Abstract
Individuals over the age of 65 comprise a substantial portion of the world population and become more susceptible to vaccine-preventable infections with age as vaccination response diminishes. The underlying reason for this impaired vaccine response in older individuals is not entirely clear. We evaluated potential differences in phenotypic and functional responses of B cells from healthy younger (22–45 years) and older (64–95 years) individuals that may associate with a diminished antibody response to influenza vaccination. We report that age is associated with expansion of atypical memory B cells (CD10−CD20+CD21−CD27−) and an age-associated B cell (ABC, CD21−T-bet+CD11c+) phenotype. Reduced expression of PAX5 was also seen in older individuals. Poor influenza-specific antibody production following vaccination was associated with low PAX5 expression and a distinct composition of the ABC compartment. Collectively, these findings demonstrate that the characteristics of the ABC populations of older individuals are associated with antibody production following influenza vaccination.
1. Introduction
B cells play a central role in orchestrating the humoral immune response. Derived from hematopoietic stem cells in the bone marrow, B cells undergo multiple rounds of selection and differentiation to become mature cells with a specific B-cell receptor (BCR). A growing body of evidence suggests unique phenotypes of mature B cells are functionally distinct [1]. Age-associated B cells (ABCs) are a recently described subset of B cell defined as CD21−T-bet+CD11c+ [2,3], which are expanded with age in both mice and humans as a result of chronic inflammation [4]. ABCs have been compared to exhausted or unresponsive B cells due to their phenotypic similarities [5] and lack of response to BCR stimulation. While initial work describing this subset utilized mice, studies in humans are limited and must be expanded to elucidate the functional relevance of this population.
The transcription factor PAX5 defines the B-cell lineage, with continued expression from initial stages of commitment in bone marrow pro-B cells [6] until terminal differentiation in the periphery [7]. PAX5 maintains commitment to the B-cell lineage through its downstream gene regulatory network, ensuring expression of lineage appropriate genes while repressing those of alternative fates. For this reason, reduced PAX5 expression in the bone marrow of older mice contributes to diminished B lymphopoiesis (reviewed in [8]) and a shift towards the myeloid lineage with age [9]. However, it is unknown to what extent early dysregulation of PAX5 disrupts B-cell identity in mature human B cells.
After initial lineage commitment, the regulatory network of PAX5 directly and indirectly influences expression of many factors necessary for B-cell signaling and differentiation to memory and plasma cells [7]. PAX5 is also involved in antibody production. Pax5 and other transcription factors form a complex with activation-induced cytidine deaminase (AID) to direct class switch recombination [10]. Therefore, dysregulation of PAX5 expression with age could adversely affect B-cell functioning and humoral response. B-cell phenotype and differentiation are regulated by the transcription factor PAX5, suggesting its involvement in the expansion of the ABC subset with age.
In this study, we evaluated the association between reduced influenza vaccine response and PAX5-mediated maintenance of B-cell identity and expansion of ABCs with age. Healthy older individuals (over age 65) were recruited to characterize a phenotype of peripheral blood B cells and its association with age and influenza vaccine response. Prevention of influenza infection is largely mediated by humoral immunity, making influenza vaccination response an ideal means to evaluate the functional role of B cells in younger and older individuals. We also investigated the role of B-cell identity in aging by examining expression of PAX5 and select members of its associated gene regulatory network; we then determined whether the distinct phenotypic changes we observed in older individuals associated with failure to seroconvert following influenza vaccination.
We demonstrate that PAX5 expression is significantly reduced in ABCs of older individuals relative to younger. Additionally, atypical memory B cells (CD21−CD27−) and ATYP ABCs are expanded in older individuals. When we examined these findings in the context of antibody production following influenza vaccination, loss of CD27+ ABCs and reduced PAX5 expression on this population was associated with failure to mount an effective response to influenza vaccination. Collectively, these findings demonstrate that diminished influenza vaccine response is associated with perturbation of B-cell phenotype that likely leads to B cell senescence. Reversing B-cell dysfunction in older individuals may be a necessary approach to enhanced effectiveness of vaccines that rely on humoral responses for protection such as the influenza vaccine.
2. Materials and methods
2.1. Cohort
All experiments were performed using blood obtained from healthy individuals (Table 1) who provided signed, informed consent, and as approved by each institutions Institutional Review Board (Rush University Medical Center’s Institutional Review Board, Louis Stokes Cleveland VA Medical Center, University of Arizona, American Red Cross Biomedical Services). Individuals were reported to have no signs of acute illness at the time of enrollment and were not taking severe immunemodulating drugs.
Table 1.
Descriptions of study cohorts. Ages and sources of samples used throughout the study. Names reference collection site are used throughout the text. SD, standard deviation.
| Cohort | Number of individuals | Age range (years) | Mean age (years) ± SD |
|---|---|---|---|
|
| |||
| Rush | 50 younger | 22–45 | 28.5 ± 1.5 |
| 35 older | 66–90 | 79 ± 8.0 | |
| VA | 48 older | 64–95 | 79.2 ± 7.9 |
| Red cross | 5 younger | 34–44 | 37.6 ± 4.0 |
| 5 older | 65–73 | 67.8 ± 3.3 | |
| Arizona | 63 older | 65–90 | 74.9 ± 6.2 |
2.2. Phenotypic characterization by flow cytometry
Whole blood from the Rush cohort was processed immediately after collection by density gradient centrifugation using Lymphocyte Separation Media (Corning, Manassas, VA) in order to collect peripheral blood mononuclear cells (PBMCs). In order to identify B-cell inhibitory and costimulatory molecules, PBMCs were stained with: anti-CD19-PECF594, anti-CD22-APC, anti-CD40-AF700, anti-CD80-APCH7, anti-CD86-V450, anti-CD72-FITC, anti-CD85j-PE, anti-HLA-DR-PECy7 (all from BD Biosciences, Franklin Lakes, New Jersey), anti-CD305-PerCP/Cy5.5 (BioLegend, San Diego, CA), and LIVE/DEAD® Fixable Aqua Stain (Thermo Fisher Scientific, Waltham, MA). B-cell subsets were defined by staining PBMCs with: anti-CD10-PE and PE-Cy7, anti-CD19-PECF594, BB151 and BV786, anti-CD20-FITC and APC, anti-CD21-APC and BV711, anti-CD24-PerCP/Cy5.5, anti-CD27-AF700 and PAC-H7, anti-CD38-V450, anti-IgD-FITC, anti-IgM-PE, anti-IgG-BV421, all from BD Biosciences, anti-FcRL4 (APC) (BioLegend), and anti-CD11c-AF700 (Thermo Fisher Scientific). For all staining, PBMCs were first incubated with LIVE/DEAD® Fixable Aqua Stain (Thermo Fisher Scientific) at a concentration of 1 μL/mL for 20 min at room temperature. Cells were then rinsed twice with phosphate-buffered saline (PBS) (Corning), and stained for 45 min at room temperature with fluorochrome conjugated antibodies. If necessary, intracellular staining was performed using the BD Cytofix/Cytoperm kit (BD Biosciences) or Transcription Factor Buffer Set (BD Biosciences) following manufacturer’s instructions. Intracellular staining was performed using anti-PAX5-PE and BV421 and anti-T-bet-PE-CF594 (all BD Biosciences). Following staining, all cells were rinsed twice with PBS, fixed in 1% paraformaldehyde (PFA), and acquired via flow cytometry using the LSRII or Fortessa flow cytometer (BD Biosciences) with all samples within a study completed on the same instrument with fluorescence parameter PMTs normalized with Rainbow Calibration Particles (Spherotech, Lake Forest, IL) prior to compensation with CompBead (BD Biosciences) and necessary antibodies. Analysis was performed using FlowJo vX (Tree Star, Ashland, OR). Gating of positive populations based on florescence minus one (FMO) controls.
2.3. Differentiation and proliferation culture
PBMCs from the Red Cross cohort were thawed and incubated with CFSE (Life Technologies, Carlsbad, CA) for 10 min as per manufacturer’s instructions. 5 mL of FBS (Gemini Bioproducts, West Sacramento, CA) was added and cells were incubated on ice for 5 min. Cells were rinsed and cultured with 10 μg/mL (Invivogen, San Diego, CA), 10 μg/mL CD40L (Invitrogen, San Diego, CA) and 2.5 ng/mL rhIL-21(Thermo Fisher Scientific), or CpG with CD40L and rhIL-21 similar to conditions described by Marasco et al. [11]. Controls with no stimuli, no CFSE, and anti-CD3/CD28 beads (Thermo Fisher Scientific) were included with each culture. Cells were then surface stained and acquired via flow cytometry using the Fortessa flow cytometer (BD Biosciences).
2.4. CMV status
Serostatus of individuals in VA and Arizona cohorts was determined using AccuDiag Cytomegalovirus IgG (CMv IgG) ELISA Kit (Diagnostic Automation Inc., Woodland Hills, CA) and CMV-specific IgG ELISA (Gold Standard Diagnostics, Davis, CA) kit respectively as per manufacturers’ instructions.
2.5. Influenza vaccination
As previously described in Van Epps et al. [12], individuals from the VA cohort received the trivalent inactivated influenza vaccination during the 2010–2011 or 2011–2012 season, for which the same A/California7/2009-like virus, A/Perth/16/2009-like virus (H3N2), and B/Brisbane/60/2008 strains were used. Vaccine response was determined by hemagglutination-inhibition assay (HI) for influenza B, A(H1N1)pdm09, and A(H3N2) viruses with seroconversion defined as four-fold or greater increase in antibody titer to 2/3 strains 2–4 months after vaccination. Average age of responders was 78.1 ± 7.0 years, with non-responders averaging 79.9 ± 8.5 years. PBMCs collected 2–4 months following vaccination were characterized by flow cytometry.
2.6. Statistics
Data was analyzed and plotted using GraphPad Prism v6 (GraphPad Software Inc., San Diego, CA). Unpaired t-Tests with Welch’s correction was used to compare means of younger and older individuals and vaccine responders and non-responders. Logistic regression was performed using the sjstats package (version 0.11.0) [13,15,16] in R (version 1.0.153) [17].
3. Results
3.1. Frequencies of atypical memory B cells and ABCs are increased in older individuals
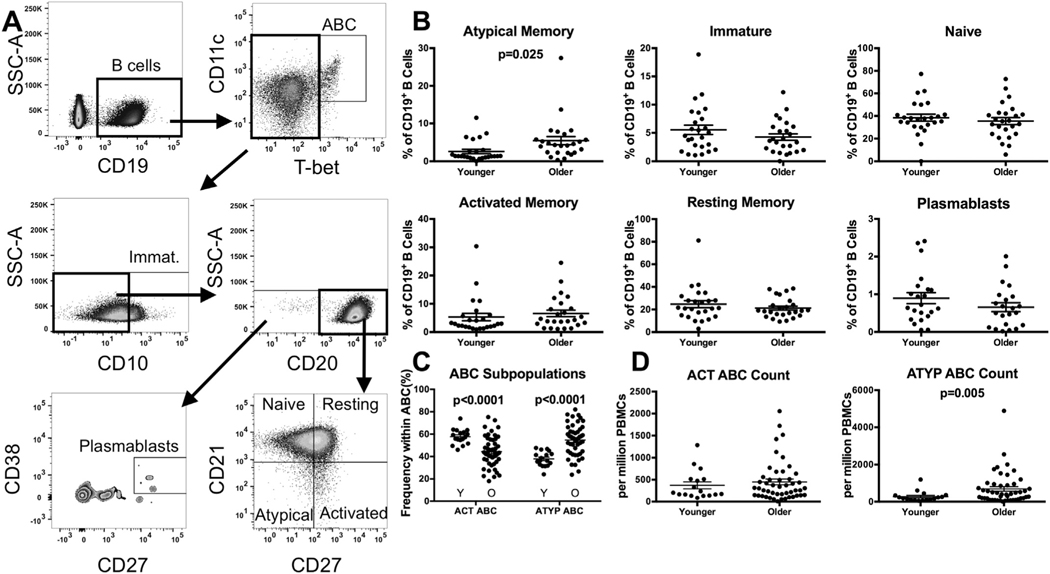
We evaluated peripheral blood B-cell phenotypes between healthy younger and older individuals by flow cytometry (gating strategy Fig. 1A). Overall, the frequency of the peripheral CD19+ B cell pool was significantly reduced with age (4.69% of lymphocytes in older individuals vs. 6.91% in younger; p = .020) (Supp. Fig. 1A). We examined the distribution of peripheral B-cell subsets within the CD19+ population: immature (CD10+), naïve (CD10−CD20+CD21+CD27−), resting memory (CD10−CD20+CD21+CD27+), activated memory (CD10−CD20+CD21−CD27+), atypical memory (CD10−CD20+CD21−CD27−), plasmablasts (CD10−CD20−CD27++CD38++) subsets. We observed a more than threefold increase in the CD21−CD27− atypical memory population, from 2.59% of peripheral B cells in younger individuals to 5.45% in older individuals (p = .025) (Fig. 1B). Expression of the inhibitory molecule Fc receptor-like protein 4 (FcRL4), which is associated the similarly defined CD21−CD27− traditional tissue-like memory cells, was similar between younger and older individuals (Supp. Fig. 1B). No significant differences in frequency of the naïve, resting memory, activated memory, or plasmablast populations were seen with age (Fig. 1B).
Fig. 1.
Increased frequency of atypical memory B cells and ATYP ABCs with age. PBMCs were stained immediately after isolation in order to identify B-cell subsets by flow cytometry. (A) Gating strategy. (B) Subset frequency within the CD19+ population. n = 25 from Rush cohort, ACT and ATYP ABC n = 17 younger, 48 older VA cohort (Unpaired t-Tests with Welch’s correction). (C) Frequency of ACT and ATYP ABC within the T-bethi CD11chi B cell pool (Unpaired t-Tests with Welch’s correction). (D) Absolute count of ACT and ATYP ABC per million PBMCs (Unpaired t-Tests with Welch’s correction). Circles represent single individuals. Bars indicate mean ± SEM. ABC, age-associated B cell. Immat., immature. Resting, resting memory. Activated, activated memory. Atypical, atypical memory. ABC, Age-associated B cells. Y, younger. O, older.
We next examined CD21−T-bet+CD11c+ cells, defined as age-associated B cells (ABCs). Further characterizing this population, we examined CD27+ ABCs similar to activated memory B cells and CD27− ABCs resembling atypical memory B cells, which we referred to as ACT and ATYP ABCs respectively. In investigating the frequencies of these populations within ABCs, we observed ACT ABCs to be significantly reduced with age (older-ACT ABC 44.4% of ABCs, younger-ACT ABC 57.8% of ABCs, p < .0001), while ATYP ABCs were significantly expanded (older-ATYP ABC 54.7% of ABCs, younger-ATYP ABC 37.9% of ABCs, p < .0001) (Fig. 1C). Examining the absolute number of these cells present per one million PBMCs, ATYP ABCs were significantly increased in number (older-ATYP ABC count 694 per million PBMCs, younger-ATYP ABC count 278 per million PBMCs, p = .005) with age, while the number of ACT ABCs remained unchanged (Fig. 1D).
3.2. Expression of inhibitory molecule CD72 is lower in older individuals
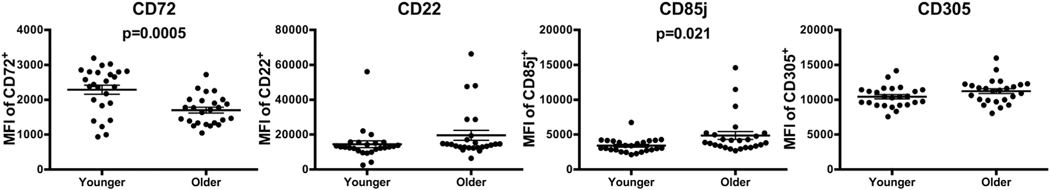
We next analyzed expression of molecules involved in regulating the BCR response. Inhibitory molecules CD22, CD72, CD85j, and CD305 were selected as their expression is reportedly elevated on a CD21− CD27− population [19]. To examine these receptors by flow cytometry, we evaluated the mean florescence intensity (MFI) of each marker. CD72 expression within the CD72+ population was significantly reduced with age (MFI of older 1701, MFI of younger 2287, p = .0005) (Fig. 2), but did not differ significantly by subset with age (Supp. Fig. 2A). Additionally, we observed an increase in MFI of CD85j on CD85j+ B cells (MFI of older 4859, MFI of younger 3406, p = .021) (Fig. 2). No significant differences were seen in expression of CD22 and CD305 with age (Fig. 2) or in frequency of any inhibitory marker (Supp. Fig. 2B). Expression of activating molecules CD40, CD80, CD86, and HLA-DR was also examined on CD19+ B cells, but no significant differences were seen in frequency (Supp. Fig. 2C) or MFI with age (Supp. Fig. 2D).
Fig. 2.
Reduced expression of inhibitory molecule CD72 with age. Surface expression of BCR inhibitory molecules was examined by flow cytometry. MFI of population of B cells positive for each marker. n = 25 from Rush cohort (Unpaired t-Tests with Welch’s correction). Circles represent single individuals. Bars indicate mean ± SEM. MFI, mean florescence intensity.
3.3. PAX5 expression is reduced in older individuals
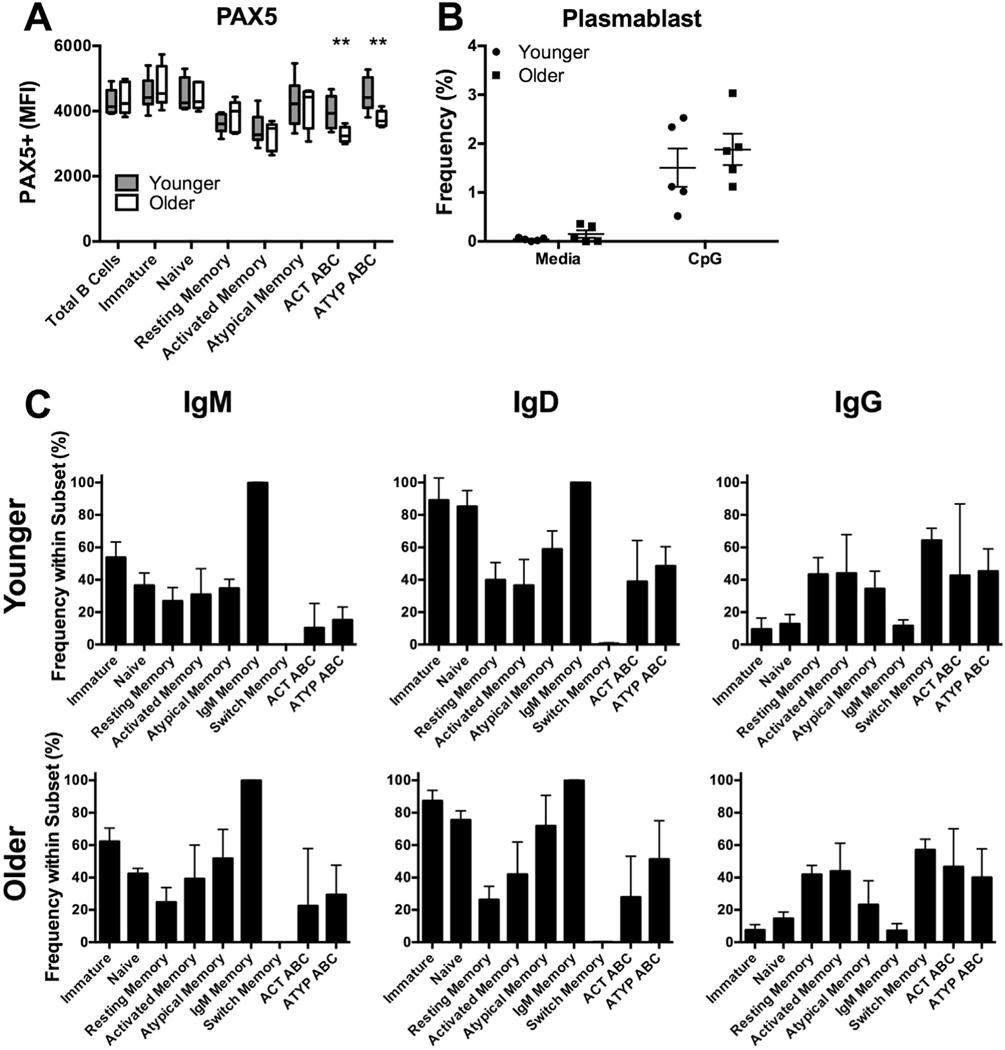
PAX5 is a key regulator of B-cell identity and differentiation. Examining expression of PAX5 across total B-cells and our previously-defined B-cell subsets in younger and older individuals (Fig. 3A) revealed a significant reduction in only ATYP ABC (older-MFI 3761, younger-MFI 4487, p = .006) and ACT ABC with age (older-MFI 3277, younger-MFI 3950, p = .007) (Fig. 3A). Frequency of PAX5+ cells did not vary with age across subsets (Supp. Fig. 3A).
Fig. 3.
PAX5 expression is decreased in ABCs of older individuals. PBMCs from younger and older individuals were stained for analysis by flow cytometry. (A) PAX5 expression across B-cell subsets. ** indicate from left to right p = .007, 0.006, n = 8 younger, 5 older from Rush cohort (Unpaired t-Tests with Welch’s correction for each subset). (B) Plasmablast (CD10−CD27++CD38++) frequency within total B cells after four days of culture of PBMCs with TLR9 agonist CpG. n = 5 from Red Cross cohort (two-way ANOVA). (C) Isotype frequency within peripheral B cell subsets. n = 5 from Red Cross cohort (two-way ANOVA). Box and whisker plot bars indicate minimum to maximum values, box indicates 25th–75th percentiles. Circles and squares represent single individuals. ABC, age-associated B cell. MFI, mean florescence intensity. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We next examined outcomes of TLR9 agonist stimulation which is known to induce plasmablast differentiation [11]. Culture with TLR9 agonist (CpG) stimulation revealed no significant difference in generation of plasmablasts (Fig. 3B) or proliferation of B-cell subsets with age (Supp. Fig. 3B).
We also examined isotype expression across peripheral B cell subsets as expression of class switch recombination initiator AID is reduced with age [20]. Therefore, we examined surface IgM, IgD, and IgG expression by flow cytometry. No significant difference was seen in immunoglobulin expression across subsets with age (Fig. 3C). We observed no quantifiable surface immunoglobulin expression on plasmablasts of younger or older individuals (data not shown).
3.4. CMV-associated phenotype of older individuals
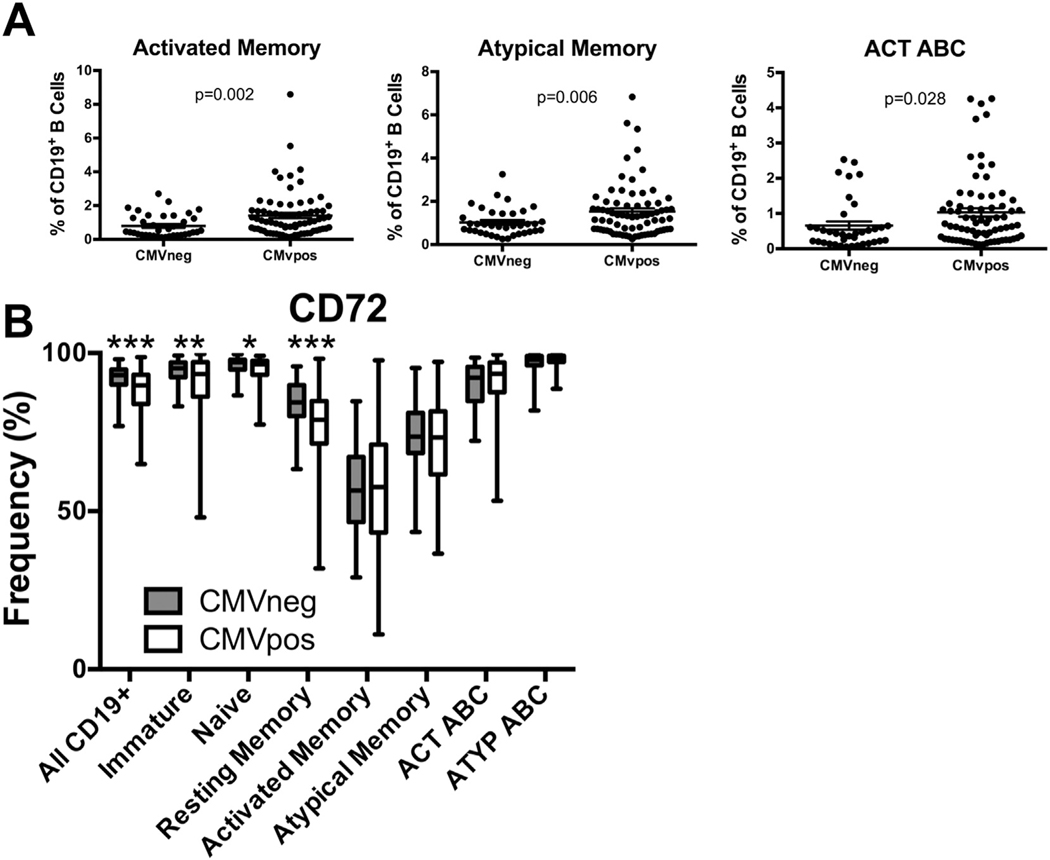
As chronic CMV infection is highly prevalent in older populations and is associated with immune senescence, we examined whether CMV infection, not age, contributed to the significant phenotypes we observed in older individuals. We first examined frequencies of B-cell subsets in CMV-positive (CMVpos) and CMV-negative (CMVneg) older individuals of pooled VA and Arizona cohorts. The activated memory, atypical memory, and ACT ABC populations were significantly expanded in CMVpos older individuals (p = .002, p = .006, p = .028 respectively) (Fig. 4A). Association of atypical memory subset frequency with CMV seropositivity was independent of cohort while Act ABC frequency was not (Supp. Fig. 4A).
Fig. 4.
Reduced CD72 expression is associated with CMV infection. (A) Activated memory, atypical memory, and ACT ABC subset frequency in cohorts of individuals were grouped based on CMV status and age. n = 37 CMVneg, 74 CMVpos combined VA and Arizona cohorts. (Unpaired t-Tests with Welch’s correction). Circles represent single individuals. (B) Frequency of CD72 positive cells within each subset. n = 37 CMVneg, 74 CMVpos combined VA and Arizona cohorts. (two-way ANOVA). Bars indicate minimum to maximum values, box indicates 25th–75th percentiles.
Frequency of CD72 expression was significantly reduced on B cells of CMVpos older individuals across multiple subsets (Fig. 4B). Association of CD72 with CMV seropositivity was independent of cohort (Supp. Fig. 4B). Expression of PAX5 did not differ by CMV serostatus (Supp. Fig. 4C).
3.5. Distinct B-cell phenotype associated with influenza vaccine response
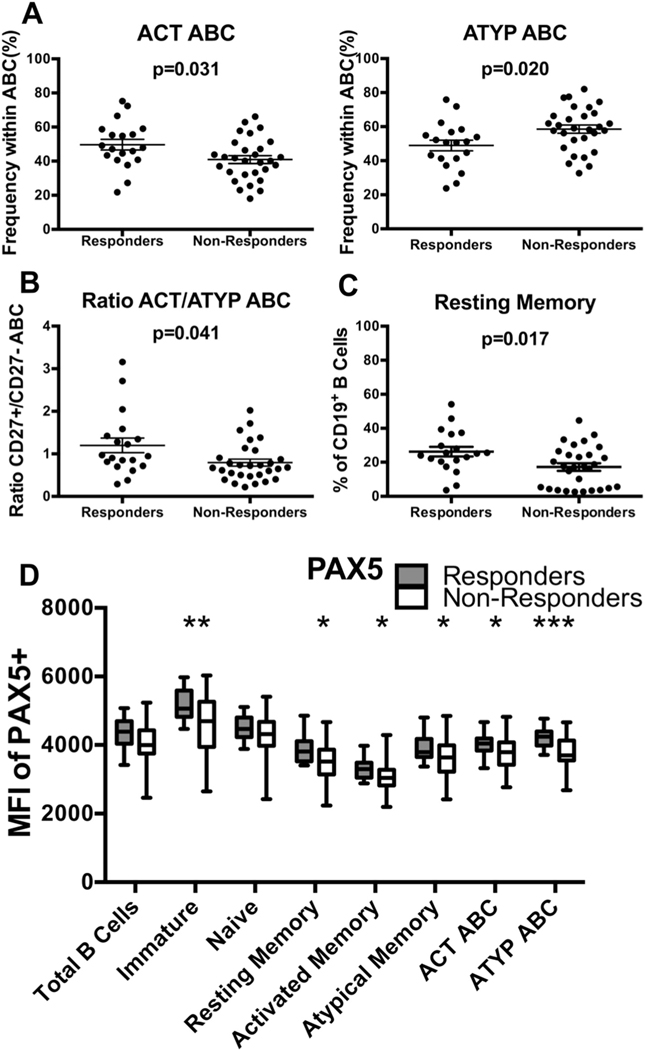
Having observed differences in phenotypic characteristics of B cells from younger and older individuals, we evaluated the relationship of these markers to influenza-specific antibody production following vaccination in older individuals. Using flow cytometry, we quantified expression of several markers of interest (CD10, CD20, CD21, CD27, CD11c, T-bet, CD72, and PAX5) on PBMCs of older individuals vaccinated for influenza 2–4 months prior. Outcomes of vaccination were measured by hemagglutination inhibition assay (HI), with responders defined by a fourfold increase in antibody titer for at least two of the three influenza strains in the vaccine as previously defined [12]. In defining the classification of non-responders, we specifically selected those with low titers before vaccination which did not increase following vaccination. This prevented the inclusion of individuals falsely deemed “non-responder” due to high antibody titers pre-vaccination. In comparing the frequency of B-cell subsets between responders and non-responders, non-responders had significantly fewer ACT ABCs (p = .031) and more ATYP ABCs (p = .020) (Fig. 5A), with the ratio of ACT/ATYP ABCs significantly higher in responders (ACT/ATYP ABC ratio non-responders 0.80, ACT/ATYP ABC ratio responders 1.20, p = .041) (Fig. 5B). ATYP ABC count was expanded in non-responders (count ATYP ABC older 864 per million PBMCs, count ATYP ABC younger 434 per million PBMCs, p = .051) (Fig. 5C). Frequency of resting memory B cells was significantly reduced in non-responders (frequency of resting memory in non-responders 17.2% vs. frequency of resting memory in responders 26.3%, p = .017) (Fig. 5D). No significant difference in the frequency of other B-cell subsets in relation to vaccine response was observed (Supp. Fig. 5A). Similarly, there was no significant association between expression of CD72 and influenza vaccine response (Supp. Fig. 5B).
Fig. 5.
Distinct older B-cell phenotype associates with poor response to influenza vaccination. PBMCs were collected 2–4 months following influenza vaccination. Response status determined by HI. (A) Frequency of ACT ABC, and ATYP ABC B cells in total ABC population as quantified by flow cytometry. n = 19 responders, 29 non-responders from VA cohort (Unpaired t-Tests with Welch’s correction). Circles represent single individuals. (B) Ratio of ACT ABC to ATYPABC as quantified by flow cytometry. n = 19, 29 (Unpaired t-Tests with Welch’s correction t). Circles represent single individuals. (C) Count of ATYP ABC per million PBMCs. n = 19 responders, 29 non-responders from VA cohort (Unpaired t-Tests with Welch’s correction). Circles represent single individuals. (D) Frequency of resting memory population in total B cells as measured by flow cytometry. n = 19, 29 (Unpaired t-Tests with Welch’s correction). Circles represent single individuals. (E) PAX5 expression across B-cell subsets. * indicate from left to right p = .002, 0.013, 0.021, 0.037, 0.031, and 0.0004, as measured by flow cytometry. n = 19, 29 (Unpaired t-Test). Box and whisker plot bars indicate minimum to maximum values, box indicates 25th–75th percentiles. Bars indicate mean ± SEM. MFI, mean florescence intensity. ABC, age-associated B cell.
As PAX5 expression was reduced in ACT and ATYP ABCs of older individuals, we evaluated its expression among influenza vaccine responders and non-responders. PAX5 expression in total B cells of non-responders was reduced in comparison to responders (PAX5+ MFI non-responder 4057 vs. PAX5+ MFI responder 4335, p = .051) (Fig. 5E). Expression of PAX5 was significantly reduced among multiple B-cell subsets of non-responders (Fig. 5E), specifically the immature, resting memory, activated memory, atypical memory, ACT ABC, and ATYP ABC subsets (Fig. 5E). PAX5 was most dramatically reduced in the ATYP ABC population (PAX5+ MFI non-responder 3815 vs. PAX5+ MFI responder 4206, p = .0004) (Fig. 5E) of non-responders.
Logistic regression analysis was performed on all variables which were significantly different between responders and non-responders to identify associations between phenotypic characteristics and antibody production following influenza vaccination (Supp. Fig. 6). Regression coefficients showed associations between ratio PAX5 expression in ATYP ABC and vaccine response (Table 2).
Table 2.
Regression analysis of vaccine response. Logistic regressions were performed for parameters which were statistically significant between responders and non-responders. n = 48 from VA cohort. CI, confidence interval (95%).
| Association of B-cell phenotype and vaccine response outcome | ||
|---|---|---|
| Regression coefficient (CI) | p value | |
|
| ||
| PAX5 MFI ATYP ABC | 0.003 (0.001) | 0.007 |
| PAX5 MFI immature | 0.002 (0.0006) | 0.007 |
| PAX5 MFI ACT ABC | 0.003 (0.001) | 0.012 |
| ATYP ABC frequency | −6.73 (2.90) | 0.020 |
| PAX5 MFI resting Mem. | 0.002 (0.0007) | 0.030 |
| Ratio ACT/ATYP ABC | 1.26 (0.61) | 0.038 |
| PAX5 MFI Atypical Mem. | 0.001 (0.0007) | 0.071 |
| PAX5 MFI activated Mem. | 0.001 (0.0007) | 0.184 |
| Resting Mem. frequency | −39.93 (31.66) | 0.207 |
| ACT ABC frequency | 3.27 (2.72) | 0.229 |
4. Discussion
As the number of individuals in need of geriatric care continues to grow, it becomes ever more apparent the older population has a unique set of healthcare needs which are not being optimally met. Currently, older individuals remain susceptible to influenza despite high compliance with vaccination, as 90% of influenza deaths occur in individuals over 65 years of age [21]. Therefore, identifying age-related deficits in the immune system, or immunosenescence, and pathways contributing to impaired humoral response with age is imperative for public health. Importantly, the characterization of B cells from older individuals may yield additional predictors of diminished vaccine responses and a potential therapeutic target.
These findings indicate that the composition of the ABC population and PAX5 expression may serve as potential biomarkers of influenza vaccine response in older individuals. This study is the first to associate expansion of specific ABC populations with a reduced influenza vaccine response in older individuals. In collecting PBMCs 2–4 months following vaccination, we demonstrate populations consistently expressed with age, and not transiently induced by vaccination. Though ABCs are associated with age, they have unique properties enabling humoral response to both foreign and self-antigens depending on the context of stimulus and regulating factors. Recent work by Lau et al. [22] suggests a similar population, CD19+CD27+CD21lo B cells with elevated Blimp-1 and T-bet, expands after vaccination and likely becomes long-lived plasma cells. This population may be similar to the expansion we observed in ACT ABC following culture with thymus-independent antigen. While further work must examine the function of this population in our study, we did see a reduction in the frequency of ACT ABCs in vaccine non-responders with age [23]. These data support the concept that a CD27+ population with elevated T-bet is integral for successful antibody production following vaccination as their loss with age and replacement with ATYP ABCs was associated with weakened humoral responses.
These changes to ABC frequencies may be indicative of humoral response due to T-bet’s involvement in antibody production. T-bet, which defines the ABC population, also facilitates expression of AID to enable immunoglobulin mutation [24]. Blomberg et al. [25] have previously associated transcriptional control of AID with weak vaccine response. While the role of T-bet in ABCs warrants further investigation, changes to the ABC population with age may ultimately affect potential for AID production and humoral response.
Though T-bet is often associated with anti-viral response [26], it also defines and controls phenotype, identity, and function of ABCs [27]. However, the association of the ABCs with failure to seroconvert following vaccination suggests the expansion of this population is not always beneficial for vaccine response despite its high expression of T-bet. This is likely due to heterogeneity within the ABC population, in which the ACT population may promote humoral response while the ATYP population does not. As we observed a significant increase in the ATYP population of ABCs with age and in vaccine non-responders, this ATYP ABC population may be expanding as the ACT ABC population decreases with age.
We also observed an expansion of the atypical memory subset outside of the ABC population. The atypical memory population of the older cohort does not resemble CD21−CD27− tissue-like memory B cells associated with exhaustion in HIV as it did not exhibit elevated expression of the inhibitory molecule FcRL4. While CD27−IgG+ B cells are suggested to resemble FcRL4+ B cells in many ways such as IgG expression levels or exhaustion [28], we did not observe a significant change in IgG expression with age. Furthermore, CD21−CD27− B cells of older individuals mayor may not be functionally exhausted. Although we examined expression of multiple inhibitory and co-stimulatory molecules in younger and older individuals, we, as well as others [29] did not observe differential expression of costimulatory molecules with age. While expression of CD72 was reduced in our cohort, this is likely a result of persistent CMV infection. Dauby et al. [30] demonstrated expression of CD72 was elevated in the atypical memory population during primary CMV relative to chronic infection. We demonstrate reduced CD72 expression across multiple B-cell subsets during CMV infection. This effect of CMV may also induce expansion of atypical memory B cells in older individuals [30]. However, as other viruses may also stress the immune system for many years, further work should explore the impact of other chronic infections on B-cell phenotype.
This is the first association of reduced PAX5 expression in B cells of humans with reduced vaccine responses. Expression of Pax5 has been demonstrated to be reduced in common lymphoid progenitors from older mice relative to younger mice, which contributes to the reduced B-cell potential seen with age [31]. Additionally, PAX5 synchronizes expression patterns of a vast gene regulatory network which ensures appropriate B-cell subset distribution, and regulates BCR signaling and terminal differentiation (reviewed in [32]). Therefore, its reduction in older individuals has the potential to drive severe disruption of B-cell identity and humoral response. Though we did not observe a reduction in frequency of plasmablasts generated with age, this may be a result of small sample size, as reduce antibody quantity with age has been described previously [23]. We also observed a reduced frequency of ACT ABCs in influenza vaccine non-responders; as this population is thought to produce antibodies [33], its reduced frequency suggests this population may become increasingly important for antibody production with age.
In our study, antibody production in response to influenza vaccination was assessed by HI. Though this measurement does not account for Fc-mediated antibody activity, HI remains the best standardized and most widely used method of assessing influenza vaccine response. While it has been suggested pre-vaccination antibody titer is the most important factor determining whether an individual is protected from influenza [12,34], this information was not available at the time of enrollment and was not used to select individuals. Individuals in this study were categorized based on titer at time 0 and increase in this titer following vaccination. While reduced IgG levels with age could affect the outcome of the HI which reports influenza-specific IgG levels, previous examination of isotype and antibody lineage by Tabibian-Keissar et al. [35] reported no significant differences with age. Similarly, we did not observe significant changes to frequencies of IgM, IgD, or IgG on peripheral B cells. However, further work examining isotype frequency in lymphoid organs with age warrants further examination to provide a more complete picture of antibody response with age.
In summary, our findings demonstrate that age-related phenotypic changes occur in B cells of healthy individuals, resulting in differential expression of molecules regulating composition of the B-cell compartment, terminal differentiation, and humoral response. We observed phenotypic changes such as expansion of ATYP ABCs and reduced PAX5 expression to be associated with age and inability to seroconvert following vaccination which were predictive of antibody production following influenza vaccination. Therefore, these age-related changes have potential value as biomarkers of immunosenescence and identify pathways of interest to improve vaccine-specific antibody production with age.
Supplementary Material
Acknowledgements
Dr. Shah’s effort was supported by the Rush Center of Excellence on Disparities in HIV and Aging (P20MD006886, PI Barnes) and the Illinois Department of Public Health. Dr. Canaday’s effort was supported by NIH AI108972 and Veterans Affairs (CX000249).
We wish to thank the individuals and the staff of the Rush Memory Clinic Data and Specimen Repository and Senior Care. Additionally, Jeffrey Martinson for flow cytometry expertise.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clim.2018.02.003.
References
- [1].Kaminski DA, Wei C, Qian Y, Rosenberg AF, Sanz I, Advances in human B cell phenotypic profiling, Front. Immunol 3 (2012) 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP, A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice, Blood 118 (2011) 1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, Marrack P, Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity, Blood 118 (2011) 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Russell Knode LM, Naradikian MS, Myles A, Scholz JL, Hao Y, Liu D, Ford ML, Tobias JW, Cancro MP, Gearhart PJ, Age-associated B cells express a diverse repertoire of VH and Vkappa genes with somatic hypermutation, J. Immunol 198 (2017) 1921–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rubtsova K, Rubtsov AV, Cancro MP, Marrack P, Age-associated B cells: a T-bet-dependent effector with roles in protective and pathogenic immunity, J. Immunol 195 (2015) 1933–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Singh H, Medina KL, Pongubala JM, Contingent gene regulatory networks and B cell fate specification, Proc. Natl. Acad. Sci. U. S. A 102 (2005) 4949–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fuxa M, Busslinger M, Reporter gene insertions reveal a strictly B lymphoid-specific expression pattern of Pax5 in support of its B cell identity function, J. Immunol 178 (2007) 3031–3037. [DOI] [PubMed] [Google Scholar]
- [8].Riley RL, Van der Put E, King AM, Frasca D, Blomberg BB, Deficient B lymphopoiesis in murine senescence: potential roles for dysregulation of E2A, Pax-5, and STAT5, Semin. Immunol 17 (2005) 330–336. [DOI] [PubMed] [Google Scholar]
- [9].van Lochem EG, van der Velden VH, Wind HK, te Marvelde JG, Westerdaal NA, van Dongen JJ, Immunophenotypic differentiation patterns of normal hematopoiesis in human bone marrow: reference patterns for age-related changes and disease-induced shifts, Cytometry B Clin. Cytom 60 (2004) 1–13. [DOI] [PubMed] [Google Scholar]
- [10].Hauser J, Grundstrom C, Kumar R, Grundstrom T, Regulated localization of an AID complex with E2A, PAX5 and IRF4 at the Igh locus, Mol. Immunol 80 (2016) 78–90. [DOI] [PubMed] [Google Scholar]
- [11].Marasco E, Farroni C, Cascioli S, Marcellini V, Scarsella M, Giorda E, Piano Mortari E, Leonardi L, Scarselli A, Valentini D, Cancrini C, Duse M, Grimsholm O, Carsetti R, B-cell activation with CD40L or CpG measures the function of B-cell subsets and identifies specific defects in immunodeficient patients, Eur. J. Immunol 47 (2017) 131–143. [DOI] [PubMed] [Google Scholar]
- [12].Van Epps P, Tumpey T, Pearce MB, Golding H, Higgins P, Hornick T, Burant C, Wilson BM, Banks R, Gravenstein S, Canaday DH, Preexisting immunity, not frailty phenotype, predicts influenza postvaccination titers among older veterans, Clin. Vaccine Immunol 24 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lüdecke D, Data Visualization for Statistics in Social Science, 2017. [Google Scholar]
- [15].Lüdecke D, sjmisc: Miscellaneous Data Management Tools, 2017. [Google Scholar]
- [16].Lüdecke D, sjstats: Statistical Functions for Regression Models, 2017. [Google Scholar]
- [17].R.C. Team, in: R.F.f.S. Computing (Ed.), R: A Language and Environment for Statistical Computing, R.C. Team, Vienna, Austria, 2017. [Google Scholar]
- [19].Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS, Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals, J. Exp. Med 205 (2008) 1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Daniela Frasca AML, Suzanne C.Lechner, Ryan John G., Schwartz Robert, Riley Richard L., Blomberg Bonnie B., Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells, J. Immunol 180 (2008) 5283–5290. [DOI] [PubMed] [Google Scholar]
- [21].CDC, in: C.f.D. Control (Ed.), Estimates of Deaths Associated with Seasonal Influenza – United States, 1976–2007 2010, pp. 1057–1062. [PubMed] [Google Scholar]
- [22].Denise Lau LY-LL, Sarah F.Andrews, Henry Carole, Rojas Karla Thatcher, Neu Karlynn E., Huang Min, Huang Yunping, DeKosky Brandon, Palm Anna-Karin E., Ippolito Gregory C., Georgiou George, Wilson Patrick C., Low CD21 expression defines a population of recent germinal center graduates primed for plasma cell differentiation, Sci. Immunol 2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stiasny K, Aberle JH, Keller M, Grubeck-Loebenstein B, Heinz FX, Age affects quantity but not quality of antibody responses after vaccination with an inactivated flavivirus vaccine against tick-borne encephalitis, PLoS One 7 (2012), e34145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Knox JJ, Buggert M, Kardava L, Seaton KE, Eller MA, Canaday DH, Robb ML, Ostrowski MA, Deeks SG, Slifka MK, Tomaras GD, Moir S, Moody MA, Betts MR, T-bet+ B cells are induced by human viral infections and dominate the HIV gp140 response, JCI Insight 2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Frasca D, Diaz A, Romero M, Phillips M, Mendez NV, Landin AM, Blomberg BB, Unique biomarkers for B-cell function predict the serum response to pandemic H1N1 influenza vaccine, Int. Immunol 24 (2012) 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Barnett BE, Staupe RP, Odorizzi PM, Palko O, Tomov VT, Mahan AE, Gunn B, Chen D, Paley MA, Alter G, Reiner SL, Lauer GM, Teijaro JR, Wherry EJ, Cutting edge: B cell-intrinsic T-bet expression is required to control chronic viral infection, J. Immunol 197 (2016) 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rubtsova K, Rubtsov AV, van Dyk LF, Kappler JW, Marrack P, T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance, Proc. Natl. Acad. Sci. U. S. A 110 (2013) E3216–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jessie GCASN, Fecteau F, A new memory CD27−IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation, J. Immunol 177 (2006) 3728–3736. [DOI] [PubMed] [Google Scholar]
- [29].Colonna-Romano G, Bulati M, Aquino A, Scialabba G, Candore G, Lio D, Motta M, Malaguarnera M, Caruso C, B cells in the aged: CD27, CD5, and CD40 expression, Mech. Ageing Dev 124 (2003) 389–393. [DOI] [PubMed] [Google Scholar]
- [30].Dauby N, Kummert C, Lecomte S, Liesnard C, Delforge ML, Donner C, Marchant A, Primary human cytomegalovirus infection induces the expansion of virus-specific activated and atypical memory B cells, J. Infect. Dis 210 (2014) 1275–1285. [DOI] [PubMed] [Google Scholar]
- [31].Lescale C, Dias S, Maes J, Cumano A, Szabo P, Charron D, Weksler ME, Dosquet C, Vieira P, Goodhardt M, Reduced EBF expression underlies loss of B-cell potential of hematopoietic progenitors with age, Aging Cell 9 (2010) 410–419. [DOI] [PubMed] [Google Scholar]
- [32].Holmes ML, Pridans C, Nutt SL, The regulation of the B-cell gene expression programme by Pax5, Immunol. Cell Biol 86 (2008) 47–53. [DOI] [PubMed] [Google Scholar]
- [33].Isnardi I, Ng YS, Menard L, Meyers G, Saadoun D, Srdanovic I, Samuels J, Berman J, Buckner JH, Cunningham-Rundles C, Meffre E, Complement receptor 2/CD21-human naive B cells contain mostly autoreactive unresponsive clones, Blood 115 (2010) 5026–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Reber AJ, Kim JH, Biber R, Talbot HK, Coleman LA, Chirkova T, Gross FL, Steward-Clark E, Cao W, Jefferson S, Veguilla V, Gillis E, Meece J, Bai Y, Tatum H, Hancock K, Stevens J, Spencer S, Chen J, Gargiullo P, Braun E, Griffin MR, Sundaram M, Belongia EA, Shay DK, Katz JM, Sambhara S, Preexisting immunity, more than aging, influences influenza vaccine responses, Open Forum Infect. Dis 2 (2015), ofv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hilla Tabibian-Keissar Lena Hazanov, Schiby Ginette, Rosenthal Noemie, Rakovsky Aviya, Michaeli Miri, Gitit Lavy Shahaf Yishai Pickman, Rosenblatt Kinneret, Melamed Doron, Deborah Dunn-Walters Ramit Mehr, Barshack I, Aging affects B-cell antigen receptor repertoire diversity in primary and secondary lymphoid tissues, Eur. J. Immunol 46 (2016) 480–492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.