Abstract
Aims
Bidirectional and durable block of mitral isthmus (MI) is essential for catheter ablation of persistent atrial fibrillation (PeAF) and perimitral flutter (PMF), but it remains a challenge. The aim of this study was to create a simple anatomical ablation strategy with minimal fluoroscopy that would yield a high success rate for MI block.
Methods and results
Patients with PeAF or PMF were included. Mitral isthmus was ablated in a stepwise strategy. In Step 1, endocardial MI linear ablation was performed; in Step 2, ablation was targeted to the posterolateral portion of the left atrium along the MI line; in Step 3, epicardial ablation within the coronary sinus (CS) was performed across the MI line to the ostium of the vein of Marshall (VOM) or performed within the VOM if available; in Step 4, the catheter was rotated and ablated in the CS to isolate the CS; and in Step 5, the early activation site with complex component potential above the MI line during distal CS pacing was considered as the ablation target. All patients were followed up. A total of 178 (17 patients with mechanical prosthetic mitral valve) were included. One hundred and sixty-six patients achieved a confirmed MI bidirectional conduction block (93%). One patient had cardiac tamponade. Four patients showed re-conduction across the MI line during a repeated ablation. In the latest follow-up [12 (7, 16) months], 161 of 178 (90%) patients maintained their sinus rhythm.
Conclusion
A simple stepwise anatomical ablation strategy for MI shows a high success rate with low fluoroscopy exposure.
Keywords: Atrial fibrillation, Catheter ablation, Mitral isthmus, Low fluoroscopy, Vein of Marshall
Graphical Abstract
Graphical Abstract.
What’s new?
A strategy is proposed to achieve a competitive mitral isthmus (MI) block via ethanol infusion of the vein of Marshall (VOM).
This strategy is applicable for patients who need MI ablation with low fluoroscopy exposure and does not involve anatomical variation that might cause failure in the ethanol infusion of the VOM.
As an anatomical ablation strategy, the proposed strategy is reproducible by other cardiac electrophysiologists.
Introduction
Although until now mitral isthmus (MI) has not been the standard strategy for catheter ablation of persistent atrial fibrillation (PeAF) and perimitral flutter (PMF), it is commonly used worldwide, especially in long-standing AF and PMF. The bidirectional and durable block of MI is essential for this strategy to achieve its full potential.1–3 However, MI block represents a technical challenge in such cases. The reported success rate of MI block varies by electrophysiologists in different electrophysiology laboratories, from 51 to 96%.4–7 Epicardial conduction through the vein of Marshall (VOM), great cardiac vein, and coronary sinus (CS) plays an important role in MI block, and the VOM is a source of arrhythmia.8 Ethanol infusion of the VOM is helpful in MI block and has been widely accepted recently. However, ethanol infusion of the VOM might fail in some cases due to variations in VOM anatomy.4,5,9,10 Based on our experience, we found that endocardial plus epicardial ablation within the CS to isolate the CS has the potential to yield a high success rate for MI block. This represents a simple anatomical ablation strategy with minimal fluoroscopy exposure.
Methods
Study population
From January 2019 to December 2021, consecutive patients with PeAF or PMF who underwent endocardial MI linear ablation for the first time were included. Patients who had received a prior MI ablation or surgical ablation procedure were excluded. This study was conducted in accordance with the Declaration of Helsinki (2000) and was approved and supervised by the Ethics Committee of Chongqing General Hospital. All patients were given full explanations of the procedures, and written informed consent was obtained from each patient.
Preoperative preparation and ablation procedure
All procedures were performed under sedation with midazolam and fentanyl. A multipolar electrode catheter was deployed in the CS. Following transseptal puncture, an 8.5-Fr SL-1 sheath (St. Jude Medical, MN, USA) was introduced into the left atrium (LA) via the right femoral vein. Heparin was administered to achieve an activated clotting time of more than 300 s after the transseptal puncture. Three-dimensional electro-anatomic mapping was performed using CARTO3 (Biosense Webster, Diamond Bar, CA, USA). Radiofrequency (RF) was performed using a standard irrigated-tip ablation catheter (Thermocool SmartTouch SF, or ThermoCool SmartTouch Navi-star, Biosense Webster).
The ablation strategies involved pulmonary vein isolation (PVI), left atrial posterior wall isolation, and MI linear ablation. Pulmonary vein isolation and posterior wall isolation were confirmed before MI ablation.
The procedural endpoint was bidirectional conduction block of the PV, left atrial posterior wall, and MI during sinus rhythm.
Stepwise mitral isthmus linear ablation
We performed MI linear ablation using a stepwise strategy (Figure 1).
Figure 1.
Overview of the stepwise strategy for MI ablation from Step 1 to Step 5. Endocardial MI and anterior antrum of LPV ablation in Step 1; posterolateral LA ablation in Step 2; epicardial ablation within CS/VOM in Step 3; CS/VOM isolation in Step 4; breakthrough site ablation in Step 5. CS, coronary sinus; LA, left atrium; LAA, left atrial appendage; LIPV, left inferior pulmonary vein; LPV, left pulmonary vein; LSPV, left superior pulmonary vein; MI, mitral isthmus; VOM, vein of Marshall.
Step 1
We conducted endocardial MI linear ablation. We first ablated the MI line point-by-point using RF at a power of 40 W in power-controlled setting. The ablation index (AI) was 520–550, and the ablation time was 30–40 s when the AI was unavailable, with an average contact force of 10–15 g for each RF application. Initial MI linear ablation was performed at the posterior-lateral line, from the 3 to 4 o’clock position of the mitral annulus (MA) to the left inferior pulmonary vein (LIPV). We treated the anterior antrum of the left PV (LPV) as part of the MI line. Thus, paralleled reinforced RF application was targeted to the anterior antral segment along the LPV. The staged endpoint for Step 1 was no high-frequency and fragmentary near-field potential recorded along the initial ablation line.
For AF, electrical cardioversion (CV) was performed. Preliminary MI block was evaluated during sinus rhythm. In cases where PMF persisted or residual MI conduction was confirmed, further ablation was applied, and Step 2 was initiated. Conduction block of the MI line was defined as conduction propagated along the MA and terminated at the MI line on the opposite site of the pacing site, without crossing the MI line, in both the endocardium and epicardium [e.g. activation from proximal CS to distal CS during left atrial appendage (LAA) pacing] (Figure 2).
Figure 2.
Definition of MI block (bidirectional block on both endocardial and epicardial surface). Pacing comes from the lateral side of the MI line by the multipolar electrode catheter in CS, while recording is done by the ablation catheter on the other site of the MI line in both the endocardium and epicardium. Then, pacing is done by the ablation catheter, and the recording is in the multipolar electrode catheter. Pacing from one side of the MI line. Activation was terminated at the MI line on the opposite site in both the endocardium and epicardium. CS, coronary sinus; LAA, left atrial appendage; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; MI, mitral isthmus.
Step 2
In this step, further RF application was targeted to the posterolateral portion of LA along the MI line, where a large and sharp atrial potential was recorded. Radiofrequency was applied to the area between the MI line and the ostium of the VOM. For instance, if CS 1–2 is deployed exactly at the MI line, then the ablation area for Step 4 will be from CS 3–4 to CS 5–6 and from the mitral valve to the LIPV. To avoid oesophageal injury, the AI was restricted to 400–450, or the ablation time was 20–25 s when AI was unavailable. The staged endpoint was a significant reduction in the local atrial potential.
If a conduction block of the MI line was not achieved or PMF was not yet terminated, Step 3 was initiated.
Step 3
Epicardial ablation within the CS was performed in Step 3. In this stage, we attempted to search for the ostium of the VOM without fluoroscopy. For patients with a large ostium of the VOM, the contact force vector of the tip of the catheter in the 3D electroanatomical system was changed, and the catheter protruded into the VOM during the slight withdrawing of the catheter from the very distal end of the CS to the near-end. The epicardial ablation was performed across the epicardial side of the intersection of the MI line and LIPV to the ostium of the VOM with the contact force vector toward the endocardium. Radiofrequency application was also performed within the VOM from the very distal-end of the VOM if the local impedance was allowed. If the VOM could not be found in the 3D electroanatomical system, we ablated within the CS from the epicardial side of the MI line to the posterior of the LA, with the force vector in the direction of the LIPV, and during this move of the catheter, the slight change of the orientation of the force vector indicated the location of the ostium of the VOM. The ablation power was 25 W, with an irrigation of 30 mL/min, and an AI of 350 or an RF application duration of 20–25 s using a temperature-controlled mode (Figure 3); thus, we could observe a significant decrease of local potential.
Figure 3.
Position of the ablation catheter from the ostium to distal-end of the VOM in Step 3. (A) Fluoroscopy and the 3D electroanatomical system showing an ablation catheter in the ostium of the VOM, and a multipolar electrode catheter in the CS/GCV. (B) Fluoroscopy and the 3D electroanatomical system showing an ablation catheter in the VOM crossed the MI line and located at the epicardial surface of the ridge between LAA and left pulmonary vein, and a multipolar electrode catheter in the CS/GCV. CS, coronary sinus; GCV, great cardiac vein; LAA, left atrial appendage; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; MI, mitral isthmus; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; VOM, vein of Marshall.
Step 4
If all abovementioned steps failed to block the MI line or terminate perimetral flutter, we relocated the ablation catheter into the CS and rotated the catheter to locate high-voltage and sharp potentials. This step was performed to eliminate the residual musculature surrounding the CS and eventually isolate the CS. Similar application settings as those performed in Step 3 were used. Focal RF application was restricted to <10 s, with a significant decrease or disappearance of local potential. At the end of this step, either no near-field potential could be recorded along the CS and VOM, or no atrium capture could be seen using distal CS pacing (Figure 4).
Figure 4.
Position and direction of the ablation catheter for CS isolation in Step 4. The ablation catheter was rotated in the CS to locate and ablate the residual musculature lateral to the MI line in order to isolate the CS. The presentation of different vector directions with near-field potential during catheter rotation was signed as the ablation target in Step 4. CS, coronary sinus; LAA, left atrial appendage; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; MI, mitral isthmus.
Step 5
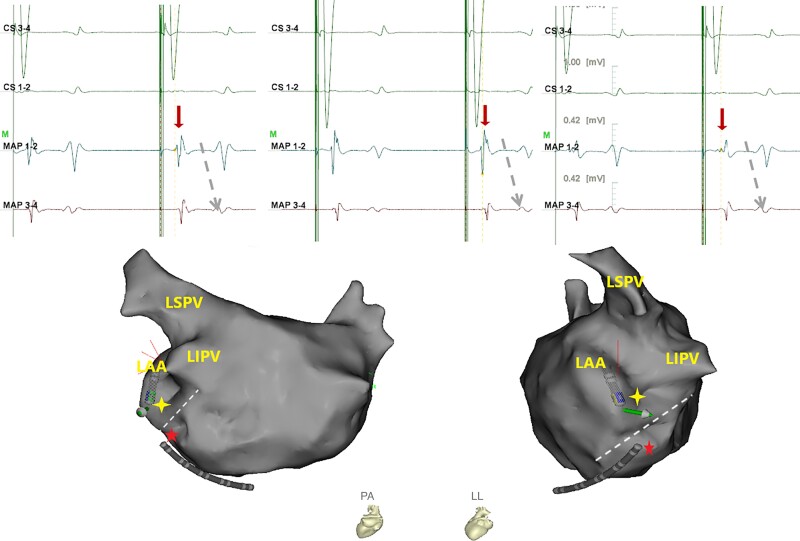
The bidirectional block of the MI line was re-evaluated from every first to fourth step, but we did not specifically perform activation mapping to identify the certain conduction gap until all of the abovementioned steps had failed and the perimetral flutter was terminated by electrical CV. Then, endocardial activation mapping above the MI line was finally performed during distal CS pacing. The early activation site with complex component potential (e.g. fragmental potential or triple-potential) was considered the conduction breakthrough site (Figure 5). Radiofrequency application was delivered as outlined in the first-step ablation. Further RF application was targeted to the epicardial side of the breakthrough site through the CS if available. To avoid steam pop and other procedural complications, endocardial ablation was not performed in the repetitive ablative site. Acute MI block was defined as meeting the criteria of differential pacing after a 30 min waiting period.
Figure 5.
Potentials of the ablation target in Step 5. In comparison with MAP 3–4, MAP 1–2 was located nearer the MI line. During the distal CS pacing, the earlier activation site with complex component potential in MAP 1–2 indicated that MAP 1–2 was on the conduction breakthrough site. MAP, mapping; CS, coronary sinus; LAA, left atrial appendage; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; MI, mitral isthmus.
Follow-up
All patients underwent a systematic follow-up schedule after the procedure. Surface electrocardiogram and 24 h Holter monitoring were recorded after the procedure at an interval of every 3 months or whenever symptoms of palpitation presented. Additionally, telephone interviews were conducted quarterly for all patients.
Statistical analysis
Continuous variables are reported as the mean ± standard deviation (SD) or as the median and interquartile range (IQR) if appropriate and compared using Student’s t-test or analysis of variance test. Categorical variables are expressed as frequencies and percentages and were compared using Fisher’s exact test. All data were processed by SPSS (version 21.0; SPSS Inc., Chicago, IL, USA). A P-value of <0.05 was considered significant.
Results
Finally, 178 patients from 5 centres using the stepwise ablation strategy for MI were included in this study (172 PeAF and 6 PMF). The characteristics of the patients are listed in Table 1. Among the 178 patients, 19 had prior MA surgery, including 17 patients with a mechanical prosthetic valve, 1 with a bioprosthetic valve, and 1 with MA plasty. Five patients had prior AF catheter ablation (PVI only).
Table 1.
Patient characteristics (n = 178)
| Sex ratio (male) | 74 (42%) |
| Age (years) | 68 ± 10 |
| History of AF (months) | 12 (7–42) |
| LA (mm) | 41 ± 7 |
| Prior AF ablation | 5 (3%) |
| Hypertension | 85 (48%) |
| Diabetes | 31 (17%) |
| Stroke or TIA | 14 (8%) |
| CAD (PCI or CABG) | 7 (4%) |
| Mitral valve surgery | 19 (11%) |
| Cardiomyopathy | 12 (7%) |
| CHF | 74 (42%) |
Values are presented as the mean ± SD, n (%), or median (IQR).
AF, atrial fibrillation; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHF, congestive heart failure; LA, left atrium; PCI, percutaneous coronary intervention; TIA, transient ischaemic attack.
Thirty-four patients with PeAF converted to PMF during ablation. Mitral isthmus block was achieved in all patients with PMF. Confirmed MI block was achieved in 166 of 178 (93%) patients. The total time for MI ablation was 11 (IQR: 8–17) min. In six patients, including two patients with prior MA surgery, the MI conduction block could not be assessed because AF was incessant during the procedure (indeterminate MI line block). After excluding these 6 patients, and of 172 patients to be able to evaluate MI block, 166 patients achieved the criteria of bidirectional conduction block (97%) at the end of the procedure, including 14 patients with prior MA surgery (88%). All MI ablation procedures were performed without fluoroscopy, except for one patient with a mechanical prosthetic valve in whom the ablation catheter was unable to protrude across the MI line in the 3D electroanatomical system, even with the help of fluoroscopy. The protocol and result of MI stepwise ablation are listed in Table 2. Patients with prior MA surgery, who had a larger LA (48 ± 7 vs. 41 ± 6 mm; P = 0.001), showed no significant difference in the failure of CV (2/19 vs. 4/159; P = 0.125) or MI block (2/17 vs. 5/155; P = 0.144) compared with those without prior MA surgery.
Table 2.
Index procedure characteristics (n = 178)
| Total MI ablation time (min) | 11 (8–17) |
| Block in Step 1 | 37 (21%) |
| ȃMI ablation time (min) | 7 (5–8) |
| Block in Step 2 | 18 (10%) |
| ȃMI ablation time (min) | 7 (6–12) |
| Block in Step 3 | 71 (40%) |
| ȃMI ablation time (min) | 13 (9–20) |
| Block in Step 4 | 24 (13%) |
| ȃMI ablation time (min) | 17 (14–26) |
| Block in Step 5 (tough MI block) | 16 (9%) |
| ȃMI ablation time (min) | 21 (18–27) |
| Failed MI block | 6 (3%) |
| Indeterminate MI block | 6 (3%) |
Values are presented as n (%) or median (IQR).
MI, mitral isthmus.
Complications
One patient had cardiac tamponade (requiring pericardiocentesis), and one patient had gastroparesis (recovered after 3 months). One patient had femoral haematoma, and one patient had femoral pseudoaneurysm. Three patients had transient hypoxaemia shortly after the bolus administration of midazolam or fentanyl. Forty-one patients who were suspected to suffer from coronary heart disease underwent coronary angiography (CAG) before and after ablation during the procedure, and no coronary artery impairment was observed by CAG.
Follow-up
As sinus rhythm could not be achieved at the end of the procedure, the patients with indeterminate MI block were followed up but unanalysed. Finally, 172 patients were followed up and analysed, with a mean follow-up time of 12 (IQR: 7–16) months (Table 3). Twenty-three patients (13%) had a recurrence of atrial arrhythmia after 3 months of blank time, including 4 patients with AF and 19 patients with atrial tachycardia (AT) or atrial flutter (AFL). Among these patients, two had failed MI block, and six achieved MI block in Step 5, which were defined as tough MI block. Moreover, recurrence was observed in 13% of patients with MI block compared with 33% patients without MI block (P = 0.184). However, patients without MI block and with tough MI block in combination were more likely to have recurrent atrial tachycardia (36 vs. 10%; P = 0.003). The recurrence rates in patients with and without prior MA surgery were 29 and 12%, respectively (P = 0.056). Among the patients who had recurrence, 5 patients with paroxysmal atrial arrhythmia received prolonged oral amiodarone administration for 2 (IQR: 1–2) months, and 13 patients with persistent atrial arrhythmia received CV or re-ablation (Table 3). Seven patients received electrical CV (all presented as persistent AT or AFL), and four of them received a repeat procedure when atrial arrhythmia recurred after CV. Two patients (one with persistent AFL, and another with persistent AF) used intravenous amiodarone for CV, and the patient with AFL received a repeat procedure when AFL recurred after CV. A total of nine patients received a repeat procedure for CV, and all were with AT or AFL. A total of four patients showed re-conduction across the MI line, three of whom were confirmed as PMF. For the patients with MI re-conduction, one patient failed in MI block, and three patients achieved MI block in Step 5 in their prior procedure; all of them achieved MI block in repeat ablation. Four patients had epicardial gaps, including one patient with both epicardial and endocardial gaps. One patient had AT recurrence after a repeat procedure, and received another repeat procedure. All nine patients maintained sinus rhythm in the latest follow-up [the follow-up duration after the latest procedure was 9 (IQR: 6–14) months].
Table 3.
Follow-up (n = 172)
| Follow-up duration (months) | 12 (7–16) |
| Freedom from AA at any time during follow-up | 141 (82%) |
| Freedom from AA after 3 months | 149 (87%) |
| Freedom from AA in the latest follow-up | 161 (94%) |
| Recurrence after 3 months | 23 (13%) |
| ȃAF | 4 (2%) |
| ȃAFL/AT | 19 (11%) |
| ȃRepeat ablation | 9 (5%) |
| ȃCV with electrical CV | 7 (4%) |
| ȃCV with AAD | 2 (1%) |
| Stroke | 2 (1%) |
| Death | 2 (1%) |
| Rehospitalization | 47 (27%) |
Values are presented as n (%) or median (IQR).
AA, atrial arrhythmia; AAD, antiarrhythmic drugs; AF, atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; CV, cardioversion.
Two patients were reported to have suffered sudden death without any premonitory symptoms in the second quarter telephone interview after the MI ablation procedure. One of them, who had chronic obstructive pulmonary disease, heart failure, and hypertension, had AF recurrence and maintenance in the last follow-up before their death. The other patient, who had hypertension, diabetes, and heart failure and who had received concomitant LAA occlusion during the MI ablation procedure, maintained sinus rhythm in the last follow-up before their death.
Two patients had ischaemic stroke. One patient, who had a prior stroke history and received concomitant LAA occlusion during the MI ablation procedure, suffered from a stroke 2 months after the procedure. She interrupted rivaroxaban 4 days before the stroke because of gastrointestinal haemorrhage and had a transient episode of AF 1 day before the stroke. Another case of stroke occurred 1 month after the MI ablation procedure in a patient with hyperthyroid cardiopathy who maintained sinus rhythm after the procedure without cessation of rivaroxaban (20 mg/day).
Overall, at the latest follow-up, 161/178 (90%) patients had achieved sinus rhythm, and 105 of 178 (59%) patients had a decreased LA diameter. From 78 patients with moderate to severe mitral valve regurgitation (MR) at baseline, 64 (82%) patients had MR amelioration.
Discussion
Mitral isthmus is an important linear ablation target for catheter ablation of PeAF and PMF, and it is widely adopted. Although its use is controversial, a durable block after MI ablation is key to the maintenance of sinus rhythm.3,11–13
The most popular MI linear ablation strategy is to identify the conduction gap across the line after the completion of initial linear ablation.7 This strategy is accepted by most electrophysiology laboratories, and it has achieved a high success rate for the majority of cases. However, for some difficult cases, it might fail and reconnection of the residual gap is common, particularly in patients with several conduction gaps and those who require multiple or prolonged ablation. Although transient bidirectional block of MI might occur, these patients are more likely to have recurrence of AFL during follow-up. Meanwhile, a significant difference in the success rate or duration in MI linear ablation using this strategy across different centres and studies is obvious. Only experienced electrophysiologists could achieve a successful result, and the results are rarely replicated.
Ethanol infusion of the VOM exhibited a higher success rate of MI block, from 80 to 100%, and has been widely accepted recently.4,9,14 However, because the VOM could not be identified or cannulated in some cases, ethanol infusion of VOM was completed only in 84–92% patients.4–6,10
The confirmed success rate of MI block in this study was 93%. However, for the six patients who could not be confirmed for MI block because AF was incessant during the procedure, we performed a more aggressive and intensive ablation that included the endocardium and epicardium of the MI line area. Because the failure of CV is more likely to be related to atrial fibrosis than to MI line un-block, it is reasonable to believe that MI block would have a higher success rate. The reasons for the high success rate of MI linear block were considered to be multifaceted. First, in Step 1, we performed solid initial ablation in the endocardial surface to achieve the transmural lesion, especially in the LPV-LAA ridge, which is an area that the VOM transverses and drains into. Then, the MI line conduction gap would most likely be conducted across the isthmus in the epicardium. Therefore, in Step 2, we eliminated the insertion of the conduction gap across the line in the posterolateral portion of LA. In Step 3, we attempted to remove the epicardial conduction directly inside CS, especially through the VOM. In Step 4, we attempted to isolate CS and VOM conduction to avoid epicardial conduction. Additionally, LA inferoposterior line ablation for posterior wall ablation could work in the MI block as there were some connections between the LA and CS in this area. Only 9% patients underwent Step 5 before MI block; thus, identification of the conduction gap was essential only in 9% patients. The residual gap was always easily identified and eliminated and was always only one gap. However, in tough MI ablation cases, the patients with block achieved in Step 5 showed a relatively higher rate of recurrence and MI line reconnection, which may be due to reversible block by oedema for repeat ablation. This indicates that MI block using anatomical ablation strategy might be more useful and more durable than using an electro-anatomic gap mapping strategy. In comparison with our previous work, in which we used the electro-anatomic gap mapping strategy for MI ablation, we experienced an improvement in success rate, efficiency, and repeatability with the use of the present strategy.
This strategy was safe with a low risk of cardiac tamponade (1/178), or impairment of the MA, phrenic nerve, or coronary artery (0/178), even with ablation inside the CS in most cases. In Step 4, we rotated and ablated in several directions beyond the endocardial direction, which may have led to complications, such as tamponade or coronary artery injury. However, the ablation was only applied when high-voltage and sharp potentials were recorded with a low energy and short duration, showing efficacy and safety. Cardiac tamponade occurred several minutes after a steam pop when ablation was performed in CS, and the force and impendence showed an increase when RF was delivered. Thus, avoiding an inappropriate force, impendence, or temperature during ablation is essential for handling this complication. The gastroparesis was most likely caused by LA posterior wall ablation and was self-limited, as reported in prior studies.15
As shown in the follow-up, our ablation strategy for PeAF was beneficial for the maintenance of sinus rhythm, LA size decreasing, and MA regurgitation ease, all of which were also shown in prior studies.16,17
For patients with prior MA surgery, the strategy was also effective for MI block and sinus rhythm maintenance.
For recurrence cases, 19 of 23 (83%) were AFL or AT. Recurrence and AFL/AT recurrence was more commonly observed in patients with MI un-block or tough MI block. In the repeat procedures, MI reconnection was seen only in patients with MI un-block or tough MI block in their prior procedure. Then, ablation from Steps 1 to 4 exhibited a highly effective and acute MI block. However, even for un-block or tough MI block cases, MI block was easily achieved in the repeat procedure.
Limitation
First, MI ablation was performed by five electrophysiologists in this study, and most cases (149/178) were completed by one of them, who guided or supervised MI ablation in the rest of the cases; thus, the efficiency and efficacy of this strategy should be reproduced and testified by more electrophysiologists. Second, half of the patients were followed up for <1 year, and only 24 h monitoring was used. A longer duration of monitoring and follow-up is necessary to evaluate sinus rhythm maintenance and durable MI block.
Conclusions
Our simplified stepwise anatomical ablation strategy for MI showed a high success rate of acute block with low fluoroscopy exposure, as well as a promising outcome in terms of safety and sinus rhythm maintenance.
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript. We thank Dr Chen Guocai (North-Kuanren General Hospital), Dr Caohua (The Ninth People’s Hospital of Chongqing’), Dr Lan Yunjing (Fuling Central Hospital), and Dr Dong Jin (Hanzhong Central Hospital) for their work in performing AF ablation and data collection.
Contributor Information
Xiaoqin Li, Department of Cardiology, Chongqing General Hospital, University of Chinese Academy of Sciences, 118 Xingguang Road, Yubei District, 401120 Chongqing, China.
Mengmeng Li, Department of Cardiology, Peking University Third Hospital, Beijing, China.
Yuan Zhang, Department of Cardiology, Chongqing General Hospital, University of Chinese Academy of Sciences, 118 Xingguang Road, Yubei District, 401120 Chongqing, China.
Hao Zhang, Department of Cardiology, Chongqing General Hospital, University of Chinese Academy of Sciences, 118 Xingguang Road, Yubei District, 401120 Chongqing, China.
Wenli Wu, Department of Cardiology, Chongqing General Hospital, University of Chinese Academy of Sciences, 118 Xingguang Road, Yubei District, 401120 Chongqing, China.
Boli Ran, Department of Cardiology, Chongqing General Hospital, University of Chinese Academy of Sciences, 118 Xingguang Road, Yubei District, 401120 Chongqing, China.
Xiaoli Li, Department of Cardiology, Chongqing General Hospital, University of Chinese Academy of Sciences, 118 Xingguang Road, Yubei District, 401120 Chongqing, China.
Qianmei Tang, Department of Cardiology, Chongqing General Hospital, University of Chinese Academy of Sciences, 118 Xingguang Road, Yubei District, 401120 Chongqing, China.
Biao Fu, Department of Cardiology, Chongqing General Hospital, University of Chinese Academy of Sciences, 118 Xingguang Road, Yubei District, 401120 Chongqing, China.
Funding
None declared.
Data availability
The relevant data in this article will be shared upon reasonable request.
References
- 1. Inoue K, Hikoso S, Masuda M, Furukawa Y, Hirata A, Egami Yet al. Pulmonary vein isolation alone vs. more extensive ablation with defragmentation and linear ablation of persistent atrial fibrillation: the EARNEST-PVI trial. Europace 2021;23:565–74. [DOI] [PubMed] [Google Scholar]
- 2. Sutter JS, Lokhnygina Y, Daubert JP, Bahnson T, Jackson K, Koontz JIet al. Safety and efficacy outcomes of left atrial posterior wall isolation compared to pulmonary vein isolation and pulmonary vein isolation with linear ablation for the treatment of persistent atrial fibrillation. Am Heart J 2020;220:89–96. [DOI] [PubMed] [Google Scholar]
- 3. Wang XH, Kong LC, Li Z, Nie P, Pu J. Mitral isthmus block is associated with favorable outcomes after reablation for long-standing persistent atrial fibrillation. Clin Cardiol 2020;43:1119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valderrábano M, Peterson LE, Swarup V, Schurmann PA, Makkar A, Doshi RNet al. Effect of catheter ablation with vein of Marshall ethanol infusion vs catheter ablation alone on persistent atrial fibrillation: the VENUS randomized clinical trial. JAMA 2020;324:1620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakashima T, Pambrun T, Vlachos K, Goujeau C, André C, Krisai Pet al. Impact of vein of Marshall ethanol infusion on mitral isthmus block: efficacy and durability. Circ Arrhythm Electrophysiol 2020;13:e008884. [DOI] [PubMed] [Google Scholar]
- 6. Ishimura M, Yamamoto M, Himi T, Kobayashi Y. Durability of mitral isthmus ablation with and without ethanol infusion in the vein of Marshall. J Cardiovasc Electrophysiol 2021;32:2116–26. [DOI] [PubMed] [Google Scholar]
- 7. Chen S, Zhou G, Lu X, Wei Y, Xu J, Cai Let al. The importance of identifying conduction breakthrough sites across the mitral isthmus by elaborate mapping for mitral isthmus linear ablation. Europace 2019;21:950–60. [DOI] [PubMed] [Google Scholar]
- 8. Chugh A, Gurm HS, Krishnasamy K, Saeed M, Lohawijarn W, Hornsby Ket al. Spectrum of atrial arrhythmias using the ligament of Marshall in patients with atrial fibrillation. Heart Rhythm 2018;15:17–24. [DOI] [PubMed] [Google Scholar]
- 9. He Z, Yang L, Bai M, Yao Y, Zhang Z. Feasibility, efficacy, and safety of ethanol infusion into the vein of Marshall for atrial fibrillation: a meta-analysis. Pacing Clin Electrophysiol 2021;44:1151–62. [DOI] [PubMed] [Google Scholar]
- 10. Derval N, Duchateau J, Denis A, Ramirez FD, Mahida S, André Cet al. Marshall bundle elimination, pulmonary vein isolation, and line completion for anatomical ablation of persistent atrial fibrillation (Marshall-PLAN): prospective, single-center study. Heart Rhythm 2021;18:529–37. [DOI] [PubMed] [Google Scholar]
- 11. Yamashita S, Tokuda M, Mahida S, Sato H, Ikewaki H, Oseto Het al. Very long term outcome after linear versus electrogram guided ablation for persistent atrial fibrillation. Sci Rep 2021;11:23591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yao Y, Hu F, Du Z, He J, Shi H, Zhang Jet al. The value of extensive catheter linear ablation on persistent atrial fibrillation (the CLEAR-AF Study). Int J Cardiol 2020;316:125–9. [DOI] [PubMed] [Google Scholar]
- 13. Lu X, Peng S, Xu J, Wang R, Pang L, Zhou Get al. Acute conduction recurrence of mitral isthmus: incidence, clinical characteristics, and implications. Pacing Clin Electrophysiol 2020;43:1564–71. [DOI] [PubMed] [Google Scholar]
- 14. Takigawa M, Vlachos K, Martin CA, Bourier F, Denis A, Kitamura Tet al. Acute and mid-term outcome of ethanol infusion of vein of Marshall for the treatment of perimitral flutter. Europace 2020;22:1252–60. [DOI] [PubMed] [Google Scholar]
- 15. Garg L, Garg J, Gupta N, Shah N, Krishnamoorthy P, Palaniswamy Cet al. Gastrointestinal complications associated with catheter ablation for atrial fibrillation. Int J Cardiol 2016;224:424–30. [DOI] [PubMed] [Google Scholar]
- 16. Wu JT, Zhao DQ, Zhang FT, Liu XJ, Hu J, Zhang LMet al. Effect of catheter ablation on clinical outcomes in patients with atrial fibrillation and significant functional mitral regurgitation. BMC Cardiovasc Disord 2021;21:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soulat-Dufour L, Lang S, Addetia K, Ederhy S, Adavane-Scheuble S, Chauvet-Droit Met al. Restoring sinus rhythm reverses cardiac remodeling and reduces valvular regurgitation in patients with atrial fibrillation. J Am Coll Cardiol 2022;79:951–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The relevant data in this article will be shared upon reasonable request.