Abstract
Physical activity (PA) is a key component for brain health and Reserve, and it is among the main dementia protective factors. However, the neurobiological mechanisms underpinning Reserve are not fully understood. In this regard, a noradrenergic (NA) theory of cognitive reserve (Robertson, 2013) has proposed that the upregulation of NA system might be a key factor for building reserve and resilience to neurodegeneration because of the neuroprotective role of NA across the brain. PA elicits an enhanced catecholamine response, in particular for NA. By increasing physical commitment, a greater amount of NA is synthetised in response to higher oxygen demand. More physically trained individuals show greater capabilities to carry oxygen resulting in greater Vo2max – a measure of oxygen uptake and physical fitness (PF). In the current study, we hypothesised that greater Vo2 max would be related to greater Locus Coeruleus (LC) MRI signal intensity. As hypothesised, greater Vo2max related to greater LC signal intensity across 41 healthy adults (age range 60–72). As a control procedure, in which these analyses were repeated for the other neuromodulators’ seeds (for Serotonin, Dopamine and Acetylcholine), weaker associations emerged. This newly established link between Vo2max and LC-NA system offers further understanding of the neurobiology underpinning Reserve in relationship to PA. While this study supports Robertson’s theory proposing the upregulation of the noradrenergic system as a possible key factor building Reserve, it also provide grounds for increasing LC-NA system resilience to neurodegeneration via Vo2max enhancement.
Keywords: Locus Coeruleus, Vo2Max, aging, brain age, neuroimaging, voxel based morphometry
Introduction
It is estimated that 40% of dementia causes are modifiable by simply addressing people’s lifestyle (Livingston et al. 2020). Of this 40%, lifetime physical activity (PA) has a crucial role in protecting against neurodegeneration and related cognitive decay (Livingston et al. 2020). It is documented that individuals with a greater physical fitness (PF) level can sustain more severe neuropathological burdens resulting in mitigated cognitive impairment (Livingston et al. 2020; Wallace et al. 2019; Erikson et al. 2018). For these reasons PA has been associated with the construct of reserve, which is defined as the individual’s ability to maintain better-than-expected cognitive and brain functions given brain insult or psychological and neurological illnesses (Stern 2000; Stern et al. 2020; Cabeza et al. 2018). Hence, it is understood that PA contributes to maintaining healthy cognition and reducing neurodegeneration (Song et al. 2022; Livingston et al. 2020; Wallace et al. 2019; Erikson et al 2018).
In the current study we investigated whether the association between physical fitness and resilience to dementia may be partially explained by the noradrenergic theory of cognitive reserve (Robertson 2013&2014), proposing that continuous activation (and the related integrity) of the Locus Coeruleus (LC) – noradrenergic system could be a key candidate affecting cognitive reserve and resilience capabilities. Specifically, it is hypothesized that the continuous upregulation of the LC-NA system over the lifespan might be one of the key neurobiological components for building cognitive reserve, given the marked noradrenergic decay observed in neurodegenerative diseases (Braak et al. 2011; Mather et al. 2016). In fact, it is documented that the LC-NA system degeneration is the primary marker of dementia and its loss of functionality and integrity has been associated with cognitive decline (Braak et al. 2011; Ehrenberg et al. 2017; Betts et al. 2017; Satoh et al. 2019; Dahl et al. 2020). In Robertson’s model, the noradrenergic up-regulation would be protective against the neurodegenerative dynamics due to the neuroprotective role of NA in brain (Heneka et al. 2002; Heneka et al. 2015). Supporting evidence has shown anti-oxidative (Troadec et al. 2005) and anti-inflammatory (Feinstain et al. 2002; Giorgi et al. 2020) properties of NA along with promotion of neurogenesis and synaptogenesis by increasing brain derived neurotrophic factor (BDNF) (Counts et al. 2010; Hassani et al. 2020; Traver et al. 2005; Mannari et al. 2008). Accordingly, in the last decades several studies have supported this model reporting that LC-NA system integrity was related to greater brain and cognitive health (Wilson et al. 2013; Elman et al. 2021; Jacobs et al. 2021; Plini et al. 2021; Dahl et al. 2022; Galgani et al. 2023), including better attentive and mnemonic functions both in healthy and clinical populations (Clewett et al. 2016; Dahl et al. 2019; Dutt et al. 2021; Plini et al. 2021; Prokopiou et al. 2022; Galgani et al. 2023).
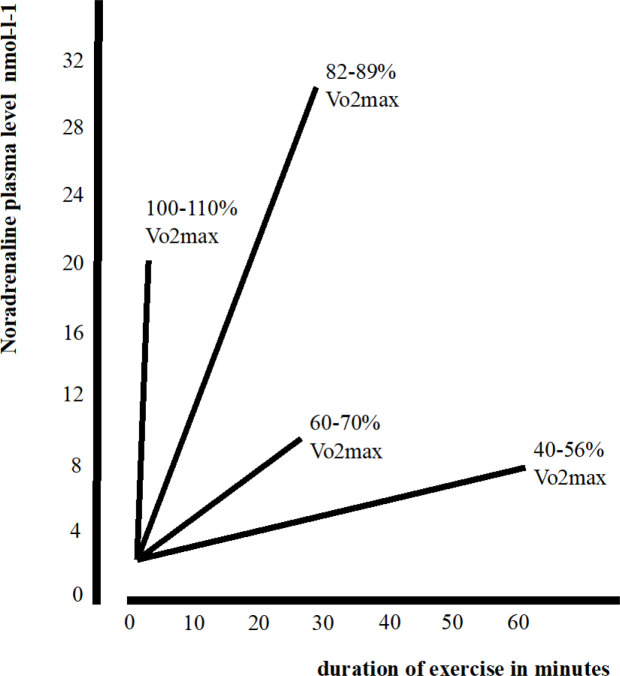
However, no studies have investigated the possible relationship between LC-NA system integrity and PA. As shown in figure 1, by increasing physical exertion, a greater amount NA is synthesized and released in response to higher oxygen demand (Kjær et al. 1987; Bekar et al. 2012; Green et al 2007; Escourrou et al. 1984; Winter et al. 2007). Maximal training intensity (between 110% and 80% of maximal oxygen consumption – Vo2max) elicits the highest NA release in comparison with lesser demanding training intensities. Indeed, the LC-NA system is involved in breath cycles and LC neurons are highly sensitive to oxygen and Co2 variations (Melnychuk et al. 2021; Melnychuk et al. 2008; Biancardi et al. 2007), and in mediating vasoconstriction and vasodilation in response to oxygen demand variations (Forense et al. 2020; Zhang et al. 2019; Bola et al. 2017; Bekar et al. 2012). Further, trained individuals show greater oxygen carrying capacity while training (Crowley et al. 2022; Milanović et al. 2015). Vo2max is a measure of oxygen uptake commonly used as a main parameter of physical fitness and cardiovascular health (Strasser and Burtscher 2018; Hanscomb et al. 2021; Xu et al. 2022; Babu et al. 2022; https://health.ucdavis.edu/sports-medicine/resources/vo2description). A large meta-analyses carried out on 102,980 individuals found that greater Vo2 max was protective against all causes of mortality, while individuals with lower physical fitness show elevated risk (Kodama et al. 2009). More recently, greater levels of Vo2max have been associated with better brain and cognitive health (Wendell et al. 2013; Hwang et al. 2017; Pantzar et al. 2018; Wallace et al. 2019; Erikson et al. 2018), greater cortical thickness (Olivo et al. 2021) and greater white matter integrity (d’Arbeloff et al. 2020; Maleki et al. 2022). Furthermore, a study conducted on 2013 healthy adults (age range 21–84) found that greater cardio respiratory fitness (CRF) was associated with greater total brain volume, and greater grey matter volume in key brain regions such as the middle temporal gyrus, the hippocampal gyrus and the orbitofrontal cortex (Wittfled at el. 2020). In the same study greater Vo2 max was also related to greater volume in the bilateral anterior cingulate cortex, an area rich in noradrenergic receptors (Palomero-Gallager et al. 2015). Correspondingly, literature on dementia reported that higher Vo2 max and overall higher levels of physical fitness were associated with better brain and cognitive health (Manning et al. 2022; Nilsson et al. 2020; Bosch et al. 2020) and stronger resilience to neurodegeneration (namely more preserved brain integrity and functioning – Olivo et al. 2021; Petkus et al. 2021; Kurl et al. 2018; Ding et al. 2018; Livingston et al. 2020).
Figure1 -.
Plasma noradrenaline concentration in relation to intensity and duration of exercise. Figure adapted from Kjaer et al. 1987 with permission of Prof. Michael Kjaer
In light of the neuroprotective effects of greater Vo2 max together with the well documented LC-NA system role in dementia progression, we investigated whether variation in Vo2max was related to the MRI signal intensity (a parameter of integrity / tissue density) of the LC-NA system. We hypothesised that greater Vo2max would be associated with greater LC signal intensity due to the neuroprotective effects of NA release. Specifically, we considered vo2max levels as a proxy measure of chronic NA up-regulation, and therefore, greater Vo2max as an outcome of the frequent and intense LC-NA system up-regulation required by strenuous PA accumulated across the lifespan. (see figure 1 adapted from Kajer et al. 1987).
To test Robertson’s model, we performed voxel-based morphometry (VBM) analyses on 41 healthy older adults provided by the Centre for BrainHealth®, The University of Texas at Dallas -USA (Chapman et al. 2016). First, the relationship between LC signal intensity and Vo2 max was investigated. Second, the relationship between Vo2max and other key neuromodulator seeds were investigated as a control procedure. Therefore the analyses were repeated for the Dopaminergic system using the Ventral Tegmental Area (VTA), for the Serotoninergic system using on the Dorsal and Median Raphe (DR and MR) and on for the Cholinergic system using the Nucleus Basalis of Meynert (NBM). Third, we investigated the relationship between biological brain maintenance – BrainPAD (Boyle et al. 2021) and the neuromodulatory subcortical system to explore a possible relationship between Vo2max and BrainPAD. Our final aim was to investigate the relationship between LC and a measure of high order cognitive control assessed with the test of strategic learning (TOSL) (Chapman et al. 2016).
Methods
Participants, Neuropsychological tests and Vo2Max assessment
The study was approved by the Institutional Review Board of the Texas Southwestern Medical Center at Dallas, University of Texas at Dallas and The Cooper Institute. All study experiments were performed in accordance with the Declaration of Helsinki and other relevant guidelines and regulations. All participants provided informed consent.
Data were provided by the Center of BrainHealth the University of Dallas Texas by Sandra Chapman and colleagues (Chapman et al. 2016). Participants were healthy older adults, 15 males and 26 females with age ranging from 60 to 72 years old. All participants underwent a preliminary screening, therefore none of them had no history of neurological or psychological illness and scored below 14 at the Beck Depression Inventory (BDI). In addition, all subjects were right-handed, not exceeded 40 of body mass index (BMI) and had as minimum education level an high school diploma. Lastly all participants’ IQ level was within the normative ranges and they scored above 26 at the Montreal Cognitive Assessment (MoCA). After the screening, participants underwent 3T high-res MRI and a neuropsychological battery which included the Test of Strategic Learning (TOSL) as primary measure for high order executive functioning. Indeed, TOSL is a tool sensitive to complex attention, memory and abstract reasoning. Subsequently, VO2max and maximum heart rate (MHR) were evaluated via electrocardiogram, a “Lode—Excalibur Sport cycle ergometer, (Groningen, Netherlands) and a Jaeger Oxycon Pro, (Hoechberg, Germany)” for Vo2max assessment. For detailed info please refer to (Chapman et al. 2016) and see table 1.
Table 1.
shows the key sociodemographic, neural indices and neuropsychological characteristics of the sample tested. Key: TIV, total intracranial volume; MaxVo2 (maximal oxygen consumption); MaxHR (maximum heart rate); BrainPAD (Brain predicted age discrepancy (biological maintenance index); TOSL (test of strategic learning).
| age | education | TIV | MaxVO2 | MaxHR | BrainPAD | TOSL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| female | male | female | male | female | male | female | male | female | male | female | male | female | male | |
| n. | 26 | 15 | 26 | 15 | 26 | 15 | 26 | 15 | 26 | 15 | 26 | 15 | 26 | 15 |
| Mean | 63.846 | 64.000 | 16.692 | 16.667 | 1356.84 | 1561.87 | 18.478 | 23.491 | 158.000 | 146.133 | −1.800 | −2.090 | 5.346 | 4.733 |
| SD | 3.552 | 3.140 | 2.035 | 1.447 | 91.432 | 110.200 | 2.972 | 4.380 | 13.345 | 14.352 | 7.878 | 11.245 | 1.832 | 2.374 |
| Min. | 57.000 | 58.000 | 12.000 | 14.000 | 1195.16 | 1401.53 | 14.350 | 14.050 | 125.000 | 125.000 | −13.048 | −30.205 | 2.000 | 1.000 |
| Max. | 71.000 | 70.000 | 18.000 | 18.000 | 1535.43 | 1792.00 | 23.000 | 29.630 | 181.000 | 169.000 | 12.398 | 16.243 | 10.000 | 10.000 |
Neuroimaging processing and analyses
41 3T T1-weighted high-res MRI images of healthy older adults (age range 60–72 - 15 males, 26 females) were processed using the standard CAT12 pipeline with a voxel size of 1mm3. The scans were modulated and oriented in MNI template and then whole brain images (MNI grey matter + white matter) were used for the formal analyses. In CAT12 a voxel-based morphometry (VBM) multiple regression model was built in order to assess the relationship between Vo2max and LC signal intensity. Age in years, gender, education, maxHR and total intracranial volume (TIV) were included as covariates and the positive relationship was tested in keeping with the main hypothesis, predicting that greater LC signal intensity would be related with higher scores Vo2 max. As a control procedure, the opposite, negative relationship was also tested. As additional control procedure, the same analyses were repeated for the neuromodulatory seed regions of the serotoninergic, dopaminergic and cholinergic systems, in order to contrast them with the noradrenergic hypothesis. The VBM analyses investigating the relationship between LC and BrainPAD and the TOSL test followed the same pipeline with the only exception being maxHR, which was not entered as a covariate.
BrainPAD methodology
Brain Predicted Age Discrepancy (BrainPAD), calculated following Boyle et al. 2020, is an objective measure reflecting how the brain is ageing. It is similar to the previously developed indices such as Brain Gap Estimation—BrainAGE by Gaser et al. 2013, but it is computed only based on grey matter rather than white (WM) and grey matter (GM) together since GM is linearly associated with aging (Ge et al. 2002; Boyle et al. 2020). BrainPAD is obtained by calculating the discrepancy between the chronological age and the biological age of the brain defined on a healthy brain ageing trajectory of typical subjects. Boyle and colleagues defined the normal trajectory of GM ageing in healthy subjects. Then, they trained an algorithm to predict the degree of GM deterioration in relation to the chronological age in a three further populations of healthy individuals. BrainPAD provides a candidate measure of brain maintenance in terms of years of accelerated or reduced brain degeneration. Higher discrepancies between the biological brain age and chronological age are indices of abnormal ageing and poorer brain maintenance In the current study we also aimed to replicate and extend the findings reported in Plini et al. 2021, where LC signal intensity was related to better brain maintenance (lower BrainPAD scores) across 686 participants, both healthy controls, mild cognitive impairments and demented subjects.
Neuromodulatory subcortical system ROI definition
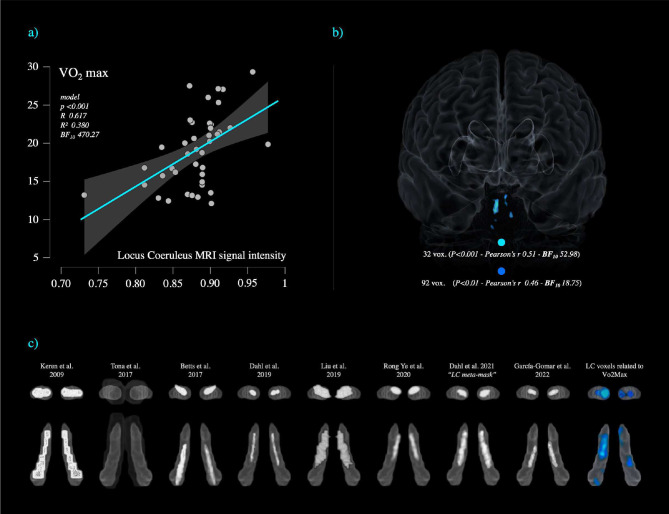
Accurate MRI localization of the Locus Coeruleus (LC) in the human brain is still lacking of agreement (Liu et al. 2019). In the last few years, several probabilistic maps of the LC have been released (Keren et al. 2009 & 2015; Tona et al. 2017; Betts et al. 2017; Dahl et al. 2019; Liu et al. 2019; Rong Ye et al. 2020; Dahl et al. 2021; García-Gomar et al. 2022), however, these probabilistic maps, by using neuromelanin sensitive MRI sequences (neuromelanin is a pigment found in catecholaminergic neurons), described inconsistent localization and spatial extent of the LC within the MNI space. These differences reflect a relatively large anatomical variability suggesting that the LC varies across the general population both during the lifespan and in response to neurodegeneration (Yeo-Jin et al 2021; Liu et al. 2019; Keren et al. 2009). With increasing age, the LC neuromelanin signal intensity tends to shift from the rostral to the caudal portion (Liu et al. 2019; Keren et al. 2009). This process might be influenced by ageing, the degree of biological brain maintenance and even dementia progression, which is likely to exacerbate this “caudal- shifting” process. Indeed, it is acknowledged that the LC-NA system is susceptible to compensatory changes involving the caudal portion of the LC and peri- coeruleus/LC-peri-dendritic regions (Epi-coeruleus and Sub-coeruleus) extending even towards the serotoninergic nuclei (Mai and Paxinos 2012; Szot et al. 2007; Szot et al. 2006; Hoogendijk et al. 1999; Szot et al. 2000; Matchett et al. 2021; Ishimatsu et al. 1996; Janitzky et al. 2020). Therefore as outlined by Yeo-Jin Yi et al. (2021), because of these dynamics, different MRI atlases (particularly the ones developed on young populations) might not reflect the actual LC extent because of the lower strength of neuromelanin signals in young subjects. In other words, the actual LC might be underestimated because not all the LC cells (voxels) would be “sufficiently labelled yet” (Yeo-Jin et al 2021). Conversely, the atlases made on older populations might exclude rostral regions more likely to be labelled in younger populations. Therefore, focusing only on one of the previously published LC atlases or on a “conservative one” might be misleading in terms of generalisation of the LC spatial localization. As observed by Yeo-Jin Yi et al. (2021), the combined LC spatial localization across different subjects and conditions exceeded the typical spatial localization of an individual LC. For these reasons, in the current study, the isolation of the LC signal intensity was achieved using the “omni-comprehensive” LC mask (Plini et al. 2021) which solves the inconsistent LC spatial localization reported by previous studies (Keren et al. 2009 & 2015; Tona et al. 2017; Betts et al. 2017; Dahl et al. 2019; Liu et al. 2019; Rong Ye et al. 2020; Dahl et al. 2021; García-Gomar et al. 2022) without encroaching upon other pontine and cerebellar regions, and without crossing the walls of the 4th ventricles. In figure 3c in the result section the different LC atlases can be visualized in comparison with the LC-omni-comprehensive mask. Further details can be found in supplementary materials of Plini et al. 2021 and at this link: https://www.youtube.com/watch?v=90bsA6Jqxs4). The MRI LC omni-comprehensive mask was manually developed (voxel by voxel in FSL eyes – edit mode) to carefully define a common space that included all the previous maps as to increase the likelihood of inclusion of the entirety of the LC rostro-caudal extent, and to properly account for the large individual variability documented in literature. By doing so, voxel-wise structural analyses isolating the LC-NA system were made possible in CAT12.
Figure 3 -.
a) shows the relationship between vo2max and Locus Coeruleus signal intensity in a sample of 41 healthy subjects (age range 60–72). Results are covaried for Age, Gender, Education, Total Intracranial Volume and max heart rate for P<0.001*. b) The significant LC voxels for P<0.01 and P<0.001 clusters are displayed in a 3D-reconstruction of the brain from a midline frontal point of view. c) The LC clusters associated with Vo2max are shown in comparison with the previously published LC atlases and masks displayed on the LC omni-comprehensive mask by Plini et al. 2021.
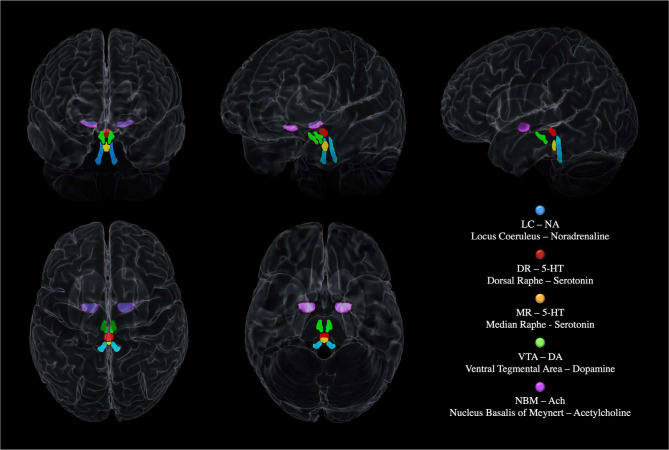
The other neuromodulatory seeds were based on previously published atlases: th MR and DR ROIs were provided by Beliveau et al. 2015, and the VTA mask was obtained by downloading the VTA MNI probabilistic map (Pauli et al. 2018) from the NeuroVault website (https://neurovault.org/ accessed on 15 December 2018). The NMB was developed on the basis of the probabilistic MNI maps of the acetylcholine cells of the Forebrain, which are provided by SPM Anatomy Toolbox 2.2c (https://www.fzjuelich.de/inm/inm1/EN/Forschung/_docs/SPMAnatomyToolbox/SPMAnatomyToolbox_node.html accessed on 15 December 2018) by Zaborszky et al. 2008 and George et al. 2011, Schulz et al. 2018, Liu et al. 2015, Kilimann et al. 2014, Koulousakis et al. 2019). These other neuromodulatory seeds were chosen on the base on their cortical and cerebellar projections. Consequently, the DR and the MR were chosen over the other Raphe nuclei because they vastly project to the cortex and the Cerebellum being the main serotoninergic nuclei of the CNS. Similarly, the VTA was chosen over the Substantia Nigra (SN) because the VTA is the main brain nucleus responsible of the cortical irroration of Dopamine (while the SN projects subcortically but not in the Cerebellum which does not shows relevant dopaminergic projections). Regarding the Cholinergic system, the NBM was chosen over the Tegmental Cholinergic Neurons, because it has the largest number of cholinergic neurons, and it projects diffusely to whole brain’s cortex (May and Paxinos 2012). Figure 2 shows the 3D MRI reconstruction of the five neuromodulators’ seeds.
Figure 2 –
The neuromodulatory subcortical system: the main nuclei synthetizing and projecting to the cortex the main neuromodulators involved in cognition. The figure shows a 3D MRI reconstruction of the five neuromodulators’ seeds within the whole brain.
Bayesian modelling, multiple regression, ANCOVA and mediation analyses
Pearson’s and Bayesian correlation matrices were built to explore the different relationships between Vo2max, neuropsychological measures and BrainPAD with the five neuromodulators’ seeds. The average signal intensity of the clusters of voxels identified by the VBM analyses was used to better isolate each neuromodulators’ effect. The average values were extracted applying the 1mm3 binary masks of the 5 ROIs on whole brain images using FSL. In FSL the flags of “fslstats” “-k” (mask) and “-m” (output mean) were used to gather the average voxel intensities. The average signal intensity was then calculated subject-by-subject for each ROI and computed in each of the following models performed in JASP (https://jasp-stats.org/).
In the Bayesian multiple regression model Vo2max was treated as dependent variable and the five neuromodulators entered as independent covariates, in order to compare their different effects against the “null model”. Age, gender, education, TIV and maxHR were treated as covariates and added to the “null model”. The analyses followed the default JASP setting with the exception being that Bayesian information criteria (BIC) was selected in the advanced option of JASP interface.
To test whether LC signal intensity variations were congruently associated with Vo2max level, in a ANCOVA model was conducted. Vo2max was treated as a factor divided in 2 levels (Vo2max above 20 [n.22) and Vo2max below 20 [n.19]). A 20 cut-off was decided on the basis of previous literature referring to the same age range of the current sample – Kurl et al. 2018; Wendell et al. 2013; Meyers et al 2017; Kodama et al. 2009). The average LC signal intensity was entered as a dependent variable and the model was controlled for age, gender, education, TIV and maxHR. Post-hoc analyses were then Bonferroni corrected with 10000 bootstrapping.
Finally, to test potential mediation effects between Vo2max and brain maintenance, a mediation analysis with parallel multiple mediators was performed using Vo2max as a predictor (Y) and BrainPAD (X) as outcome. As multiple mediators, the average signal intensity of the five neuromodulators’ seeds were entered in parallel. The model was covaried for age, gender, education, TIV and maxHR. Standard model was set with 95% confidence intervals and 5000 bootstrap samples.
Results
Vo2max and Locus Coeruleus MRI signal intensity
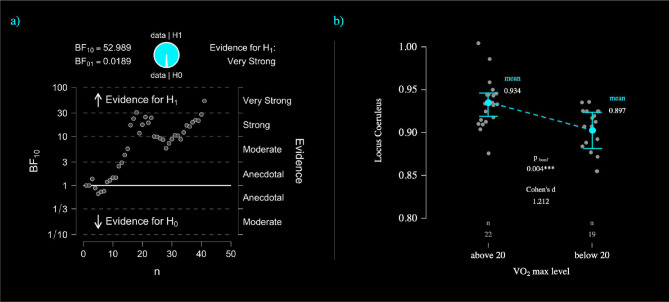
As shown in table 2, the analyses confirmed a relationship between Vo2max and LC signal intensity after controlling for age, gender, TIV, education and maxHR. 92 voxels within the LC region significantly related with vo2max, i.e., greater LC signal intensity was associated with greater vo2max values. We note that a portion of the significant LC voxels in our findings overlaps the core of the previously published LC atlases and masks (Keren et al. 2009 & 2015; Tona et al. 2017; Betts et al. 2017; Dahl et al. 2019; Liu et al. 2019; Rong Ye et al. 2020; Dahl et al. 2021; García-Gomar et al. 2022), as shown in figure 3. The LC cluster survived when the statistical threshold was increased to p<0.01 and p<0.001 with a maximal Bayesian factor (BF10) of 55.98. Other weaker but relevant associations were observed between the DR and VTA signal intensity. A cluster of 32 voxel within the serotoninergic DR region and a cluster of 30 voxels within dopaminergic VTA region, which did not survive when the statistical threshold was raised to p<0.001. Minor associations were found for the MR and NBM nuclei. When the inverse relationships were tested, negligible results were found and none survived at p<0.01 threshold.
Table 2.
shows the results for the VBM multivariate linear regression analyses testing the positive relationship between the five ROIs and vo2max.
| Neuromodulatory System | side | MNI coordinates | peak T valuea | peak Z scoreb | peak cluster Kec | p value uncorrd | FWEe | FDRf | total number of voxels for p<0.05 with max BF10g | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | Z | |||||||||
| vo2 max | |||||||||||
| Locus Coeruleus** | right | 4 | −38 | −22 | 3.72 | 3.39 | 65 | 0.000 | 1.000 | 1.005 | 92 (BF10 52.98) |
| Dorsal Raphe* | / | 0 | −28 | −10 | 2.77 | 2.61 | 5 | 0.004 | 1.000 | 1.005 | 32 (BF10 9.173) |
| Median Raphe | right | 2 | −34 | −20 | 2.01 | 1.95 | 6 | 0.632 | 1.000 | 1.005 | 6 (BF10 2.233) |
| Ventral Tegmental Area* | right | −2 | −24 | −18 | 2.61 | 2.47 | 3 | 0.446 | 1.000 | 1.005 | 30 (BF10 80.35) |
| Nucleus Basalis of Meynert | left | −16 | −8 | −8 | 1.91 | 1.86 | 3 | 0.870 | 1.000 | 1.005 | 3 (BF10 0.150) |
The results across the n.41 healthy subjects are covaried for age, gender, total intracranial volume, education and maximal heart rate (maxHR). The table reports the significant clusters of voxels predicting vo2max values for the statistical threshold of p<0.05. Bayesian Factors (BF10) are reported as parameter of strength in brackets. Cluster of voxels surviving p<0.01 are marked with *. Cluster of voxels surviving p<0.001 are marked with **. No clusters survived multiple comparison corrections (FWE).
Peak T value: T value of the most significant cluster of contiguous voxels
Peak Z-score: Z-score of the most significant cluster of contiguous voxels
Peak cluster Ke: number of voxels of the most significant cluster of contiguous voxels
P value uncorrected
FWE = family wise error correction value
FDR = false discovery rate correction value (q)
Total number of voxels outcoming in the ROI including all clusters of contiguous voxels (in brackets are reported Bayes Factors)
Factorial analysis – ANCOVA
When Vo2max values were treated as a factor divided in 2 levels (Vo2max above 20 VS Vo2max below 20), the LC average signal intensity differently variated between groups. Indeed, individuals with vo2max below 20 had significantly lower LC signal intensity in comparison with individuals with vo2max above 20 (about 1.2 standard deviations - Cohens’d 1.21). This difference remained significant after Bonferroni correction (P<0.004 – refer to table 4 and figure 4b) while controlling for age, gender, education, TIV and maxHR. This analysis is consistent with the previous multiple regression model proposing the same pattern of associations.
Table 4.
reporting the results of the ANCOOVA model treating vo2max as factor divided in 2 levels (above 20, below 20) and Locus Coeruleus average signal intensity as a dependent variable while controlling for age, gender, education, TIV and maxHR. Locus Coeruleus signal intensity significantly differs (Cohens’s d – 1.21) between the 2 groups (P<0.01 Bonferroni corrected).
| ANCOVA – Locus Coeruleus signal intensity and VO2Max level | |||||
|---|---|---|---|---|---|
|
| |||||
| Cases | Sum of Squares | df | Mean Square | F | p |
|
| |||||
| VO2Max Level | 0.011 | 1 | 0.011 | 9.847 | 0.004 |
| gender | 0.001 | 1 | 0.001 | 1.123 | 0.297 |
| age | 6.902e-4 | 1 | 6.902e-4 | 0.599 | 0.444 |
| education | 4.150e-6 | 1 | 4.150e-6 | 0.004 | 0.953 |
| TIV | 0.001 | 1 | 0.001 | 1.067 | 0.309 |
| MaxHR | 4.896e-4 | 1 | 4.896e-4 | 0.425 | 0.519 |
| Residuals | 0.039 | 34 | 0.001 | ||
| Bootstrapped Post Hoc Comparisons - Vo2Max level | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 99% bca† CI | ||||||||
|
|
|
|||||||
| Mean Difference | Lower | Upper | SE | bias | t | Cohen’s d | Pbonf | |
|
| ||||||||
| Above 20 | 0.039 | 0.014 | 0.079 | 0.012 | −2.687e-4 | 3.138 | 1.212 | 0.004*** |
Note. Type III Sum or Squares
p < .01
Note. The model is controlled for the effect of age, gender, education, TIV and maxHR.
Note. Mean Difference estimate is based on the median of the bootstrap distribution.
Note. Cohen’s d does not correct for multiple comparisons.
Note. Bootstrapping based on 1000 successful replicates.
Note. Results are averaged over the levels of: gender
Bias corrected accelerated
Figure 4 –
a) Bayesian sequential analyses for the association between Locus Coeruleus signal intensity and Vo2max. The sequential analyses reports “very strong” evidence supporting H1 (BF10 52.98). b) ANCOVA plot displays the mean difference between the 2 levels of Vo2max (individuals above Vo2max of 20 VS individual below Vo2max of 20).
Brain Maintenance (BrainPAD) and Locus Coeruleus MRI signal intensity
The VBM analyses investigating the relationship between LC and biological brain maintenance revealed a similar pattern of findings. A significant relationship was found between LC signal intensity and BrainPAD; an LC cluster of 153 voxels was associated with greater biological brain maintenance. Weaker findings were observed for the NBM (52 voxels) and the DR (33 voxels). This pattern of findings replicate our previous findings relating biological brain maintenance primarily to the noradrenergic system in a larger sample of 686 subjects (Plini et al. 2021). The LC cluster survived the statistical threshold of p<0.001. All the other ROIs showed a weaker pattern not surviving the p<0.01 threshold (see table 4 and figure 4 in the supplementary materials).
Neuropsychological Performance (TOSL) and Locus Coeruleus MRI signal intensity
In a similar vein, we observed a disproportionate link between LC and TOSL. Greater LC signal intensity related to higher scores on the TOSL, namely greater LC signal intensity was related with greater levels of high order cognitive control. A cluster of 101 voxels significantly predicted high order cognitive control performances, but did not survive multiple comparison corrections. Weaker and negligible results were observed for the other nuclei and none of them survived when the statistical threshold was increased to P<0.01 (see table 5 and figure 5 in the supplementary materials). These findings are consistent with previous work relating LC signal intensity to better cognitive performances (Clewett et al. 2016; Dahl et al. 2019; Dutt et al. 2021; Plini et al. 2021; Prokopiou et al. 2022; Galgani et al. 2023).
Multiple regressions, mediation analyses and correlation matrices
Correlation analysis revealed no relationship between the key variables investigated. As shown in table 3, the Bayesian multiple regression model including the group of five neuromodulators, showed that the combined effect of LC and VTA signal intensity is the best model relating to vo2max, followed by LC+VTA+DR. As stand-alone variable, LC is strongest predictor of vo2max followed by the VTA. These findings corroborate the VBM analyses and are consistent with our theoretical hypothesis linking the catecholaminergic involvement in physical activity to the subcortical neuromodulator’s MRI signal intensity.
Table 3.
reporting the results of the Bayesian multiple regression model investigating the associations between the five ROIs of the neuromodulatory subcortical system and vo2max by covaring for age, gender, education, total intracranial volume and max hearth rate.
| Bayesian Multiple Regression Model Comparison – vo2max and the neuromodulatory subcortical system | |||||
|---|---|---|---|---|---|
|
| |||||
| Models | P(M) | P(M|data) | BFM | BF10 | R2 |
|
| |||||
| Null model (incl. age, education, gender, TIV, MaxHR) | 0.167 | 8.737e-4 | 0.004 | 1.000 | 0.414 |
| LC + VTA | 0.017 | 0.162 | 11.381 | 1850.837 | 0.661 |
| LC + DR + VTA | 0.017 | 0.109 | 7.232 | 1249.826 | 0.685 |
| DR + VTA | 0.017 | 0.101 | 6.617 | 1154.276 | 0.653 |
| LC | 0.033 | 0.082 | 2.596 | 470.274 | 0.604 |
| VTA | 0.033 | 0.063 | 1.964 | 362.961 | 0.599 |
| LC + VTA + NBM | 0.017 | 0.056 | 3.477 | 637.007 | 0.674 |
Note. All models indude age, education, gender, TIV, MaxHR. Table displays only a subset of models; to see all models, select “No” under “Limit No. Models Shown”.
Mediation analyses showed no mediation effect for the five neuromodulators and Vo2max / BrainPAD, however, a significant direct effect between vo2max and BrainPAD was observed, leading us to infer that physical fitness might affect biological brain maintenance directly (see table 3 in supplementary materials). No other significant effects were observed for TOSL.
Discussion
In the current study we aimed to investigate a sample of 41 healthy adults to see whether vo2max was related to LC MRI signal intensity. As a secondary aim we investigated whether LC signal intensity could be related to a measure of biological brain maintenance (BrainPAD) and to higher order cognitive functions (TOSL) as already documented in literature (Plini et al. 2021). To this end, we tested the relationship between LC-NA system MRI signal intensity and vo2max values and contrasted this hypothesis against the other main neuromodulatory systems. As we anticipated, greater vo2max values were related with greater LC signal intensity. We also observed weaker but significant relationships between Vo2max and VTA and DR signal intensity. Compared to the other neuromodulatory nuclei, greater LC signal intensity disproportionally related with higher physical fitness level, better biological brain maintenance and greater cognitive control abilities (TOSL). These findings are consistent with our proposal that vo2max might be used as a proxy index of the upregulation of the LC-NA system and (possibly) its related integrity, albeit it should be noted that other unknown confounding factors may affect the nature of this relationship. In our view, the findings support the Noradrenergic theory of Reserve suggesting that greater physical activity, as indexed by higher oxygen uptake, may contribute reserve via the integrity of the LC/NA system. However, we also demonstrated a direct effect of greater vo2max upon biological brain maintenance independent of LC involvement.
Nevertheless, these preliminary findings linking Vo2max and the LC-NA system are suggestive that greater fitness levels can affect LC integrity contributing to overall LC and brain health. Given the importance of LC integrity for predicting brain maintenance and cognition, vo2max should be further considered among the resilience factors to neurodegenerative diseases and possibly a key variable to target for curtailing or preventing LC-NA degeneration across the lifespan (Braak et al. 2011; Ehrenberg et al. 2017; Betts et al. 2017; Satoh et al. 2019; Dahl et al. 2020). Our interpretation of these findings is that greater noradrenergic tone elicited by greater physical fitness can contribute to the integrity (maintenance) of the LC-NA system via diverse mechanisms. One mechanism might involve the anti-inflammatory and anti-oxidative properties of NA, namely, increased NA release following physical exercise (Kjær et al. 1987; Winter et al. 2007) could reduce overall LC and brain inflammation (Heneka et al. 2002; Heneka et al. 2012; Traver et al. 2005; Troadec et al. 2005; Feinstain et al. 2002). Similarly, increased NA might also increase the level of BDNF while reducing biomarkers of neurodegeneration within the LC and across the brain (Omulabi et al. 2021; Counts et al. 2010; Hassani et al. 2020; Traver et al. 2005; Mannari et al. 2008).
These neurobiological mechanisms might also explain the short- and long-term repercussion of physical activity on cognitive performances (Basso and Suzuki 2017; van Dogen et al. 2016; Blomstrand & Engvall 2020; Bosch et al. 2020; Nilsson et al. 2020; Winter et al. 2007), as several studies described enhanced cognitive functions immediately after physical exercise and better cognitive performances in well trained individuals (Wendell et al. 2013; Hwang et al. 2017; Pantzar et al. 2018; Wallace et al. 2019; Erikson et al. 2018). In a work from Nilsson and colleagues (Nilsson et al. 2020), the increased BDNF plasma levels following acute exercise were associated with greater cognitive training gains over a period of 12 weeks. Furthermore, another study by Engerof and colleagues (Engerof et al. 2018) found the regular physical activity led to greater bioavailability of BDNF resulting in greater brain volumes. These neurobiological processes are mediated by the LC-NA system which both underpin adaptation to physical stress and cognitive functioning (Kjær et al. 1987; Winter et al. 2007; Bekar et al. 2012; Green et al 2007; Jimenez et al. 2007; Escourrou et al. 1984; Robertson 2013&2014, Mather 2016). This interconnection was well presented empirically in a multimodal MRI study by Mather and colleagues (Mather et al. 2020), where several sets of isometric handgrip contractions for 18 seconds before cognitive tests were related to greater LC phasic activity during an fMRI attentional task, resulting in faster reaction times compared to control condition. In addition, Mather and colleagues, also reported that greater structural LC MRI contrast was related to better attentional control. Moreover, a previous study by Segal and colleagues (Segal et al. 2012) showed that 6 minutes of physical activity at 70% of vo2max enhanced memory consolidation of stimuli presented earlier both in MCI and healthy individuals. In parallel with enhanced memory, the groups which trained after stimuli presentation showed also significantly higher NA levels relative to controls. This interdependence between physical activity, cognition, catecholamines and BDNF levels was earlier demonstrated by Winter and colleagues (Winter et al. 2007) on a study on 27 healthy young subjects. Winter and colleagues measured catecholamines and BDNF plasma levels at baseline, after physical exercise and after a subsequent learning task. They found that, compared to rest- and moderate physical activity-conditions, only intense physical activity (6 min. running) elicited greater catecholamine and BDNF release and that these levels predicted greater immediate learning, and greater retention both at intermediate (1week) and long term (8–10months) stages. These studies, corroborate preceding evidence revealing 5-fold NA increase for 2 hours following exercise (Chatterton et al. 1996; Brenner et al. 1997; Brenner et al. 1998; Urhausen et al. 1995; Kjær et al. 1987), and well support the concept that enhanced NA release due physical activity might subsequently influence cognitive performance via LC-NA activity. In addition, from the reserve/resilience point of view, the long-term beneficial effect of PA on LC-NA system might also involve the chronic over-expression of neuropeptide Galanin (O’Neal et al. 2001; Van Hoomissen et al. 2004; Holmes et al. 2006). Indeed, it has been reported a dose-dependent PA-Galanin expression within the noradrenergic neurons (O’Neal et al. 2001; Holmes et al. 2006; Murray et al. 2010), which ameliorated stress-induced anxiety-like behaviour in murine models (Tillage et al. 2020). Other studies also reported a protective role PA-Galanin over-expression in maintenance of dendritic density and neurite outgrowth (Nowacka-Chmielewska et al. 2022; Sciolino et al. 2015; Holmes 2014; Hobson et al. 2013). Similarly, this effect might be combined with PA-BDNF levels, which were associated with increased LC dendrite density (Nakai et al. 2006; Matsunaga et al, 2004) as well as promoting neurogenesis in key brain structures such as the Hippocampus (Liu and Nusslock 2018).
The above extant literature might suggest that vo2max variations can affect LC-NA integrity (maintenance) in humans, possibly influencing the degree of LC-NA resilience to neurodegeneration via several pathways including NA and following BDNF and Galanin metabolism (Nakai et al. 2006; Matsunaga et al, 2004; Holmes 2014; Nowacka-Chmielewska et al. 2022; Gibbons et al. 2023 – in figure 5 we summarise the aforementioned mechanism). Our preliminary findings show that vo2max varies with signal intensity, disproportionately within the structure of the LC, and highlights a potential mechanism in which physical fitness builds Reserve through the neuroprotective role of NA metabolism across the central nervous system (Robertson, 2013). However, it is worth mentioning that, as emerged both from VBM analyses and Bayesian modelling, our findings outline how other main neuromodulators can have a relevant role in relationship to vo2max and PA. Indeed, the observed associations between the dopaminergic VTA and serotoninergic DR and MR with vo2max are suggestive that greater physical fitness might be related with greater structural integrity of such nuclei. Indeed, in a similar way to NA but in minor extent, evidence shown increased 5-HT and DA following physical training (Heijnen et al. 2016; Cordeiro et al. 2017; Kjær et al. 1987; Bekar et al. 2012; Green et al 2007; Escourrou et al. 1984; Winter et al. 2007). Consistently, animal studies also shown greater concentrations both of 5-HT and DA within the cortex and the brainstem immediately after exercise (Heijnen et al. 2016; Dey et al. 1992; Meeusen and De Meirleir 1995; Foley and Fleshner 2008) implying greater integrity and functionality of such neuromodulatory systems might result in chronic (Foley and Fleshner 2008; Heijnen et al. 2016; Meeusen 2005; Dey et al. 1992). These findings together with our results are indicative that Vo2max levels might affect 5-HT and DA nuclei integrity in humans for the same mechanisms discussed above. Nevertheless, the overall monoaminergic involvement in response to PA might be considered as factor capable to shape brain and cognitive health, and that greater Vo2max level can simultaneously stimulate Brainstem nuclei, possibly resulting in greater integrity via the neuroprotective role of NA as described by Robertson (2013, 2014). It is possible that the neuroprotective role of NA on dopaminergic and cholinergic neurons as described in literature may also partially explain the nature of the association between Vo2max and other neuromodulatory nuclei (Zhu et al. 2018; Heneka et al. 2002; Heneka et al. 2015; Troadec et al. 2005; Feinstain et al. 2002; Giorgi et al. 2020; Counts et al. 2010; Hassani et al. 2020; Traver et al. 2005; Mannari et al. 2008). However, it worth mentioning the described noradrenergically mediated mechanisms might interact with other neurochemical dynamics, or even that the observed associations may be entirely dependent on different neurobiological domains related to physical activity, such as lactate metabolism for instance (Hashimoto et al. 2021; Cai et al. 2022; Xue et al. 2022).
Figure 5 -.
proposed neurobiological mechanisms between Vo2Max, LC-NA system and brain and cognitive health. Based on the Noradrenergic theory of Cognitive Reserve (Robertson 2013 & 2014). Physical activity (measured with Vo2Max), by up-regulating the LC-NA system, would support brain and cognitive health via NA, BDNF and Galanin metabolism. In this framework, the neuroprotective and anti-inflammatory effects of NA may act in combination with Galanin and BDNF levels elicited by Physical activity. Galanin, particularly in the LC, would be protective against stress promoting dendrite density. Similarly, greater BDNF levels would contribute maintaining LC dendrites while promoting overall brain plasticity.
Limitations
The main limitation of the current preliminary study is the small sample size; a larger sample and a longitudinal design would provide more accurate casual relationships among these variables and would enable an understanding of possible trajectories across time. The number of participants may also explain why our significant associations did not survive multiple comparison corrections and the lack of mediation effects. However, our sample size is similar to other studies investigating vo2max in relationship to brain and cognitive variables (Manning et al. 2022; Nilsson et al. 2020; Bosch et al. 2020), and also matched the size of other relevant studies whose used the same methodology than ours (Olivo et al. 2021; Petkus et al. 2021). Furthermore, we have also enhanced the validity of our design by performing thorough control analyses, contrasting 9 different hypotheses to the main hypothesis, and systematically covarying for relevant confounding factors such as age, gender, education, total intracranial volume, and max heart rate. These control analyses and the employment of both null hypothesis statistical testing and Bayesian modelling, strengthens the reliability of our observations.
Another constraint involves the methodological limitations of VBM analyses in retrospective studies which should be taken into account while considering these findings. Post-mortem histological investigations would offer more precise quantifications about the relationship between vo2max and the integrity (maintenance) of the neuromodulatory subcortical system. However, within these limitations, our findings are consistent with previous in-vivo evidence using similar methodology (Plini et al. 2021; Dutt et al. 2021; Olivo 2021; Petkus et al. 2021).
Lastly, it should be noted that the current study did not control for other potential confounds, such as genetic variables, ethnicity, body parameters (ratio between body fat and lean mass) and dietary behavior which can affect Vo2max level (Santisteban et al. 2022; Williams et al. 2017; Nightingale et al. 2016; Bacon et al. 2013; Muoio et al. 1994), LC variation and brain and cognitive health as well. It is important to note that this is a preliminary investigation based on a relatively small sample focused on VBM analysis of circumscribed areas of the brainstem. Although, many of these findings are consistent with the literature, further (longitudinal) investigations in larger samples are required. Nevertheless, the current findings show consistency between multiple regression and Bayesian approaches.
Conclusions and clinical implications
This is the first preliminary evidence linking in vivo LC-NA system integrity to Vo2max in healthy older subjects. These findings provide a possible explanation behind the neurobiological mechanisms connecting physical fitness to improved cognitive functions and resilience to neurodegenerative diseases. These novel and unique results shed light on possible neurobiological dynamics underpinning the relationship between physical fitness and brain health, whilst being consistent with Robertson’s theoretical framework positing the noradrenergic system as a key neuromodulatory basis of Reserve. However, it should be noted that the integrity of the LC-NA system may be the factor which leads to a higher exercise capacity (greater vo2max), and that the current study was not designed to address this. Therefore, future studies, using physical exercise to specifically target the LC-NA system, should aim to replicate the current findings, focusing particularly on prospective investigations including biomarkers, neuropsychological testing, body metrics and other life-style factors (including dietary behaviour). The current study provides additional evidence on the growing literature outlining the pivotal role of physical activity in matter of brain maintenance and cognitive health particularly in the face of neurodegenerative diseases (Matta Mello Portugal et al. 2013; Tyndall et al. 2018; Barnes et al. 2018; Livingston et al. 2020; Basso et al. 2022). This study adds support for preventative strategies focused on physical training protocols as a practical tool for increasing brain and cognitive health and protecting against neurodegeneration (Livingston et al. 2020; Batouli & Saba 2017; Freberg & Taglialatela 2022; Gibbons et al. 2023). Indeed, several studies reported that vo2max is effectively trainable using various methodologies at different ages in healthy populations (Gormley et al. 2008; Ozaki et al. 2013; Murawska-Cialowicz et al. 2015; Scribbans et al. 2016; Menz et al. 2019; Wen et al. 2019). Physical activity interventions also can significantly improve cognitive and brain health (Chapman et al. 2016; Kovacevic et al. 2020; Nilsson et al. 2020; Blomstrand & Engvall 2020; Zhu et al. 2021; Liu et al. 2021; Upadhyay et al. 2022; Basso et al. 2022), while evidence showed that in even MCI and early stages of dementia physical exercise can alleviate cognitive impairment, possibly slowing down disease progression (Anderson et al. 2011; Segal et al. 2012; Arcoverde et al. 2014; Reiter et al. 2015; Varma et al. 2017; Barnes & Corkery 2018; Yu et al. 2020).
Funding and Acknowledgments
Project founded by the Irish Research Council—Irish Research Council Laureate Consolidator Award (2018-23) IRCLA/2017/306 to Paul Dockree.
We are grateful to Dr. Laura Defina for assistance with collection and interpretation of the physical fitness measures. This work was supported by a grant from the National Institutes of Health (RC1-AG035954)
Thanks are extended to Francesca Fabbricatore for the thoughtful comments and for proofreading the manuscript.
Footnotes
Conflict of interest
The authors declare no conflict of interest
Data availability statement
The study was approved by the ethical review board of the University of Texas Southwestern Medical Center at Dallas, University of Texas at Dallas and The Cooper Institute. All participants were informed about the study protocol and provided informed written consent prior to scanning.”
The datasets used and/or analysed during the current study available from author Jeffrey S. Spence, Center for BrainHealth, University of Texas at Dallas, on reasonable request.
The Locus Coeruleus omni-comprehensive mask and the nifty images for significant clusters are available on request. Contact Dr. Emanuele RG Plini (plinie@tcd.ie / emanuele.rg.plini@gmail.com)
References
- 1.Anderson HS, Kluding PM, Gajewski BJ, Donnelly JE, Burns JM. Reliability of peak treadmill exercise tests in mild Alheimer disease. Int J Neurosci. 2011. Aug;121(8):450–6. doi: 10.3109/00207454.2011.574762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arcoverde C, Deslandes A, Moraes H, Almeida C, Araujo NB, Vasques PE, Silveira H, Laks J. Treadmill training as an augmentation treatment for Alzheimer’s disease: a pilot randomized controlled study. Arq Neuropsiquiatr. 2014. Mar;72(3):190–6. doi: 10.1590/0004-282X20130231. [DOI] [PubMed] [Google Scholar]
- 3.Babu AS, Arena R, Myers J. Post-COVID era: Time to re-introduce “cardiorespiratory fitness” as a vital sign. EClinicalMedicine. 2022. Jul 9;51:101546. doi: 10.1016/j.eclinm.2022.101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacon AP, Carter RE, Ogle EA, Joyner MJ. VO2max trainability and high intensity interval training in humans: a meta-analysis. PLoS One. 2013. Sep 16;8(9):e73182. doi: 10.1371/journal.pone.0073182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes JN, Corkery AT. Exercise Improves Vascular Function, but does this Translate to the Brain? Brain Plast. 2018. Dec 12;4(1):65–79. doi: 10.3233/BPL-180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basso JC, Suzuki WA. The Effects of Acute Exercise on Mood, Cognition, Neurophysiology, and Neurochemical Pathways: A Review. Brain Plast. 2017. Mar 28;2(2):127–152. doi: 10.3233/BPL-160040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basso Julia & Oberlin Douglas & Satyal Medha & O’Brien Catherine & Crosta Christen & Psaras Zach & Metpally Anvitha & Suzuki Wendy. (2022). Examining the Effect of Increased Aerobic Exercise in Moderately Fit Adults on Psychological State and Cognitive Function. Frontiers in Human Neuroscience. 16. 833149. 10.3389/fnhum.2022.833149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batouli SAH, Saba V. At least eighty percent of brain grey matter is modifiable by physical activity: A review study. Behav Brain Res. 2017. Aug 14;332:204–217. doi: 10.1016/j.bbr.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Bekar LK, Wei HS, Nedergaard M. The locus coeruleus-norepinephrine network optimizes coupling of cerebral blood volume with oxygen demand. J Cereb Blood Flow Metab. 2012. Dec;32(12):2135–45. doi: 10.1038/jcbfm.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Betts M.J., Kirilina E., Otaduy M.C.G., Ivanov D., Acosta-Cabronero J., Callaghan M.F., Lambert C., Cardenas-Blanco A., Pine K., Passamonti L., et al. Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain. 2019;142:2558–2571. doi: 10.1093/brain/awz193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biancardi V., Bicego K., Almeida M.C., Gargaglioni L.H. Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflügers Arch.-Eur. J. Physiol. 2007;455:1119–1128. doi: 10.1007/s00424-007-0338-8. [DOI] [PubMed] [Google Scholar]
- 12.Blomstrand Peter & Engvall Jan. (2020). Effects of a Single Exercise Workout on Memory and Learning Functions in Young Adults – a Systematic Review. Translational Sports Medicine. 4. 10.1002/tsm2.190. [DOI] [Google Scholar]
- 13.Bola RA, Kiyatkin EA. Inflow of oxygen and glucose in brain tissue induced by intravenous norepinephrine: relationships with central metabolic and peripheral vascular responses. J Neurophysiol. 2018. Feb 1;119(2):499–508. doi: 10.1152/jn.00692.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyle R., Jollans L., Rueda-Delgado L.M., Rizzo R., Yener G.G., McMorrow J.P., Knight S.P., Carey D., Robertson I.H., Emek-Savaş D.D., et al. Brain-predicted age difference score is related to specific cognitive functions: A multi-site replication analysis. Brain Imaging Behav. 2021;15:327–345. doi: 10.1007/s11682-020-00260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner I, Shek PN, Zamecnik J, Shephard RJ. Stress hormones and the immunological responses to heat and exercise. Int J Sports Med. 1998. Feb;19(2):130–43. doi: 10.1055/s-2007-971895. [DOI] [PubMed] [Google Scholar]
- 16.Brenner IK, Zamecnik J, Shek PN, Shephard RJ. The impact of heat exposure and repeated exercise on circulating stress hormones. Eur J Appl Physiol Occup Physiol. 1997;76(5):445–54. doi: 10.1007/s004210050274. [DOI] [PubMed] [Google Scholar]
- 17.Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, Lindenberger U, Nyberg L, Park DC, Reuter-Lorenz PA, Rugg MD, Steffener J, Rajah MN. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci. 2018. Nov;19(11):701–710. doi: 10.1038/s41583-018-0068-2. Erratum in: Nat Rev Neurosci. 2018 Dec;19(12):772. Erratum in: Nat Rev Neurosci. 2018 Dec;19(12):772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai M, Wang H, Song H, Yang R, Wang L, Xue X, Sun W, Hu J. Lactate Is Answerable for Brain Function and Treating Brain Diseases: Energy Substrates and Signal Molecule. Front Nutr. 2022. Apr 28;9:800901. doi: 10.3389/fnut.2022.800901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman SB, Aslan S, Spence JS, Keebler MW, DeFina LF, Didehbani N, Perez AM, Lu H, D’Esposito M. Distinct Brain and Behavioral Benefits from Cognitive vs. Physical Training: A Randomized Trial in Aging Adults. Front Hum Neurosci. 2016. Jul 18;10:338. doi: 10.3389/fnhum.2016.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chatterton RT Jr, Vogelsong KM, Lu YC, Ellman AB, Hudgens GA. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clin Physiol. 1996. Jul;16(4):433–48. doi: 10.1111/j.1475-097x.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 21.Cordeiro LMS, Rabelo PCR, Moraes MM, Teixeira-Coelho F, Coimbra CC, Wanner SP, Soares DD. Physical exercise-induced fatigue: the role of serotonergic and dopaminergic systems. Braz J Med Biol Res. 2017. Oct 19;50(12):e6432. doi: 10.1590/1414-431X20176432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Counts S.E., Mufson E.J. Noradrenaline activation of neurotrophic pathways protects against neuronal amyloid toxicity. J. Neurochem. 2010;113:649–660. doi: 10.1111/j.1471-4159.2010.06622.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowley E., Powell C., Carson B.P., Davies R.W., “The Effect of Exercise Training Intensity on VO2max in Healthy Adults: An Overview of Systematic Reviews and Meta-Analyses”, Translational Sports Medicine, vol. 2022, Article ID 9310710, 10 pages, 2022. 10.1155/2022/9310710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.d’Arbeloff T, Elliott ML, Knodt AR, Sison M, Melzer TR, Ireland D, Ramrakha S, Poulton R, Caspi A, Moffitt TE, Hariri AR. Midlife Cardiovascular Fitness Is Reflected in the Brain’s White Matter. Front Aging Neurosci. 2021. Apr 6;13:652575. doi: 10.3389/fnagi.2021.652575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahl M.J., Mather M., Werkle-Bergner M., Kennedy B.L., Guzman S., Hurth K., Miller C.A., Qiao Y., Shi Y., Chui H.C., et al. Locus coeruleus integrity is related to tau burden and memory loss in autosomal-dominant Alzheimer’s disease. medRxiv. 2020. doi: 10.1101/2020.11.16.20232561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De la Rosa A., Solana E., Corpas R. et al. Long-term exercise training improves memory in middle-aged men and modulates peripheral levels of BDNF and Cathepsin B. Sci Rep 9, 3337 (2019). 10.1038/s41598-019-40040-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dey S, Singh RH, Dey PK. Exercise training: significance of regional alterations in serotonin metabolism of rat brain in relation to antidepressant effect of exercise. Physiol Behav. 1992. Dec;52(6):1095–9. doi: 10.1016/0031-9384(92)90465-e. [DOI] [PubMed] [Google Scholar]
- 28.Ding K, Tarumi T, Zhu DC, Tseng BY, Thomas BP, Turner M, Repshas J, Kerwin DR, Womack KB, Lu H, Cullum CM, Zhang R. Cardiorespiratory Fitness and White Matter Neuronal Fiber Integrity in Mild Cognitive Impairment. J Alzheimers Dis. 2018;61(2):729–739. doi: 10.3233/JAD-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrenberg A.J., Nguy A.K., Theofilas P., Dunlop S., Suemoto C.K., Alho A.T.D.L., Leite R.P., Rodriguez R.D., Mejia M.B., Rüb U., et al. Quantifying the accretion of hyperphosphorylated tau in the locus coeruleus and dorsal raphe nucleus: The pathological building blocks of early Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2017;43:393–408. doi: 10.1111/nan.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elman JA, Puckett OK, Beck A, Fennema-Notestine C, Cross LK, Dale AM, Eglit GML, Eyler LT, Gillespie NA, Granholm EL, Gustavson DE, Hagler DJ Jr, Hatton SN, Hauger R, Jak AJ, Logue MW, McEvoy LK, McKenzie RE, Neale MC, Panizzon MS, Reynolds CA, Sanderson Cimino M, Toomey R, Tu XM, Whitsel N, Williams ME, Xian H, Lyons MJ, Franz CE, Kremen WS. MRI-assessed locus coeruleus integrity is heritable and associated with multiple cognitive domains, mild cognitive impairment, and daytime dysfunction. Alzheimers Dement. 2021. Jun;17(6):1017–1025. doi: 10.1002/alz.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engeroff T, Füzéki E, Vogt L, Fleckenstein J, Schwarz S, Matura S, Pilatus U, Deichmann R, Hellweg R, Pantel J, Banzer W. Is Objectively Assessed Sedentary Behavior, Physical Activity and Cardiorespiratory Fitness Linked to Brain Plasticity Outcomes in Old Age? Neuroscience. 2018. Sep 15;388:384–392. doi: 10.1016/j.neuroscience.2018.07.050. [DOI] [PubMed] [Google Scholar]
- 32.Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, Macko R, Marquez DX, Petruzzello SJ, Powell KE; FOR 2018 PHYSICAL ACTIVITY GUIDELINES ADVISORY COMMITTEE*. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med Sci Sports Exerc. 2019. Jun;51(6):1242–1251. doi: 10.1249/MSS.0000000000001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Escourrou P, Johnson DG, Rowell LB. Hypoxemia increases plasma catecholamine concentrations in exercising humans. J Appl Physiol Respir Environ Exerc Physiol. 1984. Nov;57(5):1507–11. doi: 10.1152/jappl.1984.57.5.1507. [DOI] [PubMed] [Google Scholar]
- 34.Feinstein D.L., Heneka M.T., Gavrilyuk V., Russo C.D., Weinberg G., Galea E. Noradrenergic regulation of inflammatory gene expression in brain. Neurochem. Int. 2002;41:357–365. doi: 10.1016/S0197-0186(02)00049-9 [DOI] [PubMed] [Google Scholar]
- 35.Foley TE, Fleshner M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromolecular Med. 2008;10(2):67–80. doi: 10.1007/s12017-008-8032-3. [DOI] [PubMed] [Google Scholar]
- 36.Freberg E, Taglialatela G. Exercise as a Potential Therapeutic Strategy to Target the Clinical Link Between Depression and Alzheimer’s Disease: A Narrative Review. J Alzheimers Dis. 2022;89(3):759–767. doi: 10.3233/JAD-210632. [DOI] [PubMed] [Google Scholar]
- 37.Froese L, Dian J, Gomez A, Unger B, Zeiler FA. The cerebrovascular response to norepinephrine: A scoping systematic review of the animal and human literature. Pharmacol Res Perspect. 2020. Oct;8(5):e00655. doi: 10.1002/prp2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galgani A, Lombardo F, Martini N, Vergallo A, Bastiani L, Hampel H, Hlavata H, Baldacci F, Tognoni G, De Marchi D, Ghicopulos I, De Cori S, Biagioni F, Busceti CL, Ceravolo R, Bonuccelli U, Chiappino D, Siciliano G, Fornai F, Pavese N, Giorgi FS. Magnetic resonance imaging Locus Coeruleus abnormality in amnestic Mild Cognitive Impairment is associated with future progression to dementia. Eur J Neurol. 2023. Jan;30(1):32–46. doi: 10.1111/ene.15556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.García-Gomar MG, Videnovic A, Singh K, Stauder M, Lewis LD, Wald LL, Rosen BR, Bianciardi M. Disruption of Brainstem Structural Connectivity in REM Sleep Behavior Disorder Using 7 Tesla Magnetic Resonance Imaging. Mov Disord. 2022. Apr;37(4):847–853. doi: 10.1002/mds.28895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibbons TD, Cotter JD, Ainslie PN, Abraham WC, Mockett BG, Campbell HA, Jones EMW, Jenkins EJ, Thomas KN. Fasting for 20 h does not affect exercise-induced increases in circulating BDNF in humans. J Physiol. 2023. Jan 11. doi: 10.1113/JP283582. [DOI] [PubMed] [Google Scholar]
- 41.Giorgi FS, Biagioni F, Galgani A, Pavese N, Lazzeri G, Fornai F. Locus Coeruleus Modulates Neuroinflammation in Parkinsonism and Dementia. Int J Mol Sci. 2020. Nov 16;21(22):8630. doi: 10.3390/ijms21228630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gormley SE, Swain DP, High R, Spina RJ, Dowling EA, Kotipalli US, Gandrakota R. Effect of intensity of aerobic training on VO2max. Med Sci Sports Exerc. 2008. Jul;40(7):1336–43. doi: 10.1249/MSS.0b013e31816c4839. [DOI] [PubMed] [Google Scholar]
- 43.Green Howard & Duhamel Todd & Foley K & Ouyang Jing & Smith Ian & Stewart R. (2007). Glucose Supplements Increase Human Muscle Na(+)-K(+)-ATPase Activity During Prolonged Exercise. American journal of physiology. Regulatory, integrative and comparative physiology. 293. R354–62. 10.1152/ajpregu.00701.2006. [DOI] [PubMed] [Google Scholar]
- 44.O’Neal H.A., Van Hoomissen J.D., Holmes P.V., Dishman R.K., Preprogalanin mRNA levels are increased in rat locus coeruleus after treadmill exercise training, Neurosci. Lett. 299 (2001) 69–72. [DOI] [PubMed] [Google Scholar]
- 45.Hanscombe KB, Persyn E, Traylor M, Glanville KP, Hamer M, Coleman JRI, Lewis CM. The genetic case for cardiorespiratory fitness as a clinical vital sign and the routine prescription of physical activity in healthcare. Genome Med. 2021. Nov 9;13(1):180. doi: 10.1186/s13073-021-00994-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hashimoto T, Tsukamoto H, Ando S, Ogoh S. Effect of Exercise on Brain Health: The Potential Role of Lactate as a Myokine. Metabolites. 2021. Nov 29;11(12):813. doi: 10.3390/metabo11120813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hassani O.K., Rymar V.V., Nguyen K.Q., Huo L., Cloutier J.-F., Miller F.D., Sadikot A.F. The noradrenergic system is necessary for survival of vulnerable midbrain dopaminergic neurons: Implications for development and Parkinson’s disease. Neurobiol. Aging. 2020;85:22–37. doi: 10.1016/j.neurobiolaging.2019.09.014 [DOI] [PubMed] [Google Scholar]
- 48.Heijnen S, Hommel B, Kibele A, Colzato LS. Neuromodulation of Aerobic Exercise-A Review. Front Psychol. 2016. Jan 7;6:1890. doi: 10.3389/fpsyg.2015.01890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heneka MT, Galea E, Gavriluyk V, Dumitrescu-Ozimek L, Daeschner J, O’Banion MK, Weinberg G, Klockgether T, Feinstein DL. Noradrenergic depletion potentiates beta -amyloid-induced cortical inflammation: implications for Alzheimer’s disease. J Neurosci. 2002. Apr 1;22(7):2434–42. doi: 10.1523/JNEUROSCI.22-07-02434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hobson SA, Vanderplank PA, Pope RJ, Kerr NC, Wynick D. Galanin stimulates neurite outgrowth from sensory neurons by inhibition of Cdc42 and Rho GTPases and activation of cofilin. J Neurochem. 2013. Oct;127(2):199–208. doi: 10.1111/jnc.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmes PV, Yoo HS, Dishman RK. Voluntary exercise and clomipramine treatment elevate prepro-galanin mRNA levels in the locus coeruleus in rats. Neurosci Lett. 2006. Nov 6;408(1):1–4. doi: 10.1016/j.neulet.2006.04.057. [DOI] [PubMed] [Google Scholar]
- 53.Holmes PV. Trophic Mechanisms for Exercise-Induced Stress Resilience: Potential Role of Interactions between BDNF and Galanin. Front Psychiatry. 2014. Jul 28;5:90. doi: 10.3389/fpsyt.2014.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoogendijk W.J., Feenstra M.G., Botterblom M.H., Gilhuis J., Sommer I.E., Kamphorst W., Eikelenboom P., Swaab D.F. Increased activity of surviving locus ceruleus neurons in Alzheimer’s disease. Ann. Neurol. 1999;45:82–91.doi: [DOI] [PubMed] [Google Scholar]
- 55.Hwang J, Castelli DM, Gonzalez-Lima F. The positive cognitive impact of aerobic fitness is associated with peripheral inflammatory and brain-derived neurotrophic biomarkers in young adults. Physiol Behav. 2017. Oct 1;179:75–89. doi: 10.1016/j.physbeh.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 56.Ishimatsu M., Williams J.T. Synchronous Activity in Locus Coeruleus Results from Dendritic Interactions in Pericoerulear Regions. J. Neurosci. 1996;16:5196–5204. doi: 10.1523/JNEUROSCI.16-16-05196.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Hoomissen J.D., Holmes P.V., Zellner A.S., Poudevigne A.M., Dishman R.K., The effect of B-adrenergic blockade during chronic exercise on contextual fear conditioning and mRNA for galanin and brain-derived neurotrophic factor, Behav. Neurosci. 118 (2004) 1378–1390. [DOI] [PubMed] [Google Scholar]
- 58.Jacobs HIL, Becker JA, Kwong K, Engels-Domínguez N, Prokopiou PC, Papp KV, Properzi M, Hampton OL, d’Oleire Uquillas F, Sanchez JS, Rentz DM, El Fakhri G, Normandin MD, Price JC, Bennett DA, Sperling RA, Johnson KA. In vivo and neuropathology data support locus coeruleus integrity as indicator of Alzheimer’s disease pathology and cognitive decline. Sci Transl Med. 2021. Sep 22;13(612):eabj2511. doi: 10.1126/scitranslmed.abj2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Janitzky K. Impaired Phasic Discharge of Locus Coeruleus Neurons Based on Persistent High Tonic Discharge—A New Hypothesis with Potential Implications for Neurodegenerative Diseases. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.00371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jimenez C, Melin B, Savourey G, Launay JC, Alonso A, Mathieu J. Effects of passive hyperthermia versus exercise-induced hyperthermia on immune responses: hormonal implications. Eur Cytokine Netw. 2007. Sep;18(3):154–61. doi: 10.1684/ecn.2007.0101. [DOI] [PubMed] [Google Scholar]
- 61.Kjær M., Secher N., Galbo H. Physical stress and catecholamine release. Baillieres Clin. Endocrinol. Metab. 1987;1:279–298. doi: 10.1016/S0950-351X(87)80064-2. [DOI] [PubMed] [Google Scholar]
- 62.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory Fitness as a Quantitative Predictor of All-Cause Mortality and Cardiovascular Events in Healthy Men and Women: A Meta-analysis. JAMA. 2009;301(19):2024–2035. doi: 10.1001/jama.2009.681 [DOI] [PubMed] [Google Scholar]
- 63.Kovacevic A, Fenesi B, Paolucci E, Heisz JJ. The effects of aerobic exercise intensity on memory in older adults. Appl Physiol Nutr Metab. 2020. Jun;45(6):591–600. doi: 10.1139/apnm-2019-0495. [DOI] [PubMed] [Google Scholar]
- 64.Kurl S, Laukkanen JA, Lonnroos E, Remes AM, Soininen H. Cardiorespiratory fitness and risk of dementia: a prospective population-based cohort study. Age Ageing. 2018. Jul 1;47(4):611–614. doi: 10.1093/ageing/afy060. [DOI] [PubMed] [Google Scholar]
- 65.Liu A.K.L., Chang R.C.-C., Pearce R.K.B., Gentleman S.M. Nucleus basalis of Meynert revisited: Anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol. 2015;129:527–540. doi: 10.1007/s00401-015-1392-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu J, Tao J, Xia R, Li M, Huang M, Li S, Chen X, Wilson G, Park J, Zheng G, Chen L, Kong J. Mind-Body Exercise Modulates Locus Coeruleus and Ventral Tegmental Area Functional Connectivity in Individuals With Mild Cognitive Impairment. Front Aging Neurosci. 2021. Jun 14;13:646807. doi: 10.3389/fnagi.2021.646807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu K.Y., Acosta-Cabronero J., Cardenas-Blanco A., Loane C., Berry A., Betts M., Kievit R.A., Henson R., Düzel E., Howard R., et al. In vivo visualization of age-related differences in the locus coeruleus. Neurobiol. Aging. 2019;74:101–111. doi: 10.1016/j.neurobiolaging.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu PZ, Nusslock R. Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front Neurosci. 2018. Feb 7;12:52. doi: 10.3389/fnins.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu K.Y., Kievit R.A., Tsvetanov K.A. et al. Noradrenergic-dependent functions are associated with age-related locus coeruleus signal intensity differences. Nat Commun 11, 1712 (2020). 10.1038/s41467-020-15410-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020. Aug 8;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mai J.K., Paxinos G. The Human Nervous System. 3rd ed. Academic Press; Cambridge, MA, USA: 2012 [Google Scholar]
- 72.Mai J.K., Paxinos G. The Human Nervous System. 3rd ed. Academic Press; Cambridge, MA, USA: 2012 [Google Scholar]
- 73.Maleki S, Hendrikse J, Chye Y, Caeyenberghs K, Coxon JP, Oldham S, Suo C, Yücel M. Associations of cardiorespiratory fitness and exercise with brain white matter in healthy adults: A systematic review and meta-analysis. Brain Imaging Behav. 2022. Jun 30. doi: 10.1007/s11682-022-00693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mannari C., Origlia N., Scatena A., Del Debbio A., Catena M., Dell’Agnello G., Barraco A., Giovannini L., Dell’Osso L., Domenici L., et al. BDNF Level in the Rat Prefrontal Cortex Increases Following Chronic but Not Acute Treatment with Duloxetine, a Dual Acting Inhibitor of Noradrenaline and Serotonin Re-uptake. Cell. Mol. Neurobiol. 2008;28:457–468. doi: 10.1007/s10571-007-9254-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Manning JR, Notaro GM, Chen E, Fitzpatrick PC. Fitness tracking reveals task-specific associations between memory, mental health, and physical activity. Sci Rep. 2022. Aug 15;12(1):13822. doi: 10.1038/s41598-022-17781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marin Bosch B, Bringard A, Logrieco MG, Lauer E, Imobersteg N, Thomas A, Ferretti G, Schwartz S, Igloi K. Effect of acute physical exercise on motor sequence memory. Sci Rep. 2020. Sep 18;10(1):15322. doi: 10.1038/s41598-020-72108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matchett B.J., Grinberg L.T., Theofilas P., Murray M.E. The mechanistic link between selective vulnerability of the locus coeruleus and neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 2021;141:631–650. doi: 10.1007/s00401-020-02248-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mather M., Harley C.W. The Locus Coeruleus: Essential for Maintaining Cognitive Function and the Aging Brain. Trends Cogn. Sci. 2016;20:214–226. doi: 10.1016/j.tics.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mather M., Huang R., Clewett D., Nielsen S.E., Velasco R., Tu K.P., Han S., Kennedy B. Isometric exercise facilitates attention to salient events in women via the noradrenergic system. NeuroImage. 2020;210:116560. doi: 10.1016/j.neuroimage.2020.116560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsunaga W, Shirokawa T, Isobe K. BDNF is necessary for maintenance of noradrenergic innervations in the aged rat brain. Neurobiol Aging. 2004. Mar;25(3):341–8. doi: 10.1016/S0197-4580(03)00093-9. [DOI] [PubMed] [Google Scholar]
- 81.Matta Mello Portugal E, Cevada T, Sobral Monteiro-Junior R, Teixeira Guimarães T, da Cruz Rubini E, Lattari E, Blois C, Camaz Deslandes A. Neuroscience of exercise: from neurobiology mechanisms to mental health. Neuropsychobiology. 2013;68(1):1–14. doi: 10.1159/000350946. [DOI] [PubMed] [Google Scholar]
- 82.Exercise Meeusen R. and the brain: insight in new therapeutic modalities. Ann Transplant. 2005;10(4):49–51. [PubMed] [Google Scholar]
- 83.Meeusen R., De Meirleir K. (1995). Exercise and brain neurotransmission. Sports Med. 20, 160–188. 10.2165/00007256-199520030-00004 [DOI] [PubMed] [Google Scholar]
- 84.Melnychuk MC, Dockree PM, O’Connell RG, Murphy PR, Balsters JH, Robertson IH. Coupling of respiration and attention via the locus coeruleus: Effects of meditation and pranayama. Psychophysiology. 2018. Sep;55(9):e13091. doi: 10.1111/psyp.13091. [DOI] [PubMed] [Google Scholar]
- 85.Melnychuk MC, Robertson IH, Plini ERG, Dockree PM. A Bridge between the Breath and the Brain: Synchronization of Respiration, a Pupillometric Marker of the Locus Coeruleus, and an EEG Marker of Attentional Control State. Brain Sci. 2021. Oct 6;11(10):1324. doi: 10.3390/brainsci11101324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Menz V, Marterer N, Amin SB, Faulhaber M, Hansen AB, Lawley JS. Functional Vs. Running Low-Volume High-Intensity Interval Training: Effects on VO2max and Muscular Endurance. J Sports Sci Med. 2019. Aug 1;18(3):497–504. [PMC free article] [PubMed] [Google Scholar]
- 87.Milanović Z, Sporiš G, Weston M. Effectiveness of High-Intensity Interval Training (HIT) and Continuous Endurance Training for VO2max Improvements: A Systematic Review and Meta-Analysis of Controlled Trials. Sports Med. 2015. Oct;45(10):1469–81. doi: 10.1007/s40279-015-0365-0. [DOI] [PubMed] [Google Scholar]
- 88.Muoio DM, Leddy JJ, Horvath PJ, Awad AB, Pendergast DR. Effect of dietary fat on metabolic adjustments to maximal VO2 and endurance in runners. Med Sci Sports Exerc. 1994. Jan;26(1):81–8. [PubMed] [Google Scholar]
- 89.Murawska-Cialowicz E, Wojna J, Zuwala-Jagiello J. Crossfit training changes brain-derived neurotrophic factor and irisin levels at rest, after wingate and progressive tests, and improves aerobic capacity and body composition of young physically active men and women. J Physiol Pharmacol. 2015. Dec;66(6):811–21. [PubMed] [Google Scholar]
- 90.Murray P.S.; Groves J.L.; Pettett B.J.; Britton S.L.; Koch L.G.; Dishman R.K.; Holmes P.V. Locus coeruleus galanin expression is enhanced after exercise in rats selectively bred for high capacity for aerobic activity. Peptides 2010, 31, 2264–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Myers J, Kaminsky LA, Lima R, Christle JW, Ashley E, Arena R. A Reference Equation for Normal Standards for VO2 Max: Analysis from the Fitness Registry and the Importance of Exercise National Database (FRIEND Registry). Prog Cardiovasc Dis. 2017. Jun-Jul;60(1):21–29. doi: 10.1016/j.pcad.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 92.Nakai S, Matsunaga W, Ishida Y, Isobe K, Shirokawa T. Effects of BDNF infusion on the axon terminals of locus coeruleus neurons of aging rats. Neurosci Res. 2006. Mar;54(3):213–9. doi: 10.1016/j.neures.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 93.Nightingale CM, Donin AS, Kerry SR, Owen CG, Rudnicka AR, Brage S, Westgate KL, Ekelund U, Cook DG, Whincup PH. Cross-sectional study of ethnic differences in physical fitness among children of South Asian, black African-Caribbean and white European origin: the Child Heart and Health Study in England (CHASE). BMJ Open. 2016. Jun 20;6(6):e011131. doi: 10.1136/bmjopen-2016-011131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nilsson J, Ekblom Ö, Ekblom M, Lebedev A, Tarassova O, Moberg M, Lövdén M. Acute increases in brain-derived neurotrophic factor in plasma following physical exercise relates to subsequent learning in older adults. Sci Rep. 2020. Mar 10;10(1):4395. doi: 10.1038/s41598-020-60124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nowacka-Chmielewska M, Grabowska K, Grabowski M, Meybohm P, Burek M, Małecki A. Running from Stress: Neurobiological Mechanisms of Exercise-Induced Stress Resilience. International Journal of Molecular Sciences. 2022; 23(21):13348. 10.3390/ijms232113348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Olivo G., Nilsson J., Garzón B. et al. Higher VO2max is associated with thicker cortex and lower grey matter blood flow in older adults. Sci Rep 11, 16724 (2021). 10.1038/s41598-021-96138-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Omoluabi T, Torraville SE, Maziar A, Ghosh A, Power KD, Reinhardt C, Harley CW, Yuan Q. Novelty-like activation of locus coeruleus protects against deleterious human pretangle tau effects while stress-inducing activation worsens its effects. Alzheimers Dement (N Y). 2021. Dec 31;7(1):e12231. doi: 10.1002/trc2.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ozaki H., Loenneke J.P., Thiebaud R.S. et al. Resistance training induced increase in VO2max in young and older subjects. Eur Rev Aging Phys Act 10, 107–116 (2013). 10.1007/s11556-013-0120-1 [DOI] [Google Scholar]
- 99.Palomero-Gallagher N., Amunts K., Zilles K. Transmitter Receptor Distribution in the Human Brain. Brain Mapp. 2015:261–275. [Google Scholar]
- 100.Pantzar A, Jonasson LS, Ekblom Ö, Boraxbekk CJ, Ekblom MM. Relationships Between Aerobic Fitness Levels and Cognitive Performance in Swedish Office Workers. Front Psychol. 2018. Dec 20;9:2612. doi: 10.3389/fpsyg.2018.02612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Petkus AJ, Jarrahi B, Holschneider DP, Gomez ME, Filoteo JV, Schiehser DM, Fisher BE, Van Horn JD, Jakowec MW, McEwen SC, Petzinger G. Thalamic volume mediates associations between cardiorespiratory fitness (VO2max) and cognition in Parkinson’s disease. Parkinsonism Relat Disord. 2021. May;86:19–26. doi: 10.1016/j.parkreldis.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Plini ERG, O’Hanlon E, Boyle R, Sibilia F, Rikhye G, Kenney J, Whelan R, Melnychuk MC, Robertson IH, Dockree PM. Examining the Role of the Noradrenergic Locus Coeruleus for Predicting Attention and Brain Maintenance in Healthy Old Age and Disease: An MRI Structural Study for the Alzheimer’s Disease Neuroimaging Initiative. Cells. 2021. Jul 20;10(7):1829. doi: 10.3390/cells10071829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Prokopiou PC, Engels-Domínguez N, Papp KV, Scott MR, Schultz AP, Schneider C, Farrell ME, Buckley RF, Quiroz YT, El Fakhri G, Rentz DM, Sperling RA, Johnson KA, Jacobs HIL. Lower novelty-related locus coeruleus function is associated with Aβ-related cognitive decline in clinically healthy individuals. Nat Commun. 2022. Mar 23;13(1):1571. doi: 10.1038/s41467-022-28986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reiter K., Nielson K., Smith T., Weiss L., Alfini A., & Smith J. (2015). Improved Cardiorespiratory Fitness Is Associated with Increased Cortical Thickness in Mild Cognitive Impairment. Journal of the International Neuropsychological Society, 21(10), 757–767. doi: 10.1017/S135561771500079X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robertson I.H. A noradrenergic theory of cognitive reserve: Implications for Alzheimer’s disease. Neurobiol. Aging 2013, 34, 298–308 [DOI] [PubMed] [Google Scholar]
- 106.Robertson I.H. A right hemisphere role in cognitive reserve. Neurobiol. Aging 2014, 35, 1375–1385. [DOI] [PubMed] [Google Scholar]
- 107.Santisteban KJ, Lovering AT, Halliwill JR, Minson CT. Sex Differences in VO2max and the Impact on Endurance-Exercise Performance. Int J Environ Res Public Health. 2022. Apr 19;19(9):4946. doi: 10.3390/ijerph19094946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Satoh A., Iijima K.M. Roles of tau pathology in the locus coeruleus (LC) in age-associated pathophysiology and Alzheimer’s disease pathogenesis: Potential strategies to protect the LC against aging. Brain Res. 2019;1702:17–28. doi: 10.1016/j.brainres.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 109.Sciolino N.R.; Smith J.M.; Stranahan A.M.; Freeman K.G.; Edwards G.L.; Weinshenker D.; Holmes P.V. Galanin mediates features of neural and behavioral stress resilience afforded by exercise. Neuropharmacology 2015, 89, 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scribbans TD, Vecsey S, Hankinson PB, Foster WS, Gurd BJ. The Effect of Training Intensity on VO2max in Young Healthy Adults: A Meta-Regression and Meta-Analysis. Int J Exerc Sci. 2016. Apr 1;9(2):230–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Segal S.K., Cotman C.W., Cahill L.F. Exercise-Induced Noradrenergic Activation Enhances Memory Consolidation in Both Normal Aging and Patients with Amnestic Mild Cognitive Impairment. J. Alzheimer’s Dis. 2012;32:1011–1018. doi: 10.3233/JAD-2012-121078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Song S., Gaynor A.M., Gazes Y., Lee S., Xu Q., Habeck C., Stern Y., Gu Y. Physical activity moderates the association between white matter hyperintensity burden and cognitive change. Frontiers in Aging Neuroscience - 14-2022-ISSN: 1663–4365 DOI= 10.3389/fnagi.2022.945645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stern Y., Urquiljo E.M.A., Bartrés-Faz D., Belleville S., Cantillon M., Chetelat G., Ewers M., Franzmeier N., Kempermann G., Kremen W.S., et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s Dement. 2020;16:1305–1311. doi: 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Strasser B, Burtscher M. Survival of the fittest: VO2max, a key predictor of longevity? Front Biosci (Landmark Ed). 2018. Mar 1;23(8):1505–1516. doi: 10.2741/4657. [DOI] [PubMed] [Google Scholar]
- 117.Szot P., Leverenz J.B., Peskind E.R., Kiyasu E., Rohde K., Miller M.A., Raskind M.A. Tyrosine hydroxylase and norepinephrine transporter mRNA expression in the locus coeruleus in Alzheimer’s disease. Mol. Brain Res. 2000;84:135–140.doi: 10.1016/S0169-328X(00)00168-6 [DOI] [PubMed] [Google Scholar]
- 118.Szot P., White S., Greenup J., Leverenz J., Peskind E., Raskind M. Changes in adrenoreceptors in the prefrontal cortex of subjects with dementia: Evidence of compensatory changes. Neuroscience. 2007;146:471–480.doi: 10.1016/j.neuroscience.2007.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Szot P., White S.S., Greenup J.L., Leverenz J., Peskind E.R., Raskind M.A. Compensatory Changes in the Noradrenergic Nervous System in the Locus Ceruleus and Hippocampus of Postmortem Subjects with Alzheimer’s Disease and Dementia with Lewy Bodies. J. Neurosci. 2006;26:467–478. doi: 10.1523/JNEUROSCI.4265-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tarumi T, Thomas BP, Tseng BY, et al. Cerebral White Matter Integrity in Amnestic Mild Cognitive Impairment: A 1-Year Randomized Controlled Trial of Aerobic Exercise Training. Journal of Alzheimer’s Disease : JAD. 2020. ;73(2):489–501. DOI: 10.3233/jad-190875. [DOI] [PubMed] [Google Scholar]
- 121.Tarumi T., Tomoto T., Repshas J., Wang C., Hynan L. S., Cullum C. M., Zhu D. C., & Zhang R. (2021). Midlife aerobic exercise and brain structural integrity: Associations with age and cardiorespiratory fitness. NeuroImage, 225, Article 117512. 10.1016/j.neuroimage.2020.117512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tillage R.P.; Wilson G.E.; Liles L.C.; Holmes P.V.; Weinshenker D. Chronic Environmental or Genetic Elevation of Galanin in Noradrenergic Neurons Confers Stress Resilience in Mice. J. Neurosci. 2020, 40, 7464–7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Traver S., Salthun-Lassalle B., Marien M., Hirsch E., Colpaert F., Michel P.P. The Neurotransmitter Noradrenaline Rescues Septal Cholinergic Neurons in Culture from Degeneration Caused by Low-Level Oxidative Stress. Mol. Pharmacol. 2005;67:1882–1891. doi: 10.1124/mol.104.007864. [DOI] [PubMed] [Google Scholar]
- 124.Troadec J.D., Marien M., Darios F., Hartmann A., Ruberg M., Colpaert F., Michel P.P. Noradrenaline provides long-term protection to dopaminergic neurons by reducing oxidative stress. J. Neurochem. 2001;79:200–210. doi: 10.1046/j.1471-4159.2001.00556.x. [DOI] [PubMed] [Google Scholar]
- 125.Tyndall AV, Clark CM, Anderson TJ, Hogan DB, Hill MD, Longman RS, Poulin MJ. Protective Effects of Exercise on Cognition and Brain Health in Older Adults. Exerc Sport Sci Rev. 2018. Oct;46(4):215–223. doi: 10.1249/JES.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 126.Upadhyay N, Schörkmaier T, Maurer A, Claus J, Scheef L, Daamen M, Martin JA, Stirnberg R, Radbruch A, Attenberger U, Stöcker T, Boecker H. Regional cortical perfusion increases induced by a 6-month endurance training in young sedentary adults. Front Aging Neurosci. 2022. Aug 9;14:951022. doi: 10.3389/fnagi.2022.951022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Urhausen A, Gabriel H, Kindermann W. Blood hormones as markers of training stress and overtraining. Sports Med. 1995. Oct;20(4):251–76. doi: 10.2165/00007256-199520040-00004. [DOI] [PubMed] [Google Scholar]
- 128.van Dongen EV, Kersten IHP, Wagner IC, Morris RGM, Fernández G. Physical Exercise Performed Four Hours after Learning Improves Memory Retention and Increases Hippocampal Pattern Similarity during Retrieval. Curr Biol. 2016. Jul 11;26(13):1722–1727. doi: 10.1016/j.cub.2016.04.071. [DOI] [PubMed] [Google Scholar]
- 129.Varma VR, Watts A. Daily Physical Activity Patterns During the Early Stage of Alzheimer’s Disease. J Alzheimers Dis. 2017;55(2):659–667. doi: 10.3233/JAD-160582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wallace LMK, Theou O, Godin J, Andrew MK, Bennett DA, Rockwood K. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer’s disease: a cross-sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol. 2019. Feb;18(2):177–184. doi: 10.1016/S1474-4422(18)30371-5. Erratum in: Lancet Neurol. 2019 Mar;18(3):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wendell CR, Gunstad J, Waldstein SR, Wright JG, Ferrucci L, Zonderman AB. Cardiorespiratory fitness and accelerated cognitive decline with aging. J Gerontol A Biol Sci Med Sci. 2014. Apr;69(4):455–62. doi: 10.1093/gerona/glt144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Williams CJ, Williams MG, Eynon N, Ashton KJ, Little JP, Wisloff U, Coombes JS. Genes to predict VO2max trainability: a systematic review. BMC Genomics. 2017. Nov 14;18(Suppl 8):831. doi: 10.1186/s12864-017-4192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wilson R.S., Nag S., Boyle P.A., Hizel L., Yu L., Buchman A.S., Schneider J.A., Bennett D.A. Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology. 2013;80:1202–1208. doi: 10.1212/WNL.0b013e3182897103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, Krueger K, Fromme A, Korsukewitz C, Floel A, Knecht S. High impact running improves learning. Neurobiol Learn Mem. 2007. May;87(4):597–609. doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 135.Wittfeld K, Jochem C, Dörr M, Schminke U, Gläser S, Bahls M, Markus MRP, Felix SB, Leitzmann MF, Ewert R, Bülow R, Völzke H, Janowitz D, Baumeister SE, Grabe HJ. Cardiorespiratory Fitness and Gray Matter Volume in the Temporal, Frontal, and Cerebellar Regions in the General Population. Mayo Clin Proc. 2020. Jan;95(1):44–56. doi: 10.1016/j.mayocp.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 136.Xu C, Hou Y, Si K, Cao Z. Cardiorespiratory fitness, genetic susceptibility, inflammation and risk of incident type 2 diabetes: A population-based longitudinal study. Metabolism. 2022. Jul;132:155215. doi: 10.1016/j.metabol.2022.155215. [DOI] [PubMed] [Google Scholar]
- 137.Xue X, Liu B, Hu J, Bian X, Lou S. The potential mechanisms of lactate in mediating exercise-enhanced cognitive function: a dual role as an energy supply substrate and a signaling molecule. Nutr Metab (Lond). 2022. Jul 30;19(1):52. doi: 10.1186/s12986-022-00687-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yi Yeo-Jin, Lüsebrink Falk, Maaß Anne, Ziegler Gabriel, Yakupov Renat, Kreißl Michael C., Betts Matthew, Speck Oliver, Düzel Emrah, Dorothea Hämmerer bioRxiv 2021.10.01.462807; doi: 10.1101/2021.10.01.462807 [DOI] [Google Scholar]
- 139.Yu F, Salisbury D, Mathiason MA. Inter-individual differences in the responses to aerobic exercise in Alzheimer’s disease: Findings from the FIT-AD trial. J Sport Health Sci. 2021. Jan;10(1):65–72. doi: 10.1016/j.jshs.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang Q., Roche M., Gheres K.W. et al. Cerebral oxygenation during locomotion is modulated by respiration. Nat Commun 10, 5515 (2019). 10.1038/s41467-019-13523-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhu L, Yu Q, Herold F, Cheval B, Dong X, Cui L, Xiong X, Chen A, Yin H, Kong Z, Mueller N, Kramer AF, Zou L. Brain Structure, Cardiorespiratory Fitness, and Executive Control Changes after a 9-Week Exercise Intervention in Young Adults: A Randomized Controlled Trial. Life (Basel). 2021. Mar 30;11(4):292. doi: 10.3390/life11040292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhu MY. Noradrenergic Modulation on Dopaminergic Neurons. Neurotox Res. 2018. Nov;34(4):848–859. doi: 10.1007/s12640-018-9889-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study was approved by the ethical review board of the University of Texas Southwestern Medical Center at Dallas, University of Texas at Dallas and The Cooper Institute. All participants were informed about the study protocol and provided informed written consent prior to scanning.”
The datasets used and/or analysed during the current study available from author Jeffrey S. Spence, Center for BrainHealth, University of Texas at Dallas, on reasonable request.
The Locus Coeruleus omni-comprehensive mask and the nifty images for significant clusters are available on request. Contact Dr. Emanuele RG Plini (plinie@tcd.ie / emanuele.rg.plini@gmail.com)