uncertain-threat anticipation is key, it may be necessary to devise new paradigms that are more suitable for community and biobank samples—for instance, using aversive auditory stimuli or film clips.
It is important to acknowledge the modest size of the BST-disposition associations observed in the present study. This is not surprising; it is, in fact, entirely consistent with theoretical expectation and prior work focused on the extended amygdala and other isolated brain regions (37, 69). The magnitude of our core association—between N/NE and BST reactivity to Uncertain Threat—is also notably larger (5.1% variance explained) than associations between neuroticism and polygenic scores in a large GWAS study (78), and squarely in line with brain-behavior associations reported in large brain-wide association (‘BWAS’) studies (79). Collectively, these observations reinforce the value of well-chosen fMRI paradigms for probing clinically relevant phenotypes, though we acknowledge that the strength of the present findings are far too weak to be useful for screening, clinical, or treatment development purposes. In order to predict additional variance in N/NE, it will be necessary to adopt discovery-based multivoxel or multivariate machine learning approaches at the expense of neuroanatomical specificity (80).
Understanding the neural systems governing individual differences in N/NE is important. Elevated N/NE confers risk for a range of deleterious outcomes spanning health, wealth, and well-being. The present findings highlight the relevance of threat-elicited BST function to individual differences in N/NE, particularly when threat is uncertain. A relatively large and carefully phenotyped sample, well-controlled tasks, and a pre-registered, best-practices approach (e.g., spatially unsmoothed data, a priori anatomical ROIs, and repeated cross-validation framework) bolster confidence in the robustness and translational relevance of these results. These observations lay the groundwork for the kinds of prospective-longitudinal and mechanistic studies that will be necessary to determine causation and, ultimately, to develop improved interventions for extreme N/NE.
Supplementary Material
ACKNOWLEDGMENTS
Authors acknowledge assistance and critical feedback from A. Antonacci, L. Friedman, J. Furcolo, M. Gamer, C. Grubb, R. Hum, C. Kaplan, T. Kashdan, J. Kuang, C. Lejuez, D. Limon, B. Nacewicz, L. Pessoa, S. Rose, J. Swayambunathan, A. Vogel, J. Wedlock, members of the Affective and Translational Neuroscience laboratory, the staff of the Maryland Neuroimaging Center, and the Office of the Registrar at the University of Maryland. This work was partially supported by the ASAP Foundation; California National Primate Center; National Institutes of Health (DA040717, MH107444, MH121409, MH121735, MH128336, MH129851, OD011107, MH131264); National Research Foundation of Korea (2021R1F1A1063385 and 2021S1A5A2A03070229); University of California, Davis; University of Maryland; and Yonsei Signature Research Cluster Program (2021–22-0005). Authors declare no conflicts of interest.
Footnotes
RESOURCE SHARING
Raw data and select materials are publicly available at the National Institute of Mental Health Data Archive (https://nda.nih.gov/edit_collection.html?id=2447). Key neuroimaging maps have been or will be available at NeuroVault.Org. Processed neuroimaging data have also been shared with the Affective Neuroimaging Collaboratory (https://sites.dartmouth.edu/affectiveneuroimagingcollaboratory) and the ENIGMA Consortium Anxiety workgroup (https://enigma.ini.usc.edu/ongoing/enigma-anxiety).
REFERENCES
- 1.Shackman A. J. et al. , Dispositional negativity: An integrative psychological and neurobiological perspective. Psychological Bulletin 142, 1275–1314 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zentner M., Shiner R. L., “Fifty years of progress in temperament research: A synthesis of major themes, findings, and challenges and a look forward” in Handbook of temperament, Zentner M., Shiner R. L., Eds. (Guilford Press, New York, NY, 2012), pp. 673–700. [Google Scholar]
- 3.Hur J., Stockbridge M. D., Fox A. S., Shackman A. J., Dispositional negativity, cognition, and anxiety disorders: An integrative translational neuroscience framework. Progress in Brain Research 247, 375–436 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soto C. J., Do links between personality and life outcomes generalize? Testing the robustness of trait–outcome associations across gender, age, ethnicity, and analytic approaches. Social Psychological and Personality Science 12, 118–130 (2021). [Google Scholar]
- 5.Hoff K. A., Einarsdóttir S., Chu C., Briley D. A., Rounds J., Personality changes predict early career outcomes: Discovery and replication in 12-Year longitudinal studies. Psychological Science (in press). [DOI] [PubMed] [Google Scholar]
- 6.Buecker S., Maes M., Denissen J. J. A., Luhmann M., Loneliness and the Big Five personality traits: A meta-analysis. European Journal of Personality 34, 8–28 (2020). [Google Scholar]
- 7.Swider B. W., Zimmerman R. D., Born to burnout: A meta-analytic path model of personality, job burnout, and work outcomes. Journal of Vocational Behavior 76, 487–506 (2010). [Google Scholar]
- 8.Jokela M. et al. , Personality, disability-free life years, and life expectancy: Individual participant meta-analysis of 131,195 individuals from 10 cohort studies. Journal of Personality 10.1111/jopy.12513 (in press). [DOI] [PubMed] [Google Scholar]
- 9.Liu M. et al. , Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nature Genetics 51, 237–244 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puterman E. et al. , Predicting mortality from 57 economic, behavioral, social, and psychological factors. Proceedings of the National Academy of Sciences USA 117, 16273–16282 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo J. et al. , Personality and health: Disentangling their between-person and within-person relationship in three longitudinal studies. Journal of Personality and Social Psychology 122, 493–522 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rimfeld K. et al. , The winding roads to adulthood: A twin study. JCPP Advances 1, e12053 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucher M. A., Suzuki T., Samuel D. B., A meta-analytic review of personality traits and their associations with mental health treatment outcomes. Clinical Psychology Review 70, 51–63 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Brouwer M. E. et al. , Psychological theories of depressive relapse and recurrence: A systematic review and meta-analysis of prospective studies. Clinical Psychology Review 74, 101773 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Fullana M. A. et al. , Risk and protective factors for anxiety and obsessive-compulsive disorders: an umbrella review of systematic reviews and meta-analyses. Psychological Medicine 50, 1300–1315 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Kostyrka-Allchorne K., Wass S. V., Sonuga-Barke E. J. S., Research Review: Do parent ratings of infant negative emotionality and self-regulation predict psychopathology in childhood and adolescence? A systematic review and meta-analysis of prospective longitudinal studies. Journal of Child Psychology and Psychiatry 61, 401–416 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Hovenkamp-Hermelink J. H. M., Jeronimus B. F., Myroniuk S., Riese H., Schoevers R. A., Predictors of persistence of anxiety disorders across the lifespan: a systematic review. The Lancet Psychiatry 8, 428–443 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Lynch S. J., Sunderland M., Newton N. C., Chapman C., A systematic review of transdiagnostic risk and protective factors for general and specific psychopathology in young people. Clinical Psychology Review 87, 102036 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Schneider G., Köhnke C., Teismann H., Berger K., Childhood trauma and personality explain more variance in depression scores than sociodemographic and lifestyle factors - Results from the BiDirect Study. J Psychosom Res 147, 110513 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Scholten W. et al. , Recurrence of anxiety disorders and its predictors in the general population. Psychol Med 10.1017/s0033291721002877 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ten Have M. et al. , Duration of anxiety disorder and its associated risk indicators: Results of a longitudinal study of the general population. Depress Anxiety 38, 328–336 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Toenders Y. J. et al. , Predicting depression onset in young people based on clinical, cognitive, environmental, and neurobiological data. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 7, 376–384 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Waszczuk M. A. et al. , The prognostic utility of personality traits versus past psychiatric diagnoses: Predicting future mental health and functioning. Clinical Psychological Science 10.1177/21677026211056596 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eysenck H. J., The biological basis of personality (Charles C. Thomas, Springfield, IL, 1967). [Google Scholar]
- 25.Kagan J., Reznick J. S., Snidman N., Biological bases of childhood shyness. Science 240, 167–171 (1988). [DOI] [PubMed] [Google Scholar]
- 26.Spielberger C. D., “Theory and research on anxiety” in Anxiety and behavior, Spielberger C. D., Ed. (Academic Press, NY, 1966), pp. 3–22. [Google Scholar]
- 27.Goldsmith H. H. et al. , Roundtable: what is temperament? Four approaches. Child Dev 58, 505–529 (1987). [PubMed] [Google Scholar]
- 28.Shackman A. J., Fox A. S., Contributions of the central extended amygdala to fear and anxiety. Journal of Neuroscience 36, 8050–8063 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox A. S., Shackman A. J., The central extended amygdala in fear and anxiety: Closing the gap between mechanistic and neuroimaging research. Neuroscience Letters 693, 58–67 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hur J. et al. , Anxiety-related frontocortical activity is associated with dampened stressor reactivity in the real world. Psychological Science 33, 906–924 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox A. S. et al. , Intergenerational neural mediators of early-life anxious temperament. Proceedings of the National Academy of Sciences USA 112, 9118–9122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Somerville L. H., Whalen P. J., Kelley W. M., Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry 68, 416–424 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charpentier C. J. et al. , How representative are neuroimaging samples? Large-scale evidence for trait anxiety differences between fMRI and behaviour-only research participants. Social Cognitive and Affective Neuroscience (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shackman A. J. et al. , Dispositional negativity in the wild: Social environment governs momentary emotional experience. Emotion 18, 707–724 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vos T. et al. , Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet 396, 1204–1222. (interactive dashboard at https://vizhub.healthdata.org/gbd-compare/) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ACHA, American College Health Association-National College Health Assessment (ACHA-NCHA III) (American College Health Association, Silver Spring, MD, 2020). [Google Scholar]
- 37.Shackman A. J., Fox A. S., Getting serious about variation: Lessons for clinical neuroscience. Trends in Cognitive Sciences 22, 368–369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikolaidis A. et al. , Suboptimal phenotypic reliability impedes reproducible human neuroscience. bioRxiv 10.1101/2022.07.22.501193 (2022). [DOI] [Google Scholar]
- 39.Fox A. S., Lapate R. C., Davidson R. J., Shackman A. J., “The nature of emotion: A research agenda for the 21st century” in The nature of emotion. Fundamental questions, Fox A. S., Lapate R. C., Shackman A. J., Davidson R. J., Eds. (Oxford University Press, New York, NY, 2018), pp. 403–417. [Google Scholar]
- 40.Clark L. A., Cuthbert B., Lewis-Fernandez R., Narrow W. E., Reed G. M., Three approaches to understanding and classifying mental disorder: ICD-11, DSM-5, and the National Institute of Mental Health’s Research Domain Criteria (RDoC). Psychol Sci Public Interest 18, 72–145 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Miller K. L. et al. , Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci 19, 1523–1536 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sauer-Zavala S. E., Barlow D. H., Neuroticism. A new framework for emotional disorders and their treatment (Guilford Press, NY, NY, 2021). [Google Scholar]
- 43.Yarkoni T., Westfall J., Choosing Prediction Over Explanation in Psychology: Lessons From Machine Learning. Perspect Psychol Sci 12, 1100–1122 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuhn M. et al. , Caret: classification and regression training. R package version 6.0–86. 2020. URL http://CRAN.R-project.org/package=caret (2020). [Google Scholar]
- 45.Team R. C., R: A language and environment for statistical computing. Version 4.0. 2 (Taking Off Again). R Foundation for Statistical Computing, Vienna, Austria: (2020). [Google Scholar]
- 46.Soto C. J., John O. P., The next Big Five Inventory (BFI-2): Developing and assessing a hierarchical model with 15 facets to enhance bandwidth, fidelity, and predictive power. Journal of personality and social psychology 113, 117 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Soto C. J., John O. P., The next Big Five Inventory (BFI-2): Developing and assessing a hierarchical model with 15 Facets to enhance bandwidth, fidelity, and predictive power. J Pers Soc Psychol 113, 117–143 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Villalta-Gil V. et al. , Convergent individual differences in visual cortices, but not the amygdala across standard amygdalar fMRI probe tasks. Neuroimage 146, 312–319 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boissy A., Fear and fearfulness in animals. Quarterly Review of Biology 70, 165–191 (1995). [DOI] [PubMed] [Google Scholar]
- 50.Shackman A. J., Stockbridge M. D., LeMay E. P., Fox A. S., “The psychological and neurobiological bases of dispositional negativity” in The nature of emotion. Fundamental questions, Fox A. S., Lapate R. C., Shackman A. J., Davidson R. J., Eds. (Oxford University Press, New York, NY, 2018), pp. 67–71. [Google Scholar]
- 51.Duvarci S., Bauer E. P., Paré D., The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. Journal of Neuroscience 29, 10357–10361 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glover L. R. et al. , A prefrontal-bed nucleus of the stria terminalis circuit limits fear to uncertain threat. Elife 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duvarci S., Bauer E. P., Paré D., The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. J Neurosci 29, 10357–10361 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brinkmann L. et al. , Inter-individual differences in trait anxiety shape the functional connectivity between the bed nucleus of the stria terminalis and the amygdala during brief threat processing. Neuroimage 166, 110–116 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Sabatinelli D. et al. , Emotional perception: Meta-analyses of face and natural scene processing. Neuroimage 54, 2524–2533 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Hudson M. et al. , Dissociable neural systems for unconditioned acute and sustained fear. NeuroImage 10.1016/j.neuroimage.2020.116522, 116522 (in press). [DOI] [PubMed] [Google Scholar]
- 57.Hur J. et al. , Anxiety and the neurobiology of uncertain threat anticipation. bioRxiv 10.1101/2020.02.25.964734, 2020.2002.2025.964734 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mobbs D. et al. , Neural activity associated with monitoring the oscillating threat value of a tarantula. Proceedings of the National Acadademy of Sciences USA 107, 20582–20586 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shackman A. J. et al. , Heightened extended amygdala metabolism following threat characterizes the early phenotypic risk to develop anxiety-related psychopathology. Mol Psychiatry 22, 724–732 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberts B. W. et al. , A systematic review of personality trait change through intervention. Psychol Bull 143, 117–141 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Sauer-Zavala S. et al. , Does the unified protocol really change neuroticism? Results from a randomized trial. Psychol Med 10.1017/s0033291720000975, 1–10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stieger M. et al. , Changing personality traits with the help of a digital personality change intervention. Proc Natl Acad Sci U S A 118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zemestani M., Ommati P., Rezaei F., Gallagher M. W., Changes in neuroticism-related constructs over the Unified Protocol for Transdiagnostic Treatment of Emotional Disorders in patients on an optimal dose of SSRI. Personal Disord 10.1037/per0000482 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Davis M., Walker D. L., Miles L., Grillon C., Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35, 105–135 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grupe D. W., Nitschke J. B., Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci 14, 488–501 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chavanne A. V., Robinson O. J., The Overlapping Neurobiology of Induced and Pathological Anxiety: A Meta-Analysis of Functional Neural Activation. Am J Psychiatry 178, 156–164 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shackman A. J., Fox A. S., Two Decades of Anxiety Neuroimaging Research: New Insights and a Look to the Future. Am J Psychiatry 178, 106–109 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.LeDoux J. E., Anxious. Using the brain to understand and treat fear and anxiety (Viking, New York, NY, 2015). [Google Scholar]
- 69.LeDoux J. E., Pine D. S., Using Neuroscience to Help Understand Fear and Anxiety: A Two-System Framework. Am J Psychiatry 173, 1083–1093 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Mobbs D. et al. , Viewpoints: Approaches to defining and investigating fear. Nat Neurosci 22, 1205–1216 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Silverman M. H. et al. , Trait neuroticism and emotion neurocircuitry: Functional magnetic resonance imaging evidence for a failure in emotion regulation. Dev Psychopathol 31, 1085–1099 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klamer S. et al. , Association between Neuroticism and Emotional Face Processing. Sci Rep 7, 17669 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holmes A. J., Patrick L. M., The Myth of Optimality in Clinical Neuroscience. Trends Cogn Sci 22, 241–257 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwandt M. L. et al. , The CRF1 antagonist verucerfont in anxious alcohol-dependent women: Translation of neuroendocrine, but not of anti-craving effects. Neuropsychopharmacology 41, 2818–2829 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paulus M. P. et al. , The effects of FAAH inhibition on the neural basis of anxiety-related processing in healthy male subjects: a randomized clinical trial. Neuropsychopharmacology 46, 1011–1019 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kwako L. E. et al. , The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology 40, 1053–1063 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bloomfield M. A. P. et al. , The acute effects of cannabidiol on emotional processing and anxiety: a neurocognitive imaging study. Psychopharmacology (Berl) 239, 1539–1549 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagel M. et al. , Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat Genet 50, 920–927 (2018). [DOI] [PubMed] [Google Scholar]
- 79.Marek S. et al. , Reproducible brain-wide association studies require thousands of individuals. Nature 603, 654–660 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Woo C. W., Chang L. J., Lindquist M. A., Wager T. D., Building better biomarkers: brain models in translational neuroimaging. Nat Neurosci 20, 365–377 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.