Abstract
Aims
Overweight is associated with increased risk of atrial fibrillation (AF), but the impact of overweight and AF recurrence after ablation is less clear. Despite this, an increasing number of AF ablations are carried out in overweight patients. We investigated the impact of body mass index (BMI) on AF recurrence rates after ablation.
Methods and results
Through Danish nationwide registers, all patients undergoing first-time AF ablation between 2010 and 2018 were identified. Exposure of interest was BMI. The primary outcome was recurrent AF, defined from either any usage of antiarrhythmic medication, AF hospitalization, cardioversion, or re-ablation. A total of 9188 patients were included. Median age and interquartile range was 64 (60–75) in the normal-weight group and 60 (53–66) in the morbidly obese. There was an increase in comorbidity burden with increasing BMI, including a higher prevalence of heart failure, chronic obstructive pulmonary disease, diabetes, and hypertension. At 1- and 5-year follow ups, recurrence rates of AF increased incrementally by BMI categories. The hazard ratios and 95% confidence intervals of recurrent AF after ablation were 1.15 (1.07–1.23), 1.18 (1.09–1.28), and 1.26 (1.13–1.41) in overweight, obese, and morbidly obese, respectively, compared with normal-weight patients. Procedure duration and X-ray dose exposure also increased with increasing BMI.
Conclusion
Following AF ablation, recurrence rates of AF increased incrementally with increasing BMI. Therefore, aggressive weight management pre ablation in overweight patients could potentially provide substantial benefits and improve short- and long-term outcomes after ablation.
Keywords: Atrial fibrillation, Ablation, Body mass index, Recurrence of atrial fibrillation
Graphical Abstract
Graphical Abstract.
What’s new?
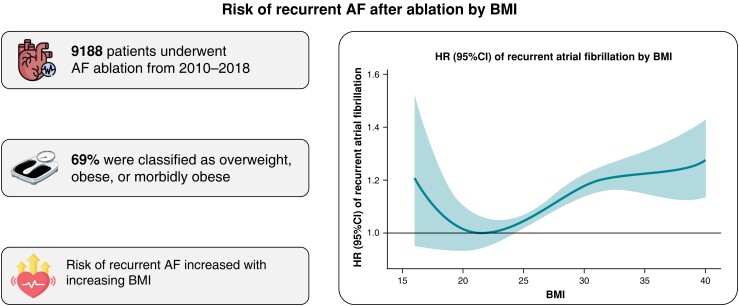
Sixty-nine per cent of atrial fibrillation (AF) ablation patients were classified as overweight, obese, or morbidly obese.
Atrial fibrillation recurrences increase incrementally with increasing body mass index after ablation.
The impact of overweight on AF recurrence after ablation yielded similar risk point estimates as being age 70 years or more, having heart failure, chronic obstructive pulmonary disease, or hypertension.
Introduction
Atrial fibrillation (AF) is a risk factor for serious morbidities such as heart failure (HF), disabling stroke, and death.1–3 Though AF is rarely acutely life-threatening, the distress caused by symptoms can be severe and result in a major reduction in quality of life.4 Identifying the optimal treatment strategy for AF is a challenge for clinicians. Treatment strategies may be influenced by underlying comorbidity, risk factors, and patient preferences as well as the clinical judgement by the treatment responsible physician. The restoration and maintenance of sinus rhythm is one of the cornerstones in the treatment of AF, and catheter ablation has become a viable first-line therapy in an increasing number of patients.5,6 However, the recurrence rates of catheter ablation often vary substantially and influenced by underlying comorbidity and individual risk factors.
The proportion of people with high body mass index (BMI) and severe obesity is increasing and will pose a further significant burden to the healthcare system in the years to come.7 Obesity is known to increase the prevalence of AF. However, studies examining the influence of high BMI on AF ablation outcomes are limited. Furthermore, current knowledge is based on studies with limited study cohorts, and diverging results have been reported.8,9 In general, there is solid evidence that weight loss has a beneficial effect on AF incidence and symptom burden,10 but data are limited when it comes to assessing the impact of BMI and obesity on ablation outcomes. Despite this, an increasing number of AF ablations is being carried out on overweight patients. Therefore, we used the nationwide Danish registries and sought to examine the impact of BMI on AF ablation clinical recurrence.
Methods
Data sources
In Denmark, every citizen is assigned a unique civil registration number at birth. Using this unique identification number, it is possible to crosslink all Danish nationwide registries at an individual level. In this study, four registries were used: the Civil Registration System, the Danish National Patient Register, the Danish National Prescription Register, and the Danish National Ablation Register. The Civil Registration System holds data on age, sex, and vital status of patients where all deaths are registered within 14 days of occurrence. The Danish National Patient Register contains data on hospital admissions, visits to outpatient clinics, and procedures or operations.11 All contacts from 1995 with the hospital system are encoded according to the International Classification of Disease, 10th revision. The Danish National Prescription Register contains information on all filled prescriptions, including drug, quantity, strength, number of packages, and dispensing date, encoded according to the Anatomical Therapeutic Chemical Classification System (ATC codes).12 All patients who have undergone ablation are enrolled in the Danish National Ablation Register, which contains information on height, weight, and procedure data. Registration in the Danish registries is mandatory and does not require patient consent, making the data sets complete with no missing data at follow up.
Study population and body mass index
All patients aged 18 years and above having undergone first-time AF ablation were identified from 1 January 2010 to 31 December 2018, with inclusion in the study at the procedure date. Exclusion criteria were not residing in Denmark, missing data, and outlier values regarding height and weight: height outside 1.30–2.25 m and weight over 180 kg were considered outliers. The patients were divided into five classes based on BMI—underweight: <18.5; normal weight: 18.5–24.9; overweight: 25.0–29.9; obese: 30.0–35.0; morbidly obese: >35. Missing data and outlier values on height and weight were few. Underweight patients were excluded due to small population size. In Denmark, all centres perform AF ablation on morbidly obese patients, limiting this potential bias.
Exposure and outcome variables
Exposure of interest was BMI. The primary outcome was recurrent AF. Recurrent AF was defined as any usage of antiarrhythmic medication, AF hospitalization, cardioversion, or re-ablation at any time after index AF ablation procedure, whichever event came first.13
Procedure data comprised X-ray dose, procedure duration, ablation duration, and X-ray exposure and duration.
Comorbidities and medications
Relevant comorbidities, including HF, ischaemic heart disease, haemorrhagic and ischaemic stroke, chronic obstructive pulmonary disease (COPD), and chronic kidney disease, were identified 5 years prior to ablation. Relevant medications including non-loop diuretics, loop diuretics, beta-blockers, calcium channel inhibitors, renin-angiotensin inhibitors, amiodarone, dronedarone, Class 1C antiarrhythmics, and digoxin were identified 180 days prior to ablation. Diabetes mellitus (DM) was defined from use of diabetes medications, and hypertension (HA) was defined as the usage of at least two antihypertensive medications.
The diagnoses and ATC codes for comorbidities and medication use are listed in Supplementary material online, Table S1.
Statistical analysis
Descriptive baseline tables were used to describe the study population at the AF ablation date with continuous variables reported by the median and interquartile range (IQR) and categorical variables summarized with counts and percentages.
The cumulative incidence of recurrent AF at 1- and 5-year follow ups, excluding the blanking period, was estimated and stratified by BMI using the Aalen–Johansen estimator, taking the competing risk of death into account. The 1-year cumulative incidence of recurrent AF by BMI was further stratified separately by procedure year.
Relative rates for the primary outcome and stratified by procedure year were examined using survival analysis by Cox proportional hazards models, presented as hazard ratios (HRs) with corresponding 95% confidence intervals (95% CIs). The relative rates for the primary outcome were depicted graphically using BMI as a continuous variable and by the five BMI classes. Tests for interaction on sex and age according to the impact of BMI were performed.
Procedure data were reported by median (IQR) and mean with standard deviations (±SDs). Procedure duration over 500 min, ablation duration over 300 min, X-ray duration over 200 min, and X-ray dose over 4000 cGy/m2 were considered significant outliers and excluded. Missing data were few and evenly distributed according to the BMI classes.
Analyses and data processing were performed with R statistics.14
Ethics
Approval for register-based studies is not required from an ethics committee in Denmark. The Danish Data Protection Agency approved the use of registry data; this study is registered with the data responsible institute (approval number: P-2019-404).
Results
Study population
A total of 9760 patients underwent first-time ablation for AF in Denmark from 2010 to 2018, out of which 9188 patients were eligible for the study (see Supplementary material online, Figure S1). About 6328 (69%) were classified as overweight, obese, or morbidly obese. Median age (IQR) decreased from 64 (57–70) in the normal-weight group to 60 (53–66) in the morbidly obese. Median BMI was 27 (24–30) with no major time-related differences. There was an increase in comorbidity burden with increasing BMI, including a higher prevalence of HF, COPD, DM, and HA. The median CHA2DS2-VASc score increased from 1 (0–2) in normal weight to 2 (1, 3) in morbidly obese. The use of amiodarone also increased with BMI, while the use of Class 1C antiarrhythmic medication was stable (Table 1).
Table 1.
Baseline characteristics of the study cohort
| BMI | 18.5–24.9 | 25.0–29.9 | 30.0–35.0 | >35.0 |
|---|---|---|---|---|
| Patients, total n | 2860 | 3814 | 1766 | 748 |
| Age, median (IQR), years | 64 (57–70) | 63 (55–68) | 61 (54–67) | 60 (53–66) |
| Males, n (%) | 1819 (63.6) | 2968 (77.8) | 1272 (72.0) | 430 (57.5) |
| CHA2DS2-VASc, median (IQR) | 1 (0–2) | 1 (0–2) | 2 (1–2) | 2 (1–3) |
| Comorbidities, n (%) | ||||
| HF | 373 (13.0) | 556 (14.6) | 311 (17.6) | 168 (22.5) |
| IHD | 346 (12.1) | 576 (15.1) | 330 (18.7) | 142 (19.0) |
| COPD | 93 (3.3) | 104 (2.7) | 57 (3.2) | 40 (5.3) |
| Ischaemic stroke | 184 (6.4) | 183 (4.8) | 77 (4.4) | 32 (4.3) |
| Haemorrhagic stroke | 10 (0.3) | 9 (0.2) | 4 (0.2) | n < 3 |
| CKD | 36 (1.3) | 55 (1.4) | 32 (1.8) | 20 (2.7) |
| Diabetes | 82 (2.9) | 178 (4.7) | 215 (12.2) | 153 (20.5) |
| Hypertension | 1040 (36.4) | 1811 (47.5) | 1089 (61.7) | 558 (74.6) |
| Medication, n (%) | ||||
| Non-loop diuretics | 415 (14.5) | 832 (21.8) | 541 (30.6) | 319 (42.6) |
| Loop diuretics | 261 (9.1) | 425 (11.1) | 390 (22.1) | 255 (34.1) |
| Beta-blockers | 1930 (67.5) | 2860 (75.0) | 1425 (80.7) | 628 (84.0) |
| Calcium channel inhibitors | 510 (17.8) | 801 (21.0) | 429 (24.3) | 200 (26.7) |
| Renin-angiotensin inhibitors | 841 (29.4) | 1533 (40.2) | 926 (52.4) | 443 (59.2) |
| Amiodarone | 443 (15.5) | 738 (19.3) | 464 (26.3) | 222 (29.7) |
| Dronedarone | 86 (3.0) | 133 (3.5) | 65 (3.7) | 27 (3.6) |
| Class 1C antiarrhythmic | 410 (14.3) | 623 (16.3) | 274 (15.5) | 121 (16.2) |
| Digoxin | 398 (13.9) | 512 (13.4) | 288 (16.3) | 128 (17.1) |
| OAC | 2733 (95.6) | 3692 (96.8) | 1720 (97.4) | 728 (97.3) |
BMI, body mass index; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥ 75, diabetes mellitus, stroke or transient ischaemic attack, vascular disease, age 65–74, sex; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease: HF, heart failure; IHD, ischaemic heart disease; IQR, interquartile range; OAC, oral anticoagulants.
Atrial fibrillation recurrence according to body mass index
One- and 5-year follow-up, blanking period excluded
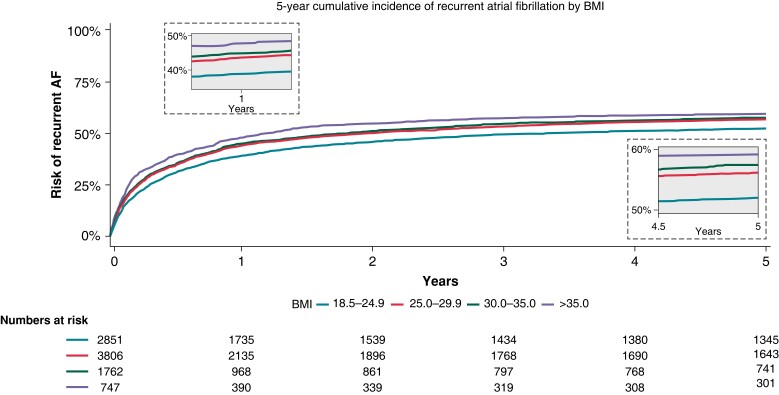
In the 1- and 5-year follow up, the incidence of AF recurrence increased substantially in the high BMI categories. Notably, at 1-year follow up, we observed an incrementally increased risk of AF recurrence by BMI of 39, 44, 45, and 48% for normal weight, overweight, obese, and morbidly obese patients, respectively. At 5-year follow up, the same incrementally increased risk of AF recurrence was found, 52, 56, 57, and 59% for normal weight, overweight, obese, and morbidly obese patients, respectively (Figure 1).
Figure 1.
The cumulative 1- and 5-year recurrent AF incidences after AF ablation by BMI. X-axis depicts time in days. Y-axis depicts the cumulative AF recurrence. AF, atrial fibrillation; BMI, body mass index.
Cox proportional hazard models
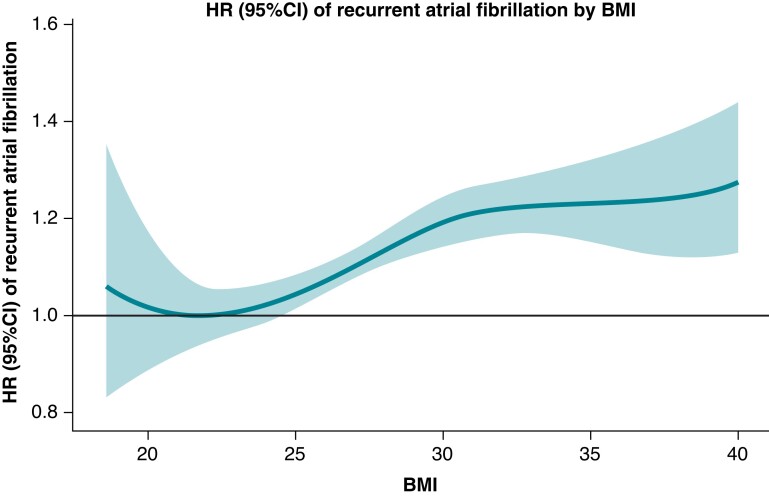
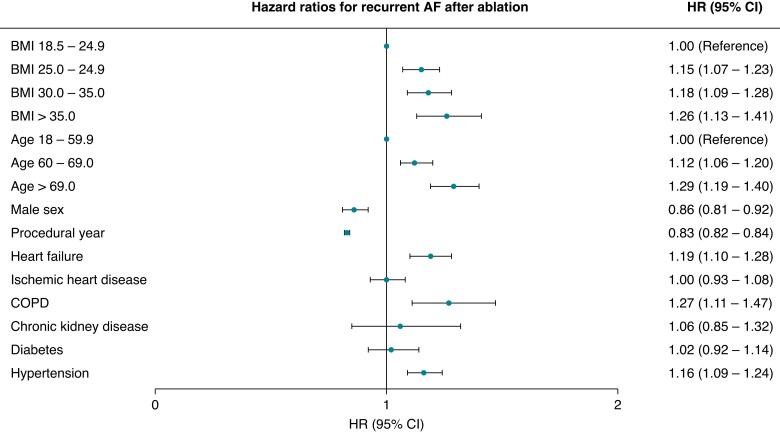
The HRs increased incrementally and significantly by BMI when compared with normal weight; HR (95% CI) 1.15 (1.07–1.23), 1.18 (1.09–1.28), and 1.26 (1.13–1.41) in overweight, obese, and morbidly obese, respectively (Figures 2 and 3). The association and influence of high BMI on AF recurrence yielded similar risk point estimates as being age 70 years or more, having HF, COPD, or HA. No interaction between sex, age, and the impact of BMI on AF recurrence was found.
Figure 2.
Hazard ratios (95% CI) of recurrent AF by BMI. X-axis depicts BMI. Y-axis depicts HR (95% CI) of recurrent AF. AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; HR, hazard ratio.
Figure 3.
Risk factors for recurrent AF after AF ablation. AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio.
Time trends
Temporal trends showed decreasing AF recurrence rates over time consistent in each BMI group. Notably, morbidly obese patients were relatively less prone to recurrent AF in the latter period (Table 2).
Table 2.
One-year cumulative incidences and hazard ratios on the impact of BMI regarding recurrent AF in two time periods
| 2010–14 | 2015–18 | |||||||
|---|---|---|---|---|---|---|---|---|
| Cumulative incidence (%) | HR | 95% CI | P-value | Cumulative incidence (%) | HR | 95% CI | P-value | |
| BMI 18.5–24.9 | 47 | 1 (Reference) | 32 | 1 (Reference) | ||||
| BMI 25.0–29.9 | 52 | 1.16 | (1.04–1.29) | 0.007 | 37 | 1.21 | (1.08–1.35) | 0.001 |
| BMI 30.0–35.0 | 54 | 1.23 | (1.08–1.41) | 0.002 | 38 | 1.20 | (1.05–1.37) | 0.009 |
| BMI > 35.0 | 61 | 1.43 | (1.20–1.71) | <0.001 | 39 | 1.20 | (1.00–1.43) | 0.05 |
AF, atrial fibrillation; BMI, body mass index; ; CI, confidence interval; HR, hazard ratio.
Procedure data according to body mass index
X-ray median (IQR) dosage was 11 (5–26) cGy/m2 in normal weight and 30 (13–66) cGy/m2 in morbidly obese. The median (IQR) ablation and procedure time was 30 (20–38) and 120 (100–154) minutes in patients with normal weight and 32 (24–42) and 128 (100–165) in morbidly obese patients (Table 3).
Table 3.
Procedure data by BMI
| BMI | 18.5–24.9 | 25.0–29.9 | 30.0–35.0 | >35.0 |
|---|---|---|---|---|
| AF ablation data | ||||
| X-ray dose in cGy/m2, median (IQR), mean (SD) | 11 (5–26), 36 (119) | 19 (7–39), 61 (213) | 24 (10–52), 81 (277) | 30 (13–66), 93 (297) |
| Procedure duration in minutes, median (IQR), mean (SD) | 120 (100–154), 129 (53) | 120 (100–160), 132 (51) | 125 (100–163), 135 (52) | 128 (100–165), 137 (55) |
| Ablation duration in minutes, median (IQR), mean (SD) | 30 (20–38), 30 (15) | 31 (21–40), 32 (16) | 32 (22–41), 33 (17) | 32 (24–42), 34 (17) |
| X-ray duration in minutes, median (IQR), mean (±SD) | 11 (7–17), 13 (10) | 11 (7–17), 14 (10) | 12 (7–18), 14 (9) | 12 (8–19), 14 (10) |
AF, atrial fibrillation; BMI, body mass index; IQR, interquartile range; SD, standard deviation.
First-reached endpoints
Table 4 depicts the distribution of the first-reached individual components of the composite endpoint of recurrent AF at 1- and 5-year follow ups. Hospital admissions for AF, including DC conversion, were the most frequent component. No difference was found between the distribution of the components of the composite endpoint across the two follow-up periods.
Table 4.
First-reached endpoint at 1- and 5-year follow ups
| BMI | Total |
|---|---|
| 1 year | |
| Amiodarone/dronedarone, n (%) | 738 (8) |
| Flecainid/propafenone, n (%) | 395 (4) |
| AF hospitalization, n (%) | 1903 (21) |
| DC conversion, n (%) | 634 (7) |
| AF re-ablation, n (%) | 251 (3) |
| 5 year | |
| Amiodarone/dronedarone, n (%) | 771 (8) |
| Flecainid/propafenone, n (%) | 418 (5) |
| AF hospitalization, n (%) | 2715 (30) |
| DC conversion, n (%) | 864 (9) |
| AF re-ablation, n (%) | 325 (4) |
AF, atrial fibrillation; BMI, body mass index.
Supplementary material online, Table S2 showed the endpoints divided by BMI, demonstrating similar trends according to BMI, though the use of amiodarone increased by BMI.
Discussion
In this nationwide study on AF patients undergoing first-time ablation, we uncovered several novel findings: (i) Almost 70% of patients undergoing ablation were either overweight, obese, or morbidly obese. (ii) Risk of AF recurrence increased incrementally with increasing BMI, both in short- and long-term follow ups, and both in adjusted and unadjusted models. The impact of BMI over 25 on recurrence risk was comparable with that of important comorbidities such as HF, COPD, HA, and age over 70 years. (iii) Finally, we found increasing procedure duration and radiation dose with increasing BMI.
The prevalence of patients with overweight or obesity and AF is rapidly increasing and is an ongoing health burden with millions of cases globally. In our national cohort, most patients were overweight or obese, emphasizing the need to improve treatment quality in overweight patients. It has been well-established that the incidence of AF increases according to BMI but the link between BMI and recurrent AF after AF ablation is less clear.
No randomized clinical trials have examined the impact of BMI on AF patients undergoing ablation. The studies examining recurrent AF by BMI after AF ablation are observational with smaller population sizes. Diverging results have been shown, ranging from no association15,16 to supporting our results, that the risk of recurrent AF increases by BMI.17–19 The follow-up methodology varied, but none of the studies used loop recorders. Instead, recurrent AF was defined from symptomatic recurrent AF or AF found in Holter monitoring with 1 day of monitoring at various time marks being most prevalent.
The randomized clinical trials currently available only examine the effect of weight loss after ablation and they are few and present conflicting results: A study where 149 of 281 overweight patients having undergone AF ablation were offered risk factor management. This led to significant weight loss and drop in blood pressure compared with the control group. At 6 months of follow up, the intervention group displayed lesser AF frequency, duration, and symptoms than the control group, emphasizing the importance of weight loss.20 The recently published SORT-AF trial included 133 patients having undergone AF ablation with BMI between 30 and 40. Patients were randomized for weight loss (n = 67) or usual care (n = 66). Though the weight loss was significant, there was no significant difference in AF burden after ablation.21 These conflicting results highlight the need for further studies.
The underlying mechanism between AF and overweight is multifactorial. Overweight is independently associated with left atrium enlargement, probably secondary to increased blood pressure.22 Further, epicardial adipose tissue (EAT) plays a significant role. It has been demonstrated that EAT is increased in overweight patients and that slow voltage and conduction slowing in the areas of EAT is present, suggesting a role in AF substrate development.23 Further, EAT induced left atrial inflammation induce atrial fibrosis, further increasing the risk of AF.24 Why weight loss leads to less AF is presently not fully understood. A recent study examined the effect of weight loss in sheep, finding that weight loss led to less inflammation, less atrial fibrosis, and improved conduction velocity compared with baseline, ultimately leading to less AF.25
Our findings that morbidly obese patients were less prone to recurrent AF over time is, to our knowledge, unique and yet to be described in other investigations. The overall temporal improvement of AF ablation has previously been established in a study using Danish registries, which used the same combined endpoint as us to detect recurrent AF13 and in two worldwide surveys.26,27
Reviewing our procedure data, we mainly found a correlation between BMI and radiation dose, secondary between BMI and procedure time. Generally, previous studies on this area are sparse. Radiation dose and procedure time are the two most investigated factors, and studies indicate a correlation between these factors and BMI.28–30
Strengths and limitations
The large study sample and nationwide unselected sample of patients are the main strengths of this study and minimize the risk of selection bias. Residual confounding is present, we did not have access to potential confounders such as paroxysmal/persistent AF and sleep apnoea. Our definition of recurrent AF may lead to less recurrent AF because asymptomatic recurrent AF may not lead to hospital admission or the start of antiarrhythmic medicine. Importantly though, previous studies did not use loop recorders. Further, information on AAD indication is not available to us, potentially overestimating AF recurrence but our AF recurrence rates are comparable with previous studies. The Danish National Ablation Register was initiated in 2010, but not all ablation centres were included until 2011, this is not believed to have any impact on our results.
Conclusions
In this nationwide study examining recurrent AF post-AF ablation, we found that recurrence rates of AF increased incrementally according to BMI, both in short- and long-term follow ups. Notably, high BMI had similar impact as other known comorbidities such as HF, COPD, HA, and age over 70 years on the risk AF recurrences after ablation. Aggressive pre-ablation weight management in overweight patients might be a target to improve short- and long-term outcomes after ablation.
Supplementary Material
Contributor Information
Jacob Tønnesen, Department of Cardiology, Herlev-Gentofte University Hospital, University of Copenhagen, Hellerup, Denmark.
Jannik Pallisgaard, Department of Cardiology, Herlev-Gentofte University Hospital, University of Copenhagen, Hellerup, Denmark.
Martin H Ruwald, Department of Cardiology, Herlev-Gentofte University Hospital, University of Copenhagen, Hellerup, Denmark.
Peter Vibe Rasmussen, Department of Cardiology, Herlev-Gentofte University Hospital, University of Copenhagen, Hellerup, Denmark.
Arne Johannessen, Department of Cardiology, Herlev-Gentofte University Hospital, University of Copenhagen, Hellerup, Denmark.
Jim Hansen, Department of Cardiology, Herlev-Gentofte University Hospital, University of Copenhagen, Hellerup, Denmark.
Rene Husted Worck, Department of Cardiology, Herlev-Gentofte University Hospital, University of Copenhagen, Hellerup, Denmark.
Christopher R Zörner, Department of Cardiology, Herlev-Gentofte University Hospital, University of Copenhagen, Hellerup, Denmark.
Lise Riis-Vestergaard, Department of Cardiology, Herlev-Gentofte University Hospital, University of Copenhagen, Hellerup, Denmark.
Charlotte Middelfart, Department of Cardiology, Herlev-Gentofte University Hospital, University of Copenhagen, Hellerup, Denmark.
Gunnar Gislason, Department of Cardiology, Herlev-Gentofte University Hospital, University of Copenhagen, Hellerup, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; The Danish Heart Foundation, Copenhagen, Denmark; The National Institute of Public Health, University of Southern Denmark, Copenhagen, Denmark.
Morten Lock Hansen, Department of Cardiology, Herlev-Gentofte University Hospital, University of Copenhagen, Hellerup, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Supplementary material
Supplementary material is available at Europace online.
Authors’ contribution
M.L.H. and J.T. conceived the study idea. J.T., M.L.H., M.H.R., P.V.R., and J.P. designed the research. J.T. performed the statistical analysis and wrote the manuscript. J.T., P.V.R., and J.P. contributed to the programming. All authors critically revised and approved the manuscript.
Funding
This project was supported by Novo Nordisk (Denmark). The funders had no role in the design, analysis, write-up, or decision to submit for publication.
Data availability
The data are stored at Statistics Denmark and research environments can apply for access.
References
- 1. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SAet al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation 2016;133:484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of left ventricular dysfunction. J Am Coll Cardiol 1998;32:695–703. [DOI] [PubMed] [Google Scholar]
- 3. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PAet al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920–5. [DOI] [PubMed] [Google Scholar]
- 4. Thrall G, Lane D, Carroll D, Lip GYH. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med 2006;119:448.e1–19. [DOI] [PubMed] [Google Scholar]
- 5. Hakalahti A, Biancari F, Nielsen JC, Raatikainen MJP. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis. Europace 2015;17:370–8. [DOI] [PubMed] [Google Scholar]
- 6. Wazni OM, Dandamudi G, Sood N, Hoyt R, Tyler J, Durrani Set al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med 2021;384:316–24. [DOI] [PubMed] [Google Scholar]
- 7. Williamson K, Nimegeer A, Lean M. Rising prevalence of BMI ≥40 kg/m2: a high-demand epidemic needing better documentation. Obes Rev 2020;21:e12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guijian L, Jinchuan Y, Rongzeng D, Jun Q, Jun W, Wenqing Z. Impact of body mass index on atrial fibrillation recurrence: a meta-analysis of observational studies. Pacing Clin Electrophysiol 2013;36:748–56. [DOI] [PubMed] [Google Scholar]
- 9. Zhuang J, Lu Y, Tang K, Peng W, Xu Y. Influence of body mass index on recurrence and quality of life in atrial fibrillation patients after catheter ablation: a meta-analysis and systematic review. Clin Cardiol 2013;36:269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CXet al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol 2015;65:2159–69. [DOI] [PubMed] [Google Scholar]
- 11. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health 2011;39:38–41. [DOI] [PubMed] [Google Scholar]
- 13. Pallisgaard JL, Gislason GH, Hansen J, Johannessen A, Torp-Pedersen C, Rasmussen PVet al. Temporal trends in atrial fibrillation recurrence rates after ablation between 2005 and 2014: a nationwide Danish cohort study. Eur Heart J 2018;39:442–9. [DOI] [PubMed] [Google Scholar]
- 14. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. http://www.R-project.org/. [Google Scholar]
- 15. Cha YM, Friedman PA, Asirvatham SJ, Shen WK, Munger TM, Rea RFet al. Catheter ablation for atrial fibrillation in patients with obesity. Circulation 2008;117:2583–90. [DOI] [PubMed] [Google Scholar]
- 16. Mohanty S, Mohanty P, Di Biase L, Bai R, Dixon A, Burkhardt Det al. Influence of body mass index on quality of life in atrial fibrillation patients undergoing catheter ablation. Heart Rhythm 2011;8:1847–52. [DOI] [PubMed] [Google Scholar]
- 17. Providência R, Adragão P, de Asmundis C, Chun J, Chierchia G, Defaye Pet al. Impact of body mass index on the outcomes of catheter ablation of atrial fibrillation: a European Observational Multicenter Study. J Am Heart Assoc 2019;8:e012253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Winkle RA, Mead RH, Engel G, Kong MH, Fleming W, Salcedo Jet al. Impact of obesity on atrial fibrillation ablation: patient characteristics, long-term outcomes, and complications. Heart Rhythm 2017;14:819–27. [DOI] [PubMed] [Google Scholar]
- 19. Sivasambu B, Balouch MA, Zghaib T, Bajwa RJ, Chrispin J, Berger RDet al. Increased rates of atrial fibrillation recurrence following pulmonary vein isolation in overweight and obese patients. J Cardiovasc Electrophysiol 2018;29:239–45. [DOI] [PubMed] [Google Scholar]
- 20. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey Det al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64:2222–31. [DOI] [PubMed] [Google Scholar]
- 21. Gessler N, Willems S, Steven D, Aberle J, Akbulak RO, Gosau Net al. Supervised Obesity Reduction Trial for AF ablation patients: results from the SORT-AF trial. Europace 2021;23:1548–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stritzke J, Markus MRP, Duderstadt S, Lieb W, Luchner A, Döring Aet al. The aging process of the heart: obesity is the main risk factor for left atrial enlargement during aging: the MONICA/KORA (monitoring of trends and determinations in cardiovascular disease/cooperative research in the region of Augsburg) study. J Am Coll Cardiol 2009;54:1982–9. [DOI] [PubMed] [Google Scholar]
- 23. Mahajan R, Nelson A, Pathak RK, Middeldorp ME, Wong CX, Twomey DJet al. Electroanatomical remodeling of the atria in obesity: impact of adjacent epicardial fat. JACC Clin Electrophysiol 2018;4:1529–40. [DOI] [PubMed] [Google Scholar]
- 24. Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol 2018;71:2360–72. [DOI] [PubMed] [Google Scholar]
- 25. Mahajan R, Lau DH, Brooks AG, Shipp NJ, Wood JPM, Manavis Jet al. Atrial fibrillation and obesity: reverse remodeling of atrial substrate with weight reduction. JACC Clin Electrophysiol 2021;7:630–41. [DOI] [PubMed] [Google Scholar]
- 26. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman Jet al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005;111:1100–5. [DOI] [PubMed] [Google Scholar]
- 27. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman Jet al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:32–8. [DOI] [PubMed] [Google Scholar]
- 28. Ector J, Dragusin O, Adriaenssens B, Huybrechts W, Willems R, Ector Het al. Obesity is a major determinant of radiation dose in patients undergoing pulmonary vein isolation for atrial fibrillation. J Am Coll Cardiol 2007;50:234–42. [DOI] [PubMed] [Google Scholar]
- 29. Glover BM, Hong KL, Dagres N, Arbelo E, Laroche C, Riahi Set al. Impact of body mass index on the outcome of catheter ablation of atrial fibrillation. Heart 2019;105:244–50. [DOI] [PubMed] [Google Scholar]
- 30. Letsas KP, Siklódy CH, Korantzopoulos P, Weber R, Bürkle G, Mihas CCet al. The impact of body mass index on the efficacy and safety of catheter ablation of atrial fibrillation. Int J Cardiol 2013;164:94–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are stored at Statistics Denmark and research environments can apply for access.