Abstract
Aims
To investigate the role of genetic testing in patients with idiopathic atrioventricular conduction disease requiring pacemaker (PM) implantation before the age of 50 years.
Methods and results
All consecutive PM implantations in Southern Switzerland between 2010 and 2019 were evaluated. Inclusion criteria were: (i) age at the time of PM implantation: < 50 years; (ii) atrioventricular block (AVB) of unknown aetiology. Study population was investigated by ajmaline challenge and echocardiographic assessment over time. Genetic testing was performed using next-generation sequencing panel, containing 174 genes associated to inherited cardiac diseases, and Sanger sequencing confirmation of suspected variants with clinical implication. Of 2510 patients who underwent PM implantation, 15 (0.6%) were young adults (median age: 44 years, male predominance) presenting with advanced AVB of unknown origin. The average incidence of idiopathic AVB computed over the 2010–2019 time window was 0.7 per 100 000 persons per year (95% CI 0.4–1.2). Most of patients (67%) presented with specific genetic findings (pathogenic variant) or variants of uncertain significance (VUS). A pathogenic variant of PKP2 gene was found in one patient (6.7%) with no overt structural cardiac abnormalities. A VUS of TRPM4, MYBPC3, SCN5A, KCNE1, LMNA, GJA5 genes was found in other nine cases (60%). Of these, three unrelated patients (20%) presented the same heterozygous missense variant c.2531G > A p.(Gly844Asp) in TRPM4 gene. Diagnostic re-assessment over time led to a diagnosis of Brugada syndrome and long-QT syndrome in two patients (13%). No cardiac events occurred during a median follow-up of 72 months.
Conclusion
Idiopathic AVB in adults younger than 50 years is a very rare condition with an incidence of 0.7 per 100 000 persons/year. Systematic investigations, including genetic testing and ajmaline challenge, can lead to the achievement of a specific diagnosis in up to 20% of patients. Heterozygous missense variant c.2531G > A p.(Gly844Asp) in TRPM4 gene was found in an additional 20% of unrelated patients, suggesting possible association of the variant with the disease.
Keywords: Pacemaker, Atrioventricular Block, Inherited heart disease, Genetics, TRPM4
What’s new?
Advanced atrioventricular block of unknown origin in young adults (<50 years) is an unusual condition and should merit appropriate diagnostic investigations
The incidence of idiopathic atrioventricular block in young adults is 0.7 per 100 000 persons/year
Systematic investigations, including genetic testing and ajmaline challenge, can lead to the achievement of a specific diagnosis in up to 20% of patients
Introduction
Advanced atrioventricular node (AVN) disease, i.e. Mobitz Type II second-degree atrioventricular block (AVB) or third-degree AVB, is an extremely rare condition at paediatric age and in young adults (<50 years old).1–4 Large epidemiological studies have been previously conducted in children with isolated complete AVB and the outcome of this patient group has been well characterized.5 AVB was considered idiopathic if a cardiac, metabolic, toxicological and infectious aetiology was excluded.
In contrast, the clinical profile of adults younger than 50 years with advanced AVN disease has been poorly defined. Recent studies have considered heterogeneous aetiologies underlying AVN disease in the young, including congenital AVB, congenital heart diseases, cardiomyopathies, infectious diseases, cardiac involvement of a muscular dystrophy and complications of cardiac surgery, radiofrequency ablation or alcohol septal ablation.4 Other causes of AVB include coronary artery disease and cardiac sarcoidosis, although they occur more frequently after the fifth decade of life.
The incidence, aetiologies, and outcome of advanced AVB of unknown origin (also termed as ‘idiopathic’) in young adults without concomitant cardiac diseases are mostly unknown. Furthermore, because this patient group requires life-long pacing which may lead to deterioration of left ventricular systolic function, there is the legitimate question whether modern pacing modalities such as cardiac resynchronization pacing (CRT) or conduction system pacing shall be considered. Indeed, past studies in older patients reported a reduction in left ventricular function already 1 year after pacemaker (PM) implantation,6 and a pacing-induced cardiomyopathy in up to 20% of patients with preserved pre-implant left ventricular function during long-term follow-up.7
The aim of this study, conducted on a statewide population, was to assess incidence and genetic features of AVB in young adults receiving their first PM implantation before the age of 50 years. Moreover, we sought to evaluate the impact of a diagnostic re-assessment over time and the long-term outcomes.
Methods
Study population
Between January 1st 2010 and December 31st 2019, a total of 2510 adult patients received their first PM implantation at one of the three implanting centers in Swiss Canton Ticino, all serving a population of about 340 000 inhabitants.8 Adult patients (≥ 18 years) with advanced AVN disease treated with PM were identified by searching the Swiss ICD and Pacemaker registry and the local implantation registry of each participating institution. The Swiss ICD and Pacemaker registry is a national data repository managed by the Working Group Pacing and Electrophysiology of the Swiss Society of Cardiology and collects all consecutive PMs and implantable cardioverter-defibrillators implanted at each Swiss center.9 In Switzerland, the indication for pacemaker implantation follows the European Society of Cardiology guidelines on cardiac pacing.10 Therefore, the indication for PM implantation in patients with AVN disease included a Mobitz Type II, advanced 2nd degree AVB, or 3rd degree AVB.
All recordings with the documentation of AVB were independently reviewed and classified by two investigators. An in-depth review of medical records and results of diagnostic work up was performed to evaluate symptoms at time of PM implantation, and the most likely aetiology of the advanced AV conduction disturbance. Available data on medical history, physical examination, metabolic and toxicological screening, baseline 12-lead ECG, two-dimensional echocardiography (2D-TTE), 24 h Holter monitoring, and a coronary angiogram were evaluated to rule out structural abnormalities or other aetiologies as cause of AVB. A careful evaluation of the presence of symptoms and prodromes suggesting a vagally mediated AVB was performed in all cases; tilt table test was conducted in specific cases. Borrelliosis was evaluated by conventional blood test panel including screening for the anti-Borrelia (Borrelia burgdorferi) IgM and IgG.
AVB was considered idiopathic if a cardiac, metabolic, toxicological and infectious aetiology was excluded. Patients with congenital cardiac disease, concomitant valvular heart disease, coronary artery disease, cardiomyopathy, cardiac sarcoidosis or cardiac involvement of a muscular dystrophy were excluded from the cohort with idiopathic AVB.
All available pre-implantation, baseline and follow-up ECGs were recorded at a paper speed of 25 mm/s and amplitude of 10 mm/mV and reviewed by two experienced electrophysiologists, independently; in case of disagreement, the ECG was reviewed by a third electrophysiologist. This analysis aimed at identifying ECG signs of inherited arrhythmia diseases. Early repolarization pattern was considered in the presence of QRS slurring (a smooth transition from the QRS segment to the ST segment) or notching (a positive J deflection of at least 1 mm inscribed on the S wave) in the inferior leads (II, III, and aVF), lateral leads (I, aVL, and V4–V6), or both. An ECG was considered diagnostic of BrS (Type 1) if a coved type ST elevation ≥2 mm was documented in ≥1 lead from V1 to V3 in the presence or absence of a sodium-channel blocker agent. Atrioventricular conduction abnormalities were considered as bundle branch block (BBB) of any type or first-degree AV block. Patients with pre-existing complete BBB were excluded. QTc measurement was performed in all patients with spontaneous AV conduction and it was considered abnormal in case of a prolongation greater than 480 ms.11 Short QT syndrome was considered in the presence of a QTc ≤360 ms.
Institutional and Ethics Committee approval was obtained (Swiss Ethics, approval number: TI3778) and all identified patients gave informed consent to participate in the study.
Pharmacological challenge with ajmaline
Ajmaline challenge was performed in all patients with spontaneous AV conduction to rule out Brugada syndrome (BrS). Ajmaline was administered intravenously over a 5 min period at a dose of 1 mg/kg. The test was considered positive for BrS only if coved type 1 ECG was documented in ≥1 right precordial leads (V1–V3).11
Pacemaker implantation and programming
A dual-chamber pacemaker was implanted in all patients. At patient’s discharge, pacemaker programming aimed to minimize the frequency of right ventricular pacing and was performed in accordance to guidelines in all patients. Ventricular minimizing pacing algorithms were systematically used. AAI-ADI/DDD mode switching or automated search of intrinsic conduction (AVD hysteresis) were activated in each patient.
Genetic analysis
All patients were offered to receive a genetic test. After obtained informed consent, genetic testing was performed using TruSight Cardio (Illumina) which contains 174 genes known to be associated to inherited cardiac diseases and high-throughput sequencing (MiSeq) (see Supplementary material online, Table).
Follow-up
Patient follow-up was conducted at the implanting center or by a cardiologist who reports to the implanting center. All follow-up visits were reviewed, PM programming was carefully analyzed, and device print outs were evaluated. Clinical follow-up of patients consisted of physical examination, ECG and device interrogation performed at least every 12 months. Percentage of atrial and ventricular pacing were reported. Atrial and ventricular arrhythmias recorded by the device were systematically captured. Each patient underwent an echocardiographic examination before implantation and at least once every year thereafter. Left ventricular ejection fraction was quantified using a modified biplane Simpson rule in 2- and 4-chamber apical view. Major cardiac events were defined as the occurrence of cardiovascular death, hospitalization for heart failure or sustained ventricular arrhythmias.
Statistics
Continuous data were presented as median (25–75 percentiles) and compared with the Mann–Whitney U test. Categorical data were expressed as number and percentage of population and compared with the Fisher exact test. We computed the yearly incidence of pacemaker implantation as an average of the number of pacemaker implantation per year and the resident population of subjects aged 50 or less, together with its exact Poisson 95% confidence interval, overall and by sex. The resident population was obtained from the official Swiss statistics. Statistical analyses were performed with Stata software (version 16, StataCorp, College Station, TX, USA). All tests were 2-sided and a P-value below 0.05 was considered statistically significant.
Results
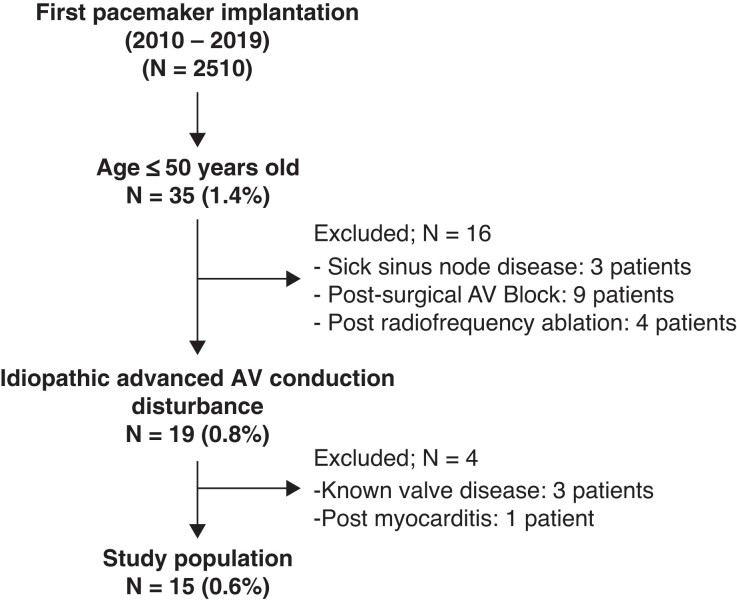
A total of 2510 PM implantations were performed in Canton Ticino (Switzerland) from 2010 to 2019 (Figure 1) including 35 patients (1.4%) < 50 years at the time of implantation. Fifteen patients (0.6% of the initial population) had a diagnosis of idiopathic AVB.
Figure 1.
Flow chart for patient’s selection.
Clinical characteristics
The clinical and demographic characteristics of the study population is presented in Table 1. The median age at first pacemaker implantation was 44 years. Male predominance was observed. Female patients tended to be on average 8 years younger than male patients. Intermittent complete AVB was the most frequent AVN conduction disturbance. All patients were symptomatic and the most common presenting symptoms were dizziness and fatigue (Table 1). None of the patients had a family history of sudden cardiac death. Median left ventricular ejection fraction before implantation was 60% (58–61).
Table 1.
Demographic and clinical characteristics of the 15 young adults with an advanced AVN disease without known cardiac disease at the time of first pacemaker implantation
| Variable | All patients | Female patients | Male patients | P valuea |
|---|---|---|---|---|
| N = 15 | N = 5 | N = 10 | ||
| Age at implant, (CI 25th–75th percentile) | 44 (31.7–46.7) | 37.5 (21.7–46.5) | 45.5 (40.5–48) | 0.51 |
| Atrioventricular conduction disturbance | 1.00 | |||
| ȃMobitz II—second-degree AV block, (%) | 2 (13.3%) | 1 (20%) | 1 (10%) | |
| ȃThird-degree AV block—intermittent, (%) | 8 (53.3%) | 3 (60%) | 5 (50%) | |
| ȃThird-degree AV block—permanent, (%) | 5 (33.3%) | 1 (20%) | 4 (40%) | |
| Presenting symptom | ||||
| ȃDizziness, (%) | 8 (53.3%) | 4 (80%) | 4 (40%) | 0.28 |
| ȃSyncope, (%) | 2 (13.3%) | 1 (20%) | 1 (10%) | 1.00 |
| ȃDyspnoea or fatigue, (%) | 6 (40%) | 3 (60%) | 3 (30%) | 0.33 |
| Comorbidity | ||||
| ȃHypertension, (%) | 3 (20%) | 1 (20%) | 2 (20%) | 1.00 |
| ȃDyslipidemia, (%) | 2 (13.3%) | 1 (20%) | 1 (10%) | 1.00 |
| ȃSmoking habit, (%) | 3 (20%) | 2 (40%) | 1 (10%) | 0.24 |
Fisher exact test for all but age (Mann Whitney U test).
Diagnostic work up
As shown in Table 2, despite extensive cardiac imaging, the aetiology of the advanced AVB remained unknown in the vast majority of cases at the time of first PM implantation. A pre-implantation magnetic resonance imaging was performed in most cases and was normal. Furthermore, in about 50% of cases, in whom either a coronary angiography or a computed tomography was performed, no coronary artery disease was found. All patients underwent genetic testing. Ajmaline challenge was performed in 10 patients (only those with intermitted AVB) still having a spontaneous AV conduction.
Table 2.
Diagnostic work up of idiopathic AVN disease
| Diagnostic exam | N = 15 |
|---|---|
| Cardiac enzymes testing | 15 (100%) |
| Lyme disease testing | 12 (80%) |
| Adenosine plasma level | 1 (6.6%) |
| Autoimmune diseases panel | 2 (13%) |
| Transthoracic echocardiography | 15 (100%) |
| Ajmaline challenge | 10 (67%)a |
| Genetic test | 15 (100%) |
| Tilt table testing | 1 (6.6%) |
| Cardiac magnetic resonance imaging | 12 (80%) |
| Coronary angiography or cardiac computed tomography | 9 (60%) |
Drug challenge was performed in all patients with spontaneous AV conduction.
All patients received a dual-chamber pacemaker programmed in DDD mode with activated ventricular minimization algorithm. The most common pacing site was a mid-septal location (13 cases), and right ventricular apex in two cases.
Genetic testing
The entire study population was systematically investigated for the most common genes associated to inherited heart diseases. Results are reported in Table 3.
Table 3.
Genetic test results
| Gender | Age at IPG implantation | PR (ms) | Intrinsic QRSa/paced QRS (ms) | Gene variant | Protein Change | NM number | Significance (ACMG criteria) | LVEF at last FU | VP% at last FU | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pt #1 | Male | 48 | – | 137/150 | TRPM4 | p.(Gly844Asp) | 017 636.3 | US | 65% | 100% |
| c.2531G > A | ||||||||||
| Pt #2 | Female | 42 | 135 | 88/145 | None | – | – | – | 63% | 8% |
| Pt #3 | Female | 19 | – | 120/160 | MYBPC3 | p.(Asp358Glu) | 000256.3 | US | 55% | 100% |
| c.1074T > A | ||||||||||
| Pt #4 | Male | 30 | 150 | 100/145 | None | – | – | – | 60% | 18% |
| Pt #5 | Female | 25 | 280 | 90/162 | SCN5A | p.(Arg1897Trp) | 198056.2 | US | 55% | 80% |
| c.5689C > T | ||||||||||
| KCNE1 | p.(Asp85Asn) | 000219.5 | ||||||||
| c.253G > A | ||||||||||
| Pt #6 | Male | 47 | 180 | 99/149 | None | – | – | – | 58% | 7% |
| Pt #7 | Male | 44 | – | 118/162 | LMNA | – | – | US | 59% | 100% |
| Pt #8 | Male | 44 | 148 | 105/151 | None | – | – | – | 56% | 9% |
| Pt #9 | Male | 26 | 137 | 96/149 | MYBPC3 | p.(Asp358Glu) | 000256.3 | US | 61% | 9% |
| c.1074T > A | ||||||||||
| Pt #10 | Male | 44 | – | 130/175 | TRPM4 | p.(Gly844Asp) | 017636.3 | US | 62% | 100% |
| c.2531G > A | ||||||||||
| Pt #11 | Male | 49 | 140 | 102/158 | None | – | – | – | 53% | 12% |
| Pt #12 | Male | 30 | 245 | 96/144 | PKP2 | p.(Lys672ArgfsTer12) | 004572.3 | Pathogenic | 58% | 9% |
| c.2013delC | ||||||||||
| Pt #13 | Female | 33 | 139 | 84/153 | None | – | – | – | 60% | 7% |
| Pt #14 | Female | 47 | 150 | 95/162 | GJA5 | p.(Arg316His) | 005266.6 | US | 61% | 5% |
| c.947G > A | ||||||||||
| Pt #15 | Male | 45 | – | 100/152 | TRPM4 | p.(Gly844Asp) | 017636.3 | US | 60% | 100% |
| c.2531G > A |
US, uncertain significance; IPG, implantable pulse generator; LVEF, left ventricle ejection fraction; VP%, ventricular pacing percentage; FU, follow-up; ACMG, American College of Medical Genetics and Genomics.
Intrinsic QRS intervals refer to measurement performed during the episode of AV block.
A pathogenic variant c.2013delC p.(Lys672ArgfsTer12) in PKP2 gene was found in a patient with no overt structural abnormalities (Figure 2, Panel B). Moreover, a variant of uncertain significance (VUS) of TRPM4, MYBPC3, SCN5A, KCNE1, LMNA, GJA5 genes was documented in other 9 cases (60%) (Table 3).
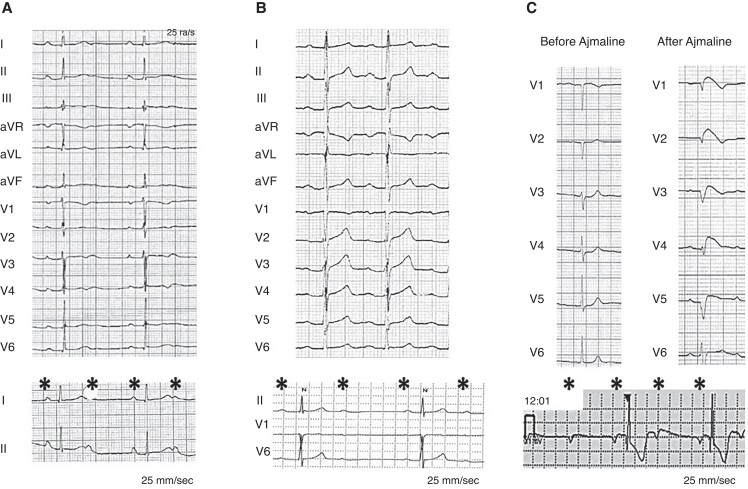
Figure 2.
Twelve-lead ECG tracings of three young symptomatic adults with atrioventricular block requiring pacemaker implantation. Panel A: patient (Pt #5) with a variant of uncertain significance of SCN5A gene had a documented long-QT interval and intermittent atrioventricular block. Panel B: patient with a pathogenic variant of PKP2 (Pt #12). Panel C: ajmaline-induced BrS patient (Pt #2) with negative genetic test.
Of note, in three unrelated patients, the mutational analysis detected the same heterozygous missense variant c.2531G > A p.(Gly844Asp) in exon 17 of the TRPM4 gene.
None of patients with a MYPBC3 variant presented phenotypic signs of hypertrophic cardiomyopathy. Among the 6 patients without genetic abnormalities there were not athletes nor patients with relevant clinical habits that may explain the AVB. Moreover, ECG recordings during AV block were reviewed to investigate the behaviour of the sinus rate contextual to the AV block and none of them suggested a possible vagally mediated AV block.
Follow-up
During a median follow-up of 72 months (25–75% 49–90 months), no cardiac events (cardiovascular death, hospitalization for heart failure or sustained ventricular arrhythmias) occurred. After the pacemaker implantation, all patients reported the resolution of the presenting symptom(s).
About 40 months after device implantation, one patient manifested a Brugada type 2 ECG (Pt #2) and underwent an ajmaline test which unmasked a Brugada type 1 ECG (Figure 2, Panel A, B); the genetic analysis did not identified any gene variant. On the basis of the absence of spontaneous Type 1 ECG documentation, and no ventricular arrhythmias at routine PM controls, no indication to ICD upgrading nor programmed ventricular stimulation at EP study was established. Moreover, the patient did not present any further syncopal event after PM implantation, supporting the bradycardic aetiology of the presenting symptoms.
In another patient (Pt#5), 2 years after the device implantation, a non-sustained ventricular tachycardia was recorded by the device and prolonged QTc (>500 ms) was found in repeated ECGs, leading to initiation of a beta-blocker therapy. This patients had a VUS of SCN5A and KCNE1 genes.
During follow-up, percentage of pacing was ≥40% in 6 patients. There was no difference in age [44 (22.7–47.2) vs. 45.5 (32.2–48.2) years, P = 0.51], gender (67% vs. 60% males), and clinical presenting symptoms between those patients with higher or lower than 40% ventricular pacing. Moreover, left ventricular ejection fraction and the left ventricular end-diastolic diameter remained unchanged during the follow-up in those patients with a ventricular pacing higher than 40%.
Disease incidence
Canton Ticino has a relatively stable resident population with an annual growth of about 0.5% per year. Indeed, in 2010 there were 335 720 inhabitants and in 2019 the official recorded number of residents was 353 343.10 As shown in Figure 1, 15 patients (42.8%) out of 35 patients with a first PM implantation at the age of 50 years or younger were identified having an advanced AVB without any underlying cardiac disease. The average incidence computed over the 2010–2019 time window was 0.7 per 100 000 persons per year (95% CI 0.4–1.2); it was 1.0 (95%CI 0.5–1.8) in men and 0.5 (95% CI 0.2–1.1) in women. The yearly incidence over the time window of first PM implantation in young adults ranged from 0 to 1.5 implants per 100 000 persons.
Discussion
To the best of our knowledge, this is the first study which systematically screened for the most common genes associated to inherited heart diseases in young adults (<50 years) with AVB requiring a pacemaker. Importantly, systematic investigations over time, including genetic testing and ajmaline challenge, led to the achievement of a specific diagnosis (Brugada syndrome, long-QT syndrome, and arrhythmogenic cardiomyopathy) in up to 20% of patients). In addition, heterozygous missense variant c.2531G > A p.(Gly844Asp) in TRPM4 gene was found in other 20% of patients, suggesting disease association of this variant with the disease.
Genetic findings
Genetic testing revealed specific findings (pathogenic variant) or VUS in 67% of patients. A patient presented with a pathogenic variant in PKP2 gene, while a VUS of TRPM4, MYBPC3, SCN5A, KCNE1, LMNA, GJA5 genes was found in 9 other cases.
Interestingly, heterozygous frame-shift variant c.2013delC p.(Lys672ArgfsTer12) in exon 10 of the PKP2 gene was documented in a patient with no overt structural cardiac abnormalities. This variant has been described before as disease causing (PP5*) and is reported in ClinVar as pathogenic.12 Moreover, its prevalence in gnomAD is very low (0.00000796, PM2*).13 Radical variants in PKP2 gene are known to be disease causing (PVS1*), therefore the variant c.2013delC p.(Lys672ArgfsTer12) in PKP2 gene is classified following the ACMG criteria as pathogenic.14 Atrioventricular conduction disorders have been described in cases with arrhythmogenic cardiomyopathy15 and bundle branch block is the most common observed conduction disorder in these cases. Therefore, the clinical presentation of this case is of utmost interest due the presence of AVB as the sole manifestation of arrhythmogenic cardiomyopathy. Genetic testing had a significant impact on this patient in terms of prognostic stratification, need of echocardiographic regular assessment, sport restriction and family members’ evaluation.
Notably, heterozygous missense variant c.2531G > A p.(Gly844Asp) in exon 17 of the TRPM4 gene was found in 3 different unrelated patients (20%). This finding highlights the possibility of disease association of the above variant with AVN disease. This variant is reported as variant of uncertain significance in ClinVar.12 It is listed in the gnomAD database with a frequency of 0.0009805 (BS2* supporting), which is not higher than the prevalence of the disease (1:1000 for conduction system disease).13 Multiple lines of computational evidence suggest no impact on the gene product (BP4*). The initial report of the variant TRPM4-c.2531G > A p.(Gly844Asp) documented co-segregation with the disease with incomplete penetrance in a large pedigree (PP1*).16 Functional studies exhibit gain of function of the calcium-activated non-selective cation channel encoded by the TRPM4 gene (PS3*) and further studies also documented several cases with cardiac conduction disorders hosting the variant TRPM4-c.2531G > A p.(Gly844Asp).17 Further disease co-segregation studies in patients’ families are warranted to upgrade this variant to likely pathogenic. Familial segregation of a VUS should be performed by a careful phenotype assessment in patient’s family members. Genetic testing for genes with limited, disputed, or refuted evidence is not performed in patients with a weak (non-definite) phenotype in the clinical setting.18
Patient-specific induced pluripotent stem cell (hiPSC-CMs) models could provide a good tool to test VUS, and confirm their relevance by assessing functional impact, as patient-derived cells recapitulate the electrophysiological features of the disorder.19
Epidemiology of AVN disease in the young (≤ 50 years)
This is one of the first studies assessing the incidence of a symptomatic AVB in young adults referred for pacemaker implantation in a European region before the age of 50. The incidence remained stable over a period of 10 years. All these findings significantly extend our current knowledge about the so-called idiopathic advanced AVN conduction disturbance in young adults, and helps in refining the nosology and pathogenesis of the disease.
Our study has some similarities but also significant differences with a recently published study by Rudbeck-Resdal et al.,4 who reported the aetiology of AVB in a nationwide cohort in patients younger than 50 years referred for pacemaker implantation. In a nationwide study over 15 years, they collected 1027 patients. About 33% of these patients had a congenital or iatrogenic atrioventricular block (complication of cardiac surgery, radiofrequency ablation or alcohol septal ablation), and about 17% had mixed causes. They showed that in about 50% of the 1027 patients implanted in Denmark during the last 15 years, the aetiology remained unknown despite extensive use of advanced cardiac diagnostic imaging, including magnetic resonance, cardiac computed tomography or cardiac angiography. Notably, genetic testing was very rarely performed in the Danish population4 accounting for 0.6% of the studied population. In contrast, all our patients underwent a genetic examination limited to the most common gene variants associated to hereditary cause of AVB or other inherited cardiac disorders. In our study, a genetic aetiology was diagnosed in 3 cases (20%). Mutations in the LMNA and SCN5A genes are among the best known hereditary causes of AVB. In our study, we could identify one patient with VUS of SCN5A gene and documented long-QT interval in repeated ECG (QTc 500 ms), intermittent AVB and episodes of non-sustained ventricular tachycardia; one patients had an ajmaline-induced BrS patient with negative genetic test; and finally, one patient with a PKP2 pathogenic variant. In all these patients, there were clinical implications, including drug-therapy initiation, life-style recommendations and screening of first-degree relatives. It should be noted that molecular–genetic testing was not nationwide implemented in Switzerland before 2008. With increased use of molecular–genetic testing, the number of patients diagnosed with a hereditary aetiology is likely to increase in the future.
According to American Heart Association Heart Disease and Stroke Statistics,3 Mobitz type II second-degree and third-degree AVB is a rare conduction disturbance affecting approximately 0.003% and 0.02% of apparently healthy adult population, respectively. Johnson et al.20 found only one case among >67 000 symptom-free US Air Force males, whereas Rose et al.21 in their study of >18 000 civil servants, did not find any cases. On the other hand, among 293 124 patients with diabetes mellitus and 552 624 with hypertension enrolled with Veterans Health Administration hospitals, third-degree AVB was present in 1.1% and 0.6%, respectively.22 In Denmark, a nation having a population of about 5.6 million inhabitants, 1027 patients required a pacemaker implantation at the age of 50 years or younger over a period of 15 years (between 1996 and 2015). Of those, in 517 patients the aetiology of AVN disease was classified as unknown. This represents an approximate incidence of 0.6 cases per 100 000 inhabitants. Consistent with this latter report, but in a more contemporary population and in a well-defined European region, we found that symptomatic advanced AVB at young age fulfil the criteria of a rare disease according to the Health Programme of the European Union (EU)22; indeed the recorded incidence in Canton Ticino (Switzerland) was 0.7 case per 100 000 inhabitants but more frequent in male (1.0; 95% CI: 0.5–1.8) than in female (0.5; 95% CI: 0.2–1.1) patients. A rare disease is defined when it affects <1 in 2000 citizens.23 Due to the low prevalence, research is limited and misdiagnosis frequent.
Our findings are in line with a recent study by Tassetti et al.24 reporting abnormal genetic findings in up to 67% of young patients with advanced AVB, and may represent a call for adoption of advanced AVN conduction disease at young age within the rare disease and orphan medicine legislations at the European level, including the EU Regulation on Orphan Medicinal Products, the EU Regulation on Pediatric Drugs, the EU Regulation on Advanced Therapies, and the Commission Communication Rare Diseases.
Practical considerations
Although no arrhythmic events occurred during ajmaline challenge, to increase its safety, it should always be performed under close supervision in an appropriate environment with all advanced life support facilities available, ideally including the possibility of performing a temporary PM implantation and venoarterial extracorporeal membrane oxygenation placement in case of a refractory episode of ventricular fibrillation.
Long-term outcomes of AVN disease in the young (≤ 50 years)
Another finding was that in this well-defined population treated with a dual-chamber PM and up-to-date programming, including activation of an algorithm to minimize right ventricular pacing, there was no deterioration of left ventricular function during a median follow-up of 6 years after PM implantation.25 These results may contribute to the controversy whether alternative pacing solutions such as conduction system pacing or cardiac resynchronization therapy are needed in patients without cardiac diseases and normal ventricular function. Several previous studies suggest that conventional right ventricular apical pacing may have a deleterious effect on left ventricular function, worsen heart failure, and increase hospitalization rate.6,7,26 In contrast to these results, our data show unchanged ventricular function and diameters of left ventricular chambers as well as no cardiac events, including hospitalization for heart failure during a long-term follow-up.
Long-term safety and efficacy data of alternative pacing modality such as conduction system pacing or CRT in young adults with advanced atrioventricular conduction disturbance and normal ejection fraction are currently missing. However, given the rarity of the disease, it is unlike to conduct a randomized controlled trial to address this issue thus, only observational data can be collected and cautiously compared.
Limitations
Our study has certain limitations. It is a retrospective study conducted, due to the rarity of the condition, in a small population of patients with heterogeneous clinical characteristics. The incidence of AVB might be underestimated since our study selected only patients who were implanted with a PM. The disease association of TRPM4 variant with AVN disease has not been investigated with further disease co-segregation studies in patients’ families. Therefore, no conclusion can be drawn on the pathogenicity of the documented VUS and the impact on prognosis. Finally, HV interval measurement was not performed during PM implantation and therefore the site of AV block was not investigated.
Conclusions
Idiopathic AVB in adults younger than 50 years is a very rare condition. Systematic investigations, including genetic testing and ajmaline challenge, can lead to the achievement of a specific diagnosis in up to 20% of patients. Heterozygous missense variant c.2531G > A p.(Gly844Asp) in TRPM4 gene was found in an additional 20% of unrelated patients, suggesting possible association of the variant with the disease.
Supplementary Material
Contributor Information
Angelo Auricchio, Division of Cardiology, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Lugano 6900, Switzerland.
Andrea Demarchi, Division of Cardiology, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Lugano 6900, Switzerland.
Tardu Özkartal, Division of Cardiology, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Lugano 6900, Switzerland; Division of Cardiology, Ospedale di Bellinzona, Bellinzona 6500, Switzerland.
Daniela Campanale, Division of Cardiology, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Lugano 6900, Switzerland.
Maria Luce Caputo, Division of Cardiology, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Lugano 6900, Switzerland.
Marcello di Valentino, Division of Cardiology, Ospedale di Bellinzona, Bellinzona 6500, Switzerland.
Andrea Menafoglio, Division of Cardiology, Ospedale di Bellinzona, Bellinzona 6500, Switzerland.
Francois Regoli, Division of Cardiology, Ospedale di Bellinzona, Bellinzona 6500, Switzerland.
Marco Facchini, Division of Cardiology, Ospedale di Locarno, Locarno 6600, Switzerland.
Alessandro Del Bufalo, Division of Cardiology, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Lugano 6900, Switzerland.
Pietro Foglia, Division of Cardiology, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Lugano 6900, Switzerland.
Nicola Ferrari, Division of Cardiology, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Lugano 6900, Switzerland.
Fulvio Bomio, Division of Cardiology, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Lugano 6900, Switzerland.
Argelia Medeiros-Domingo, Swiss DNAlysis, Cardiogenetics, Düdendorf 8600, Switzerland.
Tiziano Moccetti, Division of Cardiology, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Lugano 6900, Switzerland.
Giovanni B Pedrazzini, Division of Cardiology, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Lugano 6900, Switzerland.
Catherine Klersy, Service Clinical Epidemiology and Biometry, Fondazione IRCCS Policlinico San Matteo, Pavia 27100, Italy.
Giulio Conte, Division of Cardiology, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Lugano 6900, Switzerland.
Supplementary material
Supplementary material is available at Europace online.
Funding
This study was supported by a research grant (N° FF21084) from the Swiss Heart Foundation.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Proclemer A, et al. Registro italiano pacemaker e defibrillatori - bollettino periodico 2018. Associazione italiana di aritmologia e cardiostimolazione [the pacemaker and implantable cardioverter-defibrillator registry of the Italian association of arrhythmology and cardiac pacing - annual report 2018]. Gital Cardiol (Rome). 2020;21:157–69. [DOI] [PubMed] [Google Scholar]
- 2. Pombo Jiménez M, et al. Spanish pacemaker registry. 17th official report of the section on cardiac pacing of the spanish society of cardiology (2019). Rev Esp Cardiol (Engl Ed) 2020;73:1038–48. [DOI] [PubMed] [Google Scholar]
- 3. Benjamin EJ, et al. Heart disease and stroke statistics-2018 update: a report from the American heart association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 4. Rudbeck-Resdal J, et al. Aetiologies and temporal trends of atrioventricular block in young patients: a 20-year nationwide study. Europace 2019;21:1710–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eliasson H, et al. Outcome in young patients with isolated complete atrioventricular block and permanent pacemaker treatment: a nationwide study of 127 patients. Heart Rhythm 2015;12:2278–84. [DOI] [PubMed] [Google Scholar]
- 6. Yu CM, et al. Biventricular pacing in patients with bradycardia and Normal ejection fraction. N Engl J Med 2009;361:2123–34. [DOI] [PubMed] [Google Scholar]
- 7. Khurshid S, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy, heart rhythm 2014;11:1619–25. [DOI] [PubMed] [Google Scholar]
- 8. https://www.pxweb.bfs.admin.ch. Last access Jul 13, 2021
- 9. https://www.pacemaker.ch. Last access Jul 13, 2021
- 10. Brignole M, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2013;34:2281–329. [DOI] [PubMed] [Google Scholar]
- 11. Priori SG, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European society of cardiology (ESC) endorsed by: association for European paediatric and congenital cardiology (AEPC). Eur Heart J 2015;36:2793–867. [DOI] [PubMed] [Google Scholar]
- 12. Landrum MJ, et al. Public archive of interpretations of clinically relevant variants. Nucleic Acids Res 2016;44:D862–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karczewski KJ, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020;581:434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richards S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med 2015;17:405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang L, et al. The electrocardiographic manifestations of arrhythmogenic right ventricular dysplasia. Curr Cardiol Rev 2014;10:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu H, et al. Gain-of-Function mutations in TRPM4 cause autosomal dominant isolated cardiac conduction disease. Circ Cardiovasc Genet 2010;3:374–85. [DOI] [PubMed] [Google Scholar]
- 17. Stallmeyer B, et al. Mutational spectrum in the Ca2+-activated cation channel gene TRPM4 in patients with cardiac conductance disturbances. Hum Mutat 2012;33:109–17. [DOI] [PubMed] [Google Scholar]
- 18. Wilde AAM, et al. European heart rhythm association (EHRA)/heart rhythm society (HRS)/Asia pacific heart rhythm society (APHRS)/latin American heart rhythm society (LAHRS) expert consensus statement on the state of genetic testing for cardiac diseases. Europace 2022;24(8):1307-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moretti A, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med 2010;363:1397–409. [DOI] [PubMed] [Google Scholar]
- 20. Johnson RL, et al. Electrocardiographic findings in 67,375 asymptomatic subjects, VII: atrioventricular block. Am J Cardiol 1960;6:153–77. [DOI] [PubMed] [Google Scholar]
- 21. Rose G, et al. Prevalence and prognosis of electrocardiographic findings in middle-aged men. Br Heart J 1978;40:636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Movahed MR, et al. Increased prevalence of third-degree atrioventricular block in patients with type II diabetes mellitus. Chest 2005;128:2611–4. [DOI] [PubMed] [Google Scholar]
- 23. https://www.eurordis.org/about-rare-diseases. Last access Jul 13, 2021
- 24. Tassetti L, et al. Prevalence of inherited cardiac diseases among young patients requiring permanent pacing. Circ Arrhythm Electrophysiol 2021;14:e010562. [DOI] [PubMed] [Google Scholar]
- 25. Auricchio A, et al. Reducing ventricular pacing frequency in patients with atrioventricular block: is it time to change the current pacing paradigm? Circ Arrhythm Electrophysiol 2016;9:e004404. [DOI] [PubMed] [Google Scholar]
- 26. Resdal Dideriksen J, et al. Long-term outcomes in young patients with atrioventricular block of unknown aetiology. Eur Heart J 2021;42:2060–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.