Abstract
Aims
In catecholaminergic polymorphic ventricular tachycardia (CPVT), the exercise-stress test (EST) is the cornerstone for the diagnosis, risk stratification, and assessment of therapeutic efficacy, but its repeatability is unknown. We aimed to test the repeatability of ventricular arrhythmia characteristics on the EST in patients with CPVT.
Methods and results
EST-pairs (ESTs performed within 18 months between 2005 and 2021, on the same protocol, and without or on the exact same treatment) of patients with RYR2-mediated CPVT from two specialized centres were included. The primary endpoint was the repeatability of the maximum ventricular arrhythmia score [VAS: 0 for the absence of premature ventricular contractions (PVCs); 1 for isolated PVCs; 2 for bigeminal PVCs; 3 for couplets; and 4 for non-sustained ventricular tachycardia]. Secondary outcomes were the repeatability of the heart rate at the first PVC and the ΔVAS (the absolute difference in VAS between the EST-pairs). A total of 104 patients with 349 EST-pairs were included. The median duration between ESTs was 343 (interquartile range, 189–378) days. Sixty (17.2%) EST-pairs were off therapy. The repeatability of the VAS was moderate {Krippendorf α, 0.56 [95% confidence interval (CI), 0.48–0.64]}, and the repeatability of the heart rate at the first PVC was substantial [intra-class correlation coefficient, 0.78 (95% CI, 0.71–0.84)]. The use of medication was associated with a higher odds for a ΔVAS > 1 (odds ratio = 3.52; 95% CI, 2.46–4.57; P = 0.020).
Conclusion
The repeatability of ventricular arrhythmia characteristics was moderate to substantial. This underlines the need for multiple ESTs in CPVT patients and CPVT suspicious patients and it provides the framework for assessing the therapeutic efficacy of novel CPVT therapies.
Keywords: Catecholaminergic polymorphic ventricular tachycardia, Diagnostic test, Exercise-stress test, RYR2, Ventricular arrhythmia
Graphical Abstract
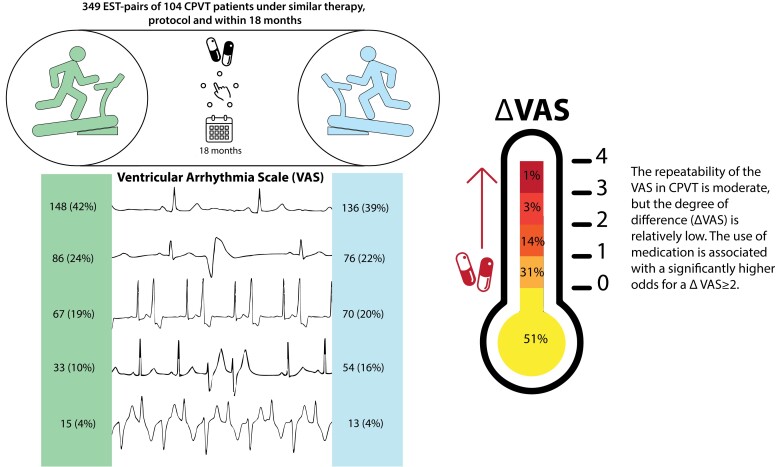
Graphical Abstract.
What’s new?
A single exercise-stress test to diagnose catecholaminergic polymorphic ventricular tachycardia (CPVT) in a suspected patient may be insufficient, because the repeatability of the ventricular arrhythmia score is moderate.
In a CPVT patient, an increase in ventricular arrhythmia score of at least two categories on a subsequent exercise-stress test under similar circumstances is likely due to progression of a disease or therapeutic inefficacy and should be addressed accordingly. Conversely, a decrease in ventricular arrhythmia score of at least two categories after a therapeutic modification suggests a positive effect.
A higher degree of difference in ventricular arrhythmia score on subsequent exercise-stress tests in CPVT patients using medication suggests the potential effect of unsatisfactory adherence to medication.
Introduction
Catecholaminergic polymorphic ventricular tachycardia (CPVT)—a rare but potentially life-threatening genetic heart rhythm disorder associated with variants in the RYR2 gene—is typically diagnosed with an exercise-stress test (EST).1 In patients with CPVT, ventricular arrhythmia, typically bidirectional ventricular tachycardia (VT) and polymorphic VT, can occur particularly during physical exercise or emotional stress. This could result in syncope, sudden cardiac arrest, or sudden cardiac death.
The EST was designed originally in 1949 as a tool to assess the presence of obstructive coronary artery disease.2 Subsequently, EST protocols were developed and improved and the EST is used in a wider spectrum of diseases,3 including the diagnosis of CPVT and the evaluation of its therapeutic interventions.1,4,5,6 In a typical CPVT patient undergoing an EST, there are no ventricular arrhythmias at rest, but premature ventricular contractions (PVCs) appear during mild exercise and the ventricular arrhythmia burden and severity increase during subsequent stages.7 Complex ventricular arrhythmias, defined as couplets or (non-)sustained VT, have been associated with future arrhythmic events8 and generally compels treatment intensification.1 In addition, studies addressing therapeutic efficacy use EST-specific endpoints, namely the occurrence and severity of ventricular arrhythmias9,10,11 Finally, EST heart rate measurements have been studied to develop potential new tools for risk stratification in patients with CPVT.12,13
Apart from our personal clinical observations that the occurrence and severity of ventricular arrhythmia on consecutive ESTs in a CPVT patient might be variable over time, a higher incidence of VT or ventricular fibrillation in the afternoon and evening hours compared with morning hours in children with CPVT has been described.14 In addition, performing follow-up ESTs in genotype-positive family members with initially negative EST results increases the likelihood of finding ventricular arrhythmias.5,15 Finally, different EST protocols may affect variability, for example, a newly published ‘burst’ EST protocol—with a sudden onset of a high workload—significantly increased the likelihood of diagnosing CPVT.16
Clearly, the EST is a very important tool for establishing the diagnosis, prognosis, and evaluation of treatment in patients with CPVT.1,6 However, the intra-patient variability—the repeatability—of the presence and severity of ventricular arrhythmias on the EST in CPVT is unknown. Here, we aimed to test the repeatability of ventricular arrhythmia characteristics on the EST in two large cohorts of patients with RYR2-mediated CPVT.
Methods
Study population
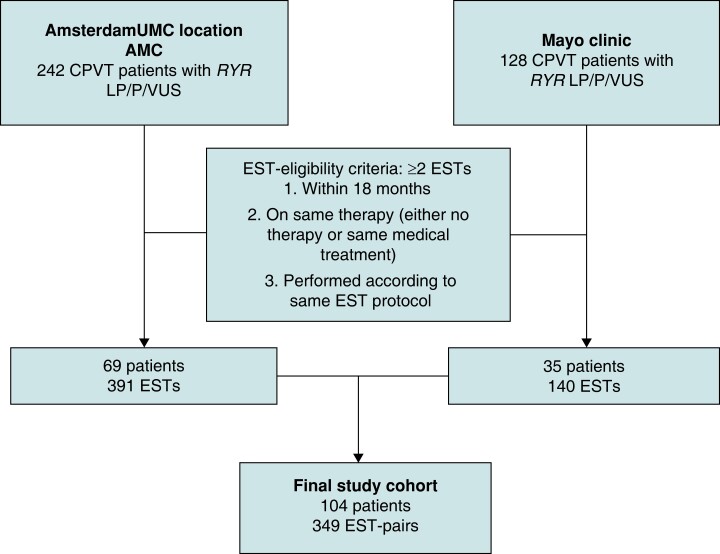
This was a retrospective cohort study including data from two tertiary referral centres for patients with CPVT: the Amsterdam UMC, location AMC, Amsterdam, the Netherlands and the Mayo Clinic, Rochester, MN, USA. Institutional review board approval and informed consent were obtained if necessary for this type of research in both centres. All ESTs from 2005 until 15 June 2021 for Amsterdam UMC CPVT patients and until 21 April 2021 for Mayo Clinic CPVT patients were checked for eligibility.
In this study, we included patients diagnosed with CPVT and with a RYR2 variant of uncertain significance or a RYR2 (likely) pathogenic variant according to ACMG criteria.17 Patients with an RYR2 variant of uncertain significance were excluded in the absence of a definite CPVT phenotype.7,18 A CPVT phenotype was defined as bigeminal ventricular premature beats or more complex VAs in index patients, and isolated ventricular premature beats or more complex VAs in family members on the EST, epinephrine challenge test, or Holter monitoring. Furthermore, patients with significant cardiac comorbidities—defined as an LVEF <40%, a (history of) significant coronary artery stenosis, or a (history of) moderate or severe mitral-, pulmonic-, or aortic valve stenosis or regurgitation—were excluded. Eligible patients had to have at least two ESTs fulfilling the following criteria: (i) performed within 18 months of each other, (ii) on exactly the same treatment on both occasions (i.e. no drugs or on exactly the same drug(s) and dosage(s) and no left cardiac sympathetic denervation performed in between subsequent ESTs), (iii) performed with the same EST protocol on both occasions. All ESTs were analyzed by either S.N.J.P. (Amsterdam UMC) or K.E.T. (Mayo Clinic) and prespecified EST baseline and outcome variables were collected using Castor EDC.19
Outcomes
The primary outcome was the occurrence and severity of ventricular arrhythmias, defined as the maximum ventricular arrhythmia score (VAS), an ordinal score with 0 for the absence of PVCs, 1 for isolated PVCs, 2 for bigeminal PVCs, 3 for couplets, and 4 for non-sustained VT, defined as at least three consecutive monomorphic or polymorphic PVCs. The secondary outcomes were the heart rate at the first PVC (HR-PVC) and the heart rate at 1 minute in the recovery phase (HR-recovery). Furthermore, we calculated the delta-VAS (ΔVAS), defined as the absolute difference in VAS between the paired ESTs. Baseline EST variables and patient demographics were also collected. The EST protocols are described in Supplemental Material online.
Statistical analyses
ESTs were paired according to the aforementioned inclusion criteria. Multiple EST-pairs per patient were allowed. Continuous variables are presented as mean ± standard deviation (SD) or median (interquartile range), as appropriate. Categorical variables are presented as numbers and percentages. The repeatability of nominal and ordinal variables was assessed using the Krippendorf α. The 95% confidence interval (CI) of Krippendorf α was calculated using non-parametric bootstrapping of 1000 samples. A value of Krippendorf α<0 reflects poor repeatability, 0–0.20 reflects slight repeatability, 0.21–0.4 reflects fair repeatability, 0.41–0.60 reflects moderate repeatability, 0.61–0.8 reflects substantial repeatability, and >0.81 reflects almost perfect repeatability.20,21 To assess the repeatability of the continuous variables we calculated the intra-class correlation coefficient (ICC) with the corresponding 95% CI. The ICC varies from 0 to 1 reflecting a degree of repeatability similar as for Krippendorf α. Furthermore, Bland–Altman plots were used to visualize whether the SD of the error was independent of the measurement value and to calculate the mean error and its 95% CI. The analyses of the association between potential risk factors and a high ΔVAS and more detailed information on the statistical analyses, including the sensitivity analyses, are described in Supplemental Material online. The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
Results
Description of the study population
From a total of 104 eligible patients, 349 EST-pairs were included and analysed (Figure 1). The median number of EST-pairs per patient was 2 (1–3), and in 45 patients (43.3%) a single EST-pair was included. The median age at diagnosis was 18 (12–39) years, 25 patients (24.0%) were probands, and 5 (4.8%) experienced a sudden cardiac arrest before diagnosis (Table 1). Sixty EST-pairs (17.2%) were off therapy. Most EST-pairs on therapy were on combination therapy with beta-blocker and flecainide. The median age at the time of the first EST was 20 (14–38) years and the median time between the paired ESTs was 343 [189–378] days (Table 2). Most ventricular arrhythmia-unrelated characteristics of the paired ESTs were substantially repeatable to almost perfectly repeatable (Table 3). Approximately half of the ESTs were performed in the morning [190 (54.4%) of EST1 and 197 (56.4%) of EST2; Krippendorf α, 0.240 (95% CI, 0.138–0.343)]. Within the EST-pairs, 131 (37.5%) changed from morning to afternoon recording or the reverse.
Figure 1.
Flowchart of included patients with RYR2-mediated CPVT. CPVT, catecholaminergic polymorphic ventricular tachycardia; EST, exercise-stress test; LP, likely pathogenic; P, pathogenic; VUS, variant of uncertain significance.
Table 1.
Patient characteristics
| Characteristic | Complete patient cohort (n = 104) |
|---|---|
| Age at diagnosis in years, median (IQR) | 18 (12–39) |
| Female, No. (%) | 58 (55.8) |
| Probands, No. (%) | 25 (24.0) |
| Family members with SCD <40 years of age, No. (%) | 19 (18.3) |
| Worst symptom before diagnosis, No. (%) | |
| ȃAsymptomatic | 69 (66.3) |
| ȃSyncope with or without seizures | 30 (28.8) |
| ȃSCA | 5 (4.8) |
| Reason of first presentation, No. (%) | |
| ȃCardiac symptoms | 27 (26.0) |
| ȃFamily screening | 74 (71.1) |
| ȃOther | 3 (2.9) |
| RYR2 variant classification, No. (%) | |
| ȃPathogenic | 53 (51.0) |
| ȃLikely pathogenic | 26 (25.0) |
| ȃUncertain significance | 25 (24.0) |
| Beta-blockera, No. (%) | 95 (91.3) |
| Flecainidea, No. (%) | 62 (59.6) |
| LCSDa, No. (%) | 23 (22.1) |
IQR, interquartile range; LCSD, left cardiac sympathetic denervation; No, number.
Number and proportion of patients who ever received this treatment during follow-up.
Table 2.
Baseline information for exercise-stress test-pairs
| Characteristic | Complete cohort of EST-pairs (n = 349) |
|---|---|
| Time between ESTs in days, median (IQR) | 343 (189–378) |
| Age at first EST in years, median (IQR) | 20 (14–38) |
| EST protocola, No. (%) | |
| ȃBruce protocol | 52 (14.9) |
| ȃSuper Bruce protocol | 142 (40.7) |
| ȃ50 linear | 34 (9.7) |
| ȃ50/25-2 linear | 25 (7.2) |
| ȃ50/25-1 linear | 17 (4.9) |
| ȃO2 protocol | 67 (19.2) |
| ȃOther | 12 (3.4) |
| Treatment at the time of EST, No. (%) | |
| ȃNone | 60 (17.2) |
| ȃBeta-blocker monotherapy | 101 (28.9) |
| ȃFlecainide monotherapy | 17 (4.9) |
| ȃBeta-blocker and flecainide combination therapy | 118 (33.8) |
| ȃBeta-blocker and LCSD | 7 (2.0) |
| ȃFlecainide and LCSD | 5 (1.4) |
| ȃBeta-blocker, flecainide, and LCSD | 41 (11.7) |
| Beta-blocker type at the time of EST, No. (%) (n = 267) | |
| ȃMetoprolol | 115 (43.1) |
| ȃAtenolol | 26 (9.7) |
| ȃBisoprolol | 23 (8.6) |
| ȃPropranolol | 43 (16.1) |
| ȃSotalol | 4 (1.5) |
| ȃNadolol | 56 (21.0) |
| Body weight difference in kilograms, median (IQR) | 0 (0–4) |
EST, exercise-stress test; IQR, interquartile range; LCSD, left cardiac sympathetic denervation.
Descriptions of the different exercise-stress test protocols are provided in Supplemental Material online.
Table 3.
Exercise-stress tests characteristics
| EST 1 | EST 2 | Measure of repeatabilitya | Mean difference (95% CI) | |
|---|---|---|---|---|
| Time of dayb, No. (%) | ||||
| ȃMorning | 190 (54.4) | 197 (56.4) | 0.24 (0.14–0.34) | NA |
| ȃAfternoon | 159 (45.5) | 152 (43.6) | ||
| Duration of EST in minutes, median (IQR) | 8.0 (6.7–10) | 8.2 (6.9–10.1) | 0.89 (0.85–0.90) | 0.1 (−2.3–2.5) |
| Maximum workload attained in METS, mean (SD) | 13.0 (4.1) | 13.1 (4.1) | 0.92 (0.90–0.93) | 0.1 (−3.1–3.4) |
| Reason for discontinuation exercise, No. (%) | ||||
| ȃFatigue, pain, or discomfort | 301 (86.2) | 326 (93.4) | 0.32 (0.15–0.48) | NA |
| ȃVentricular arrhythmias | 41 (11.7) | 17 (4.9) | ||
| ȃEST protocol completed | 7 (2.0) | 6 (1.7) | ||
| Baseline heartrate in b.p.m., mean (SD) | 70.8 (16.4) | 70.0 (15.9) | 0.74 (0.67–0.78) | −0.7 (−23.7–22.2) |
| Maximum heartrate in b.p.m., median (IQR) | 150 (130–176) | 153 (130–176) | 0.88 (0.85–0.90) | 0.7 (−25.3–26.7) |
| Body weight in kg, mean (SD) | 64.0 ± 19.3 | 65.9 ± 18.3 | 0.965 (0.945–0.977) | 1.9 (−7.2–10.9) |
b.p.m., beats per minute; CI, confidence interval; EST, exercise-stress test; IQR, interquartile range; METS, metabolic equivalents; SD, standard deviation.
For continuous variables, the intra-class correlation coefficient and its corresponding 95% confidence interval is reported. For categorical or ordinal variables, the Krippendorf α and its 95% confidence interval, obtained with 1000 bootstrap samples, is reported.
Morning was defined as the start of the recording before noon, 12:00PM, afternoon was defined as at or after 12:00PM.
Primary outcome and secondary outcomes
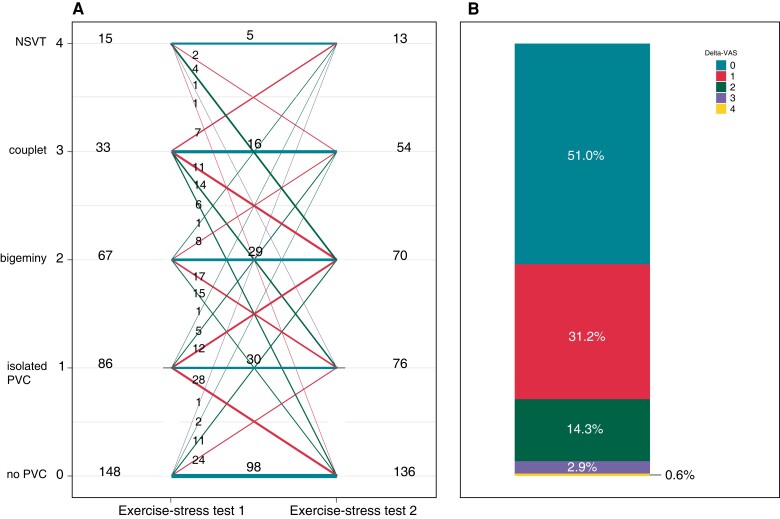
A significant number of ESTs did not show any ventricular ectopy, corresponding to a VAS of 0 [148 (42.4%) of EST1 and 136 (39.0%) of EST2], and the number of ESTs with non-sustained VT, corresponding to a VAS of 4, was relatively low [15 (4.3%) of EST1 and 13 (3.7%) of EST2; Figure 2A]. The majority of patients with a VAS of 0 were under adequate treatment (n = 71.6% for EST1 and 72.8% for EST2). The overall repeatability of the VAS between EST-pairs was moderate [Krippendorf α, 0.57 (95% CI, 0.48–0.64)]. Of the EST-pairs with a VAS above 0, the HR-PVC was 123 ± 27 beats per minute (b.p.m.) at EST1 vs. 124 ± 28 b.p.m. at EST2, reflecting substantial repeatability [ICC, 0.78 (95% CI, 0.71–0.84); mean difference, −2 (95% CI, −37.4 to 33.4) b.p.m.]. The repeatability of the HR-recovery was almost perfect [116 (102–141) b.p.m. vs. 117 (102–142) b.p.m.; ICC, 0.819 (95% CI, 0.774–0.856); mean difference, 1.0 (95% CI, −29 to 31) b.p.m.].
Figure 2.
Ventricular arrhythmia score and ΔVAS on paired exercise-stress tests. (A) Ventricular arrhythmia score (VAS) on Y-axis. Number of exercise-stress tests (ESTs) with this score on the left for EST 1 and on the right for EST 2. Horizontal lines and corresponding numbers represent EST sets with similar VAS (±1 class) on paired ESTs. Diagonal lines and corresponding numbers represent EST-pairs with changing VAS (>1 class) between paired ESTs. (B) Proportion of patients with a ΔVAS ranging from 0 to 4. ΔVAS was defined as the absolute difference in VAS between the paired ESTs. NSVT, non-sustained ventricular tachycardia; PVC, premature ventricular contraction.
ΔVAS
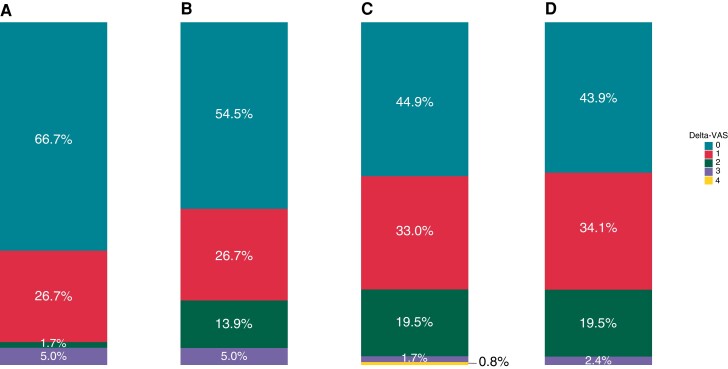
Most EST-pairs [n = 178 (51.0%)] had a ΔVAS of 0, reflecting the same VAS on both ESTs, and 109 (31.2%) had a ΔVAS of 1, reflecting a change of 1 VAS category between the paired ESTs. Only 62 EST-pairs (17.8%) had a ΔVAS > 1 (Figure 2B). Treatment at the time of the ESTs was associated with higher odds of a ΔVAS > 1 compared with no treatment [odds ratio (OR), 3.52 (95% CI, 2.46–4.57); P = 0.020; Table 4]. Accordingly, the distribution of ΔVAS in the EST-pairs without treatment differs from those with treatment, and the proportion ΔVAS < 2 was highest in the EST-pairs performed without treatment (Figure 3). Furthermore, an increase in the difference in maximum heart rate between the EST-pairs in b.p.m. was associated with a higher odds of a ΔVAS > 1 [OR, 1.04, (95%CI, 1.01–1.06); P = 0.003; Table 4]. The repeatability of the maximum heart rate in the different ΔVAS groups is described in Supplemental Material online.
Table 4.
Predictors of ΔVAS
| OR (95% CI) | P-valuea | |
|---|---|---|
| Proband | 1.25 (0.76–1.74) | 0.380 |
| Time between paired ESTs (per day change) | 1.00 (1.00–1.00) | 0.301 |
| Use of any antiarrhythmic drug vs. no treatmentb | 3.52 (2.46–4.57) | 0.020 |
| Type of antiarrhythmic treatment (reference: no treatment)b | ||
| ȃBeta-blocker monotherapy | 3.24 (2.11–4.37) | 0.041 |
| ȃBeta-blocker and flecainide | 3.96 (2.14–4.35) | 0.015 |
| ȃBeta-blocker, flecainide, and LCSD | 3.94 (1.99–4.50) | 0.032 |
| Age at the first EST in years | 1.00 (1.00–1.00) | 0.152 |
| Center: Mayo Clinic (reference: Amsterdam UMC) | 0.85 (0.36–1.34) | 0.522 |
| Change from morning to afternoon (reference: no change) | 0.91 (0.49–1.33) | 0.668 |
| Bike protocol (reference: treadmill protocol) | 1.14 (0.67–1.62) | 0.579 |
| Change in reason of stop EST (reference: no change) | 1.62 (0.85–2.39) | 0.218 |
| RYR2 variant classification (reference: uncertain significance) | ||
| ȃPathogenic | 1.27 (0.72–1.82) | 0.911 |
| ȃLikely pathogenic | 1.04 (0.41–1.67) | 0.392 |
| Body weight difference in kg | 0.98 (0.93–1.03) | 0.381 |
| Difference in maximum heart rate in b.p.m. | 1.04 (1.01–1.06) | 0.003 |
CI, confidence interval; LCSD, left cardiac sympathetic denervation; OR, odds ratio; VAS, ventricular arrhythmia score.
The P reflects the level of significance corresponding to the estimate of the cumulative link mixed model or generalized linear mixed model, as appropriate, taking repeated measures per individual into account.
OR and P-values are based on the estimate of the generalized linear mixed model, because the proportional odds assumption of the cumulative link mixed model was violated.
Figure 3.
ΔVAS in EST-pairs stratified by the use of medication. (A) EST-pairs performed without medical therapy, (B) EST-pairs performed under beta-blocker monotherapy, (C) EST-pairs performed under combination therapy of beta-blocker and flecainide, (D) EST-pairs performed under combination therapy of beta-blocker, flecainide, and left cardiac sympathetic denervation. The histogram shows the proportion of patients with a ΔVAS ranging from 0 to 4. The ΔVAS is defined as the difference in VAS between the paired ESTs. EST, exercise-stress test; VAS, ventricular arrhythmia score.
Discussion
This is the first study assessing the repeatability of the occurrence and severity of ventricular arrhythmias on the EST in patients with CPVT. In half of the EST-pairs the VAS was identical, and in only 17.8% the VAS changed two categories or more, corresponding to a moderate repeatability. The repeatability of HR-PVC and HR-recovery was substantial. Thus, the intra-patient variability in heart rate measurements during the EST is low, whereas ventricular arrhythmia severity is more variable, although the degree of difference was low in the vast majority. These results will likely contribute to improvements in the use and interpretation of the EST in CPVT, both in patient care and in research.
A reliable read-out parameter is essential to demonstrate robust evidence of treatment efficacy. In CPVT, the efficacy of beta-blockers and flecainide is mainly evaluated with the ventricular arrhythmia burden on the EST, such as the VAS.9,10,11 Attempts at a trial with clinical endpoints involving symptoms have been made,10 but small cohorts and a low burden of symptoms, pertinent to rare diseases like CPVT, preclude randomized intervention studies with these more relevant, but remote, endpoints. As new drugs with a significant potential in CPVT are on the horizon22, it is of great importance to study in detail the repeatability of the VA characteristics on EST in CPVT. Our results provide a framework for using ventricular arrhythmia characteristics on the EST as a reliable read-out parameter to assess potential therapeutic efficacy for novel CPVT therapies.
Factors possibly influencing repeatability
There are several external and internal factors potentially influencing the repeatability of ventricular arrhythmia characteristics on the EST in CPVT. The substantial to almost perfect repeatability of the baseline and maximum heart rate, EST duration, and maximally attained workload underline the identical circumstances of the paired ESTs. Our analyses showed that the odds for an increased ΔVAS were higher in the EST-pairs performed under medical therapy compared with those without. This may reflect the lower penetrance in the subset of patients without therapy, of whom most ESTs had a VAS of 0. Alternatively, this might be due to fluctuations in the drug serum levels and its corresponding antiarrhythmic effect on the EST. Non-adherence—a well-known problem in the treatment of inherited cardiac arrhythmias23—prior to the EST might underlie these fluctuations. This is in line with our observation that the difference in maximum heart rate between ESTs was associated with the odds for an increased ΔVAS and the repeatability of the maximum heart rate in the EST-pairs with a ΔVAS > 1 was substantial but almost perfect in the pairs with a ΔVAS of 0 and 1. The maximum heart rate is reduced by beta-blockers used in CPVT and therefore poor adherence or fluctuations of the serum level of the drugs will lead to a higher variability in maximum heart rate—and potentially the VAS. Future research should focus on this and explore the potential use of the variability of the EST to evaluate treatment adherence. Alternatively, the fluctuations could also be associated with body weight. Although the repeatability of body weight was almost perfect, we further assessed the potential influence of body weight changes in our sensitivity analyses and its potential association with ΔVAS. Altogether, the results suggest that body weight changes had a limited effect on our results.
Other external factors were not associated with ΔVAS. This included a change in EST recording from morning to afternoon or the reverse, excluding the circadian rhythm as a confounder of the repeatability. This is in contrast to a previous study in children with CPVT, in which the odds for VT or ventricular fibrillation on ESTs was higher in the afternoon compared with the morning.14 Future studies in larger cohorts of CPVT patients of all ages should be conducted to clarify this contrasting observation. Finally, the repeatability of the maximum heart rate and the HR-recovery was almost perfect. The difference between these two measures, the heart rate recovery, is indicative of the parasympathetic activity of the autonomic nerve system and is associated with the occurrence of symptoms in CPVT.12 Therefore, parasympathetic activity does not seem highly variable within a patient at subsequent ESTs and is not likely related to our observed variability in VAS.
Our results do not indicate an association between probands or the age at the first EST and the ΔVAS. Furthermore, the VAS both decreased and increased from EST1 to EST2. Therefore, a phenotype progression does not explain the variability of VAS. However, the age at first EST ranged from 14 to 38 years old (IQR), thus our results cannot be adopted to children, in whom a phenotype progression might play a more important role. Previous studies in two different cohorts have shown that a CPVT phenotype is not always present at the first EST performed and could develop during follow-up.5,15 The reverse, a CPVT phenotype dissolving on follow-up ESTs, has not been described, but is also not likely to be seen, because most patients with a CPVT phenotype will be treated with a beta-blocker, affecting their ventricular arrhythmia burden. This study population differs from the first multicentre observational study by Hayashi in 2009, with a higher proportion of family members with a lower burden of symptoms.8 This study population is representative of the patients currently seen at specialized arrhythmia centres, as over time, more and more family members with CPVT are being identified prior to the onset of symptoms due to cascade screening.
Study limitations
Due to the retrospective character of this study, we could not account for potential non-adherence in patients using oral medication at the time of the EST. Furthermore, the time between intake of the oral medication and EST was not regulated and might have affected the plasma levels of medication at the time of EST, but these levels could not be assessed due to the study design. However, this practice does resemble the actual clinical assessment of the efficacy of therapy in patients with CPVT. We allowed multiple EST-pairs per patient in our analyses. Therefore, the 95% CI of our main results might be tighter than in reality. However, our sensitivity analyses including only one EST-pair per patient, showed that Krippendorf α, ICC, and mean differences of our primary and secondary outcomes and baseline characteristics were consistent with the complete cohort results. Additionally, in most patients, we included only one EST-pair.
We were limited to the information on the EST reports and printouts of the ECG recordings. In both centres, incomplete ESTs are printed, which is mostly limited to one 12-lead ECG recording per minute. Therefore, the HR-PVC might not be completely accurate. Furthermore, because of this limitation, we were unable to quantitate and compare the total number of PVCs throughout the test, or the maximum number of PVCs in one minute. Although these are not the most important characteristics of a CPVT phenotype, it would have been given a more complete overview of the repeatability. Furthermore, this could have potentially led to an over- or underestimation of the VAS score of 0 or 1 in some ESTs.
Our study cohort consisted of 25 (24.0%) patients whose RYR2 variant was graded as a variant of uncertain significance. However, these RYR2 positive subjects were only included if a typical clinical phenotype was present, which highly suggests that their variant will be upgraded ultimately.18 Furthermore, there was no difference in the ΔVAS between the majority having a RYR2 likely pathogenic or pathogenic variant and this minority subset with a RYR2 variant of uncertain significance.
Clinical implications
Our results will have important implications, both in patient care and in research. The lack of perfect repeatability of the VAS underlines the need for multiple ESTs in patients suspected for CPVT. Contrarily, as only a minority of the EST-pairs (18.7%) had a ΔVAS > 1, this suggests that if a patient’s EST does not show any ventricular ectopy, the risk for couplets or (non)-sustained VT on a second EST (only 3 of the 148 ESTs in our series), and therefore the risk for arrhythmic events,8 is very low. In patients diagnosed with CPVT, follow-up ESTs with a sudden increase in VAS of at least 2 categories compared with the previous EST under identical circumstances, should urge the clinician to scale up therapy, i.e. to increase dosage, or to proceed to either combination therapy (beta-blockers plus flecainide) or even triple therapy (beta-blockers, flecainide, and left cardiac sympathetic denervation). Subsequently, after such a therapeutic change, a decrease in VAS of at least 2 categories most likely implicates a good effect of the medication, but should always be accompanied by follow-up ESTs. In the presence of complaints, such as near-syncope or palpitations, a single negative EST result does not necessarily rule out ventricular arrhythmia as a cause of these symptoms. Our results implicate that a VAS decrease of at least 2 could serve as a clinical surrogate endpoint to test the effect of potential new medications or treatments. In the design of such studies the present results, demonstrating reasonable repeatability with a low degree of difference in ventricular arrhythmia severity between ESTs in approximately 82% of the patients, should be factored into study design and power calculations. Furthermore, these studies should be designed to avert non-adherence and serum fluctuations to lower the variability of VAS. Finally, we showed that the resting heart rate, maximum heart rate, heart rate at the first PVC, and heart rate at 1 minute of recovery are internally consistent and potentially reliable measures of effect.
Supplementary Material
Contributor Information
Puck J Peltenburg, Department of Clinical and Experimental Cardiolgy, Heart Center, Amsterdam UMC, University of Amsterdam, the Netherlands; Department of Pediatric Cardiology, Emma Children’s Hospital, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 Amsterdam, AZ, The Netherlands.
Sanjeev N J Pultoo, Department of Clinical and Experimental Cardiolgy, Heart Center, Amsterdam UMC, University of Amsterdam, the Netherlands.
Kathryn E Tobert, Departments of Cardiovascular Medicine, Pediatric and Adolescent Medicine, and Molecular Pharmacology & Experimental Therapeutics, Division of Heart Rhythm Services and Pediatric Cardiology, Windland Smith Rice Sudden Death Genomics Laboratory, Mayo Clinic, Rochester, MN, USA.
J Martijn Bos, Departments of Cardiovascular Medicine, Pediatric and Adolescent Medicine, and Molecular Pharmacology & Experimental Therapeutics, Division of Heart Rhythm Services and Pediatric Cardiology, Windland Smith Rice Sudden Death Genomics Laboratory, Mayo Clinic, Rochester, MN, USA.
Krystien V V Lieve, Department of Clinical and Experimental Cardiolgy, Heart Center, Amsterdam UMC, University of Amsterdam, the Netherlands.
Michael Tanck, Department of Clinical and Experimental Cardiolgy, Heart Center, Amsterdam UMC, University of Amsterdam, the Netherlands.
Sally-Ann B Clur, Department of Pediatric Cardiology, Emma Children’s Hospital, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 Amsterdam, AZ, The Netherlands.
Nico A Blom, Department of Pediatric Cardiology, Emma Children’s Hospital, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 Amsterdam, AZ, The Netherlands; Department of Pediatric Cardiology, Willem-Alexander Children’s Hospital, Leiden University Medical Center, Albinusdreef 2, 2333 Leiden, ZA, The Netherlands.
Michael J Ackerman, Departments of Cardiovascular Medicine, Pediatric and Adolescent Medicine, and Molecular Pharmacology & Experimental Therapeutics, Division of Heart Rhythm Services and Pediatric Cardiology, Windland Smith Rice Sudden Death Genomics Laboratory, Mayo Clinic, Rochester, MN, USA.
Arthur A M Wilde, Department of Clinical and Experimental Cardiolgy, Heart Center, Amsterdam UMC, University of Amsterdam, the Netherlands.
Christian van der Werf, Department of Clinical and Experimental Cardiolgy, Heart Center, Amsterdam UMC, University of Amsterdam, the Netherlands.
Supplementary material
Supplementary material is available at Europace online.
Funding
This work was supported by ZonMW Priority Medicines for Rare Diseases and Orphan Drugs (grant 113304045 to C.v.d.W.), the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program (to M.J.A.), the Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation, the Nederlandse Federatie van Universitair Medische Centra, the Netherlands Organisation for Health Research and Development and the Royal Netherlands Academy of Sciences (CVON 2012-10 PREDICT to A.A.M.W.), E-Rare Joint Transnational Call for Proposals 2015 ‘Improving Diagnosis and Treatment of Catecholaminergic Polymorphic Ventricular Tachycardia: Integrating Clinical and Basic Science’ (to A.A.M.W.), and the AEPC junior members research grant 2019 (to P.J.P.).
Data availability statement
The data underlying this article cannot be shared publicly due to the privacy of study participants. The data will be shared on reasonable request to the corresponding author.
References
- 1. Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul Cet al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in may 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 2013;10:1932–63. [DOI] [PubMed] [Google Scholar]
- 2. Grossman M, Weinstein WW, Katz LN. The use of the exercise test in the diagnosis of coronary insufficiency. Ann Intern Med 1949;30:387–97. [DOI] [PubMed] [Google Scholar]
- 3. Guidelines for cardiac exercise testing . ESC Working group on exercise physiology, physiopathology and electrocardiography. Eur Heart J 1993;14:969–88. [PubMed] [Google Scholar]
- 4. van der Werf C, Nederend I, Hofman N, van Geloven N, Ebink C, Frohn-Mulder IMet al. Familial evaluation in catecholaminergic polymorphic ventricular tachycardia: disease penetrance and expression in cardiac ryanodine receptor mutation-carrying relatives. Circ Arrhythm Electrophysiol 2012;5:748–56. [DOI] [PubMed] [Google Scholar]
- 5. Hayashi M, Denjoy I, Hayashi M, Extramiana F, Maltret A, Roux-Buisson N. The role of stress test for predicting genetic mutations and future cardiac events in asymptomatic relatives of catecholaminergic polymorphic ventricular tachycardia probands. Europace 2012;14:1344–51. [DOI] [PubMed] [Google Scholar]
- 6. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis ABet al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. Heart Rhythm 2018;15:e190–252. [DOI] [PubMed] [Google Scholar]
- 7. Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation 1995;91:1512–9. [DOI] [PubMed] [Google Scholar]
- 8. Hayashi M, Denjoy I, Extramiana F, Maltret A, Buisson NR, Lupoglazoff JMet al. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation 2009;119:2426–34. [DOI] [PubMed] [Google Scholar]
- 9. Leren IS, Saberniak J, Majid E, Haland TF, Edvardsen T, Haugaa KH. Nadolol decreases the incidence and severity of ventricular arrhythmias during exercise stress testing compared with beta1-selective beta-blockers in patients with catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2016;13:433–40. [DOI] [PubMed] [Google Scholar]
- 10. Kannankeril PJ, Moore JP, Cerrone M, Priori SG, Kertesz NJ, Ro PS. Efficacy of flecainide in the treatment of catecholaminergic polymorphic ventricular tachycardia: a randomized clinical trial. JAMA Cardiol 2017;2:759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Werf C, Kannankeril PJ, Sacher F, Krahn AD, Viskin S, Leenhardt Aet al. Flecainide therapy reduces exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. J Am Coll Cardiol 2011;57:2244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lieve KVV, Dusi V, van der Werf C, Bos JM, Lane CM, Stokke MKet al. Heart rate recovery after exercise is associated with arrhythmic events in patients with catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol 2020;13:e007471. [DOI] [PubMed] [Google Scholar]
- 13. Franciosi S, Roston TM, Perry FKG, Knollmann BC, Kannankeril PJ, Sanatani S. Chronotropic incompetence as a risk predictor in children and young adults with catecholaminergic polymorphic ventricular tachycardia. J Cardiovasc Electrophysiol 2019;30:1923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miyake CY, Asaki SY, Webster G, Czosek RJ, Atallah J, Avasarala Ket al. Circadian variation of ventricular arrhythmias in catecholaminergic polymorphic ventricular tachycardia. JACC Clin Electrophysiol 2017;3:1308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wanguemert F, Bosch Calero C, Perez C, Campuzano O, Beltran-Alvarez P, Scornik FSet al. Clinical and molecular characterization of a cardiac ryanodine receptor founder mutation causing catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2015;12:1636–43. [DOI] [PubMed] [Google Scholar]
- 16. Roston TM, Davies B, Franciosi S, De Souza AM, Lksman ZW, Sanatani Set al. Burst exercise testing can unmask arrhythmias in patients with incompletely penetrant catecholaminergic polymorphic ventricular tachycardia. JACC Clin Electrophysiol 2021;7:437–41. [DOI] [PubMed] [Google Scholar]
- 17. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster Jet al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med 2015;17:405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giudicessi JR, Lieve KVV, Rohatgi RK, Koca F, Tester DJ, van der Werf Cet al. Assessment and validation of a phenotype-enhanced variant classification framework to promote or demote RYR2 missense variants of uncertain significance. Circ Genom Precis Med 2019;12:e002510. [DOI] [PubMed] [Google Scholar]
- 19. Castor EDC . Castor Electronic Data Capture2019. [online] Available at: https://castoredc.com. [Internet].
- 20. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- 21. Zapf A, Castell S, Morawietz L, Karch A. Measuring inter-rater reliability for nominal data - which coefficients and confidence intervals are appropriate. BMC Med Res Methodol 2016;16:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klipp RC, Li N, Wang Q. EL20, a potent antiarrhythmic compound, selectively inhibits calmodulin-deficient ryanodine receptor type 2. Heart Rhythm 2018;15:578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Donovan CE, Waddell-Smith KE, Skinner JR, Broadbent E. Predictors of beta-blocker adherence in cardiac inherited disease. Open Heart 2018;5:e000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of study participants. The data will be shared on reasonable request to the corresponding author.