Abstract
Aims
Atrial fibrillation (AF) is a global health problem with high morbidity and mortality. Catheter ablation (CA) can reduce AF burden and symptoms, but AF recurrence (AFr) remains an issue. Simple AFr predictors like P-wave duration (PWD) could help improve AF therapy. This updated meta-analysis reviews the increasing evidence for the association of AFr with PWD and offers practical implications.
Methods and results
Publication databases were systematically searched and cohort studies reporting PWD and/or morphology at baseline and AFr after CA were included. Advanced interatrial block (aIAB) was defined as PWD ≥ 120 ms and biphasic morphology in inferior leads. Random-effects analysis was performed using the Review Manager 5.3 and R programs after study selection, quality assessment, and data extraction, to report odds ratio (OR) and confidence intervals. : Among 4175 patients in 22 studies, 1138 (27%) experienced AFr. Patients with AFr had longer PWD with a mean pooled difference of 7.8 ms (19 studies, P < 0.001). Pooled OR was 2.04 (1.16–3.58) for PWD > 120 ms (13 studies, P = 0.01), 2.42 (1.12–5.21) for PWD > 140 ms (2 studies, P = 0.02), 3.97 (1.79–8.85) for aIAB (5 studies, P < 0.001), and 10.89 (4.53–26.15) for PWD > 150 ms (4 studies, P < 0.001). There was significant heterogeneity but no publication bias detected.
Conclusion
P-wave duration is an independent predictor for AF recurrence after left atrium ablation. The AFr risk is increasing exponentially with PWD prolongation. This could facilitate risk stratification by identifying high-risk patients (aIAB, PWD > 150 ms) and adjusting follow up or interventions.
Keywords: Atrial fibrillation, Ablation, P-wave, Interatrial block/conduction, Recurrence
Graphical Abstract
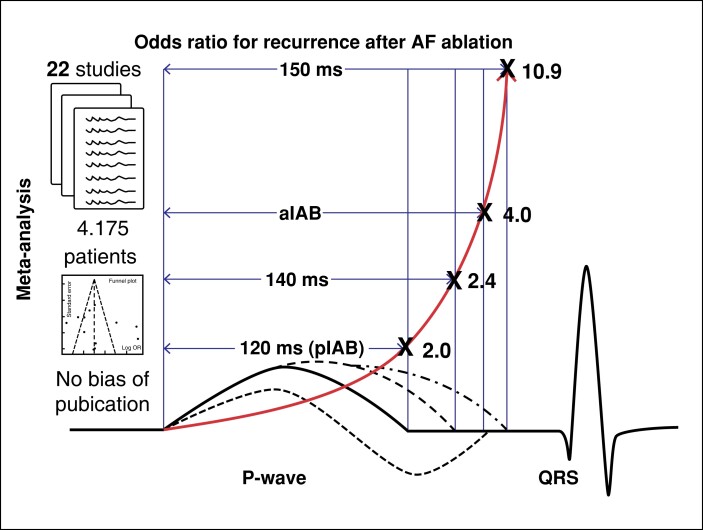
Graphical Abstract.
P-wave duration (PWD) is an independent predictor for recurrence after atrial fibrillation (AF) ablation. The risk is increasing exponentially with PWD prolongation and the presence of partial (pIAB) or advanced (aIAB) interatrial block. Thus, PW could identify high-risk patients (aIAB, PWD > 150 ms) in order to adjust follow up or interventions.
What’s new?
The risk for atrial fibrillation recurrence (AFr) after catheter ablation is increasing exponentially with longer P-wave duration (PWD) at baseline.
The odds ratio for AFr risk is 2 at 120 ms, 2.4 at 140 ms, 4 for advanced interatrial block (IAB; PWD > 120 ms, biphasic morphology in inferior leads) and 10.9 at 150 ms.
Advanced IAB has two times and PWD > 150 mg five times higher AFr risk than partial IAB.
Thus, PWD could facilitate risk stratification and adjustment of follow up or interventions.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia affecting 2% of the population with >34 million patients around the world and a prevalence that will double by 2050. Atrial fibrillation is associated with increased morbidity and mortality and a significant financial burden for the social security systems worldwide.
Catheter ablation (CA) has been established as an effective therapy reducing AF burden and symptoms. However, recurrence during follow up remains a major concern making patient selection for first or repeat ablations a very important task. Therefore, simple predictors of AF recurrence (AFr) could facilitate ablation strategy, closer follow up or prophylactic interventions and guidance of anticoagulation in such patients.
Several recent studies have shown an association between P-wave duration (PWD) and AFr after ablation. Both 12-lead and signal-averaged electrocardiogram (SAECG) has been evaluated in different populations evaluating duration or morphology of the P-waves. However, most studies are single-centre reports with limited sample sizes resulting in different PWD cut-offs and compromising its true predictive value. Moreover, several studies showed no difference in PWD for patients with AFr, reported a non-significant predictive value or failed to detect the effect size for different cut-offs.
This updated meta-analysis reviews the increasing evidence of all available studies that reported PWD prior to CA and its association with AFr during follow up in order to provide practical clinical implications.
Methods
This study was reported in adherence to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statements. We searched PubMed/Medline, Embase, and ClinicalTrials.gov databases through Cochrane Library without language restriction from database inception to January 2021. The following keywords were used as search terms: ‘P wave’, ‘P waves’, ‘interatrial block’, ‘interatrial conduction’, ‘AF recurrences’, ‘atrial fibrillation’, and ‘AF’ with filters ‘Clinical Trial’ and ‘Randomized Controlled Trial’. References of included articles were manually searched to identify additional eligible studies. No language restrictions were applied (see Supplementary material online, Table S1).
All studies were screened by three authors according to the following inclusion criteria: (i) studies including adult AF patients, (ii) PWD was measured prior to ablation, (iii) AFr after ablation was reported as an endpoint, and (iv) PWD was used as a variable to predict AFr. Case reports, reviews, letters, and editorials were excluded. The primary endpoint was AFr during follow up. Atrial fibrillation was defined as paroxysmal or persistent according to the current guidelines. Prolonged PWD > 120 ms and prolonged biphasic P-waves (in inferior leads) were defined as partial (pIAB) and advanced interatrial block (aIAB), respectively.1
Two authors independently extracted data and summarized them in a data extraction file. Any disagreement was resolved by consensus or by consulting a third author. The missing data of eligible studies were calculated by the reported continuous PWD values or by contacting the original authors. The studies selected in our meta-analysis were evaluated for methodological quality using the Newcastle–Ottawa scale (0–9 points) based on selection, comparability, and outcome.
Statistics
Data for continuous variables were pooled to calculate a weighted mean difference (WMD) and 95% confidence interval (CI). The WMD of PWD between patients with and without AFr was computed and compared. The pooled odds ratio (OR) and 95% CI of PWD per cut-off value or according to the presence of partial or advanced IAB were calculated to evaluate their prognostic value for the primary endpoint. Furthermore, forest plots were constructed to display overall effects using a random-effects model. Heterogeneity was assessed using Higgins I2 statistics, with values of 25, 50, and 75% representing low, moderate, and high heterogeneity, respectively. Sensitivity analysis was performed to evaluate the effect modification according to method (ECG or SAECG) and AF type as well as to exclude the effect of publication bias (based on Funnel plot) on the overall pooled estimates. Additionally, Egger’s and Copas tests were applied to evaluate the presence of publication bias. Review Manager 5.3 (Cochrane Collaboration, Oxford, UK), R 3.5.3 (open source), and Stata 16.0 (Stata Corp, TX, USA) were used for the analysis. A P-value <0.05 was considered statistically significant.
Results
From the initial 351 studies screened and retrieved according to the search strategy, 100 were removed as duplicates. Potentially eligible studies (n = 37) were identified after screening titles and abstracts and 15 were excluded following full-text review for not meeting the inclusion criteria. Consequently, a total of 22 studies including 4.175 AF patients were included in the final analysis (Figure 1).2–23 Quality assessment using the Newcastle–Ottawa scale showed high scores (≥7 points) in the majority of the studies enrolled in our meta-analysis (see Supplementary material online, Table S2).
Figure 1.
Flow chart of the study selection process according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines. AFr, atrial fibrillation recurrence; PWD, P-wave duration.
Baseline characteristics
All 22 included studies were single-centre cohort studies. There were 10 studies with exclusively paroxysmal and 1 with persistent AF patients. Most studies (n = 15) used ECG and 6 used SAECG for PWD measurement (Table 1).
Table 1.
Baseline characteristics of the included cohort studies
| Study | Year | Country | n | Method | PWD cut-off | Age, years | PAF, % | Male, % | HTN, % | EF, % | LAD, mm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ogawa et al. 2 | 2007 | JP | 27 | SAECG | N/A | 55± 13 | 93 | 78 | − | 65± 6 | – |
| Okumura et al. 3 | 2007 | JP | 51 | SAECG | 150 | 61± 11 | 80 | 76 | − | 68± 10 | 36± 5 |
| Masuda et al. 4 | 2012 | JP | 88 | SAECG | 130, RS20 | 64± 11 | 100 | 66 | 67 | 65± 6 | 36± 5 |
| Blanche et al. 5 | 2012 | CH | 102 | SAECG | 140 | 59± 10 | 60 | 81 | 38 | 62± 9 | 42± 5 |
| Caldwell et al. 6 | 2013 | UK | 100 | ECG | 120, 140, 150, aIAB | 58± 11 | 100 | 72 | − | 60± 5 | 41± 6 |
| Salah et al. 7 | 2013 | CN | 198 | ECG | 125 | 57± 8 | 100 | 76 | 43 | 64± 8 | 43± 5 |
| Park et al. 8 | 2015 | KR | 525 | ECG | 120 | 56± 12 | 100 | 76 | 46 | 62± 9 | – |
| Wu et al. 9 | 2015 | CN | 204 | ECG | 120, aIAB | 59± 10 | 100 | 55 | 47 | – | 39± 6 |
| Knecht et al. 10 | 2016 | CH | 129 | ECG | 120 | 61± 8 | 65 | 79 | 57 | 67± 4 | – |
| Arroja et al. 11 | 2016 | CH | 45 | SAECG | 120 | 59± 10 | 100 | 87 | − | 66± 5 | 39± 4 |
| Kanzaki et al. 12 | 2016 | JP | 79 | SAECG | 120 | 64± 9 | 100 | 71 | 52 | 62± 9 | 47± 5 |
| Gul et al. 13 | 2016 | CA | 62 | ECG | 120, aIAB | 58± 11 | 100 | 76 | 15 | – | 41± 6 |
| Mugnai et al. 14 | 2016 | BE | 201 | ECG | 120 | 56± 11 | 100 | 72 | 31 | 54± 10 | – |
| Doi et al. 15 | 2018 | JP | 205 | ECG | 120, 150 | 64± 11 | 53 | 76 | 55 | 53± 6 | – |
| Jadidi et al. 16 | 2018 | DE | 143 | ECG | 150 | 65± 10 | 0 | 36 | 32 | – | 44± 6 |
| Kaypakli et al. 17 | 2018 | TR | 114 | ECG | 120 | 61± 7 | 100 | 12 | 16 | 51± 8 | – |
| Higuchi et al. 18 | 2018 | JP | 113 | ECG | 126 | 58± 11 | 0 | 88 | 41 | 61± 9 | 43± 6 |
| Wu et al. 19 | 2018 | CN | 329 | ECG | 120, aIAB | 69± 4 | 100 | 54 | 32 | 64± 9 | 39± 5 |
| Nakatani et al. 20 | 2019 | JP | 201 | ECG | 120 | 64± 11 | 100 | 73 | 47 | 60± 9 | 39± 6 |
| Chen et al. 21 | 2019 | CN | 411 | ECG | 120 | 63± 10 | 100 | 58 | 55 | – | 40± 6 |
| Zink et al. 22 | 2020 | DE | 678 | ECG | 120 | 63± 9 | 53 | 58 | 78 | 66± 7 | – |
| Yang et al. 23 | 2020 | CN | 207 | ECG | 120, 140, aIAB | 59± 11 | 48 | 34 | 51 | 65± 6 | 38± 5 |
| Total | 4175 | 61± 10 | 72 | 62 | 45 | 62± 8 | 40± 5 |
aIAB, advanced interatrial block; EF, ejection fraction; HTN, hypertension; LAD, left atrial diameter; PAF, paroxysmal atrial fibrillation; PWD, P-wave duration; SAECG, signal-averaged electrocardiogram.
Included patients (n = 4175) had a mean age of 61 ± 10 years with a normal left ventricular function (LVEF 62 ± 8%) and a left atrium of 40 ± 5 mm. There were 62% males and 72% paroxysmal AF (PAF) patients. Among the 16 studies that reported comorbidities, the most common diseases were hypertension (45%) followed by diabetes and ischaemic heart disease. The mean baseline PWD among reported studies was 126 ms. During a mean follow-up time of 16 ± 9 months (ranging from 3 to 50), 1138 patients (27%) experienced an AFr after CA (Table 2).
Table 2.
Outcome characteristics of the included cohort studies
| Study | FUP | AFr, % | PWD AFr(+) | PWD AFr(−) | Outcome predictors of AFr | Sen. | Spec. | NPV | PPV |
|---|---|---|---|---|---|---|---|---|---|
| Ogawa et al. 2 | 16 ± 4 | 26 | 168± 10 | 161± 7 | PWD shortening | ||||
| Okumura et al. 3 | 3 | 29 | 167± 15 | 146± 13 | PWD HR: 10.3, P = 0.03 | 93 | 72 | 58 | 96 |
| Masuda et al. 4 | 16 ± 4 | 42 | 163± 21 | 158± 18 | Terminal part of filtered PW (atrial late potential) OR: 4.22, P = 0.006 | 54 | 91 | 73 | |
| Blanche et al. 5 | 12 ± 7 | 35 | 158 + 34 | 140 + 18 | PWD (no results for >/<140 ms), AUC 70%, P < 0.01 | 69 | 53 | 45 | 76 |
| Caldwell et al. 6 | 32 ± 14 | 47 | 139± 17 | 129± 14 | Max. PWD (OR: 2.4, P = 0.02), PW dispersion, LA size | 69 | 53 | 45 | 76 |
| Salah et al. 7 | 9 ± 3 | 30 | 123± 10 | 104± 14 | PWD ≥ 125 ms | 60 | 90 | 84 | 72 |
| Park et al. 8 | 21 ± 10 | 16 | 112± 18 | 112± 22 | PW amplitude (Lead I) < 0.1 mV (HR 2.16) and PAF, AUC 71% | ||||
| Wu et al. 9 | 14 ± 6 | 30 | 121± 14 | 117± 14 | PWD > 120(HR: 2.1,P = 0.04),LAD (HR: 1.05, P = 0.034) | 66 | 80 | ||
| Knecht et al. 10 | 12 | 35 | 128± 19 | 120± 16 | AF burden (HR: 2.02, P < 0.001), PWD (2.6, P = 0.01) adj. for AF type/LAD | ||||
| Arroja et al. 11 | 12 ± 5 | 49 | 142± 21 | 139± 17 | Neither SA-PWD nor EP measurements | ||||
| Kanzaki et al. 12 | 10 | 14 | 136± 13 | 128± 15 | P-wave force > 9.3 mV*ms (P < 0.001) | ||||
| Gul et al. 13 | 50 ± 22 | 52 | – | – | aIAB (OR: 3.34, P = 0.03). | ||||
| Mugnai et al. 14 | 22 ± 16 | 31 | 129± 13 | 119± 11 | PWD (HR: 1.05, P < 0.001)/dispersion (HR: 1.05, P < 0.001) adj. for LA/age | 78 | 63 | 49 | 86 |
| Doi et al. 15 | 12 | 24 | 137± 16 | 130± 13 | Terminal PW force V1 > 56.7 mV*ms (PWD*amplitude, AUC 80%, P < 0.01) | 75 | 76 | ||
| Jadidi et al. 16 | 12 | 22 | – | – | PWD > 150 ms (OR: 3.75, P = 0.0002) | 67 | 65 | ||
| Kaypakli et al. 17 | 12 | 21 | 94± 11 | 87± 11 | PWD indexed for PR in II (AUC 76%, OR: 1.143, P = 0.001, cut-off 60) and hypertension (OR = 0.194, P = 0.020) adjusted for age, diabetes, ACEI-ARB use, CHADS-VASc and HAS-BLED score, HsCRP, LA size | 75 | 69 | ||
| Higuchi et al. 18 | 22 | 50 | 133± 17 | 121± 13 | Interatrial conduction time IACT > 123 (HR 2.23, P = 0.01) | 53 | 85 | ||
| Wu et al. 19 | 17 ± 8 | 29 | 121± 14 | 117± 14 | Resting heart rate (RHR) < 50 b.p.m. (HR 1.92, P = 0.02), aIAB (HR 1.82, P = 0.02), LAD (HR 1.05, P = 0.03), adjusted for PWD/CHADS score | ||||
| Nakatani et al. 20 | 12 | 22 | 124± 18 | 126± 17 | Coefficient of variation of PWD in 12 ECG leads as conduction heterogeneity index (AUC 70%, cut-off 0.080) | 68 | 66 | 36 | 98 |
| Chen et al. 21 | 20 ± 8 | 25 | – | – | PWD ≥ 120 ms (OR = 1.69, P = 0.02) adjusted for age, sex, LAD | ||||
| Zink et al. 22 | 3 | 24 | 129± 31 | 122± 22 | Prior stroke/TIA (HR 1.54, P = 0.11), CAD (HR 1.85, P = 0.005), CV during ablation (HR 1.78, P = 0.001), age*sex (HR 1.01, P = 0.04) | ||||
| Yang et al. 23 | 12 | 32 | 109± 14 | 104± 11 | Morphology–voltage–P-wave (MVP) score > 3 (AUC79%, P < 0.001) | 53 | 90 |
aIAB, advanced interatrial block; ACEI-ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blockers; AFr, atrial fibrillation recurrence; AUC, area under curve; CA, catheter ablation; CAD, coronary artery disease; CV, cardioversion; FUP, follow up in months; HR, hazard ratio; LA(D), left atrium (diameter); N/PPV, negative/positive predictive value (%); PWD, P-wave duration; SA, signal averaged; Sen., sensitivity (%); Spec., specificity (%); TIA, transient ischaemic attack.
Longer P-wave duration in patients with atrial fibrillation recurrence
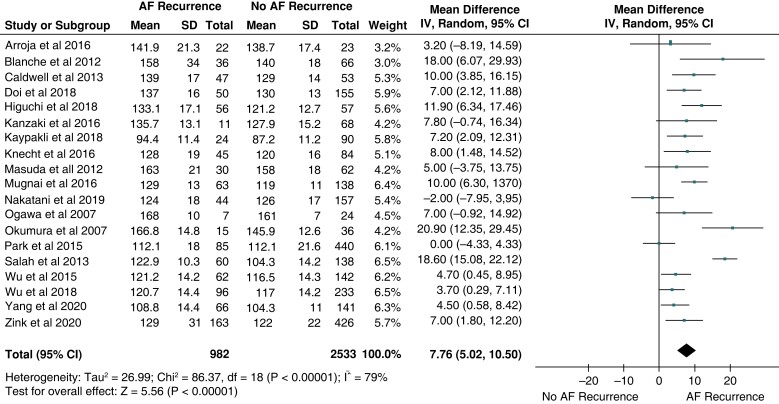
Most studies (n = 19) reported PWD in both patients with and without AFr.2–12,14,15,17–20,22,23 Patients with AFr had longer PWD with a mean pooled difference (ΔPWD) of 7.8 ms (P < 0.001, Figure 2). Subgroup analysis revealed that the ΔPWD remained significantly different in patients measured only with ECG (13 studies, ΔPWD 7.01 ms, P < 0.001) 6–10,14,15,17–20,22,23 or only with SAECG (6 studies, ΔPWD 10,22 ms, P < 0.001).2–5,11,12 The difference between ECG and SAECG studies was not statistically significant (P = 0.46). The mean ΔPWD remained significant when analysing studies with PAF only (10 studies, ΔPWD 6.6 ms, P = 0.004).4,6–9,11–14,16–21 A respective meta-analysis for persistent AF patients was not possible, since there was only one study reporting such results (ΔPWD 11.9 ms, P < 0.001).18Supplementary material online, Figure S1 depicts the respective Forrest plots for different recording methods (ECG, SAECG) and AF types.
Figure 2.
Baseline difference (mean ± SD) of PWD in patients with and without atrial fibrillation (AF) recurrence after catheter ablation. The forest plot shows the weighted pooled mean difference and 95%CI of baseline PWD between the two groups.
P-wave as atrial fibrillation recurrence predictor
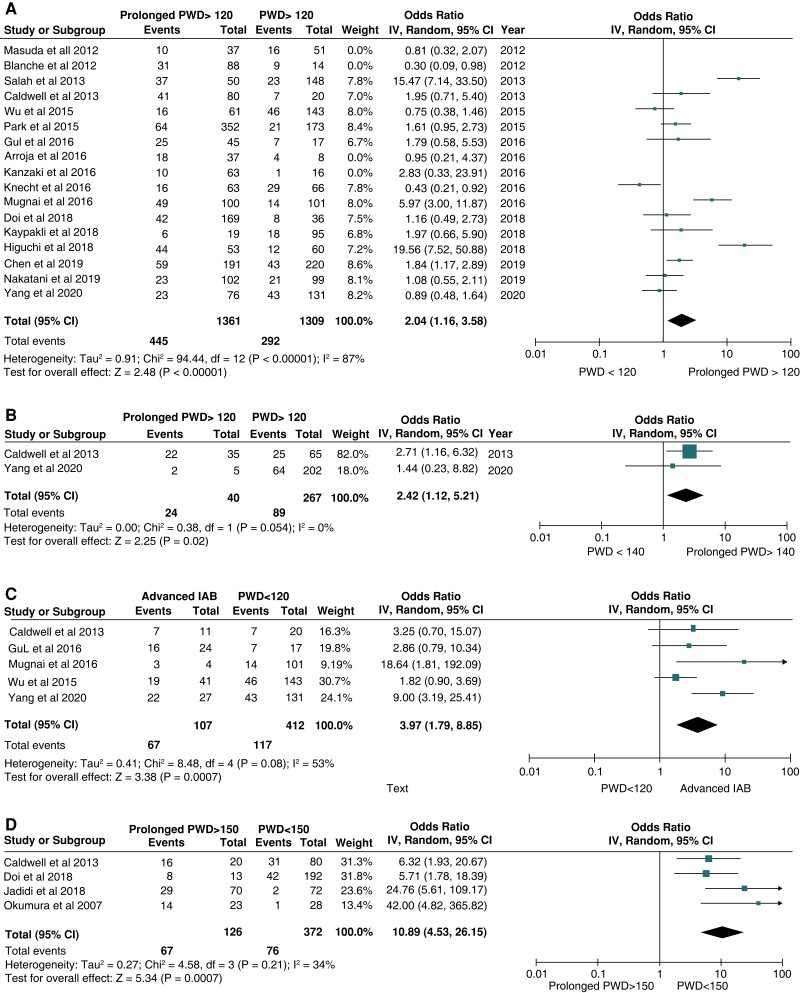
The association between PWD and the incidence of AFr was calculated after identifying the number of patients having specific PWD and AFr in each study. The pooled OR was 2.04 (1.16–3.58) for PWD > 120 ms (13 studies, P = 0.01),6–10,13–15,17–23 2.42 (1.12–5.21) for PWD > 140 ms (2 studies, P = 0.02),6,23 3.97 (1.79–8.85) for aIAB (5 studies, P < 0.001),6,13,14,19,23 and 10.89 (4.53–26.15) for PWD > 150 ms (4 studies, P < 0.001),3,6,15,16 revealing that the risk for AFr increased significantly from >120 to >130 and >140 and excited that of advanced IAB when the PWD was over >150 ms (Figure 3).
Figure 3.
Subgroup analysis demonstrates the effect of P-wave duration (PWD) for the prediction of atrial fibrillation recurrence (AFr) after catheter ablation in comparison with morphological characterization of advanced interatrial block (aIAB: PWD > 120 ms and biphasic P-wave in inferior leads). (A) Pooled odds ratio for PWD>120 ms. (B) Pooled odds ratio for PWD>140 ms. (C). Pooled odds ratio for aIAB. (D). Pooled odds ratio for PWD>150 ms.
Subgroup analysis showed that the predictive value of PWD > 120 ms was significant both for patients with paroxysmal (10 studies, OR: 2.2, P = 0.004)4,6–8,11,12,14,17,19,20 and persistent AF patients (OR: 19.6, P < 0.001).18Supplementary material online, Figure S2 depicts the Forrest plots for the subgroups of studies reporting only on pAF and persistent AF.
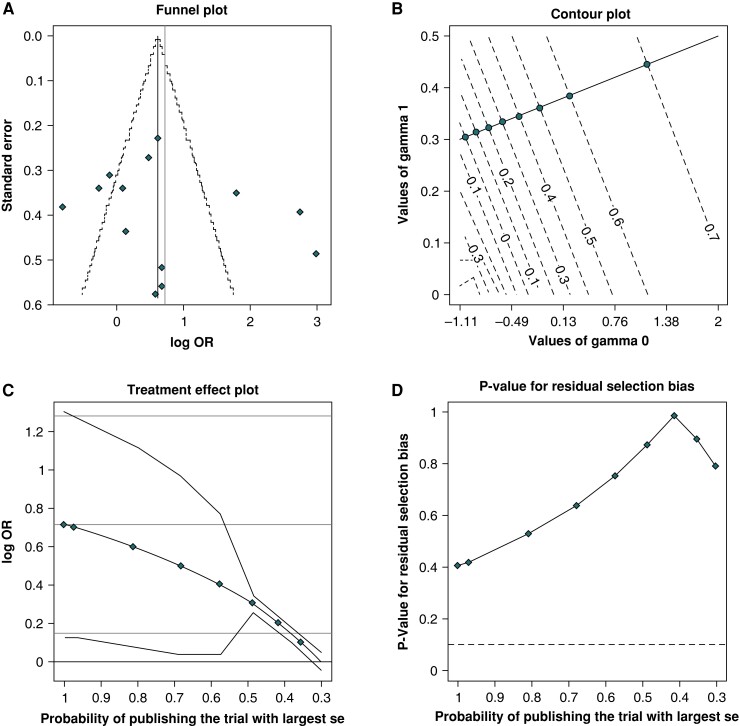
There was significant heterogeneity as revealed by the Higgins I2 statistics. Although visual inspection of the funnel plots suggested some asymmetry, Egger’s and Copas’ test revealed no evidence of publication bias and sensitivity analysis did not reveal a significant change in the results of the overall analysis (Figure 4).
Figure 4.
Copas test detected no publication bias with the (A) funnel plot, (B) contour plot, (C) treatment effect plot, or (D) residual selection bias plot.
Discussion
To our knowledge, this updated meta-analysis is the largest systematic collection and quantitative synthesis of 4.175 patients undergoing AF ablation. We revealed the strong predictive value of different pre-procedural PWD cut-offs for the recurrence of AF after CA. In specific, we found that a pre-procedural PWD > 120 ms (pIAB) doubles the risk for AFr during follow up. When this is combined with morphologic criteria (biphasic P wave in inferior leads), indicating an aIAB, the risk is four times higher. Most importantly though, further PWD prolongation to >150 ms leads to a 10 times higher risk of recurrence.
Identifying patients at risk for arrhythmia recurrence after AF ablation remains challenging. Several predictive models have been reported including clinical, anatomical, imaging, and serological characteristics. Common predictors are LA size, AF type, age, female sex, and to a lesser extent estimated glomerular filtration rate or biomarkers such as B-type natriuretic peptide. However, these models have a highly variable discriminatory ability (c-statistic) and do not characterize accurately the individual structural and electrical atrial remodelling.24 This is better assessed with pre-procedural imaging (e.g. cardiac MRI with late gadolinium enhancement) or intra-procedural mapping (low-voltage areas). These methods can depict anatomical changes and the surrogate presence of fibrosis, which are associated with higher recurrence risk, but are costly and not readily available for pre-procedural planning. Thus, in the clinical setting, there is a need for a feasible low-cost surrogate of recurrence risk that could potentially improve patient selection and translate into cost savings by avoiding unnecessary procedures. The present analysis reveals the practical implications of PWD and IAB by describing their predictive value.
P-wave duration and interatrial block
Electrocardiography is a simple and widely available tool that can predict the risk for AFr by evaluation of the PWD and its characteristics.25,26 P-wave changes have been associated with conduction changes and fibro-fatty replacement in histological studies.27 More specifically, this conduction delay at the Bachmann bundle level has been defined as pIAB or aIAB (Graphical Abstract). This results in atrial remodelling and asynchronous LA contraction,28,29 but can also appear without LA enlargement as a surrogate of AF substrate.1 In fact, aIAB has been described as a separate clinical entity, called ‘Bayes’ syndrome’, that has been associated with AF or other atrial arrhythmias and an increased stroke, dementia and mortality risk.1
Chen et al.21 reported that PWD was an independent predictor of atrial scarring, even after adjusting for age, sex, and LA diameter. Moreover, non-predictive value triggers were more common in patients with scarring, putting them at greater risk of recurrence. This is in agreement with the results of the study by Mugnai et al.,14 who found that PWD and dispersion were independent predictors of recurrence in patients with non-dilated left atria. In other words, electrical remodelling often precedes apparent structural changes and patients with normal dimensioned left atria and PAF should undergo AF ablation early, to prevent further electromechanical deterioration.
On the other hand, patients with persistent AF and advanced remodelling are more prone to AFr after CA. Jadidi et al.16 found that an amplified PWD of >150 ms signifies extended LA scar with high sensitivity and specificity. P-wave duration in these patients was the only independent AFr predictor, even after adjusting for known confounders like age, sex, LA diameter, structural cardiomyopathies, hypertension, and antiarrhythmic drugs. Consequently, as shown in our analysis, AFr risk in patients with PWD > 120 ms and persistent AF was almost 10 times higher than those with PAF (see Supplementary material online, Figure S1).
In support of these findings, we found an exponential dose–response effect between the PWD and AFr risk. While pre-procedural PWD > 120 ms (pIAB) doubles the risk for AFr (OR ∼2.0), this is slightly higher for PWD > 140 ms (OR ∼2.4) and much higher for aIAB (OR ∼4.0) and PWD of >150 ms (OR ∼10.9). Interestingly, the method of recording did not influence the outcomes. The mean PWD difference was similar whether measured by ECG or SAECG (P = 0.46). Thus, while averaging, filtering and amplifying the electrical signal of the P-wave offers more accurate measurements, practically the SAECG does not improve the predictive value of PWD. Similarly, the mean PWD difference was similar in patients with paroxysmal and persistent AF (P = 0.365). Therefore, these cut-offs could facilitate patient selection for additional substrate ablation, for patients with earlier stages of fibrosis, or alternative strategies for patients with advanced stages, as in the DECAAF II study. Given the insight that ablation is not as effective in scar, we should evaluate new approaches in such patients. Our findings for example could help select those with high scar or recurrence risk and prospectively randomize them to LA ablation (radiofrequency or pulsed field ablation) or a ‘pace and ablate’ (AV junction) strategy.
The association of PWD with AFr after ablation has also been evaluated in a recent meta-analysis of 1482 patients conducted by Pranata et al.26 The association was significant in SAECG, ECG, and PAF subgroups as well in both genders and all age groups, with or without structural heart disease. These results supplemented those from an earlier analysis of 1010 patients by Wang et al.25 and a meta-analysis of 2587 patients by Tse et al.,30 both of which did not specify the predictive value of different cut-offs, as in our analysis. The first one included only eight studies, while the later one focused more on new-onset AF and included three studies regarding AFr.30 Our findings derive from a significantly larger population and provide for the first time practical insights for different PWD cut-offs, which should be further evaluated in prospective studies.
Other P-wave characteristics
There are also several other P-wave indices that have been evaluated as predictors of AFr and in some studies overweighed the PWD. The PWD index, defined as the ratio of PWD to the PR interval in Lead II, has been described as a way to overcome the effects of the autonomic nerve system and was found to be an independent AFr predictor.17 The PWD dispersion (max–min value > 45 ms) has also shown very good discriminative predictive value in a study by Mugnai et al.14 but failed to reach significance in the study by Caldwell et al.6 A later study by Nakatani et al.20 though argued that the coefficient of variation for the PWD (>0.08), calculated by dividing the standard deviation by the mean PWD value, has the highest predictive accuracy among P-wave parameters in predicting AFr in PAF patients. Finally, the combination of PWD with other characteristics like in the morphology–voltage–PWD (MVP) score has also shown good predictive accuracy. The MVP assigns 0–2 points for each of the following factors: morphology in inferior leads, voltage in Lead 1 and PWD. Yang et al.23 found that an MVP >3 has the best predictive ability for AFr (c-statistics 0.789), but this index requires additional measurements of low-amplitude P-waves in Lead I and was not directly compared with PWD alone.
The P-wave terminal force in lead V1 (PTWFV1 > 0.04 mm*s), calculated by multiplying the duration and the amplitude of deep terminal negativity of the P-wave (prime) in Lead V1, was also found to be strongly correlated with LA enlargement and the risk of AF occurrence.31 However, in the study by Doi et al.,15 PWTFV1 did not overweight the predictive value of PWD for AFr. Kanzaki et al.12 came to a similar conclusion, with SAECG and P-wave force (the amplitude of the negative terminal phase multiplied by the filtered PWD) values >9.3 mV*ms becoming significant only when measured acutely post-procedurally. Masuda et al.4 found a simpler SAECG marker; the atrial late potential, defined as PWD ≥ 130 ms and a terminal root mean-squared voltage ≤2.0 mV, which was associated with AFr in PAF patients (OR = 4.2). Park et al.8 though proposed an easier approach and found the P-wave amplitude in Lead I (<0.1 mV) to be independently associated with AFr and linearly correlated with LA voltage and conduction velocity. The recent consensus document about P-wave parameters and indices provides a further in-depth analysis that underlines the importance of this topic.32 Taken together, these studies reveal a paucity of methods to approach P-wave morphology. Nevertheless, PWD and IAB have a higher practical value, since they are easily identifiable and simple to use and report in the majority of the studies.18
Clinical implications
Our study has shown that the OR for AFr after CA increased exponentially from 2 for PWD > 120 ms to 2.4 for PWD > 140 ms, then 4 for aIAB and 10 for PWD > 150 ms. We reviewed the evidence connecting PWD with atrial fibrosis and suggest that the considerations of this simple measurement are far more practical than other complex P-wave indices. These specific PWD cut-off limits could be used as a surrogate marker of fibrosis to better stratify patients into different treatments, leading them to ablation, when the risk of recurrence is acceptable or examining alternatives, when signs of advanced fibrosis are present. Accordingly, patients with prolonged PWD should have a closer follow-up strategy. The present findings emphasize the clinical importance of evaluating PWD prior to CA for AF and deserve further investigation.
Limitations
The variation in population characteristics, measurements, ablation techniques (radiofrequency or cryo-ablation), or strategies, endpoints and follow up has contributed to high heterogeneity (I2 = 87% for PWD > 120 ms). This was due to the widely inclusive selection criteria and was reduced as patient characteristics converged through selection (prolonged PWD). However, we used random-effects models and performed subgroup and sensitivity analysis to analyse and eliminate this heterogeneity. Additionally, the included studies had high quality according to the Newcastle–Ottawa scale (≥7 points). Although no study reported on intra- or inter-observer variability for PWD measurement, our findings were consistent and significant, regardless of the measurement method. Although the increased OR of the group with PWD >150 ms could be partially explained by older studies or selection of sicker persistent AF patients, the concurrent results, even by inclusion of only recent studies, designate an exponential relationship that has also been seen between PWD and new-onset AF. Due to limited data, comparison of the predictive value of PWD with that of LA size was not possible. Nevertheless, we found no evidence of publication bias and we quantified for the first time the prognostic value of PWD for different cut-offs and IAB definitions.
Conclusion
In this updated meta-analysis of 4175 patients, PWD was found to be an independent predictor of AFr after CA. This risk is increasing exponentially with PWD prolongation. Thus, it could facilitate risk stratification by identifying high-risk patients (aIAB, PWD > 150 ms) and adjusting follow up or interventions.
Supplementary Material
Contributor Information
Stergios Intzes, Democritus University of Thrace, Medical School, Alexandroupoli, Greece.
Konstantinos Zagoridis, Democritus University of Thrace, Medical School, Alexandroupoli, Greece.
Marianthi Symeonidou, Democritus University of Thrace, Medical School, Alexandroupoli, Greece.
Emmanouil Spanoudakis, Democritus University of Thrace, Medical School, Alexandroupoli, Greece.
Arash Arya, Department of Electrophysiology, Heart Center, University of Leipzig, Struempellstr. 39, 04289 Leipzig, Germany.
Borislav Dinov, Department of Electrophysiology, Heart Center, University of Leipzig, Struempellstr. 39, 04289 Leipzig, Germany.
Nikolaos Dagres, Department of Electrophysiology, Heart Center, University of Leipzig, Struempellstr. 39, 04289 Leipzig, Germany.
Gerhard Hindricks, Department of Electrophysiology, Heart Center, University of Leipzig, Struempellstr. 39, 04289 Leipzig, Germany.
Andreas Bollmann, Department of Electrophysiology, Heart Center, University of Leipzig, Struempellstr. 39, 04289 Leipzig, Germany.
Emmanuel Kanoupakis, Department of Cardiology, Heraklion University Hospital, Crete, Greece.
Emmanuel Koutalas, Department of Cardiology, Heraklion University Hospital, Crete, Greece.
Sotirios Nedios, Department of Electrophysiology, Heart Center, University of Leipzig, Struempellstr. 39, 04289 Leipzig, Germany.
Supplementary material
Supplementary material is available at Europace online.
Authors’ contribution
S.I., K.Z., M.S., E. Kout., S.N.: conceptualization, investigation, methodology, data analysis, visualization, writing. E.S., A.A., N.D., G.H., A.B., E. Kan., S.N.: validation, supervision, writing—review and editing.
Funding
None declared.
Data availability
Data are available upon request from the corresponding author.
References
- 1. Bayes de Luna A, Platonov P, Cosio FG, Cygankiewicz I, Pastore C, Baranowski Ret al. Interatrial blocks. A separate entity from left atrial enlargement: a consensus report. J Electrocardiol 2012;45:445–51. [DOI] [PubMed] [Google Scholar]
- 2. Ogawa M, Kumagai K, Vakulenko M, Yasuda T, Siegerman C, Garfinkel Aet al. Reduction of P-wave duration and successful pulmonary vein isolation in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2007;18:931–8. [DOI] [PubMed] [Google Scholar]
- 3. Okumura Y, Watanabe I, Ohkubo K, Ashino S, Kofune M, Hashimoto Ket al. Prediction of the efficacy of pulmonary vein isolation for the treatment of atrial fibrillation by the signal-averaged P-wave duration. Pacing Clin Electrophysiol PACE 2007;30:304–13. [DOI] [PubMed] [Google Scholar]
- 4. Masuda M, Inoue K, Iwakura K, Okamura A, Toyoshima Y, Doi Aet al. Impact of pulmonary vein isolation on atrial late potentials: association with the recurrence of atrial fibrillation. Europace 2013;15:501–7. [DOI] [PubMed] [Google Scholar]
- 5. Blanche C, Tran N, Rigamonti F, Burri H, Zimmermann M. Value of P-wave signal averaging to predict atrial fibrillation recurrences after pulmonary vein isolation. Europace 2013;15:198–204. [DOI] [PubMed] [Google Scholar]
- 6. Caldwell J, Koppikar S, Barake W, Redfearn D, Michael K, Simpson Cet al. Prolonged P-wave duration is associated with atrial fibrillation recurrence after successful pulmonary vein isolation for paroxysmal atrial fibrillation. J Interv Card Electrophysiol 2014;39:131–8. [DOI] [PubMed] [Google Scholar]
- 7. Salah A, Zhou S, Liu Q, Yan H. P wave indices to predict atrial fibrillation recurrences post pulmonary vein isolation. Arq Bras Cardiol 2013;101:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park JK, Park J, Uhm JS, Joung B, Lee MH, Pak HN. Low P-wave amplitude (<0.1 mV) in lead I is associated with displaced inter-atrial conduction and clinical recurrence of paroxysmal atrial fibrillation after radiofrequency catheter ablation. Europace 2016;18:384–91. [DOI] [PubMed] [Google Scholar]
- 9. Wu JT, Long DY, Dong JZ, Wang SL, Fan XW, Yang HTet al. Advanced interatrial block predicts clinical recurrence of atrial fibrillation after catheter ablation. J Cardiol 2016;68:352–6. [DOI] [PubMed] [Google Scholar]
- 10. Knecht S, Pradella M, Reichlin T, Muhl A, Bossard M, Stieltjes Bet al. Left atrial anatomy, atrial fibrillation burden, and P-wave duration-relationships and predictors for single-procedure success after pulmonary vein isolation. Europace 2018;20:271–8. [DOI] [PubMed] [Google Scholar]
- 11. Arroja JD, Burri H, Park CI, Giraudet P, Zimmermann M. Electrophysiological abnormalities in patients with paroxysmal atrial fibrillation in the absence of overt structural heart disease. Indian Pacing Electrophysiol J 2016;16:152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kanzaki Y, Inden Y, Ando M, Kamikubo Y, Ito T, Mizutani Yet al. An ECG index of P-wave force predicts the recurrence of atrial fibrillation after pulmonary vein isolation. Pacing Clin Electrophysiol PACE 2016;39:1191–7. [DOI] [PubMed] [Google Scholar]
- 13. Gul EE, Pal R, Caldwell J, Boles U, Hopman W, Glover Bet al. Interatrial block and interatrial septal thickness in patients with paroxysmal atrial fibrillation undergoing catheter ablation: long-term follow-up study. Ann Noninvasive Electrocardiol 2017;22:e12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mugnai G, Chierchia GB, de Asmundis C, Julia J, Conte G, Sieira-Moret Jet al. P-wave indices as predictors of atrial fibrillation recurrence after pulmonary vein isolation in normal left atrial size. J Cardiovasc Med 2016;17:194–200. [DOI] [PubMed] [Google Scholar]
- 15. Doi A, Takagi M, Katayama H, Yoshiyama T, Hayashi Y, Tatsumi Het al. Diagnostic value of electrocardiographic P-wave characteristics in atrial fibrillation recurrence and tachycardia-induced cardiomyopathy after catheter ablation. Heart Vessels 2018;33:1381–9. [DOI] [PubMed] [Google Scholar]
- 16. Jadidi A, Muller-Edenborn B, Chen J, Keyl C, Weber R, Allgeier Jet al. The duration of the amplified Sinus-P-wave identifies presence of left atrial low voltage substrate and predicts outcome after pulmonary vein isolation in patients with persistent atrial fibrillation. JACC Clin Electrophysiol 2018;4:531–43. [DOI] [PubMed] [Google Scholar]
- 17. Kaypakli O, Koca H, Sahin DY, Okar S, Karatas F, Koc M. Association of P wave duration index with atrial fibrillation recurrence after cryoballoon catheter ablation. J Electrocardiol 2018;51:182–7. [DOI] [PubMed] [Google Scholar]
- 18. Higuchi S, Ejima K, Shoda M, Yamamoto E, Iwanami Y, Yagishita Det al. Impact of a prolonged interatrial conduction time for predicting the recurrence of atrial fibrillation after circumferential pulmonary vein isolation of persistent atrial fibrillation. Heart Vessels 2019;34:616–24. [DOI] [PubMed] [Google Scholar]
- 19. Wu J, Fan X, Yang H, Yan L, Xu X, Duan Het al. Usefulness of a low resting heart rate to predict recurrence of atrial fibrillation after catheter ablation in people ≥65 years of age. Am J Cardiol 2018;122:97–101. [DOI] [PubMed] [Google Scholar]
- 20. Nakatani Y, Sakamoto T, Yamaguchi Y, Tsujino Y, Kataoka N, Kinugawa K. Coefficient of variation of P-wave duration measured using an automated measurement system predicts recurrence of atrial fibrillation. J Electrocardiol 2019;53:79–84. [DOI] [PubMed] [Google Scholar]
- 21. Chen Q, Mohanty S, Trivedi C, Gianni C, Della Rocca DG, Canpolat Uet al. Association between prolonged P wave duration and left atrial scarring in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2019;30:1811–8. [DOI] [PubMed] [Google Scholar]
- 22. Zink MD, Chua W, Zeemering S, di Biase L, Antoni BL, David Cet al. Predictors of recurrence of atrial fibrillation within the first 3 months after ablation. Europace 2020;22:1337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang N, Yan N, Cong G, Yang Z, Wang M, Jia S. Usefulness of morphology-voltage-P-wave duration (MVP) score as a predictor of atrial fibrillation recurrence after pulmonaryveinisolation. Ann Noninvasive Electrocardiol 2020;25:e12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dretzke J, Chuchu N, Agarwal R, Herd C, Chua W, Fabritz Let al. Predicting recurrent atrial fibrillation after catheter ablation: a systematic review of prognostic models. Europace 2020;22:748–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang YS, Chen GY, Li XH, Zhou X, Li YG. Prolonged P-wave duration is associated with atrial fibrillation recurrence after radiofrequency catheter ablation: a systematic review and meta-analysis. Int J Cardiol 2017;227:355–9. [DOI] [PubMed] [Google Scholar]
- 26. Pranata R, Yonas E, Vania R. Prolonged P-wave duration in sinus rhythm pre-ablation is associated with atrial fibrillation recurrence after pulmonary vein isolation – a systematic review and meta-analysis. Ann Noninvasive Electrocardiol 2019;24:e12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huo Y, Mitrofanova L, Orshanskaya V, Holmberg P, Holmqvist F, Platonov PG. P-wave characteristics and histological atrial abnormality. J Electrocardiol 2014;47:275–80. [DOI] [PubMed] [Google Scholar]
- 28. Nedios S, Lobe S, Knopp H, Seewoster T, Heijman J, Crijns Het al. Left atrial activation and asymmetric anatomical remodeling in patients with atrial fibrillation: the relation between anatomy and function. Clin Cardiol 2021;44:116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dinov B, Knopp H, Lobe S, Nedios S, Bode K, Schonbauer Ret al. Patterns of left atrial activation and evaluation of atrial dyssynchrony in patients with atrial fibrillation and normal controls: factors beyond the left atrial dimensions. Heart rhythm 2016;13:1829–36. [DOI] [PubMed] [Google Scholar]
- 30. Tse G, Wong CW, Gong M, Wong WT, Bazoukis G, Wong SHet al. Predictive value of inter-atrial block for new onset or recurrent atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol 2018;250:152–6. [DOI] [PubMed] [Google Scholar]
- 31. Huang Z, Zheng Z, Wu B, Tang L, Xie X, Dong Ret al. Predictive value of P wave terminal force in lead V1 for atrial fibrillation: a meta-analysis. Ann Noninvasive Electrocardiol 2020;25:e12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen LY, Ribeiro ALP, Platonov PG, Cygankiewicz I, Soliman EZ, Gorenek Bet al. P wave parameters and indices: a critical appraisal of clinical utility, challenges, and future research – a consensus document endorsed by the International Society of Electrocardiology and the International Society for Holter and Noninvasive Electrocardiology. Circ Arrhythmia Electrophysiol 2022;15:e010435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request from the corresponding author.