Abstract
Aims
Cardiac disease progression prior to first ventricular arrhythmia (VA) in LMNA genotype–positive patients is not described.
Methods and results
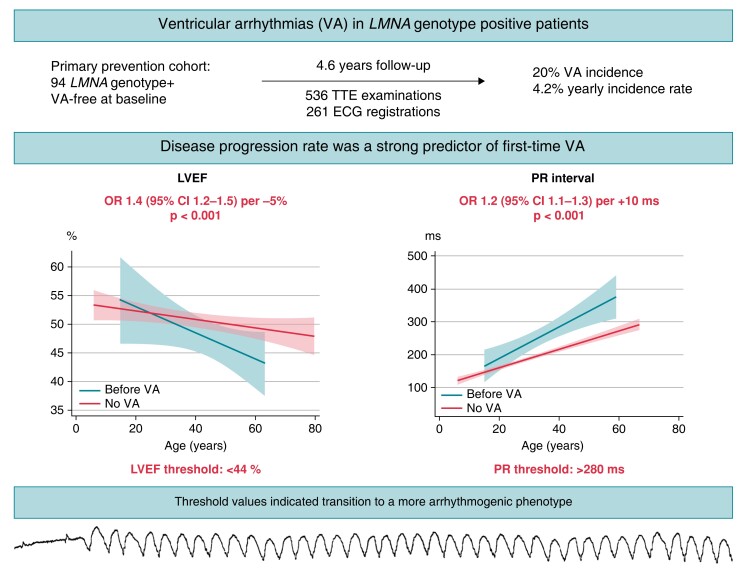
We performed a primary prevention cohort study, including consecutive LMNA genotype–positive patients from our centre. Patients underwent repeated clinical, electrocardiographic, and echocardiographic examinations. Electrocardiographic and echocardiographic disease progression as a predictor of first-time VA was evaluated by generalized estimation equation analyses. Threshold values at transition to an arrhythmic phenotype were assessed by threshold regression analyses. We included 94 LMNA genotype–positive patients without previous VA (age 38 ± 15 years, 32% probands, 53% females). Nineteen (20%) patients experienced VA during 4.6 (interquartile range 2.1–7.3) years follow up, at mean age 50 ± 11 years. We analysed 536 echocardiographic and 261 electrocardiogram examinations. Individual patient disease progression was associated with VA [left ventricular ejection fraction (LVEF) odds ratio (OR) 1.4, 95% confidence interval (CI) 1.2–1.6 per 5% reduction, left ventricular end-diastolic volume index (LVEDVi) OR 1.2 (95% CI 1.1–1.3) per 5 mL/m2 increase, PR interval OR 1.2 (95% CI 1.1–1.4) per 10 ms increase]. Threshold values for transition to an arrhythmic phenotype were LVEF 44%, LVEDVi 77 mL/m2, and PR interval 280 ms.
Conclusions
Incidence of first-time VA was 20% during 4.6 years follow up in LMNA genotype–positive patients. Individual patient disease progression by ECG and echocardiography were strong predictors of VA, indicating that disease progression rate may have additional value to absolute measurements when considering primary preventive ICD. Threshold values of LVEF <44%, LVEDVi >77 mL/m2, and PR interval >280 ms indicated transition to a more arrhythmogenic phenotype.
Keywords: LMNA cardiomyopathy, Lamin A/C, Laminopathy, Ventricular arrhythmia, Primary preventive implantable cardioverter defibrillator
Graphical Abstract
Graphical Abstract.
In a primary prevention cohort of 94 LMNA genotype–positive patients without VA at baseline, VA incidence was as high as 20% after 4.6 years follow up. Individual patient disease progression by ECG and echocardiography was a strong predictor of subsequent VA, suggesting additional value to the previously described risk factors in current guidelines for primary preventive ICD implantation. Threshold values of LVEF <44% and PR interval >280 ms indicated transition to a more arrhythmogenic phenotype. ECG, electrocardiogram; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; VA, ventricular arrhythmia.
What’s new?
We provide insights to disease progression prior to first ventricular arrhythmia in LMNA genotype–positive patients.
This study identified electrocardiographic and echocardiographic threshold values for reaching a more arrhythmic phenotype in LMNA genotype–positive patients, including left ventricular ejection fraction <44%, left ventricular end-diastolic volume index >77 mL/m2, and PR interval >280 ms.
This study is the first to report disease progression rate as a predictor for ventricular arrhythmia. Some LMNA genotype–positive patients illustrated rapid disease progression prior to first ventricular arrhythmia but had not developed other classical risk predictors at time of the arrhythmic event.
Introduction
Cardiac laminopathies are highly malignant and arrhythmogenic variants of familial dilated cardiomyopathy,1,2 with a lifetime penetrance of nearly 100%.3 The LMNA gene encode nuclear envelope proteins lamin A and C.4LMNA mutations cause a variety of phenotypes, including cardiac and skeletal muscle disease. Ventricular arrhythmias (VAs) are common, and implantation of an implantable cardioverter defibrillator (ICD) is most often necessary.4 However, ICD implantation at young age may result in long-term complications,5 highlighting the importance of correct timing to avoid premature implantation of device.
Several previous studies have described predictors of VA in LMNA genotype–positive patients.6–13 Current guidelines on ICD implantation report male sex, non-missense LMNA mutations, non-sustained ventricular tachycardia (NSVT) and left ventricular ejection fraction (LVEF) < 45% as risk factors for VA in LMNA disease.14,15 Atrioventricular (AV) block has also been described as a predictor of VA,8,13 as well as late gadolinium enhancement by cardiac magnetic resonance (CMR) imaging.8,9 Disease progression rate prior to first VA in LMNA genotype–positive patients has not been described and may provide additional value to the previously reported predictors of adverse outcome.
We aimed to describe the cardiac phenotype from baseline to time of first VA in LMNA genotype–positive patients and to explore if individual disease progression rate is a predictor of life-threatening arrhythmic events. We aimed to determine at what level of electrical, structural, and functional pathology the patients transitioned to a more arrhythmic phenotype by analysing repeated electrocardiogram (ECG) registrations and echocardiographic examinations, to optimize the prediction of VA and the correct timing of ICD implantation.
Methods
Study design and population
We performed a primary prevention cohort study, including consecutive LMNA genotype–positive patients at Oslo University Hospital, Rikshospitalet, Norway, from 2003 to 2020, expanding on a previously published data set.16 Only patients with no history of VA at baseline were included. All patients underwent ≥2 evaluations including echocardiography and ECG. The interval of follow-up examinations was individualized and usually included yearly follow ups.
Proband status was defined as the first affected individual in a family who sought medical attention due to symptoms of cardiac laminopathy and genetic testing confirmed a pathogenic LMNA genotype. Genotype-positive family members were identified by cascade genetic screening. The pathogenicity of mutations was evaluated according to the guidelines from the American College of Medical Genetics and Genomics and the Association for Molecular Pathology.17 Patients with pathogenic (P) and likely pathogenic (LP) genetic variants were considered genotype positive. Mutations were classified as missense or non-missense (nonsense, frameshift, and splice site). Patients were classified as phenotype positive if either AV block (PR interval >200 ms or higher degree), atrial fibrillation (AF), left ventricular (LV) dilatation, or LV dysfunction was present.
The study complied with the declaration of Helsinki and was approved by the Regional Medical Ethics Committee of South-Eastern Norway. All patients gave written informed consent.
Electrocardiogram
Twelve-lead ECG was obtained at time of first consultation in all patients without paced rhythm or in paced patients on clinical indication. We noted rhythm, PR interval, QRS duration, and grade of AV block. Twenty-four-hour Holter monitoring was repeated once yearly or more frequently on clinical indication.
Ventricular arrhythmias
We defined VA as sustained ventricular tachycardia (VT, runs of consecutive ventricular beats ≥120 beats per minute (b.p.m.) lasting longer than 30 s or requiring intervention earlier due to haemodynamic instability) documented on 12-lead ECG or 24 h Holter recording, ventricular fibrillation (VF), aborted cardiac arrest, or appropriate therapy from a primary preventive ICD. Appropriate ICD therapy was defined as anti-tachycardia pacing or shock therapy for documented VT or VF. Device programming was left to the physicians decision, but the majority of patients were programmed with VT zone 200–250 b.p.m. with six ATP sequences before shock therapy, and VF zone >250 b.p.m. with one ATP sequence before shock therapy. Non-sustained ventricular tachycardia, defined as ≥3 consecutive ventricular beats ≥120 b.p.m. lasting <30 s, and AF were recorded from ECG, Holter monitoring, and ICD records. Outcome was adjudicated for all patients in January 2021.
Patients were defined as VA free at baseline if there was no history of documented VA prior to enrolment and if no VA was detected at time of first consultation.
Echocardiography
All participants underwent a transthoracic echocardiographic examination at study baseline and at every follow-up visit (using Vivid 7, E9 or E95; GE Healthcare, Horten, Norway; offline data analysis, EchoPac; GE Healthcare). Left ventricular (LV) end-diastolic volume (EDV), end-systolic volume (ESV), and LVEF were calculated by modified Simpson biplane method. Left ventricular dilatation was defined as LV EDV indexed >74 mL/m2 in males or >61 mL/m2 in females, and LV dysfunction was defined as LVEF <53%.18 Left ventricular global longitudinal strain (GLS) was derived from speckle tracking analyses on 2D grey scale image loops with >50 frames per second from the three apical views, and expressed as the average peak negative strain in a 16 segment LV model. Left atrial volume (LAV) was calculated by the area length method. Right ventricular (RV) structure was assessed by RV basal diameter (RVD), and RV function was assessed by tricuspid annular plane systolic excursion (TAPSE). All echocardiographic examinations belonging to one patient was analysed by the same echocardiographer.
Statistics
Descriptive data were expressed as mean ± standard deviation (SD), frequencies (%), or median with interquartile range (IQR). Continuous variables were compared by Student’s t-test, Fisher’s exact test, or Mann–Whitney U test, as appropriate. Baseline predictors of first-time VA were assessed by univariable Cox regression analyses and were age adjusted in multivariable analyses.
Yearly incidence rate for VA was calculated as patient-year at risk with 95% confidence interval (CI). Disease progression during follow up was assessed by mixed model analyses with random effect at individual and family level and a fitting covariance structure. An interaction term between age and presence of VA was used to compare disease progression in patients without VA and disease progression prior to VA in patients with arrhythmic event during follow up. Graphical illustration of disease progression was presented as linear fit plot by patient age. We performed generalized estimating equation (GEE) analyses to assess individual patient disease progression as a predictor of life-threatening arrhythmia, with binomial family, logit link, and fitting covariance structure accounting for random effects at individual and family level. Disease progression analyses were also evaluated when excluding all examinations in patients without an ICD, and when excluding ATP as an arrhythmic endpoint. Furthermore, electrical and functional disease progression was evaluated as a predictor of VA after adjusting for presence of the established risk markers: NSVT, AV block degree II/III or LVEF < 45%. Every patient was evaluated if any of these markers were present and categorized as ‘risk markers present’ or ‘not present’.
Threshold regression analyses compared findings in non-arrhythmic patients with those who experienced first-time VA during follow up, using the last examination before VA as time of censor for threshold. To describe the clinical phenotype at time of first VA, we performed subanalyses of patients with an echocardiographic and ECG examination ±12 months of the arrhythmic event, reporting mean echocardiographic and median ECG values at time of first VA.
P-values were two-sided and values <0.05 considered significant (STATA version 16.1; StataCorp LLC, College Station, TX, USA).
Results
We included 94 LMNA genotype–positive patients without VA at baseline [age 38 (IQR 27–51) years, 32% probands, 53% females, 86% with non-missense mutations; Table 1]. Seventy-seven (82%) patients were phenotype positive at time of first consultation. A total of 261 ECG registrations and 536 echocardiographic examinations were performed during 4.6 (IQR 2.1–7.3) years follow up. Median number of examinations per patient was 5 (IQR 3–8), and median time between each examination was 1.0 (IQR 0.4–1.4) years. Fourteen different genetic variants were identified (see Supplementary material online, Table S1).
Table 1.
Baseline characteristics of 94 LMNA genotype–positive patients, and comparison of patients with and without VA at time of censor
| All patients at time of inclusion (n = 94) | Patients with VA parameters at time of VA (n = 19) | Patients without VA at time of last follow up (n = 75) | P-value | |
|---|---|---|---|---|
| Age, years | 38 (27–51) | 47 (40–57) | 44 (28–54) | 0.04 |
| Proband, n (%) | 30 (32) | 10 (53) | 20 (27) | 0.03 |
| Female, n (%) | 50 (53) | 9 (47) | 41(55) | 0.57 |
| Non-missense mutation, n (%) | 81 (86) | 18 (95) | 63 (84) | 0.23 |
| Phenotype positive, n (%) | 77 (82%) | 19 (100) | 62 (83) | 0.06 |
| AV block,an (%) | 45 (52) | 17 (94) | 34 (50) | <0.001 |
| Atrial fibrillation, n (%) | 42 (45) | 16 (84) | 42 (56) | 0.03 |
| NSVT, n (%) | 30 (32) | 13 (68) | 37 (49) | 0.07 |
| Syncope, n (%) | 20 (22) | 7 (37) | 19 (26) | 0.27 |
| NYHA functional class ≥ II, n (%) | 26 (28) | 11 (61) | 23 (32) | 0.02 |
| Beta-blocker, n (%) | 18 (19) | 15 (79) | 47 (66) | 0.28 |
| ACE/AT2 inhibitor, n (%) | 18 (19) | 15 (79) | 27 (36) | 0.001 |
| MCRA, n (%) | 4 (4) | 4 (21) | 6 (8) | 0.21 |
| Cordarone/dronedarone, n (%) | 4 (4) | 5 (26) | 1 (1) | 0.001 |
| LVEF (%) | 51 ± 12 | 40 ± 12 | 51 ± 11 | <0.001 |
| GLS (%) | −16.1 ± 4.6 | −11.7 ± 4.3 | −15.6 ± 4.2 | 0.01 |
| LV EDVi (m) | 71 ± 19 | 83 ± 22 | 67 ± 16 | <0.001 |
| LV ESVi (mL) | 36 ± 17 | 52 ± 20 | 34 ± 16 | <0.001 |
| LAVi (mL) | 41 ± 19 | 70 ± 24 | 41 ± 15 | <0.001 |
| RVD (mm) | 41 ± 8 | 47 ± 10 | 42 ± 7 | 0.03 |
| TAPSE (mm) | 23 ± 5 | 20 ± 7 | 22 ± 5 | 0.38 |
| PR interval (ms) | 178 (146–232) | 312 (204–430) | 182 (158–212) | 0.03 |
| QRS width (ms) | 90 (80–100) | 115 (93–123) | 86 (78–95) | 0.003 |
Values are given as mean ± SD, numbers with percentages or median with IQR. Time of censor for patients with VA is timing of VA (echocardiographic and ECG findings ± 12 months). Time of censor for patients without VA is last clinical evaluation.
ACE, angiotensin converting enzyme; AT2, angiotensin 2 receptor blocker; AV block, atrioventricular block; GLS, global longitudinal strain; i, indexed value; IQR, interquartile range; LAV, left atrial volume; LV EDV, left ventricular end-diastolic volume; LVEF, LV ejection fraction; LV ESV, LV end-systolic volume; MCRA, mineral corticoid receptor antagonist; NYHA, New York Heart Association; NSVT, non-sustained ventricular tachycardia; RVD, right ventricular diameter basal; SD, standard deviation; TAPSE, tricuspid annular plane systolic excursion; VA, ventricular arrhythmia.
AV block degree 1, 2, or 3.
Cardiac phenotype at time of first ventricular arrhythmia
Nineteen (20%) patients experienced VA during follow up, resulting in a yearly incidence rate of 4.2% (95% CI 2.7–6.6%). Age at first VA was 47 (IQR 40–57) years and time from study inclusion to VA was 4.1 (IQR 0.8–7.3) years. At time of VA, 12 (63%) patients had LVEF <45%, 13 (68%) had previous NSVT, and 17 (94%) patients had AV block, while RV function was preserved in most patients (Table 1).
On the other hand, 7 (37%) patients with VA had LVEF >45%, 6 (32%) had no NSVT, 1 (6%) did not have AV block, and 3 (11%) patients had none of the classical risk markers prior to first arrhythmia (LVEF >45%, no NSVT, no high-degree AV block). The three patients without classical risk markers at time of VA were all females. Two of them showed evident disease progression with LVEF reduction of −19 and −13% in absolute values during follow up but without reaching LVEF <45%. Their last clinical evaluations (including an echocardiographic examination) were performed 1.8 and 1.4 years prior to VA. The third patient was a 24-year-old woman with an exercise-related aborted cardiac arrest. She dropped out from follow up 3.2 years prior to the cardiac arrest. During previous follow ups, she had displayed mild disease progression with LVEF reduction from 60 to 53%.
Characteristics of first ventricular arrhythmia
Among the 19 patients who experienced VA during follow up, 16 had a primary preventive ICD implanted at time of the arrhythmic event. Of these, 14 patients received appropriate anti-tachycardia therapy (7 anti-tachycardia pacing, 7 defibrillation), and 2 had sustained VT in the monitoring zone. The three patients with VA during follow up without ICD implanted at time of VA were the 24-year-old woman with an exercise-related aborted cardiac arrest, a 25-year-old woman with aborted cardiac arrest while being hospitalized for heart failure work-up, and a 53-year-old woman with a self-terminating VT (190 b.p.m.) lasting for several hours.
Baseline predictors of first-time ventricular arrhythmia
Age, proband status, NSVT, AF, AV block, LV dilatation, and biventricular dysfunction at baseline were predictors for first-time VA in univariable analyses (Table 2). Proband status, AV block, LV dilatation, and biventricular dysfunction remained significant predictors when adjusted for age. Non-missense mutations and patient sex did not predict VA.
Table 2.
Baseline predictors of experiencing first-time VA (n = 19) during 4.6 years of follow up in 94 LMNA genotype–positive patients without VA at study inclusion
| Univariable HR (95% CI) | P-value | Age-adjusted HR (95% CI) | P-value | |
|---|---|---|---|---|
| Age | 1.0 (1.0–1.1) | 0.01 | ||
| Proband | 3.8 (1.5–9.4) | 0.01 | 2.9 (1.2–7.5) | 0.02 |
| Female | 0.6 (0.3–1.5) | 0.30 | ||
| Non-missense mutation | 2.2 (0.3–16.4) | 0.45 | ||
| NSVT | 3.3 (1.3–8.2) | 0.01 | 2.0 (0.7–5.4) | 0.17 |
| Syncope | 0.6 (0.2–2.1) | 0.44 | ||
| Atrial fibrillation | 3.6 (1.4–9.7) | 0.01 | 2.1 (0.6–7.0) | 0.22 |
| AV block | 6.7 (2.1–21.1) | 0.001 | 4.9 (1.1–22.8) | 0.04 |
| ȃGrade I | 1.8 (0.6–5.4) | 0.33 | ||
| ȃGrades II and III | 5.3 (1.9–14.8) | 0.001 | 3.1 (0.9–10.6) | 0.08 |
| NYHA functional class ≥ II | 2.9 (1.2–7.2) | 0.02 | 1.9 (0.7–5.2) | 0.22 |
| LVEF, −5% | 1.3 (1.1–1.6) | 0.003 | 1.3 (1.0–1.5) | 0.02 |
| GLS, % | 1.2 (1.1–1.3) | 0.001 | 1.2 (1.1–1.4) | 0.001 |
| LV EDVi, 5 mL/m2 | 1.1 (1.0–1.2) | 0.01 | 1.1 (1.0–1.2) | 0.05 |
| LAVi, 5 mL/m2 | 1.1 (1.0–1.2) | 0.01 | 1.0 (0.9–1.2) | 0.46 |
| RVD, 5 mm | 1.2 (1.0–1.4) | 0.05 | 1.0 (0.9–1.3) | 0.68 |
| TAPSE, mm | 1.1 (1.0–1.2) | 0.02 | 1.1 (1.0–1.3) | 0.01 |
AV block, atrioventricular block; CI, confidence interval; GLS, global longitudinal strain; HR, hazard ratio; i, indexed values; LAV, left atrial volume; LV EDVi, left ventricular end-diastolic volume; LVEF, LV ejection fraction; NYHA, New York Heart Association; NSVT, non-sustained ventricular tachycardia; RVD, right ventricular diameter basal; TAPSE, tricuspid annular plane systolic excursion; VA, ventricular arrhythmia.
Disease progression rates as predictors of first-time ventricular arrhythmia
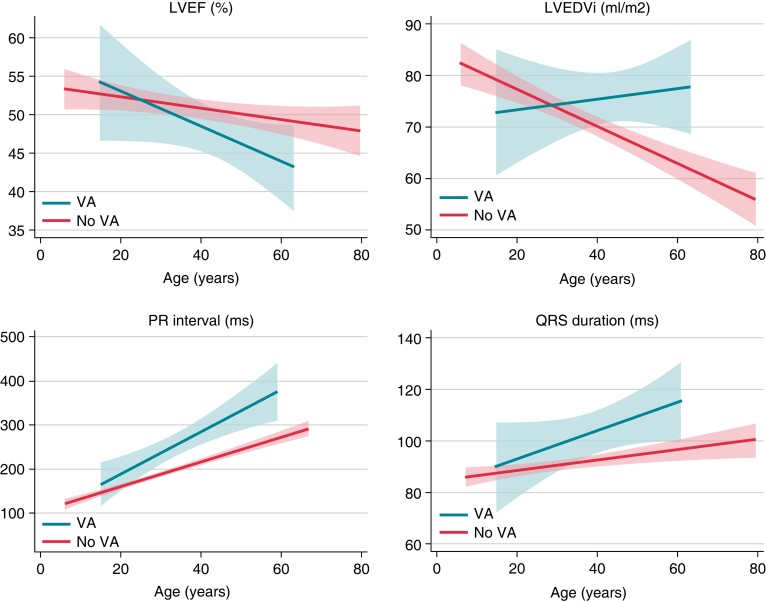
Comparison of disease progression during follow up in patients with and without VA showed evident acceleration of disease progression prior to arrhythmia in the group who experienced VA (Figure 1, Table 3). Mean yearly progression rate in LVEF was 1% in patients with VA. Maximum decline in EF during 1 year was 16%. Progression in LV dysfunction, biventricular and atrial dilatation, and increasing PR and QRS durations were all strong predictors of forthcoming first-time VA (Table 3). Disease progression implied high odds of experiencing VA before the next visit [LVEF 5% decrease OR 1.4 (95% CI 1.2–1.6) and LVEF 10% decrease OR 1.9 (95% CI 1.4–2.7), LV end-diastolic volume index (LVEDVi) 5 mL/m2 increase OR 1.2 (95% CI 1.1–1.3), PR interval 10 ms increase OR 1.2 (95% CI 1.1–1.4), and QRS interval 5 ms increase OR 1.2 (95% CI 1.1–1.4)]. The progressive increase of conduction delay was observed before initiation of antiarrhythmic medication (Table 1), and all predictors of arrhythmic events were adjusted for use of beta-blocker medication (Table 3). Disease progression as a predictor of VA was also evaluated after adjusting for the presence of any of the following: NSVT, LVEF < 45%, and presence of AV block degree II/III (see Supplementary material online, Table S2). Disease progression affecting cardiac function was a strong predictor of VA, irrespective of the presence of traditional risk markers. However, increasing PR interval and QRS duration were, as expected, outperformed by presence of the established risk factors which included AV block degree II/III.
Figure 1.
Disease progression in 94 LMNA genotype–positive patients, comparing patients with and without first-time VA during 4.6 years follow up. Disease progression by LVEF, LVEDVi, PR interval, and QRS width is illustrated as linear fit plot by patient age, comparing disease progression in patients without VA to disease progression prior to VA in patients with first arrhythmic event during follow up. Calculated from 251 ECGs and 460 echocardiographic examinations. ECG, electrocardiogram; LVEDVi, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; VA, ventricular arrhythmia.
Table 3.
Individual patient yearly disease progression rates and the association to first-time VA in 94 LMNA genotype–positive patients, by 261 ECGs and 536 echocardiographic examinations
| Progression of parameter per unit | Prediction of VA OR (95% CI) | Progression rate 1 year (SE) | ||
|---|---|---|---|---|
| Before VA n = 19 | Patients without VA n = 75 | P-value for interaction | ||
| LVEF (%) | 0.94 (0.91–0.97) | −0.8 (0.3)a | −0.2 (0.1) | 0.02 |
| GLS (%) | 1.36 (1.20–1.54) | +0.1 (0.2) | +0.1 (0.0)a | 0.40 |
| LV EDVi (mL/m2) | 1.04 (1.02–1.06) | +0.2 (0.5) | +0.2 (0.2) | 0.03 |
| LV ESVi (mL/m2) | 1.05 (1.03–1.08) | +0.8 (0.4)a | +0.2 (0.1) | 0.01 |
| LAVi (mL/m2) | 1.03 (1.01–1.05) | +2.3 (0.5)a | +0.6 (0.2)a | <0.001 |
| RVD (mm) | 1.10 (1.04–1.16) | +0.5 (0.2)a | +0.2 (0.1) | 0.13 |
| TAPSE (mm) | 0.96 (0.91–1.01) | −0.5 (0.2)a | −0.1 (0.1) | 0.01 |
| PR interval (ms) | 1.02 (1.01–1.03) | +4.3 (2.0)a | +4.6 (0.6)a | 0.28 |
| QRS width (ms) | 1.04 (1.01–1.07) | +0.3 (0.7) | +0.1 (0.2) | 0.04 |
| BSA (m2) | 5.19 (0.42–63.9) | −0.00 (0.00) | +0.01 (0.00) | 0.02 |
OR values for forthcoming VA adjusted for time, age, family clustering, and use of beta-blocker medication. Calculated by generalized estimating equations with binomial family, logit link individual intercept, and independent covariance structure. Yearly progression rate adjusted for family clustering. Calculated by linear mixed model statistics. P-value for interaction compares yearly disease progression rates in patients with and without VA. Calculated by an interaction term between age and the presence of VA. In patients with VA, only observations up till the arrhythmic event was included in the mixed model regression analyses.
ECG, electrocardiogram; GLS, global longitudinal strain; i, indexed value; LAV, left atrial volume; LV EDV, left ventricular end-diastolic volume; LVEF, LV ejection fraction; LV ESV, LV end-systolic volume; OR, odds ratio; RVD, right ventricular diameter basal; TAPSE, tricuspid annular plane systolic excursion; VA, ventricular arrhythmia.
Significant yearly progression by patient age.
By last follow up, 51 (54%) patients had received a primary preventive ICD. Re-evaluation of disease progression as a predictor of VA when only including patients with a primary preventive ICD, and when excluding ATP as an endpoint, showed similar results as in the total cohort (see Supplementary material online, Tables S3 and S4).
Threshold values for transition to a more arrhythmic phenotype
Reduced LV function was a strong predictor of first-time VA with particularly high risk when passing a threshold of LVEF <44% (Table 4). Furthermore, threshold values LVEDVi >77 mL/m2, LAVi >63 mL/m2, RV dilatation >42 mm, and TAPSE <21 mm were associated with first-time VA. PR interval threshold for first-time VA was 280 ms, and QRS threshold was 108 ms.
Table 4.
Threshold values for increased odds of experiencing first-time VA in 94 LMNA genotype–positive patients, by 241 ECGs and 441 echocardiographic examinations
| Threshold value | OR before threshold | P-value | OR after threshold | P-value | |
|---|---|---|---|---|---|
| LVEF (%, neg) | 44 | 1.02 | <0.05 | 1.10 | <0.001 |
| GLS (%) | −12.2 | 1.02 | 0.04 | 1.12 | <0.001 |
| LV EDVi (mL/m2) | 77 | 1.02 | 0.04 | 1.08 | <0.001 |
| LV ESVi (mL/m2) | 54 | 1.03 | 0.01 | 1.13 | <0.001 |
| LAVi (mL/m2) | 63 | 1.03 | 0.01 | 1.21 | <0.001 |
| RVD (mm) | 42 | 1.02 | 0.11 | 1.08 | <0.001 |
| TAPSE (mm, neg) | 22 | 1.02 | 0.12 | 1.08 | <0.001 |
| PR interval (ms) | 280 | 1.00 | 0.58 | 1.13 | <0.001 |
| QRS width (ms) | 108 | 1.02 | 0.15 | 1.19 | <0.001 |
Threshold values for reaching point of elevated increase in risk of ventricular arrhythmia, calculated by threshold regression analyses without use of random effects (univariate analysis). LVEF and TAPSE expressed as negative values.
ECG, electrocardiography; GLS, global longitudinal strain; i, indexed value; LAV, left atrial volume; LV EDV, left ventricular end-diastolic volume; LVEF, LV ejection fraction; LV ESV, LV end-systolic volume; OR, odds ratio; RVD, right ventricular diameter basal; TAPSE, tricuspid annular plane systolic excursion; VA, ventricular arrhythmia.
Discussion
Incidence of first VA in this primary prevention LMNA cohort was 20% during almost 5 years of follow up and happened at median age of 47 years. At time of first VA, most patients showed pronounced LV structural and functional pathology, and prominent conduction delay, while RV function was preserved in most patients. However, three patients experienced VA without any classical risk markers.
We present threshold values for entering a more arrhythmic phenotype that may help alert clinicians to consider a primary preventive ICD. PR prolongation and ventricular dysfunction progressed rapidly prior to the first VA, and the individual patient disease progression rate was a strong predictor of first-time VA. Our findings support the need of individualized follow up in LMNA genotype–positive patients and dynamic evaluation of patient risk to optimize timing of implantation of a primary preventive ICD.
Baseline predictors of life-threatening arrhythmia
Current guidelines for ICD implantation in LMNA genotype–positive patients are based on a limited number of studies, reporting male sex, non-missense mutations, NSVT, and LVEF <45% as risk factors for VA.14,15 Others have reported AV block as a risk factor.8,13 Our study supported NSVT, AV block, and LVEF <45% at baseline as predictors for future VA. AV block and LVEF <45% remained robust markers also when adjusted for age, emphasizing the increase in arrhythmic risk by progressive conduction disease and decreasing cardiac function. The age-related penetrance in cardiac laminopathies makes age a strong determinant for events. Hence, the fact that age out-performed NSVT as a predictor for VA in our study may not be clinically relevant.
Syncope at baseline was not a predictor of future VA. However, this result may be biased by bradycardia induced syncopes and non-cardiac syncopes, as this can be difficult to determine exactly. Syncope is an important symptom in LMNA disease and should not be disregarded. NYHA functional class did not predict VA when adjusted for age, which may be explained by the stronger effect of age compared to NYHA class in the statistical model.
This study did not support an association between VA and male sex or with non-missense mutations, adding to the debate on the role of sex and mutation type in LMNA disease. Other recent studies have also reported similar risk between males and females and between different genotypes.10,11 The previously reported arrhythmic risk of male sex should be further explored and explanations may include hormonal factors, sex-related exercise habits,19 and other unknown factors. In our study, as many as 83% of the patients had non-missense mutations. This is a higher proportion than previous reports,10,11,13 but in line with a Japanese laminopathy population.20 The skewed proportions may explain the lack of association to VA.
Disease progression prior to first life-threatening arrhythmia
Risk prediction in patients with cardiac laminopathies has been elaborated in recent years, including a large multicentre study providing robust data.13 Still, the optimal timing of primary preventive ICD implantation remains a challenge in cardiac laminopathies. Patients are followed at regular intervals, but the time of transition to higher risk of VA is unclear and may be missed. By analysing a large number of individual-level repeated ECG registrations and echocardiographic examinations, we could evaluate patient’s disease progression which added important information on arrhythmic risk. Accelerated electrical, structural, and functional disease progressions were clear markers of higher risk of VA. Importantly, 2 of 3 patients without any of the classical risk markers at time of VA showed >10% decline in LVEF prior to the event without reaching LVEF <45%, indicating that progression per se should be noted as increased arrhythmic risk, particularly when LVEF is above 45% and traditional risk markers are not fulfilled. Importantly, disease progression remained a strong predictor of VA after adjusting for the traditional risk markers NSVT, high-degree AV block, and LVEF <45%.
Disease progression was more prominent in those who later experienced VA (Figure 1). LVEDVi increased prior to first VA but visually seemed to decline by increasing age in patients without VA. However, regression analyses did not detect any decline in LVEDVi (Table 3) in non-VA patients, and the visually observed trend could possibly be explained by increasing body weight with age, combined with a slower dilatation rate in patients without VA (Table 3).
Timing of the transition to an arrhythmogenic phenotype
This study identified threshold values for increased risk of VA and were generated from repeated echocardiographic measurements from each study participant during long-term follow up, in contrast to being based on one baseline measurement from each participant.6 The threshold was determined by the value at which the slope of the risk curve changes, with a more pronounced incline in risk of VA after threshold. Importantly, several patients had arrhythmic events without having reached threshold, illustrated by the large spread in ECG and echocardiographic findings observed at time of first VA (Table 1). These findings emphasize that there are no ‘safe values’ in lamin disease. However, passing threshold represented a clear increase in arrhythmic risk and should alert the clinician to consider a primary preventive ICD.
The reported threshold values should be interpreted with care, as they do not include confidence intervals or random effects. In contrast, the results from the GEEs are robust but do not incorporate the threshold effect. Therefore, optimal risk prediction should incorporate considerations of both an approximate threshold and the individual progression rate.
Clinical implications
The present study adds to current knowledge on risk prediction in cardiac laminopathies by describing disease progression rate by ECG and echocardiography as strong predictors of VA, and by proposing threshold values indicating transition to a more arrhythmic phase of the disease (LVEF <44%, GLS worse than −12.2%, LVEDVi >77 mL/m2, LAVi >63 mL/m2, RVD >42 mm, TAPSE <21 mm, PR interval >280 ms, QRS width >108 ms).
Our results support that the LMNA cardiac phenotype requires close follow up. Patients’ LVEF showed a mean yearly decline of 1% and a maximum decline of 16%, suggesting that yearly echocardiographic examinations are appropriate. A decline in LVEF of ∼10% implied high arrhythmic risk and should alert the clinician also when not reaching <45%. Importantly, the clinician needs to be aware of the values from the previous visits to recognize changes in structural or functional parameters. Ideally, re-visits should be performed in the same centre by the same team. In case of new onset symptoms, or signs of electrical, structural, or functional disease progression, follow-up intervals should be increased to every 6 months, or more frequently as appropriate.
Limitations
The number of endpoints in this study was limited. Follow-up intervals were individualized according to clinical practice and patients with deterioration during follow up were examined at shorter intervals, providing more follow-up data than for the equivalent time period in patients with mild phenotype. This fact may have biased our data. Only three patients had no established risk factors at time of VA and the disease progression could be regarded as the only clinical event to alert risk. However, in the remaining patients, established risk markers were fulfilled and it cannot be concluded that the disease progression per se made the difference in risk prediction. Electrocardiogram registrations were predominantly performed in young patients with less pronounced phenotype due to the natural disease progression with high frequency of cardiac devices at older age. We were not able to include exercise data, or other environmental or hormonal exposures, and the impact of such data would be of great interest in future studies. Cardiac magnetic resonance imaging was not included in this study due to limited number of examinations and due to lack of repeated CMR examinations. The national referral nature of our centre may have resulted in high disease prevalence and the single centre design makes the external validity of the results undetermined. The findings of this study should be validated in larger multicentre cohorts.
Conclusions
In a primary preventive cohort of 94 LMNA genotype–positive patients, yearly incidence rate of VA was as high as 4.2%. We identified threshold values by ECG and echocardiography for transition to a more arrhythmic disease phase. At time of first VA, most patients had pronounced LV pathology and prominent conduction delay, but events occurred also in a few patients without obvious risk markers, highlighting the need for individualized follow up. Individual patient disease progression was a strong predictor of VA, showing additional prognostic value to previously reported predictors of arrhythmic outcome.
Supplementary Material
Acknowledgement
The authors are grateful for the contribution of the LMNA genotype–positive patients included in this study.
Contributor Information
Christine Rootwelt-Norberg, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, 0424 Oslo, Norway; Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway.
Eystein T Skjølsvik, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, 0424 Oslo, Norway; Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway.
Monica Chivulescu, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, 0424 Oslo, Norway; Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway.
Martin P Bogsrud, Unit for Cardiac and Cardiovascular Genetics, Oslo University Hospital, Ullevål, Norway.
Margareth P Ribe, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, 0424 Oslo, Norway; Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway.
Eivind W Aabel, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, 0424 Oslo, Norway; Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway.
Jan Otto Beitnes, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, 0424 Oslo, Norway; Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway.
Pål H Brekke, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, 0424 Oslo, Norway; Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway.
Trine F Håland, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, 0424 Oslo, Norway; Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway.
Nina E Hasselberg, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, 0424 Oslo, Norway; Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway.
Øyvind H Lie, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, 0424 Oslo, Norway; Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway.
Kristina H Haugaa, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, 0424 Oslo, Norway; Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway; Faculty of Medicine, Karolinska Institute and Cardiovascular Division, Karolinska University Hospital, Stockholm, Sweden.
Supplementary material
Supplementary material is available at Europace online.
Funding
This work was supported by Precision Health Care Center for optimized cardiac care (ProCardio) supported by the Norwegian Research Council (grant number #309762), European Research Area Network on Cardiovascular Diseases (ERA-CVD), and EMPATHY project (NFR grant number #298736).
Data availability
The data underlying this article will not be shared publicly.
References
- 1. Pasotti M, Klersy C, Pilotto A, Marziliano N, Rapezzi C, Serio Aet al. Long-term outcome and risk stratification in dilated cardiolaminopathies. J Am Coll Cardiol 2008;52:1250–60. [DOI] [PubMed] [Google Scholar]
- 2. Bondue A, Arbustini E, Bianco A, Ciccarelli M, Dawson D, De Rosa Met al. Complex roads from genotype to phenotype in dilated cardiomyopathy: scientific update from the working group of myocardial function of the European society of cardiology. Cardiovasc Res 2018;114:1287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hasselberg NE, Haland TF, Saberniak J, Brekke PH, Berge KE, Leren TPet al. Lamin A/C cardiomyopathy: young onset, high penetrance, and frequent need for heart transplantation. Eur Heart J 2018;39:853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Captur G, Arbustini E, Bonne G, Syrris P, Mills K, Wahbi Ket al. Lamin and the heart. Heart 2018;104:468–79. [DOI] [PubMed] [Google Scholar]
- 5. Olde Nordkamp LR, Postema PG, Knops RE, van Dijk N, Limpens J, Wilde AAet al. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: a systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm 2016;13:443–54. [DOI] [PubMed] [Google Scholar]
- 6. van Rijsingen IA, Arbustini E, Elliott PM, Mogensen J, Hermans-van Ast JF, van der Kooi AJet al. Risk factors for malignant ventricular arrhythmias in lamin a/c mutation carriers a European cohort study. J Am Coll Cardiol 2012;59:493–500. [DOI] [PubMed] [Google Scholar]
- 7. Anselme F, Moubarak G, Savouré A, Godin B, Borz B, Drouin-Garraud Vet al. Implantable cardioverter-defibrillators in lamin A/C mutation carriers with cardiac conduction disorders. Heart Rhythm 2013;10:1492–8. [DOI] [PubMed] [Google Scholar]
- 8. Hasselberg NE, Edvardsen T, Petri H, Berge KE, Leren TP, Bundgaard Het al. Risk prediction of ventricular arrhythmias and myocardial function in lamin A/C mutation positive subjects. Europace 2014;16:563–71. [DOI] [PubMed] [Google Scholar]
- 9. Kumar S, Baldinger SH, Gandjbakhch E, Maury P, Sellal JM, Androulakis AFet al. Long-term arrhythmic and nonarrhythmic outcomes of lamin A/C mutation carriers. J Am Coll Cardiol 2016;68:2299–307. [DOI] [PubMed] [Google Scholar]
- 10. Peretto G, Barison A, Forleo C, Di Resta C, Esposito A, Aquaro GDet al. Late gadolinium enhancement role in arrhythmic risk stratification of patients with LMNA cardiomyopathy: results from a long-term follow-up multicentre study. Europace 2020;22:1864–72. [DOI] [PubMed] [Google Scholar]
- 11. Barriales-Villa R, Ochoa JP, Larrañaga-Moreira JM, Salazar-Mendiguchía J, Díez-López C, Restrepo-Córdoba MAet al. Risk predictors in a Spanish cohort with cardiac laminopathies. The REDLAMINA registry. Rev Esp Cardiol (Engl Ed) 2021;74:216–24. [DOI] [PubMed] [Google Scholar]
- 12. Haugaa KH, Hasselberg NE, Edvardsen T. Mechanical dispersion by strain echocardiography: a predictor of ventricular arrhythmias in subjects with lamin A/C mutations. JACC Cardiovasc Imaging 2015;8:104–6. [DOI] [PubMed] [Google Scholar]
- 13. Wahbi K, Ben Yaou R, Gandjbakhch E, Anselme F, Gossios T, Lakdawala NKet al. Development and validation of a new risk prediction score for life-threatening ventricular tachyarrhythmias in laminopathies. Circulation 2019;140:293–302. [DOI] [PubMed] [Google Scholar]
- 14. Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm Jet al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace 2015;17:1601–87. [DOI] [PubMed] [Google Scholar]
- 15. Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCCet al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy: executive summary. Heart Rhythm 2019;16:e373–407. [DOI] [PubMed] [Google Scholar]
- 16. Skjølsvik ET, Haugen Lie Ø, Chivulescu M, Ribe M, Castrini AI, Broch Ket al. Progression of cardiac disease in patients with lamin A/C mutations. Eur Heart J Cardiovasc Imaging 2022;23:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster Jet al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17:405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande Let al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 19. Skjølsvik ET, Hasselberg NE, Dejgaard LA, Lie Ø H, Andersen K, Holm Tet al. Exercise is associated with impaired left ventricular systolic function in patients with lamin A/C genotype. J Am Heart Assoc 2020;9:e012937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakajima K, Aiba T, Makiyama T, Nishiuchi S, Ohno S, Kato Ket al. Clinical manifestations and long-term mortality in lamin A/C mutation carriers from a Japanese Multicenter Registry. Circ J 2018;82:2707–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will not be shared publicly.